Abstract
Background
Laser‐assisted in‐situ keratomileusis (LASIK) is a surgical procedure that corrects refractive errors. This technique creates a flap of the outermost parts of the cornea (epithelium, bowman layer, and anterior stroma) to expose the middle part of the cornea (stromal bed) and reshape it with excimer laser using photoablation. The flaps can be created by a mechanical microkeratome or a femtosecond laser.
Objectives
To compare the effectiveness and safety of mechanical microkeratome versus femtosecond laser in LASIK for adults with myopia.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2019, Issue 2); Ovid MEDLINE; Embase; PubMed; LILACS; ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We used no date or language restrictions. We searched the reference lists of included trials. We searched the electronic databases on 22 February 2019.
Selection criteria
We included randomized controlled trials (RCTs) of LASIK with a mechanical microkeratome compared to a femtosecond laser in people aged 18 years or older with more than 0.5 diopters of myopia or myopic astigmatism.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 16 records from 11 trials enrolling 943 adults (1691 eyes) with spherical or spherocylindrical myopia, who were suitable candidates for LASIK. Five hundred and forty‐seven participants (824 eyes) received LASIK with a mechanical microkeratome and 588 participants (867 eyes) with a femtosecond laser. Each trial included between nine and 360 participants. In six trials, the same participants received both interventions. Overall, the trials were at an uncertain risk of bias for most domains.
At 12 months, data from one trial (42 eyes) indicates no difference in the mean uncorrected visual acuity (logMAR scale) between LASIK with a mechanical microkeratome and LASIK with a femtosecond laser (mean difference (MD) –0.01, 95% confidence interval (CI) –0.06 to 0.04; low‐certainty evidence). Similar findings were observed at 12 months after surgery, regarding participants achieving 0.5 diopters within target refraction (risk ratio (RR) 0.97, 95% CI 0.85 to 1.11; 1 trial, 79 eyes; low‐certainty evidence) as well as mean spherical equivalent of the refractive error 12 months after surgery (MD 0.09, 95% CI –0.01 to 0.19; 3 trials, 168 eyes [92 participants]; low‐certainty evidence).
Based on data from three trials (134 eyes, 67 participants), mechanical microkeratome was associated with lower risk of diffuse lamellar keratitis compared with femtosecond laser (RR 0.27, 95% CI 0.10 to 0.78; low‐certainty evidence). Thus, diffuse lamellar keratitis was a more common adverse event with femtosecond laser than with mechanical microkeratome, decreasing from an assumed rate of 209 per 1000 people in the femtosecond laser group to 56 per 1000 people in the mechanical microkeratome group. Data from one trial (183 eyes, 183 participants) indicates that dry eye as an adverse event may be more common with mechanical microkeratome than with femtosecond laser, increasing from an assumed rate of 80 per 1000 people in the femtosecond laser group to 457 per 1000 people in the mechanical microkeratome group (RR 5.74, 95% CI 2.92 to 11.29; low‐certainty evidence). There was no evidence of a difference between the two groups for corneal haze (RR 0.33, 95% CI 0.01 to 7.96; 1 trial, 86 eyes) and epithelial ingrowth (RR 1.04, 95% CI 0.11 to 9.42; 2 trials, 102 eyes [51 participants]). The certainty of evidence for both outcomes was very low.
Authors' conclusions
Regarding the visual acuity outcomes, there may be no difference between LASIK with mechanical microkeratome and LASIK with femtosecond laser. Dry eye and diffuse lamellar keratitis are likely adverse events with mechanical microkeratome and femtosecond laser, respectively. The evidence is uncertain regarding corneal haze and epithelial ingrowth as adverse events of each intervention. The limited number of outcomes reported in the included trials, some with potentially significant risk of bias, makes it difficult to draw a firm conclusion regarding the effectiveness and safety of the interventions investigated in this review.
Plain language summary
Effectiveness and safety of two types of tools used in LASIK (a type of refractive surgery) for nearsightedness
What was the aim of this review? The aim of this Cochrane Review was to assess whether two types of tools for surgical correction of vision (laser‐assisted in‐situ keratomileusis [LASIK]) are effective and safe in people with nearsighted vision. One tool uses a high‐precision blade (mechanical microkeratome) and the other tool uses infrared waves (femtosecond laser).
What was studied in this review? Nearsightedness is a medical condition in which people can see objects near to them clearly but objects farther away are blurry. As of 2010, nearsightedness affected approximately two billion people worldwide. Nearsightedness can be treated by using either glasses, contact lenses, or surgery. Surgery for nearsightedness changes the shape of the transparent structure in the front part of the eye (cornea). LASIK is the most common surgical procedure used to correct nearsightedness.
The LASIK procedure creates a flap in the cornea in order to reshape it. This flap can be made by either a mechanical microkeratome or a femtosecond laser. The mechanical microkeratome uses a blade to make the flap and the femtosecond laser uses a laser to make the flap. The flaps made by the two methods are different in thickness and structure, and the side effects resulting from each method are also different. We collected and analyzed all relevant studies to answer this question. We found 11 studies that included 943 participants (1691 eyes).
What were the main results? There is no evidence of a difference in vision outcomes between using mechanical microkeratome or femtosecond laser. The certainty of evidence is low. However, there may be a difference in side effects between the two methods. The mechanical microkeratome group had more cases of dry eye and the femtosecond laser group had more cases of swelling of the cornea. Overall, due to the low‐certainty evidence presented, it is difficult to draw a general conclusion regarding the effectiveness and safety of these two tools.
How up‐to‐date was this review? The authors searched for trials that had been published up to 22 February 2019.
Summary of findings
Summary of findings 1. Laser‐assisted in‐situ keratomileusis (LASIK) with a mechanical microkeratome compared to LASIK with a femtosecond laser for LASIK in adults with myopia or myopic astigmatism.
| Laser‐assisted in‐situ keratomileusis (LASIK) with a mechanical microkeratome compared to LASIK with a femtosecond laser for LASIK in adults with myopia or myopic astigmatism | |||||
| Patient or population: adults (18 years) with more than 0.5 diopters of myopia or myopic astigmatism Setting: eye clinic Intervention: LASIK with a mechanical microkeratome Comparison: LASIK with a femtosecond laser | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with LASIK with a femtosecond laser | Risk with LASIK with a mechanical microkeratome | ||||
| Mean UCVA, 12 months after surgery (unit LogMAR) Follow‐up: 1 day to 12 months | The mean uncorrected visual acuity after 12 months surgery in the LASIK with a femtosecond laser group was ‐0.04 | MD 0.01 lower (‐0.06 lower to 0.04 higher) | ‐ | 42 (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
| Proportion of eyes within ± 0.5 diopters of target refraction, 12 months after surgery (unit diopters) Follow‐up: 1 day to 12 months | 925 per 1000 | 897 per 1000 (786 to 1000) | RR 0.97 (0.85 to 1.11) | 79 (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
| Mean spherical equivalent of the refractive error, 12 months after surgery (unit diopters) Follow‐up: 1 day to 12 months | The mean spherical equivalent of the refractive error 12 months after surgery in the LASIK with a femtosecond laser group ranged from ‐0.30 to ‐0.31 |
MD 0.09 higher (‐0.01 lower to 0.19 higher) | ‐ | 168 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b |
| Corneal haze, any time point | 23 per 1000 | 8 per 1000 (0 to 185) | RR 0.33 (0.01 to 7.96) | 86 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c |
| Dry eye, any time point | 80 per 1000 | 457 per 1000 (233 to 899) | RR 5.74 (2.92 to 11.29) | 183 (1 RCT) | ⊕⊕⊝⊝ Lowa,b |
| Diffuse lamellar keratitis, any time point | 209 per 1000 | 56 per 1000 (21 to 163) | RR 0.27 (0.10 to 0.78) | 134 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b |
| Epithelial ingrowth, any time point | 20 per 1000 | 20 per 1000 (2 to 185) | RR 1.04 (0.11 to 9.42) | 102 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; UCVA: uncorrected visual acuity. | |||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to imprecision. bDowngraded one level due to risk of bias. cDowngraded two levels due to very serious imprecision.
Background
Description of the condition
Refractive errors are an important cause of vision impairment and blindness (Holden 2016). Myopia (nearsightedness) is a type of refractive error that causes blurry vision at distance because abnormal structural conditions of the cornea, lens, or length of the eye prevent the images from focusing properly on the retina (Riordan‐Eva 2011). Myopia affected 1.95 billion people worldwide in 2010 (Holden 2016), with a higher prevalence in urban areas (Morgan 2012). The global economic burden generated by myopia has been estimated at USD 202 billion per annum (Smith 2009), and approximately USD 139 billion in the USA alone (NASEM 2016).
Description of the intervention
Refractive errors can be corrected through non‐surgical (eyeglasses and contact lenses) and surgical methods. The surgical methods are long‐lasting treatments that are used when a person becomes intolerant to contact lenses, encounters visual aberration from high‐powered spectacles, or desires to eliminate or reduce their dependence on glasses or contact lenses (Azar 2002). The eye is an optical system with a refractive power that can be changed by altering the curvature of its refractive surface or the location of elements of the system. Intraocular implants, such as intraocular lenses, can be used to correct refractive errors; however, the most common refractive surgeries performed in the USA are keratorefractive techniques. Keratorefractive surgeries are a group of techniques that modify the refractive power of the cornea by changing its curvature. These techniques include photorefractive keratectomy, laser subepithelial keratomileusis, intrastromal lenticule extraction, and laser‐assisted in‐situ keratomileusis (LASIK) (Bower 2001).
Due its safety and efficacy profile, LASIK is more popular compared with other surgeries. This technique creates a flap of the outermost parts of the cornea (epithelium, bowman layer, and anterior stroma) to expose the middle part of the cornea (stromal bed) and reshape it with excimer laser using photoablation (Ang 2009). LASIK comprises the creation of a corneal flap with an intended diameter that ranges from 7.8 mm to 9.8 mm and a thickness of 90 μm to 180 µm. The flap can be achieved by a mechanical microkeratome or a femtosecond laser (Farjo 2013). The mechanical microkeratome uses an oscillating blade to create the corneal flap (Bower 2001), while the femtosecond laser creates the flap with a focusable photodisruptive laser that delivers ultrashort (10–15 seconds) pulses with a wavelength within the infrared spectrum in a preset pattern (Lubatschowski 2000). This laser ionizes the tissue, causing molecular disruption within the cornea. The beam is focused on a small spot, creating electrically charged particles through multiphotonic absorption which release electrons from the atoms by a process known as avalanche ionization (Azar 2006). The free electrons transfer their energy to the surrounding medium, evaporating the adjacent tissue and forming cavitation bubbles consisting of carbon dioxide, nitrogen, and water (Bashir 2017). When the cavitation bubbles expand, they produce a regular and precise dissection of the corneal flap (Farjo 2013; Huhtala 2016; Sales 2016). The main differences between femtosecond and microkeratome flaps are the thickness and architecture. This Cochrane Review focused on the use of femtosecond laser or mechanical microkeratome in LASIK to correct myopia.
How the intervention might work
The femtosecond laser has some theoretical advantages over the use of a mechanical microkeratome in LASIK. For instance, the thickness of flaps created with the mechanical microkeratome have a significant variation (25 μm to 250 µm) compared with the femtosecond laser (78 μm to 173 µm). The predictability of the procedure to create a flap could be an important factor in the biomechanical integrity of the cornea (Flanagan 2003). Complications associated with the mechanical microkeratome are free or incomplete flaps, buttonholes, and epithelial erosions (Azar 2006). These complications are less common with the femtosecond laser, because it dissects in patterns that allow variation of flap width, depth, and diameter that may lead to better surgical results (Ang 2009; Gil‐Cazorla 2011; Issa 2011; Medeiros 2011). Other proposed benefits of the femtosecond laser use in LASIK are better uncorrected visual acuity (UCVA) (Gil‐Cazorla 2011), lower intraocular pressure during the procedure (Chaurasia 2010), and a lower incidence of dry eye (Salomão 2009).
The creation of the corneal flap with a mechanical microkeratome is free of some complications specific to LASIK with femtosecond laser, such as vertical gas breakthrough, anterior chamber bubbles, and opaque bubble layer (Azar 2006; Courtin 2015; Gatinel 2013; Moshirfar 2010; Stonecipher 2006). Another advantage of the mechanical microkeratome over the femtosecond laser is that it is fully reusable after dismantling and sterilization (the blade is the only single use part of the device), and there is no need to switch the patient to the excimer laser; these factors lower the overall cost of the procedure (Azar 2019). Other reported benefits of the mechanical microkeratome are lower risks of developing corneal haze (Patel 2008), transient light sensitivity (Stonecipher 2006), diffuse lamellar keratitis (Moshirfar 2010), and rainbow glare (Gatinel 2013).
Why it is important to do this review
The number of people with myopia globally is projected to increase to 4.76 billion by 2050 (Holden 2016). The trend has important economic (Corcoran 2015) and public health implications (NASEM 2016). LASIK is one of the most common surgical procedures used to correct refractive errors (Bower 2001). Femtosecond laser technology are often used in high‐income countries, while the mechanical microkeratome are used in low‐income countries (Salomao 2010). Both procedures have some advantages and disadvantages over the other, some of which are controversial (Ang 2009; Azar 2006; Bashir 2017; Farjo 2013). Therefore, it is important to evaluate the comparative effectiveness and safety of both procedure for myopia.
Objectives
To compare the effectiveness and safety of mechanical microkeratome versus femtosecond laser in LASIK for myopia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We did not restrict trial inclusion based on language or publication status.
Types of participants
People aged 18 years or older with more than 0.5 diopters of myopia or myopic astigmatism.
Types of interventions
We included trials that compared mechanical microkeratome with femtosecond laser in LASIK.
Types of outcome measures
Primary outcomes
Mean uncorrected visual acuity (UCVA) at 12 months after surgery. We used logMAR for visual acuity analyses.
Secondary outcomes
Mean UCVA at one and three months after surgery
Mean best corrected visual acuity (BCVA) at one, three, and 12 months after surgery
Proportion of eyes within ± 0.5 diopters of target refraction at one and 12 months after surgery
Proportion of eyes with loss of two or more lines of BCVA at 12 months after surgery
Mean spherical equivalent of the refractive error, measured in diopters, at one and 12 months after surgery
Intraoperative and postoperative pain at one day and one week, assessed with any validated measurement scale
Quality of life measures, assessed with any validated measurement scale at any point within follow‐up
Adverse outcomes
We considered adverse outcomes as reported by included trials up to 12 months after surgery. Specific adverse outcomes of interest were the following.
