Abstract
Klebsiella pneumoniae is a gram-negative bacterium found in the environment and as a commensal in humans and animals. In humans, K. pneumoniae is one of the most serious nosocomial infections encountered currently and is characterized by liver abscesses, pneumonia, and bacteremia resulting in meningoencephalitis and endophthalmitis. K. pneumoniae in veterinary medicine is rarely reported in NHP, and recent literature describing this disease is sparse. In our colony of predominantly outdoor-housed rhesus macaques (Macaca mulatta), K. pneumoniae is cultured infrequently from healthy animals during routine screening and is even rarer in sick animals. This report summarizes the clinical and postmortem findings associated with this pathogen in 9 rhesus macaques and compares these results with the disease outcomes reported for humans. In these cases, K. pneumoniae infection was confirmed through culture or PCR testing or both. In our experience, when this bacterium does cause clinical signs, the disease is rapidly progressive and severe. At necropsy of NHP, the findings are strikingly similar to opportunistic Klebsiella-associated syndromes described in humans and include liver abscesses, meningoencephalitis, and endophthalmitis. In addition, many of the affected macaques had similar risk factors to humans that succumb to disease, thus perhaps indicating that rhesus macaques could be a viable model for investigating these syndromes.
Abbreviation: KAS, Klebsiella-associated syndromes
Klebsiella pneumoniae is a ubiquitous organism found in the environment in soil and water and as an upper respiratory or gastrointestinal commensal in many mammalian species. It is often cultured from the nasopharynx or gastrointestinal tract of otherwise healthy-appearing humans and animals.6,20,23 In humans, disease due to K. pneumoniae infection is associated with risk factors including immunosuppression, long-term hospitalization, and treatment with multiple antibiotics.3 In fact, K. pneumoniae is one of the most serious nosocomial infections in humans, compounded by the recent increase in multidrug resistance of this bacterium.3 In humans, it causes a variety of Klebsiella-associated syndromes (KAS), including pyogenic liver abscesses, pneumonia, bacteremia, and urinary tract infections.3 In addition, with bacteremia, meningoencephalitis and endophthalmitis can occur as part of a systemic infection, particularly in people who live in Asian countries and have concurrent uncontrolled diabetes mellitus.7
In cultures of K. pneumoniae, the hypermucoviscous phenotype is a marker for hypervirulent forms that are associated with significant disease in immunocompetent hosts.20 The hypermucoviscous phenotype is apparent within bacterial cultures when a string test is positive.8,22 In histologic sections, individual hypermucoviscous Klebsiella organisms have a clear space surrounding them—evidence of a mucous capsule.14
Natural infections by K. pneumoniae in veterinary medicine are most often reported as a common cause of mastitis in dairy cattle.17 Although rarer, K. pneumoniae has been reported in several NHP species, including many macaques (Macaca mulatta, M. fascicularis, M. nemistrina, M. speciosa), African green monkeys (Chlorocebus aethiops), common marmosets (Callithrix jacchus), and owl monkeys (Aotus trivirgatus).6,9,10,12,19,22,23 Within NHP species, the bacterium can be subclinical or can cause pneumonia, septicemia, meningitis, and peritonitis or visceral abscesses, thus presenting similarly to human KAS. African green monkeys seem particularly susceptible to disease, developing multisystemic abscesses leading to death, and may be infected simply by proximity to K. pneumoniae carriers, including rhesus macaques.5,6 Experimentally, both rhesus and cynomolgus macaques have been found to be culture-positive for K. pneumoniae, but even with immunosuppression, these species seem more likely to be subclinical carriers than to develop any form of clinical disease.5 Given that disease associated with K. pneumoniae is uncommon in NHP, physical manifestations of infection, including pathologic findings, have not been described in detail in recent years.9,12,21 Our objectives in this retrospective case study are to describe the clinical and pathologic features of K. pneumoniae infection and disease in rhesus macaques from a large breeding colony, to compare these observations with human KAS, and to address potential risk factors and implications for colony health and experimental protocols.
Case Summary
Materials and Methods
Animals.
Between 2010 and 2015, our facility (the California National Primate Research Center, Davis, CA) had an average population of approximately 4000 NHP, comprising approximately 99% rhesus macaques and less than 1% cynomolgus macaques (M. fascicularis) and titi monkeys (Callicebus cupreus). All animals were observed daily either cageside or within outdoor enclosures. In this time period, 28 rhesus macaques cultured positive for K. pneumoniae among a total 29,285 cultures (0.03%). From this group, animals were included in our case series only when they had identifiable pathologic findings consistent with K. pneumoniae infection from a necropsy examination. Among the culture-positive animals, only 8 rhesus macaques had gross and histologic lesions consistent with K. pneumoniae infection. We also included a ninth rhesus macaque that had histologic lesions strongly consistent with K. pneumoniae infection, including identification of gram-negative bacteria with consistent morphology, and which was PCR-positive for K. pneumoniae within these same tissues. All 9 animals were euthanized after clinical diagnostic evaluation (n = 5) or were found dead (n = 4).
All animals at our facility are maintained according to the USDA Animal Welfare Act and Regulations,1,2 and the Guide for the Care and Use of Laboratory Animals.13 The animal care and use program, including protocols for the maintenance and breeding of rhesus macaque colonies of the University of California–Davis, is approved by the University of California–Davis IACUC, is fully AAALAC-accredited, is USDA-registered, and maintains a Public Health Services Assurance.18
Clinical methods.
