Abstract
The visual impairment associated with inherited retinal degeneration and age-related degeneration of photoreceptors is causing substantial challenges in finding effective therapies. However, induced pluripotent stem cell (iPSC)-derived therapeutic cells such as photoreceptor and retinal pigment epithelium (RPE) cells provide the ultimate options in the rescue of lost photoreceptors to improve the visual function in end-stage degeneration. Retinal cells derived from iPSC are therapeutic cells that could be promising in the field of cell replacement therapy and regenerative medicine. This review presents an overview of the photoreceptor degeneration, methods of iPSC generation, iPSC in retinal disease modeling, summarizes the photoreceptor differentiation protocols, and challenges remained with photoreceptor cell replacement for the treatment of retinal diseases. Thus, the burden and increased incidence of visual impairment emphasizes the need of novel therapy, where iPSC-derived photoreceptor and RPE cells proved to be promising for curing the retinal dysfunction and act as renovation in approach to improve visual function.
KEYWORDS: Induced pluripotent stem cells, Photoreceptors, Retinal degeneration, Retinal disease modeling, Transplantation
INTRODUCTION
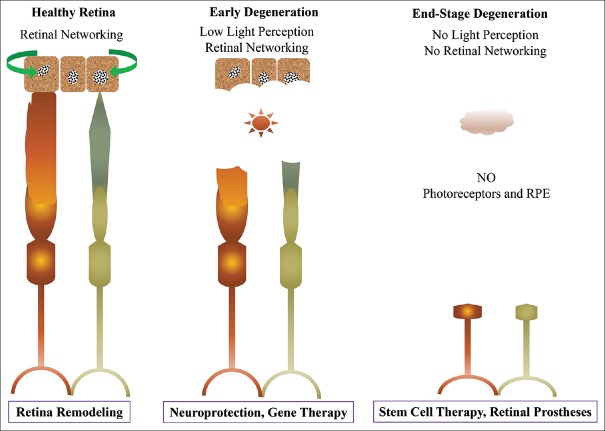
The ontogenetic of retina presents a unique structure for studying neurogenesis, cellular interactions, and gene influences. The retinal cell fates are determined by the various transcription factors and are generated from the pool of multipotent progenitor cells [1,2]. The retina is formed by the proper organization of specific neurons such as photoreceptors (rod and cone), bipolar, amacrine, horizontal, ganglion cells, and types of glial cell such as Müller glia [3]. The functional light-sensitive photoreceptor cells are responsible for the visual senses of interaction with the environments. However, vision impairment due to degeneration of photoreceptor is affecting more than 43 million people globally with an exponential increase in population with advanced age predominantly over 50 years (WHO, 2016, Visual Impairment and Blindness). The cause of visual impairment is due to the progressive loss of photoreceptors related to genetically heterogeneous disorders. The degeneration of photoreceptors associated with genetic and environmental insults leads to blindness is linked to high metabolic need in the renewal of photoreceptor outer segment discs (OSD) [4]. The photoreceptor degeneration is a complex trait altered due to a large number of variant genes, related to environmental factors and predominantly monogenic inheritance [5,6]. Numerous therapeutic strategies, such as neuroprotective agents, gene therapy, retinal prosthesis, and cell replacement therapy have been developed, and some of them already entered the clinical trials. However, cell therapy is an option for advanced retinal diseases, and challenges persist due to the limited source of therapeutic cells, such as retinal pigment epithelium (RPE) and photoreceptors as shown in Figure 1. In this regard, induced pluripotent stem cells (iPSCs) provide a unique in vitro model that allows the generation of retinal progenitor cells for modeling of retinal degenerative diseases. Retinal cell derivatives generated from iPSCs are useful for drug screening for personalized medicine and effective strategies for cellular therapy in both early and end-stage retinal diseases. Furthermore, modeling of developmental disorders is particularly amenable using iPSCs and their derivatives [7].
Figure 1.
Illustration showing progressive photoreceptor degeneration and potential therapeutic approaches
In this review, we specially focus and summarize recent perspectives for directed differentiation of photoreceptor cells from iPSC and iPSC-derived photoreceptor transplantation in retinal disease modeling and possibilities for improving the retinal functions. All the information was obtained from the reliable literature sources.
PHOTORECEPTOR DEGENERATION
The photoreceptors are exceptionally vulnerable cells in the retina, and progressive degeneration of these cells leads to the irreversible loss of vision. Usually, light-sensing photoreceptors (rods – dim and cones – bright) form the visual transduction cascade to perform specialized visual functions. These cells undergo complex phototransduction mechanism that interlinked with the metabolism of retinoid; thus, high metabolic rate is involved in the retinoid visual cycle at the cellular level, molecular level, and electrophysiology of photoreceptor function [8,9]. The metabolic alteration in retinoid contributes to a high level of susceptibility to genetic defects causing dysfunction or death of photoreceptors. Such anomalies lead to loss of inner retinal connection and alter the neuronal networking cascade. Fortunately, the transplanted photoreceptor precursors from the developing retina can contribute to making single and short synaptic interplay to the optical network for retinal modeling [10].
Several inherited retinal diseases are associated with dysfunction and progressive loss of photoreceptors, such as retinitis pigmentosa [11], age-related macular degenerations [12], and Leber’s congenital amaurosis (LCA) [13]. Among them, retinitis pigmentosa is the leading cause of untreatable blindness that is characterized by gradual constriction of visual field. Moreover, the loss of photoreceptors in inherited retinal diseases does not have genotype–phenotype correlation due to extensive genetic heterogeneity. Inherited retinal diseases, such as macular degeneration, retinitis pigmentosa, and Usher syndrome constitute a genetically heterogeneous group with almost 293 human genetic loci and more than 256 genes identified so far (Retnet; https://sph.uth.edu/retnet/sym-dis.htm) [14].
PLURIPOTENT STEM CELLS AND CELLULAR REPROGRAMMING
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and iPSCs, provide a unique in vitro model for generating the therapeutic cells, such as photoreceptor and RPE for cell replacement therapy in retinal degenerative diseases. Here, we specifically focus on iPSCs generated from somatic cells by cellular reprogramming using defined transcription factors.
