Abstract
Exosomes, 60–200-nm extracellular vesicles secreted from cells, have been used as an active pharmaceutical ingredient or drug carrier in disease treatment. Human- and plant-derived exosomes are registered in clinical trials, but more complete reports are available for human-derived exosomes. Because exosomes act as vesicles and carry cell secreting components, they have been used as drug or peptide vehicles to treat diseases. The dendritic cells (DCs) and mesenchymal stem cells (MSCs) are two popular cell sources for exosome preparation. Exosomes from DCs can initiate inflammation in patients, particularly in patients with cancer, as they contain the tumor antigen to induce specific inflammation response. A well-established cell bank of MSCs is available, and these cells can be used as an alternative source for exosome preparation. The major application of MSC-derived exosomes is in inflammation treatment. Exosomes in clinical trials need to comply with good manufacturing practice (GMP). Three important issues are prevalent in GMP for exosomes, i.e., upstream of cell cultivation process, downstream of the purification process, and exosome quality control. This paper concisely reviews exosome development, including exosome generation and clinical trial application.
KEYWORDS: Clinical trial, Exosomes, Good manufacturing practice
INTRODUCTION
The extracellular vesicles (EVs) are secreted by cells and recycled in body fluids, which are collective term covering the name of exosomes, microvesicles (MVs), microparticles, ectosomes, oncosomes, and apoptotic bodies. The difference of the above terms depends on the size. The oncosomes, ability to transfer oncogenic material, exhibit their atypical large size (1–10 μm). MVs are ranged in size from 50 to 1000 nm in diameter. The exosomes are ranged from 60 to 200 nm [1]. The isolations of apoptotic bodies or ectosomes are obtained by the procedures of ~300–500 ×g (removing cells), followed by force at ~1000 ×g to remove cellular debris, and finally followed by a longer centrifugation at higher g forces (~10,000< × < ~16,000 ×g). The most commonly used method for isolating exosomes is ultracentrifugation (UC) at 100,000–120,000 ×g [2]. The components of EVs include lipids and proteins in addition to nucleic acids. Moreover, EVs feature the property of the cell sources. The minimal information for studies of EVs 2018 (MISEV2018) has claimed some criteria for EVs. The general characterization of EVs according to MISEV2018 would contain at least three positive protein markers of EVs, including at least one transmembrane/lipid-bound protein and cytosolic protein, and at least one negative protein marker. The importance of the ratio of proteins to particles has been mentioned in the MISEV2018. Apart from the definition from MISEV2018, the other term might be more appropriate as an extracellular particle [3]. Exosomes have been used as pathological markers [4], gene carrier, and drug carrier [5]. The size of exosomes is 60-200 nm and owing to its biocompatibility, exosomes have great potential for use as anti-cancer drug vehicles. In order to ensure the biological activity of exosomes, a standardized manufacturing process, such as a process in compliance with good manufacturing practice (GMP), of exosomes is vital.
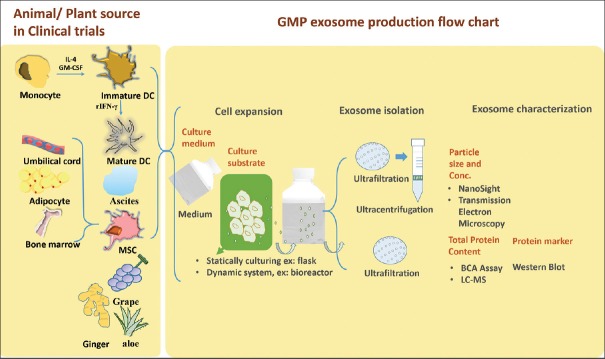
Because exosomes are secreted by cells, a production system could be established using a large-scale cell cultivation system. The downstream purification system should preferably conform to the procedures of vaccine production because of the similarity in particle size and features of secretory vesicles of the host cells. The challenge in GMP of exosomes is quality control. Although markers of exosomes have been defined by previous studies, the type of cells producing exosomes is diverse [6,7]. Most reviews have focused on the generation and application of exosomes in preclinical or clinical trials [5,8,9,10,11,12,13,14,15,16,17]. Therefore, this article concisely reviews exosomes in clinical trials and their production in compliance with GMP. Three main criteria, including upstream of cell cultivation system, downstream of the purification system, and quality control of exosomes, are discussed for GMP. Exosomes from human and plant sources involved in clinical trials are mentioned in the article [Figure 1].
