Abstract
Background
Live-attenuated influenza vaccine (LAIV) was licensed for prophylaxis of children 2–17 years old in Europe in 2012 and is administered as a nasal spray. Live-attenuated influenza vaccine induces both mucosal and systemic antibodies and systemic T-cell responses. Tonsils are the lymph nodes serving the upper respiratory tract, acting as both induction and effector site for mucosal immunity.
Methods
Here, we have studied the early tonsillar T-cell responses induced in children after LAIV. Thirty-nine children were immunized with trivalent LAIV (containing A/H1N1, A/H3N2, and B viruses) at days 3, 7, and 14 before tonsillectomy. Nonvaccinated controls were included for comparison. Tonsils and peripheral blood (pre- and postvaccination) were collected to study T-cell responses.
Results
Tonsillar and systemic T-cell responses differed between influenza strains, and both were found against H3N2 and B viruses, whereas only systemic responses were observed against A/H1N1. A significant increase in cross-reactive tonsillar CD8+ T cells recognizing conserved epitopes from a broad range of seasonal and pandemic viruses occurred at day 14. Tonsillar T cells showed significant cytokine responses (Th1, Th2, and granulocyte-macrophage colony-stimulating factor).
Conclusions
Our findings support the use of LAIV in children to elicit broadly cross-reactive T cells, which are not induced by traditional inactivated influenza vaccines and may provide protection to novel virus strains.
Keywords: children, IFN-γ, LAIV, T cells, tonsils
Our study found significant increases of cross-reactive CD8+ T cells within 14 days of LAIV in the tonsils of children. These T cells may provide broad protection, recognizing conserved epitopes from a broad range of seasonal and pandemic viruses.
Young children carry a considerable burden of influenza disease, with the World Health Organization estimating that 10%–30% of children are infected each year. Each year, influenza has been reported to cause 20.5 million cases of severe lower respiratory tract infections in children <6 years old [1]. Inactivated influenza vaccines (IIV) are approved for use in children 6 months old or older. Inactivated influenza vaccines mainly confer protective immunity by inducing strain-specific antibodies. Due to the continuous antigenic drift and occasional shift, there is an urgent need for vaccines capable of inducing broader protection. Historical evidence showed that in the absence of influenza-specific antibodies, influenza-specific CD8+ and CD4+ T cells play an important role in recovery from influenza infection [2, 3]. The importance of naturally occurring T-cell immunity in protection against seasonal and pandemic influenza was recently demonstrated in a large population-based study [4]. Furthermore, CD8+ T cells from the lungs of patients with influenza A or B were found to cross-react to influenza A, B, and C viruses [5], an important finding for the design of future universal influenza vaccines.
Moreover, CD8+ cells were associated with less severe pandemic infection in 2009 and lower viral shedding, as well as increased survival after H7N9 avian influenza infection in China [6, 7]. Furthermore, numerous studies have demonstrated that individuals have T-cell subsets with cross-reactivity to influenza A strains to which they have not been previously exposed [8–12]. Hence, cross-reactive CD8+ T cells recognizing conserved internal influenza epitopes are an interesting research focus in the development of universal vaccines.
In 2012, a live-attenuated influenza vaccine (LAIV) was licensed for children 2–17 years old in Europe. Live-attenuated influenza vaccine is administered as a nasal spray and more closely resembles a natural infection inducing long-lasting systemic humoral and cellular immune responses [13, 14]. In meta-analysis studies, the LAIV has high efficacy in children <6 years old when the vaccine strains matched the epidemic strains [3, 15, 16]. However, studies have shown protection after LAIV also in seasons with strain mismatch, indicating a broader protective effect that we are currently not able to quantitate. At this time, there are no well established immunological correlates of protection (COP) after LAIV, although induction of T cells has been highlighted. In a large field study, the majority of subjects with high frequencies of interferon (IFN)-γ-secreting cells (≥100 spot-forming cells per million lymphocytes) were protected from influenza infection, although the specific phenotype of these IFN-γ-secreting cells was not defined [3]. Furthermore, significant increases in IFN-γ + CD4+ and CD8+ T cells were observed after LAIV immunization, but not after IIV in children aged 5–9 years old. In adults, no increase in T or natural killer cells after IIV or LAIV vaccination was found [17]. Moreover, low baseline levels of T cells correlate with higher responses after vaccination [18]. When comparing the effect of different prime-boost strategies for LAIV and IIV, only regimes including LAIV induced CD4+, CD8+, and γδ + T cells specific for highly conserved influenza epitopes [19].
Intranasal administration of vaccines has several advantages over parenteral administration, with the potential of stimulating both local and systemic immune responses, as well as being easy to administer and needle-free. Human nasopharynx-associated lymphoid tissue, which consists of adenoids and tonsils, plays an important role in immune defense of the upper respiratory tract, both as an inductor and effector site of adaptive humoral and cellular immunity [20].
We have shown proof of concept that the LAIV induced systemic T cells cross-reactive to drifted strains, providing potential clinical protection [21]. An unanswered question is whether LAIV induces T cells in the local draining lymph nodes, which could provide broad protection. Our current study is a continuation of earlier work, in the same cohort, where we showed induction of tonsillar B cells and local immunoglobulin A after LAIV [22]. In this study, we focused on the early T-cell responses in the local tonsillar tissue, to investigate whether LAIV induced cross-reactive CD8+ T cells in the tonsils. To study tonsillar T cells, we vaccinated children with LAIV at specified time intervals before elective tonsillectomy, which allowed us to elucidate local, tonsillar-specific, and systemic T-cell responses. Increased understanding of these cross-protective cellular immune responses induced by LAIV may aid design of a future universal vaccine.
