Abstract
Multiple myeloma (MM) is a haematologic malignancy with significant improvements in the overall survival over the last decade. However, patients still relapse and die due to a lack of treatment options. Ultimately, novel therapies with the potential for long term remissions are needed for patients with advanced MM. Research efforts for such immune therapies were not successful until recently when the first immunotherapies for MM were approved in 2015 and many more are under development. In this review, we focus on adoptive cell therapies including CAR T-cell and CAR NK-cell therapies for patients with MM. We will provide an update on clinical and translational advances with a focus on results from ongoing clinical trials with BCMA targeted cellular therapies and the development of other novel targets, changes in the manufacturing process, trials focusing on earlier lines of therapy and combinations with other therapies as well as off the shelf products.
Keywords: Multiple Myeloma, CAR T-cell therapy, B-Cell Maturation Antigen, Chimeric Antigen Receptors, Adoptive Immunotherapy
Introduction
Multiple myeloma (MM) is a heterogenous, largely incurable haematologic malignancy and although the last decade has seen considerable improvements in treatments, there is still an unmet need for newer therapies in the relapsed refractory population(1, 2).
Patients with MM are significantly immunocompromised by the suppression of normal plasma cells and impaired immune surveillance against the MM cells as well as infections(3). Therapies that can restore anti-tumour immune effector cell function while simultaneously targeting MM cells have potential for greater efficacy. The first immunotherapies for MM were approved in 2015 with the monoclonal antibodies - daratumumab targeting CD38(4, 5) and elotuzumab targeting SLAMF7(6). More recently the field in myeloma is crowded with immune therapies that act via multiple mechanisms that include checkpoint inhibitors, antibody drug conjugates (ADCs), bispecific T cell engagers (BiTEs) and chimeric antigen receptor cells (CARs). None of these therapies are FDA approved yet but given some promising results approvals are anticipated within the next year.
CAR T-cell therapy
The adoptive transfer of antigen specific engineered T-cells combine the target specificity of monoclonal antibodies with the cytotoxicity of T-cells. These T-cells are transduced with a lentiviral or retroviral vector that carries the gene encoding a CAR, after which they are expanded manifold before they can be infused into patients. Once infused into patients, these CAR cells encounter antigen and in response release cytokines, lyse the target cells and proliferate in vivo(7).
A CAR T-cell consists of an extracellular non-MHC restricted targeting domain, usually derived from a single-chain variable fragment (scFv) of a monoclonal antibody, a spacer region, a transmembrane domain, and intracellular signalling domains including the CD3ζ activation domain and a co-stimulatory domain such as CD28 or 4-1BB(8). In MM clinical trials, most CAR constructs are derived from second generation CARs.
The effectiveness of CAR T-cell therapy is largely dependent on identifying the perfect target which is universally and exclusively expressed on cancer cells relative to normal cells to prevent on target off-tumour toxicity(9, 10). Most myeloma CAR T-cell products target B-cell maturation antigen (BCMA)(11).
B-Cell Maturation Antigen (BCMA)
BCMA, a type III transmembrane receptor, is an excellent target for immunotherapy as it is almost exclusively expressed on plasma cells compared to other immune targets such CD38 and SLAMF7(12). It is also known as tumour necrosis factor receptor superfamily member 17 (TNFRSF17) or CD269. Ligands for BCMA include A Proliferation Inducing Ligand (APRIL) and B-cell Activating Factor (BAFF) and they are produced by osteoclasts. Their interaction with BCMA induces differentiation of plasma cells and it is also involved in the pathogenesis of MM(13). Soluble BCMA is considered a marker of tumour burden and increased levels are associated with worse outcomes(14). BCMA is expressed in nearly all plasma cell neoplasms(15) however its expression is highly variable.
BCMA CAR T-cell clinical trials (table 1)
Table 1:
Summary of major BCMA CAR T-cell trials
| Trial | Dose Range | Response Rate |
VGPR or better |
PFS | CRS any grade (grade 3-4) |
Neurotoxicity any grade |
|---|---|---|---|---|---|---|
| Bb2121 (n=33) |
50-800 million cells | 85% | 72% | 11.8 months |
76% (6%) | 42% |
| JCARH125 (n=44) |
50-450 million cells |
82% | 48% | NA | 80% (9%) | 25% |
| LCAR-B38M (n=57) |
0.07 to 2.1 million cells/kg | 88% | 73% | 15 months |
90% (7%) | 2% |
| P-BCMA-101 (n=19) |
50-1143 million cells | 63% | 22% | 9.5 months |
10% (0%) | 5% |
The first anti-BCMA CAR was designed by National Cancer Institute (NCI) investigators and consisted of a murine derived scFv and a CD28 costimulatory domain transduced with a retroviral vector that showed in vivo efficacy(12). They then conducted the first-in-human phase I dose escalation clinical trial of BCMA CAR T-cells (CAR-BCMA) in relapsed refractory patients with MM with a median of 7 prior lines of therapy. The four dose levels ranged from 0.3x106 to 9x106 cells/kg. The first three dose levels did not show much toxicity or efficacy. At the highest dose level 9x106 cells/kg both toxicities and responses were seen, with the first two patients achieving a stringent CR and VGPR as well as cytokine release syndrome (CRS) and prolonged cytopenias(16). Due to this significant toxicity and the concern that the degree of CRS correlated with the tumour burden, they then limited eligibility at this highest dose level to patients with <30% bone marrow involvement. In total 16 patients with a median of 9.5 prior lines of therapy were treated with 9x106 cells/kg. They reported an ORR of 81% (≥PR) with 63% ≥VGPR and a median event-free survival of 7.1 months. There were 11 patients that responded (≥PR) and were also evaluated for minimal residual disease (MRD) status. They were all bone marrow MRD negative. At this dose, 94% patients (60% grade 3-4) developed CRS and this resolved in all patients. Peripheral blood analysis from these patients showed a peak expansion of highly differentiated CD8+ CAR-T cells 6-9 days after infusion and these higher expansion levels correlated with anti-MM responses(17) (NCT02215967).
