SUMMARY
The molecular mechanisms that govern the maturation of oligodendrocyte lineage cells remain unclear. Emerging studies have shown that N6-methyladenosine (m6A), the most common internal RNA modification of mammalian mRNA, plays a critical role in various developmental processes. Here, we demonstrate that oligodendrocyte lineage progression is accompanied by dynamic changes in m6A modification on numerous transcripts. In vivo conditional inactivation of an essential m6A writer component, METTL14, results in decreased oligodendrocyte numbers and CNS hypomyelination, although oligodendrocyte precursor cell (OPC) numbers are normal. In vitro Mettl14 ablation disrupts post-mitotic oligodendrocyte maturation and has distinct effects on OPC and oligodendrocyte transcriptomes. Moreover, the loss of Mettl14 in oligodendrocyte lineage cells causes aberrant splicing of myriad RNA transcripts, including that which encodes the essential paranodal component neurofascin 155 (NF155). Together, our findings indicate that dynamic RNA methylation plays an important regulatory role in oligodendrocyte development and CNS myelination.
Keywords: Mettl14, m(6)A, mRNA methylation, RNA epigenetic regulation, oligodendrocyte precursor cells, oligodendrocytes, oligodendrocyte development, alternative splicing, NF155
eTOC Blurb:
Xu et. al. show that oligodendrocyte development is associated with dynamic changes in posttranscriptional mRNA methylation. Moreover, they demonstrate that the m6A epigenetic RNA mark has considerable impact on the myelinating cell’s transcriptome and is essential for normal CNS myelination.
INTRODUCTION
Oligodendrocytes are glial cells in the CNS that are responsible for myelination of axons, which allows for rapid saltatory conduction (Nave and Werner, 2014; Simons and Nave, 2015). Oligodendrocytes develop from oligodendrocyte precursor cells (OPCs), which originate from discrete regions of the embryonic neural tube (Rowitch, 2004). To become mature myelinating oligodendrocytes, OPCs first exit the proliferation state and differentiate into pre-myelinating oligodendrocytes, resulting in the expression of major myelinating proteins such as myelin basic protein (MBP) and proteolipid protein (PLP). A series of morphological changes then allows these oligodendrocytes to extend a number of processes that wrap axons with the multilayered myelin sheath (Zuchero and Barres, 2015). Elucidating the key events involved in oligodendrocyte lineage progression is critical to understand the cellular and developmental biology of myelin production and regeneration.
Following a defined series of steps, oligodendrocyte lineage progression is tightly controlled in time and space (Liu et al., 2016). The exact mechanism by which oligodendrocyte lineage progression is regulated, however, has yet to be fully elucidated. A number of intrinsic and extrinsic factors have been found to be critical for regulating oligodendrocyte development. Growth factors, such as Sonic Hedgehog (SHH), bone morphogenetic proteins (BMPs), and platelet-derived growth factor (PDGF), have been shown to influence the maturation of oligodendrocyte lineage cells from early progenitors to mature, myelinating cells (Nishiyama et al., 2009). Transcription factors, such as Nkx-2.2, Olig1, Olig2, Sox10, Myrf and ZFP24, are required for maturation of oligodendrocytes (Elbaz and Popko, 2019; Mitew et al., 2014). In addition, epigenetic mechanisms including chromatin remodeling by DNA methylation, histone deacetylases, and gene silencing by non-coding RNAs have been shown to play critical roles in oligodendrocyte differentiation and function during development and remyelination (Li and Richardson, 2009; Marin-Husstege et al., 2002; Moyon and Casaccia, 2017; Ye et al., 2009; Zhao et al., 2010b).
Although reversible chemical modification of DNA and histone proteins is known to influence gene expression and a multitude of biological processes, a similar role for the chemical modification of RNA has only recently been identified (Fu et al., 2014). The discovery of reversible N6-methyladenosine (m6A) mRNA methylation has revealed a new dimension of post-transcriptional regulation of gene expression (Yue et al., 2015). Emerging studies have demonstrated this m6A “mark” influences various aspects of mRNA metabolism, including stability, translation, localization, and splicing (Roundtree et al., 2017; Wang et al., 2014; Xiao et al., 2016; Zhao et al., 2014; Zhou et al., 2018). By controlling the turnover and/or translation of transcripts during cell-state transitions, m6A modification of mRNA plays key regulatory roles during embryonic and adult stem cell differentiation (Frye et al., 2018). Recent studies have highlighted the function of m6A in lineage fate decisions during cell development, such as embryonic stem cell pluripotency exit, T cell differentiation, hematopoietic fate transition, and gametogenesis (Batista et al., 2014; Geula et al., 2015; Ivanova et al., 2017; Li et al., 2017; Weng et al., 2018b; Xu et al., 2017; Zhang et al., 2017). Importantly, a recent study in neural stem cells revealed that conditional inactivation of the gene that encodes METTL14, a core component of the m6A methyltransferase complex (Liu et al., 2014), disrupts cortical neurogenesis (Yoon et al., 2017), thus revealing a critical role of the m6A mark in CNS neuronal development.
In this study, we sought to elucidate the role that m6A mRNA methylation plays in oligodendrocyte lineage progression by conditionally inactivating the Mettl14 gene specifically in these cells using a Mettl14 conditional (floxed) mouse line in combination with oligodendrocyte Cre driver lines. In vivo, we found myelin abnormalities and altered oligodendrocyte numbers in the Mettl14 mutants. Despite these findings, OPC numbers were not affected. In vitro, OPCs lacking Mettl14 did not properly differentiate into mature oligodendrocytes, suggesting that m6A plays a critical role in oligodendrocyte differentiation. RNA-seq and m6A -seq revealed that OPC and oligodendrocyte transcripts encoding transcription factors, DNA epigenetic regulators and signaling pathways that are critical for oligodendrocyte lineage progression were m6A marked, and differentially affected by the Mettl14 deletion. We also found pervasive, aberrant mRNA splicing in the Mettl14-deleted OPCs and oligodendrocytes. Importantly, we discovered that the critical paranode component, NF155, is differentially spliced and significantly disrupted during myelination in the Mettl14 ablated mutants.
RESULTS
Oligodendrocyte lineage progression is accompanied by changes in m6A modification on numerous transcripts
To characterize changes of the m6A mark and its role in gene expression during oligodendrocyte lineage progression, we performed m6A-seq and RNA-seq on both purified OPCs and mature, cultured oligodendrocytes. Using an immunopanning approach (Emery and Dugas, 2013), we purified OPCs from neonatal mouse pups. These cells were maintained under proliferating conditions with the addition of the OPC mitogen PDGF-AA. We obtained mature oligodendrocytes by promoting OPC differentiation via removal of PDGF-AA and addition of the T3 hormone to the culture media (Fig.1 A). SMART2 single cell RNA-seq was used for m6A mRNA profiling (Picelli et al., 2014; Weng et al., 2018b), which detected 3,554 m6A marked transcripts in OPCs and 2606 m6A marked transcripts in oligodendrocytes. Gene ontology analyses indicated that these m6A marked transcripts have important functions for cell development in both OPCs (Fig.1 B) and oligodendrocytes (Fig.1 C). The m6A-seq data also revealed transcripts present in both OPCs and oligodendrocytes that were differentially marked by m6A, demonstrating the dynamic nature of this mRNA modification. We found 2,806 transcripts with the m6A mark in OPCs that were present but not marked in oligodendrocytes (Fig.1 D), and 1,626 transcripts that possessed the m6A mark in oligodendrocytes but not in OPCs (Fig.1 E). Only 23 of the shared transcripts (Fig.1 F), showed the m6A mark in both OPCs and oligodendrocytes. The dynamic nature of the m6A mark in oligodendrocyte lineage cells suggests that it may play an important role in regulating oligodendrocyte differentiation and CNS myelination.
Figure 1. Oligodendrocyte lineage progression is accompanied by changes in m6A modification on numerous transcripts.
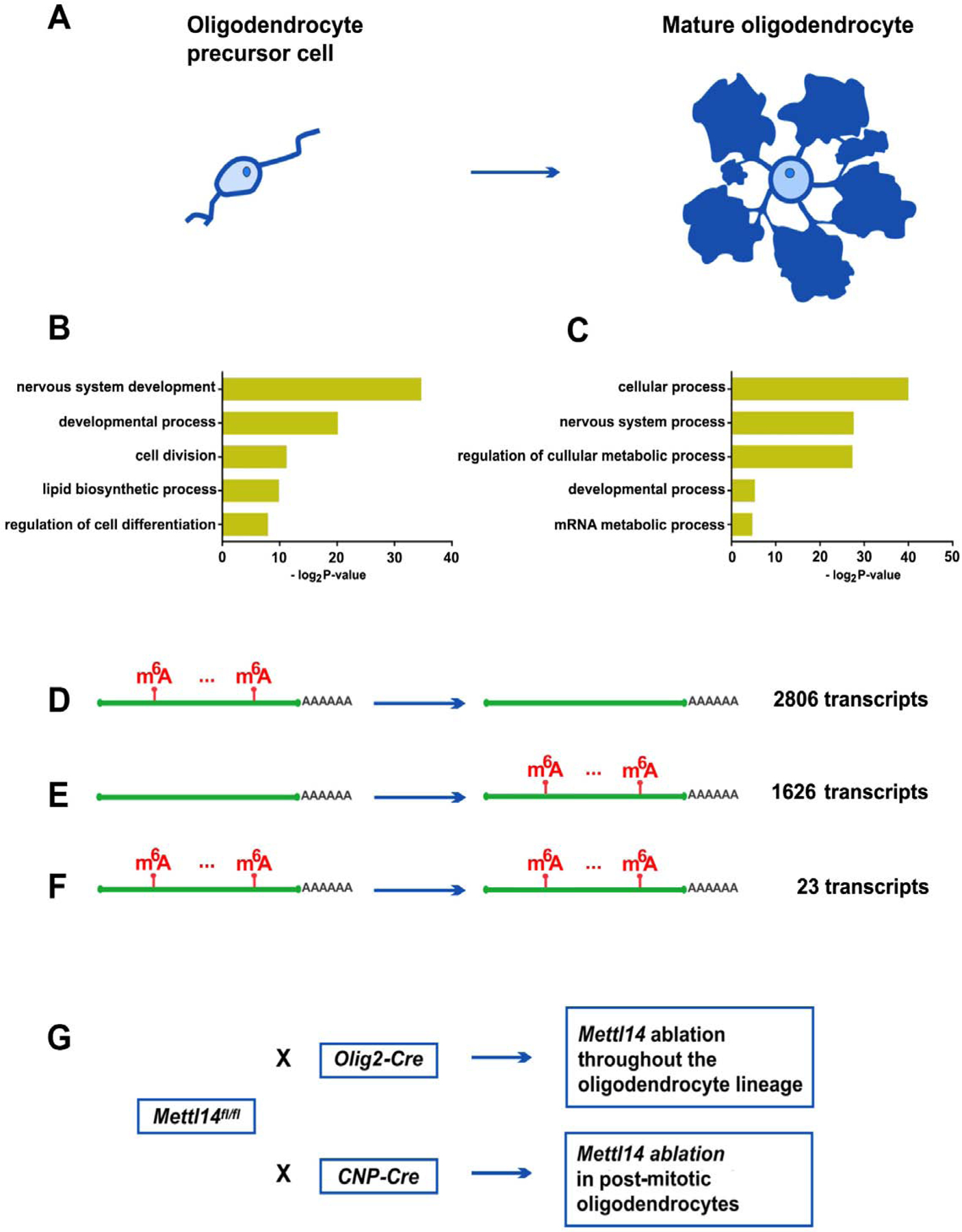
(A) Schematic drawing of an OPC and mature oligodendrocyte.
(B–C) The gene ontology categories of the m6A marked transcripts that belong to OPCs (B) and oligodendrocytes (C) (log2 |CPM|>1, Z-score>0).
(D) Of the 11,502 transcripts that are expressed both in OPCs and oligodendrocytes, 2806 transcripts bear the m6A mark in OPCs, but not in oligodendrocytes. (log2 |CPM|>1, Z score>0).
(E) Of the 11,502 transcripts that are expressed both in OPCs and oligodendrocytes, 1626 transcripts bear the m6A mark in oligodendrocytes, but not in OPCs.
(F) Of the 11,502 transcripts that are expressed both in OPCs and oligodendrocytes, 23 transcripts bear the m6A mark in both OPCs and oligodendrocytes.
(G) Mouse lines generated for this study. Mettl14fl/fl mouse line was crossed with Olig2-Cre and CNP-Cre mouse lines, to conditional eliminate Mettl14 in oligodendrocyte lineage cells and post-mitotic cells, respectively.
In order to investigate the role of the m6A mark in CNS myelinating cells, we generated mouse lines in which the gene encoding an essential m6A writer component, METTL14, was conditionally inactivated at distinct oligodendrocyte developmental stages. We crossed mice carrying a conditional allele of Mettl14 (Mettl14fl/fl) (Koranda et al., 2018) with mice expressing the Cre recombinase under the transcriptional control of oligodendrocyte transcription factor 2 (Olig2), which is expressed throughout the oligodendrocyte lineage (Schüller et al., 2008) (Fig.1 G). The Mettl14fl/fl;Olig2-Cre mouse line allows us to study the role of m6A in developing oligodendrocyte lineage cells (Bergles and Richardson, 2015). We also generated Mettl14fl/fl;CNP-Cre mice (Fig.1 G), in which Mettl14 is conditionally eliminated by Cre under the transcriptional control of the myelin protein CNP primarily in post-mitotic oligodendrocytes (Lappe-Siefke et al., 2003), allowing us to study the role of m6A in maturing oligodendrocytes.
Mettl14 ablation leads to reduction of mature oligodendrocytes but not OPCs
We first examined whether the Mettl14 gene was efficiently inactivated via the Cre-loxP genetic strategy by examining METTL14 expression in the CNS using immunohistochemistry. We observed a reduction of METTL14 expression in both Mettl14fl/fl;Olig2-Cre and Mettl14fl/fl;CNP-Cre mutants, compared to their Mettl14fl/fl littermate controls at postnatal day P12 (Fig.S5 A–F), and across different CNS white matter regions at postnatal day 18 (P18) (data not shown), a time point at which myelin is still undergoing development.
