Abstract
Background
Short-term (48-week) results of the OPTIONS trial showed that nucleoside reverse transcriptase inhibitors (NRTIs) can be safely omitted from salvage therapy as long as the regimen has a cumulative activity of >2 active antiretroviral medications. The long-term durability of this approach and outcomes in persons who have more-extensive HIV-1 drug resistance are uncertain.
Methods
Participants with virologic failure and anticipated antiretroviral susceptibility received an optimized regimen and were randomized to omit or add NRTIs. A separate group with more resistance (cumulative activity ≤2 active agents) received an optimized regimen including NRTIs.
Results
At week 96, among 360 participants randomized to omit or add NRTIs, 70% and 65% had HIV-1 RNA <200 copies/mL, respectively. Virologic failure was uncommon after week 48. Younger age and starting fewer new antiretroviral medications were associated with higher odds of virologic failure. In the highly resistant group, 53% had HIV-1 RNA <200 copies/mL at week 96.
Conclusions
HIV-1 salvage therapy can safely omit NRTIs without compromising efficacy or durability of response as long as the new regimen has a cumulative activity of >2 active drugs. Younger people and those receiving fewer new antiretrovirals require careful monitoring. Even among individuals with more-extensive resistance, most achieve virologic suppression.
Clinical Trials Registration
Keywords: HIV-1, antiretroviral therapy, treatment-experienced participants, randomized controlled trial, salvage therapy, drug resistance
HIV-1 salvage therapy can safely omit nucleoside reverse transcriptase inhibitors without compromising efficacy or durability of response as long as the new regimen has a cumulative activity of 2 or more active drugs.
(See the Major Article by Markowitz et al., on pages 1398–406.)
In people with human immunodeficiency virus-1 (HIV-1) infection (PWH) who have virologic failure on antiretroviral therapy (ART), guidelines recommend starting at least 2, and preferably 3, new active antiretroviral medications [1]. The question of whether nucleoside reverse transcriptase inhibitors (NRTIs) should be included in a new regimen when other active agents are available was addressed in the OPTIONS trial (AIDS Clinical Trials Group [ACTG] A5241) [2]. In this study, participants who were failing protease inhibitor (PI)-based therapy but whose virus was sensitive to a new regimen with a cumulative activity of >2 active agents were randomized to add or omit NRTIs from their new regimen. At week 48, the omit NRTIs group was not inferior to the add NRTIs group for the primary outcome of regimen failure [2].
The initial report of the OPTIONS trial findings focused on week 48 results (primary outcome) leaving important questions unanswered, such as the long-term durability of the 2 strategies. In addition, the impact of NRTIs on metabolic outcomes and quality of life (QOL) were not described. Now, we report on the virologic responses through 96 weeks (end of study follow-up) and factors associated with virologic failure. In participants who experienced virologic failure, we describe the frequency and type of treatment-emergent drug resistance. Because of the importance of safety and tolerability with long-term ART, we present the metabolic, renal, and self-reported QOL outcomes.
In addition to the 2 groups that were randomized to omit or add NRTIs, OPTIONS included a third, nonrandomized group with more drug resistance (sensitive only to a regimen with a cumulative phenotypic susceptibility score of ≤2 active agents as opposed to >2 in the randomized groups). Based on treatment history and resistance testing, the participants in this group were treated with a combination of active and partially active agents that included NRTIs. Here, for the first time, we report the outcomes following treatment in these individuals with highly drug-resistant HIV-1.
METHODS
The OPTIONS design, eligibility criteria, and procedures were previously described [2]. OPTIONS (NCT00537394) was an open-label, phase III, partially randomized strategy trial in treatment-experienced PWH (failing PI-based regimen with triple-class experience or drug resistance [nonnucleoside reverse transcriptase inhibitors, NNRTIs, NRTIs, and PIs]) that used a continuous phenotype susceptibility score (cPSS) to select an optimized antiretroviral regimen. The cPSS is the sum of the predicted activity of antiretrovirals (excluding NRTIs) in each study regimen [3]. An optimized regimen was the combination of antiretrovirals with the highest cPSS that was acceptable to the participant and local study investigators. Optimized regimens and NRTIs were recommended based upon treatment history, viral resistance, and coreceptor tropism test results (PhenoSense GT and Trofile, respectively; Monogram Biosciences). Participants who had previously received enfuvirtide or an integrase strand transfer inhibitor (INSTI) were presumed to be resistant to these agents. Participants with cPSS >2 were randomly assigned to receive their optimized regimen only (omit NRTIs group) or to add NRTIs (add NRTIs group) to their optimized regimen, stratified by INSTI experience and choice of maraviroc-containing study regimen. A separate group of participants with cPSS ≤2 (highly resistant group) were directly assigned to receive an optimized regimen and add NRTIs. Optimized regimens, consisting of medications available at the time of the trial, were composed of 3 or 4 of the following: ritonavir-boosted darunavir or tipranavir, raltegravir, etravirine, maraviroc, or enfuvirtide. All participants were in the United States and provided informed consent in compliance with US Department of Health and Human Services guidelines.
