Abstract
Background
Despite the absence of adequate safety or efficacy data, clindamycin is widely prescribed in the neonatal intensive care unit (NICU). We evaluated the association between clindamycin exposure and adverse events, as well as antibiotic effectiveness in infants.
Methods
This was a retrospective cohort study of infants receiving clindamycin prior to postnatal day 121 who were discharged from a Pediatrix Medical Group NICU (1997–2015). Using a previously developed pharmacokinetic model, we performed simulations to predict clindamycin exposure based on available dosing data. We used multivariable logistic regression to evaluate the association between clindamycin exposure and safety outcomes during and after clindamycin therapy. We reported the proportion of infants with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and clearance of MRSA bacteremia.
Results
A total of 4089 infants received clindamycin at a median (25th–75th percentile) dose of 15 mg/kg/day (12–16). Clearance increased with older gestational age. Infants with the highest total clindamycin exposure had marginally increased odds of NEC within 7 days (adjusted odds ratio=1.95 [1.04–3.63]), but exposure was not associated with death, sepsis, seizures, intestinal perforation, or intestinal strictures. Of 25 infants who had MRSA bacteremia, 19 (76%) cleared the infection by the end of the clindamycin course.
Conclusions
Higher clindamycin exposure was not associated with increased odds of death or non-laboratory adverse events. The use of pharmacokinetic models combined with available electronic health record data offers a valuable, cost-effective approach to analyzing the safety and effectiveness of drugs in infants when large-scale trials are not feasible.
Keywords: neonate, antibiotics, pharmacokinetics, pharmacodynamics, clindamycin
Clindamycin is the 6th most common antibiotic prescribed in the neonatal intensive care unit (NICU).1 A member of the lincosamide class of antibiotics, clindamycin works by binding to the 50S subunit of susceptible bacterial ribosomes and inhibiting protein synthesis. In infants, clindamycin has been included in guidelines as part of the regimen for treatment of complicated intra-abdominal infection.2,3 Clindamycin is also used to treat Gram-positive and anaerobic infections, particularly bacteremia and meningitis caused by Staphylococcus aureus (S. aureus), which are associated with high morbidity and mortality (~25%).4 Treatment with clindamycin is often indicated because a large fraction of S. aureus infections are resistant to methicillin.4
Despite clindamycin’s widespread use, the United States Food and Drug Administration (FDA) label does not address dosing in premature and term infants <1 month of age.5 Until recently, recommended dosing for clindamycin in infants was largely based on extrapolated adult and pediatric dosing, along with data from two pharmacokinetics studies of 12 and 40 infants published in the 1980s.6 Nevertheless, more recent population pharmacokinetic data derived from infants receiving clindamycin have become available using opportunistic study designs, and have led to new dosing recommendations.7,8 Despite this newer data, no studies large enough to evaluate clindamycin efficacy in infants have been performed, and safety data are limited. Clindamycin use in premature infants has been previously associated with the development of intestinal strictures and necrotizing enterocolitis (NEC).9,10 Of the 21 infants for which safety data were collected in a recent pharmacokinetic trial, nine (43%) experienced adverse events, though none were considered to be related to administration of clindamycin.7 Three infants experienced a seizure during the study, and two of these had a predicted exposure to clindamycin in the upper range of all infants enrolled in the pharmacokinetic study. The investigators suggested that further studies to evaluate clindamycin safety were needed.
While large-scale, prospective safety and efficacy studies are needed, such trials are difficult to perform in infants and are frequently underpowered. For example, in a partially-randomized, open-label safety trial of multiple antimicrobials for intraabdominal infection, only 46 infants were enrolled in the clindamycin arm; a larger number of infants is needed to identify rare adverse events.11–13 The Pediatrix Medical Group Clinical Data Warehouse is a rich source of safety and efficacy data that can be linked to clindamycin exposure among hospitalized infants. Our objective was to perform a pharmacoepidemiological study to evaluate the association between clindamycin exposure and adverse events, as well as antibiotic effectiveness in infants.
MATERIALS AND METHODS
Study Design and Sample
We conducted a retrospective cohort study of infants who initiated clindamycin prior to postnatal day 121 and were subsequently discharged from a NICU managed by the Pediatrix Medical Group between January 1, 1997 and December 31, 2015. The Pediatrix Medical Group Clinical Data Warehouse contains data obtained from admission notes, daily progress notes, and discharge summaries, including demographic data, medications, laboratory results, diagnoses, and procedures.12 A majority of data fields are entered via drop-down menus, while others allow free text. While the fields are not mandatory, all providers are trained to enter data, and the accuracy of the results from our database has been validated against external datasets from the Centers for Disease Control, Vermont Oxford Network, and the National Institute of Child Health & Human Development Neonatal Research Network.14–18 Clindamycin dosing information was automatically extracted from a free text field by two independent statisticians, and any conflicts were resolved by manual examination of the text field. Drug courses without complete dosing information (including amount and frequency), or those missing medication start or end dates, were excluded. Route of administration was not always available from the text field, but because we used a pharmacokinetics model based on intravenous dosing, courses that were known to be administered enterally were excluded. The first course of clindamycin meeting the inclusion criteria was included in the analysis.
