Abstract
Regular exercise reduces risk of various chronic diseases and can prevent the development and recurrence of cancer, making it a promising non-pharmacological modulator of disease. Yet the effect of regular exercise on solid organ transplant outcome remains uncertain. Using a model of voluntary wheel running exercise and skin transplantation in mice, we hypothesized that exercise strengthens the alloimmune response, leading to an increased rate of rejection. Instead, we found that regular exercise in mice resulted in prolonged graft survival, with mean allograft survival time increasing by almost 50%. We observed this graft survival extension in exercised mice despite evidence of a slightly enhanced alloimmune response, comprised of increased proliferation of alloreactive CD4+ T cells, as well as increased IFNγ production by these cells. Exercise was not associated with significant changes in numbers of conventional CD4+ or CD8+ T cells, NK cells, or Foxp3+ regulatory T cells. In conclusion, our study suggests that exercise increases skin graft resistance to a similar or slightly higher level of alloimmunity and supports regular exercise as an important beneficial pursuit for transplant recipients.
1. Introduction
Various epidemiological studies report that physical activity level is an important predictor of disease occurrence and progression. Lower physical activity level is associated with higher incidence of chronic diseases such as metabolic syndrome and cardiovascular disease (1), while regular exercise can protect against the development and recurrence of certain cancers (2, 3). Because physical inactivity is a predominant yet modifiable risk factor for disease (4), numerous studies have investigated exercise as a non-pharmacological intervention in disease, including in cancers and in autoimmune diseases (5, 6). However, whether exercise modulates transplant outcomes remains to be determined.
Recent work demonstrated that voluntary running in tumor-bearing mice resulted in an approximately 60% reduction in tumor growth and dramatically reduced frequency of metastases, due to increased epinephrine- and IL-6-mediated NK cell mobilization and tumor infiltration in exercising mice (7). Regular stretching in mice also significantly reduced tumor volume, alongside modestly increased levels of inflammatory cytokines, including IL-6 and IFNγ (8). These works and others suggest that exercise augments immunity (9, 10). Conversely, voluntary wheel running in a mouse model of colitis resulted in a significant reduction in colitis symptoms and inflammation-associated gene expression (11). In addition, acute high-intensity swimming increased levels of circulating immunosuppressive Foxp3+ regulatory T cells (Tregs) in elite adolescent swimmers (12). Hence, exercise may also have immune-dampening effects, reducing inflammation and proving beneficial for autoimmune diseases (6).
Given the conflicting roles of exercise in immune-mediated conditions, we aimed to understand how exercise affects transplant rejection. Solid organ transplantation is a common treatment for end-stage organ failure. However, in the absence of sufficient immunosuppression, recognition of the transplanted donor tissue as foreign leads to T cell-dependent graft rejection (13). The kinetics of acute rejection in the absence of immunosuppression depend mainly on the extent of genetic disparities between the donor and the recipient, although host environmental factors, such as high-fat (14) and high-salt diet (15), infection (16–19), and the microbiota (20, 21), have also been shown to impact graft survival. Using a murine model of voluntary wheel running and skin transplantation, we hypothesized that exercise strengthens the alloimmune response, ultimately leading to faster rejection. However, we instead provide evidence that voluntary wheel running prolongs skin graft survival, despite slight enhancement of the alloresponse.
2. Materials and Methods
2.1. Mice and Exercise Model
Eight-week-old female and male C57BL/6 mice (B6, purchased from Harlan–Envigo) were divided into control (non-exercising) and exercise enrichment cages. Control cages were equipped with unmodified igloos, while exercise cages contained both running wheels (diameter 11 cm) and igloos equipped with Fast-Trac (Bio-Serv) running tracks. Mice were housed in enrichment cages for four weeks prior to transplantation and until termination of the experiments after transplantation. Marilyn female mice on a CD45.1+ RAG-KO B6 background expressing a transgenic TCR specific for an H-Y antigen presented by I-Ab (22) were obtained from Charles Mainhart via Taconic Biosciences and were bred in house. 2W1S-Ova+ female B6 mice that express a ubiquitous transgene for the model antigen 2W1S (23) were obtained from James J. Moon (MGH, Boston) and were bred in house.
2.2. Weight and Blood Glucose Assessment
Body weight and heart weight were measured using a laboratory scale. Hearts were bisected to remove blood clots prior to weighing. Blood glucose was measured after 13 hours of fasting, following 3.5 weeks of habitation in control or exercise cages.
2.3. Skin Transplantation
Tail skin from control or exercised male B6 donor mice was transplanted onto the flank of control or exercised female B6 recipients, respectively. Bandages were removed after 7 days and graft survival was monitored every other day thereafter. The day at which less than 20% of viable skin tissue remained was reported as the day of rejection.
2.4. CFSE Labeling and Adoptive Transfer of Marilyn T Cells
Spleen and lymph nodes (inguinal, brachial, axillary, cervical, and mesenteric) were isolated from Marilyn mice. Spleens and lymph nodes were homogenized and splenic red blood cells were lysed using ACK lysis buffer. Spleen and lymph node homogenates were then combined and suspended (10×106 cells/ml) in sterile phosphate buffered saline (PBS) with 5μM CellTrace CFSE (Invitrogen) for 20 minutes at 37°C, then quenched with an equal volume of fetal bovine serum (FBS). Cells were washed and resuspended in sterile PBS. CFSE-labeled Marilyn cells (2×105 or 6×105 cells) were transferred intravenously (I.V.) in 200μL into female B6 mice (CD45.1−) 1 day before skin transplantation.
