Abstract
The Roux-en-Y Gastric Bypass (RYGB) mouse model is a vital tool for studying the pathophysiology of bariatric surgery and contributes greatly to research on obesity and diabetes. However, complications including postsurgical hypoglycemia can have profoundly negative effects. Unlike in humans, blood glucose (BG) is not typically managed in postoperative rodents, despite their critical role as translational models; without this management, rodents can experience hypoglycemia, potentially impairing wound healing, decreasing survivability, complicating interpretation of research data, and limiting translational utility. In this project, we sought to identify an optimal method for minimally invasive administration of dextrose in C57BL/6N (n = 16; 8 male, 8 female) mice. To do so, we characterized BG pharmacokinetic profiles after subcutaneous and oral–transmucosal (OTM) administration of dextrose. Compared with OTM dosage, the subcutaneous route provided more consistent and reliable delivery of glucose and did not cause significant adverse reactions. We then evaluated the frequency of hypoglycemic events after RYGB in C57BL/6N mice (n = 16; 8 male, 8 female) and the effects of subcutaneous dextrose supplementation on morbidity and mortality. BG measurement and behavioral pain assessment (grimace test) were performed for 3 d after surgery. Hypoglycemic (BG ≤ 60 mg/dL) animals were assigned to dose (5% dextrose SC) or no-dose treatment groups. Nearly all (87%) mice became hypoglycemic; 2 of these mice died. No significant intergroup difference in grimace score or mortality was detected. Overall, our results demonstrate that hypoglycemia is a frequent adverse event after RYGB in mice and that subcutaneous injection of dextrose is a safe and effective way to manage hypoglycemia. Further studies are necessary to optimize the intervention threshold and optimal dosage; regardless, we recommend glycemic management after RYGB surgery in mice.
Abbreviations: BG, blood glucose; OTM, oral–transmucosal; RYGB, Roux-en-Y gastric bypass
Translational animal models of bariatric surgery are a vital tool for understanding the pathophysiologic mechanisms that occur postoperatively and the factors associated with surgical efficacy. Glycemic management is imperative for optimal postoperative recovery in humans and animals alike. Both hyperglycemia and hypoglycemia can significantly influence rates of healing, morbidity, and mortality in patients.5,8,17,18,24,25,33 The physiologic effects of hypoglycemia are numerous and are commonly implicated as major factors for postoperative complications. Rodent surgical models may experience complications such as severe postoperative hypoglycemia, particularly given that blood glucose (BG) measurement and management are typically not included in routine rodent postoperative regimens. Documented literature evaluating the frequency and severity of hypoglycemia in mice after bariatric surgery is lacking and is vitally needed to improve postoperative care, specifically for the Roux-en-Y Gastric Bypass (RYGB) mouse model, which experiences more complications than Vertical Sleeve Gastrectomy surgery.
Hypoglycemia affects many physiologic functions ranging from metabolic to neurologic, all of which can have a significant impact on animal health, welfare, and study outcomes. Mild hypoglycemia stimulates the autonomic nervous system, causing increased blood flow to the brain, release of counter regulatory glucagon and epinephrine, and decreased insulin secretion.31 Prolonged hypoglycemia can lead to a catabolic state with inefficient energy for wound healing.32 With increasing severity, cardiovascular disturbances and neuroglycopenic symptoms occur, which can range from confusion and dizziness to seizures, coma, and death.8,20,31 Diabetic patients are especially vulnerable due to their already compromised glucose metabolism, making them at higher risk for impaired wound healing,28 postoperative surgical site infections,30 and mortality after surgical procedures.
The postoperative period is a particularly vulnerable time for both human and animal patients. In the days after an invasive surgical procedure, patients often experience fatigue,10 pain, and subsequent loss of appetite which can result in hypoglycemia.21 Surgery type6 and drug choice23 for analgesia may also exacerbate postoperative anorexia. These physiologic and psychologic stresses can be especially severe for laboratory mice. Mice have a higher metabolic rate than humans, so periods of inappetence can have a profound impact on glucose homeostasis.16 Mice also commonly decrease food intake during pain and stress.21 Preoperative events like fasting may increase the risk factors for postoperative hypoglycemia, particularly in diabetics.1 While human patients are closely monitored and placed on strict postoperative diets, these practices are less common in laboratory animals used to model human diseases, recovery, and treatment regimens, particularly in rodents. As a result, postoperative glycemic management is of known importance to human patients but is not well studied in mice. Mouse models for bariatric surgeries2,7,12,22,29 are likely to have significant effects on glucose metabolism and homeostasis. However, very little literature reports on the postoperative glycemic state in mice and no information on the effects of glucose supplementation on survivability. The postoperative glycemic state and efficacy of glucose supplementation should be assessed in rodents, not only to better replicate human care for translational research, but also to improve the health and welfare of research animals.
The overall objective of this study was to determine the frequency of hypoglycemia in mice after RYGB surgery and evaluate the effect of dextrose supplementation on morbidity and mortality in postoperative hypoglycemic animals. To achieve this objective we initially characterized the basic glucose pharmacokinetics in nonmanipulated adult mice after subcutaneous and oral–transmucosal (OTM) administration to determine the most appropriate minimally invasive route of administration for clinical treatment of hypoglycemia. We hypothesized that implementation of BG monitoring and treatment during the initial postoperative period in RYGB mice would improve morbidity and mortality, thus decreasing deleterious cofounding factors associated with this translatable animal model and improving animal welfare.
Materials and Methods
Animals.
