During our clinical work against the epidemic of coronavirus disease 2019 (COVID-19) in Wuhan [1], we observed a high incidence of malnutrition in critically ill patients (data unpublished). Therefore, nutritional therapy was very important. In patients with dysphagia and a very high aspiration risk, postpyloric enteral nutrition (EN) was required [2]. However, how to place the postpyloric tube was a challenge in COVID-19 patients. Patients with masks removed (to expose the nasal cavity) were seriously infectious to doctors. Besides, it was difficult to perform the tube placement bedside for doctors with heavy medical protective clothes, goggles, and face shield. Here, we shared our practice of novel placing method in Wuhan.
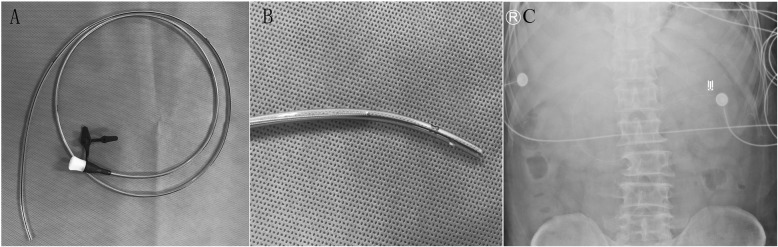
A 130-cm-long non-spiral transpyloric tube with a guide wire (CH10-130, inner diameter 2.0–2.1 mm, Flocare, Nutricia Ltd., Wuxi, China) (Fig. 1a) was used in our isolation unit. The procedure of placement was similar to the method reported by our previous study [3, 4]. Patients were placed in right decubitus position about 30–45° with bed head raised at about 30°. After esophageal placement and gastric placement, the postpyloric placement was performed by advancing the tube at 5–10 cm intervals gradually and checking its tip position each time. Subsequently, the tip position would be confirmed by abdominal plain radiographs or gastrointestinal ultrasound bedside. The tube that we used has several advantages compared with spiral tube. First, the price of Flocare tube (approximately $22) is 1/3 less compared with spiral tube (approximately $71) in China. Second, the Flocare tube has two side holes near its tip (Fig. 1b); it is less likely to be blocked. Third, the guide wire is shorter in length compared with the tube; therefore, the rigid tip could not damage the digestive tract during our placing procedure
Fig. 1.
The 130-cm-long transpyloric tube with a guide wire (CH10-130, inner diameter 2.0–2.1 mm, Flocare, Nutricia Ltd., Wuxi, China) used in our unit (a). This Flocare tube has two side holes near its tip (b). Abdominal plain radiograph showed the tip of Flocare tube was positioned near the Treitz ligament (c)
There have been three patients who received our novel method of postpyloric tube placement. The 3 cases were all successful at the first attempt (Fig. 1c). The median time of procedure was 19 (14–25) minutes, and the median insertion length was 105 (95–110) cm. No operation- and tube-related complications were found. Considering the less expensive tube and high success rate, our novel blind bedside postpyloric placement may be easier to perform in patients with COVID-19 worldwide.
Acknowledgements
Not applicable.
Authors’ contributions
Yuan ST, Zhang WH, and Zou L wrote the manuscript; Liu Y, Shi QK, and Sun JK modified the manuscript. All authors read and approved the final manuscript. The work has not been published previously nor is under consideration for publication elsewhere.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81701881) and the Nanjing Medical Science and Technology Development Foundation (No. YKK17102).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Sun JK, Wang X, Yuan ST. A novel method of blind bedside placement of postpyloric tubes. Crit Care. 2018;22(1):62. doi: 10.1186/s13054-018-1986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Zhang W, Wang X, Yuan S, Shi Q, Liu Y, Mu X. Application of blind bedside non-spiral nasointestinal tubes in critically ill patients. Chinese J Clin Nutr. 2019;27(1):42–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.