Abstract
Background
The formal risk assessment is essential in the management of acute coronary syndrome (ACS). In this study, we develop a risk model for the prediction of 3-year mortality for Chinese ACS patients with machine learning algorithms.
Methods
A total of 2174 consecutive patients who underwent angiography with ACS were enrolled. The missing data among baseline characteristics were imputed using the MissForest algorithm based on random forest method. In model development, a least absolute shrinkage and selection operator (LASSO) derived Cox regression with internal tenfold cross-validation was used to identify the predictors for 3-year mortality. The clinical performance was assessed with decision curve analysis.
Results
The average follow-up period was 27.82 ± 13.73 months; during the 3 years of follow up, 193 patients died (mortality rate 8.88%). The Kaplan–Meier estimate of 3-year mortality was 0.91 (95% confidence interval (CI): 0.890.92). After feature selection, 6 predictors were identified: Age,” “Creatinine,” “Hemoglobin,” “Platelets,” “aspartate transaminase (AST)” and “left ventricular ejection fraction (LVEF)”. At tenfold internal validation, our risk model performed well in both discrimination (area under curve (AUC) of receiver operating characteristic (ROC) analysis was 0.768) and calibration (calibration slope was approximately 0.711). As a comparison, the AUC and calibration slope were 0.701 and 0.203 in Global Registry of Acute Coronary Events (GRACE) risk score, respectively. Additionally, the highest net benefit of our model within the entire range of threshold probability for clinical intervention by decision curve analysis demonstrated the superiority of it in daily practice.
Conclusion
Our study developed a prediction model for 3-year morality in Chinese ACS patients. The methods of missing data imputation and model derivation base on machine learning algorithms improved the ability of prediction. .
Trial registration ChiCTR, ChiCTR-OOC-17010433. Registered 17 February 2017–Retrospectively registered
Keywords: Acute coronary syndrome, Risk model, Machine learning, LASSO, Random forest
Introduction
As the unstable and progressive stage of coronary heart disease (CHD), acute coronary syndrome (ACS) includes three serious and life-threating clinical manifestations: ST-segment elevation myocardial infarction (STEMI), non-STEMI, and unstable angina pectoris [1, 2]. The prognosis of ACS patients varies considerably for different pathophysiological changes in individuals based on their level of disease. Thus, a formal assessment to identify high-risk patients is essential in the management of ACS [3].
Currently, the Global Registry of Acute Coronary Events (GRACE) is the most commonly used risk assessment tool and is recommended by guidelines for predicting short- and long-term mortality [4]. However, the GRACE risk score was developed in North America, South America, and Europe but included few participants in Asia [5]. The clinical performance of this risk score has not been assessed in the Chinese population. A risk tool derived from Clinical Pathways for Acute4 Coronary Syndromes (CPACS) investigators for Chinese patients with ACS has been described previously [6]. However, this CPACS risk score only predicted hospital mortality and did not use algorithms to avoid overfitting in the model estimation. Therefore, we aim to develop a specific risk model for the prediction of long-term (3-year) mortality for Chinese ACS patients in a hospital-based dataset.
Results
Study population and outcomes
From January 2009 to September 2012, a total of 2174 ACS patients were included in this study. The average follow-up period was 27.82 ± 13.73 months; during the 3 years of follow up, 193 patients died (mortality rate 8.88%), including 121 cases of cardiac death (cardiac mortality was 5.57%) and 55 cases of non-cardiovascular death. 31 patients were categorized into unknown death. The Kaplan–Meier estimate of 3-year mortality was 0.91 (95% confidence interval (CI) 0.890.92). The baseline characteristics of this study population were stratified according to patients who survived until the end of the follow-up period and those who did not survive. The mean age of the patients was 64.54 ± 10.57 years, and the number of male patients was 1713 (78.79%). More than half of patients had hypertension (1183, 54.64%) and one in five had diabetes (470, 21.72%) or previous myocardial infarction (379, 23.9%). Meanwhile, the higher percentage of usage of evidence-based medications were found in survival group, including aspirin, clopidogrel, beta-blockers, angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers and statins. The difference between two groups is summarized in Table 1.
Table 1.
