Abstract
Background:
Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX) is a replacement for perfluorooctanoic acid in the production of fluoropolymers used in a variety of consumer products. GenX alters fetal development and antibody production and elicits toxic responses in the livers and kidneys of rodents. The GenX effect on the blood–brain barrier (BBB) is unknown. The BBB protects the brain from xenobiotic neurotoxicants and harmful endogenous metabolites.
Objectives:
We aimed to investigate the effects of GenX on the transport activity and expression of P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance–associated protein 2 (MRP2) at the BBB.
Methods:
Transporter activities were measured in isolated rat brain capillaries by a confocal microscopy–based method. ATPase (enzymatic hydrolysis of adenosine triphosphate to inorganic phosphate) levels were measured in vitro. Western blotting determined P-gp and BCRP protein levels. Cell survival after GenX exposure was determined for two human cell lines.
Results:
Nanomolar levels of GenX inhibited P-gp and BCRP but not MRP2 transport activities in male and female rat brain capillaries. P-gp transport activity returned to control levels after GenX removal. GenX did not reduce P-gp- or BCRP-associated ATPase activity in an in vitro transport assay system. Reductions of P-gp but not BCRP transport activity were blocked by a peroxisome proliferator–activated receptor () antagonist. GenX reduced P-gp and BCRP transport activity in human cells.
Conclusion:
In rats, GenX at rapidly (in 1–2 h) inhibited P-gp and BCRP transport activities at the BBB through different mechanisms. was required for the GenX effects on P-gp but not BCRP transport activity. https://doi.org/10.1289/EHP5884
Introduction
Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy) propanoate (GenX) (CAS no. 62037-80-3) is a chemical precursor used in the production of polytetrafluoroethylene (Teflon) and as a replacement for perfluorooctanoic acid (Wang et al. 2013). The production volume of GenX in the European Union is estimated to be ; however, the worldwide production volume is unknown (Beekman et al. 2016). Although GenX is unlikely to pose a significant aquatic hazard, its bioactivity and persistence in environmental media are problematic (Hoke et al. 2016). GenX is a contaminant in rivers of the Netherlands, Germany, and China (Heydebreck et al. 2015). In June 2017, GenX was detected in the Cape Fear River in eastern North Carolina, United States (Sun et al. 2016). Further surveys nearby and downstream from a PFAS manufacturing facility detected GenX in air, private well water, and some local food products including oysters and honey (Pritchett et al. 2019; Clabby 2018). Most importantly, GenX was detected in finished drinking water. Since GenX has the potential to disrupt biological signaling pathways known to regulate ABC [adenosine triphosphate (ATP) binding cassette] transporters (Conley et al. 2019; Miller and Cannon 2014; More et al. 2017; Zhang et al. 2014), we investigated its effects on three transporters at the BBB: ABCB1, [P-glycoprotein (P-gp)], ABCG2 [breast cancer resistance protein (BCRP)], and ABCC2 [multidrug resistance–associated protein 2 (MRP2)]. We hypothesized that disruption of signaling pathways by GenX would change basal levels of transporter expression and/or activity. ABC transporters are primary active transporters that derive their energy from the hydrolysis of ATP to . Within biological barriers, they function to restrict the access of toxic drugs, endobiotics, and xenobiotics to sensitive cells, tissues, and organs (Locher 2016). In tumor cells, their overexpression leads to multidrug resistance and presents major obstacles in cancer therapies (Bockor et al. 2017; Robey et al. 2018). In most human tissues, P-gp, BCRP, and ABCC2/MRP2 are ubiquitously expressed at low levels; however, in the biological barriers, they are highly expressed and function to protect the brain, retina, testes, and the developing fetus (Nagy et al. 2016). Their localization and expression levels in liver and kidney also serve to eliminate harmful agents from the body (Leslie et al. 2005). Specifically, in the canalicular membrane of liver, they transport conjugates and harmful metabolites into bile. In the proximal tubules of the kidney, they aid in excretory transport of substrates into urine. They are also expressed in the enterocytes of the intestine, where they function to limit the absorption of harmful substrates into the body (Murakami and Takano 2008). The substrates of P-gp, BCRP, and MRP2 are chemically diverse and include chemotherapeutics, lipids, steroids, bilirubin, bile acids, platelet-activating factor, dietary flavonoids, and conjugated endogenous and xenobiotic metabolites (Kim 2002).
Signaling pathways dynamically regulate the activity and expression of P-gp, BCRP, and MRP2 at the BBB (Miller and Cannon 2014). To examine the effects of GenX on these transporters, we exposed rat brain capillaries ex vivo to low nanomolar concentrations of GenX. We also dosed rats in vivo by oral gavage with 30, 300, or GenX and measured transport activity ex vivo using a steady-state confocal microscopy–based assay. Lastly, we expanded our study to humans by measuring the effect of GenX on the survival of two human cell lines exposed to increasing concentrations of cytotoxic substrates of P-gp and BCRP.
Materials and Methods
Materials
Crystalline GenX (molecular weight of 347.084 g/mol, purity] was purchased from SynQuest Labs. P-gp fluorescent substrate [] cyclosporine A (NBD-CSA) was custom synthesized by R. Wenger (Sandoz) (Schramm et al. 1995). MRP2 fluorescent substrate Texas Red®, Sigma-Aldrich (sulforhodamine MRP2 inhibitor MK-571, corn oil, and mouse monoclonal antibody A1978 were purchased from Sigma-Aldrich. P-gp inhibitor PSC-833 and BCRP inhibitor KO-134 were purchased from Tocris Bioscience. Adriamycin was the gift of the Drug Synthesis and Chemistry Branch, Developmental Therapeutic Program of the National Cancer Institute (NCI), National Institutes of Health (NIH). Mitoxantrone hydrochloride was purchased from Sigma-Aldrich. Both Adriamycin and mitoxantrone were dissolved in double-distilled water () and stored at . P-gp rabbit monoclonal antibody ab170904 was purchased from Abcam. Secondary antibodies, Alexa Fluor® 647 Goat anti-Mouse IgG and Alexa Fluor® 647 Goat anti-Rabbit IgG, were purchased from Thermo Fisher Scientific. Western blotting lysis buffer CelLytic™ MT Mammalian Tissue Lysis/Extraction Reagent with complete mini protease inhibitor was purchased from Sigma-Aldrich. The BCRP substrate BODIPY™ FL prazosin and the Western blotting materials, including 10-well Invitrogen NuPAGE 4-12% Bis-Tris Gels NP0321 and polyvinylidene difluoride (PVDF) Western blot membranes, were obtained from Invitrogen (Thermo Fisher Scientific).
Animals
Male and female Hsd:Sprague-Dawley® (SD®) rats (age 12–15 wk) were purchased from Envigo. Animals were housed in the NIEHS Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)–approved animal care facility [ humidity, room temperature, 12-h light/dark cycle, polycarbonate shoebox cages (Tecniplast), and Sani-Chip® bedding (PJ Murphy Forest Products)] for at least 1 wk prior to use and allowed access to food (NIH #31) and tap water ad libitum consumption. Animals were euthanized by inhalation followed by decapitation. All animal protocols were approved by the Animal Care and Use Committee at the NIEHS according to the guidelines from the NIH. All data are reported in compliance with the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines.
