Abstract
Hair follicle formation in developing embryonic skin requires stepwise signaling between the epithelial epidermis and mesenchymal dermis, and their specialized derivatives, the placode/germ/peg and dermal condensate/papilla, respectively. Classically, distinct stages of hair follicle morphogenesis have been defined, in the mouse model, based on 1) changes in cell morphology and aggregation, 2) expression of few known molecular markers, 3) the extent of follicle downgrowth, and 4) the presence of differentiating cell types. Refined genetic strategies and recent emerging technologies, such as live imaging and transcriptome analyses of isolated cell populations or single cells, have enabled a closer dissection of the signaling requirements at different stages of hair follicle formation, particularly early on. They have also led to the discovery of precursor cells for placode, dermal condensate and future bulge stem cells that, combined with molecular insights into their fate specification and subsequent formation, serve as novel landmarks for early hair follicle morphogenetic events and studies of the signaling networks mediating these processes. In this review, we integrate the emergence of hair follicle precursor cell states and novel molecular markers of fate and formation to update the widely used 20-year old seminal classification guide of hair follicle morphogenetic stages by Paus and colleagues. We then temporally describe the latest insights into the early cellular and molecular events and signaling requirements for hair follicle morphogenesis in relation to one another in a holistic manner.
Keywords: hair follicle morphogenesis, placode progenitors, dermal condensate, stem cell niche, dermal papilla, classification, guide
1 |. INTRODUCTION
The mature hair follicle (HF) is structurally complex, belying its small size. It is predominantly comprised of concentric rings of epithelial cells that form the hair shaft and inner root sheath (1), with reserve stem cells in the bulge region (2–7) and their progenitors, transit-amplifying matrix cells, at the bulbar base. Surrounded by the matrix is a central cluster of mesenchymal cells, the dermal papilla (DP), which acts as an instructive signaling niche (8–10) for these transit-amplifying progenitors to proliferate, migrate upwards and differentiate into the several layers of shaft and inner root sheath cell lineages during the hair growth phase (10–12). Adding to the complexity is the presence of other HF resident cell types: sebocytes that make up the mature sebaceous gland (13–15), and melanocytes that pigment the hair (16). The specification of the epithelial cell types of the HF and of the mesenchymal DP, from the embryonic placode (Pc) and dermal condensate (DC), respectively, and later emergence of other HF resident cell types, is a tightly controlled process during embryogenesis, both temporally and spatially (17).
Hair inductive capacity lies within the dermis, which has been demonstrated in “cut-and-paste” tissue recombination experiments; recombined hair-forming dermis and non-hairy glabrous epidermis, both derived from murine embryonic skin before HF morphogenesis, are able to form hair, while dermis from glabrous regions is unable to induce follicles even in hairy epidermis (18,19). Remarkably, xenograft experiments recombining feather-forming dermis from chicken with scale-forming epidermis from lizards or hair forming dermis from mouse with chicken epidermis results in the production of scale or feather structures, respectively, suggesting that the induction of epidermal appendages is controlled by the mesenchyme (19,20). While the “first dermal signal” for patterned initiation of HF morphogenesis in the epidermis remains elusive (21–23), it is known that widespread Wnt signaling activity in the upper dermis by embryonic day (E) 12.5 precedes HF formation (24) and is required for HF induction (25). This signal acts on the epidermis to induce Pc formation at ~E14.0 (17,26), which produce the “first epithelial signal” that acts on the underlying fibroblasts. One or more such signals, including FGF20 (27), lead to the formation of the DC, local condensations of upper dermal fibroblasts, at E14.5 (17). The DC then secretes still unknown “second dermal signals” to catalyze proliferation of Pc progenitors and downgrowth of the HF (17). Xenograft experiments have failed to completely form feathers, scales or HFs suggesting that species-specific signaling crosstalk between the epithelial and mesenchymal compartments initiated by the “second dermal signal” is necessary for proper downgrowth and differentiation (28,29). Complete understanding of these crucial reciprocal signals in early HF morphogenesis remains elusive, owing to the rapidity with which epithelial-mesenchymal crosstalk and subsequent morphological changes occur, although many individual components have been parsed.
The evaluation of HF morphogenetic stages has historically relied upon progressive changes in cellular shape and morphology, dynamic aggregation of cells, emergence of follicle resident cell types, extent of HF downgrowth (17,30), and the usage of few known molecular markers, IL-1RI (30), TGF-βRII (31), and Alkaline Phosphatase (AP) (30,32). In a seminal classification guide from 20 years ago, Paus and colleagues summarized these key characteristics of HF morphogenesis to provide a well-defined classification system for greater spatiotemporal clarification of the major HF morphogenesis stages (30). Since its establishment, advanced mouse genetic methods have enabled numerous functional studies that uncovered the essential roles of major signaling pathways, such as Wnt, Eda/Edar, Fgf, Bmp, Shh and TGFβ signaling (21–23,26). Furthermore, many emerging technologies such as live imaging, multicolor fluorescent labeling and isolation of distinct cell types from specific stages, as well as high sensitivity transcriptomics at both the population and single-cell level, have enabled a more fine-toothed dissection of the cellular and molecular dynamics of HF morphogenesis. Such advances have permitted the definition of molecular signatures of Pc and DC, and neighboring cell types (33–35), as well as identified migration as the main cellular mechanism of Pc and DC formation (36–38). They have also allowed for the discovery of precursors to the Pc (pre-PC) (24,39–41), multipotent fibroblasts that give rise to the DC (42) and the fated precursors of DC (pre-DC) (43), by their molecular properties and prior to identifiable changes in cell morphologies and arrangement. Finally, they have enabled identification of suprabasal SOX9+ precursors to HF stem cells after placode formation (44,45).
In this review, we update the well-established classification guide of HF morphogenesis stages by incorporating the recently discovered early precursor cell states and the many new cellular and molecular insights into early HF fate specification and formation. We then describe the current knowledge of reciprocal mesenchymal-epithelial interaction to provide a comprehensive overview of the dynamism of HF morphogenesis, focusing on early cellular, molecular and signaling events during the first wave of embryonic hair follicle formation.
2 |. UPDATED STAGING OF HF MORPHOGENESIS
2.1 -. Early Morphogenesis
To account for the recently discovered precursor cell states and new molecular events during the earliest phase of HF formation (“molecular placode” pre-Pc before morphological Pc; fated pre-DC before DC formation; HFSC precursors before bulge formation), we subdivided the previous stage 0 from the original classification (30) into 2 new stages, stages 1 and 2 (Figure 1). These are prefaced by a new stage 0 during which HF induction from the dermis is set up before any patterned molecular or cellular events. The previously classified advanced Pc/DC and germ stages then succeed the new precursor stages (Figure 1). All molecular markers related to early morphogenesis described in this review are featured in a comprehensive Figure 5 that is, for easy reference, color-coded by cell type and stage, and lists the corresponding cited publications.
Figure 1. Updated Classification of Hair Follicle Morphogenesis Stages.
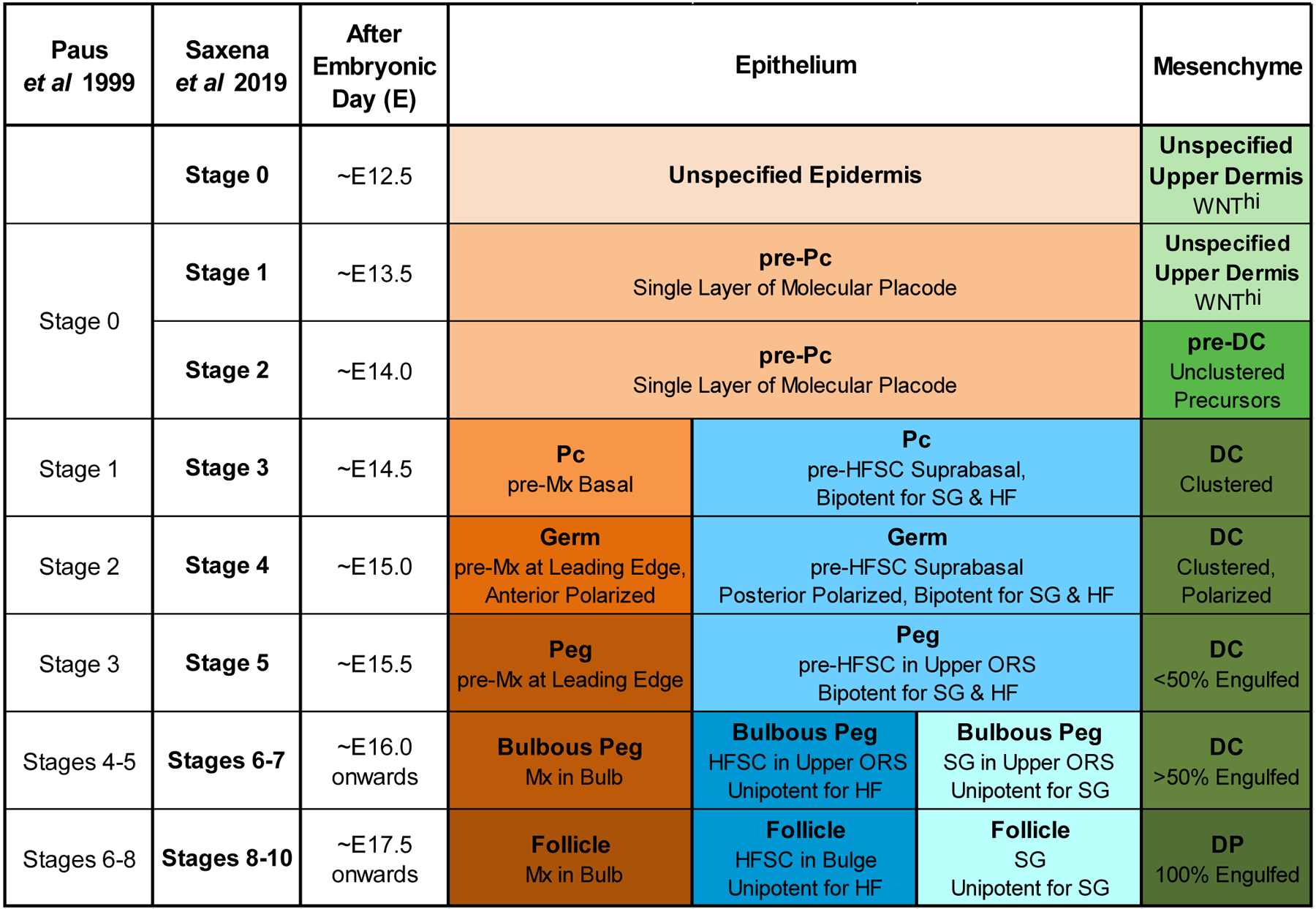
Updated hair follicle morphogenetic classification including new stages for emergence and spatial localization of recently discovered precursor cell states in the epithelial and mesenchymal compartments, including pre-Pc, pre-DC and HFSC precursors. Differentiation of the pre-Pc into the Pc, then into Mx (matrix), HFSC, and SG (sebaceous gland), as well as of the pre-DC into the DP serve as hallmarks for stage identification. The previous classification system by Paus et al, 1999 is outlined. The approximate gestational ages are provided for HF stages as they first appear during the first wave of primary guard hair formation.