MATERIAL AND METHODS
Study Design
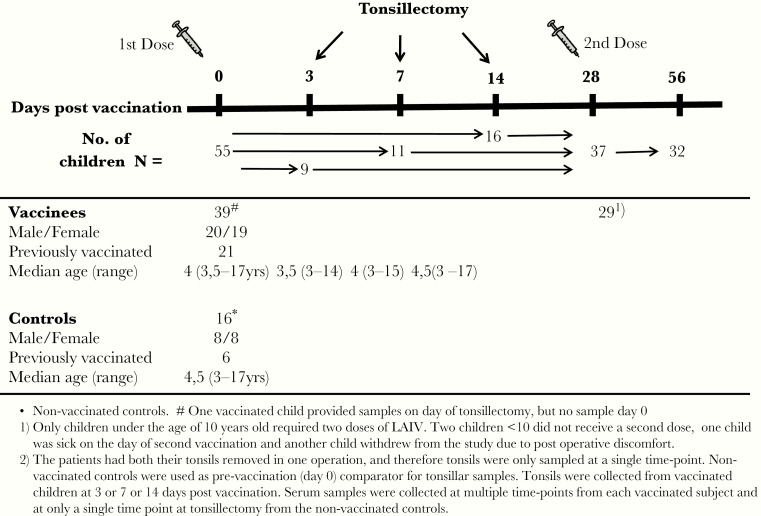
Fifty-five healthy children (3–17 years old) were recruited from the Otorhinolaryngology outpatient clinic at Haukeland University Hospital, Norway. Thirty-nine children (20 boys and 19 girls, median age 4 years) were immunized with the trivalent LAIV (Fluenz; AstraZeneca) in 2012–2013 at 3, 7, or 14 days before elective tonsillectomy. Controls consisted of age-matched unvaccinated children scheduled for tonsillectomy, providing a background comparison for postvaccination tonsillar responses (Figure 1). The study was approved by the Ethical Committee of Western-Norway and the Norwegian Medicines Agency (www.clinicaltrials.gov: NCT01866540). Demographics and inclusion and exclusion criteria for this trial have been published, and this work is a continuation of earlier findings to decipher the immune profiling after LAIV [13].
Figure 1.
Study design. Healthy, young children were recruited from the Department of Otorhinolaryngology scheduled for elective tonsillectomy and vaccinated intranasally with a live-attenuated influenza vaccine (LAIV) at 3, 7, or 14 days before tonsillectomy. Tonsils were extracted in total and collected from the operation theater from vaccinated children (n = 39) and a group of matched nonvaccinated controls (n = 16). Tonsil mononuclear cells were separated from the tonsils immediately after operation and used in the T-cell assays. Blood samples were taken before vaccination, at the time of tonsillectomy, and up to 56 days postvaccination. Peripheral blood mononuclear cells were separated and used in the T-cell assays, and plasma was stored for use in the hemagglutination inhibition assay. The number of subjects providing samples at each time point is shown.
Live-Attenuated Influenza Vaccine
Trivalent LAIV contained 107 fluorescent focus units of A/California/7/2009 (H1N1), A/Victoria/361/2011 (H3N2), and B/Wisconsin/1/2010. Live-attenuated influenza vaccine was administered intranasally as a 0.1-mL spray dose per nostril. Twenty-nine children <9 years old received 2 doses of vaccine 28 days apart as recommended by the manufacturer.
Sample Collection
Blood samples were collected pre- and postvaccination (days 0 [at tonsillectomy], 28, and 56). Cell preparation tubes (BD Biosciences) were used to separate peripheral blood mononuclear cells (PBMCs) and plasma. Peripheral blood mononuclear cells were used fresh in the T-cell assays, whereas plasma samples were aliquoted and stored at −80°C before use in the hemagglutination inhibition (HI) assay. Tonsils were collected during the operation and kept in saline, and tonsillar mononuclear cells (TMCs) were isolated by Ficoll gradient centrifugation and used directly in the T-cell assays [23].
Antigens and Peptides
Split virus antigens from the vaccine strains A/California/7/09(H1N1), A/Victoria/361/2011(H3N2) and B/Wisconsin/1/2010, were provided by GlaxoSmithKline, Belgium. By using the Immunome Epitope Data Base, a panel of cross-reactive CD4+ (33 peptides) and CD8+ (31 peptides) T-cell epitopes were selected from influenza isolates spanning from 1934 to 2009, according to sequence conservancy, human leukocyte antigen (HLA) supertype coverage, and prevalence [24]. Only the peptides with the highest conservancy score were selected among the CD4 or CD8 T-cell epitopes to detect cross-protective responses using 2 and 7 peptides from the internal proteins, respectively (Supplementary Table 1). These peptide epitopes have been empirically shown to differentiate between CD4 and CD8 T-cell responses. The peptides were chemically synthetized by Fmoc chemistry (Mimotopes, Clayton, Australia) and dissolved in 100% dimethyl sulfoxide at 20 mg/mL.
Hemagglutination Inhibition Assay
The influenza strain-specific HI antibody was measured pre- and postvaccination. Plasma samples were treated with receptor destroying enzyme ([RDE] Seiken, Japan). Duplicate samples from each subject (starting dilution of 1:10) were tested at the same time, using 8 hemagglutinating units of the homologous H1N1 and H3N2 vaccine strains or ether-treated B virus (Influenza Reagent Resources) and 0.7% turkey red blood cells [25]. An HI titer of 40 has been shown to be protective in adults.
Interferon-γ Enzyme-Linked Immunospot Assay
Secretion of IFN-γ from TMCs and PBMCs were detected as described earlier [13] by using an IFN-γ ELISpot kit (Mabtech AB, Sweden). In brief, 4 × 105 lymphocytes/well were added in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal calf serum with negative control (medium alone), split virus antigen, or peptides at a concentration of 2 µg/mL. Plates were incubated overnight (37°C, 5% CO2) and developed the next day according to the manufacturer’s instructions. Spots were counted using an Immunoscan reader and associated software (CTL Europe). The negative control (medium alone) value was subtracted from the influenza-specific (A/H1N1, A/H3N2, B, or peptide panel) responses.
Multiplex Cytokine Assay
Tonsillar mononuclear cells and PBMCs (1 × 106 cells/well) were incubated in lymphocyte medium for 72 hours in the presence of a mixture of 2.5 μg/mL of 3 split influenza antigens (A/H1N1, A/H3N2, and B), as previously described [26]. The cytokines present in the supernatants were quantified using a 10-Plex kit (LHC0001M; Thermo Fisher Scientific) using a Luminex 100 machine (Luminex Corporation) and StarStation v.3.0 Software (Applied Cytometry, UK).