Capillary Isolation and ex Vivo ABC Transport Assay
To measure ex vivo transport activity of P-gp and BCRP at the blood–brain barrier (BBB), capillaries from male and female rats were isolated as previously reported (Chan and Cannon 2017). Briefly, the brains from four to six male or female rats were harvested following euthanasia and placed in assay buffer [, pH 7.4, supplemented with of glucose and of sodium pyruvate] on ice. Cortical gray matter was isolated by discarding white matter, meninges, midbrain, choroid plexus, and olfactory lobes using dissecting forceps and a stereomicroscope. The remaining cortical gray matter enriched for capillaries was minced with a razor blade, suspended in isolation buffer, and homogenized by 40 up-and-down strokes using a Thomas (size C) mechanical tissue grinder (Thomas Scientific; catalog no. 3431E55) matched with a size C serrated pestle (Thomas Scientific; catalog no. 3431F25; clearance: 150 to ) rotating at . A final homogenization was performed by 10 up-and-down strokes using a KONTES® Dounce tissue grinder (pestle, size B; catalog no. 885300-0015; clearance: 165 to ; VWR). The resulting homogenate was suspended in an equal volume of ice-cold 30% Ficoll PM400 dissolved in assay buffer to achieve a final Ficoll PM 400 content of 15% wt/vol and centrifuged for 20 min at at 4°C using an RC-5B Centrifuge (Sorvall) with a SS-34 rotor. Following centrifugation, supernatants were removed, and pelleted capillaries were resuspended in assay buffer containing 1% bovine serum albumin (BSA) (Sigma-Aldrich) and captured by passage through a filter [pluriStrainer (Pluriselect), 43-50030-03]. BSA from Sigma was removed from isolated capillaries by three sequential centrifugations at following resuspension in isolation buffer (no BSA). After the third centrifugation, the capillaries were resuspended in assay buffer, and equal volumes were loaded into sterile borosilicate chamber slides purchased from Thermo Scientific (catalog no. 155380). The chamber slides were incubated at room temperature for 15 min to allow capillary settling and binding. Next, the chamber slides were rinsed with assay buffer. To measure transporter activity, chamber slide bound capillaries were incubated at 0–4 h in of assay buffer containing a fluorescent substrate () specific for each transporter (NBD-CSA for P-gp, BODIPY™ FL prazosin for BCRP, and Texas Red® for MRP2). Transporter activity was determined as the mean measurement of steady-state luminal fluorescence for 15–20 individual capillaries per chamber. Luminal fluorescence reaches a steady state in 30 min at room temperature (Chan and Cannon 2017). Nonspecific background fluorescence was determined by measuring the luminal fluorescence of fully inhibited capillaries. All inhibitors were administered as a 30-min pretreatment before fluorescent substrates were added. The inhibitors used were PSC-833 (, P-gp), KO134 (, BCRP), and MK-571 (, MRP2). Specific transport for each transporter was calculated as the difference between total noninhibited transport minus nonspecific inhibited transport. To measure luminal fluorescence, confocal images of capillaries were captured using a Zeiss 710 confocal microscope. Luminal fluorescence was quantified using FIJI/ImageJ (File version, ImageJ 1.52a; Java 1.8.0_112) (NIH) analysis software. The solvent [0.1% vol/vol dimethylsulfoxide (DMSO)] was used as the vehicle control (VC) to match the concentration of the treatment solvents. GenX in compared to GenX in DMSO 0.1% produced no significant effects on P-gp transport (Figure S1). For all relevant experiments, GenX was dissolved in DMSO immediately prior to use. Negative controls employed inhibitors specific for each transporter. Where relevant, GenX and the peroxisome proliferator–activated receptor () antagonist, GW9662 () were freshly dissolved in DMSO and used alone or in cotreatments (inhibitor studies) in volumes (0.1% vol/vol) to match VC volumes. When needed, staggered time courses were used to accommodate confocal image acquisitions at 10 min/dose group.
Measuring Transport Activity after GenX Removal
The reversibility assays are a modified design of the ex vivo transport assay. Briefly, isolated capillaries from four to six rats were pooled and equally distributed into chamber slides and incubated at room temperature in assay media with a VC (DMSO vol/vol 0.1%) and the P-gp- or BCRP-specific fluorescent substrate (). In our VC group, steady-state transport activity was established by measuring luminal fluorescence after 1–5 h incubation at room temperature. In a second group, GenX was added to achieve a final concentration in media containing the fluorescence substrates and incubated for 1 h for P-gp and 2 h in the BCRP transport assays. At 1–2 h, respectively, P-gp and BCRP transport activities were measured. Following transport measurements, GenX was removed by two washes (equal volumes) with assay media containing fluorescence substrates specific for each transporter. After GenX removal, the samples were incubated in assay media with appropriate substrates. Next, P-gp and BCRP transport activities were measured at 0.5, 1, 2, and 3 h after GenX removal. All transporter activities were determined as the mean measurement of steady-state luminal fluorescence minus background fluorescence for 15–20 individual capillaries per chamber. Capillaries were imaged using a Zeiss 710 confocal microscope. Luminal fluorescence was quantified using FIJI/ImageJ analysis software. All transport experiments, including the reversibility assays, were performed two times to ensure reproducibility.
ATPase Activation Assay
The ATPase assay was used to determine if GenX at concentrations of 0.001, 0.01, 0.1, and influenced the ATPase activity associated with P-gp and BCRP transport activity in a purified system. The assay is based on the spectrophotometric quantitation of Pi produced from P-gp- or BCRP ATPase-mediated conversion of ATP to when the substrate was stimulated with paclitaxel for P-gp and sulfasalazine for BCRP. To accomplish this, of membrane vesicles, provided by the assay kit, containing either of P-gp or BCRP proteins, were diluted 10-fold in assay buffer and transferred to a well of a 96-well plate in triplicate. The wells contained either a) P-gp or BCRP plus substrates (stimulated positive control), b) P-gp or BCRP plus substrates with the ATPase inhibitor vanadate () (ATPase negative control), c) P-gp or BCRP minus substrates (negative control), d) P-gp or BCRP minus substrates with GenX, or e) P-gp or BCRP plus substrates and GenX in DMSO 0.1% at 0.001, 0.01, 0.1, or final concentration. The mixtures were preincubated at 37°C for 10 min, and the reaction was started by the addition of Mg-ATP () and transporter-specific substrates or DMSO 0.1% where appropriate. The plate was incubated at 37°C for 20 min. All reactions were terminated by the addition of of 5% sodium dodecyl sulfate. Pi levels are a function of ABC transporter activity, which requires the hydrolysis of ATP to . To measure Pi, a colorimetric detection solution was prepared by mixing one part of ammonium molybdate in zinc acetate (pH 5.0) with three parts of 10% ascorbic acid. The final detection solution was mixed by inversion, and was added to each sample and incubated for 20 min at 37°C. Assays were read at on a Gemini™ spectrometer (Molecular Devices) and graphed as relative units of ATPase activity. The means of triplicate samples were calculated using Prism software (version 7.05; GraphPad). Data are expressed as .