Procedures and Outcomes
Study evaluations occurred before entry, at entry, weeks 1, 4, 8, 12, 16, and 24, and every 12 weeks thereafter through week 96. The primary efficacy outcome was regimen failure, which was a composite outcome of first confirmed virologic failure or discontinuation of NRTI assignment. Virologic failure was defined as any 1 of the following (with confirmation on repeat measurement): <1 log10 copies/mL HIV-1 RNA decrease from baseline to week 12; virologic rebound to >200 copies/mL after suppression to <200 copies/mL; lack of suppression to <200 copies/mL by week 24; or HIV-1 RNA ≥200 copies/mL at or after week 48. Following intention-to-treat principles, participants who experienced virologic failure or who discontinued their assigned NRTI strategy (primary study endpoint) continued to be followed through 96 weeks to be evaluated for secondary outcomes. Secondary outcomes included: change in CD4 cell count from baseline; occurrence of newly acquired HIV-1 drug resistance or tropism shift between baseline and confirmed virologic failure; change in lipids from baseline; change in cardiovascular risk score from baseline; and change in QOL scores from baseline. Fasting lipids were collected at entry, and weeks 24, 48, and 96. QOL was assessed at baseline, weeks 8 and 24, and every 24 weeks thereafter using the general health score, which uses a visual analog scale that ranges from 0 (worst possible health) to 100 (perfect health). Cardiovascular risk was assessed using the Framingham risk score as this study was completed in 2011 prior to the introduction of newer guidelines for assessing risk.
Statistical Analysis
Calculating percentages of participants with HIV-1 RNA below limits used 2 methods: observed analysis included only participants with an observed RNA result; imputed analysis included all participants and missing values were imputed as greater than limit.
Cumulative probability of regimen or virologic failure by 96 weeks was estimated using a stratified Kaplan–Meier estimator. Stratum-specific estimates by group used inverse variance weights. Confidence intervals (CIs) used log(–log)-transformed Greenwood-estimated variance. Participants without regimen failure were censored at last visit. Noninferiority was concluded if the upper 95% confidence bound of the treatment difference was <15%.
Secondary outcomes used marginal modeling with generalized estimating equations incorporating equicorrelation structure for continuous outcomes and independence correlation and logit link for dichotomous outcomes. Nonlinear time trends were included as suggested by goodness of fit using Quasi-AIC.
Association of baseline characteristics with observed virologic failure in the randomized groups used logistic regression, a stepwise covariate selection process, reparameterization of covariates exhibiting nonlinearity in the logit, and testing for all 2-way statistical interactions in the main-effects model.
RESULTS
Study Participants
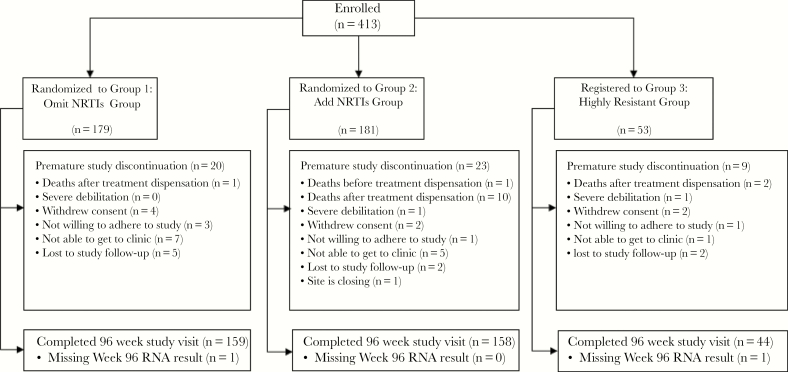
A total of 413 participants enrolled. Three hundred-sixty participants with cPSS of >2 were randomized to receive an optimized regimen without NRTIs (omit NRTIs group, n = 179) or an optimized regimen that added NRTIs (add NRTIs group, n = 181). An additional 53 participants who had highly resistant virus received an optimized regimen with a cumulative activity of 2 or fewer active agents (cPSS ≤ 2) and added NRTIs (highly resistant group). Table 1 summarizes baseline characteristics. Figure 1 shows participant disposition: 159 in the omit NRTIs group (89%), 158 in the add NRTIs group (87%), and 44 in the highly resistant group (83%) completed the study with a week 96 visit.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristic | Omit NRTIs Group, n = 179 | Add NRTIs Group, n = 181 | Randomized Groups Total, n = 360 | Highly Resistant Group, n = 53 | P Valuea |