To gain insight into how Mettl14 inactivation affects oligodendrocyte lineage cell development, we used immunohistochemistry to detect the oligodendrocyte lineage cell marker Olig2 in P18 mice (Fig.2 A, B). We found a decreased percentage of Olig2/METTL14 double positive cells (Fig.2 C) accompanied by a reduction of Olig2+ cell numbers in the corpus callosum in Mettl14fl/fl;Olig2-Cre (Fig.2 D) mutants. Similarly, P18 Mettl14fl/fl;CNP-Cre (Fig.S1 A,B) mutants also showed decreased percentage of Olig2/METTL14 double positive cells (Fig.S1 C) and decreased Olig2+ cell numbers (Fig.S1 D) and in the corpus callosum. The reduction of oligodendrocyte lineage cells in both Mettl14fl/fl;Olig2-Cre and Mettl14fl/fl;CNP-Cre mutant corpus callosum indicates that Mettl14 is important in oligodendrocyte lineage development.
Figure 2. Oligodendrocyte lineage cell-specific ablation of Mettl14 results in loss of oligodendrocytes.
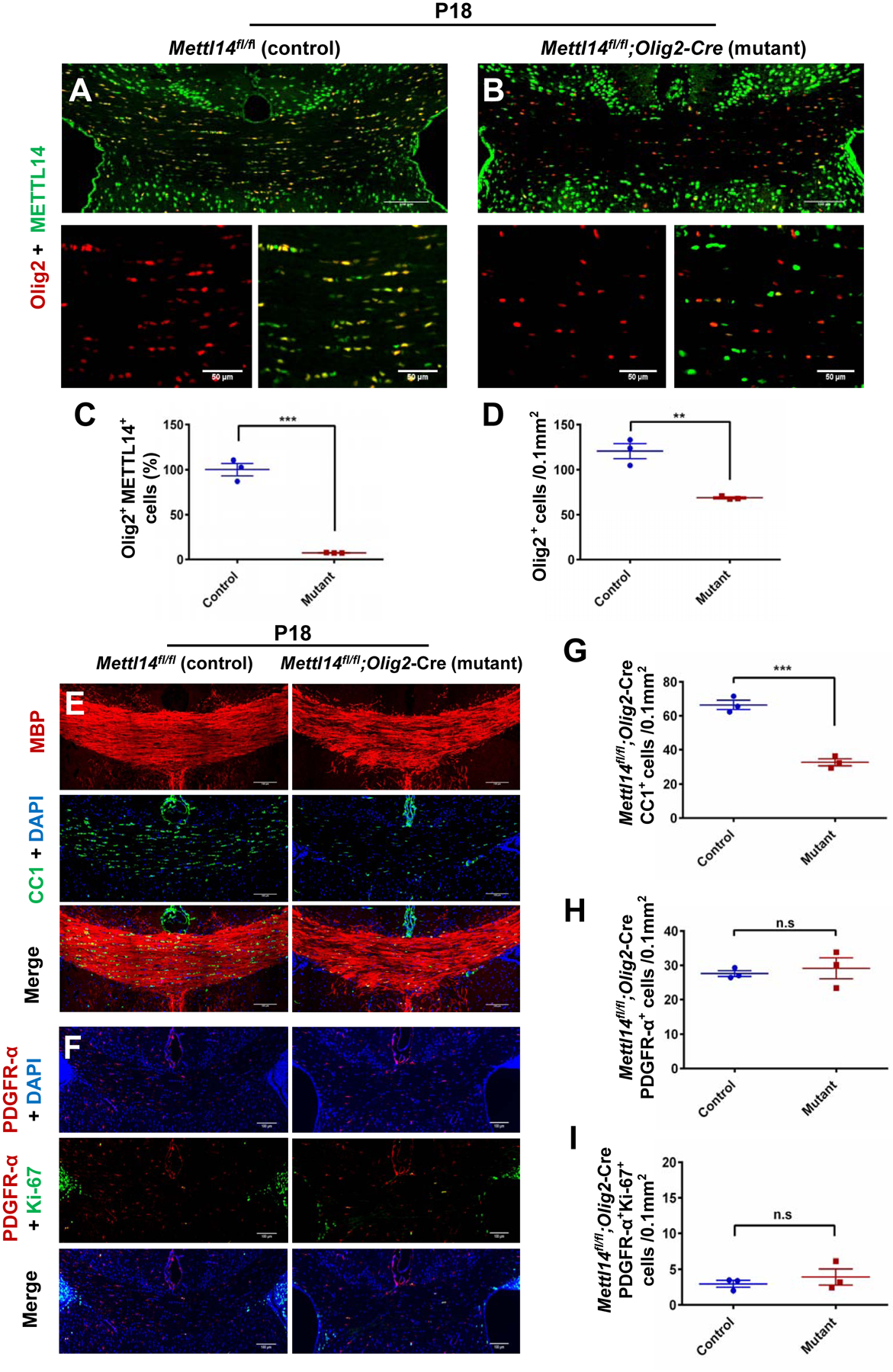
(A–B) Representative METTL14 (green) and Olig2 (red) immunostaining in the corpus callosum of P18 Mettl14fl/fl;Olig2-Cre control (A) and mutant (B) mice (Scale bar=100μm, 50μm).
(C) Quantification analysis showing a significantly reduced percentage of Olig2+/METTL14+ double positive cells in the mutants. Values represent mean ± SEM (n=3; ***p<0.001; unpaired Student’s t-test).
(D) Quantification analysis showing a statistically significant reduction of total oligodendrocyte lineage cells (Olig2+ cells). Values represent mean ± SEM (n=3; **p<0.01; unpaired Student’s t- test).
(E) Representative CC1 (green) and MBP (red) immunostaining in the corpus callosum of P18 Mettl14fl/fl;Olig2-Cre control and mutant mice. Mutant corpus callosum showed visible reduction of oligodendrocytes (CC1+ cells) and patchy myelin (MBP) (Scale bar=100μm).
(F) Representative PDGFR-α (red) and Ki-67 (green) immunostaining in the corpus callosum of P18 Mettl14fl/fl;Olig2-Cre control and mutant mice (Scale bar=100μm).
(G) Quantification showing a significant reduction of CC1+ cells (OLs) in P18 Mettl14fl/fl;Olig2-Cre mutant corpus callosum. Values represent mean ± SEM (n=3; ***p<0.001; unpaired Student’s t-test).
(H) Quantification showing no significant difference between control and mutant numbers of PDGFR-α+ cells (OPCs) in P18 Mettl14fl/fl;Olig2-Cre mice. Values represent mean ± SEM (n=3; p>0.05; unpaired Student’s t-test).
(I) Quantification showing no significant difference between control and mutant numbers of PDGFR-α+ and Ki67+ double positive cells in P18 Mettl14fl/fl;Olig2-Cre mice. Values represent mean ± SEM (n=3; p>0.05; unpaired Student’s t-test).
During early development, Olig2+ pMN progenitors produce both motor neurons and oligodendrocytes (Ravanelli and Appel, 2015). Therefore, we explored whether the Mettl14 deletion affects motor neuron development. We used choline acetyltransferase (ChAT) immunohistochemistry to identify motor neurons in the lumbar spinal cord at P12, and we found no difference in motor neuron numbers in both Mettl14fl/fl;Olig2-Cre (Fig.S7 A,B,E) and Mettl14fl/fl;CNP-Cre (Fig.S7 C,D,F) mutant mice compared to controls. In addition, motor neurons express METTL14 in the mutants of both strains (Fig.S7 B,D), suggesting that Cre recombination has limited effect in motor neurons.
To identify the oligodendrocyte lineage stage(s) that was affected by Mettl14 inactivation, we examined the number of OPCs and post-mitotic oligodendrocytes in P18 Mettl14fl/fl;Olig2-Cre and Mettl14fl/fl;CNP-Cre corpus callosum. CC1 antibody immunostaining specific for mature oligodendrocytes, showed that the mutants had significantly fewer mature oligodendrocytes as compared to controls in the corpus callosum of both Mettl14fl/fl;Olig2-Cre (Fig.2 E,G) and Mettl14fl/fl;CNP-Cre mice (Fig.S1 E,G). Interestingly, the number of cells positive for PDGF-receptor-alpha (PDGFR-α), a marker for OPCs, showed no difference in the mutants in both Mettl14fl/fl;Olig2-Cre (Fig.2 F,H) and Mettl14fl/fl;CNP-Cre mice (Fig.S1 F,H), indicating that the loss of Mettl14 does not disrupt OPC formation.
To investigate Mettl14’s role in OPC proliferation, we co-stained CNS tissue sections with Ki-67, a marker of cellular proliferation, and PDGFR-α to detect OPCs. We found no significant difference in the numbers of proliferating OPCs between mutants and controls in both P18 Mettl14fl/fl;Olig2-Cre (Fig.2 F,I) and P18 Mettl14fl/fl;CNP-Cre mice (Fig.S1 F,I). We further examined the effects of Mettl14 ablation on OPCs and proliferating OPCs at an earlier time point P12, when a larger percentage of OPCs are normally proliferative compared to P18. The quantitative analysis of P12 sections revealed results similar to those seen at P18, with no significant difference of OPC and proliferating OPC numbers between mutants and controls in both Mettl14fl/fl;Olig2-Cre (Fig. S6 C, D, K, M) and Mettl14fl/fl;CNP-Cre mice (Fig.S6 G, H, L, N). In addition, similarly to P18 mutants, both P12 Mettl14fl/fl;Olig2-Cre (Fig.S6 A, B, I) and Mettl14fl/fl;CNP-Cre (Fig.S6 E, F, J) mice showed reduced numbers of CC1+ mature oligodendrocytes in the corpus callosum.
Together, our findings demonstrate that Mettl14 ablation leads to the reduction of oligodendrocyte lineage cells, in which mature oligodendrocyte numbers, as opposed to OPC numbers, are predominantly affected.
Mettl14 ablation in oligodendrocyte lineage cells leads to hypomyelination
To characterize the pathological consequences caused by Mettl14 ablation during myelin development, we examined two different CNS regions, the corpus callosum and optic nerve, in both P18 Mettl14fl/fl;Olig2-Cre and Mettl14fl/fl;CNP-Cre mice using electron microscopy (EM). We found hypomyelination in both corpus callosum and optic nerve in Mettl14fl/fl;Olig2-Cre (Fig.3 A) and Mettl14fl/fl;CNP-Cre P18 mutants (Fig.S2 A), indicating that pathological changes start when myelin is developing. Quantitative analyses showed significantly increased g-ratios, the ratio between the inner axonal and outer total diameter of the myelin sheath, of myelinated axons (Fig.3 B, Fig.S2 B) and a significantly increased percentage of unmyelinated axons in both P18 Mettl14fl/fl;Olig2-Cre (corpus callosum: Fig.3 D, optic nerve: Fig.3 E) and Mettl14fl/fl;CNP-Cre (corpus callosum: Fig.S2 D, optic nerve: Fig.S2 E) mutants. These results revealed decreased thickness of the myelin sheath and fewer myelinated axons upon Mettl14 ablation. CNS hypomyelination was further supported by Western blot analysis, which revealed significant reductions of the myelin proteins MBP and myelin-associated glycoprotein (MAG) expression levels in the Mettl14fl/fl;Olig2-Cre (Fig.3 H, I), and Mettl14fl/fl;CNP-Cre (Fig.S2 H, I) P18 mutant brains.
Figure 3. Mettl14 ablation leads to hypomyelination.
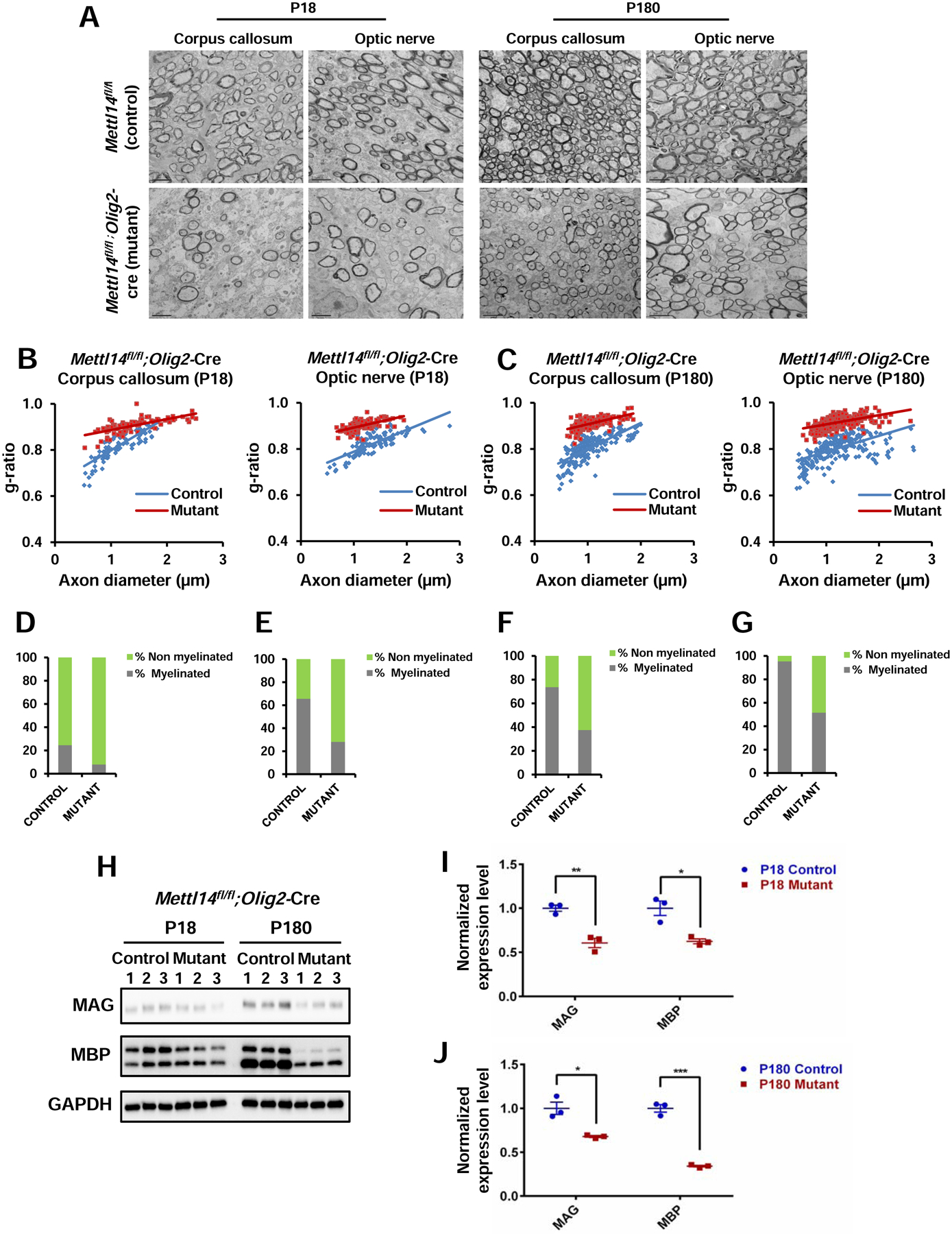
(A) Representative EM images of corpus callosum and optic nerve in P18 and P180 Mettl14fl/fl;Olig2-Cre control and mutant animals. Mutant corpus callosum and optic nerve had thinner myelin and fewer myelinated axons in both ages (Scale bar=2μm).