GenX in Vivo Dosing Studies
Male and female rats (five/dose group) received a single oral gavage dose of high-performance liquid chromatography (HPLC)–grade water as a VC or GenX in HPLC-grade water at 30, 300, or (10, 100, or , respectively). All doses were given in a volume of . Animals were euthanized at 5 h postdosing. Brains from each dose group were pooled, and capillaries were isolated as previously described above. P-gp and BCRP transport activities were measured ex vivo as described above.
GenX Cytotoxicity in Human Cells
The P-gp overexpressing the human ovarian NCI/ADR-RES cell line was obtained from NCI at Frederick Cancer Center (Scudiero et al. 1998). It was selected for its reliable high expression of P-gp. A second line, MX-MCF-7, was selected for its high expression levels of BCRP. This mitoxantrone-resistant MX-MCF-7 BCRP-expressing cell line (Nakagawa et al. 1992) was a generous gift of Dr. E. Schneider, Wadsworth Center, New York State Department of Health. Both low passage lines () were grown in Phenol Red-free RPMI media supplemented with 10% fetal bovine serum and antibiotics and used up to 15–20 passages, after which the cells were discarded and a new cell culture started from fresh frozen stock. Both cell lines were used in cytotoxicity studies that measured cell growth inhibition. First, we determined the cytotoxic effect of GenX (alone) for each line. Cell growth inhibition assays were performed for each line by plating 100,000–125,000 (NCI/ADR-RES for P-gp and MX-MCF-7 for BCRP) cells/well in triplicate. Each was grown for 18 h at 37°C and 5% to allow attachment. Next, they were grown in fresh media containing increasing concentrations of GenX ( to ). After 72 h, the cultures were washed three times with to remove nonadherent cells. The remaining adherent cells were harvested by trypsinization and counted in a Beckman Coulter Counter (Beckman).
To determine if GenX () affected the toxicity of known cytotoxic substrates for P-gp or BCRP, for each line, we plated 100,000–125,000 (NCI/ADR-RES for P-gp and MX-MCF-7 for BCRP) cells/well in triplicate. Each was grown for 18 h at 37°C and 5% to allow attachment. Next, they were grown in fresh media containing GenX and the toxic P-gp substrate, Adriamycin ( to ). MX-MCF-7 cells were grown in media for 72 h with or without GenX plus the toxic BCRP substrate mitoxantrone ( to ). After 72 h, the cultures were washed three times with to remove nonadherent cells. The remaining adherent cells were harvested by trypsinization and counted in a Beckman Coulter Counter (Beckman).
Gel Electrophoresis and Western Blotting
Isolated capillaries pooled from 6 rats were treated with VC (DMSO 0.1%) or GenX at room temperature in Falcon tubes (Fisher Scientific; catalog no. 14-959-53A) containing assay buffer (, pH 7.4, supplemented with of glucose and of sodium pyruvate). After treatment, capillaries were pelleted by centrifugation for 15 min at a centrifugal force of at 4°C and stored at until use. Membrane-containing protein lysates were isolated by adding of lysis buffer (CelLytic™ MT Cell Lysis Reagent; Sigma-Aldrich; catalog no. C3228-500; with Roche Complete Mini protease inhibitor cocktail; catalog no. 4693159001) to each pellet. Capillary pellets were kept on ice and vortexed for 30 s every 10 min for 90 min. In addition, the samples were sonicated at 4°C for 60 s at the 20-, 40-, and 60-min time points. To separate the nuclei from cytoplasm and cellular membranes, the samples were centrifuged at for 30 min. The pellets containing nuclei were discarded, and the supernatants containing cytoplasm and membranes were centrifuged at for 90 min. The liquid cytosolic fraction was removed, and the remaining membrane pellet was dissolved in MT cell (Sigma-Aldrich) lysis buffer and stored at until use. Membrane protein concentration was determined as described by the Coomassie Protein Assay Kit 23200 (Thermo Scientific), a modified version of the Bradford assay (Bradford 1976). BSA protein standards were provided by the kit. Electrophoresis and Western blotting were performed according to the manufacturer’s instructions. Briefly, of capillary membrane lysates were mixed with and (Invitrogen), loaded into NuPAGE Bis-Tris Gels (4–12%) using the XCell SureLock Mini-Cell Electrophoresis System (Invitrogen), and electrophoresed in MOPS Running Buffer (Invitrogen) for 50 min at a constant . Following electrophoresis, the resolved proteins were transferred from the gel to a PVDF membrane (Invitrogen) using the XCell II Blot Module using Invitrogen™ Bolt™ Transfer Buffer plus 10% (vol/vol) methanol. Electrotransfer was performed at constant current (0.1 up to approximately ) or voltage (10 to ) for 60 min. To visualize the P-gp-, BCRP-, and -specific bands, the membrane was incubated at room temperature for 30 min in blocking buffer (Intercept® Blocking Buffer, Licor). The membrane was washed in and hybridized overnight at 4°C in with 0.1% Tween with 1:200 vol/vol P-gp (Abcam; catalog no. 170904) and BCRP (Abcam; catalog no. 207732) primary antibodies and 1:5,000 vol/vol primary antibody (Abcam; catalog no. 8224). To remove excess antibody, the membrane was washed three times with with 0.1% Tween and incubated at room temperature for 1 h in with 1:10,000 vol/vol secondary antibodies [Odyssey Goat anti-Mouse IR Dye 800CW or Goat anti-Rat IR dye 680CW (Licor)] for an additional 60 min and washed three times with with 0.1% Tween to remove excess unbound secondary antibody. Specific protein bands were imaged and quantified for protein fluorescence using FluorChem M (ProteinSimple). For each sample, target band intensities were normalized to . Western blotting was performed in triplicate from three independent experiments. Means and standard errors of the mean (SEM) were calculated using Prism software (version 7.05; GraphPad) software. Data are expressed as a .
Statistics
Luminal fluorescence and cell survival data were analyzed and graphed using Prism software (version 7.05; GraphPad). Data are expressed as , and significant differences between the control and treated means were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. For the ex vivo transport assay, significance for each data point was determined by comparing treated to control: *; **; ***.
