Key Points
Question
Is there a benefit associated with take-home naloxone for overdose reversals supplied to patients who are receiving treatment for opioid use disorder?
Findings
This year-long cohort study enrolled 395 study participants and provided overdose education and take-home naloxone kits. After 1 year, 73 participants performed 114 opioid overdose reversals in the community.
Meaning
These findings suggest that take-home naloxone supplied to patients in opioid treatment programs may be part of a targeted harm-reduction strategy to reduce negative outcomes associated with opioid overdose in the community.
This cohort study examines the use of take-home naloxone for overdose reversals performed by study participants who are receiving treatment for opioid use disorder.
Abstract
Importance
The US opioid crisis was deemed a public health emergency in 2017. More than 130 individuals in the US die daily as a result of unintentional opioid overdose deaths.
Objective
To measure use of take-home naloxone for overdose reversals performed by study participants with opioid use disorder receiving treatment at an opioid treatment program.
Design, Setting, and Participants
In a year-long cohort study, between April 4, 2016, and May 16, 2017, 395 study participants enrolled at the University of New Mexico Addiction and Substance Abuse Opioid Treatment Program, an outpatient clinic treating substance use disorders. Inclusion criteria included all patients enrolled at University of New Mexico Addiction and Substance Abuse Opioid Treatment Program during the study enrollment period; positive history of opioid use disorder treated with methadone, buprenorphine, or naltrexone; and age 18 years or older. Exclusion criteria included allergy to naloxone and age younger than 18 years. The study closed 1 year after enrollment, on May 17, 2018. Data analysis was performed from May 2018 to July 2019.
Exposure
Two doses of take-home naloxone combined with opioid overdose education were provided to study participants.
Main Outcomes and Measures
The primary outcome was to measure the association of take-home naloxone with overdose reversals performed by patients with opioid use disorder enrolled in an opioid treatment program.
Results
We enrolled 395 study participants (270 female [68.4%]; mean [SD] age, 35.4 [12.6] years; 260 [65.8%] with Hispanic white race/ethnicity) in the 1-year prospective trial. Sixty-eight female participants (25.2% of all female participants) were pregnant at the time of enrollment. Seventy-three of the 395 study participants (18.0%) performed 114 overdose reversals in the community. All community reversals were heroin related. Most study participants (86.8%) stated that the person on whom they performed an overdose reversal was a friend, relative, acquaintance, or significant other. In the year before enrollment, only 18 study participants (4.5%) had been prescribed naloxone.
Conclusions and Relevance
Take-home naloxone as part of overdose education and naloxone distribution provided to patients in an opioid treatment program may be associated with a strategic targeted harm reduction response for reversing opioid overdose–related deaths. Policy makers may consider regulations to mandate overdose education and naloxone distribution in opioid treatment programs.
Introduction
In the US in 2018, on average, 130 individuals died every day from an unintentional overdose (OD) of prescription opioid analgesics or injection heroin.1 Opioid use disorder (OUD) may develop in many ways, including use of prescription opioid analgesics given to patients for legitimate chronic pain, misuse of prescription opioids borrowed from a friend or relative for chronic pain, and illicit use of injection heroin.2 Almost one-half of all prescription opioid–related emergency department visits are the result of nonmedical opioid use.3 Medication-assisted treatment and increased availability of naloxone are considered best practices for reducing patient mortality associated with OUD.4
Unintentional opioid OD is the single greatest cause of mortality among injection drug users in the United States and accounts for more than one-half of all deaths among people who inject opioids.5,6,7 Overdose is a common occurrence among opioid users worldwide and in the United States, with data showing that 46% to 68% of individuals with OUDs have experienced at least 1 OD.8,9 In addition, the most common cause of death for dependent opioid users is OD.7
Opioid users rarely OD while alone.10,11 The other person or people present are most commonly other injection drug users.12 In many surveys, injection drug users consistently report a high prevalence of witnessing OD events.13,14
Unfortunately, injection drug users also report reluctance to contact emergency medical services upon witnessing an OD.8,13,15,16 One community study found that, when interviewing those who performed reversal on others during an opioid OD, they were much more likely to use physical stimulation rather than traditional first aid measures.13 In addition, most injection drug users (73.2%) receive their information on how to prevent or respond to an OD from friends or other drug users.13
Naloxone, the antidote to an opioid OD, is the primary harm reduction tool used to reverse ODs in the community by bystanders, paramedics, and police officers and upon arrival at emergency departments.17,18 Although naloxone access has become more available in large urban centers, where the standing order for naloxone has been widely publicized, patients with OUD and other behavioral health conditions obtain their medications from a pharmacist less frequently than other populations.19
Take-home naloxone (THN) is the provision of naloxone directly to a patient in a clinical setting, which bypasses the pharmacy or a third party. Settings in which THN has been shown to be very useful include opioid treatment and syringe exchange programs, after release from incarceration with a diagnosis of OUD, and with police officers and first responders in the community.20 We hypothesized that THN as part of OD education and naloxone distribution would be associated with a decreased number of opioid OD deaths among study participants at University of New Mexico’s Addiction and Substance Abuse Program (UNM ASAP) and in the community of Bernalillo County, New Mexico.
