Abstract
PREMISE:
Cold tolerance is an important factor limiting the geographic distribution and growing season for many plant species, yet few studies have examined variation in cold tolerance extensively within and among closely related species and compared that to their geographic distribution.
METHODS:
This study examines cold tolerance within and among species in the genus Arabidopsis. We assessed cold tolerance by measuring electrolyte leakage from detached leaves in multiple populations of five Arabidopsis taxa. The temperature at which 50% of cells were lysed was considered the lethal temperature (LT50).
RESULTS:
We found variability within and among taxa in cold tolerance. There was no significant within-species relationship between latitude and cold tolerance. However, the northern taxa, A. kamchatica, A. lyrata subsp. petraea, and A. lyrata subsp. lyrata, were more cold tolerant than A. thaliana and A. halleri subsp. gemmifera both before and after cold acclimation. Cold tolerance increased after cold acclimation (exposure to low, but nonfreezing temperatures) for all taxa, although the difference was not significant for A. halleri subsp. gemmifera. For all taxa except A. lyrata subsp. lyrata, the LT50 values for cold-acclimated plants were higher than the January mean daily minimum temperature (Tmin), indicating that if plants were not insulated by snow cover, they would not likely survive winter at the northern edge of their range.
CONCLUSIONS:
Arabidopsis lyrata and A. kamchatica were far more cold tolerant than A. thaliana. These extremely cold-tolerant taxa are excellent candidates for studying both the molecular and ecological aspects of cold tolerance.
Keywords: Arabidopsis lyrata, Arabidopsis thaliana, Arabidopsis halleri subsp. gemmifera, Arabidopsis kamchatica, Brassicaceae, cline, cold tolerance, cold acclimation, electrolyte leakage, LT50
The low diversity of plant species in the far north may in part be due to the inherent difficulties of adapting to the stress of life at high latitudes. Overwintering structures of plants growing at high latitudes may experience extreme cold, and northern plants often experience a broad range of temperatures throughout the year. Cold tolerance increases after cold acclimation, which is induced by exposure to low but nonfreezing temperatures (Xin and Browse, 2000), and most species are more cold tolerant in the winter than during the growing season. In general, it is predicted that species living in the far north will be tolerant to more extreme cold and will exhibit greater cold acclimation.
Ecological niche modeling suggests that low temperatures, especially winter minimum temperatures (January Tmin), are one of the primary forces determining the geographic boundaries of many plant species (Pither, 2003; Hoffmann, 2005). One would expect that a species’ physiological tolerance to cold and freezing temperatures would be correlated with its geographic range, and there appears to be a strong relationship in insects (Addo-Bediako et al., 2000). In the few plant studies that have been conducted, it is not yet clear whether such a relationship exists (Hofmann et al., 2013; Hofmann and Bruelheide, 2015).
Physiological tolerance to cold also varies among populations within species (Le et al., 2008; Kreyling et al., 2012; Hofmann et al., 2015). In some species, the variation in cold tolerance is correlated with latitude or winter minimum temperatures (Tmin), forming a cline (Hannah et al., 2006; Zhen and Ungerer, 2008; Pagter et al., 2010; Kreyling et al., 2012; Zuther et al., 2012; Menon et al., 2015). The development of a cline depends on interactions between gene flow and selection (Endler, 1973; Slatkin, 1987). A latitudinal cline is expected if there is selection for increased cold tolerance in northern regions and selection against cold tolerance in southern regions, perhaps due to physiological costs of cold tolerance. Further, the differentiating effects of selection must overcome the homogenizing effects of gene flow (Endler, 1973; Slatkin, 1973, 1987). If there are no costs to cold tolerance in the south, or ample gene flow, plants should exhibit the same high level of cold tolerance across the species geographic range (Xin and Browse, 2000). The fact that cold tolerance is generally inducible (cold acclimation), suggests that it likely has a physiological cost, or else plants would always have maximum cold tolerance. Some studies in Arabidopsis suggest that there is a cost of cold tolerance (Jackson et al., 2004; Wos and Willi, 2015), but others do not (Zhen et al., 2011; Wos and Willi, 2018). Although a cline in cold tolerance has been observed in some species, such as A. thaliana (Hannah et al., 2006; Zhen and Ungerer, 2008; Zuther et al., 2012), we do not necessarily expect to see this in other species which may have different levels of gene flow and selection.
Arabidopsis thaliana is a model organism for understanding the molecular and physiological underpinnings of cold tolerance and cold acclimation in plants (Thomashow, 1999, 2010; Xin and Browse, 2000; Hannah et al., 2006; Horton et al., 2016). Many genes important for cold acclimation have been identified in A. thaliana, including INDUCER OF CBF EXPRESSION 1 (ICE1), and C-repeat binding factors (CBF genes), which regulate many downstream genes (Thomashow, 1999, 2010; Zhu et al., 2007. Cold exposure in A. thaliana causes extensive reconfiguration of the metabolome (Cook et al., 2004; Kaplan et al., 2004; Hannah et al., 2006), changes to the extracellular proteome (Takahashi et al., 2019), changes to cellular membranes (Uemura et al., 1995), the chloroplast membrane (Barnes et al., 2016) and the cell wall (Takahashi et al., 2019). Many of these important studies have spurred similar research in other species, especially crops and other economically important plants (Renaut et al., 2005; Uemura et al., 2006; Shi et al., 2018).
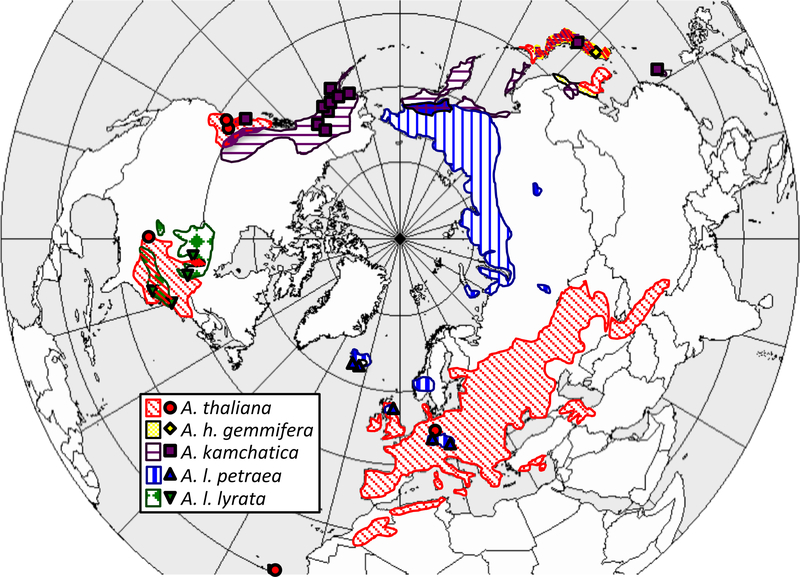
Studies of cold tolerance among members of the Arabidopsis genus have great potential to advance our understanding of the interplay among the ecology, evolution and genetics of cold tolerance. The genus is distributed throughout the northern hemisphere (Fig. 1). Ecological niche modeling shows that there are differences in the climate niches occupied by different Arabidopsis species (Hoffmann, 2005). Arabidopsis thaliana and A. kamchatica have the widest climatic amplitudes in the genus with A. thaliana growing in warmer, moister conditions and A. kamchatica growing in colder, drier conditions (Hoffmann, 2005). Arabidopsis thaliana has the widest geographic distribution in the genus (Fig. 1). It was introduced to both coasts of the United States from Europe and is found in climates ranging from the Mediterranean to the Arctic Circle in northern Europe (Hoffmann, 2005). Arabidopsis halleri subsp. gemmifera has a relatively small geographic distribution and occupies areas that are warmer and moister in summer than other taxa in the genus. Arabidopsis lyrata subsp. lyrata also occupies a small climate niche and does not occupy extremely cold areas. However, the other subspecies of the A. lyrata complex that we investigated, A.lyrata subsp. petraea, is found in some of the coldest regions occupied by any taxa in this genus, including northern and central Siberia (Hoffmann, 2005).