For all stages, we describe the updated classification in the context of the first wave of primary guard hair formation, for which most new cellular and molecular insights have been discovered in recent years, likely due to the ability to study first wave hair formation in isolation. In the experimental mouse model, the touch sensitive (tylotrich) guard hairs are induced starting at approximately embryonic day (E)13.5, before the formation of secondary, non-tylotrich coat hair types (2nd wave: awl, auchene, initiated at ~E15.5; 3rd wave: zigzag, initiated at ~E17.5-E18.5) that make up the majority of adult hairs (27,46,47). Finally, while we provide the approximate gestational ages for each HF stage as they first appear, it is important to note that, due to variability of developmental timing, the early stages of first wave hair formation co-exist in parallel (e.g. at E15.0) and can be identified and distinguished by the stage-specific criteria defined below.
Stage 0 –
Before HF morphogenesis begins at ~E13.5, basal epidermal cells are a uniform layer without any morphological signs or patterned molecular distinctions of HF formation (Figure 1, Figure 2, stage 0). At stage 0, widespread Wnt signaling activity in the upper dermis (24) is important for setting up HF inductivity by supplying the critical, but still unknown “first dermal signal”: Epidermal ablation of Wntless (Wls), a mediator of broad epidermal Wnt ligand secretion, at E13.5 and broad dermal ablation of β-catenin both result in a loss of Wnt signaling activity in the dermis and subsequent absence of Pre-Pc induction (25), demonstrating the requirement of Wnt signaling upstream of still unknown target genes that act as inductive signals towards the epidermis. Knockout of the epidermal transcription factor ΔNp63 prevents expression of Wnt target genes in early epidermal progenitors and subsequent HF formation (48), further confirming the important role of epidermis-derived Wnts and broad dermal Wnt signaling activity in pre-Pc fate specification and HF induction. Interestingly, recent single-cell RNA sequencing analyses suggest that upper dermal fibroblasts are transitioning toward DC fate specification at this early induction stage prior to morphogenesis (42).
Figure 2. Stages 0–2 of Hair Follicle Morphogenesis.
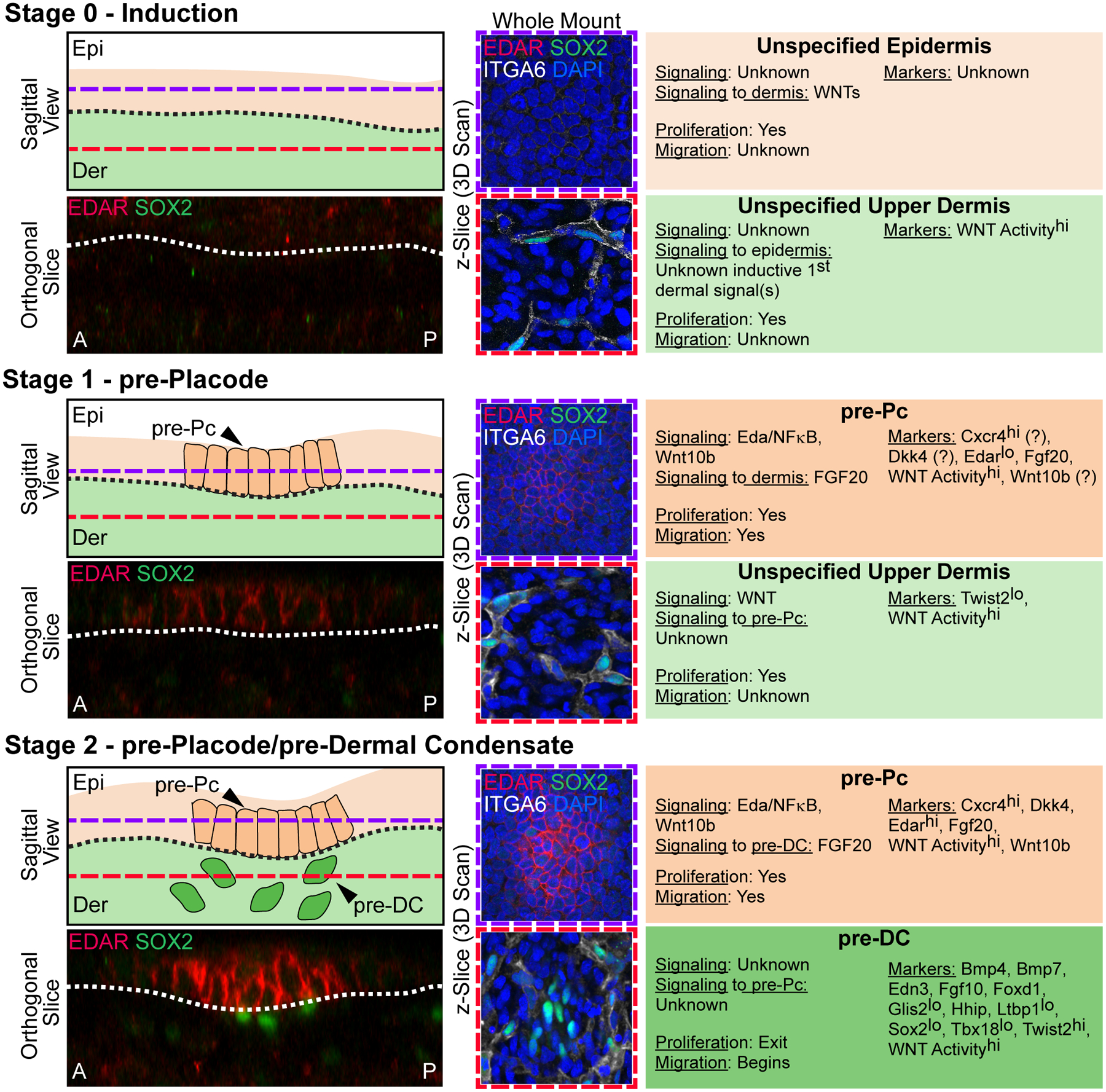
Left top: Sagittal view schematic of HF morphogenesis stages. Purple dashed lines mark the epidermal Z-plane, and red dashed lines mark the dermal Z-plane of 3D-imaged confocal scans of whole mount immunofluorescence of E15.0 back skin. A and P denote anterior and posterior orientation of embryonic skin (head is left). Left bottom: Orthogonal slice from a 3D reconstruction of whole mount immunofluorescence for EDAR and SOX2. Middle: Whole mount immunofluorescence for EDAR, SOX2, and ITGA6 in the epithelial plane (top, purple frame), and dermal plane (bottom, red frame). DAPI marks all nuclei. Right: Description of autocrine and paracrine signaling, proliferation and migration status, and markers of the relevant epithelial and mesenchymal populations at each stage.
Stage 0 – Induction. The uniform unspecified multipotent epidermis resides over an unspecified dermis. EDAR is not expressed in the epidermal compartment and only SOX2+/ITGA6+ Schwann cells are present in the dermis. Widespread Wnt signaling activity in the upper dermis sets up HF induction by the critical, but still unknown “first dermal signal(s)”.
Stage 1 – pre-Placode. Emergence of placode precursors (pre-Pc), the fated “molecular placode”, in the epidermis over an unspecified dermis. The pre-Pc in the epidermal plane expresses EDAR. Only SOX2+/ ITGA6+ Schwann cells are present in the dermal compartment. Markers with question marks refer to known expression by in situ hybridization and/or protein staining at E13.5, in which the presence of recently discovered pre-DC (stage 2) cannot be ruled out.
