Abstract
Objectives:
Rhesus (Rh) blood group with variable expression of D antigen is one of the complex systems in immunohematology. Weak D antigen is a phenotype where the D antigen is weakly expressed on red blood cells, and this antigen cannot be detected by routine methods. This study was conducted to determine the frequency of Rh D negativity and weak D antigen among healthy blood donors and to review the clinical significance of weak D antigen pertaining to Rh D-negative transfusions.
Materials and Methods:
This cross-sectional prospective study was conducted in G. B Pant Hospital from January 2016 to June 2017 in which all the blood donors from Port Blair and adjacent islands of Andaman and Nicobar were grouped for Rh D antigen and those who tested negative for the D antigen were further tested for weak D antigen by incubating for 30 min and subsequent addition of anti-human globulin sera.
Results:
Out of 6415 donors, 6085 (94.86%) were Rh D positive and 330 (05.14%) were Rh D negative. Among the Rh D-negative donors, 05 (01.51%) were positive for weak D antigen. The frequency of Rh D negativity was 25.76% in a blood group, 25.15% in B, 07.88% in AB and 41.21% in O blood group phenotype.
Conclusion:
Although the frequency of weak D antigen is low (01.51%), the strong immunogenicity of Rh D antigen discernates the need for appropriate testing for weak D antigen. This is of particular concern in Rh D-negative pregnant females as it can produce alloimmunization if accidentally given weak D antigen positive blood.
KEYWORDS: Alloimmunization, Rhesus D negative, Transfusion, Weak D antigen
INTRODUCTION
Rhesus (Rh) blood antigen were first described by Levine and Stetson in 1939 who described a patient having an antibody that agglutinated red blood cells (RBC) of 85% of ABO-compatible donors. This was a second major discovery in immunohematology after the discovery of ABO blood groups by Landsteiner in 1990. Human blood groups were divided into two major groups depending on the presence (Rh positive) or absence (Rh negative) of Rh antigen. Later, Fisher and Race published their work on Rh antigen, whereby the nomenclature of CDE was accepted. The Rh system consists of over 50 antigens, with D being the major antigen expressed by Rh D protein. Out of Rh system, 5 (D, C, c, E, and e) are important for causing clinical complications. Molecular genetics has shown that there are two Rh genes, one encoding D, the other encoding the Cc and Ee antigens [1]. Subsequent to the conflicting grouping of complex Rh system, a weak D antigen was described by Stratton in 1946.
Weak D represents a D phenotype where due to reduced D antigen expression on red cells, the antigen is not detected by routine techniques (spin tube method). However, the demonstration of this weakly expressed antigen can be undertaken by prolonged incubation and the use of anti-human globulin.
Years after the discovery of this weak D antigen, it has remained a topic of controversy whether to routinely test for weak D antigen or not. The clinical implications are of concern when dealing with pregnant women.
This study was conducted with the aim to determine the frequency of weak D phenotype among Rh D-negative individuals. The objective being, to highlight its clinical implications related to the risk of alloimmunization in RhD-negative individuals and the justification for testing it.
MATERIALS AND METHODS
This cross-sectional prospective study was conducted in Blood bank, G. B. Pant hospital, Andaman and Nicobar Islands Institute of Medical Sciences, Port Blair from January 2016 to June 2017 in which all the donors from Port Blair and adjacent islands of Andaman and Nicobar were grouped for RhD antigen by immediate spin tube technique and those who tested negative for the D antigen were further tested for weak D antigen making sure that every donor is studied only once. Weak D antigen positive cells have a weak expression of the D antigen and may be misclassified as D negative cells in routine Rh grouping procedures. However, the more elaborate indirect antiglobulin test is capable of detecting all grades of weak D antigen with the exception of the very low-grade types.
