Abstract
Social deficits are features of autism and highly heritable traits. A common variant in autism-related CNTNAP2 gene, rs2710102, has been linked with social performance, but the neural substrates are largely unknown. We investigated variations in social performance and functional connectivity (static and dynamic) in the subregions of right temporoparietal junction (RTPJ), a key node of brain social network, using resting-state magnetic resonance imaging (n = 399) by genotype at rs2710102 in healthy volunteers. Social performance was evaluated using the social domain of the Autism-Spectrum Quotient (AQ-social; n = 641) and fixation time on eye areas during an eye-tracking task (n = 32). According to previous evidence that the A-allele is the risk allele for social dysfunction, we classified participants into GG and A-allele carriers (AA/AG) groups. The A-allele carriers showed poor social performance (high AQ-social and short fixation time on eye areas) compared with the GG carriers. In the A-allele carriers, decreased stationary functional connectivity between the orbitofrontal cortex and posterior RTPJ (pRTPJ), and decreased dynamic functional connectivity (dFC) between the medial prefrontal cortex (mPFC) and pRTPJ were observed. The fixation time at eye areas positively were correlated with the pRTPJ-mPFC dFC. These findings provided insight for genetic effect on social behavior and its potential neural substrate.
Keywords: CNTNAP2, social performance, temporoparietal junction, medial prefrontal cortex, dynamic functional connectivity
Introduction
Deficits in social interaction are core features of autistic spectrum disorders, a highly heritable neurodevelopmental disorder (Ronald and Hoekstra, 2011). These social impairments typically manifest as abnormalities in social interaction and difficulties in and recognition of expression and emotion (Bauminger, 2002). For example, individuals with autism were reported less attention paid to the eyes and other core features of faces during eye-tracking studies (Pelphrey et al., 2002). Recently, genetic behavior studies suggested that social-communication abilities are heritable traits and, hence, could be modulated by genetic variations (Skuse et al., 2014; St Pourcain et al., 2014). Contactin-associated protein-like 2 (CNTNAP2) is an important gene in the human genome and has been associated with autistic spectrum disorders (Arking et al., 2008). Although the CNTNAP2 gene was initially associated with language impairments in autism (Alarcon et al., 2008), mutations in CNTNAP2 may also be involved in social dysfunction. The A allele of rs2710102, a highly circumscribed region of the CNTNAP2 gene, has been associated with social anxiety-related disorders (Stein et al., 2011), of which a key feature is difficulty with social-communication (Pickard et al., 2017). Study in children with autistic trait also suggested that the A allele of rs2710102 has also been positively associated with levels of social inhibition (Steer et al., 2010).
The neural substrates of genotype effects on social performance remain largely unexplored. Recently, several lines of evidence have suggested that modulation of the CNTNAP2 genotype affects inter-region connectivity. At the cellular level, evidence suggests that Caspr2, a protein encoded by CNTNAP2, is important for neuronal migration and subsequent laminar organization, which indicates a crucial role for CNTNAP2 in inter-region connectivity (Strauss et al., 2006). In addition, structural and resting-state functional imaging studies have also identified impaired inter-region connectivity in individuals who are carriers of the autism risk allele of CNTNAP2 (Scott-Van Zeeland et al., 2010; Tan et al., 2010). For example, a study using resting-state imaging suggested that risk carriers of common genetic variants in CNTNAP2 are associated with having impaired functional connectivity in the medial prefrontal cortex (Scott-Van Zeeland et al., 2010), a key node for reasoning about mental states (mainly reasoning about the minds of other people), which supports successful social interaction in realistic environments (Frith and Frith, 2012).
In addition to the medial prefrontal cortex, the temporoparietal junction (TPJ) is another key neural substrate involved in reasoning about mental states (Schurz et al., 2014; Apps et al., 2015). As a supramodal association area, the TPJ, and especially the right TPJ (RTPJ), contribute to several social functions, including theory of mind, social decision-making and moral judgment (Schurz et al., 2014; Ye et al., 2015; Bitsch et al., 2018), as well as other cognitive process, such as attentional processes (Krall et al., 2016). Based on task-related functional magnetic resonance imaging (MRI) data from the BrainMap database, the RTPJ is segmented into the anterior and the posterior RTPJ (aRTPJ and pRTPJ) via the method of coactivation-based parcellation (Bzdok et al., 2013). Functionally, the anterior and posterior parts of these regions are linked to antagonistic brain networks for attentional and social processing, respectively (Bzdok et al., 2013). Consistent with this, dysfunctions in the pRTPJ during social tasks have reported in social-related disorders, such as autism (Abu-Akel et al., 2017). Increasingly, imaging studies have also suggested impaired functional connectivity of the TPJ with other social brain regions in autism (Venkataraman et al., 2015; Igelstrom et al., 2017), although functional connectivity based on the subregions of the TPJ remains largely unknown.