All mice studies were approved by the IACUC of University of California, Davis and mice were maintained in accordance to the standards established by the Guide for the Care and Use of Laboratory Animals.14 Mice were SPF for the following pathogens: all ectoparasites and endoparasites, ectromelia virus, Theiler disease virus, lymphocytic choriomeningitis virus, minute virus of mice, mouse adenovirus of mice (types 1 and 2), mouse hepatitis virus, mouse parvovirus, pneumonia virus of mice, reovirus 3, rotavirus, Sendai virus, murine norovirus, Mycoplasma spp., Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Klebsiella spp., Mycoplasma arthritidis, Mycoplasma pulmonis, Pasteurella multocida, Pasteurella pneumotropica, Pseudomonas spp., Salmonella spp., Streptobacillus moniliformis, Streptococcus (β-hemolytic) spp., and Helicobacter spp. Mice were single-housed in IVC (Optimice IVC, Animal Care Systems, Centenniel, CO) on a 12:12-h (lights on, 0600) light:dark cycle at 68 to 79 °F (20.0 to 26.1 °C) and 30% to 70% humidity. All mice were fed 60% high fat diet (TD.06414, Envigo Teklad Diets, Madison, WI) without restriction for 4 wk before use. Mice were acclimated to 5 min of gentle handling daily for 5 d prior to any procedures.
Glucose pharmacokinetic study.
To determine the optimal administration route for dextrose supplementation for treatment of hypoglycemia, a prospective balanced crossover study design was conducted to evaluate subcutaneous and OTM dextrose (50% injectable; Hospira, Lake Forest, IL) administration in the same cohort of mice. C57BL/6N mice (n = 16; 8 male, 8 female; weight, 19.4 to 24.9 g; age, 7 to 8 wk) were used to establish the optimal dextrose dosing procedure in the first phase of the study. Mice were fasted for 16 h prior to dextrose administration, after which each mouse received a single dose of 50 mg dextrose administered either subcutaneously (1 mL, 5% dextrose) or OTM (two 0.05-mL drops of 50% dextrose). All mice underwent a 5-d washout period before undergoing a second trial, in which they received the opposite administration route. Treatment order was randomized. Eight mice (4 male, 4 female) were then randomly selected (4 OTM, 4 subcutaneous) and used as saline controls after an additional 5-d washout period.
The BG concentration in a drop of blood collected from the tail tip was measured by using a handheld glucometer (Nova Statstrip, Nova Biomedical, Waltham, MA). The tail tip was lanced for the initial baseline blood sample and the scab was removed for each subsequent BG measurement to avoid multiple lances. The first drop of blood was discarded during each collection to obtain a fresh sample for testing. After baseline sampling, 50 mg dextrose was administered either subcutaneously or through the OTM route. Subcutaneous dextrose (1 mL, 5% dextrose) was administered between the shoulder blades, and the OTM dose was administered by scruffing the mouse, placing the first drop (0.05 mL, 50% dextrose) of dextrose onto the gingiva and waiting approximately 3 to 5 seconds before placing the second drop (0.05 mL, 50% dextrose) to optimize mucosal uptake and minimize oral intake. BG concentrations were measured at 5, 15, 30, 60, 90, 120, and 180 min after dextrose administration. After completion of all the blood samples for the pharmacokinetics study, mice were provided high fat diet without restriction and a cup of purified soft diet (DietGel 76A, ClearH2O, Westbrook, ME).
After study periods, mice were assessed daily for dermatitis, an anticipated adverse effect of subcutaneous dextrose injection. Cases of dermatitis were treated with nail trimming and applying dilute chlorhexidine to affected areas (Vedco, Saint Joseph, MO). Cases of dermatitis were scored for severity by using a modified version of a previously established scoring system.11 The character of lesion, length of lesion, and region(s) affected were used to calculate a score from 0 (not present) to 100 (most severe).11
Glucose monitoring and supplementation in RYGB mice.
The second phase of our investigation was a prospective interventional study to evaluate the clinical benefit of glucose supplementation after RYGB surgery. A second cohort of 16 mice (8 male, 8 female; weight, 25.5 to 44.7 g; age, 7 to 9 wk) age underwent RYGB surgery, 3 d of intensive (3 times daily) postoperative monitoring, and then daily monitoring for 3 wk. Animals were not fasted prior to surgery. RYGB was performed as a clinically translational surgery similar to the human approach, in which the stomach was reduced to approximately 15% to 20% of its original capacity, and the intestines were rerouted to reduce digesta absorption. The surgical procedure was performed by using aseptic technique in a designated surgical room. Anesthesia was induced and maintained with inhalant isoflurane (2.0% to 3.0%). Standard-release injectable meloxicam (2 mg/kg SC; Loxicom, Norbrook Laboratories, Newry, Northern Ireland) was administered preoperatively. A 2-cm vertical midline incision was made through both the skin and muscle wall. The intestines were gently exteriorized, and the jejunum was bisected at the ligament of Trietz. The jejunum–jejunum anastomosis was performed by attaching the proximal jejunum in an end-to-side anastomosis 3 cm distal from its original location. The fundus was surgically separated into 2 pouches (approximately 85% and 15%) by suturing the fundus closed from the left side of the esophagus to the greater curvature. The proximal jejunum was then anastomosed to the smaller stomach pouch (15%), just below the esophageal sphincter. Prior to closing, the peritoneal space was flushed with 100 mL sterile saline, followed by administration of enrofloxacin (20 mg/kg IP; Baytril, Bayer, Pittsburgh, PA).
Once mice were sternal and ambulatory, they were administered buprenorphine (0.1 mg/kg SC) and each returned to a cage containing ALPHA-dri bedding (Shepherd Specialty Papers, Watertown, TN), a Shepherd Shack (Shepherd Specialty Papers, Watertown, TN), and a cotton square (Ancare, Bellmore, NY) for enrichment. Sustained-release meloxicam (4 mg/kg SC; ZooPharm, Windsor, CO) was administered 4 to 5 h after surgery. Enrofloxacin (20 mg/kg SC; Baytril, Bayer) was administered daily for 2 d postoperatively, with the first injection in the right shoulder and the second in the right flank, to reduce risk of localized skin irritation. For 3 d after surgery, all cages received supplemental heat, and mice received without restriction Ensure (Abbott Laboratories, Abbott Park, IL), 1 or 2 pellets of dry 60% fat diet (TD.06414, Envigo Teklad Diets, Madison, WI), and 1 cup of purified soft diet (DietGel 76A, ClearH2O, Westbrook, ME) for 3 d. The food was changed daily, to avoid spoilage and rancidity. Once the mice no longer received supplemental heat, 60% fat diet was provided without restriction.