Figure 1.
Summary of exosomes in clinical trials and flow chart for exosome production in compliance with good manufacturing practice. DC: Dendritic cells, MSC: Mesenchymal stem cell, LC-MS: Liquid chromatography–mass spectrometry
EXOSOME PRODUCTION FOLLOWING GOOD MANUFACTURING PRACTICE
An exosome is a monolayer of small vesicles with a diameter of 60-200 nm formed by cytoplasmic membrane invagination. Exosomes are widely distributed in all tissues, intercellular spaces, and body fluids. Almost all cells secrete exosomes. Exosomes, similar to a postman, can be accurately transmitted to specific organ cells through cell surface receptors for cell–cell transmission [7].
The methods for the production of GMP-grade exosomes in recent years are shown in Table 1. A GMP-grade exosome production method includes the type of cells, culture environment, cultivation system, dissociation enzyme, and culture medium. Further purification is required after production, generally divided into three-step process. The third issue in GMP of exosomes is the establishment of identification method, including physical structure and bioactivity function characteristics.
Table 1.
Summary of the methods for exosome production in compliance with good manufacturing practice
| Cell expansion | Exosome isolation | Exosome validation | Results | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell source | Cultivating substrate | Dissociation enzyme | Culture medium | 1st process Removing cells and cell debris | 2nd process Concentration of condition medium | 3rd process Exosome purification | Total protein content | Bio-characterization | Physical characterization | ||
| MDDCs | T-175 flasks | Not reported | Serum-free | 3/0.8 μm filter | 500-kDa MWCO hollow fiber membrane | Sucrose/deuterium UC at 100,000 ×g | ELISA | Tetraspanin proteins, such as CD81, CD63, CD9, and CD82; costimulatory molecule CD86; adhesion proteins, such as CD11b, CD11c, CD58, and CD54 | Not reported | Increased quantity (concentration of MHC class II) and protein characterization (using FACS) to standardize exosome vaccine | Journal of immunological methods 2002, 270 (2), 211-26 [18] |
| BM-MSCs | T225 flask | Not reported | HPL/FBS | 0.22 μm filter | UC at 30,000 × g for 20min | UC at 120,000 ×g for 3h | Not reported | CD90, CD14, CD34, CD45, CD73; HLA-II (DR); total RNA; miRNA | NTA | 10% HPL-based EV-depleted medium is appropriate for the purification of exclusively human MSC-derived EVs | Cytotherapy, 2017; 19: 458-472 [19] |
| hCPCs | CellBIND® | TrypLETM Select | Free of nonhuman animal-derived components | Centrifugation (3000 × g) and filtration (0.22 μm) | Amicon Ultra-15 (100 kDa cut-off) or Centricon Plus-70 | TFF with a 300-kDa cut-off hollow fiber cartridge | QuantiProTM BCA assay kit | GATA4, TBX5, TBX18, MESP1, TSG101, GRP94, and GAPDH | TEM | High exosome yield, and consistent removal of contaminating proteins (97%) | Front Physiol. 2018; 9: 1169 [20] |
| HEK293 cell | Hollow-fiber bioreactors (fibercell systems) | Not reported | EV-depleted cell culture medium | Differential centrifugation and filtration (0.22 μm) | TFF device (0.05 μm pore size) | UC at 110,000 ×g for 3 h SEC | Bradford assay | CD63 and calnexin | NTA; immune-TEM; LC-MS | Combination TFF and SEC for large staring volumes | J Extracell Vesicles 2018, 7 (1), 1442088 [22] |
| BM-MSCs | Hollow-fiber bioreactors (quantum bioreactor) | - | HPL for confluence then HPL-free for collection | Centrifugation (1000 × g) and 0.2-μm filters | Not applied | UC at 110,000 ×g for 3 h | MicroBCA assay | Exosome markers (CD9, CD63, CD81, and CD47); mesenchymal markers (CD29 and CD90); siRNA sequence | NanoSight; TEM | Shelf life, biodistribution, toxicology profile, and efficacy | JCI Insight. 2018 Apr 19; 3 (8) [21] |