Weak D antigen testing
Principle - The procedure was based on the principle of agglutination of RBC carrying the RhD antigen in the presence of anti-RhD antibody. Phenotyping (grouping) was done by testing the blood sample with monoclonal IgM anti D (MEDICLONE; M/s Mediclone Biotech Pvt. Ltd., Tamil Nadu, India) as per manufacturer's instructions. The presence of hemagglutination determined the positive Rh D antigen and the blood was categorized as Rh D positive. If no agglutination was obtained, then the RBCs were checked with MEDICLONE D IgG (Mediclone Biotech Pvt. Ltd., Tamil Nadu, India) as it sensitizes the Rh positive or weak D antigen cells which gave agglutination on the addition of Coomb's reagent (Indirect Coomb's test).
Sample
Anticoagulated blood of the Rh D-negative donors was the sample for weak D testing. A 5% RBC saline suspension was made by washing the RBCs with isotonic saline.
Procedure
For each sample, a tube was taken and labeled as test sample. A saline tube acted as control. One drop of MEDICLONE D (IgG) and saline were added to the test tube and control tube, respectively. One drop of 5% RBC suspension was added to each tube. After mixing, incubation was done for 30 min for sensitization. After washing the sensitized cells 3–4 times with normal saline and discarding the supernatant, two drops of Anti-human serum (Coomb's serum) were added and centrifuged for 1 min. The sediment cells were gently dislodged and examined macroscopically as well as microscopically for agglutination.
Interpretation
Agglutination of sensitized RBC with Coomb's serum was considered as weak D antigen (Du) positive, whereas Rh D-negative blood resulted in no agglutination of RBC.
Negative controls using known D-negative red cells were run in parallel; these tubes must be free of agglutination; otherwise, the whole procedures for tests and controls must be repeated. Sensitized red cells were used to confirm the validity of all negative tests; agglutination must be visible after addition of sensitized red cells and centrifugation; otherwise, the test result is invalid, and the procedures must be repeated. As this test is a very sensitive test, if any doubts arise in the interpretation, the entire test should be repeated after thoroughly washing the red cells in saline and re-suspending them before use. If the sensitized cell test is negative or nonsensitized cell test is positive, then the weak D antigen test is invalid, and it should be repeated.
Compliance with ethical standards
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Local Ethics Committee of the Institute. Informed written consent was obtained from all patients before their enrollment in this study.
RESULTS
A total of 6415 donors came for the period between January 2016 and June 2017. Of 6415 donors, 6085 (94.86%) were Rh D positive and 330 (5.144%) were Rh D negative [Table 1]. Among the 330 Rh D-negative individuals, 05 (1.51%) were positive for weak D antigen, and rest were negative [Table 2]. The frequency of RhD negativity was 25.76% among a blood group, 25.15% among B, 07.88% among AB, and 41.21% among O blood group [Figure 1]. The weak D antigen positive individuals among blood group A phenotype were 0.60% of the total Rh D negative, 0.91% among B and nil among AB and O group [Table 3].
Table 1.
Frequency of Rhesus D antigen in healthy donors
| Blood group | Number of donors | Frequency (%) |
|---|---|---|
| Rh D positive | 6085 | 94.86 |
| Rh D negative | 330 | 5.14 |
| Total | 6415 | 100 |
Rh D: Rhesus D
Table 2.
Frequency of weak D antigen in Rhesus D negative donors
| Blood group | Number of donors | Frequency (%) |
|---|---|---|
| Du positive | 5 | 1.51 |
| Du negative | 325 | 98.48 |
| Total | 330 | 100 |
Du: Weak D antigen
Figure 1.
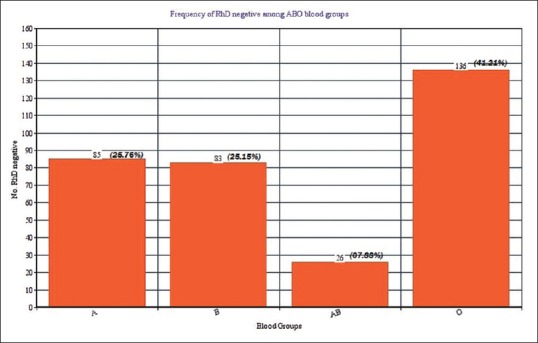
Frequency of Rh D negative among ABO blood groups
Table 3.
