Abstract
Heterologous immunity (H.I.) is a consequence of an encounter with a specific antigen, which can alter the subsequent immune response to a different antigen. This can happen at the innate immune system level—often called trained immunity or innate immune memory—and/or at the adaptive immune system level involving T memory cells and antibodies. Viruses may also induce T cell-mediated H.I., which can confer protection or drive immunopathology against other virus subtypes, related or unrelated viruses, other pathogens, auto- or allo-antigens. It is important to understand the underlying mechanisms for the development of antiviral “universal” vaccines and broader T cell responses rather than just subtype-specific antibody responses as in the case of influenza. Furthermore, knowledge about determinants of vaccine-mediated H.I. may inform public health policies and provide suggestions for repurposing existing vaccines. Here, we introduce H.I. and provide an overview of evidence on virus- and antiviral vaccine-induced T cell-mediated cross-reactive responses. We also discuss the factors influencing final clinical outcome of virus-mediated H.I. as well as non-specific beneficial effects of live attenuated antiviral vaccines such as measles and vaccinia. Available epidemiological and mechanistic data have implications both for the development of new vaccines and for personalized vaccinology, which are presented. Finally, we formulate future research priorities and opportunities.
Keywords: cross-protection, immune memory, molecular mimicry, TCR repertoire, T cell epitope, virus-induced immunity, immunopathology, immunomodulation
Introduction
Heterologous immunity (H.I.) arises from previous infections, which alter the immune response to a subsequent infection with a different pathogen (1). This mechanism is more likely to occur between closely related antigens, but may also occur among unrelated antigens, including bacteria, viruses, protozoa, and parasites. H.I. may alter the outcome of infections by providing sufficient immune protection or, in other cases, aggravating immunopathology (2).
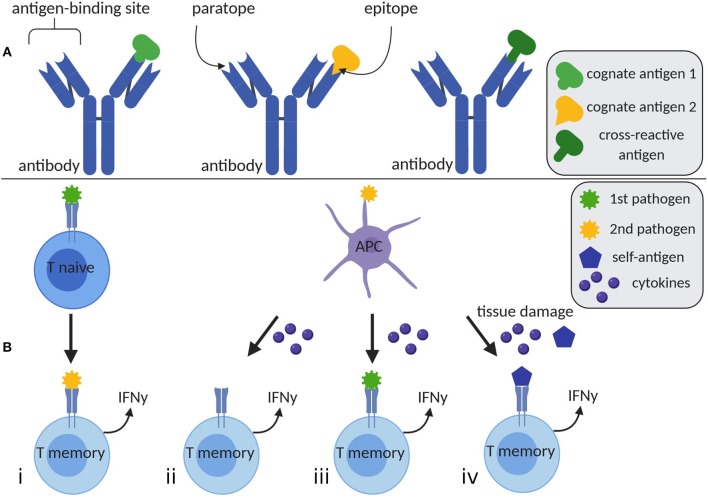
H.I. is mediated by T memory cells or antibodies (Figure 1). Immunoglobulins recognize antigens when antigenic epitopes attach to paratopes (Table 1) at the antigen-binding site. Antibodies are potentially polyspecific, capable of binding different epitopes to various antigens. Furthermore, epitopes sharing similar sequences may bind to the same paratope, providing cross-protection (4). In the context of molecular mimicry, antibodies may also react to self-antigens, eliciting autoreactive immunopathology (5).
Figure 1.
Humoral and cellular mediated heterologous immunity. (A) A single antibody has the ability to bind distinct antigens 1 and 2 by different paratopes at the antigen-binding site. Furthermore, it is able to detect a cross-reactive antigen, whose epitope is similar to the one of antigen 1. (B) (i) T memory cells may be activated by an unrelated second pathogen, which is cross-reactive with the first encountered pathogen. (ii) The appearance of a second pathogen may elevate cytokine levels, which potentially lead to TCR-independent T cell activation. (iii) Simultaneous presence of cytokines and remaining antigens of previously encountered pathogens may stimulate T cells. (iv) High levels of cytokines and tissue damage due to inflammation or chronic diseases result in increased concentrations of self-antigens, which may be engaged by T cells. Created with BioRender.com, adapted from Welsh et al. (3). APC, antigen presenting cell; IFNγ, interferon γ.
Table 1.
