Abstract
Objectives
To examine elevated neonatal inflammatory and neurotrophic proteins from children born extremely preterm in relation to later childhood brain Magnetic Resonance Imaging volumes and cognition.
Study design
We measured circulating inflammation-related proteins and neurotrophic proteins on postnatal days 1, 7, and 14 in 166 children at 10 years of age (73 males; 93 females). Top quartile levels on ≥2 days for ≥3 inflammation-related proteins and for ≥4 neurotrophic proteins defined exposure. We examined associations among protein levels, brain Magnetic Resonance Imaging volumes, and cognition with multiple linear and logistic regressions.
Results
Analyses were adjusted for gestational age at birth and sex. Children with ≥3 elevated inflammation-related proteins had smaller grey matter, brain stem/cerebellar, and total brain volumes than those without elevated inflammation-related proteins, adjusted for neurotrophic proteins. When adjusted for inflammation-related proteins, children with ≥4 neurotrophic proteins, compared with children with no neurotrophic proteins, had larger grey matter and total brain volumes. Higher grey matter, white matter, and cerebellum and brainstem volumes were significantly correlated with higher IQ. Grey and white matter volumes were correlated with each other (r = −0.18; P = .021), and cerebellum and brainstem was highly correlated with grey matter (r = 0.55; P < .001) and white matter (r = 0.29; P < .001). Adjusting for other brain compartments, cerebellum and brainstem was associated with IQ (P = .016), but the association with white matter was marginally significant (P = .051). Grey matter was not associated with IQ. After adjusting for brain volumes, elevated inflammation-related proteins remained significantly associated with a lower IQ, and elevated neurotrophic proteins remained associated with a higher IQ.
Conclusions
Newborn inflammatory and neurotrophin protein levels are associated with later brain volumes and cognition, but their effects on cognition are not entirely explained by altered brain volumes.
Among children born extremely preterm, elevated circulating, inflammation-related proteins in the first 2 postnatal weeks are associated with numerous adverse neurologic outcomes, including deficits in executive function (EF) and intelligence,1 attention deficit hyperactivity disorder symptoms,2 and social impairments3 at age 10 years, as well as motor impairment4 and microcephaly5 at 2 years of age. The mechanisms through which inflammation-associated proteins alter the risk for later adverse outcomes among those born extremely premature likely affect brain structural integrity. Structural damage may result from direct tissue destruction as well as epigenetic mechanisms, including those that influence apoptosis,6,7 affect neuronal and oligoden-droglia cell survival,8 reduce production or promote loss of myelination,9 and enhance synaptogenesis.10
Because microcephaly at age 2 years in the extremely premature population, independent of head size at birth, is associated with both neonatal inflammation as well as adverse cognitive and neurologic outcomes,11 we hypothesize that reduced brain volumes may link neonatal inflammation and adverse outcomes. Conversely, larger brain volumes might link elevated concentrations of neurotrophic-associated proteins (ie, proteins that support growth, survival, and differentiation of developing neurons) and a lower risk of cognitive impairment.12
To date, there is no evidence of a direct relationship between exposure to early life inflammation-associated and neurotrophic proteins and alteration of brain volumes in a large sample of children born extremely premature. Further, only limited data show an association between total and compartmentalized brain volumes and adverse outcomes at school age in children born extremely or very preterm.13-21
The Extremely Low Gestational Age Newborn (ELGAN) Study was designed to test the hypothesis that perinatal inflammation is associated with persisting brain structural and functional disorders in children born extremely premature. In a subsample of ELGAN Study participants, we aimed to evaluate the association of early life exposure with inflammation-related and neurotrophic proteins with deep and cortical grey matter, cerebellar and brainstem, white matter, and total solid brain volumes at 10 years of age, and to evaluate the association of subcortical and cortical grey matter, cerebellar and brainstem, white matter, and total brain Magnetic Resonance Imaging (MRI) volumes with adverse cognitive outcomes at 10 years of age.
Methods
The ELGAN Study is a multicenter, observational study of the risk of structural and functional neurologic disorders in extremely premature infants. From 2002 to 2004, women delivering at <28 weeks of gestation were asked to enroll in the study; 1249 mothers of 1506 infants consented to participate. At 10 years of age, 966 surviving children for whom we obtained neonatal blood specimens for measurement of inflammation-related and neurotrophic proteins, were targeted for recruitment. The families of 889 of these children (92%) returned for follow-up.
Of the 889 children returning at age 10 years of age, 11 were not accompanied by the parent or caregiver during the follow-up visit (hence informed consent could not be obtained), and 5 children were unable to complete the child assessments, leaving a final sample of 873 children. The institutional review boards of all participating institutions approved enrollment and consent procedures and consent was obtained from all participants in this follow-up study.
A sample of 166 children (73 males and 93 females) was drawn from the larger sample of 873 to conduct MRI studies at 1 of 12 participating institutions when the child was between 9 and 11 years of age, an age at which cognition measures more reliably indicate life-long cognitive status than at younger ages. Sampling for MRI studies was based on the level of circulating blood protein markers of inflammation in the first 2 weeks of life, before either MRI results or other outcomes were known. The children were sampled to obtain approximately equal numbers of children with high, intermediate, and low circulating inflammation-associated protein values (see Neurotrophic and Inflammatory Proteins for a description of the groups). We attempted to avoid selection bias within each of the 3 groups of the cohort by targeting for the MRI sample all infants in the ELGAN cohort who had high inflammatory protein levels, while randomly selecting from the intermediate and low inflammatory exposure groups. Although selection for MRI was conducted independent of gestational age, the distribution of gestational age among those sampled and not sampled did not differ.
Procedures for the Assessment of Cognitive Function
Child measures were selected to provide the most comprehensive assessment of cognitive function obtainable in a single testing session. Evaluations were administered by certified child psychologists blinded to clinical information in a 3- to 4-hour session that included breaks. All psychology examiners underwent a 1-day in-person training and verification of competency for administering the neurocognitive test battery. The child assessments reported here evaluated general cognitive ability with standardized tests of IQ and EF.
IQ.
IQ was assessed with the School-Age Differential Ability Scales–II (DAS-II,22 verbal and nonverbal reasoning scales). Because the DAS-II verbal and nonverbal IQ scores were strongly correlated within the sample, the mean of these 2 measures was used as an estimate of overall IQ.
EF.
Attention and EF were assessed with the DAS-II and the Developmental NEeuroPSYchological Assessment, second edition (NEPSY-II).23 The DAS-II recall of digits backward and recall of sequential order measured verbal working memory. The NEPSY-II auditory attention and auditory response set measured sustained auditory attention, set switching, and inhibition. The NEPSY-II inhibition–inhibition and inhibition–switching tasks measured simple inhibition and inhibition in the context of set shifting, respectively. NEPSY-II animal sorting measured concept generation and mental flexibility.
Derivation of Levels of Cognitive Functioning Based on IQ and EF.