| ADSC; BM-MSCs | Flasks | Trypsin-EDTA | PL | Centrifugation at 3000 × g for 20 min | Not applied | UC: 100,000 ×g for 1 h at 4°C UF: Purified by TFF | Micro BCA-protein assay kit | Cytokine quantification by; Immunogenicity and immunomodulatory properties; Secretome versus. MSC immunomodulatory properties | NTA; phospholipid quantification; FT-IR | UF lead to higher protein, lipid, cytokine, and exosome yield compared with that with UC | Nanomedicine 2019, 14 (6), 753-765 [23] |
MDDCs: Monocyte-derived dendritic cells, BM-MSCs: Bone marrow-mesenchymal stem cells, hCPCs: Human cardiac progenitor cells, ADSC: Adipose-Derived Stem Cell, PL: Platelet lysate, HPL: Human platelet lysate, FBS: Foetal bovine serum, EV: Extracellular vesicles, EDTA: Ethylenediaminetetraacetic acid, UC: Ultracentrifugation, TFF: Tangential flow filtration, SWC: Size-exclusion chromatography, UF: Ultrafiltration, NTA: Nanoparticle tracking analyzer, TEM: Transmission electron microscopy, LC-MS: Liquid chromatography-mass spectrometry, SEC: Size-exclusion chromatography, MWCO: Molecular weight cut-off, BCA: Bicinchoninic acid, FACS: Fluorescence-activated cell sorting, MHC: Major histocompatibility complex, HLA-II (DR): Human Leukocyte Antigen II – DR isotype, FT-IR: Fourier-transform infrared spectroscopy
Upstream of cell culturing system for setting an exosome-secreting environment
Five types of cells, including human cardiac progenitor cells, bone marrow mesenchymal stem cells (MSCs), adipose tissue-derived stem cells, monocyte-derived dendritic cells (DCs), and HEK293 cells, have been applied in GMP for exosome production. Cell cultivation employs static systems, such as a flask, as well as dynamic systems, such as a bioreactor. Two types of static flask system used include stand tissue culture flask [18,19] and CellBIND® surface. The CellBIND® surface is pretreated with oxygen-containing functional group and has a net negative surface charge [20]. Bioreactors are also used for large-scale production because of the dynamic monitoring system, which is beneficial for the GMP process [21,22]. Because the size of exosomes is around 60-200 nm, the hollow fiber bioreactor system with molecular weight cutoff membrane is employed for condition medium (CM) harvest. The hollow fiber bioreactor system provides a dynamic environment for cell cultivation and a continuous medium collection system. The collection system provides the reduced volume of harvested CM that benefits for downstream purification. Both animal-free [20] and animal-derived [23] dissociation enzymes are utilized in the process. The cultivation medium differs based on the source of the cells, but it can be classified into animal-free [18,20] or animal-derived [21] components. The process of GMP for exosome can be improved in many aspects to obtain more and purer exosomes. For examples, using xeno-free conditions to culture cells can reduce the doubling time and lead to high exosome yield and consistent removal of contaminating proteins up to 97% [20]. Furthermore, the 10% pooled human platelet lysate (HPL)-based EV-depleted medium, which is suitable for the production of human MSC-derived exosomes as it retains the characteristic surface marker expression, cell morphology, viability, and in vitro differentiation potential, can be used [19].
The advantage of static flask system is less skilled labor comparing to that of bioreactor. If the cultivation system requires specific parameters such as CO2, O2, pH to manipulate, the bioreactor would be an attractive method. The most common cultivation reagent of dissociation enzymes or medium should be animal free for avoiding the pathogenic source or ethical issue. In some studies have mentioned that the HPL showed the greater bioactivity than traditional medium. Therefore, the reagent choose would be considered in the further clinical application.