Previous studies on functional connectivity have mainly focused on static functional connectivity (sFC), which is based on the assumption of spatial and temporal stationarity throughout the entire scan period. This assumption may ignore the time-varying characteristics of inter-region connections, which have been implicated by other measurements with higher temporal resolutions, such as event-related potentials (Makeig et al., 2004; Onton et al., 2006). To address this issue, recent studies with resting-state functional magnetic resonance imaging (fMRI) developed dynamic functional connectivity (dFC) analysis to estimate the variability of inter-region synchronization (Allen et al., 2014). The credibility of the dFC derived from resting-state fMRI has been demonstrated through simultaneous electroencephalography/functional magnetic resonance (EEG/fMRI) studies (Britz et al., 2010; Chang et al., 2013). As previous studies suggested, inter-regions dynamic connection may reflect represent several cognitive function and underlie neural substrate of brain disease (Liao et al., 2018; Liu et al., 2018). Such connectivity dynamics is also closely associated with genetics (Gao et al., 2014). Of significance, it enables identification of the neurobiological features associated with normal brain development (Hutchison and Morton, 2015) and social-related disorders (Rashid et al., 2018). Hence, exploring dynamic connection may be conducive to reveal the neural substrate underlies the genetic effect on social function.
In the present study, we explored the effect of a common variant of the CNTNAP2 gene, rs2710102, on social behavior and the sFC and dFC of RTPJ subregions. Based on the close relationship between the A allele of rs2710102 and social disorders, we first hypothesized that individuals with the A allele of rs2710102 may present poor social performance. Social behavior was evaluated using the social sub-scales of the Autism-Spectrum Quotient (AQ) and fixation on eyes during an eye-tracking task. Human eye-to-eye contact is a primary source for social cues that play a vital role in social interaction and communication (Kendon, 1967). Gaze perception activates a social network of brain regions, including both the TPJ and medical prefrontal cortex (Carlin and Calder, 2013; von dem Hagen et al., 2014). Given that the CNTNAP2 gene potentially impacts on social-network inter-region connectivity and there are functional distinctions between the anterior and posterior RTPJ, we further assumed that a common variant of the CNTNAP2 gene, rs2710102, modulates functional connectivity (sFC and dFC) differently in the anterior and posterior RTPJ. Specifically, the significant modulatory effect may be only existent for the social seed area (the posterior RTPJ), but not the attentional seed area (the anterior RTPJ).
Materials and methods
Participants
All participants were college students from Anhui Medical University. After receiving a complete description of the study, all participants provided written informed consent. The present study was approved by the Ethics Committee of Anhui Medical University. Exclusion criteria for participants included neurological or psychological diseases, alcohol or drug abuse, traumatic brain injury or visible brain structure abnormity. Participants with a first-degree relative with a neurological or psychiatric disorder were also excluded.
First, a total of 641 eligible participants completed the AQ, which is frequently used in measuring autistic traits and consists of social and non-social aspects as described below (Davis et al., 2017). Of these participants, rs2710102 genotype data were available for 504 participants (the sample was chosen to explore the effect of genotype social function). From the participants with available behavior (AQ) and genotype data, 447 participants completed MRI scanning. After excluding participants with poor MRI data (resulting from artifacts or excessive head motion), a total of 399 participants were included in the final analysis of functional connectivity. Of these participants, 32 participants completed the eye-tracking task.
The AQ
The AQ is increasingly used in the screening of individuals for high levels of autistic traits. It consists of 50 items, which are divided into following five dimensions: communication, social skill, attention switching, imagination and attention to detail (Baron-Cohen et al., 2001). On the basis of previous studies, there are social aspects (AQ-Social, including communication, social skills, attention switching and imagination) and one non-social aspect (attention to detail) in the AQ (Davis et al., 2017). The AQ-social provided one index in our study to evaluate social performance. In present study, we used the Chinese version of AQ, which has good reliability (0.89) and validity (0.81) (Zhang et al., 2016). We adopted the 4-point Likert scoring system. Scores range from 1 to 4 for items showing autistic features: ‘definitely agree’ scored 4 points, ‘slightly agree’ scored 3 points, ‘slightly disagree’ scored 2 points and ‘definitely disagree’ scored 1 point. Some items were reverse scored.
Eye-tracking task
The eye-tracking task was performed using an integrated SMI Eye Tracking (SensoMotoric Instruments, Germany) to evaluate the fixation time on the interest areas of stimuli. All stimuli in the tracking task included 24 faces adapted from the Chinese Faces Affective Picture System (CFAPS). There were two male and two female faces for each of the six basic emotions and balanced by age, sex and emotion. Participants were instructed to look at the photographs in any manner they selected. The faces were divided into following four areas of interest (AOI): the eyes, nose, mouth and non-core feature areas (the rest area of the face). The eyes-AOI can cover the eyes of each image shape covering 3% of the surface of the image (the same as the nose-AOI (1%), mouth-AOI (2%) and the non-core feature-AOI (94%)) (Supplementary Figure S1). In present study, we mainly treated fixation time on eye-AOI as index for social performance, in contrast, the fixation time on non-core feature AOI was treated as index for non-social performance. The non-social performance may alter at different direction with the social performance. More information about the eye-tracking task could be found in the Supplementary Materials.
CNTNAP2 genotyping
Blood samples were collected to obtain DNA with standard procedures. The genotyping of all participants was determined in comparison with control DNA confirmed by sequencing in the SNP pattern. The genotype of rs2710102 is GG, GA and AA. According to previous studies suggesting that the A allele is the risk allele for social dysfunction, we classified the genotypes of rs2710102 into GG and A carriers (AA/AG) groups. More information about the gene scanning could be found in the Supplementary materials.