Glossary.
| Heterosubtypic | Referring to different serotypes of influenza A virus, which are defined based on the surface proteins hemagglutinin (HA) and neuraminidase (NA). |
| HLA molecule | The human leucocyte antigen is located on cell surfaces and may present antigenic peptides to T cells. |
| Immunodominance | Only a few (immunodominant) epitopes are preferentially targeted by the immune response. The remaining epitopes evoke barely detectable T cell responses. |
| Molecular mimicry | An alignment of pathogenic structures with those of the host, which leads to immune evasion. However, structure similarity of pathogens and self-antigens may elicit autoreactive immune responses. |
| Paratope | A segment of an antibody's antigen-binding site, which complementarily binds an epitope. |
| Private specificity of TCR repertoire | TCR repertoires, which are different among individuals. |
| TCR repertoire | All T cell receptor clonotypes expressed by an organism. |
Likewise, cellular-mediated H.I. plays a role in immunomodulation. This may be elicited via T cell receptor (TCR) cross-reactivity (one possible mechanism of H.I.), recognizing similar but distinct antigens or even autoantigens. T cells may also be activated non-specifically by cytokines [reviewed in (3)]. Cross-reactive antigens elicit an expansion of T memory cells, leading to a modified T cell memory pool, a change in patterns of immunodominance, and an altered hierarchy of T cell responses (6). This process heavily depends on the individual private specificities of TCR repertoires and ultimately results in a modified T cell response (7).
Trained immunity, also known as innate immune memory, is a recently described adaptation of innate immune cells following antigenic exposure. Epigenetic reprogramming leads to production of inflammatory mediators and a shift in cellular metabolism, providing an enhanced response to secondary stimulation [reviewed in (8)]. Thus, physiological processes such as mucosal tolerance, restriction of tissue damage, innate immunity maturation, and non-specific vaccine-mediated protection are achieved. Nevertheless, trained immunity can become maladaptive, causing immune paralysis or hyperinflammation [reviewed in (9)].
This review presents recent scientific findings regarding virus- or antiviral vaccine-induced T cell-mediated H.I. and thus provides some background for the discussion on benefits and risks of H.I. Implications for future research priorities for vaccine development are also considered.
Virus- and Antiviral Vaccine-Induced T Cell-Mediated Heterologous Immunity
Influenza Virus
Naïve T cells of donors who self-reported as having no influenza A Virus (IAV) H1N1/09 exposure or influenza symptoms can recognize unique strain-specific epitopes using tetramer staining, whereas the same donors' memory T cells recognize conserved epitopes of the surface protein hemagglutinin (HA) (10). In H1N1/09 infected or vaccinated donors, the frequency of naïve T cells recognizing unique epitopes was significantly higher compared to conserved epitope-specific T cells (10). This has also been shown for CD4+ (10) and CD8+ T cells in mice (11).
Such observations suggest that H.I. influences the severity of infection (10). An age-related dampening of T-cell mediated H.I. was observed following a second heterologous infection in ferrets, which allowed the development of significant morbidity (12). These findings are in agreement with other studies focusing on aged animals, which showed that the clinical severity of primary infection is only moderately accentuated (13–16), while heterologous secondary infection induced severe disease (12, 17, 18). The induction of influenza virus-specific memory T cells is extensively investigated as they are responsible for heterologous protection in secondary natural infections with another influenza strain [reviewed in (19)]. Tissue resident memory T cells (Trm) in the lung are particularly important in that respect as they are crucial for achieving optimal protection [reviewed in (19)]. Previous animal studies showed that a single intranasal live attenuated IAV vaccine application can evoke long-lasting protection to heterosubtypic challenge via Trm response in the lung with a similar phenotype to those of infected mice (20). Several in silico approaches are available to identify T cell immunogenic regions on virus proteins. It has been demonstrated that epitope-rich regions within the nucleoprotein (NP) of the influenza virus contain highly conserved epitopes and therefore present promising targets for a T cell-mediated vaccine due to cross-reactivity with distinct strains (21). Gutiérrez et al. developed a computational method to compare the efficacy of conserved T cell epitopes (EpiCC), which may complement current methods for selecting the best composition of an associated vaccine (22). Furthermore, CD8+ T cells recognizing different NP variants were associated with cross-reactive TCR clonotypes against distinct strains (23). This was shown for the immunodominant and abundant human epitopes NP338−346 and NP44−52 (23). A structural analysis of the associated HLA molecules revealed adoption of similar conformation as a basis for cross-recognition (23).