Stage 2 – pre-Placode/pre-Dermal Condensate. pre-Pc in the epidermis over dermal condensate precursors (pre-DC) in the dermis. The pre-Pc in the epidermal plane expresses EDAR more strongly. Pre-DC emerge as low-level SOX2+/ITGA6− unclustered cells underneath pre-Pc.
Before pre-Pc specification, neural crest-derived melanoblasts, precursors of HF-resident melanocytes, are already present in the dermis. During Stage 0, melanoblasts begin migrating upward into the epidermis (49). At this stage of skin development, melanoblasts express Sox10 (50), Mitf (51), Pax3 (52), Dct (53), Kit (54) and Tyrp1 (53).
Stage 1 –
Still unknown signals from Wnt-responsive upper dermal cells act on the uniform epidermis to induce Pc formation, termed the “first dermal signal”. At stage 1 around E13.5 – 13.75, the molecular Pc precursor (pre-Pc) cell fate is focally induced in epidermal progenitors at sites of future HF morphogenesis, prior to any morphological signs (Figure 1, Figure 2 stage 1). Several markers for the pre-Pc state (“molecular placode”) have been identified, such as active Wnt signaling (24,55,56), Edar (57–59) and Fgf20 (27). Other genes are expressed in molecular placodes, but the precise timing of their expression with relation to DC fate acquisition in Stage 2 follicles is unclear. These include Wnt10b (60), its downstream target Dkk4 (40,61), and Cxcr4 (62) (Figure 2, Figure 5). The timing of signaling pathways and other inter- and intracellular molecular machinery that regulate expression of these genes has yet to be fully dissected. At this earliest pre-Pc stage, Wnt signaling remains widely active in the upper dermis.
Stage 2 –
The establishment of the pre-Pc then sparks the induction of the DC cell fate in the closest neighboring fibroblasts at ~E14.0 that precedes stereotypic aggregation of the mesenchymal DC cluster (Figure 2, stage 2) (43). These DC precursors (pre-DC) are at a transitional state from fibroblasts towards the DC fate. Pre-DC specification requires the pre-placodal production of Fgf20, an important component of the “first epithelial signal” (43) that is also required for DC aggregation and maintenance at the following stages (36,37). At this stage, the pre-Pc shows active Wnt signaling and expression of Edar, as well as Cxcr4 (62), Dkk4 (40,61) and Wnt10b (60). Whether Shh (24,55), Pcad (34) and Lhx2 (33,34), three bona-fide stage 3 Pc markers, are already expressed at stage 1 or 2 is currently unclear.
In contrast to the other fibroblast-type cells in the mesenchyme, including fibroblasts that will transition into pre-DC, pre-DC cells are already post-mitotic, indicating that acquisition of DC fate is concomitant with the shutdown of the cell cycle machinery (37,42,43). Like the pre-Pc, there are high levels of Wnt signaling activity in pre-DC (24), when compared to other dermal fibroblasts; in fact, Wnt activation is necessary for acquisition of DC fate (42) and DC formation (46). Pre-DC cells, ranging from 1 to ~15–20 cells, are randomly dispersed amongst dermal fibroblasts and located right below pre-Pc cells (separated by the basement membrane), which by contrast form a contiguous unit (Figure 2). Pre-DC can be discerned by de novo expression of Foxd1 and Sox2, as well as by upregulation of Tbx18 and highest expression of pandermal fibroblast marker Twist2 (43), which begins ramping up expression prior to acquisition of DC fate amongst upper dermal fibroblasts (42) (Figure 2). Foxd1 (35), Sox2 (8,63), Tbx18 (64), Bmp4 (25,65,66), Bmp7 (25), Hhip (62), and Fgf10 (41) have been previously identified as highly expressed signature genes in the aggregated DC (Figure 5).
Stage 3 –
Stage 3 closely resembles Stage 1 from Paus et al (30), and both the Pc and DC are morphologically identifiable in addition to expression of several signature genes (Figure 1, Figure 3, stage 3). The Pc, starting at ~E14.5, can now be distinguished from the rest of the epidermis because it appears thicker and is comprised of larger, tightly packed vertically oriented keratinocytes that slightly invaginate into the underlying dermis with basement membrane at the leading edge (Figure 3, stage 3). Most pre-Pc genes remain expressed joining an expanded Pc signature (Figure 3) (35) (http://hair-gel.net). Intriguingly, Cxcr4 expression wanes as the Pc matures, and is completely lost by Stage 4 HF morphogenesis, although the gene, itself, appears dispensable for HF morphogenesis (62). Shh (24,55),Pcad (34) and Lhx2 (33,34), a downstream target of Edar, are now expressed within these basal matrix progenitors. At this stage, Sox9+ suprabasal HFSC precursors are specified during perpendicular asymmetric cell divisions of Shh-expressing basal Pc progenitors with high Wnt signaling activity (45,67) (Figure 1, Figure 3, stage 3), and, once suprabasal, have very low levels of Wnt signaling. Shh and Sox9 expression in basal Pc and suprabasal HFSC precursors, respectively, is largely non-overlapping (45,67). These HFSC precursors will, through the course of morphogenesis give rise to the all epithelial cells of the hair follicle, including the sebaceous gland (44,68) and Merkel cells (69) (Figure 4). Basal Pc progenitors themselves are precursors of the progenitors at the leading edge of downgrowing HFs that become replaced by HFSC progeny by the end of morphogenesis (Figure 4) (44).
Figure 3. Stages 3–4 of Hair Follicle Morphogenesis.
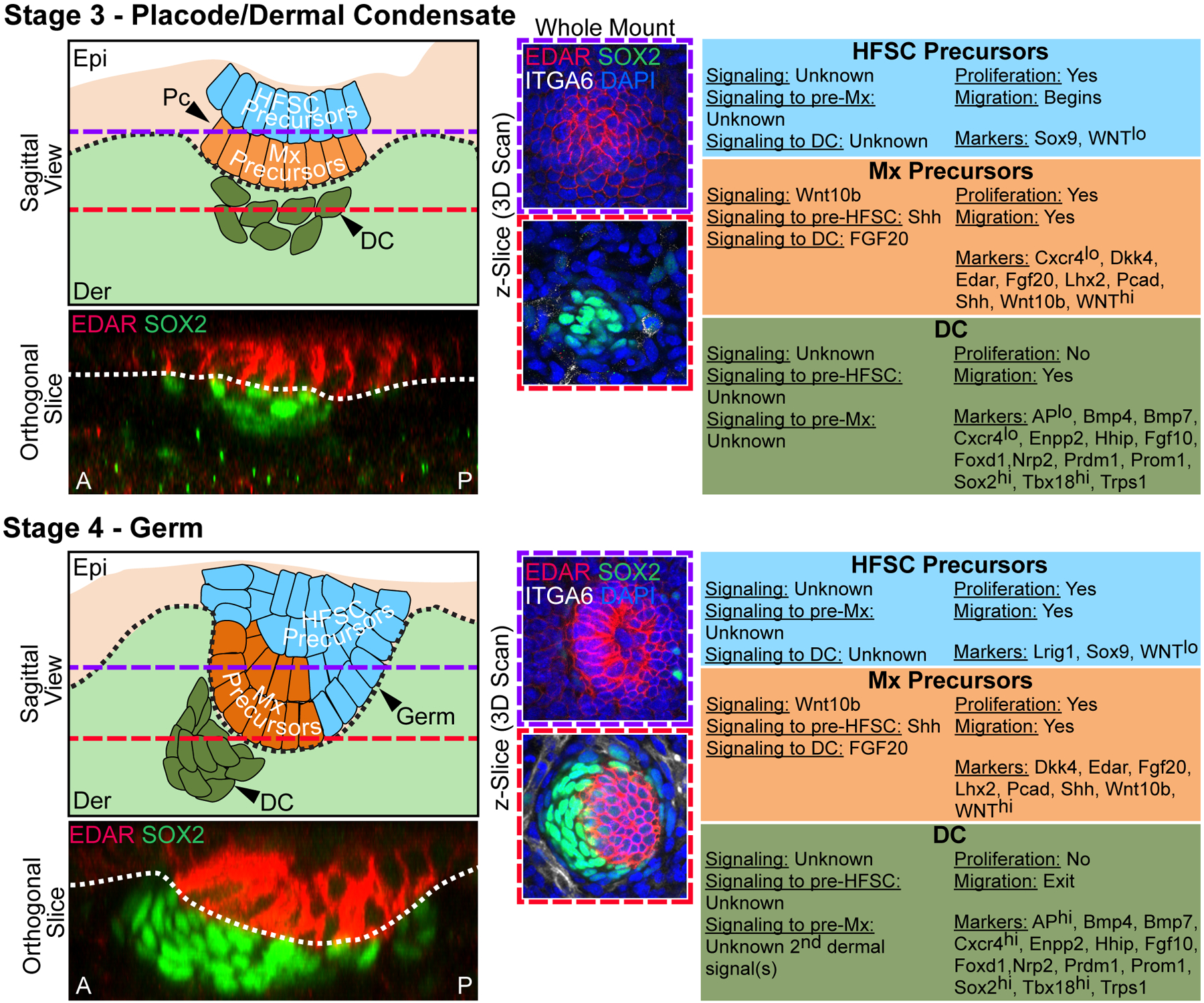
Left top: Sagittal view schematic of HF morphogenesis stages. Purple dashed lines mark the epidermal Z-plane, and red dashed lines mark the dermal Z-plane of 3D-imaged confocal scans of whole mount immunofluorescence of E15.0 back skin. A and P denote anterior and posterior orientation of embryonic skin (head is left). Left bottom: Orthogonal slice from a 3D reconstruction of whole mount immunofluorescence for EDAR and SOX2. Middle: Whole mount immunofluorescence for EDAR, SOX2, and ITGA6 in the epithelial plane (top, purple frame), and dermal plane (bottom, red frame). DAPI marks all nuclei. Right: Description of autocrine and paracrine signaling, proliferation and migration status, and markers of the relevant epithelial and mesenchymal populations at each stage.