For each mouse, BG was measured immediately prior to surgery, at 30 min after surgery, and then 3 times daily (0800, 1200, and 1600) for 3 d. Mice with BG ≤ 60 mg/dL were randomly allocated to either the dosed (D) or nondosed (ND) groups at the first measured hypoglycemic event. Mice remained in their assigned treatment group for the duration of the study. Mice in the D group received 1 mL 5% SC dextrose after each BG measurement of ≤ 60 mg/dL; mice in the ND group did not receive supplemental dextrose, regardless of the BG level. Injection sites for D group mice were alternated between the left shoulder and left flank, to decrease injection site irritation and discomfort.
Additional observations, including assessments of pain and overall wellbeing, were made daily for the full 3-wk postoperative timeframe. Pain assessment via grimace scale19 was recorded daily for 3 d postsurgery. In brief, the grimace scoring system uses mouse facial expressions (orbital tightening, nose bulge, cheek bulge, ear position, and whisker change) to score for pain on a 3-point scale (0, not present; 1, moderate pain; and 2, severe pain), which then are added together for a total score ranging from 0 to 10.19 We used multiple observers for grimace scale scoring, to recapitulate a clinical setting. Activity assessment, fecal output, dehydration, and mortality were observed and recorded daily for 3 wk. Dehydration was treated with subcutaneous saline as needed. Cases of perianal irritation from loose stools as a result of the bariatric surgery were treated by using Desitin (Johnson and Johnson, New Brunswick, NJ). Cases of dermatitis were scored and treated as described previously. Body weights were recorded prior to surgery and subsequently every 3 d for 3 wk.
Any mice found moribund at any time during the study were euthanized through CO2 inhalation. All mice found dead or euthanized were necropsied to determine cause of death.
Statistical analysis.
BG concentrations during the pharmacokinetic study were assessed by using mixed-effects linear regression, with pairwise comparisons of treatments using Bonferroni adjustment for multiple comparisons. The trapezoidal rule was used to determine the AUC, with a Kruskal–Wallis test with adjustment for multiple comparisons to compare AUC values. A log-rank test was used to compare survival between the D and ND treatment groups. Mixed-effects ANOVA was used to compare average postoperative BG between the D and ND treatment groups. A Mann–Whitney U test was used to compare the average number of hypoglycemic episodes between the D and ND treatment groups. A Kruskal–Wallis test was used to compare grimace scale scores between treatment groups. A P value of less than 0.05 was considered significant. Statistical analyses were performed by using Stata/IC (version 13.1, StataCorp, College Station, TX) and Prism (version 8.3, GraphPad Software, La Jolla, CA).
Results
Glucose pharmacokinetic study.
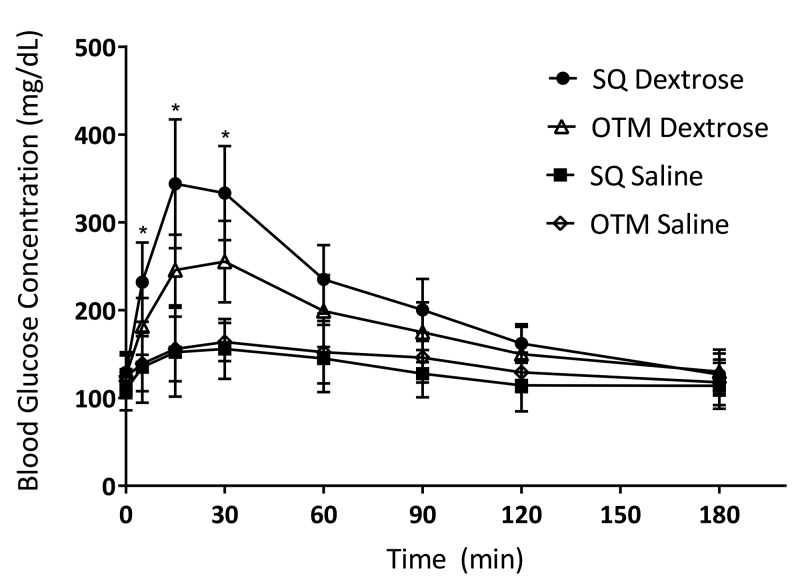
Glucose curves are illustrated in Figure 1, with pharmacokinetics parameters shown in Table 1. Baseline BG levels were not significantly different across all 4 groups (subcutaneous dextrose, OTM dextrose, subcutaneous saline, OTM saline). BG concentrations increased significantly above baseline from 5 min to 120 min after subcutaneous dextrose administration (P < 0.001 from 5 min to 90 min; P = 0.002 at 120 min), and from 5 min to 90 min after OTM dextrose administration (P < 0.001). After subcutaneous dextrose administration, peak glucose concentrations (Cmax) were reached at 15 min (343.8 ± 73.3 mg/dL) and were significantly (P = 0.023) higher than OTM Cmax peak glucose concentrations at 30 min (255.2 ± 46.5 mg/dL). Significantly higher BG was observed after subcutaneous dextrose compared with OTM dextrose between 5 and 30 min (P = 0.005 at 5 min, P < 0.001 at 15 min, P < 0.001 at 30 min). After the 30 min, BG levels declined more rapidly with subcutaneous administration than with OTM administration, with no significant differences from 60 to 180 min. The AUC0–180min for subcutaneous dextrose (38,018 ± 4656.3) was significantly (P = 0.016) higher than for OTM dextrose (33,112 ± 5748). BG levels after subcutaneous and OTM saline administration did not differ significantly different at any point during the monitoring period. All groups returned to near baseline levels by the 180 min time point. All mice tolerated all dosing events without severe complications.
Figure 1.
Blood glucose concentration (mg/dL; mean ± 1 SD) after the administration of subcutaneous or OTM administration of dextrose (50 mg) or saline. Time 0, time of administration of dextrose or saline. *, Significant (P < 0.05) difference between BG values depending on route of administration.