Definitions
A clindamycin course was defined as the receipt of clindamycin on consecutive days. For example, the course was considered to be 8 days in duration if clindamycin was started on postnatal day 14, the dose was increased on postnatal day 17, and the course was continued until postnatal day 21.
Safety outcomes of interest included the following: laboratory adverse events, seizures, intestinal perforation, intestinal strictures, NEC, sepsis, and death. A laboratory adverse event was defined as any of the following occurring on a day of clindamycin therapy, excluding the first day of clindamycin therapy: direct bilirubin >5 mg/dL, creatinine >1.7 mg/dL, aspartate aminotransferase (AST) >200 units/L, alanine aminotransferase (ALT) >100 units/L, neutropenia defined by absolute neutrophil count <1000/mcL, or thrombocytopenia defined as platelet count <50 ×109/L. Creatinine values >10 mg/dL (<0.1% of values) were not included in the analysis due to suspected errors in transcription. Seizures, intestinal perforation, and intestinal strictures were defined according to clinician diagnosis. Likewise, NEC was defined as medical or surgical NEC (≥modified Bell stage II19) as diagnosed by the clinician. An episode of sepsis was defined as a new positive culture from blood or cerebrospinal fluid for any organism not generally considered a contaminant. Probable and definite coagulase-negative Staphylococcus were included.20
Methicillin resistant S. aureus (MRSA) bacteremia was defined as a positive blood culture for MRSA obtained during clindamycin therapy or within 7 days prior to the start of clindamycin therapy. Clearance of MRSA bacteremia was defined as documentation of at least one subsequent negative blood culture, without recurrence of a positive culture before the end of clindamycin therapy.
Pharmacokinetics Analysis
Using the population pharmacokinetic model developed from a previous pharmacokinetic study8 and the software NONMEM (Icon Development Solutions, Ellicott City, MD), we performed simulations to predict clindamycin exposure in infants. Daily weight and postmenstrual age were used to derive population estimates of pharmacokinetic parameters, including clearance and volume of distribution for each exposed infant. If daily weight was not available on the start day of the course, a weight within 3 days was used (or the birth weight if prior to postnatal day 11). Dosing information, population estimates of pharmacokinetic parameters, and estimates of inter-individual variability from the population pharmacokinetic model were used in the simulations. We calculated the total area under the curve (AUC) from 0 to 24 hours (AUC24), maximum concentration (Cmax), total cumulative AUC (AUCcum), volume of distribution, and clearance for each exposed infant.
Statistical Analysis
Means and standard deviations or medians and 25th–75th percentiles were reported for continuous variables, and counts and percentages were reported for categorical variables. We reported the proportion of infants with the safety outcomes of interest during the clindamycin course (excluding the first day of therapy) and at 7 days after the course by quartile of exposure, defined as the mean AUC24 during the course. For stricture and intestinal perforation, we also reported the proportion of infants with these outcomes within 30 and 90 days after the end of the course. We reported the proportion of infants with MRSA bacteremia and clearance of MRSA bacteremia.
We used multivariable logistic regression to evaluate the association between AUCcum, maximum Cmax, and the safety outcomes of interest. Exposure covariates were evaluated continuously and by quartile of exposure. We adjusted for gestational age, small for gestational age status,21 sex, and race as fixed effects. In models of AUCcum, duration of therapy was included as a fixed effect. Treatment center was included as a random effect. For the outcome of seizures, we included intraventricular hemorrhage (grade III or IV) as an additional covariate. For the outcome of NEC, we included a previous diagnosis of NEC (prior to the start day of therapy) as an additional covariate. For the outcomes of strictures and intestinal perforation, we included a previous diagnosis of NEC (including during therapy and 7 days after) as an additional covariate. Outcomes were evaluated during therapy plus 7 days after therapy (all safety outcomes), during therapy plus 30 and 90 days after therapy (intestinal perforation and stricture), and at any point prior to discharge (death).
P-values <0.05 were considered statistically significant. Analyses were conducted using Stata 15.1 software (College Station, TX).