Methods
Between April 4, 2016, and May 16, 2017, 395 study participants voluntarily enrolled at the UNM ASAP for this prospective cohort study. The study closed 1 year after enrollment, on May 17, 2018. These study participants were current patients of UNM ASAP.
This study was approved by the University of New Mexico Human Research and Review Committee and was registered in the National Institutes of Health Clinical Trials (ClinicalTrials.gov Identifier NCT02669901). A certificate of confidentiality is on record at the National Institutes of Health for this study. Written informed consent was obtained from each individual study participant. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Each study participant completed the initial visit and at least 1 follow-up visit. Inclusion criteria included history of OUD treated with methadone, buprenorphine, or naltrexone and age 18 years or older. Exclusion criteria included allergy to naloxone and age younger than 18 years.
The UNM ASAP is a federally qualified opioid treatment program (OTP) that provides medication-assisted treatment and behavioral therapy for adults, including pregnant women, as well as adolescents. Approximately 65% of the UNM ASAP patients are women. Most (80%) of the patients had previously used heroin (compared with prescription opioids) as their drug of choice before beginning medication-assisted treatment.
Initial Visit
Data Collection
At the initial visit, study participants provided demographic data, social history, limited medical history, type of medication-assisted treatment, medication list, and history of illicit drug use.21 All 395 study participants enrolled were evaluated to determine whether they had received a naloxone prescription in the year before enrollment. First, each participant’s electronic medical record was queried to see whether a prescription for naloxone was written in the year before study enrollment. Second, every study participant was personally asked by the research coordinator at the initial study visit whether they had directly received a naloxone prescription in the year before enrollment.
OD Education and Naloxone Distribution
During the initial visit, the study participant along with his or her companion, if present, were provided with OD information and learned the importance of using naloxone. Study participants were encouraged, but not required, to bring a companion to the visit. The education included recognizing signs and symptoms of an OD, the importance of calling 911, rescue breathing techniques, and staying with the person experiencing the OD until medical help has arrived. The research coordinator distributed 1 naloxone kit (2 doses) to study participants after they felt confident with using the naloxone autoinjector.21,22 Study participants were informed that they could return to the clinic to obtain a naloxone replacement kit if needed.
Quarterly Follow-up Visits
Every 3 months, study participants were asked to follow up with the study coordinator to report on whether an OD reversal was performed and whether naloxone replacement kits were needed. Study participants could also obtain naloxone replacement kits Monday through Friday during clinic hours (7 am to 5 pm). Urine toxicology screens were obtained as part of the routine UNM ASAP clinic procedure and were reported previously.23
Statistical Analysis
Stata statistical software version 15 (Stata Corp) was used for all analyses, including distributions of study participants’ characteristics at the initial visit, relationships between community members for whom OD reversal was performed and study participants, number of OD reversals performed, and reasons for replacement of THN kits. Stata 15 was also used for χ2 tests for statistical significance of associations between companion attendance at initial opioid education and performing at least 1 OD reversal. One-sided P < .05 was considered statistically significant. Data analysis was performed from May 2018 to July 2019.
Results
Characteristics of Study Participants
Of the 395 study participants, 270 (68.4%) were female, 277 (70.1%) were aged 18 to 39 years (mean [SD] age, 35.4 [12.6] years), 260 (65.8%) were Hispanic white, 279 (70.6%) were treated with methadone, and 354 (89.6%) did not have a companion present during their naloxone training (Table 1). The demographic characteristics of the study participants were not statistically significantly different from those of the UNM ASAP clinic population.23 The sex of the study population reflected the UNM ASAP population, which had 65% female participants. Of all study female participants, 68 (25.2%) were pregnant at the time of their enrollment. The UNM ASAP preferentially accepts pregnant women with OUD and follows these patients throughout their pregnancy and as long as needed for medication-assisted treatment.
