Abstract
The Toll-like receptor 4 (TLR4) that recognizes endotoxin, a trigger of inflammation in alcoholic liver disease (ALD), activates two signaling pathways utilizing different adapter molecules: the common TLR adapter, myeloid differentiation factor 88 (MyD88), or Toll/interleukin immune-response–domain-containing adaptor inducing interferon (IFN)-β. The MyD88 pathway induces proinflammatory cytokine activation, a critical mediator of ALD. Here we evaluated the role of MyD88 in alcohol-induced liver injury in wild-type, TLR2-deficient, TLR4-deficient, or MyD88-deficient (knockout [KO]) mice after administration of the Lieber-De-Carli diet (4.5% volume/volume ethanol) or an isocaloric liquid control diet for 5 weeks. Alcohol feeding resulted in a significant increase in serum alanine aminotransferase levels, liver steatosis and triglyceride levels suggesting liver damage in WT, TLR2-KO, and MyD88-KO but not in TLR4-KO mice. Expression of inflammatory mediators (tumor necrosis factor–α and interleukin-6) and TLR4 coreceptors (CD14 and MD2) was significantly higher in livers of alcohol-fed WT, TLR2-KO, or MyD88-KO, but not in TLR4-KO mice, compared to controls. Reactive oxygen radicals produced by cytochrome P450 and the nicotinamide adenine dinucleotide phosphate complexes contribute to alcoholic liver damage. Alcohol feeding–induced expression and activation of cytochrome P450 and the nicotinamide adenine dinucleotide phosphate complex were prevented by TLR4-deficiency but not by MyD88-deficiency. Liver expression of interferon regulatory factor 3 (IRF3), a MyD88-independent signaling molecule, was not affected by chronic alcohol treatment in whole livers of WT mice or in any of the KO mice. However, the induction of IRF7, an IRF3-inducible gene, was found in Kupffer cells of alcohol-fed WT mice. Alcohol feeding also induced nuclear factor–κB activation in a TLR4-dependent MyD88-independent manner.
Conclusion:
While TLR4 deficiency was protective, MyD88 deficiency failed to prevent alcohol-induced liver damage and inflammation. These results suggest that the common TLR adapter, MyD88, is dispensable in TLR4-mediated liver injury in ALD.
Alcoholic liver disease (ALD) is characterized by a spectrum of liver pathology ranging from fatty liver, steatohepatitis, to cirrhosis, and it represents the second leading cause for liver transplantation in the United States.1 Gut-derived lipopolysaccharide (LPS), a component of the gram-negative bacterial wall, has been proposed as a key player in the pathogenesis of ALD.2–4 Exposure to LPS during chronic alcohol consumption results in increased production of inflammatory mediators, as well as in induction of reactive oxygen species (ROS), leading to progression of liver injury.5,6 Indeed, mice deficient in tumor necrosis factor-alpha (TNFα) type I receptor were protected from alcohol-induced liver injury.7 Of the different sources of ROS, the role of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation was suggested by attenuated induction of liver injury in mice deficient in p47phox, an inducible component of the NADPH oxidase complex.8 Recent studies showed that LPS induces NADPH oxidase activation.9,10 However, it remains to be determined whether LPS, alcohol, or both activate NADPH in ALD.
Recognition of pathogen-derived molecules, including endotoxin, occurs through pattern recognition receptors such as Toll-like receptors (TLR) expressed on Kupffer cells as well as on other cell types in the liver.11–13 LPS is recognized by TLR4 and its coreceptors, CD14 and MD2.12 Previous studies demonstrated that inability to induce LPS signaling due to deficiency of LPS-binding protein, CD14, or mutation in TLR4 protects mice from alcohol-induced liver damage.14–16 These observations imply that receptors in LPS-sensing and/or downstream signaling pathways may be important in the pathogenesis of ALD.
TLR4 activates two signaling pathways via recruitment of adaptor molecules.17 Recruitment of the common TLR adaptor, myeloid differentiation factor 88 (MyD88), leads to rapid activation of nuclear factor κB (NFκB) and increased TNFα production, while recruitment of Toll/interleukin–immune-response–domain-containing adaptor inducing interferon (IFN)-β (TRIF), an adapter shared by TLR4 and TLR3, activates tank-binding kinase 1/inhibitor of kappa B kinase–ϵ and interferon regulatory factor 3 (IRF3), leading to production of type I IFNs and delayed NF-κB activation.17,18 The significance of the two different downstream pathways and the role of distinct adapter molecules of TLR4 activation in alcoholic liver disease are yet to be evaluated.
Considering the importance of LPS-induced ROS and inflammatory cascade activation in ALD and the role of TLR4 signaling in LPS-induced downstream pathways, we hypothesized that TLR4 was critical in alcohol-induced liver disease and TLR4 exerted its effects via the MyD88-dependent downstream pathways. We further hypothesized that TLR4 may be critical in induction of ROS in alcoholic liver injury. Therefore, in this study, we evaluated the effect of chronic alcohol feeding on liver damage, steatosis, inflammation, and enzymes involved in ROS induction in mice deficient in TLR4, TLR2, or the common TLR adapter, MyD88.
Materials and Methods
Animal Studies.
All animals received proper care in agreement with animal protocols approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School. Six-week-old to eight-week-old, male wild-type (WT) (C57BL/6), TLR2-deficient, TLR4-deficient, and MyD88-deficient mice (all on C57BL/6 background, five to six mice/group) received the Lieber-DeCarli diet (Bio-Serv, Frenchtown, NJ) with 4.5% (volume/volume) ethanol (32.4% ethanol-derived calories) for 5 weeks; pair-fed control mice received an equal amount of calories as their alcohol-fed counterparts with the alcohol-derived calories substituted with dextran-maltose. Serum was separated from whole blood and frozen at −80°C. Livers were snap-frozen in liquid nitrogen, or stored in RNAlater (Qiagen GmbH, Hilden, Germany) for RNA extraction or fixed in 10% neutral-buffered formalin for histopathological analysis.