We evaluated cognitive outcomes using latent class analysis (LCA) classifications, which represented both IQ and EF abilities as a better predictor of functional outcome (eg, academic success) than IQ alone.24 The following ranges were derived through LCA analysis, rather than chosen by the investigators, and identified 4 subgroups of children in our cohort corresponding with overall cognitive functioning that was normal (34% of cohort, with mean IQ and EF scores within normal range on all measures), low-normal (41%, with mean IQ and EF scores ranging from 0.5 to 1.5 SD below the norm), moderately impaired (17%, with mean IQ and EF scores between 1.5 and 2.5 SDs below the norm), and severely impaired (8%, with mean IQ and EF scores 2.5 to 4.0 SDs below the norm).24
Procedures for the Assessment of Inflammation and Neurotrophic Proteins
Blood Protein Measurements.
Drops of whole blood were collected on (Schleicher & Schuell 903, GE Healthcare, Chicago, Illinois) filter paper on postnatal day 1 (range, 1-3 days), postnatal day 7 (range, 5-8 days), and postnatal day 14 (range, 12-15 days). Twenty-eight inflammatory-associated proteins taken from blood samples in the first 2 weeks of life were measured in the Laboratory of Genital Tract Biology, Brigham and Women’s Hospital. Details about the procedure for processing the blood spots and for measuring protein concentrations and absolute value ranges for 28 inflammation-regulating proteins are explained elsewhere.2,25 Volumes of blood spotted on the filter paper varied, and to standardize the inflammation protein measurements we eluted each fixed spot area in the same volume of elution buffer and normalized the concentration of each biomarker to total protein concentration. Measurements were made in duplicate, and the mean served as the basis for all tables and analyses.
Choice of Top Quartile Levels for Assigning Status of Elevated Proteins.
The protein levels varied with gestational age, and with the postnatal day of collection.25 Consequently, we divided our sample into 9 groups defined by gestational age category (23-24, 25-26, and 27 weeks) and postnatal day of blood collection (1, 7, and 14). Three considerations led us to use highest quartile protein levels for a particular gestational age and postnatal day or week of sampling as a measure of inflammation signal or elevated neurotrophic function: (1) normative data for circulating inflammation-associated proteins in ELGANs are not available, (2) circulating protein concentrations in our cohort of ELGANs varied according to gestational age and by the postnatal day or week the blood was sampled, and (3) the protein values did not conform to a normal distribution. Sustained elevation for a particular protein was defined as a protein concentration in the highest quartile on ≥2 of the 3 blood samples obtained in the first 2 weeks.
Inflammation-Associated and Neurotrophic Proteins.
Children were selected for the MRI sample based on 6 inflammation-related proteins previously reported to be associated with structural and functional neurologic outcomes in ELGAN Study analyses4,5,26: Interleukin (IL)-6, tumor necrosis factor-alpha, intercellular adhesion molecule-1, IL-8, serum amyloid A, and C-reactive protein. Individual protein levels may be correlated, and this collinearity may complicate examining effects of individual proteins. Therefore, children were classified as having low, moderate, or high levels of inflammatory proteins if they had sustained elevation on 0, 1-2, or ≥3 of these 6 proteins. These inflammatory risk groups were used both in selecting the MRI sample and in analyses.
We also categorized children based on sustained elevation of 12 neurotrophic proteins: IL-6R, regulated upon activation, and normal T-cell expressed, and (presumably) secreted (RANTES), brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor, insulin-like growth factor-1, vascular endothelial growth factor, vascular endothelial growth factor receptor-1, vascular endothelial growth factor receptor-2, placental growth factor, angiopoietin 1 (ANG-1), ANG-2, and thyroid-stimulating hormone. We summed the number of these 12 proteins with sustained elevation and categorized children as having low (0-1), moderate (2-3), or high (≥4) levels of elevated neurotrophic proteins (Table I; available at www.jpeds.com). Because the blood levels of neurotrophic proteins under study might correlate with one another, we previously conducted and reported the results of LCAs on the neurotrophin proteins. We identified 3 distinct subgroups: (1) class 1 had ≤2 elevated proteins and served as a referent group; (2) class 2 had elevations of ≥3 proteins including ≥2 of the following 3 neurotrophic proteins: RANTES, BDNF, and Ang-1; and (3) class 3 had low levels on ≥2 of the 3 neurotrophin group 2 proteins, RANTES, BDNF, and Ang-1, but had elevated levels of ≥3 of the other neurotrophic proteins.12
Table I.
Demographic and protein characteristics of sample (n = 166)
| Characteristics | MRI population |
Not in MRI sample |
P value |
|---|---|---|---|
| Sex | .042 | ||
| Female | 93 (56) | 335 (47) | |
| Male | 73 (44) | 373 (53) | |
| Gestational age, wk | .984 | ||
| 23-24 | 35 (21) | 145 (20) | |
| 25-26 | 75 (45) | 321 (45) | |
| 27 | 56 (34) | 242 (34) | |
| Birthweight z-score | .968 | ||
| Less than −2 | 9 (5) | 43 (6) | |
| Between −2 and −1 | 22 (13) | 93 (13) | |
| Greater than −1 | 135 (81) | 572 (81) | |
| Maternal race | .148 | ||
| White | 102 (62) | 442 (63) | |
| Black | 50 (30) | 176 (25) | |
| Other | 12 (7) | 82 (12) | |
| Hispanic ethnicity | .523 | ||
| Yes | 14 (8) | 71 (10) | |
| No | 152 (92) | 635 (90) | |
| Maternal education | .750 | ||
| High school or less | 66 (40) | 281 (41) | |
| Some college | 42 (25) | 156 (23) | |
| College graduate | 57 (35) | 247 (36) | |
| Insurance | .033 | ||
| Private | 96 (58) | 472 (67) | |
| Public | 70 (42) | 236 (33) | |
| Elevated inflammatory proteins | <.001 | ||
| 0 | 62 (37) | 368 (57) | |
| 1-2 | 48 (29) | 198 (31) | |
| ≥3 | 56 (34) | 79 (12) | |
| Elevated neurotrophic proteins | <.001 | ||
| 0-1 | 67 (40) | 328 (51) | |
| 2-3 | 51 (31) | 181 (28) | |
| ≥4 | 48 (29) | 137 (21) |
Values are number (%).
MRI Protocol: ELGAN Cohort
Images were acquired with MRI scanners with magnetic field strengths of 3.0 T (11 sites) and 1.5 T (1 site). The full MRI protocol, which was applied without sedation, consisted of the following scans: sagittal T1-weighted conventional spin echo, axial T2-weighted gradient echo, inversion recovery dual-echo turbo (fast) spin echo, and dual-echo turbo spin echo. The total imaging time was approximately 20 minutes. A phantom scan with the full ELGAN protocol was performed within 2 weeks preceding each child’s MRI scanning session and the phantom images were sent to our image processing laboratory for approval. In addition, all MRIs were read clinically by a neuroradiologist.