Bluebird Bio subsequently developed an anti BCMA CAR bb2121 which incorporates a 4-1BB costimulatory domain, NCI’s murine scFv and a lentiviral vector for CAR insertion(18). This product was evaluated in a non-randomized, open label, 2-part (dose escalation - 50, 150, 450 or 800 ×106 cells and dose expansion – 150 or 450 ×106 cells) multi-site study (CRB-401) in patients with relapsed/refractory MM (≥3 prior lines of therapy)(19). Data from the first 33 patients treated at varying dose levels on this study has been published with an ORR of 85% with a median PFS of 11.8 months (table 1). All patients that had a response (≥PR) and could be evaluated for MRD (16 patients), achieved MRD negative status of ≤10−4 cells. The most common adverse events were hematologic with grade ≥3 neutropenia in 85% and thrombocytopenia and anaemia in 45%. CRS was seen in 76% (grade ≥3 = 6%) and neurologic toxic effects was seen in 42% (grade ≥3 = 3%)(19). An update presented at ASCO 2018, reported on 43 patients treated in either the dose escalation or dose expansion phase of the trial showing that patients with higher doses achieved better responses and longer duration of response (50x106 cells, n=3, ORR 33%, median duration of response (mDOR) 1.9m; 150x106 cells, n=14, ORR 57.1%, mDOR non evaluable; 450-800x106 cells, ORR 95.5%, mDOR 10.8m). They therefore defined 150-800 x106 cells as an active dose(20) (NCT02658929).
A phase II single arm open-label (KarMMa) trial to evaluate bb2121 CAR T cells further in relapsed and refractory myeloma patients worldwide is ongoing. It has an estimated enrolment of 150 patients with doses ranging from 150-450x106 CAR+ T-cells. The trial has completed enrolment in North America and Europe and is currently enrolling in Japan (NCT03361748).
The CAR products derived from preclinical work at Memorial Sloan Kettering Cancer Center (MSKCC) and now being developed by Juno/Celgene include MCARH171, JCARH125 and FCARH143. They are second generation, fully human B-cell derived scFv CAR products with a 4-1BB costimulatory domain and truncated epidermal growth factor receptor(21). MCARH171 has a different scFv and a gamma-retroviral construct while JCARH125 and FCARH143 consist of an identical CAR with a lentiviral backbone and only differ in the manufacturing process.
A single institution dose expansion phase I study of MCARH171 at MSKCC followed a standard 3+3 design and 11 patients were treated at the following dose levels: 1x106 cells/kg (n=3), 150x106 (n=3), 450x106 (n=4) and 800x106 cells (n=1). The ORR was 64% with no dose limiting toxicity and more frequent durable responses at the higher doses of ≥ 450 million CAR T-cells. Higher peak expansion was noted in the three patients with the most durable outcomes(22) (NCT03070327).
The initial data on 44 patients in an ongoing multicentre phase I/II EVOLVE study of JCARH125 was presented at ASH 2018 (table 1). These patients had been treated with a median of 7 prior therapies and 64% patients had high-risk cytogenetics. They received escalating doses 50x106 cells (n=14), 150x106 cells (n=28) and 450 x 106 cells (n=2) with ORR of 79% for the first dose level and 86% for the second dose level with an ORR of 82% (48%≥VGPR) for the cohort. In this trial the ORR of 79% at 50 x 106 cells was much higher in contrast to bb2121 where the ORR was 33% at the same dose. Responses also deepened over time in 36% over the past 4 weeks at the lowest dose. Any grade CRS was observed in 80% and neurotoxicity in 25%. Although grade ≥ 3 adverse events developed in only a minor subset of patients (7% needing ICU admission) there was one death at the highest dose level secondary to Klebsiella pneumonia sepsis(23) The trial is currently enrolling on the dose escalation phase and doses up to 800x106 cells will be considered followed by which at least 75 subjects will be treated at the recommended phase 2 dose (NCT03430011).
Data from a phase I study conducted at the University of Pennsylvania, in collaboration with Novartis with a CART-BCMA (lentiviral transduction with fully human scFv, 4-1BB costimulatory domain and CD3zeta) product in 25 patients with relapsed refractory MM (median of 7 prior lines of therapy) in 3 cohorts – cohort 1 patients received 100-500x106 cells without conditioning chemotherapy, cohort 2 received cyclophosphamide followed by 10-50x106 cells, and cohort 3 (n = 11) received cyclophosphamide followed by 100-500x106 cells was recently published(24). The dose was split over 3 days (10% on day 0, 30% on day 1 and 60% on day 2). Responses were dose dependent and ORR for cohorts 1, 2 and 3 were 44%, 20% and 64% respectively. Similar to their prior data in chronic lymphocytic leukaemia they found that a higher percentage of CD27+CD45RO-CD8+ T cells (naive and stem cell memory T cells) within the leukapheresis product were associated with greater in vivo expansion as well as clinical response(25). They also noted that responders had decreased expression of BCMA and this expression increased at progression in most patients (NCT02546167).
The LCAR-B38M CAR construct was developed by Nanjing Biotech and subsequently licensed to Janssen (table 1). It consists of two llama derived variable-heavy chain only fragments that target two epitopes of BCMA.
The phase I/II multicentre study was conducted in China with an estimated enrolment of 100 patients. Although a single study each site had its own protocol for lymphodepletion and timing of CAR T-cell administration and included conditioning with Cy 300 mg/m2 alone day −5 to −3. Patients had received a median of 3 prior lines of therapy which is less than other CARs. The median dose was 0.5x106 cells/kg (0.07-2.1x106) in 3 split doses day 1 - 20%, day 3 - 30% and day 7 - 50%. Data from 57 patients treated at a single site, The Second Affiliated Hospital of Xi’an Jiaotong University, was published in 2018(26). The ORR was 88% (75% with ongoing responses at time of publication), mDOR was 16 months (MRD negative mDOR = 22 months) and median PFS was 15 months (MRD negative CR PFS = 24 months), however median OS was not reached (MRD positive OS = 8 months, MRD negative = NR). Responses were achieved at all doses and BCMA expression did not correlate with responses. It was well tolerated, 90% had CRS (≥grade 3 CRS 7%) and one patient developed neurotoxicity(27). Although this is a multiinstitution trial each academic centre is reporting the data individually which makes it difficult to fully understand. A phase 2 study will be initiated in China (CTR2018007) in 2019.
In the United States and Europe, Janssen is conducting a multicentre phase Ib/II clinical trial of this CAR construct as JNJ-68284528 (CARTITUDE-1) which is currently enrolling, and preliminary results are anticipated shortly (NCT03548207).
