Abstract
Background
Weight change may inform tuberculosis treatment response, but its predictive power may be confounded by human immunodeficiency virus (HIV).
Methods
We prospectively followed up adults with culture-confirmed, drug-susceptible, pulmonary tuberculosis receiving standard 4-drug therapy (isoniazid, rifampin, pyrazinamide, and ethambutol) in Brazil. We examined median weight change 2 months after treatment initiation by HIV status, using quantile regression, and unsuccessful tuberculosis treatment outcome (treatment failure, tuberculosis recurrence, or death) by HIV and weight change status, using Cox regression.
Results
Among 547 participants, 102 (19%) were HIV positive, and 35 (6%) had an unsuccessful outcome. After adjustment for confounders, persons living with HIV (PLWH) gained a median of 1.3 kg (95% confidence interval [CI], −2.8 to .1) less than HIV-negative individuals during the first 2 months of tuberculosis treatment. PLWH were at increased risk of an unsuccessful outcome (adjusted hazard ratio, 4.8; 95% CI, 2.1–10.9). Weight change was independently associated with outcome, with risk of unsuccessful outcome decreasing by 12% (95% CI, .81%–.95%) per 1-kg increase.
Conclusions
PLWH gained less weight during the first 2 months of tuberculosis treatment, and lack of weight gain and HIV independently predicted unsuccessful tuberculosis treatment outcomes. Weight, an easily collected biomarker, may identify patients who would benefit from alternative treatment strategies.
Keywords: Tuberculosis, HIV, body weight changes, treatment outcome, observational study
We evaluated the impact of human immunodeficiency virus (HIV) on weight gain and tuberculosis treatment outcomes. Persons living with HIV gained less weight and were more likely to have unsuccessful tuberculosis treatment outcomes (death, failure, relapse) than persons without HIV.
Tuberculosis remains a major public health problem worldwide [1–3]. According to the World Health Organization (WHO), an estimated 10 million people acquired tuberculosis and 1.3 million died of tuberculosis in 2017, making it the deadliest infectious disease in the world [1]. Predictors and surrogate end points of unsuccessful treatment outcomes are needed to allow for possible interventions to improve outcomes [4, 5].
Malnutrition is one of the main risk factors for tuberculosis, a disease characterized by wasting [6], and may negatively affect pharmacologic response to treatment [7, 8]. This vicious cycle of malnutrition and tuberculosis is further complicated by human immunodeficiency virus (HIV), a leading risk factor for tuberculosis and a predictor of tuberculosis-related death [9]. Persons living with HIV (PLWH) accounted for 9% of incident tuberculosis cases and 23% of tuberculosis deaths globally in 2017 [1]. Furthermore, the relationship between malnutrition and tuberculosis is most pronounced in PLWH [9–11].
Because of the complex relationship between tuberculosis, HIV, and malnutrition, it is important to consider HIV when examining the association between weight change and tuberculosis treatment outcome. However, previous studies that examined weight change during tuberculosis treatment, from several geographic regions and among patients with drug-susceptible or multidrug-resistant tuberculosis, have rarely addressed tuberculosis-HIV coinfection [12–18]. One study concluded that weight gain was an unreliable predictor of overall treatment response [12], whereas others found that inadequate weight gain (<5% initial body weight) after 2 months [13] or by the end of treatment [14] was associated with unsuccessful treatment outcomes among HIV-negative populations. Two additional studies suggesting that weight loss was a surrogate for tuberculosis treatment outcome included HIV-positive patients with tuberculosis, but neither examined the effect of HIV on weight change during treatment [15, 16]. Thus, we sought to examine the association between HIV and weight change during the first 2 months of antituberculosis treatment and to characterize differences in weight change patterns between PLWH and HIV-negative persons. We also assessed associations of HIV, baseline weight, and early weight change with tuberculosis treatment outcomes, including tuberculosis treatment failure, tuberculosis recurrence, and death.
METHODS
Study Design and Participants
Regional Prospective Observational Research for Tuberculosis (RePORT) Brazil is a prospective cohort study at 5 participating centers in Brazil: 3 in Rio de Janeiro (Instituto Nacional de Infectologia Evandro Chagas [INI], Clínica da Familia Rinaldo Delamare [Rocinha], and Secretaria de Saúde de Duque de Caxias [Caxias]), 1 in Salvador (Instituto Brasileiro para Investigação da Tuberculose), and 1 in Manaus (Fundação Medicina Tropical Dr Heitor Vieira Dourado). RePORT-Brazil enrolled participants ≥18 years old who started treatment for culture-confirmed pulmonary tuberculosis [19]. Participants were excluded if they had received antituberculosis treatment for ≥7 days in the past, had received >7 days of fluoroquinolone antibiotic therapy within the previous 30 days, were pregnant or breastfeeding, or did not plan to remain in the region during the 24-month follow-up period. This analysis included patients with drug-susceptible tuberculosis who were enrolled between June 2015 and 1 May 2018 and received ≥1 dose of intensive-phase standard tuberculosis therapy, with follow-up through May 2019.
