Abstract
Aims.
In a background of interest in staging models in psychiatry, we tested the validity of a simple staging model of cognitive impairment to predict incident dementia.
Method.
A large community sample of adults aged ≥55 years (N = 4803) was assessed in the baseline of a longitudinal, four-wave epidemiological enquiry. A two-phase assessment was implemented in each wave, and the instruments used included the Mini-Mental Status Examination (MMSE); the History and Aetiology Schedule and the Geriatric Mental State-AGECAT. For the standardised degree of cognitive impairment Perneczky et al's MMSE criteria were applied. A panel of psychiatrists diagnosed cases of dementia according to DSM-IV criteria, and cases and sub-cases of dementia were excluded for the follow-up waves. Competing risk regression models, adjusted by potential confounders, were used to test the hypothesised association between MMSE levels and dementia risk.
Results.
Out of the 4057 participants followed up, 607 (14.9%) were classified as ‘normal’ (no cognitive impairment), 2672 (65.8%) as ‘questionable’ cognitive impairment, 732 (18.0%) had ‘mild’ cognitive impairment, 38 (0.9%) had ‘moderate’ cognitive impairment and eight (0.2%) had ‘severe’ impairment.
Cognitive impairment was associated with risk of dementia, the risk increasing in parallel with the level of impairment (hazard ratio: 2.72, 4.78 and 8.38 in the ‘questionable’, ‘mild’ and ‘moderate’ level of cognitive impairment, respectively).
Conclusions.
The documented gradient of increased risk of dementia associated with the severity level of cognitive impairment supports the validity of the simple staging model based on the MMSE assessment.
Key words: Cognitive impairment, dementia, Mini-Mental Status Examination, staging
Introduction
Cognitive disturbance is frequent in the elderly population (Rait et al. 2005; Zhang et al. 2014) and is a source of increasing concern. Although cognitive disturbance is considered to be nuclear in the concept of dementia, including conditions such as Alzheimer's disease (APA, 2013), it is now also regarded as an index of ill-health (Regal-Ramos et al. 2005; Wiesli et al. 2005) and of frailty (Kelaiditi et al. 2013). In relation to this, it has been shown that cognitive impairment increases the mortality-risk (Nguyen et al. 2003; Schultz-Larsen et al. 2008; Park et al. 2013). We have confirmed these previous findings, but have also reported a gradient of increased mortality-risk in parallel with the severity of cognitive disturbance (Santabárbara et al. 2014) suggesting the possibility of using the staging paradigm in this area. Implicit in the philosophy of staging is the gradual progression and deterioration of the clinical syndrome and of the underlying biological processes (Rikkert et al. 2011). This model seeks to define the extent of progression of a disorder at a particular point in time and to differentiate early, milder clinical phenomena from those accompanying illness progression and chronicity. The model has been widely considered to be useful in clinical medicine (Edge & Compton, 2010), having considerable potential in psychiatry (McGorry, 2007).
The staging model has been applied in the field of dementia and different methods have been proposed (Hughes et al. 1982; Reisberg et al. 1988), although none of them has been generally accepted (Rikkert et al. 2011). These models in dementia emphasise the assessment of cognitive impairment, but we consider clinical dementia to be much broader (Semrau et al. 2015). Still, although there is no consensus about its significance, the construct global cognitive impairment has interest by itself, as shown by international groups pondering the so-called ‘cognitive frailty’ (Kelaiditi et al. 2013). The construct cognitive impairment is considered by some as a syndrome (Breitner, 2015), that may be observed in different diseases, and we have recently documented that is associated with an increased mortality-risk even after controlling for dementia (Santabárbara et al. 2014). Therefore, we still feel the construct may be a candidate for staging models.
Timesaving methods of staging cognitive impairment would be particularly useful both in clinical settings and in community studies, since scales in widespread use were developed for staging dementia (Hughes et al. 1982; Reisberg et al. 1988), and may be time-consuming. Methods based on simple instruments such as the Mini-Mental State Examination (MMSE) (Folstein et al. 1975) would be convenient in this respect. Perneczky et al. (2006), mapping MMSE scores onto CDR categories, reported that the MMSE may perform as a surrogate of the CDR for the staging of dementia in AD. Using the same MMSE staging scores reported by Perneczky et al. (2006) we were able to document the gradient of increased mortality-risk in parallel with the severity of the cognitive impairment (Santabárbara et al. 2014). However, the power of this categorisation of the MMSE to predict cognitive deterioration and dementia has not been tested previously.
A considerable number of studies, including studies conducted in the community have reported the association of cognitive impairment and dementia, including Alzheimer's disease (Ward et al. 2013), particularly in the field of the so called ‘mild cognitive impairment’ (Mitchell & Shiri-Feshki, 2009). Some of these studies used the MMSE to document cognitive disturbance (Aevarsson & Skoog, 2000; Hensel et al. 2009; Wong et al. 2013). However, none of such studies tested the staging system of the MMSE. Moreover, to our knowledge, none of the previous studies associating cognitive disturbance and dementia controlled for mortality (Mauri et al. 2012; Wong et al. 2013). Since death may prevent the occurrence of illness, particularly in the aged population, it is recommended that the effect of mortality should be taken into account in incidence studies (Berry et al. 2010).
In this context, we now aim at documenting that a simple method based on the MMSE is useful for staging cognitive disturbance at a population level. Our hypothesis is that individuals with cognitive difficulties identified by the MMSE, but not being classified as cases or subcases of dementia in the baseline study, will have an increased risk of incident dementia, the risk increasing according to the severity level of impairment.