Another Chinese CAR T-cell product with a retrovirus transduction 4-1BB with a murine scFv and tEGFR developed by HRAIN biotechnology is being evaluated in a multicentre study in China and prelim results from 20 patients were presented at ASH 2018. These patients had a median age of 57.5 years but had received a median of 5.5 prior lines of therapy and 45% patients with ECOG 3 and 10% with ECOG 4 were included. The patients received 9x106 cells/kg and had an ORR of 85% (1 sCR, 8 CR, 5 VGPR, 2 PR) and a median PFS of 15 months with 1 patient in CR with ongoing response at 18 months. CRS was seen in 45% (1 patient with grade 3 CRS) but no evidence of neurotoxicity. They also noted cytopenias in a majority of patients - anaemia 80%, leukopenia 75% and thrombocytopenia 65%(28) (NCT03093168). The third Chinese multicentre trial of a CAR T product CT053 developed by Carsgen therapeutics is being tested in a phase I study and 14 patients were evaluable at the time of presentation at ASH 2018. They had a median age of 55 years with majority of patients received a dose of 150x106 CAR T-cells. Although there was a short median follow up of 92 days, they reported an ORR of 100% with 5 CR, 6 VGPR and 3 PR(29) (NCT03302403, NCT03716856, NCT03380039).
Kite/Gilead also developed a fully-human anti-BCMA CAR T-cell therapy in relapsed/refractory MM called KITE-585 and a multicentre phase I, open-label, first-in-human study was underway(30, 31) (NCT03318861) when further development was halted in 2019.
A table and a figure comparing the various MM BCMA CAR products in much detail have been published in a prior review(11).
Beyond BCMA
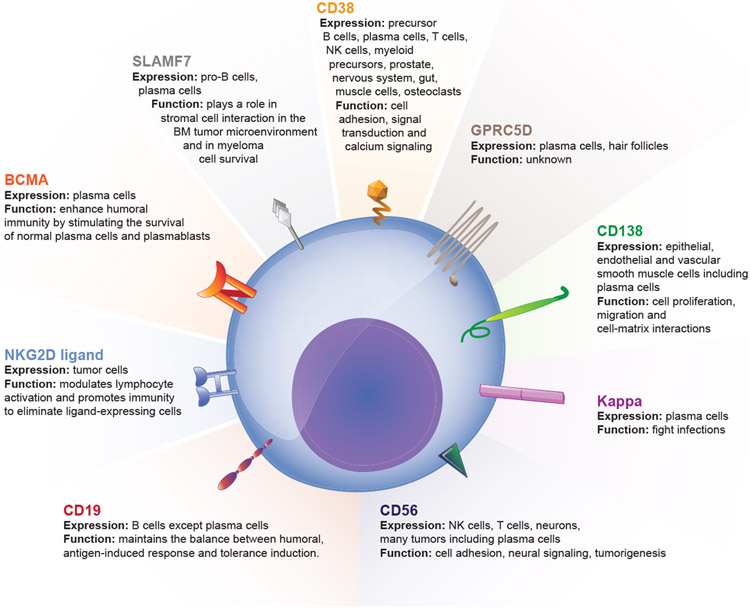
1. Other targets (figure 1)
Figure 1:
Chimeric Antigen Receptor T cell targets in Multiple Myeloma
i). G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D)
This protein is an orphan seven transmembrane G protein coupled receptor, that is highly expressed in the bone marrow with MM but not expressed on normal tissues(32) and it’s expression is associated with a poor prognosis(33). A dose escalation study of a bispecific antibody that targets CD3 and GPRC5D is already underway (NCT03399799). The first CAR T-cell clinical trial targeting this protein is scheduled to open to accrual in 2019 at MSKCC(34).
ii). CD138 (syndecan-1)
CD138 is expressed on multiple human tissues (epithelial, endothelial and vascular smooth muscle cells) including plasma cells and MM patients. Although a CD138 CAR T-cell has been studied in MM, patients had a modest response and toxicities were less than expected due to which it was hypothesized that the CAR had limited potency(35). Another trial targeting this protein is currently recruiting in the United States (NCT03672318).
iii). CD38 (ADP-Ribosyl Cyclase)
Although this glycoprotein is expressed on precursor B cells and plasma cells it is also expressed on T-cells, NK cells, myeloid precursors, prostate, nervous system, gut, muscle cells and osteoclasts(36). Despite its expression on multiple tissues, daratumumab a monoclonal antibody that targets this protein is used widely in the management of myeloma with limited side effects(4). A rational strategy for antiCD38 CAR T-cells using affinity optimization for reducing on-target off-tumour effects has been developed(37, 38). An open-label phase I single dose-escalation safety study of anti-CD38 CAR-T Cells in patients with relapsed or refractory MM is currently enrolling (NCT03464916).
iv). Signalling Lymphocytic Activation Molecule Family 7 (SLAMF7)/CD2 Subset-1(CS1)/CD319
This protein is a member of the SLAM family of transmembrane receptors that modulate the function of immune cells(39). It is expressed on pro-B cells and plasma cells especially malignant ones however its exact role in myeloma pathogenesis is unclear. Some evidence suggests it plays a role in stromal cell interaction in the BM tumour microenvironment and in myeloma cell survival. SLAMF7 CAR T-cells are effective in in vitro and in vivo models of refractory MM(40). There is an ongoing phase I trial that is currently recruiting patients targeting this receptor with an estimated enrolment of 30 patients (NCT03710421).
v). Natural Killer Group 2D (NKG2D) ligands
NK cells identify tumours using activating receptors such as NKG2D. The NKG2D ligands are found in hematologic malignancies including myelomas. CARs manufactured to express a chimeric NKG2D receptor have demonstrated preclinical efficacy in multiple tumour types by targeting the NKG2D ligands(41). Five relapsed MM patients were treated with NKG2D CARs with no responses and no dose-limiting toxicities, cytokine release syndrome, or CAR T cell–related neurotoxicity observed. Consistent with preclinical studies, NKG2D-CAR T cell–expansion and persistence were limited, and modifications are needed to boost CAR T-cell expansion and target density(42). A preclinical study showed that CAR-NKG2D activated and expanded NK cells (NKAE) cells are more effective than the CAR-NKG2D CD45RA- T cells and are the basis for development of NKG2D-CAR NK cell therapy in MM(43).
vi). CD56 (Neural Cell Adhesion Molecule 1)
CD56 is strongly expressed by malignant plasma cells in 70% of myeloma patients and CD56 targeted chimeric antigen receptor in a systemic xenograft model of myeloma showed antitumor efficacy(44).