Table 1.
BG pharmacokinetic parameters after the administration of dextrose or saline
| Route | Baseline BG (mg/dL) | Cmax (mg/dL) | Time (min) at Cmax | BG (mg/dL) at 180 min | AUC0-180 min |
| Subcutaneous dextrose | 129 ± 20 | 344 ± 73a | 15 | 127 ± 24 | 38,018 ± 4656a |
| OTM dextrose | 126 ± 26 | 255 ± 47a | 30 | 130 ± 25 | 33,112 ± 5748a |
| Subcutaneous saline | 110 ± 24 | 156 ± 34 | 30 | 114 ± 26 | 23,449 ± 5356 |
| OTM saline | 125 ± 24 | 170 ± 20 | 30 | 100 ± 22 | 24,004 ± 4602 |
Significant (P < 0.05) differences between values after subcutaneous compared with OTM routes of administration.
Skin site reactions and dermatitis.
Two mice developed mild dermatitis at the injection site after subcutaneous dextrose administration (Table 2). Both cases were scored as level 1 (mild) and resolved after nail trimming and cleaning with dilute chlorhexidine.
Table 2.
Scoring of dermatitis after subcutaneous administration of glucose during phase 1
| Animal ID | Onset of dermatitis (no. of days after pharmacokinetics study) | Character of lesion | Length of largest lesion | Region affected | Calculated severity score (/100) | No. of days until resolution |
| 1 | 3 | Punctuate crust | <1 cm | Dorsal cervical | 33 | 4 |
| 4 | 3 | Excoriation with punctuate crust | <1 cm | Left hindquarter | 33 | 6 |
Glucose monitoring and supplementation in RYGB mice.
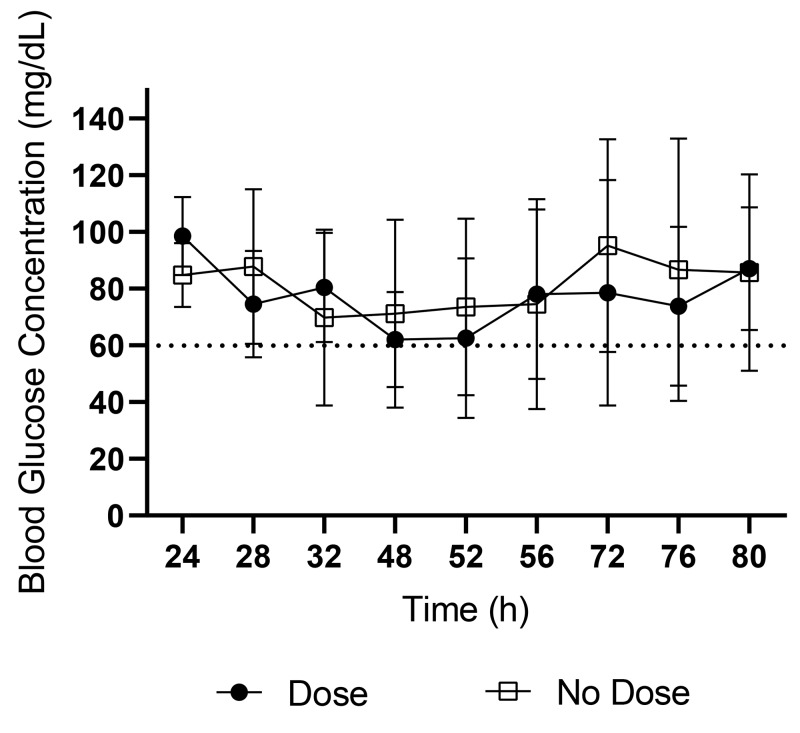
RYGB surgery was performed successfully in all 16 mice. There were no surgery-related complications in the immediate postoperative period. Several mild postoperative complications were noted during the 3-wk monitoring period, including dehydration, diarrhea, and dermatitis. During the first 3 d after surgery, 14 of the 16 mice became hypoglycemic (Table 3). Half of these mice (n = 7) were supplemented with 1 mL 5% SC dextrose when they were determined to be hypoglycemic (D group), with the other 7 receiving no supplementation (ND group). Preoperative and immediate postoperative BG concentrations were not significantly different between treatment groups. The average number of hypoglycemic episodes and average BG concentration (Figure 2) did not differ significantly between treatment groups. Two mice did not fall below the hypoglycemia threshold during the 3-d period, with their lowest BG measurements being 76 and 86 mg/dL.
Table 3.
Incidence and prevalence of hypoglycemic episodes during the 3-d glucose monitoring period after RYGB surgery
| Animal ID | Onset of hypoglycemia (no. of days after surgery) | No. of episodes of hypoglycemia |
| Dosed treatment group | ||
| 7 | 1 | 4 |
| 1 | 2 | 2 |
| 9 | 2 | 2 |
| 12 | 2 | 2 |
| 14 | 2 | 2 |
| 5 | 1 | 1 |
| 6 | 1 | 1 |
| Total | 14 | |
| No-dose treatment group | ||
| 8 | 1 | 7 |
| 10 | 1 | 3 |
| 15 | 2 | 3 |
| 2 | 2 | 2 |
| 3 | 2 | 2 |
| 11 | 1 | 2 |
| 13 | 2 | 1 |
| Total | 20 | |
| Not enrolleda | ||
| 4 | not applicable | not applicable |
| 16 | not applicable | not applicable |
Animals that failed to reach the hypoglycemia threshold (60 mg/dL) during the 3-d examination period were not enrolled in either treatment group.
Figure 2.
Average BG concentrations (mg/dL; mean ± 1 SD) for D and ND groups during the first 3 d after RYGB surgery. Time 0, time of surgery. No significant differences were present at any time point.
Pain and grimace score.