Biochemical Assays.
Serum alanine aminotransferase (ALT) was determined using a kinetic method (Advanced Diagnostics Inc., South Plainfield, NJ), endotoxin levels were measured using the Limulus amebocyte lysate assay (Lonza Ltd., Swizerland) and alcohol content using an alcohol analyzer (Analox Lunenberg, MA). Liver triglyceride levels were assessed using the L-Type Triglyceride H kit (Wako Chemicals USA Inc., VA).
RNA Analysis.
RNA was purified using the RNeasy kit (Qiagen Sciences, Maryland, USA) and on-column DNA digestion. cDNA was transcribed with the Reverse Transcription System (Promega Corp., Madison, WI). Real-time quantitative polymerase chain reaction was performed using the iCycler (Bio-Rad Laboratories Inc., Hercules, CA), as described19; primer sequences are shown in Table 1.
Table 1.
Real-Time PCR Primers
| Target Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| 18S | gta acc cgt tga acc cca tt | cca tcc aat cgg tag tag cg |
| CD-14 | gga agc cag aga aca cca tc | cca gaa gca aca gca aca ag |
| MD-2 | gac gct gct ttc tcc cat a | cat tgg ttc ccc tca gtc tt |
| IRF3 | aac cgg aaa gaa gtg ttg cg | gca ccc aga tgt acg aag tcc |
| IRF7 | aca ggg cgt ttt atc ttg cg | tcc aag ctc ccg gct aag t |
| TNFα | cac cac cat caa gga ctc aa | agg caa cct gac cac tct cc |
| IL-6 | aca acc acg gcc ttc cct act t | cac gat ttc cca gag aac atg tg |
| CYP2E1 | aag cgc ttc ggg cca g | tag cca tgc agg acc acg a |
| p22phox | tgg agc gat gtg gac aga ag | ccc gga cgt agt aat tcc tgg |
| p47phox | cca ggg cac tct cac tga ata | atc agg ccg cac ttt gaa gaa |
| p67phox | gct gcg tga aca cta tcc tgg | agg tcg tac ttc tcc att ctg ta |
| gp91phox | ctg ctc tcc ttt ctc agg ggt | gtg tgc agt gct atc atc caa |
| Nox4 | agg att gtg ttt aag cag agc at | ccg gca cat agg taa aag gat g |
Western Blot and NF-κB Activity Analysis.
Whole-cell lysates or nuclear fractions were extracted from liver, as described.19 Samples with equal amounts of protein were separated in a 10% polyacrylamide gel, and identified on the nitrocellulose membrane with specific primary antibodies followed by horseradish peroxidase–labeled secondary antibodies and detection using the chemiluminescence assay. The NF-κB activity was measured in whole liver extracts as described.19
Histopathological Analysis.
Sections of formalin-fixed livers were stained with hematoxylin and eosin or for 4-hydroxynonenal and analyzed by microscopy.
Membrane Preparations.
Total liver cell membranes were isolated by the method of Nagamatsu et al.20 with slight modifications. Briefly, liver tissue was homogenized in 1 mL of 10 mM Tris-HCl, pH 7.4, 1 mM ethylene diamine tetraacetic acid, 200 mM sucrose, and 1 mM phenylmethylsulfonyl fluoride; the nuclei and cell debris were removed by centrifugation at 900g for 10 minutes at 4°C; and the resulting supernatant was centrifuged at 110,000g for 75 minutes at 4°C. The membrane pellet was solubilized in 10 mM Tris-HCl, pH 7.4, 1 mM ethylene diamine tetraacetic acid, 0.5% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride for 1 hour at 4°C, centrifuged at 14,000g for 10 minutes at +4°C, and 1 μg/mL aprotinin was added to solubilized membrane samples prior to analysis.
Microsomal Preparations.
Liver tissue was homogenized in cold KP buffer (0.1 M K2HPO4/KH2PO4, pH 7.4). Homogenates were sonicated (40% duty cycle, four output) for 20 seconds on ice followed by centrifugation at 12,000g for 15 minutes at +4°C; pellets were washed with KP buffer at 100,000g for 1 hour in +4°C and assayed for protein content using the BioRad Protein Assay.
Isolation of Kupffer Cells and Hepatocytes.
Mice received anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg); the livers were perfused with saline solution for 5 minutes followed by in vivo digestion with liberase enzyme (20 mg/L) for 5 minutes and in vitro digestion for additional 30 minutes at 37°C. The hepatocytes were separated by centrifugation for 5 minutes at 300g, while the nonhepatocyte content was loaded on the top of the 50%–25% Percoll gradient and centrifuged for 60 minutes at 800g. The intercushion fraction was washed and adhered to plastic in Dulbecco’s modified Eagle’s medium +5% fetal bovine serum; the nonadherent fraction was washed and the adherent Kupffer cell population was adjusted to 2 ×106/mL in Dulbecco’s modified Eagle’s medium +10% fetal bovine serum.
Statistical Analysis.
Statistical significance was determined using the nonparametric Kruskal-Wallis test followed by the Mann-Whitney test. Data are shown as mean ± standard deviation and were considered statistically significant at P < 0.05.
Results
Chronic Alcohol Feeding Induces Liver Damage in MyD88-Deficient but not in TLR4-Knockout Mice.
