Abstract
AIM
To conduct a systematic review and Meta-analysis to examine the association between uveitis and psoriatic disease, and to evaluate whether one condition predisposes individuals to the other.
METHODS
We performed a comprehensive search of PubMed and EMBASE to identify cohort studies examining the association between uveitis and psoriatic disease [psoriasis and/or psoriatic arthritis (PsA)]. We used a random-effects model to calculate the pooled relative risks (RRs) adjusted for confounders, along with the 95% confidence intervals (CIs).
RESULTS
This Meta-analysis included a total of 6 studies and a maximum of 80 178 648 participants. Compared with non-psoriatic controls, uveitis risk was significantly elevated in patients with psoriasis (RR=1.49; 95%CI: 1.08-2.07), and PsA (RR=3.16; 95%CI: 2.16-4.63). Furthermore, pre-existing uveitis was associated with a significantly increased risk of psoriasis (RR=1.62; 95%CI: 1.44-1.83), and PsA (RR=4.44; 95%CI: 3.52-5.60).
CONCLUSION
The results of this systematic review and Meta-analysis suggest an overall positive bidirectional association between uveitis and psoriatic disease (psoriasis and PsA), warranting increased awareness among clinicians involved in the management of these two conditions. Therefore, there remains a need for more detailed studies of the possible common pathogenesis of psoriatic disease and uveitis.
Keywords: Meta-analysis, psoriasis, psoriatic arthritis, risk, uveitis
INTRODUCTION
Uveitis is a common inflammatory ocular disease comprising multifarious clinical entities that threaten vision[1]–[2], with symptoms that include blurred vision, ocular pain, and photophobia. Untreated uveitis may result in various ocular complications, such as glaucoma, keratosis, cystic macular edema, post-adhesions, cataracts, and permanent vision loss[3]. It is estimated that uveitis affects over 2 million people worldwide, constituting the third leading cause of vision loss, and responsible for approximately 15% of preventable blindness[2]–[5]. Uveitis is associated with multiple disorders, including many systemic autoimmune conditions such as psoriasis, inflammatory bowel disease, ankylosing spondylitis and Behcet's disease. Thus, uveitis can be a vital warning sign that allows early diagnosis and medical intervention[3],[6]–[7].
Psoriasis is a chronic complex systemic autoimmune inflammatory disease. It is characterized by the distinct silvery scales shielded erythematous plaques that show up in different areas of the skin[8]–[9]. Psoriasis affects over 125 million people worldwide[7]–[10], and sometimes occurs in the setting of other immune-mediated diseases, such as multiple sclerosis, rheumatoid arthritis, Crohn's disease, and type-1 diabetes[8],[10]–[13]. Psoriatic arthritis (PsA) is a chronic progressive inflammatory arthritis that can result in severe disability[14]–[16]. PsA reportedly affects 10%-40% of individuals with psoriasis, and it usually presents synchronously with, or after, psoriasis onset[15]–[16]. The pathogenesis of psoriatic disease is complex, and the exact mechanism remains elusive. It is believed that helper T cells 1 (Th1) and Th17 promote the inflammatory response in psoriasis[17]–[18]. Additionally, certain cytokines, including interleukin 23 (IL-23), IL-17, tumor necrosis factor-α (TNF-α), and IL-6 appear to play important roles in psoriatic disease pathogenesis, and are also elevated in uveitis[18]–[24].
Increasing numbers of studies have reported the co-occurrence of uveitis and psoriasis[25]–[26], or have found that one condition seems to predispose to the other[27]–[32]. In contrast, some studies demonstrate no significantly increased incidence or prevalence of uveitis among psoriasis patients, and vice versa[7],[11]. However, no systematic analysis or review has yet been performed. Further analysis of the available evidence regarding the link between uveitis and psoriasis will improve our understanding of the causes and consequences of both conditions, and provide a reference for better healthcare practices. Therefore, in the present study, we aimed to analyze published data regarding the risk for incident (new-onset) uveitis in patients with psoriasis and PsA, as well as the corresponding risk of psoriatic diseases among patients with uveitis. To our knowledge, this is the first systematic review and Meta-analysis on this topic.
MATERIALS AND METHODS
We performed a systematic review and Meta-analysis of cohort studies on the association between uveitis and psoriatic disease according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)[33] and Meta-analysis Of Observational Studies in Epidemiology (MOOSE)[34] guidelines.
Search Strategy
We searched PubMed and EMBASE databases for studies published from inception to August 31, 2019, using the queries in Table 1. References of all the related reviews and included studies were hand-searched in case of omission.
Table 1. Search strategy.