Downstream of purification system for exosomes
In general, there are three steps of purification, including filtration for removing the cell debris, concentrating the CM, and exosome isolation from the concentrated CM. The differential centrifugation is the common strategy for the concentration of CM and exosome isolation from the concentrated CM. Although a less additive reagent is added during differential centrifugation comparing to that of sucrose gradient method, workforce, and labor are the disadvantages of the differential centrifugation purification [24].
Tangential flow filtration (TFF) is an alternative method for the concentration of CM and purification of exosomes in recent years owing to the advantages of less time and workforce for large-scale purification. In addition, the performance of TFF and UC has been compared. Exosomes obtained from TFF exhibit higher immunomodulatory potency than those from UC. Moreover, the immunomodulatory potency of exosomes from TFF is similar to that of the parental cells, confirming the rationality of replacing cells with their secreted exosome. The reports showed that more soluble factors, such as cytokines, DNA, RNA, proteins, or lipids, are contained in the exosomes obtained from TFF than in those from UC. EV aggregation or destruction is observed after harvesting exosomes by UC because elevated shear forces in UC may break down the exosomes and thus, the proteins released from exosomes [23]. In addition, size-exclusion chromatography (SEC) is a method developed based on the size exclusion theory for exosome purification. Compared to exosomes from UC, a 100-fold reduction in ferritin, a major protein complex contaminant, concentration is observed in SEC-purified exosomes [22].
We specifically explored whether to use commercially available ExoQuick™ as a purification process, but none of the other GMP production processes discussed in this article were verified. According to our unpublished research results, the purification method using ExoQuick™ is fast and convenient, but the purified sample still contains the contaminate proteins from culturing medium; therefore, it is only suitable for exosome preparation in the research stage.
The purification of exosomes involved the criteria of recovery rate and specificity. In general, to achieve a higher recovery would decrease the specificity and vice versa. This is because higher specificity achievement should follow step-by-step purification procedure to remove un-purity matters. The advantages of differential centrifugation are to obtain a high purity of exosomes; however, the recovery rate would be lost in each step of differential centrifugation and time-consuming of differential centrifugation is one of the disadvantages. Therefore, sucrose gradient centrifugation overcomes the time consuming and maintenance of purity of exosomes. The one disadvantage of sucrose gradient centrifugation is the residues of sucrose reagent. The system of ultrafiltration brings more attractive features in overcoming of time-consuming, increased specificity, and recovery rate. However, the protein may suffer in-stable in the ultrafiltration system because the CM is concentrated in the purification process and thus may cause a raised osmotic pressure.
Exosome characterization – Physicochemical and biological properties
The adsorption of protein and protein content in exosomes is determined by ELISA and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) [18]. Another alternative method using a BCA-protein assay kit has been developed for protein quantification [20,21,23]. With the progress in technology, a microfluidic electrophoresis analyzer [19] and liquid chromatography–mass spectrometry [22] have been established for the analysis of exosome components. To quantify the cytosolic proteins from parent cells and exosome markers, such as CD9, CD63, and CD81, flow cytometry is used.
Recent literature has indicated that physicochemical properties of exosomes, such as particle size and concentration, can be determined using NanoSight instruments and transmission electron microscopy (TEM) to observe the structure and size of exosomes. In addition, studies have described methods such as phospholipid quantification, cytokine quantification, and immunomodulatory properties for identifying exosomes [23].
EXOSOMES IN CLINICAL TRIALS
Two categories of exosomes are applied in clinical trial, namely, exosomes derived from plants and human specimen [Figure 1]. Until now, completed results of clinical trials using exosomes from human specimens have been reported; by contrast, plant derived exosomes are in the beginning stage, patients were not yet recruited in clinical trials. Due to the vesicle structure of exosomes, using exosomes as a drug carrier has also been performed in clinical trials. Table 2 describes the exosome applied in clinical trials having complete clinical reports, and Table 3 summaries the development of exosomes in clinical trials in the recruiting-status or not-recruiting status.
Table 2.
