Abstract
Cardiogenic shock is associated with significant morbidity and mortality, and clinicians have increasingly used short-term mechanical circulatory support (MCS) over the last 15 years to manage outcomes. In general, the provision of greater hemodynamic support comes with device platforms that are more complex and potentially associated with more adverse events. In this review, we compare and contrast the available percutaneous and surgically placed device types used in cardiogenic shock and discuss the associated clinical and hemodynamic data to support device use.
Keywords: cardiogenic shock, mechanical circulatory support, intra-aortic balloon pump, Impella, extracorporeal membrane oxygenation, ECMO, TandemHeart, CentriMag
INTRODUCTION
Cardiogenic shock (CS) is a state of low cardiac output associated with hypotension and evidence of end-organ hypoperfusion. Confirmatory hemodynamic criteria include systolic blood pressure < 90 mm Hg, cardiac index ≤ 2.2 L/min/m2, and an elevated pulmonary capillary wedge pressure > 15 mm Hg.1 The leading cause of CS has been acute myocardial infarction (AMI); however, non–AMI-related CS is reported to be on the rise.2 Unfortunately, CS continues to be associated with significant morbidity and mortality.
There has been an overall increase in the use of temporary mechanical circulatory support (MCS) to try and improve CS outcomes, and multiple short-term device options are available that provide left- and/or right-sided support. The most widely used short-term device has been the intra-aortic balloon pump (IABP), although more robust devices are being used with increased frequency.3,4 This review highlights the available percutaneous and surgical options for temporary MCS and the associated clinical and hemodynamic data that support the use of these various devices.
INDICATIONS AND BRIDGE SUPPORT STRATEGIES
The available short-term MCS devices used to treat CS are listed and illustrated in Table 1 and Figure 1,5 while indications for use are defined in Table 2.6 These devices are often used as a bridge until next steps are determined (ie, the patient recovers or worsens to the point of needing a durable ventricular assist device or transplantation).
Table 1.
Short-term mechanical circulatory support options divided by side of support (left side, right side, biventricular) and whether they are inserted percutaneously or surgically. IABP: intra-aortic balloon pump; VA ECMO: venoarterial extracorporeal membrane oxygenation; LV-Ao: left ventricle-aorta; RA-PA: right atrium–pulmonary artery; LA-Ao: left atrium-aorta; RV: right ventricle; LV: left ventricle
| SHORT-TERM MECHANICAL CIRCULATORY SUPPORT | PERCUTANEOUS OPTIONS | SURGICAL OPTIONS |
|---|---|---|
| Left-sided |
|
|
| Right-sided |
|
|
| Biventricular |
|
|
Figure 1.
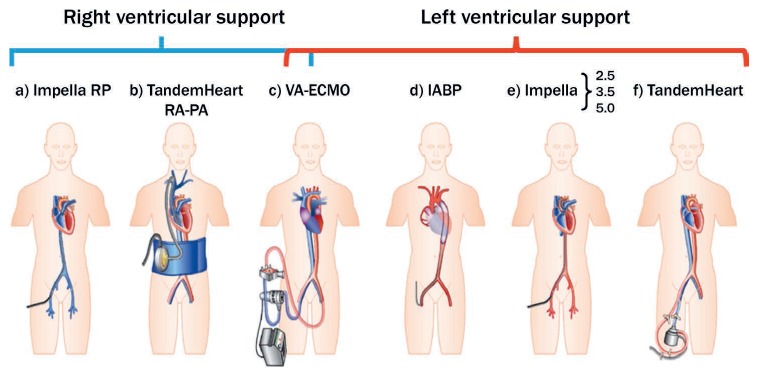
Schematic drawing of current commercially available percutaneous mechanical support devices for cardiogenic shock. On the left are devices for right ventricular support, and on the right are those for left ventricular support. (a) Impella® RP, (b) TandemHeart™ RA-PA (right atrium–pulmonary artery), (c) venoarterial (VA) extracorporeal membrane oxygenation (ECMO), (d) intra-aortic balloon pump, (e) Impella, (f) TandemHeart. Adapted from H. Thiele et al. with permission from Oxford University Press.5
Table 2.