Corneal haze
Dry eye
Visual symptoms (double images, glares, halos, starburst)
Flap displacement
Flap melt
Diffuse lamellar keratitis
Infectious keratitis
Epithelial ingrowth
Corneal ectasia
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist (Lori Rosman) searched the following electronic databases for randomized controlled trials. There were no restrictions on language or year of publication. The electronic databases were last searched on 22 February 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 22 February 2019; Appendix 1).
MEDLINE Ovid (1946 to 22 February 2019; Appendix 2).
Embase.com (1947 to 22 February 2019; Appendix 3).
PubMed (1948 to 22 February 2019; Appendix 4).
LILACS (Latin American and Caribbean Health Science Information Database (1982 to 22 February 2019; Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicalTrials.gov; searched 22 February 2019; Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 22 February 2019; Appendix 7).
Searching other resources
We searched the reference lists of reports from trials we included in the review to look for additional trials. We did not conduct manual searches of conference proceedings or abstracts specifically for this review.
Data collection and analysis
Selection of studies
Two review authors (NKL, AN) assessed the titles and abstracts of articles identified through the literature search against inclusion criteria (listed in the Criteria for considering studies for this review section) and independently classified these as 'definitely relevant', 'possibly relevant', or 'definitely not relevant'. We used Covidence software to manage the screening process (Covidence). Any disagreement was resolved by a third review author (EGH). We obtained the full‐text copies of all trials classified as 'definitely relevant' or 'possibly relevant'. Each review author independently assessed each trial for inclusion and labeled it as either 'include' or 'exclude'. We contacted the authors of the primary trials via email for clarification whenever necessary. If there was no response within three weeks, we assessed the trial based on the information available. A third review author (EGH) resolved any disagreement. We documented the reason for exclusion of each trial excluded after reviewing the full report in a Characteristics of excluded studies table. We used Google Translate to assess trials written in languages other than English and Spanish. We used a PRISMA flowchart to depicts the flow of information through the different phases of the systematic review (Moher 2009).
Data extraction and management
Two review authors (NKL, AN) independently extracted data from the included trials using data extraction forms developed by Cochrane Eyes and Vision and accessed via Covidence (Appendix 8). A third review author (EGH) resolved any disagreements. We contacted authors of the primary trials via email to obtain missing information or to clarify data. We waited three weeks for a response; in the absence of a response, we used the available information, as provided in published reports. One review author (NKL) entered data into Review Manager 5 (Review Manager 2014), and a second review author (AN) verified the data entered.
Assessment of risk of bias in included studies
Two review authors (NKL, AN) evaluated the risk of selection (random sequence generation and allocation concealment before randomization), performance (masking of trial participants and personnel), detection (masking of outcome assessors), attrition (missing data and absence of an intention‐to‐treat analysis), reporting (selective outcome reporting), and other potential sources of bias using the Cochrane 'Risk of bias' assessment tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We classified the risk of bias as 'low', 'high', or 'unclear' (insufficient information for assessment). We contacted authors of the primary trials when methods were unclear or when additional information about trial design or methods was required to assess the risk of bias. We waited three weeks for a response; in the absence of a response, we assessed the risk of bias based on descriptions provided in published reports. A third review author (EGH) resolved any disagreement between review authors.
Measures of treatment effect
We calculated mean differences (MDs) with 95% confidence intervals (CIs) for continuous outcomes such as mean UCVA after surgery, BCVA after surgery, and mean spherical equivalent of refractive error after surgery. For dichotomous outcomes (e.g. proportion of eyes within 0.5 diopters of target refraction after surgery), we calculated risk ratios (RRs) with 95% CIs. We used logMAR (logarithm of the minimum angle of resolution) for the outcomes that included visual acuity.
Unit of analysis issues
The participant was the primary unit of analysis whenever: only one eye per participant was enrolled in the trial; or two eyes of a participant were treated as a single unit after being administered the same treatment (e.g. mean values, binocular visual acuity). If neither of these conditions was met, the eye was considered the unit of analysis. If two eyes of the same participant were randomized to two different interventions, the correlation between the two eyes must be accounted for in the analysis; and in such case, we would only use the results that have accounted for this correlation.
Dealing with missing data
We contacted trial authors to obtain missing data or data reported unclearly in the trial reports. We allowed three weeks for trial authors to respond and used the available information whenever there was no response. We did not impute missing participant data for analysis.
Assessment of heterogeneity
We compared the participant characteristics, trial interventions, and outcomes across trials to assess for clinical and methodological heterogeneity. We used a visual inspection of forest plots and Chi² test statistics to assess statistical heterogeneity among estimates of effect size from the included trials. We used the I² statistic, which estimates the proportion of variation in observed effects not due to chance, to identify inconsistency among trials; an I² statistic value greater than 50% represented substantial heterogeneity (Higgins 2017).
Assessment of reporting biases
We did not perform a meta‐analysis with 10 or more trials, therefore a visual inspection of funnel plots of the intervention effect estimates for evidence of asymmetry was not meaningful. An asymmetric funnel plot may suggest small‐study effects, which could be the result of reporting bias, heterogeneity, or differences in the methodological quality of trials. We assessed selective outcome reporting as part of the 'Risk of bias' assessment among individual trials.
Data synthesis
We combined the effect estimates from individual trials using the random‐effects model when trials had no substantial clinical or methodological heterogeneity.
Subgroup analysis and investigation of heterogeneity
There were insufficient data from the trials to examine findings by the degree of myopia at baseline among the trial participants: low to moderate myopia (less than 6.0 diopters) and high myopia (6.0 diopters or more).
Sensitivity analysis
We performed sensitivity analyses for primary and secondary outcomes to explore the effects of restricting our analyses to trials in which the authors received no financial compensation from the industry. We also performed a sensitivity analysis for the trials that had at least 80% follow‐up of participants in each group in the trials with high risk of bias and to explore the effects of fixed‐effect versus random‐effects meta‐analyses. Post hoc, we planned to conduct a sensitivity analysis to examine the effects of restricting our analyses to trials for which the unit of analysis was the patient (one eye per participant) or trials that used paired‐eye design and accounted for correlation between the two eyes in their analysis. However, we did not perform this because the only meta‐analysis performed involved only two trials, both of which were paired‐eye design and did not report whether they accounted for correlation between the two eyes in their analysis.
Summary of findings
We summarized the findings of the review using the GRADE approach to assess the strengths and limitations of evidence for both primary and secondary outcomes using the GRADEpro Guideline Development Tool (GDT) software (GRADEpro 2015). Using this approach, we classified the evidence for each outcome as 'high', 'moderate', 'low', or 'very low' and documented reasons for our judgments. We included the following seven outcomes in Table 1.
Mean UCVA, 12 months after surgery
Proportion of eyes within ± 0.5 diopters of target refraction, 12 months after surgery
Mean spherical equivalent of the refractive error, 12 months after surgery
Corneal haze, at any time point
Dry eye, at any time point
Diffuse lamellar keratitis, at any time point
Epithelial ingrowth, at any time point
In our protocol, we planned to include the following outcomes in this review, but there were insufficient data to include them in the 'Summary of findings' table.
Proportion of eyes with loss of two or more lines of BCVA, 12 months after surgery
Postoperative pain, within one week after surgery
Quality of life score, 12 months after surgery
Results
Description of studies
Results of the search
As of February 2019, the electronic searches resulted in 6560 titles and abstracts (Figure 1). After removal of duplicates, we screened 4802 records from which we identified 74 trials for full‐text review. We then excluded 57 records after full‐text review because they were either non‐randomized trials (56 records) or did not compare a femtosecond laser with a mechanical microkeratome (1 record). We classified one study as ongoing. There were five records from the Patel_group 2010 and two records from the Manche_group 2008. Overall, we included 16 records from 11 trials that met the inclusion criteria for the review (see Characteristics of included studies table).
1.
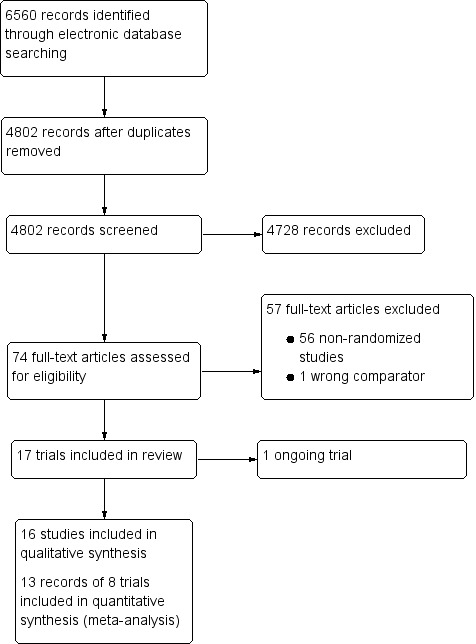
Trial flow diagram.
Included studies
Participant selection
All included trials enrolled adults (aged > 18 years old) with spherical or spherocylindrical myopia who were suitable candidates for Laser‐assisted in‐situ keratomileusis (LASIK) after examination (Buzzonetti 2008; Durrie 2005; Gui‐Hong 2018; Hasimoto 2013; Manche_group 2008; Patel_group 2010; Salomão 2009; Tan 2007; Tran 2005; Zhai 2013; Zhou 2012).There were 943 participants (1691 eyes) enrolled across all trials; 547 participants (824 eyes) received LASIK with a mechanical microkeratome and 588 participants (867 eyes) received LASIK with a femtosecond laser. Each trial included between 9 and 360 participants. Six trials randomized one eye to one intervention and the fellow eye to another (Durrie 2005; Hasimoto 2013; Manche_group 2008; Patel_group 2010; Tan 2007; Tran 2005). The trials were conducted in the USA (5 trials), China (3 trials), and one each conducted in Brazil, Italy and Singapore. Preoperative refraction of participants was up to 7.50 diopters of sphere and 3 diopters of cylinder. The year of publication ranged from 2005 to 2018, with follow‐up time between one day and five years.
Interventions
Trials included in this review assigned participants to receive LASIK with a mechanical microkeratome or a femtosecond laser. One trial randomized 81 participants (161 eyes) into three intervention groups (Zhai 2013), of which two of the three intervention arms involving 60 participants (117 eyes), were relevant to this review. Three trials did not report review specific outcomes of interest (Tan 2007; Zhai 2013; Zhou 2012), therefore we did not include them in the analysis. The remaining eight trials included 482 participants (772 eyes) enrolled across all trials; 270 participants (365 eyes) received LASIK with a mechanical microkeratome and 312 participants (407 eyes) with a femtosecond laser. Among the eight trials that reported review specific outcomes of interest, two trials used a parallel‐trial design (Buzzonetti 2008; Gui‐Hong 2018), and six trials used a within‐person trial design (Durrie 2005; Hasimoto 2013; Manche_group 2008; Patel_group 2010; Salomão 2009; Tran 2005). All eight trials were similar in treatment setting.
Outcomes
Three trials reported the mean uncorrected visual acuity (UCVA) after surgery (Durrie 2005; Gui‐Hong 2018; Patel_group 2010). One trial reported best corrected visual acuity (BCVA) after surgery (Patel_group 2010). Five trials reported the mean spherical equivalent of the refractive error after surgery (Buzzonetti 2008; Durrie 2005; Gui‐Hong 2018; Manche_group 2008; Patel_group 2010), two of these trials reported proportion of eyes within 0.5 diopters of target refraction after surgery (Durrie 2005; Manche_group 2008). Four trials reported adverse events (Hasimoto 2013; Manche_group 2008; Salomão 2009; Tran 2005). We considered the methods used to measure the outcomes to be consistent across trials. None of the trials assessed proportion of eyes with loss of two or more lines of BCVA from preoperative visual acuity, pain, quality of life, visual symptoms, flap displacement, flap melt, infectious keratitis, or corneal ectasia.
Funding sources
Four trials reported source of funding. One trial was funded by the IRCCS‐Casa Sollievo della Sofferenza Hospital and Institue of Ophthalmology of Catholic University (Buzzonetti 2008), another was funded by the National Institutes of Health, Research to Prevent Blindness, and the Mayo Foundation (Patel_group 2010). Salomão 2009 was supported by US Public Health Service grants from the National Eye Institute, and Research to Prevent Blindness. IntraLase Corp funded one trial (Tran 2005), and the remaining seven trials did not report funding sources.
Ongoing Study
We identified one ongoing trial (PACTR201708002498199).
Excluded studies
We reviewed and excluded 57 records. We described the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
A summary of the risk of bias for included trials is presented in Figure 2 and a risk of bias graph is presented in Figure 3.
2.

3.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included trials
Allocation
We judged four trials at low risk of bias for random sequence generation because the trial investigators described a random component in the sequence generation process (Durrie 2005; Hasimoto 2013; Manche_group 2008; Zhou 2012). The remaining seven trials did not specify the method for randomization, therefore, we judged the risk to be unclear (Buzzonetti 2008; Gui‐Hong 2018; Patel_group 2010; Salomão 2009; Tan 2007; Tran 2005; Zhai 2013).
Two trials reported using sealed and opaque envelopes to conceal treatment allocation (Hasimoto 2013; Manche_group 2008), and we judged them at low risk of bias. The remaining nine trials did not describe the method of allocation concealment and we judged them at unclear risk of bias
Blinding
One trial specified masking of both participants and trial personnel (Manche_group 2008), and another reported masking of participants (Hasimoto 2013), and we judged them at low risk of bias. Patel_group 2010 stated that "it was not possible to mask patients…", therefore we judged this trial at high risk of bias. The remaining trials included in the review did not specify how masking of participants and personnel was achieved and we judged them at unclear risk of bias.
Three trials described adequate masking of the outcome assessors (Durrie 2005; Hasimoto 2013; Patel_group 2010), and we judged them at low risk of detection bias. The remaining eight trials did not provide a clear description of the measures used to mask outcome assessors or did not address this issue, therefore, we judged them at unclear risk of bias.
Incomplete outcome data
We judged five trials at low risk of bias; Patel_group 2010, Durrie 2005, and Salomão 2009 reported no missing outcome data. Manche_group 2008 described missing outcome data that we considered balanced in numbers across intervention groups with similar reasons for missing data across the two groups. Tran 2005 reported one amblyopic eye that was dropped from the analysis; we considered that reason for missing data unlikely to be related to the outcomes being explored in the review.
