Abstract
Indolent B-cell lymphomas other than follicular lymphoma account for up to 10% of all B-cell neoplasms. While they typically follow a slowly progressive course, some patients experience rapid progression and early mortality. Prognostic scoring systems have not been adopted, hindering the ability of clinicians or researchers to predict outcomes, or risk-stratify patients during clinical trials. To address this, we evaluated the utility of existing prognostic indices and novel early disease-related outcomes to predict subsequent long term survival. Baseline characteristics and outcomes data were generated from a longitudinal cohort study that prospectively enrolled patients 623 newly diagnosed with marginal zone lymphoma, lymphoplasmacytic lymphomas, or B-cell lymphomas not otherwise specified, beginning in 2002. The International Prognostic Index (IPI), Follicular Lymphoma International Prognostic Index (FLIPI), and MALT International prognostic index (MALT-IPI) demonstrated c-statistics ranging from 0.593–0.612 for event-free survival (EFS), and 0.683–0.714 for overall survival (OS). Patients who attained event-free survival at 12 months (EFS12) experienced similar mortality to the US general population (standardized mortality ratio (SMR) 1.19; 95% CI 0.95–1.46). Patients who did not attain EFS12 had subsequent worse morality (SMR 3.14 (95% CI 2.05–4.59). The MALT-IPI demonstrated utility in predicting subsequent long-term outcomes among patients with non-follicular indolent B-cell lymphomas. This index should be used by clinicians giving guidance to patients at the time of initial diagnosis, and risk stratification during clinical studies. The divergent long-term outcomes experienced by patients who do or do not attain EFS12 suggest there exists a subset of patients who harbor high-risk disease. Future research efforts should focus on methods to identify these patients at the time of diagnosis, in order to enable risk-tailored therapy.
Introduction
Indolent (low-grade) B cell lymphomas are a pathologically diverse group of lymphomas that share the clinical features of slow progression, minimal initial clinical symptoms, and a relatively favorable prognosis. These include nodal marginal zone (NMZL) and extranodal marginal zone lymphomas (EMZL), splenic marginal zone (SMZL), lymphoplasmacytic lymphomas (LPL), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and follicular lymphoma (FL). Prognosis of DLBCL is informed by the International Prognostic Index (IPI),[1] while FL prognosis is typically evaluated using the follicular lymphoma international prognostic index (FLIPI).[2] Similarly, CLL/SLL, mantle cell lymphoma, and plasma cell myeloma each have well-established disease-specific prognostic indices.[3–5] A recent prognostic model for mucosal associated marginal zone lymphoma has been developed (MALT-IPI).[6] Additionally, two prognostic scoring systems have been developed specifically for splenic marginal zone lymphomas.[7, 8] However, relatively little is known about how the most informative prognostic models perform when applied to marginal zone lymphomas (splenic, nodal and EMZL), lymphoplasmacytic lymphomas, as well as a range of clinical entities that are difficult to definitively categorize beyond unclassifiable low grade B cell lymphomas NOS (referred to as “B-NOS” throughout this manuscript). We refer to this collection of subtypes, which in aggregate represent up to 10% of B cell neoplasms,[9] as non-follicular indolent B cell lymphomas (NFIBLs).
Clinical outcomes research among patients with NFIBLs is also hindered by a lack of distinctive clinical endpoints. The relatively good median overall survival (OS) of patients with indolent lymphomas paradoxically complicates the usage of OS as an endpoint in clinical trials and observational studies, by necessitating impractically long observation periods. Several recent publications have demonstrated the utility of earlier endpoints such as event-free survival at 12 (EFS12) or 24 (EFS24) months in multiple lymphoma subtypes, demonstrating strong correlations with long-term outcomes.[10–13] These early endpoints help clinicians recognize susceptible patients early in their treatment course, enabling management strategies tailored to their risk level, and can further focus clinical research efforts onto cohorts with identified unmet needs. Finally, such endpoints can serve as significant milestones for patients, by identifying those expected to achieve subsequent OS indistinguishable from that of the general population.
We designed the current study to elucidate the utility of existing prognostic indices and early clinical endpoints for predicting subsequent long-term outcomes of patients with NFIBLs. To accomplish this, we utilized a prospective observational study initiated in 2002, which enrolled newly diagnosed lymphoma patients with a protocol-specified methodology for capturing baseline clinical data, serial management strategy, and clinical events such as deaths, relapses, transformations, and treatment responses.[14]
Methods
Study Population
Patients were selected from the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE; CA97274) from September 2002 through December 2012. Full details of this prospective cohort study of Hodgkin Lymphoma (HL), Non-Hodgkin Lymphoma (NHL) and CLL/SLL outcomes have been previously published.[14] Briefly, enrollment into the MER is offered to consecutive patients newly diagnosed with HL, NHL or CLL/SLL who are evaluated at Mayo Clinic Rochester or the University of Iowa within 9 months of diagnosis, are age 18 years or older, and are US residents at time of diagnosis.