Data Collection and Definitions
Standard tuberculosis therapy was the combination of isoniazid, rifampin or rifabutin, pyrazinamide, and ethambutol for 2 months, followed by isoniazid and rifampin for 4 months. Antimicrobial drug susceptibility testing was performed to assess drug resistance. At all study sites, participants are treated for tuberculosis according to guidelines by the Brazilian Ministry of Health; recommendations for treatment length, directly observed therapy (DOT), and programmatic support are the same regardless of HIV.
Demographic, clinical, and diagnostic information was collected during 3 clinical visits (baseline, 2 months after treatment initiation and at the end of treatment) and through telephone contact every 6 months thereafter through 24 months. Chest radiography at baseline was used to characterize the extent of lung disease and identify the presence or absence of lung cavitation. In accordance with the Brazilian National tuberculosis program, all participants underwent HIV testing, unless they already had a positive test result. In participants with a positive HIV test result, CD4 cell counts and HIV-1 RNA levels were obtained if none were available within the previous 6 months.
Study Outcomes and Variables of Interest
The primary outcome was change in weight, measured in kilograms, between treatment initiation and 2 months of follow-up. Weight was measured using the same scale during all visits. The exposure of interest was HIV status, based on HIV testing results at baseline unless the participant was known to have HIV. Unsuccessful treatment outcomes, including tuberculosis treatment failure, tuberculosis recurrence, or death during follow-up, were defined according to WHO guidelines [20]. Treatment failure was defined as sputum smear or culture positive for Mycobacterium tuberculosis at 5 months of treatment or later. Tuberculosis recurrence was defined as a case considered cured at treatment completion but with later symptoms of active tuberculosis and a sputum smear or culture positive for M. tuberculosis.
We examined age, sex, race (white or other, black, or mixed), household income (minimum wage or below, above minimum wage, or not reported/none), self-reported directly DOT (yes or no), tobacco use (current, former, or never), alcohol use (current, former, or never), current marijuana use, current cocaine use, baseline body mass index (BMI), baseline sputum smear result, lung cavitation on chest radiograph, glycated hemoglobin A1c, and hemoglobin as potential risk factors and confounders. BMI was categorized by WHO criteria as underweight (<18.5 kg/m2), normal weight (18.5–25 kg/m2), or overweight (≥25 kg/m2). Hemoglobin A1c indicated diabetes status: normal (<5.7%), prediabetes (5.7%–6.5%), or diabetes (≥6.5%) [21]. Hemoglobin indicated anemia severity: mild (11.00 to <13 g/dL for men; 11.00 to <12 g/dL for women), moderate (8.00 to <11 for both sexes), or severe (<8 g/dL for both sexes) [22].
Among PLWH, we also assessed CD4 cell count (>50/μL or ≤50/μL), viral load (unsuppressed or suppressed: ≥400 vs <400 copies/mL), timing of antiretroviral therapy (ART), and ART regimen. The timing of ART was categorized into before tuberculosis treatment initiation, early (≤2 weeks after treatment initiation), or late (>2 weeks after treatment initiation). ART regimens were categorized as protease inhibitor based, integrase inhibitor based, or nonnucleotide reverse-transcriptase based. CD4 cell count, and HIV-1 load were used as markers for the severity of HIV infection. Baseline CD4 cell counts include the result closest to tuberculosis treatment initiation but between 6 months before and 1 month after initiation of tuberculosis treatment. Baseline HIV-1 loads include the result closest to tuberculosis treatment initiation but between 6 months and 1 week after tuberculosis treatment initiation.
Statistical Analysis
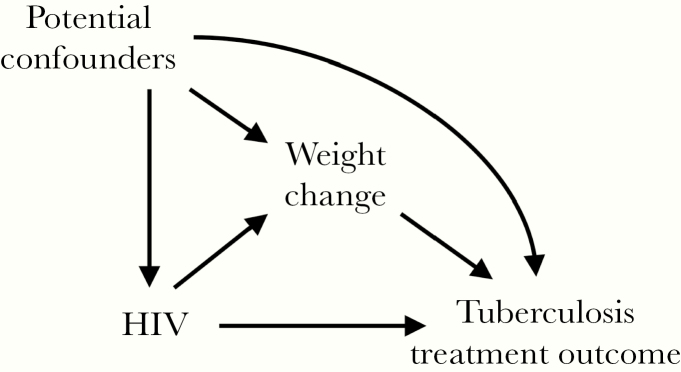
Baseline characteristics of participants were summarized by HIV status and compared using Wilcoxon rank sum test for continuous and χ 2 test for categorical variables. The primary analysis compared median weight change between PLWH and HIV-negative participants, using bootstrapped quantile regression. We identified covariates for inclusion in multivariable models using a directed acyclic graph approach [23] (Figure 1). A priori identified confounders of the association between HIV and weight change included baseline weight, age, sex, study site, smear status, and hemoglobin level. We also examined the association between severity of HIV infection (viral load and CD4 cell count) on weight change, by further categorizing PLWH according to HIV-related characteristics and comparing them with HIV-negative participants. There was a minimal amount of missingness for smear status (n = 3 [<1%]) and hemoglobin level (n = 5 [<1%]), so missing values were singly imputed before multivariable modeling, using the mode and median values, respectively.