Hasimoto 2013 reported missing outcome data of three participants (6 eyes), one participant (2 eyes) had a head trauma (intervention group was not specified), another participant (1 eye) presented with diffuse lamellar keratitis (femtosecond laser group), and one other participant (1 eye) presented with bleeding from limbal vessels (intervention group was not specified). We considered the missing outcome data likely to be related to true outcomes, therefore we judged this trial at high risk of bias.
The remaining five trials had insufficient reporting of incomplete outcome data and we judged them at unclear risk of bias.
Selective reporting
Outcomes reported in the different reports of the Patel_group 2010 were not prespecified in trial registration, therefore we judged it at unclear risk of bias. We also judged the remaining 10 trials at unclear risk of bias for selective reporting because we did not identify trial protocols.
Other potential sources of bias
Two trials declared financial compensations from the industry directly related to one of the intervention groups (Durrie 2005; Tran 2005), and we judged them to be at high risk of other bias. The remaining trials appear to be free from other sources of bias, and we judged them at low risk for other bias.
Effects of interventions
See: Table 1
See: Table 1
Mean uncorrected visual acuity (UCVA) after surgery
One trial provided data of 42 eyes for the primary outcome (Patel_group 2010). The mean difference (MD) of UCVA at 12 months after surgery was –0.01, (95% confidence interval (CI) –0.06 to 0.04). Three trials provided data from 192 eyes for the mean UCVA at one month (Durrie 2005; Gui‐Hong 2018; Patel_group 2010) (MD 0.13, ‐0.08 to 0.33). Two trials provided data of mean UCVA at three months after surgery (Durrie 2005; Patel_group 2010). There was low statistical heterogeneity between the two trials, the I² statistic was 0% and the Chi² test for heterogeneity was not statistically significant (P = 1.00). Therefore, we combined the data of UCVA at three months in a meta‐analysis (72 eyes). The observed MD of UCVA was 0.00 (95% CI –0.03 to 0.03; Analysis 1.1; Figure 4). We did not perform an overall UCVA analysis because two groups included data from the same trial and were based on two clinically different time points. The certainty of evidence for this outcome across the various trials was low; we downgraded for risk of bias and imprecision.
1.1. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 1: Mean uncorrected visual acuity after surgery
4.

Forest plot of comparison: 1 LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, outcome: 1.1 Mean uncorrected visual acuity after surgery [logMAR].
Proportion of eyes within ± 0.5 diopters of target refraction after surgery
Data from the trial by Manche_group 2008 comprised 79 eyes and found that the proportion of eyes 0.5 diopters within target refraction was 3% lower after LASIK with mechanical microkeratome 12 months after surgery: risk ratio (RR) 0.97, 95% CI 0.85 to 1.11; low‐certainty evidence; Analysis 1.2; we downgraded for risk of bias and imprecision. Two other trials with 125 participants (Durrie 2005; Patel_group 2010), reported the proportion of eyes within 0.5 diopters of target refraction after LASIK one month after surgery. We combined the data of the two trials because heterogeneity was low, the I² statistic was 0% and the Chi² test for heterogeneity was not statistically significant (P = 0.67). There was no difference between LASIK with a mechanical microkeratome or a femtosecond laser of achieving 0.5 diopters within target refraction one month after surgery (Analysis 1.2; Figure 5). We did not perform an overall analysis because the groups were based on two clinically different time points. However, the certainty of evidence for this outcome across the various time points and trials was low; we downgraded for risk of bias and imprecision.
1.2. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 2: Proportion of eyes within ± 0.5 diopters of target refraction after surgery
5.

Forest plot of comparison: 1 LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, outcome: 1.2 Proportion of eyes within ± 0.5 diopters of target refraction after surgery.
Mean spherical equivalent of the refractive error after surgery
Durrie 2005, Patel_group 2010, and Gui‐Hong 2018 reported mean spherical equivalent of refractive error one month after surgery. There was considerable heterogeneity (I² = 96%, Chi² test for heterogeneity was statistically significant, P < 0.0001) thus, rendered a summary of the pooled effect estimate inappropriate. Point estimates from the three trials showed no evidence of a difference between the two groups: Durrie 2005 (102 eyes, 51 participants); MD 0.40, 95% CI 0.29 to 0.51; Gui‐Hong 2018: (240 eyes, 120 participants); MD –0.01, 95% CI –0.05 to 0.03; Patel_group 2010: (42 eyes, 21 participants); MD –0.06, 95% CI –0.24 to 0.12; Analysis 1.3; Figure 6). However, data from Durrie 2005, which seem to be an outlier, indicate that participants undergoing femtosecond LASIK seem to do better in spherical equivalent of the refractive error. The possible explanation probably has to do with the flap thickness predictability and biomechanics of the corneal flap, which is more efficiently created with the femtosecond laser, and has low spherical aberrations compared to microkeratome LASIK (Bashir 2017). The certainty of evidence across the included trials was low; we downgraded for risk of bias and imprecision.
1.3. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 3: Mean spherical equivalent of the refractive error after surgery
6.

Forest plot of comparison: 1 LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, outcome: 1.3 Mean spherical equivalent of the refractive error after surgery [diopters].
Three trials reported mean spherical equivalent of refractive error 12 months after surgery (Buzzonetti 2008; Manche_group 2008; Patel_group 2010; 168 eyes, 92 participants). We combined the data of the three trials in a meta‐analysis and observed no evidence of a difference in mean spherical equivalent of refractive error between the two groups (MD 0.09, 95% CI –0.01 to 0.19; I² = 0%; Analysis 1.3; Figure 6). The certainty of evidence was low; we downgraded for risk of bias and imprecision.
Corneal haze
Based on the trial by Manche_group 2008 (86 eyes), the RR of corneal haze was 77% lower with the mechanical microkeratome group compared with the femtosecond laser group (RR 0.33, 95% CI 0.01 to 7.96; Figure 7). The certainty of evidence was very low; we downgraded for risk of bias and very serious imprecision.
7.

Forest plot of comparison: 1 LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, outcome: 1.4 Corneal haze.
Dry eye
The trial by Salomão 2009 (183 eyes, 183 participants) had a RR of 5.74 for the participants assigned to the mechanical microkeratome group compared with the femtosecond laser group (95% CI 2.92 to 11.29; Figure 8). The certainty of evidence was low; we downgraded for risk of bias and imprecision. The trial by Manche_group 2008 described a self‐reported mean dry eye score at preoperative, and one, three, six, and 12 months postoperative time points. We did not include the trial in the quantitative synthesis because 100% of the participants reported dry eye preoperatively. The findings reflected an increase in the mean dry eye score postoperatively in the femtosecond laser group one month after surgery (P = 0.02), the score returned to baseline by six months after surgery. The certainty of evidence was low; we downgraded for risk of bias and imprecision.
8.

Forest plot of comparison: 1 LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, outcome: 1.5 Dry eye.
Diffuse lamellar keratitis
Manche_group 2008, Hasimoto 2013, and Tran 2005 (134 eyes, 67 participants) reported diffuse lamellar keratitis. We combined the data of the trials in a meta‐analysis since there was no heterogeneity (I² = 0%, Chi² P = 0.89). The RR for the participants assigned to the mechanical microkeratome group was 73% lower compared with the femtosecond laser group (RR 0.27, 95% CI 0.10 to 0.78; Analysis 1.6; Figure 9). The certainty of evidence was low; we downgraded for risk of bias and imprecision.
1.6. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 6: Diffuse lamellar keratitis
9.

Forest plot of comparison: 1 LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, outcome: 1.6 Diffuse lamellar keratitis.
Epithelial ingrowth
Two trials reported epithelial ingrowth (Manche_group 2008; Tran 2005; 102 eyes, 51 participants). We combined data in a meta‐analysis and observed a RR of 1.04 for the participants assigned to the mechanical microkeratome group compared with the femtosecond laser group (95% CI 0.11 to 9.42; I² = 0%; Analysis 1.7; Figure 10). The certainty of evidence was very low; we downgraded for risk of bias and very serious imprecision.
1.7. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 7: Epithelial ingrowth
10.

Forest plot of comparison: 1 LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, outcome: 1.7 Epithelial ingrowth.
Sensitivity analysis
The results of the sensitivity analyses of the trials in which the authors received financial compensation from the industry, trials that had at least 80% follow‐up of participants in each group, or trials with high risk of bias, or the use of fixed‐effect versus random‐effects meta‐analysis showed no difference in the findings (data not shown).
Discussion
Summary of main results
We included 16 reports of 11 trials in this review representing 943 participants (1691 eyes). The trials allocated adults 18 years and older, with spherical or spherocylindrical myopia considered suitable candidates for Laser‐assisted in‐situ keratomileusis (LASIK) after examination, to a mechanical microkeratome group or a femtosecond laser group. Based on our assessment of the trials that reported uncorrected visual acuity (UCVA), there was no evidence of a difference in UCVA after LASIK in eyes with the flap created by a femtosecond laser compared to eyes with the flap created by a mechanical microkeratome. There was also no evidence of a difference between the two treatments in terms of achieving within 0.5 diopters of target refraction or in mean spherical equivalent of refractive error 12 months after surgery. The association between corneal haze and epithelial ingrowth with the interventions was uncertain. We observed a lower risk of diffuse lamellar keratitis in the mechanical microkeratome group compared with the femtosecond laser group when combining the results of three trials. Only one trial reported dry eye after the intervention. This trial showed that there may be a higher risk of dry eye in the mechanical microkeratome compared to the femtosecond laser group. We found no trials that reported proportion of eyes with loss of two or more lines of best corrected visual acuity (BCVA) from preoperative visual acuity, pain, quality of life, visual symptoms, flap displacement, flap melt, infectious keratitis, or corneal ectasia. These findings were robust to our sensitivity analysis.
Overall completeness and applicability of evidence
The trials included in the review were not substantially heterogeneous from a clinical point of view. We considered that the trials identified were not sufficient to address all the objectives of the review. For instance, relevant outcomes such as, quality of life, proportion of eyes with loss of two or more lines of BCVA from preoperative visual acuity, as well as intraoperative and postoperative pain should have been investigated.
Potential biases in the review process
Two review authors independently assessed the titles and abstracts and full‐text reports of potentially eligible trials; a third review author resolved any conflicts. The extraction of data and assessment of risk of bias for all trials included in this review were done in the same manner. During the review process, we changed four outcomes in the 'Summary of findings' table specified in the protocol due to insufficient data available from the trials. All outcomes incorporated in Table 1 were prespecified secondary outcomes in the protocol. We are aware of no other potential biases in the review process.
Certainty of the evidence
We graded five outcomes reported in Table 1 at low‐certainty evidence and two at very low‐certainty evidence. All the trials that reported the seven outcomes had serious risk of bias and we downgraded them. We also downgraded the certainty of evidence of all the adverse events outcomes due to serious or very serious concerns related to imprecision in the results. We identified trial registration in only one trial and there were inconsistencies between trial outcomes defined in the protocol and those reported in the trial publications. Two trials were sponsored by companies related to one of the interventions under investigation and had roles in the design or analysis of these trials (Durrie 2005; Tran 2005). Some investigators reported financial relationships with the company.
Agreements and disagreements with other studies or reviews
Chen 2012 published one systematic review and meta‐analysis comparing IntraLase femtosecond laser versus mechanical microkeratomes in LASIK for myopia. The purpose of the review was to evaluate the safety, efficacy, and predictability of the two interventions. The trials included were randomized and non‐randomized trials. The primary outcomes were loss of more than two lines of corrected distance visual acuity, uncorrected distance visual acuity 20/20 or better, manifest refraction spherical equivalent within 0.50 diopters, final refractive spherical equivalent, and astigmatism. The secondary outcomes were flap thickness predictability, changes in higher‐order aberrations, and complications.
The trial investigators concluded the results showed no differences in visual acuity‐related outcomes between the IntraLase group and the mechanical microkeratomes group. These findings are consistent with the results of this review. Regarding complications, Chen 2012 reported higher events of diffuse lamellar keratitis in the femtosecond laser group, higher risk of loose epithelium in one or two quadrants in the group of mechanical microkeratome, and no significant difference in epithelial ingrowth between groups. Our results were consistent with a higher risk of diffuse lamellar keratitis in the femtosecond laser group as we observed a RR that was 73% lower in the mechanical microkeratome group compared with the femtosecond laser group (Analysis 1.6; Figure 9). Concerning intraoperative epithelial defects, we did not include this adverse event in our prespecified outcomes, therefore, we did not include it in the review. The findings of Chen 2012 regarding epithelial ingrowth were consistent with those presented in this review (Figure 10).
Authors' conclusions
Implications for practice.
Based on the findings of this review, there is no evidence of a difference in visual acuity outcomes in laser‐assisted in‐situ keratomileusis (LASIK) with a mechanical microkeratome or a femtosecond laser. Each intervention may be related to a higher risk of adverse events compared to the other; dry eye with the mechanical microkeratome and diffuse lamellar keratitis with the femtosecond laser. These findings should be reviewed by patients and physicians to choose the intervention that best suits their needs.
Implications for research.
There is a clear need for future research comparing mechanical microkeratome with femtosecond laser in LASIK. An important number of included trials had unclear or high risk of bias and each outcome explored in this review was not reported in more than three trials. In order to produce trials with low risk of bias with the possibility of comparing patient‐centered outcomes across trials, we recommend future researchers to:
involve patients in defining outcomes;
clearly describe a random component in the sequence generation process, the method of concealment prior to assignment of participants, and the process of masking participants and evaluators;
clearly describe the amount, nature, and handling of incomplete data;
specify the funding source;
register a protocol and follow the prespecified methods and outcomes;
evaluate participants at one, three, six, and 12 months for any outcome (e.g. visual acuity, refractive error, etc.);
document and report adverse events;
evaluate patient satisfaction and quality of life with a validated instrument (e.g. Quality of Life Impact of Refractive Correction [QIRC] questionnaire) at 12 months after surgery;
Describe the planned flap and excimer laser (e.g. wavefront) characteristics; and
stratify patients into degrees of myopia (e.g. low, moderate, high).
As noted previously, we observed a higher risk of diffuse lamellar keratitis with the femtosecond laser. The etiology of this sterile inflammation is suggested to be due to any debris on the interface that initiate an inflammatory reaction (Randleman 2012). Theoretically, the absence of metallic debris from the mechanical microkeratome should reduce the risk of this complication, but we observed the contrary in this review. This finding needs further basic and clinical research.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2020 | Amended | Issue 7 2020: Number of participants that contributed data for diffuse lamellar keratitis and epithelial ingrowth corrected in the Abstract and Effects of interventions section. |
History
Protocol first published: Issue 2, 2018 Review first published: Issue 4, 2020
Acknowledgements
We thank Cochrane Eyes and Vision (CEV) and the editorial team for their support, guidance, and comments.