Statistical Analysis
Differences between pre- and postvaccination responses were analyzed by non-parametric Kruskal-Wallis multiple comparisons test or the Mann-Whitney test using GraphPad Prism version 6 for Mac OS X. The correlation analysis was performed by non-parametric Spearman correlation. P < .05 was considered significant.
RESULTS
Early Hemagglutination Inhibition Antibody Responses After Live-Attenuated Influenza Vaccine
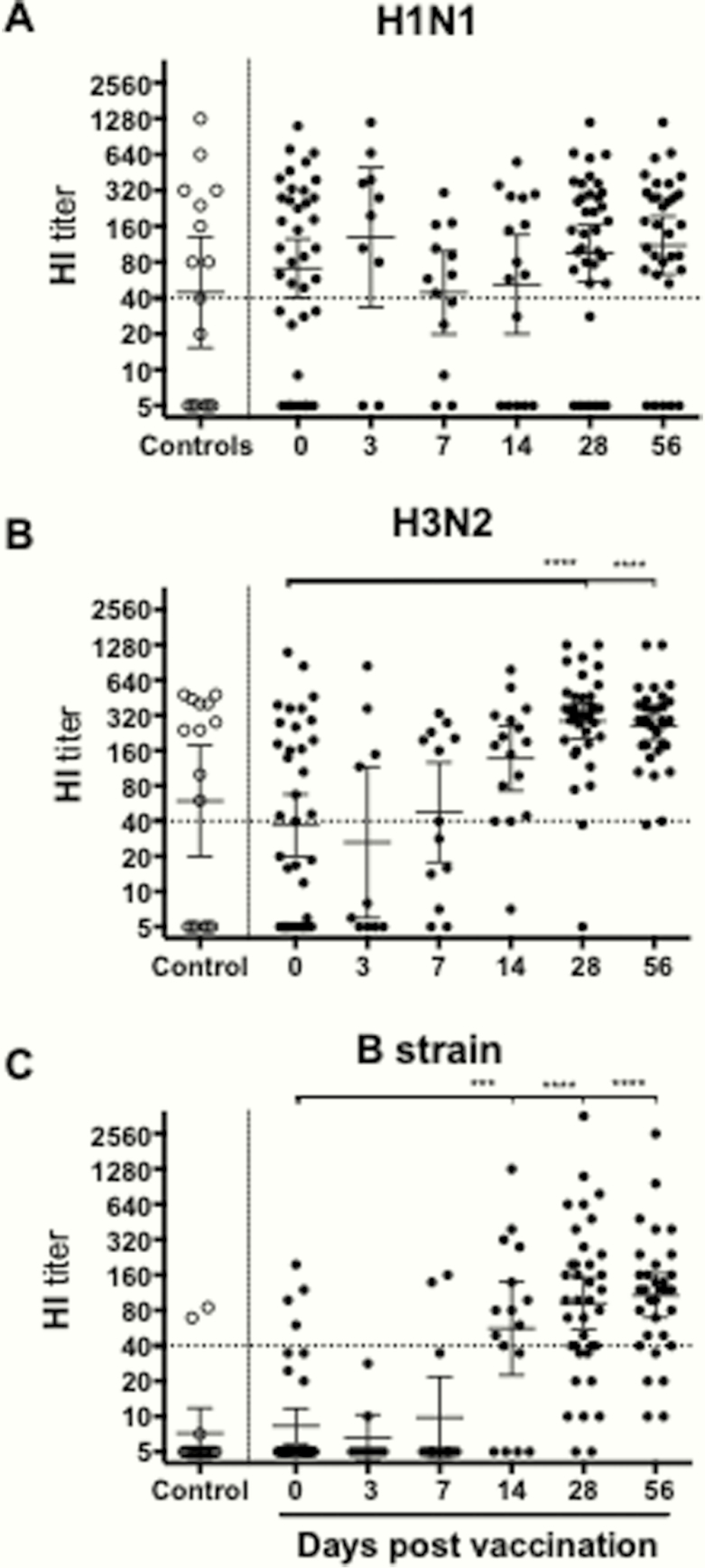
The influenza-specific HI responses were measured after LAIV. The majority of subjects (59%) had prevaccination HI titers ≥40 to H1N1, and no increase in antibodies was observed after vaccination, except for 1 subject (Figure 2). H3N2-specific HI titers ≥40 were observed in 49% of children prevaccination, and titers increased from day 14 with all subjects having titers ≥40 at day 56. Most children (89%) had no prevaccination HI antibodies to influenza B, but antibodies increased at day 14. By 56 days postvaccination, 84% of children had titers ≥40. The nonvaccinated controls had similar antibody titers to the prevaccination titers of the vaccinees, supporting their use as relevant controls for analysis of tonsillar T cells.
Figure 2.
Serum hemagglutination inhibition (HI) antibody response after live-attenuated influenza vaccine (LAIV). Plasma was collected pre- and postvaccination including at the time of tonsillectomy from children vaccinated with LAIV. The data show the influenza A H1N1 (A), influenza A H3N2 (B), and (C) B-strain specific HI responses of each individual subject. Influenza strain-specific HI antibody was measured by HI assay, prevaccination (day 0), the day of tonsillectomy (day 3, 7, or 14), and days 28 and 56 postvaccination. Control refers to the nonvaccinated group, which had similar HI titers as the day 0 vaccinees supporting their use as controls for the tonsillar results. The horizontal lines represent the geometric mean titers ± 95% confidence interval. The dotted line represents an HI titer of 40 regarded as protective antibody titers.
