Abstract
Cardiogenic shock in the setting of acute myocardial infarction remains a major cause of morbidity and mortality. In fact, acute myocardial infarction accounts for 81% of patients in cardiogenic shock. Despite advances in pharmacologic and device-based approaches to support patients with cardiogenic shock, no significant improvement in mortality has been observed over the past 20 years, although multiple registries are providing new insight into this complex syndrome. Key elements for optimal treatment include integration of hemodynamic and metabolic data for diagnosis and risk stratification, early evaluation and appropriate initiation of acute mechanical circulatory support devices, and an organized algorithmic approach to decision making.
Keywords: cardiogenic shock, acute myocardial infarction, acute mechanical circulatory support, shock classification, early revascularization, risk assessment
INTRODUCTION
Approximately every 40 seconds, someone in the United States experiences a myocardial infarction. Acute myocardial infarction (AMI) accounts for roughly 80% of patients in cardiogenic shock (CS).1 In this scenario, CS commonly manifests with left ventricular failure. However, in 20% of cases, CS is due to a complication associated with AMI, such as acute mitral regurgitation, ventricular septal defect, or subacute or acute free wall rupture. Right heart failure may also contribute to CS and is more commonly associated with right ventricular myocardial infarction.
Of the 805,000 heart attacks that occur each year in the United States, an estimated 38% are due to ST-elevation myocardial infarction (STEMI).2 Electrocardiographic criteria for STEMI have been well defined by the American College of Cardiology, American Heart Association, European Society of Cardiology, and World Heart Federation (Table 1).3,4 STEMI is associated with a 2-fold increased risk for developing CS.5
Table 1.
| ST-ELEVATION MYOCARDIAL INFARCTION (STEMI) |
The American College of Cardiology, American Heart Association, European Society of Cardiology, and World Heart Federation committee established the following electrocardiogram (ECG) criteria for STEMI:
|
A new left bundle branch block is considered a STEMI equivalent. Patients with a pre-existing left bundle branch block can be further evaluated using Scarbossa's criteria:
|
| NON–ST-ELEVATION MYOCARDIAL INFARCTION (NSTEMI) |
| NSTEMI is diagnosed in patients who have symptoms consistent with acute cardiogenic shock and troponin elevation but without ECG changes consistent with STEMI. Unstable angina and NSTEMI differ primarily in the presence or absence of detectable troponin leak. Myocardial injury is expected at troponin levels exceeding the 99th percentile upper reference limit, which is specified for each individual assay. Specific cutoffs can be found in manufacturers' package inserts, in peer-reviewed publications, and on the International Federation of Clinical Chemistry and Laboratory Medicine website. |
PATIENT OUTCOMES IN CARDIOGENIC SHOCK
Recent analyses of the National Inpatient Sample report that the incidence of CS among STEMI patients increased from 6.5% to 10.1% between 2003 and 2010. It further showed that in-hospital mortality for these patients decreased from 44.6% to 33.8% over the same time period.6 Similar findings were reported in an analysis of the same database from 2003 to 2011. In this analysis, the National Cardiovascular Data Registry (NCDR) reported that 12.2% of STEMI patients experienced CS between 2007 and 2011, with an in-hospital mortality rate of 33.1% for STEMI complicated by CS. The authors also observed that CS was less prevalent (4.3%) among non-STEMI patients but was associated with a higher in-hospital mortality rate of 40.8%.7
Despite these registry data, randomized clinical trials continue to report mortality rates between 40% and 60% for patients with AMI and shock (Table 2). In 1999, the SHOCK (Should we Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial reported 30-day mortality rates of 41% for patients younger than age 75 who received early revascularization and 75% for patients older than 75. While the SHOCK trial failed to show improvement in its primary end point of 30-day mortality, it was the first trial to report improved 6-month outcomes (secondary end point) associated with early revascularization among the entire patient cohort. However, among patients older than age 75, both 30-day and 6-month mortality were worse when receiving early revascularization compared to medical therapy alone.8 The long-term benefits of early revascularization were confirmed by the same group at 10 years of follow-up.9
Table 2.