RESULTS
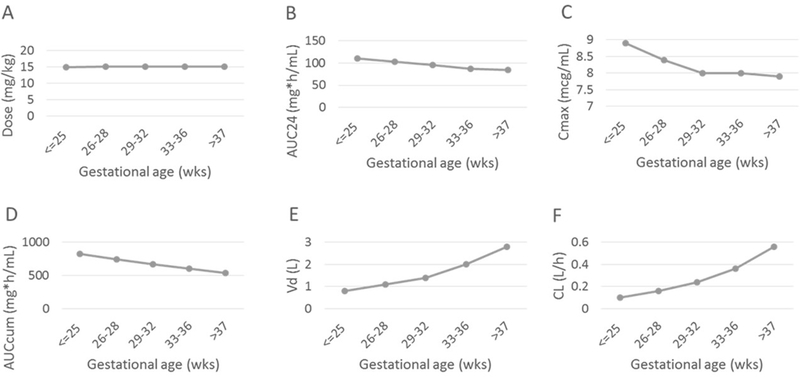
Of 7410 infants prescribed clindamycin, 4089 were prescribed a clindamycin course that met criteria for inclusion in the analysis. The median gestational age and birth weight of the infants were 30 weeks (25th–75th percentile: 26–34) and 1280 g (860–1940), respectively (Table 1). A total of 2299/4089 (56%) infants were male, and 693/4079 (17%) were small for gestational age. Of 3990 infants with known race/ethnicity, 1670 (42%) were White, 845 (21%) were Black, 1350 (34%) were Hispanic, and 125 (3%) were of other races or ethnicities. The median weight at the start of clindamycin course was 1555 g (1068–2210). Clindamycin courses started on a median postnatal age of 12 days (6–26) and were continued for a median duration of 7 days (4–10). The median daily dose of clindamycin was 15 mg/kg/day (12–16) (Supplemental Table 1). Overall, AUC24 and maximum Cmax decreased with gestational age, while volume of distribution and clearance increased (Figure 1).
Table 1.
Demographics and outcomes overall and by quartile of exposure (mean area under the plasma concentration time curve over the 24-hour dosing period [AUC24]).
| Total N=4089 | Quartile 1 N=1031 | Quartile 2 N=1010 | Quartile 3 N=1019 | Quartile 4 N=1029 | |
|---|---|---|---|---|---|
| AUC24 (mcg*h/mL)* | 96 (80–115) | 69 (61–75) | 89 (85–92) | 104 (99–109) | 134 (124–150) |
| Gestational age (wks)* | 30 (26–34) | 32 (28–35) | 31 (27–35) | 29 (26–32) | 27 (25–31) |
| Birth weight (g)* | 1280 (860–1940) | 1555 (990–2225) | 1450 (930–2140) | 1190 (835–1703) | 1040 (780–1590) |
| Postnatal age at start of course (days)* | 12 (6–26) | 9 (5–19) | 12 (5–25) | 14 (7–29) | 17 (7–32) |
| Postmenstrual age at start of course (wks)* | 32 (29–35) | 34 (31–37) | 34 (31–36) | 31 (29–33) | 31 (27–35) |
| Weight at start of course (g)* | 1555 (1068–2210) | 1700 (1153–2380) | 1731 (1200–2338) | 1435 (1047–1940) | 1385 (930–2065) |
| Duration of course (days)* | 7 (4–10) | 7 (4–10) | 7 (4–10) | 7 (4–10) | 7 (4–10) |
| Laboratory adverse event | |||||
| During course | 891/3771 (24) | 213/924 (23) | 208/931 (22) | 211/959 (22) | 259/957 (27) |
| During course + 7 days | 1142/3964 (29) | 266/990 (27) | 267/976 (27) | 274/996 (28) | 335/1002 (33) |
| Sepsis | |||||
| During course | 175 (4) | 41 (4) | 31 (3) | 44 (4) | 59 (6) |
| During course + 7 days | 278/3566 (8) | 74/861 (9) | 56/842 (7) | 67/928 (7) | 81/935 (9) |
| NEC | |||||
| During course | 148 (4) | 28 (3) | 39 (4) | 46 (5) | 35 (3) |
| During course + 7 days | 196/3561 (6) | 42/855 (5) | 49/839 (6) | 54/929 (6) | 51/938 (5) |
| Intestinal perforation | |||||
| During course | 43 (1) | 12 (1) | 11 (1) | 12 (1) | 8 (0.8) |
| During course + 7 days | 62/3552 (2) | 18/854 (2) | 17/838 (2) | 15/927 (2) | 12/933 (1) |
| During course + 30 days | 78/2025 (4) | 24/428 (6) | 20/416 (5) | 17/566 (3) | 17/615 (3) |
| During course + 90 days | 84/503 (17) | 24/97 (25) | 20/107 (19) | 19/134 (14) | 21/165 (13) |
| Seizures | |||||
| During course | 41 (1) | 8 (0.8) | 7 (0.7) | 15 (1) | 11 (1) |
| During course + 7 days | 66/3553 (2) | 17/855 (2) | 9/837 (1) | 20/925 (2) | 20/936 (2) |
| Stricture | |||||
| During course | 4 (0.1) | 1 (0.1) | 2 (0.2) | 1 (0.1) | 0 (0) |
| During course + 7 days | 4/3545 (0.1) | 1/851 (0.1) | 2/836 (0.2) | 1/925 (0.1) | 0/933 (0) |
| During course + 30 days | 16/2014 (0.8) | 2/423 (0.5) | 5/413 (1) | 6/563 (1) | 3/615 (0.5) |
| During course + 90 days | 20/469 (4) | 2/81 (2) | 6/101 (6) | 9/129 (7) | 3/158 (2) |
| Death | |||||
| During course | 0 | 0 | 0 | 0 | 0 |
| During hospitalization | 319/3671 (9) | 77/925 (8) | 72/898 (8) | 77/917 (8) | 93/931 (10) |
Median (25th-75th percentile)
AUC24 indicates area under the curve from 0 to 24 hours; NEC, necrotizing enterocolitis
Figure 1. Dosing and pharmacokinetic parameters by gestational age.