| PubMed | EmBase |
| #1 “Uveitis”[Mesh] | #1 ‘uveitis’/exp |
| #2 Uveitis | #2 ‘uveitis’ |
| #3 Uveitides | #3 ‘uveitides’ |
| #4 “Panuveitis”[Mesh] | #4 ‘panuveitis’ |
| #5 Panuveitis | #5 ‘sympathetic ophthalmia’/exp |
| #6 “Ophthalmia, Sympathetic”[Mesh] | #6 ‘sympathetic ophthalmia’ |
| #7 Sympathetic Ophthalmia | #7 ‘pars planitis’/exp |
| #8 “Pars Planitis”[Mesh] | #8 ‘pars planitis’ |
| #9 Pars Planitis | #9 ‘panophthalmitis’/exp |
| #10 “Panophthalmitis”[Mesh] | #10 ‘panophthalmitis’ |
| #11 Panophthalmitis | #11 ‘panophthalmitides’ |
| #12 Panophthalmitides | #12 ‘iridocyclitis’/exp |
| #13 “Iridocyclitis”[Mesh] | #13 ‘iridocyclitis’ |
| #14 Iridocyclitis | #14 ‘heterochromic cyclitis’ |
| #15 Heterochromic Cyclitis | #15 ‘anterior scleritis’ |
| #16 anterior scleritis | #16 ‘iritis’/exp |
| #17 “Iritis”[Mesh] | #17 ‘iritis’ |
| #18 Iritis | #18 ‘iritides’ |
| #19 Iritides | #19 ‘choroiditis’/exp |
| #20 “Choroiditis”[Mesh] | #20 ‘choroiditis’ |
| #21 Choroiditis | #21 ‘choroiditides’ |
| #22 Choroiditides | #22 ‘chorioretinitis’/exp |
| #23 “Chorioretinitis”[Mesh] | #23 ‘chorioretinitis’ |
| #24 Chorioretinitis | #24 Chorioretinitides |
| #25 Chorioretinitides | #25 ‘uveoretinitis’ |
| #26 uveoretinitis | #26 ‘vitritis’ |
| #27 vitritis | #27 ‘retinitis’/exp |
| #28 “Retinitis”[Mesh] | #28 ‘retinitis’ |
| #29 Retinitis | #29 ‘neuroretinitis’ |
| #30 Neuroretinitis | #30 (#1-29/OR) |
| #31 (#1-30/OR) | #31 ‘psoriasis’/exp |
| #32 “Psoriasis”[Mesh] | #32 ‘psoriasis’ |
| #33 Psoriasis | #33 ‘psoriases’ |
| #34 Psoriases | #34 ‘pustulosis of palms and soles’ |
| #35 Pustulosis of Palms and Soles | #35 ‘pustulosis palmaris et plantaris’ |
| #36 Pustulosis Palmaris et Plantaris | #36 ‘palmoplantaris pustulosis’ |
| #37 Palmoplantaris Pustulosis | #37 ‘pustular psoriasis of palms and soles’ |
| #38 Pustular Psoriasis of Palms and Soles | #38 ‘psoriatic arthritis’/exp |
| #39 “Arthritis, Psoriatic”[Mesh] | #39 ‘psoriatic arthritis’ |
| #40 Arthritic Psoriasis | #40 ‘psoriasis arthropathica’ |
| #41 Psoriatic Arthritis | #41 ‘psoriatic arthropathy’ |
| #42 Psoriasis Arthropathica | #42 ‘psoriatic arthropathies’ |
| #43 Psoriatic Arthropathy | #43 (#31-42/OR) |
| #44 Psoriatic Arthropathies | #44 (#43 AND #30) |
| #45 (#32-44/OR) | |
| #46 (#31 AND #45) |
Study Selection
We included studies that met the following inclusion criteria: 1) cohort studies; 2) risk estimates with corresponding 95% confidence interval (CIs) on the association between psoriatic disease and uveitis reported, or provided sufficient information for calculating these effect sizes; 3) the case group consisted with patients with uveitis (psoriatic disease), and the control group consisted of non-uveitis individuals (non-psoriatic patients) or the general population; 4) written in English. We excluded any reviews, case reports and conference abstracts.
The retrieved records were imported into Endnote for management. After the removal of duplications, the titles and abstracts of the unique publications were screened to exclude irrelevant studies. The full texts of remained publications were further carefully reviewed, and studies that met the pre-set selection criteria were included. Two authors (Li CR, Chen L) independently performed the study selection, and a third acted as a moderator in case of any discrepancies.
Data Extraction
We extracted from every article: first author, year of publication, the region where the study was carried out, study design, definition criteria for uveitis and psoriatic disease, the sample size and demographical characteristics (age and sex) of each group, follow-up duration, risk estimates, and adjusted covariates.
Quality Evaluation
The methodologic quality of included studies was assessed by two independent reviewers with the Newcastle-Ottawa scale. This scale consists of three domains: selection, comparability between the case and control groups, and ascertainment of the outcome of interest, with a maximum score of 9 points. Studies with a score ≥6 were considered of good quality. Any disagreement was addressed by discussion, and a third author was consulted when necessary.
Meta-analysis
The Meta-analyses were conducted with the STATA software version 12 (STATA Corporation, College Station, TX, United States). PsA and psoriasis without PsA were separately analyzed. If more than one effect sizes were reported in a study, we adopted the one with the most adjusted covariates. Mantel-Haenszel weighting and random effects models were used for all the Meta-analyses due to anticipated heterogeneity. Statistical heterogeneity across studies was measured using the I2 statistic (>50% considered significant). All statistical tests were two-sided using an α level of 0.05. Subgroup analysis was performed according to the region where the study was conducted, the age of the participants and the regression analysis model (Cox proportional hazards regression model and Poisson regression model). Sensitivity analyses were used to explore the source of heterogeneity and evaluate the robustness of the results by removing each study in turn if there were at least 3 studies available. Publication bias was not performed due to the small number of included studies.
RESULTS
Study Selection and Characteristics
Our systematic search with the defined parameters retrieved 5763 records. Removal of duplicates resulted in 5017 potentially relevant articles. Screening of titles and abstracts led to the exclusion of 4958 studies unrelated to our topic. We selected 59 articles for full-text review, and 53 articles were excluded due to lack of controls, no useful data, or repeated datasets from the same authors. Thus, our final analysis included a total of 6 cohort studies (Figure 1)[11],[28]–[32].
Figure 1. Flowchart for the selection of eligible studies.
Table 2 summarizes the characteristics of the included studies. All were published in the last five years. They were conducted in the USA (n=3), UK (n=1), Denmark (n=1), and Taiwan, China (n=1). Three studies utilized large-scale health-claims databases, and the other three were based on nationwide population databases. Five studies reported uveitis risk in PsA, and four reported the corresponding risk in psoriasis without PsA. Two studies analyzed the PsA risk in cases of uveitis, and two evaluated the risk of psoriasis without PsA among individuals with uveitis. Four studies recruited adults, one focused on children, and one had no age restriction. Three studies calculated risk estimates using the Cox proportional hazards regression model, while the other three employed the Poisson regression model. All studies adjusted for age and sex between the case and control groups, with the exception of one study that focused on the association between uveitis and psoriasis without PsA.