Recent clinical trials evaluating treatment options in cardiogenic shock. IABP: intra-aortic balloon pump; PCI: percutaneous coronary intervention; RRT: renal replacement therapy; SHOCK: Should we Emergently Revascularize Occluded Coronaries for Cardiogenic Shock trial; IABP-SHOCK II: Intra-Aortic Balloon Pump Support for Myocardial Infarction with Cardiogenic Shock trial; IMPRESS: Impella Versus IABP Reduces Mortality in STEMI Patients Treated with Primary PCI in Severe Cardiogenic Shock trial; CULPRIT SHOCK: Culprit Lesion-Only PCI Versus Multivessel PCI in Cardiogenic Shock trial; DCSI: Detroit Cardiogenic Shock Initiative
| TRIAL | YEAR | PATIENTS | PATIENT POPULATION | TREATMENT ARMS | PRIMARY END POINT(S) | RESULTS |
|---|---|---|---|---|---|---|
| SHOCK | 1999 | 302 | Shock due to left ventricular failure complicating myocardial infarction | Emergency revascularization or initial medical stabilization | 30-day all-cause mortality | No significant difference in mortality between treatment groups (46.7% vs 56.0%; P = .11) |
| IABP – SHOCK II | 2012 | 600 | Shock complicating acute myocardial infarction | Intra-aortic balloon counterpulsation or no intra-aortic balloon counterpulsation | 30-day all-cause mortality | No significant difference in mortality between treatment groups (39.7% vs 41.3%; P = .69) |
| IMPRESS | 2016 | 48 | Severe shock complicating acute myocardial infarction | Impella CP or intra-aortic balloon pump | 30-day all-cause mortality | No significant different in mortality between Impella CP and IABP (46% vs 50%, P = .96) |
| CULPRIT SHOCK | 2017 | 706 | Shock presenting with multivessel disease and acute myocardial infarction | Culprit-lesion-only PCI or immediate multivessel PCI | 30-day mortality and renal failure requiring RRT | Culprit-lesion-only PCI was associated with slightly decreased risk of mortality (OR: 0.84, P = .03) compared to multivessel PCI, but the risk of RRT did not differ (OR: 0.71, P = .07) |
| DCSI | 2018 | 41 | Acute myocardial infarction complicated by cardiogenic shock | Single treatment arm of invasive hemodynamic monitoring and rapid initiation of non-IABP MCS | Survival to explant and change in CPO | Survival to explant was 85% vs 51% in historical controls (P < .001), and post-procedure CPO increased 67% compared to pre-procedure CPO (P < .001) |
More recently, the IABP-SHOCK II (Intra-Aortic Balloon Pump Support for Myocardial Infarction with Cardiogenic Shock) trial reported that the use of an intra-aortic balloon pump for AMI (both STEMI and non-STEMI) was not superior to medical therapy alone in reducing 30-day mortality. Mortality rates in this trial ranged between 39.7% and 41.3% in both treatment arms. In 2017, the CULPRIT SHOCK (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) trial reported that culprit-lesion-only intervention was associated with reduced 30-day mortality (43.3%) compared to multivessel intervention (51.6%) for AMI complicated by CS (AMI-CS) (P = .03).10 The Detroit Cardiogenic Shock Initiative, a single-arm trial, found that early mechanical circulatory support (MCS) intervention was associated with improved survival to explant compared to historical controls (85% vs 51%, P < .001).11 Despite this promising risk reduction, collective trial data are not conclusive and suggest that mortality due to AMI with CS remains largely unchanged over the past two decades.
CLASSIFICATION OF CARDIOGENIC SHOCK
One explanation for varying results for AMI-CS outcomes in clinical trials may be the lack of clear definitions for CS severity. The original Killip and Diamond-Forrester classifications for shock severity in AMI used physical exam findings and hemodynamic data to assess perfusion and congestion. The more recent Diamond-Forrester classification defined four clinical subsets of shock: (I) no pulmonary congestion or peripheral hypoperfusion, (II) isolated pulmonary congestion, (III) isolated peripheral hypoperfusion, and (IV) both pulmonary congestion and hypoperfusion. These subsets correlated with cardiac index and pulmonary capillary wedge pressure and were associated with increasing mortality (Table 3).12,13 Since the initial development of this classification system, several consensus statements have highlighted a further need for improved definitions for diagnosing CS, additional classification systems to gauge the severity of CS, algorithms for using acute MCS (AMCS) devices, and a better understanding of hemodynamic-guided decision making in CS.