Interferon-γ T-Cell Responses in Tonsils and Blood
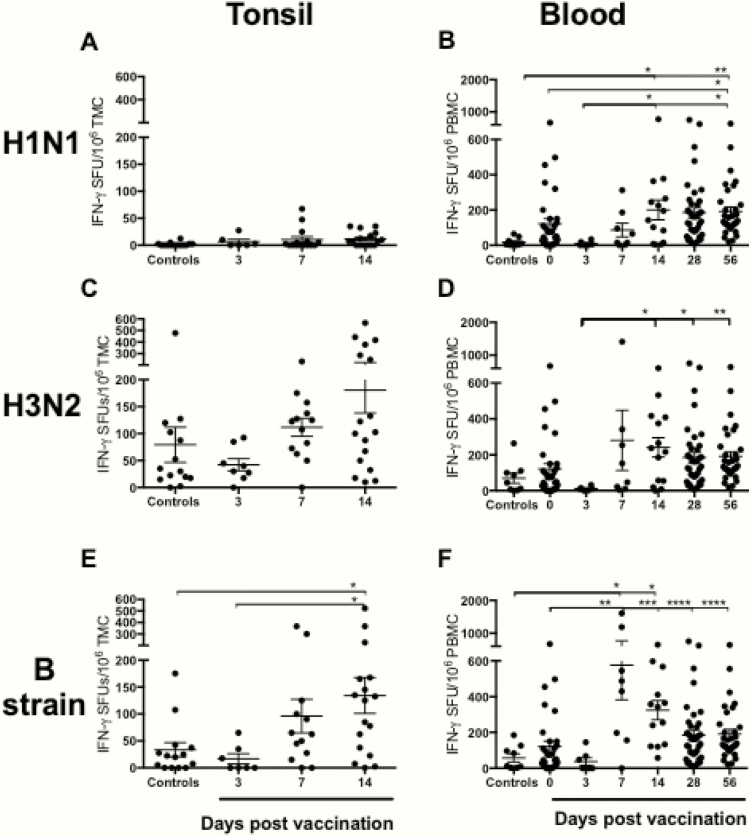
Antigen-specific IFN-γ responses were measured in TMCs and PBMCs from LAIV-vaccinated and control subjects after stimulation with either split antigens (Figure 3) or peptides representing conserved CD4+ and CD8+ T-cell epitopes (Figure 4). Low levels of H1N1-specific IFN-γ-secreting TMCs were detected in nonvaccinated controls, and no increase in IFN-γ-secreting TMC response was detected after vaccination. These findings were confirmed by using CD4+ and CD8+ H1N1-specific peptides (Supplementary Figure 1). In contrast, the H1N1-specific IFN-γ response in PBMCs was significantly enhanced from day 0 and 3 to 56 days postvaccination (means = 58–167 and 9–167 spot-forming units [SFU]/1 × 106 cells, respectively), with a peak reached at day 14 (mean 200 SFU/1 × 106 cells). The H3N2-specific IFN-γ response of TMC was higher 14 days postvaccination compared with controls (day 14 mean = 181 and control = 80 IFN-γ SFU/1 × 106 cells, respectively), although not statistically significant. Vaccination did not significantly enhance the H3N2-specific IFN-γ response in PBMCs. In contrast, both the tonsillar and the systemic PBMC B-strain-specific IFN-γ responses were significantly higher 14 days postvaccination compared with the nonvaccinated subjects (tonsillar mean = 134 and systemic mean 325 versus nonvaccinated tonsillar mean = 18 and systemic mean = 58 IFN-γ SFU/1 × 106 cells, respectively).
Figure 3.
Strain-specific T-cell responses in tonsils and peripheral blood mononuclear cells (PBMCs) after live-attenuated influenza vaccination (LAIV). The influenza H1N1, H3N2, and B strain-specific interferon (IFN)-γ responses in tonsillar mononuclear cells ([TMC] A, C, and E) and PBMCs (B, D, and F) were determined by IFN-γ enzyme-linked immunospot in nonvaccinated controls and subjects vaccinated with the LAIV. Each symbol represents the influenza-specific IFN-γ response (spot-forming units [SFU] per 1 × 106 cells) after stimulation with split virus antigens. The horizontal bars represent the mean IFN-γ response for each time point ± standard error of the mean. Statistical significance was determined by the non-parametric Kruskal-Wallis multiple comparisons test (*, P < .05; **, P < .005).
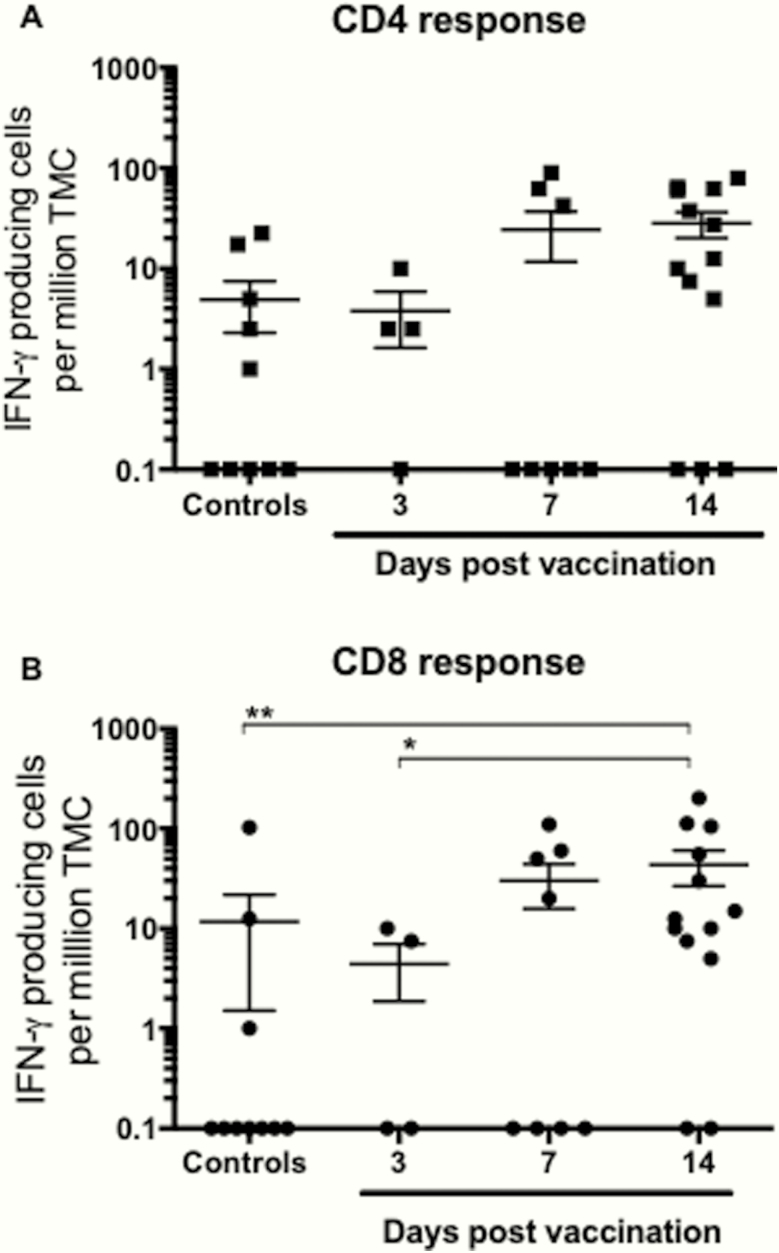
Figure 4.