Dosing and pharmacokinetic parameters by gestational age: A) median daily dose (mg/kg); B) AUC24: mean area under the plasma concentration time curve over the 24 hour dosing period (mcg*h/mL); C) Cmax: maximum concentration (mcg/mL); D) AUCcum: total cumulative AUC (mcg*h/mL); E) Vd: volume of distribution (L); and F) CL: clearance (L/h).
At least one of the six laboratory measurements of interest was obtained in 3771 infants during clindamycin therapy, and 891/3771 (24%) had a laboratory adverse event during therapy (Table 1). Among infants with a laboratory adverse event, 668/881 (76%) had thrombocytopenia, 289/848 (34%) had neutropenia, 218/616 (35%) had elevated direct bilirubin, 49/285 (17%) had elevated ALT, 107/699 (15%) had elevated creatinine, and 33/272 (12%) had elevated AST. Other safety outcomes were less common during therapy, including sepsis (175/4089, 4.3%), NEC (148/4089, 3.6%), intestinal perforation (43/4089, 1.1%), seizures (41/4089, 1.0%), and strictures (4/4089, 0.1%).
In adjusted analysis of outcomes occurring during clindamycin therapy plus 7 days after therapy, exposure in either Quartile 3 or Quartile 4 of AUCcum, relative to exposure in Quartile 1, was associated with decreased odds of sepsis: odds ratio (OR) (95% confidence interval [CI]) 0.60 (0.39–0.91) and 0.56 (0.34–0.93), respectively (Table 2). Exposure in Quartile 2 of AUCcum was associated with marginally decreased odds of having a laboratory adverse event (OR 0.77 [0.60–0.98]). Exposure in Quartile 4 of AUCcum was associated with marginally increased odds of NEC (OR 1.95 [1.04–3.63]). AUCcum was not associated with seizures, intestinal perforation, or stricture during clindamycin therapy plus 7 days after therapy. During this time period, an increase in maximum concentration of 1 mcg/mL was associated with borderline increased odds of NEC (OR 1.05 [1.00–1.10]). Neither AUCcum nor maximum Cmax were associated with intestinal perforation or stricture in the 30 or 90 days after therapy (Table 3).
Table 2.
Laboratory and clinical outcomes during and seven days after clindamycin therapy. Values given are odds ratios and 95% confidence interval estimates.
| Continuous | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|
| Laboratory adverse event | |||||
| AUCcum | 1.01 (0.98–1.03) | Ref | 0.77 (0.60–0.98) | 0.83 (0.63–1.08) | 1.05 (0.75–1.47) |
| Maximum Cmax | 1.02 (0.99–1.05) | Ref | 0.97 (0.78–1.21) | 1.04 (0.84–1.30) | 1.15 (0.92–1.44) |
| Sepsis | |||||
| AUCcum | 0.99 (0.95–1.03) | Ref | 0.82 (0.56–1.21) | 0.60 (0.39–0.91) | 0.56 (0.34–0.93) |
| Maximum Cmax | 0.99 (0.94–1.04) | Ref | 0.73 (0.50–1.06) | 0.80 (0.55–1.17) | 0.83 (0.57–1.20) |
| NEC | |||||
| AUCcum | 1.00 (0.95–1.04) | Ref | 1.40 (0.82–2.40) | 1.36 (0.78–2.37) | 1.95 (1.04–3.63) |
| Maximum Cmax | 1.05 (1.00–1.10) | Ref | 1.33 (0.84–2.09) | 1.23 (0.77–1.96) | 1.52 (0.96–2.42) |
| Intestinal perforation | |||||
| AUCcum | 0.97 (0.91–1.04) | Ref | 1.45 (0.48–4.42) | 2.17 (0.76–6.19) | 2.23 (0.72–6.91) |
| Maximum Cmax | 0.99 (0.89–1.10) | Ref | 0.64 (0.30–1.39) | 0.88 (0.42–1.83) | 0.74 (0.34–1.59) |
| Seizures | |||||
| AUCcum | 1.02 (0.95–1.10) | Ref | 0.96 (0.44–2.07) | 0.88 (0.39–2.02) | 1.02 (0.38–2.73) |
| Maximum Cmax | 1.02 (0.94–1.12) | Ref | 0.62 (0.29–1.31) | 0.67 (0.32–1.38) | 1.07 (0.56–2.06) |
| Stricture | |||||
| AUCcum | 0.84 (0.63–1.13) | Ref | 6.87 (0.14–346.96) | 8.60 (0.31–241.72) | - |
| Maximum Cmax | 0.73 (0.39–1.35) | Ref | 0.31 (0.03–3.49) | - | 0.35 (0.03–4.18) |
AUCcum indicates total area under the curve during entire course (mcg*h/100mL); Cmax: maximum concentration (mcg/mL)
Table 3.