|---|---|---|---|---|---|
| Age, y, median (Q1, Q3) | 46 (40, 51) | 46 (41, 52) | 46 (40, 52) | 43 (40, 50) | .233d |
| Female sex, No. (%) | 47 (26) | 46 (25) | 93 (26) | 6 (11) | .021e |
| Race/ethnicity, No. (%) | |||||
| White non-Hispanic | 55 (31) | 59 (33) | 114 (32) | 18 (35) | .885e |
| Black non-Hispanic | 69 (39) | 79 (44) | 148 (41) | 19 (37) | |
| Hispanic | 46 (26) | 37 (21) | 83 (23) | 14 (27) | |
| Other | 8 (4) | 4 (2) | 12 (3) | 1 (2) | |
| Baseline CD4+, cells/mm3, median (Q1, Q3) | 212 (105, 348) | 193 (104, 376) | 207 (104, 363) | 85 (25, 232) | <.001d |
| Baseline HIV-1 RNA, (log10 copies/mL, median (Q1, Q3) | 4.2 (3.6, 4.6) | 4.2 (3.6, 4.7) | 4.2 (3.6, 4.6) | 4.4 (4.1, 4.8) | .023d |
| Years of previous ARV exposure, median (Q1, Q3) | 12 (9, 16) | 10.7 (7.5, 14.0) | 11.4 (0.5, 25.0) | 13.1 (10.7, 16.5) | .016d |
| Previous enfuvirtide or integrase inhibitor exposure, No. (%) | 32 (18) | 34 (19) | 66 (18) | 40 (75) | <.001e |
| Screening HIV-1 tropism, No. (%) | |||||
| CCR5 | 88 (49) | 89 (49) | 177 (49) | 10 (19) | <.001e |
| Dual/mixed | 72 (40) | 71 (39) | 143 (40) | 31 (58) | |
| CXCR4 | 8 (4) | 10 (6) | 18 (5) | 8 (15) | |
| Nonreportable | 11 (6) | 11 (6) | 22 (6) | 4 (8) | |
| Number of active NRTIs chosen prior to randomization, No. (%)b | |||||
| 0 | 18 (10) | 21 (12) | 39 (11) | 6 (11) | .476e |
| 1 | 100 (56) | 103 (57) | 203 (56) | 34 (64) | |
| 2 or 3 | 61 (34) | 57 (31) | 118 (33) | 13 (25) | |
| Total number of new ARVs, including NRTIs, started following randomization, No. (%) | |||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 9 (17) | <.001e |
| 1–2 | 17 (9) | 9 (5) | 26 (7) | 15 (28) | |
| 3 | 138 (77) | 92 (51) | 230 (64) | 17 (32) | |
| 4–6 | 24 (13) | 80 (44) | 104 (29) | 12 (23) | |
| Total cholesterol from all samples, mg/dL | |||||
| Median (Q1, Q3) | 164 (140, 187) | 164 (137, 192) | 164 (139, 191) | 178 (133, 207) | .128d |
| Number missing | 16 | 19 | 35 | 5 | |
| Non-HDL cholesterol from fasting samples, mg/dL | |||||
| Median (Q1, Q3) | 124 (101, 149) | 131 (102, 156) | 126 (101, 152) | 140 (109, 171) | .053d |
| Number missing | 27 | 28 | 55 | 8 | |
| LDL cholesterol from all samples, mg/dL | |||||
| Median (Q1, Q3) | 90 (69, 115) | 97 (69, 120) | 93 (69, 117) | 88 (62, 126) | .963d |
| Number missing | 30 | 30 | 60 | 10 | |
| Framingham risk score, % | |||||
| Median (Q1, Q3) | 7.4 (3.4, 13.2) | 8.5 (3.7, 13.3) | 8.1 (3.6, 13.3) | 8.6 (5.5, 14.5) | .200d |
| Number missing | 12 | 18 | 30 | 4 | |
| Calculated creatinine clearance, mL/min | |||||
| Median (Q1, Q3) | 108.4 (86.5, 134.4) | 107.0 (88.4, 127.3) | 107.3 (87.1, 130.7) | 105.3 (97.1, 132.2) | .419d |
| Number missing | 1 | 0 | 1 | 0 | |
| QOL score, point categories, No. (%)c | |||||
| 0–60, quartile 1 | 47 (26) | 51 (28) | 98 (27) | 10 (19) | .123e |
| 61–75, quartile 2 | 42 (23) | 38 (21) | 80 (22) | 9 (17) | |
| 76–100, quartiles 3 and 4 | 83 (46) | 89 (49) | 172 (48) | 34 (64) | |
| Missing | 7 (4) | 3 (2) | 10 (3) | 0 (0) |
Baseline characteristics include the entire study sample except in cases where missing values are noted.