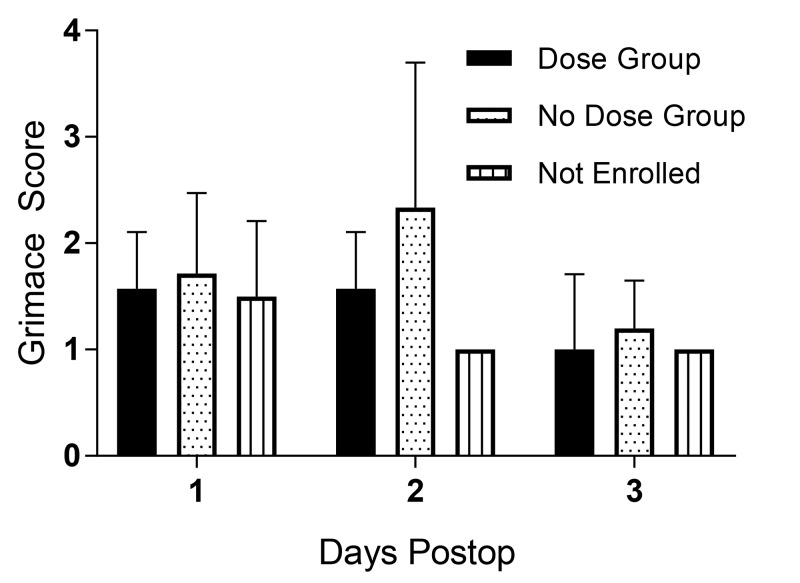
Pain was assessed daily according to the grimace score for 3 d after surgery. There was no significant difference in grimace scores between D and ND groups (Figure 3).
Figure 3.
Average grimace scores (mean ± 1 SD) for D and ND groups during the first 3 d after RYGB surgery. No significant differences were present at any time point.
Skin site reactions and dermatitis.
After surgery, 8 mice developed dermatitis (Table 4); all lesions resolved after nail trimming and cleaning with dilute chlorhexidine.
Table 4.
Scoring of dermatitis after RYGB surgery in phase 2.
| Mouse | Date of onset (postoperative day) | Character of lesion | Length of largest lesion | Region | Calculated severity score (/100) | Time (d) to resolution | |
| Dose treatment group | |||||||
| 1 | 14 | Punctuate crust | <1 cm | Dorsal cervical | 33 | 4 | |
| 7 | 6 | Multiple punctuate crusts | <1 cm | Dorsal cervical | 44 | 4 | |
| 12 | 5 | Punctuate crust | <1 cm | Dorsal cervical | 33 | 5 | |
| No-dose treatment group | |||||||
| 3 | 3 | Ulcerated | <1 cm | Dorsal cervical | 56 | 9 | |
| 4 | 4 | Punctuate crust | <1 cm | Dorsal cervical | 33 | 9 | |
| 11 | 6 | Punctuate crust | <1 cm | Left thorax | 33 | 3 | |
| 15 | 5 | Punctuate crust | <1 cm | Right thorax | 33 | 5 | |
| Not enrolleda | |||||||
| 16 | 5 | Punctuate crust | <1 cm | Dorsal cervical | 33 | 4 | |
Animals that failed to fall below the 60-mg/dL hypoglycemia threshold during the 3-d examination period were not enrolled in either treatment group.
Fecal output.
Feces were present in the cages of 12 of 16 mice the morning after surgery. By postoperative day 2, feces were present in the cages of 11 of 16 mice. By postoperative day 3, feces were present in the cages of all 13 surviving animals.
Animal body weights.
By postoperative day 3, surviving animals lost between 2.2 and 6.9 g body weight, representing 7.6% to 21.8% of their preoperative weight. At the end of the 21-d monitoring period, surviving animals had lost between 1.1 and 8.8 g body weight, representing 3.3% to 26.6% of their preoperative weight.
Survival outcomes.
Recovery from surgery was 100% (n = 16). In total, 7 of the 16 mice were either found dead (n = 3) or euthanized (n = 4) during the 3-wk postoperative monitoring period. As summarized in Table 5, only 2 deaths were attributed to hypoglycemia; the other 5 deaths were due to other causes. Hypoglycemia was implicated as the cause of death in 2 mice (1 in D group, 1 in ND group); for both mice, the last BG measurement was below the limit of detection (less than 15 mg/dL) of the glucometer. After the low BG reading, the mouse in the D group was dosed with dextrose but then was found dead 4 h later. The mouse in the ND group had been hypoglycemic for 3 consecutive time points before becoming moribund; it was therefore euthanized. Both mice died 2 d after surgery. The 9 surviving mice were euthanized and necropsied at set time points of 1, 2, and 2.5 mo after surgery.
Table 5.
Causes of postoperative death as determined through necropsy
| Before surgery | ||||||||
| Cause of death | Mouse | Weight (g) | BG (mg/dL) | Treatment group | Date of death (d after surgery) | Last BG (mg/dL); day and time | Second to last BG (mg/dL); day and time | Weight (g) prior to weight on day of death |
| Aspiration pneumonia | 13 | 30.96 | 152 | ND | 2 | 98 day 2, 1200 | 59 day 2, AM | NR |
| 14 | 31.72 | 185 | D | 4 | 61 day 3, PM | 21 day 3, 1200 | 24.81, day 3 | |
| Bedding impaction | 6 | 41.95 | 148 | D | 4 | 75 day 3, PM | 80 day 3, 1200 | 36.11, day 3 |
| Hypoglycemia | 9 | 37.33 | 192 | D | 2 | Low day 2, 1200 | 84 day 2, AM | NR |
| 10 | 42.34 | 163 | ND | 2 | Low day 2, AM | 16 day 1, PM | NR | |
| Surgery site leakage | 2 | 38.42 | 268 | ND | 10 | 60 day 3, PM | 65 day 3, 1200 | 27.08, day 9 |
| 5 | 44.67 | 154 | D | 11 | 71 day 3, PM | 76 day 3, PM | 28.94, day 11 | |
NR, not recorded
Low is a readout on the glucometer when BG is below the limit of quantification
Discussion
This study established hypoglycemia as a common and potentially fatal complication after RYGB surgery in mice. The study also characterized the pharmacokinetics profiles of 2 minimally invasive dextrose administration routes in mice to assess the efficacy of glucose supplementation in hypoglycemic RYGB mice after bariatric surgery. Considering the ease of administration, pharmacokinetic profile, and severity of side effects of each administration technique, we selected subcutaneous as the optimal supplementation route. Moreover, we identified 24 to 48 h after surgery as the most critical timeframe for the occurrence of hypoglycemia. Immediate treatment is warranted to prevent the occurrence of severe and irreversible hypoglycemia.