Image data acquisition
Structural and functional MRI for each participant was obtained using a 3-T scanner (Discovery GE750w) at University of Science and Technology of China. During functional MRI scanning, participants were instructed to keep their eyes closed, but not to fall asleep and not specifically try to think of anything in particular. Functional images were acquired composed of 217 echo-planar imaging volumes with the following parameters: repetition time = 2400 ms; echo time = 30 ms; flip angle = 90°; matrix size = 64 × 64, field of view = 192 × 192 mm2; slice thickness = 3 mm; 46 continuous slices (one voxel = 3 × 3 × 3 mm3). High spatial resolution T1-weighted anatomic images with 188 slices were also acquired in sagittal orientation (TR = 8.16 ms; TE = 3.18 ms; flip angle = 12; field of view = 256 × 256 mm2; slice thickness = 1 mm; and voxel size = 1 × 1 × 1 mm3).
Functional data preprocessing
Functional MRI data were preprocessed with the Data Processing Assistant for Resting-State Functional MR Imaging toolkit (Chao-Gan and Yu-Feng, 2010). For each participant, we applied the following processing steps: discarding of the first five volumes to achieve a steady-state, slice timing correction, realignment, spatial normalization based on the unified segmentation of structural images, nuisance regressors with 24 Friston motion parameters, white matter high signal, cerebrospinal fluid signal and global signals as regressors, filter with a temporal band-pass of 0.01–0.1 Hz and spatial smoothing (Gaussian kernel = 4 × 4 × 4 mm). Finally, motion scrubbing was conducted using the method of cubic spline to minimize the influence of the time points with high motion (defined as frame-wise displacement > 0.5), as well as one time point prior to and two time points after each of these high-motion time points.
Static and dFC
The RTPJ subregions underwent a recent data-driven characterization that revealed a subspecialization in the RTPJ using coactivation-based parcellation (Bzdok et al., 2013; http://anima.fz-juelich.de/). sFC was calculated using DPARSF software. For each individual, Pearson’s correlation coefficients were computed between the mean time series of each ROI and the time series of each voxel in the remainder of the brain. To improve normality, correlation coefficients were converted to z-values using Fisher’s r-to-z transformation and results were displayed using sFC maps for each participant.
dFC was calculated using DynamicBC software with a sliding-window approach (Liao et al., 2014). In this approach, window length is an important parameter in the computation of dFC. As a previous study has suggested, the window length should optimize the balance between acquiring reliable inter-region connectivity (with longer windows) and capturing fast shifting dynamic relationships (with shorter windows) (Leonardi and Van De Ville, 2015). In present study, the window length of 50 TRs (120 s) was selected based on the recommendation of a previous resting-state dynamic analysis (Leonardi and Van De Ville, 2015; Li et al., 2018) and the window was shifted by five TRs (12 s). In our data, the full-length time series was comprised of 212 TRs, so, there were 33 windows for each participant. For the time series in each window, the Pearson’s correlation coefficient of the TPJ with subregions with all other voxels was calculated and Fisher’s z-transformed, yielding a sliding-window z-value map. Then, for each participant, a set of sliding-window beta maps was used to calculate the dFC map (standard deviation in beta values at each voxel). Finally, the dFC maps were converted to z-values using Fisher’s r-to-z transformation to improve normality. Two supplementary window lengths [40 TRs (96 s) and 60 TRs (144 s)] were also chosen to validate our findings of temporal variability of the TPJ subregion connectivity.
Statistical analysis
The Pearson’s χ2 test was applied to compare sex differences and two-sample t-tests were applied to check for differences in age, the non-social AQ aspect (attention to detail) and social AQ aspects (AQ-social), as well as the subscales (social skills, communication, attention switching and imagination) between the two genotype groups (AA/AG vs GG).Voxel-wise two-sample t-tests with gender, age and mean head motion as covariates were used to quantitatively compare the differences in the sFC and dFC of the RTPJ subregions between the two genotype groups within the grey matter mask using dpabi software. All statistical maps were corrected using the Gaussian Random Field (GRF) method at the threshold for voxel P < 0.001 and cluster P < 0.05. The BrainNet Viewer package was used to map the remaining regions onto cortical surfaces (Xia et al., 2013). Spearman’s correlation analyses were performed to explore the associations between the significantly different RPTJ connectivity between groups (static and dynamic) and behavioral performance within all participants or each group. Significance was determined by P < 0.05 (two-tailed), with no correction. In consideration of the strong impact of singular values on correlation analyses, we excluded singular data defined as greater than three standard deviations from the mean. Six participants were excluded when performing correlation analysis because of singular data for mean connectivity. Finally, there were 393 participants included in the correlation analysis for the subscales of the AQ and 32 participants for analysis of fixation time at eye-AOI and non-core feature AOI.
Results
Genotype effects on behavior performance
The genotype distribution of rs2710102 in the current study (AA = 98, AG = 240 and GG = 166) was consistent with a previous report of variations of this gene in a healthy Chinese Han population (Ji et al., 2013). There were no significant differences in terms of sex and age between two genotype groups (AA/AG and GG group) (Supplementary Table S1).