Abbreviations: ARV, antiretroviral; CCR5, C-C chemokine receptor 5-tropic virus; CXCR4, chemokine receptor type 4; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NRTI, nucleoside reverse transcriptase inhibitor; QOL, quality of life.
aStatistical comparisons of baseline characteristics between combined randomized groups and highly resistant group.
bAn active NRTIs is defined to be either partially sensitive or sensitive from a net assessment by Monogram PhenoSense GT testing at screening.
cQuality of life categories defined by grouped quartiles as informed by correlates of virologic failure analysis.
dTwo sample Wilcoxon test with continuity correction.
e Χ 2 test.
Figure 1.
Participant disposition. Abbreviation: NRTI, nucleoside reverse transcriptase inhibitor.
There were fewer deaths following treatment initiation in the omit NRTIs group than in the add NRTIs group (Figure 1): 1 and 10, respectively; cumulative probability of death through 96 weeks was 0.6% (95% CI, 0.1%–4%) and 5.7% (95% CI, 3.1%–10.3%), respectively. Because of the small number of events, the 95% CIs on the cumulative probabilities of death overlap. The cumulative probability of death through 96 weeks (2 deaths) in the highly resistant group (also receiving NRTIs) was 4% (95% CI, 1%–15.1%). The causes and timing of death were heterogeneous and there was no pattern suggesting a common mechanism or specific etiology.
Regimen and Virologic Failure
At week 96, 70% of all 179 participants in the omit NRTIs group and 65% of all 181 participants in the add NRTIs group had HIV-1 RNA <200 copies/mL; while 61% and 59% had HIV-1 RNA <50 copies/mL, respectively (Supplementary Table 1). Among the 158 participants in each randomized group who had a week 96 HIV-1 RNA value (observed analysis), 79% in the omit NRTIs group and 75% in the add NRTIs group had HIV-1 RNA <200 copies/mL; while 69% and 68% had HIV-1 RNA <50 copies/mL, respectively.
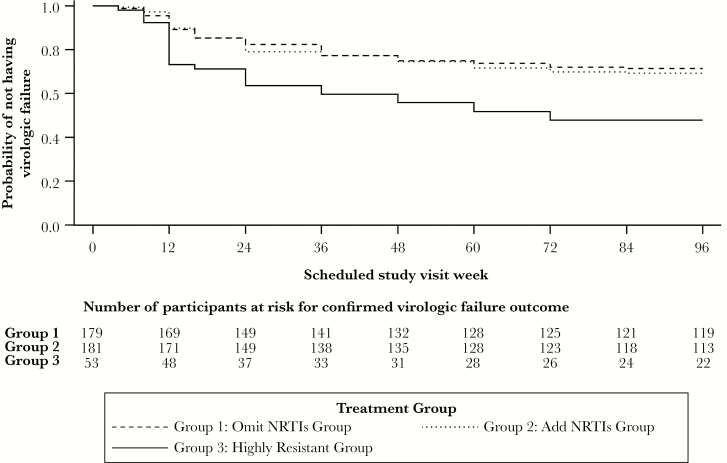
The cumulative probability for regimen failure (virologic failure or discontinuation of NRTI assignment) at week 96 was 33.6% in the omit NRTIs group and 31.3% in the add NRTIs group. The upper bound of the 95% CI on the difference in regimen failure between randomized groups (omit–add) was 11.5% and, thus, noninferiority of omit versus add NRTIs (compared to the prespecified bound of 15%) was achieved. Most regimen failures were due to virologic failure (46 of 60 in the omit NRTIs group, 50 of 57 in the add NRTIs group). By week 96, the estimated cumulative probability for virologic failure was 28.2% in the omit NRTIs group and 30.2% in the add NRTIs group (Figure 2); the upper bound of the 95% CI on the difference between groups was 7.4% and, thus, the omit NRTIs group can be concluded to be noninferior to the add NRTIs group at a lower noninferiority threshold of 10%. Most virologic failures occurred in the first 48 weeks: only 15 of 104 (14%) virologic failures occurred in the randomized groups after week 48.
Figure 2.
Cumulative probability of virologic failure over time by treatment group. Abbreviation: NRTI, nucleoside reverse transcriptase inhibitor.