Material and methods
Study design and sample
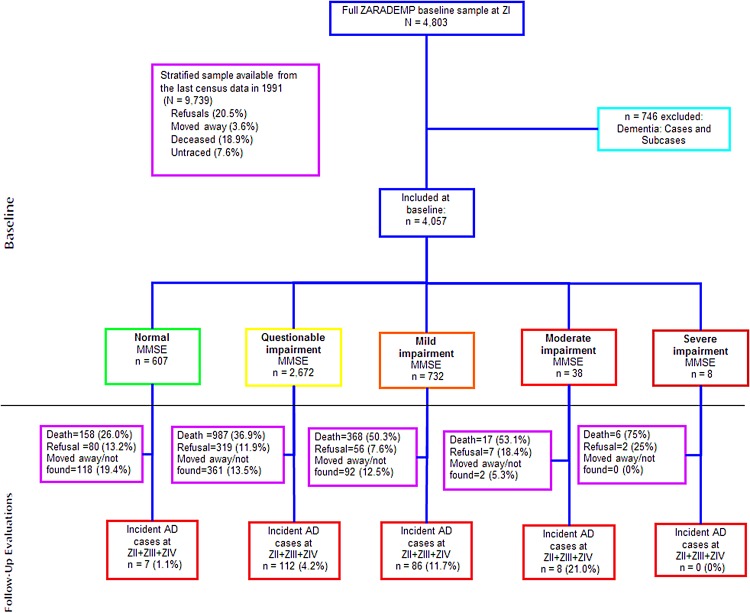
The ZARAgoza DEMentia DEPression Project (ZARADEMP Project) was designed as a longitudinal, community-based study to examine the incidence of dementia and the risk factors in incident cases of dementia. It was carried out in Zaragoza, a large city in Spain, with an important proportion of inhabitants coming from rural areas (Lobo et al. 2005). A stratified random sample of individuals 55 years of age and older, with proportional allocation by age and sex, drawn from the eligible individuals (n = 157 787) in the Spanish official census lists of 1991, was invited to participate in the baseline examination. In the baseline study, 4803 individuals were interviewed. For the follow-up, because we were interested in cognitively intact individuals, we excluded subjects considered to be cases or subcases of dementia at baseline (see definitions below; n = 746), for a starting sample of 4057 participants. The ZARADEMP Study participants underwent a baseline assessment (Wave I, starting in 1994) and three follow-up visits, starting in 1997 (Wave II), in 1999 (Wave III) and in 2006 (Wave IV) (Fig. 1). Further details of the objectives and methods of the project have been described elsewhere (Lobo et al. 2005, 2011). The Ethics Committee of the University of Zaragoza and the Fondo de Investigación Sanitaria approved this study, according to Spanish Law, and all individuals provided written informed consent.
Fig. 1.
Study flow chart.
Data collection
Several standard tools, previously validated in Spain, were incorporated in the ZARADEMP interview, including the Spanish version of the MMSE (Folstein et al. 1975; Lobo et al. 1999) for the screening of cognitive function. Both validity coefficients and population norms in this official version of the MMSE are very similar to those reported in the USA (Crum et al. 1993). The mental state of the study participants was assessed using the Geriatric Mental State B (GMS-B)-AGECAT (Copeland et al. 1987; Lobo et al. 1995), a semi-structured standardised clinical interview that may be used by lay interviewers. The GMS-B includes neuropsychological items and provides a ‘threshold global score’ that discriminates between ‘non-cases’, ‘subcases ’ and ‘cases’ of dementia. Psychiatric history was taken using the History and Aetiology Schedule (Dewey & Copeland, 2001), a standardised method accompanying the GMS. Instrumental and basic activities of daily living were assessed using the Lawton and Brody scale (Lawton & Brody, 1969; Tárraga, 1995); and Katz's Index (Katz et al. 1963; Álvarez et al. 1992), respectively. Information on medical conditions considered to be risk factors of dementia was collected using the EURODEM Questionnaire (Launer et al. 1992). This Questionnaire was developed to assess risk factors of dementia by EURODEM, a research consortium for European Studies of dementia. The authors performed a pooled analysis of European population-based prospective studies of individuals 65 years and older, with 528 incident dementia patients and 28 768 person-years of follow-up (Launer et al. 1999). Each item in the ZARADEMP interview has been operationally defined, according to previously agreed EURODEM criteria. Detailed information on medical conditions in this study has been reported elsewhere (Lobo-Escolar et al. 2008). Systematic checks on the reliability of the assessments were implemented to prevent the ‘reliability-drift’.
Dementia case finding
A two-phase epidemiological case-finding process for dementia was implemented in the baseline study (Wave I) and a similar method in the follow-up waves (Waves II, III and IV). In phase I in each wave, well-trained and regularly supervised lay interviewers (senior medical students) conducted the ZARADEMP interview at the subject's homes or place of residence. Interviewers in the follow-up waves were unaware of the results of the baseline interview. Outside caregivers were interviewed when the participant was considered to be unreliable (in cases of dementia and approximately 10% of subcases of dementia). Participants were nominated as ‘probable cases’ on the basis of GMS threshold ‘global’ score (1/2) and/or Mini-Mental (23/24) standard cut-off points.
In phase II, the ‘probable cases’ of dementia were reassessed at the subjects’ homes or place of residence by research psychiatrists. The same assessment instruments and methods were used, and a neurological examination was performed to help in the diagnostic process. The validity of this diagnostic process has been previously documented (Lobo et al. 1995). Identified cases of dementia were presented to a panel of four research psychiatrists. Variables in the ZARADEMP interview were operationalised to conform to the DSM-IV criteria used to diagnose the cases. For the diagnosis of ‘incident case’ of dementia, agreement by at least three of the psychiatrists was required. To document the accuracy of the panel diagnosis of dementia, a proportion of cases were invited for a hospital diagnostic work-up, which included neuroimage studies and a complete neuropsychological diagnostic battery.
Assessment of stages of cognitive impairment
Information coming from the MMSE was used to classify subjects according to their scores into corresponding degrees of cognitive impairment. Standardised degrees of cognitive impairment in the MMSE validated by Perneczky et al. (2006) have been used in this study: ‘normal’ (scores 30); ‘questionable’ (scores 26–29); ‘mild’ (scores 21–25); ‘moderate’ (scores 11–20) and ‘severe’ (scores 0–10).
Ascertainment of mortality
All-cause mortality of the ZARADEMP-Project respondents was ascertained through a reliable source, the official population registry in the city. Information in the registry was completed and verified via death certificate, which provides accurate information, including day, month and year of death. Days from birth to the date of death were calculated for each subject, and those individuals remaining alive in 1st January 2007 or missing (refusal, emigrated, not localisable) were included in the analysis as censured.
