Abstract
Introduction
Bilateral paralysis of the diaphragm may be an idiopathic clinical condition or associated with several diseases such as trauma, surgery, viral infections, neurologic disorders. The diaphragm is the main respiratory muscle. It is a cupoliform muscle-tendon structure, innervated bilaterally by phrenic nerve, which originates from C3-C5 nerve roots. Diaphragmatic paralysis is a clinical disorder that generates hypoventilation and basal pulmonary atelectasis, predisposing to hypercapnic respiratory failure. The clinic manifestations mimic cardio-respiratory pathologies, therefore often misdiagnosticated.
Case presentation
A 55-year-old man with a previous C6-7 traumatic fracture, referred multiple accesses to the emergency room for acute nocturnal dyspnoea, treated with antibiotic therapy, diuretic therapy and long-term oxygen therapy, without beneficial effects. He referred to our pulmonary clinic for evaluation of persistent and worsening orthopnoea due to unknown cause for about 2 years. Clinical examination, respiratory functional tests and diaphragm ultrasound revealed a strong suspicion of diaphragmatic deficit, confirmed by electromyography.
Conclusions
The patient accesses to the emergency room numerous times and the clinical frame have been always oriented towards a cardio-respiratory origin. From the onset of the symptom to the respiratory evaluation, about 2.5 years have passed. The manifestation of clear orthopnoea has addressed the functional respiratory study towards a more thorough diaphragmatic evaluation assessed by ultrasound.
Key words: Diaphragm paralysis, dyspnoea, respiratory failure, diaphragmatic ultrasound
Case presentation
A 55-year-old man with no previous smoking history, has a remarkable medical history for hypertension, dyslipidaemia, carotid vasculopathy, diabetes mellitus type II, symmetric axonal diabetic polyneuropathy. He reported a previous C6-7 traumatic fracture, he fell from a tree while he was working, treated with anterior arthrodesis and positioning of intersomatic cage in two phases (June-August 2015); an episode of stroke cerebri (August 2015), a hospitalization for heart failure with bilateral and symmetrical foot oedema (February 2017). Multiple accesses to the emergency room for acute nocturnal dyspnoea, treated with antibiotic therapy, diuretic therapy and long-term oxygen therapy. He referred to our pulmonary clinic for evaluation of persistent and worsening orthopnoea, asphyxiation perception in clinostatic position and daytime sleepiness due to unknown cause since 2016. The examination was unremarkable. Body Mass Index was 38. Respiratory function tests (RFT) in orthostatic position showed: total lung capacity 44.2%; residual volume 127%; vital capacity 37.6%; forced vital capacity 38.6%; forced expiratory volume during the first second 39.3%; Tiffeneau index 81.1%; diffusing lung CO 88%; diffusing lung CO/Va 80%; maximal inspiratory pressure 36 cmH2O; maximal expiratory pressure 70 cm H2O; sniff nasal inspiratory pressure 21. RFT in clinostatic position showed a 20% reduction in vital capacity. A blood gas analysis was performed in room air: pH=7.42 pO2=63 pCO2=54 HCO3-=40.9 SaO2=88.2% Lac=0.8: respiratory failure type II. Echocardiogram is within limits with EF=60%. The posteroanterior (PA) chest X-ray showed opacification of cost-phrenic sinuses (Figure 1).
Moreover, a cardiorespiratory monitoring was performed in room air and showed an apnea-hypopnea index of 23.9. Diaphragmatic ultrasonography was performed with a convex probe in a semi-sitting position, showing a diaphragmatic excursion of 1 cm and with a linear probe, showing a thickening fraction of 27% (Figure 2).
The functional tests and the ultrasonographic findings raised a strong suspicion of diaphragmatic dysfunction, confirmed by an electromyography examination of the phrenic nerve with the complete absence of motor unit action potentials (MUAPs). A longterm non-invasive ventilation was prescribed, with an evident clinical improvement.