Laboratory and clinical outcomes during, 30 days after, and 90 days after clindamycin therapy. Values given are odds ratios and 95% confidence interval estimates.
| Continuous | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|
| 30 days after therapy | |||||
| Intestinal perforation | |||||
| AUCcum | 0.96 (0.88–1.04) | Ref | 1.04 (0.38–2.87) | 1.88 (0.75–4.76) | 1.56 (0.55–4.41) |
| Maximum Cmax | 0.99 (0.89–1.09) | Ref | 0.66 (0.31–1.38) | 0.87 (0.43–1.77) | 0.75 (0.36–1.58) |
| Stricture | |||||
| AUCcum | 0.94 (0.76–1.16) | Ref | 0.32 (0.05–1.99) | 0.66 (0.13–3.26) | 0.26 (0.03–2.32) |
| Maximum Cmax | 1.03 (0.86–1.24) | Ref | 1.24 (0.27–5.62) | 0.29 (0.03–2.91) | 2.06 (0.44–9.62) |
| 90 days after therapy | |||||
| Intestinal perforation | |||||
| AUCcum | 0.96 (0.88–1.04) | Ref | 0.97 (0.34–2.75) | 1.58 (0.57–4.39) | 1.11 (0.34–3.64) |
| Maximum Cmax | 0.96 (0.87–1.07) | Ref | 0.73 (0.33–1.62) | 0.84 (0.39–1.83) | 0.76 (0.35–1.65) |
| Stricture | |||||
| AUCcum | 0.91 (0.74–1.11) | Ref | 0.45 (0.07–2.75) | 0.82 (0.13–5.11) | 0.12 (0.01–1.89) |
| Maximum Cmax | 0.95 (0.77–1.16) | Ref | 2.49 (0.49–12.72) | 0.31 (0.03–3.67) | 2.06 (0.34–12.38) |
AUCcum indicates total area under the curve during entire course (mcg*h/100mL); Cmax: maximum concentration (mcg/mL)
No infants died during clindamycin therapy, and 319/3671 (9%) died prior to discharge. Compared to exposure in Quartile 1 of AUCcum, exposure in either Quartile 3 or Quartile 4 was associated with decreased odds of death prior to discharge (Table 4). Infants with exposure in Quartile 4 of maximum Cmax, compared to the 1st quartile also had decreased odds of death. A total of 25 infants had a positive blood culture for MRSA, and 19/25 (76%) cleared the infection by the end of the clindamycin course. Clearance occurred in 3/5 (60%) with mean AUC24 in Quartile 1, 2/2 (100%) in Quartile 2, 4/7 (57%) in Quartile 3, and 10/11 (91%) in Quartile 4.
Table 4.
Death outcomes during hospitalization. Values given are odds ratios and 95% confidence interval estimates.
| Continuous | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|
| Death | |||||
| AUCcum | 0.97 (0.93–1.01) | Ref | 0.47 (0.32–0.68) | 0.50 (0.33–0.74) | 0.49 (0.31–0.78) |
| Maximum Cmax | 0.95 (0.90–1.01) | Ref | 0.97 (0.67–1.39) | 0.79 (0.54–1.14) | 0.67 (0.46–0.98) |
AUCcum indicates total area under the curve during entire course (mcg*h/100mL); Cmax: maximum concentration (mcg/mL)
DISCUSSION
In this large cohort of >4000 infants hospitalized in the NICU, we were able to use available dosing data to evaluate the association between calculated drug exposure and hospital outcomes. When evaluating drug safety and effectiveness, calculation of actual drug exposure is superior to simply observing receipt of the drug or duration of therapy, because age-associated body composition and organ function can be taken into account.22 Our method capitalized on the existence of a large electronic health record with granular dosing information. Given the difficulty in performing the necessary trials in infants large enough to evaluate safety and efficacy,23,24 there has been an increase in studies using electronic health record data to support validation of pharmacokinetic models and assess safety in the infant population.25–27 Cohort studies using electronic health record data to compare different therapies can be substantially biased, as many factors, such as clinical indication and severity of condition, contribute to a clinician’s decision to prescribe a particular medication. Since clindamycin can be prescribed for multiple indications, and specific indication was not available from our database, we designed our study to examine the association between a range of clindamycin exposures and outcomes, which minimized the bias of the clinician’s choice of treatment.