Induced pluripotent stem cells
iPSC was an innovative discovery by Takahashi and Yamanaka in 2006, where mouse embryonic/skin fibroblasts and adult human fibroblasts were converted into PSCs by the overexpression of defined transcription factors, such as Oct4, Sox2, Klf4, and c-Myc using the retroviral system [15,16]. These cells were morphologically identical and showed similar pluripotent gene expression like in ESCs system [15,16]. Furthermore, Yu et al. used other sets of defined factors, such as Oct4, Sox2, Nanog, and LIN28 using lentivirus to generate iPSCs from foreskin fibroblasts [17]. These iPSCs showed the expression of pluripotency genes and potential to differentiate into developmental germ layers (endoderm, mesoderm, and ectoderm) investigated using standard in vivo teratoma assay and alternative in vitro embryoid body formation [16]. iPSCs have been generated from somatic cells of different mammals, such as mice [18], human [16], monkeys [19], and pigs [20]. These iPSCs showed similar characteristic features of PSCs; however, cell reprogramming efficiency differs among different cell origin, cell types, and no consensus on the most consistent protocol for generating the reliable and safest iPSCs [21]. Still, iPS technology has been revolutionizing the stem cell research and therapy for regenerative medicine.
Alternative methods for induced pluripotent stem cell generation
Since the discovery of iPSC technology, reprogramming protocol improvements are increasing to achieve efficient derivation and to maintain the normal genomic integrity. Recently, iPSC methods are available as commercial kit, but they do require reliability with regard to efficiency and reproducibility of reprogramming somatic cells into iPS for biomedical uses in routine work [22]. Different methods for cellular reprogramming are summarized and extensively reviewed [23] [Table 1].
Table 1.
Methods and transcription factors for the generation of induced pluripotent stem cells
| Methods | Factors | Reference |
|---|---|---|
| Single lentiviral vectors expressing “stem cell cassette” with Cre-Lox transgene excision system | Oct4, Klf4, Sox2, cMyc | [24] |
| Cre-recombinase excisable DOX-inducible lentiviral vectors system | Oct4, Klf4, Sox2, cMyc | [25] |
| Repeated transfection of two expression plasmids without viral vectors system | (cDNA of Oct3/4, Sox2, Klf4) and cDNA of cMyc | [26] |
| Nonintegrating adenoviral vectors system | Oct4, Sox2, Klf4, cMyc | [27] |
| Nonintegrating Sendai virus-based vector system | Oct4, Sox2, Klf4, cMyc | [28] |
| Recombinant reprogramming proteins system (fusion of poly-arginine protein transduction domain to the C terminus of transcription factors) | Oct4, Sox2, Klf4, cMyc | [29] |
| Human artificial chromosomes (HACs) vectors (iHAC1 and iHAC2) system | Oct4, Sox2, Klf4, cMyc | [30] |
| Synthetic modified mRNA transfection system | Oct4, Sox2, Klf4, cMyc | [31] |
| Direct transfection of mature double-strand microRNAs (miRNAs) system | Combination of mir-200c, mir-302 s and mir-369 s | [32] |
| Doxycycline-inducible factors delivered by “piggyback” transposition system | Oct4, Sox2, Klf4, cMyc | [33] |
| Nonviral minicircle DNA vectors system | Oct4, Sox2, Nanog, Lin28 | [34] |
| Nonintegrating episomal plasmid vectors system | Oct4, Sox2, Nanog, Lin28, Klf4, cMyc | [35] |
Molecules for enhancing reprogramming efficiency
Generation of iPSCs is time-consuming, and the efficiency varies with cell types and without consistent protocols. Thus, for clinical application of iPSCs, it is necessary to overcome the limitations of low efficiency. Numerous lists of small molecules, growth factors, microRNAs, and siRNAs have been suggested to enhance the efficiency of cellular reprogramming based on several well-known mechanisms [36,37]. Some of the commonly used small molecules are listed in Table 2.
Table 2.
Small molecules and the mechanism that promotes reprogramming efficiency
| Molecules | Mechanism | Reference |
|---|---|---|
| VPA or NaB | Inhibition of HDAC | [38,39] |
| BIX-01294 (BIX) | Inhibition of G9a histone methyltransferase (G9a HMTase) | [40] |
| SB431542 plus PD0325901 | Inhibition of TGFβ receptor and MEK signaling pathway | [41] |
| SB431542 or ALK5 (A-83-01) | Inhibition of Type 1 TGFβ receptor | [42,43] |
| Vitamin C | Alleviates cell senescence and promotes epigenetic modifications | [44] |
| Y-27632 | Inhibition of the ROCK pathway | [45] |
| PS48 | Activation of PDK1 | [46] |
VPA: Valproic acid, NaB: Sodium butyrate, HDAC: Histone deacetylase, Rho-associated Kinase, PDK1: 3′-phosphoinositide-dependent kinase-1, MEK: MAPK/ERK Kinase
Molecular basis of genomic instability of induced pluripotent stem cell
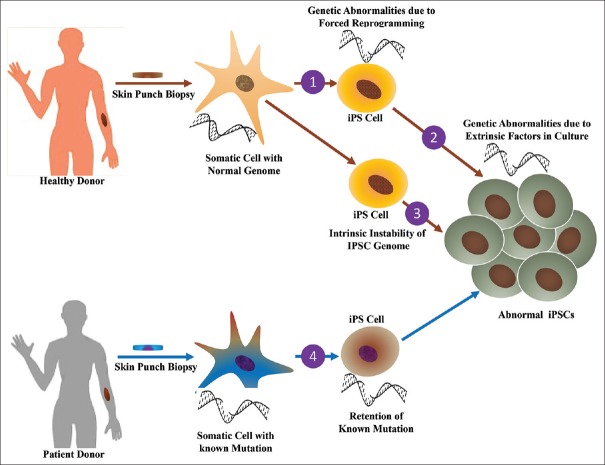
Genomic instability acquired during forced reprogramming, cultural adaptation, passage-induced mutation, and viral integration has been reported [47,48]. The maintenance of genomic stability is essential to produce distinct cell lineages because differentiated cells inherit harmful effects such as chromosomal aberrations, copy number variations (CNV), and single-nucleotide variant [48]. Such effects remained significant challenges to be addressed before used in clinics.