Figure 1.
Map of range distributions for A. kamchatica, A. lyrata subsp. lyrata, A. lyrata subsp. petraea, A. thaliana, and A. halleri subsp. gemmifera. Points represent collections used in this study. Map from Smolensk (2011). Species ranges from Hoffmann (2005).
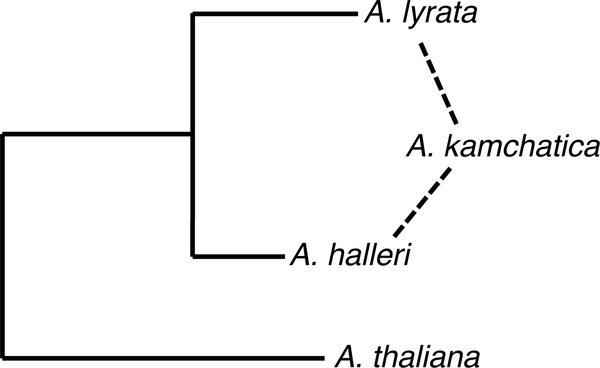
The evolutionary history of the genus Arabidopsis has been well studied (Novikova et al., 2016). It is thought that A. lyrata and A. halleri diverged from A. thaliana 4 to 7 million years ago (Ma) (Hohmann et al., 2015; Novikova et al., 2016). Subsequently, A. lyrata and A. halleri diverged from each other between 0.4 and 2 Ma, although there may have been more recent admixture in East Asia (Castric et al., 2008; Hohmann et al., 2015; Novikova et al., 2016). Arabidopsis lyrata can be separated into at least two subspecies: A. lyrata subsp. lyrata in North America and A. lyrata subsp. petraea in Iceland and Eurasia. North American A. lyrata subsp. lyrata (hereafter A. l. lyrata) is thought to have been derived from A. lyrata subsp. petraea (A. l. petraea) via a founder event ~260,000 years ago (190,000–310,000) (Wright et al., 2003; Mattila et al., 2017). Arabidopsis halleri also has multiple subspecies, with A. halleri subsp. halleri and A. halleri subsp. ovirensis in Europe, and A. halleri subsp. gemmifera (A. h. gemmifera) in the Russian Far East and East Asia. Arabidopsis kamchatica is an allotetraploid that originated ~64,000–145,000 years ago from hybridization of diploid A. h. gemmifera and A. l. petraea (Shimizu-Inatsugi et al., 2009; Paape et al., 2018). It is likely to have spread outward from Japan to eastern Russia, across the Bering land bridge to Alaska, and down the western edge of Canada (Shimizu-Inatsugi et al., 2009).
Our study investigates differences in cold tolerance and acclimation capacity within and among five Arabidopsis taxa. Our first goal was to determine whether Arabidopsis taxa differed in cold tolerance. We predicted that taxa with northern distributions, such as A. kamchatica and A. l. petraea, would be more cold tolerant than taxa like A. thaliana and A. h. gemmifera, that have more southern distributions (Fig. 2). The second goal of our study was to compare the cold tolerance of populations originating from different latitudes to determine whether there was a relationship between latitude and cold tolerance within each species. We predicted that northern populations would be more cold tolerant than southern populations.
Figure 2.
Relationships among Arabidopsis species used. Neighbor joining tree (Jukes–Cantor correction) of diploid taxa was made using ITS accessions DQ528813.1, DQ528913.1, and DQ528880.1 from Koch and Matschinger (2007) in GenBank. Branch lengths for allotetraploid A. kamchatica are not representative of genetic distance.
MATERIALS AND METHODS
Study species
We assessed freeze damage before and after cold acclimation in plants from five Arabidopsis taxa: A. thaliana, A. kamchatica, A. lyrata subsp. lyrata, A. l. subsp. petraea, and A. halleri subsp. gemmifera. The species range of each taxa and collection localities used in this study are described in Table 1 and Fig. 2.
Table 1.
Arabidopsis taxa, locations of population tested for cold tolerance, and number of individuals per population. A. thaliana was obtained from The Arabidopsis Information Resource (TAIR).