(B) g-ratio analyses showing significantly higher g-ratios in both P18 Mettl14fl/fl;Olig2-Cre mutant corpus callosum (Mutant g ratio=0.91, control g ratio=0.80) and optic nerve (mutant g ratio= 0.90, control g ratio= 0.82), (n=3; ***p<0.001; unpaired Student’s t test).
(C) g-ratio analyses showing significantly higher g-ratios in both P180 Mettl14fl/fl;Olig2-Cre mutant corpus callosum (Mutant g ratio=0.91, control g ratio=0.80) and optic nerve (mutant g ratio= 0.91, control g ratio= 0.83), (n=3; ***p<0.001; unpaired Student’s t test).
(D–G) Percentage of myelinated axons in corpus callosum and optic nerve (D. P18 Mettl14fl/fl;Olig2-Cre corpus callosum, E. P18 Mettl14fl/fl;Olig2-Cre optic nerve, F. P180 Mettl14fl/fl;Olig2-Cre corpus callosum, G. P180 Mettl14fl/fl;Olig2-Cre optic nerve). (n=3, ***p<0.001; unpaired Student’s t test).
(H) Western blot showing myelin protein expression (MAG, MBP) levels in both P18 and P180 Mettl14fl/fl;Olig2-Cre control and mutant animals (n=3).
(I–J) Quantification of immunoblots. MAG and MBP expression levels were normalized to GAPDH expression levels. Both MAG and MBP were significantly reduced in P18 (I) and P180 (J) Mettl14fl/fl;Olig2-Cre mutants. Values represent mean ± SEM (n=3; *p<0.05; **p<0.01; ***p<0.001; unpaired Student’s t test).
See also Figure S2.
To explore whether the pathological change caused by Mettl14 ablation persists in adulthood, we analyzed adult CNS regions using EM. The Mettl14fl/fl;Olig2-Cre mutant animals are clinically normal until about 6 months (P180) of age, when they begin to display occasional hind limb flexion, slight ataxia, and mild tremor. The Mettl14fl/fl;CNP-Cre mutant animals start to display tremor and hind limb clenching at around 4 months of age, with symptoms becoming progressively worse (e.g. ataxic phenotype). The earlier appearance of clinical symptoms in the Mettl14fl/fl;CNP-Cre mutant animals is likely the result of Cre expression in Schwann cells in the peripheral nervous system of the CNP-Cre mice (Brockschnieder et al., 2004). Similar to P18 animals, both Mettl14fl/fl;Olig2-Cre (P180) and Mettl14fl/fl;CNP-Cre (P150) adult mutants displayed myelin abnormalities (Fig.3 A, Fig.S2 A), with increased g-ratios (Fig.3 C) and non-myelinated axon percentages in both corpus callosum (Fig.3 F, Fig.S2 F) and optic nerve (Fig.3 G, Fig.S2 G). Western blot analysis also revealed decreased myelin protein levels in both P180 Mettl14fl/fl;Olig2-Cre (Fig.3 H, J) and P150 Mettl14fl/fl;CNP-Cre (Fig.S2 H, J) mutants. Together, our results demonstrate that Mettl14 is important in CNS myelination.
Mettl14 ablation prevents oligodendrocyte differentiation
To further determine the role of Mettl14 in different oligodendrocyte lineage stages, we turned to in vitro cultures. We found that the Mettl14 mutant OPCs were isolated as efficiently as control OPCs, and that the mutant cells displayed similar mitotic activity and bipolar morphology in the presence of PDGF-AA (Fig.4 A, B). Interestingly, the Mettl14 deleted cells did not develop into MBP+ mature oligodendrocytes after 5 days of differentiation following removal of PDGF-AA from the culture media (Fig.4 D). The mutant cells did not send out the extensive membrane structure seen with control oligodendrocytes (Fig.4 C). Indeed, only rare cells (less than 7%, data not shown) that escaped Cre recombination and were METTL14+ in the mutant cell cultures developed into MBP+ cells (Fig.4 E). Our Western blot data confirmed the almost complete elimination of METTL14 in the mutant oligodendrocytes, as well as the dramatic reduction of major myelin protein (MAG and MBP) expression (Fig.4 F, G). These results correlate with our in vivo findings, strongly suggesting that Mettl14 is critical for oligodendrocyte maturation.
Figure 4. Mettl14 ablated OPCs fail to develop into mature oligodendrocytes in vitro.
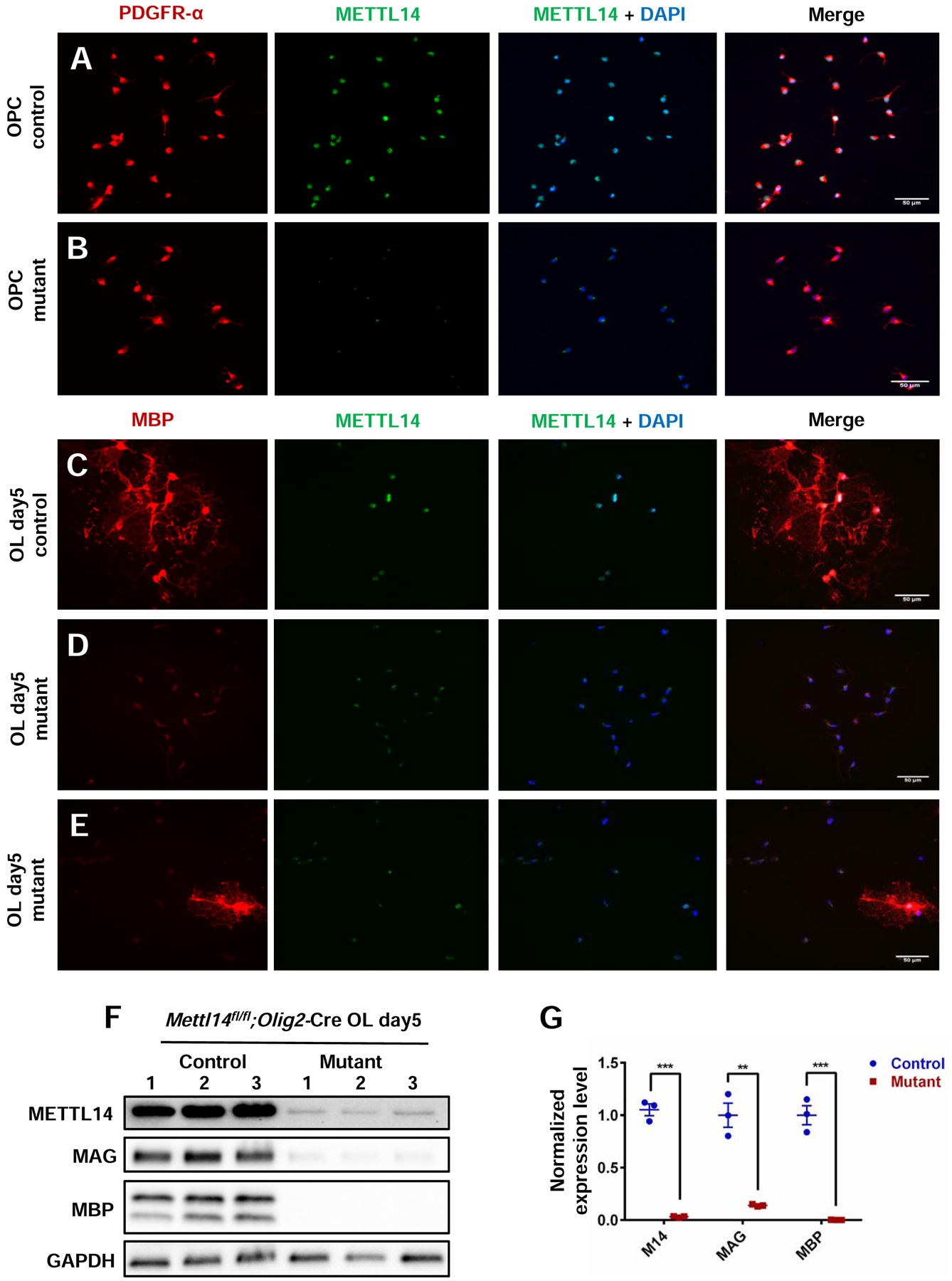
(A–B) PDGFR-α and METTL14 immunostaining of Mettl14fl/fl;Olig2-Cre control and mutant OPCs in culture. METTL14 was eliminated from the mutant OPCs, which showed no morphological changes compared to control OPCs (Scale bar=50μm).
(C–D) MBP and METTL14 immunostaining of Mettl14fl/fl;Olig2-Cre control and mutant oligodendrocytes that had been cultured in differentiation media for 5 days (oligodendrocyte day5). Mutant cells fail to develop into MBP-positive cells (Scale bar=50μm).
(E) Only rare cells (white arrow pointed) that had escaped Cre-mediated recombination and thus expressed METTL14 in the mutant day5 OL group successfully differentiated into MBP expressing oligodendrocytes (Scale bar=50μm).
(F) Western blot showing METTL14, MAG, MBP and GAPDH expression levels in control and mutant OL day5 groups.
(G) Quantification of immunoblots showing significant reduction of METTL14, MAG and MBP expression in mutant OL day5 group. METTL14, MAG and MBP expression levels were normalized to GAPDH expression level. Values represent mean ± SEM (n=3; **p<0.01; ***p<0.001, unpaired Student’s t test).
See also Figure S4.
We next examined cell morphology and maturation from early to late post-mitotic differentiation stages of oligodendrocytes in vitro to further explore the effects of Mettl14 on oligodendrocyte lineage cell maturation. We used O1, an antibody specific for the myelin galactolipid galactocerebroside, to detect oligodendrocyte morphology (Sommer and Schachner, 1981) from day 1 to day 5 after cells were plated in differentiation media. Interestingly, Mettl14 deleted cells (Fig.5 C) showed O1 immunoreactivity but did not display the morphological changes of control cells, which progressively extended their membrane structures to form complex membrane sheets (Fig.5 A). The Mettl14 ablated cells did not express appreciable levels of MBP (Fig.5 D), whereas control cells matured gradually from day 1 to day 5 with increasing MBP expression (Fig.5 B). These results together with our in vivo data strongly suggest that Mettl14 plays an important role in post mitotic oligodendrocyte maturation.
Figure 5. Mettl14 ablation prevents oligodendrocyte differentiation.
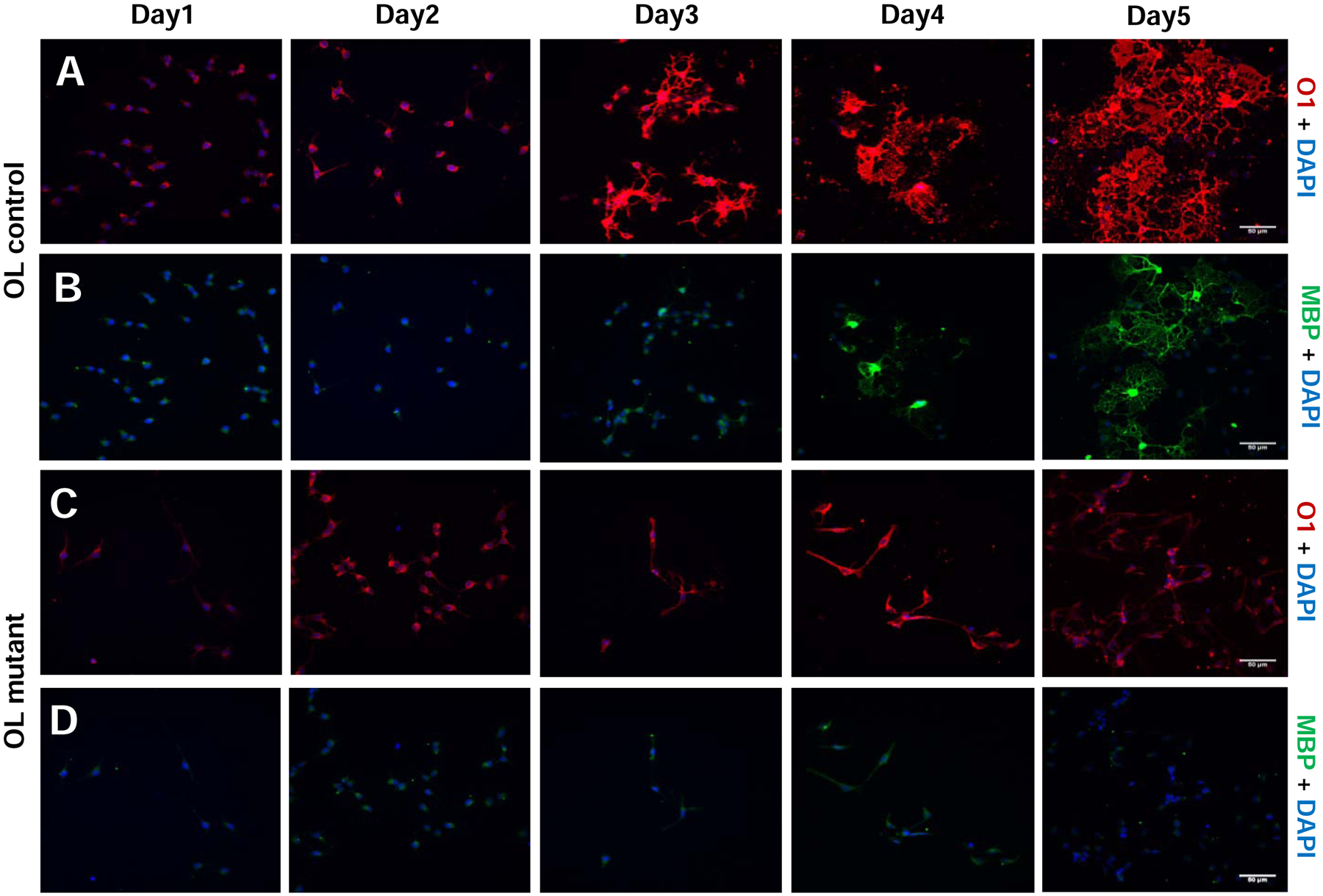
(A–B) O1 and MBP immunostaining of Mettl14fl/fl (control) oligodendrocytes that had been seeded in differentiation media for 1–5 days (day1–5). Control cells progressively differentiated into mature oligodendrocytes (Scale bar=50μm).