Figure 1.
Directed acyclic graph for the primary and secondary analyses. The primary analysis examined the association between human immunodeficiency virus (HIV) and weight change, while adjusting for potential confounders. The secondary analysis examined the independent effect of HIV and weight change on tuberculosis treatment outcome and used mediation analysis to determine the total, direct, and indirect effects of HIV and weight change on tuberculosis treatment outcome.
In secondary analyses, we also examined the effect of HIV, baseline weight, and weight change on unsuccessful tuberculosis treatment outcomes (treatment failure, tuberculosis recurrence, or death). Using Cox proportional hazards regression, we examined hazard ratios for unsuccessful treatment outcome by baseline weight, weight change during the first 2 months of treatment, and HIV status. Follow-up times were calculated from tuberculosis treatment initiation until unsuccessful outcome or last contact. For each Cox model, we calculated crude and adjusted estimates, adjusting for a limited number of a priori considered confounders owing to the low outcome rate.
We also conducted mediation analysis to examine the total effect of HIV on unsuccessful treatment outcome, while evaluating the role of weight change as a potential mediator and distinguishing the direct effect of HIV on unsuccessful treatment outcome from the indirect effect mediated through weight change. Causal mediation analysis assumes a correctly specified causal model, no unmeasured confounding, and no uncontrolled confounding between exposure and mediator, between mediator and outcome, and between exposure and outcome. Mediation analysis was carried out using the paramed Stata software package (StataCorp), which used logistic regression to estimate parameters [24].
In sensitivity analysis, missing CD4 cell count (n = 11) and HIV-1 RNA levels (n = 32) were multiply imputed and results were compared with the primary analysis. In addition, because all but 8 PLWH were from INI and Manaus, we conducted sensitivity analyses for all analyses restricted to those sites. All analyses used StataIC 15.0 software (StataCorp), and differences were considered significant at P < .05.
Ethical Approval
The protocol, informed consent, and study documents were approved by the institutional review boards at study sites and Vanderbilt University Medical Center. Participation was voluntary, and written informed consent was obtained from all participants.
RESULTS
Among 598 participants enrolled in RePORT-Brazil during the study period, 547 (91%) also had weight measured at 2 months and were included in the study. Reasons for exclusion (n = 51) include death (n = 13) or loss to follow-up (n = 28) before month 2, and missing weight measurement at month 2 (n = 10). Excluded participants were more likely to be HIV positive (30% vs 19%; P = .06), of mixed race (63% vs 46%; P = .02), or current or former smokers (current, 35% vs 22%; former, 37% vs 26%; P ≤ .01) than those included. None of the excluded participants were overweight, versus 11% of the included participants, but there was not a significant difference in median baseline BMI between the 2 groups (19.4 vs 20.3; P = .15)
Overall, participants had a median age of 37 years (interquartile range [IQR], 37–49 years), and 20% had HIV. Almost half of participants were either prediabetic or diabetic, 59% had some degree of anemia at baseline, and more than half received DOT, based on self-report. PLWH were significantly more likely to be male than HIV-negative participants and had higher rates of anemia. As expected, PLWH had lower rates of sputum smear positivity and lung cavitation on chest radiograph. There were additional differences in racial distribution, alcohol use, tobacco use, and hemoglobin between PLWH and HIV-negative participants (Table 1). The duration of follow-up also differed between these groups (median, 12 [IQR, 5.9–17.8] vs 18 [12–24] months; P < .01).
Table 1.
Baseline Clinical and Demographic Characteristics of the Study Population by Human Immunodeficiency Virus Status
| Study Participants, No. (%)a | |||
|---|---|---|---|
| Characteristic at Baseline | HIV Negative (n = 445) | HIV Positive (n = 102) | P Valueb |
| Age at enrollment, median (IQR), y | 37 (26–51) | 37 (29–45) | .60 |
| Male sex | 276 (62) | 76 (75) | .02 |
| Site | |||
| Manaus | 38 (9) | 69 (68) | <.01 |