The 5 macaques that were evaluated clinically were sedated (ketamine, 10 mg/kg IM) and underwent full physical exams, CBC and serum chemistry analysis, and imaging. Treatments were varied and often were given only once before euthanasia was elected due to the severity of clinical signs. These treatments may have included: fluid therapy (Lactated Ringers Solution, 6 to 30 mL/kg/h, with additional bicarbonate at 20 mEq/500 mL of fluids or with 5% dextrose), antibiotics (enrofloxacin [5 mg/kg IM BID, Baytril, Bayer AG, Shawnee, KS] and ceftriaxone [299 mg IV once]), pain medications (buprenorphine [0.03 mg/kg IM once] or oxymorphone [0.15 mg/kg IM once]), and antiinflammatory drugs (dexamethasone 1.5 mL IV once).
Necropsy and histology.
Routine diagnostic necropsies were performed on all macaques, and tissue samples were collected in 10% neutral buffered formalin. After thorough fixation, tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H and E). Gram stains were performed according to the standard protocol.4
Culture.
Culture material was obtained by using culturette swabs obtained antemortem (n = 2) or during necropsy (n = 7); one animal was cultured at both times. Swabs were streaked onto a sheep blood agar plate and a MacConkey agar plate. The identification of Klebsiella pneumoniae is based on colony morphology, Gram stain, and confirmatory identification with manual biochemical testing and API20E identification strips (bioMerieux, St Louis, MO). Colonies with the hypermucoviscous phenotype were verified by using a string test.8
PCR analysis.
Klebsiella PCR screening on scrolls from formalin-fixed paraffin-embedded tissues was performed by a commercial laboratory (Charles River Research Animal Diagnostic Services, Wilmington, MA) to detect Klebsiella pneumoniae and K oxytoca.
Results
Animals.
Spanning the time period (2010 through 2015) with no apparent seasonality, 9 (0.3%) rhesus macaques among the 2967 colony necropsies performed fit the search criteria. Signalment, housing, and risk factor data are presented in Table 1. Predominantly, affected macaques were either infants (age: average, 46 d; range, 13 to 75 d; n = 5) or geriatric animals (age: average, 19.4 y; range, 18.7 to 20.3 y; n = 3); the remaining animal was middle-aged (4.5 y). Two infants and 3 of the adult animals had concurrent disease(s)—an additional risk factor—including severe lung mite infestation (n = 1), pathogenic diarrhea (n = 1, Campylobacter jejuni), endometriosis (n = 1), trauma (n = 2), amyloidosis (n = 2), and neoplasia (n = 2). The 4 adult macaques all had seropositivity (presumed latent) to viral infections (rhesus cytomegalovirus or herpes B virus or both). All adults were female, but none of them was pregnant or nursing. None of the macaques was participating in a study at the time of clinical presentation or death.
Table 1.
Signalment for the 9 rhesus macaques in our series
| Case no. | Age at diagnosis | Sex | Housing | SPF statusa | Positive viral testing | Other risk factors |
| 1 | 13 d | Female | Outdoor | Level 1 | Not available | |
| 2 | 27 d | Male | Outdoor | None | Not available | |
| 3 | 1.7 mo | Female | Outdoor | Level 2 | Not available | Pathogenic diarrhea (C. jejuni) |
| 4 | 2.1 mo | Male | Outdoor | None | Not available | Trauma |
| 5 | 2.5 mo | Male | Outdoor | None | Not available | |
| 6 | 4.5 y | Female | Outdoor | Level 1 | Cytomegalovirus | |
| 7 | 18.8 y | Female | Indoor | None | Cytomegalovirus Herpes B virus | Thyroid carcinoma Lung mites |
| 8 | 19.2 y | Female | Outdoor | Level 1 | Cytomegalovirus | Hospitalization Multiple antibiotics Gastrointestinal adenocarcinoma Hepatic amyloidosis |
| 9 | 20.3 y | Female | Outdoor | None | Cytomegalovirus Herpes B virus Rhesus rhadinovirus | Enteric and Renal amyloidosis Enteritis Trauma |
SPF Level 1: negative for SIV, SRV, STLV, and herpes B virus. SPF Level 2: negative for all level 1 viruses, rhesus rhadinovirus, and foamy virus
Clinical presentation.
Significant clinical findings are presented in Table 2. All 4 animals that had no recorded clinical signs (and thus were found dead) were infants. Four adult animals presented less than 12 h before euthanasia was elected due to poor prognosis and response to therapy. Clinical signs at presentation included weakness (n = 4), unresponsiveness (n = 3), hypothermia (temperature less than 98 °F, n = 2), visual abnormalities (n = 2), neurologic abnormalities (n = 2), painful abdomen (n = 2), and dyspnea (n = 1).
Table 2.