Stage 3 – Placode/Dermal Condensate. Pc in the epithelium, with matrix (Mx) precursors at the basal layer and HFSC precursors in the suprabasal layer, above an aggregating DC in the dermis. Pronounced EDAR expression is detectable in maturing placodes. Pronounced SOX2 expression is present in the maturing DC.
Stage 4 – Germ. Downgrown germ in the epithelium, with matrix (Mx) precursors at the basal layer and HFSC precursors in the suprabasal layers, above an aggregating and polarized DC in the dermis. At this stage, the DC has more cells and is further condensed. Note anterior polarization (towards the head) of the downgrowing germ with the DC at the leading edge. EDAR continues to be highly expressed at the germ stage. SOX2 is highly expressed in the DC.
Figure 4. Stages 5–10 of Hair Follicle Morphogenesis.
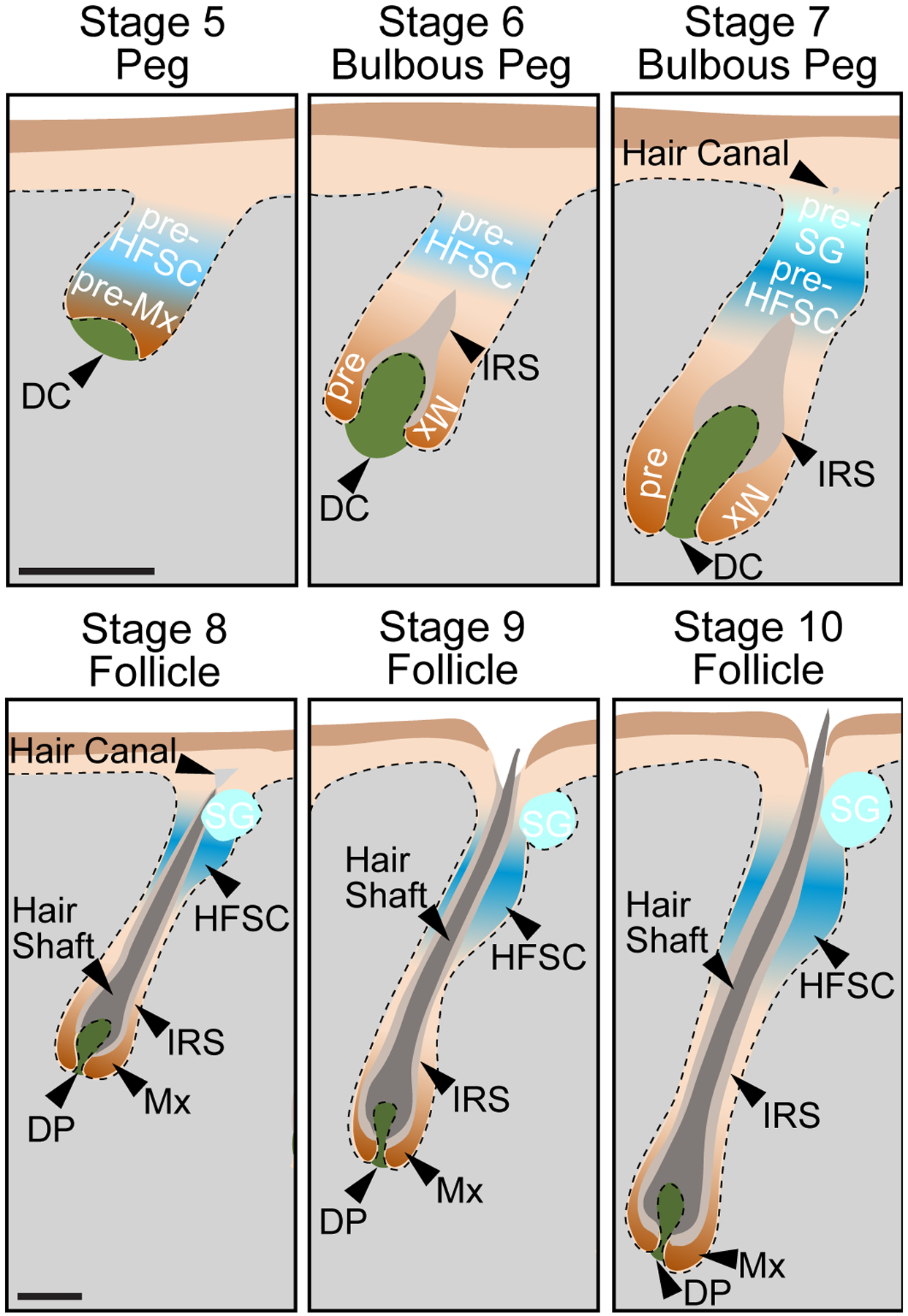
Sagittal view schematics for the late stages of HF morphogenesis. Pseudoscale bars represent the relative scale for stages 5–7 and stages 8–10.
Stage 5 – Peg. Downgrowth of the hair peg, comprised of Mx precursors at the leading edge, and HFSC precursors above them. The DC is beginning the be engulfed.
Stage 6 – Bulbous Peg. At the bulbous peg stage, Mx precursors engulf the DC by > 50% in the bulb region. HFSC precursors remain in the upper ORS. The IRS is beginning to form.
Stage 7 – Bulbous Peg. At this later bulbous peg stage, Mx precursors almost entirely engulf the DC. Bipotent HFSC precursors bifurcate into unipotent HFSC precursors, which reside in the presumptive bulge area, and sebocytes that will make up the sebaceous gland (pre-SG), which reside in the upper part of the follicle. The hair canal is first becoming visible.
Stage 8 – Follicle. At the follicle stage, the Mx now completely engulfs the DP. The IRS reaches the hair canal, and the lineages of the hair shaft are being produced. HFSC reside in the bulge. The sebaceous gland (SG) is formed.
Stage 9 – Follicle. The follicle further elongates into the dermis. The tip of the hair shaft leaves the IRS and enters the hair canal. The DP is even more fully narrowed.
Stage 10 – Follicle. The hair shaft emerges from the epidermis. The follicle has reached its maximum length expanding into the subcutaneous adipose layers.
The DC can now be recognized as an early cluster of aggregating cells. At this stage, the DC contains a greater number of cells (~35+) than pre-DC (Figure 3, stage 3). It begins to become polarized along the anterior-posterior (A-P) axis; this polarization becomes more prominent at stage 4, described below. They are also molecularly distinct from pre-DC: Sox2, Foxd1, Tbx18 expression is further upregulated and Twist2 expression is lost (43) (Figure 3). Earlier molecular studies of aggregated DC at E14.5 showed upregulated expression of genes associated with cell migration, axon guidance, canonical Wnt signaling, and Notch signaling (35), as well as additional molecular signature genes that can be queried at the accompanying web database (http://hair-gel.net) (Figure 3, Figure 5): Cxcr4 (62), Enpp2 (70), Nrp2 (71), Prdm1 (72,73), Sdc1 (74), Prom1 (75), and Trps1 (76). At this stage, DC weakly express AP, which is readily used as a marker for mature DP in postnatal skin (30).
Figure 5. Molecular Markers for Early Hair Follicle Morphogenesis Cell States and Types.

Marker expression for cell types at the key stages of early hair follicle morphogenesis. Genes are color-coded for expression in respective compartments; high (hi) and low (lo) expression is noted where it is known. Grey boxes with question marks refer to unknown expression by in situ hybridization and/or protein staining, at related neighboring stages. Color boxes with question markers (Cxcr4, Dkk4, Wnt10b) in Stage 1 pre-Pc refers to known expression by in situ hybridization or protein staining at E13.5, where the presence of recently discovered pre-DC (stage 2) cannot be ruled out. References for each marker are on the right.
Stage 4 –
By Stage 4, the Pc has elongated into the hair germ at ~E15.0, which has a more pronounced invagination into the dermis (Figure 1, Figure 3, stage 4). During Pc downgrowth, the germ is now prominently polarized as, through PCP-dependent cell rearrangements, the Sox9+ HFSC precursors for the entire pilosebaceous unit migrate toward the posterior side of the developing HF with Pcad, Shh, Lhx2 expressing matrix precursors at the leading anterior edge (77). Bipotent HFSC precursors, for both the future bulge stem cells and sebaceous gland, additionally express Lrig1 (68,78). DC at this stage are positioned on the anterior cap of the developing HF, which is crucial for maintaining appropriate spatial organization of HFSC precursors (77). DC at Stage 4 are further clustered than DC at Stage 3, and are comprised of more aggregating non-proliferative cells (up to 90+ cells). Though, they are also molecularly distinct, although many markers are shared (35,43) (Figure 3, Figure 5). Finally, they express AP more highly, indicating differentiation toward the mature DP (30).