| Taxon | Source locality | Latitude | Longitude | Num plants | Collector/Source |
|---|---|---|---|---|---|
| A. kamchatica | Taiwan | 24 | 121 | 2 | Hsu, J.B.Beck |
| A. kamchatica | Lake Biwa, Japan | 35.4444444 | 136.05 | 1 | H.Marui |
| A. kamchatica | Strathcona Park, BC | 49.82915 | −125.8728 | 12 | J.A.Steets, D.E.W |
| A. kamchatica | Grant Lagoon, Kodiak, AK | 57.37 | −154.65 | 1 | C.Parker |
| A. kamchatica | Portage Glacier, AK | 60.7916167 | −148.90213 | 12 | N.T., D.E.W. |
| A. kamchatica | Thompson Pass, AK | 61.13 | −145.73 | 1 | N.T., D.E.W. |
| A. kamchatica | Exit Glacier, AK, USA | 60.18795 | −149.6312833 | 3 | N.T., D.E.W. |
| A. kamchatica | Goodnews Bay, AK | 59.1166667 | −161.58333 | 1 | C.Parker |
| A. kamchatica | Rainbow Ridge, AK | 63.32 | −145.64 | 1 | N.T., D.E.W. |
| A. kamchatica | Ptarmigan Creek, AK | 65.4529 | −145.5075657 | 12 | N.T.,D.E.W |
| A. lyrata subsp. lyrata | Cedar Mountain, NC | 36.426667 | −79.93777778 | 1 | R.Mauricio |
| A. l yrata subsp. lyrata | New York | 40 | −74 | 1 | Mitchell-Olds |
| A. lyrata subsp. lyrata | Presque Isle, PA | 42.14 | −80.11 | 1 | J.A.Steets |
| A. lyrata subsp. lyrata | Bailey’s Harbor, WI | 45.07036N | −87.1711 | 1 | A.Breen |
| A. lyrata subsp. petraea | Plech, Germany | 49.65 | 11.47 | 1 | Mitchell-Olds |
| A. lyrata subsp. petraea | Exeter McNair, England | 50.72 | −3.53 | 1 | Mitchell-Olds |
| A. lyrata subsp. petraea | Braemer, Scotland | 57.01 | −3.4 | 1 | R.Ennos |
| A. lyrata subsp. petraea | Esja Mountain, Iceland | 64.2 | −21.7 | 1 | M.Schierup |
| A. lyrata subsp. petraea | Reykjavik, Iceland | 64° N | −21 | 1 | M.Schierup |
| A. thaliana Cvi-0 | Cape Verde Islands | 15° N | −23 | 1 | TAIR |
| A. thaliana Col-0 | Landsberg, Germany | 52° N | 10 | 1 |
TAIR |
| A. thaliana Seattle-0 | Seattle, WA | 47° N | −122 | 1 |
TAIR |
| A. thaliana Van-0 | Vancouver, BC | 49° N | −123 | 1 |
TAIR |
| A. thaliana Ler-1 | Landsberg, Germany | 52° N | 10 | 1 |
TAIR |
| A. halleri subsp. gemmifera | Fujita (Gifu), Japan | 34.93 | 133.63 | 3 | Fujita Corp. |
All taxa studied are perennials except A. thaliana. Arabidopsis thaliana can have either a summer annual or winter annual life history throughout most of its range (Griffith et al., 2004), but plants at the northern limit are primarily winter annuals due to a short growing season (Kuittinen et al., 1997a, b; Hoffmann, 2002). Perennials and winter annuals overwinter as rosettes and flower in the spring. Leaves are retained throughout winter and are likely to be important in storing resources needed for new growth and flowering soon after snowmelt because there is no obvious underground storage structure. For this reason, cold tolerance of leaves is likely a good proxy of overall cold tolerance for both actively growing plants and overwintering plants. Because rosettes are small and low to the ground, snow is likely to provide substantial insulation from extremely cold air temperatures in winter, and mid-winter snowmelt will expose plants to subsequent cold snaps.
Electrolyte leakage assay
The measurement of electrolyte leakage in detached leaves is a common method to estimate freeze tolerance in plants (Hannah et al., 2006). When cell membranes are damaged due to cold exposure, the cell contents leak out and are measured after being dispersed in water. Electrolyte leakage is expressed in terms of relative conductivity, where conductivity is measured after exposure to cold temperatures (initial electrolyte leakage; ELI), then the total conductivity (total electrolyte leakage; ELT) is measured after the sample has been autoclaved to lyse the remaining cells (Prasil and Zamecnik, 1998). The relative leakage (ELR) is ELI/ELT, and the lethal temperature (LT50) is the temperature that causes 50% of electrolytes to leak from cells (or 50% cell lysis), which is commonly used to estimate the temperature at which tissues die.
We measured electrolyte leakage and LT50 according to the methods of Armstrong et al. (2015), with a few modifications. Briefly, leaves were cooled at a rate of 4°C/h. We ensured that leaves froze, rather than supercooling, by nucleating with ice chips. Three replicate leaves per plant were removed at 6–7 different temperatures to assay the amount of cellular damage: 0, −2, −6, −10, −14 and −18°C for non-acclimated plants, and 0, −4, −11, −18, −25, −32 and −35˚ C for acclimated plants. Thus, each assay included 18–21 similarly-sized leaves per plant. While temperatures during the growing season are unlikely to be as low as −18°C, it is necessary to include colder temperatures to estimate the LT50. Even colder temperatures were used for cold-acclimated plants because our preliminary studies showed that they suffered very little damage at the temperatures used for non-acclimated plants (Armstrong et al., 2015).
To minimize variation due to the hydration status of the leaves, we watered plants 24 h before the leakage assays. We assayed three replicate leaves per plant for each temperature. Since A. thaliana plants are small and did not have enough leaves, we treated leaves from each inbred accession as if all were from the same individual. Perennials were grown in the greenhouse for at least 1 yr before testing for electrolyte leakage from non-acclimated plants. After initial measurements of “non-acclimated” electrolyte leakage, all plants were cold acclimated at 4°C with 8 h of soft-white fluorescent light per day for 4 wk, then used for the” acclimated” electrolyte leakage trial.
Data analyses
To estimate the point at which 50% of electrolyte leakage would occur (LT50), we fit the data to a sigmoidal curve in which the relative leakage ELR at the temperature T follows a two-parameter logistic model:
where the inflection point, d, gives the LT50 estimate, and s is a scale parameter. With this method, the lower asymptote of the curve approaches an ELR of 0 at temperatures that are too warm to cause damage to the leaf tissue, the upper asymptote approaches an ELR of 1, corresponding to 100% cell lysis due to freeze damage.
The sigmoidal curve fitting method is a much more statistically rigorous method of estimating the LT50 than other methods because it utilizes all of the electrolyte leakage data. Some methods of reporting freeze damage such as simply assuming that LT50 is 50% of the maximum leakage obtained from measurements (Lipp et al., 1994; Loik and Redar, 2003) are based on a single point rather than including the electrolyte leakage data from multiple temperatures to estimate the LT50, and a linear relationship between temperature and electrolyte leakage was assumed. Other studies reported only the percentage of electrolyte leakage at one or a few temperatures and did not attempt to estimate LT50 (Nunes and Smith, 2003; Hasdai et al., 2006). Estimates based on a single point are less accurate than the method we used. Most importantly, our method allows direct statistical comparisons of LT50 across multiple species.
Population structure within taxa and the sigmoidal nature of the data made it necessary for us to use a nonlinear mixed-effects model to analyze the electrolyte leakage data. We used the nlme package (Pinheiro and Bates, 2000) implemented in the R statistical environment (R Core Team, 2011). The dependent variable was the relative electrolyte leakage (ELR). Our independent variables, taxon and acclimation treatment, were treated as fixed effects; and population, family, individual, and date of EL measurement were modeled as random effects. In this model, these independent variables were allowed to have linear effects on the two parameters of the logistic function. We first conducted a model selection (Faraway, 2006) to include the relevant random effects by the Akaike information criterion (AIC), using the method of Pinheiro and Bates (2000). To test the significance of fixed effects, i.e., whether acclimation and/or taxa influenced the shape of the electrolyte leakage response curve, we performed likelihood ratio tests to compare models with and without a fixed effect (including the interaction of taxon and acclimation effects). The test statistic reported for the likelihood ratio tests is D, where D is twice the difference in the log likelihoods of two models: D = −2 [ln (Likelihood for a simpler model) – ln (Likelihood for a model with more parameters)].