Clinical characteristics of the study population
| Characteristics | Total | Patients survived | Patients died | P Value |
|---|---|---|---|---|
| No. of patients | N = 2174 | N = 1981 | N = 193 | |
| Age | 64.54 ± 10.57 | 63.96 ± 10.54 | 70.54 ± 8.83 | <0.001 |
| Gender, man, n (%) | 1713 (78.79%) | 1572 (79.35) | 141 (73.06) | 0.041 |
| Medical history | ||||
| Pre-hypertension, n (%) | 1183 (54.64) | 1072 (54.31) | 111 (58.12) | 0.313 |
| Pre-diabetes mellitus, n (%) | 470 (21.72) | 412 (20.88) | 58 (30.37) | 0.002 |
| Pre-heart failure, n (%) | 83 (5.3) | 60 (4.3) | 23 (12.2) | <0.001 |
| Pre-myocardial infarction, n (%) | 379 (23.9) | 329 (23.6) | 50 (26.3) | 0.475 |
| At admission | ||||
| HR, beats/min | 74.99 ± 26.09 | 74.51 ± 26.69 | 79.96 ± 18.05 | <0.001 |
| SBP, mm Hg | 130.51 ± 22.1 | 130.68 ± 21.52 | 128.79 ± 27.43 | 0.263 |
| DBP, mm Hg | 76.29 ± 12.8 | 76.52 ± 12.64 | 73.88 ± 14.11 | <0.001 |
| LVEF, % | 50.52 ± 24.62 | 50.55 ± 24.95 | 50.22 ± 19.41 | 0.896 |
| Risk assessment | ||||
| GRACE risk score | 92.95 ± 26.1 | 91.35 ± 25.64 | 109.44 ± 25.2 | <0.001 |
| TIMI classification | 4.58 ± 2.03 | 4.39 ± 1.94 | 5.94 ± 2.20 | <0.001 |
| Laboratory values | ||||
| Serum creatinine, μmol/L | 94.44 ± 51.3 | 92.69 ± 47.87 | 112.37 ± 76 | <0.001 |
| Blood glucose, mmol/L | 7.17 ± 3.3 | 7.03 ± 3.11 | 8.59 ± 4.6 | <0.001 |
| Total cholesterol, mmol/L | 4.13 ± 1.11 | 4.13 ± 1.1 | 4.09 ± 1.2 | 0.602 |
| WBC, n × 109/L | 7.92 ± 5.66 | 7.79 ± 5.75 | 9.23 ± 4.43 | <0.001 |
| RBC, n × 1012/L | 4.45 ± 1.11 | 4.47 ± 1.15 | 4.16 ± 0.65 | <0.001 |
| Hemoglobin, g/L | 134.22 ± 35.39 | 135.17 ± 36.42 | 124.42 ± 19.89 | <0.001 |
| Platelets, n × 109/L | 162.28 ± 61.05 | 161.79 ± 60.75 | 167.33 ± 63.95 | 0.229 |
| AST, U/L | 56.91 ± 102.95 | 52.55 ± 81.82 | 102.8 ± 222.7 | <0.001 |
| Serum K+, mmol/L | 3.96 ± 0.47 | 3.96 ± 0.45 | 4 ± 0.62 | 0.261 |
| Serum Ca2+, mmol/L | 2.32 ± 4.4 | 2.34 ± 4.61 | 2.16 ± 0.18 | 0.619 |
| Fibrinogen, g/L | 3.83 ± 11.22 | 3.8 ± 11.46 | 4.18 ± 8.23 | 0.679 |
| Discharge medications | ||||
| Aspirin, n (%) | 2005 (94.18%) | 1865 (96.68%) | 140 (70.0%) | <0.001 |
| Clopidogrel, n (%) | 2002 (94.03%) | 1854 (96.11%) | 148 (74.0%) | <0.001 |
| ACEI/ARBs, n (%) | 1228 (57.73%) | 1140 (59.16%) | 88 (44.0%) | <0.001 |
| Beta-blockers, n (%) | 1434 (67.45%) | 1340 (69.57%) | 94 (47.0%) | <0.001 |
| Statins, n (%) | 1949 (91.55%) | 1807 (93.68%) | 142 (71.0%) | <0.001 |
Data are expressed as mean ± SD or counts and percentages, as appropriate
HR heart rate, SBP systolic blood pressures, DBP diastolic blood pressure, LVEF left ventricular ejection fraction, GRACE Global Registry of Acute Coronary Events, TIMI thrombolysis in myocardial infarction, WBC white blood cell, RBC red blood cell, AST aspartate transaminase, ACEI angiotensin-converting-enzyme inhibitors, ARB angiotensin II receptor blockers
The Killip classifications were excluded as predictors in the model because of a large amount of missing data and the difficulty of conducting accurate measurements. The details of missing data among baseline characteristics are listed in Additional file 1.