We thank the Covidence team for their support.
We thank the Cochrane support team for helping us with technical issues.
We acknowledge Lori Rosman for developing the search strategy.
We thank Jimmy Le, Anupa Shah, Riaz Qureshi, Tianjing Li, Samuel Abariga, and Kristina Lindsley for their great support provided during the review process.
We thank our peer reviewers: Vishal Jhanji (University of Pittsburgh), Shameema Sikder (Wilmer Eye Institute), Terry Kungel, and Nancy Fitton (Cochrane Consumer Network).
This work was presented at the Association for Research in Vision and Ophthalmology (ARVO) 2019 Annual Meeting.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Myopia] explode all trees #2 myop* #3 (short near/3 sight*) or ("near" near/3 sight*) #4 nearsighted* #5 MeSH descriptor: [Refractive Errors] this term only #6 (Refract*) near/3 (error* or disorder*) #7 {or #1‐#6} #8 MeSH descriptor: [Keratomileusis, Laser In Situ] explode all trees #9 Keratomileus* #10 LASIK #11 Femto‐lasik or Femtolasik #12 (Femtosecond near/3 laser*) #13 Microkeratom* #14 (refract* near/3 surg*) #15 corneal flap* #16 MeSH descriptor: [Refractive Surgical Procedures] this term only #17 MeSH descriptor: [Corneal Surgery, Laser] this term only #18 MeSH descriptor: [Cornea] explode all trees and with qualifier(s): [Surgery ‐ SU] #19 {or #8‐#18} #20 #7 and #19
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp myopia/ 13. myop*.tw. 14. ((short or near) adj3 sight*).tw. 15. nearsighted*.tw. 16. Refractive Errors/ 17. (Refract* adj3 (error* or disorder*)).tw. 18. or/12‐17 19. exp Keratomileusis, Laser In Situ/ 20. Keratomileus*.tw. 21. LASIK.tw. 22. (Femto‐lasik or Femtolasik).tw. 23. (Femtosecond adj3 laser*).tw. 24. Microkeratom*.tw. 25. (refract* adj3 surg*).tw. 26. corneal flap*.tw. 27. Refractive Surgical Procedures/ 28. Corneal Surgery, Laser/ 29. exp Cornea/su [Surgery] 30. or/19‐29 31. 18 and 30 32. 11 and 31
Appendix 3. Embase search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'myopia'/exp #34 'high myopia'/exp #35 myop*:ab,ti #36 ((short NEAR/3 sight*):ab,ti) OR ((near NEAR/3 sight*):ab,ti) #37 nearsighted*:ab,ti #38 'refraction error'/de #39 (refract* NEAR/3 (error* OR disorder*)):ab,ti #40 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 #41 'keratomileusis'/exp #42 keratomileus*:ab,ti #43 lasik:ab,ti #44 'femto‐lasik':ab,ti OR femtolasik:ab,ti #45 (femtosecond NEAR/3 laser*):ab,ti #46 microkeratom*:ab,ti #47 (refract* NEAR/3 surg*):ab,ti #48 'corneal flap*':ab,ti #49 'refractive surgery'/de #50 'laser refractive surgery'/de #51 'cornea'/exp AND 'surgery'/lnk #52 #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 #53 #40 AND #52 #54 #32 AND #53
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 myop*[tw] #3 (short[tw] OR near[tw]) AND sight*[tw] #4 nearsighted*[tw] #5 (Refract*[tw]) AND (error*[tw] OR disorder*[tw]) #6 #2 OR #3 OR #4 OR #5 #7 Keratomileus*[tw] #8 LASIK [tw] #9 Femto‐lasik[tw] OR Femtolasik[tw] #10 Femtosecond[tw] AND laser*[tw] #11 Microkeratom*[tw] #12 refract*[tw] AND surg*[tw] #13 corneal flap*[tw] #14 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 #15 #1 AND #6 AND #14 #16 Medline[sb] #17 #15 NOT #16
Appendix 5. LILACS search strategy
(Myop$ OR Miopía OR Miopia OR MH:C11.744.636 OR ((short or near) AND sight$) OR nearsighted$ OR (refract$ AND (error$ OR disorder$)) OR "Errores de Refracción" OR "Erros de Refração" OR MH: C11.744) AND (Keratomileus$ OR "Queratomileusis por Láser In Situ" OR "Ceratomileuse Assistida por Excimer Laser In Situ" OR LASIK OR MH:E02.594.480.750$ OR MH:E04.014.520.480.750$ OR MH:E04.540.825.437.374$ OR "Femto‐lasik" OR Femtolasik OR (Femtosecond laser$) OR Microkeratom$ OR (refractive surg$) OR (corneal flap$) OR "Procedimientos Quirúrgicos Refractivos" OR "Procedimentos Cirúrgicos Refrativos" OR MH:E04.540.825 OR MH:E02.594.480 OR MH:E04.014.520.480 OR MH:E04.540.825.437 OR mh:("Cornea/SU"))
Appendix 6. ClinicalTrials.gov search strategy
(myopia OR refractive errors) AND (LASIK OR keratomileusis OR femtosecond laser OR microkeratome OR refractive surgery OR corneal flap OR corneal laser surgery)
Appendix 7. WHO ICTRP search strategy
myopia AND LASIK OR myopia AND keratomileusis OR myopia AND femtosecond laser OR myopia AND microkeratome OR myopia AND refractive surgery OR myopia AND corneal flap OR myopia AND corneal laser surgery OR refractive error AND LASIK OR refractive error AND keratomileusis OR refractive error AND femtosecond laser OR refractive error AND microkeratome OR refractive error AND refractive surgery OR refractive error AND corneal flap OR refractive error AND corneal laser surgery
Appendix 8. Data extraction form on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
|
Exclusions after randomization Losses to follow‐up Number randomized/analyzed How were missing data handled? e.g. available‐case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or unit of randomization/unit of analysis |
|
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up only, please indicate | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n = ) Comparator (n = ) See MECIR 65 and 70 |
|
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 |
List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
Data and analyses
Comparison 1. LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean uncorrected visual acuity after surgery | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 At 1 month | 3 | 384 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.08, 0.33] |
| 1.1.2 At 3 months | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 1.1.3 At 12 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 1.2 Proportion of eyes within ± 0.5 diopters of target refraction after surgery | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 At 1 month | 2 | 144 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.77, 0.99] |
| 1.2.2 At 12 months | 1 | 79 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.85, 1.11] |
| 1.3 Mean spherical equivalent of the refractive error after surgery | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 At 1 month | 3 | 384 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.18, 0.40] |
| 1.3.2 At 12 months | 3 | 168 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.19] |
| 1.4 Corneal haze | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.4.1 Any time point | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Dry eye | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.5.1 Any time point | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Diffuse lamellar keratitis | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Any time point | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.10, 0.78] |
| 1.7 Epithelial ingrowth | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 Any time point | 2 | 102 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.11, 9.42] |
| 1.8 Best corrected visual acuity after surgery | 1 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 1.8.1 At 1 month | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 1.8.2 At 3 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 1.8.3 At 12 months | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.09, 0.03] |
1.4. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 4: Corneal haze
1.5. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 5: Dry eye
1.8. Analysis.

Comparison 1: LASIK with a mechanical microkeratome versus LASIK with a femtosecond laser, Outcome 8: Best corrected visual acuity after surgery
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Buzzonetti 2008.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Number randomly assigned: 47 eyes, 28 participants Exclusions after randomization: not reported Number analyzed: not reported Unit of analysis: eye Losses to follow‐up: not reported Handling of missing data: not reported Power calculation: "Given the expected difference between the mean aberration values of 20% and the expected SD from the mean value for each group of 20%, the sample size considered in this trial (n = 24 eyes in the mechanical microkeratome group and n = 23 eyes in femtosecond laser group) provided a power of 85% at a level of 0.02." Study dates: June 2004 to November 2005 |
|
| Participants |
Country: Italy Overall mean age: 38.7 years (SD 9.8) Age range: not reported Gender: not reported Setting: Institute of Ophthalmology, Catholic University, Rome Equivalence of baseline characteristics: yes Inclusion criteria: people with spherical and spherocylindrical myopia, preoperative astigmatism value 2.00 D and mean spherical equivalent defect of –5.2 D (SD 3.3) Exclusion criteria: not reported |
|
| Interventions |
Laser for ablation: Bausch & Lomb Technolas 217 excimer laser Intervention 1 Intervention: LASIK with a Hansatome microkeratome Flap dimensions: 160 μm thickness, 9.0 mm diameter, superior hinge Number of people randomized: 24 eyes, 15 participants Length of follow‐up: Planned: not reported Actual: 12 months Intervention 2 Intervention: LASIK with an IntraLase femtosecond laser Flap dimensions: 120 μm thickness, 9.0 mm diameter, and 50 degree, superior hinge angle Number of people randomized: 23 eyes, 13 participants Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes |
Mean UCVA after surgery
BCVA after surgery
Mean spherical equivalent of the refractive error after surgery
Adverse events reported: no |
|
| Identification |
Sponsorship source: IRCCS‐Casa Sollievo della Sofferenza Hospital ans Institue of Ophthalmology of Catholic University Country: Italy Setting: Institute of Ophthalmology Comments: none Author's name: Luca Buzzonetti Institution: Ophthalmology Department, IRCCS–Casa Sollievo della Sofferenza Hospital, Institute of Ophthalmology, Catholic University Email: lbuzzonetti@ tibernet.it Address: Via Sabotino, 2‐00195 Rome, Italy |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to either the mechanical microkeratome or IntraLase." Comment: randomization method not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no clear description of the measures used to mask trial participants and personnel from knowledge of which intervention a participant received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of the measures used to mask outcome assessors from knowledge of which intervention a participant received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unclear whether all participants randomized were also analyzed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Durrie 2005.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: within person Number randomly assigned: 102 eyes, 51 participants Exclusions after randomization: 0 Number analyzed: 102 eyes, 51 participants Unit of analysis: eye Losses to follow‐up: 0 Handling of missing data: no missing data Power calculation: not reported Study dates: July 2003 to August 2003 |
|
| Participants |
Country: USA Overall mean age: 34.7 years (SD 7.7) Age range: not reported Gender: women 69%, men 31% Setting: not reported Equivalence of baseline characteristics: yes Inclusion criteria: people with a manifest myopic refractive error up to 7.00 D sphere with < 0.50 D astigmatism in both eyes or aberrometer dilated refraction up to 7.50 D sphere with < 1.50 D astigmatism, age ≥ 21 years, a normal ophthalmic examination except for refractive error, normal topography, no history of ocular surgery, stable refraction within 60.50 D over the past year, no ongoing use of ophthalmic or systemic medications, and no history of autoimmune disease Exclusion criteria: not reported |
|
| Interventions |
Laser for ablation: LADARVision 4000 Intervention 1 Intervention: LASIK with a Hansatome microkeratome Flap dimensions: 180 μm thickness, 9.5 mm diameter, superior hinge Number of people randomized: 51 eyes, 51 participants Length of follow‐up: Planned: not reported Actual: 3 months Intervention 2 Intervention: LASIK with an IntraLase femtosecond laser Flap dimensions: 118 μm thickness, 9.5 mm diameter, and 55 degree superior hinge Number of people randomized: 51 eyes, 51 participants Length of follow‐up: Planned: not reported Actual: 3 months |
|
| Outcomes |
Mean UCVA after surgery
Mean spherical equivalent of the refractive error after surgery
Proportion of eyes within ± 0.5 D of target refraction after surgery
Adverse events reported: no |
|
| Identification |
Sponsorship source: not reported Country: USA Setting: Institute of Ophthalmology Comments: none Author's name: Guy M Kezirian Institution: SurgiVision Consultants, Inc. Email: guy1000@surgivision.net Address: 2183 Hathaway Avenue, Westlake Village, CA 91362‐5170 |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The selection of the flap creation method was done at the time of enrollment using a predefined randomization schedule." |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: trial did not address this risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinical examinations were performed by optometrists trained in LASIK evaluations who were blinded to which eye had the femtosecond laser flap." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients (51/51, 100%) appeared for the 1‐ and 3‐month visits." |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | High risk | Quote: "Dr. Durrie is a paid consultant to IntraLase Corporation and Alcon Laboratories, Inc. Dr. Kezirian received financial compensation from IntraLase Corporation for his assistance with data analysis and preparation of the manuscript" |
Gui‐Hong 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Number randomly assigned: 240 eyes, 120 participants Exclusions after randomization: not reported Number analyzed: 240 eyes, 120 participants Unit of analysis: eye Losses to follow‐up: not reported Handling of missing data: not reported Power calculation: not reported Study dates: June 2014 to May 2015 |
|
| Participants |
Country: China Overall mean age: 27.2 years (SD 3.2) Age range: 19 to 44 years Gender: women 54%, men 46% Setting: not reported Equivalence of baseline characteristics: yes Inclusion criteria: people with myopia Exclusion criteria: not reported |
|
| Interventions |
Laser for ablation: not reported Intervention 1 Intervention: LASIK with a microkeratome Flap dimensions: not reported Number of people randomized: 120 eyes, 60 participants Length of follow‐up: Planned: not reported Actual: 6 months Intervention 2 Intervention: LASIK with a femtosecond laser Flap dimensions: not reported Number of people randomized: 120 eyes, 60 participants Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes |
Mean UCVA after surgery
Mean spherical equivalent of the refractive error after surgery
|
|
| Identification |
Sponsorship source: not specified Country: China Setting: Institute of Ophthalmology Comments: none Author's name: Xiao – Hong Gu Institution: Gu's Eye Hospital Email: 3235745327@qq. com Address: Luohe 462000, Henan Province, China |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: trial did not describe masking participants or trial personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: trial did not describe masking of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unclear whether all participants randomized were analyzed |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Hasimoto 2013.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: within person Number randomly assigned: 38 eyes, 19 participants Exclusions after randomization: 6 eyes, 3 participants Number analyzed: 32 eyes, 16 participants Unit of analysis: eye Losses to follow‐up: 0 Handling of missing data: eyes with missing data excluded from analysis Power calculation: not reported Study dates: July 2010 to September 2010 |
|
| Participants |