Table 2. Characteristics of included studies.
| Study | Region | Setting | Follow-up (median, y) | Exposure/outcome | Sample size/age range (y)/male (%) |
Analysis model | RR (95%CI) | Covariates adjustment | |
| Exposed | Control | ||||||||
| Aletaha-2019 | USA | Health-claims database | 2006-2015/2.5-2.8 | PsO/UV | 115141/18-64/NM | 74228131/18-64/NM | Cox proportional hazards model | 1.4 (1.2-1.7) | Age, sex, insurance, region |
| PsA/UV | 8406/18-64/NM | 5380715/18-64/NM | 4.0 (2.9-5.6) | ||||||
| UV/PsO | 34422/18-64/NM | 22166447/18-64/NM | 1.6 (1.3-1.8) | ||||||
| UV/PsA | 4.8 (3.9-6.0) | ||||||||
| Brandon-2018 | USA | Health-claims database | 2000-2013/5.3 | PsO/UV | 4312/0-16/34.4 | 43120/0-16/34.4 | Poisson regression model | 1.35 (0.52-2.94) | Age, sex |
| PsA/UV | 212/0-16/44.8 | 2120/0-16/44.8 | 22.36 (7.04-54.75) | ||||||
| Charlton-2018 | UK | Population-based | 1998-2014/NMa | PsA/UV | 6783/18-89/49.5 | 27132/18-89/49.5 | Poisson regression model | 3.55 (2.21-5.70) | Age, sex, smoking, BMI |
| Chi-2017 | Taiwan, China | Population-based | 2000-2011/7.3 | PsO/UV | 137847/all/58.6 | 147954/All/58.8 | Cox proportional hazards model | 1.16 (1.06-1.26) | Age, sex, comorbidities |
| PsA/UV | 10107/all/62.3 | 1.95 (1.61-2.37) | |||||||
| Egeberg-2015 | Denmark | Population-based | 1997-2011/NMb | PsO/UV | 67394/≥18/48.9 | 5434749/≥18/49.4 | Poisson regression model | 2.13 (1.76-2.59)c | Age, sex, comorbidities, socioeconomic status |
| PsA/UV | 6735/≥18/43.7 | 2.50 (1.53-4.08) | |||||||
| UV/PsO | 13114/≥18/48.6 | 5495764/≥18/49.4 | 1.73 (1.46-2.04)c | ||||||
| UV/PsA | 3.77 (2.66-5.34) | ||||||||
| Kaine-2018 | USA | Health-claims database | 2008-2015/3.0 | PsA/UV | 14898/≥18/44.6 | 35037/≥18/44.4 | Cox proportional hazards model | 2.21 (1.64-2.98) | Age, sex, calendar year of diagnosis, region |
BMI: Body mass index; NM: Not mention; PsA: Psoriatic arthritis; PsO: Psoriasis; RR: Rate ratio; UV: Uveitis. awith a mean follow-up of 5.5y; bwith a maximum follow-up of 15y; ccrude RR.
Quality Evaluation
In terms of the association between uveitis and psoriatic disease, all studies were deemed to be of “good quality”, with a median score of 8 (range: 6-9).
Risk of Uveitis in Psoriasis
Four studies, including a total of 80 178 648 participants, reported the risk of uveitis in patients with psoriasis (Table 2)[11],[28],[30]–[31]. The pooled analysis revealed a significantly increased uveitis risk among psoriasis patients (RR=1.49; 95%CI: 1.08-2.07; Figure 2), and there was substantial heterogeneity among the studies (I2=91.1%). Therefore, the subgroup analyses were carried out and the results are presented in Table 3. The risk of uveitis in cases of psoriasis remained increased across all analyzed subgroups, but this increment was no longer statistically significant in the subgroup comprising studies performed outside of the USA nor the subgroup consisting of only children. Additionally, we found no heterogeneity between the studies conducted in the USA (I2=0), and between the studies that applied the Poisson regression model (I2=0). These findings suggest that the heterogeneity among studies may be partly derived from the different regions and the different analysis models.
Figure 2. Forest plots on the risk of uveitis in psoriasis without psoriatic arthritis.
Table 3. Subgroup analyses on the risk of uveitis in psoriatic arthritis.