We processed all scans to align them in the same plane, and these formed 288 × 288 × 70 matrix size axial datasets. The images were processed on a pixel by pixel basis to generate quantitative T2 and proton density (PD) maps. The T2 maps were generated with the standard dual echo formula and PD maps were generated by reversing the T2-weighting and further normalized to unity, relative to the maximum PD value of intraventricular cerebrospinal fluid.
The PD and T2 maps of each child were used to segment all intracranial soft tissues, referred to as intracranial matter. The cerebellum and brainstem were manually segmented as a single entity using the image editor of ImageJ (National Institutes of Health, Bethesda, Maryland). The total cerebral segment was generated by algebraic subtraction (intracranial matter minus cerebellum and brainstem) performed on a pixel-by-pixel basis. Using PD and T2 thresholding quantitative MRI segments, the cerebrum was then automatically subsegmented into white matter, grey matter, and cerebrospinal fluid. The deep grey matter segment, which encompassed the striatum, pallidum, and thalamus, was generated via a combination of fast and manual segmentation with ImageJ and refined by PD and T2 thresholding. Finally, the cortical grey matter segment was generated by subtracting the deep grey matter from the cerebral grey matter segment.
Structural segments were read sequentially by a Mathcad-programmed algorithm (PTC, Needham, Massachusetts), which counted the non-null pixels of each segment and multiplied the final count by the voxel volume, giving segmental volumes expressed in cubic centimeters. These computational steps were fully automatic and did not require input from the operator.
Data Analyses
We tested 2 hypotheses. First, we hypothesized that subcortical and cortical grey matter, cerebellum and brainstem, white matter, and total brain MRI volumes among those with elevated levels of circulating inflammation-associated and/or elevated circulating neurotrophic proteins in the first 2 weeks of life differ from those without such neonatal protein elevated levels. Second, we hypothesized that larger subcortical and cortical grey matter, cerebellum and brainstem, white matter, and total brain MRI volumes are associated with a decreased risk of cognitive impairment.
Statistical Analyses
Analyses proceeded in 3 phases. First, we examined whether inflammatory and neurotrophic proteins were associated with brain volumes using multiple linear regression models. Inflammatory and neurotrophic levels were categorized in these analyses, and slopes were interpreted as the expected adjusted differences in brain volume (in cubic centimeters) relative to reference groups. Because total brain volumes differ between boys and girls, all analyses included sex as a covariate. Second, we examined whether grey matter, white matter, and cerebellum and brainstem volumes were associated with composite IQ using linear regression models. These models treated both brain volume and IQ as continuous measures, and results were scaled so that slopes could be interpreted as expected differences in IQ points associated with a one SD decrease in brain volume. Third, we examined whether previously reported associations between elevated proteins and cognitive function remained after adjusting for brain volume as a potential mediator. Multiple linear regression was used with composite IQ as the cognitive outcome, and multiple multinomial logistic regression was used for the outcome of categorized cognitive function based on a LCA of IQ and EF.
Results
MRI Sample Characteristics in Relation to Total Cohort Characteristics
Children who underwent MRI differed from those who were not in the MRI sample in having a lower proportion of males and private insurance, and higher values of inflammatory and neurotrophic proteins (Table I). The MRI study selection process was designed to yield an enriched number of children with a high number of elevated circulating inflammation-associated proteins for imaging.
Brain Volume as a Function of Inflammation-Related and Neurotrophic Protein Status
Brain segmental volumes were examined as correlates of inflammation-associated protein exposure, and each of the 3 inflammation-associated strata (≥3,1-2, or 0 proteins) was subcategorized further according to neurotrophic-associated protein status (≥4, 2-3, or 0-1 proteins). The actual mean brain volumes adjusted for sex are shown in Table II (available at www.jpeds.com). Table III highlights the difference in mean brain volume for each protein-related group, defined by number of proteins, relative to the reference group, adjusting for the other protein status and sex.
Table II.
Brain volumes (cm3)*
| Mean (95% CI) Volumes (cm3) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protein risk groups | Total brain matter | Cerebral matter | Grey matter | Cortical grey matter |
Deep grey matter |
White matter | Cerebellum and brainstem |
Row n |
| ≥3 inflammation-related proteins | ||||||||
| Overall | 1211 (1166-1256) | 1358 (1310-1405) | 864 (821-907) | 810 (770-851) | 39 (37-41) | 347 (322-371) | 117 (110-125) | 56 |
| ≥4 neurotrophic proteins | 1277 (1210-1345) | 1413 (1341-1485) | 904 (837-970) | 867 (808-927) | 42 (39-45) | 374 (337-411) | 132 (120-144) | 24 |
| 2-3 neurotrophic proteins | 1154 (1082-1227) | 1325 (1248-1402) | 848 (777-919) | 746 (682-810) | 37 (34-40) | 307 (267-346) | 105 (92-118) | 21 |
| 0-1 neurotrophic proteins | 1174 (1074-1274) | 1299 (1193-1404) | 810 (712-908) | 807 (711-902) | 39 (34-44) | 364 (310-419) | 110 (92-127) | 11 |
| 1-2 inflammation-related proteins | ||||||||
| Overall | 1279 (1231-1327) | 1413 (1362-1464) | 926 (879-972) | 867 (824-909) | 43 (41-45) | 352 (325-378) | 145 (136-153) | 48 |
| ≥4 neurotrophic proteins | 1267 (1184-1350) | 1419 (1332-1507) | 905 (824-986) | 882 (811-954) | 45 (41-48) | 357 (312-403) | 141 (127-156) | 16 |
| 2-3 neurotrophic proteins | 1329 (1240-1418) | 1453 (1359-1547) | 965 (878-1052) | 914 (837-991) | 43 (39-47) | 364 (315-413) | 149 (134-165) | 14 |
| 0-1 neurotrophic proteins | 1251 (1173-1329) | 1375 (1293-1458) | 914 (838-990) | 812 (743-881) | 42 (39-46) | 337 (294-379) | 144 (130-157) | 18 |
| 0 inflammation-related proteins | ||||||||
| Overall | 1348 (1306-1391) | 1499 (1454-1544) | 979 (938-1020) | 878 (840-915) | 43 (41-45) | 369 (346-393) | 146 (139-154) | 62 |
| ≥4 neurotrophic proteins | 1368 (1251-1486) | 1573 (1449-1697) | 1012 (898-1127) | 905 (804-1007) | 46 (41-51) | 356 (292-420) | 149 (128-169) | 8 |
| 2-3 neurotrophic proteins | 1363 (1280-1446) | 1531 (1443-1618) | 958 (877-1039) | 868 (794-942) | 43 (40-47) | 405 (360-450) | 149 (135-164) | 16 |
| 0-1 neurotrophic proteins | 1337 (1284-1391) | 1470 (1413-1527) | 980 (928-1033) | 876 (830-922) | 42 (40-44) | 357 (328-386) | 145 (136-155) | 38 |
values are mean (95% CI).
For the number of elevated values of neurotrophic proteins (≥4, 2-3, 0-1) at the 3 levels of inflammatory protein exposures (≥4, 1-3, and 0), adjusted for sex. The volumes include total brain matter (total brain matter), cerebral matter, total, deep, and cortical grey matter, white matter, and cerebellum and brain stem.
