Abstract
Objectives
Relapse following remission is common in ANCA-associated vasculitis (AAV), particularly with ANCA directed at proteinase 3 (PR3-ANCA). We evaluated the association of a PR3-ANCA level rise with subsequent relapse.
Methods
Data from the rituximab versus cyclophosphamide/azathioprine for AAV (RAVE) trial were utilized. Starting from time of achieving complete remission, serial measurements by direct and capture ELISA were analyzed in 93 patients with PR3-ANCA, using Cox proportional hazards regression.
Results
A rise in PR3-ANCA was identified in 58/93 subjects (62.4%) by direct ELISA, and 59/93 (63.4%) by capture ELISA. Relapses occurred in 55/93 (59.1 %) subjects, with 25/55 within one year after a rise by direct ELISA and 21/55 by capture ELISA. A rise by direct ELISA was associated with subsequent severe relapses (P<0.001, HR 4.57), particularly in patients presenting with renal involvement (P<0.001, HR 7.94) and alveolar hemorrhage (P<0.001, HR 24.19). Both assays identified increased risk for severe relapse in the rituximab group (direct:P=0.002, HR 5.80; capture:P=0.007, HR 4.54), but not the cyclophosphamide/azathioprine group (P=0.103, P=0.197).
Conclusions
The association of PR3-ANCA rise with subsequent relapse risk is partially affected by the PR3-ANCA detection methodology, disease phenotype and remission induction treatment. A rise in PR3-ANCA during complete remission conveys an increased risk of relapse, particularly severe relapse, among patients with renal involvement or alveolar hemorrhage and those treated with rituximab; serial measurements of PR3-ANCA may be informative in this subset of patients but the risk of relapse must be weighed carefully against the risks associated with therapy.
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a heterogenous group of diseases including granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). AAV is defined by inflammation and necrosis predominantly of small blood vessels, along with necrotizing granulomatous tissue inflammation in GPA(1,2). Acute morbidity, mortality and irreversible organ damage are attributable to the underlying disease and to complications of immunosuppressive therapy(3–6). Remission is achieved in up to 90% of patients with induction therapy, but more than half of patients with severe disease experience relapses(5–7). In the absence of definitive indicators of oncoming relapses, balancing the risks of immunosuppression with the benefits of disease control remains challenging(8,9).
The diagnostic utility of ANCA testing has been widely accepted, whereas their clinical utility as a biomarker of disease activity and predictor of relapses has remained controversial despite numerous investigations(10–32). A clear role for serial ANCA measurements in AAV as a whole has not been established, and it is not advised to make treatment decisions based on changes in ANCA titers alone(33,34). Methodologic issues including the specific assays and the definitions of an ANCA rise, the ANCA type, the disease phenotype and the treatment chosen to induce or maintain remission all deserve consideration when assessing the clinical utility of serial ANCA monitoring(9,33). It has been well-documented that patients with ANCA targeting proteinase 3 (PR3-ANCA) have a higher risk of relapse than patients with ANCA toward myeloperoxidase (MPO-ANCA)(7,31,35–38). Most recently, a single center study looking at 166 AAV patients found that a rise in ANCA (PR3- or MPO-ANCA) was predictive of relapses, particularly in patients who had presented with renal involvement, and in those with nonrenal severe disease(15).
Consequently, we hypothesized that methodology used to measure ANCA levels, the disease phenotype, and the remission induction regimen all impact the clinical utility derived from serial measurements of ANCA titers. To address this hypothesis, we evaluated the relationship between PR3-ANCA titers and the risk of relapse within one year of a rise in a well-defined cohort of patients with severe AAV.
Patients and Methods
Patients
This study utilized serum samples and clinical data collected during the rituximab versus cyclophosphamide for AAV (RAVE) trial. Details of the RAVE trial protocol have been described elsewhere(4,7). Briefly, patients were randomized to rituximab or cyclophosphamide, along with a prespecified prednisone taper, for induction therapy and were followed prospectively according to protocol at baseline; at weeks 1, 2, 3, and 4; at months 2, 4, and 6, and every 3 months until month 18. Thereafter, patients were seen every 6 months until common close out of the trial (which was the month 18 time point of the last patient enrolled). Additional visits occurred at the discretion of patients and providers to evaluate disease activity or medication toxicity. With each visit, the Birmingham Vasculitis Activity Score for Wegener granulomatosis (BVAS/WG) score was used in conjunction with clinical assessment to evaluate disease activity, and serum samples were collected and stored at −80°C(4,7).
Each of the 197 trial participants had either new or relapsing disease at the time of enrollment. A positive serum assay for PR3-ANCA or MPO-ANCA was required for enrollment. The present study focused on the 131 patients having PR3-ANCA because of the higher number of patients and relapses in this group. In contrast, of the 66 MPO-ANCA positive patients, 52 achieved complete remission, and only 15 experienced a relapse. (4,7).
Disease activity
Assessments
The BVAS/WG instrument was utilized to document disease activity at each study visit. A score of 1 or more reflects active disease within the 28 days prior to assessment, while 0 indicates remission(39). Complete remission was defined as a BVAS/WG of 0, along with a prednisone dose of 0mg. Disease relapse was defined as any new disease activity, with an increase in BVAS/WG of 1 or more points, after achievement of complete remission. A relapse was considered severe if BVAS/WG was greater than 3, if a new major item was present, or if, in the judgment of the clinician, reinitiation of induction therapy was warranted(40).
Disease manifestations and disease phenotype categories
The organ manifestations present at enrollment and at each study visit were recorded by expert clinicians (study investigators) using the BVAS/WG instrument. The disease phenotype categories used for this analysis are based on the BVAS/WG items recorded at the time of enrollment. BVAS/WG items considered to reflect underlying necrotizing granulomatous inflammation included mouth ulcers, retroorbital mass/proptosis, bloody nasal discharge, sinus involvement, salivary gland enlargement, subglottic inflammation, conductive deafness, other major or minor ENT, pulmonary nodule/cavity, endobronchial involvement, meningitis, and cord lesion. In contrast, capillaritis was defined as the presence of one or more of the following BVAS/WG items: cutaneous purpura, scleritis, retinal hemorrhage or exudate, sensorineural deafness, mesenteric ischemia, alveolar hemorrhage, hematuria, red blood cell casts on urinalysis or glomerulonephritis, rise in creatinine, sensory peripheral neuropathy, or motor mononeuritis multiplex. Patients were considered to have renal disease if any renal item on the BVAS/WG (hematuria, red blood cell casts or glomerulonephritis, rise in creatinine, or “other”) was scored. A patient was categorized as having alveolar hemorrhage only if that item was scored on the BVAS/WG. All other BVAS/WG items cannot be clearly attributed to either necrotizing granulomatous inflammation or capillaritis and were, therefore, not considered to categorize the patient one way or another. Using this approach all patients could be clearly assigned to one or more of five groups subjected to analysis: “granulomatous disease only,” “any granulomatous disease,” “any capillaritis,” “renal involvement,” “alveolar hemorrhage” at enrollment.