The MER collects clinical, pathologic, demographic and epidemiologic data at enrollment. Central pathology review in accordance with current WHO classification is performed by MER-affiliated hematopathologists. Patients are systematically followed every 6 months for the first 3 years and annually thereafter to assess events characterized as death, disease progression and retreatment which, initially patient reported, are verified through medical record review. For decedents, copies of the death certificate and/or medical records associated with death are obtained. Study physicians assign a cause of death using definitions developed for the Eastern Cooperative Oncology Group (ECOG) Intergroup trial 4494.[15] This study is reviewed and approved by the Human Subjects Institutional Review Board at the Mayo Clinic and the University of Iowa; written informed consent is obtained from all participants in accordance with the Declaration of Helsinki.
Statistical analysis
IPI, FLIPI, and MALT-IPI scores were assigned according to the algorithms defined by Shipp et al., Solal-Celigny et al., and Thieblemont et al. respectively as the sum of each of the included risk factors at the time of diagnosis.[1, 2, 6]
Overall survival (OS) was defined as time from diagnosis until death due to any cause. Event free survival (EFS) was defined as time from diagnosis until relapse or progression, unplanned retreatment of lymphoma after initial management, or death due to any cause. EFS indicators at predefined timepoints (i.e., EFS at 12 months (EFS12) or EFS at 24 months (EFS24)) were defined based on EFS status at the indicated timepoint after date of diagnosis. Kaplan-Meier curves were used to display survival curves. Expected survival accounting for age and sex was generated in R using the general US (survexp.us) population as reference groups.[16] Observed vs. expected OS was plotted using a conditional approach and summarized using standardized mortality ratios (SMR) of observed to expected deaths.[17–19] Concordance statistics (C-statistics) were generated for each respective index to measure their ability to discriminate EFS and OS. C-statistics quantify the area under receiver operator characteristic (ROC) curves, with C=0.5 indicating the model is no better than chance and C=1.0 indicating a perfect prediction rate. All analyses were performed using SASv9.4 and Rv3.4.2.
Results
Patient characteristics and patterns of clinical presentation
Six-hundred twenty-three patients with newly diagnosed NFIBL were enrolled in the MER from 2002–2012. The following types of lymphoma were included in our analysis: Extranodal Marginal Zone (EMZL; N=258), Gastric MALT lymphomas (MALT; N=41), Unclassifiable Low-grade B-cell Lymphoma (B-NOS; N=121), Lymphoplasmacytic Lymphoma (LPL; N=78), Nodal Marginal Zone (NMZL; N=51), and Splenic Marginal Zone (SMZL; N=74). Demographic and baseline clinical characteristics are demonstrated in Table 1. Gastric MALT lymphomas were considered separate from EMZL for analysis of baseline characteristics and initial treatment approaches, but thereafter were pooled with EMZL for survival analyses. Median age was 63 years (range 18–92 years) without notable variance among subtypes. For the majority of the analyzed subsets, gender was evenly distributed with males representing 52% of the population. There was, however, a stronger male predominance (74%) noted for patients with LPL.
Table 1:
Demographic and treatment information
| Extranodal MZL/MALT (N=258) | Gastric MALT (N=41) | B-NOS (N=121) | LPL (N=78) | Nodal MZL (N=51) | Splenic MZL (N=74) | Total (N=623) | |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| F | 149 (58%) | 16 (39%) | 51 (42%) | 20 (26%) | 23 (45%) | 38 (51%) | 297 (48%) |
| M | 109 (42%) | 25 (61%) | 70 (58%) | 58 (74%) | 28 (55%) | 36 (49%) | 326 (52%) |
| Age | |||||||
| Mean(SD) | 60 (13) | 67 (10) | 66 (11) | 62 (10) | 60 (11) | 64 (12) | 62 (12) |
| Median | 61 | 68 | 66 | 64 | 63 | 64 | 63 |
| Q1, Q3 | 53, 70 | 59, 76 | 58, 75 | 56, 68 | 53, 68 | 56, 73 | 54, 71 |
| Range | 18 – 88 | 50 – 87 | 35 – 85 | 31 – 88 | 36 – 80 | 28 – 92 | 18 – 92 |
| Age | |||||||
| <=60 | 128 (50%) | 12 (29%) | 38 (31%) | 33 (42%) | 21 (41%) | 30 (41%) | 262 (42%) |