Clinical findings and treatments
| Case no. | Clinical signs | CBC | Serum chemistry | Imaging | Culture | Treatment |
| 1 | Not available | Not available | Not available | Not available | Not available | Not available |
| 2 | Not available | Not available | Not available | Not available | Not available | Not available |
| 3 | Seizures Avisual Diarrhea | Leukocytosis with neutrophilia, left degenerative shift, and toxic change; Hyperfibrinogenemia | Hypoglycemia | Not available | Rectal: C. jejuni | Fluids with Dextrose Enrofloxacin |
| 4 | Not available | Not available | Not available | Not available | Not available | Not available |
| 5 | Not available | Not available | Not available | Not available | Not available | Not available |
| 6 | Unresponsive, weak, dilated pupils, painful abdomen, hypothermic (97.9 °F) | Leukocytosis with neutrophilia; Mild anemia | Hyponatremia; Hypochloremia; Hypokalemia; Hyperphosphatemia; mild increased AST, ALP, LDH; Hypoalbuminemia | Radiography: increased Pulmonary Pathology Ultrasonography: normal | Endotracheal tube: K. pneumoniae | Oxymorphone Ceftriaxone Dexamethasone |
| 7 | Weak, hypothermic (97.3 °F), laterally recumbent, neurologic signs, acute dyspnea | Neutrophilia with mild left shift; Hyperfibrinogenemia; Thrombocytopenia | Hyponatremia; Hypochloremia; Hypokalemia; mild increased AP; Hypoalbuminemia | Radiography: increased Pulmonary Pathology Ultrasonography: cysts near uterus | Gingiva: negative | IV fluids Enrofloxacin Flunixin Buprenorphine |
| 8 | ~1-mo history of unresponsive diarrhea; ~1d acutely weak, hunched, hepatomegaly, crackles in lungs | Leukocytosis with neutrophilia; Hyperfibrinogenemia; Mild anemia | Not available | Not available | Nasal: K. pneumoniae Rectal: negative | None |
| 9 | Weak, not moving, painful abdomen | Leukopenia; Anemia; Hypoproteinemia | Hyponatremia; Hypochloremia; Hypokalemia | Radiography: Obscured cardiac silhouette, increased interstitial density in lungs and pleural cavity, large liver | Not available | IV fluids with bicarbonate buprenorphine |
One adult animal (no. 8) had a prolonged hospitalization (33 d) for uncontrollable diarrhea and was treated with 4 different antibiotics (azithromycin [40 mg/kg PO QD for 5 d], tylosin [20 mg/kg IM QD for 10 d], metronidazole [50 mg/kg SC QD for 5 d], and doxycycline [5 mg/kg PO BID for 10 d]). On day 34, she presented with peracute (less than 12 h) clinical signs, including a sharp decline in appetite, weakness, palpable hepatomegaly, and crackles in the lungs. Euthanasia was elected at this time due to poor prognosis.
Clinical diagnostic testing.
Abnormal findings are summarized in Table 2. All animals evaluated by the veterinary staff (n = 5) had bloodwork performed including whole-blood analysis (NOVA-CCX Stat Analyzer, Nova Biomedical, Waltham, MA), hematology, and serum biochemistry profiles. Significantly, animals had leukocytosis with neutrophilia (n = 4), hyperfibrinogenemia (n = 3), and electrolyte imbalances (n = 3). Three animals were radiographed, which showed pulmonary changes including increased interstitial density and opacities (Figure 1). One animal underwent abdominal ultrasonography, and cystic masses were noted and were interpreted as endometriosis. Culture swabs from 2 animals (1 nasal swab, 1 endotracheal tube swab) were obtained during clinical evaluation, but the animals declined rapidly, and euthanasia for humane reasons was elected before the culture results were available. Both of these animals were positive for Klebsiella pneumoniae according to the antemortem swabs.
Figure 1.
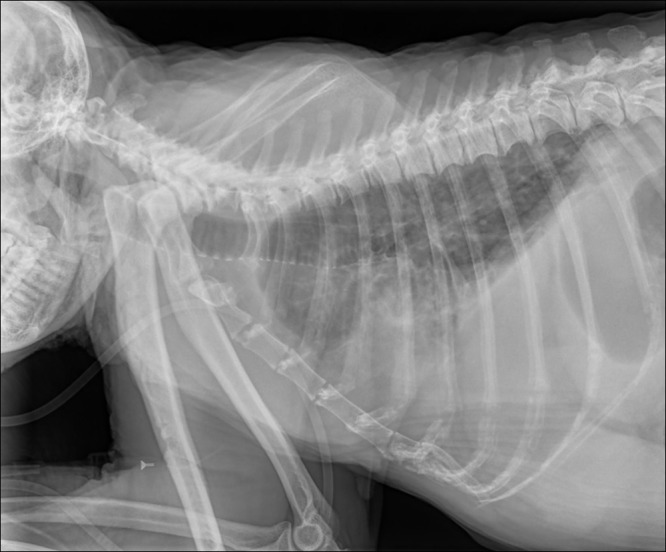
Case no. 9. Thoracic radiograph (lateral) reveals an obscured cardiac silhouette by increased interstitial density in the lungs and pleural cavity.
Clinical treatments.
Treatments included fluid therapy, antibiotics, analgesics, and antiinflammatory drugs (Table 2). All animals that received treatment, including 3 that were given broad-spectrum antibiotics, were nonresponsive and ultimately were euthanized.
Necropsy findings.