(C–D) O1 and MBP immunostaining of Mettl14fl/fl;Olig2-Cre (mutant) oligodendrocytes that had been seeded in differentiation media for 1–5 days (day1–5). Mutant cells did not differentiate into MBP positive oligodendrocytes, and never formed membrane sheath structures like control cells (Scale bar=50μm).
Mettl14 ablation differentially alters the OPC and oligodendrocyte transcriptomes
To elucidate the effects of Mettl14 on oligodendrocyte lineage cell gene expression during development at the transcriptome level, we performed RNA-seq with both purified OPCs and cultured mature oligodendrocytes from Mettl14fl/fl;Olig2-Cre control and mutant mice. The quantification of differential expression in the Mettl14 mutant transcriptome revealed distinct differences in the OPCs and myelinating oligodendrocytes. For quantification analysis we defined significance of differentially expressed transcripts using the following three criteria: 1. a 99.9% confidence interval, adjusted for false discovery, as a q-value using methods previously described (Benjamini and Yekutieli, 2005); 2. fold changes (FC) that exceeded 2.0 fold (log2 |FC|>1) in expression and 3. an expression level that exceeded two counted transcripts per million (log2 |CPM|>1). Of the 11,809 transcripts present in the OPC transcriptome, 586 were expressed at significantly higher levels and 177 were expressed at significantly lower levels in the mutant cells (Fig.6 A). Among the 12,542 transcripts present in mature oligodendrocytes, 1,388 transcripts were significantly upregulated and 1,247 were downregulated in the mutant cells (Fig.6 B). Interestingly, among the significantly downregulated oligodendrocyte transcripts, many are normally highly expressed in myelinating oligodendrocytes, such as Mbp, Mog, Mag, Plp1, and Cnp (Fig.6 B). The downregulation of these myelin transcripts correlates with the downregulation of myelin protein expression observed in the Mettl14 ablated mutant animals.
Figure 6. Mettl14 deletion differentially alters OL and OPC transcriptome.
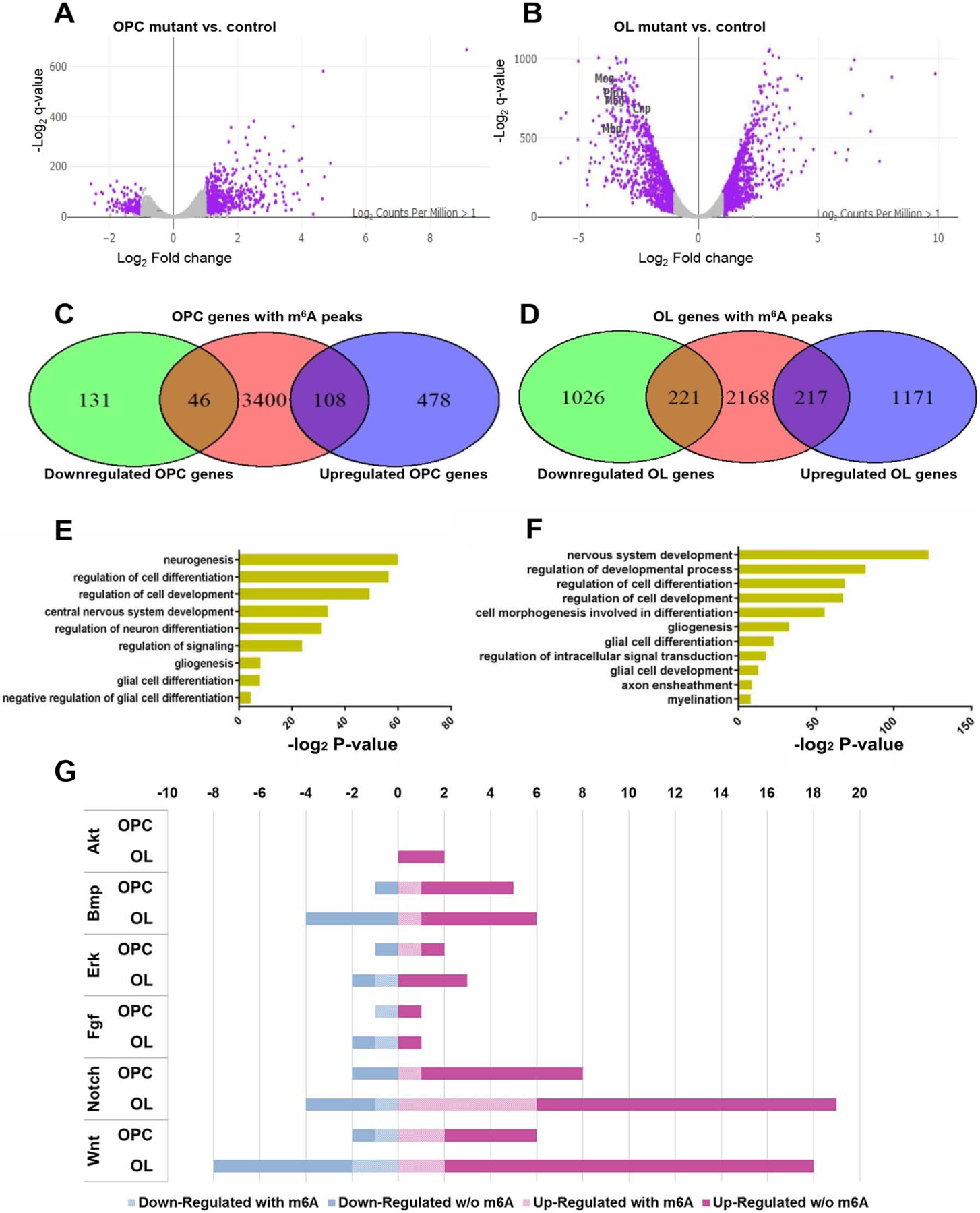
(A–B) Volcano plots display the differentially expressed genes in the Mettl14fl/fl;Olig2-Cre OPCs (A) and oligodendrocytes (B) mutants versus controls (n=3). The highlighted genes (purple) are significantly (q-value<0.001, log2 |CPM|>1) regulated and have a notable fold change (log2 |FC|>1) in their expression in the mutants. Selected myelin genes are labeled.
(C–D) Venn diagram shows the numbers of significantly downregulated or upregulated OPC (C) and oligodendrocyte (OL) (D) transcripts that also have the m6A mark.
(E–F) The ontology categories of the m6A marked transcripts that are significantly altered in the OPCs (E) and oligodendrocytes (F). (log2 |FC|>1, log2 |CPM|>1, q-value <0.001, Z-score>0).
(G) Bar graph shows the number of m6A marked transcripts in the selected altered signaling pathways in OPCs and oligodendrocytes. (log2 |FC|>1, log2 |CPM|>1, q-value<0.001, Z-score>0).
The m6A mark has been shown to play a role in reducing the stability of m6A-containing transcripts (Wang et al., 2014, 2018; Weng et al., 2018a; Yoon et al., 2017; Zhang et al., 2017). Accordingly, many transcripts in the Mettl14fl/fl;Olig2-Cre mutants had higher relative expression levels. We compared the m6A-seq data and RNA-seq data, and found that among the 3,554 m6A marked OPC transcripts, 46 transcripts had significantly downregulated expression levels, and 108 transcripts had significantly upregulated expression levels (Fig.6 C). Among the 2,606 m6A marked oligodendrocyte transcripts, 221 had significantly downregulated expression levels, and 217 transcripts had significantly upregulated expression levels (Fig.6 D). Gene ontology analysis of significantly altered m6A marked transcripts revealed many important functions such as glia cell development in OPCs (Fig.6 E) and myelination in oligodendrocytes (Fig.6 F). These results indicate that the m6A mark differentially regulates the OPC and oligodendrocyte transcriptomes.
Mettl14 regulates OPC and oligodendrocyte transcripts that are critical for oligodendrocyte lineage progression
In order to find clues of how the m6A mark regulates oligodendrocyte lineage development, we examined the expression of factors that play a critical cell-autonomous role in oligodendrocyte lineage progression by cross-comparing our m6A-seq and RNA-seq datasets. We identified a number of transcripts that encode transcriptional factors implicated in oligodendrocyte lineage progression as being dynamically marked by m6A at different oligodendrocyte lineage stages. For example, Hey1, Klf19, Sox2, Sox5, Srebf1, Tcf19, Zeb2 are marked by m6A in OPCs, but not in oligodendrocytes; Hes1, Nkx6.2, Olig2 and Yy1 are marked by m6A only in oligodendrocytes, but not in OPCs (Table S1). The dynamic m6A marked status of these transcription factor transcripts suggests a time-specific, post-transcriptional regulatory role of m6A during oligodendrocyte lineage progression and may contribute to the differentially altered transcriptome in OPCs and oligodendrocytes following Mettl14 deletion.
Studies have shown that DNA epigenetic regulation mechanisms, such as chromatin remodeling and histone modifications, are important for oligodendrocyte lineage progression (Koreman et al., 2018). Our RNA-seq and m6A-seq analyses revealed that many transcripts encoding histone modification regulators bear an m6A mark, and were significantly differentially expressed in the mutant transcriptome (Table S2). We detected transcripts of histone “writers” such as histone acetyltransferases (HATs) Hat1, histone methyltransferases (HMTs) Smyd2, Prdm2, Setdb1, Suv39h1, Ash1l, Dot1l; histone “erasers” such as histone deacetylases (HDACs) Hdac3, Hdac7, Hdac8, Hdac9, and lysine demethylases (KDMs) Kdm2b, Kdm5c, Kdm3b, Kdm4a, Kdm4c, Kdm6a that had the m6A mark and had significantly altered mRNA levels in the Mettl14 ablated mutants: these transcripts encode proteins with important regulator functions in oligodendrocyte development (Hernandez and Casaccia, 2015). Thus, our findings suggest a possible link between m6A RNA modification and histone modifications in the regulation of oligodendrocyte lineage development.
We also examined key signaling pathways that are critically involved in oligodendrocyte lineage progression. We found transcripts that were significantly altered by Mettl14 ablation in the bone morphogenetic proteins (BMPs), ERK/MAPK, fibroblast growth factor families (FGFs), Notch/Delta, Sonic hedgehog (Shh) and Wnt signaling pathways in OPCs (Table S3); and P13K/AKT/mTOR, BMPs, ERK/MAPK, insulin-like growth factor-1 (IGF-1), Notch/Delta, Shh and Wnt signaling pathways in oligodendrocytes (Table S4). The alternation of critical gene expression levels in these signaling pathways provided us with important clues regarding disruption of oligodendrocyte maturation displayed by the Mettl14fl/fl;Olig2-Cre and Mettl14fl/fl;CNP-Cre animals. Indeed, many transcripts that encode critical components of these signaling pathways have the m6A mark (Fig.6 G) (Table S3, S4), suggesting that m6A may regulate these signaling pathways to promote oligodendrocyte lineage progression.
Mettl14’s possible mechanism of actions in oligodendrocyte lineage cells
We also wished to explore potential mechanism(s) of action of the m6A mark in regulating oligodendrocyte lineage cell development and function in addition to the disruption of the cells’ transcriptomes discussed above. Previous studies have shown m6A’s role in increasing the translational efficiency of the marked transcripts in various systems (Coots et al., 2017; Shi et al., 2017; Wang et al., 2015; Zhou et al., 2018). In order to investigate this potential mechanism, we compared transcriptional and translational levels of a subset of the m6A marked transcripts that encode proteins critical for oligodendrocyte development (Bujalka et al., 2013; Zhou and Anderson, 2002). We found significantly decreased levels of these proteins both in vivo (Fig.3 H,I,J) and in vitro (Fig.4 F,G; Fig.S8 A,B), however, the observed reductions correlated with the levels of mRNA reduction found in the oligodendrocyte transcriptome (Table S5), suggesting that translational regulation may not be a key feature of m6A gene regulation in oligodendrocyte lineage cells. Nevertheless, a more comprehensive proteomics assessment will be required to determine the global impact of the m6A mark on translational efficiency in oligodendrocytes.
MBP, a predominant and critical protein of myelin, is translated locally in the myelin compartment (Colman et al., 1982), which requires the active transport of its mRNA into oligodendrocyte processes (Carson et al., 1997). This transport requires the RNA binding protein heterogeneous nuclear ribonucleoprotein (hnRNP) A2 (Hoek et al., 1998; Müller et al., 2013). Importantly, it was recently discovered that hnRNPA2B1, an isoform of hnRNPA2 that is expressed in oligodendrocyte lineage cells (Han et al., 2010), is an m6A reader (Alarcón et al., 2015), suggesting that the m6A mark might have a role in regulating Mbp mRNA transport in oligodendrocytes. In order to investigate this possibility, we used the RNAscope approach to determine the distribution of Mbp and Myrf mRNA in oligodendrocytes of the corpus callosum. In controls, Myrf mRNA is localized in the oligodendrocyte cell bodies; whereas, Mbp mRNA distributes to the complex, web-like oligodendrocyte processes (Fig.S3 A). In P18 Mettl14fl/fl;Olig2-Cre mutant oligodendrocytes, Myrf and Mbp mRNA levels are clearly reduced (Fig.S3 B), as expected, but the distribution of these transcripts does not appear altered, suggesting that the absence of the m6A mark has not disrupted the transport of the Mbp mRNA into the myelin compartment. In addition to MBP mRNA, a number of key oligodendrocyte transcripts has been shown to be localized in the myelin sheath (Thakurela et al., 2016). A more extensive analysis of the myelin mRNA content of the Mettl14 mutants will help determine the role of the m6A mark in the establishment of myelin transcriptome.