| Subgroup analysis | No. of datasets | Pooled estimates (random-effects model) |
||
| Relative risk (95%CI) | P | I2 | ||
| Psoriasis without psoriatic arthritis | 4 | 1.49 (1.08-2.07) | 0.016 | 91.1% |
| Region | ||||
| USA | 2 | 1.43 (1.20-1.69) | 0.000 | 0 |
| Others | 2 | 1.56 (0.86-2.85) | 0.147 | 96.9% |
| Age | ||||
| Children | 1 | 1.35 (0.52-3.51) | 0.538 | - |
| Adults | 2 | 1.74 (1.18-2.58) | 0.006 | 89.1% |
| Children and adults | 1 | 1.16 (1.06-1.26) | 0.001 | - |
| Statistical analysis | ||||
| Cox-proportional hazards model | 2 | 1.27 (1.03-1.56) | 0.024 | 78.0% |
| Poisson regression model | 2 | 2.10 (1.74-2.53) | 0.000 | 0 |
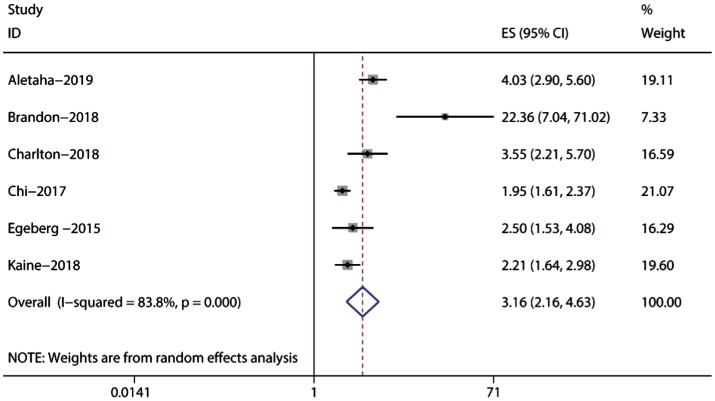
| Psoriatic arthritis | 6 | 3.16 (2.16-4.63) | 0.000 | 83.8% |
| Region | ||||
| USA | 3 | 4.63 (2.11-10.16) | 0.000 | 89.2% |
| Others | 3 | 2.46 (1.71-3.55) | 0.000 | 64.3% |
| Age | ||||
| Children | 1 | 22.36 (7.04-71.02) | 0.000 | - |
| Adults | 4 | 2.97 (2.16-4.08) | 0.000 | 62.8% |
| Children and adults | 1 | 1.95 (1.61-2.37) | 0.000 | - |
| Statistical analysis | ||||
| Cox-proportional hazards model | 3 | 2.55 (1.69-3.86) | 0.000 | 85.7% |
| Poisson regression model | 3 | 4.89 (2.03-11.78) | 0.000 | 82.9% |
Risk of Uveitis in Psoriatic Arthritis
Six studies, including a total of 11 074 848 participants, reported the risk of uveitis in cases of PsA (Table 2)[11],[28]–[32]. Data synthesis revealed a significant association between PsA and uveitis risk (RR=3.16; 95%CI: 2.16-4.63), with significant heterogeneity (I2=83.8%; Figure 3). Subgroup analyses indicated that the research region and analysis model did not modify the association between uveitis and PsA risk (Table 3). However, we detected significant heterogeneity across all subgroups.
Figure 3. Forest plots on the risk of uveitis in psoriatic arthritis.
Risk of Psoriasis/Psoriatic Arthritis in Uveitis
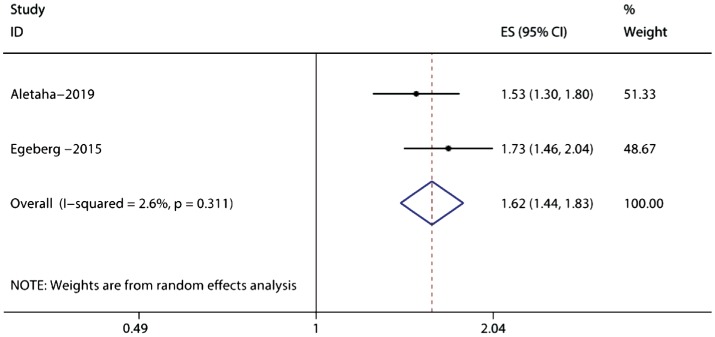
Two cohort studies, including 27 709 747 samples, examined the risk of psoriasis and PsA in cases of uveitis[28],[31]. The combined evidence revealed a significantly increased risk of psoriasis (RR=1.62; 95%CI: 1.44-1.83), without significant heterogeneity (I2=2.6%; Figure 4). These same cohort studies also demonstrated that patients with uveitis were more likely to develop subsequent PsA (RR=4.44; 95%CI: 3.52-5.60), without significant heterogeneity (I2=29.9%; Figure 5). These two studies both included adult only, where one was carried out in USA while the other one in Denmark. The two studies applied different analysis models.
Figure 4. Forest plots on the risk of psoriasis without psoriatic arthritis in uveitis.
Figure 5. Forest plots on the risk of psoriatic arthritis in uveitis.
Sensitivity Analysis
To explore the potential effects of any single study on heterogeneity and on the risk estimates, we performed a sensitivity analyses by recalculating the pooled estimate, omitting one study each time. The results showed that no single study made an inordinate contribution to the significant heterogeneity across the studies. Moreover, analysis of the remaining studies yielded similar findings regarding the association between PsA and uveitis risk. However, upon removal of the study conducted by Aletaha et al[28], the pooled RR of uveitis in psoriasis was no longer statistically significant.
DISCUSSION
Our present Meta-analysis included 6 studies, with a maximum of 80 178 648 participants for analyzing. The results demonstrate increased uveitis risks in cases of psoriasis and PsA, with a higher risk in PsA than psoriasis [RR=1.49; 95%CI: 1.08-2.07 (psoriasis/UV) and RR=3.16; 95%CI: 2.16-4.63 (PsA/UV)]. Additionally, patients with uveitis exhibit overall increased risks for psoriasis and for PsA [RR=1.62; 95%CI: 1.44-1.83 (UV/psoriasis) and RR=4.44; 95%CI: 3.52-5.60 (UV/PsA)]. These results suggest an overall positive bidirectional association between psoriatic disease and uveitis. To our knowledge, this was the first Meta-analysis performed to examine the evidence of the bidirectional relationship between uveitis and psoriatic disease, including psoriasis and PsA.
Uveitis specifically refers to inflammation of the uveal tract tissues, such as the choroid, ciliary body and iris[35]. Uveal inflammation can be unilateral or bilateral, and its natural course can be chronic, recurrent, or acute[3]. Based on the anatomic location of the intraocular inflammation[2]–[3],[35]–[36], anterior uveitis involves the ciliary body and iris, and is typically associated with redness and pain[1],[4],[37]. While both posterior (involves the uvea and choroid) and intermediate uveitis (characterized by vitreous humor inflammation)[38] are primarily accompanied by visual symptoms, like blind spots or floaters, and may involve impacts on central vision[39]. Due to its significant affiliation with systemic disease, as we demonstrated in this study, uveitis may be particularly suited for classification according to etiological criteria[6]. Uveitis can be caused by both infectious and noninfectious factors[2],[39], with noninfectious uveitis being most common, especially in developed countries (almost 81% of all cases)[40]. Noninfectious uveitis is thought to result from inappro-priate immune system activation[3], and most commonly (40%) presents in the context of another systemic autoimmune disease, such as PsA, psoriasis, reactive arthritis, inflammatory bowel disease, ankylosing spondylitis, sarcoidosis, relapsing polychondritis, systemic lupus erythematosus, and Behcet's disease[27],[39]–[41]. Infectious uveitis is more commonly observed in developing and tropical countries (accounting for up to 50% of cases), most often in the background of toxoplasmosis, tuberculosis, and cytomegalovirus infection[39]–[42].