Model derivation
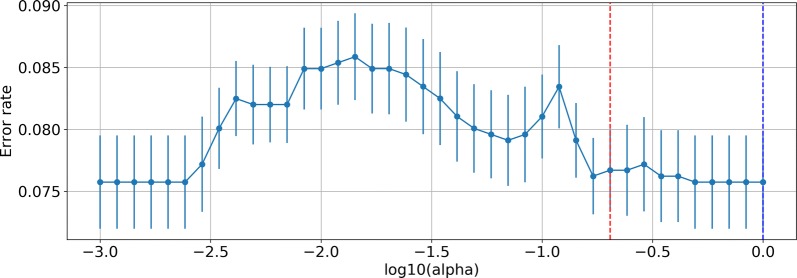
First, we conducted Cox regression with the least absolute shrinkage and selection operator (LASSO) penalization to perform predictor selection, which can help reduce the dimensions of a prediction model. To determine the penalty factor (lambda), a tenfold cross-validated error plot of the lasso model was constructed as shown in Fig. 1. The optimal lambda was determined by choosing the most regularized and parsimonious model within 1 standard error from the minimum.
Fig. 1.
10-fold cross-validated error plot: The blue dot line equals lambda with the minimum error, whereas the red dot line is the lambda we manually choose
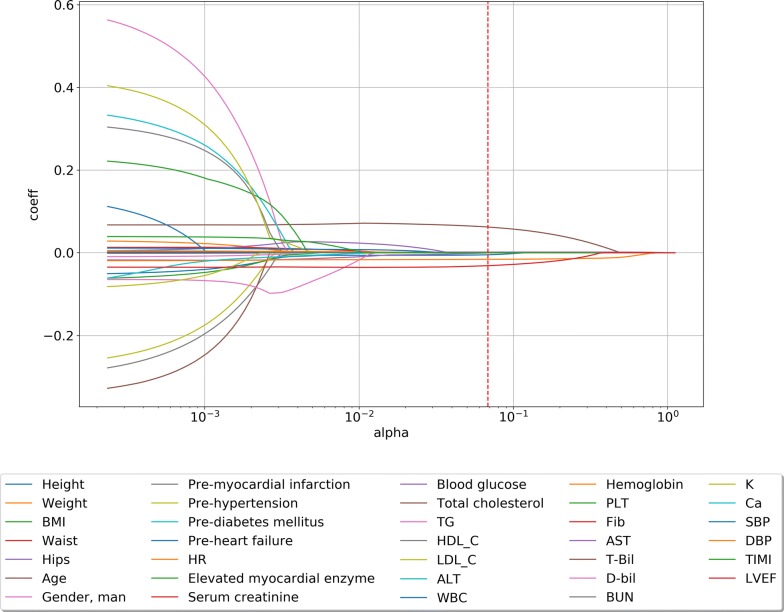
Because of the imbalance in our data, even the most parsimonious model with 0 characters was less than 7.7%, and that model was also within 1 standard error. To balance the power of the lasso penalty and the accuracy of our model, after some experiments with different lambdas, we manually choose a proper lambda that is still within 1 standard error and provided good results. The lambda is shown in Fig. 1. The LASSO path of all coefficients of predictors at varying log-transformed lambda values is shown in Fig. 2. We added the thrombolysis in myocardial infarction (TIMI) classification in predictor selection just as reference.
Fig. 2.
LASSO path of all coefficients of predictors at varying log-transformed lambda values: The red dot line is the lambda we manually choose. LASSO least absolute shrinkage and selection operator, BMI body mass index, HR heart rate, SBP systolic blood pressures, DBP diastolic blood pressure, LVEF left ventricular ejection fraction, WBC white blood cell, RBC red blood cell, AST aspartate transaminase, ALT alanine transaminase, BUN blood urea nitrogen, T-Bil total bilirubin, D-Bil direct bilirubin, HDL-C high-density lipoprotein cholesterol, LDL-C low density lipoprotein cholesterol, TG triglyceride, PLT platelets, Fib Fibrinogen, TIMI thrombolysis in myocardial infarction
The final LASSO model with the optimal lambda included the following 6 non-zero variables: “Age,” “Serum creatinine,” “Hemoglobin,” “Platelets,” “aspartate transaminase (AST),” and “left ventricular ejection fraction (LVEF)”. After we determined the most important predictors, the prediction model was developed using normal Cox regression without penalization. Under the proportional hazard assumption, the baseline hazard function can be estimated by Breslow’s Estimator. The formula for predicting the risk of 3-year mortality is as follows:
where We created an Excel file of this formula to favor workability in daily practice. Additionally, the use of this predictive model was demonstrated in 5 patients from this study population (Additional file 2).