2.5. Leukocyte Isolation
Skin graft-draining lymph nodes (axillary, brachial, and inguinal) were isolated at the indicated times, resuspended in complete DMEM (Corning, containing 10% FBS, 1% penicillin/streptomycin, 1% L-glutamine, 1% non-essential amino acids, 1% HEPES and 50μM β-mercaptoethanol), and counted using an Accuri C6 Plus Flow Cytometer (BD Biosciences). For skin leukocyte isolation, either skin graft or shaved flank was harvested. The skin was cut into small pieces, resuspended in 2.5mL RPMI medium (Gibco) supplemented with Liberase (0.4mg/mL, Roche) and DNAse I (0.01%, MP Biomedicals), and then incubated for 2 hours while shaking at 37°C. After incubation, the skin was homogenized using a 70μm cell strainer and syringe plunger. Single-cell suspensions were centrifuged, and pellets were resuspended in complete DMEM.
2.6. Flow Cytometry
Spleen and lymph node cells were stained with Fixable Aqua Dead Cell Stain (Invitrogen), then stained with fluorophore-labeled antibodies against CD4, CD8, TCRβ, CD44, CD45.1, NK1.1, or PD-1. Cells were then fixed and permeabilized using Foxp3/Transcription Factor Staining kit (eBioscience) and stained with fluorophore-conjugated antibodies against Foxp3, Ki67, or IFNγ. In some experiments, cells were stained using fluorophore-conjugated 2W1S:I-Ab tetramers prior to surface antibody staining. Graft-infiltrating cells were stained with Fixable Aqua Dead Cell Stain prior to phenotypic staining, then with fluorophore-conjugated antibodies against CD4, CD8, CD44, CD45.1, and PD-1, and finally fixed, permeabilized, and stained with anti-Foxp3 antibody. All fluorophore-conjugated antibodies were from Invitrogen, eBioscience, or BD Pharmingen. Flow cytometry was performed on an LSR Fortessa (BD Biosciences) and analyzed using FCS Express 6 Flow software.
2.7. Ex vivo stimulation of Marilyn T Cells
Single cell suspensions from skin-draining lymph nodes isolated 4 days post-transplantation were plated in 24-well tissue culture plates (2 or 5×106 cells/well). Anti-CD3 (2C11, 5μg/mL), anti-CD28 (PV.1, 1μg/mL), and brefeldin A (BioLegend, 5μg/ml) were added into each well, and cells were incubated for 24 hours at 37°C in 5% CO2. Stimulated cells were then harvested and stained as described above.
2.8. Donor Splenocyte Transfusion (DST) Immunization
Following 4 weeks of habitation in control or exercise cages, female B6 mice were each immunized I.V. with one quarter of a homogenized spleen from a 2W1S-Ova+ female B6 mouse. Spleens from recipients were harvested one week later, and leukocytes were isolated and stained as described above.
2.9. Statistical Analysis
Graft survival curves were compared using Kaplan-Meier plots and log-rank (Mantel-Cox) tests. Comparisons between control and exercised cohorts were analyzed with two-tailed unpaired t test. P values of less than 0.05 were considered to be statistically significant. Statistical analyses were performed using GraphPad Prism 7.
2.10. Study Approval
Mouse studies were approved by the Animal Resources Center of the University of Chicago under IACUC guidelines (protocol 71095) and according to the NIH guidelines for animal use.
3. Results
3.1. Model of voluntary exercise in female B6 mice
We began by establishing a model of voluntary wheel-running exercise in mice. Weightmatched male and female B6 mice were divided into control (non-exercising) or exercise enrichment cages where they were maintained for four weeks prior to skin transplantation of male skin to female recipients. The recipients remained in their assigned enrichment cages until termination of the experiments, which allowed them to continue exercising after transplantation (Figure 1A).
Figure 1. Model of voluntary exercise in female B6 mice.