Covariates
Potentially confounding factors assessed at baseline included socio-demographic characteristics (sex and education), medical risk factors (vascular disease, hypertension and diabetes), functional status and affective diagnosis (anxiety and depression). Education was categorised into three levels: illiterate (unable to read and write, and <2 years of formal education), primary (complete or incomplete) and secondary school or higher. Functional status was based on the Katz Index (Katz et al. 1963), and the Instrumental Activities of Daily Living Scale (Lawton & Brody, 1969). For this study, scores on each scale were dichotomised into disability and no disability. The diagnosis of depression and anxiety were based on the AGECAT computer system. Blood pressure (BP) was measured during the interview using a standard manual tensiometer, using the average of 2 BP readings; hypertension was considered when BP >140/99 mmHg or if the participant reported being treated for hypertension. The presence of vascular risk factors and diabetes was based on the medical history obtained using the EURODEM Risk Factors Questionnaire (Launer et al. 1992). Vascular diseases were dichotomised, distinguishing between vascular disease (angina and/or myocardial infarct and/or stroke) and no history of vascular disease. Diabetes was dichotomised into persons with a previous medical diagnosis or receiving treatment for diabetes and the absence of diabetes.
Statistical procedures
The two-tailed Cochran–Armitage (Armitage, 1955) test for trend was used to seek a linear trend in proportions across MMSE stages, and two-tailed analysis of variance test was used to seek linear trend in means of continuous measures.
We used a multivariate survival analysis with age as timescale (Thiébaut & Bénichou, 2004) to study the specific hypothesis that standardised stages of cognitive impairment (Perneczky et al. 2006) are associated with an increased risk of dementia. The cumulative incidence function (CIF) approach (Fine & Gray, 1999) was used to display the risk of patients experiencing the event of interest (i.e., dementia), taking into account the competing event (death) as time progressed (Pintilie, 2006; Putter et al. 2007). In order to support using competing risks regression model, we assessed the difference between the CIF of dementia and death by means of the Kochar–Lam–Yip's test (Kochar et al. 2002).
In a first step of the survival analysis, we built CIF (Scrucca et al. 2007) for the MMSE degrees of cognitive impairment to estimate the probability of incident dementia. Then, we test for equality of CIF across groups (Gray, 1988). The different degrees of cognitive impairment were compared with the ‘normal’ performance group in the MMSE.
In a second step of the survival analysis, in order to explore mechanisms explaining the association between MMSE stages and risk of dementia, we used a multivariate model in which we controlled for potential modifiers: sociodemographic factors (sex and education), functional status, affective diagnosis (anxiety and depression) and medical risk factors (vascular disease, hypertension and diabetes).
To examine the assumption of proportional distribution hazards, we tested the time-varying effect of each covariate using the Scheike and Zhang test (Scheike & Zhang, 2008).
For all analyses, we used R software (http://www.r-project.org), with its cmprsk and timereg packages for survival analyses and coin package for Cochran–Armitage test for trend.
Results
Out of the 4057 participants in baseline, 607 (14.9%) were classified as ‘normal’ (no cognitive impairment), 2672 (65.8%) as ‘questionable’ cognitive impairment, 732 (18.0%) had ‘mild’ cognitive impairment, 38 (0.9%) had ‘moderate’ cognitive impairment and 8 (0.2%) had ‘severe’ impairment (Fig. 1). The median follow-up time was 8.4 years (Interquartile range: 3.1–11.4). During the follow-up period, 1516 (37.3%) individuals died and 213 (5.2%) were considered to have incident dementia.
Table 1 shows the sociodemographic characteristics of the participants by level of cognitive impairment. The participants in the higher level of cognitive impairment (MMSE) were significantly older and, as the severity of the impairment increased, participants were more likely to be women and illiterate. Moreover, between the subgroups classified by level of impairment, significant differences were observed in most clinical variables (Table 1).
Table 1.
Characteristics of participants at baseline, by level of cognitive impairment
| Normal (n = 607) | Questionable (n = 2672) | Mild (n = 732) | Moderate (n = 38) | Severe (n = 8) | p-valuea | |
|---|---|---|---|---|---|---|
| MMSE, mean ± s.d. | 30 ± 0.0 | 27.7 ± 1.0 | 23.4 ± 1.3 | 17.2 ± 2.6 | 3.6 ± 4.0 | <0.001 |
| Age, year, mean ± s.d. | 67.6 ± 7.0 | 71.4 ± 8.7 | 77.7 ± 9.4 | 77.4 ± 9.7 | 76.0 ± 9.0 | <0.001 |
| Women, n (%) | 300 (49.4) | 1417 (53.0) | 482 (65.8) | 29 (76.3) | 1 (12.5) | <0.001 |
| Educational level, n (%) | <0.001 | |||||
| Illiterate | 15 (2.4) | 173 (6.5) | 110 (15.0) | 14 (36.8) | 3 (37.5) | |
| Primary school | 362 (59.6) | 2036 (76.2) | 596 (81.4) | 20 (52.6) | 5 (62.5) | |
| Secondary school or higher | 227 (37.4) | 442 (16.5) | 18 (2.4) | 3 (7.8) | 0 (0) | |
| Functional status | ||||||
| Basic ADLs, n (%) | 18 (3.0) | 159 (6.0) | 92 (14.3) | 5 (42.2) | 3 (89.3) | <0.001 |
| Instrumental ADLs, n (%) | 30 (5.1) | 282 (10.7) | 183 (30.4) | 13 (70.3) | 4 (95.0) | <0.001 |
| Medical risk factors | ||||||
| Hypertension, n (%) | 405 (66.8) | 184 (66.8) | 525 (73.0) | 26 (67.6) | 7 (52.3) | 0.025 |
| Diabetes, n (%) | 62 (10.2) | 309 (11.7) | 124 (16.2) | 6 (13.8) | 0 (10.2) | <0.001 |
| Vascular disease, n (%) | 47 (8.3) | 285 (11.3) | 93 (14.9) | 1 (18.3) | 0 (33.6) | 0.035 |
| Affective diagnosis | ||||||
| Anxiety, n (%) | 162 (26.6) | 733 (27.3) | 176 (23.2) | 6 (9.1) | 0 (7.4) | 0.052 |
| Depression, n (%) | 45 (7.4) | 266 (9.9) | 134 (18.8) | 9 (13.9) | 1 (3.7) | <0.001 |
Cochran–Armitage trend test or analysis of variance linear trends.