Main necropsy findings, including histologic diagnoses, are presented in Table 3. Comorbidities discovered or confirmed at necropsy included a single case each of trauma, hepatic amyloidosis with large intestinal adenocarcinoma, enteric and renal amyloidosis with enteritis and trauma, pathogenic bacterial diarrhea, and thyroid carcinoma with endometriosis. Related to K. pneumoniae infection, the abdominal cavities of 2 macaques were filled with yellow to tan, thick fluid, consistent with suppurative peritonitis (Figure 2). One animal had grossly visible multifocal hepatic abscesses (Figure 3). Histologically, these lesions were large, distorted the architecture (Figure 4, inset), and were filled with necrotic cellular debris and neutrophils. Within the inflammation, myriad short bacillar bacteria with 3- to 5-µm clear capsules (Figure 4) were present and were gram-negative (Figure 4, inset). Another animal had multifocal suppurative hepatitis within a background of significant amyloid deposition (Figure 5). Two macaques had grossly identifiable pleuropneumonia or pulmonary abscesses (Figure 6), and an additional 4 animals had pneumonia on histology. Histologically, pneumonia was most often characterized by large aggregates of neutrophils and macrophages that filled airways and distorted the normal pulmonary architecture (Figure 6, inset). CNS changes included meningitis (n = 4), meningoencephalitis (n = 3), meningomyelitis (n = 1), and often concurrent optic neuritis (n = 3) or endopthalmitis (n = 1). Although meningitis or encephalitis was suspected grossly in 3 cases (Figure 7), inflammation of the CNS was found histologically in 7 (78%) of the 9 total cases, including both infant and adult animals. Meningitis was characterized by marked expansion of the meninges by large numbers of neutrophils with intracytoplasmic and intralesional bacteria as seen in liver. Encephalitis and myelitis involved areas of malacia and hemorrhage admixed with neutrophils and prominent perivascular cuffing by neutrophils and macrophages (Figure 8). In some cases (nos. 6 and 7), neuropil lesions were associated with fibrinoid vasculitis (Figure 9), supporting the pathogenesis of bacteremia. According to lesion distribution and histologic findings, septicemia or bacteremia was confirmed in 8 of the 9 cases.
Table 3.
Necropsy, culture, and PCR test results
| Case no. | Necropsy findings | Tissue culture-positive for K. pneumoniae | PCR test for K. pneumoniae |
| 1 | Meningoencephalitis, endophthalmitis | Lung, pleura | ± |
| 2 | Peritonitis, pneumonia, liver abscesses, meningoencephalitis, endophthalmitis | Brain, peritoneum, bone marrow | Not available |
| 3 | Meningoencephalitis | Not available | + |
| 4 | Trauma, gastric rupture, lung edema, meningoencephalitis | Lung | Not available |
| 5 | Pneumonia | Lung | ± |
| 6 | Pneumonia, meningoencephalitis, meningomyelitis | Lung, brain, bone marrow | + |
| 7 | Pneumonia, lung mites, meningoencephalitis, optic neuritis, thyroid tumor, endometriosis | Lung, brain | Not available |
| 8 | Hepatic amyloid, colonic adenocarcinoma, pneumonia, liver abscesses | Not available | Not available |
| 9 | Pneumonia, peritonitis, meningoencephalitis, enteric and renal amyloidosis | Pleura, nasal fluid, brain, liver, bone marrow | Not available |
Figure 2.
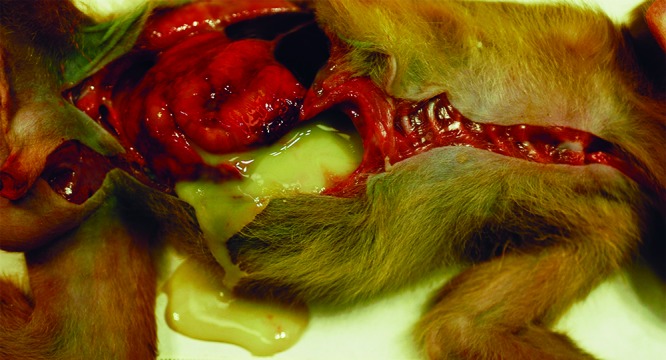
Case no. 2. Purulent material fills the abdominal cavity, and the serosal surfaces of the abdominal viscera are congested and inflamed.
Figure 3.
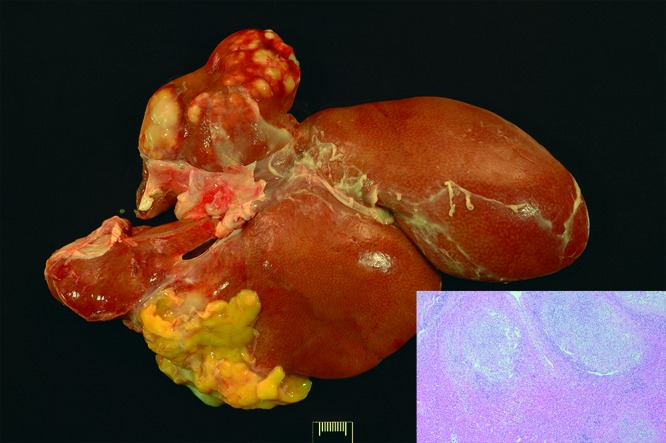
Case no. 9. The left lateral lobe of the liver contains multifocal abscesses. Inset: Disrupting the hepatic architecture are multiple areas of necrosis admixed with neutrophils and macrophages, with peripheral fibrosis (abscesses). Hematoxylin and eosin staining; magnification, 40×.
