Abstract
Cardiogenic shock (CS) is a complex syndrome of end-organ hypoperfusion that requires timely and thorough decision making. While many pathophysiologic and technical principles have been delineated in this issue, the purpose of this case-based report is to reflect upon some of these principles in the context of real-life scenarios. Given the obvious lacuna of evidence-based recommendations in CS, the authors provide a rationale for their decision-making process. The first case is a young post-heart-transplant patient with graft failure who was in a state of biventricular failure and restrictive physiology and required acute mechanical circulatory support (MCS). The second case is a patient who suffered a mechanical complication after experiencing an acute myocardial infarction that required MCS.
Keywords: heart failure, cardiogenic shock, mechanical support devices, pressure volume loop physiology
INTRODUCTION
Cardiogenic shock (CS) is a complex syndrome that may present as an acute or acute over chronic process. Although initial compensatory physiologic mechanisms allow transient stabilization of hemodynamics, these mechanisms become pathological over time and lead to disease progression. Therefore, management of CS requires timely and proficient decision making to optimize care. The following discussion reflects upon some of the principles of CS in the context of real-life case scenarios. A simulation software was used to represent pressure-volume loops in each case; these are for educational purposes only and should not be used to guide clinical decision making.1
CASE 1. CARDIOGENIC SHOCK DUE TO POST-HEART-TRANSPLANT GRAFT FAILURE
A 23-year-old female presented with symptoms of fatigue and shortness of breath 1 year post heart transplantation for hypertrophic cardiomyopathy. Associated symptoms included nausea, vomiting, and diarrhea for a few weeks prior to presenting with the same symptoms. Her initial vitals were blood pressure 89/69 mm Hg, heart rate 138 bpm, and oxygen saturation 97% on room air, and her lower extremities were cold to the touch. Her laboratory data included tacrolimus level 6 mg/dL (goal 10–12 mg/dL), creatinine 2.3 mg/dL (baseline 1 mg/dL), alanine aminotransferase 56 mg/dL, aspartate transaminase 55 mg/dL, total bilirubin 1.4 mg/dL, and lactic acid 3.5 mg/dL. Her chest x-ray showed mild cardiomegaly and pulmonary edema, and echocardiogram demonstrated new severe biventricular dysfunction with a left ventricular ejection fraction (LVEF) of approximately 25% and moderate-to-severe right ventricular dysfunction. Right heart catheterization showed a right atrial pressure of 20 mm Hg, pulmonary artery pressure of 28/20 with a mean of 23 mm Hg, and pulmonary capillary wedge pressure (PCWP) of 19 mm Hg with a pulmonary artery saturation of 27.6% (assumed arterial saturation was 98% with hemoglobin level of 11.4 mg/dL); her cardiac output calculated by the Fick method was 2.7 L/min and her cardiac index was 1.3 L/min/m2.
Comment
The patient's hemodynamics reflect biventricular failure (elevated right- and left-sided pressures, decreased cardiac output/index, pulmonary artery pulsatility index of 0.4,2 and right atrial/PCWP ratio 1.05) with equalization of pressures and tachycardia, which is a typical presentation of a significant rejection episode. It is necessary to consider biventricular mechanical circulatory support (MCS). However, although percutaneous biventricular support is a reasonable option to consider in this case, the presence of a small restricted heart (Figure 1 and Online Video 1) makes the placement of left and right MCS challenging. (For more information about calculating pressure-volume loops such as those in Figure 1, refer to “Pathophysiology and Advanced Hemodynamic Assessment of Cardiogenic Shock” by Brener, Rosenblum, and Burkhoff on page 7–15 of this issue.)
Figure 1.
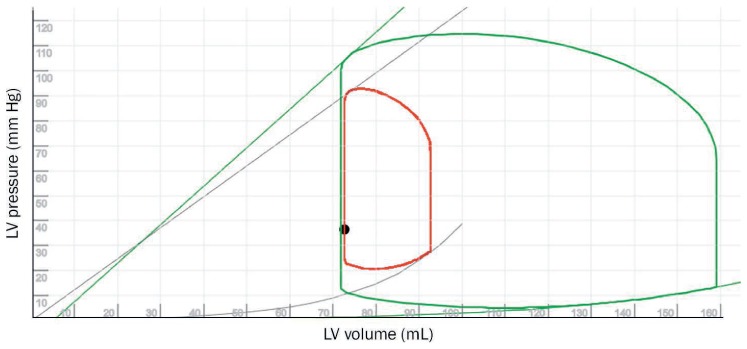
Pressure-volume (PV) loop showing a normal left ventricle (LV; green loop) and LV with acute cardiogenic shock with a comparatively restrictive pattern (red loop). The grey and green lines represent contractility (end-systolic PV relationship; ESPVR) and grey and green curves represent ventricular elastance (end-diastolic PV relationship; EDPVR). See Online Video 1.