Table 1. Demographic Characteristics of Participants Enrolled at the University of New Mexico Addiction and Substance Abuse Opioid Treatment Program.
| Characteristic | Participants, No. (%) (N = 395) |
|---|---|
| Sex | |
| Female | 270 (68.4) |
| Male | 125 (31.6) |
| Pregnancy status of female participants | |
| Pregnant women of childbearing age | 68 (25.2) |
| Nonpregnant women of childbearing age | 165 (61.1) |
| Women not of childbearing age | 37 (13.7) |
| Age, y | |
| 18-19 | 13 (3.3) |
| 20-29 | 154 (39.0) |
| 30-39 | 110 (27.8) |
| 40-49 | 49 (12.4) |
| 50-59 | 48 (12.2) |
| ≥60 | 21 (5.3) |
| Race/ethnicity | |
| Hispanic white | 260 (65.8) |
| Non-Hispanic white | 100 (25.3) |
| American Indian or Alaska Native | 16 (4.1) |
| Black or African American | 9 (2.3) |
| Asian | 1 (0.3) |
| Not reported | 9 (2.3) |
| Medication-assisted treatment | |
| Methadone | 279 (70.6) |
| Buprenorphine | 109 (27.6) |
| Naltrexone (oral or intramuscular) | 7 (1.8) |
| Companion for naloxone education | |
| Present | 33 (8.4) |
| Not present | 354 (89.6) |
| Unknown | 8 (2.0) |
Naloxone Reversals Performed on Community Members Overdosing on Heroin
All OD reversals performed in the community were the result of heroin OD according to the study participants who performed these reversals. Study participants who completed at least 1 follow-up visit and/or came to the study coordinator requesting at least 1 naloxone replacement kit were included in the 336 participants counted as having at least 1 follow-up visit, for an 85% retention rate.
No study participant reported performing a naloxone reversal for a prescription opioid OD. Seventy-three study participants (18.0%) performed 114 OD reversals in the community. Two study participants overdosed during the study period, and naloxone was administered to them after a family member called 911. Three study participants died during the study: 1 from liver disease, 1 from a confirmed stroke, and 1 from an unknown cause. Replacement kits were given to study participants without question whenever requested.
Most participants (86.8%) stated that the person on whom they performed an OD reversal was a friend (55.3%), relative (17.5%), acquaintance (9.6%), or significant other (4.4%). Only 13.2% of the reported reversals were performed on strangers (Table 2).
Table 2. Community Members Whose Overdoses Were Reversed by Naloxone Administered by Study Participants.
| Relationship of Community Member to Study Participant | Community Members, No. (%) (N = 114) |
|---|---|
| Friend | 63 (55.3) |
| Relative | 20 (17.5) |
| Stranger | 15 (13.2) |
| Acquaintance | 11 (9.6) |
| Significant other | 5 (4.4) |
Naloxone Replacement Kits Requested
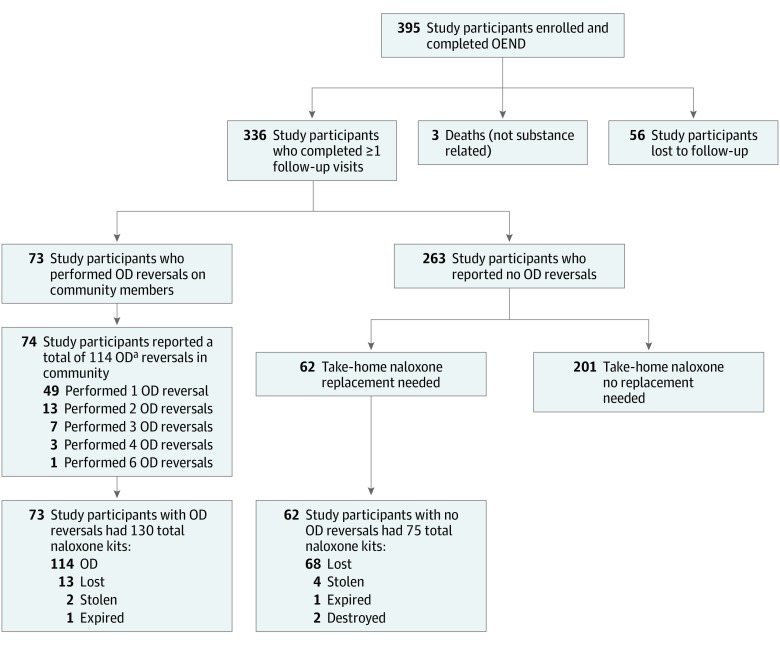
One hundred thirty replacement kits were requested by the 73 study participants who performed OD reversals for the 114 community members. These replacement kits were requested because of OD, being lost, stolen, or expired. For the 263 study participants who reported no reversal in the community, 62 study participants requested a total of 75 replacement kits (Figure).