Model validation
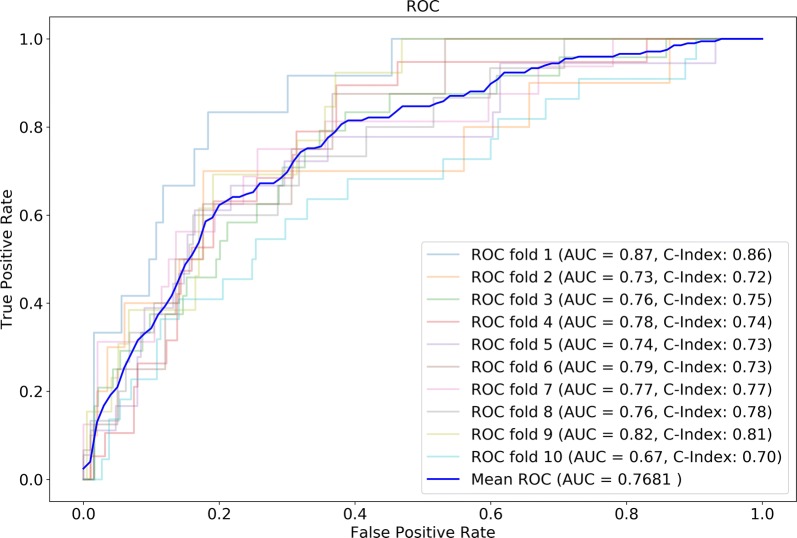
The discrimination by tenfold cross-validation and receiver operating characteristic curve (ROC) analysis result of our risk model for the prediction of 3-year mortality was good (area under curve (AUC) = 0.7681) (Fig. 3). However, the GRACE score had relatively poor power in the discriminative ability (AUC = 0.709). The Harrell’s C-statistic was 0.7601 for this risk model. We found good agreement between the predicted and observed 3-year risk of mortality. The calibration slope was approximately 0.711, as calculated by linear least-squares regression of the given points in the calibration plot (Fig. 4). The calibration slope for the GRACE score was 0.203. Thus, our model exhibited better calibration than the GRACE score.
Fig. 3.
Tenfold cross-validation and ROC analysis result of our model for the prediction of 3-year mortality. ROC receiver operating characteristic curve
Fig. 4.
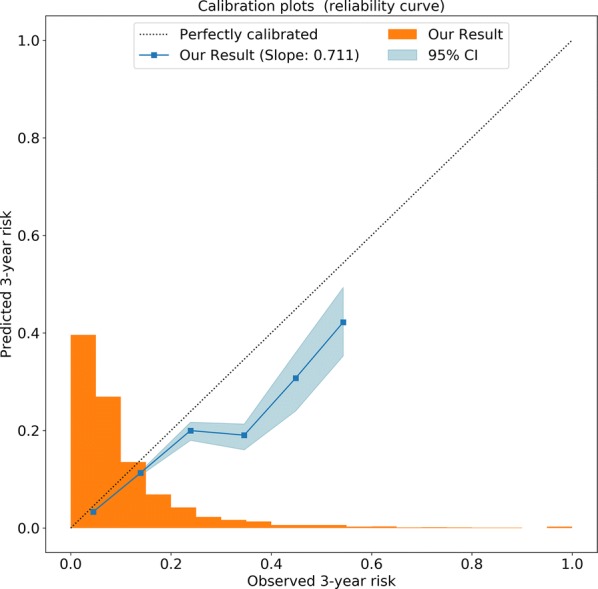
Calibration plot: Calibration plot showing the agreement between predicted (x-axis) and observed (y-axis) 3-year risk of the mortality. Squares represent binned Kaplan–Meier estimates with 95% confidence filled with the blue area. The dotted line represents perfect calibration. The bar histogram on the x-axis reflects the percentage of patients with a predicted risk corresponding to the x-axis
Clinical performance
We performed a decision curve analysis to compare the clinical utility of our risk model and the GRACE score. Because all of the treatments for ACS, including percutaneous coronary intervention (PCI) and thrombolysis, involve some harm for patients, the optimal decision threshold was > 0%. We observed the highest net benefit of our model within the entire range of threshold probability for clinical intervention (Fig. 5). This indicates the superiority of our risk model in clinical performance, regardless of the risk threshold for PCI or thrombolysis.
Fig. 5.