Initial Clinical Course
In the setting of acute post-transplant rejection, CS can potentially be reversed if treated early, appropriately, and aggressively. Therefore, inotropes were initiated and an intraaortic balloon pump (IABP) was placed. Endomyocardial biopsy was performed, which showed acute cellular graft rejection (grade 2R); immunohistochemical staining for C4d was present in < 10% of myocardial capillaries. The patient received pulse doses of steroids and thymoglobulin. Although the patient received appropriate treatment for rejection while supporting her heart, her pulmonary artery oxygen saturation improved to 58% but subsequently dropped to 36%. A repeat echocardiogram confirmed worsening biventricular function. After a multidisciplinary team discussed other possible biventricular support options, arteriovenous extracorporeal membrane oxygenation (ECMO) was placed using the IABP arterial access point.
While the configuration of peripheral arteriovenous ECMO is effective in supporting systemic circulation, the increase in afterload (Figure 2 and Online Video 2) may lead to a reduction of cardiac output, especially when heart performance is suboptimal. In an extreme state, this can lead to a lack of aortic valve opening (Figure 3) with stagnation of blood in the left ventricle (LV) (Figure 4) and arch, resulting in pulmonary congestion and adverse neurological outcomes.
Figure 2.
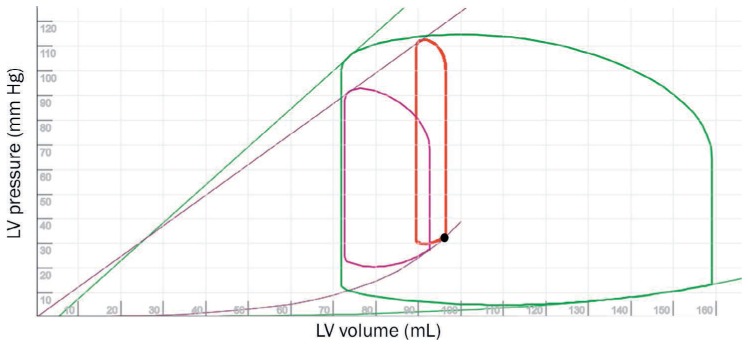
Pressure-volume (PV) loop showing the impact of extracorporeal membrane oxygenation in a poorly functioning left ventricle (LV; red loop). While the systemic arterial pressure has increased compared to the cardiogenic shock state (purple loop), the end-diastolic volume has increased with a significant increase in afterload (red loop). The green loop represents a normally functioning heart. Purple and green lines represent contractility (end-systolic PV relationship; ESPVR) and purple and green curves represent ventricular elastance (end-diastolic PV relationship; EDPVR). See Online Video 2.
Figure 3.
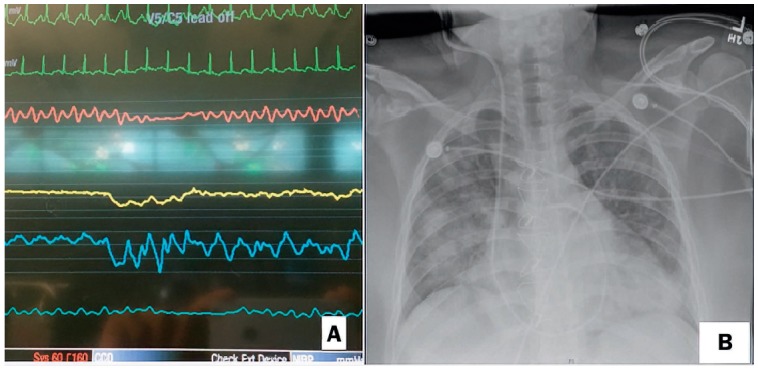
(A) Bedside monitor shows intermittent loss of pulsatility with extracorporeal membrane oxygenation and inotrope support. (B) Chest x-ray shows congested lungs due to high left ventricular end-diastolic pressure.
Figure 4.