2.2 -. Late Morphogenesis
Late morphogenetic events of Stage 5, beginning at ~E15.5, through Stage 10 closely resemble Stage 3 to Stage 8 in the original classification guide (30) (Figure 1). As such, we will reinforce the previously established staging, but will integrate the previously undescribed Sox9+ HFSC precursors colonizing the bulge region. (Figure 4).
Stage 5 –
This stage is marked by more pronounced downgrowth of the HF, from the hair germ stage to the hair peg stage (Figure 1, Figure 4, stage 5). This is associated with an elongated epithelial cell morphology and concentric orientation around the axis of the future HF. Basal Pc cells continue to express Shh and Pcad, while the entire developing outer root sheath (ORS) expresses K5 (79). The upper portion of the stage 5 HF, where the eventual bulge is formed, co-expresses Sox9 and Lrig1 (44,78). The DC remains on the leading edge of the downgrowing hair peg and assumes a more rounded morphology, preceding its eventual engulfment by the HF matrix and transition into the mature DP.
Stage 6 –
By Stage 6, the hair peg begins to resemble a mature HF more closely. The lower portion of the hair peg, in closest contact with the DC, begins to form a bulb-like shape (Figure 1, Figure 4, stage 6). The HF lineages are also becoming specified; at this stage the inner root sheath (IRS) begins to develop, expressing Blimp1 in the Henle layer (72) and Gata3 in its Huxley and cuticle layers (80,81). Sox9 and Lrig1-expressing HFSC bipotent precursors remain in the upper ORS (78). The DC is in the process of engulfment; by Stage 6 the DC is more than 50% engulfed by the surrounding matrix cells at the base of the hair peg. The morphology of the DC is also becoming more akin to the mature DP; the DC is elongating and is longer than it is wide. Sox10+ melanoblasts begin migrating into the developing HF from the epidermis (50,82) while melanoblasts at the epidermis gradually disappear (49).
Stage 7 –
During Stage 7, the IRS is elongating up through the developing follicle, and the hair canal begins developing (Figure 1, Figure 4, stage 7). The hair shaft begins to form and expresses nuclear Lef1 and has active Wnt signaling in its pre-cortex (83). In pigmented mice, melanin granules are also detectable in the differentiating precortex region, above the DC. Sox9+ HFSC precursors reside in the location of the future bulge of the mature HF in the upper ORS. The sebaceous gland lineage is delineated at this stage; Oil Red O+ Lrig1+ sebocytes are first visible in the upper part of the hair follicle, at the site of the future sebaceous gland (pre-SG) (30,78,84). The DC is almost entirely engulfed by the surrounding matrix.
Stage 8 –
The growing HF has elongated past the boundary of the lower dermis, and the hair canal is morphologically visible (Figure 1, Figure 4, stage 8). The IRS has reached the level of the hair canal and starts to express trichohyalin, detected by AE15 antibodies (85), also expressed in the medulla of the hair shaft (80)(81). The maturing hair shaft can be recognized by AE13-stained hair keratins and marking of the cortex by Foxn1 (86). Other markers including K71 (K6irs1/Krt2–6g) (87), Cutl1 (88), and Hoxc13 (89) are expressed by the IRS at later stages, but it is unclear at which stage they first appear. The companion layer, which expresses K6 (81), can be found between the IRS and the K5+ ORS. Sebocytes form the sebaceous gland (SG), just above the bulge on the posterior side of the HF. The DP is now fully engulfed by matrix cells, and appears morphologically thinner than in Stage 7 DC. Melanoblasts are separated into two populations at two distinct locations: Sox10− precursors to melanocyte stem cells localize at the bulge region and Sox10+ melanocyte precursors localize next to the DP in the hair matrix compartment (49,50,82).
Stage 9 –
By Stage 9 of HF development, the tip of the hair shaft leaves the IRS and enters the hair canal (Figure 1, Figure 4, stage 9). The DP is even more fully narrowed.
Stage 10 –
The HF has reached maximal length by Stage 10; it extends to the subcutaneous level (Figure 1, Figure 4, stage 10). The hair shaft emerges through the epidermis.
3 -. ESSENTIAL SIGNALING PATHWAYS FOR HAIR FOLLICLE MORPHOGENESIS
3.1 -. Initial HF induction
Broad dermal Wnt signaling activity is the upstream initiating event for HF morphogenesis. Subsequent Wnt signaling activation, alternating in epidermal Pc and dermal DC, leads to downstream signaling events to control HF formation. Several additional pathways, such as Eda, Fgf, Bmp and Shh signaling, have been identified over the past two decades to be essential in either compartment and their stage-specific roles will be described in the following.
3.1.1 -. Sequential Wnt activity in dermis and epidermis
The importance of Wnt/β-catenin signaling in HF formation has been well demonstrated over the years (21–23,26,90–92). Mutant mice with eliminated expression of the transcription factor Lef1, a β-catenin binding partner, lack HF as well as other skin appendages such as teeth and mammary gland (93). Conversely, transgenic epidermal overexpression of Lef1 leads to abnormal HF clustering and ectopic HF formation in hairless epithelium (94), while mice expressing stabilized β-catenin in the epidermis exhibited de novo HF morphogenesis (95). Wnt10b expression (60) and Wnt signaling is localized in the nascent stage 1 and 2 pre-Pc (83), and it is essential for Pc induction as epidermal β-catenin ablation (24,55) prevented Pc formation. Likewise, forced broad epidermal misexpression of Wnt inhibitors Dkk1, normally found in the dermal fibroblasts surrounding early HFs, and Dkk2, a surrogate for pre-Pc marker Dkk4, both blocked Pc formation (40,56). In fact, the balance between Wnt signaling activators and inhibitors is thought to limit the number of HFs by setting up a pre-pattern through lateral inhibition that follows the reaction-diffusion model (40,96), famously proposed by Alan Turing nearly 70 years ago (97,98). In this model, short-range activation signals are counteracted by long-range inhibition signals, consistent with a wider diffusion range of smaller Dkks compared to larger, hydrophobic Wnts (96,99,100), thereby limiting the HF induction field. Besides driving Pc initiation, localized epidermal Wnt activity is also required for Pc formation during the transition from pre-Pc (stages 1 and 2) to Pc (stage 3) by coordinating cell migration (36): live imaging and tracking cell divisions during early Pc morphogenesis demonstrated that Wnt and Eda signaling mediate Pc formation through directed migration and cytoskeletal rearrangements, rather than cell proliferation.
Preceding focal Wnt signaling and Pc formation, broad uniform Wnt signaling activity in the upper dermis (24) is essential for pre-Pc induction at stage 0: dermal specific β-catenin ablation blocked localized Pc Wnt signaling and subsequent Pc formation (25). While broad dermal Wnt signaling is an absolute requirement for Pc fate specification, the Wnt target gene(s) that serve as key Pc inductive signal(s), i.e. the first dermal signal(s) is/are still unknown. Dermal Wnt activity itself requires production of epidermal Wnt ligands, as blocking Wnt ligand secretion in Wntless (Wls) mutants results in a failure of dermal Wnt signaling (25). Epidermal Wnt ligand expression is controlled by the transcription factor ΔNp63 (48), a key regulator of epidermal fate specification (101,102).
Finally, after HF induction and following localized Wnt signaling in the pre-Pc and Pc, intensified Wnt activity was also found in the early clustered DC of stage 3 HFs, which is required for HF progression (24,46). Also at stage 3, localized high Wnt signaling in basal Pc progenitors together with active Shh signaling (45) and a suprabasal Wntlow signaling environment (67) are essential for suprabasal Sox9+ HFSC fate acquisition before HF downgrowth in the following stages. For this process, asymmetric cell division in the basal layer of stage 3 Pcs, perpendicular to the basal-suprabasal plane, is required for the emergence of suprabasal SOX9+ HFSC precursors (45).
3.1.2 -. Eda signaling
Upon binding of TNF family member ligand Ectodysplasin (Eda) to its receptor Edar, downstream NFκB activation triggers transcriptional regulation essential for placode development (103,104). Mutations in Eda (tabby) and Edar (downless) in both humans and mouse models fail to form skin appendages such as HFs and teeth (105,106). During HF initiation, Eda is uniformly expressed throughout the epidermis, while Edar expression is confined to the stage 1 and 2 pre-Pc and later to the stage 3 Pc (39). Eda signaling is downstream of Wnt signaling; abolishing Wnt activity in the epidermis eliminates Edar expression and NFκB activation, while Wnt activity persists even after genetic abrogation of Eda signaling (24). Although Edar is one the earliest markers for pre-Pc, Eda/Edar signaling appears to be dispensable for pre-Pc induction of first wave HF. In the absence of Eda pre-Pcs remain stuck at the stage 1–2 stage, and is therefore required for further development to stage 3 Pc (24). Second and third wave HFs did form, but third wave HFs lost the characteristic zigzag shape, suggesting that Eda signaling also plays a role in establishing the molecular mechanism for hair shaft bending (107). In addition, this also indicates that molecular controls of HF morphogenesis can have intrinsic differences between first, second and third wave HFs.