Suggested indications for short-term mechanical circulatory support. Adapted from Rihal et al. with permission from Elsevier.6 AMI: acute myocardial infarction; MCS: mechanical circulatory support; PCI: percutaneous coronary intervention; LV: left ventricular; RV: right ventricular; VAD: ventricular assist device; INTERMACS: interagency registry for mechanically assisted circulatory support; ECMO: extracorporeal membrane oxygenation; EF: ejection fraction; VT: ventricular tachycardia
| INDICATION | COMMENTS |
|---|---|
| Complications of AMI | Ischemic mitral regurgitation is particularly well suited to these devices because the hemodynamic disturbance is usually acute and substantial. Acutely depressed LV function from large AMIs during and after primary PCI is an increasing indication for temporary MCS use. Cariogenic shock from RV infarction can be treated with percutaneous RV support. |
| Severe heart failure in the setting of nonischemic cardiomyopathy | Examples include severe exacerbations of chronic systolic heart failure as well as acutely reversible cardiomyopathies such as fulminant myocarditis, stress cardiomyopathy, or peripartum cardiomyopathy. In patients presenting in INTERMACS profiles 1 or 2, MCS can be used as a bridge to destination VAD placement or as a bridge to recovery if the ejection fraction rapidly improves. |
| Acute cardiac allograft failure | Primary allograft failure (adult or pediatric) may be caused by acute cellular- or antibody-mediated rejection, prolonged ischemic time, or inadequate organ preservation. |
| Post-transplant RV failure | Acute RV failure has several potential causes, including recipient pulmonary hypertension, intraoperative injury/ischemia, and excess volume/blood product resuscitation. MCS support provides time for the donor right ventricle to recover function, often with the assistance of inotropic and pulmonary vasodilator therapy. |
| Patients slow to wean from cardiopulmonary bypass following heart surgery | Although selected patients may be transitioned to a percutaneous system for additional weaning, this is rarely done. |
| Refractory arrhythmias | Patients can be treated with a percutaneous system that is somewhat independent of the cardiac rhythm. For recurrent, refractory ventricular arrhythmias, ECMO may be required for biventricular failure. |
| Prophylactic use for high-risk PCI | Seen particularly in patients with severe LV dysfunction (EF < 20%–30%) and complex coronary artery disease involving a large territory (sole remaining vessel, left main or three-vessel disease). |
| High-risk or complex ablation of VT | Similar to high-risk PCI, complex VT ablation can be made feasible with percutaneous support. MCS use allows the patient to remain in VT longer during arrhythmia mapping without as much concern about systemic hypoperfusion. |
| High-risk percutaneous valve interventions | These evolving procedures may be aided by MCS. |
Intra-aortic Balloon Pump
The intra-aortic balloon pump is a polyethylene balloon attached to a double-lumen catheter (7–8F) and a pump console. The balloon is advanced over a guidewire through an introducer sheath until the proximal tip of the IABP is just below the ostium of the left subclavian artery. The pump provides counterpulsation therapy with inflation (diastole) and deflation (systole) of the balloon and is synchronized with either electrocardiogram (ECG) or pressure trigger for timing. Optimal timing of balloon inflation is at the onset of diastole or timed to the dicrotic notch on the arterial waveform. Generally, 1:1 IABP support, or one inflation per cardiac cycle, is used, and support can be weaned by changing the frequency of inflation to 1:2 and 1:3 levels. Therapeutic anticoagulation is recommended to reduce thrombotic complications.7 There are several different IABP sizes ranging from 25 cm3 to 50 cm3, and selection is typically based on the patient's height. The larger-capacity 50 cm3 IABP provides greater diastolic augmentation and systolic unloading.8–10
Although the femoral artery is commonly used for access, the positive safety profile and feasibility of transthoracic IABPs has been reported by several investigators.11,12 IABPs can be placed surgically by attaching a Gore-Tex graft to the subclavian or axillary artery. Alternatively, Estep et al. published a percutaneous approach using a micropuncture guidewire roadmap technique that permits placement of a sheath into the axillary artery without needing a surgical cut down or graft conduit. Based on several case series, including 163 bridge-to-transplantation patients, 141 patients (86.5%) were successfully transplanted with support that ranged from 3 to 152 days. The most frequent complications attributed to extended support were device malfunction or migration necessitating exchange or repositioning (37.3%).12 The axillary site can be considered in patients with severe peripheral artery disease (PAD) or in those with extended support needs measured in several days to weeks. Axillary support is considered a viable placement option because it permits upright sitting and ambulation and has a low infection rate due to the transthoracic location (Figure 2).
Figure 2.