| Caxias | 111 (25) | 4 (4) | |
| INI | 63 (14) | 27 (27) | |
| Rocinha | 84 (19) | 1 (1) | |
| Salvador | 149 (34) | 1 (1) | |
| Race | |||
| White or other | 112 (25) | 24 (24) | <.01 |
| Black | 142 (32) | 15 (15) | |
| Mixed | 191 (43) | 63 (62) | |
| Household income | |||
| Minimum wage or below | 204 (46) | 37 (36) | .21 |
| Above minimum wage | 154 (35) | 42 (41) | |
| None or not reported | 87 (20) | 23 (23) | |
| Tobacco use | |||
| Current | 102 (23) | 17 (17) | <.01 |
| Former | 98 (22) | 42 (41) | |
| Never | 245 (55) | 43 (42) | |
| Alcohol use | |||
| Current | 215 (48) | 30 (29) | <.01 |
| Former | 151 (34) | 63 (62) | |
| Never | 79 (18) | 9 (9) | |
| BMI, median (IQR), kg/m2 | 20.3 (18.4–22.5) | 20.5 (18.4–22.9) | .61 |
| BMI group | |||
| Underweight (<18.5 kg/m2) | 121 (27) | 29 (28) | .55 |
| Normal weight (18.5–25 kg/m2) | 278 (63) | 59 (58) | |
| Overweight (≥25 kg/m2) | 46 (10) | 14 (14) | |
| HbA1c, median (IQR), % (n = 591) | 5.8 (5.4–6.3) | 5.7 (5.2–6.2) | .03 |
| HbA1c category | |||
| Normal (<5.7%) | 217 (49) | 52 (52) | .24 |
| Prediabetes (5.7%–6.5%) | 126 (29) | 34 (34) | |
| Diabetes (≥6.5%) | 97 (22) | 15 (15) | |
| Hemoglobin, median (IQR), g/dL (n = 593) | 12.4 (11.3–13.5) | 10.4 (8.7–11.8) | <.01 |
| Anemia categoryc | |||
| None | 214 (49) | 15 (15) | <.01 |
| Mild | 136 (31) | 25 (25) | |
| Moderate | 83 (19) | 46 (45) | |
| Severe | 7 (2) | 16 (16) | |
| Self-reported DOT (n = 594) | 224 (51) | 63 (62) | .05 |
| Positive smear result (n = 595) | 375 (85) | 66 (65) | <.01 |
| Lung cavitation (n = 588) | 272 (62) | 28 (29) | <.01 |
Abbreviations: BMI, body mass index; DOT, directly observed therapy; HbA1c, glycosylated hemoglobin A1c; HIV, human immunodeficiency virus; INI, Instituto Nacional de Infectologia Evandro Chagas; IQR, interquartile range.
aData represent no. (column %) of participants unless otherwise specified.
b P values determined with Wilcoxon rank sum test for continuous and χ 2 test for categorical variables, comparing persons with and without HIV.
cAnemia categories were defined as follows: none, ≥13 g/dL for men and ≥12 g/dL for women; mild, 11–13 g/dL for men and 11–12 g/dL for women; moderate, 8–11 g/dL for both sexes; and severe,<8 g/dL for both sexes.
Among the 102 PLWH, the median CD4 cell count was 97/μL (IQR, 49–282/μL), and 24 (26%) had baseline CD4 cell counts >50/μL. The median viral load was 18 572 copies/mL (IQR, 566–162 035/copies mL), and 57 (76%) were not virologically suppressed. Just more than one-third of PLWH were receiving ART before antituberculosis treatment initiation, 28 (28%) started within 2 weeks, 30 (29%) started between 2 weeks and 2 months, and 7 (7%) never started ART. Most PLWH received an integrase inhibitor–based (37%) or nonnucleotide reverse-transcriptase–based (45%) ART regimen, and 10% were started on a protease inhibitor–based regimen.
Primary Analysis: Weight Change
During the first 2 months of antituberculosis treatment, the overall median weight change was a gain of 1.7 kg. This differed significantly by HIV status, however. PLWH had a median weight gain of 0.1 kg (IQR, −3.2 to 2.6), compared with 2 kg (0.4–4) for HIV-negative persons. Among the 102 PLWH, 28% lost ≥5% of their baseline weight, 47% had stable weight (change between −5% and 5%), and 26% gained ≥5% of their baseline weight. Notably, among the 445 HIV-negative participants, only 3% had weight loss ≥5%, 60% had stable weight, and 36% had weight gain ≥5% (Table 2).
Table 2.
Patterns of Weight Change From Baseline to Month 2 in the Study Population, by Human Immunodeficiency Virus Status
| Study Participants | |||
|---|---|---|---|
| Weight or Weight Change | HIV Negative (n = 445) | HIV Positive (n = 102) | P Valuea |
| Absolute weight, median (IQR), kg | |||
| Baseline | 57 (50.2 to −65) | 55.1 (49.7–66) | .72 |
| At 2-mo follow-up | 59.5 (52–67.4) | 56.1 (49–65.4) | .02 |
| Weight change, median (IQR) | |||
| Absolute, kg | 2 (0.4–4) | 0.1 (−3.2 to 2.6) | <.01 |
| Relative, % | 3.6 (0.7–7) | 0.1 (−5.1 to 5.5) | <.01 |
| Weight change category, no. (%)b | |||
| Weight loss ≥5% | 15 (3) | 28 (28) | <.01 |
| Stable weight | 268 (60) | 48 (47) | |
| Weight gain ≥5% | 162 (36) | 26 (26) | |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
a P values determined with Wilcoxon rank sum test for continuous and χ 2 test for categorical variables.
bParenthetical percentages are column percentages.