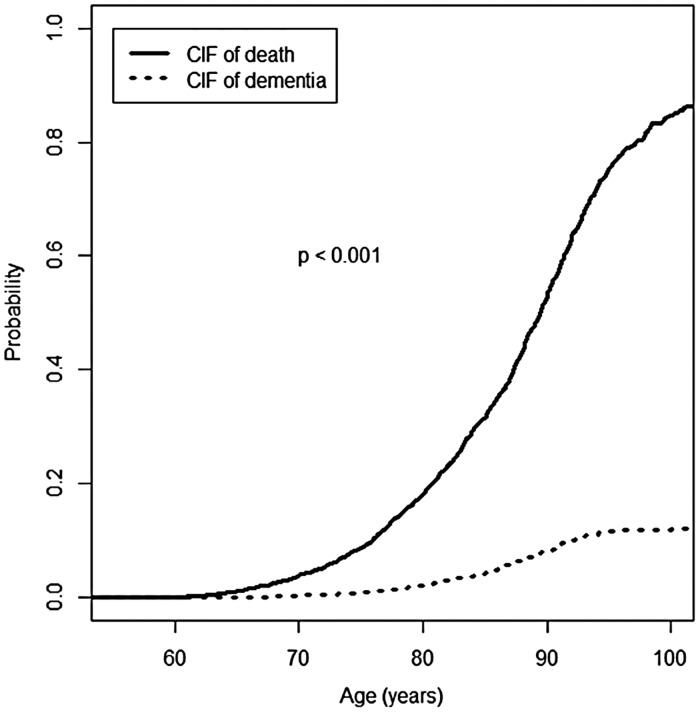
As Fig. 2 shows, a significantly higher probability of death compared to the probability of dementia was observed, according to the Kochar–Lam–Yip test (p < 0.001). Furthermore, the CIF for death is was above the CIF for dementia.
Fig. 2.
Probability of incident dementia and probability of death in the community sample. CIF: cumulative incidence function.
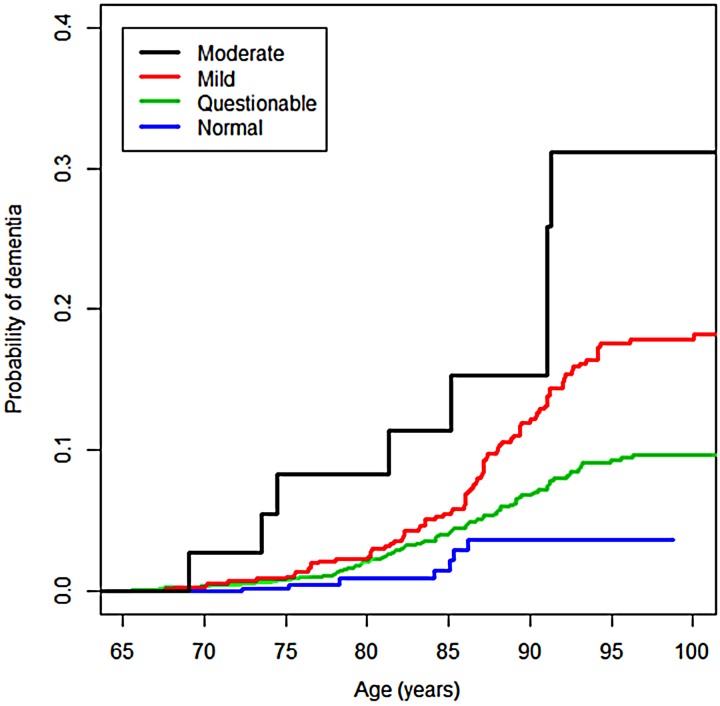
The crude comparison of the CIF by the MMSE level of impairment shows that the probability of dementia is higher in individuals with cognitive impairment when compared with the ‘normals’, the probability increasing with the severity of the impairment (p < 0.001) (Fig. 3). For example, for individuals of 85 years old, the probability (in percentage) of dementia in the ‘normal’ group was 1.5% (95% confidence intervals (CI): 0.5–3.9%), significantly lower than 4.3% (95% CI: 3.3–5.4%) for subjects in the ‘questionable’ degree, 5.9% (95% CI: 4.1–8.1) for subjects in the ‘mild’ degree and 11.7% (95% CI: 3.6–25.1%) for subjects in the ‘moderate’ degree.
Fig. 3.
Probability of incident dementia by degree of cognitive impairment in the community sample.
Table 2 shows the risk of dementia in relation to the level of cognitive impairment. The proportion of incident cases of dementia increases gradually as the MMSE scores decrease (p < 0.001). Table 2 also shows the results of the competing-risk regression analysis of the dementia risk associated with the different levels of cognitive impairment. The association with dementia risk increases by level of cognitive impairment. In the final multivariate model, model 2, with the inclusion of all potential confounding factors, the association between MMSE levels of cognitive impairment and dementia risk was: hazard ratio (HR) = 2.78 in the ‘questionable’; HR = 4.78 in the ‘mild’; and HR = 8.38 in the ‘moderate’ degree of cognitive impairment. The eight individuals with ‘severe’ cognitive impairment were not included in the analysis, since six of them had died at follow-up, and the remaining two were lost.
Table 2.
Risk of dementia associated with level of cognitive impairment
| Degrees of cognitive impairment | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| Incident dementia cases, n (%) | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Normal (n = 607) | 7 (1.1) | 1 | – | 1 | – |
| Questionable (n = 2672) | 112 (4.2) | 2.50 (1.15–5.43) | 0.021 | 2.72 (1.17–6.28) | 0.019 |
| Mild (n = 732) | 86 (11.7) | 4.52 (2.02–10.10) | <0.001 | 4.78 (2.01–11.35) | <0.001 |
| Moderate (n = 38) | 8 (21.0) | 7.93 (2.86–22.00) | <0.001 | 8.38 (2.88–24.35) | <0.001 |
Fine and Grey regression model, HR, CIs, and p-values based on ‘normal approximation’ of Wald χ2 test with 1 df are shown for all variables analysed. Model 1: included terms for sex and education; Model 2: included the terms in model 1 plus terms for functional status, affective syndromes (anxiety and depression) and medical risk factors (vascular disease, hypertension and diabetes).