Frequency of weak D antigen positive among ABO blood groups
| ABO negative group | Du | Frequency (%) of total Rh D negative |
|---|---|---|
| A negative | 2 | 0.60 |
| B negative | 3 | 0.91 |
| AB negative | 0 | 0 |
| O negative | 0 | 0 |
| Total | 5 | 1.51 |
Du: Weak D antigen, Rh D: Rhesus D
DISCUSSION
The weak D phenotype is a weakened form of D antigen that in routine D antigen testing will react with some anti-D but not with others (when 37 C incubation or an immediate spin is given). Weak D RBC has D antigen but fewer in number as compared to normal Rh D-positive red cells. By definition, weak D red cells express all epitopes of D at a low level and individuals with weak D phenotype cannot make anti-D. Red cells that have the weak D phenotype should, for most transfusion purposes, be regarded as Rh D positive. Weak D red cells have fewer D sites per cell than normal Rh D positive red cells [1].
Molecular basis of weak D antigen
Rh antigens are encoded by two closely linked genes with 92% sequence homology. RhD encodes the D antigen and RHCE the Cc and Ee antigens. Each consists of 10 exons and unusually for homologous genes, the two genes are in opposite orientation on the chromosome. Each gene encodes a 416-amino-acid polypeptide of 30–32 kDa that is palmitoylated but not glycosylated. The polypeptides encoded by RhD and RHCE differ by 31–35 amino acids, depending on the RHCE genotype [1].
The Rh D-negative phenotype is usually associated with the absence of the whole D protein from the red cell membrane. This explains why D is so immunogenic, as the D antigen comprises numerous epitopes on the external domains of the D protein. In white people, the D-negative phenotype almost always results from homozygosity for a complete deletion of RhD. D positives are either homozygous or heterozygous for the presence of RhD. In Africans, in addition to the deletion of RhD, D negative often results from an inactive RhD containing translation stop codons within the reading frame. Other genes containing inactivating mutations are also found in D negative Africana and Asians [2].
Weak D and partial D are the two variants of D antigen depending on the variable expression of D antigen due to a large number of RhD alleles. Weak D and partial D result in quantitative as well as qualitative changes in Rh protein respectively [3]. Flegel [4] pointed that weak D types 1, 2, 3, 4.0, 4.1, and 5 can be regarded as Rh D positive and transfused with Rh-positive blood. However, weak D types 4.2–11 and 15 should be regarded as Rh D negative and transfused with Rh-negative blood. Partial D can produce specific antibody production. Thus, partial D should be considered Rh D negative in such situations [5]. In our center, the molecular tests for determination of weak D types were not available.
Weak D phenotype individuals may show amino acid substitution in intracellular and transmembrane protein segment of Rh antigen [6]. A study in Taiwan using polymerase chain reaction (PCR)-restriction fragment length polymorphism and direct sequencing revealed four types of mutations that were in relation to weak D antigen [7]. While some workers observed that aberrant RhD proteins due to RhD alleles may lead to altered antigens [6], some other workers said that it was the reduced expression of RhD mRNA that led to weak D phenotype [8].
It is important that anti – D typing reagents should detect most weak D phenotypes, especially in blood donors, although very weak forms of D will be typed as Rh D negative. The weakest form of weak D, named DEL, can only be detected serologically by absorption and elution tests.
When a person's Rh phenotype is known, the probable genotype can be discerned and its likelihood calculated from known genotype frequencies within the same population. It is very important that the ethnic origin of the person is known when probable genotype determinations are carried out, as figures for one population will not apply to people of other populations. For example, in white populations, dce is 15 times more common than Dce, whereas in African populations Dce has a slightly higher frequency than dce [1].
In the UK, the recommended method for D typing of patients requires direct agglutination tests, in duplicate, with potent IgM monoclonal anti-D reagents. An antiglobulin test is not required. This means that very weak D red cells will be typed as D negative. This is not considered important, as the patient will be harmlessly transfused with D-negative red cells. Donors are not typed any longer for D by an antiglobulin test, as this is not necessary because it is unlikely that transfusion of very weak D red cells to a D-negative patient will result in immunization of the patient [1].