Chromosomal aberrations occurred due to selective pressure either from parental inheritance or from forced reprogramming as shown in Figure 2. A forced expression of the proto-oncogene, such as c-Myc used in reprogramming, is associated with inactivation of p53 causing genomic aberrations in iPSCs [49]. Furthermore, the demand of high metabolic rate associated with induction of oncogenic transcription factors causes elevation of oxidative stress (ER), which leads to genomic aberrations. Such aberrations can be reduced by the use of antioxidant in growth media that significantly lower the CNVs [50]. Likewise, parental cells harboring protein-coding mutations that cause cancer, remain permanent in the iPS cell line populations [51]. In addition, only 20% of mutations are parental inheritance, while approximately 75% of coding point mutations are associated with reprogramming and persist during the subsequent passages [52].
Figure 2.
Illustration showing the factors associated with genomic instability in iPS cells
In clinical applications, the genomic instability is of major concern which may induce teratoma, but the key factors that regulate genomic instability in iPSCs are unclear [47]. Furthermore, the level of genomic instability observed in iPSCs is comparable with the signs perceived in cancer cells [53]. Despite the instability concern, such transient aberrations and negligible report on genomic aberrations with malignant transformation will compromise the safety of iPS-derived cell therapy in clinic. Thus, the appropriate technique should be applied to distinguish harmless aberrations and those causing tumor formations. For such classification of mutation pattern, high-resolution single-nucleotide polymorphism genotyping is recommended to monitor the acquired mutations to minimize the effects on cell therapy [54].
APPLICATION OF INDUCED PLURIPOTENT STEM CELL TECHNOLOGY
iPSC technology can be directly used for developmental biology, disease modeling, drug discovery and efficacy testing, and cell transplantation. Various patient-specific iPSC lines have been generated for modeling of retinal dystrophies, such as retinitis pigmentosa, LCA, gyrate atrophy, Stargardt’s macular dystrophy, bestrophinopathies, and choroideremia [55]. Furthermore, the previous study demonstrated that rod cells derived from iPSC of retinitis pigmentosa patients showed differential responsiveness to α-tocopherol (Vitamin E) [56]. The study of human organogenesis is difficult due to inaccessibility of organs which develops in early embryos. The tracking of early eye development and organoidogenesis with fully developed laminated and functional retinal structure was generated using iPSC [57]. In addition, iPSC technology has opened new possibilities for generating continuous sources of therapeutic cells, such as RPE, retinal ganglion cells, and photoreceptors. Recently, several preclinical and clinical trials have demonstrated the safety, survival, integration, and rescue effects of clinical-grade RPE cells derived from patient-specific iPSC [58,59]. Therefore, iPSCs are promising cell sources for autologous cell transplantation, which minimizes the risks of immune rejection and the development of self-organized three-dimensional tissues using iPSC open the future possibilities for next-generation organ transplantation.
PATIENT-SPECIFIC INDUCED PLURIPOTENT STEM CELL-BASED EYE DISEASE MODELING
Recent advances in in vitro iPSC model system are helpful resources for generating enough cells to create distinctive platforms for disease modeling, drug screening, and cell-based therapy [60]. Furthermore, human cellular models have potential benefits that overcome the limitation of the animal model, which cannot faithfully mimic human physiology. The self-renewal properties of iPSCs are promising for modeling various diseases, as other specialized primary cell lines stop proliferation after few passages in laboratory culture. Several studies used iPSCs for a wider variety of human diseases, such as genetic disorders (Down’s syndrome), neurological disorders (Parkinson’s disease), and inherited metabolic syndrome (juvenile diabetes) [60].
Usually, retinal biopsies are difficult to take or inaccessible to investigate the pathophysiology of inherited retinal diseases. Such pathological investigation became possible using patient-specific iPSCs to identify novel gene expression and silencing, drug screening and development, and cell transplantation in advanced retinal degeneration. iPSCs were used for exploring the structure of eye, such as limbal-like epithelium and corneal epithelial cells [61,62], lens-specific differentiation [63], trabecular meshwork [64], optic vesicle-like structures [65], retinal ganglion cells [66], photoreceptor cells [7], and retinal pigment epithelial cells [67]. Besides, patient-specific iPSCs were used for several inherited retinal diseases, such as age-related macular degeneration, retinitis pigmentosa, and glaucoma [68,69,70]. Human iPSCs were used to study early eye development and retinogenesis that recapitulate the in vivo developmental timeline using iPSC-derived retinal organoids. All retinal neurons were appropriately arranged in their proper layer, in which photoreceptors achieved sensory cilia with prominent OSD that responded the light [57]. Furthermore, RGCs were generated from iPSCs using fibroblasts from normal tension glaucoma patient carrying a duplication of TBK1 gene [71] and multilayered optic cups from keratinocyte-derived iPSCs of patients with retinitis pigmentosa [72]. The retina precursor cells from the optic cup revealed their ability to integrate into developing mouse retina [72]. Advanced retinal diseases with severe loss of photoreceptor cells have no benefits with gene therapy, where cell transplantation remains an ultimate strategy. Therefore, the use of iPSCs-derived photoreceptors and RPE is under intense investigation to restore visual network or to arrest the loss of vision [67,72,73].
ESTABLISHMENT OF INDUCED PLURIPOTENT STEM CELL-DERIVED PHOTORECEPTOR AS THERAPEUTIC CELLS
In vitro development of light-sensitive functional photoreceptor cells is challenging, but essential therapeutic cells for retinal degenerative diseases. In early age-related macular degeneration that is associated with RPE dysfunction, transplantation of RPE cells might be beneficial for the functional recovery of native RPE; for diseases with end-stage degeneration with loss of photoreceptors, the ultimate option is photoreceptor cell transplantation. Therefore, photoreceptors derived from iPSCs using somatic cells either from patients or from healthy donor could be effective strategies for cell-based therapy. Patient-specific photoreceptor cells can also be used to investigate the role of the mutant gene and drug screening for personalized medicine. Meanwhile, iPSC-derived photoreceptor cells from a healthy donor could be promising sources for allogeneic cell transplantation. Furthermore, the sequential development of retinal neurons provides the benefit to target disease progression at different stages in retinal degeneration [74]. Numerous studies explored the potential of photoreceptor cells generated from human iPSC as listed in Table 3. iPSC-derived photoreceptor cells have been established from various cell sources such as blood, fibroblast, and keratinocytes [56,68,72,91]. However, there is no report of iPSC-derived photoreceptor cell transplantation in human trail for the vision correction due to difficulty in establishing the robust, efficient, and stable methods for generation and purification of photoreceptor cells.