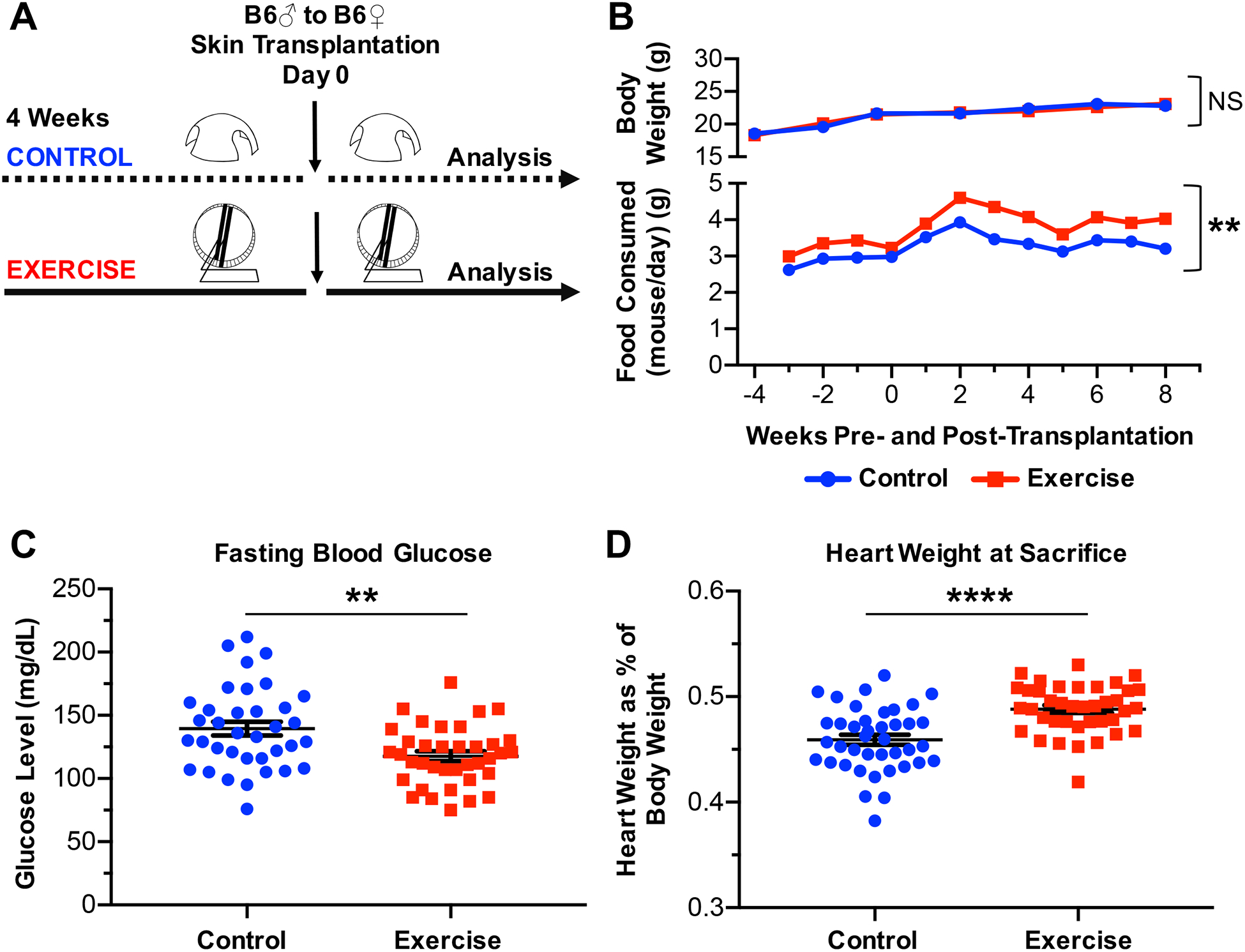
(A) Experimental design. (B) Average body weight of female B6 control (n=11) or exercised (n=10) mice, and average food consumed per mouse per day for female B6 control (n=11) or exercised (n=10) mice, recorded over experimental time. (C) Fasting blood glucose levels of female B6 control (n=36) or exercised (n=37) mice, following 3.5 weeks of habitation in control or exercised cages. (D) Heart weight as percent of body weight upon sacrifice of female B6 control (n=40) or exercised (n=39) mice. (C-D) Each point represents a single mouse. Results are displayed as mean ± SEM. (B-D) Comparisons were performed using a two-tailed unpaired t test. **p<0.01, ****p<0.0001, NS = not significant. Results were combined from 4 independent experiments.
Average body weights of female control or exercised mice remained comparable through 12 weeks of housing under experimental conditions (p=0.9705, NS), though exercised mice consistently consumed more food than control mice (p=0.0039, Figure 1B). Moreover, fasting blood glucose levels were significantly higher among control than exercised mice (p=0.0015), and heart weights upon sacrifice were significantly higher among exercised than control mice (p <0.0001) (Figures 1C and 1D). These results confirmed that exercised mice were more physically active and validated our model of voluntary wheel-running exercise.
3.2. Exercise results in prolonged allograft survival but does not reduce allogeneic T cell priming
Using our model of voluntary exercise, we investigated the impact of exercise on transplant rejection and the alloimmune response. First, to examine the effect of exercise on the kinetics of graft rejection, control or exercised female B6 mice were transplanted with skin grafts from control or exercised male B6 mice, respectively, and were monitored until rejection. Surprisingly, exercised mice experienced prolonged graft survival (p=0.0386), with the mean allograft survival time increasing from 29.8±8.195 days in control mice to 43.9±20.44 days in exercised mice (Figure 2A). Of note, two of the exercised mice failed to reject their allografts by the time of sacrifice on day >80 post-transplantation. Although these two mice had 2 of the largest hearts at sacrifice, suggesting they exercised more, other mice with similarly large hearts displayed shorter graft survival. Overall, there was no correlation between either heart size, or fasting glucose level, and skin graft survival duration (data not shown), suggesting that it is not the amount of exercise that matters, but rather the presence or absence of any exercise.
Figure 2. Exercise results in prolonged allograft survival.