Discussion
The results support the hypothesis that individuals with cognitive difficulties identified by the MMSE, but not being classified as cases or subcases of dementia have an increased risk of incident dementia, the risk increasing according to the severity level of impairment: in the final multivariate statistical model, after adjusting by all the potential confounding factors, the association between the ‘questionable’ MMSE degree of cognitive impairment and dementia risk was increased (HR = 2.78) when compared with the ‘cognitively normal’ but the risk was higher in the ‘mild’ degree of cognitive impairment (HR = 4.18) and particularly in the ‘moderate’ degree of impairment (HR = 8.38). The short number of individuals in the ‘severe’ level of impairment impeded statistical calculations, but the outcome was quite negative, since the great majority of them (75%) had died at follow-up and the remaining were lost. Therefore, despite the fact that the risk of dementia for different severity levels in the MMSE overlaps, the results in this study support for the first time a staging model in the prediction of incident dementia, since there is a progression of risk in parallel with the severity level of impairment, a clear gradient, a ‘dose–response’ relationship.
Some previous studies reporting the association of cognitive impairment and incident dementia used the MMSE, but none of them controlled by mortality, and none tested a staging system of the MMSE (Aevarsson & Skoog, 2000; Hensel et al. 2009; Wong et al. 2013). In a previous article, we also documented a gradient of increased mortality-risk in individuals with the same cognitive impairment model (Santabárbara et al. 2014). Therefore, we argue in favor of maintaining the usefulness of this staging model, since predictions of both mortality and incident dementia may be helped by knowing the level of cognitive performance of the elderly.
The usefulness of staging models in the management of medical diseases has considerable support both in the literature and in clinical practice (Edge & Compton, 2010), and McGorry (2007) has extensively advocated its use in the field of psychiatry. Staging models have certainly been promoted in the area of dementia (Reisberg et al. 1988), but cognitive impairment cannot be confounded with dementia, since dementing diseases are quite complex and need to be approached from several dimensions different from the nuclear cognitive disturbance (Rikkert et al. 2011). In fact, we are now involved in the development of a multi-dimensional scale for staging clinical needs in individuals with dementia (Semrau et al. 2015).
On the other hand, there is some controversy about the meaning of the construct cognitive impairment (Dartigues & Amieva, 2014), and new research is needed to ascertain its nature. Cognitive impairment is nuclear in the present concept of dementia (WHO, 1992), but both constructs are not equivalent, as shown by the fact that even controlling by dementia, cognitive impairment is associated with increased mortality (Santabárbara et al. 2014). This last construct is also considered to be an index of general ill health (Regal-Ramos et al. 2005) or of frailty (Kelaiditi et al. 2013). The staging model of cognitive impairment we propose here may stimulate both clinical and basic research. The model implies clinical progression, but also the progression of underlying biological pathological processes (Rikkert et al. 2011) that may therefore be investigated in parallel.
In relation to the clinical applicability of the method, particularly for the high risk groups, it is remarkable that, after the exclusion at baseline not only the cases of dementia, but also the subcases, it was possible to identify individuals with minimal cognitive difficulties, ‘questionable’ according to Perneczky et al's criteria (Perneczky et al. 2006), and individuals with ‘mild’ difficulties with a risk of dementia almost three times higher and almost five times higher respectively than the individuals with intact performance in the MMSE. Moreover, we have observed that these individuals with cognitive difficulties are different from the cases of mild cognitive impairment, MCI according to either classical, Petersen et al.’ criteria (Petersen et al. 1999) or DSM-5 criteria (Lopez-Anton et al. 2015). MCI cases are characterised by most authors as cases with subjective memory difficulties and objective measures of cognitive impairment. On the contrary, the individuals in the ‘questionable’ or in the ‘mild’ stage of disturbance in this study rarely had subjective complaints. Since more severe stages of cognitive disturbance have also been identified with this simple method, it may be recommended not only for the early identification of individuals at risk, but also for monitoring the cognitive performance in follow-up, longitudinal studies, as well as in clinical practice.
We believe this study with a long follow-up period of 12 years, which is part of the ZARADEP Project, has some additional advantages. In support of the generalisability of the findings, it was conducted in a large and representative community sample. By excluding at baseline subcases of dementia, the possibility of overestimating the dementia risk is minimised, and the robustness of the results is supported by the fact that the individuals with normal cognitive performance were used as the reference in the statistical, regression models. Moreover, the MMSE severity degrees of cognitive impairment used here have been validated against well-known scales such as the Clinical Dementia Rating (Hughes et al. 1982), which have also been used to assess cognitive impairment (Modrego & Ferrández, 2004; Sartorius et al. 2013).
An important advantage in this report, and contrary to previous similar studies, relates to the use of the competing risk model in analysing cognitive impairment as a risk factor for dementia. Traditional models (e.g., Kaplan–Meier and Cox regression) may overestimate the risk of the disease (dementia) in the presence of high rates of mortality, since they do not take into account the competing mortality risk. The competing risk approach is particularly appropriate when the expected mortality is high (Berry et al. 2010) such as in the elderly individuals. Moreover, cognitive impairment in this age group has been associated with increased mortality (Kryscio et al. 2013) as previously reported in this same population (Santabárbara et al. 2014). In addition, and contrary to previous studies using time-on-study (i.e., time since inclusion date), which apply age as a covariate in the survival models (Mauri et al. 2012; Wong et al. 2013), we used exact age as timescale. In samples of older adults age is strongly associated with some covariates (for example, chronic diseases) and therefore this method should be preferred, since the bias on effect estimates can be avoided (Thiébaut & Bénichou, 2004).