To estimate the confidence intervals of the estimated parameters for each taxon, we conducted nonparametric bootstrapping for 1000 iterations (Mooney and Duval, 1993; Efron and Tibshirani, 1998; Roberts and Fan, 2004), where each observation (the relative electrolyte leakage measurement) was the unit of resampling, and the total numbers of observations per species were constrained to match the data. Briefly, nonlinear mixed-effects models were fitted to each bootstrapped data set, and confidence intervals were calculated from the distribution of parameter estimates. We checked for normality and homogeneity of errors by inspections of plots of residuals against fitted values and quantile–quantile (QQ) plots. If the assumptions were violated, we attempted to fit an extended nonlinear mixed-effect model, where appropriate variance functions can be used to model heteroscedasticity of within group errors (Chapter 8: Pinheiro and Bates, 2000). The variance function we used was “varPower(fixed = 0.5, form = ~ fitted(.) - fitted(.)^2))”. However, if this correction failed to converge due to added complexity, we were forced to use the original models without the correction.
For within-taxon comparisons, our methodology for estimating the LT50 for each population was identical to among-taxa comparisons except that the independent variables were family, individual, and date of electrolyte leakage measurement as the random effects and population as the fixed effect. For A. h. gemmifera, only one population was available for testing, so it was excluded from the population-level comparisons.
We used analysis of covariance to test whether acclimation treatment and the latitude of origin of the population influenced LT50, as estimated for each population with the nonlinear mixed-effects models described above. The independent variables were latitude, taxon, and acclimation. We chose a model by AIC-based stepwise selection (Hastie and Pregibon, 1992; Venables and Ripley, 2002), implemented with step() function of R (R Core Team, 2011). Box–Cox transformation of the dependent variable was used to reduce non-normal distribution of residuals (Box and Cox, 1964; Fox and Weisberg, 2011). All statistical analysis was done in the R statistical environment (R Core Team, 2011). LT50 values for each population are provided in Appendix S2, and raw electrolyte leakage values are provided in Appendix S3.
RESULTS
Variation among taxa
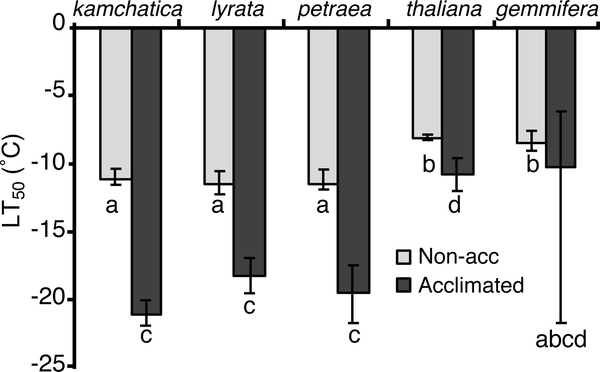
There was significant variation among taxa in cold tolerance, measured as the temperature of 50% cell lysis (LT50). This variation was significant for both non-acclimated and acclimated plants (Fig. 3; non-acclimated: D = 10.138, df = 11, p = 0.0382; acclimated: D = 9.534, df = 12, p = 0.049). Non-acclimated Arabidopsis taxa had LT50 values ranging from −8.1° to −11.1°C. “Acclimated” values are lower, ranging from −10.2° to −21.1°C (Fig. 3). Interestingly, “cold-acclimated” LT50 values appear to be more variable among taxa than non-acclimated LT50. Post hoc comparisons among taxa to determine which taxa are significantly different from each other are not well developed under this nlme statistical framework. Bootstrapped confidence intervals of LT50 estimates (Fig. 3) show that A. kamchatica, A. l. lyrata, and A. l. petraea were more cold tolerant than A. thaliana, both when non-acclimated and acclimated. It is difficult to judge whether acclimated A. h. gemmifera is different from other taxa due to extremely large confidence intervals, due in part to a small sample size. However, the best estimates of both non-acclimated and acclimated A. h. gemmifera are similar to those of A. thaliana.
Figure 3.
Mean lethal temperature (LT50) of each Arabidopsis species before (gray) and after (black) cold acclimation. LT50 values were estimated using a logistic curve fitted by nonlinear mixed modeling analysis on electrolyte leakage measurements. Means with the same letters were not significantly different. Error bars represent 95% confidence intervals around the means.
Most of the Arabidopsis taxa showed evidence of acclimation in response to cold temperatures (Fig. 3). We measured each taxon’s capacity for cold acclimation as the difference between the non-acclimated and acclimated LT50 values. There were differences among taxa in the amount of cold acclimation, as evidenced by a significant interaction between taxa and acclimation (D = 11.629, df = 26, p < 0.02). Therefore, we tested the effect of acclimation on LT50 by analyzing each taxon separately. All taxa except for A. h. gemmifera had a significant decrease in LT50 following exposure to cold temperatures, indicating that they do cold acclimate (A. kamchatica: D = 77.513, df = 10, p < 0.0001; A. l. lyrata: D = 29.658, df = 5, p < 0.0001; A. l. petraea: D = 145.867, df = 7, p < 0.0001; A. h. gemmifera: D = 1.10, df = 5, p = 0.2942; A. thaliana: D = 16.965, df = 5, p < 0.0001). The lack of a significant acclimation effect in A. h. gemmifera is most likely due to the wide confidence interval for the estimate of acclimated LT50 rather than a complete lack of acclimation. Inspection of bootstrapped confidence intervals of LT50 suggests that the three most cold-tolerant taxa have much greater capacity for cold acclimation (A. kamchatica: −10.0°C, A. l. lyrata: −6.7°C, and A. l. petraea: −8.0°C), whereas the other two taxa acclimated relatively little (A. thaliana: −2.8°C and A.h. gemmifera: −1.7°C).
Variation within taxa
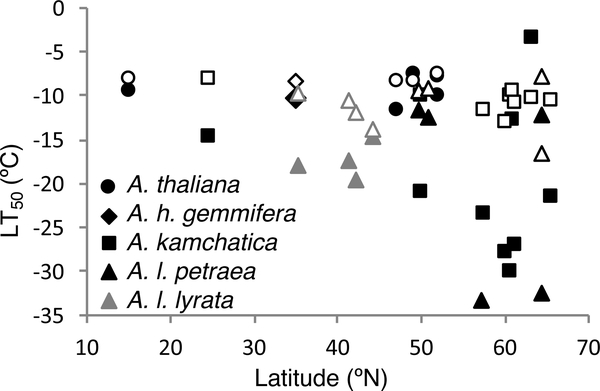
To examine cold-tolerance variation within taxa and its relationship with latitude, we estimated LT50 values in multiple populations per taxon (only one population was used for A. h. gemmifera; Figs. 4 and 5). The AIC-based stepwise selection chose a model that included taxon and acclimation effects (F4, 42 = 2.92, p < 0.03 for taxon effect and F1, 42 = 15.59, p < 0.001 for acclimation effect), indicating that there were differences among taxa, and that acclimation influenced LT50. However, latitude did not have a statistically significant effect on LT50 among populations. The relationship between LT50 and latitude was also not statistically different between the acclimation treatments. Despite the lack of within-taxon effects, there was a clear trend for more northern plants to have higher cold tolerance when all species are combined, but this is due to differences among taxa (Fig. 4).
