Abstract
AIM
To measure the concentration of vascular endothelial growth factor-A (VEGF-A), and placental growth factor (PLGF) in aqueous humor of uveal melanoma patients before and after Iodine-125 plaque therapy (IPT), determine the postoperative fluctuation and evaluate associated factors in vivo.
METHODS
Participants were 18 Chinese patients with uveal melanoma who were elected to IPT. Undiluted aqueous humor samples were collected at Iodine plaque implant and removal time, then stored immediately at −80°C until assayed. The concentration of VEGF-A, PLGF and other 7 cytokines comprising interleukin-2 (IL-2), IL-8, IL-10, interferon (IFN)-γ, programmed death (PD)-1, transforming growth factor (TGF)-β1 and insulin-like growth factor (IGF)-1 in aqueous humor was measured using Raybiotech immunoassay kit, a high throughput strategy. The VEGF-A and PLGF levels were compared across preoperation and postoperation subgroups, as well as those of other 7 interleukins. Correlation and grouped analyses were conducted to determine the independent effects of clinical parameters and other cytokines on VEGF-A and PLGF concentration or fluctuation. This study set a self-control design.
RESULTS
VEGF-A (P=0.038) and PLGF (P=0.026) were the only two increased cytokines after IPT. Preoperative and postoperative level of VEGF-A and PLGF (r=0.575, P=0.013; r=0.987, P<0.001) correlated with each other significantly. Level of VEGF-A (r=0.626, P=0.005; r=0.588, P=0.01) and PLGF (r=0.616, P=0.007; r=0.588, P=0.01) had positive correlation with tumor thickness consistently. Elevated VEGF-A or PLGF level were strong predictive factors of each other (P=0.007, OR=60.0). The elevated VEGF-A group showed a higher postoperative level of IFN-γ (P=0.005), IL-2 (P<0.001) and IL-10 (P=0.004) in aqueous humor. When the elevated PLGF group got similar results that a higher postoperative level of IFN-γ (P=0.007), IL-2 (P<0.001) and IL-10 (P=0.013) in aqueous humor.
CONCLUSION
This study reveals that VEGF-A and PLGF in aqueous humor significantly increased with tumor thickness and radiation process in uveal melanoma patients. VEGF-A and PLGF may be crucial in uveal melanoma genesis and radiotherapy reactions. Immune mediators comprised IFN-γ, IL-2 and IL-10 could play roles in the link between inflammation and angiogenesis in uveal melanoma when exposed to radiotherapy.
Keywords: uveal melanoma, vascular endothelial growth factor-A, radioplaque therapy, placental growth factor
INTRODUCTION
Uveal melanoma is the most common primary intraocular malignancy in adults, and with an obviously ethnic difference that is approximately 5-8 times more frequent in Caucasian than Asian[1]. Radiation treatment has held the dominant position for decades using isotopes such as Ruthenium (Ru)-106, Iodine (I)-125, and charged particle-like proton beam therapy or carbon ion therapy[2]–[4]. A considerable amount of clinical achievements have been made in diagnosis and treatment even the radiation-related complications for uveal melanoma[5]–[6]. However, our knowledge about the molecular biology underlying such a primary malignancy and radiotherapy reactions is quite limited. Inflammation and angiogenesis are two hallmarks of solid tumor[7]–[8], which had been well established for uveal melanoma in a deal of studies in vitro or in vivo already[9]–[10]. Researches using eyeball or ocular tissue, vitreous or aqueous humor and serum provided primary insights into the individual molecular characteristics of uveal melanoma and found that vascular endothelial growth factor-A (VEGF-A) has a validated role in its tumorigenesis, vasculogenesis and even metastatic prognosis[10]–[14]. But few has focused on the cases following brachytherapy. This time we use multiplex immunoassay and quantified analysis to investigate the aqueous humor concentration change and associative factors of VEGF-A and its homologous protein placental growth factor (PLGF) in Chinese uveal melanoma patients who treated by Iodine-125 plaque therapy (IPT).
SUBJECTS AND METHODS
Ethical Approval
This study was performed in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Beijing Tongren Hospital (TRECKY2015-017). All patients had been fully informed of the purpose and methods of the present study and provided written informed consent from themselves.