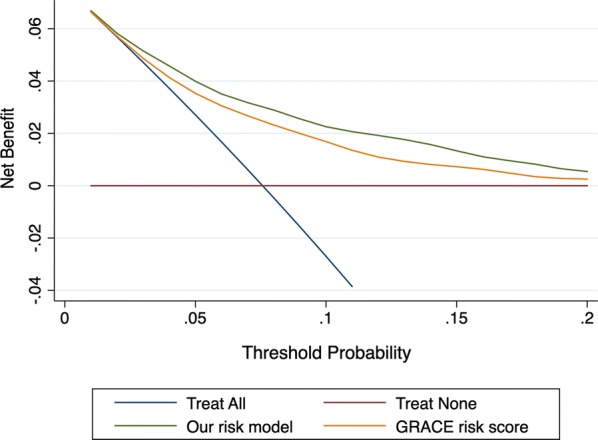
Decision curve analysis: Decision curve analysis comparing the clinical performance of our risk model (the green line) and the GRACE risk score (the yellow line). For risk of 3-year mortality, our risk model showed the highest net benefit for all potential thresholds (ranging from 0% to 20%). This demonstrated that our model would result in the highest weighted balance of clinical intervention for ACS patients, regardless of the risk threshold. ACS: acute coronary syndrome, GRACE: Global Registry of Acute Coronary Events
Discussion
In this study, we developed a risk model to predict the long-term mortality in Chinese ACS patients and performed internal validation of this model. Compared to the GRACE risk score, our risk model demonstrated better discriminative ability, improved calibration and a greater net benefit for clinical performance. Furthermore, we used machine learning methods such as random forest imputation and a penalty algorithm to maintain statistical power and avoid overfitting during model derivation. To the best of our knowledge, this is the first prediction model for long-term mortality in Chinese ACS patients.
The predictors selected by this risk model include “Age,” “Creatinine,” “Hemoglobin,” “Platelets,” “AST,” and “LVEF”. These risk factors could be supported by existing theories and research. Usually, older patients are more fragile and have more comorbidities. Many studies have considered age to be an independent predictor of ACS, and in studies focusing on other risk factors, age usually needs to be adjusted [7]. Creatinine or eGFR levels are thought to be associated with mid- and long-term mortality in ACS patients, and ACS patients with renal insufficiency are more likely to experience bleeding and other complications when given invasive treatment [8, 9]. In previous studies, baseline hemoglobin levels or anemia status were predictors of 30-day and 1-year mortality in patients with ACS or STEMI [10], while hemoglobin levels of 1416 g/dl resulted in the lowest risk of death [11]. Studies have reported that AST is associated with microvascular obstruction in ACS patients, and its predictive value is even better than that of NT-proBNP [12]. A meta-analysis of 8 studies indicated that high baseline platelet levels would increase short-term and long-term mortality in ACS patients [13], which may be related to the pathological basis of coronary heart disease involving the platelet-granulocytic system and acute pathogenesis of ACS involving intravascular inflammatory mechanisms [14]. Finally, the LVEF is considered as a marker of cardiac function in heart disease, and the guidelines also recommend ultrasound or angiography for NST-ACS patients to evaluate left ventricular function [3]. Low baseline LVEF is a predictor of mortality and MACE in ACS patients [15].
The GRACE risk score was developed based on 123 hospitals in 14 countries but only involved a small number of Chinese patients [4]. Most of the related studies on risk assessment of Chinese ACS patients investigated the domestic optimization or application of GRACE risk score. Previous CPACS studies have only reported patients with in-hospital mortality [6]. Therefore, there is no long-term risk prediction tool for the Chinese ACS population. This study is the first attempt for this purpose, and several new machine learning algorithms were used to improve the accuracy of the model.
This prediction model was established according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement [16], and we also referred to the opinion of ABCD proposed by Ewout W for validation [17]. Additionally, we did not use the conventional multiple imputations for missing value processing but applied a novel random forest algorithm. The random forest algorithm has been demonstrated as an efficient method to handle missing data. It can manage different types of missing data and can scale to high dimensions [18]. Several different imputation algorithms based on random forests have been developed, and among them, MissForest was found to have a noticeable improvement on performance compared to other methods such as the k-nearest neighbors and parametric MICE methods [19]. In this dataset, random forest imputation had a higher statistical power and better accuracy for prediction than complete-case analyses (AUC of the ROC for tenfold cross-validation, 0.744).