Table 3.
Differentiation factors, sequential expression of induced pluripotent stem cell-derived photoreceptor markers, and transplantation outcomes
| Disorder | Affected gene | Cell Source | Method | Differentiation factor | Cell types and markers | Drug testing/transplantation | Functional assessment | Reference |
|---|---|---|---|---|---|---|---|---|
| Retinitis Pigmentosa | USH2A variant (Arg4192His) | Keratinocytes | Sendai virus OCT4, SOX2, KLF4, c-MYC | Noggin, Dkk-1, IGF-1, bFGF, DAPT, aFGF, B27, N2 | Multilayer eyecup-like structures and retinal rosettes (recoverin, rhodopsin, B-opsin, R/G-opsin) Protein misfolding and ER stress in differentiated cells | Subretinal space; P4 immune compromised Rag1-/- x Crb1-/- mice | Extensive cellular integration and differentiated to photoreceptor cells Axonal projections toward the inner plexiform layer and the RPE layers | [72] |
| Retinitis Pigmentosa | Mutations (RP1, RP9, PRPH 2 or RHO gene) | Fibroblasts | Retroviral transduction OCT3/4, SOX2, KLF4, c-MYC | Lefty-A, Dkk-1, RA, Taurine, N2 | Immature photoreceptors (CRX, recoverin); Rod cells (rhodopsin); cone cells (opsin). ER stress in rod cells | Drug screening; Anti-oxidant vitamins (alpha-tocopherol, ascorbic acid, beta-carotene) | α-tocopherol is beneficial to RP9- mutated rod cells survival. No effect of ascorbic acid and β-carotene | [56] |
| Retinitis Pigmentosa | RHO-G188R mutation | Fibroblasts | Sendai virus OCT3/4, SOX2, KLF4, c-MYC | Lefty-A, Dkk-1, RA, Taurine | Photoreceptors precursors (recoverin, CRX); Rod cells (rhodopsin) DNA damage-inducible transcript (BiP and CHOP) expressed | Not performed | N/A | [68] |
| Undefined | Normal | Fibroblasts | Lentiviral transduction OCT4, NANOG, LIN28, SOX2 | Noggin, Dkk-1, IGF-1, N2, B27 | Photoreceptor precursors (Ot×2, Crx, recoverin, AIP1); Rod cells (rhodopsin); cone cells (S-opsin) | Subretinal Space; Adult wild-type mice along with Cyclosporine A (10 mg/kg/day) | FACS purified photoreceptor cells survived and integrated into the host retina after transplantation | [7] |
| Enhanced S-cone sensitivity syndrome (ESCS) | NR2E3 mutations | Fibroblasts | Sendai virus OCT4, SOX2, KLF4, c-MYC | IWR1e, CHIR, SAG, N2, DAPT | Photoreceptor precursors (OTX2, CRX, recoverin) Rod cells (NRL, NR2E3, RORβ, SW opsin) | Subretinal injection; Immune compromised SCID mice. | cGMP grade photoreceptor precursors cells were safe, and no intraocular tumor formed | [75] |
| MAK-associated Retinitis Pigmentosa | Homozygous for Alu insertion in MAK lacking exon 9 transcript | Fibroblasts | Lentiviral transduction OCT4, SOX2, KLF4, c-MYC | B27, N2, Noggin, Dkk-1, IGF1, bFGF, DAPT, aFGF | Photoreceptor precursors (OTX2, recoverin) | Not performed | MAK exon 12 is retina-specific, but not MAK exon 9 Point mutations in exon 9 and 12 are not a common cause | [76] |
| Undefined | N/A | Fibroblasts | Retroviral transduction OCT4, SOX2, KLF4, MYC | CKI-7, SB-431542, N2, RA, Taurine | Photoreceptor precursors (CRX, NRL recoverin) Rod cells (rhodopsin) | Not performed | N/A | [77] |
| Undefined | N/A | Fibroblasts | Retroviral transduction OCT4, SOX2, KLF4, MYC | Dkk-1, Lefty A, N2, RA, Taurine | Photoreceptor precursors (CRX, recoverin) Rod cells (rhodopsin) | Not performed | N/A | [78] |
| Undefined | N/A | Fibroblasts | Retroviral transduction OCT4, SOX2, KLF4, LIN-28 | B27, N2, Noggin, Dkk-1, Lefty A, IGF1, SHH, T3, RA, Taurine | Photoreceptor precursors (CRX, recoverin, arrestin 3) Rod cells (Rhodopsin); cone-specific opsin blue and opsin red/green | Not performed | N/A | [79] |
| Undefined | N/A | Corneal fibroblast | STEMCAA lentivirus OCT4, SOX2, KLF4, MYC | B27, N2, RA, Taurine, bFGF, aFGF, SHH | Photoreceptor precursors (CRX, recoverin) Rod cells (NRL, NR2E3, RORβ, SW opsin) | Not Performed | N/A | [80] |
| Undefined | N/A | hiPSC lines; fibroblasts, cord blood, keratinocytes | Lentivirus SOX2, OCT4, NANOG, LIN28. Episomes SOX2, OCT4, KLF4, MYC, NANOG, LIN28, SV40T | Heparin, N2, B27, Taurine, RA | Photoreceptor precursors (Ot×2, recoverin) Rod cells (rhodopsin); cone cells (S-opsin, L/M-opsin) | Not Performed | Retinal organoids acquired mature photoreceptors with functional domains containing an outer segment disc that were responsive to lights | [57] |
| Healthy donor | N/A | Blood (activated T cells) | Retroviral transduction; 3 bicistronic MMLV constructs: (OCT4, SOX2), (c-MYC, KLF4), and (NANOG, LIN28) | Heparin, N2, B27 | Photoreceptor precursors (CRX, recoverin) Rod cells (rhodopsin); cone cells (S-opsin) | Not performed | In vitro synaptogenesis was recapitulated in hiPSC-derived optic vesicles. CX36 required for electrical synapses were upregulated in optic vesicles | [65] |
| Gyrate atrophy | N/A | hiPSC lines, gyrate atrophy fibroblasts | Lentiviral transduction OCT4, SOX2, NANOG, LIN28, c-MYC, KLF4 | Noggin, Dkk-1 B27, N2 | Photoreceptor precursors (OTX2, CRX, NRL recoverin) Phototransduction (CNGA1, A3, B1 and B3, RETGC, ODE6B, arrestin). Photoreceptor-like cells showed electrophysiological responses | Not Performed | Gene-corrected hiPSC-RPE showed normal level of ornithine-δ-aminotransferase (OAT) activity | [81] |
| Retinitis pigmentosa | Rhodopsin mutation (E181K) | Fibroblasts | Retroviral transduction OCT3/4, SOX2, KLF4, c-MYC | N2, Noggin, Dkk-1, IGF-1 | NRL-positive rod cells DNA damage-inducible transcript (BiP and CHOP) expressed, Rhodopsin E181K mutation was responsible for the loss of rod cells | Drug Screening: mTOR inhibitors (rapamycin and PP242), AMPK activators (AICAR), ASK1 inhibitors (NQDI-1), and inhibitors for eIF2α phosphatase and protein synthesis (salubrinal) | Suppression of E181K mutant rhodopsin-related cell loss by mTOR inhibition, AMPK activation, ASK1 inhibition, or the suppression of protein synthesis | [82] |