Spleen cells from IAV-infected animals showed enhanced IFNγ production after ex vivo stimulation with the hepatitis C virus (HCV) derived peptide NS31073 (24). Such findings suggest a private repertoire of pre-existing memory T cells, which are reactivated after HCV infection (25). Cross-reactivity was also demonstrated in human peripheral blood mononuclear cells (PBMCs) of HCV positive patients with severe disease which responded to the IAV-specific peptide NA231−239 (25). Additionally, PBMCs of hepatitis B virus patients were incubated with Epstein-Barr virus EBV-BMLF1280−288 and IAV-M158−66 labeled tetramers and subsequently stained for TCR clones (26). The TCR repertoire of cross-reactive T cells recognizing IAV and EBV epitopes was broader compared to non-cross-reactive T cells and varied among individuals, further supporting an underlying private specificity (26). The concept of H.I. has recently been expanded to include allergens, following demonstration of IAV-mediated protection against allergen-induced experimental asthma (mediated by memory T cells) in a murine model (27).
Flaviviruses
The high degree of genetic sequence similarity among flaviviruses is known either to have a protective effect or to dampen the elicited secondary immune response [reviewed in (28)]. For Dengue virus (DENV), it is well-known that an infection with one serotype induces strong and long-lasting protective immunity against that specific serotype, whereas a second infection with a heterotypic virus commonly results in severe disease [reviewed in (29)]. Sub-neutralizing antibody concentrations from the first infection facilitate virus entry by promoting Fcγ-receptor uptake, resulting in antibody-dependent enhancement (ADE) of the infection. However, there is increasing evidence of a cross-protective cellular immune response between DENV and Zika virus (ZIKV) [reviewed in (29)]. Memory T cells isolated from DENV seropositive patients recognize both DENV- and ZIKV-associated peptides (30). Furthermore, DENV positive patients responded more strongly to a ZIKV infection compared to DENV negative subjects when assessed using T cell stimulation assays (30, 31). Mouse experiments have also shown, that DENV-exposed pregnant animals were protected against subsequent maternal and fetal ZIKV infection (32). This protection was conferred by CD8+ T cells, limiting trans-placental transmission of ZIKV (32). Although cross-reactivity between DENV and ZIKV is the most prominent example, other flaviviruses, such as yellow fever virus (YFV) and Japanese encephalitis virus, also prime T cell responses toward a subsequent heterologous DENV infection in mice (33). In this context, the investigators identified homologous sequences between the flavivirus polyproteins. Peptides derived from the aforementioned sequences were used to prime antigen presenting cells, which were subsequently used to stimulate splenocytes of DENV immunized mice. Some of these peptides induced enrichment of T memory cells as well as IFNγ production and proliferation, confirming cross-reactivity (33).
Human Immunodeficiency Virus
Human immunodeficiency virus 1 (HIV-1)-specific CD8+ T cell clones showed cross-reactivity against some of the other investigated HIV-1 epitopes (34). Additionally, three HIV-1-specific T cell clones recognized the A*02 restricted IAV matrix epitope GILGFVFTL (34). Furthermore, a sequence similarity between the known HIV-1 epitope HIV-Gag [SLYNTVATL [HIV-SL9]] and the HCV epitope HCV-NS5b [ALYDVVSKL [HCV-AL9]] has been observed. HIV-SL9 specific T cells of HIV-1 patients, who were not co-infected with HCV, recognized the aforementioned HCV epitope and responded with IFNγ production and expansion (35).
Hepatitis C Virus
Cross-genotype protective immunity against HCV was first described in 2003 by Lanford et al. who showed that chimpanzees, which recovered from a genotype 1 infection, were subsequently protected from infection with other genotypes (including genotype 4 and combinations of genotypes 1–4). These genotypes express proteins of up to 30% amino acid variance (36). This finding, however, has been challenged by other investigators who showed that chimpanzees developed chronic disease after being re-challenged with other genotypes (37).
CD8+ T cell cross-reactivity to NS3 epitopes of two different genotypes (1 and 3) was observed in a study with 53 anti-HCV positive injection drug users. Interestingly, CD8+ T cells recognizing both genotypes were more frequent among HCV RNA negative patients than in those with detectable viremia, implying that CD8+ T cell-mediated cross-reactivity may protect against chronic infection (38).