We found that clindamycin dosing and exposure was variable among infants during the study period. The median AUC24 of 96 mcg*h/mL in our population was comparable to the AUC24 achieved in adults who receive 600 mg clindamycin every 8 hours, 116 mcg*h/mL.28,29 Exposure (AUC24 and Cmax) decreased with increasing gestational age, which was related to higher volume of distribution and clearance. Since similar doses were used across gestational age groups, these differences were a result of the inclusion of size and maturation, due to inclusion of weight and postmenstrual age in the pharmacokinetic model in the setting of similar doses.
The laboratory abnormalities evaluated in our study were selected based on availability in our database and adverse reactions listed in the clindamycin FDA label, which indicates that jaundice, liver function test abnormalities, renal dysfunction, leukopenia, and thrombocytopenia have been observed during clindamycin therapy, although a direct etiologic relationship has not been established for these events.30 Small case reports in adults31,32 and children33 have suggested a relationship between clindamycin and hepatotoxicity, with normalization after discontinuation of clindamycin therapy. In a pharmacokinetic and safety study of 21 term and preterm infants who received clindamycin, two (9.5%) infants experienced a laboratory adverse event during the study (anemia), and neither event was attributed to clindamycin.7 In our study, 24% of infants had a laboratory adverse event during the clindamycin course. After adjustment for confounding variables, we found a marginally decreased odds of laboratory adverse events in Quartile 2 of cumulative exposure compared to Quartile 1, but this association was not seen in Quartiles 3 or 4. This absence of dose effect, combined with marginal significance, suggests that the observed association was likely due to chance. Thrombocytopenia was the most common adverse event; this is not surprising, as thrombocytopenia is commonly associated with sepsis and intra-abdominal infection,34,35 both indications for clindamycin. Additionally, in our study, direct hyperbilirubinemia was also common; in many cases, direct hyperbilirubinemia occurred without substantially elevated liver enzymes. These findings are less consistent with a drug hepatotoxic effect and more consistent with cholestasis associated with sepsis or parenteral nutrition.
After adjustment for potential confounding variables, higher clindamycin exposure was associated with decreased odds of sepsis during therapy and the 7 days after therapy, as well as decreased odds of death overall. Perhaps higher exposure to clindamycin was directly responsible for these findings, but there are also other possible explanations. Because of the extreme variability in type and duration of infant exposure to other medications, our analyses did not incorporate concomitant use of such medications, including other antibiotics, which may have affected the likelihood of sepsis or death. Higher exposure to clindamycin could also have been an indicator of a more aggressive treatment strategy overall, leading to improved outcomes. Whatever the etiology of this apparent protective effect, it remains important to note that higher clindamycin exposures were not associated with an increased odds of sepsis or death.
The lack of association between clindamycin exposure and other adverse events such as seizures, intestinal perforation, and strictures was also suggestive of clindamycin safety. We did not include a comparator “control” arm of infants who did not receive clindamycin; however, infants with very high clindamycin exposure did not have an increased risk compared to infants with very low clindamycin exposure. Prior evidence linking clindamycin exposure to adverse intestinal outcomes was limited to older studies of a small numbers of infants.9,10 A case-control study of 124 infants with NEC matched to 248 control infants suggested that clindamycin use was associated with the development of NEC (unadjusted OR, 4.16 [1.29–13.44]).9 While increased AUCcum had a borderline association with NEC in our cohort, we speculate that this finding was a result of the use of higher doses in infants with suspected intra-abdominal processes who were ultimately diagnosed with NEC during clindamycin therapy. Unfortunately, we were unable to verify this theory, because the indication for clindamycin therapy was not captured in our database. A possible link between clindamycin treatment and the development of intestinal strictures was reported in a randomized, controlled trial published in 1988 comparing ampicillin/gentamicin (n=22) vs. ampicillin/gentamicin/clindamycin (n=20) for treatment of NEC in premature infants.10 In that study, 6/15 surviving infants who received clindamycin developed strictures, compared to 1/18 survivors who did not receive clindamycin (P=0.02). The dose in the older study (20 mg/kg/day divided every 8 hours) was higher than the median dose in our study (15 mg/kg/day). In our cohort, the development of strictures was uncommon, and there was no apparent clindamycin dose effect. Our study’s finding is consistent with the preliminary results of a partially-randomized trial in infants with intra-abdominal infection comparing the following antibiotic regimens: ampicillin/gentamicin/metronidazole; ampicillin/gentamicin/clindamycin; and piperacillin-tazobactam/gentamicin.11 In this ongoing study, 2/46 (4%) infants who received clindamycin developed strictures, and this frequency was similar among the three regimens.