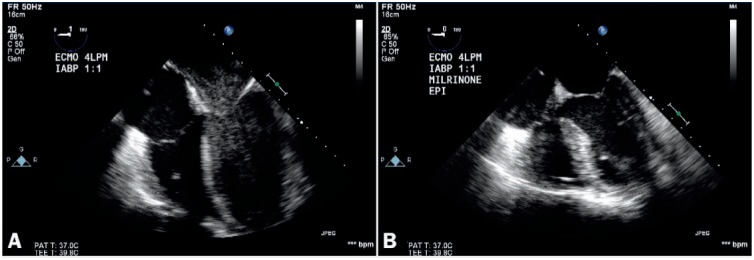
Transesophageal echocardiogram, midesophageal four-chamber view, of a patient on extracorporeal membrane oxygenation (without an unloading device) showing (A) stagnation with smoke at baseline that (B) resolves on promoting contractility with milrinone, reflecting the importance of aortic valve opening.
Subsequent Clinical Course
In order to promote pulsatility and LV unloading, inotrope support was augmented and an IABP was placed to decrease afterload. These interventions improved the hemodynamics transiently, but due to lack of myocardial improvement, the patient was relisted for transplant, which was successfully performed 2 weeks later.
Discussion
This case demonstrates some of the many complex aspects of managing CS, such as restrictive physiology, biventricular failure, and the need to constantly assess and plan MCS configuration and adequacy. However, a key learning point is the concept of unloading the LV during ECMO support.3–5 In this case, it is important to differentiate between systemic perfusion and LV afterload as reflected in Figure 2 and Online Video 2.6
CASE 2. CARDIOGENIC SHOCK IN THE SETTING OF AN ACUTE SHUNT
A 72-year-old female with no previous medical history presented to an outside hospital emergency room with hypotension and was found to have an anterior ST-elevation myocardial infarction. She was started on vasopressors and underwent an emergent angiogram, which demonstrated critical stenosis in the mid portion of the left anterior descending artery. Although revascularization was performed, there was poor residual perfusion distally because the vessel was diffusely diseased (Figure 5). The patient's ventriculogram revealed severe LV dysfunction and she was transferred to our facility on increasing doses of dobutamine and norepinephrine.
Figure 5.
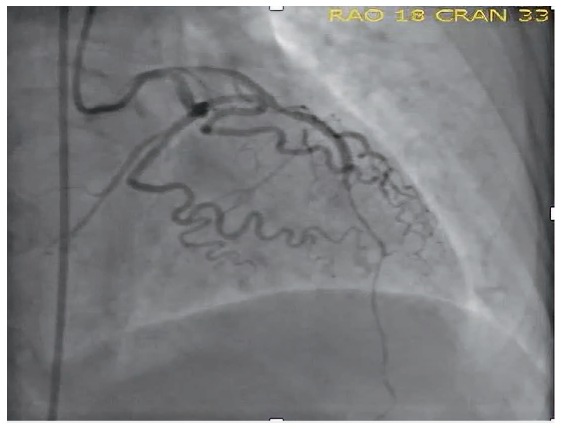
Coronary angiography of patient's left coronary system. Note the residual poor perfusion in the diffusely diseased distal left anterior descending artery.
A physical exam upon her arrival revealed a blood pressure of 80/50 mm Hg, heart rate of 110 bpm in sinus tachycardia, and oxygen saturation of 90% on 2 L of oxygen delivered by nasal cannula. She had bibasilar fine rales in two-thirds of her lung, elevated jugular venous pressure, and a thrill palpable along the left sternal border. Cardiac auscultation found a harsh, high-frequency holosystolic murmur at the left lower sternal border. There was no lower-extremity edema, and her peripheral pulses were palpable throughout.
An echocardiogram revealed a mid-septum ventricular septal defect (VSD) (Figure 6). The stroke volume from left and right ventricular outflow was used to calculate the noninvasive pulmonary/systemic output (Qp/Qs) of 3.01. The VSD flow was calculated with continuous wave Doppler and was 5.4 L/min applying Bernoulli's equation.
Figure 6.
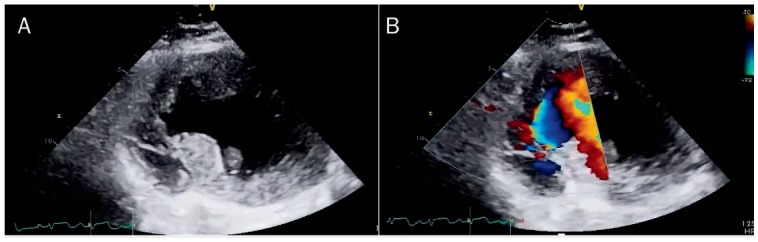
Transthoracic echocardiogram, parasternal short-axis view, demonstrates (A) evidence of interruption of the interventricular septum and (B) color Doppler through the existent gap.