Table III.
Association between elevated inflammatory and neurotrophic proteins*
| Variables | Total brain matter |
Cerebral matter | Grey matter | Cortical grey matter |
Deep grey matter |
White matter | Cerebellum and brainstem |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female | −121 (27)† | −136 (28)† | −75 (26)‡ | −62 (23)‡ | −2 (1) | −48 (15)‡ | −11 (5)§ |
| Male | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Inflammation-related proteins number | |||||||
| 0 | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 1-2 | −82 (33)* | −107 (35)‡ | −61 (32)¶ | −25 (29) | 0 | −23 (18) | −4 (6) |
| ≥3 | −157 (34)† | −175 (35)† | −126 (33)† | −88 (30)‡ | −5 (1)‡ | −31 (18) | −32 (6)† |
| Neurotrophic proteins number | |||||||
| 0-1 | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 2-3 | 20 (32) | 52 (34) | 11 (32) | 6 (29) | 0 (1) | 8 (18) | 0 (6) |
| ≥4 | 60 (34)¶ | 91 (36)§ | 34 (33) | 62 (30)§ | 3 (1)§ | 24 (19) | 10 (6)¶ |
Ref, Reference.
Slopes are the mean difference in brain volume vs the reference category.
Using number of inflammatory and neurotrophic proteins as risk categories, and MRI volumetrics (cm3), for sex for total brain matter, cerebral matter; total, deep, and cortical grey matter; white matter; and cerebellum and brain stem. Slopes (SE) from multiple linear regression models that include sex and inflammatory and neurotrophic proteins.
P < .001.
P < .01.
P < .05.
P < .10.
As is the case for the general population, volumes of all brain compartments in our extremely preterm cohort, were smaller in females compared with males (Table III). There were no significant interactions, however, between sex and elevated inflammatory proteins or between sex and neurotrophic proteins (all P > .10). In relation to proteins, for total brain volume, those with ≥3 elevated inflammatory proteins had total brain volumes that were 157 cm3 smaller than those with 0 elevated inflammatory proteins, adjusting for sex and neurotrophic proteins (Table III). Similarly, the presence of ≥3 inflammation-associated proteins was associated with a decreased total grey matter, cortical grey matter, deep grey matter, and cerebellum and brainstem volumes. Although white matter volumes were decreased with the presence of ≥3 proteins, the differences were not statistically significant (P > .10; Table III). The presence of ≥4 neurotrophic proteins was associated with significantly higher cortical and deep grey matter volumes and intracranial matter volumes. Although total brain matter, total grey matter, white matter, and cerebellum and brainstem volumes were greater so larger in the presence of ≥4 neurotrophic proteins, these differences were not statistically significant (Table III). When considering neurotrophins according to LCA-derived categories, all brain volumes were increased in class 2 and class 3 relative to the referent group (class 1), although only the association of the presence of class 3 neurotrophins was significantly associated with preserved cortical gray matter (data not shown).
We found no significant interaction between inflammatory and neurotrophic proteins. The Figure provides a graphical presentation of the mean grey matter and cerebellum and brainstem volumes as a function of number of elevated inflammatory and neurotrophic proteins.
Figure.
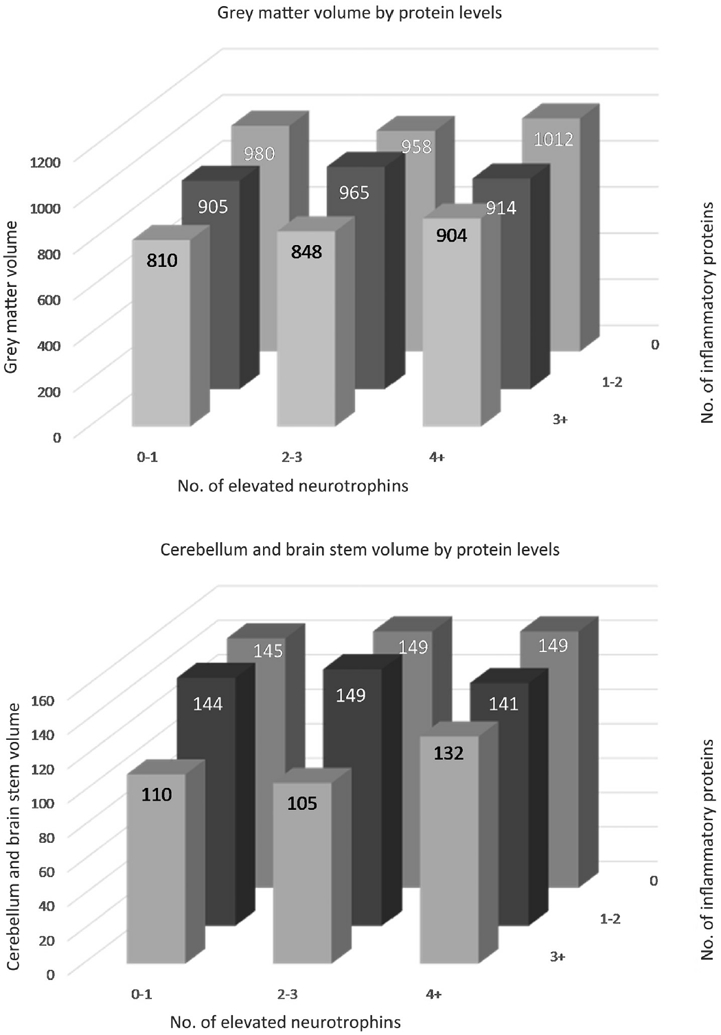
Mean grey matter and mean cerebellum and brain stem volumes depicted in Table III, adjusted for sex.
Cognitive Outcome as a Function of Brain MRI Compartment Volumes: IQ
Because we oversampled children with inflammation early in life, theoretically, cognition in the MRI sample may differ from the general ELGAN population. The minimum, first quartile, median, third quartile, and maximum IQ in the MRI sample were 31, 80, 92, 101, and 129, nearly superim-posable on the overall sample distribution (31, 80, 92, 102, and 129). Eleven percent had an IQ of <70, and 34% had an IQ of <85. When adjusting for sex and treating both volumes and IQ as continuous variables, higher grey matter, white matter, and cerebellum and brainstem volumes were significantly correlated with higher IQ (Table IV).
Table IV.
Association with IQ, controlling for sex (n = 164) after adjusting for other brain compartments
| Adjusting only for sex* |
Adjusting for sex and other brain volumes† |
|||
|---|---|---|---|---|
| Brain compartment | Slope‡ (SE) | P value | Slope (SE) | P value |
| Grey matter | −2.67 (1.21) | .029 | −1.05 (1.52) | .492 |
| White matter | −3.36 (1.15) | .004 | −2.50 (1.27) | .051 |
| Cerebellum and brainstem | −5.11 (1.15) | <.001 | −3.80 (1.56) | .016 |
Separate regression analyses for each brain volume, controlling for sex.