Figure. Flowchart of Participant Enrollment.
OD indicates overdose; OEND, overdose education and naloxone distribution.
aAll were heroin ODs.
Low Companion Attendance at Initial OD Education and Naloxone Distribution Training
There were 33 (8.4%) study participants with companions at the initial OD education and naloxone distribution training. Of these, 9 performed 1 or more OD reversals in the community. There was no association between having a companion present and performing at least 1 OD reversal (P = .18).
Prescription Data for Year Before Study Enrollment
Of the 395 study participants, 18 (4.5%) received a naloxone prescription in the year before study enrollment. Of these, 9 (50.0%) answered positively to an initial visit question, “Has someone ever used naloxone on you to prevent an overdose?” Six study participants (33.0%) stated that they had witnessed an OD reversal in the last year.
Discussion
Rationale for THN Targeting Individuals With OUDs
The large number (114) of ODs reversed in Bernalillo County, New Mexico, after providing OD education and naloxone distribution at an OTP highlights the need for providing naloxone training directly to people who use opioids. This study demonstrates that people with OUD appear very willing to administer naloxone during an OD if it is made available to them directly.8,15 Targeted THN distribution and OD training programs may be an appropriate intervention to reduce opioid OD deaths. Indeed, small prospective pilot studies15,24,25 show that over one-half of injection drug users who received OD education and naloxone distribution training reported using naloxone during 3- or 6-month follow-up periods, and the proportion of participant-confirmed reversals ranged from 74%24 to 100%.15,25 One large study26 found that naloxone was used by injection drug users for a companion (friend or spouse) in 36% of cases and for a stranger in 15% of cases. In a 2013 interrupted time series study in Massachusetts,27 the vast majority of naloxone reversal attempts were successful and were performed by friends in private settings. In most OD reversals, naloxone is used on someone other than the patient given the naloxone prescription. Community and family members of those with OUD frequently educate others on how to use naloxone in the case of an OD.8 These data, and our current year-long prospective trial, demonstrate that naloxone distribution and OD training programs targeted toward people with OUD may be helpful in preventing OD-related mortality.
Heroin-Related OD Reversals
All OD reversals were performed on community members who were using heroin at the time of their OD. The 73 study participants who performed OD reversals for the 114 community members had themselves previously used either heroin or prescription opioid analgesics, or both. The finding that every community member whose OD was reversed by the 73 study participants was using heroin at the time of the OD reversal highlights the close social network surrounding injection drug use.
Decrease in Heroin Death Rates in Bernalillo County, New Mexico, 2016 to 2018
In Bernalillo County, where the project was implemented, the age-adjusted death rate from heroin increased from 9.1 deaths per 100 000 population in 2012 to 11.2 deaths per 100 000 in 2015.28 However, from 2016 to 2018 when the study took place, the age-adjusted death rate from heroin decreased by 36% from 10.0 per 100 000 population to 6.4 per 100 000 population.28 The decrease statewide during the same period was not nearly as precipitous (15% decrease from 8.4 deaths per 100 000 population to 7.2 deaths per 100 000 population).28 In addition, of the counties surrounding Bernalillo with sufficient numbers for reporting, Valencia County saw a 125% increase, and Sandoval County saw a 253% increase in heroin OD death rates.28 Although we cannot directly attribute the work of our project to this reduction in the heroin OD death rate in the county, it is possible that our study contributed in part to this decrease.