Results
Transporter Activity in Rat Brain Capillaries Treated with GenX
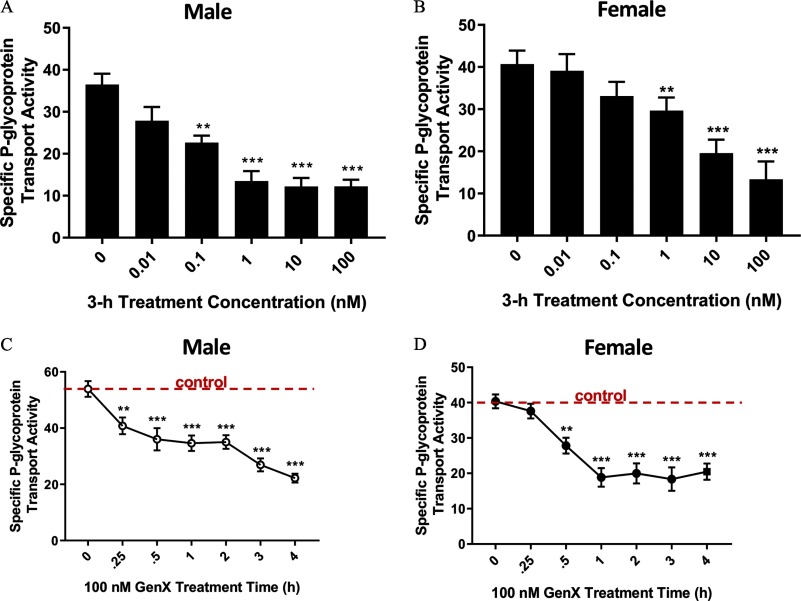
We determined the rapid effects of GenX exposure on the BBB by measuring GenX-mediated changes in ex vivo transport activity of three well-characterized ABC transporters (P-gp, BCRP, and MRP2). To accomplish this, we used an established steady state–based confocal microscopy assay (Chan and Cannon 2017). To determine the effect of increasing concentrations of GenX on the ABC transporters at the BBB, we exposed isolated capillaries from male and female rat brains to GenX for 3 h and measured transport activity. In males, P-gp transport activity was lowered but not significantly () by GenX exposure. P-gp transport activity was significantly lower in male capillaries exposed to GenX (Figure 1A). In females, P-gp transport activity was unchanged by GenX exposures but significantly lowered by GenX (Figure 1B). Next, we treated capillaries with GenX for 1–4 h and examined the hourly changes in P-gp transport. We chose GenX because it produced the greatest reduction in P-gp transport activity for both sexes. Results shown in Figure 1C,D show that GenX significantly lowered P-gp transport activity in 15 and 30 min in male and female rat brain capillaries, respectively.
Figure 1.
Changes in P-glycoprotein (P-gp) transport activity in brain capillaries from six rats treated with ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX). (A) Male and (B) female are graphs of P-glycoprotein (P-gp) transport activity at increasing doses of GenX in brain capillaries of Hsd:Sprague-Dawley® (SD®) rats. (C) Male and (D) female are graphs of the GenX-dependent reductions in P-gp transport activity over time. Dotted horizontal line denotes vehicle control (no GenX) levels. error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Significance is as compared to control unless otherwise specified: **; ***.
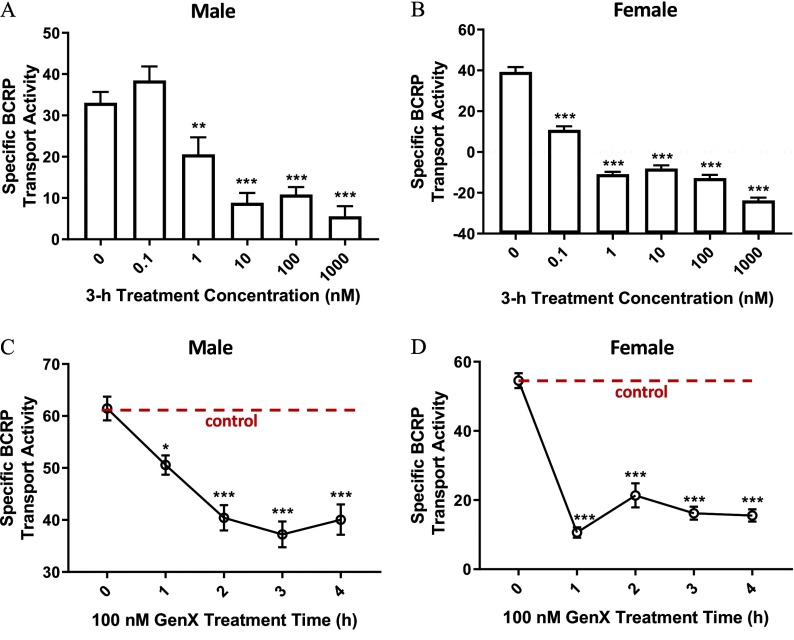
Next, we examined the effect of GenX on BCRP transport activity. We measured BCRP transport activity after treating male and female brain capillaries with GenX () for 3 h (Figure 2A,B). BCRP transport activity in males was significantly lower in samples treated with GenX. In contrast, female BCRP transport activity was significantly lower in capillaries treated with GenX. We also examined the hourly changes in BCRP transport in capillaries following exposure to GenX for 1–4 h (Figure 2C,D). Both male and female BCRP transport activity was significantly lower at 1 h. Of note, the reduction in BCRP transport activity in females was more significant than males at the 1-h time point; furthermore, GenX-dependent transport inhibition persisted throughout the 3-h assay time for both sexes.
Figure 2.
Changes in breast cancer resistance protein (BCRP) transport activity in brain capillaries from six rats treated with ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX). (A) Male and (B) female are graphs of BCRP transport activity at increasing doses of GenX in brain capillaries of Hsd:Sprague-Dawley® (SD®) rats. (C) Male and (D) female are graphs of the GenX-dependent reductions in BCRP transport activity over time. Dotted horizontal line denotes vehicle control (no GenX) levels. error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Significance is as compared to control unless otherwise specified: *; **; ***.
Lastly, we determined the GenX effect on MRP2 transport in identically designed experiments. We observed no GenX effect with regard to dose and time on MRP2 transport activity (Figure S2A–D).
Reversibility Assays for P-gp and BCRP Transport
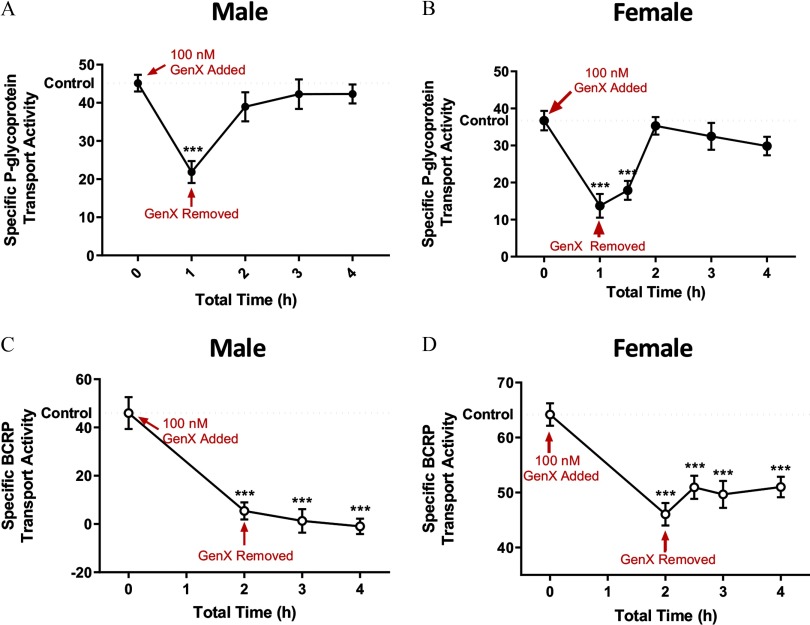
Previous transport studies from our laboratory have shown that chemical perturbation of signal transduction pathways can lead to rapid changes in transport activity independently of protein degradation or expression (Banks et al. 2018; Cannon et al. 2012). In general, these chemical-induced changes in transport activity revert to control levels upon chemical removal. Knowing this, we performed a reversibility assay by exposing male and female rat brain capillaries to GenX (). Shown in Figure 3, P-gp transport activity in males (Figure 3A) and females (Figure 3B) rapidly reverted to control levels within 1 h after GenX removal. In contrast to P-gp, GenX-mediated decreases in BCRP transport did not revert for either sex (Figure 3C,D).