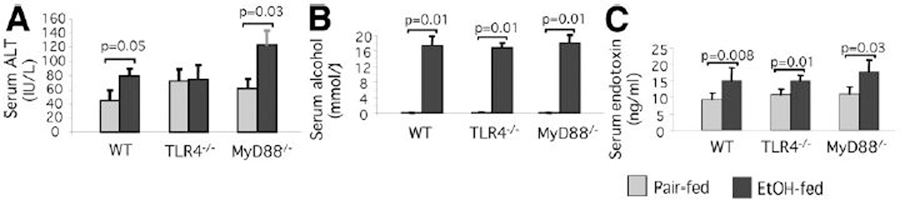
Chronic alcohol feeding of mice with the Lieber-DeCarli diet resembles several features of human alcoholic hepatitis, including hepatocyte damage, steatosis, and inflammatory cell activation.4–8,14–16 Here, we fed Lieber-DeCarli or isocaloric control (pair feeding) diets to WT, TLR4-deficient, and MyD88-deficient mice to induce early alcoholic liver damage. Chronic alcohol feeding resulted in liver injury as indicated by higher serum ALT levels in alcohol-fed compared to pair-fed mice in WT and MyD88-knockout (KO), but not in TLR4-KO mice (Fig. 1A). While there was no increase in serum ALT indicative of liver damage in the TLR4-KO mice, serum alcohol concentrations were comparable in all animals (Fig. 1B). Further, we found a moderate but significant increase in serum endotoxin levels in all alcohol-fed mice compared to the pair-fed controls, regardless of genetic background (Fig. 1C).
Fig. 1.
TLR4-deficiency but not MyD88-deficiency protects against alcohol-induced liver damage. C57BL/6 (WT), TLR4-deficient, or MyD88-deficient mice (five to six per group) received the Lieber-DeCarli diet with 4.5 volume/volume% of ethanol or isocaloric liquid control diet for 5 weeks. After the 5-week feeding period, the mice were sacrificed. Serum was separated from whole blood and analyzed for (A) ALT, (B) alcohol, and (C) endotoxin levels. Mean values ± standard deviation (SD) are shown.
TLR4 Deficiency But Not MyD88 Deficiency Protects Against Alcohol-Induced Steatosis.
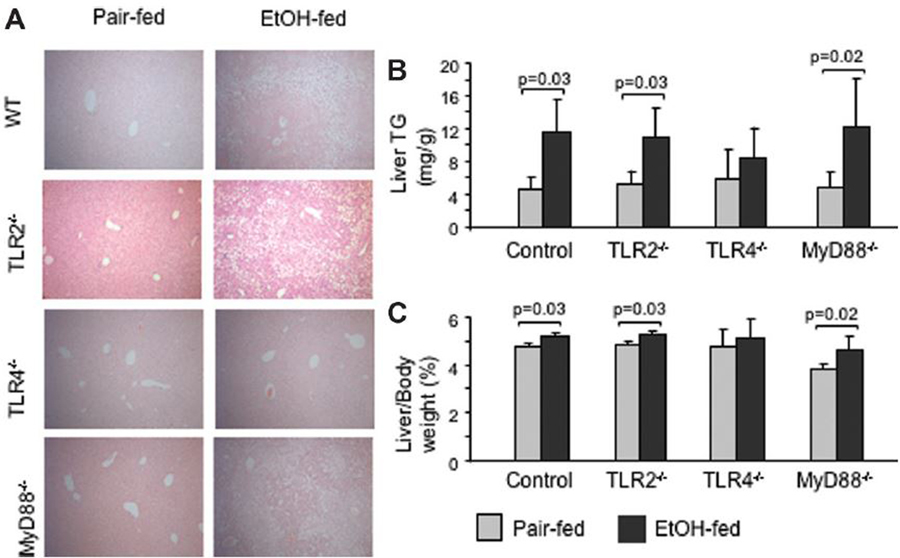
Histopathological analysis revealed that chronic alcohol feeding induced microvesicular and macrovesicular steatosis and inflammatory-cell recruitment in chronic ethanol-fed WT and MyD88-deficient but not in TLR4-KO mice (Fig. 2A; Supplementary Fig. 1). Consistent with the histopathology findings, liver triglyceride levels were significantly higher in alcohol-fed WT and MyD88-deficient mice, but not in the TLR4-KO mice, compared to the pair-fed controls (Fig. 2B). To further evaluate the role of MyD88, we also tested TLR2-deficient mice, considering that TLR2-induced signaling is solely MyD88-dependent.11 Similar to MyD88-deficient mice, the TLR2-deficient mice were not protected from alcohol-induced liver steatosis (Fig. 2A,B). Chronic alcohol feeding also resulted in increased liver/body weight ratio in WT, TLR2-deficient, and MyD88-deficient, but not in TLR4-KO mice (Fig. 2C). Collectively, these data indicated that chronic alcohol feeding induced features of ALD in WT and in MyD88-deficient, but not in the TLR4-deficient animals.
Fig. 2.
Chronic alcohol feeding induces liver steatosis in WT, TLR2-deficient, and MyD88-deficient mice, but not in TLR4-deficient mice. C57BL/6 (WT), TLR2-deficient, TLR4-deficient, or MyD88-deficient mice (five to six per group) received the Lieber-DeCarli diet as described in Materials and Methods. (A) Representative sections of formalin-fixed, paraffin-embedded livers stained with hematoxylin and eosin of each group are shown at ×100 (higher magnification is provided in Supplementary Fig. 1). (B) Liver triglyceride levels and (C) liver/body weight ratios are shown as mean ± standard deviation (SD) values from N = 5 mice/group.