Functional variant isoforms of myelin proteins are generated by alternative splicing to ensure precise oligodendrocyte lineage progression (Montague et al., 2006; Zhang et al., 2014; Zhao et al., 2010a). Previous studies have shown that m6A plays a critical role in regulating mRNA splicing in various cellular systems (Haussmann et al., 2016; Xiao et al., 2016; Zhao et al., 2014; Zhou et al., 2019). In order to investigate the potential role of the m6A mark in regulating differential splicing during oligodendrocyte development, we used LeafCutter (Li et al., 2018) to identify altered splicing events in OPC and oligodendrocyte transcriptomes. LeafCutter identifies alternatively excised intron clusters and compares differentially excised intron levels between controls and mutants. Differential splicing is measured by changes in the percent spliced in (change, or delta, dPSI) (Li et al., 2018). Global comparison of alternative splicing events revealed numerous statistically significant changes in Mettl14 ablated mutants versus controls (Stouffer’s Z-score = 40.86 in OPCs and 105.76 in oligodendrocytes). In addition, we found that 1,372 splicing events in 364 genes in OPCs, and 1,930 splicing events in 485 genes in oligodendrocytes were differentially spliced upon Mettl14 deletion (q<0.01). A number of significant differentially alternative spliced transcripts were previously shown to encode proteins with important functions in the myelinating process, such as protein tyrosine phosphate receptor type Z1(Ptprz) in OPCs (Harroch et al., 2002) and neurofascin (Nfasc) in oligodendrocytes (Table.S6,S7). Interestingly, the neurofascin protein (NF), which is essential in the establishment and maintenance of node of Ranvier domains (Howell et al., 2006; Pillai et al., 2009; Sherman et al., 2005; Thaxton et al., 2010; Zonta et al., 2008), has the most significantly altered isoforms and bears the highest differential dPSI level in the oligodendrocyte transcriptome (Fig.7 A, Table.S7).
Figure 7. Mettl14 deletion differentially alters Nfasc155 alternative splicing and expression.
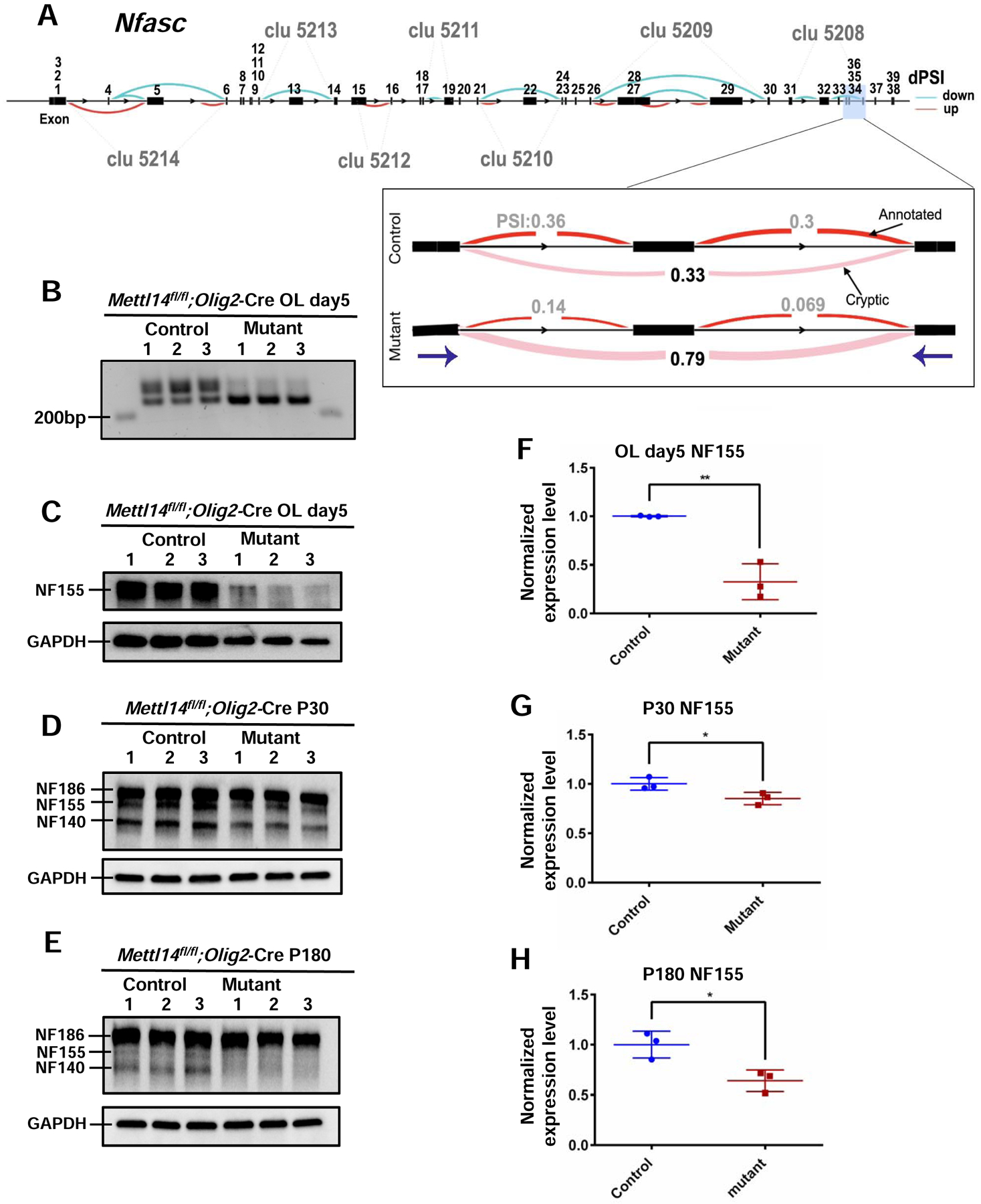
(A) Schematic view of differentially spliced sites in the Nfasc gene in control versus Mettl14fl/fl;Olig2-Cre mutant day5 oligodendrocytes. The 39 Nfasc exons are labeled above the exons. Each cluster (i.e. abbreviated as “clu X”) represents a group of introns that display alternative excision events. Specifically, these are introns that share a donor site (canonical 5’ splice site, AT) or acceptor site (canonical 3’ splice site, GA). Blue curves represent cases that have fewer splicing events in the mutants, while the red represent cases with more splicing events in the mutants (p<0.05). The magnified window shows the sample cluster (clu 5208) that we examined for the presence of aberrant spliced isoforms in the mutants in panel B. Purple arrows represent the start points for reverse and forward primers that we used for RT-PCR in (B).
(B) Differentially spliced Nfasc isoform were detected by RT-PCR and agarose gel electrophoresis in the Mettl14fl/fl;Olig2-Cre day5 oligodendrocyte mutants (218kb). (Primers used: Forward: ACTGGGAAAGCAGATGGTGG Reverse: ACATGAGCCCGATGAACCAG).
(C–E) Western blot results of NFASC in vitro (C) and in vivo (D:P30, E:P180)
(F–H) Quantification of NF155 expression in vitro (F) and in vivo (G:P30, H:P180). NF155 expression level was normalized to GAPDH expression level. NF155 had significant reduction in both P30 and P180 Mettl14fl/fl;Olig2-Cre mutants. Values represent mean ± SEM (n=3; *p<0.05; **p<0.01; unpaired Student’s t test).
To further investigate the role of m6A mRNA methylation in regulating the distribution of NF isoforms during development, we used RT-PCR to confirm the differential distribution of distinct splicing products (Fig.7 B) in purified oligodendrocyte mRNA from mutants and controls. This analysis confirmed the presence of the predicted altered spliced Nfasc mRNA product in the mutant oligodendrocytes. Since NF155 is the glial NF isoform that is required for the assembly of paranodal domains (Sherman et al., 2005), we examined NF155 expression levels in different developmental stages in vitro and in vivo. Mettl14fl/fl;Olig2-Cre mutant oligodendrocytes that had been cultured in differentiation media for 5 days had a significant reduction of NF155 expression compared to controls (Fig.7 C,F). In vivo, 1-month old animals (P30) showed significantly decreased NF155 levels in the Mettl14fl/fl;Olig2-Cre mutants (Fig.7 D,G). To further analyze nodal and paranodal domains with immunohistochemistry we used antibodies to the voltage gated sodium channel (NaCh) and Caspr to identify the nodal and paranodal domains, respectively (Fig.8 A,B). We found a significant reduction in the number of nodes at P30 in the mutants compared to controls (Fig.8 E). We also used pan-NF antibody to characterize NF localization and morphology in both nodal and paranodal domains (Fig.8 A,B). We found no difference in node and paranodal size in the mutants as compared to controls (Fig.8 F).
Figure 8. Mettl14 deletion results in aberrant node and paranode morphology.
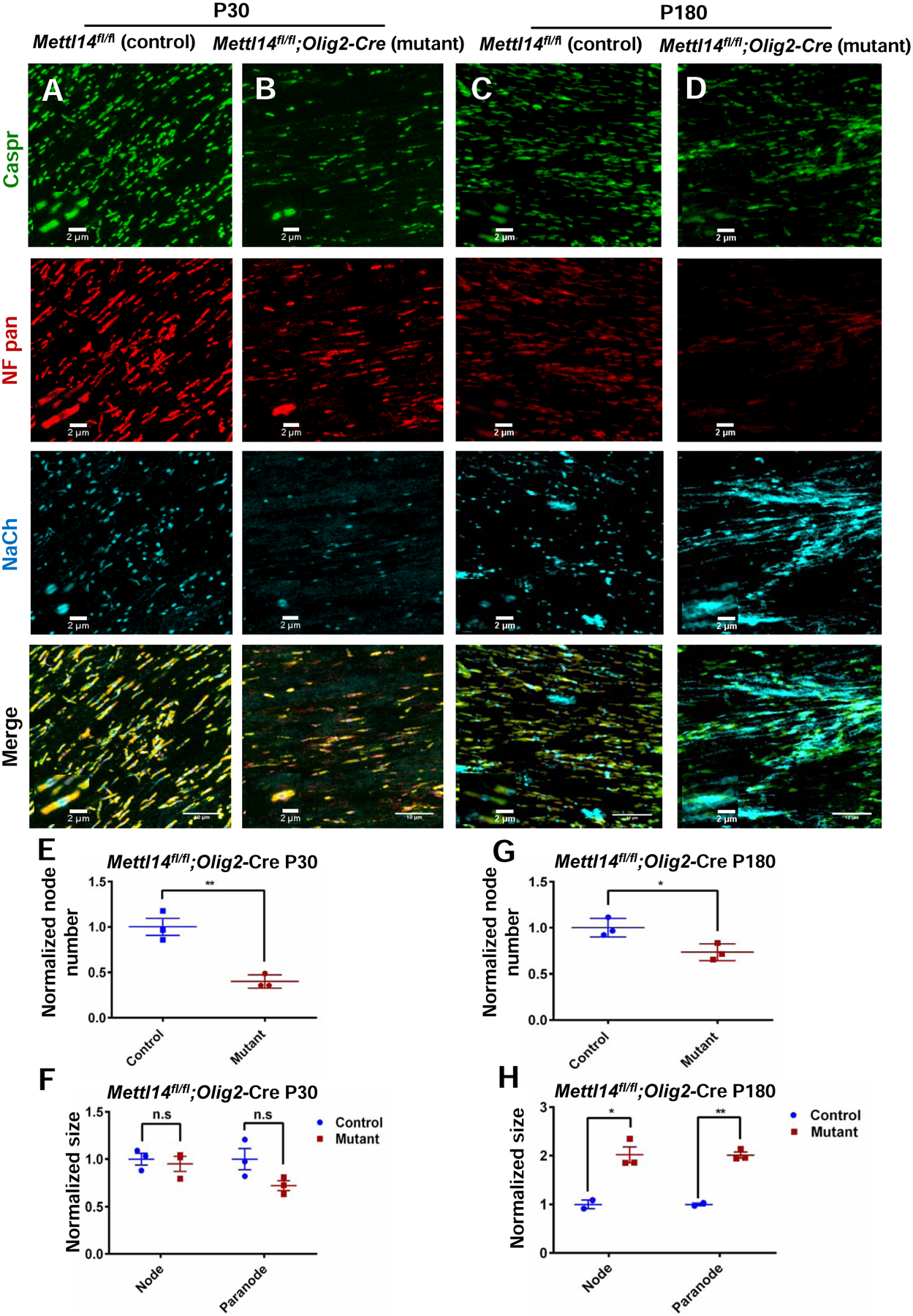
(A–B) Representative immunostaining with Caspr, Nfasc and NaCh in P30 Mettl14fl/fl;Olig2-Cre control and mutant corpus callosum. Representative node(s) of Ranvier are shown in magnified windows. (Scale bar=10μm, 2μm)
(C–D) Representative immunostaining with Caspr, Nfasc and NaCh in P180 Mettl14fl/fl;Olig2-Cre control and mutant corpus callosum. Representative node(s) of Ranvier are are shown in magnified windows. (Scale bar=10μm, 2μm)
(E, G) Quantification of node number (NaCh positive) in P30(E) and P180(G) Mettl14fl/fl;Olig2-Cre control and mutant corpus callosum. Normalized number = mutant count / control count (=1) (n=3; *p<0.05; **p<0.01, unpaired Student’s t-test).
(F, H) Quantification of node (Caspr, NaCh positive) and paranode (Nfasc, Caspr double positive) size in P30 (F) and P180 (H) Mettl14fl/fl;Olig2-Cre control and mutant corpus callosum. Normalized size = mutant size / control size (=1) (control n=2 mutant n=3; *p<0.05; **p<0.01, unpaired Student’s t-test).
We further investigated whether NF155 expression abnormalities persist to adulthood (P180) in Mettl14fl/fl;Olig2-Cre mutants. Western blot results revealed significantly reduced expression levels of NF155 in P180 mutants (Fig.7 E,H). Next, we measured nodal and paranodal domains via immunohistochemistry in these animals (Fig.8 C,D). We found significant reduction of node numbers in the mutants (Fig.8 G). In addition, both nodal and paranodal domains showed significantly increased sizes in the mutants (Fig.8 H), suggesting widespread pathological changes at the node of Ranvier in adult Mettl14fl/fl;Olig2-Cre mutants.