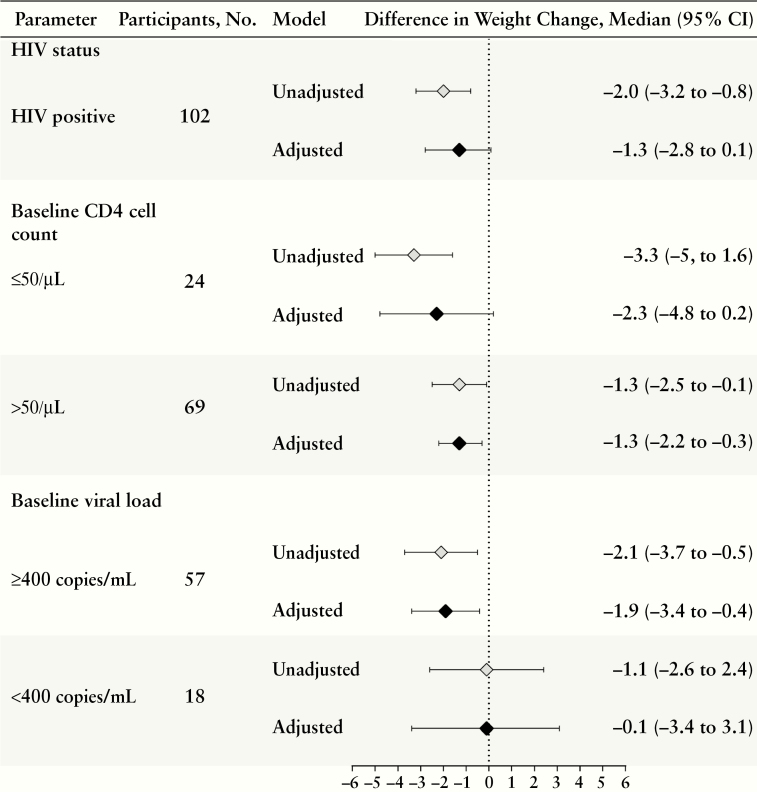
PLWH experienced less weight gain than HIV-negative persons, with an estimated median difference of −1.3 kg (95% confidence interval [CI], −2.8 to 0.1), with adjustment for baseline weight, age, sex, site, smear status, and hemoglobin. The differences in weight change were even more pronounced among PLWH with low CD4 cell counts and unsuppressed viral load (Figure 2) but were relatively similar with regard to ART timing and regimen (data not shown). Aside from HIV, other factors associated with weight change included site, sex, self-reported DOT, BMI category, baseline smear status, and anemia. Neither smear nor culture results at month 2 were associated with weight change among a subset of participants with available smear (n = 423) or culture (n = 421) results.
Figure 2.
Crude and adjusted analysis of association between human immunodeficiency virus (HIV)–related characteristics and weight change during the first 2 months of tuberculosis treatment, using bootstrapped quantile regression Adjusted models control for baseline weight, age, sex, site, smear status, and hemoglobin level. Boldface indicates statistical significance (P < .05). Abbreviation: CI, confidence interval.
Secondary Analysis: Unsuccessful Tuberculosis Treatment Outcomes
The risk of unsuccessful outcome for antituberculosis treatment was significantly associated with weight change during the first 2 months of treatment and with HIV status (Table 3). Overall, 35 participants had an unsuccessful treatment outcome, corresponding to an incidence rate of 4.1 per 1000 person-years. The rates of death (n = 13), failure (n = 17), and recurrence (n = 5) correspond to incidences of 1.5, 2.0, and 0.6 per 1000 person-years, respectively. Participants with an unsuccessful outcome lost a median of 0.4 kg (IQR, −3.4 to 2), compared with a median gain of 1.9 kg (0.1–4) among those with a successful treatment outcome. Of the 188 participants with weight gain >5%, only 6 (3%) had an unsuccessful treatment outcome, compared with 6% of those with stable weight change and 21% with weight loss >5%.
Table 3.
Cox Proportional Hazards Regression for Predictors of Unsuccessful Treatment Outcome (Death, Treatment Failure, and Tuberculosis Recurrence)
| Participants | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| Factor by Population | Total, No. | Poor Outcome, No. (%) | Crude | Adjusted |
| Primary analysis population | ||||
| HIV status | ||||
| Positive | 102 | 18 (18) | 6.14 (3.12–12.10)a | 4.79 (2.10–10.91)a,b |
| Negative | 445 | 17 (4) | Reference | Reference |
| Baseline weight (per 1-kg increase) | 547 | 35 (6) | 1.0 (.99–1.03) | 0.99 (.96–1.02)c |
| Weight at 2 mo (per 1-kg increase) | 547 | 35 (6) | 0.97 (.94–1.01) | 0.97 (.94–1.01)c |
| Absolute weight change (per 1-kg increase) | 547 | 35 (6) | 0.83 (.77–0.89)a | 0.88 (.81–0.95)a,d |
| Relative weight change (per 1% increase) | 547 | 35 (6) | 0.89 (.86–0.93)a | 0.92 (.88–0.96)a,d |
| Full population | ||||
| HIV status | ||||
| Positive | 117 | 25 (21) | 5.63 (3.20–9.90)a | 3.88 (1.81–8.34)a,b |
| Negative | 481 | 24 (5) | Reference | Reference |
| Baseline weight (per 1-unit increase) | 598 | 49 (8) | 0.98 (.96–1.01) | 0.98 (.95–1.01)c |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
aStatistically significant at P < .05.
bAdjusted for sex and hemoglobin level.
cAdjusted for HIV status, and sex.
dAdjusted for baseline weight, HIV status, and sex.