Summary of the exosome used in clinical trials with the complete reported results
| Indication | Year, phase, patients | Source | Dose | Administration | Purification | Characterization | Bioactivity | Exosome manipulation | Results |
|---|---|---|---|---|---|---|---|---|---|
| Melanoma [35] | 2000, Phase 1, (n=15) | imDC, autologous | 4×1013 or 1.3 × 1013 MHC Class II molecules | SC (90% of the volume) and ID (10%) injections weekly for 4 weeks | 500-kDa concentration and UC with D2O/sucrose cushion | CD81 tetraspanin | SEE test of potency | Pulsed with MAGE 3 tumor peptides | No Grade II toxicity; No detected MAGE3-specific CD4+ and CD8+ T cells |
| Non-small cell lung cancer [25] | Not reported. Phase 1, (n=4) | imDCs, autologous | 1.3×1013 MHC Class II molecules | SC (90% of the volume) and ID (10%) injections weekly for 4 weeks | 500-kDa concentration and UC with D2O/sucrose cushion | Not reported | MHC Class II molecules; ELISPOT for peptide-specific immune response | Pulsed with MAGE-A3, -A4, -A10, and MAGE-3DPO4 tumor peptide | Well-tolerated and only Grade 1-2 adverse events; MAGE-specific T-cell responses in 1/3 patients; increased NK lytic activity in 2/4 patients |
| Non-small cell lung cancer [26] [NCT01159288] | May 2010, Phase 2, (n=22) | Mature- dendritic (mDCs), autologous (induced by rIFN-γ) | 8.5×1011-1.0× 1013 MHC Class II molecules | Four ID at 1-week intervals | UF; DF and UC through a 1.21g/mL sucrose cushion | Exosome marker: Tetraspanin | Activation of LT11 cells Function: MHC class II molecules and CD40, CD86, and ICAM-1/CD54 | Pulsed with MAGE-A1, -A3, NY-ESO-1, Melan-A/MART1, MAGE-A3-DP04, EBV tumor peptides | One patient had Grade 3 hepatotoxicity; boosting the NK cell arm of antitumor immunity |
| Colon cancer [27] | Not reported, Phase 1, (n=40) | Ascites, autologous | 100-500 μg of protein | Four SC at weekly intervals | Differential centrifugation + sucrose/D2O density gradient UC | Exosome marker: HSPs (including HSC70, HSP70, and HSP90), CD80, ICAM-1, CD71), and LAMP-3; EM | Function: Tumor-associated carcinoembryonic antigen, MHC-I, and MHC-II molecules | ±GM-CSF | Safe, well-tolerated; tumor-specific antitumor CTL response in exosome plus GM-CSF group |
| Chronic kidney diseasesc [29] | April 2014, Phase 2/3, (n=40) | MSCs, allogeneic | 100 μg/kg/dose | Two doses of MSC-EVs, intraarterial and intravenous injections | UC at 100,000 ×g | CD9, CD63; EM | CD45, CD73 | Unmodified | Safe, well-tolerated; improved kidney function; decreased inflammation |
UC: Ultracentrifugation, imDCs: Immature-dendritic cells, SC: Subcutaneous, ID: Intradermal, MSCs: Mesenchymal stem cells, UF: Ultrafiltration, DF: Diafiltration, EM: Electron microscopy, CTL: Cytotoxic T lymphocyte, GM-CSF: Granulocyte-macrophage colony-stimulating factor, NK: Natural killer, MHC: Major histocompatibility complex, ICAM-1: Intercellular adhesion molecule 1, LAMP-3: Lysosomal-associated membrane protein 3, HSP: Heat shock protein, rIFN-γ: Recombinant interferon-γ, EV: Extracellular vesicles
Table 3.