| Undefined | N/A | hiPS cell lines (IMR90 clone 4) | N/A | N2, Heparin, B27, BMP4, Wnt3A, SU5402 | Photoreceptor precursors (CRX, recoverin) Cone-specific opsin | Not performed | N/A | [83] |
| Undefined | N/A | hiPS cell lines | mRNA reprogramming Kit, Episomal vectors | B27, N2, Insulin, Noggin, BDNF, CNTF, RA, DAPT | Photoreceptor progenitors (CRX, NRL, NR2E3), Rod cells (rhodopsin, recoverin, and phosphodiesterase 6 alpha) | Subretinal space; Rd1 mice with end-stage retinal degeneration | Differentiated into cell photoreceptors, formed cell layer, interact with host retina and visual functions was partially restored | [74] |
| Undefined | N/A | Fibroblasts | OriP/EBNA1-based episomal vectors pEP4EO2SEN2K, pEP4EO2SET2K, and pCEP4-M2L | N2, FGF2, DAPT | Photoreceptor precursors (OTX2, CRX, NRL recoverin) Rod cells (rhodopsin); cone cells (blue opsin, R/G-opsin, cone arrestin) | Not performed | Notch pathway inhibition accelerated the generation of photoreceptor precursors | [84] |
| None | N/A | hiPS cell line (NCL1), cord blood-derived CD34+ | Episomal plasmids | N1, Dkk-1, Noggin, IGF1, IWR1, SB431542, LDN193189 | Photoreceptor precursors (OTX2, CRX, BLIMP1, AIPL1, recoverin) Rod cells (NRL); cone cells (RXRγ) | Subretinal space; IL2rγ-/- mice (B6.129S4-Il2rgtm1Wjl/J) | Photoreceptor precursor cells integrated into the host retina with synaptic end-feet to plexiform layer | [85] |
| None | N/A | hiPS cell line (IMR90-4) | N/A | B27, Taurine, RA, N2 | Photoreceptor precursors (Recoverin) Rod cells (CD133/CD73, Rhodopsin); cone cells (S-opsin, L/M-opsin, cone arrestin) | Not performed | Bioreactor culture conditions improved retinal lamination and increased the yield of photoreceptors | [86] |
| None | N/A | hiPS cell line (H9, H1, IMR90-4) | N/A | N2, B27, Taurine, RA | Photoreceptor precursors (OTX2, CRX, NRL recoverin) Rod cells (rhodopsin); cone cells (S-opsin, L/M-opsin, arrestin 3, RXRγ) | Subretinal space; Cone cells transplantation into Nrl-/-, and Aipl1-/- mice | Cone precursors incorporated into host cone-rich retina. Also, cones have the potential to replace lost photoreceptors in end-stage retinal degeneration | [87] |
| None | N/A | hiPS cell line (hiPSC-2 clone), fibroblasts | N/A | N2, B27, FGF2 | Photoreceptor precursors (CRX, Recoverin, CD73) Rod cells (rhodopsin); cone cells (blue opsin, R/G-opsin) | Trans-vitreal injection; hemizygous P23H rats along Cyclosporine A (210mg/L) | Transplantation of CD73+cells showed no significant functional improvement | [88] |
| Healthy donor | N/A | hiPS cell lines (UE017, UE022, and UC005), urine cells | N/A | B27, N2, Taurine | Photoreceptor precursors (OTX2) Rod cells (rhodopsin); cone cells (S-opsin, L/M-opsin) | Not performed | Matured rod and cone cells acquisition in retinal organoids without the addition of RA | [89] |
| None | N/A | NHEK primary cell line | Sendai virus OCT4, SOX2, KLF4, c-MYC | B27, N2, DAPT, Taurine, RA | Photoreceptor precursors (OTX2, CRX, recoverin) Rods cells (rhodopsin); cone cells (B-opsin, R/G-opsin) | Not performed | Aberrant-hiPSC differentiated into retinal organoids that acquired matured photoreceptors | [90] |
N/A: Not available
Human iPSC-derived photoreceptor cells have been generated from various diseases, such as retinitis pigmentosa, enhanced S-cone sensitivity syndrome, and gyrate atrophy as listed in Table 3. Derivative photoreceptor cells revealed the characteristic expressions, such as cell lineage markers (OTX2, CRX, RECOVERIN, RHO, B-OPSIN, and R/G OPSIN) [7,56,72], expression of proteins (VGLUT1 and SNAP-25) responsible for neurotransmitter release [65], the presence of light-responsive sensory cilia in the outer segment disc [72], and electrophysiology responses [56]. iPSC-derived photoreceptor cells were generated using somatic cells of a patient with retinitis pigmentosa harboring different mutations (RP1, RP9, PRPH2, RHO, and USH2A). Such mutations lead to degeneration of photoreceptor cells over time due to ER-stress [56,72]. Interestingly, the different types of mutation causing retinitis pigmentosa subsequently decreased in iPSC-derived rod photoreceptor cells [56,68]. However, mutant iPSC-derived rod cells undergo degeneration in in vitro culture that expressed the oxidative and ER stress marker responsible for the presence of disease phenotypes [56,68]. This evidence further suggests that the loss of photoreceptors in retinitis pigmentosa is postdevelopmental degeneration due to the ER stress and the unfolded protein response [68]. Furthermore, iPSC-derived retinal organoids carrying TRNT1 mutation cause high-level expression of oxidative stress molecules and abnormal accumulation of LC3-II, leading to defective autophagy [92]. The high expression of ER stress and apoptotic markers is associated with poor survival of photoreceptor cells with the rhodopsin mutation (E181K). The use of rapamycin, PP242, AICAR, NQDI-1, and salubrinal reduces the cellular stress enhancing the better survival of iPSC-derived photoreceptor precursors [82]. The data provide a novel approach for screening new drugs to attenuate degeneration in iPSC-derived photoreceptor cells, but it is still elusive whether such drugs could recover the light-responsive function of photoreceptor cells.