Only 4% of HIV-negative persons had an unsuccessful treatment outcome, compared with 18% among PLWH, corresponding to a hazard ratio of 4.79 (95% CI, 2.10–10.91), after adjustment for sex and hemoglobin levels. Weight change was also associated with unsuccessful treatment outcome. For each 1-kg increase in weight, the risk of unsuccessful treatment outcome decreased by 12% (hazard ratio, 0.88; 95% CI, .81–.95). Neither baseline weight nor weight at month 2 were predictive of treatment outcome (Table 3). We assessed the effect of both HIV status and baseline weight on unsuccessful outcome in the full population (including the 51 participants without a month 2 visit who were excluded from the primary analysis) and found that the results were similar to those of the primary analysis (Table 3).
Mediation Analysis
From the mediation analysis, the increased risk of unsuccessful tuberculosis treatment outcome among HIV-positive persons yielded an odds ratio of 4.96 (95% CI, 2.39–10.27). The controlled direct effect of HIV on unsuccessful outcome was 3.6 (95% CI, 1.64–7.82), which expressed how much the risk of the outcome would change on average if weight change were fixed at a uniform level for HIV-positive and HIV-negative persons. The indirect effect was 1.38 (95% CI, 1.08–1.78), which was the effect of HIV on unsuccessful treatment outcome for any level of weight change. This suggests that 35% of the effect of HIV on an unsuccessful outcome was mediated through weight change, suggesting that 35% of the effect of the exposure would be reduced if the pathway through the mediator (weight change) were blocked.
Sensitivity Analyses
In sensitivity analyses restricted to INI and Manaus, the impact of HIV on weight change remained similar, but the effect of HIV on unsuccessful outcome was slightly attenuated (Supplementary Tables 1 and 2). Imputing CD4 cell counts and HIV-1 RNA viral loads or using values from outside the recommended date window did not substantively alter the results (Supplementary Table 3).
Discussion
In this prospective study of culture-positive, drug-susceptible patients with pulmonary tuberculosis treated with standard antituberculosis therapy, we found an association between HIV infection and weight change during the first 2 months of antituberculosis treatment, including in site-restricted sensitivity analyses. In addition, PLWH and persons who did not gain adequate weight during the first 2 months of antituberculosis treatment had increased risk of unsuccessful tuberculosis treatment outcomes. Conversely, few participants who gained ≥5% of their baseline weight after 2 months of antituberculosis treatment had an unsuccessful treatment outcome, suggesting the benefit of adequate weight gain in early tuberculosis treatment. Results of mediation analysis highlighted the association between HIV and unsuccessful treatment outcomes, and comparison of direct and indirect effects indicated that more than one-third of poor treatment outcome risk due to HIV was mediated by weight change. If the assumptions of causal mediation analysis are met (see Methods), these results suggest that it may be possible to prevent unsuccessful outcomes with an intervention to promote weight gain during tuberculosis treatment among PLWH. However, this finding must be carefully investigated in settings with multiple measures of weight before outcome in larger and more diverse populations, including intervention studies to promote weight gain.
Weight gain is an essential part of recovery during antituberculosis treatment, especially among PLWH, who are more likely to have unsuccessful tuberculosis treatment outcomes [25–28]. However, to our knowledge, no previous studies have quantified differences in weight change during antituberculosis treatment for drug-susceptible tuberculosis by HIV status and examined subsequent effects on treatment outcome. A prospective cohort study in Tanzania found no differences in fat mass and muscle mass changes during the first 2 months of antituberculosis treatment by HIV status [29]. We found that PLWH receiving ART before antituberculosis treatment initiation had minimal excess weight gain compared to PLWH not receiving ART at baseline, and any association would likely be the result of receiving ART and having less severe consequences of the tuberculosis-HIV burden. Similarly to our analysis, a study in Ethiopia found that HIV-negative patients gained more weight than PLWH during tuberculosis treatment [30]. Although those authors did not examine overall treatment outcomes (death, failure, or recurrence), they noted a strong inverse association between body weight gain and sputum conversion, which has been used previously as a predictor or surrogate for tuberculosis treatment outcome [30]. Of note, we did not find an association between weight change and smear or culture conversion at 2 months.
Other studies examining weight change during antituberculosis treatment and impact on treatment outcome were conducted among HIV-negative persons or populations with low rates of HIV and were thus unable to assess differences in weight change during treatment by HIV status [13, 15, 18, 31, 32]. These studies focused mostly on baseline weight, baseline disease severity, DOT, age, and adherence as predictors of weight change, many of which were also predictors in our analysis but not as strongly as HIV. Notably, 2 studies found that lack of “adequate” weight gain, defined as gain ≥5% of baseline weight during the first 2 months of antituberculosis treatment, was a predictor of unsuccessful outcome [13, 18]. We confirmed this finding.