On the other hand, this study was more stringent than previous ones in controlling for potentially confounding factors. We controlled by demographic factors and specifically low education, known to be associated with an increased risk of dementia (de Pedro-Cuesta et al. 2009), and the results were maintained when controlling by affective syndromes, namely depression and anxiety. While some previous reports are controversial (Schmand et al. 1995), a number of studies have shown the association of both depression (Chen et al. 2008; Goveas et al. 2011; Wong et al. 2013) and anxiety (Gallacher et al. 2009) with dementia. Nonetheless, these studies used instruments describing symptoms and/or syndromes of anxiety and/or depression, but we used Stage II AGECAT diagnostic categories. We also controlled for potential medical risk factors, including vascular disease, hypertension and diabetes. We did not incorporate specifically obesity or head trauma for which there is less support in the literature (Lobo et al. 2010), but believe their inclusion would not modify the results in a substantial way. It might be argued that new covariate alterations could occur in the long follow-up period in this particular study. Nonetheless, in such case the group with cognitive disturbance would probably be more exposed to the covariate alterations and, consequently, the main results and conclusions of this study would be reinforced.
Limitations
The validity of instruments used and diagnostic processes implemented here have been supported in previous studies, but putative misclassification of cognitive disturbance cannot be totally eliminated, since the sensitivity and specificity of the MMSE is not optimal (Folstein et al. 1975; Lobo et al. 1999). Likewise, while the validity of the diagnosis of incident dementia in the participants’ homes has been supported in our previous studies (Lobo et al. 2005) a full hospital protocol including neuroimaging assessments could not be completed in all cases. Furthermore, potential risk factors of dementia such as the APO-e-4 (Tai et al. 2015), co-morbidity (Cunningham & Hennessy, 2015) or the diet (Morris, 2012), were not controlled in this study.
Conclusion
In conclusion, this study has found support for the hypothesis that the staging model of cognitive impairment proposed is valid, since a clear gradient of dementia risk is shown, the risk increasing in parallel with the severity of impairment. It is remarkable that the proposed staging model based on the assessment with the MMSE, a simple, 10-min, bed-side or office cognitive test, that may be given by non-specialised researchers and health professionals, shows that, compared with an individual with good cognitive performance, an individual of the same age, sex, physical and mental conditions, has a risk of developing dementia close to three times higher in cases of ‘questionable’ cognitive disturbance, the risk progressing to more than four times and eight times higher in cases of ‘mild’ or ‘moderate’ degree of impairment, respectively. This is of major significance for clinical work in psychiatry or in settings such as primary care or geriatric facilities, and has also relevance for public health work and may be important in selecting areas for future research.
Acknowledgements
The following members of the ZARADEMP Workgroup participated in this manuscript: Pedro Saz, M.D., Ph.D.; Tirso Ventura, M.D., Ph.D.; Miguel Angel Quintanilla, M.D., Ph.D.; Juan Francisco Roy, Ph.D.; Antonio Campayo, M.D., Ph.D.; José Luis Día, M.D., Ph.D. The authors acknowledge the contribution of the lay interviewers, senior medical students who participated in the field work for this study.
Financial Support
Supported by Grants from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain (grants 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128) and Gobierno de Aragón/ Programa Operativo Fondo Social Europeo 2007–2013.
Conflict of Interest
We declare that C. De-la-Cámara has received financial support to attend scientific meetings from Janssen-Cilag, Almirall, Eli Lilly, Lundbeck, Rovi, Esteve, Novartis, and Astrazeneca. P. Gracia-García has received Grant support from Janssen, AstraZeneca, and the Ilustre Colegio de Médicos de Zaragoza; she has received Honorarium from AstraZeneca, Lilly, Janssen and Servier; she has received travel support from Lilly, Almirall, Lundbeck, Rovi, Pfizer, and Janssen. A. Lobo had a consultancy with Janssen, and he has received honorarium or travel support from Eli Lilly and Bial. None of these activities is related to the current project. For the remaining authors, none was declared.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Aevarsson O, Skoog I (2000). A longitudinal population study of the mini-mental state examination in the very old: relation to dementia and education. Dementia and Geriatric Cognitive Disorders 11, 166–175. [DOI] [PubMed] [Google Scholar]
- Alvarez Solar M, de Alaiz Rojo AT, Brun Gurpegui E, Cabañeros Vicente JJ, Calzón Frechoso M, Cosío Rodríguez I, García López P, García-Cañedo Fernández R, Pardo González I, Suárez-González A (1992). Functional capacity of patients over 65 according to the Katz index. Reliability of the method. Atencion Primaria/Sociedad Española de Medicina de Familia y Comunitaria 10, 812–816. [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn. (DSM-5). American Psychiatric Publishing: Washington, DC. [Google Scholar]
- Armitage P (1955). Tests for linear trends in proportions and frequencies. Biometrics 11, 375–386. [Google Scholar]
- Berry SD, Ngo L, Samelson EJ, Kiel DP (2010). Competing risk of death: an important consideration in studies of older adults. Journal of the American Geriatrics Society 58, 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC (2015). Observations on DSM-5 Mild neurocognitive disorder vs. its predecessor, mild cognitive impairment. Acta Psychiatrica Scandinavica 131, 15–17. [DOI] [PubMed] [Google Scholar]
- Chen R, Hu Z, Wei L, Qin X, McCracken C, Copeland JR (2008). Severity of depression and risk for subsequent dementia: cohort studies in China and the UK. British Journal of Psychiatry 193, 373–377. [DOI] [PubMed] [Google Scholar]
- Copeland JRM, Dewey ME, Wood N, Searle R, Davidson IA, McWilliam C (1987). Range of mental illness amongst the elderly in the community: prevalence in Liverpool using the GMS-AGECAT package. British Journal of Psychiatry 150, 815–823. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF (1993). Population-based norms for the Mini-Mental State Examination by age and educational level. Journal of the American Medical Association 269, 2386–2391. [PubMed] [Google Scholar]