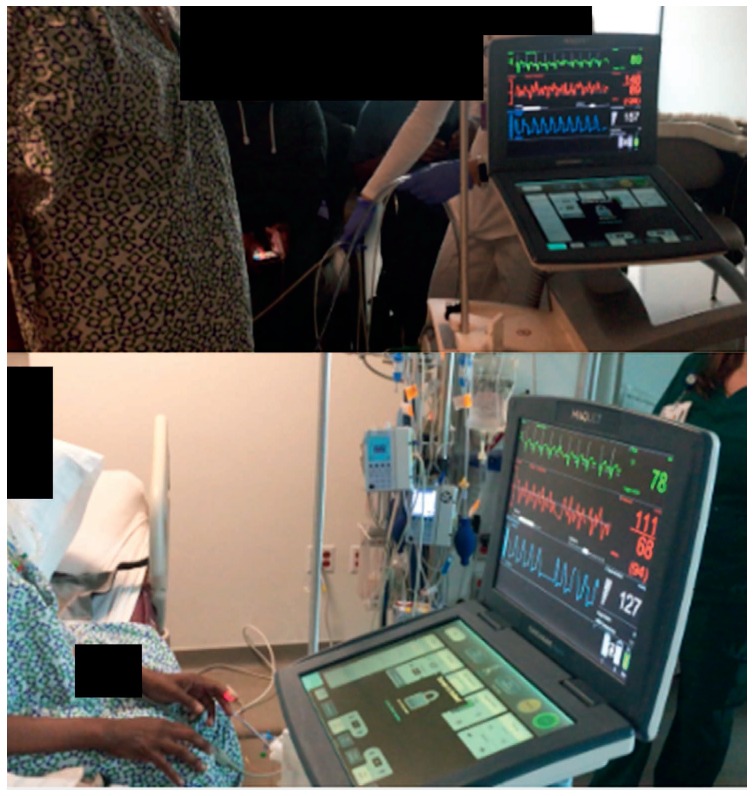
Percutaneous intra-aortic balloon pump in axillary/subclavian position permits ambulation.
The primary hemodynamic effects of IABP support include a decrease in afterload and myocardial oxygen consumption, an increase in cardiac output (up to 20%), and augmentation of coronary perfusion. Compared to the other available temporary MCS options, IABP provides the least amount of support. Specific contraindications for IABP use include aortic dissection, greater than mild aortic insufficiency, and severe PAD when using a femoral access approach. Major complications occur in 2.6% of cases and include limb ischemia, vascular injury, sepsis/bacteremia, balloon rupture, and embolic complications.13
IABP Clinical Data. The 2012 IABP-SHOCK II (Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock) trial demonstrated that IABP use compared to medical therapy immediately before revascularization did not reduce 30-day mortality in patients with AMI complicated by CS.14 While overall utilization of IABPs in the United States has been on the decline, they continue to be used in the management of hemodynamically unstable patients due to their wide availability, beneficial hemodynamic effects, and relative ease of insertion,5 and there has been an uptick in IABP use as a bridge to heart transplantation to support patients with end-stage heart failure.4
Impella Devices
The Impella (Abiomed) is a transvalvular continuous micro-axial flow device designed to propel blood from the left ventricle (LV) into the aorta via an Archimedes screw pump. In the setting of CS, the Impella device unloads the ventricle, reduces ventricular end-diastolic pressure, increases mean arterial pressure, and decreases myocardial oxygen consumption.15 Impella has five systems that can be placed percutaneously or surgically and are approved for use in the United States (Table 3).5,16 The Impella 2.5 can provide approximately 2.5 L/min of flow, and the Impella CP is an intermediate device that can provide up to 4.0 L/min. Both of these LV support systems are commonly placed percutaneously in the femoral artery using an introducer sheath and advanced in a retrograde fashion across the aortic valve under fluoroscopic guidance. In contrast, the LV Impella 5.0 (21F motor) requires a surgical cut down, insertion of a vascular graft to the axillary artery, and use of a 23F introducer sheath. Most recently, Abiomed's newest heart pump, the Impella 5.5 with SmartAssist, has received premarket approval from the US Food and Drug Administration (FDA) for safety and efficacy in the therapy of cardiogenic shock up to 14 days. The Impella 5.5 with SmartAssist delivers peak flows > 6 L/min. The motor housing is thinner and 45% shorter than the Impella 5.0 and can improve ease of pump insertion through the vasculature. The SmartAssist also integrates data informatics including left ventricular pressure (LVP), end-diastolic pressure (EDP), and cardiac power output (CPO) to support weaning algorithms and potentially optimize native heart recovery. In addition, the SmartAssist fiber optic pressure sensor allows for more precise pump positioning, management, and bedside repositioning. Repositioning of the Impella device is often performed at bedside under ultrasound guidance.