The in vitro degeneration of retinal cells and ER stress demonstrate its utility for disease modeling that recapitulates the disease phenotypes. Derivative retinal cells can be corrected for functional defect either using gene correction or pharmacological means. The previous study revealed the restoration of ornithine-δ-aminotransferase (OAT) activity in (A226V) OAT iPSC-RPE by supplementation of 600 μM Vitamin B6 and gene-corrected hiPSC-RPE cells [81]. The patient-specific iPSC carrying rhodopsin mutation (E181K) corrected using adenoviral gene transfer, which showed the ability to differentiate into rod photoreceptors [82]. Furthermore, patient-specific photoreceptor precursor cells showed the ability to integrate into the dystrophic mouse retina and develop into mature photoreceptor cells [72]. Thus, the combination of iPSCs and gene therapy approach could be used in treating various degenerative diseases causing blindness.
INDUCED PLURIPOTENT STEM CELL-DERIVED PHOTORECEPTOR CELL TRANSPLANTATION
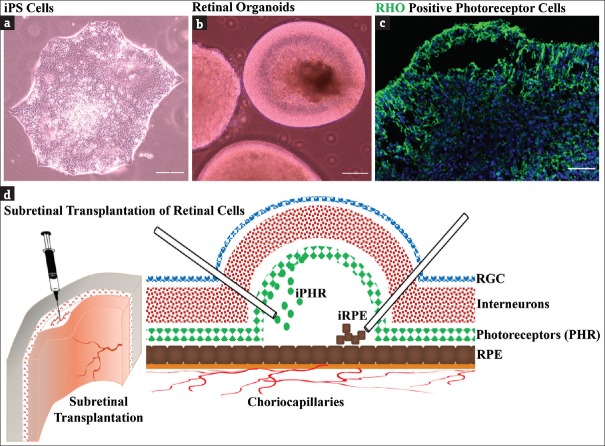
Effective transplantation of derivative photoreceptor cells should meet basic criteria for high-quality clinical-grade manufacturing, safety, and efficacy. Worthwhile rapid, efficient, and consistent differentiation protocol is necessary for replacement therapy in visually challenged patients. The acquisition of mature photoreceptors in iPSC-derived retinal organoids and strategic approaches for retinal cell transplantation is shown in Figure 3a-d. However, the use of animal-derived growth factors, viral vectors during reprogramming, genomic instability and prolonged time for differentiation limit their clinical application. The use of small molecules to overcome the cross-species antigenic contamination has been suggested [77]. Furthermore, numerous studies revealed the improved differentiation protocols using chemically defined optimal culture condition for the development of retinal cell lineages and photoreceptor precursors that showed sequential expression of retinal cell markers [68,78]. Although the efficiency of differentiation was better, there was no consistency in defined molecules for induction of photoreceptors from iPSC. Thus, the optimization of minimal culture conditions which endogenously upregulate the specific transcription factors of retinal cells is necessary. The key factors such as B27, N2, DKK1, and Noggin enhance the early stage differentiation of iPSC into retinal and photoreceptor precursors [79]. However, with improvement in the methodology for iPSC generation without viral vectors [Table 1], feeder-free culture conditions and chemically defined directed differentiation approaches for efficient generation of photoreceptor precursor cells have offered the platform for modeling human diseases.
Figure 3.
Generation of induced pluripotent stem cell-derived retinal organoids with the acquisition of photoreceptor cells. (a) Induced pluripotent stem cell colony with tightly packed cells with defined boundaries, (b) induced pluripotent stem cell-derived retinal organoids, (c) Retinal organoids with RHO-positive photoreceptor cells at apical part and (d) representative illustration showing subretinal transplantation of induced pluripotent stem cell-derived RPE and photoreceptor cells. Scale bars: 150 μm (c), 200 μm (a and b)
The transplantation of postnatal photoreceptor precursors and PSC-derived photoreceptor precursors showed the proof of its potential benefit for the restoration of visual functions [7,93,94]. Transplantation of photoreceptor cells derived from iPSC in rd1 mouse model of end-stage retinitis pigmentosa revealed the robust cell survival, developed a mature outer nuclear layer (ONL), and connected to interneurons improving the visual function [74]. However, the functional restoration after transplantation could be associated with RNA and/or protein transfer between graft and host photoreceptors; instead of transplanted photoreceptors migrating and integrating into the photoreceptor layer of recipients [95,96], these observations raise the possibility of material transfer as an alternative strategy for the treatment of retinal disorders. Surprisingly, CD73+ photoreceptor precursors derived from human iPSC transplanted into rat eyes revealed the capacity to survive and mature in proximity to host inner retina without significant functional improvement by electroretinography [88]. They suggested that more suitable transplantation strategies and closely related species (such as nonhuman primates) may improve the functionality of CD73+ cells after transplantation [88]. Further, the purification of transplantable photoreceptors using fluorescence-activated cell sorting and magnetic-activated cell sorting can be effective strategies for obtaining approximately 90% of pure cells, avoiding the possible risk of teratoma formation [7,88]. In addition, PSC-derived retinal organoids in three-dimensional culture systems offer the accessibility to the human cells such as photoreceptors and RPE for future clinical applications in degenerative diseases [97,98]. Furthermore, transplantable photoreceptor cells could be isolated from heterogeneous cells layered in the retinal organoids using novel label-free sorting approaches based on the inherent characteristics, such as mechanical and morphorheological properties [97]. Moreover, transplantation studies have revealed that photoreceptor precursors can differentiate into mature photoreceptor cells, and migrate to host ONL, in which donor cells acquire synaptogenic potential and initiate retinal synaptic circuitry with naïve photoreceptors via cellular integration [97,98]. While sensory photoreceptor cell transplantation has been the center of focus, it is essential to include RPE cells for successful and sustainable recovery of vision in certain diseases [99]. Thus, cotransplantation of RPE and photoreceptors provides a favorable environment for incorporation of donor cells as shown in Figure 3d.