vii). CD19
This target is absent on the dominant MM cells but maybe present on minor subsets of myeloma propagating cells that have a B cell phenotype(45). Researchers at the University of Pennsylvania hypothesized that targeting these CD19 positive myeloma propagating cells with CAR T-cells against CD19 (CTL019) may potentially lead to remissions. Ten patients with relapsed/refractory MM who had previously undergone an autologous stem cell transplantation (ASCT) with less than 1-year progression free survival (PFS) were given autologous CTL019 cells following salvage ASCT. Two of ten subjects experienced significantly prolonged PFS after CTL019 and ASCT compared to their first ASCT (remission inversion). This low response rate was thought to be due to inadequate in vivo engraftment secondary to 10-fold lower cell doses and 12-day delay between lymphodepleting chemotherapy. Another possible explanation was that these myeloma propagating cells may not be CD19 positive in all patients (46, 47).
viii). Kappa light chain
Since the paraprotein kappa is expressed exclusively on the malignant clone in kappa restricted patients, this light chain has been targeted with CAR T-cell therapy in a phase 1 trial for patients with MM or non-Hodgkin lymphoma/chronic lymphocytic leukaemia (NHL/CLL). A total of 7 patients with MM were treated and 4 had stable disease lasting 2-17 months. Not all patients received lymphodepleting chemotherapy (low dose cyclophosphamide) given some had received their myeloma specific therapy recently. The non-responders had not received low dose cyclophosphamide prior to infusion. This low overall response rate is thought to be secondary to kappa light chain being secreted and not retained on the surface of the plasma cells making this an ineffective target (48).
2. Advances in manufacturing
i). Newer transduction systems
To insert the CAR into T-cells, well-established viral transduction systems with either retroviral or lentiviral methods that are replication-incompetent and self-inactivating have been used in most CAR products to date given that they have a high transduction efficiency(49). Newer non-viral methods of transduction such as the transposon transposase piggyBac system are also being evaluated(50). This method has a shorter processing time, costs less and results in a higher number of memory stem cells (TSCM). It also has a very large cargo capacity (potentially >20x lentivirus) therefore can potentially accommodate more CARs into a CAR T-cell product in the future.
One such CAR product being studied in MM is the P-BCMA-101(51, 52). This CAR T-cell product is being studied in a phase I trial with five dose levels between 0.75x106 cells/kg up to 15x106 cells/kg. For 19 evaluable patients the ORR was 63% (100% for the 3 patients treated at the highest dose level) with a median PFS of 9.5 months(51) (NCT03288493) (table 1).
ii). Costimulatory domains
The first anti-BCMA CAR developed by the NCI as well as the KITE-585 CAR contain a CD28 costimulatory domain(17, 31). CD28 expressing CARs have higher potency and signalling kinetics however this also leads to increased cytokine production and T-cell exhaustion which in turn leads to reduced antitumor activity. Hence, most CARs in MM contain a 4-1BB costimulatory domain which has a lower signalling intensity leading to a memory cell-like phenotype and less exhaustion with improved efficacy(53, 54).
iii). Alternatives to single chain variable fragment (scFv)
An scFv is a fusion protein of the variable regions of the heavy (V H) and the light (V L) immunoglobulins that are connected by a short flexible peptide linker. Earlier designs of CAR T-cells contained a single chain variable fragment (scFv) derived from murine antibodies. Some patients developed anti-murine antibodies targeted towards the CARs that limited persistence and efficacy of reinfusions in non-Hodgkin’s lymphoma(55, 56) and renal cell carcinoma(57). Many newer CAR designs therefore contained human antibody derived scFvs(21) except for the LCAR-B38M that contains two llama derived V H only peptides(26).
As a next step to their murine CD28 CAR, NCI investigators developed a fully human variable heavy (VH) chain only (no light chain and no linker) BCMA targeted CAR(58). It is hypothesized that it will be less immunogenic, and it is being tested in a phase I trial for BCMA positive relapsed refractory MM patients with a target enrolment of 42 patients. Five dose levels ranging from 0.75x106-12x106/kg will be tested (NCT03602612).
The P-BCMA-101 CAR T-cell has another unique feature, the binding molecule is not an scFv but is a small fully human fibronectin domain (Centyrin) with high specificity binding to BCMA(52).
iv). Defined T cell ratios/populations
Most CAR T-cell studies infuse products that have an undefined ratio of CD4 to CD8 T-cells. This ratio is determined by the CD4:CD8 ratio in the sample collected during leukapheresis. Therefore, the proportion of these subsets can vary greatly at the time of infusion. Given this heterogeneity in the CAR product the optimal ratio or method of manufacturing is not known. In a hope to improve efficacy there are multiple fixed ratio CAR trials ongoing in MM, given they have already shown efficacy in adult B cell ALL patients(59).
The JCARH125 product is manufactured using a predefined CD4: CD8 ratio in the leukapheresis product prior to manufacturing(23). A single institution phase I study with separate CD4 and CD8 manufacturing followed by infusion of a defined 1:1 CD4:CD8 T cell ratio (+/−15%) of FCARH143 is currently enrolling and preliminary results for 11 patients were presented at ASH 2018(60). This ratio was achieved in 7 patients. Another unique feature of this trial is that it includes patients relapsed post-prior BCMA CAR T-cell therapy and post-allogeneic transplant, all of whom responded. Patients will be treated at - 50x106, 150x106, 450x106, 800x106 cells and responses were noted at the lowest dose. They had an ORR and MRD negativity rate at day 28 of 100% with 9 of 11 patients still in remission at the time of reporting. CRS developed in 91% patients and only 1 patient developed grade 3 CRS(61) (NCT03338972). Another phase I study by Cartesian Therapeutics is evaluating a CD8 only BCMA CAR T-cell product (Descartes-08) in MM (NCT03448978).
v). Improving CAR T-cell persistence
Although most anti-BCMA CAR trials have shown an unprecedented response rates in this relapsed and refractory population, most patients eventually relapse to this therapy as well and methods to improve CAR T-cell persistence are needed. It has been noted that this limited persistence may arise from tumour- and host- derived factors including antigen, inflammation and metabolic stress(62) as well as chronic (and in some cases, tonic) signalling of CARs(63), or their development from autologous partially or terminally exhausted patient T-cells(64-66).
To improve persistence, the next product from Bluebird Bio bb21217, contains the bb2121 CAR and the T cells are co-cultured with a PI3K inhibitor bb007. These T cells are exposed to the PI3K inhibitor during the manufacturing process to generate a product with enhanced memory-like (CD27+ and CD62L+) phenotype with the aim of improving CAR T-cell persistence in patients and thus efficacy(67). Preliminary results of 12 patients treated at the lowest CAR T-cell dose of 150x106 on the CRB-402 phase I trial were presented at ASH 2018. They had an ORR of 83% (≥VGPR 50%, ≥CR 25%) with a median follow up of 26 weeks. Side effects of CRS were seen in 67% patients and neurotoxicity in 25% patients. The estimated enrolment is up to 50 patients and as yet the data is not mature enough to determine if this PI3K inhibitor improves durability of responses(68) (NCT03274219).
vi). Faster manufacturing
CAR T-cell manufacturing is a complex multistep process that takes weeks before the cells are ready to be reinfused into the patients(69). Research into more efficient methods to produce these cells is ongoing.