Country: Brazil Overall mean age: not reported Age range: not reported Gender: not reported Setting: refractive surgery service of the Eye Hospital of Paraná Equivalence of baseline characteristics: baseline characteristics not reported Inclusion criteria: myopia < 6.00 D, astigmatism < 3.00, and hyperopia < 5.00 D, stable refraction, corneal diameter < 11 mm, discontinuation of contact lens 7 days before the preoperative evaluation, BCVA of 20/20 in both eyes Exclusion criteria: use of rigid gas permeable lens, dry eye and severe blepharitis, anterior segment abnormalities (cataract, corneal scar, neovascularization within 1 mm of the ablation area), recurrent corneal erosion, membrane disease severe basal, keratoconus or progressive or unstable myopia, corneal thickness resulting in < 250 μm in the residual bed, topography or preoperative aberrations (or both) indicating unfit eyes surgery for visual correction of LASIK, intraocular surgery, history of herpes keratitis, use of systemic corticosteroid, immunocompromised patients with significant atopic disease, connective tissue disease, diabetes mellitus, macular abnormalities, lactation, participation in other clinical trials |
|
| Interventions |
Laser for ablation: WaveLight Allegretto Wave EyeQ Laser Intervention 1 Intervention: LASIK with a Hansatome microkeratome Flap dimensions: 160 μm thickness, 9.5 mm diameter, superior hinge Number of people randomized: 19 eyes, 19 participants Length of follow‐up: Planned: not reported Actual: 3 months Intervention 2 Intervention: LASIK with a femto LDVTM Ziemer femtosecond laser Flap dimensions: 110 μm thickness, 9.5 mm diameter, and superior hinge Number of people randomized: 19 eyes, 19 participants Length of follow‐up: Planned: not reported Actual: 3 months |
|
| Outcomes |
Adverse events reported: yes Diffuse lamellar keratitis
|
|
| Identification |
Sponsorship source: not specified Country: Brazil Setting: Institute of Ophthalmology Comments: none Author's name: Alexander Rodrigo Hasimoto Institution: Hospital de Olhos do Paraná – HOP, Curitiba (PR) Email: tosa_alex@hotmail.com Address: Rua Benjamin Constant, 788 – Ponta Grossa (PR) – 84010‐380 |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The choice of eye and surgical technique for inclusion followed a random order (through opaque envelopes)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The choice of eye and surgical technique for inclusion followed a random order (through opaque envelopes enumerated by order of arrival of patients, containing two papers with the name of the technique to be used and another opaque envelope containing two papers with right eye and left eye for both to be drawn; once a surgical technique was defined for a given eye, the contralateral eye received the other technique)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants did not know which surgical technique they were receiving in each eye." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcome assessor, had no prior knowledge of the surgical technique administered in each eye." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of a total of 19 patients proposed for the trial, 3 patients (6 eyes) were discontinued because they had complications. One patient suffered blunt trauma to one eye in the 10th postoperative day. Immediately the patient underwent surgical intervention to reposition the flap. At the end of the third month, there was an uncorrected visual acuity of 20/16 in this eye. One patient had diffuse lamellar keratitis (DLK) in only one eye (femtosecond group) at the end of the third day. Surgical intervention was required, showing good evolution, with uncorrected visual acuity of 20/25 in this eye. And the last patient presented blood cells at the flap interface, which required flap washing, with good evolution and visual acuity without correction of 20/20 at the end of the trial. Thus, every trial was based on the evaluation of 16 patients." |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Manche_group 2008.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: within person Number randomly assigned: 102 eyes, 51 participants Exclusions after randomization: 16 eyes, 8 participants Number analyzed: 86 eyes, 43 participants Unit of analysis: eye Losses to follow‐up: 2 participants, 4 eyes Handling of missing data: eyes with missing data excluded from analysis Power calculation: not reported Study dates: May 2004 to November 2005 |
|
| Participants |
Country: USA Overall mean age: 39.7 years (SD 7.8) Age range: 25 to 59 years Gender: women 69%, men 31% Setting: Department of Ophthalmology, Stanford University School of Medicine Equivalence of baseline characteristics: yes Inclusion criteria: people with no more than 6.00 D of spherical myopia, no more than 3.00 D of refractive astigmatism, stable refraction (0.50 D of sphere or cylinder), corneal diameter < 11.0 mm to allow for suction ring fixation, discontinuation of soft contact wear ≤ 7 days before the preoperative evaluation, visual acuity correctable to at least 20/20 in both eyes, age > 21 years, and ability to participate in follow‐up examinations for 12 months after LASIK Exclusion criteria: rigid gas‐permeable contact lens use; severe dry eye; severe blepharitis; anterior segment abnormalities (i.e. cataracts, corneal scarring, or neovascularization within 1 mm of the intended ablation zone); recurrent corneal erosion; severe basement membrane disease; progressive or unstable myopia or keratoconus; unstable corneal mires on central keratometry results; corneal thickness in which the LASIK procedure could result in < 250 μm of remaining posterior corneal thickness below the flap postoperatively; baseline standard manifest refraction exhibiting > 0.75 D more minus in sphere power or a difference > 0.50 D in cylinder power, or a different type of astigmatism, i.e. 'with‐the‐rule', 'against‐the‐rule', or 'oblique' when the cylinder was > 0.50 D compared with the baseline standard cycloplegic refraction; preoperative assessment of ocular topography or aberrations (or both) indicating that either eye is not a suitable candidate for the LASIK vision correction procedure (i.e. forme fruste keratoconus, corneal warpage, or pellucid marginal degeneration); previous intraocular or corneal surgery; history of herpes zoster or herpes simplex virus keratitis; current use of systemic corticosteroid or immunosuppressive therapy; immunocompromise or clinically significant atopic disease; connective tissue disease; diabetes mellitus; steroid response; macular abnormality; pregnancy or lactation; sensitivity to the planned trial concomitant medications; or participation in another clinical trial of an ophthalmic drug or device |
|
| Interventions |
Laser for ablation: Star S4Excimer Laser System Intervention 1 Intervention: LASIK with a Hansatome microkeratome Flap dimensions: superiorly hinged, created using a 160 μm head and a 9.0 mm ring Number of people randomized: 51 eyes, 51 participants Length of follow‐up: Planned: not reported Actual: 12 months Intervention 2 Intervention: LASIK with an IntraLase femtosecond laser Flap dimensions: 120 μm thickness, 9.0 mm diameter, and superior hinge angle, degrees not reported Number of people randomized: 51 eyes, 51 participants Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes |
Mean spherical equivalent of the refractive error after surgery
Proportion of eyes within ± 0.5 D of target refraction after surgery
Adverse events reported: yes Corneal haze
Diffuse lamellar keratitis
Epithelial ingrowth
Dry eye
|
|
| Identification |
Sponsorship source: not reported Country: USA Setting: Institute of Ophthalmology Comments: none Author's name: Edward E Manche Institution: Department of Ophthalmology, Stanford University School of Medicine Email: edward.manche@stanford.edu Address: 900 Blake Wilbur Drive, Third Floor, Stanford, CA 94305 |
|
| Notes | Trial registration number: NCT00691431 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was performed by assigning the dominant eye to one keratotomy method and the fellow eye to the other method according to a prepared randomization schedule." |
| Allocation concealment (selection bias) | Low risk | Quote: "envelope containing the assignment was opened." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patient and the physician did not learn which eye would be treated with which keratome until the day of surgery." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: masking of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Four patients (5 eyes) discontinued the trial 6 months after surgery or later and required retreatment with good outcomes. Two patients (4 eyes) were lost to follow‐up. Because of the excimer laser software upgrade after the first 8 patients underwent LASIK, only the remaining 43 patients are included in the refractive outcomes and patient preference analyses to rule out any issues related to the initial version of excimer laser software." |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Patel_group 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: within person Number randomly assigned: 42 eyes, 21 participants Exclusions after randomization: 0 Number analyzed: 42 eyes Unit of analysis: eye Losses to follow‐up: 1 participant, 2 eyes Handling of missing data: eyes with missing data excluded from analysis Power calculation: "The trial was powered a priori to detect a difference of 0. 15 logMAR in UCVA or BCVA at 3 years after LASIK by assuming that the standard deviation of the difference in visual acuity would be 0.15 logMAR. This required a minimum sample size of 16 subjects ( 0.05/6, 0.20, paired test)." Study dates: 2004 to 2005 |
|
| Participants |
Country: USA Overall mean age: 41.5 years (SD 10) Age range: 29 to 55 years Gender: not reported Setting: refractive surgery service at Mayo Clinic Equivalence of baseline characteristics: yes Inclusion criteria: people with myopia or myopic astigmatism (D not reported) Exclusion criteria: any corneal abnormalities; a history of ocular disease, trauma, or surgery; or diabetes mellitus or other systemic disease known to affect the eye; or if they used ocular medications. Systemic medications were permitted unless they were known to affect the cornea or anterior segment. |
|
| Interventions |
Laser for ablation: VISX Star S4 excimer laser Intervention 1 Intervention: LASIK with a Hansatome microkeratome Flap dimensions: 160 μm thickness, 9.0 mm diameter, superior hinge Number of people randomized: 21 eyes, 21 participants Length of follow‐up: Planned: not reported Actual: 36 months Intervention 2 Intervention: LASIK with an IntraLase femtosecond laser Flap dimensions: 120 μm thickness, diameter not reported, and superior hinge, degrees not reported Number of people randomized: 21 eyes, 21 participants Length of follow‐up: Planned: not reported Actual: 36 months |
|
| Outcomes |
Mean UCVA after surgery
BCVA after surgery
Proportion of eyes within ± 0.5 D of target refraction after surgery
Mean spherical equivalent of the refractive error after surgery
Sub‐basal nerve density
Corneal sensitivity
Adverse events reported: no |
|
| Identification |
Sponsorship source: National Institutes of Health, Bethesda, Maryland (Grant EY 02037; W.M.B.); Research to Prevent Blindness, Inc, New York, NY (S.V.P. as Olga Keith Wiess Special Scholar, and an unrestricted departmental grant); and Mayo Foundation, Rochester, MN Country: USA Setting: Institute of Ophthalmology Comments: none Author's name: Sanjay V Patel Institution: Department of Ophthalmology, Mayo Clinic Email: patel.sanjay @mayo.edu Address: 200 First St SW, Rochester, MN 55905 |
|
| Notes | Trial registration number: NCT00350246 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were stratified by ocular dominance and then 1 eye of each patient was randomized to LASIK with the flap created by a femtosecond laser, and the other eye to LASIK with the flap created by a mechanical microkeratome." Comment: random component in the sequence generation process not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It was not possible to mask patients as to which treatment was received in each eye." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The videokeratography maps of each cornea were examined by 1 masked observer, and the map with the most complete image and the smallest nondigitized areas was selected for assessment of wavefront errors from the anterior corneal surface." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All subjects were included for analysis through 12 months of follow‐up after LASIK. After 1 year, 4 eyes of 2 patients required enhancement procedures for mild undercorrections, which were similar in the fellow eyes; data for these eyes were retained in the analysis at 36 months. Visual acuity and whole‐eye aberrometry data were excluded in both eyes of 1 patient at 36 months because of the presence of visually significant nuclear sclerotic cataracts; corneal topography data for this patient were included. One eye of 1 patient experienced trauma‐induced recurrent erosions between 13 and 22 months after surgery; no erosions occurred after that time and data for this eye were included at 36 months." |
| Selective reporting (reporting bias) | Unclear risk | Comment: some outcomes reported in the different reports of the trial were not prespecified in trial registration (www.clinicaltrials.gov/ct2/show/trial/NCT00350246) |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Salomão 2009.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: within person Number randomly assigned: 183 eyes, 183 participants Exclusions after randomization: 0 Number analyzed: 183 eyes, 183 participants Unit of analysis: eye Losses to follow‐up: 0 Handling of missing data: no missing data Power calculation: not reported Study dates: 2005 to 2007 |
|
| Participants |
Country: USA Overall mean age: 44 years (SD not reported) Age range: 20 to 72 years Gender: women 53.5%, men 46.5% Setting: Cole Eye Institute, Cleveland, OH Equivalence of baseline characteristics: yes Inclusion criteria: people with low‐to‐moderate myopia with no symptoms or signs of dry eye before LASIK Exclusion criteria: previous eye surgery, topical ocular medications before surgery, and other ocular conditions, such as ocular rosacea or chronic blepharitis |
|
| Interventions |
Laser for ablation: Visx Star S4 IR or LADARWave 600 Intervention 1 Intervention: LASIK with a Hansatome microkeratome Flap dimensions: 180 μm thickness, 9.5 mm diameter, superior hinge Number of people randomized: 70 eyes, 70 participants Length of follow‐up: Planned: not reported Actual: 9 months Intervention 2 Intervention: LASIK with an IntraLase FS femtosecond laser Flap dimensions: 100 μm to 110 μm thickness, 9.0 mm to 9.3 mm diameter, and superior hinge Number of people randomized: 113 eyes, 113 participants Length of follow‐up: Planned: not reported Actual: 9 months |
|
| Outcomes |
Adverse events reported: yes Dry eye
|
|
| Identification |
Sponsorship source: United States Public Health Service grants EY010056 and EY015638 from the National Eye Institute, Bethesda, Maryland, and Research to Prevent Blindness, New York, NY, USA Country: USA Setting: Institute of Ophthalmology Comments: none Author's name: Steven E Wilson Institution: Cole Eye Institute, I‐32, Cleveland Clinic Email: wilsons4@ccf.org Address: 9500 Euclid Avenue, Cleveland, OH 44195 |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "One eye of each patient was randomly chosen for inclusion in the trial." Comment: random component in the sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: trial did not address this risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: trial did not address this risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: trial did not address this risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Tan 2007.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: within person Number randomly assigned: 82 eyes, 41 participants Exclusions after randomization: 0 Number analyzed: 82 eyes, 41 participants Unit of analysis: eye Losses to follow‐up: 0 Handling of missing data: no missing data Power calculation: not reported Study dates: not reported |