Figure 4.
Mean lethal temperature (LT50) of each population before (open symbols) and after acclimation (closed symbols) by latitude of origin.
In A. kamchatica, all populations had a high acclimation capacity except for the one at 63°N (Rainbow Ridge; Fig. 4; Appendix S1a, b). This population had very wide confidence intervals both before and after acclimation. The LT50 estimates for several populations had very wide confidence intervals, partially because several LT50 estimates were based on single plant assays. The Japanese A. kamchatica population was excluded from further analysis because of problems with LT50 estimation; non-acclimated and acclimated LT50 for this population were estimated to be −105.1°C and −28.8°C, respectively, and −105.1°C is below the lowest temperature tested. Due to the nature of nonlinear mixed-effect models, estimation of an inflection point (LT50) that is outside of the measured range is highly unreliable.
Arabidopsis l. petraea from the far north had some of the lowest acclimated LT50 values (Fig. 4, Appendix S1f). Yet surprisingly, plants from two different northern populations, which are geographically very close (both in Iceland at ~65°N), showed very different acclimated LT50 values. The plant from the Reykjavik population had a high capacity for cold acclimation, whereas the plant from Esja Mountain had almost the same LT50 before and after acclimation. The nonlinear mixed-effect model did not converge with the non-acclimated data for the population from Braemer, Scotland, so it was excluded from analysis.
DISCUSSION
Variation among taxa
A great deal is known about the molecular basis of cold tolerance and acclimation in Arabidopsis thaliana (Thomashow, 1999, 2010; Xin and Browse, 2000; Hannah et al., 2006; Horton et al., 2016). However, we showed that it is not nearly as cold tolerant as its close relatives, A. lyrata and A. kamchatica, for both non-acclimated, actively growing plants, and cold-acclimated plants. Arabidopsis lyrata and A. kamchatica also had a much greater capacity for cold acclimation. A distant Arabidopsis relative, Eutrema salsugineum (=Thellungiella salsuginea O.E.Schultz; Brassicaceae), has been described as exhibiting extreme cold tolerance. Its non-acclimated LT50 estimates range from −9 to −13°C, while acclimated LT50 values range from −17.4 to −21.9°C, (Griffith et al., 2007; Khanal, Moffatt, and Gray, 2015). Thus, the cold tolerance and acclimation capacity levels of A. lyrata and A. kamchatica are similar to those of E. salsugineum. However, their closer relationship to A. thaliana makes the transfer of molecular tools much easier. Arabidopsis lyrata and A. kamchatica have the potential to provide a valuable complement to A. thaliana for cold tolerance research.
As we expected, Arabidopsis taxa with more northerly distributions, that experience colder winter temperatures (A. lyrata and A. kamchatica), to exhibit more cold tolerance and more acclimation capacity than the more southerly taxa (A. thaliana and A. h. gemmifera) (Figs. 3 and 4). Climatic range estimates suggest that A. lyrata and A. kamchatica occur in colder regions than A. thaliana and A. h. gemmifera. The January mean daily minimum temperature (Tmin) in the coldest regions where A. thaliana occurs is −31°C, and Tmin for A. h. gemmifera was −25°C, whereas the lowest Tmin for A. kamchatica is −40°C and Tmin for A. lyrata is −48°C (Hoffmann, 2005).
When we separate the two A. lyrata subspecies (A. l. lyrata and A. l. petrea), the results are less congruent with climatic estimates. The European subspecies, A. l. petraea, has a much more northerly distribution and occupies much colder regions than the North American subspecies, A. l. lyrata; the lowest January Tmin for A. l. petraea is −48°C, whereas Tmin for A. l. lyrata is only −15°C (Hoffmann, 2005). However, there was little apparent difference between the subspecies; both taxa were very cold tolerant. The North American subspecies, A. l. lyrata is thought to be recently derived from A. l. petraea, via a founder event from Europe (Wright et al., 2003; Mattila et al., 2017). It is also likely that A. l. lyrata experienced a much colder climate during the last glacial period because much of its current range is within previously glaciated regions (Hoffmann, 2005). There may not have been sufficient time or selection pressures for cold tolerance to be lost in A. l. lyrata. Thus, the historical climate and biogeographic history are likely to explain the high cold tolerance of A. l. lyrata despite the relatively warm conditions in its current range. Further, the high cold tolerance of A. l. lyrata suggests that this taxon may not fully occupy its potential climate niche and that it may have the capacity to migrate into much colder regions.
It is notable that for all taxa except A. l. lyrata, the mean acclimated LT50 values are higher than the January Tmin, indicating that if plants were not insulated by snow cover, they would not likely survive winter at the northern edge of their range. Snow is a powerful insulator. Arabidopsis species overwinter as low-lying rosettes. In the coldest climates, plants are insulated by snow cover throughout winter, greatly reducing the severity of cold that plants are exposed to. For instance, two winters of temperature measurements show that A. kamchatica in interior Alaska was never exposed to temperatures below −11°C even when the air temperatures plummeted below −40°C (Armstrong et al., 2015). However, in warmer regions, the snow frequently melts during winter, and rosettes are directly exposed to freezing air temperatures during subsequent cold snaps. This exposure may also be responsible for the retention of cold tolerance in A. l. lyrata. It also suggests that as winter temperatures in the far north increase and snow melting events become more common, plants may paradoxically require higher levels of cold tolerance.
Variation within taxa
It is interesting that differences among taxa were related to latitude and climate, but within-taxon variation was not. The lack of a cline for cold tolerance in A. thaliana was especially surprising, as cold tolerance has previously been shown to be correlated with both latitude (Zhen and Ungerer, 2008) and January minimum temperatures (Tmin) in this species (Hannah et al., 2006). Our failure to observe a cline may be due to the limited number of A. thaliana accessions in our study (N = 5). Sample sizes in A. lyrata (N = 9) and A. kamchatica (N = 46) were larger, so the lack of a latitudinal cline is more likely to be real.