Table 3.
Comparison of commercially available devices for short-term mechanical circulatory support. Modified from Guglin et al. with permission from Elsevier.5,16 CO2: carbon dioxide; FDA: US Food and Drug Administration; IABP: intra-aortic balloon pump; LV: left ventricle/ventricular; RV: right ventricle; VA-ECMO: venoarterial extracorporeal membrane oxygenation
| DEVICE | IABP | IMPELLA (2.5, CP, RP) | TANDEMHEART | VA-ECMO |
|---|---|---|---|---|
| Flow, L/min | 0.5–1 | 2.5–5 | 4–6 | 4–6 |
| Cannula size, F | 7–8 | 12–23 | Inflow 21 Outflow 15–19 | Inflow 18–23 Outflow 15–29 |
| Duration of support, FDA approved | 9 days | 4 days (2.5, CP) 14 days (5.0) 14 days (RP) | 21 days | 6 hours (limited by oxygenator durability) |
| Ventricles supported | LV | LV or RV | LV or RV | LV and RV |
| Additional requirements | Surgical cutdown for Impella 5 | Transseptal puncture | Potential need for LV venting, possible cutdown | |
| Advantages | Easy to place Good safety profile Fewer side effects, especially vascular | Multiple device options | Highest cardiac output Comparable to VA-ECMO No LV distension | Highest cardiac output Complete cardiopulmonary support (including oxygenation and CO2 removal) |
| Disadvantages | Limited hemodynamic support Contraindicated in severe aortic regurgitation | More invasive and complex to implant than the IAPB Unstable position Frequent hemolysis Vascular complications | Need tertiary or quaternary specialized care center Necessitates atrial transseptal puncture with its potential complications Vascular complications Retrograde blood flow | Requires more resources and support staff than other devices Retrograde blood flow with worsening of afterload (LV distention) Vascular complications Thrombocytopenia |
The RP Impella provides right ventricular support via a 22F motor attached to an 11F catheter that is placed through a 23F peel-away sheath in the femoral vein. With a unique 3-dimensional shape, the RP Impella is advanced in an antegrade fashion through the venous system across the tricuspid and pulmonary valves. The inflow portion of the pump is situated in the inferior vena cava and the outflow portion is positioned in the pulmonary artery.
Contraindications for left-sided Impella systems include severe aortic stenosis with aortic valve area ≤ 0.6 cm2, LV thrombus, mechanical aortic valve, and severe PAD (when using a femoral access approach). Contraindications for the RP Impella include right-sided mechanical valves, severe tricuspid or pulmonary stenosis, thrombus, and the presence of an inferior vena cava filter. General complications related to the Impella systems include device migration too far into the LV or underneath the mitral valve apparatus, placing the patient at risk for perforation/tamponade, ventricular arrhythmias, acute mitral regurgitation, hemolysis, and thrombotic complications.
Impella Clinical Data. The safety and feasibility of the Impella 2.5 and CP devices has been reported in large registries.17,18 The ISAR-SHOCK (Efficacy Study of LV Assist Device to Treat Patients With Cardiogenic Shock) trial showed that the use of Impella support was safe and provided more hemodynamic support than IABP, but there was no difference in mortality.19 These devices were also studied in the setting of high-risk percutaneous coronary intervention (PCI) in the PROTECT II trial, and the degree of hemodynamic support was noted to be greater than the IABP. However, no difference was seen in major cardiac events at 30 days compared to IABP support.20
More recently, the IMPRESS (Impella Versus IABP Reduces Mortality in STEMI Patients Treated with Primary PCI in Severe Cardiogenic Shock) trial compared mortality and safety of the Impella CP with IABP in critically ill patients with AMI-CS. At both 30 days and 6 months, mortality rates were high and similar for both groups (~50% at 6 months).21,22 It is important to highlight that this trial was significantly underpowered to detect a difference in the primary end point, and the population included critically ill patients on MCS and those with neurologic injury. Even so, a recent matched-pair analysis of 237 Impella-treated versus 237 IABP-treated patients with AMI-CS also demonstrated a lack of mortality benefit with the Impella device (30-day mortality 48.5% vs. 46.4%, P = .64).21
In the RECOVER RIGHT Trial, Anderson et al. showed that the Impella RP was a safe and reliable way to improve hemodynamics in select patients with refractory RV failure.23 The study population included two cohorts: 18 patients with RV failure after LV assist device (LVAD) implantation, and 12 patients with RV failure after cardiotomy or myocardial infarction. Overall survival at 30 days was 73.3%, and all discharged patients were alive at 180 days. In early 2019, the FDA published an advisory noting that an interim post-approval study suggested higher rates of mortality for patients treated with the Impella RP than previously observed. Further analysis of this study, however, indicates that the difference in mortality was likely related to a sicker patient population, which included patients with CS for > 48 hours and those who experienced inhospital cardiac arrest.24