Figure 4.
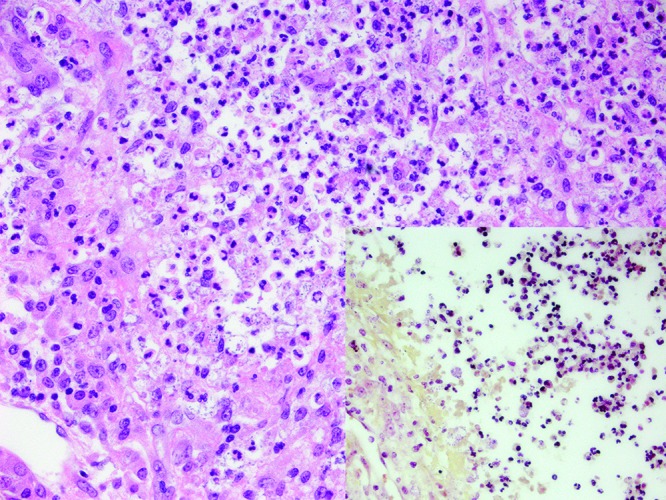
Case no. 9. Within the abscesses are large numbers of intracellular and extracellular bacillar bacteria. Hematoxylin and eosin staining; magnification, 400×. Inset: These bacteria are gram-negative. magnification, Brown and Brenn Gram staining; magnification, 400×.
Figure 5.
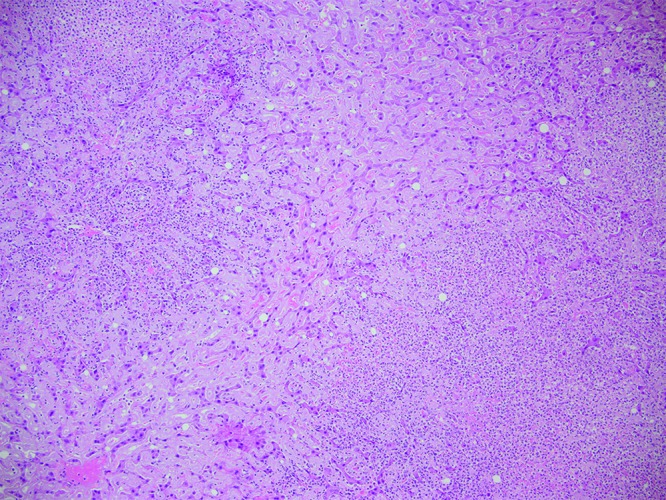
Case no. 8. Diffusely the spaces of Dissé are expanded by amyloid, and there is compression of the adjacent hepatic cords. Multifocally, there are regions of hepatic parenchyma infiltrated by large numbers of neutrophils. Hematoxylin and eosin staining; magnification, 100×.
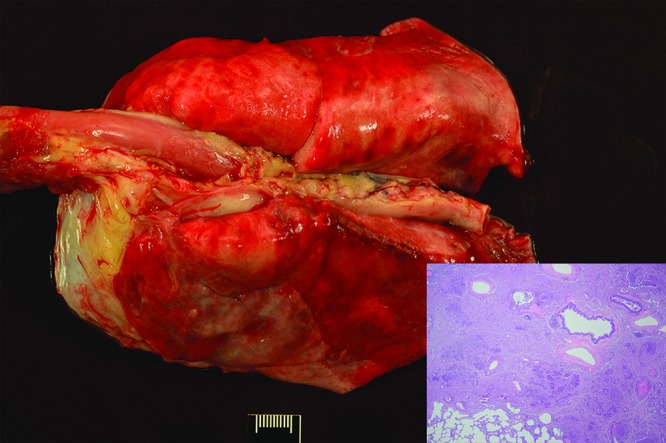
Figure 6.
Case no. 9. Throughout the lung lobes are multifocal pulmonary abscesses. The pleural surfaces are covered with mats of fibrin (pleuritis). Inset: Within these abscesses, the alveoli and bronchioles are filled with neutrophils, obliterating the majority of the normal air spaces. Hematoxylin and eosin staining; magnification, 40×.
Figure 7.
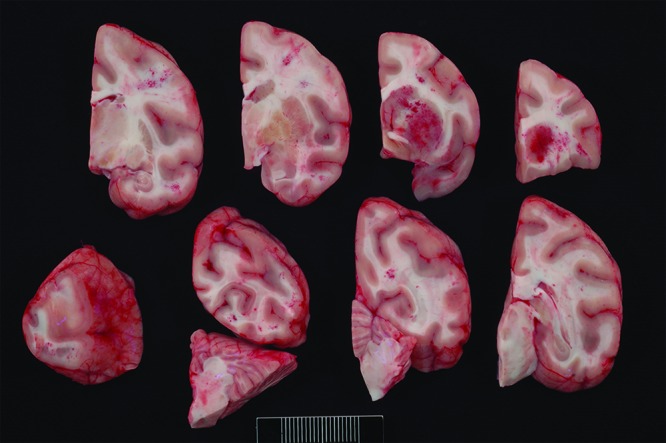
Case no. 7. Multifocally within the meninges and neuropil are areas of hemorrhage. The basal ganglia are affected most severely.