Cross-reactive CD4+ and CD8+ T-cell responses after live-attenuated influenza vaccination (LAIV) vaccination. The T-cell immune response was evaluated by measuring the number of influenza-specific interferon (IFN)-γ-secreting T cells (spot-forming units [SFU]) after LAIV, using the enzyme-linked immunospot assay. Tonsillar mononuclear cells (TMC) isolated from tonsils were tested for responses against panels of peptides representing conserved T-cell epitopes (A and B). Responses to CD4 T-cell epitopes (major histocompatibility complex [MHC] class II restriction) are shown to the left, and responses to CD8 epitopes (MHC class I restriction) are shown to the right of the figure. Each symbol represents the number of influenza-specific SFU per million TMC for each child with the mean and stand error of the mean shown. Statistical differences between vaccinated and nonvaccinated subjects were determined by the non-parametric Kruskal-Wallis (*, P < .05; **, P < .005).
Interferon-γ CD4+ and CD8+ T-Cell Responses in Tonsils
To further determine the CD4+ and CD8+ T cells with cross-reactive potential, elicited by LAIV, we used peptide epitopes from internal influenza antigens, which are highly conserved among viral strains over several decades. After stimulation with the conserved influenza-specific CD4+ (Figure 4A) or CD8+ (Figure 4B) peptides, a significant increase in CD8+ T-cell responses between the nonvaccinated controls and the LAIV immunized children at 14 days postvaccination was seen, indicating cross-reactive responses (means = 12 and 45 IFN-γ SFU/1 × 106 cells, respectively). Likewise, a nonsignificant trend towards increased CD4+ T-cell responses was observed (means = 5 and 28 IFN-γ SFU/1 × 106 cells, at days 0 and 14, respectively). Although the TMC numbers are low, they represent a substantial number of T cells, because the total number of lymphocytes in the tonsils is large (109). Some subjects remained nonresponders to the peptides, indicating that the donor is antigenically naive or lacks peptide presentation due to HLA mismatch.
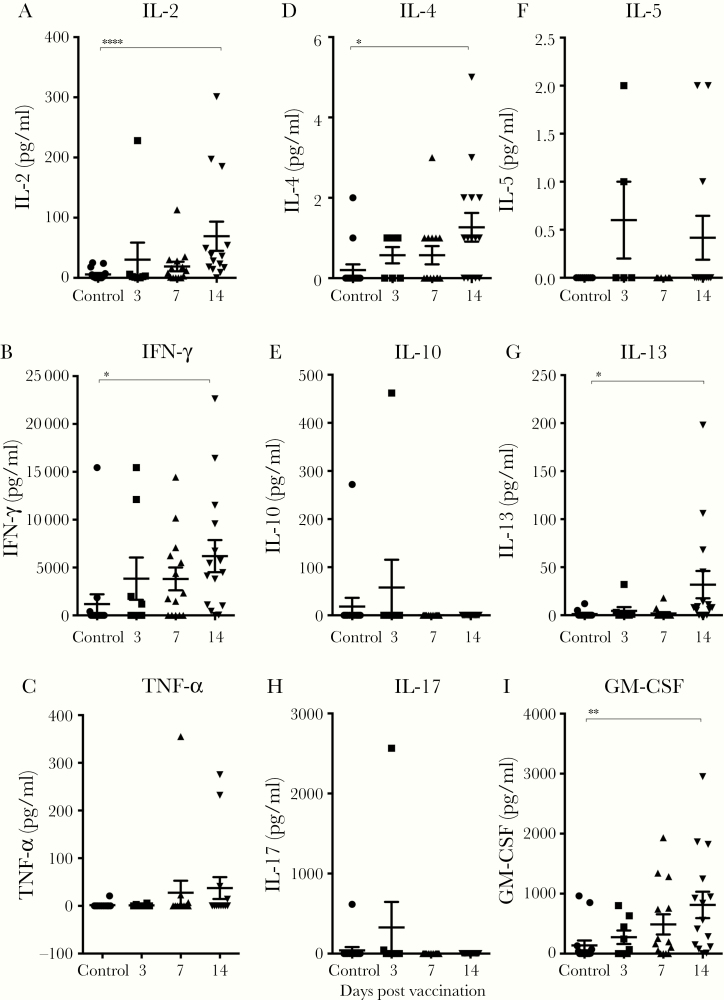
Cytokine Responses in Tonsils and Blood After Live-Attenuated Influenza Vaccine
Tonsillar mononuclear cells and PBMCs were stimulated with a mixture of the trivalent split vaccine antigens, and the cytokine responses detected after LAIV were grouped as Th1 (interleukin [IL]-2, IFN-γ, and tumor necrosis factor [TNF]-α), Th2 (IL-4, IL-5, IL-10, and IL-13), Th17 (IL-17), or granulocyte-macrophage colony-stimulating factor (GM-CSF) (Figure 5). Low levels of Th1-cytokines (IL-2, IFN-γ, and TNF-α) were observed in the unvaccinated controls, whereas a significant increase of IL-2 and IFN-γ levels was detected at day 14. The levels of Th2 cytokines (IL-4 and IL-13) as well as GM-CSF increased over time postvaccination, with day 14 levels being significantly higher than the controls. However, no significant increases were observed for IL-10, IL-5, or IL-17 during the same observation period. No significant increases in cytokine responses in PBMCs (Th1, Th2, Th17, or GM-CSF) were observed postvaccination compared with prevaccination (data not shown).
Figure 5.