TandemHeart System
The TandemHeart system (Cardiac Assist, Inc.) is a percutaneous centrifugal ventricular assist device that unloads the failing LV by pumping oxygenated blood directly from the left atrium into the distal descending aorta. There are four major components of this device: the control console, a 21F transseptal inflow cannula positioned in the left atrium, an arterial outflow cannula (15–19F), and the centrifugal pump. The pump has a brushless electromagnetic motor and provides up to 5.0 L/min of flow at a maximum speed of 7500 rpm.6,25 The venous and arterial cannulas are secured in the groin to prevent cannula migration. This device requires highly trained operators who are skilled in transseptal and large-bore access to insert the system under fluoroscopic guidance. Due to the parallel configuration relative to the native heart, both the TandemHeart device and the native LV may contribute to forward flow, creating the net effect of reductions in LV pressure, volume, and stroke volume.6,15
TandemHeart Clinical Data. Relatively little data is available on the benefits of the TandemHeart. In 2005 and 2006, two studies examined the hemodynamic benefit of this device in patients with CS. Those who were randomized to the TandemHeart arm had improved hemodynamics (ie, higher cardiac power index and lower pulmonary capillary wedge pressure) but more complications (ie, severe bleeding and limb ischemia) compared to the IABP arm.26,27 Additional reported complications include migration of the inlet cannula from the left atrium back into the right atrium, with subsequent right-to-left shunting and hypoxia, tamponade, and strokes.25 Contraindications to this device include severe PAD, atrial thrombus, and an underlying coagulopathy.
TandemHeart is not FDA approved for RV support (RA to pulmonary artery), but cases showing feasibility have been reported. Indications for RV MCS include cardiogenic shock related to RV myocardial infarction, acute pulmonary embolus, severe pulmonary hypertension, and post-LVAD RV failure.28,29
PROTEK Duo System
An alternative to the Impella RP and off-label use of the TandemHeart is the dual-lumen PROTEK Duo cannula (CardiacAssist, Inc.) coupled with the TandemHeart centrifugal pump or the CentriMag (see below).30,31 The PROTEK Duo 29F cannula is inserted into the right internal jugular vein and permits simultaneous drainage and reinfusion of blood from the right atrium into the pulmonary artery. Data regarding the safety and efficacy of this device is limited.
CentriMag
The CentriMag (Abbott) is a robust temporary ventricular assist device that can provide up to 10 L/min of flow. The system includes a centrifugal pump, flow probe, and console with the capability of adding a membrane oxygenator, and the device is surgically inserted via a median sternotomy or a lateral thoracotomy. John et al. published results of a study that used the CentriMag MCS in patients with post-cardiotomy shock.32 Out of 12 total patients, 8 were successfully bridged to durable MCS with the HeartMate VAD, 2 were successfully bridged to recovery, and 2 died while on CentriMag support.33
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) provides robust biventricular support in patients with severe refractory CS. ECMO was initially described by Hill and colleagues in the 1970s as a technique for temporary oxygenation and pulmonary support, and it has evolved greatly since then.4,34
The venoarterial ECMO (VA-ECMO) circuit is a form of cardiopulmonary bypass and consists of a centrifugal flow pump, membrane oxygenator, controller, and venous inflow/arterial outflow cannulas. Blood is pulled out of the venous system with a 15F to 29F cannula, oxygenated and warmed in the gas exchange unit, and pumped back into the systemic circulation via the femoral artery. While VA-ECMO can provide between 4 L/min and 6 L/min of flow, it also increases systemic afterload and thereby will increase LV end-diastolic pressure. This added afterload on the LV will further depress native cardiac output and could potentiate a persistent congested state unless the LV is decompressed by an unloading strategy.16
VA-ECMO can be inserted percutaneously via femoral vein/artery access or surgically with central cannulation. Contraindications to VA-ECMO include severe PAD (percutaneous only) and ≥ moderate aortic insufficiency. Although ECMO provides the highest level of support, it can lead to significant complications including pump thrombosis, bleeding, ischemic limbs, and Harlequin syndrome (differential oxygenation). With all large-bore femoral arterial cannulations, an antegrade sheath can be placed in the superficial femoral artery to improve distal limb perfusion.