Naloxone Replacement Kits Requested
It is difficult to draw any definite conclusions on the number of naloxone kits needed to perform an OD reversal in a targeted setting, or on the potential reasons why so many naloxone kits were lost, especially in the cohort who did not report an OD in the community. It is possible that naloxone kits may have been given to friends and family, and other kits may have been used for an OD reversal with the study participant being hesitant to report the event.
Companion Attendance at Initial Visit
Fewer than 9% of study participants had a companion with them at the initial visit that provided OD education and naloxone distribution training. In addition, only 9 of the 33 study participants who had a companion in the initial study visit performed an OD reversal in the community. This finding suggests that having a companion at the initial training may not be necessary. The concepts of a naloxone standing order, naloxone coprescription, and over-the-counter naloxone all validate the idea that patients with OUD, or their family members, are competent to acquire naloxone medication and use it on at-risk individuals.29
Limited Impact of Prescribing Naloxone
In the year before enrollment, only 18 study participants (4.5%) had a naloxone prescription written for them by a medical practitioner. Although there is a promising trend in naloxone prescriptions dispensed in retail pharmacies throughout the United States, it is still unclear whether these medications are reaching the population with OUD.19,30 Reasons may include stigma, lack of access to care, and lack of pharmacies in many rural locations. According to data presented by the US Department of Health and Human Services,31 the rate of naloxone coprescribing for patients taking a morphine milliequivalent dose greater than 50 was 0.3%; in addition, of those prescribed naloxone, 40% never picked up the prescription. Similarly, Medicare data32 show that the rate of naloxone cofilling was 1.3% to 5.2% among individuals receiving a morphine milliequivalent dose greater than 50.
Indeed, if there had been no OD education and naloxone distribution provided to the study participants, it is unlikely that the 114 OD reversals would have been performed in the community. National data33 show that although bystanders are present in 40% to 45% of all OD mortalities, naloxone is administered by these bystanders in only 0.8% to 4.3% of these ODs.
Take-home naloxone provides a strategic targeted response and reduces barriers in this population of community members with OUD. This patient population with OUD may need multiple methods of harm reduction and treatment before maintaining sobriety.
Increased Retention Rates in OTP
Our study also revealed that 336 study participants returned at least once during the year-long study at UNM ASAP to participate in the quarterly follow-up visit and/or to request a naloxone replacement kit. We hypothesize that having THN at UNM ASAP was a benefit for the study participants and contributed to the 85% retention rate, which is higher than those for most OTPs reported by other studies.34 This retention rate is especially high given that UNM ASAP is part of a safety-net hospital, with a patient population with high levels of socioeconomic comorbidities.
Future Treatment for Opioid OD Survivors
Facilitating treatment entry after OD reversal is a critical component for combating the opioid epidemic, considering that each nonfatal OD increases the risk of a future fatal OD.35 D’Onofrio et al36 found that emergency department–initiated buprenorphine treatment can significantly increase treatment retention among individuals with OUD. One approach may be to use a specific OD emergency response team that ensures a timely start of buprenorphine treatment after an OD reversal, engagement in continuing treatment, and access to harm-reduction interventions, including naloxone provision, testing for infectious diseases, and referral to appropriate treatment.
Limitations
This study examined the benefits associated with THN as part of OD education and naloxone distribution provided to study participants receiving medication-assisted treatment for OUD in an OTP. Although a randomized clinical trial is the reference standard for a typical medication trial, this would not be ethical for patients or community members who need naloxone to survive an opioid OD. The UNM ASAP is part of New Mexico’s only safety-net hospital that accepts pregnant women with OUD into their clinic. This OTP has a higher percentage of women than many other substance use programs around the country and may affect the study’s generalizability, especially because there is a higher prevalence of heroin addiction among men in the United States. This study may also be open to respondent bias because people in a substance use (or syringe exchange) program who are willing to accept THN may be more likely to interact with someone who has overdosed compared with people in a substance use program who are not willing to accept THN. However, it is not feasible to confirm this outcome bias without obtaining medical record data from the person whose OD was reversed with naloxone. This is primarily because of Health Insurance Portability and Accountability Act patient privacy considerations and investigational review board prohibition.