3. Earlier lines of therapy
Up until now, all CAR T-cell therapies have been studied in a highly relapsed and refractory population. Given the safety and efficacy of these products’ possible areas that CAR T-cell therapies need evaluation include in the peri autologous transplant period as well as in comparison to upfront transplantation. Other trials in patients with fewer than 1-3 median prior lines of treatment would also be important to evaluate if it leads to better disease control at earlier stages of disease. The frequency of early memory T cell phenotype, CD4/CD8 ratio and the magnitude of expansion is higher in products obtained after induction compared to the relapsed/refractory setting(70). KarMMa-2 and KarMMa-3 aims to address some of these important questions.
Given the poorer outcomes with high risk patients they are also studying this product bb2121 in high risk MM with the KarMMa-2 study. It is a multi-cohort, open-label, multicentre phase II study with an estimated enrolment of 181 patients into the following cohorts - ≥ 3 prior anti-myeloma regimens (cohort 1), or with R-ISS stage III and 1 prior anti-myeloma therapy with progressive disease within 18 months, either post-autoSCT (cohort 2a), having not had prior autoSCT (cohort 2b), or having had inadequate response to initial therapy (cohort 2c). The trial is currently recruiting, and the cohorts will enrol independently in parallel (NCT03601078). KarMMa-3 is a multicentre, randomized, open-label, phase 3 study comparing the efficacy and safety of bb2121 (254 subjects) versus standard triplet regimens (127 subjects) such as daratumumab, pomalidomide, dexamethasone (DPd); daratumumab, bortezomib, and dexamethasone (DVd); or ixazomib, lenalidomide, and dexamethasone (IRd) in subjects with 2-4 lines of prior therapy (NCT03651128).
A phase 2 study of JNJ-68284528 is estimated to enrol 40 participants into two cohorts – Cohort A with progressive disease after 1-3 prior lines of therapy and cohort B with early relapse after front-line therapy (CARTITUDE-2) (NCT04133636).
4. Combination approaches
i). Combinations with other drugs
Strategies to improve ORRs and durability of responses include combining CAR T-cell therapy with standard and/or novel myeloma therapies (IMiDs, daratumumab, elotuzumab) or with therapies that may improve persistence such as immune (checkpoint inhibitors) or epigenetic modulation (HDAC inhibitors).
Lenalidomide is being tested with MCARH171 in a phase I dose escalation study(NCT03070327). Preclinical data showing that lenalidomide improves anti-myeloma properties of CS1-directed CAR T cells has been published(71).
Sequential administration of checkpoint inhibitors after CAR T-cell therapy was evaluated in 5 subjects who progressed after BCMA targeted CAR T-cell therapy were given a PD1 inhibitor (pembrolizumab based) combination as their next therapy. One of these patients had a significant CAR T-cell expansion but the response was short-lived with progression 1 week later and return to baseline CAR T-cell levels at week 5(72). Based on this observation it is plausible that a subset of patients may respond to immune modulating approaches following CAR T-cell therapy.
Patients who relapse post BCMA CAR T-cell therapy may have partial or complete downregulation of BCMA expression(17, 73) or there may be no change(22). Given this downregulation and variable BCMA expression in patients a FCARH143 BCMA CAR T-cell therapy trial with a gamma secretase inhibitor (JSMD194) that reduces BCMA cleavage and thus increases cell surface expression of BCMA on tumour cells(74) is currently enrolling (NCT03502577).
ii). CAR product combinations
Given many relapses post CAR-T cell therapies may in part be due to antigen negative disease escape, CAR combinations and dual targeting CAR-T approaches are being developed to maximize initial treatment effect and minimize the risk of relapse.
A single arm phase 2 trial in China administered a combination of humanized anti-CD19 CAR T cells (1 × 106 cells per kg) and murine anti-BCMA CAR T cells (1 × 106 cells per kg) to 21 patients. They had an ORR of 95% although there was 1 death related to cerebral haemorrhage from sustained thrombocytopenia(75). University of Pennsylvania is also currently recruiting for a clinical trial involving two separate CAR products - CART-BCMA with or without huCART19 upfront in high-risk MM (NCT03549442).
iii). Dual targeting CARs
Preclinical data from an APRIL ligand-based dual targeting CAR (as opposed to an scFv) for binding BCMA and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) showed in vitro and in vivo efficacy(76). Based on this data Autolus Limited is conducting a multicentre phase I/II trial of this dual targeting CAR (AUTO2) with an estimated enrolment of 80 patients in Europe (NCT03287804). Similar preclinical in vivo studies of dual targeting CAR T-cells against the antigens BCMA and CS1(77) as well as BCMA and GPRC5D(78) showed superior in vivo survival.
Other dual targeting trials underway in China include - CD19 and BCMA dual targeting CARs (NCT03455972, NCT03767725), CD138 and BCMA targeting (NCT03196414) and CD38 and BCMA targeting (NCT03767751). Multi CAR T-cell therapy in which T-cells are genetically modified to specifically target several MM surface antigens, including BCMA, CD38, CD56, CD138 or alternative MM surface antigens is also being tested (NCT03271632).
5. Off the shelf/allogeneic CARs
Until now, CAR T-cells have been autologous products where a patient’s own T cells are collected and reinfused back after CAR insertion in the laboratory. More recently, allogeneic CAR T-cell products from healthy donors that do not require HLA matching are being manufactured with an attempt to make off the shelf products so that the cost and manufacturing delays with individualization of these products can be avoided. Given T-cell dysfunction in a cancer patient is also a potential issue leading sub optimal CAR products, allogeneic products may enhance functionality(79). Although this therapy seems promising there are numerous challenges that may need to be overcome including graft vs host disease, host T cell vs CAR, host NK cell vs CAR related side effects.
P-BCMA-ALLO1, an allogeneic product with the same inherent properties and functions as P-BCMA-101 but with the added advantages of treatment efficiency and ability to scale more rapidly to the needs of patients will be evaluated in a phase I clinical trial that should open shortly by Poseida therapeutics(51), but with the benefits of scale and administration efficiency that come from an allogeneic product. Preclinical data from Allogene regarding their allogeneic CAR T-cell that induced sustained in vitro and in vivo responses was recently published(80) and the clinical trial will also open shortly. Precision Biosciences, a genome editing company are developing off the shelf CAR therapies as well(81, 82).