Figure 8.
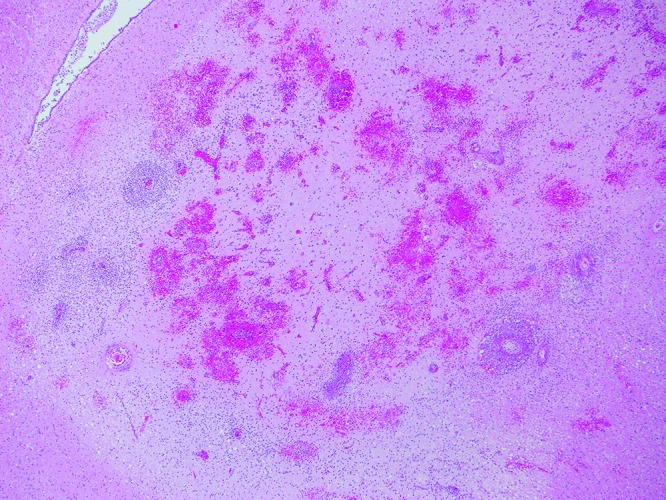
Case no. 7. Throughout the neuropil are large areas of hemorrhage and prominent perivascular cuffs. Hematoxylin and eosin staining; magnification, 40×.
Figure 9.
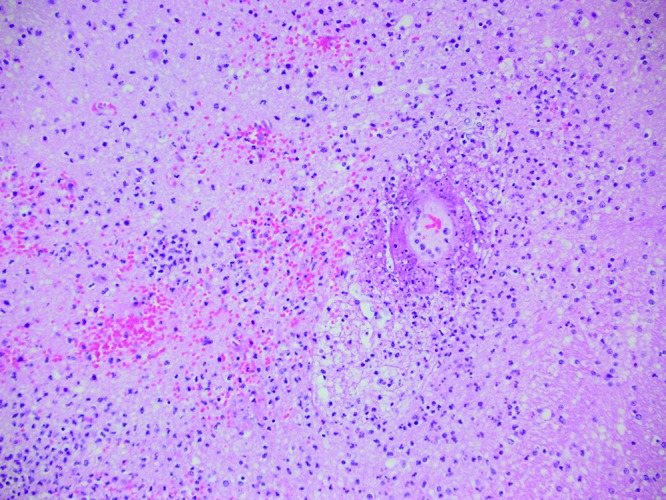
Case no. 7. Perivascular cuffs are composed predominantly of neutrophils, and there is multifocal fibrinoid necrosis of the vessels. Hematoxylin and eosin staining; magnification, 200×.
Culture results.
Six animals were cultured at necropsy, one macaque was cultured both antemortem and at necropsy (no. 6), and one macaque (no. 8) underwent antemortem nasal culture only (Tables 2 and 3). The remaining animal (no. 3) had no cultures performed. All 8 cultured animals were positive for Klebsiella pneumoniae from lung (n = 5), brain (n = 4), pleura or peritoneum (n = 2), nasal swab (n = 2), or liver (n = 1); 4 of these 8 animals were positive from multiple sites. All cultures yielded positive string tests. From 2010 through 2015, a total of 29,485 requests for culture were submitted, of which 2847 (9.7%) were from postmortem sampling.
PCR testing.
PCR testing to identify K. pneumoniae was performed on formalin-fixed, paraffin-embedded tissues from 4 animals to confirm the presence of this bacterium within observed lesions (Table 3). Two macaques (nos. 3 and 6) were positive within tissues with histologically confirmed lesions, including the one animal with no culture samples (no. 3). Two macaques (nos. 1 and 5) had equivocal results, with PCR product copy numbers too low to conclusively verify K. pneumoniae. All 4 of these animals were negative for K. oxytoca.
Discussion
The clinical and postmortem findings in these 9 rhesus macaques are similar to human presentations of opportunistic K. pneumoniae infection and lesions consistent with KAS. All of the 9 macaques succumbed quickly to the disease, with severe clinical signs presenting 12 h or less before euthanasia was elected. Although 5 macaques were thought clinically to have a bacterial infection in light of changes in bloodwork (neutrophilia with degenerative left shift) or identified pneumonia, none was suspected to have K. pneumoniae. Broad-spectrum antibiotics were administered in 3 cases with no clinically appreciable effect. However, even when K. pneumonia infection is suspected clinically, treatment options are limited, given that KAS in people has a severe clinical presentation and a high mortality rate,14 and treatment is highly intensive, often involving lengthy hospitalization and multiple simultaneous intravenous antibiotic treatments for weeks,11,15 with significant morbidity, such as neurologic deficits, on recovery.20
Similar to human KAS and previous reports in NHP,3,6,9,12,19 the macaques in this series had pulmonary or hepatic abscesses and evidence of septicemia with meningitis or endophthalmitis or both. Interestingly, liver abscesses are often associated with diabetes mellitus or liver cirrhosis in humans, which was not identified in any of our macaques, although one animal with liver abscesses did have extensive hepatic amyloidosis. Urinary tract infections were not identified in these macaque cases, although the urinary bladder was histologically examined in only 2 animals (nos. 3 and 6). In humans, septicemia due to K. pneumoniae is most often associated with liver abscesses or pneumonia,3 however, in 3 of our cases (nos. 1, 3, and 4), meningoencephalitis was present without liver or lung involvement. These cases were all infants, perhaps suggesting that they developed septicemia quickly and therefore did not have sufficient time to mount an inflammatory response in other viscera.