Only 25 infants had positive blood cultures for MRSA in our cohort, which limited our ability to assess clindamycin’s effectiveness in this subset of patients. In addition, blood cultures were not obtained at standard intervals, and some infants may have cleared infection without documentation of negative blood culture. Finally, we may have underestimated the effectiveness of clindamycin because the drug may have been discontinued or switched prior to achievement of clearance for reasons unrelated to effectiveness (e.g. clinician preference, suspected adverse drug reactions). In children, clindamycin has been shown in observational studies to be effective in the treatment of invasive MRSA infection.36 Resistance to clindamycin varies, but has been reported to be <10% in different pediatric populations with MRSA bacteremia.36,37 In our cohort, resistance of MRSA isolates to clindamycin was not consistently tested or documented.
We acknowledge our study has several limitations. Notably, our ascertainment of adverse events was limited to those that had been previously reported; therefore, adverse events specific to neonates or only detectable in a larger sample size may have been missed by our approach. Clindamycin is highly protein bound (78–94%),38–40 and albumin and alpha-1 acid glycoprotein values were significant covariates in an infant pharmacokinetic model for clindamycin volume of distribution.7 Unfortunately, these laboratory values were unavailable for our cohort. Additionally, these values shift substantially depending on the age and health status of an infant, so such laboratory values would have shifted across the study.41,42 Consequently, we relied on a previous model that was based on data from both infants and older children.8 Unavailable protein concentrations would have most likely impacted Cmax calculations; since clearance models did not include these parameters, AUC calculations were not affected. We reported total concentrations of clindamycin in our cohort, because calculation of free concentrations would have required us to apply a uniform correction factor based on normal protein values in this population. Application of such a correction factor would not have changed our results and may have been misleading since we were not able to calculate true free concentrations. Additionally, although we attempted to adjust for factors that may have affected infant outcomes, there may have been other unmeasured confounders that could have influenced the association between exposure and the outcomes described above. Finally, infants who were discharged or transferred could have developed adverse events that were not captured in our study.
In conclusion, in this large cohort of infants who received clindamycin, calculated clindamycin exposure was comparable to target exposures in adult populations. Exposure was higher in smaller, less mature infants. Higher clindamycin exposure was not associated with negative outcomes including increased odds of death, NEC, intestinal perforations, strictures, or seizures. Higher clindamycin exposure was associated with decreased odds of sepsis during and the 7 days after therapy. A large-scale randomized clinical trial would provide further evidence to validate the safety and effectiveness of clindamycin. In the absence of large-scale trials given their difficulty and cost to perform, the use of validated pharmacokinetic models combined with available electronic health record data offers a valuable, cost-effective approach to evaluating safety and effectiveness of drugs in infants.
Supplementary Material
Acknowledgments
Funding: This project was supported by the Division of Microbiology and Infectious Diseases (DMID), National Institute of Allergy and Infectious Diseases (NIAID) of NIH through the Vaccine and Treatment Evaluation Units (VTEU), and the US Department of Health and Human Services under contracts HHS (Duke University HHSN272201300017I)
Disclosures: RGG has received support from industry for research services (https://dcri.org/about-us/conflict-of-interest/).
REFERENCES
- 1.Hsieh EM, Hornik CP, Clark RH, et al. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. [DOI] [PubMed] [Google Scholar]
- 3.Bell MJ, Ternberg JL, Bower RJ. The microbial flora and antimicrobial therapy of neonatal peritonitis. J Pediatr Surg. 1980;15:569–573. [DOI] [PubMed] [Google Scholar]
- 4.Shane AL, Hansen NI, Stoll BJ, et al. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129:e914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fresenius Kabi USA, LLC. CLINDAMYCIN - clindamycin phosphate injection, solution drug label. NIH U.S. National Library of Medicine DailyMed; web site. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bd1c1648-4733-4233-8337-469fe11bd0cb. Updated October 25, 2016 Accessed March 25, 2019. [Google Scholar]
- 6.Koren G, Zarfin Y, Maresky D, Spiro TE, MacLeod SM. Pharmacokinetics of intravenous clindamycin in newborn infants. Pediatr Pharmacol (New York). 1986;5:287–292. [PubMed] [Google Scholar]
- 7.Gonzalez D, Delmore P, Bloom BT, et al. Clindamycin pharmacokinetics and safety in preterm and term infants. Antimicrob Agents Chemother. 2016;60:2888–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faix RG, Polley TZ, Grasela TH. A randomized, controlled trial of parenteral clindamycin in neonatal necrotizing enterocolitis. J Pediatr. 1988;112:271–277. [DOI] [PubMed] [Google Scholar]
- 11.Smith MJ, Autmizaguine J, Hudak M, et al. Antibiotic safety and efficacy in infants with complicated intra-abdominal infections. Oral presentation at: Pediatric Academic Societies Meeting; May, 2018; Toronto, Canada. [Google Scholar]
- 12.Spitzer AR, Ellsbury DL, Handler D, et al. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 13.ClinicalTrials.gov Antibiotic Safety (SCAMP). ClinicalTrials.gov web site. https://clinicaltrials.gov/ct2/show/NCT01994993. Accessed September 13, 2019.