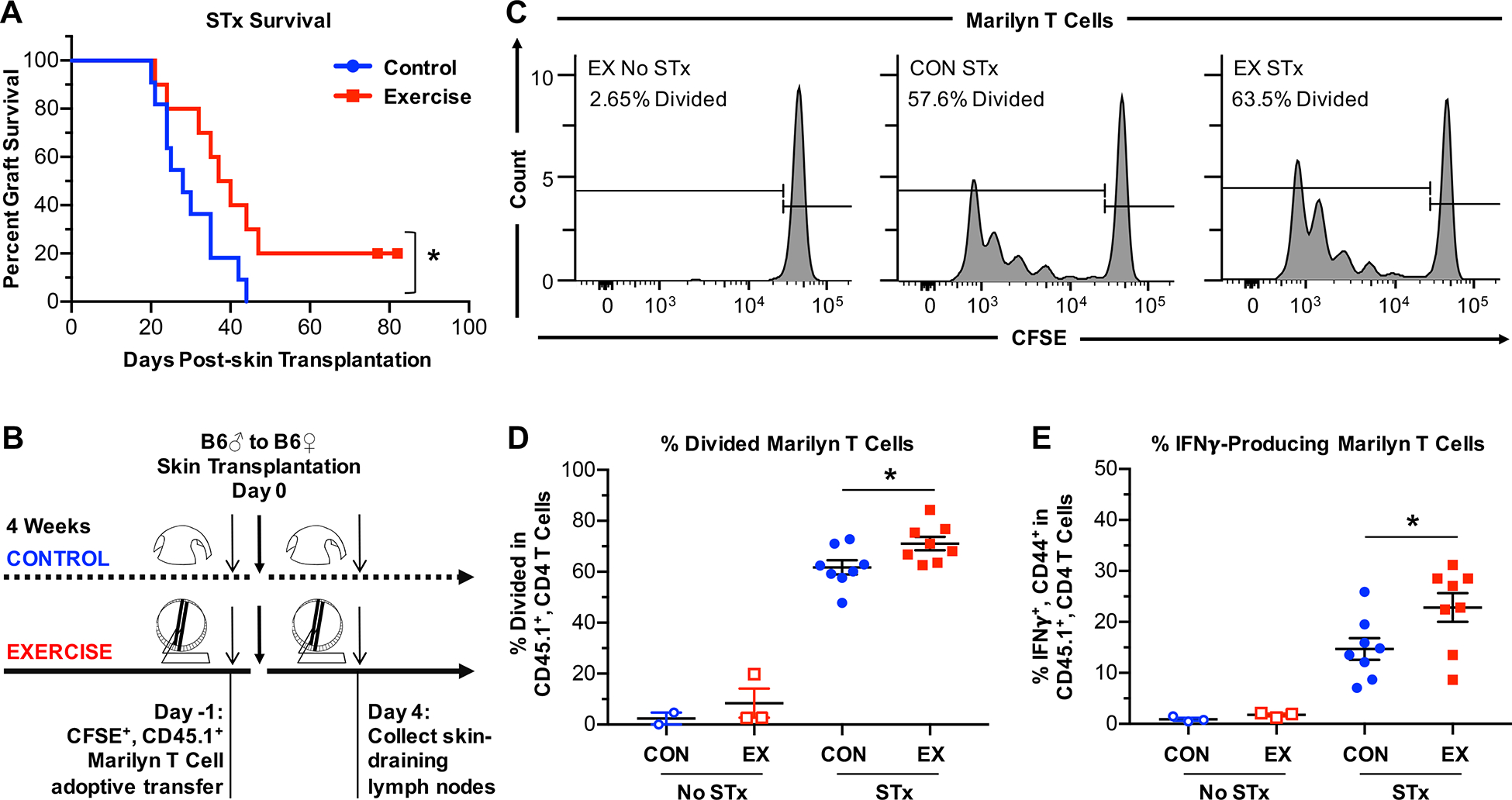
(A) Rejection kinetics of minormismatched skin allografts in control (n=11) or exercised (n=10) female B6 mice. Survival curve comparisons were performed using Mantel-Cox test. (B) Experimental design to examine priming in the dLNs using H-Y–specific, CD45.1+ congenic, CD4+ Marilyn T cells. Marilyn T cells were labeled with CFSE and transferred (2×105 or 6×105 cells/mouse) into control or exercised female B6 mice 1 day prior to transplantation with male B6 skin grafts. Mice were sacrificed 4 days after transplantation, and cells were isolated from the graft dLNs (axillary, brachial, and inguinal) for analysis of CFSE dilution. (C) Representative plots of CFSE dilution and divided Marilyn T cells. (D) Quantitation of divided Marilyn T cells in Marilyn-gated T cells on day 4 post-transplantation. (E) Percentage of IFNγ+ cells among Marilyn T cells after restimulation with anti-CD3 and anti-CD28 for 24 hours. (B-E) CON = control, EX = exercise, No STx = no skin transplantation, STx = skin transplantation from male B6 mouse. (D-E) Each point represents a single mouse. Results are displayed as mean ± SEM. Comparisons were performed using a two-tailed unpaired t test. *p<0.05. Results were combined from 2 independent experiments.