A single multiple regression model with grey matter, white matter, cerebellum and brainstem, and sex as independent variables.
Slopes are scaled to reflect the difference in IQ associated with a 1-SD decrease in brain compartment volume.
To determine whether brain compartments provide independent information, we first looked at correlations between compartments. We found that grey and white matter volumes were not strongly correlated (r = −0.18; P = .021, adjusting for sex). Cerebellum and brainstem, however, were highly correlated with grey matter (r = 0.55; P < .001) and moderately correlated with white matter (r = 0.29; P < .001). Beyond intercompartment correlations, we examined the association between IQ and each brain compartment individually (Table IV). We found that decreases in grey matter (P = .029), white matter (P = .004), and cerebellum and brainstem (P < .001) volumes were all significantly associated with lower IQ. Because of the compartment intercorrelations, it is difficult to separate out the independent effects of the different brain compartments on cognition. In a multiple regression model predicting IQ from the cerebellum and brainstem, white matter, and grey matter compartments (Table IV), cerebellum and brainstem was significantly associated with IQ (P = .016), white matter of borderline significance (P = .051), and grey matter not significantly related to IQ (P = .492; Table IV).
Is the Association Between Neonatal Proteins and School-Age Cognition Explained by MRI Brain Volume?
To determine whether previously reported associations between early proteins and later cognition1 could be accounted for by the association between proteins and brain volumes, we fit multiple linear regression models predicting IQ from brain volumes, early proteins, and sex (Table V). Because of the correlation between brain volumes, we fit separate models accounting for grey matter, white matter, and cerebellum and brainstem. Elevated inflammatory proteins were significantly associated with lower IQ, and elevated number of neurotrophic proteins were significantly associated with higher IQ, after adjusting for brain volumes (Table V). When repeating this analysis using elevation of neurotrophin class categories instead of number of neurotrophic protein elevations, class 2 was significantly associated with better cognition in all models considered (Table V).
Table V.
Association between brain volumes, proteins, and IQ*
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Risk predictors of IQ | Slope (SE) | P value | Slope (SE) | P value | Slope (SE) | P value |
| Grey matter volume† | 1.47 (1.19) | .222 | — | — | — | — |
| White matter volume† | — | — | 2.83 (1.14) | .014 | — | — |
| Cerebellum and brainstem volume† | — | — | — | — | 3.81 (1.22) | .002 |
| Inflammatory proteins | ||||||
| 0 | Ref | — | Ref | — | Ref | — |
| 1-2 | −4.45 (2.87) | .123 | −4.38 (2.81) | .121 | −4.51 (2.77) | .106 |
| ≥3 | −10.65 (3.01) | <.001 | −10.86 (2.86) | <.001 | −8.10 (3.04) | .009 |
| Neurotrophic proteins | ||||||
| 0-1 | Ref | — | Ref | — | Ref | — |
| 2-3 | 1.93 (2.79) | .489 | 1.83 (2.75) | .507 | 2.18 (2.72) | .423 |
| ≥4 | 8.29 (2.93) | .005 | 7.84 (2.90) | .008 | 7.57 (2.87) | .009 |
| Grey matter volume† | 1.63 (1.19) | .172 | — | — | — | — |
| White matter volume† | — | — | 2.79 (1.14) | .016 | — | — |
| Cerebellum and brainstem volume† | — | — | — | — | 3.99 (1.21) | .001 |
| Inflammatory proteins | ||||||
| 0 | Ref | — | Ref | — | Ref | — |
| 1-2 | −4.46 (2.88) | .124 | −4.38 (2.83) | .123 | −4.56 (2.77) | .102 |
| ≥3 | −10.00 (2.96) | <.001 | −10.27 (2.82) | <.001 | −7.39 (2.98) | .014 |
| Neurotrophic proteins | ||||||
| Category 1 | Ref | — | Ref | — | Ref | — |
| Category 2 | 8.93 (2.97) | .003 | 8.21 (2.95) | .006 | 8.45 (2.89) | .004 |
| Category 3 | 3.85 (3.04) | .208 | 3.41 (3.01) | .260 | 3.37 (2.96) | .257 |
Controlling for sex, excluding 2 children with an IQ of <40.
Category 1, ≥2 elevated neurotrophic proteins; category 2, 2 + of Ang-1, BDNF, RANTES; category 3, ≥3 elevated, not category 2.
Multiple linear regression models with IQ as the dependent variable (n = 164), using number of inflammatory and neurotrophic proteins as risk categories in the upper half of the table and using neurotrophic category categories as delineated through latent profile analysis in the bottom half of the table.
Brain volumes scaled so that slope reflects difference in IQ associated with a 1-SD increase in volume.
Cognitive Outcome as a Function of Brain MRI Compartment Volumes: IQ and EF as Determined by LCA
We considered cognition status as a composite of IQ and EF, based on latent profile analysis.24 In this sample of 166 children, when applying the composite IQ and EF outcome derived from LCA, 52 (31%) had normal cognition, 71 (43%) had low normal cognition, and 43 (26%) had cognitive impairment.
In multiple logistic regression models adjusting for sex, grey matter volume was not significantly associated with cognitive impairment, and lower white matter volume was associated with higher odds of cognitive impairment (OR, 1.69; 95% CI, 1.10-2.63 for a 1-SD decrease in volume), as was lower cerebellum and brainstem volume (OR, 2.08; 95% CI, 1.28-3.45).
We fit multiple logistic regression models predicting LCA class from brain volumes, early proteins, and sex to determine whether associations between early protein elevations and cognitive impairment could be accounted for by brain volumes (Table VI). Because of the correlation between brain volumes, we fit separate models accounting for grey matter, white matter, and cerebellum and brainstem. Adjusting for brain volumes, elevated inflammatory proteins were associated with lower IQ, and elevated neurotrophic proteins were associated with higher IQ (Table VI).
Table VI.