In the highly resistant group, at week 96, 53% (of 53 participants) had HIV-1 RNA <200 copies/mL and 47% had HIV-1 RNA <50 copies/mL. Among 43 participants who had a week 96 HIV-1 RNA value (observed analysis), 65% had HIV-1 RNA <200 copies/mL and 58% had HIV-1 RNA <50 copies/mL.
Change in CD4 Cell Count
At week 96, the mean CD4 cell count was 391/mm3 (95% CI, 357–425) for the omit NRTIs group and 428/mm3 (95% CI, 383–473) for the add NRTIs group. Mean increases in CD4 cell count from baseline to week 96 were 143/mm3 (95% CI, 115–170) and 174/mm3 (95% CI, 145–203), respectively. In the highly resistant group, the mean CD4 cell count at week 96 was 307/mm3 and the mean increase from baseline to week 96 was 133/mm3.
Baseline Factors Associated With Virologic Failure in the Randomized Groups
The following factors were significantly and independently associated with virologic failure in the randomized groups: age, number of active NRTIs chosen prior to randomization (regardless of treatment arm), total number of new antiretrovirals started following randomization, and QOL score (Supplementary Table 2). Younger participants (age 16–46 years) had significantly higher odds of virologic failure compared to older participants (age 47–69 years) (adjusted odds ratio [AOR], 4.4; 95% CI, 2.4–8.2). The number of active NRTI in the regimen chosen prior to randomization (reflecting the extent of resistance to this class) was associated with virologic failure; in general, having 1 active NRTI was associated with the lowest odds of virologic failure (Supplementary Table 2). Participants who started fewer new antiretrovirals had higher odds of virologic failure (1–2 new medications vs 4–6, AOR, 6.9 [95% CI, 2.0–24.0]; 3 vs 4–6, AOR, 3.0 [95% CI, 1.4–6.5]). QOL score was not significantly associated with virologic failure in the omit NRTIs group; however, in the add NRTIs group, the AOR of virologic failure was higher in participants with low baseline QOL scores (quartile 1, 0–60 points) versus high QOL scores (quartiles 3 and 4, 76–100 points): AOR, 5.1 (95% CI, 2.0–13.2); or medium (quartile 2: 61–75 points) versus high scores: AOR, 3.4 (95% CI, 1.2–9.3).
Tropism Changes at Virologic Failure
A total of 177 randomized participants had C-C chemokine receptor 5 (CCR5)-tropic virus at screening; most received a regimen containing maraviroc (71% in the add NRTIs group; 69% in the omit NRTIs group). Within the R5 subgroup, 27 of 89 participants in the add NRTIs group (30%) and 22 of 88 participants in the omit NRTIs (25%) experienced virologic failure. At virologic failure, only 5 of 45 (11%) who had a tropism result had non-R5 virus.
Treatment-Emergent Resistance Among Participants With Virologic Failure
Among the 131 participants across all 3 groups who experienced virologic failure, 9 did not have resistance test results. For the 122 participants with results, we assessed changes in HIV-1 sensitivity to NRTIs, NNRTIs, and PIs using phenotypic testing (Monogram PhenoSense GT) for the randomized groups and changes in INSTI resistance using genotyping (for participants who received raltegravir) for all groups. For phenotypic testing, a drug was considered susceptible if the individual’s net assessment from the report was either partially sensitive or sensitive. The findings are summarized by antiretroviral class.
NRTI
Treatment-emergent phenotypic resistance to NRTIs at virologic failure was uncommon. For example, for tenofovir, in the add NRTIs group, 6 participants (11%) with virologic failure had an increase in fold-change resistance and 2 (4%) had reversion to less resistance; in the omit NRTIs group, 2 participants (4%) had an increase in resistance and 5 (10%) had reversion to less resistance.
NNRTI
Eighty-two percent of randomized participants received an antiretroviral regimen containing etravirine. Of the 82 etravirine-exposed participants who experienced virologic failure and had resistance data, 13 (16%) developed treatment-emergent etravirine resistance.
PI
Eighty-six percent of participants in the randomized groups with virologic failure received ritonavir-boosted darunavir. Treatment-emergent darunavir resistance was rare: of the 89 darunavir-exposed participants with virologic failure, only 3 (3.4%) developed treatment-emergent darunavir resistance.
INSTI
Among the 131 participants with virologic failure, 116 received raltegravir; of these, 104 had integrase genotyping completed at baseline and 104 had testing completed at time of virologic failure. At baseline, 4 participants (all in the highly resistant group) had ≥1 major primary integrase resistance mutation (Supplementary Table 3) [4]; 15 participants had ≥1 major accessory integrase resistance mutation; and 88 participants had no mutations. At time of virologic failure, 24 participants had ≥1 major primary or major accessory mutation; 11 participants had both major primary and major accessory mutations (8 of these were in the highly resistant group), 4 participants had 1 major primary mutation (1 in the highly resistant group), and 9 participants had ≥1 major accessory mutations (none from the highly resistant group). The rate of treatment-emergent major primary integrase resistance among participants who did not have such a mutation at baseline was 11% (11/100).