Summary of exosomes used in clinical trials (source: clinical trials.com)
| Indication/year | Year, phase, patients | EV source | EV dose | Administration | EV manipulation | Results/status |
|---|---|---|---|---|---|---|
| Malignant ascites and pleural effusion (NCT01854866) | May 2013, Phase 2, (n=30) | Tumor-derived | Not reported | Perfused to the pleural or peritoneal cavity, 4 times/week | Loaded with chemotherapeutic drugs | Unknown status |
| Malignant pleural effusion (NCT02657460) | January 2016, Phase 2, (n=90) | Malignant pleural effusion | Not reported | Not reported | Loaded with methotrexate | Recruiting |
| Metastatic pancreatic cancer (NCT03608631) | March 2020, Phase 1, (n=28) | MSCs, allogeneic | Not reported | IV on days 1, 4, and 10. Treatment repeated every 14 days for up to 3 courses | KrasG12D siRNA (iExosomes) | Not yet recruiting |
| Bronchopulmonary dysplasia (NCT03857841) | June 2019, Phase 1, (n=18) | MSCs | -200 pmol phospholipid/kg | Intravenous | Not specified (UNEX-42) | Recruiting |
| Type 1 diabetes (NCT02138331) | April 2014, Phase 1, (n=20) | MSCs, allogeneic | (1.22-1.51)×106 cells/kg, Day 0 and 7 | Intravenous | Unmodified | Unknown |
| Macular holes (NCT03437759) | March 2017, Phase 1, (n=44) | MSCs, allogeneic | 50 μg or 20 μg | Dripped into vitreous cavity | Unmodified | Recruiting |
| Acute ischemic stroke (NCT03384433) | April 2019, Phase 1/2, (n=5) | MSCs, allogeneic | 200 μg | Stereotaxic injection | Enriched by miR-124 | Not yet recruiting |
| Colon cancer (NCT01294072) | January 2011, Phase 1, (n=35) | Plant-derived | Not reported | Tablets taken daily for 7 days | Loaded with curcumin | Active, not recruiting |
| Radiation- and chemotherapy-induced oral mucositis (NCT01668849) | August 2012, Phase 1, (n=60) | Grape derived | Not reported | Oral administration daily for 35 days | Unmodified | Active, not recruiting |
| Insulin resistance and chronic inflammation in polycystic ovary syndrome (NCT03493984) | May 2018, not applicable | Plant-derived (ginger and/or aloe) | Not reported | - | Unmodified | Not yet recruiting |
IV: Intravenous, EV: Extracellular vesicles, MSCs: Mesenchymal stem cells
Exosomes from human specimen
Three major sources, namely DCs, MSCs, and patient-derived tumor cells, of obtaining exosomes, are subjected to clinical trials. Exosomes are purified and processed to concentrated form by UF or differential centrifugation followed by UC with sucrose cushioning. Physical characterization is performed by electron microscopy or by detecting exosome markers, such as CD9, CD81, tetraspanins, heat shock 70 kDa protein 8 (HSC70), heat shock protein (HSP) 70, HSP90, CD80, intercellular adhesion molecule 1, CD71, lysosomal-associated membrane protein 3, CD63, Alix, and tumor susceptibility gene 101 [Table 2]. Moreover, bioactivity is characterized by the exosome derived source or by methods such as determining the immunogenicity. The application of human-derived exosome is major in cancer indication, and some in inflammatory or chronic disease. The exosomes could contain tumor antigen to induce anti-tumor immunity in a patient or anticancer drug to cause cytotoxicity for the treatment of patients with cancer. Moreover, utilization MSC featuring inflammatory regulation is a strategy to treat inflammatory or chronic disease. The following sections describe the detail information of the application of exosomes.
Cancer indication
Exosomes containing tumor antigen to induce antitumor immunity in a patient
Exosomes from DC could be either from immature or mature DCs activated by cytokines, such as recombinant interferon-γ. The injection dose ranges from 8.5 × 1011 to 4.0 × 1013 exosomes with MHC class II molecules. To induce the immunity of a patient diagnosed with cancer, the DC-derived exosome harbors tumor peptides to be injected subcutaneously. Trials employing immature DC-derived exosomes have been applied for melanoma and non-small cell lung cancer, for which the results of safety are similar, but in case of non-small cell lung cancer, MAGE-specific T-cell responses have been observed [25]. To advance T-cell stimulation, the strategy of DC maturation has been designed for patients with nonsmall cell lung cancer. However, one patient had Grade 3 hepatotoxicity, and only 32% of patients experienced stabilization for more than 4 months of progression-free survival, which is less than their primary endpoint of 50% [26]. Because tumor antigens, such as carcinoembryonic antigen, can be directly derived from a patient with cancer, ascites-derived exosomes from patients were harvested. Safety and well-tolerance in phase I trial have been reported, and a tumor-specific antitumor cytotoxic T lymphocyte response has been observed in the ascites-derived exosomes plus granulocyte-macrophage colony-stimulating factor group [27].