Association between brain volumes, proteins, and LCA cognitive function class *
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| Risk predictors of cognition | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Grey matter† | ||||||
| Low normal vs normal | 0.95 | 0.61-1.39 | — | — | — | — |
| Impaired vs normal | 1.08 | 0.73-1.83 | — | — | — | — |
| White matter† | ||||||
| Low normal vs normal | — | — | 1.46 | 0.98-2.18 | — | — |
| Impaired vs normal | — | — | 1.65 | 1.03-2.65 | — | — |
| Cerebellum and brainstem† | ||||||
| Low normal vs normal | — | — | — | — | 1.16 | 0.73-1.85 |
| Impaired vs normal | — | — | — | — | 1.72 | 1.01-2.87 |
| Inflammatory proteins | ||||||
| 0 | ||||||
| Low normal vs normal | Ref | — | Ref | — | Ref | — |
| Impaired vs normal | Ref | — | Ref | — | Ref | — |
| 1-2 | ||||||
| Low normal vs normal | 1.95 | 0.77-5.09 | 1.73 | 0.67-4.50 | 1.85 | 0.72-4.76 |
| Impaired vs normal | 2.62 | 0.87-7.94 | 2.48 | 0.81-7.53 | 2.65 | 0.88-8.04 |
| ≥3 | ||||||
| Low normal vs normal | 5.97 | 2.00-17.8 | 5.38 | 1.85-15.6 | 5.11 | 1.68-15.5 |
| Impaired vs normal | 6.81 | 1.95-23.8 | 6.99 | 2.04-24.0 | 4.76 | 1.32-17.2 |
| Neurotrophic proteins | ||||||
| 0-1 | ||||||
| Low normal vs normal | Ref | — | Ref | — | Ref | — |
| Impaired vs normal | Ref | — | Ref | — | Ref | — |
| 2-3 | ||||||
| Low normal vs normal | 0.43 | 0.16-1.14 | 0.46 | 0.17-1.23 | 0.58 | 0.22-1.59 |
| Impaired vs normal | 0.62 | 0.21-1.81 | 0.65 | 0.22-1.92 | 0.33 | 0.10-1.06 |
| ≥4 | ||||||
| Low normal vs normal | 0.25 | 0.09-0.70 | 0.27 | 0.10-0.76 | 0.26 | 0.09-0.72 |
| Impaired vs normal | 0.19 | 0.06-0.63 | 0.20 | 0.06-0.67 | 0.20 | 0.06-0.67 |
Bolded values represent associations that achieve significance at the P < .05 level.
Multiple logistic regression models with LCA class as the dependent variable (n = 164). Controlling for sex, excluding 2 children with an IQ of <40.
ORs for brain volume give expected difference in odds of impairment for a 1-SD decrease in brain volume.
Discussion
Elevated values of circulating inflammation-associated protein levels in the first 2 weeks of life are associated with reduced total brain, cortical and deep grey matter, and cerebellum and brainstem volumes, but not white matter volumes. In contrast, high levels of circulating neurotrophic proteins are associated with preserved cortical and deep grey matter and cerebellum and brainstem volumes. Additionally, reduced cerebellum and brainstem volume and white matter volume are associated with low IQ and more broadly impaired cognition (IQ and EF) independent of elevated inflammatory or neurotrophic protein status in the first weeks of life. Consequently, the effect of elevated proteins on cognitive function cannot be fully explained by their association with brain volumes.
We hypothesized that perinatal increases in circulating inflammation-associated and neurotrophic proteins alter or preserve brain structures with consequent effects on cognitive outcomes. Stated otherwise, we posited that altered brain volumes lie on the pathway between risk proffered by circulating proteins in early life and later cognition. What we found is somewhat more complex with a number of unexpected findings.
First, although circulating inflammation-associated proteins in the first 2 weeks of life are associated with overall reduced brain volumes at 10 years of age, white matter volumes do not seem to be associated with inflammation-related proteins. This observation is surprising given that early life imaging studies generally show that structural brain alterations in extremely premature newborns are predominantly of white matter.11,27 One possible explanation for this inconsistency is that brain volume does not fully capture the inflammation-associated impact on brain structure. Substantial evidence implicates residual disorganization of white matter tracts as important, if not more important for white matter function than standard imaging or through volume measures alone.28
A second unexpected finding was the particularly strong association of cerebellum and brainstem volume with later cognition more so than either white matter or gray matter (nonsignificant). Cerebellar injury in extremely preterm infants has been associated with adverse cognitive and other neurologic outcomes, 18,29-33 although cerebellar abnormalities become more predictive of adverse cognitive outcome at later school age, as in our study, than in the first years of life.18
There are several possible considerations to explain why cerebellum and brainstem volume might have the largest influence on cognitive outcome. First, substantial evidence indicates that cognitive functions are influenced strongly by cerebellar functions, and insults to the cerebellum alone can be associated with mutism and other higher cognitive functions,34-36 possibly mediated by widespread cerebellar–cortical connections.37 Second, involvement of cerebellar structures may indicate a tip of the iceberg effect in that its involvement signals the presence of a more profound and/or widespread damage than if the cerebellar structures are spared. A number of MRI studies in childhood among those born very preterm document reduced cerebellar volumes in the presence of cerebral abnormalities, even in the absence of identifiable direct cerebellar injury.38,39 Finally, the presence of a cerebellar injury may impede the neurore-covery of a cerebral insult by hindering the establishment of alternate pathways for preserving cognition.
A third unexpected finding is that the risk for impaired cognition posed by early circulating inflammation-associated proteins seems to be accounted for by its association with reduced brain volumes as well as through other mechanisms that seem to be independent of brain volume alterations. This observation suggests that mechanisms for adverse function might include alterations in microstructure not captured in quantified volumes, but possibly identifiable in connectome analyses. Alternatively, it might suggest other mechanisms that affect neuronal function and integrity, including epigenetic mechanisms associated with maternal risks linked to socioeconomic, toxic, or inflammatory exposures.40-43
The Potential Impact of Sample Selection
We derived the 166 patients who underwent MRI evaluations from the larger 10-year follow-up ELGAN cohort of 889 children. For the MRI studies, we oversampled children with elevated levels of inflammatory proteins early in life, before knowing the outcomes. We targeted 3 groups: all participants with high protein values as well as a random sample of those with intermediate and with low values of circulating inflammation-related proteins. Although, the MRI sample still may differ from the general ELGAN population (Table I), the oversampling strategy should not bias analyses that examine the effect of early life inflammation on later outcomes, or that control for inflammatory proteins as a covariate.
We used sex-adjusted absolute brain compartment volume values, rather than relative compartment volumes as a proportion of total brain matter volume. Both of these approaches have advantages and shortcomings. One argument for using the proportion approach is that normal compartment and total brain volumes may vary even at an age when brain growth changes are minimal. This approach works well if the brain pathology under consideration does not affect total brain matter volume while considering alterations in compartmental volumes. If both compartment and total brain matter volumes are affected comparably, as may be the case in children born extremely preterm, then the relative ratio of the two may not differ, even if there are substantial disease-related volume differences. The normal volume variability disadvantage when using absolute values ought to be minimal because volumetric variability should be comparable in the 3 protein risk groups, which were assigned without consideration of MRI volumetric or cognitive outcomes at 10 years. On balance, we thought that absolute values would be most informative in our analyses.
Our study has several strengths. We included a large number of infants, collected our data prospectively, and had little attrition across 10 years of follow-up. Examiners at 2 and 10 years were not aware of the medical histories of the children they examined, and our analyses of protein data are of high quality, with high content validity.44-46
Although we sampled a wide range of inflammation-associated proteins known to be associated with neurologic damage as well as a number of neurotrophic proteins, we did not evaluate all known inflammation-associated or neurotrophic proteins. We selected proteins on the basis of likely involvement in the fetal/neonatal inflammatory response and/or brain-protective properties. Rather than reporting absolute protein concentration values, we used a distribution-based definition of protein elevation based on gestational age, postnatal day, and the interval between processing blood samples, because normal values are not known and values seem to be influenced by these factors. In addition, our analysis does not give information on how individual specific proteins affect clinical or neuroimaging outcomes, but rather sheds light on the associations of inflammatory or neurotrophic proteins as a whole. Finally, our statistical modeling of associations between brain volume and IQ and cognitive function focus on linear associations, which may underestimate or miss true, more complex, associations between these factors.