There was a significant difference between genotype groups for AQ-social scores (t = 2.092, P = 0.037; Figure 1A). Specially, this difference was mainly derived from the sub-domain of communication (t = 2.620, P = 0.009). There was no significant difference between genotype groups for the non-social AQ subscale (attention to detail) or the other AQ-social domains (social skills, attention switching and imagination) (Supplementary Table S1). For the eye-tracking task, the A-allele carriers of CANTNP2 rs2710102 (18 A-allele carriers and 14 GG carriers) showed shorter fixation times on the eye-AOI at trend level (t = 2.028, P = 0.054) and longer times on the non-core feature AOI (t = 2.438, P = 0.023) (Figure 1B, Supplementary Table S2). No significant difference was found in terms of sex, age, fixation times on the nose-AOI or mouth-AOI between genotype groups (Supplementary Table S2).
Fig. 1.
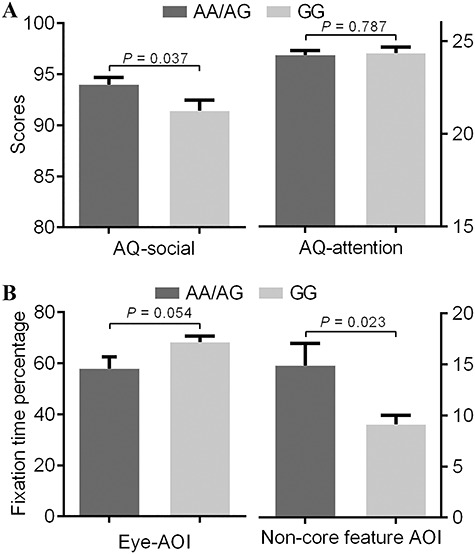
Modulatory effect of rs2710102 on social performance. (A) Mean score in the sub-aspects of AQ in two rs2710102 genotype groups. Carriers of the A allele showed poor social performance (high AQ-social score) compared to those with the GG genotype. There was no significant difference between two groups in the non-social sub-aspects (AQ-attention). (B) Fixation time percentage on different AOIs during eye-tracking task in two rs2710102 genotype groups. Carriers of the A allele showed shorter fixation time on eyes-AOI compared to those with the GG genotype and longer fixation time on non-core feature AOI. Error bars depict one standard error of the mean.
Genotype effects on the sFC of RTPJ subregions
Of the 504 participants included in the behavior analysis, 399 participants were included in the MRI analysis (AA/AG = 271, GG = 128). There were no significant differences between the genotype groups for gender, age, or head motion indexed by frame-wise displacement (Supplementary Table S3; Jenkinson et al., 2002).
We investigated the influence of the CANTNP2 rs2710102 variation on the sFC of the anterior and posterior RTPJ for whole grey matter, respectively. We first performed a one-sample t-test for the whole group, AA/AG group and GG group. Results revealed similar anterior and posterior RTPJ sFC for all three groups (Supplementary Figure S2). Group comparisons using two samples t-tests suggested that there was no significant difference in anterior RTPJ sFC between the AA/AG group and GG group. However, participants in AA/AG group exhibited lower sFC for the posterior RTPJ with the OFC (peak voxel MNI coordinate: x = −39, y = 39, z = −9; T value = −4.20; cluster size = 16) than the GG group (Figure 2).
Fig. 2.
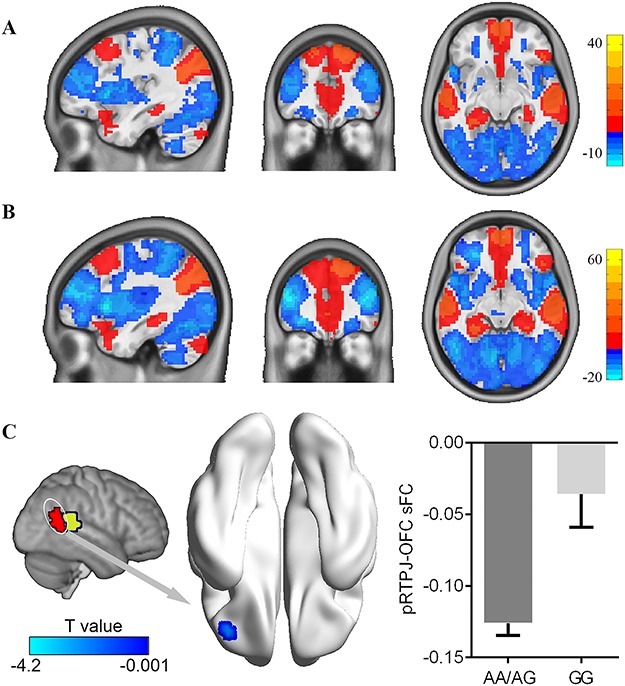
Modulatory effect of rs2710102 on the stationary functional connectivity of pRTPJ. The stationary functional connectivity pattern of pRTPJ was obtained by one-sample t-tests for rs2710102 GG homozygotes group (A) and AA/AG group (B). (C) Groups comparison revealed a decreased pRTPJ stationary connectivity with the orbital frontal cortex for the risk allele (AA/AG) compared with GG individuals. All threshold for comparisons were set as whole brain GRF correction (voxel P < 0.001, cluster P < 0.05).
Genotype effects on the dFC of RTPJ subregions
We conducted group-level comparisons to investigate the influence of the CANTNP2 rs2710102 variation on the dFC of anterior and posterior RTPJ. No significant difference was found for anterior RTPJ dFC between the AA/AG group and GG group. Compared with participants in the GG group, participants in the AA/AG group showed decreased dFC between posterior RTPJ and medial prefrontal cortex (mPFC) (peak voxel MNI coordinate: x = −3, y = 51, z = 24; T value = −4.46; cluster size = 18) than the GG group (Figure 3). The validation analysis with different sliding-window lengths also revealed decreased dFC between the posterior RTPJ and mPFC in the AA/AG group (Supplementary Figure S3).