Prolonged survival of minor mismatched skin allografts may correlate with reduced alloreactivity, as measured by proliferation of alloreactive T cells in the skin graft-draining lymph nodes (dLNs), the site of initial T cell priming following skin transplantation (20). To determine if exercise resulted in diminished responses of donor-reactive T cells, H-Y–specific, CD45.1+ RAG-KO Marilyn T cells were CFSE-labeled and adoptively transferred into CD45.1− control or exercised female B6 mice one day prior to male skin transplantation. In contrast to expectations, Marilyn T cells isolated four days post-transplantation from the dLNs displayed slightly enhanced rather than reduced proliferation in transplanted exercised mice compared to transplanted control mice (p=0.0282, Figures 2C and 2D). In addition, Marilyn T cells isolated 4 days post-transplantation from the dLNs and restimulated with anti-CD3 and anti-CD28 displayed increased production of the inflammatory cytokine IFNγ in transplanted exercised mice compared to transplanted control mice (p=0.0364, Figure 2E). Thus, exercise did not result in reduced priming of, or IFNγ production by, graft-reactive T cells in the dLNs, and changes in the early priming phase of the alloimmune response in the dLNs failed to explain the observed prolongation of graft survival in exercised mice.
Moreover, exercise did not affect the percentages or total numbers of endogenous conventional CD4+ or CD8+ T cells or NK cells in the dLNs at 4 days post-transplantation (Supplementary Figure 1). An alternate model of alloimmunity using donor splenocyte transfusion (DST) from 2W1S-Ova+ B6 female mice to immunize control or exercised female B6 mice supported this observation. Leukocytes were isolated from the spleens of recipient mice one week after immunization and stained with fluorescent 2W1S:I-Ab tetramers to measure the expansion of endogenous, polyclonal donor-reactive T cells (Supplementary Figure 2A). Exercise did not reduce alloimmune T cell responses, neither changing the percentages and total counts of 2W1S tetramer-binding CD4+ T cells, nor their expression of the antigen-experience marker CD44 or of the proliferation marker Ki67 following DST immunization (Supplementary Figure 2). Hence, exercise does not reduce endogenous T or NK cell responses in either a solid organ transplantation or an immunization model of alloimmunity.
3.3. Exercise does not reduce the T cell effector response to the allograft
As priming of the alloimmune response appeared intact in exercised mice, we next asked if exercise inhibits the effector phase of the immune response to the allograft. To determine if exercise reduces T cell recruitment into the graft or prevents T cell proliferation in the graft, animals seeded with Marilyn T cells prior to male B6 skin transplantation were sacrificed 10 days post-transplantation and cells were isolated from both the dLNs and the skin allograft (Figure 3A). Marilyn T cells were identified as CD44hi, CD45.1+ cells (Figure 3B).
Figure 3. Exercise does not reduce T cell infiltration into the graft.
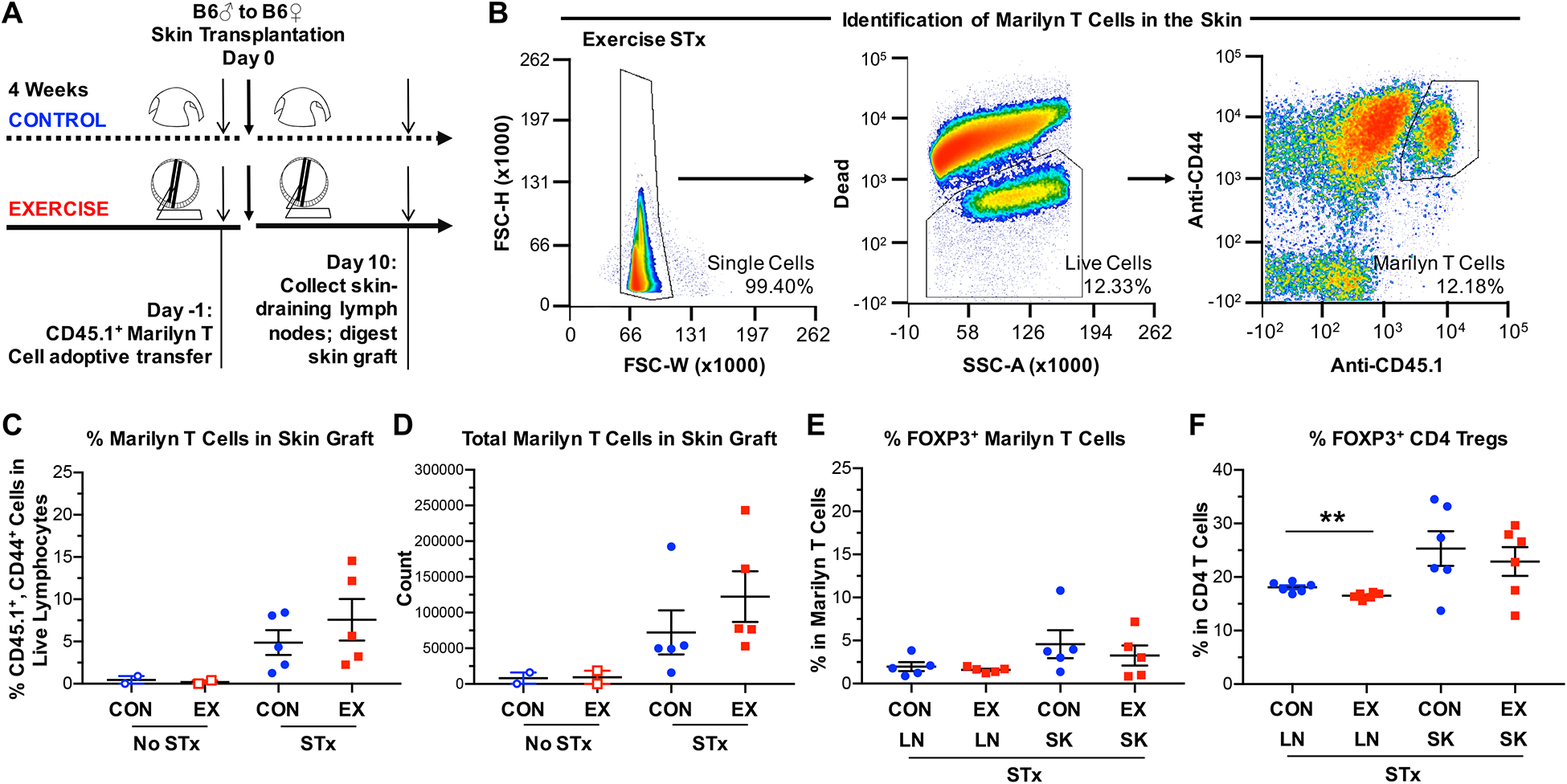