CAR NK-cell therapy
Natural killer (NK) cells are critical to the innate immune system and are also a part of the adaptive immune system. They can recognize stressed cells in the absence of antibodies and major histocompatibility complex (MHC) molecules leading to a much faster immune response.
CAR NK-cell therapy is not limited by autologous manufacturing since it is not HLA restricted and can therefore be produced from NK cell lines (most often NK92), umbilical cord blood or induced pluripotent stem cells (iPSCs)(83). CAR NK-cells have only been recently brought to clinical trials(83, 84) given some inherent advantages such as off the shelf products, reduced toxicity and cost over CAR T-cells (table 2). The first CAR NK cell trial to open in the United States targeting CD19(85) is currently enrolling with a target accrual of 36 patients (NCT03056339).
Table 2:
| CAR T-cell therapy | CAR NK-cell therapy | |
|---|---|---|
| Data currently available | Clinical data | Preclinical data |
| In-vivo persistence | Better | Worse |
| CRS/Cytopenia | More likely | Less likely |
| Viral transduction efficiency | Higher | Lower |
| Allogeneic/off-the-shelf product | Risk of GVHD if allogeneic | No risk of GVHD |
| Tumour recognition | Solely dependent on CAR | Multiple mechanisms including native receptor and CAR |
| CAR antigen downregulation | More likely | Less likely |
In multiple myeloma, preclinical data showing the genetic modification of NK-92MI cells with an anti-CD138 CAR shows enhanced cytotoxicity(86) and a viral construct of a CS1-specific CAR expressed in human NK-92 cells significantly suppressed the growth and prolonged survival in xenograft models(87). Preclinical data shows that NKG2D-CAR NK cells and BCMA-CAR NK cells are equally efficient to eradicate diverse MM cells(88).
A trial of 20 patients with relapsed/refractory MM with BCMA expression has begun enrolling in China in 2019 and patients will be treated with BCMA CAR NK-92 cells developed by Asclepius Technology Company Group (Suzhou) Co., Ltd (NCT03940833)
Summary
Although CAR T-cell therapies have generated a lot of enthusiasm in the myeloma community it must be tempered by the fact that most remissions are not cures and patients eventually relapse. Researchers are working via multiple avenues and pathways to improve this therapy given research in this field is still nascent. Numerous issues such as cost, competition from other immunotherapies (such as bispecific antibodies and antibody drug conjugates) that may have added advantages such as ease of administration with outpatient intravenous or subcutaneous dosing may pose a threat to the widespread use of CAR T-cell therapies. Although given the chronic and heterogeneous nature of this disease one size does not fit all and there will likely be a population of patients who will benefit from CAR based therapies. Currently however, the data is too premature to draw conclusions about the long-term use, but these therapies offer hope that myeloma patients will have more treatment options.
Practice Points.
Immunotherapy shows promise for patients with multiple myeloma and BCMA appears to be the most promising target currently.
The ongoing BCMA CAR T-cell trials report high overall response rates of >80%.
Cytokine release syndrome with BCMA targeted CAR T-cell therapies although common is usually manageable and serious neurotoxicity is uncommon.
Research Agenda.
Although response rates are unprecedented in this highly refractory and relapsed population the median PFS is ≤ 12 months therefore improvements in CAR T-cell persistence and duration of responses are needed.
Randomized clinical trials comparing CAR T-cells in earlier lines of therapy with other standard chemotherapy or immunotherapy combinations are ongoing.
CAR targets other than BCMA are also being evaluated and additional targets are needed.
Off the shelf CAR products such as allogeneic CAR T-cells and CAR NK-cells are areas of active research.
Acknowledgements
Not applicable
Footnotes
Conflict of Interest Statement
Urvi Shah has received research support from Celgene and honoraria from Physicians’ Education Resource. Sham Mailankody has received grant support from National Cancer Institute, research support from Takeda Oncology, Juno/Celgene, Janssen and Allogene Therapeutics and honoraria from Physicians’ Education Resource.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, et al. Trends in overall survival and costs of multiple myeloma, 2000-2014. Leukemia. 2017;31(9):1915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Anderson KC. Immune Therapies in Multiple Myeloma. Clin Cancer Res. 2016;22(22):5453–60. [DOI] [PubMed] [Google Scholar]
- 4.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–60. [DOI] [PubMed] [Google Scholar]
- 5.Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. New England Journal of Medicine. 2015;373(7):621–31. [DOI] [PubMed] [Google Scholar]
- 7.Ma T, Shi J, Liu H. Chimeric antigen receptor T cell targeting B cell maturation antigen immunotherapy is promising for multiple myeloma. Ann Hematol. 2019. [DOI] [PMC free article] [PubMed]
- 8.Ghosh A, Mailankody S, Giralt SA, Landgren CO, Smith EL, Brentjens RJ. CAR T cell therapy for multiple myeloma: where are we now and where are we headed? Leuk Lymphoma. 2018;59(9):2056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. The Journal of Gene Medicine. 2012;14(6):405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah UA, Smith EL. Multiple Myeloma, Targeting B-Cell Maturation Antigen With Chimeric Antigen Receptor T-Cells. Cancer J. 2019;25(3):208–16. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hengeveld PJ, Kersten MJ. B-cell activating factor in the pathophysiology of multiple myeloma: a target for therapy? Blood Cancer J. 2015;5:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho SF, Anderson KC, Tai YT. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front Immunol. 2018;9:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khattar P, Pichardo J, Jungbluth A, Gao Q, Smith EL, Roshal M, et al. B- Cell Maturation Antigen Is Exclusively Expressed in a Wide Range of B-Cell and Plasma Cell Neoplasm and in a Potential Therapeutic Target for Bcma Directed Therapies. Blood. 2017;130(Suppl 1):2755-. [Google Scholar]