Fig. 3.
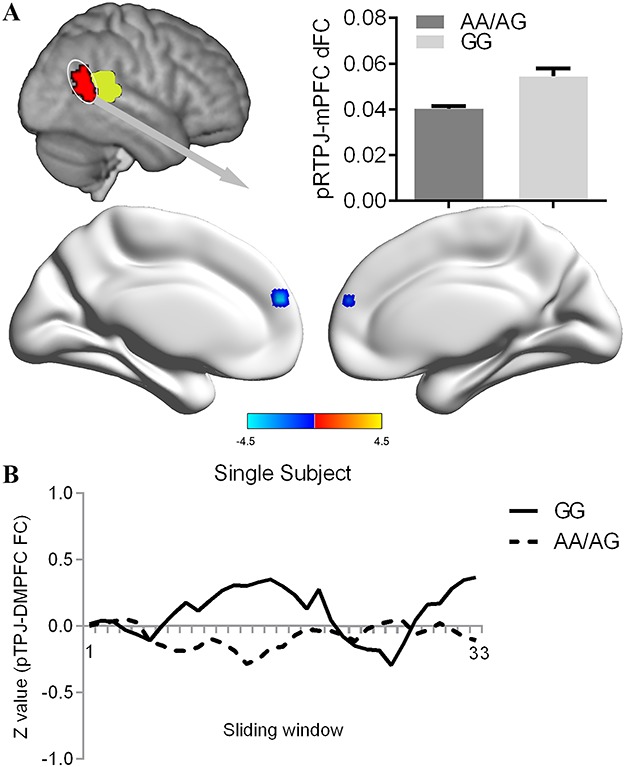
Modulatory effect of rs2710102 on the dFC of pRTPJ. (A) Groups comparison revealed a decreased pRTPJ dynamic connectivity with the medial prefrontal cortex for the risk allele (AA/AG) compared with GG individuals. Threshold for comparison was set as whole brain GRF correction (voxel P < 0.001, cluster P < 0.05). (B) The pRTPJ-mPFC functional connectivity values were displayed for a single participant in the AA/AG group and a single participant in the GG group across the 33 sliding windows.
Correlation analyses
The correlation between the posterior RTPJ-OFC sFC and any behavior performance outcome was not significant. It was also not significant between the posterior RTPJ-mPFC dFC and any behavior performance outcome among AQ and its sub-domains among all participants or each groups. However, it is notable that dFC between the posterior RTPJ and mPFC was significantly associated with the fixation time at the eye-AOI (r = 0.502, P = 0.003) and the fixation time on the non-core feature AOI (r = −0.493, P = 0.004) (Figure 4). For the correlation within the A-allele and GG carriers respectively, posterior RTPJ-mPFC dFC was significantly associated with the eye-AOI fixation time (r = 0.596, P = 0.025) and the non-core feature AOI fixation time (r = −0.572, P = 0.033) among the GG carriers, but not the A-allele carriers.
Fig. 4.
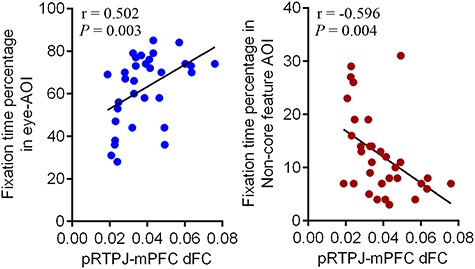
Relationship between social performance and dFC of pRTPJ with medial prefrontal cortex. Individual dynamic pRTPJ-mPFC functional connectivity was positively correlated with individual fixation time on eyes-AOI (A) and negatively correlated with individual fixation time on non-core feature AOI (B).
Discussion
In the current study, we identified the behavioral effects of the CANTNP2 rs2710102 variation on social performance and modulatory effects on brain social-network connectivity. Specifically, the rs2710102A-allele carriers had poor social function (shown by high AQ-social scores, shorter fixation times on eye areas) and diminished posterior RTPJ-OFC sFC and posterior RTPJ-mPFC dFC. The RTPJ-mPFC dFC were positively associated with social behavior.
The important role of CANTNP2 in social behavior is being increasingly implicated by research. CNTNAP2 genetic alterations have been identified in social-related disorders (mainly autistic spectrum disorders) (Bakkaloglu et al., 2008; Rodenas-Cuadrado et al., 2014). Intriguingly, the common genetic variants in CNTNAP2, including rs2710102 investigated in the current study, are also correlated with an increased risk of social-related disorders (Alarcon et al., 2008; Steer et al., 2010; Stein et al., 2011). It is notable that the CNTNAP2 rs2710102 was traditionally frequently associated with language impairments in autism (Alarcon et al., 2008). Indeed, the G allele of rs2710102 has been deemed as a risk allele for language impairments (Vernes et al., 2008; Riva et al., 2018). Accordingly, the G allele of rs2710102 has been considered as a risk allele for autism in multiple genetic imaging studies (Scott-Van Zeeland et al., 2010; Dennis et al., 2011). However, there are also findings that support the risk role of the A allele for social-related dysfunction (Steer et al., 2010; Stein et al., 2011). For example, the A allele of rs2710102 has been positively associated with the extent of social inhibition, which it contributes to independently of the trait for autism (Steer et al., 2010). Broadly consistent with these findings, our results showed that rs2710102 A-allele carriers had poor social function (demonstrated through high AQ-social scores and shorter fixation times on eye areas).