Discussion and Conclusions
Bilateral diaphragmatic paralysis is a condition that occurs when traumatic injury, systemic disease, or neurologic process results in the loss of control of the hemidiaphragms [3]. The diaphragm is the primary muscle of respiration, so the major clinical manifestations of this condition are severe orthopnoea and/or respiratory failure. Lung volumes and maximal static respiratory pressure are non-invasive parameters to assess global respiratory muscle functions and to detect dysfunctions [2,4]. Although the gold standard diagnostic tool is electromyography, there are a lot of diagnostic modalities to evaluate accurately diaphragmatic dysfunction such as plain chest radiographs, fluoroscopy and ultrasound [5]. Actually, the long-term non-invasive ventilation is the only therapeutic method to reverse the hypercapnic respiratory failure, typical in patients affected by bilateral diaphragmatic paralysis [6]. The diaphragm paralysis can be easily misdiagnosed and confused with clinical conditions of cardio-respiratory origin, above all for the unclear origin of dyspnoea as emerging symptom. Our patient had a bilateral traumatic lesion of phrenic nerve roots occurred during work. The patients manifested severe orthopnoea, multiple nocturnal accesses to the emergency room due to ventilator failure during sleep, associated with heart failure, symmetrical foot oedema and also an episode of ictus cerebri. The functional tests showed a moderate restrictive pattern. They were reassessed in the supine position, highlighting a significant vital capacity reduction between the two positions (ΔVC 20%). The reduction of diaphragmatic excursion, found as an ultrasonographic finding, raised a suspicion of diaphragmatic dysfunction. All these signs explained the inspiratory functional deficit, exacerbated in the supine position with hypercapnic respiratory failure and nocturnal intermittent desaturation episodes. Then the diagnosis of certainty was given by electromyography. Long-term intermittent non-invasive ventilation is effective in reversing ventilatory failure, improving respiratory muscle function and gas exchanges.
In conclusion, the patient accesses to the emergency room numerous times and the frame have always been oriented towards a cardio-respiratory origin. From the onset of the symptom to the respiratory evaluation, about 2.5 years have passed. The manifestation of clear orthopnoea lead to a deeper examination of the diaphragm. The functional respiratory study in the two positions and an accurate assessment of the diaphragm’s dynamic function through the use of the ultrasound, essential, simple and common tools, were decisive for the correct clinical setting.
Figure 1.
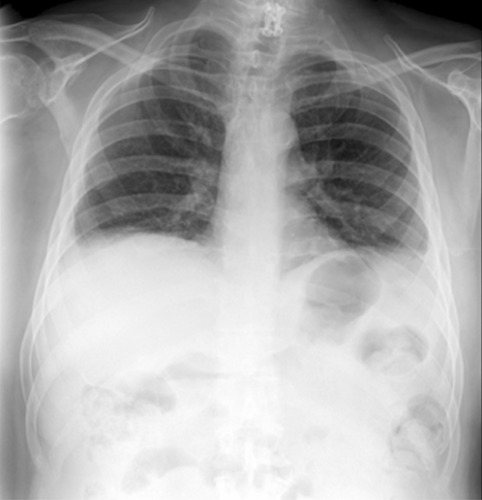
Chest X-ray: opacification of cost-phrenic sinuses in the posteroanterior (PA) projection.
Figure 2.
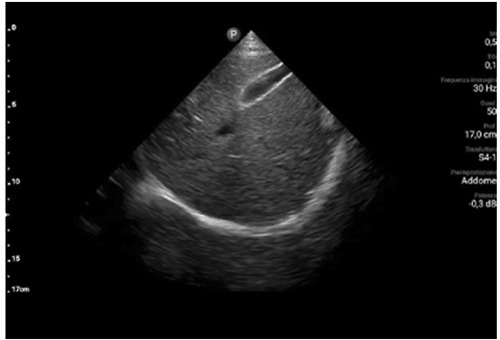
Diaphragmatic excursion in subcostal approach performed by ultrasound technic.
Acknowledgements
The authors would like to thank the patient for giving his consent and making his own data useful for the literature.
List of abbreviations
- RFT
Respiratory function tests
- PA
postero-anterior
- MUAPs
Motor unit action potentials
Funding Statement
Funding: There is no funding source.
References
- 1.Podnar S. Idiopathic phrenic neuropathies: A case series and review of the literature. Muscle Nerve 2015;52:986-92. [DOI] [PubMed] [Google Scholar]
- 2.Fromageot C, Lofaso F, Annane D, Falaize L, Lejaille M, Clair B, et al. Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 2001;82:123-8. [DOI] [PubMed] [Google Scholar]
- 3.Ko MA, Darling GE. Acquired paralysis of the diaphragm. Thorac Surg Clin 2009;19:501-10. [DOI] [PubMed] [Google Scholar]
- 4.Caruso P, Albuquerque ALP de, Santana PV, Cardenas LZ, Ferreira JG, Prina E, et al. Diagnostic methods to assess inspiratory and expiratory muscle strength. J Bras Pneumol 2015;41:110-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokatnur L, Rudrappa M. Diaphragmatic palsy. Diseases 2018;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, Toubes ME, Riveiro V, Valdés L. Diaphragmatic dysfunction. Pulmonology 2019;25:223-35. [DOI] [PubMed] [Google Scholar]