Incidence of Rh negativity and weak D phenotype
The incidence of Rh negativity varies from 5% to 25% worldwide, and that of weak D antigen ranges from 0.2% to 1%. In our study, the Rh-negativity was 5.14% whereas it was 19.4% in a study from Lahore [9] and only 2.7% in a study from Northern Nigeria [10]. The results of our study are comparable to 6.3% Rh negativity in a similar study from India [11]. Another study showed 12.62% Rh negativity in Uttarakhand, India [12]. 0.3%–0.5% Indian population is found to have weak D, while the incidence in Europe is 0.23%–0.5% and 3% in the USA [13].
In our study, the incidence of weak D phenotype was slightly higher (1.51%). Similar studies showed incidence as 0.189% in India, and a single case of weak D positive in a study from Uttarakhand India [12] giving the incidence as 0.135%. High frequency of weak D was seen in a study from Nigeria [11]. The use of potent monoclonal anti-D reagents can account for the slightly higher incidence of weak D antigen. High sensitive monoclonal reagents detect Rh D-positive cells that would be difficult to detect with low sensitive polyclonal reagents [14].
Applied aspects and molecular techniques
As the RhD antigen is highly immunogenic, the molecular determination of RhD allele status is very important, given the high rate of immunization of Rh D-negative individuals with Rh D-positive transfused RBC [5,15]. Most Rh D-negative individuals lack the whole Rh D protein from their red cells and when immunized by Rh D-positive red cells can make antibodies to it.
Serological weak D phenotype is estimated to be 0.2%–1.0% in the United States; a policy to determine RhD phenotypes in potential transfusion recipients could potentially decrease the need for tens of thousands of units of Rh-negative RBC annually in the United States (12 million U/year times 15% Rh-negative times 1% serological weak D phenotypes times 95% of RhD genotypes that do not require Rh negative times 1–2 U transfused per patient) [16].
In a study done in Northern Europe, 8442 D-negative blood donations were screened by RhD PCR sequence-specific priming (PCR-SSP). RhD PCR-positive samples were further characterized by RhD exon specific PCR-SSP or sequencing. 50 RhD-positive samples were detected. Fifteen samples harbored one of three new D el alleles. Thirty samples were due to 14 different D-negative alleles, only 5 of which were previously known. The cumulative population frequency of the 14 D-negative alleles was 1:1500. Five samples represented a D± chimera, a weak D and three partial D, which had been missed by routine serology; two recipients transfused with blood of the D± chimera donor became anti-D immunized [17]. However, these molecular techniques for RhD phenotypes are still a vision of the near future in developing countries like India.
The testing of weak D antigen is of particular importance in patients with chronic requirements for blood transfusion such as thalassemia, sickle cell anemia, chronic renal failure, HIV/AIDS. If these Rh D-negative individuals are found to be positive for weak D antigen, they can be transfused with Rh D-positive blood. This applied aspect can be of particular importance in northern India where thalassemia is prevalent. However, care should be taken that weak D antigen testing is done under strictly controlled laboratory settings as false positive results may result in inadvertent transfusion of Rh D-positive blood to such Rh D-negative cases with grave immunological and clinical consequences.
CONCLUSION
Although the weak D antigen frequency is low, its strong immunogenicity makes applied aspects of utmost clinical importance. The risk of alloimmunization remains if weak D antigen positive blood is transfused to Rh D-negative individual. This becomes a major concern if the recipient is of childbearing age and can result in hemolytic disease of the newborn in subsequent pregnancy. It is recommended to consider individuals with a weak D antigen as Rh D positive when presenting as a donor and Rh D negative when confronted as a recipient.
Limitations of the study
Study was done in a hospital based setting with healthy donors, predominantly males. Further cross-sectional studies in a larger community-based representation are warranted to confirm the true prevalence. Rare partial D phenotypes may show a strong reactivity with some monoclonal sera and may not be differentiated from true D-positive phenotype.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Mr. C. P. Paul for his tremendous work and valuable technical support throughout the study.