In addition to providing behavioral evidence, our results also provided imaging evidence supporting the modulatory effect of rs2710102 on brain social function. Specially, we found aberrant functional connectivity within the social network (diminished posterior RTPJ-OFC sFC and posterior RTPJ-mPFC dFC) in the A allele of rs2710102 carriers. It is well-known that the mPFC and RTPJ are regions the most associated with the inference of mental state (Schurz et al., 2014; Koster-Hale et al., 2017), which is essential to guide social behavior in real-life situations. In accord with the idea that the eyes provide a ‘window to the mind,’ eye-to-eye contact also activates regions implicated in the inference of mental states, including the mPFC and TPJ. Additionally, during resting states, connectivity between the TPJ and mPFC are also linked with social cognition and behavior (Brauer et al., 2016; Van Overwalle and Marien, 2016). In contrast, the OFC is traditionally deemed to be a key node of reward networks (Wallis, 2007). Recently, the impact of motivational factors on the social function has of increasing interest (Tabibnia and Lieberman, 2007; Lin et al., 2012). Chevallier C et al. proposed a social motivation theory of autism, which suggests that deficits in social cognition may be a consequence of disrupted social motivation but not the cause (Chevallier et al., 2012). Intriguingly, evidence from resting-state functional connectivity analysis also reveals that the interactions within the TPJ, mPFC and OFC contribute to social cognition (Van Overwalle and Marien, 2016).
However, previous study based on the seed of the mPFC did not report mPFC-pRTPJ connectivity differences between the genotypes of rs2710102. Besides the difference in genotype grouping, the dFC as adopted in our study may provide another potential explanation. Traditional functional connectivity assessment assumes that region coupling is stationary over time. Although convenient, this assumption may unfortunately overlook the varying nature of brain region interactions, which enable the brain to dynamically integrate and coordinate the cerebral regions responding to internal and external stimuli. Thus, the assessment of dFC here provided insights into subtle and flexible brain connectomes, which may contribute to multiple neurological and psychiatric disorders, as well as various forms of cognition. It has been demonstrated that dynamic connectomic could significantly predict cognitive performance in healthy individuals and pathological states (Liao et al., 2018; Liu et al., 2018). In the present study, we found that carriers of the risk allele for social dysfunction showed blunt variation in regional interaction (pRTPJ-mPFC). The blunt pathological states may contribute to the impairments in brain information integration and switching between cognitive processes (Liao et al., 2017; Cohen, 2018). Coincidentally, dynamic region interaction was positively associated with social performance. These findings provided novel evidence supporting the argument that dynamic interactions within the social brain network influence social performance and this effect may be modulated by genes.
It is worth noting that a relatively low effect size was observed in our study for the effect of genotype on social performance, as well as the correlation between dFC and social performance. For example, the difference for AQ-social between genotype groups just achieved statistical significance at the 0.05 level in our study and we did not find significant relationship between functional connectivity and social performance with AQ. The gross assessment method (AQ-social scale) and potential ceiling effect in participants (healthy college students) may contribute to the low effect size. In addition, we also considered a P-value > 0.05 (P-value = 0.054) as a ‘trend’ for difference on FTE. This low effect size may be caused by the small sample size (n = 32). Significantly, we found a neural feature (pRTPJ-mPFC dFC) associated with this behavior. Of course, future studies with larger sample sizes and clinical samples are necessary to validate the modulatory effect of gene on the FTE and also the neural substrate.
There are several additional limitations to this study that should be considered. First, the repetition time (2400 ms) used in present data may be not short enough to fully depict the dynamic features of brain. A multiband sequence with shorter repetition time may be a solution for use in future confirmatory research. Second, social performance in our study was measured using off-line brain signals acquisition. The relationships were obscure, although significant associations were identified. Task-based functional MRI research is needed to replicate our findings. Third, the seeds defined by population-level, not individual-level atlases likely eroded actual individual variation (Wang et al., 2015), which may lessen the correlations between pRTPJ-mPFC dFC and social performance. Finally, the absence of a long scan duration reduced the reliability of measuring stationary and dFC.
Conclusion
Our results provided novel evidences support that common variant of CNTNAP2 gene has a modulatory effect on the social performances and dynamic interaction of brain social network (pRTPJ-mPFC dFC). The dynamic interaction of brain social network is associated with social performances. These findings provided insight for the genetic-brain inaction mechanism for the social impairment in several disorders, such as autism.
Supplementary Material
Acknowledgements
We would like to thank all the participants enrolled in our study. We also acknowledge all operators of Center for Biomedical Engineering, University of Science and Technology of China to help for the MRI-data scanning.