|
| Participants |
Country: Singapore Overall mean age: 31,4 (SD 5.3) Age range: 21 to 42 years Gender: women 82.9%, men 17.1% Setting: The Eye Institute at Tan Tock Seng Hospital Equivalence of baseline characteristics: Yes Inclusion criteria: "All participants had not undergone any previous ocular surgery to either eye" Exclusion criteria: "...were excluded from the trial if they had serious preexisting ocular pathology" |
|
| Interventions |
Laser for ablation: Technolas Z100 Excimer laser Intervention 1 Intervention: LASIK with a Zyoptix XP microkeratome Flap dimensions: 120 μm thickness, 8.5 mm diameter, superior hinge Number of people randomized: 41 eyes, 41 participants Length of follow‐up: Planned: not reported Actual: 1 day Intervention 2 Intervention: LASIK with a Intralase laser Flap dimensions: 120 μm thickness, 8.5 mm diameter, superior hinge Number of people randomized: 41 eyes, 41 participants Length of follow‐up: Planned: not reported Actual: 1 day |
|
| Outcomes | Light perception
|
|
| Identification |
Sponsorship source: not reported Country: Singapore Setting: Institute of Ophthalmology Comments: none Author's name: Hung‐Ming Lee Institution: The Eye Institute, Tan Tock Seng Hospital Email: Hung_Ming_Lee@ttsh.com.sg Address: 11 Jalan Tan Tock Seng, Singapore 308433, Singapore |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: random component in the sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: trial did not address this risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: trial did not address this risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: trial did not address this risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Tran 2005.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: within person Number randomly assigned: 18 eyes, 9 participants Exclusions after randomization: 1 eye, 1 participant Number analyzed: 14 eyes, 7 participants Unit of analysis: eye Losses to follow‐up: 0 reported Handling of missing data: not reported Power calculation: not reported Study dates: not reported |
|
| Participants |
Country: USA Overall mean age: 37 years (SD 9.5) Age range: 23 to 50 years Gender: women 23%, men 77% Setting: private practice refractive surgery center, Irvine, CA Equivalence of baseline characteristics: yes Inclusion criteria: people with myopia or myopic astigmatism, visual acuity correctable to ≥ 20/40 in both eyes, stable refraction ≤ 4.00 D of spherical myopia, and no more than 2.00 D of refractive astigmatism in both eyes. Differences between fellow eyes had to be < 0.75 D in sphere and 0.50 D in cylinder. Exclusion criteria: residual, recurrent, or active ocular disease; previous ocular surgery; corneal topographic or pachymetry (or both) findings suspicious for keratoconus; and systemic autoimmune, connective tissue, or atopic disease |
|
| Interventions |
Laser for ablation: Technolas 217A Intervention 1 Intervention: LASIK with a Hansatome microkeratome Flap dimensions: 160 μm thickness, 9.5 mm diameter, superior hinge Number of people randomized: 9 eyes, 9 participants Length of follow‐up: Planned: not reported Actual: 3 months Intervention 2 Intervention: LASIK with an IntraLase FS femtosecond laser Flap dimensions: 120 μm thickness, 8.8 mm diameter, and 45 degree superior hinged Number of people randomized: 9 eyes, 9 participants Length of follow‐up: Planned: not reported Actual: 3 months |
|
| Outcomes |
Adverse events reported: yes Diffuse lamellar keratitis
Epithelial ingrowth
|
|
| Identification |
Sponsorship source: IntraLase Corp Country: USA Setting: private practice refractive surgery center Comments: none Author's name: Dan B Tran Institution: Coastal Vision Medical Group Email: dr.tran@coastallaservision Address: 709 East Anaheim Street, Long Beach, CA 90813, USA |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each eye of each patient was randomized to receive mechanical (Hansatome)." Comment: random component in the sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: masking of participants and trial personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: masking of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One eye of 1 patient was mildly amblyopic (BSCVA 20/25). The postoperative manifest refraction showed a high degree of variability in the amblyopic eye. This patient was dropped from the analysis." |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | High risk | Quote: "Funded by IntraLase Corp., Irvine, California, USA. Drs. Tran, Sarayba, Bor, and Duh are paid consultants to IntraLase Corp, an industry directly related to one of the intervention groups evaluated. |
Zhai 2013.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Number randomly assigned: 117 eyes, 60 participants Exclusions after randomization: none Number analyzed: 117 eyes, 60 participants Unit of analysis: eye Losses to follow‐up: not reported Handling of missing data: not reported Power calculation: not reported Study dates: December 2011 to August 2012 |
|
| Participants |
Country: China Overall mean age: 28.57 years (SD 4.83) Age range: 24 to 47 years Setting: Ophthalmic Center, Beijing Tongren Hospital Equivalence of baseline characteristics: yes Inclusion criteria: > 18 year old patients with myopia or myopic astigmatism, stable refraction for more than two years Exclusion criteria: not reported |
|
| Interventions |
Laser for ablation: not reported Intervention 1 Intervention: LASIK with a M2 Moria Microkeratome Flap dimensions: 110 μm thickness, 8.5 mm diameter, superior hinge Number of people randomized: 58 eyes, 30 participants Length of follow‐up: Planned: not reported Actual: 1 month Intervention 2 Intervention: LASIK with Wavelight FS200 Femtosecond laser Flap dimensions: 110 μm thickness, 8.7 mm diameter, superior hinge Number of people randomized: 59 eyes, 30 participants Length of follow‐up: Planned: not reported Actual: 1 month |
|
| Outcomes | Central corneal flap thickness
|
|
| Identification |
Sponsorship source: not reported Country: China Setting: Institute of Ophthalmology Comments: none Author's name: Zhai Chang‐Bin Institution: Ophthalmic Center, Beijing Tongren Hospital Email: zhaicbj@163.com Address: Capital Medical University, Beijing 100730, China |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: masking of participants and trial personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: masking of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
Zhou 2012.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Number randomly assigned: 720 eyes, 360 participants Exclusions after randomization: Number analyzed: 720 eyes, 360 participants Unit of analysis: eye Losses to follow‐up: not reported Handling of missing data: not reported Power calculation: not reported Study dates: December 2009 to July 2010 |
|
| Participants |
Country: China Overall mean age: 25.91 years (SD 5.03) Age range: not reported Gender: not reported Setting: Ophthalmic Center, Beijing Tongren Hospital, Capital Medical University Equivalence of baseline characteristics: yes Inclusion criteria: not reported Exclusion criteria: patients with ocular pathologies such as keratoconus, corneal scars, corneal dystrophies, previous ocular surgery, glaucoma, diabetes, or other systemic diseases known to affect the eye were excluded |
|
| Interventions |
Laser for ablation: Visx S4 excimer laser Intervention 1 Intervention: LASIK with a M2 Moria Microkeratome Flap dimensions: 110 μm thickness, 8.5 mm diameter, superior hinge Number of people randomized: 360 eyes, 180 participants Length of follow‐up: Planned: not reported Actual: 1 week Intervention 2 Intervention: LASIK with an Ziemer LDV femtosecond laser Flap dimensions: 110 μm thickness, 8.5 mm diameter, superior hinge Number of people randomized: 360 eyes, 180 participants Length of follow‐up: Planned: not reported Actual: 1 week |
|
| Outcomes | Central corneal flap thickness
Mean corneal flap thickness
Nasal and temporal flap thickness
Flap dimensions and regularity
Flap thickness accuracy
|
|
| Identification |
Sponsorship source: not reported Country: China Setting: Institute of Ophthalmology Comments: none Author's name: Yuehua Zhou Institution: Ophthalmic Center, Beijing Tongren Hospital Email: yh0220@yahoo.com Address: No. 1 Dongjiaomin Ln, Dongchong District, Beijing, China 100730 |
|
| Notes | Trial registration number: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Based on a randomization table, 180 patients were assigned to the Ziemer LDV (FS laser group) and 180 patients were assigned to the Moria M2 (microkeratome group) for bilateral LASIK" |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: masking of participants and trial personnel not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: masking of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no published trial protocol with which to compare |
| Other bias | Low risk | Comment: trial appeared free of other sources of bias |
BCVA: best corrected visual acuity; D: diopter; IOP: intraocular pressure; LASIK: laser‐assisted in‐situ keratomileusis; SD: standard deviation; UCVA: uncorrected visual acuity.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AlArfaj 2014 | Non‐randomized trial |
| Alió 2008 | Non‐randomized trial |
| Avetisov 2016 | Non‐randomized trial |
| Brar 2008 | Non‐randomized trial |
| Cañadas 2013 | Non‐randomized trial |
| Cosar 2013 | Non‐randomized study |
| Elmohamady 2018 | Non‐randomized study |
| Erie 2006 | Non‐randomized study |
| Grewal 2011 | Non‐randomized study |
| Hamilton 2008 | Non‐randomized study |
| He 2017 | Non‐randomized study |
| Hosny 2013 | Non‐randomized study |
| Hu 2015 | Non‐randomized study |
| Hussain 2015 | Non‐randomized study |
| ISRCTN43661922 | Non‐randomized study |
| Jia 2014 | Non‐randomized study |
| Jiang 2015 | Non‐randomized study |
| Kanellopoulos 2013 | Non‐randomized study |
| Kasetsuwan 2016 | Non‐randomized study |
| Kezirian 2004 | Non‐randomized study |
| Kostin 2012 | Non‐randomized study |
| Kouassi 2012 | Non‐randomized study |
| Krueger 2007 | Non‐randomized study |
| Lee 2005 | Non‐randomized study |
| Lei 2016 | Non‐randomized study |
| Li 2007 | Non‐randomized study |
| Li 2010 | Non‐randomized study |
| Li 2012 | Non‐randomized study |
| Lian 2013 | Non‐randomized study |
| Lim 2006 | Non‐randomized study |
| Lin 2012 | Non‐randomized study |
| Lin 2016 | Non‐randomized study |
| Mai 2012 | Non‐randomized study |
| Malhotra 2015 | Non‐randomized study |
| Manche 2005 | Non‐randomized study |
| McLaren 2007 | Non‐randomized study |
| Medeiros 2007 | Non‐randomized study |
| Montés Micó 2007a | Non‐randomized study |
| Montés Micó 2007b | Non‐randomized study |
| Muñoz 2010 | Non‐randomized study |
| Nau 2006 | Non‐randomized study |
| Nau 2007 | Non‐randomized study |
| NCT03193411 | Non‐randomized study |
| NCT03484468 | Non‐randomized study |
| NCT03597906 | Not an intervention or comparator of interest |
| Patel 2006 | Non‐randomized study |
| Patel 2008 | Non‐randomized study |
| Rosa 2009 | Non‐randomized study |
| Shetty 2012 | Non‐randomized study |
| Sonigo 2006 | Non‐randomized study |
| Torky 2017 | Non‐randomized study |
| von Jagow 2009 | Non‐randomized study |
| Xia 2015 | Non‐randomized study |
| Xie 2014 | Non‐randomized study |
| Zhang 2011 | Non‐randomized study |
| Zhang 2012 | Non‐randomized study |
| Zhang 2013 | Non‐randomized study |
Characteristics of ongoing studies [ordered by study ID]
PACTR201708002498199.
| Study name | Mechanical versus femtolaser corneal flaps assessment |
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Number randomly assigned: 160 eyes, 80 participants Exclusions after randomization: not reported Number analyzed: not reported Unit of analysis: eye Losses to follow‐up: not reported Handling of missing data: not reported Power calculation: not reported Study dates: not reported |
| Participants |
Country: Egypt Overall mean age: not reported Age range: 19 to 45 years Gender: not reported Setting: Tiba Eye Center Equivalence of baseline characteristics: not reported Inclusion criteria: "Myopic patients with refraction less than ‐6.00 D, astigmatism less than ‐3.00 D and a stable keratometry after cessation of soft contact lens wear for at least 2 weeks or rigid gas‐permeable contact lens wear for at least 4 weeks with K reading value ranging between 41.50 and 45.00 D". Exclusion criteria: "Corneal pachymetry was < 520 μm at the thinnest location. Patients who do not fit the inclusion criteria or with history of herpetic eye disease, corneal dystrophy, corneal scarring because of infection or trauma, keratoconus, severe dry eye, collagen vascular disease, cataract, retinal disease, diabetes mellitus, pregnancy and who showed intraoperative and postoperative complications". |
| Interventions |
Laser for ablation: not reported Intervention 1 Intervention: LASIK with a Moria microkeratome Flap dimensions: not reported Number of people randomized: 80 eyes, 40 participants Length of follow‐up: Planned: not reported Actual: not reported Intervention 2 Intervention: LASIK with a femtosecond laser (commercial name not reported) Flap dimensions: not reported Number of people randomized: 80 eyes, 40 participants Length of follow‐up: not reported Planned: not reported Actual: not reported |
| Outcomes | Corneal flap thickness
Corneal flap symmetry
|
| Starting date | |
| Contact information |
Principal investigator: Abdel Rahman El Sebaey Sarhan email: rahman_sebaey@yahoo.com |
| Notes | Trial registration number:PACTR201708002498199 |
D: diopter; LASIK: laser‐assisted in‐situ keratomileusis.
Differences between protocol and review
The use of either mechanical microkeratome with LASIK or femtosecond with LASIK as standard of care, varies widely by geography. Whereas femtosecond laser is the standard of care in high‐income countries, the mechanical microkeratome remains the standard of care in low‐income countries (Salomao 2010). During the process of completing the review, we changed the title from: 'Femtosecond laser versus mechanical microkeratome use for laser‐assisted in‐situ keratomileusis (LASIK) in adults with myopia' to 'Laser‐assisted in‐situ keratomileusis (LASIK) with a mechanical microkeratome compared to LASIK with a femtosecond laser for LASIK in adults with myopia or myopic astigmatism' to reflect the comparison between microkeratome with LASIK as the intervention to femtosecond with LASIK (as the standard of care), as used in high‐income countries. During the review process we changed four outcomes in the 'Summary of findings' table specified in the protocol due to insufficient data available from the trials. All outcomes incorporated in the 'Summary of findings' table were prespecified secondary outcomes in the protocol.
The following outcomes were projected in the protocol to be included in this review but there were insufficient data to include them in the 'Summary of findings' table.
Proportion of eyes with loss of two or more lines of best corrected visual acuity (BCVA) 12 months after surgery.
Postoperative pain within one week after surgery.
Quality of life score 12 months after surgery.
We had indicated in the protocol that the participant was the primary unit of analysis (only one eye per participant) and when two eyes of the same participant were randomized to two different interventions (paired‐eye design) we used only the results that accounted for this correlation. However, post hoc we decided to include results from studies that used paired‐eye design without accounting for correlation in their analysis.
Contributions of authors
NKL developed and designed the protocol with input from AN, CCS, EGH, AJC, and AI.
NKL, AN, CCS, EGH, AJC, and AI contributed to the content and writing of the protocol.