TLR4, But Not TLR2 or MyD88, Deficiency Protects from Alcohol-Induced Increase of Inflammatory Cytokine and TLR4 Coreceptor Expression.
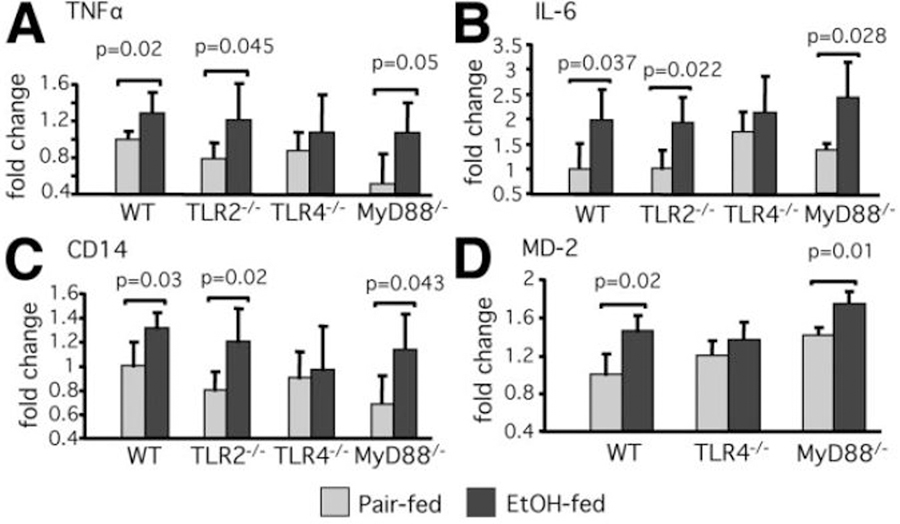
The LPS-induced, TLR4-mediated MyD88-dependent signaling pathway results in production of proinflammatory cytokines.14,17,18 We found that the expression of TNFα and IL-6 messenger RNA (mRNA) in the liver was significantly higher in alcohol-fed WT and MyD88-deficient mice compared to the pair-fed controls. In contrast, there was no increase in liver TNFα or IL-6 mRNA in TLR4-deficient mice (Fig. 3A,B). Similar to the MyD88-KO mice, TLR2-KO mice had increased TNFα and IL-6 mRNA levels in the liver after alcohol feeding compared to the pair-fed controls (Fig. 3A,B).
Fig. 3.
Chronic alcohol feeding increases liver mRNA expression of TNFα and IL-6 and TLR4 coreceptors, CD14 and MD2, in WT, TLR2-deficient, and MyD88-deficient mice, but not in TLR4-KO mice. C57BL/6, TLR2-deficient, TLR4-deficient, or MyD88-deficient mice received the Lieber-DeCarli diet. Liver RNA levels of (A) TNFα, (B) IL-6, (C) CD14, and (D) MD2 were analyzed by real-time quantitative polymerase chain reaction (qPCR). The values were normalized to 18S and are shown as the fold increase over the pair-fed WT control group from N = 5–6 per group.
Previous studies suggested that the mechanisms of liver injury and inflammatory responses to chronic ethanol consumption involve upregulation of genes involved in LPS recognition and LPS-triggered signaling,21–24 We found that chronic alcohol feeding increased liver mRNA levels of both TLR4 coreceptors, CD14 and MD2, in WT, MyD88-KO, and TLR2-KO mice, while there was no increase in TLR4-KO mice compared to the pair-fed controls (Fig. 3C,D).
Chronic Ethanol Ingestion Augments the Expression of CYP2E1 Levels in Livers of WT and MyD88-Deficient Mice, But Not in TLR4-KO Mice.
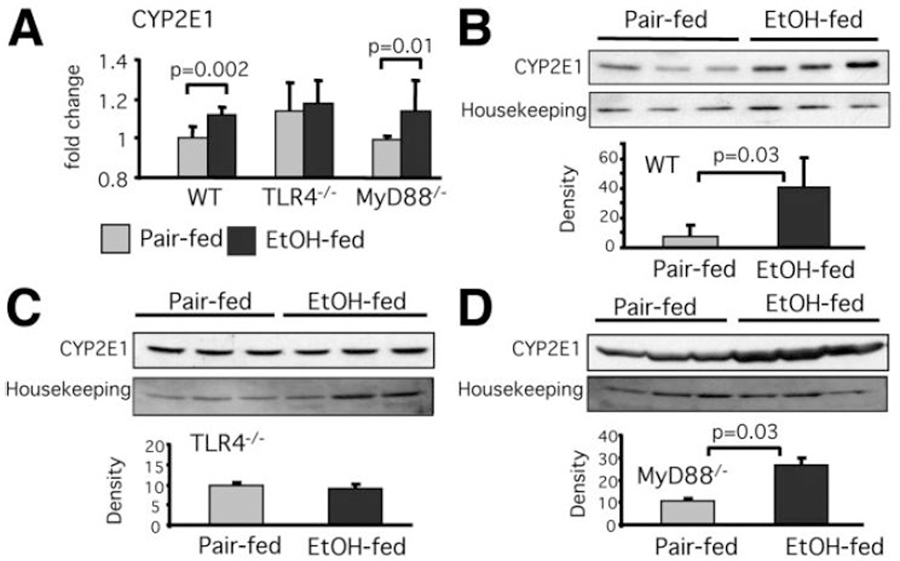
Induction of oxidative stress in ethanol-induced liver injury involves upregulation of the CYP2E1, an enzyme with powerful ability to metabolize ethanol and produce alcohol-derived toxic substrates.8,25,26 Furthermore, recent studies suggest that CYP2E1 is also upregulated by LPS.26,27 Thus, we investigated the effect of chronic alcohol exposure on CYP2E1 in livers of TLR4-deficient and MyD88-deficient mice. We found that liver mRNA levels of CYP2E1 were upregulated in WT and MyD88-deficient but not in TLR4-KO mice after chronic alcohol feeding (Fig. 4A). Consistent with this, liver microsomal CYP2E1 protein levels were significantly higher in alcohol-fed WT and MyD88-deficient mice compared to the pair-fed controls (Fig. 4B,D). In contrast, alcohol feeding induced no increase in liver CYP2E1 protein levels in TLR4-deficient mice (Fig. 4C).