DISCUSSION
RNA modifications have recently emerged as critical post-transcriptional regulatory mechanism to modulate gene expression (Frye et al., 2018). Among all post-transcriptional mRNA modifications, m6A is the most abundant internal alteration found in eukaryotic mRNA (Yue et al., 2015). In contrast to DNA and protein methylation, m6A methylation has the potential to have a very rapid influence on transcriptome changes during cell state transitions (Frye et al., 2018; Zhao and He, 2017).
In this report we show that oligodendrocyte lineage progression is accompanied by changes in m6A modification on numerous transcripts. We also show that Mettl14, which encodes an essential m6A writer component, is critical in regulating oligodendrocyte development and CNS myelination. We demonstrate altered oligodendrocyte numbers and hypomyelination in both oligodendrocyte lineage cell specific Mettl14 ablated mice. Nevertheless, OPC numbers were not altered by Mettl14 ablation. We also show that Mettl14 ablated OPCs lacked the ability to differentiate into mature MBP-positive myelin-forming oligodendrocytes in vitro. These results indicate that the m6A RNA modification is essential for post-mitotic oligodendrocyte differentiation.
Interestingly, our data revealed a more severe developmental phenotype in vitro than in vivo, suggesting communication with other CNS cell types may mitigate the effects of the Mettl14 deletion in vivo. In addition, it is curious that when we cultured Mettl14fl/fl;CNP-Cre OPCs (data not shown), in which the Mettl14 gene is inactivated later in the oligodendrocyte lineage, the mutant cells had the capacity to differentiate into MBP-positive, mature oligodendrocytes as efficiently as control cells, suggesting that the severe block in maturation that occurs in the Mettl14fl/fl;Olig2-Cre OPCs in vitro is the result of an m6A deficiency early in the oligodendrocyte lineage. This is supported by the failure of enforced METTL14 expression in the Mettl14fl/fl;Olig2-Cre OPCs to fully rescue the severe in vitro differentiation phenotype (Fig. S4). Our future efforts will be devoted to elucidating the effects of the m6A mark at distinct stages of the oligodendrocyte lineage.
Oligodendrocyte differentiation involves many steps that must be regulated in time and space (Zuchero and Barres, 2013). Our RNA-seq and m6A-seq data revealed changes of the m6A marked status on numerous transcripts that encode critical transcription factors in OPCs and oligodendrocytes, suggesting that m6A mRNA modification contributes to transcriptional changes during oligodendrocyte development. Indeed, emerging studies have shown that the turnover and/or translation of transcripts during cell-state transitions regulated by the m6A mark represent an important developmental mechanism (Frye et al., 2018).
The functional role of various histone modifiers in oligodendrocyte differentiation is stage-dependent, yet the underlying regulatory role of these factors is unknown (Copray et al., 2009; Hernandez and Casaccia, 2015). Our study revealed that transcripts that encode a number of histone modifiers are dynamically marked by m6A in OPCs and oligodendrocytes (Table S2), suggesting that m6A RNA modifications may play a role in regulating the expression of epigenetic modifiers at distinct oligodendrocyte lineage stages. Consistent with this possibility, a recent study revealed cross-talk between m6A RNA modification and histone modification (Wang et al., 2018).
In addition, we also found numerous oligodendrocyte lineage signaling pathway transcripts that are dynamically marked by m6A and expressed at significantly altered levels in the absence of METTL14. The alternation of m6A marked transcripts is accompanied by significant alterations of other important pathway transcripts that do not bear an m6A mark, suggesting that m6A RNA modification may have a primary or secondary effect on gene expression. Together, our RNA-seq and m6A-seq results indicate that m6A RNA modifications modulate the expression of multiple transcriptional regulators, DNA epigenetic modifiers and signaling pathways to facilitate oligodendrocyte lineage progression.
In addition to the effect on mRNA levels discussed above, we examined several additional potential functions of the m6A mark on gene expression in oligodendrocyte lineage cells. We explored whether the m6A mark affects translational efficiency of important myelin genes and their regulators, since it had previously been shown that translational efficiently of marked transcripts is increased (Shi et al., 2017; Wang et al., 2015). Surprisingly, we did not detect dramatic alternations of protein levels when compared with mRNA levels of m6A marked transcripts. The m6A mark has also been shown to play a role in intracellular mRNA transport (Roundtree et al., 2017). Therefore, we examined the transport of Mbp mRNA into oligodendrocyte processes, which has been shown to be critical for CNS myelination. Nevertheless, using RNAscope we were unable to detect a significant decrease in the efficiency with which the Mbp mRNA is transported into the myelin domain in the Mettl14 mutant animals. Although these initial efforts did not reveal a role for the m6A mark in mRNA translation or sub-cellular transport, more thorough analyses may uncover alterations in these processes in the Mettl14 mutant oligodendrocytes.
We did, however, detect widespread changes in the Mettl14 mutant oligodendrocyte lineage cells that were related to aberrant RNA splicing. In fact, 283 out of 364 (~78%) aberrantly spliced OPC transcripts, and 311 out of 485 (~64%) aberrantly spliced oligodendrocyte transcripts are marked by m6A. Many of these altered transcripts have been described as crucial for oligodendrocyte lineage development and function, such as Ptprz1, Gsn (Brown and Verden, 2017) and Map2 (Müller et al., 1997). Importantly, we discovered the transcript encoding NF, a critical cell adhesion protein involved in node of Ranvier establishment and maintenance, was differentially spliced under the regulation of m6A. The mouse Nfasc gene contains 39 exons, and the inclusion or exclusion of different Nfasc exons results in transcripts that encode functionally distinct isoforms (Suzuki et al., 2017). NF186 is expressed by neurons and is critical for node assembly, and NF155, which is expressed by the myelinating cells, is critical to the stability of the paranodal domain (Howell et al., 2006; Kawamura et al., 2013; Kira et al., 2018; Pomicter et al., 2010). The disruption of NF isoform distribution results in pathological changes in myelinated axons (Howell et al., 2006; Pillai et al., 2009; Thaxton et al., 2010). We identified aberrantly spliced Nfasc RNA isoforms in the Mettl14 deleted oligodendrocytes, and provided in vivo evidence of altered NF155 protein expression that correlated with morphological abnormalities of the paranodal domain. In particularly, the CNS nodes of Ranvier of adult Mettl14fl/fl;Olig2-Cre mutants displayed widespread abnormalities, strikingly reminiscent of NF155-deficient mice (Pillai et al., 2009). These results indicate m6A RNA methylation regulates Nfasc155 splicing, and plays a role in establishing and maintaining normal function of critical axonal-oligodendrocyte interactions. Interestingly, changes in NF155 expression have recently been suggested to be central to adult myelin remodeling associated with altered impulse transmission (Fields and Dutta, 2019). This raises an intriguing possibility that the m6A epigenetic mark plays a critical role in activity-dependent myelin remodeling.
A recent study reported that the proline rich coiled-coil 2 A (Prrc2a) protein is an m6A reader that participates in oligodendrocyte specification and myelination by regulating the stability of its critical downstream target Olig2 (Wu et al., 2019). Nevertheless, the clinical and pathological phenotypes of the oligodendrocyte-specific mouse mutants of Prrc2a are considerably more severe than that observed for the Mett14 writer mutants described here, and the alteration in Olig2 expression is also much more significant in the Prrc2a mutants. This raises the possibility that Prrc2a participates in other functions in addition to its putative role as an m6A reader in oligodendrocyte lineage cells.
In conclusion, our study demonstrates that m6A RNA modification is essential for normal oligodendrocyte maturation and CNS myelination. We show that the m6A mark plays an important role in regulating various aspects of gene expression in oligodendrocyte lineage cells, with the most profound effects on mRNA levels and splicing. Rapid alterations to the m6A landscape have the potential to quickly modify a cell’s phenotypic properties (Geula et al., 2015; Licht and Jantsch, 2016; Yoon et al., 2017). Therefore, in addition to its critical role during development, the m6A mark may participate in oligodendrocyte plasticity in adults. Future characterization of m6A RNA epigenetic regulation should provide important insight to our growing understanding of the myelination process and demyelinating diseases.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Brian Popko (brian.popko@northwestern.edu)
Materials Availability Statement
This study did not generate new unique reagents.
ANIMALS
All animals were housed under pathogen-free conditions, and all animal procedures and animal care were conducted in accordance with guidelines approved by the University of Chicago’s Institutional Animal Care and Use Community (IACUC). All mice were on the C57BL/6 background, and both female and male mice were used.
Mettl14fl/fl mice (Koranda et al., 2018; Weng et al., 2018b; Yoon et al., 2017) were crossed with Olig2-Cre mice (Schüller et al., 2008), and CNP-Cre mice (Lappe-Siefke et al., 2003). Mettl14fl/fl;Olig2-Cre and littermate control Mettl14fl/fl mice as well as Mettl14fl/fl;CNP-Cre and littermate control Mettl14fl/fl mice were used for experiments.
METHOD DETAILS
OPC isolation and culture
OPCs were isolated and purified (95% purity) from postnatal day 6 (P6) mouse brains using an immunopanning protocol (Emery and Dugas, 2013). In brief, mice pups (both female and male) were genotyped and marked at P4–P5. At P6, pups were deeply anaesthetized on ice and cortices were collected, diced and digested with papain at 37° C. Cells were then triturated into a single cell suspension, then sequentially immunopanned in Ran-2, GalC, and O4 antibodies from hybridomal supernatant. The remaining O4+GalC− cells (OPCs) were then trypsinized and plated in poly-d-Lysine (PDL)-coated plates with proliferation media. Once OPCs numbers reach sufficient amount, they were split and plated in differentiation media.
OPC electroporation
Amaxa cell nucleofector II device and an electroporation kit for primary mammalian glial cells (Lonza, Cat# VPI-1006) were used as per the manufacturer’s instructions for OPC electroporation. For each biological replicate, 5 million OPCs were collected and transfected with both pmaxGFP Vector (Lonza, Cat# VPI-1006) and Mettl14 plasmid (Origene, Cat# MR207291) or pmaxGFP Vector alone. Transfected OPCs were then plated in PDL coated plates with differentiation media. Transfection efficiency was about 40%, measured by GFP positive cell number versus total cell number after 96 hours of electroporation.
Immunohistochemistry and cell counts
Mice were deeply anaesthetized with 2.5% avertin (Cat#T48402, Sigma Aldrich) in dH2O. Upon the loss of nociceptive reflexes, mice were transcardially perfused with 0.9% saline followed by ice-cold 4% paraformaldehyde (PFA). Brains were collected and post-fixed overnight in 4% PFA at 4°C, followed by incubation in 30% sucrose until saturation. Tissues were then embedded in optimal cutting temperature compound (OCT) and sectioned at 10μm. Prior to Rabbit-anti-METTL14 (1:300, Sigma, Cat# HPA038002), Goat-anti-PDGFR-α (1:100, R and D systems, Cat# AF1062), Rabbit-anti-Ki67 (1:100, Abcam, Cat# ab15580), Chicken-anti-Neurofascin (1:50, R and D systems, Cat# AF3235), Mouse-anti-sodium channel (1:100, Sigma, Cat# S8809) and Rabbit-anti-Caspr (1:300, Abcam, Cat# ab34151) immunostaining, tissue sections were processed with an antibody retrieval protocol in which sections were treated with 10 mM trisodium citrate buffer (pH 6.0) at 90 °C for 30 minutes. After cooling at room temperature (RT) for 30 minutes, sections were then incubated in 10 mM glycine (in TBS with 0.25% Triton X-100) for 1hour at RT. Slices were then blocked with TBS containing 5% normal donkey serum, 1% BSA and 0.25% Triton X-100 (blocking buffer) for 2 hours at RT, followed by incubation in primary antibody(s) diluted in blocking buffer for 48 hours at 4°C. Immunohistochemistry with Rabbit-anti-MBP (1:500, Abcam, Cat# ab40390), Mouse-anti-CC1 (1:50, Milipore, Cat# OP80), Mouse-anti-Olig2 (1:100, Milipore, Cat# MABN50) and Rabbit-anti-ChAT (1:300, Milipore, Cat# AB144P) antibodies, were followed by immunostaining protocol without antigen retrieval.
Stained tissue sections were imaged with a Mariana Yokogawa-type spinning disk confocal microscope or Leica TCS SP5 two-photon confocal microscope. All experimental and littermate control tissues were imaged with the same parameters, followed with the same adjustments in Image J (NIH). Cells counts data was converted to cells/100μm2.
Immunocytochemistry
Cells cultured on cover slips were rinsed with PBS and fixed with ice cold 4% PFA for 10 minutes at RT, washed with PBS, then air dried and stored at −80°C until immunostaining. The primary antibodies used were Rabbit-anti-MBP (1:500, Abcam, Cat# ab40390), Goat-anti-PDGFR-α (1:100, R and D systems, Cat# AF1062) and Mouse-anti-O1 (1:100, R and D systems, Cat# MAB1327). Immunocytochemistry was conducted using the normal immunostaining protocol as described above, without the antigen retrieval process.
RNA scope
Mouse Myrf mRNA probe (ACDbio, Cat# 524061), mouse Mbp mRNA probe (ACDbio, Cat# 451491) and RNAscope Multiplex Fluorescent Reagent Kit V2 assay (ACDbio, Cat# 323110) were purchased from ACDbio company. Fluorophores were purchased from Akoya biosciences (Opal 520: Cat# FP1487001KT, Opal 620: Cat# FP1495001KT). Mice were processed for RNAscope as follows. Deeply anesthetized animals were transcardially perfused with 0.9% saline followed by ice-cold 4% PFA as described above. Brains were immediately dissected out and transferred to 10% NB formalin solution (Sigma, Cat# HT5011) at RT for exactly 24 hours. Brains were then transferred to freshly made 70% ethanol for 24 hours at RT and processed for paraffin embedding. Sections were cut at 5 mm. RNAscope assay was performed as per manufacturer’s specifications.