ANCA testing
Assays
Standardized direct enzyme-linked immunosorbent assays (ELISA) for PR3-ANCA and MPO-ANCA were performed on the baseline serum samples of all 197 patients(28; Euroimmun, Lubeck, Germany). For patients found to have PR3-ANCA, serial samples were tested with the direct ELISA, as well as a capture ELISA developed in our laboratory and described previously, utilizing a monoclonal antibody to PR3 (MCPR3-2), was performed on all samples(41). For each ELISA described here, stored serum samples from serial visits for each individual patient were run on the same assay plate at a single laboratory from the second thaw cycle of each sample. The definitions used for a rise by each assay were selected to be outside the intra- and inter-assay coefficients of variability, which have been published elsewhere(Euroimmun assay test instruction sheet; 14), and these definitions are consistent with prior publications using these assays(7,14). The value at each visit was compared to the lowest value within the preceding 6 months.
Definitions
For the direct ELISA, a PR3-ANCA titer of ≥ 20 units was considered positive. A rise in PR3-ANCA was defined as a doubling of the result, or an increase to at least 40 units if the assay had previously become negative, within the preceding 6 months(7).
For the capture ELISA, a level, expressed as net absorbance, of ≥ 0.10 was considered positive. A rise in PR3-ANCA was defined as a doubling of the result, with an absolute increase of at least 0.40, within the preceding 6 months(14).
Statistical analysis
All analyses were performed using SAS, version 9.3 (SAS Institute, Cary, North Carolina).
Descriptive data were summarized with mean (and standard deviation), median (and interquartile range), or percentages.
Cox proportional hazards models were used to assess whether a rise in PR3-ANCA was associated with subsequent relapse. Separate analyses were performed with the event of interest being “any” relapse or “severe” relapse. For these analyses the date of complete remission was used as time zero. All patients who experienced a rise in PR3-ANCA without previously experiencing the specified type of relapse event were identified. A rise in PR3-ANCA was modeled using a binary time-varying covariate. For a given patient, this variable has a value of “0” from time zero to the date that a rise in PR3-ANCA was detected and a value of “1” following this date. Using this approach, rises detected concurrent with a relapse event are treated as if no rise occurred. Since the primary question of interest was whether patients who experience an increase of PR3-ANCA are at increased risk for relapse during the first 12 months following the increase, the primary analyses were performed with data censored at last follow-up for patients who did not experience an increase in PR3-ANCA, and, for patients with an increase in PR3-ANCA, at 12 months following the increase or at last follow-up (whichever was shorter).
Findings from the proportional hazards regression are summarized using the hazard ratio (HR) with corresponding 95% confidence interval. As the prospective, time-dependent survival analysis precludes the calculation of sensitivity and specificity of a PR3-ANCA titer rise for relapse in this data set, we calculated the concordance index (c-index), which is an extension of the concept of the receiver operator characteristic (ROC) curve providing a measure of predictive discrimination(42). Similar to the interpretation of the area under the ROC curve, a c-index of 0.5 indicates no discrimination, and a c-index between 0.7 and 0.8 is considered to indicate adequate discrimination(43).
To further characterize the cumulative percentage of patients who relapsed following an increase in PR3-ANCA, a Kaplan-Meier analysis was performed which included only patients who experienced an increase or PR3-ANCA. For this analysis, the date of the increase was used as time zero.
All analyses were performed for the entire cohort and for patient subsets defined according to disease phenotype categories, new diagnosis versus relapsing disease, and treatment groups. In all cases, two-tailed p-values <0.05 were considered statistically significant.
Results
Baseline characteristics
Of the 131 patients with PR3-ANCA at the time of enrollment in the RAVE trial, 93/131 (80.0%) achieved complete remission on the therapy to which they were originally randomized. The remaining 38 patients were excluded from this analysis(Figure 1).
Figure 1.
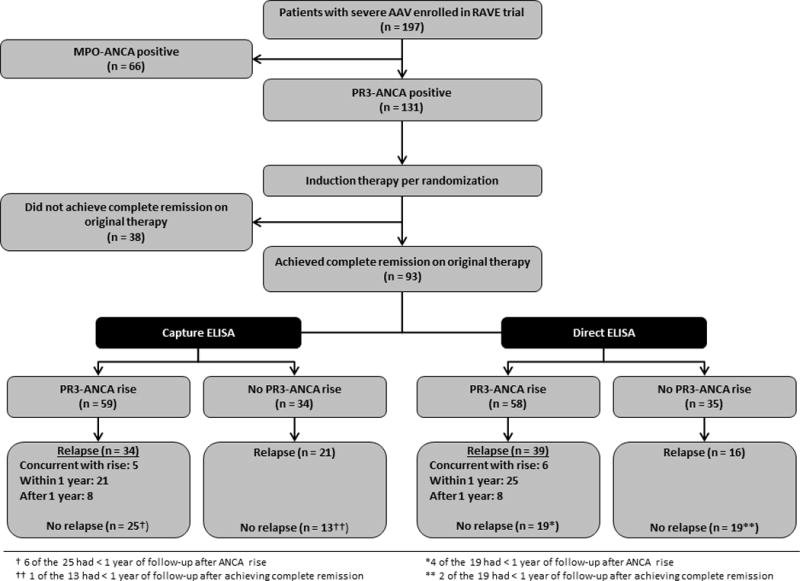
Patient selection and outcomes
Mean age of the 93 patients was 50.1 ± 14.2 years. The majority of the patients was male (n=56, 60.2%) and of white race (n=87, 93.5%); more than half had relapsing disease rather than a new diagnosis of AAV at enrollment (n=52 vs. 41, 55.9% vs. 44.1%). Median follow-up from the time of complete remission was 35.6 months, with 87/93 (93.5%) patients having follow-up of at least one year after achievement of complete remission. Additional baseline characteristics for our cohort are presented in Table 1.
Table 1.