3.1.3 -. Fgf20 signaling
After pre-Pc fate initiation at stage 1, a “first epithelial signal” leads to specification and formation of the underlying DC. So far, Fgf20 has been the only identified epithelial signal that is directly required for DC formation; upon gene ablation of this Pc-derived factor, formation of aggregated DC and subsequent HF morphogenesis was abolished in all first and most second wave HFs (27). The same group very recently demonstrated that formation of the clustered DC is achieved through Fgf20-dependent cell migration and aggregation, and not proliferation (37). The intercellular machinery driving migration to form the condensed DC remains unknown, but recent profiling of DC suggested that actin remodeling and, intriguingly, axonal guidance genes may play a role (35). Preceding its role in DC cluster formation (stage 3), very recent work demonstrated that Fgf20 is already required for DC fate specification from fibroblasts before aggregation takes place (43). DC precursors, or pre-DC, are unclustered cells at stage 2 underneath pre-Pcs that transition from a fibroblast fate to acquire the DC gene expression program. In the absence of Fgf20, pre-DC fail to become specified.
Besides failure of DC specification and formation, Pc morphology was also severely altered in Fgf20 mutants, with Pcs forming stripe-like pattern instead of rounded shapes (27). Pc expansion beyond normal size may be due to impaired lateral inhibition from the KO pre-Pcs and the absent DCs that normally produce Dkk4 and Bmp2, and Bmp4, respectively. While Fgf20 is a downstream target of Wnt and Eda signaling, Edar expression levels were also severely reduced in Fgf20 knockout Pcs (27). Both cases raise the question of whether Fgf20 acts cell-autonomously on the pre-Pc, or whether perturbed placode development could be a secondary consequence of altered signaling inputs from the pre-DC (stage 2) or DC (stage 3). Taken together, as the first known epithelial signal from the pre-Pc (stage 1) towards the dermis, Fgf20 is responsible for promoting the transition of fibroblasts to the DC fate (43). Then Fgf20 instructs pre-DC cells to migrate and aggregate to form the clustered DC (37).
3.1.4 -. Bmp signaling
While Wnt, Eda and Fgf signaling promote HF morphogenesis, BMP signaling acts in an inhibitory fashion, likely to fine-tune and reinforce the lateral inhibition already set up by Wnt/Dkk diffusion gradients for proper spacing of HFs in the reaction-diffusion model (97). During HF morphogenesis, the ligands Bmp2 and Bmp4 are enriched in Pc and DC (25), respectively, and thought to inhibit a HF fate in neighboring epidermis where the receptor Bmpr1a is expressed (65). Conversely, the BMP inhibitor Noggin is enriched in the DC and thought to activate and promote Pc formation as short-range Bmp inhibitory signal: Overexpression of Noggin results in formation of excessive Pcs (108), while secondary HF fail to form in Noggin null mice (109,110).
3.2 -. Hair follicle downgrowth
After initial HF induction, Pc and DC are formed after sequential first dermal and epithelial signals, respectively. The DC then produces the secondary dermal signal, which triggers proliferation of Pc progenitors for HF downgrowth. Continued signal crosstalk between the epidermal and dermal compartments is thought to be crucial for HF formation. To date, none of the second dermal signals have been identified yet, but epithelial Shh, Pdgfa, and Tgfβ signals have been shown to act on the dermal compartment during subsequent signal interplay for HF downgrowth (111–114). Shh signaling was also shown to be key for regulating specification of Sox9+ suprabasal future bulge SCs (45), as well as for maintaining the proper cellular movement of developing Pc cells (77).
3.2.1 -. Shh signaling
Shh is expressed in the pre-Pc and Pc at all stages, while the receptor Patched (Ptch) can be found in both epidermal and dermal compartments (113). Shh null mice display an arrest of HF development at the stage 4 germ, despite normal induction of both Pc and DC (111,112). Dermal specific ablation of Shh pathway component Smo abolished dermal Shh signaling and was shown to be important for Noggin expression in the DC and for maintaining the DC (115). Conversely, Pcs in Noggin null skins failed to express Shh mRNA and protein (110), suggesting that Shh signaling in DCs and Noggin-mediated BMP signaling inhibition in Pcs establish a positive feedback loop in developing HFs.
In addition to its role in maintaining the DC, two recent studies have shown that Shh is also indispensable for the development of the HF epithelial fate and formation. Ouspenskaia and colleagues demonstrated that Shh signaling is essential for the expansion of suprabasal Sox9+ HFSC precursors (45). In Shh-null mice, there was a significant decrease in both the number of Sox9+ suprabasal cells and levels of Sox9 expression. Moreover, high Wnt activity in basal Pc progenitors, the precursors of future matrix cells, is required for SHH expression, which then triggers symmetric divisions of overlying Sox9+ suprabasal cells. Besides establishing HFSC, Shh signaling is also essential for placode invagination and counter-rotational placode cell movements that set up polarization during the stage 3 to stage 4 transition of early HF morphogenesis (Figure 4) (77). Sox9+ suprabasal cells, which first appear around the Shh-expressing basal cells, migrate to the posterior position, while Shh-expressing cells move anteriorly. This cellular rearrangement is also DC dependent, as DC laser ablation resulted in aberrant Sox9 expression in anterior placode cells (77).
3.2.2 -. PDGFA signaling
During HF morphogenesis, the ligand Pdgfa is broadly expressed in the epidermis while the receptor, Pdgfra, is uniformly expressed in the dermis (113). Pdgfa ablation revealed a requirement of Pdgfa signaling for HF downgrowth; despite normal HF induction, the mice had a sparse hair coat due to retarded HF development (113). However, whether this a specific HF development defect has been called into question with a more recent study: Rezza and colleagues reported unperturbed HF induction and development following dermal specific Pdgfra ablation (116). As arrested HF phenotypes were only found in Pdgfa mutants with a severe systemic phenotype (113), and given that Pdgfa signaling is essential for many developmental aspects (117), the retarded HF development could stem from secondary effects of abrograted systemic Pdgfa signaling.
3.2.3 -. TGFβ signaling
Besides Shh signaling, Tgfβ signaling has also been implicated as crucial for HF downgrowth. The ligand TGFβ2 is secreted by the dermis and acts on the receptor expressed by the epithelium (31,118,119). Studies in Tgfβ2 null mice revealed a requirement of Tgfβ signaling for HF downgrowth as HF development was arrested early (114). A second study of Tgfb receptor ablation demonstrated fewer and growth-retarded HFs (120). Interestingly, a recent study placed Tgfβ signaling downstream of Eda signaling in a NF-κB/Lhx2/Tgfβ signaling axis (33). Both mice with suppressed NF-κB activity and knockouts of NF-κB target Lhx2 have impaired TGFβ signalling, demonstrating that Eda signaling is not only important for Pc maintenance during stage 1 and 2 of HF formation, but is also essential for HF downgrowth by activating TGFβ signaling.
In summary, after the first dermal signals kick-start the initiation of HF morphogenesis, activation of Wnt and Eda signaling promotes pre-Pc formation and Pc stabilization. The nascent pre-Pc in turn provides the first epithelial signals, such as Fgf20, for inducing DC fate in unclustered precursors, and then aggregation of the maturing DC. At the same time, inhibitory signals including Dkk4 and Bmp4 from the Pc and DC, respectively, suppress HF formation in the interfollicular area to maintain even spacing between established HF. Second dermal signals from the DC then promote the proliferation of Pc progenitors for HF downgrowth. Continued signal interplay through Shh and Tgfb signaling further promotes HF development towards the formation of the mature HF.
4 –. CONCLUDING REMARKS
The HF morphogenesis staging guide described by Paus and colleagues in 1999 has been widely used for 20 years; it has served as an important standard of HF development for classifying and comparing HF morphogenetic defects across numerous HF morphogenesis studies. In light of the recent discoveries of specific early precursor cell states in both the epithelial and mesenchymal HF compartments, as well as of the myriad novel molecular insights driving the classification of new stages, we propose this update here to the classical staging guide. To account for Pc induction at a “molecular placode” pre-Pc stage, for DC cell fate acquisition and pre-DC specification, and for the emergence of precursors to HFSC, we subdivided the previously defined stage 0 into 2 new stages that are prefaced by a new stage 0 with no specific patterned molecular or cellular events, while at the same time preserving previous established advanced stages that succeed the new precursor stages.
Continued and concerted efforts over the past 20 years to parse out essential signals and controls of HF initiation, in both the epidermal and dermal compartments, have provided many important insights that we summarized here. Nevertheless, many details regarding the initiating events of the “first dermal signal” and relevant crosstalk between the Pc and DC in the “first epithelial signal” and “second dermal signal” remain elusive and the full spatiotemporal account of all HF morphogenetic signaling is complete. The near simultaneous activity of many known signaling pathways, in both the epidermis and dermis, and interplay of positive and negative regulation between them further complicates the story. A more granular perspective of early HF morphogenetic events, in combination with integrated in vivo, in vitro, and in silico approaches may aid in deconvoluting the roles of many of these signaling pathways to paint a more complete picture.
Technological advances have permitted the identification of stage-specific marker genes in embryonic epidermis and dermis for identification and isolation of relevant cell types, as well generation of more specific genetic drivers for in vivo study. Critically, single-cell RNA-sequencing has allowed for a more dynamic understanding of developmentally associated transcriptional dynamics, through the use of computational modeling, as well as identification of cellular heterogeneity. Since pseudotemporal ordering of cell fates in the dermis allowed for revealing a putative differentiation trajectory from upper dermal fibroblasts to pre-DC to Stage 3 and 4 DC (42,43), it is conceivable that cognate analyses of epidermal differentiation will add in the future increased temporal resolution and, in combination with dermal differentiation analyses, will shed light on critical signaling crosstalk. Through observations of in vivo phenomena in genetic abrogation studies, substantial insights into the staging of early HF morphogenesis have been made, to drive understanding of signaling necessity. And in combination with the modeling ability of in silico approaches, we predict that we will continue to move steadily toward gaining a clearer picture of the identity of crucial dermal and epithelial signals.