Exosomes containing anti-cancer drug to cause cytotoxicity for the treatment of patients with cancer
In addition to carrying tumor antigen, exosomes containing chemo drug or siRNA have been used in the treatment of cancer. There are two clinical trials (NCT01854866 and NCT02657460) using chemo drug to treat patients diagnosed with malignant pleural effusion. In the preclinical trial and trial of NCT01854866, they used methotrexate (MTX) and cisplatin as the anticancer drugs, respectively. The survival ratio was higher when MTX was used as the anticancer drug in the preclinical trials [28]. In the trail of NCT02657460, they used MTX as the encapsulating anticancer drug and cisplatin as the comparator. KrasG12D siRNA has been promoted as another anticancer drug type for the treatment of patients with metastatic pancreas cancer, and the mesenchymal stromal cells-derived exosomes have been proposed in the clinical trial number NCT03608631.
Other indications
There have been few clinical trials that employ DC-derived exosomes after 2013 [Table 2]. The application of MSC-derived exosomes in clinical trials began in 2014 [Table 2], and the complete report was available in 2016 [29]. Most clinical trials using MSC-derived exosomes are applied for chronic diseases, immunity diseases, and acute ischemic stroke. Only one case of a clinical trial using MSC-derived exosomes encapsulating KrasG12D siRNA has been reported. Two clinical trials have reported MSC-derived EVs in treating chronic diseases, namely chronic kidney disease, and bronchopulmonary dysplasia. Due to the less degree of manipulation in exosomes, the characteristics of exosomes can be determined by exosome and MSC markers.
Plant source
Three sources of plant namely grape (NCT01668849) and ginger or aloe (NCT03493984), have been registered in clinical trial from the same sponsor, University of Louisville, but the status of clinical trials of plant-derived exosomes is under the not recruiting phase [Table 3]. The application of grape-derived exosomes is in treating diseases due to radiation- and chemotherapy-induced oral mucositis. In a previous preclinical study, Songwen Ju et al. demonstrated that grape-derived exosomes could renew the processes of intestinal tissue and participate in the process of tissue remolding when the tissue suffers from pathological damage [30]. Another study by Henry Bohler et al. used ginger or aloe to produce exosomes for treating patients diagnosed with polycystic ovary syndrome, expecting it to mitigate insulin resistance and chronic inflammation (trial number NCT03493984). Moreover, Donald Miller et al. used plant-derived exosomes as a hydrophobic drug delivery carrier to encapsulate curcumin, owing to the hydrophobic character of molecules, for treating intestinal diseases (trial number NCT01294072).
The exosomes from plant feature the advantages of animal-free issue and Chinese herbal medicine theory to support the basic scenario, but the life cycle of the plant too long and less information to provide in the exosome production and characterization. The less doubling time of mammalian cells, around 24–48 h, attractive the development of clinical trials. There is less information on GMP production for plant-derived exosome, but there are some preclinical studies investigated. The majority difference between animal and plant-derived in the production process is the medium harvest. The exosome from the animal-derived is to harvest the medium in the cultivation process. In contrast, the plant-derived exosomes are to extract the apoplastic vesicles, such as leaf, rice shoot, sunflower seed, and root, or the exosome-like vesicles from fruit juices [31,32,33]. Unfortunately, the characterizations of plant-derived exosome have less information, in particular of specific marker [34]. However, the most common methods, such as TEM, nanoparticle tracking analysis, and SDS-PAGE, for plant-derived exosome characterizations are similar to that of animal-derived exosome. Therefore, the development of GMP-grade plant-derived exosome, in particular of purification and characterization, may refer to that of animal-derived exosome.