Percutaneous MCS Recommendations
A 2015 consensus statement supported by the Society for Cardiovascular Angiography and Interventions (SCAI), American College of Cardiology (ACC), Heart Failure Society of America (HFSA), and Society of Thoracic Surgeons (STS) highlights that the choice of short-term MCS devices is contingent upon vascular anatomy, local expertise, and availability.6 Percutaneous MCS may be considered in select patients with refractory CS based on the patient's age, comorbidities, and neurological function without any preference for device selection (IIa C recommendation). Below we discuss the guidelines for MCS related to high-risk PCI, AMI, and acute or chronic heart failure complicated by CS.
High-Risk PCI. The ACC, SCAI, and American Heart Association (AHA) published expert consensus documents and clinical practice guidelines on hemodynamic support device considerations in the setting of high-risk PCI with CS. In general, elective insertion of an appropriate hemodynamic support device (eg, Impella and Tandem devices) as an adjunct to PCI may be reasonable in carefully selected high-risk patients, and alternative LV assist devices for circulatory support may be considered in patients with refractory CS (IIb C recommendation).6
Acute Myocardial Infarction. Until 2012, the United States and European guidelines, largely based on registry data, had given a class IB and class IC recommendation for using IABP in the treatment of CS. In 2013, the American College of Cardiology Foundation (ACCF)/AHA guidelines for the management of ST-elevation myocardial infarction (STEMI) downgraded the routine use of IABP to class IIa (level of evidence B). Alternative MCS devices can be considered in patients with refractory cardiogenic shock (class IIb, level of evidence C).35,36 In the 2017 European Society of Cardiology guideline recommendations, IABP use should be considered in patients with hemodynamic instability/CS due to mechanical complications (class IIa, level of evidence C), whereas routine IABP use is not indicated in patients with CS and acute MI or acute or chronic HF complicated by CS (class III, level of evidence B).37,38 In contrast, ACCF/AHA guidelines for the management of STEMI do not recommend against IABP use in these CS patient populations. Other common scenarios for the use of an IABP include refractory angina awaiting revascularization (class I recommendation in STEMI and class IIa for non-STEMI/unstable angina) and arrhythmogenic or mechanical complications of AMI.35
Acute or Chronic Heart Failure. The 2013 ACC/AHA guidelines and a more recent scientific statement from the AHA in 2017 provide a class IIa (level of evidence B) recommendation for nondurable short-term MCS devices to help manage patients with acute on chronic HF and CS.39,40 It is important to highlight that recommendations regarding use of an IABP are largely based on examinations of patients with AMI CS. Randomized controlled trials that evaluate the impact of IABPs in non-AMI related CS are lacking. There is an evolving body of data to help define the role of IABPs in stabilizing patients with acute or chronic heart failure complicated by CS while more definitive solutions such as LVAD or transplant are considered.41–43
CONCLUSION
Percutaneous and surgical options for MCS have evolved and can be rapidly deployed in patients with different etiologies and severities of CS. Device selection is guided by hemodynamic support needs, operator/institutional experience, and device-specific complications and risks. Proper understanding of each MCS device and its intended hemodynamic consequences along with careful patient selection and a multidisciplinary team approach can potentially optimize outcomes for patients on temporary MCS.
KEY POINTS
Temporary mechanical circulatory support (MCS) devices are being used with increasing frequency to treat cardiogenic shock.
A number of MCS devices are currently available; selection of device is guided by level of hemodynamic support needed, institutional experience, and device-specific risks.
Careful patient selection using a multidisciplinary team approach and proper understanding of the hemodynamic impact of each MCS device can potentially optimize outcomes with temporary MCS.
Footnotes
Conflict of Interest Disclosure:
Dr. Estep is a medical advisor to Medtronic Inc. and a consultant for Abbott.