Arabidopsis kamchatica recently crossed the Bering land bridge from eastern Russia into Alaska and southward into western Canada, and there is little genetic differentiation among populations in North America (Shimizu-Inatsugi et al., 2009). Most of our A. kamchatica samples were from North America. These plants would have required high cold tolerance when crossing the Bering land bridge, and there may not have been sufficient time for loss of cold tolerance in plants that migrated southward, even if there was selection against cold tolerance. Consistent with selection for cold tolerance when A. kamchatica crossed the land bridge, the southernmost sample in our analysis, from Taiwan, was less cold tolerant than most North American samples. An investigation of A. kamchatica cold tolerance in Asian and Russian, where genetic diversity is higher (Shimizu-Inatsugi et al., 2009), would help clarify the evolution of cold tolerance in this species.
Other studies of A. lyrata have had mixed results. Like us, Davey et al. (2018) failed to find a correlation between cold tolerance and latitude in A. l. petraea. However, Wos and Willi (2015) did find that northern populations had greater cold tolerance than southern populations. Thus, although some measures of cold tolerance may reveal a latitudinal cline in A. lyrata, our results do not. Unlike populations of A. thaliana and A. kamchatica, A. lyrata populations are highly differentiated (Wright et al., 2003; Mattila et al., 2017), and they show strong local climate adaptation (Leinonen et al., 2009, 2013; Leinonen et al., 2011; Hämälä et al., 2018; Lucek et al., 2019). The counter-adaptive effects of gene flow or recent divergence are not a likely explanation for the lack of a latitudinal cline in A. lyrata. Perhaps there is no selection against cold tolerance in the south.
Some studies on both A. thaliana and A. lyrata suggest that there may be a cost of cold tolerance (Jackson et al., 2004; Wos and Willi, 2015), but conclusions depend on how costs are measured. Zhen (2011) found no evidence of a cost in the absence of freezing stress, and Wos and Willi (2018) found that the ability to acclimate did not reduce fitness in any environment. Thus, there may or may not be selection against cold tolerance in regions that do not experience cold winters. Alternatively, southern plants, may have less persistent winter snow cover, so that they experience just as much cold as northern plants, which are insulated by snow.
Accuracy of freeze tolerance (LT50) estimates
Our study found lower LT50 values than some other studies in the same Arabidopsis species. We found slightly more freeze tolerance in A. thaliana (Hannah et al., 2006; Reyes-Diaz et al., 2006; Zuther et al., 2012), and considerably more freeze tolerance in A. lyrata (Davey et al., 2018). Our methodologies differed in a number of ways from Davey et al. (2018), primarily in how total electrolyte leakage (ELT) was estimated. Total electrolyte leakage (ELT) is intended to estimate EL when all cells have been lysed, so that LT50 represents the point when 50% of cells have been lysed. To estimate the reference conductivity corresponding to 100% lysing, Davey exposed leaves to −80°C for 3 h, whereas we autoclaved leaves. Our preliminary tests found that autoclaving leaves gave a higher ELT than freezing at −80°C, indicating that freezing did not lyse all cells in these highly freeze-tolerant plants. Because our ELT estimates were higher, more cell damage and lower temperatures were necessary to reach LT50. Thus, we believe that our LT50 estimates are reliable although we need to be cautious with the comparison among different studies.
CONCLUSIONS
We examined cold tolerance within and among Arabidopsis taxa. Taxa with northern distributions (A. lyrata and A. kamchatica) were generally more cold tolerant than those with southern distributions (A. thaliana and A. halleri subsp. gemmifera) both before and after acclimation and had a higher capacity to acclimate. Cold tolerance increased after cold acclimation (exposure to low, but non-freezing temperatures) for all taxa, although the difference was not significant for A. h. gemmifera. For all taxa except A. l. lyrata, acclimated LT50 values are higher than the January mean daily minimum temperature (Tmin), suggesting that if plants were not insulated by snow cover, they would not likely survive winter at the northern edge of their range. Arabidopsis lyrata subsp. lyrata, on the other hand, has higher cold tolerance than is needed, even at the northern edge of its range. This subspecies was recently derived in North America by a founder event from European A. lyrata subsp petraea (Wright et al., 2003; Mattila et al., 2017). It appears to have retained its ancestral high levels of cold tolerance and should have the capacity to expand northward. Since molecular tools are easy to transfer from A. thaliana to its relatives, these extremely cold-tolerant taxa (A. lyrata and A. kamchatica) are excellent candidates for studying both the molecular and ecological aspects of cold tolerance.
Supplementary Material
Appendix S1. Relationships between latitude and population cold tolerance for each taxon.
Appendix S2. The estimated cold tolerance (LT50) values for each population.
Appendix S3. Raw electrolyte leakage (EL) data.
ACKNOWLEDGMENTS
The authors thank T. Sformo for advice regarding methods and experimental design. We thank B. M. Barnes and J. G. Duman for allowing us to use their laboratory space and equipment, M. V. Wright and the staff at the Institute of Arctic Biology Greenhouse at the University of Alaska Fairbanks for helping keep plants alive, and we thank A. N. Powell and the members of the 2011 Scientific Writing, Revising and Editing class, C. P. H. Mulder, and anonymous reviewers for thoughtful comments and edits to this manuscript. This work was supported by the Alaska Experimental Program to Stimulate Competitive Research, National Science Foundation and the State of Alaska, USA (grant no. EPS-0701898); the Cooperative Institute for Arctic Research International Polar Year Student Traineeship through the National Oceanic and Atmospheric Administration (grant no. NA17RJ1224); the University of Alaska Fairbanks Center for Global Change Student Award funded by the International Arctic Research Center through the National Science Foundation (grant no. ARC-0327664); and Alaska IDeA Network of Biomedical Research Excellence, National Institutes of Health (grant no. 5P20RR016466). The contents are the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information section at the end of the article.