Psoriatic disease is an organ-specific autoimmune disease triggered by immune system activation[9],[15],[18]. Over the decades, uveitis has been described as a complication or comorbidity in an estimated 7%-20% of psoriatic disease cases[43]. However, limited data is available regarding the specific characteristics of uveitis in the context of psoriatic disease. It has been noted that psoriatic uveitis is usually chronic, bilateral, and severe[7],[27]. In both PsA- and psoriasis-related uveitis, the main symptom is “dry eye” due to preferential involvement of the anterior part of the uvea[44]–[46]. However, pan-uveitis and posterior uveitis are not uncommon in psoriatic disease, with their rates reaching 22%-44% in some studies[7],[44]. Psoriatic uveitis affects older people are more likely to require treatment with nonsteroidal anti-inflammatory drugs compared to idiopathic forms of uveitis. Uveitis complications, such as retinal vasculitis and macular edema, have also been reported during the course of psoriatic uveitis[44].
Findings in both experimental animal models and humans suggest that Th1 and Th17 cells play fundamental roles in the immunopathogenesis of uveitis and psoriatic disease[3],[7],[17],[23],[47]–[48]. Th1 and Th17 cells are both subtypes of CD4+ cells, which are major regulators of adaptive immune responses and inflammatory diseases[49]. In the presence of IL-6, IL-1β, and transforming growth factor-β (TGF-β), Th17 cells develop from naïve CD4+ cells. Then they mature and expand in the presence of IL-23. Th17 cells are mainly responsible for producing IL-17 among other cytokines[49]–[50]. IL-17 recruits neutrophils, monocytes, and Th1 cells to target tissues, and synergistically acts with other cytokines, particularly IL-1β and TNF-α[18],[49]. Elevated IL-17 levels have been reported in both serum and aqueous humor of autoimmune uveitis patients[20]–[21]. On the other hand, Th1 cells are characterized by interferon-gamma (INF-γ) secretion, and represent the main cell type promoting cellular immunity[51]–[52].
Although both Th1 and Th17 are activated in autoimmune uveitis[20],[53], some people propose that Th17 may be predominantly responsible for early-stage inflammation, while increased Th1 expression is involved in the later stages, including disease resolution[48]–[49]. This hypothesis regarding psoriatic disease has been described in the “IL-23/Th17 axis” model[18],[54]. In this model, IL-23 is secreted by dermal dendritic cells (DDCs), and induces Th17 cell activation and consequent release of proinflammatory cytokines, such as IL-17, IL-26 and IL-22. Subsequently, the DDC-activated Th1 cells produce IFN-γ and TNF-α[18],[49], contributing to the disease pathogenesis. Th17 cells recruit neutrophils and cathelicidin to activate plasmacytoid dendritic cells (PDCs); produce vascular endothelial growth factor (VEGF) resulting in angiogenesis; and interact with skin-resident cells in a manner that contributes to skin lesions and arthritis[49]. Elevated IL-23 levels have been detected in vitreous from cases of posterior uveitis, and in serum samples from patients with active autoimmune uveitis, compared to the levels in control subjects[22]–[24],[48],[55]–[56]. Moreover, IL-23 plays important roles in Th17 cell maturation and expansion. Without IL-23, Th17 cells still mature but their role becomes homeostatic rather than pathogenic[48],[54].
IL-6 is another cytokine that plays important roles in both uveitis and psoriasis. It is a well-known pro-inflammatory cytokine that promotes the differentiation of Th-17 cells from naïve CD4+ T cells[49]–[50],[57]. Elevated IL-6 levels have been detected in autoimmune uveitis patients' aqueous humor[20],[52],[57]–[58]. Moreover, the presence of IL-6 appears to be associated with macular edema and vascularization, which are complications of uveitis[20].
Furthermore, some authors have suggested that the subclinical inflammation in the psoriatic disease patients causes the breakdown of the blood-aqueous humor barrier, such that activated neutrophils from peripheral blood are able to induce anterior uveitis[7],[47],[53]. This would explain the common observation that psoriasis is followed by uveitis, with subsequent occurrence of PsA[44],[46]. Importantly, in patients with psoriasis, ocular involvement should not be overlooked since uveitis could constitute an early indication of inflammation, and a possible warning sign of PsA[45]. Therefore, with the common inflammatory cells and cytokines changes, it further proved our findings of the overall positive bidirectional association between psoriatic disease and uveitis.
Since uveitis and psoriasis have positive bidirectional associations and share common pathogenetic mechanisms, they may both benefit from the same biologic target treatments under some circumstances[59]. Antagonists of TNF-α were originally approved for comorbidity psoriasis, and exhibited efficacy against uveitis even before official FDA approval[60]–[61]. Starting in 2016, adalimumab, infliximab, and golimumab have been successfully used as off-label treatments for uveitis[48],[60]–[64]. Ustekinumab (a human mAb against subunit p40, which is the common subunit of IL-12 and IL-23) is currently used for treating psoriasis and active PsA, and two phase II clinical trials are underway to investigate its use for uveitis treatment: STELARA (NCT0291116) and STELABEC (NCT02648581)[56]. Although no results have yet been published, initial case reports showed encouraging results[56],[65]–[66]. Anti-IL-6 agents, such as tocilizumab, sarilumab, and sirukumab, have also shown promise in terms of efficacy and side effect profiles for the treatment of non-infectious uveitis[57]. On the other hand, secukinumab is a human IL-17A antibody with FDA approval for the treating psoriasis and PsA, and phase III dose-dependent studies have failed to show its efficacy against uveitis[48],[67]. However, emerging evidence indicates that the secukinumab trial failure may have been related to trial design deficiencies, as an early study[68] proved that intravenous secukinumab administration yields a higher rate of improvement compared to subcutaneous administration. Thus, further studies are needed to examine the efficacy of IL-17 antagonists against uveitis.