Effect of NRTIs on Metabolic and Renal Outcomes
We examined the effect of NRTIs on lipids by comparing the randomized groups. There was a greater increase in total cholesterol from baseline in the omit NRTIs group compared to the add NRTIs group (omit NRTIs group estimated changes 17 mg/dL higher than add NRTIs group; P = .0007), non-high-density lipoprotein (HDL) cholesterol from fasting samples (omit NRTIs estimated changes 17 mg/dL higher than add NRTIs group P = .0013), and low-density lipoprotein (LDL) cholesterol (omit NRTIs estimated changes 13 mg/dL higher than add NRTIs group, P = .0026). Ninety-five percent of participants in the add NRTIs group received tenofovir disoproxil fumarate (TDF), which decreases lipids [5, 6].
We also assessed the Framingham risk score in the randomized groups (this was the most widely used cardiovascular risk prediction tool at the time of the study). The omit NRTIs group had increasingly higher proportions (39% at week 24, 43% at week 48, 46% at week 96) of participants with moderate-to-high (>10%) risk scores compared to the add NRTIs group (38% at week 24, 40% at week 48, 43% at week 96) (P = .04 for treatment-by-time interaction), perhaps related to differences in lipids between the groups.
We examined changes in estimated creatinine clearance among participants in the randomized groups. There was greater decline in creatinine clearance from baseline in the add NRTIs group than in the omit NRTIs group at week 96: mean −2.7% vs +1.7% (P = .037).
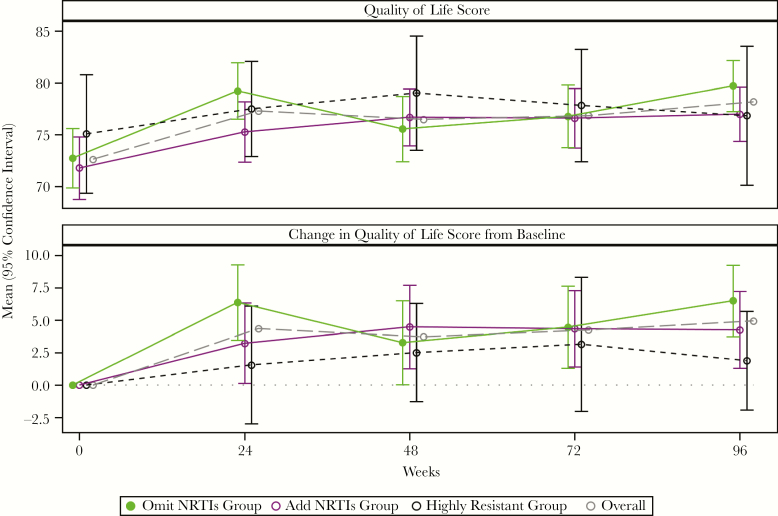
Quality-of-Life Scores
In all 3 groups, the mean QOL score significantly increased from baseline to week 96 (Figure 3). There were no significant differences between randomized treatment groups in change in QOL from baseline over 96 weeks (P = .41).
Figure 3.
Mean quality of life score and change in quality of life score over time by treatment group. Quality of life was assessed using the general health score, which uses a visual analog scale that ranges from 0 (worst possible health) to 100 (perfect health). Abbreviation: NRTI, nucleoside reverse transcriptase inhibitor.
DISCUSSION
The primary results of the OPTIONS trial demonstrated that in PWH who have virologic failure on ART and who start a regimen with a cumulative activity of >2 active antiretroviral medications omitting NRTIs did not result in inferior rates of regimen (mostly virologic) failure compared to adding NRTIs by 48 weeks. Now, we report the 96-week results of the trial, which confirm that HIV-1 salvage therapy can safely omit NRTIs without compromising regimen efficacy or durable virologic response as long as the new regimen contains a sufficient number of active drugs. The observation that virologic failure was uncommon after week 48 (>85% of virologic failures occurred before this time point) indicates that, even in highly treatment-experienced persons who have drug-resistant HIV-1, once virologic suppression is achieved, it is typically sustained.
The number of deaths between treatment initiation and 96 weeks was lower in the omit NRTIs group than in the add NRTIs group but the 95% CIs on the cumulative probability of death for this timeframe overlapped. The causes of death were heterogeneous and there was no pattern to suggest a common mechanism or specific etiology for the imbalance. Additional investigations of mitochondrial function and inflammation in the 2 groups are underway and will be the topic of a separate report.