(A) Experimental design to examine T cell recruitment into the allograft using H-Y–specific, CD45.1+ congenic, CD4+ Marilyn T cells. Marilyn T cells were transferred (2×105 cells/mouse) into control or exercised female B6 mice 1 day prior to transplantation with male B6 skin grafts. Mice were sacrificed 10 days after transplantation, and cells were isolated from both the dLNs and the skin graft. For mice that did not receive a skin graft, shaved flank skin was harvested. (B) Gating strategy for the identification of Marilyn T cells in the skin. Single cells were pre-gated on lymphocytes and Marilyn T cells identified as CD44hi, CD45.1+ cells. (C) Percentages and (D) total numbers of CD44hi, CD45.1+ cells (Marilyn T cells) in the skin allograft at 10 days post-transplantation, shown normalized per gram of skin graft. (E) Percentages of Foxp3+ cells among CD44hi, CD45.1+ cells (Marilyn T cells) in both the dLNs and skin allograft at 10 days post-transplantation. (F) Percentages of Foxp3+ cells among bulk CD4+ T cells in both the dLNs and skin allograft at 10 days post-transplantation (p=0.0059). (C-F) LN = draining lymph nodes, SK = skin allograft. Each point represents a single mouse. Results are displayed as mean ± SEM. Comparisons were performed using a two-tailed unpaired t test. **p<0.01. Results were combined from 2 independent experiments.
Surprisingly, both the percentages and total numbers of Marilyn T cells were similar in the skin grafts of exercised and control animals (Figures 3C and 3D). Additionally, exercise did not alter the percentages or numbers of endogenous CD4+ or CD8+ T cells or NK cells in the skin-dLNs or skin graft at 10 days post-transplantation (Supplementary Figure 3), nor the expression levels of the inhibitory receptor program death-1 (PD-1) on either Marilyn T cells or endogenous CD4+ or CD8+ T cells (not shown). These data suggest that exercise-mediated prolongation of graft survival was not due to reduced T cell recruitment into the graft.
To determine if exercise promoted graft survival by increasing regulatory T cell populations, we examined Foxp3+ Tregs in both the dLNs and the skin allograft ten days post-transplantation. Among Marilyn T cells and endogenous CD4+ T cells, the percentages of Foxp3+ cells were similar in transplanted exercised mice compared to transplanted control mice in either the dLNs or skin allograft (Figure 3E–F). These results suggest that exercise does not lead to prolonged graft survival by promoting the induction, recruitment, or expansion of Tregs.
4. Discussion
Our results suggest that exercise may be beneficial for allograft outcomes. Using a murine model of voluntary exercise, our observations help elucidate the complex effect of exercise on alloimmunity and graft fitness. Our data show an extension in minor mismatched graft survival despite mildly enhanced alloimmune responses, suggesting increased resistance of the skin graft to a similar or slightly enhanced level of alloimmunity. Whether these results extend to major mismatched skin grafts remains to be determined.
Previous reports describing the effects of exercise on various aspects of immunity have been somewhat inconsistent. We observed increased production of IFNγ in the graft-reactive T cells of transplanted exercised over transplanted control mice, consistent with previous reports describing enhanced IFNγ expression in murine models of physical activity (7, 8). Research using a similar murine exercise model noted enhanced mobilization and infiltration of NK cells into tumors among exercised mice as a result of catecholamine-induced IL-6 production (7), and some NK cells can be inhibitory in transplantation (24), but increased NK cell infiltration did not occur in the allografts. While the beneficial effect of exercise on allograft survival was unexpected given that exercise enhances anti-tumor immunity, it is possible that exercise induces similar proximal signaling pathways in both models, which diverge depending on the target and context of the immune response. Whether catecholamines contribute to prolonged allograft survival in exercising mice remains to be determined.