Baseline Demographic and Clinical Characteristics
| Variable | |
|---|---|
| Age at enrollment, years [mean (SD)] | 50.1 (14.2) |
| Gender [n (%)] | |
| Female | 37 (39.8) |
| Male | 56 (60.2) |
| Race or ethnic group [n (%)] | |
| White | 87 (93.5) |
| Black | 3 (3.2) |
| Other | 3 (3.2) |
| Ethnic group [n (%)] | |
| Not Hispanic or Latino | 90 (96.7) |
| Hispanic or Latino | 2 (2.2) |
| Unknown | 1 (1.1) |
| ANCA-associated vasculitis type [n (%)] | |
| Granulomatosis with polyangiitis | 90 (96.7) |
| Microscopic polyangiitis | 3 (3.2) |
| Newly diagnosed at enrollment [n (%)] | 41 (44.1) |
| Organ involvement at enrollment, by BVAS/WG [n (%)] | |
| Ear, nose, and throat | 70 (75.3) |
| Constitutional | 65 (69.9) |
| Renal | 60 (64.5) |
| Pulmonary | 50 (53.7) |
| Mucous membranes and eyes | 31 (33.3) |
| Cutaneous | 20 (21.5) |
| Nervous system | 19 (20.4) |
| Cardiovascular | 1 (1.1) |
| Gastrointestinal | 1 (1.1) |
| Other | 7 (7.5) |
| BVAS/WG at enrollment [mean (SD)] | 8.1 (3.1) |
| ESR at enrollment, mm/hr [mean (SD)] | 36.0 (28.6) |
| CRP at enrollment, mg/dL [mean (SD)] | 5.0 (12.4) |
| Creatinine at enrollment, mg/dL [mean (SD)] | 1.0 (0.65) |
| Treatment group | |
| Rituximab [n (%)] | 50 (53.7) |
| Cyclophosphamide [n (%)] | 43 (46.2) |
| Time to CR, months [median (IQR)] | 5.9 (5.8 – 6.2) |
| Duration of follow-up after CR, months [median (IQR)] | 35.6 (24.6 – 42.6) |
SD, standard deviation; n, number; ANCA, antineutrophil cytoplasmic antibody; BVAS/WG, Birmingham Vasculitis Activity Score for Wegener Granulomatosis; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CR, complete remission; IQR, interquartile range (25-75%)
Relationship of PR3-ANCA titer increases and subsequent relapses
A rise in PR3-ANCA was identified in 58/93 subjects (62.3%) by direct ELISA, and in 59/93 (63.4%) by capture ELISA(Figure 1); 46 patients experienced a rise using both assays, though not necessarily at the same visit. To estimate the relative increase in risk of a disease relapse within one year conveyed by a PR3-ANCA titer increase, and to assess how well the model discriminates those at increased risk for relapse, we calculated hazard ratios and the c-indices, respectively(Table 2). The number of patients with a PR3-ANCA rise followed by a relapse and the timing of the relapses are listed for the entire cohort(Figure 1) and for each subgroup(Table 3). Median time to relapse and proportion of patients experiencing relapse by specific time points following a PR3-ANCA titer rise are presented in Table 4.
Table 2.
Rise in PR3-ANCA level and relapse within 1 year
| Capture ELISA | Direct ELISA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N* | HR | 95% CI | P | c-index | HR | 95% CI | P | c-index | |
|
|
|||||||||
| All subjects | 93 | ||||||||
| Any relapse | 55 | 1.15 | (0.62, 2.13) | 0.648 | 0.50 | 2.24 | (1.24, 4.08) | 0.008 | 0.59 |
| Severe relapse | 42 | 1.71 | (0.80, 3.71) | 0.169 | 0.55 | 4.57 | (2.16, 10.37) | <0.001 | 0.67 |
| Granulomatous only at enrollment | 15 | ||||||||
| Any relapse | 13 | 0.36 | (0.09, 1.38) | 0.136 | 0.38 | 0.65 | (0.17, 2.54) | 0.532 | 0.49 |
| Severe relapse | 11 | 0.48 | (0.11, 2.16) | 0.340 | 0.41 | 1.01 | (0.23, 4.48) | 0.989 | 0.53 |
| Renal involvement at enrollment | 60 | ||||||||
| Any relapse | 28 | 1.02 | (0.44, 2.33) | 0.954 | 0.48 | 2.16 | (1.00, 4.64) | 0.049 | 0.59 |
| Severe relapse | 21 | 2.33 | (0.82, 6.93) | 0.116 | 0.58 | 7.94 | (2.72, 29.18) | <0.001 | 0.71 |
| Alveolar hemorrhage at enrollment | 24 | ||||||||
| Any relapse | 14 | 1.69 | (0.48, 6.24) | 0.414 | 0.54 | 9.45 | (2.58, 34.63) | <0.001 | 0.76 |
| Severe relapse | 11 | 3.18 | (0.78, 14.94) | 0.118 | 0.61 | 24.19 | (3.05, 447.2) | <0.001 | 0.81 |
| According to treatment group | |||||||||
| Cyclophosphamide | 43 | ||||||||
| Any relapse | 24 | 0.63 | (0.22, 1.70) | 0.370 | 0.42 | 1.51 | (0.59, 4.17) | 0.400 | 0.55 |
| Severe relapse | 19 | 0.40 | (0.09, 1.47) | 0.197 | 0.42 | 2.84 | (0.87, 11.40) | 0.103 | 0.62 |
| Rituximab | 50 | ||||||||
| Any relapse | 31 | 1.90 | (0.85, 4.32) | 0.117 | 0.57 | 3.09 | (1.37,7.06) | 0.006 | 0.60 |
| Severe relapse | 23 | 4.54 | (1.61, 15.05) | 0.007 | 0.68 | 5.80 | (2.06, 19.77) | 0.002 | 0.68 |
PR3-ANCA, antineutrophil cytoplasmic antibodies directed at proteinase 3; ELISA, enzyme-linked immunosorbent assay; HR, hazard ratio per Cox proportional hazards regression; CI, confidence interval; c-index, concordance index;
N = total number of first relapse events of the specified type after complete remission for each subcategory during follow-up
Table 3.