Comment
Initially an acute VSD causes a reduction in LV afterload; therefore, the end-systolic and end-diastolic pressure-volume relationships remain unaltered. There is also an increase in overall LV stroke volume (not aortic) and shorter isovolumetric contraction as the VSD creates a vent leading to an overall lower afterload for the LV (Figure 7 and Online Video 3). This case illustrates a mechanical complication after an acute anterior large myocardial infarction in which vasopressor agents that increase myocardial oxygen consumption and increase afterload can, in fact, worsen the shunt and further decrease the forward output. The impact of increasing afterload in a patient with acute VSD is evident in Figure 8 and Online Video 4. As reflected by the pressure-volume loops, it is important to recognize that the myocardial contractility does not change in this context but is an interplay of changing afterload for the ventricle. Early recognition and aggressive implantation of MCS are obvious imperative steps, but the existence of a VSD makes the choice of support device complicated.
Figure 7.
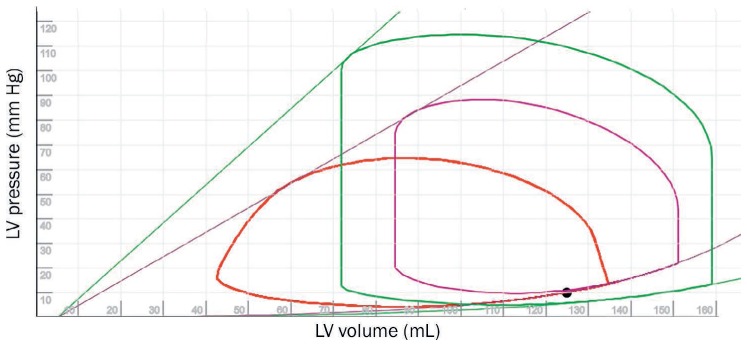
Pressure-volume (PV) loops illustrating the physiology of a ventricular septal defect (VSD). The green PV loop represents the normal state, the purple loop represents an acute heart failure state, and the red loop represents a VSD in the setting of acute heart failure. Note that the onset of a VSD has changed the shape of the PV loop, reflective of decreased afterload, shortened isovolumetric time, and higher stroke volume. Although the lines for end-systolic PV relationship (ESPVR; straight lines) and end-diastolic pressure-volume relationship (EDPVR; bottom curves) are different compared to normal PV loop, they are the same for acute heart failure with and without a VSD, suggesting that the true myocardial properties have not changed. See Online Video 3. LV: left ventricle.
Figure 8.
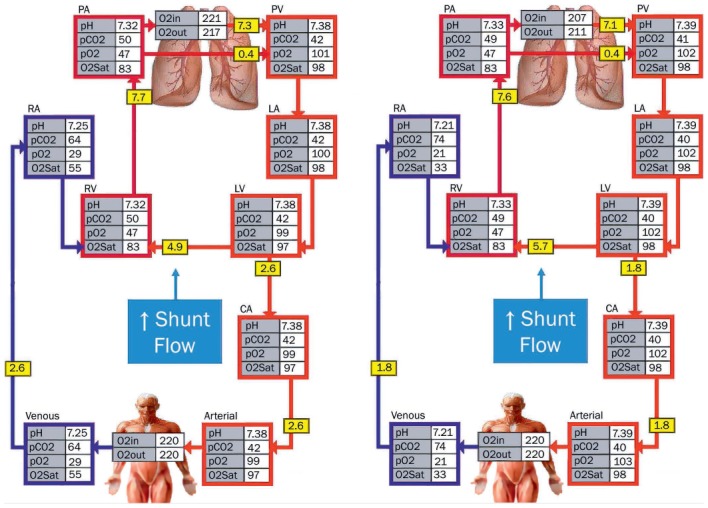
(A) Physiological schematic representing a ventricular septal defect (VSD) with shunt flow of 4.9 L/min with a step-up in oxygenation from the left to right ventricle. (B) With increasing norepinephrine and afterload on the left ventricle, the shunt flow increases to 5.7 L/min while the forward cardiac output to the systemic circulation drops from 2.6 L/min at baseline to 1.8 L/min. See Online Video 8.