Circulating neonatal inflammatory and neurotrophic protein levels are associated both with altered brain volumes and cognition at 10 years of age in extremely preterm born children. The association of early life circulating inflammation-related proteins with later impaired cognition is not fully explained by reduced brain volumes. This finding suggests that mechanisms other than those that lead to brain destruction or impaired brain growth associate inflammation-related proteins with later cognitive impairment. Beyond that, the risk on adverse cognition posed by reduced brain volumes is primarily attributable to reduced cerebellar and brain stem volumes and, surprisingly, not grey or white matter volumes. A key unanswered question in these analyses is whether the lack of an association of cerebral white matter and grey matter volumes with cognitive outcomes might be explained by microstructural or organizational abnormalities to which our volumetric measures were not sensitive. This finding suggests the potential value of connectome-level analyses in future studies. ■
Acknowledgments
We are grateful to the ELGAN Study participants and their families for their willingness to be engaged in the study for these many years and for the commitment and extra efforts that have made this work possible. We also acknowledge the inspiration, guidance, and collaboration of Alan Levition and Elizabeth Allred in conducting the ELGAN Study.
Supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-09), the Office of the Director of National Institutes of Health (1UG3OD023348-01), the National Institute of Child Health and Human Development (5P30HD018655-28), and the National Eye Institute (R01EY021820-01A1). J.F. has received research support from Fulcrum Therapeutics, F. Hoffman-LaRoche,Ltd, Janssen Research and Development, SyneuRex International Corp, and Neuren Pharmaceuticals. H.J. is named inventor in several patents (Boston Medical Center Assignee) related to quantitative MRI pulse sequences and quantitative MRI processing and has received research support for GE Healthcare. The other authors declare no conflicts of interest.
Glossary
- ANG
Angiopoietin
- BDNF
Brain-derived neurotrophic factor
- EF
Executive function
- ELGAN
Extremely Low Gestational Age Newborn
- MRI
Magnetic Resonance Imaging
- LCA
Latent class analysis
- NEPSY
NEeuroPSYchological Assessment
- PD
Proton density
- RANTES
Regulated upon activation, normal T-cell expressed, and secreted
Appendix
List of additional ELGAN Study collaborators
Boston Children’s Hospital, Boston, Massachusetts
vJanice Ware, PhD
Taryn Coster, BA
Brandi Hanson, PsyD
Rachel Wilson, PhD
Kirsten McGhee, PhD
Patricia Lee, PhD
Aimee Asgarian, PhD
Anjali Sadhwani, PhD
Tufts Medical Center, Boston, Massachusetts
Ellen Perrin, MD
Emily Neger, MA
Kathryn Mattern, BA
Jenifer Walkowiak, PhD
Susan Barron, PhD
Baystate Medical Center
Bhavesh Shah, MD
Rachana Singh, MD, MS
Anne Smith, PhD
Deborah Klein, BSN, RN
Susan McQuiston, PhD
University of Massachusetts Medical School, Worcester, Massachusetts
Lauren Venuti, BA
Beth Powers, RN
Ann Foley, Ed M
Brian Dessureau, PhD
Molly Wood, PhD
Jill Damon-Minow, PsyD
Yale University School of Medicine, New Haven, Connecticut
Richard Ehrenkranz, MD
Jennifer Benjamin, MD
Elaine Romano, APRN
Kathy Tsatsanis, PhD
Katarzyna Chawarska, PhD
Sophy Kim, PhD
Susan Dieterich, PhD
Karen Bearrs, PhD
Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina
Nancy Peters, RN
Patricia Brown, BSN
Emily Ansusinha, BA
Ellen Waldrep, PhD
Jackie Friedman, PhD
Gail Hounshell, PhD
Debbie Allred, PhD
University Health Systems of Eastern Carolina, Greenville, North Carolina
Stephen C. Engelke, MD
Nancy Darden-Saad, BS, RN, CCRC
Gary Stainback, PhD
North Carolina Children’s Hospital, Chapel Hill, North Carolina
Diane Warner, MD, MPH
Janice Wereszczak, MSN, PNP
Janice Bernhardt, MS, RN
Joni McKeeman, PhD
Echo Meyer, PhD
Helen DeVos Children’s Hospital, Grand Rapids, Michigan
Steve Pastyrnak, PHD
Julie Rathbun, BSW, BSN, RN
Sarah Nota, BS
Teri Crumb, BSN, RN, CCRC
Sparrow Hospital, Lansing, Michigan
Madeleine Lenski, MPH
Deborah Weiland, MSN
Megan Lloyd, MA, EdS
University of Chicago Medical Center, Chicago, Illinois
Scott Hunter, PhD
Michael Msall, MD
Rugile Ramoskaite, BA
Suzanne Wiggins, MA
Krissy Washington, MA
Ryan Martin, MA
Barbara Prendergast, BSN, RN
Megan Scott, PhD
William Beaumont Hospital, Royal Oak, Michigan
Judith Klarr, MD
Beth Kring, RN
Jennifer DeRidder, RN
Kelly Vogt, PhD
Brigham and Women’s Hospital, Boston, Massachusetts
Hidemi Yamamoto, BA
Stanthia Ryan, MD Candidate
Damilola Junaid, BS
Hassan Dawood, BS
Noah Beatty, BS
Ngan Luu, BA Candidate
Vanessa Tang, MD
Rosaria Rita Sassi, MD
Jenna-Malia Pasicznyk, RN
References
- 1.Kuban KC, Joseph RM, O’Shea TM, Heeren T, Fichorova RN, Douglass L, et al. Circulating inflammatory-associated proteins in the first month of life and cognitive impairment at age 10 years in children born extremely preterm. J Pediatr 2017;180:116–23.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allred EN, Dammann O, Fichorova RN, Hooper SR, Hunter SJ, Joseph RM, et al. Systemic inflammation during the first postnatal month and the risk of attention deficit hyperactivity disorder characteristics among 10 year-old children born extremely preterm. J Neuroimmune Pharmacol 2017;12:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korzeniewski SJ, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Elevated protein concentrations in newborn blood and the risks of autism spectrum disorder, and of social impairment, at age 10 years among infants born before the 28th week of gestation. Transl Psychiatry 2018;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuban KCK, O’Shea TM, Alled EN, Paneth N, Hirtz D, Fichorova RN, Leviton A. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J Child Neurol 2014;29:1692–8.24646503 [Google Scholar]
- 5.Leviton A, Kuban KC, Allred EN, Fichorova RN, O’Shea TM, Paneth N, et al. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum Dev 2011;87:325–30. [DOI] [PubMed] [Google Scholar]
- 6.Kumral A, Tuzun F, Tugyan K, Ozbal S, Yilmaz O, Yesilirmak CD, et al. Role of epigenetic regulatory mechanisms in neonatal hypoxic-ischemic brain injury. Early Hum Dev 2013;89:165–73. [DOI] [PubMed] [Google Scholar]
- 7.Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK. Effect of maternal diabetes on the embryo, fetus, and children: congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today 2015;105:53–72. [DOI] [PubMed] [Google Scholar]