In the model derivation, we used the LASSO-Cox method to estimate the relationship between predictors and time-event. LASSO regularization is a method to manage overfitting and perform variable selection and has been widely used many types of machine learning algorithms [20]. It adds the L1 norm of coefficients as the penalty term to the loss function and hence adds constraints to the coefficients. In contrast to ridge regularization, LASSO regularization performs different degrees of shrinkage on variables and pushes some coefficients to zero. When adding the LASSO method to the Cox model, the estimation variance is reduced, and a subset of predictors is selected while providing an interpretable Cox model [21]. To ensure the accuracy of the model, we did not use a nomogram to simplify the parameters in the model presentation but to estimate the patient’s death risk through the cumulative hazard using the Cox model. This model showed good consistency (AUC of the ROC for tenfold cross-validation and C-statistic) for patients who died within 3 years and good agreement (slope and plot) for the actual and predicted 3-year mortality risk. The clinical usefulness of this model mainly lies in its ability to quantify the long-term mortality of patients by combining baseline data before angiography at an individual level. DCA could be used to evaluate whether our model is more advantageous for clinical applications than the GRACE model, which is currently widely used in clinical research [22]. This method could help physician to assess the value of information provided by a risk assessment tool or test by weighted the potential risk and benefit [23]. For all risk thresholds > 0%, our model showed a higher net benefit than the GRACE model. Therefore, we believe that our model can better help patients understand the disease and help doctors make clinical decisions. Particularly for patients with a high risk of ACS, doctors can use this model to assess whether patients can benefit from treatment.
There were some limitations of our study. First, the present study lacked external validation. In addition, due to the number of samples, there were relatively few death events in this dataset. However, careful statistical methods were used for the machine learning and penalty algorithms to ensure the accuracy of the model and prevent overfitting.
Conclusion
Our study developed a prediction model based on machine learning for 3-year morality in Chinese ACS patients. The external validation and further studies are needed to confirm the usefulness of it.
Methods
Study population
The data source for this investigation was the West China Hospital CHD database. This single center database prospectively includes all CHD or high-risk patients undergoing angiography in West China Hospital affiliated to Sichuan University. For this analysis, we enrolled consecutive CHD patients from January 2009 to September 2012 who were included in the database. ACS patients were eligible for inclusion if they had (1) angiographic evidence of ≥ 50% stenosis in ≥ 1 coronary vessel; (2) ischemic chest discomfort that increased or occurred at rest; and/or (3) electrocardiography or cardiac biomarker criteria consistent with ACS. The exclusion criteria were malignancies, pregnancy, end stage renal disease and severe liver or hematological diseases. These inclusion and exclusion criteria were met by 2406 continuous CHD patients enrolled from the database. After excluding patients with loss of follow-up (n = 192) and much missing data (n = 40), 2174 patients were included in the data analysis. The study protocol was approved by the local institutional review boards in accordance with the Declaration of Helsinki. All subjects provided written informed consent before enrolment.
Baseline characteristics
Demographic data, medical history, cardiovascular risk factors, vital signs at admission, medication at discharge, and the final diagnosis were obtained from the patients’ electronic medical records and reviewed by a trained study coordinator. Blood samples were collected at admission and before angiography, and plasma biomarkers including Fib, liver and kidney function, blood glucose, and serum lipids were analyzed in the Department of Laboratory Medicine, West China Hospital, accredited by the College of American Pathologists. The Elevated myocardia enzyme is defined as the cardiac troponin T or Creatine kinase-MB raised beyond the upper limit of laboratory reference values. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mm Hg, diastolic blood pressure (DBP) ≥ 90 mm Hg and/or patients receiving antihypertensive medications. Diabetes mellitus was diagnosed in patients who had previously undergone dietary treatment for diabetes, had received additional oral antidiabetic or insulin medications or had a current fasting blood glucose level of ≥ 7.0 mmol/L or a random blood glucose level ≥ 11.1 mmol/L. The GRACE risk prediction tool used for analysis of mortality has been described previously [4]. The calculation of the GRACE risk score was performed using an online program (http://www.outcomes-umassmed.org/grace).
Follow-up and study outcome
The follow-up period ended in January 2013. Follow-up information was collected through contact with the patients’ physicians, patients or their family. All data were corroborated with the hospital records. The primary endpoint of this study was all-cause mortality, and the secondary endpoint was cardiovascular death, as documented in the database. Death was considered to be cardiac death when it was caused by acute myocardial infarction (MI), significant arrhythmias, or refractory heart failure. Sudden unexpected death occurring without another explanation was considered cardiovascular death.
Statistical analyses
Baseline demographics and clinical characteristics were compared between non-surviving patients and survivors. Continuous variables are expressed as the mean ± standard deviation (SD), and categorical variables are reported as counts and percentages. T-tests and Chi squared tests were used to evaluate differences between groups for continuous and categorical variables, respectively. The Kaplan–Meier method was used to calculate the rate of cumulative events during the follow-up period.
Missing data
To avoid loss of statistical power, all missing data among baseline characteristics were assumed to be missing at random and imputed using a random Forest-based imputation method [18, 24]. Specifically, the “MissForest” method was used. MissForest handles missing data by iteratively using Random Forests. It starts by imputing the missing values of the candidate column, which is the column with the least missing values. Then, the imputer fills other missing values of the remaining columns with a mean imputation and uses them as predictors to perform a random Forest model with the candidate column as output. The missing values of the candidate column are imputed according to the prediction made from the fitted random Forest. This process starts over again for the remaining columns and repeats over multiple times until a certain stopping criterion is met.