Patients
This prospectively consecutive randomized study included 18 patients who were eligible for IPT with unilateral primary choroidal melanoma evaluated at Tongren Eye Center between June 5, 2017 and September 25, 2017, then operated by the same surgeon. The exclusion criteria are as follows: 1) previous interventions of uveal melanoma; 2) any ophthalmic surgery history of the affected eye; 3) coexisting ocular disorders in the affected eye that could confound interpretation of cytokines' level, such as vitreous hemorrhage, diabetic retinopathy, and aged-related macular degeneration; 4) pregnancy or suckling phase for women, or a survival compromise status for any reason.
Data was recorded after thorough clinical check and laboratory or imaging examination, all baseline findings were documented with ultrasonography (Mylab 90, Esoate, Italy), orbital magnetic resonance imaging, fundus photography, optical coherence tomography (Heidelberg Spectralis HRA+OCT, Heidelberg, Germany). Fluorescein angiography and indocyanine green angiography were assisting tools in the differential diagnosis. All the diagnostic procedures were in accordance with findings of the Collaborative Ocular Melanoma Study (COMS) and Shields[5].
IPT was managed by a lead alloy COMS-type plaque[3],[15]. An episcleral radiation plaque was placed to cover the entire base of tumor and a 2-mm margin beyond. Amount of 125I seeds and plaque carrying time were adopted to dosimetric consideration for 100 Gy to tumor apex. Following up has been arranged 1mo after plaque removal then every 3mo routinely, and a thoroughly ophthalmic examination would be conducted. Systemic monitoring and screening for metastasis were recommended to be done twice-a-year.
Aqueous Humor Sampling and Multiplex Analysis
Undiluted aqueous humor samples were collected at Iodine plaque implant surgery and plaque removal time by puncturing to the anterior chamber using a 30-guage injector through the corneal limbus. No less than 50 µL aqueous humor sample at one time and then it will be stored at -80°C immediately until assayed. Raybiotech immunoassay kit QAH-CUST-1 (Raybiotech, USA) was used to determine the aqueous humor concentration of 9 immune mediators comprising VEGF-A, PLGF, interleukin-2 (IL-2), IL-8, IL-10, interferon-γ (IFN-γ), programmed death-1 (PD-1), transforming growth factor (TGF)-β1 and insulin-like growth factor (IGF)-1. A multiplexed sandwich ELISA-based quantitative array platform was used to enable accurately determining the concentration of cytokines simultaneously and could accomplish similar sensitivity as traditional ELISA. The lowest detectable concentration (LOD, pg/mL) of the assay was predetermined based on the concentration of array specific cytokine standards. In this assay, it was 142.1 for IFN-γ, 1.9 for IL-10, 11.5 for IL-2, 1.9 for IL-8, 26.2 for PD-1, 265.9 for TGF-β1, 2.0 for PLGF, 619.7 for IGF-I, 13.9 for VEGF-A. Cytokines' detectable concentration below LOD was set to its nonlinear output value. Concentration that recorded as “zero” meant undetectable, such frequency reached a peak (40%) for IGF-I, and PLGF (19%), PD-1 (18.0%), IFN-γ (9.1%), TGF-β1 (9.1%), IL-2 (9.0%).
Statistical Analysis
Statistical analyses were performed using SPSS 25.0 for Windows (IBM/SPSS, Chicago, Illinois, USA). Wilcoxon Signed Rank Test was used to compare cytokines level before and after IPT. Pearson correlation, control variables analysis and logistic regression were employed to confirm correlations between cytokines' level and clinical parameters. Wilcoxon rank-sum test was served for grouped analyses. P-value of 2-tailed below 0.05 was thought of significance.
RESULTS
A total of 18 subjects (18 eyes) who fulfilled the inclusion criteria enrolled in this study and undergone an entire follow-up period more than 18mo. The mean age was 47±14.3 years old and no one has ciliary body involved. Of 11 patients had the right eye affected and 7 cases were on the left. Half the patients were female. The mean largest basal diameter and tumor thickness at diagnosis were 12.0±1.66 mm and 6.9±2.27 mm, respectively. Radioactive plaque carrying time ranged from 7 to 35d and was 19.1d on average. No clinically significant complications associated with anterior chamber paracentesis had been observed. Three cases developed rubeosis iris or neovascular glaucoma (NVG) at 14mo after IPT on average and two had received eye enucleation for failed pharmacotherapy. We made the follow-up end at September 30, 2019. No signs of metastasis or other survival comprised events were observed among these 18 patients. Patients' clinical features are summarized in Table 1.