Fig. 4.
TLR4 deficiency protects from alcohol-induced increase of liver CYP2E1 expression. C57BL/6, TLR4-deficient, or MyD88-deficient mice (five to six per group) received the Lieber-DeCarli diet. (A) Liver RNA levels of CYP2E1 were analyzed by real-time quantitative polymerase chain reaction (qPCR). CYP2E1 protein level in liver microsomal fractions of (B) WT, (C) TLR4-deficient, and (D) MyD88-deficient mice was analyzed by western blot; calnexin expression was used as housekeeping control. A representative blot (top) and the densitometric analysis normalized to loading control from N = 3 (bottom) is shown.
TLR4-Dependent But MyD88-Independent Induction of NADPH-Complex by Alcohol Feeding.
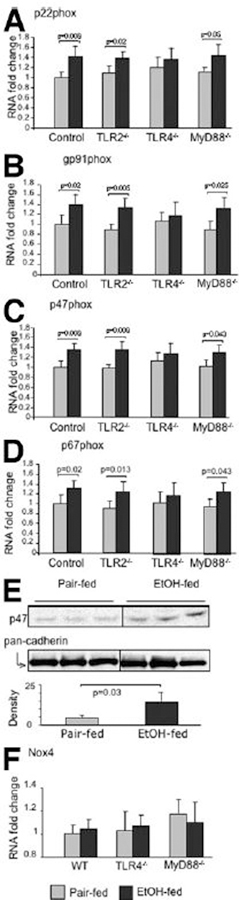
LPS and other microbial pathogens activate the NADPH complex in phagocytic cells to produce ROS, and upon ethanol treatment the NADPH oxidase complex generates ROS in Kupffer cells.8,9,28 The classic NADPH complex is composed of at least six protein components, which include two transmembrane flavocytochrome b components (gp91phox and p22phox) and four cytosolic components (p47phox, p67phox, p40phox, and Rac-1 protein).29,30 LPS is a strong inducer of NADPH transcription and functional activity.9,10,28 We found that in alcohol-fed WT, MyD88-KO, and TLR2-KO mice (Fig. 5A–D), liver mRNA levels of NADPH oxidase subunits, namely p22phox, gp91phox, p47phox, and p67phox, were significantly higher compared to pair-fed controls (Fig. 5A–D). In contrast, no such changes were observed in alcohol-fed TLR4-KO mice compared to pair-fed controls (Fig. 5A–D). In response to a wide range of stimuli, the cytosolic subunits of the NADPH complex are phosphorylated and translocated to the plasma membrane, forming an active complex and generating superoxide anion.29,30 We found increased p47phox protein levels in plasma membranes in livers of alcohol-fed mice compared to pair-fed mice, providing evidence for functional activation of the NADPH oxidase (Fig. 5E).29–31 In agreement with the role of NADPH in TLR4-dependent but MyD88-independent alcohol-induced liver injury, we identified increased staining with 4-hydroxynonenal, suggestive of oxidative tissue damage, in alcohol-fed livers of WT, TLR2-deficient, and MyD88-deficient, but not TLR4-deficient animals, compared to pair-fed controls (Supplementary Fig. 2).
Fig. 5.
Chronic alcohol feeding increases liver mRNA expression of the NADPH-complex subunits in WT, TLR2-deficient and MyD88-deficient mice but not in TLR4-KO mice. C57BL/6, TLR2-deficient, TLR4-deficient, or MyD88-deficient mice (five to six per group) received the Lieber-DeCarli diet. Liver RNA levels of (A) p22phox, (B) gp91phox, (C) p47phox, (D) p67phox, and (F) Nox4 were analyzed by real-time quantitative polymerase chain reaction (qPCR). The values were normalized to 18S and shown as fold increase over the pair-fed WT control group. (E) p47phox protein expression in whole liver membrane fractions was analyzed by western blot in alcohol-fed and pair-fed WT mice; pancadherin expression was used as a loading control. The densitometric analysis of p47phox protein expression (normalized to pan-cadherin; N = 3) is shown.
While the NADPH complex in nonphagocytic cells is structurally and functionally similar to NADPH oxidase of phagocytes,29,31 several homologs of NADPH oxidase have been identified. One of the gp91phox homologs expressed in hepatocytes is NADPH oxidase 4 (Nox4).32 However, there was no increase in liver mRNA levels of Nox4 either in alcohol-fed mice or pair-fed mice regardless of their genetic background (Fig. 5F). These data suggest that classic NADPH oxidase in liver macrophages may play a role in liver injury after chronic ethanol treatment.