REFERENCES
- 1.Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999 Aug 26;341(9):625–34. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 2.Puymirat E, Fagon JY, Aegerter P et al. Cardiogenic shock in intensive care units: evolution of prevalence, patient profile, management and outcomes, 1997–2012. Eur J Heart Fail. 2017 Feb;19(2):192–200. doi: 10.1002/ejhf.646. Collège des Utilisateurs de Bases de données en Réanimation (CUB-Réa Group [Intensive Care Database User Group]) [DOI] [PubMed] [Google Scholar]
- 3.Ouyang D, Gulati G, Ha R, Banerjee D. Incidence of temporary mechanical circulatory support before heart transplantation and impact on post-transplant outcomes. J Heart Lung Transplant. 2018 Sep 1;37(9):1060–6. doi: 10.1016/j.healun.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Shah M, Patnaik S, Patel B et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018 Apr;107(4):287–303. doi: 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 5.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019 Aug 21;40(32):2671–83. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 6.Rihal CS, Naidu SS, Givertz MM et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015 May 19;65(19):e7–e26. doi: 10.1016/j.jacc.2015.03.036. Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC) [DOI] [PubMed] [Google Scholar]
- 7.Brown JL, Estep JD. Temporary Percutaneous Mechanical Circulatory Support in Advanced Heart Failure. Heart Fail Clin. 2016 Jul;12(3):385–98. doi: 10.1016/j.hfc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Baran DA, Visveswaran GK, Seliem A, DiVita M, Wasty N, Cohen M. Differential responses to larger volume intra-aortic balloon counterpulsation: Hemodynamic and clinical outcomes. Catheter Cardiovasc Interv. 2018 Oct 1;92(4):703–10. doi: 10.1002/ccd.27387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majithia M, Jumean M, Shih H et al. The hemodynamic effects of the MEGA intra-aortic balloon counterpulsation pump. J Heart Lung Transplant. 2013 Apr;32(4S):S226. [Google Scholar]
- 10.Kapur NK, Paruchuri V, Majithia A et al. Hemodynamic effects of standard versus larger-capacity intraaortic balloon counterpulsation pumps. J Invasive Cardiol. 2015 Apr;27(4):182–8. [PubMed] [Google Scholar]
- 11.Nwaejike N, Son AY, Milano CA, Daneshmand MA. Is there a role for upper-extremity intra-aortic balloon counterpulsation as a bridge-to-recovery or a bridge-to-transplant in the treatment of end-stage heart failure? Interact Cardiovasc Thorac Surg. 2017 Oct 1;25(4):654–8. doi: 10.1093/icvts/ivx165. [DOI] [PubMed] [Google Scholar]
- 12.Estep JD, Cordero-Reyes AM, Bhimaraj A et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail. 2013 Oct;1(5):382–8. doi: 10.1016/j.jchf.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson JJ, 3rd, Cohen M, Freedman RJ, Jr et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol. 2001 Nov 1;38(5):1456–62. doi: 10.1016/s0735-1097(01)01553-4. [DOI] [PubMed] [Google Scholar]
- 14.Thiele H, Zeymer U, Neumann FJ et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012 Oct 4;367(14):1287–96. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 15.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol. 2015 Dec 15;66(23):2663–74. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Guglin M, Zucker MJ, Bazan VM et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019 Feb 19;73(6):698–716. doi: 10.1016/j.jacc.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Vetrovec GW, Anderson M, Schreiber T et al. The cVAD registry for percutaneous temporary hemodynamic support: A prospective registry of Impella mechanical circulatory support use in high-risk PCI, cardiogenic shock, and decompensated heart failure. Am Heart J. 2018 May;199:115–21. doi: 10.1016/j.ahj.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill WW, Schreiber T, Wohns DH et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014 Feb;27(1):1–11. doi: 10.1111/joic.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seyfarth M, Sibbing D, Bauer I et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008 Nov 4;52(19):1584–8. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill WW, Kleiman NS, Moses J et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012 Oct 2;126(14):1717–27. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 21.Schrage B, Ibrahim K, Loehn et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation. 2019 Mar 5;139(10):1249–58. doi: 10.1161/CIRCULATIONAHA.118.036614. [DOI] [PubMed] [Google Scholar]