- Cunningham C, Hennessy E (2015). Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheimer's Research and Therapy 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartigues JF, Amieva H (2014). Letter to the editor: cognitive frailty: rational and definition from an (I.a.N.a./i.a.g.g.) international consensus group. Journal of Nutrition, Health, and Aging 18, 95. [DOI] [PubMed] [Google Scholar]
- de Pedro-Cuesta J, Virues-Ortega J, Vega S, Seijo-Martinez M, Saz P, Rodriguez F, Rodriguez-Laso A, Reñe R, de las Heras SP, Mateos R, Martinez-Martin P, Manubens JM, Mahillo-Fernandez I, Lopez-Pousa SA, Reglà JL, Gascón J, García FJ, Fernández-Martínez M, Boix R, Bermejo-Pareja F, Bergareche A, Benito-León J, de Arce A, del Barrio JL (2009). Prevalence of dementia and major dementia subtypes in Spanish populations: a reanalysis of dementia prevalence surveys, 1990–2008. BMC Neurology 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey ME, Copeland JR (2001). Diagnosis of dementia from the history and aetiology schedule. International Journal of Geriatric Psychiatry 16, 912–917. [DOI] [PubMed] [Google Scholar]
- Edge SB, Compton CC (2010). The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology 17, 1471–1474. [DOI] [PubMed] [Google Scholar]
- Fine JP, Gray RJ (1999). A proportional hazards model for the subdistribution of competing risk. Journal of the American Statistical Association 94, 496–509. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975). Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gallacher J, Bayer A, Fish M, Pickering J, Pedro S, Dunstan F, Ebrahim S, Ben-Shlomo Y (2009). Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosomatic Medicine 71, 659–666. [DOI] [PubMed] [Google Scholar]
- Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM (2011). Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women's Health Initiative Memory Study. Journal of the American Geriatrics Society 59, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RJ (1988). A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics 16, 1141–1154. [Google Scholar]
- Hensel A, Luck T, Luppa M, Glaesmer H, Angermeyer MC, Riedel-Heller SG (2009). Does a reliable decline in Mini Mental State Examination total score predict dementia? Diagnostic accuracy of two reliable change indices. Dementia and Geriatric Cognitive Disorders 27, 50–58. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry: The Journal of Mental Science 140, 566–572. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963). Studies of illness in the aged. The Index of ADL, a standardized measure of biological and psychosocial function. Journal of the American Medical Association 185, 914–919. [DOI] [PubMed] [Google Scholar]
- Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, Ritz P, Duveau F, Soto ME, Provencher V, Nourhashemi F, Salvà A, Robert P, Andrieu S, Rolland Y, Touchon J, Fitten JL, Vellas B (2013). Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. Journal of Nutrition, Health, and Aging 17, 726–734. [DOI] [PubMed] [Google Scholar]
- Kochar SC, Lam KF, Yip PS (2002). Generalized supremum tests for the equality of cause specific hazard rates. Lifetime Data Analysis 8, 277–288. [DOI] [PubMed] [Google Scholar]
- Kryscio RJ, Abner EL, Lin Y, Cooper GE, Fardo DW, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Wan L, Schmitt FA (2013). Adjusting for mortality when identifying risk factors for transitions to mild cognitive impairment and dementia. Journal of Alzheimers Disease 35, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Brayne C, Breteler MM (1992). Epidemiologic approach to the study of dementing diseases: a nested case-control study in European incidence studies of dementia. Neuroepidemiology 11, 114–118. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A (1999). Rates and risk factors for dementia and Alzheimer's disease. Neurology 52, 78–84. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM (1969). Assessment of older people, self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186. [PubMed] [Google Scholar]
- Lobo A, Saz P, Marcos G, Día JL, De-la-Cámara C (1995). The prevalence of dementia and depression in the elderly community in a Southern European population: the Zaragoza study. Archives of General Psychiatry 52, 497–506. [DOI] [PubMed] [Google Scholar]
- Lobo A, Saz P, Marcos G, Día JL, de la Cámara C, Ventura T, Morales Asín F, Fernando Pascual L, Montañés JA, Aznar S (1999). Revalidation and standardization of the cognition mini-exam (first Spanish version of the Mini-Mental Status Examination) in the general geriatric population. Medicina Clínica 112, 767–774. [PubMed] [Google Scholar]
- Lobo A, Saz P, Marcos G, Día JL, De la Cámara C, Ventura T, Montañés JA, Lobo-Escolar A, Aznar S, ZARADEMP Workgroup (2005). The ZARADEMP-Project on the incidence, prevalence and risk factors of dementia (and depression) in the elderly community: II. Methods and first results. European Journal of Psychiatry 19, 40–54. [Google Scholar]
- Lobo A, Saz P, Quintanilla MA (2010). Dementia In Textbook of Psychosomatic Medicine (ed. Levenson JL), pp. 115–152. American Psychiatric Association: Washington. [Google Scholar]
- Lobo A, Lopez-Anton R, Santabárbara J, de-la-Cámara C, Ventura T, Quintanilla MA, Roy JF, Campayo AJ, Lobo E, Palomo T, Rodriguez-Jimenez R, Saz P, Marcos G (2011). Incidence and lifetime risk of dementia and Alzheimer's disease in a Southern European population. Acta Psychiatrica Scandinavica 124, 372–383. [DOI] [PubMed] [Google Scholar]
- Lobo-Escolar A, Saz P, Marcos G, Quintanilla MA, Campayo A, Lobo A; ZARADEMP Workgroup (2008). Somatic and psychiatric comorbidity in the general elderly population: results from the ZARADEMP Project. Journal of Psychosomatic Research 65, 347–355. [DOI] [PubMed] [Google Scholar]
- Lopez-Anton R, Santabárbara J, De-la-Cámara C, Gracia-García P, Lobo E, Marcos G, Pirez G, Saz P, Haro JM, Rodríguez-Mañas L, Modrego PJ, Dewey ME, Lobo A (2015). Mild cognitive impairment diagnosed with the new DSM-5 criteria: prevalence and associations with non-cognitive psychopathology. Acta Psychiatrica Scandinavica 131, 29–39. [DOI] [PubMed] [Google Scholar]
- Mauri M, Sinforiani E, Zucchella C, Cuzzoni MG, Bono G (2012). Progression to dementia in a population with amnestic mild cognitive impairment: clinical variables associated with conversion. Functional Neurology 27, 49–54. [PMC free article] [PubMed] [Google Scholar]