Electron Microscopy (EM) and analysis
Mice were deeply anesthetized with 2.5% avertin, followed by perfusion with 0.9% saline and 0.1M sodium cacodylate buffer containing 4% PFA and 2.5% glutaraldehyde (EM buffer). Corpus callosum and optic nerve were then post-fixed overnight at 4°C. Tissues were dissected and washed with 0.1M sodium cacodylate buffer for 3 times, followed by post fixation with 1% osmium tetroxide (diluted with 0.1M sodium cacodylate) for 2 hours and another 3 times of wash with 0.1M sodium cacodylate buffer. These tissue samples then went through dehydration steps with 30%, 50%, 70%, 90%, 95%,100% ethanol and propylene oxide (PO), followed by permeation with 1:1 PO/Epon 812 and 1:2 PO/Epon 812 for 2 hours each, and in Epon 812 overnight at RT. The next morning, samples were permeated with Epon 812 for another 4 hours at RT, and then embedded with labeled paper strips in fresh Epon 812. These samples were then cured for 48 hours in a 60°C oven. After EM processing steps, samples were sectioned (1μm) and stained with toluidine blue, before sectioned into ultrathin slices (60–90nm) and stained with uranyl acetate-lead citrate. FEI Tecnai F30 scanning transmission electron microscope (FEI company) was used to take EM images. Image J was used to analyze the EM images for g-ratio and axon counting.
Total protein isolation
Cells in culture were rinsed with sterile PBS 2 times, then lysed with ice-cold RIPA buffer containing protease inhibitors (Thermo Fisher Scientific, Cat# 78430) and phosphatase inhibitors (Sigma, Cat# P2850 and P5726)(lysis buffer), and then scraped and collected in microcentrifuge tubes for 10 min incubation on ice. Cell lysates were then centrifuged at 13,000 g for 15 min at 4°C, and supernatant was collected and stored in −80°C until measurement. To collect brain tissues, mice were deeply anesthetized with 2.5% avertin and perfused with ice-cold sterile PBS, followed by brain isolation into microcentrifuge tubes and immediately frozen in liquid nitrogen. Brain samples were then stored in −80°C until homogenization. Brain tissue protein lysates were prepared as follows: homogenized in lysis buffer, incubated on ice for 15 min and centrifuged at 13,000g for 15 min at 4°C, t hen collect supernatants. Protein concentration was determined by using a BCA Protein Assay Kit (Thermo Fisher Scientific, Cat# 23255) as per the manufacturer’s instructions.
Western blot
Protein lysates were boiled for 5 min in Laemmli sample buffer (Bio-Rad, Cat# 161–0737) with β-mercaptoethanol (Sigma, Cat# M6250), seperated by SDS-PAGE, transferred to nitrocellulose membrane and immunoblotted. The primary antibodies used were Rabbit-anti-METTL14 (1:1000, Sigma, Cat# HPA038002); Rabbit-anti-MBP (1:1000, Abcam, Cat# ab40390), Rabbit-anti-MAG (1:1000, Thermo Fisher Scientific, Cat# AB_2533179), Mouse-anti-Olig2 (1:1000, Millipore, Cat# MABN50), Mouse-anti-MYRF (1:5000, gift from Dr. Ben Emery), GAPDH(1:2000, Cell signaling, Cat# 2118S). Western blot bands were analyzed in Image Lab software (Bio-Rad laboratories).
RNA isolation
Cell and tissue RNA samples were prepared and isolated following the manufacture’s protocol (Bio-Rad, Cat# 732–6820). RNA quality was confirmed by 2100 Bioanalyzer using a model 6000 Nano kit (Agilent technologies, Cat# 5067–1511). Samples with RNA integrity number >8 were used.
RNA-seq and analysis
Bulk RNA-seq was performed on RNA isolated from cultured OPCs and oligodendrocytes as previously described (Aaker et al., 2016). Libraries were prepared and sequenced using the Illumina HiSeq 4000 at the University of Chicago Genomics Core facility. Reads were mapped using both STAR v2.6.1a and Kallisto v.0.44.0 using bowtie 2 aligner (Bray et al., 2016; Dobin et al., 2013). Mapped reads were further analyzed with the Bioconductor suite v3.7 by the University of Illinois at Chicago Bioinformatics Core facility (Huber et al., 2015).Q-values were determined as false discovery rate adjusted p-values using the method previously described (Benjamini and Yekutieli, 2005). Results were compared with the m6A-SMART-Seq analysis and visualized in R v.3.5.1 using the plot.ly, ggplot2, and venn.diagram packages. Values for expression, fold change and statistical significance were adapted for visualization using a log2 transformation.
m6A-SMART-seq and analysis
mRNA from total RNA of OPCs and oligodendrocytes was purified with Dynabeads Oligo (dT)25 (Thermo Fisher Scientific, Cat# 61006). The purified mRNA was then processed for m6A-SMART-seq and analyzed as previously described (Weng et al., 2018b). Z scores were calculated for each m6A mark and filtered with a threshold value of 0.
Differential alternative splicing analysis
Differential splicing analysis was performed between OPCs versus OPCs lacking Mettl14 and oligodendrocytes versus oligodendrocytes lacking Mettl14. In brief, exon-exon junctions from mapped RNA-seq reads, which are representative of introns that are removed from pre-mRNA, were extracted. Next, alternatively excised introns, which are comprised of two more overlapping introns (e.g. introns that share a splice site), were clustered together. Finally, differential intron excision events across conditions were tested using LeafCutter (Li et al., 2018).
Statistical analysis
All immunohistochemistry and electron microscopy data obtained from experimental and control mice were compared with a two-tailed unpaired Student’s t-test. Data were presented as mean + SEM. A p value of less-than 0.05 was considered significant. Analysis was done using GraphPad Prism version 6.00 for Windows (GraphPad Software) and Microsoft Office Excel 2010.
Data Availability
The sequencing data have been deposited to the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under accession number: GSE124244.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-METTL14 | Sigma-Aldrich | Cat# HPA038002, RRID:AB_10672401 |
| Goat anti-PDGFR-α | R and D systems | Cat# AF1062, RRID:AB_2236897 |
| Rabbit anti-MBP | Abcam | Cat# ab40390, RRID:AB_1141521 |
| Mouse anti-CC1 | Milipore | Cat# OP80, RRID:AB_2057371 |
| Mouse anti-Olig2 | Milipore | Cat# MABN50, RRID:AB_10807410 |
| Mouse anti-O1 | R and D systems | Cat# MAB1327, RRID:AB_357618 |
| Rabbit anti-Ki67 | Abcam | Cat# AB15580, RRID:AB_805388 |
| Rabbit anti-MAG | Thermo Fisher Scientific | Cat# 34–6200, RRID:AB_2533179 |
| Mouse anti-MBP | Biolegend | Cat# SMI 99, RRID:AB_2314771 |
| Mouse anti-MYRF | Generous gift from Dr. Ben Emery | Cat# 4G4, RRID:AB_2814997 |
| Mouse anti-GAPDH | Cell signaling | Cat# 2118, RRID:AB_561053 |
| Chicken anti-Neurofascin, pan | R and D systems | Cat# AF3235, RRID:AB_10890736 |
| Rabbit anti-Caspr | Abcam | Cat# ab34151, RRID:AB_869934 |
| Mouse anti-Sodium channel, pan | Sigma-Aldrich | Cat# S8809, RRID:AB_477552 |
| Rabbit anti-Choline acetyltransferase(ChAT) | Milipore | Cat# AB144P, RRID:AB_2079751 |
| Goat anti-mouse IgG+IgM | Jackson ImmunoResearch | Cat# 115-055-044, RRID:AB_2338532 |
| Goat anti-mouse IgM | Jackson ImmunoResearch | Cat# 115-005-020, RRID:AB_2338450 |
| Mouse IgG HRP Linked Whole Antibody | GE Healthcare | Cat# NA931, RRID:AB_772210 |
| Rabbit IgG HRP-Linked Whole Antibody | GE Healthcare | Cat# NA934, RRID:AB_772206 |
| Rabbit anti-Chicken IgY (H+L) Secondary Antibody, HRP | Thermo Fisher Scientific | Cat# 31401, RRID:AB_228385 |
| Donkey Anti-mouse IgG (H+L), Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21202, RRID:AB_141607 |
| Donkey Anti-Rabbit IgG (H+L) Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-21206, RRID:AB_2535792 |
| Donkey Anti-Mouse IgG (H+L), Alexa Fluor 594 | Thermo Fisher Scientific | Cat# A-21203, RRID:AB_2535789 |
| Donkey Anti-Goat IgG (H+L), Alexa Fluor 594 | Thermo Fisher Scientific | Cat# A-11058, RRID:AB_2534105 |
| Donkey Anti-Rabbit IgG (H+L), Alexa Fluor 594 | Thermo Fisher Scientific | Cat# A-21207, RRID:AB_141637 |
| Goat anti-Chicken IgY (H+L) Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | Cat# A-11042, RRID:AB_2534099 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RNAscope® Probe –Mm-Myrf | ACD bio | Cat# 524061 |
| RNAscope® Probe –Mm-Mbp | ACD bio | Cat# 451491 |
| Dynabeads Oligo(dT)25 | Thermo Fisher Scientific | Cat# 61006 |
| Dynabeads Protein A | Thermo Fisher Scientific | Cat# 1001D |
| N6-Methyladenosine 5′-monophosphate sodium salt | Sigma-Aldrich | Cat# M2780 |
| Poly-D-lysine | Sigma-Aldrich | Cat# P6407 |
| Platelet derived growth factor | PeoroTech | Cat# 100–13A |
| Neurotrophin-3 | PeproTech | Cat# 450–03 |
| Ciliary neurotrophic factor | PeproTech | Cat# 450–13 |
| Forskolin | Sigma-Alderich | Cat# F6886 |
| B27 | Life technologies | Cat# 17504044 |
| Fetal bovine serum | Altanta biologicals | Cat# S11050 |
| Normal donkey serum | Jacskon Immunoresearch | Cat# 017-000-121 |
| Protease inhibitor cocktail | Thermo Fisher Scientific | Cat# 78430 |
| Phosphatase inhibitors | Sigma-Aldrich | Cat# P2850 and P5726 |
| Laemmli sample buffer | Bio-Rad Laboratories | Cat# 161–0737 |
| β-mercaptoethanol | Sigma-Aldrich | Cat# M6250 |
| Trizol reagent | Thermo Fisher Scientific | Cat# 15596018 |
| Triiodothyronine | Sigma-Aldrich | Cat# T6397 |
| Trypsin 0.05% | Thermo Fisher Scientific | Cat# 25300–054 |
| Trypsin 2.5% | Thermo Fisher Scientific | Cat# 15090046 |
| Trypsin inhibitor | Worthington | Cat# LS003086 |
| Deoxyribonuclease I | Worthington | Cat# LS002007 |
| Papain | Worthington | Cat# LS003126 |
| Apo transferrin | Sigma-Aldrich | Cat# T1147 |
| ProLong gold abtifade reagent with DAPI | Life technologies | Cat# P36931 |
| Paraformadehyde | Thermo Fisher Scientific | Cat# T353–500 |
| Osmium Tetroxide | Electron microscopy Science | Cat# 19152 |
| Propylene Oxide | Electron microscopy Science | Cat# 20401 |
| Sodium Cacodylate Buffer | Electron microscopy Science | Cat# 11652 |
| Epon 812 | Electron microscopy Science | Cat# 14900 |
| Critical Commercial Assays | ||
| BCA Protein Assay Kit | Thermo Fisher Scientic | Cat# 23255 |
| Aurum Total RNA mini Kit | Bio-Rad Laboratories | Cat# 732–6820 |
| Agilent RNA 6000 Nano kit with chips | Agilent | Cat# 5067–1511 |
| RNA Clean & Concentrator | Zymo | Cat# R1015 |
| NEBNext Ultra RNA library Prep kit for Illumina | New England Biolabs | Cat# 61011 |
| RNA Fragmentation Reagents | Thermo Fisher Scientific | Cat# AM8740 |
| Dynabeads Oligo(dT)25 | Thermo Fisher Scientific | Cat# 61006 |
| SuperSignal West Dura Extended Duration Substrate | Thermo Fisher Scientific | Cat# 34076 |
| Pierce™ ECL Western Blotting Substrate | Thermo Fisher Scientific | Cat# 32209 |
| SMARTScribe Reverse Transcriptase | Clontech | Cat# 639537 |
| Protein A Dynabeads | Thermo Fisher Scientific | Cat# 0002D |
| Advantage 2 Polymerase Mix | Thermo Fisher Scientific | Cat# 639201 |
| Agencourt AMPure XP | Beckman Coulter | Cat# A63880 |
| RNAScope® Multiplex Fluorescent V2 Assay kit | ACD bio | Cat# 323110 |
| Basic NucleofectorTM Kit for Primary Mammalian Glial Cells | Lonza | Cat# VPI-1006 |
| Opal™ 520 | Akoya Biosciences | Cat# FP1487001KT |
| Opal™ 620 | Akoya Biosciences | Cat# FP1495001KT |
| Deposited Data | ||
| Raw and analyzed data | This paper | GEO: GSE124244 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Mettl14fl/fl | Generous gift from Dr. Xiaoxi Zhuang | ( Koranda et al., 2018; Weng et al., 2018b; Yoon et al., 2017) |
| Mouse: Olig2-Cre | Generous gift from Dr. David Rowitch | The Jackson Laboratory: 011103 |
| Mouse: CNP-Cre | Generous gift from Dr. Klaus Amin Nave | MGI: 3051635 |
| Oligonucleotides | ||
| RT-PCR primers for Nfasc aberrant spliced locus, see Figure7. Forward: ACTGGGAAAGCAGATGGTGG Reverse: ACATGAGCCCGATGAACCAG |
This paper | N/A |
| Recombinant DNA | ||
| Mettl14 (NM_201638) Mouse Tagged ORF Clone | OriGene | Cat# MR207291 |
| pmaxGFP™ vector | Lonza | Cat# VPI-1006 |
| Software and Algorithms | ||
| Image J | National Institutes of Health | RRID:SCR_003070 |
| Image Lab | Bio-Rad Laboratories | RRID:SCR_014210 |
| R v3.5.1 | R core team | RRID:SCR_001905 |
| Bioconductor v.3.7 | (Huber et al., 2015) | RRID:SCR_006442 |
| STAR v2.6.1a | (Dobin et al., 2013) | RRID:SCR_015899 |
| Trimmomatic | (Bolger et al., 2014) | RRID:SCR_011848 |
| Kallisto v0.44.0 | (Bray et al., 2016) | RRID:SCR_016582 |
| GraphPad Prism 6 | GraphPad Software | RRID:SCR_002798 |
| LeafCutter | (Li et al.,2018) | RRID:SCR_017639 |
Highlights:
Oligodendrocyte maturation is accompanied by modifications in m6A mRNA methylation
m6A mRNA methylation is required for oligodendrocyte maturation and CNS myelination
m6A mRNA methylation regulates the transcriptomes of oligodendrocyte lineage cells
Proper neurofascin mRNA splicing in oligodendrocytes requires m6A methylation
Acknowledgements
This work was supported by the NIH (R01NS520340 to B.P., R35NS097370 to G-l.M.), the National Multiple Sclerosis Society (RG-1501-02797 to B.P.), and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation to B.P. and G.-l.M. We thank the Genomics facility at the University of Chicago, and the Bioinformatics core at the University of Illinois of Chicago for assistance. The Bioinformatics analysis in the project described was performed by the UIC Research Informatics Core, supported in part by NCATS through Grant UL1TR002003. We also thank Dr. Ben Emery for generously providing MYRF antibody for this project; and Dr. Vytas Bindokas, Dr. Xiaochang Zhang, Erdong Liu, Gloria Wright and Ani Solanki for critical advice and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Aaker JD, Elbaz B, Wu Y, Looney TJ, Zhang L, Lahn BT, and Popko B (2016). Transcriptional fingerprint of hypomyelination in zfp191null and shiverer (mbpshi) mice. ASN Neuro 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, and Tavazoie SF (2015). HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, et al. (2014). m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, and Yekutieli D (2005). False discovery rate–adjusted multiple confidence intervals for selected parameters. J. Am. Stat. Assoc 100, 71–81. [Google Scholar]
- Bergles DE, and Richardson WD (2015). Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect. Biol 8, a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, and Pachter L (2016). Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol 34, 525–527. [DOI] [PubMed] [Google Scholar]