| >60 | 130 (50%) | 29 (71%) | 83 (69%) | 45 (58%) | 30 (59%) | 44 (59%) | 361 (58%) |
| Ann Arbor Stage | |||||||
| I-II | 151 (60%) | 39 (100%) | 21 (19%) | 9 (12%) | 11 (22%) | 6 (8%) | 237 (39%) |
| III-IV | 100 (40%) | 0 (0%) | 91 (81%) | 69 (88%) | 38 (78%) | 66 (92%) | 364 (61%) |
| LDH | |||||||
| <=Normal | 194 (88%) | 24 (86%) | 75 (82%) | 49 (83%) | 29 (76%) | 45 (68%) | 416 (83%) |
| >Normal | 27 (12%) | 4 (14%) | 16 (18%) | 10 (17%) | 9 (24%) | 21 (32%) | 87 (17%) |
| # of Extranodal Sites | |||||||
| <=1 | 225 (87%) | 40 (98%) | 114 (94%) | 76 (97%) | 47 (92%) | 74(100%) | 576 (92%) |
| >1 | 33 (13%) | 1 (2%) | 7 (6%) | 2 (3%) | 4 (8%) | 0 (0%) | 47 (8%) |
| PS | |||||||
| <2 | 243 (96%) | 41 (100%) | 115 (97%) | 73 (94%) | 46 (92%) | 65 (88%) | 583 (95%) |
| >=2 | 10 (4%) | 0 (0%) | 3 (3%) | 5 (6%) | 4 (8%) | 9 (12%) | 31 (5%) |
| Hemoglobin | |||||||
| <12 g/dL | 43 (18%) | 6 (15%) | 40 (36%) | 43 (57%) | 11 (22%) | 46 (63%) | 189 (32%) |
| >=12 g/dL | 201 (82%) | 33 (85%) | 72 (64%) | 32 (43%) | 39 (78%) | 27 (37%) | 404 (68%) |
| Number of Nodes | |||||||
| <=4 | 187 (94%) | 34 (100%) | 76 (90%) | 46 (92%) | 23 (70%) | 53 (93%) | 419 (92%) |
| >4 | 12 (6%) | 0 (0%) | 8 (10%) | 4 (8%) | 10 (30%) | 4 (7%) | 38 (8%) |
| Initial Treatment | |||||||
| Alkylator | 34 (13%) | 5 (12%) | 23 (19%) | 24 (31%) | 16 (31%) | 5 (7%) | 107 (17%) |
| Anthracycline | 13 (5%) | 1 (2%) | 7 (6%) | 4 (5%) | 2 (4%) | 2 (3%) | 29 (5%) |
| Active surveillance | 90 (35%) | 6 (15%) | 62 (51%) | 18 (23%) | 18 (35%) | 41 (55%) | 235 (38%) |
| Other* | 13 (5%) | 17 (41%) | 7 (6%) | 9 (12%) | 3 (6%) | 1 (1%) | 50 (8%) |
| R-Mono | 34 (13%) | 3 (7%) | 15 (12%) | 19 (24%) | 8 (16%) | 7 (9%) | 86 (14%) |
| Surgery | 9 (3%) | 1 (2%) | 1 (0.8%) | 0 (0%) | 1 (2%) | 17 (23%) | 29 (5%) |
| XRT only | 65 (25%) | 8 (20%) | 6 (5%) | 4 (5%) | 3 (6%) | 1 (1%) | 87 (14%) |
Includes H. pylori-directed therapies.
LDH; lactate dehydrogenase. PS; Eastern Cooperative Oncology Group performance status, R-mono; rituximab monotherapy, XRT; radiation therapy.
Some notable differences were identified in clinical presentation among the subtypes that affected prognostic index scoring. Patients with EMZL presented more often with localized disease and thus presented with a lower Ann Arbor stage (60% stage I-II) as compared to NMZL (22% stage I-II) or SMZL (8% stage I-II). NMZL and SMZL also presented with the highest frequencies of abnormal LDH (24% and 32%, respectively), and anemia was most common among patients with SMZL or LPL (63% and 57%, respectively). Adverse performance status (≥ 2) occurred infrequently, but more often in patients presenting with NMZL and SMZL (8%; 12%).
Choices for Initial Management
Initial management and treatment strategies for patients with NFIBL are described in Table 1. Looking at the full cohort of 623 patients, 235 (38%) were initially followed with active surveillance, with no initial treatment given. 87 (14%) patients received radiation therapy, while 107 (17%) received alkylator therapy, and 86 (14%) received rituximab monotherapy. Only 5% of aggregate patients, and less than 10% in each subtype, received an anthracycline as part of their initial treatment strategy. Initial treatment patterns varied substantially based on lymphoma subtype. Patients with SMZL had the highest rates of surgical resection (n= 17, 23%) while patients with EMZL had the highest frequency of radiation as their initial treatment strategy (n = 65, 23%). Alkylator based chemotherapy was given with the highest frequency to patients with LPL (n = 24, 31%) or NMZL (n = 16, 31%).
Patient outcomes based on histology
Differences in baseline characteristics were reflected in patient’s prognostic indices at the time of presentation. As demonstrated in Figure 1, essentially half of all patients presented with low-risk disease as assessed by FLIPI or IPI, while MALT-IPI differentiated patients into more equivalent groups of low, intermediate, and high risk. Only 8 of 623 patients presented with high risk IPI. Differentiated by histologic subtype, patients with SMZL most frequently presented with high risk disease while patients with EMZL most frequently presented with low risk categorization.