Table 3.
| CLASS | CARDIAC INDEX (MM/MIN) | PCWP (MM HG) | MORTALITY (%) |
|---|---|---|---|
| I | > 2 | < 18 | 3 |
| II | > 2 | > 18 | 9 |
| III | < 2 | < 18 | 23 |
| IV | < 2 | > 18 | 51 |
| I | > 2 | < 18 | 3 |
| II | > 2 | > 18 | 9 |
| III | < 2 | < 18 | 23 |
| IV | < 2 | > 18 | 51 |
In response to these unmet needs, the Society for Cardiovascular Angiography and Interventions (SCAI) recently proposed a staging system for CS based on expert consensus opinion. The purpose of the SCAI classification scheme is to facilitate patient care and research in CS by providing a uniform system for identifying patients who have an increased risk of dying (Table 4).14 The SCAI system included five classes of CS: (A) at risk for CS, (B) beginning CS, (C) classic CS, (D) deteriorating CS, and (E) extreme CS. Each stage is defined by a physical exam along with biochemical and hemodynamic findings. These definitions were intentionally left more general to accommodate the variability of clinical parameters available at the time of presentation. Stages C, D, and E include patients who require inotrope, vasopressor, or short-term MCS therapy for CS. Stage D patients represent Stage C patients with clinical deterioration, and Stage E patients are those who have deteriorated even further.14 The clinical utility of the SCAI staging system is currently under investigation.
Table 4.
The Society for Cardiovascular Angiography and Interventions classification scheme.13 CS: cardiogenic shock; JVP: jugular venous pressure; SBP: systolic blood pressure; CVP: central venous pressure; PA sat: pulmonary artery saturation; BNP: brain natriuretic peptide; MAP: mean arterial pressure; PCWP: pulmonary capillary wedge pressure; RAP: right atrial pressure; PAPI: pulmonary artery pulsatility index; GFR: glomerular filtration rate; LFT: liver function test; CPR: cardiopulmonary resuscitation; ECMO: extracorporeal membrane oxygenation; BiPaP: bilevel positive airway pressure
| STAGE | DESCRIPTION | PHYSICAL EXAM/BEDSIDE FINDINGS | BIOCHEMICAL MARKERS | HEMODYNAMICS |
|---|---|---|---|---|
| A At risk | Patient is not currently experiencing signs or symptoms of CS but is at risk for its development. Patient may have large acute myocardial infarction or prior infarction and/or acute on chronic heart failure symptoms. |
|
|
Normotensive (SBP ⩾ 100 mm Hg or normal) If hemodynamics done:
|
| B Beginning CS | Patient has clinical evidence of relative hypotension or tachycardia without hypoperfusion. |
|
|
SBP < 90 mm Hg or MAP < 60 mm Hg or > 30 mm Hg drop from baseline Pulse ≥ 100 If hemodynamics done:
|
| C Classic CS | Patient manifests with hypoperfusion that requires intervention (inotrope, pressor, or mechanical support, including ECMO) beyond volume resuscitation to restore perfusion. These patients typically present with relative hypotension. |
May include:
|
May include:
|
May include: SBP < 90 mm Hg or MAP < 60 mm Hg or > 30 mm Hg drop from baseline and drugs/device used to maintain BP above these targets Hemodynamics:
|
| D Deteriorating/doom | Patient is similar to category C but is getting worse and failing to respond to initial interventions. | Any of stage C | Any of Stage C and: deteriorating | Any of Stage C and: requiring multiple pressors or addition of mechanical circulatory support devices to maintain perfusion |
| E Extremis | Patient is experiencing cardiac arrest with ongoing CPR and/or ECMO, being supported by multiple interventions. |
|
“Trying to die” CPR (A-modifier) pH ⩽ 7.2 Lactate ⩾ 5 mmol/L |
|
Several registries have also begun to provide new insight into CS. The Cardiogenic Shock Working Group (CSWG) is a national multicenter retrospective registry that uses real-world experiences of patients with CS. Initiated in 2017, the CSWG registry now includes more than 1,500 patients and contains hemodynamic data for analysis of more than 1,000 patients. This is the largest contemporary US registry for any-cause CS and includes experiences with all AMCS device platforms. Due to its diverse patient profile and availability of hemodynamic data, this registry could provide valuable insight by stratifying mortality risk by different etiologies, comorbidities, and treatments of shock to inform future randomized clinical trials.15
The National Cardiogenic Shock Initiative (NCSI) is a prospective registry that will determine if implementation of an algorithm to identify CS—using hemodynamic data and early initiation of AMCS with the Impella CP device—improves clinical outcomes among patients with AMI-CS. A recent analysis from the NCSI registry involving 171 patients with AMI-CS reported 72% survival-to-discharge rate; it also identified predictors of in-hospital mortality, including age > 70 years, creatinine > 2.0, lactate > 4.0, and cardiac power output < 0.6 W.16
More recently, a group of physicians at the Inova Heart and Vascular Institute tested whether an algorithm focused on identification, stratification, and early initiation of AMCS in CS improves clinical outcomes (Figure 1).17 One year after implementing the algorithm, they reported a significant increase in 30-day survival (44% to 82%) among patients presenting with AMI-CS. They further determined that elevated lactate levels, vasopressor use, diabetes, dialysis, age, and low cardiac power output or pulmonary artery pulsatility index levels were associated with increased 30-day mortality.