PR3-ANCA rises and relapses by subgroup
| Capture ELISA | Direct ELISA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Rise | 10 | CYC, RTX (3, 7) |
No rise | 5 | CYC, RTX (3, 2) |
Rise | 11 | CYC, RTX (5, 6) |
No rise | 4 | CYC, RTX (1, 3) |
|
|
|
|
|
|
|||||||||
|
Granulomatous Only (N = 15) |
Relapse | 8 | (3, 5) | Relapse | 5 | (3, 2) | Relapse | 9 | (5, 4) | Relapse | 4 | (1, 3) |
| Concurrent | 1 | (0, 1) | Concurrent | 1 | (1, 0) | |||||||
| ≤ 1 year | 4 | (1, 3) | ≤ 1 year | 5 | (2, 3) | |||||||
| > 1 year | 3 | (2, 1) | > 1 year | 3 | (2, 1) | |||||||
| No relapse | 2 | (0, 2) | No relapse | 0 | (0, 0) | No relapse | 2 | (0, 2) | No relapse | 0 | (0, 0) | |
|
| ||||||||||||
|
Renal Involvement (N = 60) |
Rise | 33 |
CYC, RTX (15, 18) |
No rise | 27 |
CYC, RTX (14, 13) |
Rise | 36 |
CYC, RTX (16, 20) |
No rise | 24 |
CYC, RTX (13, 11) |
|
|
|
|
|
|||||||||
| Relapse | 14 | (4, 10) | Relapse | 14 | (7, 7) | Relapse | 19 | (6, 13) | Relapse | 9 | (5, 4) | |
| Concurrent | 2 | (1, 1) | Concurrent | 5 | (0, 5) | |||||||
| ≤ 1 year | 10 | (3, 7) | ≤ 1 year | 13 | (6, 7) | |||||||
| > 1 year | 2 | (0, 2) | > 1 year | 1 | (0, 1) | |||||||
| No relapse | 19† | (11, 8) | No relapse | 13†† | (7, 6) | No relapse | 17* | (10, 7) | No relapse | 15** | (8, 7) | |
|
| ||||||||||||
|
Alveolar Hemorrhage (N = 24) |
Rise | 14 |
CYC, RTX (2, 12) |
No rise | 10 |
CYC, RTX (6, 4) |
Rise | 18 |
CYC, RTX (5, 13) |
No rise | 6 |
CYC, RTX (3, 3) |
|
|
|
|
|
|||||||||
| Relapse | 8 | (0, 8) | Relapse | 6 | (4, 2) | Relapse | 14 | (4, 10) | Relapse | 0 | (0, 0) | |
| Concurrent | 1 | (0, 1) | Concurrent | 3 | (0, 3) | |||||||
| ≤ 1 year | 6 | (0, 6) | ≤ 1 year | 10 | (4, 6) | |||||||
| > 1 year | 1 | (0, 1) | > 1 year | 1 | (0, 1) | |||||||
| No relapse | 6† | (2, 4) | No relapse | 4 | (2, 2) | No relapse | 4* | (1, 3) | No relapse | 6 | (3, 3) | |
|
| ||||||||||||
|
Rituximab (N = 50) |
Rise | 35 | No rise | 15 | Rise | 32 | No rise | 18 | ||||
|
|
|
|
|
|||||||||
| Relapse | 22 | Relapse | 9 | Relapse | 23 | Relapse | 8 | |||||
| Concurrent | 3 | Concurrent | 5 | |||||||||
| ≤ 1 year | 14 | ≤ 1 year | 14 | |||||||||
| > 1 year | 5 | > 1 year | 4 | |||||||||
| No relapse | 13† | No relapse | 6†† | No relapse | 9* | No relapse | 10** | |||||
|
| ||||||||||||
|
Cyclophosphamide (N = 43) |
Rise | 24 | No rise | 19 | Rise | 26 | No rise | 17 | ||||
|
|
|
|
|
|||||||||
| Relapse | 12 | Relapse | 12 | Relapse | 16 | Relapse | 8 | |||||
| Concurrent | 2 | Concurrent | 1 | |||||||||
| ≤ 1 year | 7 | ≤ 1 year | 11 | |||||||||
| > 1 year | 3 | > 1 year | 4 | |||||||||
| No relapse | 12† | No relapse | 7 | No relapse | 10* | No relapse | 9** | |||||
5 (2, 3) of the 19 had < 1 year of follow-up after PR3-ANCA rise
1 (0,1) of the 13 had < 1 year of follow-up after complete remission
4 (1, 3) of the 17 had < 1 year of follow-up after PR3-ANCA rise
2 (1, 1) of the 15 had < 1 year of follow-up after complete remission
1 (0, 1) of the 6 had < 1 year of follow-up after PR3-ANCA rise
1 (0, 1) of the 4 had < 1 year of follow-up after PR3-ANCA rise
4 of the 13 had < 1 year of follow-up after PR3-ANCA rise
1 of the 6 had < 1 year of follow-up after complete remission
3 of the 9 had < 1 year of follow-up after PR3-ANCA rise
1 of the 10 had < 1 year of follow-up after complete remission
2 of the 12 had < 1 year of follow-up after PR3-ANCA rise
1 of the 10 had < 1 year of follow-up after PR3-ANCA rise
1 of the 9 had < 1 year of follow-up after complete remission
Table 4.
Kaplan-Meier estimates for time to relapse following a rise in PR3-ANCA*
| Direct ELISA
|
|||||
|---|---|---|---|---|---|
| Median time to relapse | Cumulative Relapse, % (95% C.I.)
|
||||
| N | 6 months | 12 months | 18 months | ||
| All subjects with rise in PR3-ANCA | |||||
| Any relapse | 52 | 11.8 mo. | 33 (19, 45) | 50 (33, 63) | 60 (43, 72) |
| Severe relapse | 60 | 19.1 mo. | 27 (15, 38) | 43 (29, 55) | 49 (34, 61) |
| Renal involvement at enrollment | |||||
| Any relapse | 31 | 19.1 mo. | 30 (12, 45) | 46 (24, 61) | 46 (24, 61) |
| Severe relapse | 37 | 22.5 mo. | 25 (9, 38) | 40 (21, 55) | 44 (24, 59) |
| Alveolar hemorrhage at enrollment | |||||
| Any relapse | 15 | 5.2 mo. | 53 (20, 73) | 69 (32, 86) | 77 (39, 91) |
| Severe relapse | 17 | 8.5 mo. | 41 (12, 60) | 61 (28, 79) | 67 (34, 84) |
| Rituximab | |||||
| Any relapse | 27 | 11.5 mo. | 35 (14, 51) | 57 (32, 63) | 65 (40, 80) |
| Severe relapse | 34 | 11.8 mo. | 30 (13, 45) | 50 (29, 65) | 57 (36, 71) |
| Cyclophosphamide | |||||
| Any relapse | 25 | 17.2 mo. | 32 (11, 48) | 44 (21, 61) | 54 (29, 71) |
| Severe relapse | 26 | 22.5 mo. | 23 (5, 38) | 35 (14, 51) | 40 (17, 56) |
Analyses include only individuals who experienced a rise in PR3-ANCA during follow-up while at risk for the specified type of relapse; rises detected concurrent with the specified type of relapse were treated as if no rise had occurred. Time zero corresponds to the date of the rise in PR3-ANCA level. The number of patients with a rise before “severe relapse” is higher than that before “any relapse” because for each grouping, one or more patients experienced a rise in PR3-ANCA level concurrent with or after a non-severe relapse, while still “at risk” for (before the occurrence of) a severe relapse.