Typically, dextrose is administrated to rodents through oral (gavage), intraperitoneal, or intravenous routes,4 none of which would be ideal for rodents recovering from a bariatric procedure. We chose to investigate subcutaneous and OTM dextrose administration routes to avoid direct manipulation of vessels, the gastrointestinal tract, and the peritoneal cavity. Most bariatric surgeries (vertical sleeve, lap band, and RYGB) aim to significantly reduce stomach volume, thus making oral gavage less suitable. Dextrose administered in drinking water would lack dose control due to potential inappetence and reduced gut capacity and would risk possible hyperglycemia, or the animal may have inadequate dosing with reduced water intake. Intraperitoneal injection is contraindicated, because it requires inserting needles near a healing surgical site. Intravenous dextrose is typically administered through surgically implanted catheters to avoid vasculature damage in diabetic animals.27 Furthermore, intravenous administration of dextrose causes BG concentrations to spike rapidly and return to baseline quickly, typically after approximately 30 min,4 which is too short-acting for the clinical needs of postoperative RYGB rodents. The subcutaneous and OTM routes are minimally invasive and complied with our criteria for safe administration after bariatric surgery.
We determined that subcutaneous dosing was the easier, more reliable administration route. All subcutaneous doses were administered accurately, and mice received precise volumes of dextrose. Conversely, OTM dosing was unreliable and difficult to perform due to the small oral mucosal surface size of the mouse. The OTM dextrose often dripped out of the mouse's mouth or was ingested, which led to incomplete and untargeted dosing. The inability to administer the OTM dose without administering some volume orally likely affected the glucose profile. In addition, our saline cohorts established that while the handling and restraint for administration was mildly stressful for the mice and resulted in a small transient increase in BG, neither technique was significantly more stressful than the other.
We hypothesized that OTM dextrose administration would cause BG to peak earlier and have overall lower sustainability than subcutaneous administration. Transmucosal drug delivery into the bloodstream is thought to be nearly immediate, with a fast absorption, due to extensive vascularization.36 In contrast, subcutaneous tissue is much less vascular, causing slower absorption; consequently we anticipated a more sustained effect.34 Our results showed that subcutaneous administration achieved peak concentrations faster than OTM, higher BG levels, and larger AUC from 5 to 30 min. Our OTM dosing challenges explain the slower-than-anticipated timeframe to reach peak BG, given that some dextrose was ingested and some was lost. Inaccurate dosing resulting in oral absorption is not desirable due to potential insulin release and subsequent decrease in BG.4 After 30 min there were no significant differences between subcutaneous and OTM dextrose, suggesting similar sustainability. The relative similarity in sustainability of the glucose profiles suggested that both methods could be viable options for supplementation on the level of glucose uptake.
We selected subcutaneous dextrose administration as the optimal route for this study, despite the risk of tissue irritation after injection. In humans, extravasation of 10% and 50% dextrose solutions is known to cause tissue damage, edema, and necrosis.9,35 There is no literature outlining the risks of subcutaneous dextrose in rodents. Although we used a low concentration of dextrose (5%), we suspect that subcutaneous dextrose could increase risk of dermatitis given that C57BL/6 mice are predisposed to developing ulcerative dermatitis, etiologies for which are numerous but not well understood.11 In the pharmacokinetic phase of our study, only 2 mice (13%) were observed to have dermatitis, whereas in the surgery phase, 8 mice (50%) developed dermatitis. Although there may be an association between subcutaneous dextrose and dermatitis, it is difficult to determine a causative relationship or risk assessment because dermatitis in C57BL/6 mice is often multifaceted and idiopathic.11 In addition, for the surgery phase of the study, a potential effect of Baytril, meloxicam, and sustained-release Meloxicam may have influenced the emergence of dermatitis. All cases of dermatitis in our study were relatively mild and resolved without causing marked discomfort to the mice. The reliability of both the dosing technique and stability of the glucose profile suggest that minor, resolvable dermatitis is an acceptable consequence of a superior dextrose supplementation route.
This study has provided clear evidence that rodents experience severe hypoglycemia after RYGB surgery. This finding supports glucose monitoring and supplementation in postoperative RYGB mice, despite finding no significant differences between treatment group grimace scores, number of hypoglycemic episodes, or mortality. Hypoglycemia is known to cause reduced movement and other depressive-like behaviors in mice26 as well as impaired healing;32 therefore untreated hypoglycemia may cause additional discomfort. In addition, only 2 mice (1 in the D group; 1 in the ND group) died after severe hypoglycemic events, thus complicating generalization regarding the effects of dextrose supplementation. The D group mouse that died was severely hypoglycemic and agonal (BG < 15 mg/dL) prior to receiving its final dextrose dose, and the intervention was likely too late to have an effect. The remaining 7 deaths could not be directly linked to hypoglycemia. Typical fasting BG levels for mice are 63 to 130 mg/dL (fast duration of 4 to 29 h) and normal resting BG is 109 to 173 mg/dL.16 Our study's threshold for hypoglycemia (BG ≤ 60 mg/dL) was selected on the basis of the low end of the reference fasting BG level. We recommend using a higher threshold for intervention (for example, 80 mg/dL), because our threshold for hypoglycemia might have been too low to maintain sufficient and sustained BG levels yet prevent the effects of hyperglycemia. Although we administered a consistent dose of 1 mL of 5% dextrose to all animals with BG ≤ 60 mg/dL, tailoring the dose of dextrose depending on the severity of hypoglycemia may improve glycemic control. In addition, increasing the number of daily BG sampling times would allow for tighter glycemic control; however, the drawback of increased handling stress must be considered. Overall, based on our study results, we strongly recommend BG monitoring for at least 3 d after RYGB surgery in mice—even in mice that appear well at 24 h after surgery, given that they may still experience a decrease in BG on the next day. In addition, we recommend extended monitoring for animals that have not been euglycemic for at least 24 h. Although no significant differences in mortality were found in this study, intervention with subcutaneous dextrose in animals of clinical concern is strongly recommended. Further studies are needed to reach a conclusion regarding dosing regimens.