Cytokine responses after live-attenuated influenza vaccination (LAIV) in tonsillar mononuclear cells (TMCs). The TMCs were isolated from nonvaccinated controls and from vaccinees at 3, 7, and 14 days after immunization with LAIV. The TMCs were stimulated for 72 hours with a mixture of split virus antigens from influenza A H1N1, influenza A H3N2, and B vaccine strains, and supernatants were analyzed by multiplex for the presence of cytokines. The Th1 (interleukin [IL]-2 [A], interferon [IFN]-γ [B], and tumor necrosis factor [TNF]-α [C]), Th2 (IL-4 [D], IL-5 [E], IL-10 [F], and IL-13 [G]), and Th17 (IL-17 [H], granulocyte-macrophage colony-stimulating factor [GM-CSF] [I]). Each symbol shows the influenza-specific cytokine response of 1 subject, and the horizontal lines represent the mean ± standard error of the mean. Statistical significance between the cytokine responses in nonvaccinated controls and vaccinated subjects was determined by the non-parametric Kruskal-Wallis multiple comparisons test. *, P < .01.
DISCUSSION
T cells contribute to the protection against severe influenza illness and in recovery from infection [2]. Influenza-specific cytotoxic CD8+ T cells have been linked to reduced viral shedding and increased survival after H7N9 avian influenza infection [6, 7]. Furthermore, pre-existing T-cell immunity has been found to protect against confirmed influenza disease in the community [4]. T cells often provide broad cross-reactive immune responses due to preferential recognition of epitopes from conserved internal influenza antigens [8–12]. Our study is the first to describe the induction of influenza cross-reactive CD8 T cells in the tonsils of healthy children after LAIV immunization. We used peptides representing conserved CD8 T-cell epitopes, and we found that tonsillar CD8+ T cells with cross-protective potential were elicited as early as 7–14 days postvaccination. This early mucosal T-cell response, close to the anatomical site of vaccine application, may provide protection at a population level, lessening the societal burden from drifted or shifted strains.
Interferon-γ has powerful antiviral activity, and increased levels may help prevent severe influenza illness [27, 28]. Elevated numbers of IFN-γ-producing T cells were detected in the blood after LAIV vaccination, lasting up to 1 year as previously described [13, 17, 19, 29, 30]. In this study, we have assessed the local influenza-specific IFN-γ response by enzyme-linked immunospot (ELISPOT) using split virus antigens, which mainly detect CD4+ T-cell responses. Differences in the IFN-γ T-cell responses were observed between the 3 vaccine strains. Responses to the B strain were significantly elevated in both tonsils and blood, whereas the increase detected against H3N2 occurred in the tonsils but did not reach significance. In contrast, the H1N1-specific response increased only in the blood. The study was conducted 3 years postpandemic, and the immune response appears to be influenced by the higher prevaccination HI titers towards H1N1, which could have reduced viral replication and hence the local tonsillar immune response. Lower prevaccination titers were found towards the influenza H3N2 and B strains, with the strongest tonsillar response towards the B strain, suggesting less exposure to influenza B and efficient replication of the B strain. Differences in LAIV effectiveness data between the United States and Europe have been found, which may be due to regional differences in vaccination strategies and infection pressure [31–33].
We observed a mixed cytokine response in blood and tonsils of both Th1 and Th2 signatures, indicating the vaccine stimulates a broad immune response, with a wide range of immune competent cells involved. More important, we did not see significant increases in influenza-specific cytokine levels in the blood up to day 14 postvaccination, corresponding to the findings of low reactogenicity after vaccination. However, we have previously observed a significantly elevated systemic multifunctional CD4+ response (IFN-γ, IL-2, and TNF-α) after 2 doses of LAIV in children [13]. This suggests that cytokine responses can be detected earlier in tonsils compared with blood. In agreement with this, we have previously found an early increase in tonsillar B cells and Tfh cells after LAIV [22, 34].
A postpandemic study found that the level of pre-existing cellular immunity was inversely correlated to HI antibodies [35]. This raised concerns that vaccination of children with IIV may not induce cross-reactive T-cell immunity, because IIVs primarily induce antibodies and not cellular responses [36]. However, this concern does not apply to LAIV, supported by our findings in the pediatric population that LAIV induced both broad cellular and humoral immune responses. Studies have also shown that animals challenged with heterosubtypic influenza strains were protected after LAIV [37–40]. More important, human studies have found that LAIV provided protection in children against a drifted H3N2 variant virus, naturally occurring during the studies and not contained in the vaccine [19, 41]. Furthermore, we have previously shown proof of concept that LAIV boosts cross-reactive, protection associated systemic CD8+ T-cell responses after LAIV, which could provide broad immunity to drifted and shifted influenza strains [21]. However, it is not known whether these T cells provide protection at the site of infection in the upper respiratory tract.
The use of conserved peptide epitope panels provides an important tool to evaluate cross-protective, T-cell responses after LAIV vaccination. Due to the high conservancy score of the universal epitopes used in this study, we conclude that LAIV vaccination of children can induce cross-reactive mucosal T-cell responses that cover a wide range of seasonal and potential pandemic strains. This important knowledge supports the use of LAIV in this age group to elicit cross-protective cellular immune responses. Together with our previous findings of systemic CD8+ T cells, this new knowledge indicates a broader protective immune response after LAIV than previously acknowledged. These findings might provide the immunological basis to explain the protection observed in the absence of protective HI antibodies (HI) and the lower hospitalization rates after childhood LAIV vaccination in the United Kingdom [41, 42].
The HI is commonly used as a COP when evaluating inactivated vaccines. However, induction of HI antibodies after mucosal LAIV does not sufficiently reflect the immune responses, due to the compartmentalization of the immune system. A body of experimental data supports the introduction of cellular assays as a relevant correlate of protection, although they are not yet fully accepted by the regulatory authorities for approval of vaccines [43]. A large efficacy trial in children suggested IFN-γ ELISPOT counts of ≥100 SFU/million PBMCs, as a correlate of protection after LAIV. Furthermore, background levels of <20 SFU/million PBMCs have been found in a United Kingdom child cohort [4]. However, prevaccination levels of >100 SFU/million PBMCs were found in our Norwegian cohort [21], probably due to previous infection. The IFN-γ ELISPOT assays may have a great potential as a correlate of protection, but this is laborious in a clinical setting [3, 44, 45]. A consensus has not been reached within the field, and the number of 100 IFN-γ-producing cells is considered arbitrary [3]. Advances in the research field may lead to the development of a more rapid and convenient assay.