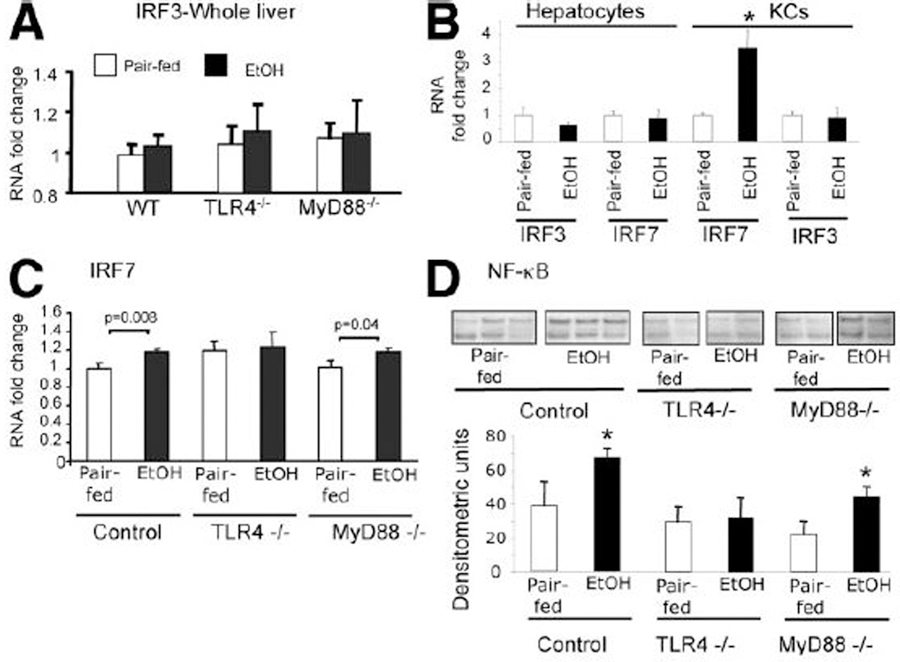
Chronic Alcohol Feeding Modulates MyD88-Independent TLR4 Signaling.
Our results so far suggested that TLR4 was critical in all examined aspects of ALD, but in the two downstream signaling pathways initiated by TLR4, MyD88-dependent signaling was dispensable. IRF3 is a marker of MyD88-independent, TLR4-mediated and TLR3-mediated signaling.17 Activation of the MyD88-independent pathway leads to IRF3 activation and results in late NFκB activation and production of type I IFN.17,18 We found that total liver IRF3 mRNA levels were comparable in all analyzed animals regardless of genetic deficiency or chronic alcohol feeding (Fig. 6A). To evaluate possible cell-specific upregulation of IRF3, we also tested IRF3 mRNA expression in isolated hepatocytes and Kupffer cells and found no difference after alcohol feeding compared to controls (Fig. 6B). However, the IRF3-inducible gene, IRF7, was upregulated in isolated Kupffer cells but not in hepatocytes in livers of alcohol-fed WT mice compared to the pair-fed controls (Fig. 6B). Consistent with the lower abundance of Kupffer cells in the total liver, total liver IRF7 mRNA showed a minimal but statistically significant increase in alcohol-fed WT mice (Fig. 6C). Relevant to our hypothesis that TLR4-dependent MyD88-independent signaling is important in ALD, we found increased IRF7 in livers of alcohol-fed MyD88-deficient mice but not in those of TLR4-deficient mice compared to pair-fed controls (Fig. 6C).
Fig. 6.
Chronic alcohol feeding results in activation of TLR4-mediated MyD88-independent pathways. C57BL/6, TLR4-deficient, or MyD88-deficient mice (five to six per group) received the Lieber-DeCarli or a pair-fed diet for 5 weeks. (A) IRF3 and (C) IRF7 RNA levels in the whole liver, and (B) IRF3 and IRF7 levels in the purified hepatocytes and Kupffer cells were analyzed by real-time quantitative polymerase chain reaction (qPCR). (D) NF-κB activity in the whole livers was analyzed using electrophoretic mobility shift assay; a representative gel (top) and the densitometric analysis from N = 5 mice/group (bottom) are shown. *P < 0.05 compared to the corresponding pair-fed control.
Finally, we asked if TLR4 or MyD88 deficiency would affect the activation of NF-κB during ALD. NF-κB is activated via both MyD88-dependent and MyD88-independent pathways, the former being more robust and immediate, and the latter accounting for a modest degree in a delayed fashion.11 The alcohol diet led to a moderate induction of NF-κB activation in the in whole livers of WT and MyD88-deficient mice compared to pair-fed controls (Fig. 6D). In contrast, NF-κB activation was not observed upon alcohol feeding in livers of TLR4-deficient mice (Fig. 6D). These data support the contention that TLR4, but not MyD88, leads to activation of signaling mechanisms, including the NF-κB pathway, during ALD.
Discussion
LPS-induced activation of inflammatory pathways and reactive oxygen radical generation are pivotal components of ALD.2–9 In this study, we confirmed protection from alcohol-induced liver damage in TLR4-deficient mice, as has been shown by others,16 and we showed for the first time that TLR4-dependent protection from ALD occurs independent of the expression of the common TLR adapter, MyD88.