Figure 1.
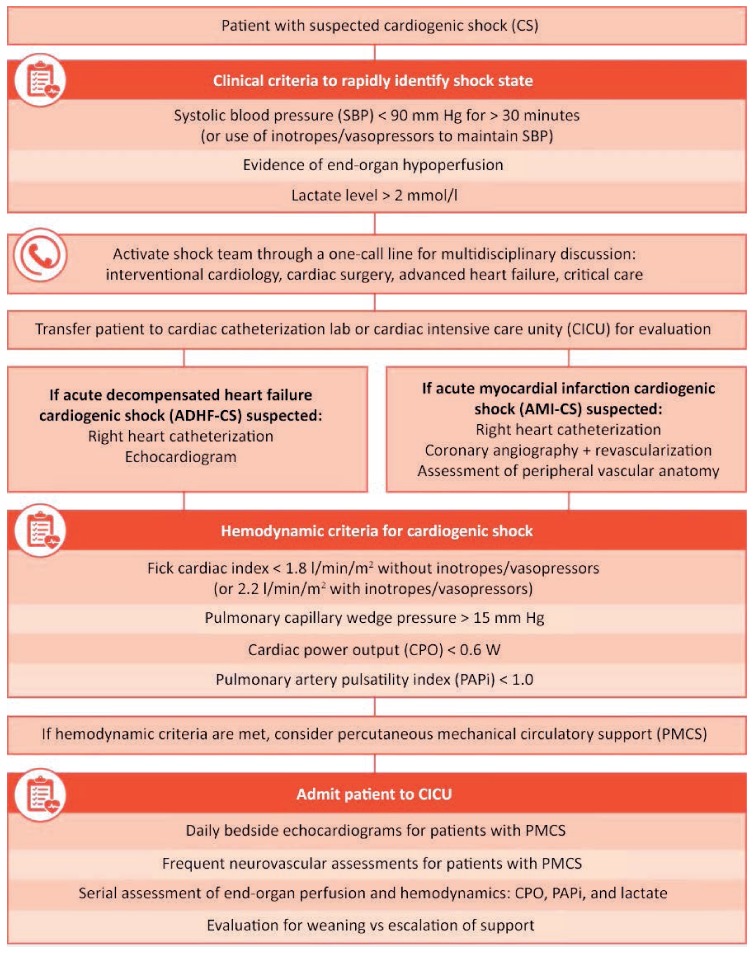
Cardiogenic Shock Algorithm developed by the INOVA Heart and Vascular Institute. Republished with permission of Elsevier Science & Technology Journals, from Standardized Team-Based Care for Cardiogenic Shock, Tehrani et al., 73(13), 2019.17
SUMMARY
The relatively minor improvements in CS outcomes over the past decades warrant a more rigorous scientific exploration of the syndrome. Past studies have helped identify elements necessary to specify and validate the optimal treatment of CS; these include integration of hemodynamic and metabolic data for diagnosis and risk stratification, early evaluation and appropriate initiation of AMCS devices, and an organized algorithmic approach to decision making. The lack of a standard CS treatment protocol may be a barrier to widespread use of a single classification system, but the diversity of classification systems being developed is no doubt a step towards broader implementation of CS classification. Recent initiatives, such as the SCAI Shock Classification Scheme, the Cardiogenic Shock Working Group Registry, the National Cardiogenic Shock Initiative, and the Inova Heart and Vascular Institute prospective registry, will provide much needed insight that may improve patient outcomes and the development of prospective studies testing the clinical utility of various treatment approaches.