In this study, we have used a relatively small cohort of children, but the data shown here are in line with previous published results for LAIV vaccination [46–48]. The ELISPOT analysis was done based on unfractionated tonsil cells, and the distinction made between CD8+ and CD4+ T-cell responses was based on the selective ability of the peptide epitopes used to be presented by major histocompatibility complex class I or II molecules as empirically verified [49]. A low level of additional cross-presentation can nonetheless not be excluded.
Children are the main transmitters of influenza in the community, and, when infected, they shed virus for a longer period compared with adults. Hence, childhood vaccination campaigns could limit the spread of influenza in the community. Indeed, after the United Kingdom commenced LAIV vaccination of children, signs of herd immunity have been observed in areas with widespread vaccination, such as reduced hospital admission of children [42]. In Japan, childhood IIV vaccination was found to have an indirect effect with a reduction in mortality rates in adults [50]. Successful LAIV immunization requires replication of the LAIV viruses in the mucosa of the upper airways to induce protection. Pre-existing antibodies or local cellular immunity could inhibit replication and hence immune response. The LAIV may be most suitable in the youngest children, with a naive immune response, and perhaps subsequent booster vaccinations should be with IIV. This would ensure a broad cellular and humoral response after LAIV, which can be further expanded by IIV immunizations to secure neutralizing immunity [35].
CONCLUSIONS
In this study, we provide the first evidence of LAIV eliciting cross-reactive CD8+ T-cell responses in the tonsils of young children. These T cells have the potential to provide broad protection against seasonal and pandemic viruses, supporting the use of LAIV as a childhood vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Table 1. Set of Conserved CD4o and CD8 or AH1N1pdm09-Specific Epitopesa
aPeptides covering unique CD4 and CD8 epitopes from the A(H1N1)pdm09 influenza virus. All of these epitopes are conserved in all 4 swine-origin H1N1 strains (A/California/07/2009, A/England/195/2009, A/Mexico-city/004/2009, and A/Paris/2592/2009). Epitopes are listed in the order of their selection by a greedy algorithm (Order). For each epitope, its estimated response frequency from the literature (ie, Prevalence), fraction of strains that contain 100% matches of the epitope (Conservancy), and the fraction of predicted HLA supertype coverage (S-type_coverage) are also listed in the table.
Supplementary Figure 1. Cross-reactive CD4+ and CD8+ T-cell responses to H1N1pdm09 after LAIV vaccination. The T-cell immune response was evaluated by measuring the number of influenza-specific IFN-γ-secreting T cells (spot forming units [SFU]) after LAIV, using the ELISPOT assay. TMCs isolated from tonsils were tested for responses against panels of peptides representing conserved T-cell epitopes of H1N1pdm09 influenza strain (A and B). Low levels of H1N1-specific IFN-γ-secreting TMCs were detected in nonvaccinated controls, and no increase in IFN-γ-secreting TMC response was detected after vaccination. Each symbol represents the influenza-specific SFU per million TMC for each child with the mean and stand error of the mean (SEM) shown. Statistical differences between vaccinated and nonvaccinated subjects were determined by the nonparametric Kruskal-Wallis (*, P < .05; **, P < .005).
Notes
Acknowledgments. We thank the children and their parents who altruistically participated in this study, the staff at Haukeland University Hospital (the Pediatric Clinical Trials Unit and the Department of Otorhinolaryngology), Dr. Siri Mjaaland and colleagues at the Norwegian Institute of Public Health, and the staff at the Influenza Centre, Department of Clinical Sciences, University of Bergen, Norway.
Financial support. This study was funded intramurally by the Influenza Centre at the University of Bergen and Haukeland University Hospital. The Influenza Centre is funded by the Ministry of Health and Care Services, Norway, the Norwegian Research Council Globvac (284930), the European Union (EU IMI 115672, FLUCOP), EU Nanomedicines Flunanoair (ERA-NETet EuroNanoMed2, JTC2016), and the K.G. Jebsen Centre for Influenza Vaccine Research.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Nair H, Brooks WA, Katz M, et al. . Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917–30. [DOI] [PubMed] [Google Scholar]
- 2. McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med 1983; 309:13–7. [DOI] [PubMed] [Google Scholar]
- 3. Forrest BD, Pride MW, Dunning AJ, et al. . Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol 2008; 15:1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayward AC, Wang L, Goonetilleke N, et al. . Natural T cell-mediated protection against seasonal and pandemic influenza. Results of the flu watch cohort study. Am J Respir Crit Care Med 2015; 191:1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koutsakos M, Illing PT, Nguyen TH, et al. . Human CD8(+) T cell cross-reactivity across influenza A, B and C viruses. Nat Immunol 2019; 20:613–25. [DOI] [PubMed] [Google Scholar]
- 6. Sridhar S, Begom S, Bermingham A, et al. . Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med 2013; 19:1305–12. [DOI] [PubMed] [Google Scholar]
- 7. Wang Z, Wan Y, Qiu C, et al. . Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8⁺ T cells. Nat Commun 2015; 6:6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sridhar S, Begom S, Bermingham A, et al. . Predominance of heterosubtypic IFN-gamma-only-secreting effector memory T cells in pandemic H1N1 naive adults. Eur J Immunol 2012; 42:2913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheible K, Zhang G, Baer J, et al. . CD8+ T cell immunity to 2009 pandemic and seasonal H1N1 influenza viruses. Vaccine 2011; 29:2159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jameson J, Cruz J, Ennis FA. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J Virol 1998; 72:8682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee LY, Ha do LA, Simmons C, et al. . Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest 2008; 118:3478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quiñones-Parra S, Grant E, Loh L, et al. . Preexisting CD8+ T-cell immunity to the H7N9 influenza A virus varies across ethnicities. Proc Natl Acad Sci U S A 2014; 111:1049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohn KG, Bredholt G, Brokstad KA, et al. . Longevity of B-cell and T-cell responses after live attenuated influenza vaccination in children. J Infect Dis 2015; 211:1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Islam S, Mohn KG, Krammer F, et al. . Influenza A haemagglutinin specific IgG responses in children and adults after seasonal trivalent live attenuated influenza vaccination. Vaccine 2017; 35:5666–73. [DOI] [PubMed] [Google Scholar]