- 22.Ouweneel DM, Eriksen E, Sjauw KD et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017 Jan 24;69(3):278–87. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Anderson MB, Goldstein J, Milano C et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant. 2015 Dec;34(12):1549–60. doi: 10.1016/j.healun.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 24.U. S. Food and Drug Administration [Internet] Silver Spring, MD: U.S. Food and Drug Administration; c2019. Maisel W. Increased Rate of Mortality in Patients Receiving Abiomed Impella RP System - Letter to Health Care Providers; 2019 Feb 4 [cited 2019 Nov 27]. Available from: https://www.fda.gov/medical-devices/letters-health-care-providers/increased-rate-mortality-patients-receiving-abiomed-impella-rp-system-letter-health-care-providers. [Google Scholar]
- 25.Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 2011 Feb 8;57(6):688–96. doi: 10.1016/j.jacc.2010.08.613. [DOI] [PubMed] [Google Scholar]
- 26.Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW, TandemHeart Investigators Group A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006 Sep;152(3):469.e1–8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005 Jul;26(13):1276–83. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 28.Kapur NK, Paruchuri V, Jagannathan A et al. Mechanical circulatory support for right ventricular failure. JACC Heart Fail. 2013 Apr;1(2):127–34. doi: 10.1016/j.jchf.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Kapur NK, Esposito ML, Bader Y et al. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation. 2017 Jul 18;136(3):314–26. doi: 10.1161/CIRCULATIONAHA.116.025290. [DOI] [PubMed] [Google Scholar]
- 30.Schmack B, Farag M, Kremer J et al. Results of concomitant groin-free percutaneous temporary RVAD support using a centrifugal pump with a double-lumen jugular venous cannula in LVAD patients. J Thorac Dis. 2019 Apr;11(Suppl 6):S913–S920. doi: 10.21037/jtd.2018.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazui T, Tran PL, Echeverria A et al. Minimally invasive approach for percutaneous CentriMag right ventricular assist device support using a single PROTEKDuo Cannula. J Cardiothorac Surg. 2016 Aug 4;11(1):123. doi: 10.1186/s13019-016-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.John R, Long JW, Massey HT et al. Outcomes of a multicenter trial of the Levitronix CentriMag ventricular assist system for short-term circulatory support. J Thorac Cardiovasc Surg. 2011 Apr;141(4):932–9. doi: 10.1016/j.jtcvs.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Abbott [Internet] Abbott Park, IL: Abbott; c2019. CentriMag Circulatory Support System; 2019 (cited 2019 Nov 26). Available from: https://www.cardiovascular.abbott/us/en/hcp/products/heart-failure/centrimag-acute-circulatory-support-system.html. [Google Scholar]
- 34.Hill JD, O'Brien TG, Murray JJ et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972 Mar 23;286(12):629–34. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 35.Griffin B, Menon V. Manual of Cardiovascular Medicine. 5th ed. Philadelphia: Wolters Kluwer Health; 2019. p. 1172. p. [Google Scholar]
- 36.Katz SD, Smilowitz NR, Hochman JS. Another Nail in the Coffin for Intra-Aortic Balloon Counterpulsion in Acute Myocardial Infarction With Cardiogenic Shock. Circulation. 2019 Jan 15;139(3):404–6. doi: 10.1161/CIRCULATIONAHA.118.038279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibanez B, James S, Agewall S et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018 Jan 7;39(2):119–77. doi: 10.1093/eurheartj/ehx393. ESC Scientific Document Group. [DOI] [PubMed] [Google Scholar]
- 38.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016 Jul 14;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. ESC Scientific Document Group. [DOI] [PubMed] [Google Scholar]
- 39.van Diepen S, Katz JN, Albert NM et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017 Oct 17;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. [DOI] [PubMed] [Google Scholar]
- 40.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15;128(16):e240–327. doi: 10.1161/CIR.0b013e31829e8776. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. [DOI] [PubMed] [Google Scholar]
- 41.Fried JA, Nair A, Takeda K et al. Clinical and hemodynamic effects of intra-aortic balloon pump therapy in chronic heart failure patients with cardiogenic shock. J Heart Lung Transplant. 2018 Nov;37(11):1313–21. doi: 10.1016/j.healun.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sintek MA, Gdowski M, Lindman BR et al. Intra-Aortic Balloon Counterpulsation in Patients With Chronic Heart Failure and Cardiogenic Shock: Clinical Response and Predictors of Stabilization. J Card Fail. 2015 Nov;21(11):868–76. doi: 10.1016/j.cardfail.2015.06.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeVore AD, Hammill BG, Patel CB et al. Intra-Aortic Balloon Pump Use Before Left Ventricular Assist Device Implantation: Insights From the INTERMACS Registry. ASAIO J. 2018 Mar-Apr;64(2):218–24. doi: 10.1097/MAT.0000000000000629. [DOI] [PubMed] [Google Scholar]