Conclusions
In 2017, the New Mexico legislature unanimously passed House Bill 370.37 The bill mandates OD education and THN, along with a prescription of naloxone to each patient in an OTP and every inmate leaving incarceration with an OUD. The bill also mandates that every New Mexico police officer carries 2 doses of naloxone while on active duty.37
The results of this year-long prospective study demonstrate that THN given to patients in an OTP may be associated with public health harm reduction in reversing opioid ODs in the community. We suggest that policy makers may consider regulations to mandate THN as part of OD education and naloxone distribution in OTPs.
References
- 1.Rudd R, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths: United States, 2010-2015. https://www.cdc.gov/mmwr/volumes/65/wr/mm655051e1.htm. Published December 30, 2016. Accessed June 9, 2018. [DOI] [PubMed]
- 2.Bose J, Hedden SL, Lipari RN, Park-Lee E. Key substance use and mental health indicators in the united states: results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18-5068, NSDUH Series H-53). https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm. Published September 2018. Accessed July 11, 2019.
- 3.Lovegrove MC, Dowell D, Geller AI, et al. US emergency department visits for acute harms from prescription opioid use, 2016-2017. Am J Public Health. 2019;109(5):-. doi: 10.2105/AJPH.2019.305007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton WM, Volkow ND, Throckmorton DC, Lurie P. Expanded access to opioid overdose intervention: research, practice, and policy needs. Ann Intern Med. 2013;158(1):65-66. doi: 10.7326/0003-4819-158-1-201301010-00013 [DOI] [PubMed] [Google Scholar]
- 5.Latkin CA, Hua W, Tobin K. Social network correlates of self-reported non-fatal overdose. Drug Alcohol Depend. 2004;73(1):61-67. doi: 10.1016/j.drugalcdep.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Sherman SG, Cheng Y, Kral AH. Prevalence and correlates of opiate overdose among young injection drug users in a large U.S. city. Drug Alcohol Depend. 2007;88(2-3):182-187. doi: 10.1016/j.drugalcdep.2006.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clausen T, Waal H, Thoresen M, Gossop M. Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction. 2009;104(8):1356-1362. doi: 10.1111/j.1360-0443.2009.02570.x [DOI] [PubMed] [Google Scholar]
- 8.Strang J, Manning V, Mayet S, et al. Overdose training and take-home naloxone for opiate users: prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses. Addiction. 2008;103(10):1648-1657. doi: 10.1111/j.1360-0443.2008.02314.x [DOI] [PubMed] [Google Scholar]
- 9.Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103(6):979-989. doi: 10.1111/j.1360-0443.2008.02182.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darke S, Hall W. Heroin overdose: research and evidence-based intervention. J Urban Health. 2003;80(2):189-200. doi: 10.1093/jurban/jtg022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sporer KA. Acute heroin overdose. Ann Intern Med. 1999;130(7):584-590. doi: 10.7326/0003-4819-130-7-199904060-00019 [DOI] [PubMed] [Google Scholar]
- 12.Strang J, Powis B, Best D, et al. Preventing opiate overdose fatalities with take-home naloxone: pre-launch study of possible impact and acceptability. Addiction. 1999;94(2):199-204. doi: 10.1046/j.1360-0443.1999.9421993.x [DOI] [PubMed] [Google Scholar]
- 13.Tracy M, Piper TM, Ompad D, et al. Circumstances of witnessed drug overdose in New York City: implications for intervention. Drug Alcohol Depend. 2005;79(2):181-190. doi: 10.1016/j.drugalcdep.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 14.Pollini RA, McCall L, Mehta SH, Celentano DD, Vlahov D, Strathdee SA. Response to overdose among injection drug users. Am J Prev Med. 2006;31(3):261-264. doi: 10.1016/j.amepre.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 15.Galea S, Worthington N, Piper TM, Nandi VV, Curtis M, Rosenthal DM. Provision of naloxone to injection drug users as an overdose prevention strategy: early evidence from a pilot study in New York City. Addict Behav. 2006;31(5):907-912. doi: 10.1016/j.addbeh.2005.07.020 [DOI] [PubMed] [Google Scholar]
- 16.Tobin KE, Davey MA, Latkin CA. Calling emergency medical services during drug overdose: an examination of individual, social and setting correlates. Addiction. 2005;100(3):397-404. doi: 10.1111/j.1360-0443.2005.00975.x [DOI] [PubMed] [Google Scholar]