Figure 3.
Transport reversibility following ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX) removal. (A) Male and (B) female are graphs denoting P-glycoprotein (P-gp) transport activities, and (C) male and (D) female are graphs denoting breast cancer resistance protein (BCRP) transport activities in brain capillaries from six rats before and after GenX () removal. error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Significance is as compared to control unless otherwise specified: ***.
The Effect of GenX on P-gp and BCRP ATPase Activity in Vitro
To determine if GenX directly inhibited ATP hydrolysis or was a P-gp or BCRP substrate in vitro, we used a reconstituted transport assay system containing vesical membranes and purified P-gp or BCRP transport proteins. Using this assay, we compared the control basal levels of substrate-stimulated P-pg or BCRP ATPase activities with and without GenX at 0.001, 0.01, 0.1, and (Figure 4A,B). GenX at 0.001, 0.01, 0.1, and did not affect the levels of ATPase activity associated with P-gp or BCRP transport when the substrate was stimulated. Negative controls show the relative ATPase levels were fully inhibited by the ATPase pan-inhibitor, vanadate (). Next, we determined if GenX could stimulate ATP hydrolysis by measuring the effect of GenX on each transporter without a substrate (Figure 4A,B). We saw no significant differences in ATPase hydrolysis for P-gp or BCRP, indicating that GenX was not a substrate for either transporter in this system.
Figure 4.
![Figures 4A and 4B titled P-gp ATPase Activity and BCRP ATPase Activity, respectively, are bar graphs plotting ATPase activity ranging from 0.0 to 0.4 in increments of 0.1 and from 0.0 to 1.0 (y-axis), respectively, for SS Control, Vanadate, No substrate (NS), NS plus GenX times 0.1 micromolar, GenX times 0.001 micromolar, GenX times 0.01 micromolar, GenX times 0.1 micromolar, and GenX times 1.0 micromolar (x-axis). There is an arrow pointing right labelled as Increasing [GenX], above the bar graphs.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ab50/7137913/b0f9b2829e8f/ehp-128-037002-g004.jpg)
Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX) effects on transport-associated ATPase activity in vitro. (A) Graph denotes rat P-glycoprotein (P-gp) ATPase activity, and (B) graph denotes rat breast cancer resistance protein (BCRP) ATPase activity. All assays were performed in triplicate. error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Note: NS, no substrate added; SS control, substrate-stimulated vehicle control; vanadate (), ATPase inhibitor. Significance is as compared to control unless otherwise specified: ***.
P-gp and BCRP Protein Levels following GenX Exposure
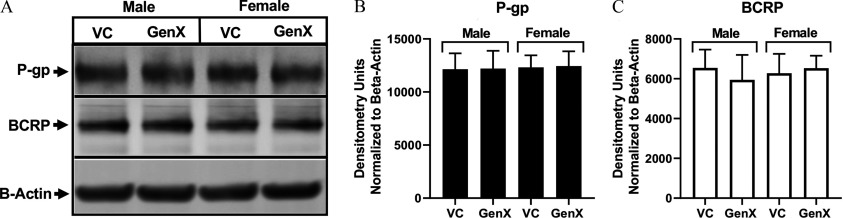
To determine if the GenX-mediated decreases in P-gp and BCRP transport activity were associated with decreases in transporter protein levels, we treated male and female rat brain capillaries for 4 h with GenX and measured P-gp and BCRP protein levels by Western blotting. Pictured in Figure 5A are representative Western blots showing the P-gp and BCRP protein levels in GenX-treated compared to nontreated male and female rat capillaries. We detected no significant differences in P-gp or BCRP protein levels in GenX () or VC-treated capillaries from three independent blots (Figure 5B,C).
Figure 5.
P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) protein levels after ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX) treatment. Representative Western blotting (left, Panel A) determined P-gp and BCRP protein levels in GenX- and vehicle control–treated Hsd:Sprague-Dawley® (SD®) rats () brain capillary membrane lysates. Mean values are from three independent experiments. P-gp, Panel B and BCRP, Panel C levels in Western blotting (right) were determined by protein densitometry (ImageJ software) normalized to actin levels to remove sample loading variabilities. error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Significance is as compared to controls.
Inhibition of in GenX-Treated Rat Brain Capillaries
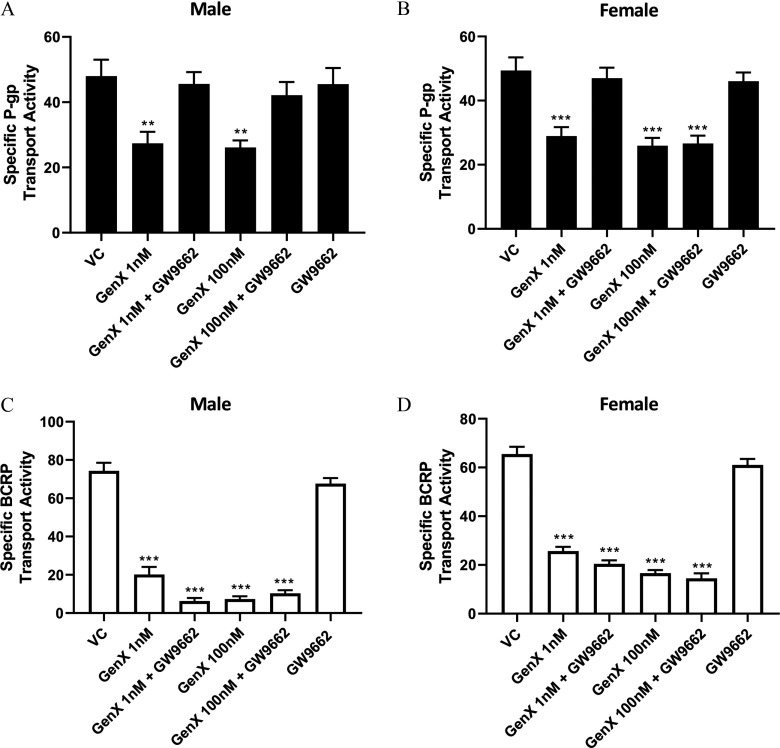
We investigated the role of in the GenX-dependent reductions of P-gp and BCRP transport. To accomplish this, isolated capillaries from male and female rats were cotreated for 4 h with GenX ( and ) with and without the inhibitor GW9662 (). Following treatment, P-gp and BCRP transport activities were determined. Shown in Figure 6A, P-gp transport in males was significantly reduced by GenX exposures of and . However, cotreatments with GW9662 () and GenX (1 and ) blocked the reductions in P-gp transport activity in females (Figure 6B), In females, GenX treatments of and also lowered P-gp transport activity relative to controls, but in contrast to males, the antagonist, GW9662, did not block the GenX decreases in P-gp transport. Capillaries cotreated with GenX and GW9662 () remained significantly lower than their vehicle-treated controls.