Table 1. Clinical features of 18 Chinese uveal melanoma patients.
| Characteristics | No. of patients | % |
| Tumor stagea | ||
| Stage I | 1 | 5.6 |
| Stage II | 17 | 94.4 |
| Tumor configuration | ||
| Mushroom | 9 | 50 |
| Flat | 1 | 5.6 |
| Hemisphere | 8 | 44.4 |
| Quadrantic location of tumor epicenter | ||
| Superior | 3 | 16.7 |
| Nasal | 4 | 22.2 |
| Inferior | 2 | 11.1 |
| Temporal | 2 | 11.1 |
| Superior temporal | 3 | 16.7 |
| Superior nasal | 1 | 5.5 |
| Inferior temporal | 3 | 16.7 |
| Anterior margin of tumor | ||
| Ora serrata to equator | 2 | 11.1 |
| Equator to macula | 15 | 83.3 |
| Macula | 1 | 5.6 |
| Associated serous retinal detachment | ||
| No | 5 | 27.8 |
| Yes | 13 | 72.2 |
| Subretinal fluid within 3 mm2 around fovea | ||
| No | 7 | 38.9 |
| Yes | 11 | 61.1 |
aTumor stage classification refers to the 7th edition validation of American Joint Committee on Cancer[16].
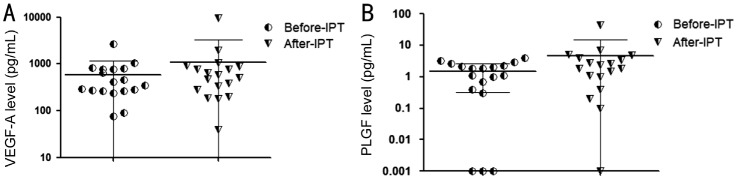
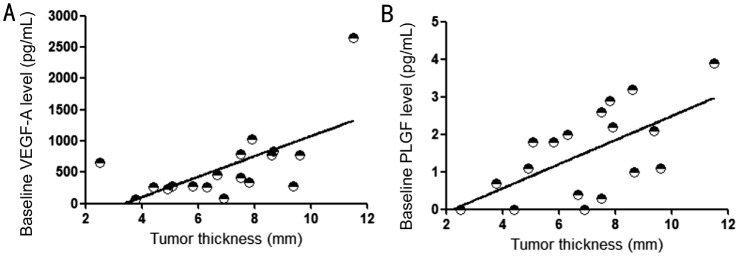
Concentration of the assayed cytokines was summarized in Table 2. Increasing expression of VEGF-A (Z=−2.069, P=0.038) and PLGF (Z=−2.187, P=0.026) were of statistical significance after plaque removal when compared with preoperative data (Figure 1). No significant post-radiation decrease of cytokines' level was observed. Baseline concentration of VEGF-A (r=0.626, P=0.005) and PLGF (r=0.616, P=0.007) had positive correlation with tumor thickness (Figure 2) and kept this significance by post-radiation phase (r=0.588, P=0.01 for VEGF-A; r=0.588, P=0.01 for PLGF). Using partial correlation analysis to control variable effect of retinal detachment, this significance maintained between level of VEGF-A and tumor thickness (pre-radiation: r=0.63, P=0.007; post-radiation: r=0.575, P=0.016). And so was for PLGF (pre-radiation: r=0.497, P=0.042; post-radiation: r=0.579, P=0.015). The concentration of VEGF-A and PLGF (pre-radiation: r=0.575, P=0.013; post-radiation: r=0.987, P<0.001) correlated with each other consistently (Table 3).