Author contributions
T.B., L.Z. and K.W. designed the study. L.G.X., Z., G.X., W.H., M.Z., X.X., P.H. and C.Z., acquired behavior and imaging data. L.Z., L.W. and X.Z. collected and analyzed genetic data. T.B., L.Z., G.J., B.Q. and Y.T. analyzed imaging data, T.B., L.Z. and K.W. wrote this article, which all authors have reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding
This study was funded by the Natural Science Foundation of China (91432301, 31571149, 81171273, 31970979 and 91232717 to K.W., 81671354, 91732303 to Y.T., 31800909 to L.Z.), the National Basic Research Program of China (2015CB856405, 2012CB720704 and 2011CB707805 to K.W.) and the Science Fund for Distinguished Young Scholars of Anhui Province (1808085J23 to Y.T.).
Conflict of interest. None declared.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Abu-Akel A.M., Apperly I.A., Wood S.J., Hansen P.C. (2017). Autism and psychosis expressions diametrically modulate the right temporoparietal junction. Social Neuroscience, 12(5), 506–18. [DOI] [PubMed] [Google Scholar]
- Alarcon M., Abrahams B.S., Stone J.L., et al. (2008). Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American Journal of Human Genetics, 82(1), 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. (2014). Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex, 24(3), 663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps M.A., Tajadura-Jimenez A., Sereno M., Blanke O., Tsakiris M. (2015). Plasticity in unimodal and multimodal brain areas reflects multisensory changes in self-face identification. Cerebral Cortex, 25(1), 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking D.E., Cutler D.J., Brune C.W., et al. (2008). A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. American Journal of Human Genetics, 82(1), 160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkaloglu B., O'Roak B.J., Louvi A., et al. (2008). Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. American Journal of Human Genetics, 82(1), 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. [DOI] [PubMed] [Google Scholar]
- Bauminger N. (2002). The facilitation of social-emotional understanding and social interaction in high-functioning children with autism: intervention outcomes. Journal of Autism and Developmental Disorders, 32(4), 283–98. [DOI] [PubMed] [Google Scholar]
- Bitsch F., Berger P., Nagels A., Falkenberg I., Straube B. (2018). The role of the right temporo-parietal junction in social decision-making. Human Brain Mapping, 39(7), 3072–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer J., Xiao Y., Poulain T., Friederici A.D., Schirmer A. (2016). Frequency of maternal touch predicts resting activity and connectivity of the developing social brain. Cerebral Cortex, 26(8), 3544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J., Van De Ville D., Michel C.M. (2010). BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage, 52(4), 1162–70. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Langner R., Schilbach L., et al. (2013). Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. NeuroImage, 81, 381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J.D., Calder A.J. (2013). The neural basis of eye gaze processing. Current Opinion in Neurobiology, 23(3), 450–5. [DOI] [PubMed] [Google Scholar]
- Chang C., Liu Z., Chen M.C., Liu X., Duyn J.H. (2013). EEG correlates of time-varying BOLD functional connectivity. NeuroImage, 72, 227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. (2010). DPARSF: a MATLAB toolbox for "pipeline" data analysis of resting-State fMRI. Frontiers in Systems Neuroscience, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E.S., Schultz R.T. (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16(4), 231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R. (2018). The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. NeuroImage, 180(Pt B), 515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., McKone E., Zirnsak M., et al. (2017). Social and attention-to-detail subclusters of autistic traits differentially predict looking at eyes and face identity recognition ability. British Journal of Psychology, 108(1), 191–219. [DOI] [PubMed] [Google Scholar]
- Dennis E.L., Jahanshad N., Rudie J.D., et al. (2011). Altered structural brain connectivity in healthy carriers of the autism risk gene, CNTNAP2. Brain Connectivity, 1(6), 447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2012). Mechanisms of social cognition. Annual Review of Psychology, 63(1), 287–313. [DOI] [PubMed] [Google Scholar]
- Gao W., Elton A., Zhu H., et al. (2014). Intersubject variability of and genetic effects on the brain's functional connectivity during infancy. Journal of Neuroscience, 34(34), 11288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen E.A., Stoyanova R.S., Rowe J.B., Baron-Cohen S., Calder A.J. (2014). Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cerebral Cortex, 24(6), 1485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Morton J.B. (2015). Tracking the Brain's functional coupling dynamics over development. Journal of Neuroscience, 35(17), 6849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom K.M., Webb T.W., Graziano M.S.A. (2017). Functional connectivity between the Temporoparietal cortex and cerebellum in autism Spectrum disorder. Cerebral Cortex, 27(4), 2617–27. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–41. [DOI] [PubMed] [Google Scholar]
- Ji W., Li T., Pan Y., et al. (2013). CNTNAP2 is significantly associated with schizophrenia and major depression in the Han Chinese population. Psychiatry Research, 207(3), 225–8. [DOI] [PubMed] [Google Scholar]
- Kendon A. (1967). Some functions of gaze-direction in social interaction. Acta Psychologica, 26(1), 22–63. [DOI] [PubMed] [Google Scholar]
- Koster-Hale J., Richardson H., Velez N., Asaba M., Young L., Saxe R. (2017). Mentalizing regions represent distributed, continuous, and abstract dimensions of others' beliefs. NeuroImage, 161, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall S.C., Volz L.J., Oberwelland E., Grefkes C., Fink G.R., Konrad K. (2016). The right temporoparietal junction in attention and social interaction: a transcranial magnetic stimulation study. Human Brain Mapping, 37(2), 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi N., Van De Ville D. (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage, 104, 430–6. [DOI] [PubMed] [Google Scholar]