- McGorry PD (2007). Issues for DSM-V: Clinical staging: a heuristic pathway to valid nosology and safer, more effective treatment in psychiatry. American Journal of Psychiatry 164, 859–860. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Shiri-Feshki M (2009). Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica 119, 252–265. [DOI] [PubMed] [Google Scholar]
- Modrego PJ, Ferrández J (2004). Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Archives of Neurology 61, 1290–1293. [DOI] [PubMed] [Google Scholar]
- Morris MC (2012). Nutritional determinants of cognitive aging and dementia. Proceedings of the Nutrition Society 71, 1–13. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS (2003). Cognitive impairment and mortality in older Mexican Americans. Journal of the American Geriatrics Society 51, 178–183. [DOI] [PubMed] [Google Scholar]
- Park MH, Kwon DY, Jung JM, Han C, Jo I, Jo SA (2013). Mini-Mental Status Examination as predictors of mortality in the elderly. Acta Psychiatrica Scandinavica 127, 298–304. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A (2006). Mapping scores onto stages: Mini-Mental State Examination and Clinical Dementia Rating. American Journal of Geriatric Psychiatry 14, 139–144. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999). Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology 56, 303–308. [DOI] [PubMed] [Google Scholar]
- Pintilie M (2006). Competing Risks: A Practical Perspective. Wiley: Chichester. [Google Scholar]
- Putter H, Fiocco M, Geskus RB (2007). Tutorial in biostatistics: competing risks and multi-state models. Statistics in Medicine 26, 2389–2430. [DOI] [PubMed] [Google Scholar]
- Rait G, Fletcher A, Smeeth L, Brayne C, Stirling S, Nunes M, Breeze E, Ng ES, Bulpitt CJ, Jones D, Tulloch AJ (2005). Prevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the community. Age and Ageing 34, 242–248. [DOI] [PubMed] [Google Scholar]
- Regal-Ramos RJ, Salinero-Fort MA, Cruz-Jentoft AJ (2005). Mortality predictive factors of a clinical cohort of elderly patients. Atencion Primaria/Sociedad Española de Medicina de Familia y Comunitaria 36, 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Crook T (1988). Global deterioration scale (GDS). Psychopharmacology Bulletin 24, 661–663. [PubMed] [Google Scholar]
- Rikkert MG, Tona KD, Janssen L, Burns A, Lobo A, Robert P, Sartorius N, Stoppe G, Waldemar G (2011). Validity, reliability, and feasibility of clinical staging scales in dementia: a systematic review. American Journal of Alzheimer's Disease and Other Dementias 26, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santabárbara J, Lopez-Anton R, Marcos G, De-la-Cámara C, Lobo E, Saz P, Gracia-García P, Ventura T, Campayo A, Rodríguez-Mañas L, Olaya B, Haro JM, Salvador-Carulla L, Sartorius N, Lobo A (2014). Degree of cognitive impairment and mortality: a 17-year follow-up in a community study. Epidemiology and Psychiatric Sciences 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius N, Semrau M, Burns A, Lobo A, Rikkert MO, Robert P, Stoppe G (2013). Symposium on the development of an instrument allowing the staging of care for patients with dementia. In World Psychiatric Association Congress.
- Scheike TH, Zhang MJ (2008). Flexible competing risks regression modeling and goodness-of-fit. Lifetime Data Analysis 14, 464–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Lindeboom J, Hooijer C, Jonker C (1995). Relation between education and dementia: the role of test bias revisited. Journal of Neurology Neurosurgery and Psychiatry 59, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Larsen K, Rahmanfard N, Kreiner S, Avlund K, Holst C (2008). Cognitive impairment as assessed by a short form of MMSE was predictive of mortality. Journal of Clinical Epidemiology 61, 1227–1233. [DOI] [PubMed] [Google Scholar]
- Scrucca L, Santucci A, Aversa F (2007). Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplantation 40, 381–387. [DOI] [PubMed] [Google Scholar]
- Semrau M, Burns A, Djukic-Dejanovic S, Eraslan D, Han C, Lecic-Tosevski D, Lobo A, Mihai A, Morris J, Palumbo C, Robert P, Stiens G, Stoppe G, Volpe U, Rikkert MO, Sartorius N; International Dementia Alliance (IDEAL) study group (2015). Development of an international schedule for the assessment and staging of care for dementia. Journal of Alzheimers Disease 44, 139–151. [DOI] [PubMed] [Google Scholar]
- Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, Collins N, Ben-Aissa M, Lei AZ, Bahroos N, Green SJ, Hendrickson B, Van Eldik LJ, LaDu MJ (2015). APOE-modulated Ab-induced neuroinflammation in Alzheimer's disease: current landscape, novel data, and future perspective. Journal of Neurochemistry 133, 465–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tárraga LL (1995). Evaluación del deterioro cognitivo y funcional de la demencia. Escalas de mayor interés en la Atención Primaria In El médico ante la demencia y su entorno, Módulo 1 (ed. Boada M and Tárraga L), pp. 37–50. Bayer SA: Barcelona. [Google Scholar]
- Thiébaut AC, Bénichou J (2004). Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study. Statistics in Medicine 23, 3803–3820. [DOI] [PubMed] [Google Scholar]
- Ward A, Tardiff S, Dye C, Arrighi HM (2013). Rate of conversion from prodromal Alzheimer's disease to Alzheimer's dementia: a systematic review of the literature. Dementia and Geriatric Cognitive Disorders Extra 28, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesli P, Schwegler B, Schmid B, Spinas GA, Schmid C (2005). Mini-Mental State Examination is superior to plasma glucose concentrations in monitoring patients with suspected hypoglycaemic disorders during the 72-hour fast. European Journal of Endocrinology/European Federation of Endocrine Societies 152, 605–610. [DOI] [PubMed] [Google Scholar]
- Wong CHY, Leung GTY, Fungible AWT, Chan WC, Lam LCW (2013). Cognitive predictors for five-year conversion to dementia in community-dwelling Chinese older adults. International Psychogeriatrics 25, 1125–1134. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (1992). International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). WHO: Geneva. [Google Scholar]
- Zhang Y, Shi Z, Liu M, Liu S, Yue W, Liu S, Xiang L, Lu H, Liu P, Wisniewski T, Wang J, Ji Y (2014). Prevalence of cognitive impairment no dementia in a rural area of Northern China. Neuroepidemiology 15, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]