Table 1 shows the physiological impact of different devices on VSD flow. Although an IABP theoretically decreases the afterload, the limited capacity for afterload reduction seems practically meaningless for a clinically significant VSD. While ECMO is an efficient and quick way to support the entire cardiopulmonary circulation, the increase in afterload associated with ECMO appears to worsen the VSD flow. Furthermore, whereas increased shunt flow might be tolerated in the setting of an ECMO circuit, there is no data on safety or success of such a strategy. There might be an opportunity to use ECMO to increase the flow combined with an unloading device, thus decreasing the afterload and hence not increasing the Qp/Qs. Positioning of a transaortic pump can be challenging in the context of a small LV. The same reasoning may also apply to using a transaortic device (Impella) as a primary strategy. Also, per current recommendations, Impella use is contraindicated in the presence of a VSD. A TandemHeart (CardiacAssist, Inc.) has a cannula positioned through a transseptal puncture in the left atrium (LA), where oxygenated blood is drawn and delivered to the descending aorta through a cannula positioned in the femoral artery; this has been used successfully in patients with a VSD complicating acute myocardial infarction.7 This LA device unloads the LV and can work effectively in the setting of a VSD to decrease the Qp/Qs. As the LA unloading device pushes blood into the arterial circulation (like ECMO) it could increase the LV afterload, and hence close attention must be paid to maintaining aortic valve opening (Figure 9 and Online Video 5).
Table 1.
Simulation of 10-mm ventricular septal defect (VSD) on Harvi software showing the quantified impact of mechanical circulatory support devices. CO: cardiac output; PA: pulmonary artery; IABP: intra-aortic balloon pump; ECMO: extracorporeal membrane oxygenation
| DEVICE TYPE | DEVICE FLOW (L/MIN) | CO (L/MIN) | VSD FLOW (L/MIN) | PA FLOW (L/MIN) | QP/QS |
|---|---|---|---|---|---|
| None | n/a | 2.69 | 5.4 | 8.07 | 3.01 |
| IABP 1:1 (40 cc) | n/a | 3.03 | 5.04 | 8.05 | 2.65 |
| ECMO | 3.37 | 0.51 | 7.41 | 7.89 | 15.49 |
| Tandem | 4.2 | 0.00 | 4.4 | 8.64 | 0 |
| Impella CP | 3.55 | 0.00 | 4.66 | 8.17 | 0 |
| Impella 5.0 | 4.6 | 0.00 | 3.43 | 8.00 | 0 |
Figure 9.
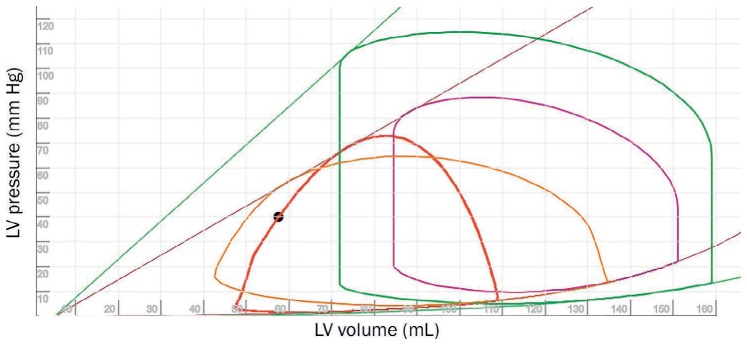
Pressure-volume (PV) loops illustrating the physiology of a ventricular septal defect (VSD). The green PV loop represent a normal state, purple PV loop represents acute heart failure, and the orange loop represents VSD on the setting of acute heart failure. When a TandemHeart device is placed in a patient with VSD, the PV loop (red) takes on a triangular shape secondary to proper unloading of the left-sided preload. The purple and green lines represent contractility (end-systolic PV relationship; ESPVR) and grey and green curve represent ventricular elastance (end-diastolic PV relationship; EDPVR). See Online Video 9. LV: left ventricle.
Subsequent Clinical Course
This patient received a TandemHeart device and was successfully weaned off all vasopressors with hemodynamic stability. While recuperating and waiting for a surgical intervention, she developed acute limb ischemia in one of her lower extremities after a week. This led to rhabdomyolysis and worsening multiorgan failure, and the family chose to pursue nonaggressive measures, finally leading to withdrawal of care.