Finally, Tregs were not strongly affected by our exercise regimen. In published literature, one study reported significantly increased levels of Tregs in adolescent humans following acute high-intensity exercise (12), while another study in mice reported finding increased levels of Tregs over control only following six weeks of high-intensity exercise but not following moderate-intensity or slow exercise (25).
The conflicting descriptions of the effects of exercise on the immune system, and on NK cells and Tregs in particular, suggest that the immune system is sensitive to the duration and intensity of training as well as the length of time elapsed post-exercise. Given that the exercise in our model was voluntary, we were unable to perform assays immediately following exercise among all mice, perhaps leading to loss of detection of such fluctuations in immune and other mediators. Thus, future studies of the impact of exercise on alloimmunity could focus on timed analyses during and post-forced rather than voluntary exercise, though studies suggest that the impact of forced exercise on the immune system differs from that of voluntary exercise (11).
We found no overall correlation between time of allograft survival and heart weight or fasting glucose levels as indicators of physical activity level. This suggests that prolonged graft survival does not require strenuous activity and supports some clinical data suggesting similar health benefits of shorter versus longer bouts of daily running (26), an important consideration for the clinical translation of our results, as patients may not tolerate high intensity exercise. Another important point is whether exercise must precede transplantation in order to provide clinical benefit. Physical activity may be challenging for many patients awaiting transplantation though clinical trials of pre-transplantation physical conditioning are underway https://clinicaltrials.gov/ct2/show/NCT02957955?term=exercise&cond=heart+transplantation&rank=18.
Our work demonstrated specifically that voluntary wheel running mildly enhanced proliferation and IFNγ production by graft-reactive T cells without expanding Tregs, supporting the general conclusion that exercise strengthens immune responses. Yet this observation was at odds with the observed prolonged graft survival, a phenomenon usually correlated with reduced alloreactivity (20). This incongruity suggests that the effect of voluntary exercise to prolong graft survival is due to other effects of exercise on the graft recipient and/or donor. Exercise may prevent graft rejection by inducing acquisition by donor-reactive T cells of inhibitory mediators that were not measured in our study, or by promoting the differentiation of inhibitory cells such as regulatory macrophages and myeloid-derived suppressor cells. However, these cells suppress immune responses through mechanisms that commonly result in promotion of Treg differentiation and/or reduction in conventional T cell expansion (27, 28), which were not observed in our study. Alternatively, exercise may prevent graft rejection by promoting overall fitness of the graft and enhancing its resistance to rejection.
Voluntary exercise resulted in greater proliferation and IFNγ production by donor-reactive T cells following skin transplantation, but not after injection of donor splenocytes. Although the reason behind these differences is not clear, one possibility is that proliferative differences may be magnified by the continuous exposure to donor antigen from a minor mismatched skin graft that persists for a few weeks but not when antigen is quickly eliminated following transfer of donor splenocytes.
Exercise is celebrated as having numerous health benefits, including reduced risk of chronic diseases such as cardiovascular disease, type 2 diabetes mellitus, and osteoporosis, as well as protection against cancer development and recurrence, retinal degeneration, and memory loss. Exercise is also beneficial to mental health, improving anxiety, and depression (29). These benefits of course carry over to transplant recipients, but our data suggest that exercise may not only improve the overall quality of life and health of transplant recipients, but also be beneficial for the transplant itself. Our studies suggest a beneficial effect of exercise on transplant outcome and support a clinical recommendation to exercise following transplantation.
Supplementary Material
Acknowledgements
We acknowledge Charles Mainhart and Dr. James J. Moon for the generous gifts of Marilyn mice and 2W1S-Ova+ transgenic mice, respectively, and thank Dr. Anita Chong and Dr. James Young for their insightful comments and discussion. This work was supported by grant R01AI115716 to MLA and T32 HD007009 to CMM. VER designed and performed experiments, data analysis, and wrote the manuscript. LC performed the skin transplantation, and CMM assisted with ex vivo experiments and leukocyte isolation and analysis. CMM and MLA designed the studies and edited the manuscript.