CONCLUSIONS
iPSC-derived photoreceptor cells are promising and future hope for effective cell-based therapy in end-stage retinal diseases. However, based on numerous publications, the hype has been created in the scientific community and to the public indicating the success of photoreceptor cell transplantation. Till date, there is no evidence on human studies showing successful and effective photoreceptor transplantation that reversed the loss of vision. Furthermore, similar data on transplantation, such as cell survival, integration, and trophic support for improving visual functions, were interpreted differently. More preclinical studies are required to claim the science behind the success of iPSC derivative transplantation.
Financial support and sponsorship
The Buddhist Tzu Chi Medical Foundation grants (TCMMP104-05-01 and TCMF-EP-108-01) supported Institute of Eye Research, Hualien Tzu Chi Hospital, Hualien, Taiwan.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Heavner W, Pevny L. Eye development and retinogenesis. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008391. pii: a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassett EA, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012;35:565–73. doi: 10.1016/j.tins.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 4.Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–72. doi: 10.1146/annurev-neuro-060909-153227. [DOI] [PubMed] [Google Scholar]
- 5.Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- 6.Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: Genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–84. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 7.Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: The interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 9.Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol. 2010;20:R114–24. doi: 10.1016/j.cub.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 11.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 12.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1:381–96. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 13.Traboulsi EI. The Marshall M. Parks memorial lecture: Making sense of early-onset childhood retinal dystrophies – The clinical phenotype of leber congenital amaurosis. Br J Ophthalmol. 2010;94:1281–7. doi: 10.1136/bjo.2009.165654. [DOI] [PubMed] [Google Scholar]
- 14.Ratnapriya R, Swaroop A. Genetic architecture of retinal and macular degenerative diseases: The promise and challenges of next-generation sequencing. Genome Med. 2013;5:84. doi: 10.1186/gm488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 18.Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Martin G, Kupriyanov S, et al. Generation of mice derived from induced pluripotent stem cells. J Vis Exp. 2012;69:e4003. doi: 10.3791/4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–91. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 20.Ezashi T, Telugu BP, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A. 2009;106:10993–8. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochiduki Y, Okita K. Methods for iPS cell generation for basic research and clinical applications. Biotechnol J. 2012;7:789–97. doi: 10.1002/biot.201100356. [DOI] [PubMed] [Google Scholar]
- 23.González F, Boué S, Izpisúa Belmonte JC. Methods for making induced pluripotent stem cells: Reprogramming Á la carte. Nat Rev Genet. 2011;12:231–42. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 24.Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–9. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 27.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–62. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiratsuka M, Uno N, Ueda K, Kurosaki H, Imaoka N, Kazuki K, et al. Integration-free iPS cells engineered using human artificial chromosome vectors. PLoS One. 2011;6:e25961. doi: 10.1371/journal.pone.0025961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, et al. PiggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narsinh KH, Jia F, Robbins RC, Kay MA, Longaker MT, Wu JC. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat Protoc. 2011;6:78–88. doi: 10.1038/nprot.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebrahimi B. Reprogramming barriers and enhancers: Strategies to enhance the efficiency and kinetics of induced pluripotency. Cell Regen (Lond) 2015;4:10. doi: 10.1186/s13619-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4:301–12. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Ware CB, Wang L, Mecham BH, Shen L, Nelson AM, Bar M, et al. Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells. Cell Stem Cell. 2009;4:359–69. doi: 10.1016/j.stem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–75. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y, Do JT, Desponts C, Hahm HS, Schöler HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–8. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–8. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–9. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, et al. A small-molecule inhibitor of tgf-beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–9. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 46.Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–5. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic instability of iPSCs: Challenges towards their clinical applications. Stem Cell Rev. 2017;13:7–16. doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang G, Zhang Y. Genetic and epigenetic variations in iPSCs: Potential causes and implications for application. Cell Stem Cell. 2013;13:149–59. doi: 10.1016/j.stem.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasi CE, Dereli-Öz A, Negrini S, Friedli M, Fragola G, Lombardo A, et al. Genomic instability in induced stem cells. Cell Death Differ. 2011;18:745–53. doi: 10.1038/cdd.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji J, Sharma V, Qi S, Guarch ME, Zhao P, Luo Z, et al. Antioxidant supplementation reduces genomic aberrations in human induced pluripotent stem cells. Stem Cell Reports. 2014;2:44–51. doi: 10.1016/j.stemcr.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji J, Ng SH, Sharma V, Neculai D, Hussein S, Sam M, et al. Elevated coding mutation rate during the reprogramming of human somatic cells into induced pluripotent stem cells. Stem Cells. 2012;30:435–40. doi: 10.1002/stem.1011. [DOI] [PubMed] [Google Scholar]
- 53.Pera MF. Stem cells: The dark side of induced pluripotency. Nature. 2011;471:46–7. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- 54.Peterson SE, Loring JF. Genomic instability in pluripotent stem cells: Implications for clinical applications. J Biol Chem. 2014;289:4578–84. doi: 10.1074/jbc.R113.516419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foltz LP, Clegg DO. Patient-derived induced pluripotent stem cells for modelling genetic retinal dystrophies. Prog Retin Eye Res. 2019;68:54–66. doi: 10.1016/j.preteyeres.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Jin ZB, Okamoto S, Osakada F, Homma K, Assawachananont J, Hirami Y, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One. 2011;6:e17084. doi: 10.1371/journal.pone.0017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–46. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 59.Sharma R, Khristov V, Rising A, Jha BS, Dejene R, Hotaling N, et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat5580. pii: eaat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowe RG, Daley GQ, et al. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20:377–88. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashi R, Ishikawa Y, Ito M, Kageyama T, Takashiba K, Fujioka T, et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One. 2012;7:e45435. doi: 10.1371/journal.pone.0045435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sareen D, Saghizadeh M, Ornelas L, Winkler MA, Narwani K, Sahabian A, et al. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl Med. 2014;3:1002–12. doi: 10.5966/sctm.2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiu X, Yang J, Liu T, Jiang Y, Le Q, Lu Y. Efficient generation of lens progenitor cells from cataract patient-specific induced pluripotent stem cells. PLoS One. 2012;7:e32612. doi: 10.1371/journal.pone.0032612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding QJ, Zhu W, Cook AC, Anfinson KR, Tucker BA, Kuehn MH. Induction of trabecular meshwork cells from induced pluripotent stem cells. Invest Ophthalmol Vis Sci. 2014;55:7065–72. doi: 10.1167/iovs.14-14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, et al. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53:2007–19. doi: 10.1167/iovs.11-9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N. Generation of retinal ganglion cells with functional axons from mouse embryonic stem cells and induced pluripotent stem cells. Invest Ophthalmol Vis Sci. 2016;57:3348–59. doi: 10.1167/iovs.16-19166. [DOI] [PubMed] [Google Scholar]
- 67.Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med. 2013;2:384–93. doi: 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin ZB, Okamoto S, Xiang P, Takahashi M. Integration-free induced pluripotent stem cells derived from retinitis pigmentosa patient for disease modeling. Stem Cells Transl Med. 2012;1:503–9. doi: 10.5966/sctm.2012-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saini JS, Corneo B, Miller JD, Kiehl TR, Wang Q, Boles NC, et al. Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell Stem Cell. 2017;20:635–47. doi: 10.1016/j.stem.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohlemacher SK, Sridhar A, Xiao Y, Hochstetler AE, Sarfarazi M, Cummins TR, et al. Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells. 2016;34:1553–62. doi: 10.1002/stem.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tucker BA, Solivan-Timpe F, Roos BR, Anfinson KR, Robin AL, Wiley LA, et al. Duplication of TBK1 stimulates autophagy in iPSC-derived retinal cells from a patient with normal tension glaucoma. J Stem Cell Res Ther. 2014;3:161. doi: 10.4172/2157-7633.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, et al. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife. 2013;2:e00824. doi: 10.7554/eLife.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2:205–18. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnea-Cramer AO, Wang W, Lu SJ, Singh MS, Luo C, Huo H, et al. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci Rep. 2016;6:29784. doi: 10.1038/srep29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, et al. CGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci Rep. 2016;6:30742. doi: 10.1038/srep30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tucker BA, Scheetz TE, Mullins RF, DeLuca AP, Hoffmann JM, Johnston RM, et al. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2011;108:E569–76. doi: 10.1073/pnas.1108918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- 78.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–31. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 79.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30:673–86. doi: 10.1002/stem.1037. [DOI] [PubMed] [Google Scholar]
- 80.Boucherie C, Mukherjee S, Henckaerts E, Thrasher AJ, Sowden JC, Ali RR. Brief report: Self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells. 2013;31:408–14. doi: 10.1002/stem.1268. [DOI] [PubMed] [Google Scholar]
- 81.Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–18. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida T, Ozawa Y, Suzuki K, Yuki K, Ohyama M, Akamatsu W, et al. The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa. Mol Brain. 2014;7:45. doi: 10.1186/1756-6606-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, et al. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A. 2014;111:8518–23. doi: 10.1073/pnas.1324212111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu J, Reynolds J, Garcia T, Cifuentes H, Chew S, Zeng X, et al. Generation of transplantable retinal photoreceptors from a current good manufacturing practice-manufactured human induced pluripotent stem cell line. Stem Cells Transl Med. 2018;7:210–9. doi: 10.1002/sctm.17-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ovando-Roche P, West EL, Branch MJ, Sampson RD, Fernando M, Munro P, et al. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther. 2018;9:156. doi: 10.1186/s13287-018-0907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Cordero A, Kruczek K, Naeem A, Fernando M, Kloc M, Ribeiro J, et al. Recapitulation of human retinal development from human pluripotent stem cells generates transplantable populations of cone photoreceptors. Stem Cell Reports. 2017;9:820–37. doi: 10.1016/j.stemcr.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gagliardi G, Ben M’Barek K, Chaffiol A, Slembrouck-Brec A, Conart JB, Nanteau C, et al. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Reports. 2018;11:665–80. doi: 10.1016/j.stemcr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li G, Xie B, He L, Zhou T, Gao G, Liu S, et al. Generation of retinal organoids with mature rods and cones from urine-derived human induced pluripotent stem cells. Stem Cells Int 2018. 2018:4968658. doi: 10.1155/2018/4968658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shrestha R, Wen YT, Ding DC, Tsai RK. Aberrant hiPSCs-derived from human keratinocytes differentiates into 3D retinal organoids that acquire mature photoreceptors. Cells. 2019;8 doi: 10.3390/cells8010036. pii: E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raab S, Klingenstein M, Liebau S, Linta L. A comparative view on human somatic cell sources for iPSC generation. Stem Cells Int. 2014;2014:768391. doi: 10.1155/2014/768391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma TP, Wiley LA, Whitmore SS, Anfinson KR, Cranston CM, Oppedal DJ, et al. Patient-specific induced pluripotent stem cells to evaluate the pathophysiology of TRNT1-associated retinitis pigmentosa. Stem Cell Res. 2017;21:58–70. doi: 10.1016/j.scr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 93.Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31:741–7. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat Commun. 2016;7:13028. doi: 10.1038/ncomms13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearson RA, Gonzalez-Cordero A, West EL, Ribeiro JR, Aghaizu N, Goh D, et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun. 2016;7:13029. doi: 10.1038/ncomms13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gasparini SJ, Llonch S, Borsch O, Ader M. Transplantation of photoreceptors into the degenerative retina: Current state and future perspectives. Prog Retin Eye Res. 2019;69:1–37. doi: 10.1016/j.preteyeres.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Gagliardi G, Ben M’Barek K, Goureau O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: A pluripotent stem cell-based approach. Prog Retin Eye Res. 2019;71:1–25. doi: 10.1016/j.preteyeres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Lorach H, Kang S, Bhuckory MB, Trouillet A, Dalal R, Marmor M, et al. Transplantation of mature photoreceptors in rodents with retinal degeneration. Transl Vis Sci Technol. 2019;8:30. doi: 10.1167/tvst.8.3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]