FIGURE 1A:
Distribution of Risk Categories using IPI FLIPI or MALT-IPI for the entire NFIBL cohort and subtypes. Gastric MALT is included in EMZL. B-G, Kaplan Meyer Estimates for the entire NFIBL cohort of OS (left) and EFS (right), as distinguished by IPI (top row), FLIPI (middle row), and MALT-IPI (bottom row). EFS, event-free survival; EMZL, extranodal marginal zone lymphomas; IPI FLIPI, International Prognostic Index Follicular Lymphoma International Prognostic Index; NFIBLs, non-follicular indolent B cell lymphomas; OS, overall survival
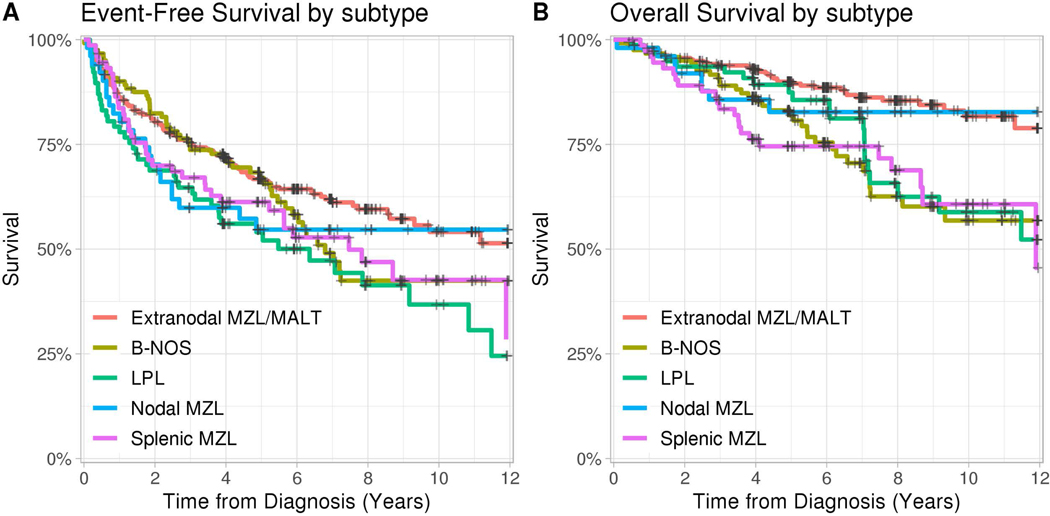
At a median follow-up of 83 months (range 5–168), 264 (42 %) patients had had an event and 127 patients (20%) had died. EFS and OS, stratified by performance index, are illustrated in figure 1, and stratified by histological subtype in Figure 2. 8 year OS for all subtypes ranged from 66% (LPL) to 85% (MALT). Of the 127 deaths, 106 had records available to verify their cause of death. Among this subset the cause of death was lymphoma (n=38) or therapy-related (n=6) for 42% of patients, while 47 patients (45%) died from other causes. Death was attributed to subsequent malignancy in 13% of patients.
FIGURE 2:
Kaplan Meyer Estimates of (A) EFS and (B) OS by NFIBL histological subtype. EFS event-free survival; OS, overall survival
Patient outcomes based on prognostic indices
IPI, FLIPI and MALT-IPI all demonstrated utility in differentiating outcomes among the cohort patients as assessed by EFS or OS based on initial prognostic index scores. For the outcome of EFS, the c-statistic was 0.593 for the IPI, 0.598 for the FLIPI and 0.612 for the MALT-IPI. All indices performed somewhat better when evaluating OS as the outcome, with c-statistics of 0.689 for the IPI, 0.683 for the FLIPI, and again MALT-IPI having the highest value of 0.714.