Abbreviations:
- dLN
skin-draining lymph node
- DST
donor splenocyte transfusion
- PD-1
program death receptor-1
- Treg
regulatory T cell
Footnotes
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care 2002;25(9):1612–1618. [DOI] [PubMed] [Google Scholar]
- 2.Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr Physiol 2012;2(4):2775–2809. doi: 2710.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol 2014;25(5):947–958. doi: 910.1093/annonc/mdt1551 Epub 2014 Jan 1098. [DOI] [PubMed] [Google Scholar]
- 4.Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W et al. The economic burden of physical inactivity: a global analysis of major noncommunicable diseases. Lancet 2016;388(10051):1311–1324. doi: 1310.1016/S01406736(1316)30383-X Epub 32016 Jul 30328. [DOI] [PubMed] [Google Scholar]
- 5.Jones LW, Alfano CM. Exercise-oncology research: past, present, and future. Acta Oncol 2013;52(2):195–215. doi: 110.3109/0284186X.0282012.0742564 Epub 0282012 Dec 0284117. [DOI] [PubMed] [Google Scholar]
- 6.Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun Rev 2018;17(1):53–72. doi: 10.1016/j.autrev.2017.1011.1010. Epub 2017 Nov 1013. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab 2016;23(3):554–562. doi: 510.1016/j.cmet.2016.1001.1011 Epub 2016 Feb 1016. [DOI] [PubMed] [Google Scholar]
- 8.Berrueta L, Bergholz J, Munoz D, Muskaj I, Badger GJ, Shukla A et al. Stretching Reduces Tumor Growth in a Mouse Breast Cancer Model. Sci Rep 2018;8(1):7864. doi: 7810.1038/s41598-41018-26198-41597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 2000;80(3):1055–1081. doi: 1010.1152/physrev.2000.1080.1053.1055. [DOI] [PubMed] [Google Scholar]
- 10.Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun 2014;39:160–71.(doi): 10.1016/j.bbi.2013.1010.1030. Epub 2013 Nov 1015. [DOI] [PubMed] [Google Scholar]
- 11.Cook MD, Martin SA, Williams C, Whitlock K, Wallig MA, Pence BD et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun 2013;33:4656.(doi): 10.1016/j.bbi.2013.1005.1005. Epub 2013 May 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson LD, Zaldivar FP, Schwindt CD, Wang-Rodriguez J, Cooper DM. Circulating T-regulatory cells, exercise and the elite adolescent swimmer. Pediatr Exerc Sci 2009;21(3):305317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurley KE, Lowry RP, Forbes RD. Immune mechanisms in organ allograft rejection. II. T helper cells, delayed-type hypersensitivity, and rejection of renal allografts. Transplantation 1983;36(4):401–405. [PubMed] [Google Scholar]
- 14.Molinero LL, Yin D, Lei YM, Chen L, Wang Y, Chong AS et al. High-Fat Diet-Induced Obesity Enhances Allograft Rejection. Transplantation 2016;100(5):1015–1021. doi: 1010.1097/TP.0000000000001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safa K, Ohori S, Borges TJ, Uehara M, Batal I, Shimizu T et al. Salt Accelerates Allograft Rejection through Serum- and Glucocorticoid-Regulated Kinase-1-Dependent Inhibition of Regulatory T Cells. J Am Soc Nephrol 2015;26(10):2341–2347. doi: 2310.1681/ASN.2014090914 Epub 2014092015 Apr 2014090911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vilchez RA, Dauber J, McCurry K, Iacono A, Kusne S. Parainfluenza virus infection in adult lung transplant recipients: an emergent clinical syndrome with implications on allograft function. Am J Transplant 2003;3(2):116–120. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed EB, Wang T, Daniels M, Alegre ML, Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant 2011;11(5):936–946. doi: 910.1111/j.1600-6143.2011.03476.x Epub 02011 Mar 03430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Chen L, Ahmed EM, Ma L, Yin D, Zhou P et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol 2008;180:5991–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant 2010;10(7):1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei YM, Chen L, Wang Y, Stefka AT, Molinero LL, Theriault B et al. The composition of the microbiota modulates allograft rejection. J Clin Invest 2016;126(7):2736–2744. doi: 2710.1172/JCI85295 Epub 82016 Jun 85220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntosh CM, Chen L, Shaiber A, Eren AM, Alegre ML. Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome 2018;6(1):96. doi: 10.1186/s40168-40018-40474-40168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007;109(12):5346–5354. doi: 5310.1182/blood-2006-5310-051318 Epub 052007 Feb 051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dileepan T, Linehan JL, Moon JJ, Pepper M, Jenkins MK, Cleary PP. Robust antigen specific th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS Pathog 2011;7(9):e1002252. doi: 1002210.1001371/journal.ppat.1002252 Epub 1002011 Sep 1002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med 2005;11(10):1059–1065. Epub 2005 Sep 1011. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Song H, Tang X, Yang Y, Vieira VJ, Niu Y et al. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports 2012;22(5):643–652. doi: 610.1111/j.1600-0838.2010.01288.x Epub 02011 Mar 01216. [DOI] [PubMed] [Google Scholar]
- 26.Lee DC, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 2014;64(5):472–481. doi: 410.1016/j.jacc.2014.1004.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribechini E, Hutchinson JA, Hergovits S, Heuer M, Lucas J, Schleicher U et al. Novel GM-CSF signals via IFN-gammaR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood Adv 2017;1(14):947–960. doi: 910.1182/bloodadvances.2017006858 eCollection 2017002017 Jun 2017006813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conde P, Rodriguez M, van der Touw W, Jimenez A, Burns M, Miller J et al. DCSIGN(+) Macrophages Control the Induction of Transplantation Tolerance. Immunity 2015;42(6):1143–1158. doi: 1110.1016/j.immuni.2015.1105.1009 Epub 2015 Jun 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruegsegger GN, Booth FW. Health Benefits of Exercise. Cold Spring Harb Perspect Med 2018;8(7).(pii):cshperspect.a029694. doi: 029610.021101/cshperspect.a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