CONCLUSION
Plant- and human tissue-derived exosomes have been registered in clinical trials. Exosomes derived from human tissue are established with more complete reports and data than those of plant-derived exosomes. Most studies describe human tissue-derived exosomes, complying with GMP, to satisfy the application requirements of clinical trials. Exosomes production by cell culture has to be subjected to exosome purification and characterization. A hollow fiber-based bioreactor for cell culture is an attractive strategy for exosome production because of the advantage that decreased volume of CM can be harvest from the filtrated fiber. Moreover, exosomes purified by UF for avoiding bioactive protein release from vesicle of exosomes have higher benefit than those of UC. The determination of biofunctions, such as biomarker of exosomes and properties derived from parental cells, are the two major issues for characterization of exosomes before application in clinical trials. Overall, the development of exosomes may be an alternative candidate for treating diseases of unstratified fields, such as cancer, inflammation diseases, and chronic diseases.
Financial support and sponsorship
Financial support was received from the Bioinnovation Center, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan, Republic of China (MF00A130SS05, MF00A130SS01, MF00A130SS02), and Department of Medical Research, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan, Republic of China.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Willms E, Cabañas C, Mäger I, Wood MJ, Vader P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738. doi: 10.3389/fimmu.2018.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanninen KM, Bister N, Koistinaho J, Malm T. Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta. 2016;1862:403–10. doi: 10.1016/j.bbadis.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aav8521. pii: eaav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang XX, Sun C, Wang L, Guo XL. New insight into isolation, identification techniques and medical applications of exosomes. J Control Release. 2019;308:119–29. doi: 10.1016/j.jconrel.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8 doi: 10.3390/cells8070727. pii: E727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiner AT, Witwer KW, van Balkom BW, de Beer J, Brodie C, Corteling RL, et al. Concise review: Developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl Med. 2017;6:1730–9. doi: 10.1002/sctm.17-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohde E, Pachler K, Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21:581–92. doi: 10.1016/j.jcyt.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials - An ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–41. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–8. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: Current perspectives. Proteomics. 2008;8:4083–99. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 14.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O, et al. Dendritic cell-derived exosomes for cancer immunotherapy: What's next? Cancer Res. 2010;70:1281–5. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 16.Taïeb J, Chaput N, Zitvogel L. Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol. 2005;25:215–23. doi: 10.1615/critrevimmunol.v25.i3.30. [DOI] [PubMed] [Google Scholar]
- 17.Burke J, Kolhe R, Hunter M, Isales C, Hamrick M, Fulzele S, et al. Stem cell-derived exosomes: A Potential alternative therapeutic agent in orthopaedics. Stem Cells Int. 2016;2016:5802529. doi: 10.1155/2016/5802529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–26. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 19.Pachler K, Lener T, Streif D, Dunai ZA, Desgeorges A, Feichtner M, et al. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy. 2017;19:458–72. doi: 10.1016/j.jcyt.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Andriolo G, Provasi E, Lo Cicero V, Brambilla A, Soncin S, Torre T, et al. Exosomes from human cardiac progenitor cells for therapeutic applications: Development of a GMP-grade manufacturing method. Front Physiol. 2018;9:1169. doi: 10.3389/fphys.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3 doi: 10.1172/jci.insight.99263. pii: 99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson DC, Yung BC, Bergamaschi C, Chowdhury B, Bear J, Stellas D, et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles. 2018;7:1442088. doi: 10.1080/20013078.2018.1442088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bari E, Perteghella S, Catenacci L, Sorlini M, Croce S, Mantelli M, et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (Lond) 2019;14:753–65. doi: 10.2217/nnm-2018-0240. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Rawat S, Arora V, Kottarath SK, Dinda AK, Vaishnav PK, et al. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:180. doi: 10.1186/s13287-018-0923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–90. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 29.Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345–57. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutter BD, Innes RW. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017;173:728–41. doi: 10.1104/pp.16.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanly C, Fiume I, Capasso G, Pocsfalvi G. Isolation of exosome-like vesicles from plants by ultracentrifugation on sucrose/Deuterium oxide (D2O) density cushions. Methods Mol Biol. 2016;1459:259–69. doi: 10.1007/978-1-4939-3804-9_18. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woith E, Melzig MF. Extracellular vesicles from fresh and dried plants-simultaneous purification and visualization using gel electrophoresis. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20020357. pii: E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]