Table 2. Aqueous humour cytokines level of 18 uveal melanoma patients before and after IPT.
| Cytokines | Before IPT |
After IPT |
Pa | ||
| Median | Mean±SD | Median | Mean±SD | ||
| VEGF-A | 376.3 | 583.7±587.89 | 537.3 | 1094.6±2167.11 | 0.038 |
| PLGF | 1.5 | 1.5±1.18 | 2.1 | 4.7±10.10 | 0.026 |
| TGF-β1 | 499.6 | 542.6±260.41 | 401.3 | 451.8±308.19 | 0.088 |
| IFN-γ | 458.9 | 404.5±206.18 | 353.0 | 364.9±216.79 | 0.468 |
| PD-1 | 31.4 | 40.2±38.52 | 28.7 | 30.5±28.69 | 0.298 |
| IL-2 | 8.1 | 8.9±6.36 | 8.6 | 9.1±6.06 | 0.899 |
| IL-10 | 1.1 | 1.0±0.37 | 1.2 | 1.0±0.52 | 0.686 |
| IL-8 | 20.7 | 52.5±66.82 | 22.6 | 47.8±59.75 | 0.742 |
| IGF-I | 214.8 | 556.4±775.34 | 200.3 | 673.1±865.93 | 0.389 |
IPT: Iodine-125 plaque therapy; SD: Standard deviation. aWilcoxon signed rank test was used and P-value with exact 2-tailed was shown.
pg/mL
Figure 1. A significant increase of VEGF-A level in aqueous humor after patients' plaque removal (A; Wilcoxon signed rank test: Z=−2.069, P=0.038), and increase of PLGF level (B; Wilcoxon signed rank test: Z=−2.187; P=0.026).
Figure 2. Positive correlation between tumor thickness and aqueous humor VEGF-A level at baseline (A; r=0.626, P=0.005), and between tumor thickness and baseline level of PLGF (B; r=0.616, P=0.007).
Table 3. Correlations analyses between aqueous humour cytokines level before IPT.
| Cytokines | PLGF | TGF-β1 | IFN-γ | PD-1 | IL-2 | IL-10 | IL-8 | IGF-I |
| VEGF-A | 0.013a | 0.359 | 0.606 | 0.68 | 0.193 | 0.35 | 0.529 | 0.46 |
| PLGF | 0.856 | 0.157 | 0.268 | 0.826 | 0.559 | 0.935 | 0.875 | |
| TGF-β1 | 0.073 | 0.003b | 0.053 | 0.032a | 0.29 | 0.212 | ||
| IFN-γ | 0.004b | 0.017a | 0.001b | 0.57 | 0.123 | |||
| PD-1 | 0.185 | 0.134 | 0.783 | 0.024a | ||||
| IL-2 | 0.001b | 0.642 | 1 | |||||
| IL-10 | 0.154 | 0.188 | ||||||
| IL-8 | 0.933 |
IPT: Iodine-125 plaque therapy. aPositive correlation with 2-tailed P-values <0.05; bPositive correlation with 2-tailed P-values <0.01.
A further comparison has been performed between groups with the increasing magnitude of VEGF-A or PLGF concentration in aqueous humor reached a 15% point or not. It was relatively higher in postoperative concentration of IFN-γ (P=0.005), PD-1 (P=0.035), IL-2 (P<0.001) and IL-10 (P=0.004) for the elevated VEGF-A group (Table 4). For the elevated PLGF group, the postoperative concentration of IFN-γ (P=0.007), IL-2 (P<0.001) and IL-10 (P=0.013) was higher (Table 4). No positive difference has been observed in baseline tumor dimensions, preoperative cytokines level and these radiation features between different groups. Results of binary logistic regression showed with elevated VEGF-A more than 15% was the strongest risk factor for elevated PLGF more than 15% (B=4.094, P=0.007, OR: 60.0) and vice versa.
Table 4. Grouped analyses of aqueous humour cytokines level differentiated by the increasing magnitude of VEGF-A or PLGF met a 15% criterion than baseline.
| Cytokines | VEGF-A |
P | PLGF |
P | ||
| Increase (n=11) | Decrease (n=7) | Increase (n=11) | Decrease (n=7) | |||
| VEGF-A | 1379±2773 | 648±333 | 0.407 | 1403±2765 | 610±332 | 0.465 |
| PLGF | 6±13 | 2±2 | 0.357 | 7±13 | 1±1 | 0.286 |
| TGF-β1 | 533±305 | 324±287 | 0.166 | 492±284 | 389±357 | 0.507 |
| IFN-γ | 471±180 | 198±160 | 0.005b | 467±184 | 204±166 | 0.007b |
| PD-1 | 11±42 | 7±13 | 0.035a | 39±30 | 18±22 | 0.134 |
| IL-2 | 11±13 | 7±3 | <0.001b | 13±4 | 3±3 | <0.001b |
| IL-10 | 11±0.4 | 7±0.5 | 0.004a | 1±0.4 | 1±0.5 | 0.013a |
| IL-8 | 11±51 | 7±43 | 0.745 | 63±72 | 24±21 | 0.187 |
| IGF-I | 11±601 | 7±786 | 0.673 | 705±768 | 623±1066 | 0.851 |
SD: Standard deviation. aPositive correlation with 2-tailed P-values <0.05; bPositive correlation with 2-tailed P-values <0.01.