Human patients receive supportive care, dietary regimens, and intensive monitoring after surgical procedures. As a result, rapid onset hypoglycemia is usually not seen in human RYGB patients. However, the same postoperative management afforded to human patients is not often reflected in rodent models recovering from the same procedures. Our results indicate that hypoglycemia is a common adverse event in mice after RYGB, manifesting in nearly 90% of the cohort within 3 d of surgery. Major abdominal surgeries are painful, and pain is known to cause inappetence in rodents,21 which can then result in hypoglycemia. Mice have a significantly higher metabolic rate than humans, increasing the risk of hypoglycemia after relatively short periods without food.16 Some published mouse RYGB protocols include preoperative fasting,12,22 which is required for human patients to reduce the risk of aspiration pneumonia. However, rodents cannot vomit,13 making aspiration pneumonia less of an issue. Instead, preoperative fasting can leave mice particularly vulnerable to hypoglycemia.16 Our results showed that most mice became hypoglycemic on day 2 after surgery. Given that the mice were not fasted preoperatively, leftover digesta in the gastrointestinal tract may have allowed them to remain euglycemic for the first postoperative day, even when no food was actively consumed. We were unable to estimate when the mice started eating after surgery; we did not measure food intake due to concerns of inaccuracy given that, beginning immediately after recovery, animals had unrestricted access to liquid Ensure, 1 or 2 pellets of dry feed, and diet gel. We therefore recorded fecal output as a secondary indicator of feeding. Because a mouse's gastrointestinal transit time is 6 to 8 h,25 most (if not all) feed consumed preoperatively is likely to be evacuated by day 2 after surgery, despite alterations in gastrointestinal motility from surgery and analgesics. As a result, mice became vulnerable to developing hypoglycemia when they were not actively ingesting feed. By day 3, all mice had positive fecal output, indicating active consumption of food. This state likely made them at decreased risk for hypoglycemia. Interestingly, fecal output was not a useful indicator of sufficient food consumption to prevent decreased BG levels. During the 3 d after surgery, 14 of the 16 mice became hypoglycemic even though feces were observed in 12 of the animals’ cages on day 1, in 11 cages on day 2, and in all 16 cages on day 3.
To combat pain, we administered buprenorphine as analgesia. However, opioids such as buprenorphine can further exacerbate inappetence in mice by causing abdominal discomfort, constipation23 and pica despite readily available food sources.15 Buprenorphine-induced pica might have contributed to the death of the mouse that died from ingesting bedding. Clearly postoperative management is essential for both human and animal patients, and care must be taken to encourage feeding and dissuade pain.
Postoperative regimens for humans and mice are dissimilar in several ways, and closing the gap will improve translatability in rodent models. To advance the clinical care of rodents after surgery, evidence-based clinical decisions need to be guided by prospective hypothesis-driven studies, in addition to clinical case evaluations. Unlike mice, humans can articulate their need for analgesia and follow a strict diet plan after bariatric surgery,3 which can combat fluctuations in pain, appetite and resultant BG levels. Although several published RYGB protocols in mice are available, they differ widely in surgical procedure, postoperative care, and mortality rates.2,7,12,29 No previous studies have included glycemic monitoring in the postoperative period in mice, and hypoglycemia is not mentioned as a possible complication, despite the strong likelihood that it did affect (if only peripherally) the overall healing, health and survivability of the animal. We conclude that hypoglycemia in mice after RYGB is a common adverse event and potential welfare issue that can be an obstacle toward creating a robust translational model. Although our study shows no difference in survivability between treatment groups, further research with larger sample sizes is necessary. Considering that surgical mouse models are time-, labor-, and financially intensive, any improvements can be substantial, particularly those that lead to a more translatable model. Given the ease and low cost of BG measurement and dextrose supplementation as well as the clinical need for glucose management, we strongly recommend their inclusion in the mouse RYGB protocol. It is critical to identify and resolve glycemic fluctuations to have mouse models that remain translatable and robust and to ensure quality animal welfare.
Acknowledgments
We are grateful for the technical support and services provided to our research by the Mouse Biology Program (www.mousebiology.org) at the University of California Davis and Dr Philip Kass for his invaluable statistical analysis. This study was supported by the Mouse Biology Program, the Metabolic Mouse Phenotyping Center (MMPC; University of California Davis; NIH-NIDDK grant no. DK092993, RRID: SCR_015357), and the UC Davis Students Training in Advanced Research (STAR) Program (NIH STAR T35 OD010956).