In another study, an HLA-restricted epitope (HCV NS3-1406) and its naturally occurring variants from different genotypes showed that the frequency of cross-reactivity between variants as well as their T cell priming capacities varied, depending on the genotype pair (39). Fytili et al. performed a similar study for another dominant HLA-dependent HCV CD8+ T cell epitope (HCV NS3-1073), which was associated with clearance of acute infection, and detected cross-reactivity between the genotype 1 variant and variants of genotypes 4, 5, and 6 but not 2 and 3 (40). The level of cross-reactivity observed in this study could be predicted through in silico analyses of peptide-MHC complexes and TCR-interacting surfaces based on topology and electrostatic features (41).
The same dominant T cell epitope (HCV NS3-1073) was also found to induce immune response in approximately a third of >100 seronegative individuals upon ex vivo stimulation. The presence of CD8+ T cells specific for that epitope was attributed to cross-reactivity with epitopes derived from other pathogens. These cells not only reacted to different genotype variants of that epitope but also to epitopes with little sequence similarity of other, unrelated viruses (cytomegalovirus, IAV, EBV) (42). Immunization with a recombinant adenovirus vector containing mycobacteria, Ebola and HIV antigens also led to T cell responses against HCV alongside the transgenic antigens (43). Cross-reactivity between an HCV and a human herpes virus peptide has also previously been demonstrated (44).
Other Viruses
Severe hand, foot and mouth disease is caused among others by enterovirus 71. A dominant capsid T cell epitope, which is highly conserved among enteroviruses, was identified and found to yield a cross-reactive, HLA-DR restricted response of human CD4+ T cells to the poliovirus variant of this epitope (45). Human RV-specific CD4+ T cells were shown to recognize epitopes shared among different RV strains (46). Human circulating RV-specific CD4+ T cells recognized conserved RV capsid protein epitopes, and T cell-mediated cross-reactivity between different strains was demonstrated (47). Zhao et al. showed that airway CD4+ T memory cells specific for a dominant, conserved epitope (SARS-N353) protect against both SARS- and MERS-CoVs and also against bat CoV in HLA transgenic murine models (48). Hepatitis E virus (HEV)-specific CD4+ and CD8+ T cell responses against different peptide pools from HEV1 were detected in acute HEV3 patients. A similar response against HEV3- and HEV1-peptide pools was detected in one subjectwith HEV1 infection (49). Finally, H.I. between the arenaviruses lymphocytic choriomeningitis virus and Pichinde virus was demonstrated in murine models and found to be T cell epitope and MHC class dependent (50).
Discussion
Protection vs. Immunopathology
Overall, virus-induced H.I. appears to be an important determinant for the final outcome of infections and of a plethora of dysregulated immune responses such as in autoimmunity and allograft rejection. In this context, prior antigenic exposures may boost protective responses [e.g., (27)] or induce immunopathology depending on the balance between antigen load and efficiency of effector T cells, which in turn is influenced by a number of factors. For example, in the case of flaviviruses, it has recently become evident that distinct T cell populations, virus serotypes, sequence, and number of infections, and HLA background all shape the immunodominance pattern (29). Additionally, patterns of T cell cytokine response among patients with a secondary DENV infection were associated with severe (51, 52) or mild dengue (53, 54). Although heterotypic antigens were addressed only in one of these studies (52), such observations may indicate involvement of cross-reactive T cells in the clinical manifestation of DENV infections.
In addition to natural viral infections, antiviral vaccines may also drive T cell-mediated H.I. and have a major impact not only against the vaccine antigens but also on completely unrelated pathogens or other antigens. To date, epidemiological evidence supporting the role of live attenuated vaccines in T cell-mediated H.I. is associated with the measles (55–62), the vaccinia (63–66), and the oral polio vaccine (67–69). These vaccines reduced overall mortality and/or risk for asthma, malignancies, and unrelated infections. Furthermore, they induced changes in the numbers or proportions of T and B cells, which, depending on persistence of effects, may influence differentiation, proliferation or survival of associated cells. Non-specific effects of vaccines have often been found to be sex-specific and influenced by revaccination as well as maternal priming. In this regard, knowledge on the potential of specific T cell epitopes (for any given HLA background) to offer protection or cause pathology is crucial for vaccine design including elimination or inclusion of such peptides.
Implications for Vaccine Development
The ability to predict the magnitude and mechanism of T cell-mediated H.I. (Figure 1B) is crucial for specific vaccine design but also for decisions on public health and vaccination policies. Structural similarity between T cell epitopes seems to be important for eliciting cross-reactive responses. Nevertheless, seemingly distinct epitopes may also bind to the same TCR and induce H.I. This may be explained by the fact that sequence similarity is also dependent on the presence of biochemically similar amino acid substitutions (70). In the context of developing broadly cross-reactive vaccines against viruses with great antigenic heterogeneity, regions of highly conserved proteins among serotypes may elicit cross-reactive T memory cell responses. This approach along with large scale systematic monitoring of circulating strains, as in the case of influenza (in order to minimize mismatch with vaccine-contained strains) may increase vaccine effectiveness.