KEY POINTS
Despite advances in pharmacologic and device-based approaches to support patients in cardiogenic shock, no significant improvement in mortality has been observed over the past 20 years.
Randomized clinical trials have failed to show clear superiority of therapeutic strategies. The heterogeneity of trial and registry data is due to an unmet need for appropriate shock classification criteria.
Key elements for the optimal treatment of cardiogenic shock include integration of hemodynamic and metabolic data for diagnosis and risk stratification, early evaluation and appropriate initiation of acute mechanical circulatory support devices, and an organized algorithmic approach to decision making.
Footnotes
Conflict of Interest Disclosure:
Dr. Kapur is a formal advisor for and conducts research on behalf of Abbott, Abiomed, Boston Scientific, LivaNova, Medtronic, MD Start, and preCARDIA.
REFERENCES
- 1.Harjola VP, Lassus J, Sionis A et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015 May;17(5):501–9. doi: 10.1002/ejhf.260. CardShock Study Investigators; GREAT network. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group M. Mozaffarian D, Benjamin EJ et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Foth C, Mountfort S. Acute Myocardial Infarction ST Elevation (STEMI) [Internet] Treasure Island, FL: StatPearls Publishing LLC; 2019. Jan, [cited 2019 Oct 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532281. [PubMed] [Google Scholar]
- 4.Thygesen K, Alpert JS, Jaffe AS et al. Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018 Nov 13;138(20):e618–e651. doi: 10.1161/CIR.0000000000000617. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. [DOI] [PubMed] [Google Scholar]
- 5.Vahdatpour C, Collins D, Goldberg S. Cardiogenic Shock. J Am Heart Assoc. 2019 Apr 16;8(8) doi: 10.1161/JAHA.119.011991. e011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolte D, Khera S, Aronow WS et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014 Jan 13;3(1) doi: 10.1161/JAHA.113.000590. e000590-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson ML, Peterson ED, Peng SA et al. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: A report from NCDR. Circ Cardiovasc Qual Outcomes. 2013 Nov;6(6):708–15. doi: 10.1161/CIRCOUTCOMES.113.000262. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999 Aug 26;341(9):625–34. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 9.Hochman JS, Sleeper LA, Webb JG et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006 Jun 7;295(21):2511–5. doi: 10.1001/jama.295.21.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiele H, Akin I, Sandri M et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017 Dec 21;377(25):2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 11.Basir MB, Schreiber T, Dixon S et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheter Cardiovasc Interv. 2018;91(3):454–61. doi: 10.1002/ccd.27427. [DOI] [PubMed] [Google Scholar]
- 12.Forrester JS, Diamond GA, Swan HJC. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol. 1977 Feb;39(2):137–45. doi: 10.1016/s0002-9149(77)80182-3. [DOI] [PubMed] [Google Scholar]
- 13.Killip T, 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. Am J Cardiol. 1967 Oct;20(4):457–64. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 14.Baran DA, Grines CL, Bailey S et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019 Jul 1;94(1):29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 15.Morine K, Jorde L, Razavi A et al. TCT-492 Multimodality Management of Cardiogenic Shock in the United States: Insights from the Cardiogenic Shock Working Group Registry. J Am Coll Cardiol. 2018 Sep;72(13 Suppl B):B197. [Google Scholar]
- 16.Basir MB, Kapur NK, Patel K et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019 Jun 1;93(7):1173–83. doi: 10.1002/ccd.28307. National Cardiogenic Shock Initiative Investigators. [DOI] [PubMed] [Google Scholar]
- 17.Tehrani BN, Truesdell AG, Sherwood MW et al. Standardized Team-Based Care for Cardiogenic Shock. J Am Coll Cardiol. 2019 Apr 9;73(13):1659–69. doi: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]