EFS12 and subsequent overall survival
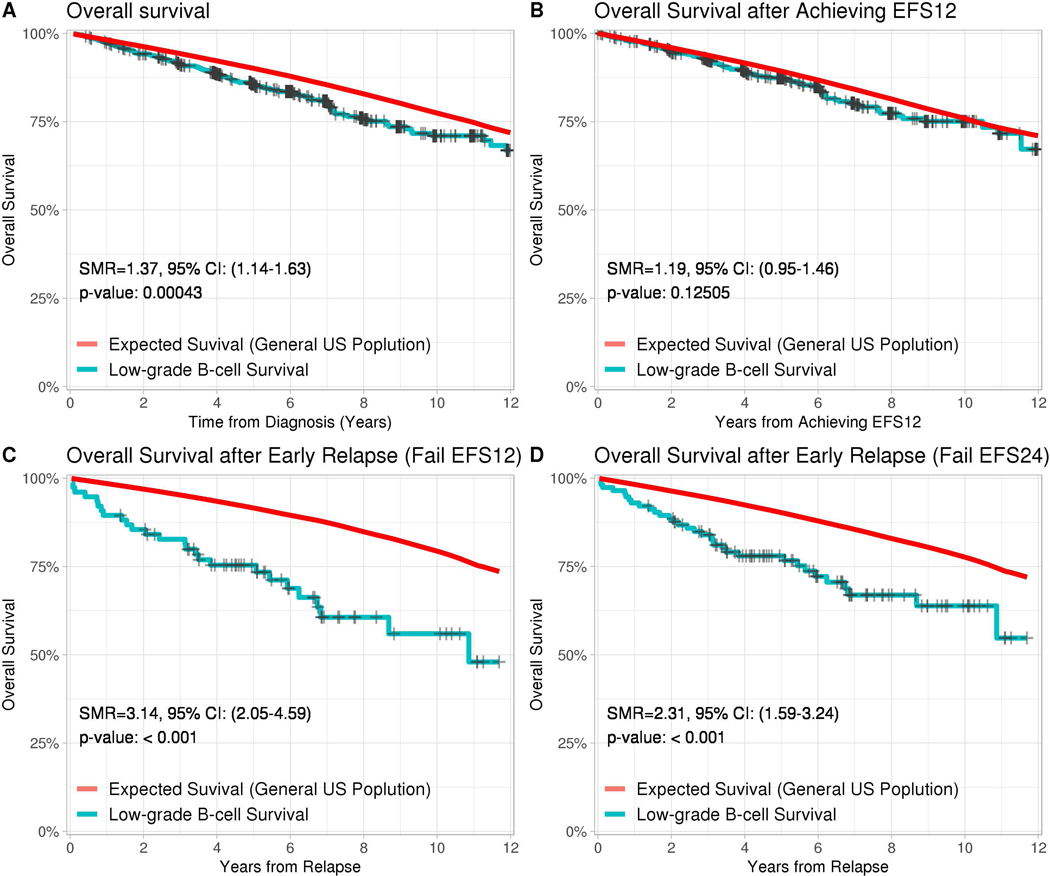
Because of the prevalence of non-lymphoma mortality over time, we sought to explore more precise predictors of premature, lymphoma-related mortality. For the entire cohort, survival was slightly lower than that of the comparable age- and sex- matched United States general population, with a standardized mortality ratio (SMR) of 1.37 (95% CI 1.14–1.63; Figure 3A). Kaplan-Meier estimates of patients achieving EFS12 and EFS24 were 85% (95% CI 82–88%) and 77% (95% CI 74–80%), respectively. Patients who attained event-free survival for the first 12 months following diagnosis (EFS12) subsequently experienced overall survival that was not statistically different from the general population (SMR 1.19; 95% CI 0.95–1.46; Figure 3B). Conversely, patients who did not attain EFS12 experienced significantly worse subsequent overall survival than the general population (SMR 3.14; 95% CI 2.05–4.59). Similar outcomes were observed for patients who did not attain EFS24 (SMR 2.31; 95% CI 1.59–3.24)
FIGURE 3:
Kaplan Meyer Estimates compared to age and gender matched general US population for (A) the entire NFIBL cohort, (B) patients who achieved EFS12, (C) patients who did not achieve EFS12, and (D) patients who did not achieve EFS24. EFS12, event-free survival at 12 months; NFIBL, non-follicular indolent B cell lymphoma
Discussion:
We report the clinical presentation, initial management, and outcomes of a large prospective cohort of patients in the United States with marginal zone, lymphoplasmacytic, or low grade B-cell lymphomas NOS. These patients were treated in the immunochemotherapy era, reflecting modern treatment practices. One exception may be the initial management of SMZL patients; the 23% observed rate of splenectomy as initial management of SMZL is likely higher than current rates and a reflection of practices prior to large published reports in 2012 and 2013 suggesting rituximab monotherapy may be a better choice for many patients.[20, 21] Overall however, our findings suggest that for prognostic purposes, these subtypes are similar enough to consider as a single category, which we refer to as NFIBLs. We additionally demonstrate that IPI, FLIPI, and MALT-IPI all have significant utility in predicting long-term prognosis among this cohort with MALT-IPI having perhaps the most advantages in terms of distribution among risk categories and prognostic distinction as measured by c-indices. Finally, we are the first to report that patients with NFIBL lymphomas who attain EFS12 can anticipate subsequent survival similar to a comparable general population.
An important question in the management of NFIBL is when to treat or when to defer therapeutic intervention at the time of diagnosis. Our data confirm that a diagnosis of NFIBL very modestly impacts mortality from time of diagnosis as compared to age-matched controls, and treating all asymptomatic patients may not yield meaningful clinical benefits. A subset of patients with a more aggressive disease course and shorter survival would ideally be identified early. Our data suggest early reassessment of EFS can facilitate identification of a small group of patients with NFIBL who are at higher risk for early mortality and therefore may benefit from more aggressive therapeutic interventions.