ACKNOWLEDGEMENTS
We thank all lab members for helpful comments on the manuscript and valuable discussions. N.S. was supported by NIH/NIAMS grant R01AR071047 and by a fellowship of the Training Program in Stem Cell Research from the New York State Department of Health (NYSTEM-C32561GG). K.W.M. was supported by The Science Appearance Career Development Award fellowship from the Dermatology Foundation. M.R. was supported by grants from NIH/NIAMS (R01AR071047; R01AR063151) and New York State Department of Health (NYSTEM-C029574: NYSTEM-C32561GG), and a fellowship from the Irma T. Hirschl Trust. We apologize to all colleagues whose relevant work we could not discuss due to space limitations.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicting interests.
REFERENCES
- 1.Sperling LC Hair anatomy for the clinician. J Am Acad Dermatol 1991: 25: 1–17. [DOI] [PubMed] [Google Scholar]
- 2.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990: 61: 1329–37. [DOI] [PubMed] [Google Scholar]
- 3.Oshima H, Rochat A, Kedzia C et al. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 2001: 104: 233–45. [DOI] [PubMed] [Google Scholar]
- 4.Taylor G, Lehrer MS, Jensen PJ et al. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000: 102: 451–61. [DOI] [PubMed] [Google Scholar]
- 5.Tumbar T, Guasch G, Greco V et al. Defining the epithelial stem cell niche in skin. Science 2004: 303: 359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris RJ, Liu Y, Marles L et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 2004: 22: 411–7. [DOI] [PubMed] [Google Scholar]
- 7.Blanpain C, Lowry WE, Geoghegan A et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004: 118: 635–48. [DOI] [PubMed] [Google Scholar]
- 8.Clavel C, Grisanti L, Zemla R et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev Cell 2012: 23: 981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan BA The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med 2014: 4: a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H, Adam RC, Ge Y et al. Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell 2017: 169: 483–496.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legué E, Nicolas J-F. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 2005: 132: 4143–54. [DOI] [PubMed] [Google Scholar]
- 12.Sequeira I, Nicolas J-F. Redefining the structure of the hair follicle by 3D clonal analysis. Development 2012: 139: 3741–51. [DOI] [PubMed] [Google Scholar]
- 13.Montagna W An introduction to sebaceous glands. J Invest Dermatol 1974: 62: 120–3. [DOI] [PubMed] [Google Scholar]
- 14.Thody a J, Shuster S. Control and function of sebaceous glands. Physiol Rev 1989: 69: 383–416. [DOI] [PubMed] [Google Scholar]
- 15.Deplewski D, Rosenfield RL. Role of hormones in pilosebaceous unit development. Endocr Rev 2000: 21: 363–92. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Wortsman J, Plonka PM et al. Hair follicle pigmentation. J Invest Dermatol 2005: 124: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy MH The secret life of the hair follicle. Trends Genet 1992: 8: 55–61. [DOI] [PubMed] [Google Scholar]
- 18.Kollar EJ The induction of hair follicles by embryonic dermal papillae. J Invest Dermatol 1970: 55: 374–8. [DOI] [PubMed] [Google Scholar]
- 19.Dhouailly D Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. J Embryol Exp Morphol 1973: 30: 587–603. [PubMed] [Google Scholar]
- 20.Dhouailly D Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds and mammals. Wilhelm Roux’s Arch Dev Biol 1975: 177: 323–340. [DOI] [PubMed] [Google Scholar]
- 21.Biggs LC, Mikkola ML. Early inductive events in ectodermal appendage morphogenesis. Semin Cell Dev Biol 2014: 25–26: 11–21. [DOI] [PubMed] [Google Scholar]
- 22.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol 2012: 23: 917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol 2009: 19: R132–42. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Tomann P, Andl T et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell 2009: 17: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D, Jarrell A, Guo C et al. Dermal -catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 2012: 139: 1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millar SE Molecular mechanisms regulating hair follicle development. J Invest Dermatol 2002: 118: 216–225. [DOI] [PubMed] [Google Scholar]
- 27.Huh S-HH, Närhi K, Lindfors PH et al. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Genes Dev 2013: 27: 450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maderson PFA Morphogenesis of Skin. Q Rev Biol 1977: 52: 417–417. [Google Scholar]
- 29.Dhouailly D, Olivera-Martinez I, Fliniaux I et al. Skin field formation: morphogenetic events. Int J Dev Biol 2004: 48: 85–91. [PubMed] [Google Scholar]
- 30.Paus R, Müller-Röver S, Van Der Veen C et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol 1999: 113: 523–32. [DOI] [PubMed] [Google Scholar]
- 31.Paus R, Foitzik K, Welker P et al. Transforming growth factor-β receptor type I and type II expression during murine hair follicle development and cycling. J Invest Dermatol 1997: 109: 518–526. [DOI] [PubMed] [Google Scholar]
- 32.Handjiski BK, Eichmüller S, Hofmann U et al. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol 1994: 131: 303–10. [DOI] [PubMed] [Google Scholar]
- 33.Tomann P, Paus R, Millar SE et al. Lhx2 is a direct NF-κB target gene that promotes primary hair follicle placode down-growth. Development 2016: 143: 1512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science 2006: 312: 1946–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sennett R, Wang Z, Rezza A et al. An Integrated Transcriptome Atlas of Embryonic Hair Follicle Progenitors, Their Niche, and the Developing Skin. Dev Cell 2015: 34: 577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahtiainen L, Lefebvre S, Lindfors PH et al. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev Cell 2014: 28: 588–602. [DOI] [PubMed] [Google Scholar]
- 37.Biggs LC, Mäkelä OJM, Myllymäki S-M et al. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. Elife 2018: 7: 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glover JD, Wells KL, Matthäus F et al. Hierarchical patterning modes orchestrate hair follicle morphogenesis. PLoS Biol 2017: 15: e2002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurikkala J, Pispa J, Jung H-S et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development 2002: 129: 2541–53. [DOI] [PubMed] [Google Scholar]
- 40.Sick S, Reinker S, Timmer J et al. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 2006: 314: 1447–50. [DOI] [PubMed] [Google Scholar]
- 41.Mailleux AA, Spencer-Dene B, Dillon C et al. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 2002: 129: 53–60. [DOI] [PubMed] [Google Scholar]
- 42.Gupta K, Levinsohn J, Linderman G et al. Single-Cell Analysis Reveals a Hair Follicle Dermal Niche Molecular Differentiation Trajectory that Begins Prior to Morphogenesis. Dev Cell 2019: 48: 17–31.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mok K-W, Saxena N, Heitman N et al. Dermal Condensate Niche Fate Specification Occurs Prior to Formation and Is Placode Progenitor Dependent. Dev Cell 2019: 48: 32–48.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowak JA, Polak L, Pasolli HA et al. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 2008: 3: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouspenskaia T, Matos I, Mertz AF et al. WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation Cell 2016: 164: 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai S-Y, Sennett R, Rezza A et al. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev Biol 2014: 385: 179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlake T Determination of hair structure and shape. Semin Cell Dev Biol 2007: 18: 267–73. [DOI] [PubMed] [Google Scholar]
- 48.Fan X, Wang D, Burgmaier JE et al. Single Cell and Open Chromatin Analysis Reveals Molecular Origin of Epidermal Cells of the Skin. Dev Cell 2018: 47: 21–37.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu W, Chuong C, Lei M. Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials Exp Dermatol 2018: : exd.13856. [DOI] [PMC free article] [PubMed]
- 50.Osawa M, Egawa G, Mak S-S et al. Molecular characterization of melanocyte stem cells in their niche. Development 2005: 132: 5589–99. [DOI] [PubMed] [Google Scholar]
- 51.Steingrímsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet 2004: 38: 365–411. [DOI] [PubMed] [Google Scholar]
- 52.Goding CR Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev 2000: 14: 1712–28. [PubMed] [Google Scholar]
- 53.Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development 1992: 115: 1111–9. [DOI] [PubMed] [Google Scholar]
- 54.Jordan SA, Jackson IJ. Melanocortin receptors and antagonists regulate pigmentation and body weight. Bioessays 1998: 20: 603–6. D [DOI] [PubMed] [Google Scholar]
- 55.Huelsken J, Vogel R, Erdmann B et al. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001: 105: 533–545. [DOI] [PubMed] [Google Scholar]
- 56.Andl T, Reddy ST, Gaddapara T et al. WNT signals are required for the initiation of hair follicle development. Dev Cell 2002: 2: 643–53. [DOI] [PubMed] [Google Scholar]
- 57.Barsh G Of ancient tales and hairless tails. Nat Genet 1999: 22: 315–6. [DOI] [PubMed] [Google Scholar]
- 58.Headon DJ, Overbeek PA. Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet 1999: 22: 370–4. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt-Ullrich R NF- B transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development 2006: 133: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 60.Reddy S, Andl T, Bagasra A et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev 2001: 107: 69–82. [DOI] [PubMed] [Google Scholar]
- 61.Fliniaux I, Mikkola ML, Lefebvre S et al. Identification of dkk4 as a target of Eda-A1/Edar pathway reveals an unexpected role of ectodysplasin as inhibitor of Wnt signalling in ectodermal placodes. Dev Biol 2008: 320: 60–71. [DOI] [PubMed] [Google Scholar]