- 16.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. Journal of Clinical Oncology. 2018;36(22):2267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, et al. Effective Targeting of Multiple B-Cell Maturation Antigen-Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum Gene Ther. 2018;29(5):585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380(18):1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raje NS, Berdeja JG, Lin Y, Munshi NC, Siegel DSD, Liedtke M, et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: Updated results from a multicentre phase I study. Journal of Clinical Oncology. 2018;36(15_suppl):8007-. [Google Scholar]
- 21.Smith EL, Staehr M, Masakayan R, Tatake IJ, Purdon TJ, Wang X, et al. Development and Evaluation of an Optimal Human Single-Chain Variable Fragment-Derived BCMA-Targeted CAR T Cell Vector. Mol Ther. 2018;26(6):1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mailankody S, Ghosh A, Staehr M, Purdon TJ, Roshal M, Halton E, et al. Clinical Responses and Pharmacokinetics of MCARH171, a Human-Derived Bcma Targeted CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma: Final Results of a Phase I Clinical Trial. Blood. 2018;132(Suppl 1):959-. [Google Scholar]
- 23.Mailankody S, Htut M, Lee KP, Bensinger W, Devries T, Piasecki J, et al. JCARH125, Anti-BCMA CAR T-cell Therapy for Relapsed/Refractory Multiple Myeloma: Initial Proof of Concept Results from a Phase 1/2 Multicenter Study (EVOLVE). Blood. 2018;132(Suppl 1):957-. [Google Scholar]
- 24.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukaemia. Nat Med. 2018;24(5):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao W-H, Liu J, Wang B-Y, Chen Y-X, Cao X-M, Yang Y, et al. Updated Analysis of a Phase 1, Open-Label Study of LCAR-B38M, a Chimeric Antigen Receptor T Cell Therapy Directed Against B-Cell Maturation Antigen, in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2018;132(Suppl 1):955-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Chen Z, Fang H, Wei R, Yu K, Jiang S, et al. Durable Remission Achieved from Bcma-Directed CAR-T Therapy Against Relapsed or Refractory Multiple Myeloma. Blood. 2018;132(Suppl 1):956-. [Google Scholar]
- 29.Jiang S, Jin J, Hao S, Yang M, Chen L, Ruan H, et al. Low Dose of Human scFv-Derived BCMA-Targeted CAR-T Cells Achieved Fast Response and High Complete Remission in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2018;132(Suppl 1):960-. [Google Scholar]
- 30.Adams GB, Feng J, Ghogha A, Mardiros A, Murakami J, Phung T, et al. Abstract 4979: Development of KITE-585: A fully human BCMA CAR T-cell therapy for the treatment of multiple myeloma. Cancer Research. 2017;77(13 Supplement):4979-. [Google Scholar]
- 31.Cornell RF, Locke FL, Bishop MR, Orlowski RZ, Larson SM, Borrello I, et al. A phase 1 multicentre study evaluating KITE-585, an autologous anti-BCMA CAR T-cell therapy, in patients with relapsed/refractory multiple myeloma. Journal of Clinical Oncology. 2018;36(15_suppl):TPS3103–TPS. [Google Scholar]
- 32.Cohen Y, Gutwein O, Garach-Jehoshua O, Bar-Haim A, Kornberg A. GPRC5D is a promising marker for monitoring the tumour load and to target multiple myeloma cells. Hematology. 2013;18(6):348–51. [DOI] [PubMed] [Google Scholar]
- 33.Atamaniuk J, Gleiss A, Porpaczy E, Kainz B, Grunt TW, Raderer M, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur J Clin Invest. 2012;42(9):953–60. [DOI] [PubMed] [Google Scholar]
- 34.Smith EL, Harrington K, Staehr M, Masakayan R, Jones J, Long TJ, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Science Translational Medicine. 2019;11(485):eaau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bo Guo MC, Qingwang Han, 1, Fan Hui, Hanren Dai, Wenying Zhang, Yajing Zhang, Yao Wang, Hongli Zhu, Weidong Han. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)- modified T cells for multiple myeloma. Journal of Cellular Immunotherapy. 2016. [Google Scholar]
- 36.Deaglio S, Mehta K, Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk Res. 2001;25(1):1–12. [DOI] [PubMed] [Google Scholar]
- 37.Drent E, Groen RW, Noort WA, Themeli M, Lammerts van Bueren JJ, Parren PW, et al. Pre- clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica. 2016;101(5):616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drent E, Themeli M, Poels R, de Jong-Korlaar R, Yuan H, de Bruijn J, et al. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol Ther. 2017;25(8):1946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malaer JD, Mathew PA. CS1 (SLAMF7, CD319) is an effective immunotherapeutic target for multiple myeloma. Am J Cancer Res. 2017;7(8):1637–41. [PMC free article] [PubMed] [Google Scholar]
- 40.Gogishvili T, Danhof S, Prommersberger S, Rydzek J, Schreder M, Brede C, et al. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7(+) normal lymphocytes. Blood. 2017;130(26):2838–47. [DOI] [PubMed] [Google Scholar]
- 41.Murad JM, Baumeister SH, Werner L, Daley H, Trebeden-Negre H, Reder J, et al. Manufacturing development and clinical production of NKG2D chimeric antigen receptor-expressing T cells for autologous adoptive cell therapy. Cytotherapy. 2018;20(7):952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol Res. 2019;7(1):100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leivas A, Rio P, Mateos R, Paciello ML, Garcia-Ortiz A, Fernandez L, et al. NKG2D-CAR Transduced Primary Natural Killer Cells Efficiently Target Multiple Myeloma Cells. Blood. 2018;132(Supplement 1):590-. [Google Scholar]
- 44.Benjamin R, Condomines M, Gunset G, Sadelain M. Abstract 3499: CD56 targeted chimeric antigen receptors for immunotherapy of multiple myeloma. 2012;72(8 Supplement):3499-. [Google Scholar]
- 45.Nerreter T, Letschert S, Gotz R, Doose S, Danhof S, Einsele H, et al. Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat Commun. 2019;10(1):3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373(11):1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight. 2019;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. The Journal of Clinical Investigation. 2016;126(7):2588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooray S, Howe SJ, Thrasher AJ. Retrovirus and lentivirus vector design and methods of cell conditioning. Methods Enzymol. 2012;507:29–57. [DOI] [PubMed] [Google Scholar]