Discussion
Although the incidence of VSD complicating acute myocardial infarction using current reperfusion therapies strategies is only 0.17% to 0.3%, inpatient mortality remains high at between 30% and 41%.8–11 As reflected in this case, the complexity of its pathophysiology makes it an extremely challenging clinical scenario. It might be possible to overcome such dismal outcomes with early recognition combined with an ideal MCS configuration that both unloads the LV and decreases the shunt fraction to effectively bridge the patient to a surgical or interventional correction. This case illustrates that not all CS patients can be treated with the same MCS device; understanding the physiology of each scenario, and determining the most effective MCS device to use, is necessary for best outcomes.
CONCLUSION
These cases illustrate the challenges of early identification of CS in patients with acute graft rejection and with a VSD complicating an acute myocardial infarction. Aggressive treatment with inotropes can sometimes exacerbate the vicious circle of neurohormonal activation, and the use of MCS devices should be considered as a first option. Selecting the most appropriate MCS device may be challenging given the different ways each one impacts the circulatory system in terms of preload, afterload, and contractility, and understanding the physiology of cardiogenic shock should form the basis of treatment choices.
KEY POINTS
Early recognition of cardiogenic shock and the choice of mechanical support (MCS) devices is challenging in patients with a small left ventricle with restrictive physiology.
The maintenance of pulsatility prevents stagnation and adverse neurological outcomes in patients with MSC devices.
There are a variety of MCS devices, and selecting the most appropriate one for patients with a ventricular septal defect is determined by the mechanical size of the defect.
Extracorporeal membrane oxygenation (ECMO) increases afterload, and use of a left ventricular unloading system should be considered in patients on ECMO.
Footnotes
Conflict of Interest Disclosure:
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Harvi Online [Internet] Burkhoff D, Dickstein ML, Schleicher T. c2020 [cited 2019 Dec 30]. Available from: https://harvi.online.
- 2.Kapur NK, Esposito ML, Bader Y et al. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation. 2017 Jul 18;136(3):314–26. doi: 10.1161/CIRCULATIONAHA.116.025290. [DOI] [PubMed] [Google Scholar]
- 3.Pappalardo F, Schulte C, Pieri M et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail. 2017 Mar;19(3):404–12. doi: 10.1002/ejhf.668. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko D, Takahashi M, Fukutomi M, Funayama H, Kario K. Additional Use of a 6F Intra-Aortic Balloon Pump on Extracorporeal Membrane Oxygenation Was Effective in a Patient with Cardiogenic Shock with Low Pulse Pressure. Int Heart J. 2019 Sep 27;60(5):1184–8. doi: 10.1536/ihj.18-643. [DOI] [PubMed] [Google Scholar]
- 5.Cevasco M, Takayama H, Ando M, Garan AR, Naka Y, Takeda K. Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. J Thorac Dis. 2019 Apr;11(4):1676–83. doi: 10.21037/jtd.2019.03.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber C, Deppe AC, Sabashnikov A et al. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion. 2018 May;33(4):283–8. doi: 10.1177/0267659117745369. [DOI] [PubMed] [Google Scholar]
- 7.Gregoric ID, Bieniarz MC, Arora H, Frazier OH, Kar B, Loyalka P. Percutaneous ventricular assist device support in a patient with a post-infarction ventricular septal defect. Tex Heart Inst J. 2008;35(1):46–9. [PMC free article] [PubMed] [Google Scholar]
- 8.French JK, Hellkamp AS, Armstrong PW et al. Mechanical complications after percutaneous coronary intervention in ST-elevation myocardial infarction (from APEX-AMI) Am J Cardiol. 2010 Jan 1;105(1):59–63. doi: 10.1016/j.amjcard.2009.08.653. [DOI] [PubMed] [Google Scholar]
- 9.López-Sendón J, Gurfinkel EP, Lopez de Sa E et al. Factors related to heart rupture in acute coronary syndromes in the Global Registry of Acute Coronary Events. Eur Heart J. 2010 Jun;31(12):1449–56. doi: 10.1093/eurheartj/ehq061. Global Registry of Acute Coronary Events (GRACE) Investigators. [DOI] [PubMed] [Google Scholar]
- 10.Singh V, Rodriguez AP, Bhatt P et al. Ventricular Septal Defect Complicating ST-Elevation Myocardial Infarctions: A Call for Action. Am J Med. 2017 Jul;130(7):863.e1–863.e12. doi: 10.1016/j.amjmed.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Crenshaw BS, Granger CB, Birnbaum Y et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation. 2000 Jan 4–11;101(1):27–32. doi: 10.1161/01.cir.101.1.27. [DOI] [PubMed] [Google Scholar]