Disease relapse occurred in 55 of 93 (59.1%) subjects. A rise in PR3-ANCA titer as measured by direct ELISA was associated with an increased risk for any relapse within one year (P=0.008), and for a severe relapse within one year (P<0.001). However, a rise detected by capture ELISA was not associated with an increased risk for either type of relapse (P=0.648, P=0.169, respectively)(Table 2). Moreover, the c-indices of 0.59 for any relapse and 0.67 for severe relapse detected by direct ELISA indicate that the model approached an adequate level of discrimination only for severe relapses(Table 2). Thirty-nine of the 58 patients (67.2%) who had a PR3-ANCA rise detected by direct ELISA had a subsequent relapse during the entire observation period, and only 25 had a relapse within one year(Figure 1). Median time to any relapse following a rise by direct ELISA was 11.8 months(Table 4). Sixteen of the 35 patients (45.7%) who did not have a PR3-ANCA rise by direct ELISA also experienced a relapse(Figure 1). Subsequent declines in PR3-ANCA value of ≥ 50% occurred in 7 of the 58 patients with a rise by direct ELISA and in 3 of 59 using capture ELISA; none of these patients experienced a relapse within one year of the rise.
Factors affecting the risk of relapse following a PR3-ANCA titer increase
We further investigated the relative increase in risk of relapse conveyed by a PR3-ANCA titer increase in patient subsets defined according to various factors of clinical relevance.
PR3-ANCA detection methodology
The method of PR3-ANCA measurement used to quantify PR3-ANCA titer changes over time was a crucial determinant of risk associated with a titer increase. An increase of PR3-ANCA detected by direct ELISA was consistently associated with an increased risk of severe relapse within one year throughout all analyzed disease subsets except for patients who only had disease manifestations attributable to necrotizing granulomatous inflammation at enrollment. In contrast, such an association was detected by capture ELISA only in patients randomized to rituximab(Table 2).
Type of relapse
The type of relapse screened for also seems to be a relevant factor affecting the relationship. The increase in risk for severe relapse within one year following a rise in PR3-ANCA detected by direct ELISA was consistently higher than that for any relapse throughout all subgroups(Table 2). Of the 42 patients experiencing a severe relapse in this cohort, 32 had a preceding rise in PR3-ANCA by direct ELISA, with 23 relapsing within one year, and 9 relapsing later. Four patients had a rise detected at the time of relapse, while only 6 of the 42 patients (14.3%) had a severe relapse without a previous or concurrent rise in PR3-ANCA. This compares to 16 of the 55 patients (29.1%) with any flare not having experienced a preceding or concurrent PR3-ANCA increase(Figure 1).
Clinical phenotype
To determine whether the risk of relapse conveyed by a PR3-ANCA rise differs by clinical disease phenotype at presentation, we categorized patients into 5 clinically distinguishable groups, those that had granulomatous disease only (n=15), those that had any granulomatous disease manifestations with or without additional manifestations attributable to capillaritis (n=76), those with any capillaritis with or without additional granulomatous disease manifestations (n=77), patients with renal involvement (n=60), and those with alveolar hemorrhage (n=24) at enrollment. As expected, there was significant overlap between the three middle groups, but mutual exclusivity between the patients with granulomatous disease only and the alveolar hemorrhage group. We observed a gradient of c-indices for the associations of PR3-ANCA with subsequent severe relapses ranging from 0.53 for those with granulomatous disease only, to 0.65 for those with any granulomatous disease, to 0.71 for both any capillaritis and renal involvement, to 0.81 for the alveolar hemorrhage group. The c-indices for the association with any relapse were similar, ranging from 0.43 for granulomatous disease only to 0.76 for alveolar hemorrhage. Data for the clinically most distinguishable groups are shown in Tables 2 and 3 and data for additional groups in the Supplement Table.
In patients with renal involvement or alveolar hemorrhage at presentation, the increased risk for a severe flare within one year following a PR3-ANCA rise detected by direct ELISA was higher than for the entire cohort and higher than for any relapse(Table 2). This relationship was most pronounced for patients with alveolar hemorrhage at the time of enrollment (n=24). Of these, 14 (58.3%) experienced disease relapse. The median time to any relapse following a rise by direct ELISA was 5.2 months in this subgroup, and the time to severe relapse was 8.5 months. As shown in Table 3, no relapses occurred in this group without a preceding or concurrently detected rise in PR3-ANCA using direct ELISA, while 4 patients had a rise without relapse during follow-up. In contrast, for patients who only had granulomatous disease manifestations at presentation, the risk of relapse was very high, but completely unrelated to PR3-ANCA levels.
Treatment regimen
The association of PR3-ANCA increases with subsequent relapse within one year was different between patients randomized to cyclophosphamide followed by azathioprine (conventional immunosuppression) versus those who received only rituximab(Table 2). In contrast to the patients randomized to rituximab, no increase in risk for relapse within one year following a PR3-ANCA rise could be detected in patients treated with conventional immunosuppression by either assay methodology. In patients randomized to rituximab, 31 of 50 (62.0%) experienced disease relapse. Table 3 shows the rises and relapses for patients receiving rituximab and cyclophosphamide/azathioprine, respectively. Among those receiving rituximab, 23 experienced a severe relapse, with 18 of these being preceded by a rise in PR3-ANCA determined by direct ELISA (14 occurring within one year of the rise, 4 more than one year after the rise). Three patients had a rise detected at the time of the severe relapse, and only 2 patients had a severe relapse without a preceding or concurrent rise in PR3-ANCA by direct ELISA.
Discussion
The clinical utility of serial ANCA measurements in AAV has remained controversial for almost three decades. Our analysis of serial PR3-ANCA measurements obtained in the context of a large randomized, double-blind, double-dummy controlled treatment trial provides novel insights with bearing on clinical practice, looking at the risk of relapse within one year of a rise. The information that can be derived from serial PR3-ANCA measurements is not only influenced by the assay methodology used, but more importantly by the patient-specific clinical context. Specifically, the risk of relapse conveyed by a PR3-ANCA titer increase depends on the type of relapse to be predicted, the clinical presentation of the patient, and the treatment chosen for the patient. Serial PR3-ANCA testing may be useful for the anticipation of severe relapses in patients presenting with disease manifestations caused by capillaritis, such as renal involvement or alveolar hemorrhage, or those treated with rituximab.