There are at least two possible explanations for the observation of MyD88-independent but TLR4-mediated alcohol-induced liver damage: first, that the MyD88-independent TLR4 signaling pathway is involved in alcoholic liver disease; second, that TLR4 activation by ethanol bypasses the MyD88 adapter to result in proinflammatory activation. Our data showing increased levels of IRF7, an IRF3-induced gene, in mice after chronic alcohol feeding provided support for the first scenario. Activation of the MyD88-independent pathway by LPS is mediated by recruitment of the TRAM (Toll/IL-1-resistance domain-containing adaptor-inducing 1FNβ-related adaptor molecule)/TRIF adapters of which TRIF is also utilized by TLR3, leading to activation and nuclear translocation of IRF3, resulting in induction of IRF3-inducible genes such as IRF7 and type I IFN.17,18,33 Activation of the MyD88-independent pathway can also result in late activation of NFκB.17,18 Previous studies showed that chronic alcohol feeding increased NF-κB activation in whole livers and in Kupffer cells.34,35 Here we found that NF-κB activation reflected the protective role of TLR4, but not MyD88, against ALD. In this context, only mice who exhibited proximal defect of TLR4-dependent activation pathway; that is, TLR4-deficient mice, but not WT, TLR2-deficient, and MyD88-deficient mice, showed lack of NF-κB activation in the liver upon alcohol feeding. These results suggested that in ALD the activation of NF-κB could occur independent of MyD88. Further, we identified activation of the IRF3-dependent pathway in ALD. While there were no differences in the mRNA of IRF3 in livers after alcohol feeding compared to pair-fed controls, the IRF7, an IRF3-dependent gene, was upregulated in total liver and to a greater extent in isolated Kupffer cells but not in hepatocytes, suggesting a functional activation of the MyD88-independendent IRF3-dependent pathway. The link between the MyD88-independent pathway activation and TLR4 was further suggested by our observation that IRF7 increase occurred in MyD88-deficient but not in TLR4-deficient mice after alcohol feeding. Together, these results pointed to a role of TLR4 in MyD88-independent liver injury during ALD, possibly involving both NF-κB and IRF3/IRF7 pathways.
The second possible explanation for the involvement of LPS-induced TLR4-dependent MyD88-independent pathways is that alcohol results in signaling that bypasses MyD88 for induction of inflammatory pathways. Recent studies indicate cross-talk between the MyD88-dependent and MyD88-independent pathways,36 which may also occur in the alcoholic liver. Furthermore, LPS-in-duced activation of the MyD88-independent pathway has been specifically linked to participation of the CD14 coreceptor.37 Importantly, CD14 deficiency can be protective from alcohol-induced liver injury15; however, the involvement of specific components of the MyD88-independent pathway in the pathogenesis of ALD awaits investigation.
Oxidative stress has been identified as a critical contributor in ALD.8,25 Consistent with chronic alcohol consumption, we found that the levels of CYP2E1, a microsomal enzyme involved in alcohol metabolism, was upregulated in WT and MyD88-KO mice. However, there was no difference in CYP2E1 levels in the TLR4-KO mice with and without alcohol feeding, suggesting the novel finding that TLR4-deficiency may protect from alcohol-induced CYP2E1-mediated oxidative stress. This observation is consistent with the reported induction of CYP2E1 by LPS that occurred both at transcriptional and posttranscriptional levels.27 Furthermore, CYP2E1 induction was shown to amplify LPS-induced liver injury.26 Reports of increased CyP2E1 in inducible nitric oxide synthase (iNOS)-KO38 and early growth response 1 (Egr1)-KO mice39 reflect the protective effect of these molecules that are downstream from TLR4; thus, these molecules could allow LPS-TLR4-mediated upregulation of CyP2E1. Consistent with the role of TLR4 in alcohol-induced ROS activation, we also found protection of TLR4-KO mice from activation of the NADPH oxidase complex. Alcohol feeding not only induced the mRNA levels of the components of the NADPH complex but it also resulted in recruitment of the regulatory component, p47phox, to the plasma membrane. This recruitment of the p47phox to the plasma membrane was prevented by TLR4 deficiency, suggesting a likely communication between TLR4 and NADPH oxidase activation in alcoholic liver disease. NADPH activation has been linked to activation of both of the MyD88-dependent and MyD88-independent TLR4 signaling pathways in other studies,40–43 suggesting a potential link in ALD between NADPH oxidase activation and TLR4. Although TLR4 interacts with Nox4,9 the predominant NADPH isoform found in hepatocytes,44 our observation of no change in Nox4 but upregulation of p47phox and other phagocyte-associated NADPH oxidase components suggests that activation of the NADPH complex in non-parenchymal cells rather than in hepatocytes could be involved in ALD.
Finally, our results confirmed reports that increased gut permeability results in increased endotoxin levels during ALD. However, our novel findings suggest that the protective effects of TLR4-deficiency do not occur at the level of gut permeability, as we found moderately increased LPS levels upon alcohol-induced liver injury regardless of TLR4 or MyD88 expression. These data also suggest that the protective effect of TLR4 deficiency occurs at the LPS-targeted cell level in the liver, possibly in Kupffer cells and hepatocytes.
Taken together, our data demonstrate that TLR4 has a critical role in alcohol-induced liver damage at the level of ROS and inflammatory cytokine induction, independent of the expression of the common TLR adapter, MyD88. These observations suggest that TLR4-induced and MyD88-independent pathways may play an important role in the pathogenesis of ALD.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health (NIH) grant AA11576 (to G.S.). Istvan Hritz is currently affiliated with the 2nd Department of Medicine, Semmelweis University, Budapest, Hungary.
Abbreviations:
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- b.w.