REFERENCES
- 1.Hoffbrand AV, Higgs DR, Keeling DM, Mehta AB. Postgraduate hematology. 7th ed. Hoboken, New Jersey: John Wiley and Sons, Ltd; 2016. pp. 252–5. [Google Scholar]
- 2.Murphy MF, Pamphilon DH, Heddle NM. Practical transfusion medicine. 4th ed. Hoboken, NJ: Wiley-Blackwell; 2013. [Google Scholar]
- 3.Rizzo C, Castiglia L, Arena E, Gangi S, Mazzola G, Caruso C, et al. Weak D and partial D: Our experience in daily activity. Blood Transfus. 2012;10:235–6. doi: 10.2450/2012.0060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegel WA. Blood group genotyping in in Germany. Transfusion. 2007;47:47S–53S. doi: 10.1111/j.1537-2995.2007.01310.x. [DOI] [PubMed] [Google Scholar]
- 5.Flegel WA. Molecular genetics and clinical applications for RH. Transfus Apher Sci. 2011;44:81–91. doi: 10.1016/j.transci.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner FF, Frohmajer A, Ladewig B, Eicher NI, Lonicer CB, Müller TH, et al. Weak D alleles express distinct phenotypes. Blood. 2000;95:2699–708. [PubMed] [Google Scholar]
- 7.Lin IL, Shih MC, Hsieh MH, Liu TC, Chang SE, Lin CL, et al. Molecular basis of weak D in Taiwanese. Ann Hematol. 2003;82:617–20. doi: 10.1007/s00277-003-0711-4. [DOI] [PubMed] [Google Scholar]
- 8.Avent ND, Reid ME. The Rh blood group system: A review. Blood. 2000;95:375–87. [PubMed] [Google Scholar]
- 9.Saqlain N, Ahmed A, Fateen T, Ahmed N. D antigen: Chances of finding weak D antigen and re-evaluation of its clinical significance as a routine blood bank procedure. Prof Med J. 2016;23:1395–9. [Google Scholar]
- 10.Kumar H, Mishra DK, Sarkar RS, Jaiprakash M. Difficulties in immunohaematology: The weak D antigen. Med J Armed Forces India. 2005;61:348–50. doi: 10.1016/S0377-1237(05)80062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed S, Kagu M, Abjah U. The role of Du testing in scaling down the burden of rhesus-D negative transfusion in Northern Nigeria. Internet J Third World Med. 2008:8. [Google Scholar]
- 12.Acharya S, Kumar R, Acharya R, Kudesia S, Kishore S. Weak D antigen – Revisited. Indian Med Gaz. 2011;9:342–5. [Google Scholar]
- 13.Aslam A, Azmi R, Sheikh MZ, Javaid I. Frequency of weak expression of ‘D ALLELE’ among healthy blood donors. Pak J Physiol. 2015;11:22–4. [Google Scholar]
- 14.Williams M. Monoclonal reagents for rhesus-D typing of Irish patients and donors: A review. Br J Biomed Sci. 2000;57:142–9. [PubMed] [Google Scholar]
- 15.Kumpel B. Are weak D RBCs really immunogenic? Transfusion. 2006;46:1061–2. doi: 10.1111/j.1537-2995.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 16.Sandler SG, Roseff SD, Domen RE, Shaz B, Gottschall JL. Policies and procedures related to testing for weak D phenotypes and administration of Rh immune globulin: Results and recommendations related to supplemental questions in the Comprehensive Transfusion Medicine Survey of the College of American pathologists. Arch Pathol Lab Med. 2014;138:620–5. doi: 10.5858/arpa.2013-0141-CP. [DOI] [PubMed] [Google Scholar]
- 17.Wagner FF, Frohmajer A, Flegel WA. RHD positive haplotypes in D negative Europeans. BMC Genet. 2001;2:10. doi: 10.1186/1471-2156-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