Survival outcomes in NFIBL and the utility of existing prognostic indices
The three prognostic indices used in this report, the IPI, FLIPI, and MALT-IPI harbor subtle differences in their included risk factors and stratification schemes. Briefly, all three indices assign a point for elevated LDH or stage III-IV disease, as well as for elevated age (>60 years for the IPI and FLIPI, and >70 for the MALT-IPI). The IPI also assigns a point each for ≥2 extranodal sites or ECOG PS >2, while the FLIPI assigns a point each for ≥4 nodal sites or hemoglobin <12 g/dL.
Each index has been previously evaluated in subsets of NFIBL. Among 144 German patients with MZL (96 extra-nodal, 32 nodal, and 16 splenic), the FLIPI was able to distinguish cohorts based upon 5 year PFS and OS, for patients with extra-nodal or splenic subtypes, but not nodal subtypes.[22] This was valid only when such patients were dichotomized to either low/intermediate or high risk groups. In a subsequent study of 143 Austrian patients with EMZL, both IPI and FLIPI scores significantly correlated with time to relapse, but the FLIPI was again able to differentiate patients when dichotomized as in the German study.[23] The MALT-IPI has been validated in two different studies of MALT lymphomas, although the most recent of these required high-risk patients be merged with intermediate-risk patients in order to discriminate outcomes.[24, 25]
While this manuscript was in preparation, another retrospective study was published, which examined the occurrence of high-grade transformation in MZL and its effects on subsequent PFS and OS.[26] The authors demonstrated that dichotomized IPI, FLIPI, and MALT-IPI scores are predictive of shorter PFS and OS. Our study contributes to the field by demonstrating that several non-follicular indolent lymphomas can be considered in aggregate for prognostic purposes, and by validating EFS12 as a key early endpoint that identifies those patients most in need of more aggressive therapies. Unlike the studies described above, the FLIPI was able to distinguish OS between low, intermediate, and high risk groups with no need for dichotomization. This may reflect the larger panel, longer follow up, or the expanded analysis of several subtypes in addition to MZL. In our series, IPI effectiveness is limited by the relative infrequency of poor performance status (4%) and multifocal extranodal disease (8%) resulting in very infrequent high risk scores, while FLIPI is limited primarily by infrequent multifocal nodal disease (9%). MALT-IPI performs optimally in this collection of patients with a robust distribution of prognostic scores across risk categories. Given the relatively marginal differences in C-statistics between the 3 indices in this study, each appears to have comparable utility.
Survival, whether measured by EFS or OS, was found to be similar regardless of subtype, with somewhat worse trends for splenic MZL, LPL or Low-grade B NOS subtypes. As a whole, diagnosis of NFIBL conveys a predicted overall survival only slightly distinguishable from the general population, likely reflecting the later age at diagnosis and typically indolent course.
Existing prognostic indices and alternative prognostic factors
Independent predictive factors in various NFIBL subsets have been previously proposed. A French series identified the presence of a serum M-protein or an immunological event as prognostic for shortened PFS in splenic MZL, while noting those same factors plus elevated ß2-microglobulin and leukocytosis were predictive for shortened OS.[27] Additionally, expression of proliferative marker Ki67 or the plasmacytic differentiation marker IRF4 (Mum1) have been found to correlate with a poorer prognosis amongst patients with lymphoplasmacytic or marginal zone lymphomas.[28] Additionally, p53 abnormalities, while rare, have been found to be associated with poor survival among patients with splenic marginal zone lymphomas.[29]
In addition, prognostic models unique to marginal zone subsets have been previously described. A marginal zone prognostic index (MZLIPI) incorporating nodal disease status, ECOG PS, and stage, was applied to a cohort of 205 Korean patients with non-gastric marginal zone lymphoma, and successfully discriminated PFS and OS across low, intermediate, and high risk groups.[30] The Intergruppo Italiano Linformi proposed a three parameter model of hemoglobin <12 g/dL, serum albumin <3∙5 g/dL and LDH >ULN, which accurately predicted cause-specific survival amongst 309 patients with splenic marginal zone lymphoma.[31] Future efforts may focus on comparing the performance of the above described prognostic indices with the MALT-IPI in NFIBL possibly with incorporation of novel prognostic factors, although our data suggests value in reassessing prognosis at the 12-month mark regardless of estimates at the time of diagnosis. Other approaches include the incorporation of gene expression profiling, which has shown promise in follicular lymphoma.[32]
Utility of the category “B-NOS”
The differential diagnosis of the NFIBLs requires integration of clinical, morphological, immunophenotypic and genetic data, yet even then diagnostic reproducibility is poor with only 56% concordance for LPL, and 63% for NMZL among experts in the Non-Hodgkin’s Lymphoma Classification Project.[1] Small B-cell lymphomas with plasmacytic differentiation frequently present diagnostic challenges,[33, 34] and marginal zone lymphoma may be mimicked closely by a variety of indolent B-cell neoplasms.[35] This appropriately leads to use of terms such as “low-grade B-cell lymphoma not otherwise specified (NOS)” or “small B-cell lymphoma with plasmacytic differentiation” as a diagnosis. This entity is reported in modern series with an incidence of 1–3% of NHL.[36, 37] In our series, low-grade B-cell lymphoma NOS represents 18% of the NFIBL’s and 1∙5% of NHL cases enrolled in the MER. Notably the median age is slightly higher for these patients than in the remainder of the NFIBL cohort and initial treatment is deferred in 60% of cases. Distribution of FLIPI, IPI, and MALT-IPI scores is similar to other NFIBL patients, but overall rates of death were slightly higher for this category. Among the classifiable LGL’s, these patients have clinical presentations most closely resembling splenic MZL except for the use of splenectomy in management.