body weight
- CYP2E1
cytochrome P450
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneal
- IRF
interferon regulatory factor
- KO
knockout
- LPS
lipopolysaccharide
- mRNA
messenger RNA
- MyD88
myeloid differentiation factor 88
- NADPH
nicotinamide adenine dinucleotide phosphate
- Nox4
NADPH oxidase 4
- NF-κB
nuclear factor κB
- OD
optical density
- ROS
reactive oxygen species
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TRIF
Toll/IL-immune response–domain-containing adaptor inducing IFN-β
- WT
wild-type
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Liver Transplantation for Alcoholic Liver Disease. Proceedings of a meeting. Bethesda, Maryland, December 6–7, 1996. Liver Transpl Surg 1997; 3:197–350. [PubMed] [Google Scholar]
- 2.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res 2005; 29(Suppl):166S–171S. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler MD. Endotoxin and Kupffer cell activation in alcoholic liver disease. Alcohol Res Health 2003;27:300–306. [PMC free article] [PubMed] [Google Scholar]
- 4.Nanji AA. Role of Kupffer cells in alcoholic hepatitis. Alcohol 2002;27: 13–15. [DOI] [PubMed] [Google Scholar]
- 5.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. HEPATOLOGY 2006;43(Suppl 1):S63–S74. [DOI] [PubMed] [Google Scholar]
- 6.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003;124:778–790. [DOI] [PubMed] [Google Scholar]
- 7.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 1999;117:942–952. [DOI] [PubMed] [Google Scholar]
- 8.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest 2000;106:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 2004;173:3589–3593. [DOI] [PubMed] [Google Scholar]
- 10.Pacquelet S, Johnson JL, Ellis BA, Brzezinska AA, Lane WS, Munafo DB, et al. Cross-talk between IRAK-4 and the NADPH oxidase. Biochem J 2007;403:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda K, Akira S. TLR signaling pathways. Semin Immunol 2004;16: 3–9. [DOI] [PubMed] [Google Scholar]
- 12.Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, et al. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. HEPATOLOGY 2000;31: 932–936. [DOI] [PubMed] [Google Scholar]
- 13.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. HEPATOLOGY 2006;44:287–298. [DOI] [PubMed] [Google Scholar]
- 14.Uesugi T, Froh M, Arteel GE, Bradford BU, Wheeler MD, Gabele E, et al. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol 2002;168:2963–2969. [DOI] [PubMed] [Google Scholar]
- 15.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol 2001;166:4737–4742. [DOI] [PubMed] [Google Scholar]
- 16.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. HEPATOLOGY 2001;34:101–108. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. TLR signaling. Semin Immunol 2007;19:24–32. [DOI] [PubMed] [Google Scholar]
- 18.Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004;113:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romics L Jr, Kodys K, Dolganiuc A, Graham L, Velayudham A, Mandrekar P, et al. Diverse regulation of NF-kappaB and peroxisome proliferator-activated receptors in murine nonalcoholic fatty liver. HEPATOLOGY 2004;40:376–385. [DOI] [PubMed] [Google Scholar]
- 20.Nagamatsu S, Kornhauser JM, Burant CF, Seino S, Mayo KE, Bell GI. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem 1992;267: 467–472. [PubMed] [Google Scholar]
- 21.Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int 2006;26:1021–1028. [DOI] [PubMed] [Google Scholar]
- 22.Deaciuc IV, Arteel GE, Peng X, Hill DB, McClain CJ. Gene expression in the liver of rats fed alcohol by means of intragastric infusion. Alcohol 2004;33:17–30. [DOI] [PubMed] [Google Scholar]
- 23.Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology 2007;132:687–697. [DOI] [PubMed] [Google Scholar]
- 24.Romics L Jr, Mandrekar P, Kodys K, Velayudham A, Drechsler Y, Dolganiuc A, et al. Increased lipopolysaccharide sensitivity in alcoholic fatty livers is independent of leptin deficiency and toll-like receptor 4 (TLR4) or TLR2 mRNA expression. Alcohol Clin Exp Res 2005;29:1018–1026. [DOI] [PubMed] [Google Scholar]
- 25.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. HEPATOLOGY 2006;43(Suppl 1):S63–S74. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Cederbaum AI. Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. HEPATOLOGY 2006;44:263–274. [DOI] [PubMed] [Google Scholar]
- 27.Abdulla D, Goralski KB, Renton KW. The regulation of cytochrome P450 2E1 during LPS-induced inflammation in the rat. Toxicol Appl Pharmacol 2006;216:1–10. [DOI] [PubMed] [Google Scholar]
- 28.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol 2006;79: 1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 2004;76:760–781. [DOI] [PubMed] [Google Scholar]
- 30.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci 2003;28:502–508. [DOI] [PubMed] [Google Scholar]
- 31.De Minicis S, Bataller R, Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology 2006;131:272–275. [DOI] [PubMed] [Google Scholar]
- 32.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001;269:131–140. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 2007;7:353–364. [DOI] [PubMed] [Google Scholar]
- 34.Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2006; 291:G681–G688. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto H, Sato Y, Azumi J, Kato J, Niitsu Y, Tamaki K. Role of endotoxin in NF-kappaB activation by ethanol in rat hepatocytes. Alcohol Clin Exp Res 2002;26(Suppl):6S–10S. [DOI] [PubMed] [Google Scholar]
- 36.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol 2007;178:1164–1171. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol 2005;6: 565–570. [DOI] [PubMed] [Google Scholar]
- 38.McKim SE, Gäbele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology 2003;125:1834–1844. [DOI] [PubMed] [Google Scholar]
- 39.McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology 2005;128:2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, et al. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol 2006;26:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laroux FS, Romero X, Wetzler L, Engel P, Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J Immunol 2005;175:5596–5600. [DOI] [PubMed] [Google Scholar]
- 42.Kumatori A, Yang D, Suzuki S, Nakamura M. Cooperation of STAT-1 and IRF-1 in interferon-gamma-induced transcription of the gp91(phox) gene. J Biol Chem 2002;277:9103–9111. [DOI] [PubMed] [Google Scholar]
- 43.Luo W, Skalnik DG. Interferon regulatory factor-2 directs transcription from the gp91phox promoter. J Biol Chem 1996;271:23445–23451. [DOI] [PubMed] [Google Scholar]
- 44.Murillo MM, Carmona-Cuenca I, Del Castillo G, Ortiz C, Roncero C, Sanchez A, et al. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem J 2007;405:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.