LITERATURE CITED
- Addo-Bediako A, Chown SL, and Gaston KJ 2000. Thermal tolerance, climatic variability and latitude. Proceedings of the Royal Society of London, B, Biological Sciences 267: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JJ, Takebayashi N, Sformo T, and Wolf DE 2015. Cold tolerance in Arabidopsis kamchatica. American Journal of Botany 102: 439–448. [DOI] [PubMed] [Google Scholar]
- Barnes AC, Benning C, and Roston RL 2016. Chloroplast membrane remodeling during freezing stress is accompanied by cytoplasmic acidification activating SENSITIVE TO FREEZING2. Plant Physiology 171: 2140–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, and Cox DR 1964. An analysis of transformations. Journal of the Royal Statistical Society, B, Methodological 26: 211–246. [Google Scholar]
- Castric V, Bechsgaard J, Schierup MH, and Vekemans X 2008. Repeated adaptive introgression at a gene under multiallelic balancing selection. PLoS Genetics 4: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Fowler S, Fiehn O, and Thomashow MF 2004. A prominent role for the CBF cold response pathway in configuring the low temperature metabolome of Arabidopsis. Proceedings of National Academy of Sciences, USA 101: 15243–15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MP, Palmer BG, Armitage E, Vergeer P, Kunin WE, Woodward FI, and Quick WP 2018. Natural variation in tolerance to sub-zero temperatures among populations of Arabidopsis lyrata subsp. petraea. BMC Plant Biology 18: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, and Tibshirani RJ 1998. An introduction to the bootstrap. Chapman and Hall/CRC, NY, NY, USA. [Google Scholar]
- Endler JA 1973. Gene flow and population differentiation. Science 179: 243–250. [DOI] [PubMed] [Google Scholar]
- Fox J, and Weisberg S 2011. An R companion to applied regression, 2nd ed Sage, Thousand Oaks, CA, USA. [Google Scholar]
- Griffith C, Kim E, and Donohue K 2004. Life-history variation and adaptation in the historically mobile plant Arabidopsis thaliana (Brassicaceae) in North America. American Journal of Botany 91: 837–849. [DOI] [PubMed] [Google Scholar]
- Griffith M, Timonin M, Wong ACE, Gray GR, Akhter SR, Saldanha M, Rogers MA, et al. 2007. Thellungiella: an Arabidopsis-related model plant adapted to cold temperatures. Plant Cell and Environment 30: 529–538. [DOI] [PubMed] [Google Scholar]
- Hämälä T, Mattila TM, and Savolainen O 2018. Local adaptation and ecological differentiation under selection, migration, and drift in Arabidopsis lyrata. Evolution 72: 1373–1386. [DOI] [PubMed] [Google Scholar]
- Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, and Hincha DK 2006. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiology 142: 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasdai M, Weiss B, Levi A, Samach A, and Porat R 2006. Differential responses of Arabidopsis ecotypes to cold, chilling and freezing temperatures. Annals of Applied Biology 148: 113–120. [Google Scholar]
- Hastie TJ, and Pregibon D 1992. Generalized linear models In Chambers JM and Hastie TJ [eds.], Statistical models in S, 13–44. Wadsworth and Brooks/Cole, Pacific Grove, CA, USA. [Google Scholar]
- Hoffmann MH 2002. Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). Journal of Biogeography 29: 125–134. [Google Scholar]
- Hoffmann MH 2005. Evolution of the realized climatic niche in the genus Arabidopsis (Brassicaceae). Evolution 59: 1425–1436. [PubMed] [Google Scholar]
- Hofmann M, and Bruelheide H 2015. Frost hardiness of tree species is independent of phenology and macroclimatic niche. Journal of Biosciences 40: 147–157. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Butof A, Welk E, and Bruelheide H 2013. Relationship between fundamental and realized niches in terms of frost and drought resistance. Preslia 85: 1–17. [Google Scholar]
- Hofmann M, Durka W, Liesebach M, and Bruelheide H 2015. Intraspecific variability in frost hardiness of fagus sylvatica L. European Journal of Forest Research 134: 433–441. [Google Scholar]
- Hohmann N, Wolf EM, Lysak MA, and Koch M 2015. A time-calibrated road map of Brassicaceae species radiation and evolutionary history. Plant Cell 27: 2770–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MW, Willems G, Sasaki E, Koornneef M, and Nordborg M 2016. The genetic architecture of freezing tolerance varies across the range of Arabidopsis thaliana. Plant Cell and Environment 39: 2570–2579. [DOI] [PubMed] [Google Scholar]
- Jackson MW, Stinchcombe JR, Korves TM, and Schmitt J 2004. Costs and benefits of cold tolerance in transgenic Arabidopsis thaliana. Molecular Ecology 13: 3609–3615. [DOI] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, and Guy CL 2004. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology 136: 4159–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal N, Moffatt BA, and Gray GR 2015. Acquisition of freezing tolerance in Arabidopsis and two contrasting ecotypes of the extremophile Eutrema salsugineum (Thellungiella salsuginea). Journal of Plant Physiology 180: 35–44. [DOI] [PubMed] [Google Scholar]
- Koch MA, and Matschinger M 2007. Evolution and genetic differentiation among relatives of Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 104: 6272–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling J, Wiesenberg GLB, Thiel D, Wohlfart C, Huber G, Walter J, Jentsch A, et al. 2012. Cold hardiness of Pinus nigra Arnold as influenced by geographic origin, warming, and extreme summer drought. Environmental and Experimental Botany 78: 99–108. [Google Scholar]
- Kuittinen H, Mattila A, and Savolainen O 1997. Genetic variation at marker loci and in quantitative traits in natural populations of Arabidopsis thaliana. Heredity 79: 144–152. [DOI] [PubMed] [Google Scholar]
- Kuittinen H, Sillanpaa MJ, and Savolainen O 1997. Genetic basis of adaptation: flowering time in Arabidopsis thaliana. Theoretical and Applied Genetics 95: 573–583. [Google Scholar]
- Le MQ, Engelsberger WR, and Hincha DK 2008. Natural genetic variation in acclimation capacity at sub-zero temperatures after cold acclimation at 4 °C in different Arabidopsis thaliana accessions. Cryobiology 57: 104–112. [DOI] [PubMed] [Google Scholar]
- Leinonen PH, Remington DL, Leppälä J, and Savolainen O 2013. Genetic basis of local adaptation and flowering time variation in Arabidopsis lyrata. Molecular Ecology 22: 709–723. [DOI] [PubMed] [Google Scholar]
- Leinonen PH, Remington DL, and Savolainen O 2011. Local adaptation, phenotypic differentiation and hybrid fitness in diverged natural populations of Arabidopsis lyrata. Evolution 65: 90–107. [DOI] [PubMed] [Google Scholar]
- Leinonen PH, Sandring S, Quilot B, Clauss MJ, Mitchell-Olds T, Agren J, and Savolainen O 2009. Local adaptation in European populations of Arabidopsis lyrata (Brassicaceae). American Journal of Botany 96: 1129–1137. [DOI] [PubMed] [Google Scholar]
- Lipp CC, Goldstein G, Meinzer FC, and Niemczura W 1994. Freezing tolerance and avoidance in high elevation Hawaiian plants. Plant Cell and Environment 17: 1035–1044. [Google Scholar]