The methodology used for ANCA-detection affects the clinical utility of serial PR3-ANCA testing. Methods with high analytical sensitivity are very useful for initial diagnostic screening, whereas analytical specificity, which often comes at the expense of analytical sensitivity, is the basis for diagnostic accuracy. While accurate determination of PR3-ANCA positivity is important for diagnostic purposes, titers are relevant as patient-specific baseline. Assays with the highest sensitivity may not provide as much of an amplitude change over baseline compared to less sensitive assays. Our data obtained in the RAVE cohort revealed no difference in sensitivity at baseline between direct ELISA and capture ELISA (data not shown), but far fewer patients turned negative for PR3-ANCA with complete remission (12/93) by capture ELISA than by direct ELISA (36/93). This is consistent with the recognized specific performance characteristics of the two methodologies(28,44,45). Although the total number of rises detected by each assay was similar, the proportion of patients who did not go on to have a disease relapse after a rise was consistently higher using the capture ELISA, as was the number of patients who experienced disease relapse without a rise. Our findings obtained with capture ELISA in the RAVE trial are consistent with our previous observations in the WGET cohort(14). Compared to capture ELISA, the direct ELISA was better at gauging the risk for relapses following a PR3-ANCA titer rise throughout most of the clinical subsets.
The PR3-ANCA response and the associated likelihood of relapses appear to be related to the disease phenotype. We observed no association between PR3-ANCA rise and relapse risk in patients who only had disease manifestations of necrotizing granulomatous inflammation at enrollment. In contrast, the highest c-indices for any relapse and severe relapses were found in patients with alveolar hemorrhage at baseline, and for severe relapses in patients with renal disease at baseline, both disease manifestations that are most clearly linked to capillaritis, rather than larger vessel or granulomatous involvement. Our findings are consistent with the recent report by Kemna et al. for patients with active renal involvement at baseline(15). Taken together, these data imply a better relationship between PR3-ANCA levels and capillaritis compared to necrotizing granulomatous inflammation. This is consistent with experimental investigations of the pathogenic role of ANCA. In vitro studies have shown that ANCA can induce neutrophil adhesion to endothelial cells, as well as neutrophil degranulation(46,47), both hallmarks of capillaritis. Moreover, some animal models of PR3-ANCA disease develop capillaritis involving the kidneys and lungs but not granulomatous inflammation(48,49).
In this study, the risk for relapse following a PR3-ANCA rise was higher in the rituximab group than in the cyclophosphamide/azathioprine group regardless of disease phenotype. Since most PR3-ANCA rises in the rituximab group occurred following B-cell reconstitution this finding might also suggest that a PR3-ANCA rise occurring in a patient off immunosuppressive therapy is of different clinical significance than a rise occurring in a patient receiving maintenance immunosuppression. For patients treated with cyclophosphamide and azathioprine, our study confirms the previous studies of such patients that have led to the widely accepted conclusion that when a patient is in complete remission intensification of conventional immunosuppression is not justified in response to a PR3-ANCA titer rise(14,16,19,22,24,25). However, in the patient subset presenting with renal disease or alveolar hemorrhage, a PR3-ANCA rise may convey an increased risk of subsequent flare regardless of therapy choice.
Our study is the first to formally evaluate the clinical utility of serial PR3-ANCA testing following remission induction with rituximab in a prospective blinded treatment trial. Even though the risk of relapse was higher in rituximab-treated patients following a PR3-ANCA titer rise compared to the conventional treatment group, a sizable proportion of PR3-ANCA titer increases were not followed by a relapse during the observation period. The risk of retreatment thus needs to be carefully weighed against the risk associated with continued observation in this treatment group, too. The role of rituximab for remission maintenance remains under investigation. The scheduled application of 500 mg of rituximab every 6 months after remission induction with cyclophosphamide has been shown to be superior to remission maintenance with azathioprine(50). The scheduled dosing of 1000 mg of rituximab applied every 4 months regardless of B-cell counts and ANCA levels, versus azathioprine for maintenance of remission following induction with rituximab, in relapsing patients is the subject of an ongoing remission maintenance trial [ClinicalTrials.gov Identifier: NCT01697267]. Our findings in the rituximab-treated patients of the RAVE trial suggest that another approach consisting of individually timed retreatment with rituximab prompted by PR3-ANCA rises in the context of B lymphocyte monitoring might be an alternative that minimizes the cumulative dose of rituximab with potential to improve the balance between disease control and immunosuppression. This approach has been used successfully at one center for chronically relapsing PR3-ANCA positive patients(51), and an ongoing randomized controlled trial compares the individually timed application of rituximab to the fixed interval dosing [ClinicalTrials.gov Identifier: NCT01731561].
Our study has several limitations. First, the noted distinctions among various disease phenotypes are based on BVAS/WG forms completed during the RAVE trial, per the judgment of expert clinicians. We were not able to verify these further for the present analysis with additional clinical data such as bronchoalveolar lavage findings in patients noted to have alveolar hemorrhage. Second, our study was not designed to address the effect of rare events such as a substantial disease phenotype change between baseline and relapse. Third, the strategy of running all serum samples from a specific patient on a single assay plate for each ELISA decreases the coefficient of variability and allows the most accurate assessment of the relative change over time. Yet, this is not typical for serial testing performed in routine clinical practice, where samples are rarely tested in parallel with preceding samples. It is therefore important for the appropriate interpretation of clinical test results to be aware of the published inter- and intra-assay variability of each assay used. The specific assays evaluated here, the MCPR3-2 capture ELISA and the Euroimmun direct ELISA, allow for comparison of our findings with previous reports obtained with these well characterized assays(14,28,52), but should not simply be extrapolated to other assays. Fourth, the intervals between PR3-ANCA measurements in our patients were 3 months up to month 18, and 6 months thereafter. Since the goal was to determine the utility of PR3-ANCA levels to predict relapses, concurrently detected rises were treated as if no rise had occurred for all analyses. It is possible that more frequent measurements of PR3-ANCA could have identified a rise earlier in these patients as suggested by the 2012 meta-analysis of the role of serial ANCA measurements which found that a higher frequency of testing was associated with better prediction of relapses(34). This might be particularly useful for rituximab treated patients as all but one of the relapses with concurrently detected PR3-ANCA rise occurred in rituximab treated patients. Additional study is needed to determine the impact of more frequent measurements or alternative means of comparing a change in levels over time. Finally, in the present study, we examined only patients with PR3-ANCA as the greater number of patients and relapse events in this group better allowed us to evaluate the prediction of relapses. Further study is needed to determine the relationship between MPO-ANCA levels and disease relapse.