mean±SD, pg/mL
DISCUSSION
Radiotherapy is a curative therapy for about 40% of cancer patients, and is widely used in uveal melanoma for decades[4],[17]. Radiation can induce direct and indirect cancer cell death, for it's always so proliferative that have more chance to be handicapped in DNA repairment than host cells[18]–[19]. Tumor cell and the stroma grows, so-called tumor microenvironment (TME)[17] has been recognized as a central role in vasculogenesis, oxygen load, immunomodulation and radioresistance. Researches towards radiation on TME were conducted in many cancers[20]–[22]. But it's rarely known for uveal melanoma that what biological effects can radiation induce since tissue necrosis and failure in gene expression profile would often hamper further findings[23]. Several centers referred to uveal melanoma's ocular fluids research, considering that soluble mediators in continuously replenished aqueous humour and less mobile vitreous gel were optimal materials for revealing the proteomic mechanisms. And Boyd et al[24] have recorded a good consistency in VEGF concentration between aqueous and vitreous humor.
In the current study, we observed that post-radiation level of VEGF-A and PLGF elevated significantly, and concentration of the two homogenous growth factors had correlation consistently. There were reports on VEGF-A elevated expression after radiotherapy in uveal melanoma patients' aqueous humor but most were about NVG patients[24]. And data for PLGF was absent. VEGF's production by oxygen-deprived tumor cells and tumor-associated macrophages (TAMs) had been demonstrated by intensive studies in vitro[25]–[26]. VEGF is detectable in vitreous and aqueous humor of uveal melanoma containing eyes and may have prognostic prediction value[10],[12],[24]. Uveal melanoma is a hypoxic tumor like most malignancy and abnormal vasculature with high tumor proliferative capability renders a steady source for VEGF[17]. PLGF belongs to VEGF superfamily, shares 42% amino acid sequence, specific receptor, e.g. VEGF-R1/Flt-1 and can be also induced by TNF-α and hypoxia[27]. It's possible that exposure of tumor and ocular tissue to γ-rays can destroy most radiosensitive living cells and results in intensive disturbance of local environment, which exacerbates local oxidative stress, induces immediate tissue repairment and inflammatory response[17],[19],[28], and thus causes such an elevation.
Tumor dimensions were strong predictors for radiotherapy complications and worse prognosis[5]. In our research, we found that the aqueous humor level of VEGF-A and PLGF elevated with tumor prominence persistently. Previous studies had also shown a good correlation of VEGF-A level with uveal melanoma prominence[24]. However, we failed to observe a significant correlation between concentration of VEGF-A, PLGF and the main radiotherapy parameters such as seeds number, radio plaque carrying time (d) and radioactive energy of 125I seeds (mCi). In a previous study about aqueous cytokines' changes of uveal melanoma undergone Ru-106 brachytherapy with adjunctive transpupillary thermotherapy, no obvious correlation has been found between VEGF-A expression and radiation interference[13]. Maybe it was due to our set of 100-120 Gy to the tumor apex was a much relatively stable dose.