References
- 1.Aldasouqi S, Sheikh A, Klosterman P, Kniestedt S, Schubert L, Danker R, Hershey DS. 2013. Hypoglycemia in patients with diabetes who are fasting for laboratory blood tests: the Cape Girardeau Hypoglycemia en route prevention program. Postgrad Med 125:136–143. 10.3810/pgm.2013.01.2629. [DOI] [PubMed] [Google Scholar]
- 2.Ayer A, Borel F, Moreau F, Prieur X, Neunlist M, Cariou B, Blanchard C, Le May C. 2017. Techniques of sleeve gastrectomy and modified Roux-en-Y gastric bypass in mice. J Vis Exp (121).1–9. 10.3791/54905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosnic G. 2014. Nutritional requirements after bariatric surgery. Crit Care Nurs Clin North Am 26:255–262. 10.1016/j.ccell.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM. 2014. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol 222:G13–G25. 10.1530/JOE-14-0182. [DOI] [PubMed] [Google Scholar]
- 5.Brady CA, Hughes D, Drobatz KJ. 2004. Association of hyponatremia and hyperglycemia with outcome in dogs with congestive heart failure. J Vet Emerg Crit Care (San Antonio) 14:177–182. 10.1111/j.1534-6935.2004.00118.x. [DOI] [Google Scholar]
- 6.Bragg D, El-Sharkawy AM, Psaltis E, Maxwell-Armstrong CA, Lobo DN. 2015. Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr 34:367–376. 10.1016/j.clnu.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Bruinsma BG, Uygun K, Yarmush ML, Saeidi N. 2015. Surgical models of Roux-en-Y gastric bypass surgery and sleeve gastrectomy in rats and mice. Nat Protoc 10:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brutsaert E, Carey M, Zonszein J. 2014. The clinical impact of inpatient hypoglycemia. J Diabetes Complications 28:565–572. 10.1016/j.jdiacomp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Gault DT. 1993. Extravasation injuries. Br J Plast Surg 46:91–96. 10.1016/0007-1226(93)90137-Z. [DOI] [PubMed] [Google Scholar]
- 10.Hackett AF, Yeung CK, Hill GL. 1979. Eating patterns in patients recovering from major surgery–a study of voluntary food intake and energy balance. Br J Surg 66:415–418. 10.1002/bjs.1800660613. [DOI] [PubMed] [Google Scholar]
- 11.Hampton AL, Hish GA, Aslam MN, Rothman ED, Bergin IL, Patterson KA, Naik M, Paruchuri T, Varani J, Rush HG. 2012. Progression of ulcerative dermatitis lesions in C57BL/6Crl mice and the development of a scoring system for dermatitis lesions. J Am Assoc Lab Anim Sci 51:586–593. [PMC free article] [PubMed] [Google Scholar]
- 12.Hao Z, Zhao Z, Berthoud HR, Ye J. 2013. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS One 8:1–9. 10.1371/journal.pone.0052922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PL. 2013. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One 8:1–16. 10.1371/journal.pone.0060537.Erratum in PLoS One 8: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 15.Jacobson C. 2000. Adverse effects on growth rates in rats caused by buprenorphine administration. Lab Anim 34:202–206. 10.1258/002367700780457509. [DOI] [PubMed] [Google Scholar]
- 16.Jensen TL, Kiersgaard MK, Sorensen DB, Mikkelsen LF. 2013. Fasting of mice: a review. Lab Anim 47:225–240. 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- 17.Kobayakawa K, Kumamaru H, Saiwai H, Kubota K, Ohkawa Y, Kishimoto J, Yokota K, Ideta R, Shiba K, Tozaki-Saitoh H, Inoue K, Iwamoto Y, Okada S. 2014. Acute hyperglycemia impairs functional improvement after spinal cord injury in mice and humans. Sci Transl Med 6:256ra137 10.1126/scitranslmed.3009430. [DOI] [PubMed] [Google Scholar]
- 18.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. 2013. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg 257:8–14. 10.1097/SLA.0b013e31827b6bbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 20.Maheandiran M, Mylvaganam S, Wu C, El-Hayek Y, Sugumar S, Hazrati L, del Campo M, Giacca A, Zhang L, Carlen PL. 2013. Severe hypoglycemia in a juvenile diabetic rat model: presence and severity of seizures are associated with mortality. PLoS One 8:1–13. 10.1371/journal.pone.0083168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer J. 2007. Use of behavior analysis to recognize pain in small mammals. Lab Anim (NY) 36:43–48. 10.1038/laban0607-43. [DOI] [PubMed] [Google Scholar]
- 22.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. 2014. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 3:191–201. 10.1016/j.molmet.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson AD, Camilleri M. 2016. Opioid-induced constipation: advances and clinical guidance. Ther Adv Chronic Dis 7:121–134. 10.1177/2040622315627801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouattara A, Lecomte P, Le Manach Y, Landi M, Jacqueminet S, Platonov I, Bonnet N, Riou B, Coriat P. 2005. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology 103:687–694. 10.1097/00000542-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Padmanabhan P, Grosse J, Asad AB, Radda GK, Golay X. 2013. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res 3:1–8. 10.1186/2191-219X-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MJ, Yoo SW, Choe BS, Dantzer R, Freund GG. 2012. Acute hypoglycemia causes depressive-like behaviors in mice. Metabolism 61:229–236. 10.1016/j.metabol.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rask-Madsen C, King GL. 2013. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17:20–33. 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg CS. 1990. Wound healing in the patient with diabetes mellitus. Nurs Clin North Am 25:247–261. [PubMed] [Google Scholar]
- 29.Seyfried F, Lannoo M, Gsell W, Tremoleda JL, Bueter M, Olbers T, Jurowich C, Germer CT, le Roux CW. 2012. Roux-en-Y gastric bypass in mice–surgical technique and characterisation. Obes Surg 22:1117–1125. 10.1007/s11695-012-0661-9. [DOI] [PubMed] [Google Scholar]
- 30.Shilling AM, Raphael J. 2008. Diabetes, hyperglycemia, and infections. Best Pract Res Clin Anaesthesiol 22:519–535. 10.1016/j.bpa.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Snell-Bergeon JK, Wadwa RP. 2012. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther 14 Suppl 1:S51–S58. 10.1089/dia.2012.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terranova A. 1991. The effects of diabetes mellitus on wound healing. Plast Surg Nurs 11:20–25. 10.1097/00006527-199121000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RE, Broussard EK, Flum DR, Wisse BE. 2016. Perioperative glycemic control during colorectal surgery. Curr Diab Rep 16:32 10.1007/s11892-016-0722-x. [DOI] [PubMed] [Google Scholar]
- 34.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 35.Wiegand R, Brown J. 2010. Hyaluronidase for the management of dextrose extravasation. Am J Emerg Med 28:257.E1–257.E2. 10.1016/j.ajem.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Zhang J, Streisand JB. 2002. Oral mucosal drug delivery: clinical pharmacokinetics and therapeutic applications. Clin Pharmacokinet 41:661–680. 10.2165/00003088-200241090-00003. [DOI] [PubMed] [Google Scholar]