Our study had several limitations. First, our analysis included only studies published in English, which may limit the results. Second, the individual studies analyzed did not specify any uveitis classifications, preventing the analysis of risks associated with different types of uveitis. Since uveitis comprises multiple clinical entities, it is possible that the underlying immunopathogenic mechanism differs depending on the causative autoantigen or pathogen. Third, a large patient sample was collected via administrative claims data, which have weaknesses regarding clinical detail and accuracy. In particular, the use of claims data limits the ability to distinguish diagnostic uncertainty or a new manifestation of an existing disease. Nevertheless, the assessment criteria and data coverage varied between the analyzed studies. Finally, we found clear heterogeneity among the included studies, which may be partially attributed to the different study designs and settings.
In summary, the results of this systematic review and Meta-analysis suggest an overall positive bidirectional association between psoriatic disease and uveitis. We demonstrate a significantly increased risk of uveitis among psoriasis patients, and psoriasis disease among uveitis patients. Based on these results, we encourage ophthalmologists and internist to seek treatment options for patients with either condition, rather than simply attempting to suppress uveitis or psoriatic disease. Understanding these comorbidity profiles may provide insight regarding the effects of comorbid conditions on disease management, treatment choices, and the healthcare burden of these diseases. Further detailed studies are needed to examine the possible common pathogenesis of psoriatic disease and uveitis.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81570847); the Natural Science Foundation of Hunan Province (No.2016JJ4095); the Programs of Science-Technology Commission of Hunan Province (No.2015JC3036).
Conflicts of Interest: Li CR, None; Chen L, None; Wang LF, None; Yan B, None; Liang YL, None; Luo J, None.
REFERENCES
- 1.Agrawal RV, Murthy S, Sangwan V, Biswas J. Current approach in diagnosis and management of anterior uveitis. Indian J Ophthalmol. 2010;58(1):11–19. doi: 10.4103/0301-4738.58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23(5):705–717. doi: 10.5301/ejo.5000278. [DOI] [PubMed] [Google Scholar]
- 3.Krishna U, Ajanaku D, Denniston AK, Gkika T. Uveitis: a sight-threatening disease which can impact all systems. Postgrad Med J. 2017;93(1106):766–773. doi: 10.1136/postgradmedj-2017-134891. [DOI] [PubMed] [Google Scholar]
- 4.Foster CS, Kothari S, Anesi SD, Vitale AT, Chu D, Metzinger JL, Cerón O. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv Ophthalmol. 2016;61(1):1–17. doi: 10.1016/j.survophthal.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Gritz DC, Wong IG. Incidence and prevalence of uveitis in northern California; the northern California epidemiology of uveitis study. Ophthalmology. 2004;111(3):491–500. discussion 500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Deschenes J, Murray PI, Rao NA, Nussenblatt RB, International Uveitis Study Group International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2. doi: 10.1080/09273940801899822. [DOI] [PubMed] [Google Scholar]
- 7.Fraga NA, Oliveira Mde F, Follador I, Rocha Bde O, Rêgo VR. Psoriasis and uveitis: a literature review. An Bras Dermatol. 2012;87(6):877–883. doi: 10.1590/S0365-05962012000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazsó A, Szodoray P, Szappanos Á, Korda J, Pálfi P, Kiss E, Poór G. Systemic autoimmune, rheumatic diseases and coinciding psoriasis: data from a large single-centre registry and review of the literature. Mediators Inflamm. 2015;2015:657907. doi: 10.1155/2015/657907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 10.Binus AM, Han J, Qamar AA, Mody EA, Holt EW, Qureshi AA. Associated comorbidities in psoriasis and inflammatory bowel disease. J Eur Acad Dermatol Venereol. 2012;26(5):644–650. doi: 10.1111/j.1468-3083.2011.04153.x. [DOI] [PubMed] [Google Scholar]
- 11.Brandon TG, Manos CK, Xiao R, Ogdie A, Weiss PF. Pediatric psoriatic arthritis: a population-based cohort study of risk factors for onset and subsequent risk of inflammatory comorbidities. J Psoriasis Psoriatic Arthritis. 2018;3(4):131–136. doi: 10.1177/2475530318799072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho S, Cho SB, Choi MJ, Zheng Z, Bang D. Behçet's disease in concurrence with psoriasis. J Eur Acad Dermatol Venereol. 2013;27(1):e113–e118. doi: 10.1111/j.1468-3083.2012.04559.x. [DOI] [PubMed] [Google Scholar]
- 13.Ottaviano G, Salvatore S, Salvatoni A, Martelossi S, Ventura A, Naviglio S. Ocular manifestations of paediatric inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2018;12(7):870–879. doi: 10.1093/ecco-jcc/jjy029. [DOI] [PubMed] [Google Scholar]
- 14.Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017;17(1):65–70. doi: 10.7861/clinmedicine.17-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eder L, Haddad A, Rosen CF, Lee KA, Chandran V, Cook R, Gladman DD. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68(4):915–923. doi: 10.1002/art.39494. [DOI] [PubMed] [Google Scholar]
- 16.Nas K, Capkin E, Dagli AZ, Cevik R, Kilic E, Kilic G, Karkucak M, Durmus B, Ozgocmen S, Anatolian Group for the Assessment in Rheumatic Diseases (ANGARD) Gender specific differences in patients with psoriatic arthritis. Mod Rheumatol. 2017;27(2):345–349. doi: 10.1080/14397595.2016.1193105. [DOI] [PubMed] [Google Scholar]