- 8.Evrard P, Marret S, Gressens P. Environmental and genetic determinants of neural migration and postmigratory survival. Acta Paediatr Suppl 1997;422:20–6. [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Oishi K, Skranes J, Buchthal S, Cunningham E, Yamakawa R, et al. Sex-Specific alterations of white matter developmental trajectories in infants with prenatal exposure to methamphetamine and tobacco. JAMA Psychiatry 2016;73:1217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calfa G, Chapleau CA, Campbell S, Inoue T, Morse SJ, Lubin FD, et al. HDAC activity is required for BDNF to increase quantal neurotransmitter release and dendritic spine density in CA1 pyramidal neurons. Hippocampus 2012;22:1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuban KC, Allred EN, O’Shea TM, Paneth N, Westra S, Miller C, et al. Developmental correlates of head circumference at birth and two years in a cohort of extremely low gestational age newborns. J Pediatr 2009;155: 344–9.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuban KCK, Heeren T, O’Shea TM, Joseph RM, Fichorova RN, Douglass L, et al. Among children born extremely preterm a higher level of circulating neurotrophins is associated with lower risk of cognitive impairment at school age. J Pediatr 2018;201:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr 2008;152:513–20.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Inder TE, Neil JJ, Dierker DL, Alexopoulos D, Anderson PJ, et al. Cortical structural abnormalities in very preterm children at 7 years of age. Neuroimage 2015;109:469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northam GB, Liegeois F, Chong WK, Wyatt JS, Baldeweg T. Total brain white matter is a major determinant of IQ in adolescents born preterm. Ann Neurol 2011;69:702–11. [DOI] [PubMed] [Google Scholar]
- 16.Ment LR, Kesler S, Vohr B, Katz KH, Baumgartner H, Schneider KC, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics 2009;123:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, et al. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr 2004;145:242–9. [DOI] [PubMed] [Google Scholar]
- 18.Matthews LG, Inder TE, Pascoe L, Kapur K, Lee KJ, Monson BB, et al. Longitudinal preterm cerebellar volume: perinatal and neurodevelopmental outcome associations. Cerebellum 2018;17:610–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemola S, Oser N, Urfer-Maurer N, Brand S, Holsboer-Trachsler E, Bechtel N, et al. Effects of gestational age on brain volume and cognitive functions in generally healthy very preterm born children during school-age: a voxel-based morphometry study. PLoS One 2017;12:e0183519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh WY, Anderson PJ, Cheong JLY, Spittle AJ, Chen J, Lee KJ, et al. Neonatal basal ganglia and thalamic volumes: very preterm birth and 7-year neurodevelopmental outcomes. Pediatr Res 2017;82:970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botellero VL, Skranes J, Bjuland KJ, Haberg AK, Lydersen S, Brubakk AM, et al. A longitudinal study of associations between psychiatric symptoms and disorders and cerebral gray matter volumes in adolescents born very preterm. BMC Pediatr 2017;17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott CD. Differential ability scales. 2nd ed. San Antonio (TX): The Psychological Corporation; 2007. [Google Scholar]
- 23.Korkman M, Kirk U, Kemp S. NEPSY-II, second edition: clinical and interpretive manual; 2007. [Google Scholar]
- 24.Heeren T, Joseph RM, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Cognitive functioning at age 10 years among children born extremely preterm: a latent profile approach. Pediatr Res 2017;82:614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leviton A, Allred EN, Yamamoto H, Fichorova RN , ELGAN Study Investigators. Relationships among the concentrations of 25 inflammation-associated proteins during the first postnatal weeks in the blood of infants born before the 28th week of gestation. Cytokine 2012;57:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr 2012;160:395–401.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics 2008;122:e662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gozdas E, Parikh NA, Merhar SL, Tkach JA, He L, Holland SK. Altered functional network connectivity in preterm infants: antecedents of cognitive and motor impairments? Brain Struct Funct 2018;223:3665–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zayek MM, Benjamin JT, Maertens P, Trimm RF, Lal CV, Eyal FG. Cerebellar hemorrhage: a major morbidity in extremely preterm infants. J Perinatol 2012;32:699–704. [DOI] [PubMed] [Google Scholar]
- 30.Anderson PJ, Treyvaud K, Neil JJ, Cheong JLY, Hunt RW, Thompson DK, et al. Associations of newborn brain magnetic resonance imaging with long-term neurodevelopmental impairments in very preterm children. J Pediatr 2017;187:58–65.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitai Y, Hirai S, Ohmura K, Ogura K, Arai H. Cerebellar injury in preterm children with cerebral palsy after intraventricular hemorrhage: prevalence and relationship to functional outcomes. Brain Dev 2015;37:758–63. [DOI] [PubMed] [Google Scholar]
- 32.Steinlin M Cerebellar disorders in childhood: cognitive problems. Cerebellum 2008;7:607–10. [DOI] [PubMed] [Google Scholar]
- 33.Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD. Cerebellar contribution to social cognition. Cerebellum 2016;15:732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum 2014;13:151–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex 2014;24:728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007;120:584–93. [DOI] [PubMed] [Google Scholar]
- 37.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013;80:807–15. [DOI] [PubMed] [Google Scholar]
- 38.Shah DK, Anderson PJ, Carlin JB, Pavlovic M, Howard K, Thompson DK, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res 2006;60:97–102. [DOI] [PubMed] [Google Scholar]
- 39.Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 2005;115:688–95. [DOI] [PubMed] [Google Scholar]
- 40.Qiu A, Shen M, Buss C, Chong YS, Kwek K, Saw SM, et al. Effects of antenatal maternal depressive symptoms and socio-economic status on neonatal brain development are modulated by genetic risk. Cereb Cortex 2017;27:3080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bommarito PA, Martin E, Fry RC. Effects of prenatal exposure to endocrine disruptors and toxic metals on the fetal epigenome. Epigenomics 2017;9:333–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Claud EC. Intrauterine inflammation, epigenetics, and microbiome influences on preterm infant health. Curr Pathobiol Rep 2018;6:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rock KD, Patisaul HB. Environmental mechanisms of neurodevelopmental toxicity. Curr Environ Health Rep 2018;5:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fichorova RN, Onderdonk AB, Yamamoto H, Delaney ML, DuBois AM, Allred E, et al. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. MBio 2011;2 e00280–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leviton A, Fichorova R, Yamamoto Y, Allred EN, Dammann O, Hecht J, et al. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine 2011;53:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McElrath TF, Fichorova RN, Allred EN, Hecht JL, Ismail MA, Yuan H, et al. Blood protein profiles of infants born before 28 weeks differ by pregnancy complication. Am J Obstet Gynecol 2011;204: 418.e1–12. [DOI] [PubMed] [Google Scholar]