Conceiving the review: NKL, AN
Designing the review: NKL
Co‐ordinating the review: NKL
Data collection for the review:
designing search strategies: Lori Rosman
undertaking searches: Lori Rosman
screening search results: NKL, AN, EGH
organizing retrieval of papers: Lori Rosman
screening retrieved papers against inclusion criteria: NKL, AN, EGH
appraising risk of bias in trials: NKL, AN, AI
extracting data from papers: NKL, AN, AI
writing to authors of papers for additional information: NKL
Data management for the review:
entering data into Review Manager 5: NKL
Analysis of data: NKL
Interpretation of data:
providing a methodologic perspective: NKL, CCS
providing a clinical perspective: NKL, AN, EGH
Writing the review: NKL
Providing general advice on the review: CCS
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Eyes and Vision (CEV) US Project, supported by grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA
-
National Institute for Health Research (NIHR), UK
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
NKL: none AN: none CCS: none EGH: none AJC: none AI: none
Edited (no change to conclusions)
References
References to studies included in this review
Buzzonetti 2008 {published data only}
- Buzzonetti L, Petrocelli G, Valente P, Tamburrelli C, Mosca L, Laborante A, et al. Comparison of corneal aberration changes after laser in situ keratomileusis performed with mechanical microkeratome and IntraLase femtosecond laser: 1-year follow-up. Cornea 2008;27(2):174-9. [DOI] [PubMed] [Google Scholar]
Durrie 2005 {published data only}
- Durrie DS, Kezirian GM. Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis: prospective contralateral eye study. Journal of Cataract and Refractive Surgery 2005;31(1):120-6. [DOI] [PubMed] [Google Scholar]
Gui‐Hong 2018 {published data only}
- Gui-Hong Xu, Zhen-Zhen Wu, Xiao-Hong Gu. Influence of microkeratome and femtosecond laser on vision and corneal flap thickness used in corneal flap making. Guoji Yanke Zazhi 2018;18(5):894-6. [Google Scholar]
Hasimoto 2013 {published data only}
- Hasimoto AR, Gomes MF, Siqueira MA, Moreira H. Femtosecond laser versus mechanical microkeratome for LASIK flap creation. Arquivos Brasileiros de Oftalmologia 2013;76(6):335-8. [DOI] [PubMed] [Google Scholar]
Manche_group 2008 {published data only}
- Chan A, Ou J, Manche EE. Comparison of the femtosecond laser and mechanical keratome for laser in situ keratomileusis. Archives of Ophthalmology 2008;126(11):1484-90. [DOI] [PubMed] [Google Scholar]
- Golas L, Manche EE. Dry eye after laser in situ keratomileusis with femtosecond laser and mechanical keratome. Journal of Cataract and Refractive Surgery 2011;37(8):1476-80. [DOI] [PubMed] [Google Scholar]
Patel_group 2010 {published data only}
- Calvo R, McLaren JW, Hodge DO, Bourne WM, Patel SV. Corneal aberrations and visual acuity after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. American Journal of Ophthalmology 2010;149(5):785-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler KN, McLaren JW, Bourne WM, Patel SV. Corneal endothelial cell changes 5 years after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. Journal of Cataract and Refractive Surgery 2012;38(12):2125-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren JW, Bourne WM, Maguire LJ, Patel SV. Changes in keratocyte density and visual function five years after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. American Journal of Ophthalmology 2015;160(1):163-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome for LASIK: a randomized controlled study. Ophthalmology 2007;114(8):1482-90. [DOI] [PubMed] [Google Scholar]
- Patel SV, McLaren JW, Kittleson KM, Bourne WM. Subbasal nerve density and corneal sensitivity after laser in situ keratomileusis: femtosecond laser vs mechanical microkeratome. Archives of Ophthalmology 2010;128(11):1413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salomão 2009 {published data only}
- Salomão MQ, Ambrósio R Jr, Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. Journal of Cataract and Refractive Surgery 2009;35(10):1756-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2007 {published data only}
- Tan CS, Au Eong KG, Lee HM. Visual experiences during different stages of LASIK: Zyoptix XP microkeratome vs IntraLase femtosecond laser. American Journal of Ophthalmology 2007;143(1):90-6. [DOI] [PubMed] [Google Scholar]
Tran 2005 {published data only}
- Tran DB, Sarayba MA, Bor Z, Garufis C, Duh YJ, Soltes CR, et al. Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes: potential impact on wavefront-guided laser in situ keratomileusis. Journal of Cataract and Refractive Surgery 2005;31(1):97-105. [DOI] [PubMed] [Google Scholar]
Zhai 2013 {published data only}
- Zhai CB, Tian L, Zhou YH, Zhang QW, Zhang J. Comparison of the flaps made by femtosecond laser and automated keratomes for sub-bowman keratomileusis. Chinese Medical Journal 2013;126(13):2440-4. [PubMed] [Google Scholar]
Zhou 2012 {published data only}
- Zhou Y, Zhang J, Tian L, Zhai C. Comparison of the Ziemer FEMTO LDV femtosecond laser and Moria M2 mechanical microkeratome. Journal of Refractive Surgery 2012;28(3):189-94. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AlArfaj 2014 {published data only}
- AlArfaj K, Hantera MM. Comparison of LASEK, mechanical microkeratome LASIK and femtosecond LASIK in low and moderate myopia. Saudi Journal of Ophthalmology 2014;28(3):214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alió 2008 {published data only}
- Alió Jl, Piñero DP. Very high-frequency digital ultrasound measurement of the LASIK flap thickness profile using the IntraLase femtosecond laser and M2 and Carriazo-Pendular microkeratomes. Journal of Refractive Surgery 2008;24(1):12-23. [DOI] [PubMed] [Google Scholar]
Avetisov 2016 {published data only}
- Avetisov SE, Mamikonyan VR, Shmeleva-Demir OA, Karamyan AA, Bubnova IA, Kazaryan EE, et al. Intraocular pressure, ocular blood flow, and corneal biomechanics changes after LASIK surgery for myopia. Vestnik Oftalmologii 2016;132(4):24-8. [DOI] [PubMed] [Google Scholar]
Brar 2008 {published data only}
- Brar GS, Grewal DS, Jain R, Grewal SP. Changes in elevation and corneal volume following LASIK with flap creation using IntraLase, mechanical keratome and LASEK. Investigative Ophthalmology and Visual Science 2008;49:ARVO E – abstract 2441. [Google Scholar]
Cañadas 2013 {published data only}
- Cañadas P, Benito-Llopis L, Hernández-Verdejo JL, Teus MA. Comparison of keratocyte density after femtosecond laser vs mechanical microkeratome from 3 months up to 5 years after LASIK. Graefe's Archive for Clinical and Experimental Ophthalmology 2013;251(9):2171-9. [DOI] [PubMed] [Google Scholar]
Cosar 2013 {published data only}
- Cosar CB, Gonen T, Moray M, Sener AB. Comparison of visual acuity, refractive results and complications of femtosecond laser with mechanical microkeratome in LASIK. International Journal of Ophthalmology 2013;6(3):350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmohamady 2018 {published data only}
- Elmohamady MN, Abdelghaffar W, Daifalla A, Salem T. Evaluation of femtosecond laser in flap and cap creation in corneal refractive surgery for myopia: a 3-year follow-up. Clinical Ophthalmology 2018;12:935-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erie 2006 {published data only}
- Erie JC, Patel SV, McLaren JW, Bourne WM. Corneal nerve morphology and function after bladeless and microkeratome LASIK. A randomized-controlled study. Investigative Ophthalmology and Visual Science 2006;47:ARVO E – abstract 516. [Google Scholar]
Grewal 2011 {published data only}
- Grewal DS, Brar GS, Grewal SP. Posterior corneal elevation after LASIK with three flap techniques as measured by Pentacam. Journal of Refractive Surgery 2011;27(4):261-8. [DOI] [PubMed] [Google Scholar]
Hamilton 2008 {published data only}
- Hamilton DR, Johnson RD, Lee N, Bourla N. Differences in the corneal biomechanical effects of surface ablation compared with laser in situ keratomileusis using a microkeratome or femtosecond laser. Journal of Cataract and Refractive Surgery 2008;34(12):2049-56. [DOI] [PubMed] [Google Scholar]
He 2017 {published data only}
- He F, Tan HX. Comparative study of clinical efficacy after LASIK with corneal flap created by femtosecond laser and microkeratome. International Eye Science 2017;17(11):2120-22. [Google Scholar]
Hosny 2013 {published data only}
- Hosny M, Zaki RM, Ahmed RA, Khalil N, Mostafa HM. Changes in retinal nerve fiber layer thickness following mechanical microkeratome-assisted versus femtosecond laser-assisted LASIK. Clinical Ophthalmology 2013;7:1919-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2015 {published data only}
- Hu L, Xie W, Tang L, Chen J, Zhang D, Yu P, et al. Corneal subbasal nerve density changes after laser in situ keratomileusis with mechanical microkeratome and femtosecond laser. Chung-Hua Yen Ko Tsa Chih 2015;51(1):39-44. [PubMed] [Google Scholar]
Hussain 2015 {published data only}
- Hussain M, Tanaka TS, Greene JB, Mian S, Shtein RM. Long-term ocular surface outcomes and in vivo corneal confocal microscopy in patients with mechanical microkeratome vs femtosecond laser-assisted LASIK. Investigative Ophthalmology and Visual Science 2015;56(7):3912. [Google Scholar]
ISRCTN43661922 {published data only}
- ISRCTN43661922. Comparison of dry eye syndrome and corneal sensation after femtosecond- and microkeratome-assisted LASIK. isrctn.com/ISRCTN43661922 (first received 28 February 2011).
Jia 2014 {published data only}
- Jia BB, Zhang Y, Gao DM, Pang YZ. Changes of tear film after LASIK with corneal flap created by femtosecond laser and microkeratome. International Eye Science 2014;14(9):1730-2. [Google Scholar]
Jiang 2015 {published data only}
- Jiang HS, Wu WH, Wang WW. Visual quality analysis of femtosecond LASIK and iris location guided mechanical SBK for high myopia. International Eye Science 2015;15(7):1168-71. [Google Scholar]
Kanellopoulos 2013 {published data only}
- Kanellopoulos AJ, Asimellis G. Three-dimensional LASIK flap thickness variability: topographic central, paracentral and peripheral assessment, in flaps created by a mechanical microkeratome (M2) and two different femtosecond lasers (FS60 and FS200). Clinical Ophthalmology 2013;7:675-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasetsuwan 2016 {published data only}
- Kasetsuwan N, Satitpitakul V, Puangsricharern V, Reinprayoon U, Pariyakanok L. Comparison of performances of femtosecond laser and microkeratome for thin-flap laser in situ keratomileusis. Lasers in Surgery and Medicine 2016;48(6):596-601. [DOI] [PubMed] [Google Scholar]
Kezirian 2004 {published data only}
- Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. Journal of Cataract and Refractive Surgery 2004;30(4):804-11. [DOI] [PubMed] [Google Scholar]
Kostin 2012 {published data only}
- Kostin OA, Rebrikov SV, Ovchinnikov AI, Stepanov AA. Corneal flap analysis after LASIK and femto-LASIK using optical coherence tomography and optical sections. Vestnik Oftalmologii 2012;128(5):3-5. [PubMed] [Google Scholar]
Kouassi 2012 {published data only}
- Kouassi FX, Blaizeau M, Buestel C, Schweitzer C, Gallois A, Colin J, et al. Comparison of Lasik with femtosecond laser versus Lasik with mechanical microkeratome: predictability of flap depth, corneal biomechanical effects and optical aberrations. Journal Francais d'Ophtalmologie 2012;35(1):2-8. [DOI] [PubMed] [Google Scholar]
Krueger 2007 {published data only}
- Krueger RR, Dupps WJ Jr. Biomechanical effects of femtosecond and microkeratome-based flap creation: prospective contralateral examination of two patients. Journal of Refractive Surgery 2007;23(8):800-7. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee HM, Koh A, Heng WJ, Fam HB. IntraLase femtosecond laser and hansatome microkeratome for LASIK: a comparative study. In: American Academy of Ophthalmology. 2005:240.
Lei 2016 {published data only}
- Lei XH, Yu CT, Zhang Y, Li J, Ma M. Effect of laser in situ keratomileusis with femtosecond laser on visual quality. International Eye Science 2016;16(6):1120-3. [Google Scholar]
Li 2007 {published data only}
- Li H, Sun T, Zhao J. Femtosecond laser vs. mechanical keratome in thin flap LASIK for correction of high myopia. In: American Academy of Ophthalmology. 2007:251.
Li 2010 {published data only}
- Li H, Sun T, Wang M, Zhao J. Safety and effectiveness of thin-flap LASIK using a femtosecond laser and microkeratome in the correction of high myopia in Chinese patients. Journal of Refractive Surgery 2010;26(2):99-106. [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li FS, Zhang J, Yin HZ, Zhou YH. Comparison of LASIK flap thickness created with Ziemer LDV femtosecond laser and Moria M2 mechanical microkeratome. International Eye Science 2012;12(6):1056-8. [Google Scholar]
Lian 2013 {published data only}
- Lian JC, Zhang SS, Zhang J, Ye S. Comparison of cornea flap made by femtosecond laser and microkeratome in laser in situ keratomileusis. Chung-Hua Yen Ko Tsa Chih 2013;49(4):305-8. [PubMed] [Google Scholar]
Lim 2006 {published data only}
- Lim T, Yang S, Kim M, Tchah H. Comparison of the IntraLase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. American Journal of Ophthalmology 2006;141(5):833-9. [DOI] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin MY, Chang DC, Hsu WM, Wang IJ. Cox proportional hazards model of myopic regression for laser in situ keratomileusis flap creation with a femtosecond laser and with a mechanical microkeratome. Journal of Cataract and Refractive Surgery 2012;38(6):992-9. [DOI] [PubMed] [Google Scholar]
Lin 2016 {published data only}
- Lin MY, Chang DC, Shen YD, Lin YK, Lin CP, Wang IJ. Factors influencing intraocular pressure changes after laser in situ keratomileusis with flaps created by femtosecond laser or mechanical microkeratome. PloS One 2016;11(1):e0147699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mai 2012 {published data only}
- Mai ZB, Liu SB, Nie XL, Tang XX, Xin BL. Comparative observation of confocal microscopy between femtosecond laser LASIK and Hansatome microkeratome LASIK. Zhonghua Shiyan Yanke Zazhi 2012;30(7):633-7. [Google Scholar]
Malhotra 2015 {published data only}
- Malhotra C, Jain AK, Veluswami J, Ram J, Gupta R, Kumar P. Higher order aberrations and visual outcomes in wavefront-optimized sub-Bowman keratomileusis: flap creation using femtosecond laser versus mechanical microkeratome. Asia-Pacific Journal of Ophthalmology 2015;4(4):197-203. [DOI] [PubMed] [Google Scholar]
Manche 2005 {published data only}
- Manche EE. A prospective randomized eye-to-eye comparison: IntraLase vs. hansatome in myopic LASIK with CustomVue. In: American Academy of Ophthalmology. 2005:174.