- 14.Ellsury DL, Clark RH, Ursprung R, et al. A multifaceted approach to improving outcomes in the NICU: the Pediatrix 100 000 Babies Campaign. Pediatrics. 2016;137. [DOI] [PubMed] [Google Scholar]
- 15.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129:1019–1026. [DOI] [PubMed] [Google Scholar]
- 16.Stoll BJ, Hansen NI, Walsh MC, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko JY, Patrick SW, Tong VT, et al. Incidence of neonatal abstinence syndrome - 28 states, 1999–2013. MMWR Morb Mortal Wkly Rep. 2016;65:799–802. [DOI] [PubMed] [Google Scholar]
- 18.Tolia VN, Patrick SW, Bennett MM, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372:2118–2126. [DOI] [PubMed] [Google Scholar]
- 19.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88 Suppl 2:S69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen IE, Groveman SA, Lawson ML, et al. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–224. [DOI] [PubMed] [Google Scholar]
- 22.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. [DOI] [PubMed] [Google Scholar]
- 23.Laventhal N, Tarini BA, Lantos J. Ethical issues in neonatal and pediatric clinical trials. Pediatr Clin North Am. 2012;59:1205–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward RM, Kern SE. Clinical trials in neonates: a therapeutic imperative. Clin Pharmacol Ther. 2009;86:585–587. [DOI] [PubMed] [Google Scholar]
- 25.Salerno S, Hornik CP, Cohen-Wolkowiez M, et al. Use of population pharmacokinetics and electronic health records to assess piperacillin-tazobactam safety in infants. Pediatr Infect Dis J. 2017;36:855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornik CP, Benjamin DK Jr, Smith PB, et al. Electronic health records and pharmacokinetic modeling to assess the relationship between ampicillin exposure and seizure risk in neonates. J Pediatr. 2016;178:125–129.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge S, Beechinor RJ, Hornik CP, et al. External evaluation of a gentamicin infant population pharmacokinetic model using data from a national electronic health record database. Antimicrob Agents Chemother. 2018;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaPlante KL, Leonard SN, Andes DR, et al. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob Agents Chemother. 2008;52:2156–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis RE, Klepser ME, Ernst EJ, et al. Evaluation of low-dose, extended-interval clindamycin regimens against Staphylococcus aureus and Streptococcus pneumoniae using a dynamic in vitro model of infection. Antimicrob Agents Chemother. 1999;43:2005–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aurobindo Pharma Limited. CLINDAMYCIN PALMITATE HYDROCHLORIDE (PEDIATRIC) - clindamycin palmitate hydrochloride (pediatric) solution drug label. NIH U.S. National Library of Medicine DailyMed; web site. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0bfe1e25-f0a1-4e11-9827-82912557c46b. Updated September 10, 2018 Accessed March 25, 2019. [Google Scholar]
- 31.Hinthorn DR, Baker LH, Romig DA, et al. Endocarditis treated with clindamycin: relapse and liver dysfunction. South Med J. 1977;70:823–826. [DOI] [PubMed] [Google Scholar]
- 32.Elmore M, Rissing JP, Rink L, et al. Clindamycin-associated hepatotoxicity. Am J Med. 1974;57:627–630. [DOI] [PubMed] [Google Scholar]
- 33.Kauffman RE, Shoeman DW, Suk-Han-Wan, et al. Absorption and excretion of clindamycin-2-phosphate in children after intramuscular injection. Clin Pharmacol Ther. 1972;13:704–709. [DOI] [PubMed] [Google Scholar]
- 34.Song R, Subbarao GC, MAheshwari A. Haematological abnormalities in neonatal necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2012;25 Suppl 4:22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guida JD, Kunig AM, Leef KH, et al. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. 2003;111:1411–1415. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Aguilar G, Hammerman WA, Mason EO Jr, et al. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus in children. Pediatr Infect Dis J. 2003;22:593–598. [DOI] [PubMed] [Google Scholar]
- 37.Pérez G, Martiren S, Reijtman V, et al. Community-acquired Staphylococcus aureus bacteremia in children: a cohort study for 2010–2014. Arch Argent Pediatr. 2016;114:508–513. [DOI] [PubMed] [Google Scholar]
- 38.Son DS, Osabe M, Shimoda M, et al. Contribution of α1-acid glycoprotein to species difference in lincosamides-plasma protein binding kinetics. J Vet Pharmacol Ther. 1998;21:34–40. [DOI] [PubMed] [Google Scholar]
- 39.Zhanel GG, Kirkpatrick ID, Hoban DJ, et al. Influence of human serum on pharmacodynamic properties of an investigational glycopeptide, LY333328, and comparator agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:2427–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flaherty JF Jr, Gatti G, White J, et al. Protein binding of clindamycin in sera of patients with AIDS. Antimicrob Agents Chemother. 1996;40:1134–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSci. 2002;4:E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cartlidge PH, Rutter N. Serum albumin concentrations and oedema in the newborn. Arch Dis Child. 1986;61:657–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.