- 17.Diani M, Altomare G, Reali E. T cell responses in psoriasis and psoriatic arthritis. Autoimmun Rev. 2015;14(4):286–292. doi: 10.1016/j.autrev.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Deng YX, Chang C, Lu QJ. The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol. 2016;50(3):377–389. doi: 10.1007/s12016-016-8535-x. [DOI] [PubMed] [Google Scholar]
- 19.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 20.El-Asrar AM, Struyf S, Kangave D, Al-Obeidan SS, Opdenakker G, Geboes K, van Damme J. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139(2):177–184. doi: 10.1016/j.clim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Jawad S, Liu BY, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocul Immunol Inflamm. 2013;21(6):434–439. doi: 10.3109/09273948.2013.815786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang SH, Liu XL, Luo LX, Qu B, Huang XK, Xu L, Lin Y, Ye SB, Liu YZ. Elevated serum IL-23 correlates with intraocular inflammation after cataract surgery in patients with Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2010;94(8):1078–1082. doi: 10.1136/bjo.2009.169052. [DOI] [PubMed] [Google Scholar]
- 23.Fotiadou C, Lazaridou E, Sotiriou E, Gerou S, Kyrgidis A, Vakirlis E, Ioannides D. IL-17A, IL-22, and IL-23 as markers of psoriasis activity: a cross-sectional, hospital-based study. J Cutan Med Surg. 2015;19(6):555–560. doi: 10.1177/1203475415584503. [DOI] [PubMed] [Google Scholar]
- 24.Velez G, Roybal CN, Colgan D, Tsang SH, Bassuk AG, Mahajan VB. Precision medicine: personalized proteomics for the diagnosis and treatment of idiopathic inflammatory disease. JAMA Ophthalmol. 2016;134(4):444–448. doi: 10.1001/jamaophthalmol.2015.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghalamkarpour F, Baradaran-Rafii A, Sadoughi MM, Abdollahimajd F, Younespour S, Zargari O, Rudolph RI. Ocular findings in patients with psoriasis: is it related to the side effects of treatment or to psoriasis itself? A case-control study. J Dermatol Treat. 2020;31(1):27–32. doi: 10.1080/09546634.2019.1577947. [DOI] [PubMed] [Google Scholar]
- 26.Egeberg A. Psoriasis and comorbidities. Epidemiological studies. Dan Med J. 2016;63(2):pii: B5201. [PubMed] [Google Scholar]
- 27.Rosenbaum JT. Uveitis in spondyloarthritis including psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease. Clin Rheumatol. 2015;34(6):999–1002. doi: 10.1007/s10067-015-2960-8. [DOI] [PubMed] [Google Scholar]
- 28.Aletaha D, Epstein AJ, Skup M, Zueger P, Garg V, Panaccione R. Risk of developing additional immune-mediated manifestations: a retrospective matched cohort study. Adv Ther. 2019;36(7):1672–1683. doi: 10.1007/s12325-019-00964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlton R, Green A, Shaddick G, Snowball J, Nightingale A, Tillett W, Smith CH, McHugh N, PROMPT study group Risk of uveitis and inflammatory bowel disease in people with psoriatic arthritis: a population-based cohort study. Ann Rheum Dis. 2018;77(2):277–280. doi: 10.1136/annrheumdis-2017-212328. [DOI] [PubMed] [Google Scholar]
- 30.Chi CC, Tung TH, Wang J, Lin YS, Chen YF, Hsu TK, Wang SH. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135(5):415–422. doi: 10.1001/jamaophthalmol.2017.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151(11):1200–1205. doi: 10.1001/jamadermatol.2015.1986. [DOI] [PubMed] [Google Scholar]
- 32.Kaine J, Song X, Kim G, Hur P, Palmer JB. Higher incidence rates of comorbidities in patients with psoriatic arthritis compared with the general population using US administrative claims data. J Manag Care Spec Pharm. 2019;25(1):122–132. doi: 10.18553/jmcp.2018.17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 35.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trusko B, Thorne J, Jabs D, Belfort R, Dick A, Gangaputra S, Nussenblatt R, Okada A, Rosenbaum J, Standardization of Uveitis Nomenclature (SUN) Project The Standardization of Uveitis Nomenclature (SUN) Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med. 2013;52(3):259–265. S1–6. doi: 10.3414/ME12-01-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampaio-Barros PD, Pereira IA, Hernández-Cuevas C, Berman A, Burgos-Vargas R, Gutierrez MA, Barcelos A, Chávez-Corrales JE, Moreno M, Palleiro DR, Saénz-Castro R, Stekman I, Azevedo VF, Braga-da-Silva JA, Citera G, Flores-Alvarado D, Gonçalves CR, Graf C, Nitsche A, Saavedra J, Ximenes AC, Vázquez-Mellado J, Collantes-Estevez E, Respondia Group An analysis of 372 patients with anterior uveitis in a large Ibero-American cohort of spondyloarthritis: the RESPONDIA Group. Clin Exp Rheumatol. 2013;31(4):484–489. [PubMed] [Google Scholar]
- 38.Deschenes J, Murray PI, Rao NA, Nussenblatt RB. International uveitis study group (IUSG) clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2. doi: 10.1080/09273940801899822. [DOI] [PubMed] [Google Scholar]
- 39.Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol. 2014;8:1891–1911. doi: 10.2147/OPTH.S47778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Maghraoui A. Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur J Intern Med. 2011;22(6):554–560. doi: 10.1016/j.ejim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 41.London NJ, Rathinam SR, Cunningham ET., Jr The epidemiology of uveitis in developing countries. Int Ophthalmol Clin. 2010;50(2):1–17. doi: 10.1097/IIO.0b013e3181d2cc6b. [DOI] [PubMed] [Google Scholar]
- 42.Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012;7:57. doi: 10.1186/1750-1172-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knox DL. Psoriasis and intraocular inflammation. Trans Am Ophthalmol Soc. 1979;77:210–224. [PMC free article] [PubMed] [Google Scholar]