McLaren 2007 {published data only}
- McLaren JW, Patel SV, Nau CB, Winter EJ, Bourne WM. Corneal wavefront errors one year after LASIK: a paired comparison between flap cut with a femtosecond laser and with a mechanical microkeratome. Investigative Ophthalmology and Visual Science 2007;48:ARVO E – abstract 2360. [Google Scholar]
Medeiros 2007 {published data only}
- Medeiros FW, Stapleton WM, Hammel J, Krueger RR, Netto MV, Wilson SE. Wavefront analysis comparison of LASIK outcomes with the femtosecond laser and mechanical microkeratomes. Journal of Refractive Surgery 2007;23(9):880-7. [DOI] [PubMed] [Google Scholar]
Montés Micó 2007a {published data only}
- Montés-Micó R, Rodríguez-Galietero A, Alió JL, Cerviño A. Contrast sensitivity after LASIK flap creation with a femtosecond laser and a mechanical microkeratome. Journal of Refractive Surgery 2007;23(2):188-92. [DOI] [PubMed] [Google Scholar]
Montés Micó 2007b {published data only}
- Montés-Micó R, Rodríguez-Galietero A, Alió JL. Femtosecond laser versus mechanical keratome LASIK for myopia. Ophthalmology 2007;114(1):62-8. [DOI] [PubMed] [Google Scholar]
Muñoz 2010 {published data only}
- Muñoz G, Albarrán-Diego C, Ferrer-Blasco T, García-Lázaro S, Cerviño-Expósito A. Long-term comparison of corneal aberration changes after laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser flap creation. Journal of Cataract and Refractive Surgery 2010;36(11):1934-44. [DOI] [PubMed] [Google Scholar]
Nau 2006 {published data only}
- Nau CB, McLaren JW, Maguire LJ, Patel, SV, Bourne WM. Keratocyte density after LASIK: does cutting the flap with a femtosecond laser make a difference? Investigative Ophthalmology and Visual Science 2006;47:ARVO E – abstract 542. [Google Scholar]
Nau 2007 {published data only}
- Nau CB, Patel SV, McLaren JW, Maguire LJ, Bourne WM. Central epithelial and flap thickness after LASIK: femtosecond laser vs. mechanical microkeratome. Investigative Ophthalmology and Visual Science 2007;48:ARVO E – abstract 5329.
NCT03193411 {published data only}
- NCT03193411. Visumax femtolasik versus moria M2 microkeratome in myopia. clinicaltrials.gov/ct2/show/NCT03193411 (first received 20 June 2017).
NCT03484468 {published data only}
- NCT03484468. Femtosecond laser versus microkeratome in creating corneal flaps in LASIK. clinicaltrials.gov/ct2/show/NCT03484468 (first received 30 March 2018).
NCT03597906 {published data only}
- NCT03597906. Topography guided LASIK by different protocols for treatment of astigmatism. clinicaltrials.gov/ct2/show/NCT03597906 (first received 24 July 2018).
Patel 2006 {published data only}
- Patel SV, McLaren JW, Maguire LJ, Bourne WM. A randomized-controlled study of bladeless and microkeratome LASIK. Investigative Ophthalmology and Visual Science 2006;47:ARVO E – abstract 4331. [Google Scholar]
Patel 2008 {published data only}
- Patel S, Alió JL, Artola A. Changes in the refractive index of the human corneal stroma during laser in situ keratomileusis. Effects of exposure time and method used to create the flap. Journal of Cataract and Refractive Surgery 2008;34(7):1077-82. [DOI] [PubMed] [Google Scholar]
Rosa 2009 {published data only}
- Rosa AM, Neto Murta J, Quadrado MJ, Tavares C, Lobo C, Van Velze R, et al. Femtosecond laser versus mechanical microkeratomes for flap creation in laser in situ keratomileusis and effect of postoperative measurement interval on estimated femtosecond flap thickness. Journal of Cataract and Refractive Surgery 2009;35(5):833-8. [DOI] [PubMed] [Google Scholar]
Shetty 2012 {published data only}
- Shetty R, Malhotra C, D'Souza S, Wadia K. WaveLight FS200 vs Hansatome LASIK: intraoperative determination of flap characteristics and predictability by hand-held bioptigen spectral domain ophthalmic imaging system. Journal of Refractive Surgery 2012;28(11 Suppl):S815-20. [DOI] [PubMed] [Google Scholar]
Sonigo 2006 {published data only}
- Sonigo B, Iordanidou V, Chong-Sit D, Auclin F, Ancel JM, Labbé A, et al. In vivo corneal confocal microscopy comparison of IntraLase femtosecond laser and mechanical microkeratome for laser in situ keratomileusis. Investigative Ophthalmology and Visual Science 2006;47(7):2803-11. [DOI] [PubMed] [Google Scholar]
Torky 2017 {published data only}
- Torky MA, Al Zafiri YA, Khattab AM, Farag RK, Awad EA. Visumax femtolasik versus Moria M2 microkeratome in mild to moderate myopia: efficacy, safety, predictability, aberrometric changes and flap thickness predictability. BMC Ophthalmology 2017;17(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Jagow 2009 {published data only}
- Jagow B, Kohnen T. Corneal architecture of femtosecond laser and microkeratome flaps imaged by anterior segment optical coherence tomography. Journal of Cataract and Refractive Surgery 2009;35(1):35-41. [DOI] [PubMed] [Google Scholar]
Xia 2015 {published data only}
- Xia LK, Yu J, Chai GR, Wang D, Li Y. Comparison of the femtosecond laser and mechanical microkeratome for flap cutting in LASIK. International Journal of Ophthalmology 2015;8(4):784-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2014 {published data only}
- Xie W, Zhang D, Chen J, Liu J, Yu Y, Hu L. Tear menisci after laser in situ keratomileusis with mechanical microkeratome and femtosecond laser. Investigative Ophthalmology and Visual Science 2014;55(9):5806-12. [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang ZH, Jin HY, Suo Y, Patel SV, Montés-Micó R, Manche EE, et al. Femtosecond laser versus mechanical microkeratome laser in situ keratomileusis for myopia: metaanalysis of randomized controlled trials. Journal of Cataract and Refractive Surgery 2011;37(12):2151-9. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang F, Deng S, Guo N, Wang M, Sun X. Confocal comparison of corneal nerve regeneration and keratocyte reaction between FS-LASIK, OUP-SBK, and conventional LASIK. Investigative Ophthalmology and Visual Science 2012;53(9):5536-44. [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang Y, Chen YG, Xia YJ. Comparison of corneal flap morphology using AS-OCT in LASIK with the WaveLight FS200 femtosecond laser versus a mechanical microkeratome. Journal of Refractive Surgery 2013;29(5):320-4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
PACTR201708002498199 {published data only}
- PACTR201708002498199. Mechanical versus femtolaser corneal flaps assessment. pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=2498 (first received 3 August 2017).
Additional references
Ang 2009
- Ang EK, Couper T, Dirani M, Vajpayee RB, Baird PN. Outcomes of laser refractive surgery for myopia. Journal of Cataract and Refractive Surgery 2009;35(5):921-33. [DOI] [PubMed] [Google Scholar]
Azar 2002
- Azar DT, Koch D. LASIK (Laser in Situ Keratomileusis): Fundamentals, Surgical Techniques, and Complications. Boca Raton (FL): CRC Press, 2002. [Google Scholar]
Azar 2006
- Azar DT, Gatinel D, Hoang-Xuan T. Refractive Surgery. 2nd edition. St Louis (MO): Elsevier Mosby, 2006. [Google Scholar]
Azar 2019
- Azar DT. Refractive Surgery E-Book. Elsevier Health Sciences, 2019. [Google Scholar]
Bashir 2017
- Bashir ZS, Ali MH, Anwar A, Ayub MH, Butt NH. Femto-LASIK: the recent innovation in laser assisted refractive surgery. Journal of the Pakistan Medical Association 2017;67(4):609-15. [PubMed] [Google Scholar]
Bower 2001
- Bower KS, Weichel ED, Kim TJ. Overview of refractive surgery. American Family Physician 2001;64(7):1183-90. [PubMed] [Google Scholar]
Chaurasia 2010
- Chaurasia SS, Luengo Gimeno F, Tan K, Yu S, Tan DT, Beuerman RW, et al. In vivo real-time intraocular pressure variations during LASIK flap creation. Investigative Ophthalmology and Visual Science 2010;51(9):4641-5. [DOI] [PubMed] [Google Scholar]
Chen 2012
- Chen S, Feng Y, Stojanovic A, Jankov MR 2nd, Wang Q. IntraLase femtosecond laser vs mechanical microkeratomes in LASIK for myopia: a systematic review and meta-analysis. Journal of Refractive Surgery 2012;28(1):15-24. [DOI] [PubMed] [Google Scholar]
Corcoran 2015
- Corcoran KJ. Macroeconomic landscape of refractive surgery in the United States. Current Opinion in Ophthalmology 2015;26(4):249-54. [DOI] [PubMed] [Google Scholar]
Courtin 2015
- Courtin R, Saad A, Guilbert E, Grise-Dulac A, Gatinel D. Opaque bubble layer risk factors in femtosecond laser-assisted LASIK. Journal of Refractive Surgery 2015;31(9):608-12. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed after 13 December 2018.Available at covidence.org.
Farjo 2013
- Farjo AA, Sugar A, Schallhorn SC, Majmudar PA, Tanzer DJ, Trattler WB, et al. Femtosecond lasers for LASIK flap creation: a report by the American Academy of Ophthalmology. Ophthalmology 2013;120(3):e5-20. [DOI] [PubMed] [Google Scholar]
Flanagan 2003
- Flanagan GW, Binder PS. Precision of flap measurements for laser in situ keratomileusis in 4428 eyes. Journal of Refractive Surgery 2003;19(2):113-23. [DOI] [PubMed] [Google Scholar]
Gatinel 2013
- Gatinel D, Saad A, Guilbert E, Rouger H. Unilateral rainbow glare after uncomplicated femto-LASIK using the FS-200 femtosecond laser. Journal of Refractive Surgery 2013;29(7):498-501. [DOI] [PubMed] [Google Scholar]
Gil‐Cazorla 2011
- Gil-Cazorla R, Teus MA, Benito-Llopis L, Mikropoulos DG. Femtosecond laser vs mechanical microkeratome for hyperopic laser in situ keratomileusis. American Journal of Ophthalmology 2011;152(1):16-21.e2. [DOI] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 17 December 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Holden 2016
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123(5):1036-42. [DOI] [PubMed] [Google Scholar]
Huhtala 2016
- Huhtala A, Pietilä J, Mäkinen P, Uusitalo H. Femtosecond lasers for laser in situ keratomileusis: a systematic review and meta-analysis. Clinical Ophthalmology 2016;10:393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Issa 2011
- Issa A, Al Hassany U. Femtosecond laser flap parameters and visual outcomes in laser in situ keratomileusis. Journal of Cataract and Refractive Surgery 2011;37(4):665-74. [DOI] [PubMed] [Google Scholar]
Lubatschowski 2000
- Lubatschowski H, Maatz G, Heisterkamp A, Hetzel U, Drommer W, Welling H, et al. Application of ultrashort laser pulses for intrastromal refractive surgery. Graefes Archive for Clinical and Experimental Ophthalmology 2000;238(1):33-9. [DOI] [PubMed] [Google Scholar]
Medeiros 2011
- Medeiros FW, Sinha-Roy A, Alves MR, Dupps WJ Jr. Biomechanical corneal changes induced by different flap thickness created by femtosecond laser. Clinics 2011;66(6):1067-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher David, Liberati Alessandro, Tetzlaff Jennifer, Altman Douglas G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [PMC free article] [PubMed] [Google Scholar]
Morgan 2012
- Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet 2012;379(9827):1739-48. [DOI] [PubMed] [Google Scholar]
Moshirfar 2010
- Moshirfar M, Gardiner JP, Schliesser JA, Espandar L, Feiz V, Mifflin MD, et al. Laser in situ keratomileusis flap complications using mechanical microkeratome versus femtosecond laser: retrospective comparison. Journal of Cataract and Refractive Surgery 2010;36(11):1925-33. [DOI] [PubMed] [Google Scholar]
NASEM 2016
- National Academies of Sciences, Engineering, Medicine, Teutsch SM, McCoy MA, Woodbury RB, Welp A, editor(s). Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington (DC): National Academies Press, 2016. [PubMed] [Google Scholar]
Randleman 2012
- Randleman JB, Shah RD. LASIK interface complications: etiology, management, and outcomes. Journal of Refractive Surgery 2012;28(8):575-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riordan‐Eva 2011
- Riordan-Eva P. Chapter 21. Optics & refraction. In: Riordan-Eva P, Emmett CT, editors(s). Vaughan & Asbury's General Ophthalmology. 18th edition. New York (NY): McGraw-Hill Companies, 2011. [Google Scholar]
Sales 2016
- Sales CS, Manche EE. Comparison of self-reported quality of vision outcomes after myopic LASIK with two femtosecond lasers: a prospective, eye-to-eye study. Clinical Ophthalmology 2016;10:1691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salomao 2010
- Salomao MQ, Wilson SE. Femtosecond laser in laser in situ keratomileusis. Journal of Cataract and Refractive Surgery 2010;36(6):1024-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009
- Smith TS, Frick KD, Holden BA, Fricke TR, Naidoo KS. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bulletin of the World Health Organization 2009;87(6):431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stonecipher 2006
- Stonecipher KG, Dishler JG, Ignacio TS, Binder PS. Transient light sensitivity after femtosecond laser flap creation: clinical findings and management. Journal of Cataract and Refractive Surgery 2006;32(1):91-4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kahuam‐López 2018
- Kahuam-López N, Navas A, Castillo-Salgado C, Graue-Hernandez EO, Jimenez-Corona A, Ibarra A. Femtosecond laser versus mechanical microkeratome use for laser-assisted in-situ keratomileusis (LASIK). Cochrane Database of Systematic Reviews 2018, Issue 2. [DOI: 10.1002/14651858.CD012946] [DOI] [PMC free article] [PubMed] [Google Scholar]