Strengths and weaknesses
Strengths of this study include the prospective cohort design of consecutively enrolled, newly diagnosed lymphoma patients; central pathology review; systematically collected clinical data; virtually complete follow-up of the cohort for disease progression and death; and medical record validation of these events. The collection of 623 patients is a large series and all patients were managed in the current immunochemotherapy era – largely prior to incorporation of b-cell receptor targeting agents. The major limitations include the observational design and treatment and clinical follow-up based on routine practice, without prescribed re-biopsy criteria. Our follow-up, while longer than most previous series at a median of 83 months, remains modest for lymphoma subtypes with a long natural history, and continued follow-up will be necessary to understand long-term outcomes. While the MER is not a population-based sample, the vast majority of our patients are from the local region and the clinical characteristics of our patients parallel those of population-based data with the exception of fewer very elderly patients.
Conclusion.
This large prospective collection of data on patients with NFIBL presenting to academic medical centers provides a contemporary picture of presentation and management patterns useful to investigators designing clinical trials as well as for clinicians counseling patients. We find that the MALT-IPI has more prognostic utility than the FLIPI or IPI in this setting, but of greatest value in identifying patients at risk of early death is reassessment of disease behavior after 12 months. These clinical data and associated biologic specimens are an open resource for collaboration. As outcomes mature, comparative effectiveness measures should be applied to further advance prognostic ability.
Acknowledgements:
Funding provided by the National Institutes of Health (National Institutes of Health (Lymphoma SPORE (P50 CA CA97274)), Lymphoma Epidemiology of Outcomes (U01 CA195568) and the Predolin Foundation).
Sponsored by a National Institutes of Health grant (P50 CA97274) to the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence and the Henry J. Predolin Foundation.
Footnotes
The corresponding author confirms that he had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The authors have no conflicts of interest to disclose.
References:
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood, 1997. 89(11): p. 3909–18. [PubMed] [Google Scholar]
- 2.Solal-Celigny P, et al. , Follicular lymphoma international prognostic index. Blood, 2004. 104(5): p. 1258–65. [DOI] [PubMed] [Google Scholar]
- 3.Hoster E, et al. , A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood, 2008. 111(2): p. 558–65. [DOI] [PubMed] [Google Scholar]
- 4.International, C.L.L.I.P.I.w.g., An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol, 2016. 17(6): p. 779–790. [DOI] [PubMed] [Google Scholar]
- 5.Munshi NC, et al. , Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood, 2011. 117(18): p. 4696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thieblemont C, et al. , A MALT lymphoma prognostic index. Blood, 2017. 130(12): p. 1409–1417. [DOI] [PubMed] [Google Scholar]
- 7.Arcaini L, et al. , Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood, 2006. 107(12): p. 4643–9. [DOI] [PubMed] [Google Scholar]
- 8.Montalbán C, et al. , Risk stratification for Splenic Marginal Zone Lymphoma based on haemoglobin concentration, platelet count, high lactate dehydrogenase level and extrahilar lymphadenopathy: development and validation on 593 cases. Br J Haematol, 2012. 159(2): p. 164–71. [DOI] [PubMed] [Google Scholar]
- 9.Morton LM, et al. , Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood, 2006. 107(1): p. 265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casulo C, et al. , Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol, 2015. 33(23): p. 2516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hapgood G, et al. , Evaluation of the Risk of Relapse in Classical Hodgkin Lymphoma at Event-Free Survival Time Points and Survival Comparison With the General Population in British Columbia. J Clin Oncol, 2016. 34(21): p. 2493–500. [DOI] [PubMed] [Google Scholar]
- 12.Maurer MJ, et al. , International Assessment of Event-Free Survival at 24 Months and Subsequent Survival in Peripheral T-Cell Lymphoma. J Clin Oncol, 2017. 35(36): p. 4019–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer MJ, et al. , Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol, 2014. 32(10): p. 1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerhan JR, et al. , Cohort Profile: The Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) Cohort Study. Int J Epidemiol, 2017. 46(6): p. 1753–1754i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habermann TM, et al. , Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol, 2006. 24(19): p. 3121–7. [DOI] [PubMed] [Google Scholar]