Various risk factors have been identified in human K. pneumoniae cases, and the same factors were present in our cases. Affected macaques tended to be infants (nos. 1 through 5; age, 13 through 75 d) or geriatric (nos. 7 through 9; age, 18.7 through 20.3 y), whereas only one animal was a middle-aged adult (no. 6, 4.5 y old). All animals that died spontaneously without clinical signs were infants (nos. 1, 2, 4, and 5). Adult macaques (nos. 6 through 9) tended to present in extreme distress, either weak or obtunded with neurologic signs and pain that was unresponsive to a myriad of treatments. The 3 geriatric animals had significant comorbidities (no. 7 with pulmonary Pneumonyssus infection and thyroid neoplasm; no. 8 with colonic adenocarcinoma and hepatic amyloidosis; and no. 9 with enteric and renal amyloidosis, enteritis, and trauma) that may have made them more susceptible to infection (e.g., decreased gastrointestinal immunity, additional lung infection). All 4 adults were seropositive for endemic viruses (cytomegalovirus, n = 4; herpes B virus, n = 2; and rhesus rhadinovirus, n =1), which may have caused immune modulation and increased susceptibility to K. pneumoniae infection. In addition, one macaque (no. 8) had long-term hospitalization with multiple antibiotic treatments due to unresolved diarrhea (likely due to the colonic adenocarcinoma); both conditions are recognized as associated risk factors in the human literature.3
Although all 8 of our culture-positive animals had identifiable risk factors, they also all were infected with hypermucoviscous K. pneumoniae, as identified through a positive string test. This form of K. pneumoniae, also called hypervirulent K. pneumoniae, is capable of causing disease in immunocompetent human hosts and potentially can explain the one young-adult macaque in the group that succumbed to infection despite having no risk factors. Unfortunately, for the cases in this series, testing for virulence factors could not be performed due to inadequate saved material. Freezing bacterial colonies is important for full characterization of this organism, because capsule typing and PCR tests for virulence factors are available commercially but can only be performed on culture material and not on formalin-fixed tissues. In addition, antibiotic sensitivity testing should be strongly considered, because multidrug resistance is increasing in human K. pneumoniae infections.3,16
Of the 28 animals that had positive K. pneumoniae cultures during the 6-y study period, only 8 rhesus macaques had clinical signs or lesions associated with infection, which ultimately led to their deaths. The other 20 animals were considered to be carriers, because they had low numbers of cultured organisms and never exhibited clinical signs associated with K. pneumoniae infection. Because subclinical Klebsiella pneumoniae infection has been shown to alter cytokine response5 and might affect the outcomes of immunologic research, routine culturing (rectal, nasal, or both) is recommended to identify carriers prior to project enrollment. In addition, previous publications have suggested that the stress of participation in experimental studies can be a risk factor for K. pneumoniae infections in NHP.12 However, experiments aimed at simulating stressful situations by administering dexamethasone failed to elicit disease in K. pneumoniae culture-positive rhesus macaques.5 In addition, as in humans, the underlying health status of the animal may play a large role in how the body responds to infection. Animals with a compromised immune system, due to immaturity or aging, may be less capable of controlling the spread from the respiratory tract to other organs. When following animals with subclinical infection, one study5 reported that only one animal that was culture-positive for K. pneumoniae succumbed to disease; this animal had been experimentally infected with monkeypox 22 d earlier, which likely caused severe immunosuppression.
Overall, clinical K. pneumoniae infection is rare (9 clinical cases in 6 y within a stable population of nearly 4000 NHP with 2967 colony necropsies and 29,485 total cultures performed) but should be considered in animals with rapidly progressive clinical signs associated with suspected bacterial infection. Broad-spectrum antibiotics can be administered, but the prognosis is guarded for survival and complete return to health. The gross necropsy findings can be subtle, but histopathology is reliable for diagnosis, with prominent encapsulated Gram-negative bacillar bacteria identified within most lesions. Cultures of lung and brain of sudden infant deaths can aid with diagnosis, because most infants with this disease do not present with clinical signs. Importantly, string tests, antibiotic sensitivity tests, and PCR tests for virulence factors should be considered for Klebsiella pneumoniae culture-positive animals, whether incidental or associated with clinical signs or lesions.
Acknowledgments
We thank Sarah Lockwood for her technical support with digital imaging, Abbie Spinner and Christy Johnson for their expertise in microbiology and analyzing culture data, and Primate Medicine Services for their care and dedication to the animals. This research was supported by the CNPRC base operating grant (no. P51 ODO11107-56).