In conclusion, risk of relapse following a PR3-ANCA titer increase during complete remission is partially dependent on the methodology used to monitor PR3-ANCA, the phenotype of disease presentation and the treatment chosen to induce remission. Within the overall study population, a rise in ANCA titer was poorly predictive of a subsequent disease flare. However, among the subset of patients with renal disease or alveolar hemorrhage, and among patients treated with rituximab, a rise in ANCA titer had greater predictive value for subsequent relapse. These findings should be confirmed in an independent cohort. Furthermore, while serial measurements of PR3-ANCA portend an increased risk of relapse in this subset of patients with AAV, the risk of relapse needs to be weighed carefully against any risks associated with therapy.
Supplementary Material
Supplement Table. Rise in PR3-ANCA level and relapse within 1 year for additional groups
Acknowledgments
Financial Support
The original rituximab versus cyclophosphamide for ANCA-associated vasculitis trial, from which this data was obtained, was supported by a grant from the National Institute of Allergy and Infectious Diseases to the Immune Tolerance Network (N01-AI-15416; protocol no. ITN021AI). Genentech, Inc. and Biogen IDEC, Inc. provided the study medications and partial funding. At the Mayo Clinic and Foundation, the trial was supported by a Clinical and Translational Science Award from the National Center for Research Resources (NCRR) (RR024150-01); at Johns Hopkins University, by grants from the NCRR (RR025005) and career development awards (K24 AR049185, to Dr. Stone, and K23 AR052820, to Dr. Seo); and at Boston University, by a Clinical and Translational Science Award (RR 025771), grants from the National Institutes of Health (M01 RR00533) and a career development award (K24 AR02224, to Dr. Merkel), and an Arthritis Foundation Investigator Award (to Dr. Monach). Enzyme-linked immunosorbent assay kits for antineutrophil cytoplasmic antibody testing were provided by Euroimmun (Lubeck, Germany).
This project was made possible by the CTSA Grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Langford C. Clinical features and diagnosis of small-vessel vasculitis. Cleve Clin J Med. 2012;79(Suppl 3):S3–7. doi: 10.3949/ccjm.79.s3.01. [DOI] [PubMed] [Google Scholar]
- 3.Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET) Arthritis Rheum. 2005;52(7):2168–78. doi: 10.1002/art.21117. [DOI] [PubMed] [Google Scholar]
- 4.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352(4):351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 7.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–27. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhold-Keller E, Beuge N, Latza U, de Groot K, Rudert H, Nolle B, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. 2000;43(5):1021–32. doi: 10.1002/1529-0131(200005)43:5<1021::AID-ANR10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Specks U. Accurate Relapse Prediction in ANCA-Associated Vasculitis-the Search for the Holy Grail. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1(8426):425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 11.Tervaert JW, van der Woude FJ, Fauci AS, Ambrus JL, Velosa J, Keane WF, et al. Association between active Wegener’s granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149(11):2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum. 1998;41(9):1521–37. doi: 10.1002/1529-0131(199809)41:9<1521::AID-ART2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Finkielman JD, Lee AS, Hummel AM, Viss MA, Jacob GL, Homburger HA, et al. ANCA are detectable in nearly all patients with active severe Wegener’s granulomatosis. Am J Med. 2007;120(7):643.e9–14. doi: 10.1016/j.amjmed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, St Clair EW, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147(9):611–9. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kemna MJ, Damoiseaux J, Austen J, Winkens B, Peters J, van Paassen P, et al. ANCA as a Predictor of Relapse: Useful in Patients with Renal Involvement But Not in Patients with Nonrenal Disease. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tervaert JW, Huitema MG, Hene RJ, Sluiter WJ, The TH, van der Hem GK, et al. Prevention of relapses in Wegener’s granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. 1990;336(8717):709–11. doi: 10.1016/0140-6736(90)92205-v. [DOI] [PubMed] [Google Scholar]
- 17.Gaskin G, Savage CO, Ryan JJ, Jones S, Rees AJ, Lockwood CM, et al. Anti-neutrophil cytoplasmic antibodies and disease activity during long-term follow-up of 70 patients with systemic vasculitis. Nephrol Dial Transplant. 1991;6(10):689–94. doi: 10.1093/ndt/6.10.689. [DOI] [PubMed] [Google Scholar]
- 18.Pettersson E, Heigl Z. Antineutrophil cytoplasmic antibody (cANCA and pANCA) titers in relation to disease activity in patients with necrotizing vasculitis: a longitudinal study. Clin Nephrol. 1992;37(5):219–28. [PubMed] [Google Scholar]
- 19.Kerr GS, Fleisher TA, Hallahan CW, Leavitt RY, Fauci AS, Hoffman GS. Limited prognostic value of changes in antineutrophil cytoplasmic antibody titer in patients with Wegener’s granulomatosis. Arthritis Rheum. 1993;36(3):365–71. doi: 10.1002/art.1780360312. [DOI] [PubMed] [Google Scholar]
- 20.Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120(1):12–7. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Jayne DR, Gaskin G, Pusey CD, Lockwood CM. ANCA and predicting relapse in systemic vasculitis. Qjm. 1995;88(2):127–33. [PubMed] [Google Scholar]
- 22.Kyndt X, Reumaux D, Bridoux F, Tribout B, Bataille P, Hachulla E, et al. Serial measurements of antineutrophil cytoplasmic autoantibodies in patients with systemic vasculitis. Am J Med. 1999;106(5):527–33. doi: 10.1016/s0002-9343(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 23.Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, Kallenberg CG, et al. Prediction of relapses in Wegener’s granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthritis Rheum. 2000;43(9):2025–33. doi: 10.1002/1529-0131(200009)43:9<2025::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Girard T, Mahr A, Noel LH, Cordier JF, Lesavre P, Andre MH, et al. Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener’s granulomatosis? A prospective study. Rheumatology (Oxford) 2001;40(2):147–51. doi: 10.1093/rheumatology/40.2.147. [DOI] [PubMed] [Google Scholar]
- 25.Nowack R, Grab I, Flores-Suarez LF, Schnulle P, Yard B, van der Woude FJ. ANCA titres, even of IgG subclasses, and soluble CD14 fail to predict relapses in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2001;16(8):1631–7. doi: 10.1093/ndt/16.8.1631. [DOI] [PubMed] [Google Scholar]