We found that level of IFN-γ, IL-2 and IL-10 shows a significant post-radiation variation between the elevated and not elevated subgroups of VEGF-A and PLGF in aqueous humor. It seemed like that radiation exposure makes something different. IL-2 performs multi roles in antigen-mediated T lymphocyte differentiation and excitation and enhances the cell-killing activity of NK cell and CD8+ T cell[29]–[30]. As a crucial component binding inherent and acquired immunity, IFN-γ is in charge of immune regulation, especially promotes Th1 cell differentiation and enhances macrophages' killing ability[30]–[32]. There're many common aspects between functions of IL-2 and IFN-γ. One is activating CD8+ T cell, and IFN-γ can be induced by NK cell[33]. IL-10 is believed a dual-role regulatory factor in the immune system, but in TME it's more like an immune stimulation component for upregulation of IFN-γ expression by CD8+ T cell and in assistance with IL-2 to mediate anti-tumor capability[34]. Malignant tumors are always accompanied with unproperly local immune environment or immunosuppression of the whole organism[35]. Lee et al[13] had observed that uveal melanoma containing eyes had a relatively lower level of IL-2, IL-10, TNF-α and a higher level of IL-8, IFN-γ. Accumulating evidence showed radiotherapy can lead to more release of tumor-derived antigens, merge effective antigen-presenting cells, and trigger corresponding cytokine cascades, all of which are participants in tumor-related immune response[17],[36]. Given IL-2 is crucial in cellular immunity and T cell activation, and IFN-γ is believed having antiangiogenic potentials[37], both of them are used in immunotherapy for strengthening anti-tumor property[30],[38].
VEGF-A is active both in the primary tumor and irradiated tumors. It's not simply a bystander of inflammatory reaction that can recruit macrophages and regulatory T cells (Tregs) to tumor tissue. It also contributes to vascular abnormity which increases tumor hypoxia and radioresistance[17],[26]. Anti-VEGF therapy is efficacious to tumor vessel normalization and may have the potential to enhance radiotherapy efficiency[39]–[40], though the precise timing and dose are being investigated[17]. It is well known that PLGF promotes vascular genesis in abnormal conditions such as ischemic disease. But it's undetermined in tumor's angiogenesis because controversial notions are keeping in sight[39]. The interplay is real a complexity of tumor-related immunostimulatory or immunoregulatory cells, and downstream cytokines or chemokines expression profile of TME[17],[26],[40]. How it made ties at the post-radiation phase between VEGF-A, PLGF and other cytokines like IL-2, IFN-γ and IL-10 required to be investigated for further. Whereas the current research may reveal certain fluctuation of VEGF-A and PLGF after therapeutic radiation of uveal melanoma. But what's the association between this short-term variation and clinical outcomes for uveal melanoma patients is poorly understood so far and we failed to get a pleasing result for this time. For limited sample size and just restricted to protein analyses, may there are other implicative factors out of account kept unknown. As a self-control study, we choose previous studies as a comparator but find a relatively lower VEGF-A level in aqueous humor than we did[13],[24]. It perhaps because of different detection strategies.
In conclusion, this study demonstrates a notable elevation of VEGF-A and PLGF level in aqueous humour after IPT of uveal melanoma patients, and sheds some light on the molecular features indicating uveal melanoma's radiotherapy reactions. Increased level of VEGF-A, PLGF with tumor prominence may be useful for predicting disease severity and long-term radiation-related outcomes. Post-radiative concentration variations of IFN-γ, IL-2 and IL-10 between different groups with VEGF-A and PLGF elevated or not may indicate their roles in radiation-induced immune response. Modulation of these factors might be therapeutic for uveal melanoma, radiative side-effect or radioresistance. More approaches and additional studies are needed to investigate what's the specific manner and schedule of these mediators' evolution modulated by radiation in the future.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81570891); Beijing Natural Science Foundation (No.7151003).
Conflicts of Interest: Chen MX, None; Liu YM, None; Li Y, None; Yang X, None; Wei WB, None.