Figure 6.
Inhibition of peroxisome proliferator–activated receptor gamma () in the ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX)–treated brain capillaries from six rats. P-glycoprotein (P-gp) transport activity in (A) male and (B) female rat brain capillaries treated with 1 and GenX with or without the peroxisome inhibitor GW9662 (). Breast cancer resistance protein (BCRP) transport activity in (C) male and (D) females rat brain capillaries treated with 1 and GenX with or without the inhibitor GW9662 (). error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Significance was determined by comparing treatment to its respective vehicle control: **; ***.
In similarly designed experiments, we investigated the involvement of on GenX reductions of BCRP transport. Isolated capillaries from male and female rats were cotreated for 4 h with and GenX with or without the inhibitor GW9662 (). Following treatments, BCRP transport activities were measured. Shown in Figure 6C,D, BCRP transport activity in male and female capillaries treated with and GenX were significantly lower than their vehicle-treated controls. In contrast to our findings with P-gp, cotreating with GenX and GW9662 () had no effect on the GenX-mediated reductions in BCRP transport activities in either sex.
In Vivo Exposures to GenX by Oral Gavage Dosing in Sprague-Dawley Rats
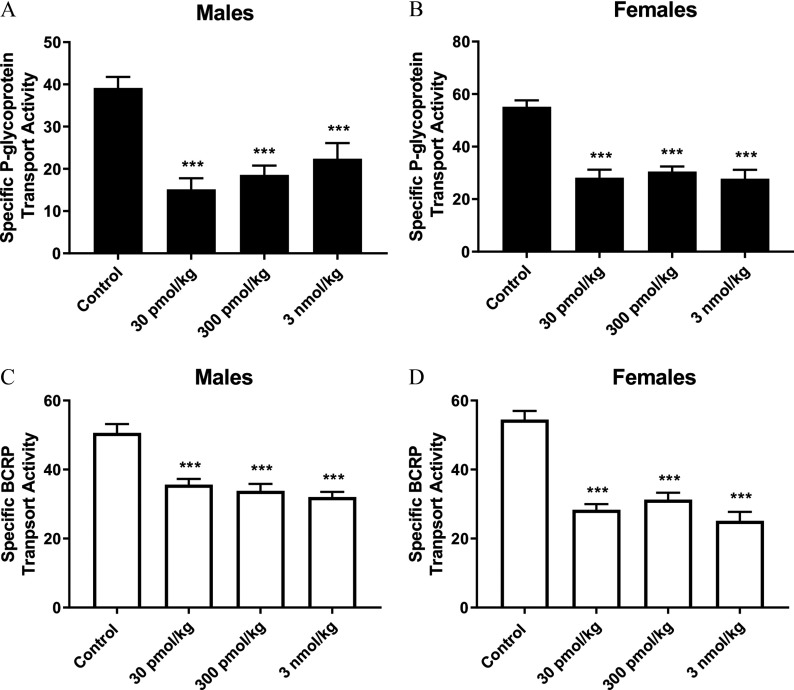
To validate our ex vivo experiments, we dosed male and female SD rats with GenX at , , and by oral gavage and measured P-gp and BCRP transport in isolated rat brain capillaries activity 5 h later. Shown in Figure 7A–D, all GenX dose groups produced significant decreases in P-gp and BCRP transport activity in both male (Figure 7A,C) and female (Figure 7B,D) rat brain capillaries.
Figure 7.
Dosing ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX) in vivo by oral gavage. Ex vivo determination of P-glycoprotein (P-gp) transport activity in (A) males and (B) females and breast cancer resistance protein (BCRP) transport activity in (C) males and (D) females following in vivo dosing of rats with GenX. error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Significance is as compared to control: ***. Each dose group contained capillaries isolated from five rats.
Cytotoxicity of GenX in Human Cells
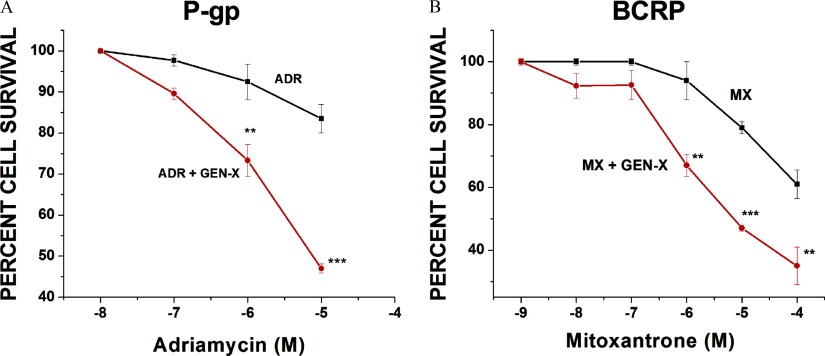
To determine if the GenX effects we observed in rats occurred in human cells, we performed cell growth inhibition assays on two human-derived cell lines (NCI/ADR-RES and MX-MCF-7). NCI/ADR-RES are human-derived ovarian cells that express higher levels of P-gp relative to BCRP. MX-MCF7 are human-derived mammary epithelial cells that express higher levels of BCRP relative to P-gp. To assess the toxicity of GenX, each line was grown in media with increasing concentrations of GenX (), and percent cell survival was determined. We saw no change in percent cell survival for either line grown in the presence of GenX (Figure S3). Having established that GenX was not toxic to either cell line, we grew each for 72 h in complete media with or without GenX and a cytotoxic substrate. The P-gp substrate Adriamycin () was used for the NCI/ADR-RES cell line, and the BCRP substrate mitoxantrone () was used with the MX-MCF-7 cell line. We reasoned if GenX inhibited P-gp or BCRP transport, it would increase substrate toxicity and significantly reduce cell survival. Shown in Figure 8A, cotreatments with GenX and Adriamycin reduced NCI/ADR-RES cell survival from 85% (no GenX) to 45% (with GenX). Similarly, cotreating MX-MCF-7 human cells with mitoxantrone and GenX significantly reduced cell survival from 63% (no GenX) vs. 37% (with GenX).
Figure 8.
Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX) toxicity in vitro. (A) Graph representing the percent of NCI/ADR-RES cells surviving 72-h Adriamycin () exposures with (red line) or without (black line) GenX (). (B) Graph representing the percent of MX-MCF-7 cells surviving 72-h mitoxantrone () exposures with (red line) or without (black line) GenX (). Values represent three separate experiments carried out in triplicate. error (SE) is shown. SE and significance were determined by one-way analysis of variance (ANOVA) and Tukey multiple comparison. Significance is as compared to control: **; ***.
Discussion
In the watershed of the Cape River in North Carolina, GenX is a contaminant in finished drinking water and has the capacity to alter important biological signaling pathways (Hopkins et al. 2018; Sun et al. 2016). In this report, we investigated the effects of GenX on the expression and activity of three important ABC efflux transporters (P-gp, BCRP, and MRP2) of the BBB. BBB function relies on the concerted action of endothelial cell tight junctions and luminal efflux transporters to limit the paracellular and transcellular passage of harmful xenobiotics and endogenous metabolites into the brain (Daneman 2012). We showed that exposing rat brain capillaries ex vivo to low nanomolar concentrations of GenX rapidly reduced the transport activity of P-gp and BCRP but not MRP2. The lack of change in MRP2 transport activity indicates that GenX affects the ABC transporters of the BBB selectively. Furthermore, it shows that the GenX treatments are not causing capillary leakage. If GenX caused capillary leakage, luminal fluorescence would decrease and measurable transport activity for MRP2 would be reduced.
To complement our ex vivo GenX treatments in isolated capillaries, we dosed rats with GenX by oral gavage at , , or and measured their P-gp and BCRP transport activity ex vivo. We found that in vivo exposure to GenX also lowered P-gp and BCRP transport levels at all three dose groups in both male and female rats.
Our reversibility experiments using rat brain capillaries indicated that GenX inhibition of P-gp and BCRP transport is mechanistically different. P-gp transport levels returned to control levels after GenX removal in both sexes. However, BCRP transport did not return to control levels for either sex after GenX removal. We also noted significant differences between P-gp and BCRP in our inhibitor studies. For both sexes, the antagonist, GW9662, blocked the GenX reductive effect on P-gp transport. Interestingly, GW9662 blocked the GenX-dependent reductions of P-gp transport in males but not in females. This may result from differences in reported sex-specific expression levels of (Bagal and Bungay 2014). More work is needed to fully understand this difference. Lastly, we observed no effect of GW9662 on GenX-dependent reductions of BCRP transport. Our findings suggest that nanomolar levels of GenX inhibit P-gp and BCRP transport by different mechanisms and implicates the requirement for in the reductive effect of GenX on P-gp transport.
Data from the in vitro purified ATPase assay show that GenX at concentrations between 1 and did not alter the levels of ATPase activity associated with P-gp or BCRP transport. They also suggest that GenX did not inhibit transport through direct contact with the P-gp or BCRP transporter proteins; therefore, we conclude that GenX is not a substrate inhibitor or an ATPase inhibitor for either transporter.
Our Western blotting data indicate that protein turnover/degradation was not a contributing factor to the GenX-dependent decreases in P-gp or BCRP transport. Quantitation from three independent protein blots showed that 4-h treatment of GenX did not significantly alter P-gp or BCRP protein levels. The absence of changes in P-gp or BCRP protein levels by GenX treatments lends support to the idea that GenX affects transport activity and not protein expression. These experiments cannot eliminate the possibility that protein subcellular localization is contributing to GenX inhibition of P-gp or BCRP transport.
Although this work is largely focused on the transporters of the BBB, P-gp and BCRP are expressed in many human tissues, where they contribute to barrier functions of the body, e.g., testis, retina, and placenta (Liu 2019). Independent of the biological barriers, P-gp and BCRP function in the liver and kidney to remove drugs, harmful toxicants, and metabolites from the body (Leslie et al. 2005). Clinically, they are important in the absorption and distribution of prescription drugs. In addition, their activity contributes to chemoresistance in cancer therapies (Gottesman and Ling 2006; Qosa et al. 2015). They also serve to protect the brain and limit neurotoxicity during prolonged cancer chemotherapies, thus reducing the risk of therapy-related cognitive dysfunctions (Leslie et al. 2005; Miller 2015). To extend the relevance to humans and increase the importance of our findings in rats, we showed that GenX alone was not toxic to two human cell lines; however, when co-dosed with cytotoxic substrates for P-gp or BCRP, respectively, substrate toxicity increased. These data suggest that GenX can inhibit P-gp and BCRP transport in human cells.
Presently, there are no blood or urine concentration data available in the U.S. population, even though humans were likely exposed to GenX in finished drinking water. In 2017, finished drinking water from eastern North Carolina wastewater treatment plants (Pender County and Sweeney, North Carolina) contained concentrations of GenX at (). Additional human exposures to GenX occur through contaminated air and food. The current provisional health goal for GenX in North Carolina is or (Hopkins et al. 2018), which is four times higher than our lowest effect level in male rats. Based on an acute and chronic aquatic toxicity study, GenX was not toxic and did not bioaccumulate in any of the species tested (Daphnia, trout, carp, and algae) (Hoke et al. 2016). However, GenX is persistent in the environment, and this study and others show it interacts with signaling molecules to produce important biological changes (Conley et al. 2019; Li et al. 2019).
In summary, our findings in rats suggest that low nanomolar levels of GenX can affect BBB function by inhibiting two important transporters: P-gp and BCRP. It also implicates the involvement of for the effect of GenX on P-gp. This work is significant because inhibition of P-gp and BCRP transport function at the BBB can increase the risk of brain exposure to toxic xenobiotic agents (Roulet et al. 2003). Additionally, inhibition of P-gp and BCRP in the intestine, liver, and kidney can lead to significant changes in the absorption and elimination of harmful xenobiotics, drugs, and metabolites.
Supplementary Material
Acknowledgments
The authors would like to thank the Fluorescence Microscopy and Imaging Core at the NIEHS for technical assistance. This research was supported by the Intramural Research Program of NIH/NCI (Project ZIA BC 011476).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5884).
These authors contributed equally to this work.
Current address: Alicia C. Richards, SRC, Arlington, VA, USA.