- 44.Durrani K, Foster CS. Psoriatic uveitis: a distinct clinical entity? Am J Ophthalmol. 2005;139(1):106–111. doi: 10.1016/j.ajo.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 45.Abbouda A, Abicca I, Fabiani C, Scappatura N, Peña-García P, Scrivo R, Priori R, Paroli MP. Psoriasis and psoriatic arthritis-related uveitis: different ophthalmological manifestations and ocular inflammation features. Semin Ophthalmol. 2017;32(6):715–720. doi: 10.3109/08820538.2016.1170161. [DOI] [PubMed] [Google Scholar]
- 46.Rehal B, Modjtahedi BS, Morse LS, Schwab IR, Maibach HI. Ocular psoriasis. J Am Acad Dermatol. 2011;65(6):1202–1212. doi: 10.1016/j.jaad.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 47.Forrester JV, Kuffova L, Dick AD. Autoimmunity, autoinflammation, and infection in uveitis. Am J Ophthalmol. 2018;189:77–85. doi: 10.1016/j.ajo.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein JE, Pepple KL. Cytokines in uveitis. Curr Opin Ophthalmol. 2018;29(3):267–274. doi: 10.1097/ICU.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 50.Guedes MC, Borrego LM, Proença RD. Roles of interleukin-17 in uveitis. Indian J Ophthalmol. 2016;64(9):628–634. doi: 10.4103/0301-4738.194339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang ZC, Wang YQ, Zhu GJ, Gu YF, Mao LP, Hong M, Li YL, Zheng MQ. Imbalance of Th17/Treg cells in pathogenesis of patients with human leukocyte antigen B27 associated acute anterior uveitis. Sci Rep. 2017;7:40414. doi: 10.1038/srep40414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W, Zhao B, Jiang R, Zhang R, Wang Y, Wu H, Gordon L, Chen L. Cytokine expression profile in aqueous humor and sera of patients with acute anterior uveitis. Curr Mol Med. 2015;15(6):543–549. doi: 10.2174/1566524015666150731100012. [DOI] [PubMed] [Google Scholar]
- 53.Caspi RR. Understanding autoimmunity in the eye: from animal models to novel therapies. Discov Med. 2014;17(93):155–162. [PMC free article] [PubMed] [Google Scholar]
- 54.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung JH, Song GG, Kim JH, Seo YH, Choi SJ. The association between genetic polymorphisms of the interleukin-23 receptor gene and susceptibility to uveitis: a meta-analysis. BMC Ophthalmol. 2017;17(1):81. doi: 10.1186/s12886-017-0477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pepple KL, Lin P. Targeting interleukin-23 in the treatment of noninfectious uveitis. Ophthalmology. 2018;125(12):1977–1983. doi: 10.1016/j.ophtha.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin P. Targeting interleukin-6 for noninfectious uveitis. Clin Ophthalmol. 2015;9:1697–1702. doi: 10.2147/OPTH.S68595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernández Garfella ML, Palomares Fort P, Román Ivorra JA, Cervera Taulet E. Aqueous humor levels of different interleukins 1-β, 2, 6 and 10, tumor necrosis factor-α and vascular endothelial growth factor in uveitis treated with adalimumab. J Ophthalmic Vis Res. 2015;10(1):49–54. doi: 10.4103/2008-322X.156110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fotiadou C, Lazaridou E. Psoriasis and uveitis: links and risks. Psoriasis (Auckl) 2019;9:91–96. doi: 10.2147/PTT.S179182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fotiadou C, Lazaridou E, Kemanetzi C, Kyrmanidou E, Ioannides D. Recalcitrant psoriatic uveitis and anti-tumor necrosis factor-α monoclonal antibodies: experience from a psoriasis referral center. Int J Dermatol. 2015;54(9):1105–1108. doi: 10.1111/ijd.12744. [DOI] [PubMed] [Google Scholar]
- 61.Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–796.e3. doi: 10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 62.Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, Barisani-Asenbauer T, Franco P, Heiligenhaus A, Scales D, Chu DS, Camez A, Kwatra NV, Song AP, Kron M, Tari S, Suhler EB. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932–943. doi: 10.1056/NEJMoa1509852. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, Schlaen A, Pavesio C, Cimino L, van Calster J, Camez AA, Kwatra NV, Song AP, Kron M, Tari S, Brézin AP. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388(10050):1183–1192. doi: 10.1016/S0140-6736(16)31339-3. [DOI] [PubMed] [Google Scholar]
- 64.Cordero-Coma M, Calvo-Río V, Adán A, Blanco R, Álvarez-Castro C, Mesquida M, Calleja S, González-Gay MA, Ruíz de Morales JG. Golimumab as rescue therapy for refractory immune-mediated uveitis: a three-center experience. Mediators Inflamm. 2014;2014:717598. doi: 10.1155/2014/717598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baerveldt EM, Kappen JH, Thio HB, van Laar JA, van Hagen PM, Prens EP. Successful long-term triple disease control by ustekinumab in a patient with Behcet's disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72(4):626–627. doi: 10.1136/annrheumdis-2012-202392. [DOI] [PubMed] [Google Scholar]
- 66.Mugheddu C, Atzori L, Del Piano M, Lappi A, Pau M, Murgia S, Zucca I, Rongioletti F. Successful ustekinumab treatment of noninfectious uveitis and concomitant severe psoriatic arthritis and plaque psoriasis. Dermatol Ther. 2017;30(5) doi: 10.1111/dth.12527. [DOI] [PubMed] [Google Scholar]
- 67.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, Androudi S. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777–787. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 68.Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL, AIN457A2208 Study Group Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939–948. doi: 10.1016/j.ophtha.2014.12.033. [DOI] [PubMed] [Google Scholar]