Correspondingly, our results may be explained by the finding that tuberculosis increases resting metabolic rate, a measure of the cost of sustained immune activation, by 14%, which rises to about 30% among persons with tuberculosis and HIV [33, 34]. The impairment of net protein anabolism from increased energy expenditures to fight infection is increased in patients with both tuberculosis and HIV compared with those with either tuberculosis or HIV alone [11]. Owing to increased immune activation and resulting energy consumption, it may take longer for HIV-positive patients with tuberculosis to sustain adequate weight gain even after initiation of antituberculosis therapy. Another study found that lack of 5% weight gain by the end of tuberculosis treatment was most predictive of a unsuccessful treatment outcome [14]. However, given their homogenous study population, those authors recommended future analyses to examine weight changes among subsets of patients, including PLWH. As seen in our study, using end-of-treatment weight change as a proxy for unsuccessful treatment outcome would fail to capture deaths that occurred before that point.
Our study had limitations. First, 51 participants had insufficient follow-up to assess weight change at month 2. Second, we examined weight change at 2-month follow-up. It is possible that weight change differences by HIV status vary as tuberculosis treatment duration increases, with gradual improvement in host immune function. However, the first 2 months of antituberculosis treatment represent a critical time in the course of disease, and losses to follow-up and events thereafter may strongly influence conclusions about weight changes between baseline and the end of treatment.
In sensitivity analyses, we examined the impact of baseline weight on unsuccessful treatment outcome and found little effect, which further highlights the importance of weight change during the first 2 months of treatment. It is also difficult to discern key causative factors in our analysis: do unsuccessful tuberculosis outcomes occur because participants do not gain weight, or do participants not gain weight because they are not responding to therapy? Future studies of potential interventions to promote weight gain during the first 2 months of treatment could provide insights. Translational studies may be helpful in assessing immune function pertaining to M. tuberculosis and HIV in persons who do and those who do not gain weight during antituberculosis therapy.
This study also had several strengths, including its prospective design, active follow-up for recurrence, death, and treatment failure, and collection of data regarding tuberculosis and HIV clinical characteristics. We were able to compare weight change and tuberculosis treatment outcomes by HIV status, and among PLWH we examined the impact of HIV severity on weight change and subsequent treatment outcomes. Finally, we present results that should be generalizable to other low-resource countries with tuberculosis-HIV rates similar to those in Brazil, which has a high tuberculosis-HIV burden [1].
In conclusion, weight change in early tuberculosis treatment and HIV status have the potential to be effective, readily available, and inexpensive surrogate markers of unsuccessful tuberculosis treatment outcome and could trigger evaluation of interventions among patients at highest risk for these outcomes. More research is needed to identify the types of interventions that would best promote weight gain during tuberculosis treatment, especially among PLWH.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. We are grateful for the support and dedication of study staff at each enrollment site who made the study possible. We thank study coordinators (Aline Benjamin, Adriana Rezende, Jamile Garcia, Michael Rocha, and Alexandra Brito), nurses (Quezia Medeiros, André Bezerra, Martinelle Godinho, Vanessa Nascimento, and Amanda Rabelo), and physicians (Flavia Sant’ Anna, Anna Carvalho, João Marine, Nelia Araujo, and Izabella Safe). We also thank the Regional Prospective Observational Research for Tuberculosis (RePORT)–Brazil study participants.
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Brazilian Ministry of Health, the National Institutes of Health, the National Institutes of Allergy and Infectious Diseases, or the National Center for Advancing Translational Sciences.
Financial support. This work was supported by the Departamento de Ciência e Tecnologia, Secretaria de Ciência e Tecnologia, Ministério da Saúde, Brazil (grant 25029.000507/2013-07 to V. C. R.), the National Institutes of Allergy and Infectious Diseases (grants U01-AI069923 and K01-AI131895 to P. F. R), and the National Center for Advancing Translational Sciences (Clinical and Translational Science Award (CTSA) award TL1TR000447 to L. S. P.).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Association for Clinical and Translational Science Annual Meeting, 6 March 2019, Washington, DC; Society for Epidemiologic Research, 20 June 2019, Minneapolis, MN.