REFERENCES
- 1.Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 2005;140(4):612–617. doi: 10.1016/j.ajo.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Lin AJ, Rao YJ, Acharya S, Schwarz J, Rao PK, Grigsby P. Patterns of care and outcomes of proton and eye plaque brachytherapy for uveal melanoma: review of the National Cancer Database. Brachytherapy. 2017;16(6):1225–1231. doi: 10.1016/j.brachy.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Liu YM, Li Y, Wei WB, Xu X, Jonas JB. Clinical characteristics of 582 patients with uveal melanoma in China. PLoS One. 2015;10(12):e0144562. doi: 10.1371/journal.pone.0144562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Char DH, Kroll S, Phillips TL, Quivey JM. Late radiation failures after iodine 125 brachytherapy for uveal melanoma compared with charged-particle (proton or helium ion) therapy. Ophthalmology. 2002;109(10):1850–1854. doi: 10.1016/s0161-6420(02)01174-0. [DOI] [PubMed] [Google Scholar]
- 5.Shields CL, Kels JG, Shields JA. Melanoma of the eye: revealing hidden secrets, one at a time. Clin Dermatol. 2015;33(2):183–196. doi: 10.1016/j.clindermatol.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Finger PT, Chin KJ, Semenova EA. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol. 2016;26(1):60–66. doi: 10.5301/ejo.5000670. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Bremnes RM, Al-Shibli K, Donnem T, Sirera R, Al-Saad S, Andersen S, Stenvold H, Camps C, Busund LT. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol. 2011;6(4):824–833. doi: 10.1097/JTO.0b013e3182037b76. [DOI] [PubMed] [Google Scholar]
- 9.Bronkhorst IH, Jager MJ. Uveal melanoma: the inflammatory microenvironment. J Innate Immun. 2012;4(5-6):454–462. doi: 10.1159/000334576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunavoelgyi R, Funk M, Sacu S, Georgopoulos M, Zlabinger G, Zehetmayer M, Schmidt-Erfurth U. Intraocular activation of angiogenic and inflammatory pathways in uveal melanoma. Retina. 2012;32(7):1373–1384. doi: 10.1097/IAE.0b013e318239e299. [DOI] [PubMed] [Google Scholar]
- 11.Wierenga APA, Cao J, Mouthaan H, et al. Aqueous humor biomarkers identify three prognostic groups in uveal melanoma. Invest Ophthalmol Vis Sci. 2019;60(14):4740–4747. doi: 10.1167/iovs.19-28309. [DOI] [PubMed] [Google Scholar]
- 12.Nagarkatti-Gude N, Bronkhorst IH, van Duinen SG, Luyten GP, Jager MJ. Cytokines and chemokines in the vitreous fluid of eyes with uveal melanoma. Invest Ophthalmol Vis Sci. 2012;53(11):6748–6755. doi: 10.1167/iovs.12-10123. [DOI] [PubMed] [Google Scholar]
- 13.Lee CS, Jun IH, Kim TI, Byeon SH, Koh HJ, Lee SC. Expression of 12 cytokines in aqueous humour of uveal melanoma before and after combined Ruthenium-106 brachytherapy and transpupillary thermotherapy. Acta Ophthalmol. 2012;90(4):e314–e320. doi: 10.1111/j.1755-3768.2012.02392.x. [DOI] [PubMed] [Google Scholar]
- 14.Barak V, Pe'er J, Kalickman I, Frenkel S. VEGF as a biomarker for metastatic uveal melanoma in humans. Curr Eye Res. 2011;36(4):386–390. doi: 10.3109/02713683.2010.534573. [DOI] [PubMed] [Google Scholar]
- 15.American Brachytherapy Society - Ophthalmic Oncology Task Force. Electronic address: paulfinger@eyecancer.com, ABS-OOTF Committee. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13(1):1–14. doi: 10.1016/j.brachy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 16.AJCC Ophthalmic Oncology Task Force. International validation of the American joint committee on cancer's 7th edition classification of uveal melanoma. JAMA Ophthalmol. 2015;133(4):376–383. doi: 10.1001/jamaophthalmol.2014.5395. [DOI] [PubMed] [Google Scholar]
- 17.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groenewald C, Konstantinidis L, Damato B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye (Lond) 2013;27(2):163–171. doi: 10.1038/eye.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagliara MM, Tagliaferri L, Azario L, Lenkowicz J, Lanza A, Autorino R, Caputo CG, Gambacorta MA, Valentini V, Blasi MA. Ruthenium brachytherapy for uveal melanomas: factors affecting the development of radiation complications. Brachytherapy. 2018;17(2):432–438. doi: 10.1016/j.brachy.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Lauber K, Ernst A, Orth M, Herrmann M, Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol. 2012;2:116. doi: 10.3389/fonc.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madani I, De Neve W, Mareel M. Does ionizing radiation stimulate cancer invasion and metastasis? Bull Cancer. 2008;95(3):292–300. doi: 10.1684/bdc.2008.0598. [DOI] [PubMed] [Google Scholar]