- Li J., Duan X., Cui Q., Chen H., Liao W. (2018). More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. Psychological Medicine, 49, 852–60. [DOI] [PubMed] [Google Scholar]
- Liao W., Wu G.R., Xu Q., et al. (2014). DynamicBC: a MATLAB toolbox for dynamic brain connectome analysis. Brain Connectivity, 4(10), 780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Cao M., Xia M., He Y. (2017). Individual differences and time-varying features of modular brain architecture. Neuro Image, 152, 94–107. [DOI] [PubMed] [Google Scholar]
- Liao W., Li J., Duan X., Cui Q., Chen H., Chen H. (2018). Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Human Brain Mapping, 39(10), 4105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Adolphs R., Rangel A. (2012). Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience, 7(3), 274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liao X., Xia M., He Y. (2018). Chronnectome fingerprinting: identifying individuals and predicting higher cognitive functions using dynamic brain connectivity patterns. 39(2), 902–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S., Debener S., Onton J., Delorme A. (2004). Mining event-related brain dynamics. Trends in Cognitive Sciences, 8(5), 204–10. [DOI] [PubMed] [Google Scholar]
- Onton J., Westerfield M., Townsend J., Makeig S. (2006). Imaging human EEG dynamics using independent component analysis. Neuroscience and Biobehavioral Reviews, 30(6), 808–22. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Sasson N.J., Reznick J.S., Paul G., Goldman B.D., Piven J. (2002). Visual scanning of faces in autism. Journal of Autism and Developmental Disorders, 32(4), 249–61. [DOI] [PubMed] [Google Scholar]
- Pickard H., Rijsdijk F., Happé F., Mandy W. (2017). Are social and communication difficulties a risk factor for the development of social anxiety? Journal of the American Academy of Child and Adolescent Psychiatry, 56(4), 344–351.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid B., Blanken L.M.E., Muetzel R.L., et al. (2018). Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Human Brain Mapping, 39(8), 3127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva V., Cantiani C., Benasich A.A., et al. (2018). From CNTNAP2 to early expressive language in infancy: the mediation role of rapid auditory processing. Cerebral Cortex, 28(6), 2100–8. [DOI] [PubMed] [Google Scholar]
- Rodenas-Cuadrado P., Ho J., Vernes S.C. (2014). Shining a light on CNTNAP2: complex functions to complex disorders. European Journal of Human Genetics, 22(2), 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A., Hoekstra R.A. (2011). Autism spectrum disorders and autistic traits: a decade of new twin studies. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics, 156b(3), 255–74. [DOI] [PubMed] [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews, 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland A.A., Abrahams B.S., Alvarez-Retuerto A.I., et al. (2010). Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Science Translational Medicine, 2(56), 56ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse D.H., Lori A., Cubells J.F., et al. (2014). Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proceedings of the National Academy of Sciences of the United States of America, 111(5), 1987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pourcain B., Skuse D.H., Mandy W.P., et al. (2014). Variability in the common genetic architecture of social-communication spectrum phenotypes during childhood and adolescence. Mol Autism, 5(1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C.D., Golding J., Bolton P.F. (2010). Traits contributing to the autistic spectrum. PLoS One, 5(9), e12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Yang B.Z., Chavira D.A., et al. (2011). A common genetic variant in the neurexin superfamily member CNTNAP2 is associated with increased risk for selective mutism and social anxiety-related traits. Biological Psychiatry, 69(9), 825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss K.A., Puffenberger E.G., Huentelman M.J., et al. (2006). Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. The New England Journal of Medicine, 354(13), 1370–7. [DOI] [PubMed] [Google Scholar]
- Tabibnia G., Lieberman M.D. (2007). Fairness and cooperation are rewarding: evidence from social cognitive neuroscience. Annals of the New York Academy of Sciences, 1118, 90–101. [DOI] [PubMed] [Google Scholar]
- Tan G.C.Y., Doke T.F., Ashburner J., Wood N.W., Frackowiak R.S.J. (2010). Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. NeuroImage, 53(3), 1030–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Marien P. (2016). Functional connectivity between the cerebrum and cerebellum in social cognition: a multi-study analysis. NeuroImage, 124(Pt A), 248–55. [DOI] [PubMed] [Google Scholar]
- Venkataraman A., Duncan J.S., Yang D.Y., Pelphrey K.A. (2015). An unbiased Bayesian approach to functional connectomics implicates social-communication networks in autism. Neuroimage Clin, 8, 356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernes S.C., Newbury D.F., Abrahams B.S., et al. (2008). A functional genetic link between distinct developmental language disorders. New England Journal of Medicine, 359(22), 2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J.D. (2007). Orbitofrontal cortex and its contribution to decision-making. Annual Review of Neuroscience, 30, 31–56. [DOI] [PubMed] [Google Scholar]
- Wang D., Buckner R.L., Fox M.D., et al. (2015). Parcellating cortical functional networks in individuals. Nature Neuroscience, 18(12), 1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. (2013). BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One, 8(7), e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H., Chen S., Huang D., Zheng H., Jia Y., Luo J. (2015). Modulation of neural activity in the temporoparietal junction with Transcranial direct current stimulation changes the role of beliefs in moral judgment. Frontiers in Human Neuroscience, 9, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Sun Y., Chen F., et al. (2016). Psychometric properties of the autism-Spectrum quotient in both clinical and non-clinical samples: Chinese version for mainland China. BMC Psychiatry, 16, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