- 16.Therneau T, A Package for Survival Analysis in S. 2013. [Google Scholar]
- 17.Breslow NE, et al. , Multiplicative Models and Cohort Analysis. Journal of the American Statistical Association, 1983. 78(381): p. 1–12. [Google Scholar]
- 18.Thernau T, et al. , Expected Survival Based on Hazard Rates. Technical report series; Section of biostatistics, 1994. 54. [Google Scholar]
- 19.Verheul HA, et al. , Background mortality in clinical survival studies. Lancet, 1993. 341(8849): p. 872–5. [DOI] [PubMed] [Google Scholar]
- 20.Else M, et al. , Rituximab, used alone or in combination, is superior to other treatment modalities in splenic marginal zone lymphoma. Br J Haematol, 2012. 159(3): p. 322–8. [DOI] [PubMed] [Google Scholar]
- 21.Kalpadakis C, et al. , Treatment of splenic marginal zone lymphoma with rituximab monotherapy: progress report and comparison with splenectomy. Oncologist, 2013. 18(2): p. 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heilgeist A, et al. , Prognostic value of the Follicular Lymphoma International Prognostic Index score in marginal zone lymphoma: an analysis of clinical presentation and outcome in 144 patients. Cancer, 2013. 119(1): p. 99–106. [DOI] [PubMed] [Google Scholar]
- 23.Troch M, Wohrer S, and Raderer M, Assessment of the prognostic indices IPI and FLIPI in patients with mucosa-associated lymphoid tissue lymphoma. Anticancer research, 2010. 30(2): p. 635–9. [PubMed] [Google Scholar]
- 24.Hong J, et al. , Validation of the Marginal Zone Lymphoma International Prognostic Index. Ann Hematol, 2019. 98(2): p. 457–464. [DOI] [PubMed] [Google Scholar]
- 25.Salar A, et al. , Long-term results of a phase 2 study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma. Blood, 2017. 130(15): p. 1772–1774. [DOI] [PubMed] [Google Scholar]
- 26.Alderuccio JP, et al. , Risk Factors for Transformation to Higher-Grade Lymphoma and Its Impact on Survival in a Large Cohort of Patients With Marginal Zone Lymphoma From a Single Institution. J Clin Oncol, 2018: p. JCO1800138. [DOI] [PubMed] [Google Scholar]
- 27.Thieblemont C, et al. , Treatment of splenic marginal zone B-cell lymphoma: an analysis of 81 patients. Clinical lymphoma, 2002. 3(1): p. 41–7. [DOI] [PubMed] [Google Scholar]
- 28.Petit B, et al. , Indolent lymphoplasmacytic and marginal zone B-cell lymphomas: absence of both IRF4 and Ki67 expression identifies a better prognosis subgroup. Haematologica, 2005. 90(2): p. 200–6. [PubMed] [Google Scholar]
- 29.Gruszka-Westwood AM, et al. , p53 abnormalities in splenic lymphoma with villous lymphocytes. Blood, 2001. 97(11): p. 3552–8. [DOI] [PubMed] [Google Scholar]
- 30.Oh SY, et al. , Nongastric marginal zone B-cell lymphoma: a prognostic model from a retrospective multicenter study. Cancer letters, 2007. 258(1): p. 90–7. [DOI] [PubMed] [Google Scholar]
- 31.Arcaini L, et al. , Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood, 2006. 107(12): p. 4643–9. [DOI] [PubMed] [Google Scholar]
- 32.Huet S, et al. , A gene-expression profiling score for prediction of outcome in patients with follicular lymphoma: a retrospective training and validation analysis in three international cohorts. Lancet Oncol, 2018. 19(4): p. 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina TJ, et al. , Marginal zone lymphomas with plasmacytic differentiation and related disorders. American journal of clinical pathology, 2011. 136(2): p. 211–25. [DOI] [PubMed] [Google Scholar]
- 34.Lin P, et al. , Lymphoplasmacytic lymphoma and other non-marginal zone lymphomas with plasmacytic differentiation. American journal of clinical pathology, 2011. 136(2): p. 195–210. [DOI] [PubMed] [Google Scholar]
- 35.Piris MA, et al. , A marginal zone pattern may be found in different varieties of non-Hodgkin’s lymphoma: the morphology and immunohistology of splenic involvement by B-cell lymphomas simulating splenic marginal zone lymphoma. Histopathology, 1998. 33(3): p. 230–9. [DOI] [PubMed] [Google Scholar]
- 36.Arora N, Manipadam MT, and Nair S, Frequency and distribution of lymphoma types in a tertiary care hospital in South India: analysis of 5115 cases using the World Health Organization 2008 classification and comparison with world literature. Leukemia & lymphoma, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Laurini JA, et al. , Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood, 2012. 120(24): p. 4795–801. [DOI] [PubMed] [Google Scholar]