- Loik ME, and Redar SP 2003. Microclimate, freezing tolerance, and cold acclimation along an elevation gradient for seedlings of the great basin desert shrub, Artemisia tridentata. Journal of Arid Environments 54: 769–782. [Google Scholar]
- Lucek K, Hohmann N, and Willi Y 2019. Postglacial ecotype formation under outcrossing and self-fertilization in Arabidopis lyrata. Molecular Ecology 28: 1043–1055. [DOI] [PubMed] [Google Scholar]
- Mattila TM, Tyrmi J, Pyhäjärvi T, and Savolainen O 2017. Genome-wide analysis of colonization history and concomitant selection in Arabidopsis lyrata. Molecular Biology and Evolution 34: 2665–2677. [DOI] [PubMed] [Google Scholar]
- Menon M, Barnes WJ, and Olson MS 2015. Population genetics of freeze tolerance among natural populations of Populus balsamifera across the growing season. New Phytologist 207: 710–722. [DOI] [PubMed] [Google Scholar]
- Mooney CZ, and Duval RD 1993. Bootstrapping: a nonparametric approach to statistical inference. Sage, Newbury Park, CA, USA. [Google Scholar]
- Novikova PY, Hohmann N, Nizhynska V, Tsuchimatsu T, Jamshaid A, Muir G, Guggisberg A, et al. 2016. Sequencing of the genus Arabidopsis identifies a complex history of nonbifurcating speciation and abundant trans-specific polymorphism. Nature Genetics 48:1077–1082. [DOI] [PubMed] [Google Scholar]
- Nunes MES, and Smith GR 2003. Electrolyte leakage assay capable of quantifying freezing resistance in rose clover. Crop Science 43: 1349–1357. [Google Scholar]
- Paape T, Briskine RV, Halstead-Nussloch G, Lischer HEL, Shimizu-Inatsugi R, Hatakeyama M, Tanaka K, et al. 2018. Patterns of polymorphism and selection in the subgenomes of the allopolyploid Arabidopsis kamchatica. Nature Communications 9: 3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagter M, Kristoffersen A, Bronnum P, and Jensen M 2010. Phenotypic differences in development of cold hardiness in three latitudinal populations of Acer platanoides L. Scandinavian Journal of Forest Research 25: 412–420. [Google Scholar]
- Pinheiro JC, and Bates DM 2000. Mixed-effects models in S and S-plus Springer Verlag, NY, NY, USA. [Google Scholar]
- Pither J 2003. Climate tolerance and interspecific variation in geographic range size. Proceedings of the Royal Society of London, B, Biological Sciences 270: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasil I, and Zamecnik J 1998. The use of a conductivity measurement method for assessing freezing injury I. Influence of leakage time, segment number, size and shape in a sample on evaluation of the degree of injury. Environmental and Experimental Botany 40: 1–10. [Google Scholar]
- R Core Team. 2011. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Website: http://www.R-project.org. [Google Scholar]
- Renaut J, Hoffmann L, and Hausman JF 2005. Biochemical and physiological mechanisms related to cold acclimation and enhanced freezing tolerance in poplar plantlets. Physiologia Plantarum 125: 82–94. [Google Scholar]
- Reyes-Diaz M, Ulloa N, Zuniga-Feest A, Gutierrez A, Gidekel M, Alberdi M, Corcuera LJ, and Bravo LA 2006. Arabidopsis thaliana avoids freezing by supercooling. Journal of Experimental Botany 57: 3687–3696. [DOI] [PubMed] [Google Scholar]
- Roberts JK, and Fan X 2004. Bootstrapping within the multilevel/hierarchical linear modeling framework: a primer for use with SAS and SPLUS. Multiple Linear Regression Viewpoints 30: 23–34. [Google Scholar]
- Shi Y, Ding Y, and Yang S 2018. Molecular regulation of CBF signaling in cold acclimation. Trends in Plant Science 23: 623–637. [DOI] [PubMed] [Google Scholar]
- Shimizu-Inatsugi R, Lihova J, Iwanaga H, Kudoh H, Marhold K, Savolainen O, Watanabe K, et al. 2009. The allopolyploid Arabidopsis kamchatica originated from multiple individuals of Arabidopsis lyrata and Arabidopsis halleri. Molecular Ecology 18: 4024–4048. [DOI] [PubMed] [Google Scholar]
- Slatkin M 1973. Gene flow and selection in a cline. Genetics 74: 733–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin M 1987. Gene flow and the geographic structure of natural-populations. Science 236: 787–792. [DOI] [PubMed] [Google Scholar]
- Smolensk AD 2011. Projection. The Tao of D&D 6 September 2011. Website: http://tao-dnd.blogspot.com/2011/09/projection.html. [Google Scholar]
- Takahashi D, Gorka M, Erban A, Graf A, Kopka J, Zuther E, and Hincha DK 2019. Both cold and sub-zero acclimation induce cell wall modification and changes in the extracellular proteome in Arabidopsis thaliana. Scientific Reports 9: 2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF 1999. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology 50: 571–599. [DOI] [PubMed] [Google Scholar]
- Thomashow MF 2010. Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiology 154: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Joseph RA, and Steponkus PL 1995. Cold acclimation of Arabidopsis thaliana: effect on plasma membrane lipid composition and freeze-induced lesions. Plant Physiology 109: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Tominaga Y, Nakagawara C, Shigematsu S, Minami A, and Kawamura Y 2006. Responses of the plasma membrane to low temperatures. Physiologia Plantarum 126: 81–89. [Google Scholar]
- Venables WN, and Ripley BD 2002. Modern applied statistics with S, 4th ed Springer, NY, NY, USA. [Google Scholar]
- Wos G, and Willi Y 2015. Temperature-stress resistance and tolerance along a latitudinal cline in north american Arabidopsis lyrata. PloS ONE 10: e0131808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wos G, and Willi Y 2018. Thermal acclimation in Arabidopsis lyrata: genotypic costs and transcriptional changes. Journal of Evolutionary Biology 31: 123–135. [DOI] [PubMed] [Google Scholar]
- Wright SI, Lauga B, and Charlesworth D 2003. Subdivision and haplotype structure in natural populations of Arabidopsis lyrata. Molecular Ecology 12: 1247–1263. [DOI] [PubMed] [Google Scholar]
- Xin Z, and Browse J 2000. Cold comfort farm: the acclimation of plants to freezing temperatures. Plant Cell and Environment 23: 893–902. [Google Scholar]
- Zhen Y, and Ungerer MC 2008. Clinal variation in freezing tolerance among natural accessions of Arabidopsis thaliana. New Phytologist 177: 419–427. [DOI] [PubMed] [Google Scholar]
- Zhen Y, Dhakal P, and Ungerer MC 2011. Fitness benefits and costs of cold acclimation in Arabidopsis thaliana. American Naturalist 178: 44–52. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Dong CH, and Zhu JK 2007. Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Current Opinion in Plant Biology 10: 290–295. [DOI] [PubMed] [Google Scholar]
- Zuther E, Schulz E, Childs LH, and Hincha DK 2012. Clinal variation in the non-acclimated and cold-acclimated freezing tolerance of Arabidopsis thaliana accessions. Plant, Cell and Environment 35: 1860–1878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Relationships between latitude and population cold tolerance for each taxon.
Appendix S2. The estimated cold tolerance (LT50) values for each population.
Appendix S3. Raw electrolyte leakage (EL) data.