Model derivation and validation
The development and validation of this risk model followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement [16]. The independent predictors of 3-year mortality were identified among baseline characteristics using a Cox proportional-hazards regression model. The proportional hazard assumption was verified using the Schoenfeld residuals method.
When performing model estimation, the LASSO method was applied to avoid the overfitting, and the penalty parameter was selected by cross-validation [20, 21]. According to design of our study, only the clinical characteristic before intervention were put into LASSO path and feature selection. The LASSO method is a shrinkage regression technique using L1 regularization and designed for high-dimensional data. Furthermore, this algorithm shrinks the coefficients of noninfluential predictors to zero and thus excludes them from the final model. This technique has been widely used in both machine learning and clinical practice. The estimated risk of mortality of a given patient was calculated from the cumulative hazard function of the Cox model as follows:
In this equation, is the baseline hazard function of time t, and is the linear product of the predictors and associated coefficients for a patient.
The model was validated with tenfold cross-validation [25]. In the assessment of the discrimination ability of the prediction model, Harrell’s C-statistic was used to estimate the degree of discrimination, and ROC analysis was conducted for visual inspection. Furthermore, the calibration was investigated using a calibration plot by plotting the predicted and observed probabilities of events across increasing levels of predicted risk.
Clinical performance
To assess the utility of our model in clinical practice, we compared this risk model to the GRACE score. First, we sought to examine the difference in the AUC of ROC between these two risk-assessment tools. Second, we performed decision-curve analysis to quantify the clinical usefulness of our prediction model (which was also compared with that of the GRACE score) [26]. This analysis was used to assess the net true-positive classification rate using a model over a range of thresholds. The values (from 0 to 1) represent the benefit from clinical intervention, and higher values indicate more significant benefit.
Data analyses were performed using Python (version 3.6) with the scientific libraries “scikit-learn”, “scikit-survival”, “lifelines” and Stata Statistical Software (Release 15. College Station, TX: StataCorp LLC).
Supplementary information
Additional file 1: The details of missing data among variables.
Additional file 2: The risk formula of our model and the demo of use it.
Acknowledgements
Not applicable.
Abbreviations
- CHD
Coronary heart disease
- ACS
Acute coronary syndrome
- STEMI
ST-Segment elevation myocardial infarction
- GRACE
Global Registry of Acute Coronary Events
- CPACS
Clinical pathways for acute coronary syndromes
- HR
Heart rate
- SBP
Systolic blood pressures,
- DBP
Diastolic blood pressure
- SD
Standard deviation
- TRIPOD
Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis
- LASSO
Least Absolute Shrinkage And Selection Operator
- ROC
Receiver operating characteristic curve
- AUC
Area under curve
- CI
Confidence interval
- LVEF
Left ventricular ejection fraction
- TIMI
Thrombolysis in myocardial infarction
- WBC
White blood cell
- RBC
Red blood cell
- AST
Aspartate transaminase
- PCI
Percutaneous coronary intervention
- ACEI
Angiotensin-converting-enzyme inhibitors,
- ARB
Angiotensin II receptor blockers
Authors’ contributions
YML, ZLL, PY and MC participated in study conception and design. FC and QL performed the acquisition of data. YML and ZLL participated in data analysis and were major contributions in coding for model development. All the authors participated in drafting the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Sichuan Science and Technology Program (Grant Numbers: 2018SZ0385) (Sichuan, China); the National Natural Science Foundation of China (Grant Number: 81400267, Beijing, China); “13th Five-Year” National key Research and Development Program of China (2016YFC1102204, 2017YFC1104204).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted after the acquisition of written informed consent from the participating patients and upon the approval by the ethics committee of West China Hospital, Sichuan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi-ming Li and Zhuo-lun Li contributed equally to this work
Contributor Information
Yong Peng, Email: pengyongcd@126.com.
Mao Chen, Email: hmaochen@vip.sina.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12967-020-02319-7.