- 22.El Chediak A, Shamseddine A, Bodgi L, Obeid JP, Geara F, Zeidan YH. Optimizing tumor immune response through combination of radiation and immunotherapy. Med Oncol. 2017;34(9):165. doi: 10.1007/s12032-017-1025-z. [DOI] [PubMed] [Google Scholar]
- 23.Dogrusöz M, Kroes WG, van Duinen SG, et al. Radiation treatment affects chromosome testing in uveal melanoma. Invest Ophthalmol Vis Sci. 2015;56(10):5956–5964. doi: 10.1167/iovs.15-17092. [DOI] [PubMed] [Google Scholar]
- 24.Boyd SR, Tan D, Bunce C, Gittos A, Neale MH, Hungerford JL, Charnock-Jones S, Cree IA. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol. 2002;86(4):448–452. doi: 10.1136/bjo.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch KR, Refaian N, Hos D, Schlereth SL, Bosch JJ, Cursiefen C, Heindl LM. Autocrine impact of VEGF-A on uveal melanoma cells. Invest Ophthalmol Vis Sci. 2014;55(4):2697–2704. doi: 10.1167/iovs.13-13254. [DOI] [PubMed] [Google Scholar]
- 26.el Filali M, Missotten GS, Maat W, Ly LV, Luyten GP, van der Velden PA, Jager MJ. Regulation of VEGF-A in uveal melanoma. Invest Ophthalmol Vis Sci. 2010;51(5):2329–2337. doi: 10.1167/iovs.09-4739. [DOI] [PubMed] [Google Scholar]
- 27.Failla CM. Placenta Growth Factor. 2017;v.53:2896–2898. [Google Scholar]
- 28.Good JS, Harrington KJ. The hallmarks of cancer and the radiation oncologist: updating the 5Rs of radiobiology. Clin Oncol (R Coll Radiol) 2013;25(10):569–577. doi: 10.1016/j.clon.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Santos JM, Cervera-Carrascon V, Havunen R, Zafar S, Siurala M, Sorsa S, Anttila M, Kanerva A, Hemminki A. Adenovirus coding for interleukin-2 and tumor necrosis factor alpha replaces lymphodepleting chemotherapy in adoptive T cell therapy. Mol Ther. 2018;26(9):2243–2254. doi: 10.1016/j.ymthe.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauldin IS, Wages NA, Stowman AM, et al. Intratumoral interferon-gamma increases chemokine production but fails to increase T cell infiltration of human melanoma metastases. Cancer Immunol Immunother. 2016;65(10):1189–1199. doi: 10.1007/s00262-016-1881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 32.Bhat P, Leggatt G, Waterhouse N, Frazer IH. Interferon-γ derived from cytotoxic lymphocytes directly enhances their motility and cytotoxicity. Cell Death Dis. 2017;8(6):e2836. doi: 10.1038/cddis.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong JL, Mailliard RB, Moschos SJ, Edington H, Lotze MT, Kirkwood JM, Kalinski P. Helper activity of natural killer cells during the dendritic cell-mediated induction of melanoma-specific cytotoxic T cells. J Immunother. 2011;34(3):270–278. doi: 10.1097/CJI.0b013e31820b370b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumm JB, Oft M. Pegylated IL-10 induces cancer immunity: the surprising role of IL-10 as a potent inducer of IFN-γ-mediated CD8(+) T cell cytotoxicity. Bioessays. 2013;35(7):623–631. doi: 10.1002/bies.201300004. [DOI] [PubMed] [Google Scholar]
- 35.Chellappa S, Aandahl EM, Taskén K. Cancer immunity and immune evasion mechanisms. In: Akslen L., Watnick R., editors. Biomarkers of the Tumor Microenvironment. Cham: Springer; 2017. pp. 195–233. [Google Scholar]
- 36.Ma JL, Jin L, Li YD, He CC, Guo XJ, Liu R, Yang YY, Han SX. The intensity of radiotherapy-elicited immune response is associated with esophageal cancer clearance. J Immunol Res. 2014;2014:794249. doi: 10.1155/2014/794249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wigginton JM, Gruys E, Geiselhart L, Subleski J, Komschlies KL, Park JW, Wiltrout TA, Nagashima K, Back TC, Wiltrout RH. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest. 2001;108(1):51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dillman RO, Depriest C, McClure SE. High-dose IL2 in metastatic melanoma: better survival in patients immunized with antigens from autologous tumor cell lines. Cancer Biother Radiopharm. 2014;29(2):53–57. doi: 10.1089/cbr.2013.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 40.Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, Griffin RJ. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res. 2007;13(11):3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]