Several characteristics were associated with virologic failure in the randomized groups in OPTIONS. Compared to older participants, younger participants were more likely to experience virologic failure. Previous studies have shown that younger people have greater difficulties with adherence [7, 8], suggesting enhanced adherence support is needed to improve outcomes in this high-risk group. As in previous studies of second-line therapy (EARNEST, SECOND-LINE, ACTG A5273), in OPTIONS having virus with less NRTI resistance at time of regimen selection was associated with higher odds of virologic failure, perhaps related to poorer adherence [9–11]. Finally, starting fewer new antiretroviral medications was associated with a higher likelihood of virologic failure, emphasizing the importance of using new classes of active medications as part of salvage regimens whenever possible.
The importance of active agents in achieving virologic suppression was further demonstrated in the highly resistant group who were directly assigned to receive active and partially active medications. As expected, this group had lower rates of virologic suppression than the randomized groups, where the cumulative activity of the regimen was higher. Nevertheless, even in the highly resistant group, over half of participants achieved HIV-1 RNA <200 copies/mL at week 96, indicating that virologic suppression is possible in this difficult-to-treat population. Current regimens may yield even more favorable results. In OPTIONS, the only integrase inhibitor available was raltegravir. Based on results of the SAILING trial [12], which showed that dolutegravir was superior to raltegravir in participants with previous virologic failure, one would anticipate that regimens with dolutegravir would be associated with even better virologic outcomes than those seen in OPTIONS.
The OPTIONS trial also confirmed that the frequency of treatment-emergent resistance varies by antiretroviral class. In participants who received the PI, darunavir, only 3.4% of those with virologic failure developed treatment-emergent darunavir resistance, a remarkably low proportion and consistent with the high barrier to resistance of this class even in highly treatment-experienced patients. By contrast, 16% of those with virologic failure developed treatment-emergent etravirine resistance. The rate of treatment-emergent primary major INSTI resistance on raltegravir was similar (11%). These results comport to the higher barrier to resistance of boosted PIs as compared to NNRTI or first-generation INSTIs, like raltegravir.
We also evaluated QOL scores, which significantly improved after starting a new regimen, demonstrating a strong link between effective treatment and better QOL. Participants in the add NRTIs group who had lower QOL at baseline had higher likelihood of virologic failure; this association was not observed in the omit NRTIs group. One potential explanation is that participants with lower QOL were less able to tolerate NRTIs leading to higher rates of virologic failure.
Finally, we found expected changes in metabolic and renal parameters. Total cholesterol, non-HDL cholesterol, and LDL cholesterol levels rose in the omit NRTIs group compared to the add NRTIs group, most likely because 95% of those in the latter group received TDF, which lowers lipids [5, 6]. There was a small decline in creatinine clearance (−2.7%) in the add NRTI group, possibly from TDF, which affects renal function [13, 14].
The OPTIONS trial is unique in several aspects: participants received 2–3 active agents in the randomized arms and did not receive NRTIs in 1 arm. In contrast, recycling of NRTIs was a component of most previous treatment-experienced trials: in the DUET, RESIST, POWER, MOTIVATE, and BENCHMRK trials [15–19], treatment-experienced participants received an optimized background regimen with or without a single new agent; response rates varied from 34% to 72% at 48 weeks and 58% to 62% at 96 weeks. OPTIONS demonstrated sustained virologic responses in the majority of participants even without recycling NRTIs—a finding that changed treatment guidelines [1].
A limitation of this analysis is that most participants (82%) in the add NRTIs group received TDF/emtricitabine; the lipid and renal effects we observed would likely not be seen with tenofovir alafenamide or abacavir. Strengths of the study include the large sample size and the long duration of follow-up.
In conclusion, the 96-week results confirm and extend the original findings of the OPTIONS trial: HIV-1 salvage therapy can safely omit NRTIs without compromising regimen efficacy or durable virologic response as long as the new regimen contains a sufficient number of active drugs. We have identified specific subgroups at a higher risk of virologic failure; based on these findings, more careful attention to younger people and those receiving fewer new antiretroviral medications is warranted. Ultimately, including newer agents in salvage regimens, like second-generation integrase inhibitors or drugs against novel targets, are likely to improve virologic outcomes even further, leading to sustained virologic suppression in the vast majority of treatment-experienced people with HIV-1.