- 50.Vargas JE, Chicaybam L, Stein RT, Tanuri A, Delgado-Canedo A, Bonamino MH. Retroviral vectors and transposons for stable gene therapy: advances, current challenges and perspectives. J Transl Med. 2016;14(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregory T, Cohen AD, Costello CL, Ali SA, Berdeja JG, Ostertag EM, et al. Efficacy and Safety of P- Bcma-101 CAR-T Cells in Patients with Relapsed/Refractory (r/r) Multiple Myeloma (MM). Blood. 2018;132(Suppl 1):1012-. [Google Scholar]
- 52.Hermanson DL, Barnett BE, Rengarajan S, Codde R, Wang X, Tan Y, et al. A Novel Bcma-Specific, Centyrin-Based CAR-T Product for the Treatment of Multiple Myeloma. Blood. 2016;128(22):2127-. [Google Scholar]
- 53.Salter AI, Ivey RG, Kennedy JJ, Voillet V, Rajan A, Alderman EJ, et al. Phosphoproteomic analysis of chimeric antigen receptor signalling reveals kinetic and quantitative differences that affect cell function. Sci Signal. 2018;11(544). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sommermeyer D, Hill T, Shamah SM, Salter AI, Chen Y, Mohler KM, et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia. 2017;31(10):2191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117(1):72–82. [DOI] [PubMed] [Google Scholar]
- 58.Lam N, Alabanza L, Trinklein N, Buelow B, Kochenderfer JN. T Cells Expressing Anti-B-Cell Maturation Antigen (BCMA) Chimeric Antigen Receptors with Antigen Recognition Domains Made up of Only Single Human Heavy Chain Variable Domains Specifically Recognize Bcma and Eradicate Tumors in Mice. Blood. 2017;130(Suppl 1):504-. [Google Scholar]
- 59.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee DH, Cervantes-Contreras F, Lee SY, Green DJ, Till BG. Improved Expansion and Function of CAR T Cell Products from Cultures Initiated at Defined CD4:CD8 Ratios. Blood. 2018;132(Suppl 1):3334-. [Google Scholar]
- 61.Green DJ, Pont M, Sather BD, Cowan AJ, Turtle CJ, Till BG, et al. Fully Human Bcma Targeted Chimeric Antigen Receptor T Cells Administered in a Defined Composition Demonstrate Potency at Low Doses in Advanced Stage High Risk Multiple Myeloma. Blood. 2018;132(Suppl 1):1011-. [Google Scholar]
- 62.Delgoffe GM, Powell JD. Feeding an army: The metabolism of T cells in activation, anergy, and exhaustion. Mol Immunol. 2015;68(2 Pt C):492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ajina A, Maher J. Strategies to Address Chimeric Antigen Receptor Tonic Signalling. Mol Cancer Ther. 2018;17(9):1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545(7655):452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghoneim HE, Zamora AE, Thomas PG, Youngblood BA. Cell-Intrinsic Barriers of T Cell-Based Immunotherapy. Trends Mol Med. 2016;22(12):1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klebanoff CA, Crompton JG, Leonardi AJ, Yamamoto TN, Chandran SS, Eil RL, et al. Inhibition of AKT signalling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah N, Alsina M, Siegel DS, Jagannath S, Madduri D, Kaufman JL, et al. Initial Results from a Phase 1 Clinical Study of bb21217, a Next-Generation Anti Bcma CAR T Therapy. Blood. 2018;132(Suppl 1):488-. [Google Scholar]
- 69.Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dancy E, Garfall AL, Cohen AD, Fraietta JA, Davis M, Levine BL, et al. Clinical Predictors of T Cell Fitness for CAR T Cell Manufacturing and Efficacy in Multiple Myeloma. Blood. 2018;132(Supplement 1):1886-. [Google Scholar]
- 71.Wang X, Walter M, Urak R, Weng L, Huynh C, Lim L, et al. Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor-Redirected T Cells Against Multiple Myeloma. Clin Cancer Res. 2018;24(1):106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernabei L, Garfall AL, Melenhorst JJ, Lacey SF, Stadtmauer EA, Vogl DT, et al. PD-1 Inhibitor Combinations As Salvage Therapy for Relapsed/Refractory Multiple Myeloma (MM) Patients Progressing after Bcma-Directed CAR T Cells. Blood. 2018;132(Suppl 1):1973-. [Google Scholar]
- 73.Cohen AD, Garfall AL, Stadtmauer EA, Lacey SF, Lancaster E, Vogl DT, et al. Safety and Efficacy of B-Cell Maturation Antigen (BCMA)-Specific Chimeric Antigen Receptor T Cells (CART-BCMA) with Cyclophosphamide Conditioning for Refractory Multiple Myeloma (MM). Blood. 2017;130(Suppl 1):505-. [Google Scholar]
- 74.Pont MJ, Hill T, Cole GO, Abbott JJ, Kelliher J, Salter AI, et al. gamma-secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019. [DOI] [PMC free article] [PubMed]
- 75.Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019;6(10):e521–e9. [DOI] [PubMed] [Google Scholar]
- 76.Lee L, Draper B, Chaplin N, Philip B, Chin M, Galas-Filipowicz D, et al. An APRIL-based chimeric antigen receptor for dual targeting of BCMA and TACI in multiple myeloma. Blood. 2018;131(7):746–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen KH, Wada M, Pinz KG, Liu H, Shuai X, Chen X, et al. A compound chimeric antigen receptor strategy for targeting multiple myeloma. Leukemia. 2018;32(2):402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernandez de Larrea C, Staehr M, Lopez A, Chen Y, Purdon TJ, Ng KY, et al. Optimal Dual- Targeted CAR Construct Simultaneously Targeting Bcma and GPRC5D Prevents Bcma-Escape Driven Relapse in Multiple Myeloma Blood. 2019. [DOI] [PMC free article] [PubMed]
- 79.Graham C, Jozwik A, Pepper A, Benjamin R. Allogeneic CAR-T Cells: More than Ease of Access? Cells. 2018;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sommer C, Boldajipour B, Kuo TC, Bentley T, Sutton J, Chen A, et al. Preclinical Evaluation of Allogeneic CAR T Cells Targeting BCMA for the Treatment of Multiple Myeloma. Mol Ther. 2019;27(6):1126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCreedy BJ, Senyukov VV, Nguyen KT. Off the shelf T cell therapies for hematologic malignancies. Best Pract Res Clin Haematol. 2018;31(2):166–75. [DOI] [PubMed] [Google Scholar]
- 82.MacLeod DT, Antony J, Martin AJ, Moser RJ, Hekele A, Wetzel KJ, et al. Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells. Mol Ther. 2017;25(4):949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rezvani K Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transplant. 2019;54(Suppl 2):785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther. 2017;25(8):1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8(2):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28(4):917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maroto-Martin E, Encinas J, Garcia-Ortiz A, Alonso R, Leivas A, Paciello ML, et al. NKG2D and BCMA-CAR NK Cells Efficiently Eliminate Multiple Myeloma Cells. A Comprehensive Comparison Between Two Clinically Relevant CARs. European Hematology Association Library. 2019.