References
- 1.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC Guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 5.Fox KA, Eagle KA, Gore JM, Steg PG, Anderson FA. Grace, Investigators G: the Global Registry of Acute Coronary Events, 1999 to 2009–GRACE. Heart. 2010;96:1095–1101. doi: 10.1136/hrt.2009.190827. [DOI] [PubMed] [Google Scholar]
- 6.Peng Y, Du X, Rogers KD, Wu Y, Gao R, Patel A. Clinical pathways for acute coronary syndromes in China I: predicting in-hospital mortality in patients with acute coronary syndrome in China. Am J Cardiol. 2017;120:1077–1083. doi: 10.1016/j.amjcard.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Henderson RA, Jarvis C, Clayton T, Pocock SJ, Fox KA. 10-Year mortality outcome of a routine invasive strategy versus a selective invasive strategy in non-ST-segment elevation acute coronary syndrome: the British Heart Foundation RITA-3 Randomized Trial. J Am Coll Cardiol. 2015;66:511–520. doi: 10.1016/j.jacc.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 8.Wong JA, Goodman SG, Yan RT, Wald R, Bagnall AJ, Welsh RC, Wong GC, Kornder J, Eagle KA, Steg PG, et al. Temporal management patterns and outcomes of non-ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J. 2009;30:549–557. doi: 10.1093/eurheartj/ehp014. [DOI] [PubMed] [Google Scholar]
- 9.McCullough PA. Treatment disparities in patients with acute coronary syndromes and kidney disease. Eur Heart J. 2009;30:526–527. doi: 10.1093/eurheartj/ehp028. [DOI] [PubMed] [Google Scholar]
- 10.Brener SJ, Mehran R, Dangas GD, Ohman EM, Witzenbichler B, Zhang Y, Parvataneni R, Stone GW. Relation of baseline hemoglobin levels and adverse events in patients with acute coronary syndromes (from the acute catheterization and urgent intervention triage strategY and Harmonizing Outcomes with RevasculariZatiON and Stents in Acute Myocardial Infarction Trials) Am J Cardiol. 2017;119:1710–1716. doi: 10.1016/j.amjcard.2017.02.052. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- 12.Feistritzer HJ, Reinstadler SJ, Klug G, Reindl M, Wohrer S, Brenner C, Mayr A, Mair J, Metzler B. Multimarker approach for the prediction of microvascular obstruction after acute ST-segment elevation myocardial infarction: a prospective, observational study. BMC Cardiovasc Disord. 2016;16:239. doi: 10.1186/s12872-016-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Wu H, Mueller C, Gibson CM, Murphy S, Shi Y, Xu G, Yang J. Baseline platelet count and clinical outcome in acute coronary syndrome. Circ J. 2012;76:704–711. doi: 10.1253/circj.CJ-11-0707. [DOI] [PubMed] [Google Scholar]
- 14.Martin JF, Kristensen SD, Mathur A, Grove EL, Choudry FA. The causal role of megakaryocyte-platelet hyperactivity in acute coronary syndromes. Nat Rev Cardiol. 2012;9:658–670. doi: 10.1038/nrcardio.2012.131. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee JT, Beshansky JR, Ruthazer R, Alkofide H, Ray M, Kent D, Manning WJ, Huggins GS, Selker HP. In-hospital measurement of left ventricular ejection fraction and one-year outcomes in acute coronary syndromes: results from the IMMEDIATE Trial. Cardiovasc Ultrasound. 2016;14:29. doi: 10.1186/s12947-016-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins GS, Reitsma JB, Altman DG, Moons KGM. members of the Tg: transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Eur Urol. 2015;67:1142–1151. doi: 10.1016/j.eururo.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–1931. doi: 10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breiman L. Random Forests. Mach Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 19.Waljee AK, Mukherjee A, Singal AG, Zhang Y, Warren J, Balis U, Marrero J, Zhu J, Higgins PD. Comparison of imputation methods for missing laboratory data in medicine. BMJ Open. 2013;3:e002847. doi: 10.1136/bmjopen-2013-002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tibshirani R. Regression shrinkage and selection via the Lasso. J Roy Stat Soc: Ser B (Methodol) 1996;58:267–288. [Google Scholar]
- 21.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–395. doi: 10.1002/(SICI)1097-0258(19970228)16:4<385::AID-SIM380>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Kerr KF, Brown MD, Zhu K, Janes H. Assessing the clinical impact of risk prediction models with decision curves: guidance for correct interpretation and appropriate use. J Clin Oncol. 2016;34:2534–2540. doi: 10.1200/JCO.2015.65.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313:409–410. doi: 10.1001/jama.2015.37. [DOI] [PubMed] [Google Scholar]
- 24.Shah AD, Bartlett JW, Carpenter J, Nicholas O, Hemingway H. Comparison of random forest and parametric imputation models for imputing missing data using MICE: a CALIBER study. Am J Epidemiol. 2014;179:764–774. doi: 10.1093/aje/kwt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank E, Harrell J. Regression modeling strategies. Newyork: Springer; 2006. [Google Scholar]
- 26.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: The details of missing data among variables.
Additional file 2: The risk formula of our model and the demo of use it.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.