- 62.Sennett R, Rezza AA, Dauber KL et al. Cxcr4 is transiently expressed in both epithelial and mesenchymal compartments of nascent hair follicles but is not required for follicle formation. Exp Dermatol 2014: 23: 748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Driskell RR, Giangreco A, Jensen KB et al. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 2009: 136: 2815–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grisanti L, Clavel C, Cai X et al. Tbx18 targets dermal condensates for labeling, isolation, and gene ablation during embryonic hair follicle formation. J Invest Dermatol 2013: 133: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 1995: 172: 126–38. [DOI] [PubMed] [Google Scholar]
- 66.Bazzi H, Fantauzzo KA, Richardson GD et al. Transcriptional profiling of developing mouse epidermis reveals novel patterns of coordinated gene expression. Dev Dyn 2007: 236: 961–970. [DOI] [PubMed] [Google Scholar]
- 67.Xu Z, Wang W, Jiang K et al. Embryonic attenuated Wnt/β-catenin signaling defines niche location and long- term stem cell fate in hair follicle. Elife 2015: 4: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol 2012: 23: 928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen MB, Cohen I, Kumar V et al. FGF signalling controls the specification of hair placode-derived SOX9 positive progenitors to Merkel cells. Nat Commun 2018: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grisanti L, Rezza A, Clavel C et al. Enpp2/autotaxin in dermal papilla precursors is dispensable for hair follicle morphogenesis. J Invest Dermatol 2013: 133: 2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hillman RT, Feng BY, Ni J et al. Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev 2011: 25: 2333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horsley V, O’Carroll D, Tooze R et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 2006: 126: 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robertson EJ, Charatsi I, Joyner CJ et al. Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development 2007: 134: 4335–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson GD, Fantauzzo KA, Bazzi H et al. Dynamic expression of Syndecan-1 during hair follicle morphogenesis. Gene Expr Patterns 2009: 9: 454–460. [DOI] [PubMed] [Google Scholar]
- 75.Ito Y, Hamazaki TS, Ohnuma K et al. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol 2007: 127: 1052–60. [DOI] [PubMed] [Google Scholar]
- 76.Fantauzzo KA, Tadin-Strapps M, You Y et al. A position effect on TRPS1 is associated with Ambras syndrome in humans and the Koala phenotype in mice. Hum Mol Genet 2008: 17: 3539–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cetera M, Leybova L, Joyce B et al. Counter-rotational cell flows drive morphological and cell fate asymmetries in mammalian hair follicles. Nat Cell Biol 2018: 20: 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frances D, Niemann C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev Biol 2012: 363: 138–146. [DOI] [PubMed] [Google Scholar]
- 79.Moll R, Dhouailly D, Sun TT. Expression of keratin 5 as a distinctive feature of epithelial and biphasic mesotheliomas - An immunohistochemical study using monoclonal antibody AE14. Virchows Arch B Cell Pathol Incl Mol Pathol 1989: 58: 129–145. [DOI] [PubMed] [Google Scholar]
- 80.Kobielak K, Pasolli HA, Alonso L et al. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol 2003: 163: 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaufman CK, Zhou P, Pasolli HA et al. GATA-3: An unexpected regulator of cell lineage determination in skin. Genes Dev 2003: 17: 2108–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harris ML, Buac K, Shakhova O et al. A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors. PLoS Genet 2013: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999: 126: 4557–68. [DOI] [PubMed] [Google Scholar]
- 84.Jensen KB, Collins CA, Nascimento E et al. Lrig1 Expression Defines a Distinct Multipotent Stem Cell Population in Mammalian Epidermis. Cell Stem Cell 2009: 4: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lynch MH, O’Guin WM, Hardy C et al. Acidic and basic hair/nail (“hard”) keratins: Their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “Soft” keratins. J Cell Biol 1986: 103: 2593–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee D, Prowse DM, Brissette JL. Association between mouse nude gene expression and the initiation of epithelial terminal differentiation. Dev Biol 1999: 208: 362–374. [DOI] [PubMed] [Google Scholar]
- 87.Kikkawa Y, Oyama A, Ishii R et al. A Small Deletion Hotspot in the Type II Keratin Gene mK6irs1 / Krt2–6g of the Caracul (Ca) Mutation. Genetics 2003: 165: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellis T, Gambardella L, Horcher M et al. The transcriptional repressor CDP (Cutl1) is essential for epithelial cell differentiation of the lung and the hair follicle. Genes Dev 2001: 15: 2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tkatchenko AV, Visconti RP, Shang L et al. Overexpression of Hoxc13 in differentiating keratinocytes results in downregulation of a novel hair keratin gene cluster and alopecia. Development 2001: 128: 1547–58. [DOI] [PubMed] [Google Scholar]
- 90.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev 2003: 17: 1189–200. [DOI] [PubMed] [Google Scholar]
- 91.Lien W-H, Fuchs E. Wnt some lose some: transcriptional governance of stem cells by Wnt/ -catenin signaling. Genes Dev 2014: 28: 1517–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuchs E Scratching the surface of skin development. Nature 2007: 445: 834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Genderen C van, Okamura RM, Fariñas I et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 1994: 8: 2691–703. [DOI] [PubMed] [Google Scholar]
- 94.Zhou P, Byrne C, Jacobs J et al. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 1995: 9: 700–13. [DOI] [PubMed] [Google Scholar]
- 95.Gat U, DasGupta R, Degenstein L et al. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998: 95: 605–14. [DOI] [PubMed] [Google Scholar]
- 96.Maini PK, Baker RE, Chuong CM. The turing model comes of molecular age. Science (80- ) 2006: 314: 1397–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li A, Lai YC, Figueroa S et al. Deciphering principles of morphogenesis from temporal and spatial patterns on the integument. Dev Dyn 2015: 244: 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turing AM The Chemical Basis of Morphgenesis. Philos Trans R Soc London Ser B, Biol Sci 1952: 237: 37–72. [Google Scholar]
- 99.Farin HF, Jordens I, Mosa MH et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 2016: 530: 340–343. [DOI] [PubMed] [Google Scholar]
- 100.Mills KM, Szczerkowski JLA, Habib SJ. Wnt ligand presentation and reception: From the stem cell niche to tissue engineering. Open Biol 2017: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mills AA, Zheng B, Wang XJ et al. P63 Is a P53 Homologue Required for Limb and Epidermal Morphogenesis. Nature 1999: 398: 708–713. [DOI] [PubMed] [Google Scholar]
- 102.Yang A, Schweitzer R, Sun D et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999: 398: 714–8. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt-Ullrich R, Aebischer T, Hülsken J et al. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development 2001: 128: 3843–53. [DOI] [PubMed] [Google Scholar]
- 104.Naito A, Yoshida H, Nishioka E et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci 2002: 99: 8766–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferguson BM, Brockdorff N, Formstone E et al. Cloning of Tabby, the murine homolog of the human EDA gene: evidence for a membrane-associated protein with a short collagenous domain. Hum Mol Genet 1997: 6: 1589–94. [DOI] [PubMed] [Google Scholar]
- 106.Srivastava AK, Pispa J, Hartung AJ et al. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci 1997: 94: 13069–13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duverger O, Morasso MI. Epidermal patterning and induction of different hair types during mouse embryonic development. Birth Defects Res Part C - Embryo Today Rev 2009: 87: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plikus M, Wang WP, Liu J et al. Morpho-Regulation of Ectodermal Organs. Am J Pathol 2004: 164: 1099–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Botchkarev VA, Botchkareva NV., Roth W et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol 1999: 1: 158–64. [DOI] [PubMed] [Google Scholar]
- 110.Botchkarev VA, Botchkareva NV., Sharov AA et al. Modulation of BMP Signaling by Noggin is Required for Induction of the Secondary (Nontylotrich) Hair Follicles. J Invest Dermatol 2002: 118: 3–10. [DOI] [PubMed] [Google Scholar]
- 111.St-Jacques B, Dassule HR, Karavanova I et al. Sonic hedgehog signaling is essential for hair development. Curr Biol 1998: 8: 1058–1069. [DOI] [PubMed] [Google Scholar]
- 112.Chiang C, Swan RZ, Grachtchouk M et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol 1999: 205: 1–9. [DOI] [PubMed] [Google Scholar]
- 113.Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development 1999: 126: 2611–21. [DOI] [PubMed] [Google Scholar]
- 114.Foitzik K, Paus R, Doetschman T et al. The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol 1999: 212: 278–89. [DOI] [PubMed] [Google Scholar]
- 115.Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev 2012: 26: 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rezza A, Sennett R, Tanguy M et al. PDGF signalling in the dermis and in dermal condensates is dispensable for hair follicle induction and formation. Exp Dermatol 2015: 24: 468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Betsholtz C Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev 2004: 15: 215–228. [DOI] [PubMed] [Google Scholar]
- 118.Heine U Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol 1987: 105: 2861–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pelton RW Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol 1991: 115: 1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qiu W, Li X, Tang H et al. Conditional Activin Receptor Type 1B (Acvr1b) Knockout Mice Reveal Hair Loss Abnormality. J Invest Dermatol 2011: 131: 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]