Current address: Andrew W. Trexler, Campbell University School of Osteopathic Medicine, Lillington, NC, USA.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Bagal S, Bungay P. 2014. Restricting CNS penetration of drugs to minimise adverse events: role of drug transporters. Drug Discov Today Technol 12:e79–e85, PMID: 25027378, 10.1016/j.ddtec.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Banks DB, Chan GN, Evans RA, Miller DS, Cannon RE. 2018. Lysophosphatidic acid and amitriptyline signal through LPA1R to reduce P-glycoprotein transport at the blood-brain barrier. J Cereb Blood Flow Metab 38(5):857–868, PMID: 28447863, 10.1177/0271678X17705786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, Zweers P, Muller A, de Vries W, Janssen P, Zeilmaker M. 2016. Evaluation of Substances Used in the GenX Technology by Chemours, Dordrecht. RIVM Letter Report 2016–0174. Bilthoven, Netherlands: National Institute for Public Health and the Environment Ministry of Health, Welfare and Sport. [Google Scholar]
- Bockor L, Bortolussi G, Vodret S, Iaconcig A, Jašprová J, Zelenka J, et al. 2017. Modulation of bilirubin neurotoxicity by the Abcb1 transporter in the Ugt1-/- lethal mouse model of neonatal hyperbilirubinemia. Hum Mol Genet 26(1):145–157, PMID: 28025333, 10.1093/hmg/ddw375. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248– 254, PMID: 942051, 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. 2012. Targeting blood-brain barrier sphingolipid signaling reduces basal p-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA 109(39):15930–15935, PMID: 22949658, 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GNY, Cannon RE. 2017. Assessment of ex vivo transport function in isolated rodent brain capillaries. Curr Protoc Pharmacol 76:7.16.1–7.16.16, PMID: 28306152, 10.1002/cpph.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clabby C. 2018. GenX questions continue: what about food? Coastal Review Online, News and Features section. 2 May 2018. https://www.coastalreview.org/2018/02/genx-questions-continue-food/ [accessed 18 March 2020].
- Conley JM, Lambright CS, Evans N, Strynar MJ, McCord J, McIntyre BS, et al. 2019. Adverse maternal, fetal, and postnatal effects of hexafluoropropylene oxide dimer acid (GenX) from oral gestational exposure in Sprague-Dawley rats. Environ Health Perspect 127(3):37008, PMID: 30920876, 10.1289/EHP4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R. 2012. The blood-brain barrier in health and disease. Ann Neurol 72(5):648– 672, PMID: 23280789, 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Ling V. 2006. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett 580(4):998–1009, PMID: 16405967, 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Heydebreck F, Tang J, Xie Z, Ebinghaus R. 2015. Alternative and legacy perfluoroalkyl substances: differences between European and Chinese river/estuary systems. Environ Sci Technol 49(14):8386–8395, PMID: 26106903, 10.1021/acs.est.5b01648. [DOI] [PubMed] [Google Scholar]
- Hoke RA, Ferrell BD, Sloman TL, Buck RC, Buxton LW. 2016. Aquatic hazard, bioaccumulation and screening risk assessment for ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate. Chemosphere 149:336–342, PMID: 26874062, 10.1016/j.chemosphere.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Hopkins ZR, Sun M, Dewitt JC, Knappe DRU. 2018. Recently detected drinking water contaminants: GenX and other per-and polyfluoroalkyl ether acids. J Am Water Works Assoc 110(7):13–28, 10.1002/awwa.1073. [DOI] [Google Scholar]
- Kim RB. 2002. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev 34(1–2):47–54, PMID: 11996011, 10.1081/dmr-120001389. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. 2005. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204(3):216–237, PMID: 15845415, 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Li CH, Ren XM, Guo LH. 2019. Adipogenic activity of oligomeric hexafluoropropylene oxide (perfluorooctanoic acid alternative) through peroxisome proliferator-activated receptor gamma pathway. Environ Sci Technol 53(6):3287–3295, PMID: 30785727, 10.1021/acs.est.8b06978. [DOI] [PubMed] [Google Scholar]
- Liu X. 2019. ABC family transporters. Adv Exp Med Biol 1141:13–100, PMID: 31571164, 10.1007/978-981-13-7647-4_2. [DOI] [PubMed] [Google Scholar]
- Locher KP. 2016. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol 23(6):487–493, PMID: 27273632, 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- Miller DS, Cannon RE. 2014. Signaling pathways that regulate basal ABC transporter activity at the blood-brain barrier. Curr Pharm Des 20(10):1463–1471, PMID: 23789954, 10.2174/13816128113199990457. [DOI] [PubMed] [Google Scholar]
- Miller DS. 2015. Regulation of ABC transporters blood-brain barrier: the good, the bad, and the ugly. Adv Cancer Res 125:43–70, PMID: 25640266, 10.1016/bs.acr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- More VR, Campos CR, Evans RA, Oliver KD, Chan GN, Miller DS, et al. 2017. PPAR-α, a lipid-sensing transcription factor, regulates blood–brain barrier efflux transporter expression. J Cereb Blood Flow Metab 37(4):1199–1212, PMID: 27193034, 10.1177/0271678X16650216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Takano M. 2008. Intestinal efflux transporters and drug absorption. Expert Opin Drug Metab Toxicol 4(7):923–939, PMID: 18624680, 10.1517/17425255.4.7.923. [DOI] [PubMed] [Google Scholar]
- Nagy I, Toth B, Gáborik Z, Erdo F, Krajcsi P. 2016. Membrane transporters in physiological barriers of pharmacological importance. Curr Pharm Des 22(35):5347–5372, PMID: 27464727, 10.2174/1381612822666160726101748. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Schneider E, Dixon KH, Horton J, Kelley K, Morrow C, et al. 1992. Reduced intracellular drug accumulation in the absence of P-glycoprotein (mdr1) overexpression in mitoxantrone-resistant human MCF-7 breast cancer cells. Cancer Res 52(22):6175–6181, PMID: 1358431. [PubMed] [Google Scholar]
- Pritchett JR, Rinsky JL, Dittman B, Christensen A, Langley R, Moore Z, et al. 2019. Notes from the field: targeted biomonitoring for GenX and other per- and polyfluoroalkyl substances following detection of drinking water contamination–North Carolina, 2018. MMWR Morb Mortal Wkly Rep 68(29):647–648, PMID: 31344024, 10.15585/mmwr.mm6829a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Miller DS, Pasinelli P, Trotti D. 2015. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res 1628(Pt B):298–316, PMID: 26187753, 10.1016/j.brainres.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. 2018. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18(7):452–464, PMID: 29643473, 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulet A, Puel O, Gesta S, Lepage J-F, Drag M, Soll M, et al. 2003. MDR1-deficient genotype in Collie dogs hypersensitive to the P-glycoprotein substrate ivermectin. Eur J Pharmacol 460(2–3):85–91, PMID: 12559367, 10.1016/s0014-2999(02)02955-2. [DOI] [PubMed] [Google Scholar]
- Schramm U, Fricker G, Wenger R, Miller DS. 1995. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol 268(1 Pt 2):F46–F52, PMID: 7840247, 10.1152/ajprenal.1995.268.1.F46. [DOI] [PubMed] [Google Scholar]
- Scudiero DA, Monks A, Sausville EA. 1998. Cell line designation change: multidrug-resistant cell line in the NCI anticancer screen. J Natl Cancer Inst 90(11):862, PMID: 9625176, 10.1093/jnci/90.11.862. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River Watershed of North Carolina. Environ Sci Technol Lett 3:415–419, 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int 60:242–248, PMID: 24660230, 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ren XM, Wan B, Guo LH. 2014. Structure-dependent binding and activation of perfluorinated compounds on human peroxisome proliferator-activated receptor gamma. Toxicol Appl Pharmacol 279(3):275–283, PMID: 24998974, 10.1016/j.taap.2014.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.