- Brockschnieder D, Lappe-Siefke C, Goebbels S, Boesl MR, Nave K-A, and Riethmacher D (2004). Cell depletion due to diphtheria toxin fragment A after Cre-mediated recombination. Mol. Cell. Biol 24, 7636–7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TL, and Verden DR (2017). Cytoskeletal regulation of oligodendrocyte differentiation and myelination. J. Neurosci 37, 7797–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujalka H, Koenning M, Jackson S, Perreau VM, Pope B, Hay CM, Mitew S, Hill AF, Lu QR, Wegner M, et al. (2013). MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol. 11, e1001625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JH, Worboys K, Ainger K, and Barbarese E (1997). Translocation of myelin basic protein mRNA in oligodendrocytes requires microtubules and kinesin. Cell Motil. Cytoskeleton 38, 318–328. [DOI] [PubMed] [Google Scholar]
- Colman DR, Kreibich G, Frey AB, and Sabatini DD (1982). Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol 95, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coots RA, Liu X-M, Mao Y, Dong L, Zhou J, Wan J, Zhang X, and Qian S-B (2017). m6A Facilitates eIF4F-Independent mRNA Translation. Mol. Cell 68, 504–514.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray S, Huynh JL, Sher F, Casaccia-Bonnefil P, and Boddeke E (2009). Epigenetic mechanisms facilitating oligodendrocyte development, maturation, and aging. Glia 57, 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz B, and Popko B (2019). Molecular control of oligodendrocyte development. Trends Neurosci. 42, 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, and Dugas JC (2013). Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb. Protoc 2013, 854–868. [DOI] [PubMed] [Google Scholar]
- Fields RD, and Dutta DJ (2019). Treadmilling model for plasticity of the myelin sheath. Trends Neurosci. 42, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Harada BT, Behm M, and He C (2018). RNA modifications modulate gene expression during development. Science 361, 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Dominissini D, Rechavi G, and He C (2014). Gene expression regulation mediated through reversible m6A RNA methylation. Nat. Rev. Genet 15, 293–306. [DOI] [PubMed] [Google Scholar]
- Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. (2015). Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347, 1002–1006. [DOI] [PubMed] [Google Scholar]
- Han SP, Friend LR, Carson JH, Korza G, Barbarese E, Maggipinto M, Hatfield JT, Rothnagel JA, and Smith R (2010). Differential subcellular distributions and trafficking functions of hnRNP A2/B1 spliceoforms. Traffic 11, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harroch S, Furtado GC, Brueck W, Rosenbluth J, Lafaille J, Chao M, Buxbaum JD, and Schlessinger J (2002). A critical role for the protein tyrosine phosphatase receptor type Z in functional recovery from demyelinating lesions. Nat. Genet 32, 411–414. [DOI] [PubMed] [Google Scholar]
- Haussmann IU, Bodi Z, Sanchez-Moran E, Mongan NP, Archer N, Fray RG, and Soller M (2016). m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature 540, 301–304. [DOI] [PubMed] [Google Scholar]
- Hernandez M, and Casaccia P (2015). Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage. Glia 63, 1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek KS, Kidd GJ, Carson JH, and Smith R (1998). hnRNP A2 selectively binds the cytoplasmic transport sequence of myelin basic protein mRNA. Biochemistry 37, 7021–7029. [DOI] [PubMed] [Google Scholar]
- Howell OW, Palser A, Polito A, Melrose S, Zonta B, Scheiermann C, Vora AJ, Brophy PJ, and Reynolds R (2006). Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain 129, 3173–3185. [DOI] [PubMed] [Google Scholar]
- Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, et al. (2015). Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, Monahan J, Carrieri C, Enright AJ, and O’Carroll D (2017). The RNA m6A Reader YTHDF2 Is Essential for the Post-transcriptional Regulation of the Maternal Transcriptome and Oocyte Competence. Mol. Cell 67, 1059–1067.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura N, Yamasaki R, Yonekawa T, Matsushita T, Kusunoki S, Nagayama S, Fukuda Y, Ogata H, Matsuse D, Murai H, et al. (2013). Anti-neurofascin antibody in patients with combined central and peripheral demyelination. Neurology 81, 714–722. [DOI] [PubMed] [Google Scholar]
- Kira J-I, Yamasaki R, and Ogata H (2018). Anti-neurofascin autoantibody and demyelination. Neurochem. Int 104360. [DOI] [PubMed] [Google Scholar]
- Koranda JL, Dore L, Shi H, Patel MJ, Vaasjo LO, Rao MN, Chen K, Lu Z, Yi Y, Chi W, et al. (2018). Mettl14 is essential for epitranscriptomic regulation of striatal function and learning. Neuron 99, 283–292.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreman E, Sun X, and Lu QR (2018). Chromatin remodeling and epigenetic regulation of oligodendrocyte myelination and myelin repair. Mol. Cell. Neurosci 87, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, and Nave K-A (2003). Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet 33, 366–374. [DOI] [PubMed] [Google Scholar]
- Li H, and Richardson WD (2009). Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration. Nat. Neurosci 12, 815–817. [DOI] [PubMed] [Google Scholar]
- Li YI, Knowles DA, Humphrey J, Barbeira AN, Dickinson SP, Im HK, and Pritchard JK (2018). Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet 50, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, et al. (2017). FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6-Methyladenosine RNA Demethylase. Cancer Cell 31, 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht K, and Jantsch MF (2016). Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. J. Cell Biol 213, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. (2014). A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol 10, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Moyon S, Hernandez M, and Casaccia P (2016). Epigenetic control of oligodendrocyte development: adding new players to old keepers. Curr. Opin. Neurobiol 39, 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, and Casaccia-Bonnefil P (2002). Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J. Neurosci 22, 10333–10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, and Emery B (2014). Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 276, 29–47. [DOI] [PubMed] [Google Scholar]
- Montague P, McCallion AS, Davies RW, and Griffiths IR (2006). Myelin-associated oligodendrocytic basic protein: a family of abundant CNS myelin proteins in search of a function. Dev Neurosci 28, 479–487. [DOI] [PubMed] [Google Scholar]
- Moyon S, and Casaccia P (2017). DNA methylation in oligodendroglial cells during developmental myelination and in disease. Neurogenesis (Austin) 4, e1270381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C, Bauer NM, Schäfer I, and White R (2013). Making myelin basic protein -from mRNA transport to localized translation. Front. Cell Neurosci 7, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Heinrich M, Heck S, Blohm D, and Richter-Landsberg C (1997). Expression of microtubule-associated proteins MAP2 and tau in cultured rat brain oligodendrocytes. Cell Tissue Res. 288, 239–249. [DOI] [PubMed] [Google Scholar]
- Nave K-A, and Werner HB (2014). Myelination of the nervous system: mechanisms and functions. Annu. Rev. Cell Dev. Biol 30, 503–533. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, and Zhu X (2009). Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci 10, 9–22. [DOI] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Björklund AK, Winberg G, Sagasser S, and Sandberg R (2014). Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc 9, 171–181. [DOI] [PubMed] [Google Scholar]
- Pillai AM, Thaxton C, Pribisko AL, Cheng J-G, Dupree JL, and Bhat MA (2009). Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J. Neurosci. Res 87, 1773–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomicter AD, Shroff SM, Fuss B, Sato-Bigbee C, Brophy PJ, Rasband MN, Bhat MA, and Dupree JL (2010). Novel forms of neurofascin 155 in the central nervous system: alterations in paranodal disruption models and multiple sclerosis. Brain 133, 389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanelli AM, and Appel B (2015). Motor neurons and oligodendrocytes arise from distinct cell lineages by progenitor recruitment. Genes Dev. 29, 2504–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Luo G-Z, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al. (2017). YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH (2004). Glial specification in the vertebrate neural tube. Nat. Rev. Neurosci 5, 409–419. [DOI] [PubMed] [Google Scholar]
- Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han Y-G, Huillard E, Sun T, Ligon AH, Qian Y, et al. (2008). Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJH, Cottrell DF, et al. (2005). Neurofascins are required to establish axonal domains for saltatory conduction. Neuron 48, 737–742. [DOI] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, and He C (2017). YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 27, 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, and Nave K-A (2015). Oligodendrocytes: myelination and axonal support. Cold Spring Harb. Perspect. Biol 8, a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer, and Schachner (1981). Monoclonal Antibodies (01 to 04) to Oligodendrocyte Cell Surfaces: An lmmunocytological Study in the Central Nervous System. Dev. Biol 83. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Ayukawa N, Okada C, Tanaka M, Takekoshi S, Iijima Y, and Iijima T (2017). Spatio-temporal and dynamic regulation of neurofascin alternative splicing in mouse cerebellar neurons. Sci. Rep 7, 11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakurela S, Garding A, Jung RB, Müller C, Goebbels S, White R, Werner HB, and Tiwari VK (2016). The transcriptome of mouse central nervous system myelin. Sci. Rep 6, 25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Pillai AM, Pribisko AL, Labasque M, Dupree JL, Faivre-Sarrailh C, and Bhat MA (2010). In vivo deletion of immunoglobulin domains 5 and 6 in neurofascin (Nfasc) reveals domain-specific requirements in myelinated axons. J. Neurosci 30, 4868–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. (2014). N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, and He C (2015). N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ, Zhang Z, Ogawa Y, Kellis M, Duester G, et al. (2018). N6-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat. Neurosci 21, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, Shi H, Skibbe J, Shen C, Hu C, et al. (2018a). METTL14 Inhibits Hematopoietic Stem/Progenitor Differentiation and Promotes Leukemogenesis via mRNA m6A Modification. Cell Stem Cell 22, 191–205.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y-L, Wang X, An R, Cassin J, Vissers C, Liu Y, Liu Y, Xu T, Wang X, Wong SZH, et al. (2018b). Epitranscriptomic m6a regulation of axon regeneration in the adult mammalian nervous system. Neuron 97, 313–325.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Li A, Sun B, Sun J-G, Zhang J, Zhang T, Chen Y, Xiao Y, Gao Y, Zhang Q, et al. (2019). A novel m6A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 29, 23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Adhikari S, Dahal U, Chen Y-S, Hao Y-J, Sun B-F, Sun H-Y, Li A, Ping X-L, Lai W-Y, et al. (2016). Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 61, 507–519. [DOI] [PubMed] [Google Scholar]
- Xu K, Yang Y, Feng G-H, Sun B-F, Chen J-Q, Li Y-F, Chen Y-S, Zhang X-X, Wang C-X, Jiang L-Y, et al. (2017). Mettl3-mediated m6A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 27, 1100–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao X, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, et al. (2009). HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci 12, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K-J, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim N-S, Zhu Y, Zheng L, et al. (2017). Temporal control of mammalian cortical neurogenesis by m6a methylation. Cell 171, 877–889.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Liu J, and He C (2015). RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 29, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chen Y, Sun B, Wang L, Yang Y, Ma D, Lv J, Heng J, Ding Y, Xue Y, et al. (2017). m6A modulates haematopoietic stem and progenitor cell specification. Nature 549, 273–276. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BS, and He C (2017). Gamete On” for m(6)A: YTHDF2 Exerts Essential Functions in Female Fertility. Mol. Cell 67, 903–905. [DOI] [PubMed] [Google Scholar]
- Zhao L, Mandler MD, Yi H, and Feng Y (2010a). Quaking I controls a unique cytoplasmic pathway that regulates alternative splicing of myelin-associated glycoprotein. Proc. Natl. Acad. Sci. USA 107, 19061–19066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi Q-S, Xin M, et al. (2010b). MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65, 612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang Y, Sun B-F, Shi Y, Yang X, Xiao W, Hao Y-J, Ping X-L, Chen Y-S, Wang W-J, et al. (2014). FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 24, 1403–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, and Anderson DJ (2002). The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wan J, Shu XE, Mao Y, Liu X-M, Yuan X, Zhang X, Hess ME, Brüning JC, and Qian S-B (2018). N6-Methyladenosine Guides mRNA Alternative Translation during Integrated Stress Response. Mol. Cell 69, 636–647.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Ż, Pan JN, He C, Parisien M, and Pan T (2019). Regulation of Co-transcriptional Pre-mRNA Splicing by m6A through the Low-Complexity Protein hnRNPG. Mol. Cell 76, 70–81.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, and Brophy PJ (2008). Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J. Cell Biol 181, 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, and Barres BA (2013). Intrinsic and extrinsic control of oligodendrocyte development. Curr. Opin. Neurobiol 23, 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, and Barres BA (2015). Glia in mammalian development and disease. Development 142, 3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data have been deposited to the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under accession number: GSE124244.