References
- 1.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159 [Google Scholar]
- 2.Animal Welfare Regulations. 2013. 9 CFR § 3.129. [Google Scholar]
- 3.Broberg CA, Palacios M, Miller VL. 2014. Klebsiella: a long way to go towards understanding this enigmatic jet-setter. F1000Prime Rep 6:1–12. 10.12703/P6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JH, Brenn L. 1931. A method for the differential staining of Gram-positive and Gram-negative bacteria in tissue sections. Bull Johns Hopkins Hosp 48:69–73. [Google Scholar]
- 5.Burke RL, West MW, Erwin-Cohen R, Selby EB, Fisher DE, Twenhafel NA. 2010. Alterations in cytokines and effects of dexamethasone immunosuppression during subclinical infections of invasive Klebsiella pneumoniae with hypermucoviscosity phenotype in rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Comp Med 60:62–70. [PMC free article] [PubMed] [Google Scholar]
- 6.Burke RL, Whitehouse CA, Taylor JK, Selby EB. 2009. Epidemiology of invasive Klebsiella pneumoniae with hypermucoviscosity phenotype in a research colony of nonhuman primates. Comp Med 59:589–597. [PMC free article] [PubMed] [Google Scholar]
- 7.Chung DR, Lee SS, Kim HB, Choi HJ, Eom JS, Choi YH, Lee JS, Chung MH, Kim YS, Lee H, Lee MS, Park CK, Korean Study Group for Liver Abscess. 2007. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect 54:578–583. 10.1016/j.jinf.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Cox BL, Schiffer H, Dagget G, Jr, Beierschmitt A, Sithole F, Lee E, Revan F, Halliday-Simmons I, Beeler-Marfisi J, Palmour R, Soto E. 2015. Resistance of Klebsiella pneumoniae to the innate immune system of African green monkeys. Vet Microbiol 176:134–142. 10.1016/j.vetmic.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Giles RC, Jr, Hildebrandt PK, Tate C. 1974. Klebsiella air sacculitis in the owl monkey (Aotus trivirgatus). Lab Anim Sci 24:610–616. [PubMed] [Google Scholar]
- 10.Good RC, May BD. 1971. Respiratory pathogens in monkeys. Infect Immun 3:87–93. 10.1128/IAI.3.1.87-93.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang CT, Chen YC, Chang SC, Sau WY, Luh KT. 2000. Klebsiella pneumoniae meningitis: timing of antimicrobial therapy and prognosis. QJM 93:45–53. 10.1093/qjmed/93.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Fox JG, Rohovsky MW. 1975. Meningitis caused by Klebsiella spp in two rhesus monkeys. J Am Vet Med Assoc 167:634–636. [PubMed] [Google Scholar]
- 13.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 14.Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 58:1054–1073. 10.1111/j.1365-2958.2005.04918.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee B, Yeroushalmi K, Me Me H, Sojitra P, Jilani U, Iqbal S, Ahmed S, Verley J, Akella J. 2018. Community acquired Klebsiella pneumoniae meningitis: a case report. Germs 8:92–95. 10.18683/germs.2018.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez LL, Rusconi B, Gildersleeve H, Qi C, McLaughlin M, Scheetz MH, Seshu J, Eppinger M. 2016. Genome sequences of five clinical isolates of Klebsiella pneumoniae. Genome Announc 4:1–2. 10.1128/genomeA.00040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz MA, Ahlström C, Rauch BJ, Zadoks RN. 2006. Fecal shedding of Klebsiella pneumoniae by dairy cows. J Dairy Sci 89:3425–3430. 10.3168/jds.S0022-0302(06)72379-7. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health. 2002. Public health service policy on humane care and use of laboratory animals. Bethesda (MD): Office of Laboratory Animal Welfare [Google Scholar]
- 19.Pisharath HR, Cooper TK, Brice AK, Cianciolo RE, Pistorio AL, Wachtman LM, Mankowski JL, Newcomer CE. 2005. Septicemia and peritonitis in a colony of common marmosets (Callithrix jacchus) secondary to Klebsiella pneumoniae infection. Contemp Top Lab Anim Sci 44:35–37. [PubMed] [Google Scholar]
- 20.Pomakova DK, Hsiao CB, Beanan JM, Olson R, MacDonald U, Keynan Y, Russo TA. 2012. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumoniae: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis 31:981–989. 10.1007/s10096-011-1396-6. [DOI] [PubMed] [Google Scholar]
- 21.Snyder SB, Lund JE, Bone J, Soave OA, Hirsch DC. 1970. A study of Klebsiella infections in owl monkeys (Aotus trivirgatus). J Am Vet Med Assoc 157:1935–1939. [PubMed] [Google Scholar]
- 22.Twenhafel NA, Whitehouse CA, Stevens EL, Hottel HE, Foster CD, Gamble S, Abbott S, Janda JM, Kreiselmeier N, Steele KE. 2008. Multisystemic abscesses in African Green Monkeys (Chlorocebus aethiops) with invasive Klebsiella pneumoniae—identification of the hypermucoviscosity phenotype. Vet Pathol 45:226–231. 10.1354/vp.45-2-226. [DOI] [PubMed] [Google Scholar]
- 23.Whitehouse CA, Keristead N, Taylor J, Reinhardy JL, Beierschmitt A. 2010. Prevalence of hypermucoid Klebsiella pneumoniae among wild-caught and captive vervet monkeys (Chlorocebus aethiops sabaeus) on the island of St Kitts. J Wildl Dis 46:971–976. 10.7589/0090-3558-46.3.971. [DOI] [PubMed] [Google Scholar]