SUPPLEMENTARY DATA
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are particularly grateful to Dr Richard Haubrich, the cochair of the ACTG A5241 trial. We thank all members of the ACTG A5241 study team, especially Drs Karin Klingman, Timothy Wilkin, Victoria Johnson, Sally Hodder, and Jorge Santana. We appreciate the staff at the ACTG sites that conducted the trial as well as all of the study participants. We thank Delaney Taylor for her assistance in preparing this manuscript.
Disclaimer. The views expressed are those of the authors and do not necessarily represent the views of the NIH or Department of Health and Human Services.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant numbers AI-68634 to Statistical and Data Management Center, AI-069501 to Case Clinical Trials Unit, Cincinnati Clinical Research Site, and AI-68636 to AIDS Clinical Trials Group); Boehringer Ingelheim, Janssen, Merck, ViiV Healthcare, AbbVie, and Roche provided study medications; Monogram Biosciences provided resistance and tropism tests; and Merck supported integrase genotypic resistance testing.
Potential conflicts of interest. R. T. G.’s institution has received educational grants from Gilead, ViiV, Janssen, Theratechnologies, and Merck; and he has served on scientific advisory boards for Merck, Gilead, and Theratechnologies. K. T. T.’s institution receives research grants from ViiV, Janssen, Merck, and Gilead; and she has served as an advisor for Gilead Sciences. J. J. E. is an ad hoc consultant to Merck, Gilead Sciences, Janssen, and ViiV Healthcare and receives contract support from Gilead Sciences, Janssen, and ViiV Healthcare for work unrelated to this study. C. J. F. receives grants to his institution for research and education from Gilead, ViiV, Janssen, Merck, Amgen, Cytodyn, and is on the speakers bureau for Clinical Care Options. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV Department of Health and Human Services; https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/15/virologic-failure. Accessed 24 December 2017. [Google Scholar]
- 2. Tashima KT, Smeaton LM, Fichtenbaum CJ, et al. ; A5241 Study Team HIV salvage therapy does not require nucleoside reverse transcriptase inhibitors: a randomized, controlled trial. Ann Intern Med 2015; 163:908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tashima KT, Mollan KR, Na L, et al. Regimen selection in the OPTIONS trial of HIV salvage therapy: drug resistance, prior therapy, and race-ethnicity determine the degree of regimen complexity. HIV Clin Trials 2015; 16:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanford University. HIV drug resistance database https://hivdb.stanford.edu/. Accessed 5 June 2019.
- 5. Tungsiripat M, Kitch D, Glesby MJ, et al. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS 2010; 24:1781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santos JR, Saumoy M, Curran A, et al. ; Tenofovir/emtricitabine influence on lipid metabolism (TULIP) Study Group The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61:403–8. [DOI] [PubMed] [Google Scholar]
- 7. Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002; 34:1115–21. [DOI] [PubMed] [Google Scholar]
- 8. Beer L, Skarbinski J. Adherence to antiretroviral therapy among HIV-infected adults in the United States. AIDS Educ Prev 2014; 26:521–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. La Rosa AM, Harrison LJ, Taiwo B, et al. ; ACTG A5273 Study Group Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): a randomised, phase 3, non-inferiority study. Lancet HIV 2016; 3:e247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyd MA, Moore CL, Molina JM, et al. ; SECOND-LINE study group Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: an exploratory analysis. Lancet HIV 2015; 2:e42–51. [DOI] [PubMed] [Google Scholar]
- 11. Paton NI, Kityo C, Thompson J, et al. ; Europe Africa Research Network for Evaluation of Second-line Therapy (EARNEST) Trial Team Nucleoside reverse-transcriptase inhibitor cross-resistance and outcomes from second-line antiretroviral therapy in the public health approach: an observational analysis within the randomised, open-label, EARNEST trial. Lancet HIV 2017; 4:e341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cahn P, Pozniak AL, Mingrone H, et al. ; extended SAILING Study Team Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 13. Mocroft A, Kirk O, Reiss P, et al. ; EuroSIDA Study Group Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS 2010; 24:1667–78. [DOI] [PubMed] [Google Scholar]
- 14. Scherzer R, Estrella M, Li Y, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 2012; 26:867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eron JJ, Cooper DA, Steigbigel RT, et al. ; BENCHMRK Study Teams Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis 2013; 13:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katlama C, Clotet B, Mills A, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antivir Ther 2010; 15:1045–52. [DOI] [PubMed] [Google Scholar]
- 17. Hicks CB, Cahn P, Cooper DA, et al. ; RESIST investigator group Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet 2006; 368:466–75. [DOI] [PubMed] [Google Scholar]
- 18. Gulick RM, Lalezari J, Goodrich J, et al. ; MOTIVATE Study Teams Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 2008; 359:1429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clotet B, Bellos N, Molina JM, et al. ; POWER 1 and 2 study groups Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 2007; 369:1169–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.