- 26.Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL. Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int. 2003;63(3):1079–85. doi: 10.1046/j.1523-1755.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanders JS, Huitma MG, Kallenberg CG, Stegeman CA. Prediction of relapses in PR3-ANCA-associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology (Oxford) 2006;45(6):724–9. doi: 10.1093/rheumatology/kei272. [DOI] [PubMed] [Google Scholar]
- 28.Damoiseaux J, Dahnrich C, Rosemann A, Probst C, Komorowski L, Stegeman CA, et al. A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis. 2009;68(2):228–33. doi: 10.1136/ard.2007.086579. [DOI] [PubMed] [Google Scholar]
- 29.Terrier B, Saadoun D, Sene D, Ghillani P, Amoura Z, Deray G, et al. Antimyeloperoxidase antibodies are a useful marker of disease activity in antineutrophil cytoplasmic antibody-associated vasculitides. Ann Rheum Dis. 2009;68(10):1564–71. doi: 10.1136/ard.2008.094714. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen N, Salmela A, Ekstrand A, de Groot K, Gregorini G, Cohen Tervaert JW, et al. Changes in proteinase 3 anti-neutrophil cytoplasm autoantibody levels in early systemic granulomatosis with polyangiitis (Wegener’s) may reflect treatment rather than disease activity. Clin Exp Rheumatol. 2013;31(1 Suppl 75):S38–44. [PubMed] [Google Scholar]
- 31.Alberici F, Smith RM, Jones RB, Roberts DM, Willcocks LC, Chaudhry A, et al. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thai LH, Charles P, Resche-Rigon M, Desseaux K, Guillevin L. Are anti-proteinase-3 ANCA a useful marker of granulomatosis with polyangiitis (Wegener’s) relapses? Results of a retrospective study on 126 patients. Autoimmun Rev. 2014;13(3):313–8. doi: 10.1016/j.autrev.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Birck R, Schmitt WH, Kaelsch IA, van der Woude FJ. Serial ANCA determinations for monitoring disease activity in patients with ANCA-associated vasculitis: Systematic review. Am J Kidney Dis. 2006 Jan;47(1):15–23. doi: 10.1053/j.ajkd.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis–a meta-analysis. Rheumatology (Oxford) 2012;51(1):100–9. doi: 10.1093/rheumatology/ker280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franssen C, Gans R, Kallenberg C, Hageluken C, Hoorntje S. Disease spectrum of patients with antineutrophil cytoplasmic autoantibodies of defined specificity: distinct differences between patients with anti-proteinase 3 and anti-myeloperoxidase autoantibodies. J Intern Med. 1998;244(3):209–16. doi: 10.1046/j.1365-2796.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 36.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143(9):621–31. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 37.Lionaki S, Blyth ER, Hogan SL, Hu Y, Senior BA, Jennette CE, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64(10):3452–62. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao Y, Tian Z, Li W, Ma L, Yu Y, Ren W. Predictors of treatment resistance and relapse in Chinese patients with antineutrophil cytoplasmic antibody-associated disease. J Rheumatol. 2014;41(5):916–22. doi: 10.3899/jrheum.130758. [DOI] [PubMed] [Google Scholar]
- 39.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44(4):912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Outcomes of non-severe relapses in ANCA-associated vasculitis treated with glucocorticoids. Arthritis Rheumatol. 2015 doi: 10.1002/art.39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J, Fass DN, Hudson JA, Viss MA, Wieslander J, Homburger HA, et al. Capture-ELISA based on recombinant PR3 is sensitive for PR3-ANCA testing and allows detection of PR3 and PR3-ANCA/PR3 immunecomplexes. J Immunol Methods. 1998;211(1-2):111–23. doi: 10.1016/s0022-1759(97)00203-2. [DOI] [PubMed] [Google Scholar]
- 42.Kremers WK. Concordance for survival time data: Fixed and time-dependent covariates and possible ties in predictor and time. Department of Health Science Research, Mayo Clinic; Rochester, Minnesota: 2007. (Technical Report Series No. 80). << http://www.mayo.edu/research/documents/biostat-80pdf/doc-10027891>>. [Google Scholar]
- 43.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd. New York (NY): John Wiley & Sons, Inc; 2000. p. 162. [Google Scholar]
- 44.Gisslen K, Wieslander J, Westberg G, Herlitz H. Relationship between anti-neutrophil cytoplasmic antibody determined with conventional binding and the capture assay, and long-term clinical course in vasculitis. J Intern Med. 2002;251(2):129–35. doi: 10.1046/j.1365-2796.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 45.Westman KW, Selga D, Bygren P, Segelmark M, Baslund B, Wiik A, et al. Clinical evaluation of a capture ELISA for detection of proteinase-3 antineutrophil cytoplasmic antibody. Kidney Int. 1998;53(5):1230–6. doi: 10.1046/j.1523-1755.1998.00873.x. [DOI] [PubMed] [Google Scholar]
- 46.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990;87(11):4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taekema-Roelvink ME, Kooten C, Kooij SV, Heemskerk E, Daha MR. Proteinase 3 enhances endothelial monocyte chemoattractant protein-1 production and induces increased adhesion of neutrophils to endothelial cells by upregulating intercellular cell adhesion molecule-1. J Am Soc Nephrol. 2001;12(5):932–40. doi: 10.1681/ASN.V125932. [DOI] [PubMed] [Google Scholar]
- 48.Primo VC, Marusic S, Franklin CC, Goldmann WH, Achaval CG, Smith RN, et al. Anti-PR3 immune responses induce segmental and necrotizing glomerulonephritis. Clin Exp Immunol. 2010;159(3):327–37. doi: 10.1111/j.1365-2249.2009.04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Little MA, Al-Ani B, Ren S, Al-Nuaimi H, Leite M, Jr, Alpers CE, et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One. 2012;7(1):e28626. doi: 10.1371/journal.pone.0028626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371(19):1771–80. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 51.Cartin-Ceba R, Golbin JM, Keogh KA, Peikert T, Sanchez-Menendez M, Ytterberg SR, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single center. Arthritis Rheum. 2012;64(11):3770–8. doi: 10.1002/art.34584. [DOI] [PubMed] [Google Scholar]
- 52.Noel N, Andre C, Bengoufa D, Dehoulle C, Mahler M, Limal N, et al. Performance evaluation of three assays for the detection of PR3-ANCA in granulomatosis with polyangiitis in daily practice. Autoimmun Rev. 2013;12(12):1118–22. doi: 10.1016/j.autrev.2013.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Table. Rise in PR3-ANCA level and relapse within 1 year for additional groups