- 17.Rando J, Broering D, Olson JE, Marco C, Evans SB. Intranasal naloxone administration by police first responders is associated with decreased opioid overdose deaths. Am J Emerg Med. 2015;33(9):1201-1204. doi: 10.1016/j.ajem.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 18.Wakeman SE, Bowman SE, McKenzie M, Jeronimo A, Rich JD. Preventing death among the recently incarcerated: an argument for naloxone prescription before release. J Addict Dis. 2009;28(2):124-129. doi: 10.1080/10550880902772423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CM, Lurie PG, Compton WM. Increase in naloxone prescriptions dispensed in US retail pharmacies since 2013. Am J Public Health. 2016;106(4):689-690. doi: 10.2105/AJPH.2016.303062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gertner AK, Domino ME, Davis CS. Do naloxone access laws increase outpatient naloxone prescriptions? evidence from Medicaid. Drug Alcohol Depend. 2018;190:37-41. doi: 10.1016/j.drugalcdep.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 21.Katzman JG, Takeda MY, Bhatt SR, Moya Balasch M, Greenberg N, Yonas H. An innovative model for naloxone use within an OTP setting: a prospective cohort study. J Addict Med. 2018;12(2):113-118. doi: 10.1097/ADM.0000000000000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evzio [package insert]. Richmond, VA: Kaleo Pharma Inc; 2014. [Google Scholar]
- 23.Katzman JG, Greenberg NH, Takeda MY, Moya Balasch M. Characteristics of patients with opioid use disorder associated with performing overdose reversals in the community: an opioid treatment program analysis. J Addict Med. 2019;13(2):131-138. doi: 10.1097/ADM.0000000000000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner KD, Valente TW, Casanova M, et al. Evaluation of an overdose prevention and response training programme for injection drug users in the Skid Row area of Los Angeles, CA. Int J Drug Policy. 2010;21(3):186-193. doi: 10.1016/j.drugpo.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piper TM, Stancliff S, Rudenstine S, et al. Evaluation of a naloxone distribution and administration program in New York City. Subst Use Misuse. 2008;43(7):858-870. doi: 10.1080/10826080701801261 [DOI] [PubMed] [Google Scholar]
- 26.Enteen L, Bauer J, McLean R, et al. Overdose prevention and naloxone prescription for opioid users in San Francisco. J Urban Health. 2010;87(6):931-941. doi: 10.1007/s11524-010-9495-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New Mexico Department of Health . New Mexico death certificate data, 2012-2018. https://nmhealth.org/about/erd/bvrhs/hsp/. Accessed December 11, 2019.
- 29.Lewis DA, Park JN, Vail L, Sine M, Welsh C, Sherman SG. Evaluation of the overdose education and naloxone distribution program of the Baltimore Student Harm Reduction Coalition. Am J Public Health. 2016;106(7):1243-1246. doi: 10.2105/AJPH.2016.303141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohn M, Talbert JC, Huang Z, Lofwall MR, Freeman PR. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215. doi: 10.1001/jamanetworkopen.2019.6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giroir BP. The opioid epidemic and emerging public health policy priorities. https://www.ama-assn.org/system/files/2019-02/19-nac-opioid-epidemic-presentation_0.pdf. Published February 13, 2019. Accessed January 23, 2020.
- 32.U.S. Department of Health and Human Services . Report on pain management best practices: updates, gaps, inconsistencies, and recommendations. https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html. Published 2019. Accessed July 11, 2019.
- 33.Mattson CL, O’Donnell J, Kariisa M, Seth P, Scholl L, Gladden RM. Opportunities to prevent overdose deaths involving prescription and illicit opioids, 11 states, July 2016-June 2017. MMWR Morb Mortal Wkly Rep. 2018;67(34):945-951. doi: 10.15585/mmwr.mm6734a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79-87. doi: 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warner-Smith M, Darke S, Day C. Morbidity associated with non-fatal heroin overdose. Addiction. 2002;97(8):963-967. doi: 10.1046/j.1360-0443.2002.00132.x [DOI] [PubMed] [Google Scholar]
- 36.D’Onofrio G, Chawarski MC, O’Connor PG, et al. Emergency department-initiated buprenorphine for opioid dependence with continuation in primary care: outcomes during and after intervention. J Gen Intern Med. 2017;32(6):660-666. doi: 10.1007/s11606-017-3993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.State of New Mexico . Criminal expungement act. https://legiscan.com/NM/text/HB370/2019. Published 2017. Accessed January 21, 2020.