References
- 1. Global tuberculosis report 2018 Geneva, Switzerland: World Health Organization; 2018. https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf [Google Scholar]
- 2. Wallis RS, Doherty TM, Onyebujoh P, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 2009; 9:162–72. [DOI] [PubMed] [Google Scholar]
- 3. Dheda K, Barry CE 3rd, Maartens G. Tuberculosis. Lancet 2016; 387:1211–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raviglione M, Marais B, Floyd K, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 2012; 379:1902–13. [DOI] [PubMed] [Google Scholar]
- 5. Uplekar M, Weil D, Lonnroth K, et al. ; WHO’s Global TB Programme WHO’s new end TB strategy. Lancet 2015; 385:1799–801. [DOI] [PubMed] [Google Scholar]
- 6. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8:286–98. [PubMed] [Google Scholar]
- 7. Schwenk A, Hodgson L, Wright A, et al. Nutrient partitioning during treatment of tuberculosis: gain in body fat mass but not in protein mass. Am J Clin Nutr 2004; 79:1006–12. [DOI] [PubMed] [Google Scholar]
- 8. Koethe JR, von Reyn CF. Protein-calorie malnutrition, macronutrient supplements, and tuberculosis. Int J Tuberc Lung Dis 2016; 20:857–63. [DOI] [PubMed] [Google Scholar]
- 9. Semba RD, Darnton-Hill I, de Pee S. Addressing tuberculosis in the context of malnutrition and HIV coinfection. Food Nutr Bull 2010; 31:S345–64. [PubMed] [Google Scholar]
- 10. Swaminathan S, Padmapriyadarsini C, Sukumar B, et al. Nutritional status of persons with HIV infection, persons with HIV infection and tuberculosis, and HIV-negative individuals from southern India. Clin Infect Dis 2008; 46:946–9. [DOI] [PubMed] [Google Scholar]
- 11. Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. Tuberculosis and nutrition. Lung India 2009; 26:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kennedy N, Ramsay A, Uiso L, Gutmann J, Ngowi FI, Gillespie SH. Nutritional status and weight gain in patients with pulmonary tuberculosis in Tanzania. Trans R Soc Trop Med Hyg 1996; 90:162–6. [DOI] [PubMed] [Google Scholar]
- 13. Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med. 2006; 174:344–8. [DOI] [PubMed] [Google Scholar]
- 14. Krapp F, Véliz JC, Cornejo E, Gotuzzo E, Seas C. Bodyweight gain to predict treatment outcome in patients with pulmonary tuberculosis in Peru. Int J Tuberc Lung Dis 2008; 12:1153–9. [PubMed] [Google Scholar]
- 15. Bernabe-Ortiz A, Carcamo CP, Sanchez JF, Rios J. Weight variation over time and its association with tuberculosis treatment outcome: a longitudinal analysis. PLoS One. 2011; 6:e18474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung-Delgado K, Revilla-Montag A, Guillén-Bravo S, Bernabe-Ortiz A. Weight variation over time and its relevance among multidrug-resistant tuberculosis patients. Int J Infect Dis 2014; 23:20–4. [DOI] [PubMed] [Google Scholar]
- 17. Vasantha M, Gopi PG, Subramani R. Weight gain in patients with tuberculosis treated under directly observed treatment short-course (DOTS). Indian J Tuberc 2009; 56:5–9. [PubMed] [Google Scholar]
- 18. Hoa NB, Lauritsen JM, Rieder HL. Changes in body weight and tuberculosis treatment outcome in Viet Nam. Int J Tuberc Lung Dis 2013; 17:61–6. [DOI] [PubMed] [Google Scholar]
- 19. Hamilton CD, Swaminathan S, Christopher DJ, et al. RePORT international: advancing tuberculosis biomarker research through global collaboration. Clin Infect Dis. 2015; 61:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Definitions and reporting framework for tuberculosis—2013 revision Updated December 2014. Available from: https://apps.who.int/iris/bitstream/handle/10665/79199/9789241505345_eng.pdf;jsessionid=8A4F1CACB7370B4DEE32270838E8C117?sequence=1. Accessed 11 February 2019.
- 21. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(suppl 1):81–90. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 23. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10:37–48. [PubMed] [Google Scholar]
- 24. Emsley R, Liu H.. PARAMED: Stata module to perform causal mediation analysis using parametric regression models. Chestnut Hill, MA: Boston College Department of Economics: Statistical Software Components; 2013. [Google Scholar]
- 25. Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg 2002; 96:291–4. [DOI] [PubMed] [Google Scholar]
- 26. Koethe JR, Chi BH, Megazzini KM, Heimburger DC, Stringer JSA. Macronutrient supplementation for malnourished HIV-infected adults: a review of the evaluation in resource-adequate and resource-constrained settings. Clin Infect Dis 2011; 49:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu E, Spiegelman D, Semu H, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis 2011; 204:282–90. [DOI] [PubMed] [Google Scholar]
- 28. Schmaltz CA, Sant’Anna FM, Neves SC, et al. Influence of HIV infection on mortality in a cohort of patients treated for tuberculosis in the context of wide access to HAART, in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr 2009; 52:623–8. [DOI] [PubMed] [Google Scholar]
- 29. PrayGod G, Range N, Faurholt-Jepsen D, et al. Predictors of body composition changes during tuberculosis treatment in Mwanza, Tanzania. Eur J Clin Nutr 2015; 69:1125–32. [DOI] [PubMed] [Google Scholar]
- 30. Filate M, Mehari Z, Alemu YM. Longitudinal body weight and sputum conversion in patients with tuberculosis, Southwest Ethiopia: a retrospective follow-up study. BMJ Open 2018; 8:e019076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vasantha M, Gopi PG, Subramani R. Weight gain in patients with tuberculosis treated under directly observed treatment short-course (DOTS). Indian J Tuberc 2009; 56:5–9. [PubMed] [Google Scholar]
- 32. Phan MN, Guy ES, Nickson RN, Kao CC. Predictors and patterns of weight gain during treatment for tuberculosis in the United States of America. Int J Infect Dis 2016; 53:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization. Guideline: nutritional care and support for patients with tuberculosis Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 34. Melchior JC, Raguin G, Boulier A, et al. Resting energy expenditure in human immunodeficiency virus-infected patients: comparison between patients with and without secondary infections. Am J Clin Nutr 1993; 57:614–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.