- 15. Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2012: CD004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44. [DOI] [PubMed] [Google Scholar]
- 17. He XS, Holmes TH, Zhang C, et al. . Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol 2006; 80:11756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He XS, Holmes TH, Sasaki S, et al. . Baseline levels of influenza-specific CD4 memory T-cells affect T-cell responses to influenza vaccines. PLoS One 2008; 3:e2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoft DF, Babusis E, Worku S, et al. . Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 2011; 204:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandtzaeg P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am J Respir Crit Care Med 2011; 183:1595–604. [DOI] [PubMed] [Google Scholar]
- 21. Mohn KG, Zhou F, Brokstad KA, Sridhar S, Cox RJ. Boosting of cross-reactive and protection-associated T cells in children after live attenuated influenza vaccination. J Infect Dis 2017; 215:1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohn KG, Brokstad KA, Pathirana RD, et al. . Live attenuated influenza vaccine in children induces B-cell responses in tonsils. J Infect Dis 2016; 214:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis 1995; 171:198–203. [DOI] [PubMed] [Google Scholar]
- 24. Savic M, Dembinski JL, Kim Y, et al. . Epitope specific T-cell responses against influenza A in a healthy population. Immunology 2016; 147:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madhun AS, Akselsen PE, Sjursen H, et al. . An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine 2010; 29:266–73. [DOI] [PubMed] [Google Scholar]
- 26. Eriksson JC, Cox RJ, Szyszko E, Davidsson A, Brokstad KA. Local and systemic cytokine and chemokine responses after parenteral influenza vaccination. Influenza Other Respir Viruses 2007; 1:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hill DA, Baron S, Perkins JC, et al. . Evaluation of an interferon inducer in viral respiratory disease. JAMA 1972; 219:1179–84. [PubMed] [Google Scholar]
- 28. Richman DD, Murphy BR, Baron S, Uhlendorf C. Three strains of influenza A virus (H3N2): interferon sensitivity in vitro and interferon production in volunteers. J Clin Microbiol 1976; 3:223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao RG, Suarez NM, Obermoser G, et al. . Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children. J Infect Dis 2014; 210:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu W, Higgs BW, Morehouse C, et al. . A whole genome transcriptional analysis of the early immune response induced by live attenuated and inactivated influenza vaccines in young children. Vaccine 2010; 28:2865–76. [DOI] [PubMed] [Google Scholar]
- 31. Caspard H,Gaglani M, Clipper L, et al. . Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children 2–17 years of age in 2013–2014 in the United States. Vaccine 2016; 34:77–82. [DOI] [PubMed] [Google Scholar]
- 32. Mohn KG, Smith I, Sjursen H, Cox RJ. Immune responses after live attenuated influenza vaccination. Hum Vaccin Immunother 2018; 14:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pebody R, McMenamin J, Nohynek H. Live attenuated influenza vaccine (LAIV): recent effectiveness results from the USA and implications for LAIV programmes elsewhere. Arch Dis Child 2018; 103:101–5. [DOI] [PubMed] [Google Scholar]
- 34. Lartey S, Zhou F, Brokstad KA, et al. . Live attenuated influenza vaccine induces tonsillar follicular T helper cell responses that correlate with antibody induction. J Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sridhar S, Begom S, Hoschler K, et al. . Longevity and determinants of protective humoral immunity after pandemic influenza infection. Am J Respir Crit Care Med 2015; 191:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bodewes R, Fraaij PL, Geelhoed-Mieras MM, et al. . Annual vaccination against influenza virus hampers development of virus-specific CD8⁺ T cell immunity in children. J Virol 2011; 85:11995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng X, Zengel JR, Suguitan AL Jr, et al. . Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis 2013; 208:594–602. [DOI] [PubMed] [Google Scholar]
- 38. Rekstin A, Isakova-Sivak I, Petukhova G, et al. . Immunogenicity and cross protection in mice afforded by pandemic H1N1 live attenuated influenza vaccine containing wild-type nucleoprotein. Biomed Res Int 2017; 2017:9359276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jang YH, Seong BL. Cross-protective immune responses elicited by live attenuated influenza vaccines. Yonsei Med J 2013; 54:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Isakova-Sivak I, Korenkov D, Smolonogina T, et al. . Comparative studies of infectivity, immunogenicity and cross-protective efficacy of live attenuated influenza vaccines containing nucleoprotein from cold-adapted or wild-type influenza virus in a mouse model. Virology 2017; 500:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Belshe RB, Gruber WC, Mendelman PM, et al. . Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr 2000; 136:168–75. [DOI] [PubMed] [Google Scholar]
- 42. Pebody R, Sile B, Warburton F, et al. . Live attenuated influenza vaccine effectiveness against hospitalisation due to laboratory-confirmed influenza in children two to six years of age in England in the 2015/16 season. Euro Surveill 2017; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. La Gruta NL, Turner SJ. T cell mediated immunity to influenza: mechanisms of viral control. Trends Immunol 2014; 35:396–402. [DOI] [PubMed] [Google Scholar]
- 44. Wilkinson TM, Li CK, Chui CS, et al. . Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274–80. [DOI] [PubMed] [Google Scholar]
- 45. Sridhar S, Brokstad KA, Cox RJ. Influenza vaccination strategies: comparing inactivated and live attenuated influenza vaccines. Vaccines (Basel) 2015; 3:373–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mohn KG, Zhou F, Brokstad KA, Sridhar S, Cox RJ. Live attenuated influenza vaccination boosts durable cross-reactive and protection-associated T-cells in children. J Infect Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mohn KG, Bredholt G, Brokstad KA, et al. . Longevity of B -cell and T-cell responses after live attenuated influenza vaccination in children. J Infect Dis 2015; 211:1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mohn KG, Brokstad KA, Pathirana RD, et al. . Live attenuated influenza vaccine in children induces B-cell responses in tonsils. J Infect Dis 2016; 214:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Savic MD, Dembinski JL, Kim Y, et al. . Epitope specific T-cell responses against influenza A in a healthy population. Immunology 2016; 147:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001; 344:889–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.