Besides their specific effect, it is now known that vaccines may also exert a non-specific influence on the immune system (71). For the diphtheria-tetanus-pertussis and measles vaccines, it was shown that the order of vaccination has an impact on overall morbidity and mortality (72). The concept that the most recently administered vaccine leaves a non-specific immunological imprint until subsequent immunization may guide changes in the recommended order of childhood vaccinations. Such changes could result in beneficial non-specific effects with minor changes of existing national vaccination schemes. Similarly, age at the time of (initial or booster) immunization with each existing vaccine may need to be reconsidered based on the accumulating knowledge on immunosenescence and effects of age on virus-induced H.I. Accordingly, time of vaccination has been linked to differences in T cell populations and strength and type of heterologous immune response (73, 74). Sex-specific differences in terms of protective non-specific effects of vaccines such as measles and vaccinia (64, 75–80) have also been described. Modification of vaccine composition (e.g., enrichment of particular proteins or epitopes) or conditions of administration (e.g., age, dose, number of immunizations) could potentially help us achieve the beneficial heterologous effects of vaccines without compromising their primary protective effects (vaccine specific). Indeed, adequate application of knowledge regarding vaccine-mediated H.I. brings us a step closer to precision medicine and personalized vaccinology. Administration of live attenuated vaccines to women as part of preconception health counseling is another measure, which could enhance protection of offspring in the first months of life.
The potential of virus- and antiviral vaccine-induced immunomodulation may also be exploited for novel applications such as preventing infections among elderly and immunocompromised populations or non-infectious inflammatory diseases. In this respect, the choice of a particular adjuvant or pharmacological modulator is also important since these may polarize T cell immune responses toward a specific cytokine output depending on the desired outcome, e.g., induction of T1 type of response for prevention of infection as well as allergies.
Future Research Priorities
The need for new vaccines with higher efficacy and broader and longer-lasting protection is driven by the moderate protection provided by current seasonal influenza vaccines against the included strains, zoonotic and pandemic influenza threats, and the challenge of complying with annual vaccinations. Several approaches are currently being investigated with varying results and distance from truly universal vaccines. The use of adjuvants, addition of neuraminidase, and inclusion of specific strains induce broader reactive immune responses albeit within the same virus subtype. Additionally, immunogenic influenza HA-stem constructs induce B cells which produce cross-protective antibodies, at least within a group of viruses. A particular promising approach for the development of truly universal influenza vaccines seems to be the induction of T cells reactive to internal viral proteins, primarily of Trm in the respiratory mucosa for timely control of viral replication. Such approaches could also prove useful for developing vaccines against other respiratory viruses such as rhinoviruses. Similarly, knowledge gained from current studies of T cell responses against DENV/ZIKV infections at several time points, and with different clinical presentations and history of infection may inform strategies for developing pan-flavivirus vaccines. Indeed, there is already evidence for cross-reactive immunogenic epitopes contained in these viruses.
Properties of virus-induced H.I. may be leveraged beyond infection protection. We have previously shown an influenza virus-mediated protection over development of experimental asthma in a murine model. The protection was conferred by CD4+ and CD8+ T memory cells, which were transferred from animals previously infected with influenza or immunized with cross-reactive influenza peptides to sensitized mice before challenge with an allergen. Given the global prevalence of allergies, peptide immunization strategies early in life could potentially induce protective cellular immune responses against viruses and allergen-induced asthma, and complement existing vaccination schedules. Importantly, directing non-specific beneficial effects of existing live attenuated viral vaccines against other inflammatory disorders including cardiovascular disease and cancer could be a quantum leap in the fight against non-communicable diseases (65, 81–84).
Further immunological and clinical studies are needed to decipher vaccine-induced H.I.-mediated mechanisms and impact on morbidity and mortality contributing to health promotion. Associated potentiators such as booster vaccinations and maternal priming need to be examined carefully in different socioeconomic settings and with a sex-differential analysis (85).
Author Contributions
CS and PN planned, structured, and edited the manuscript. PN searched the literature and integrated all contributions. All authors wrote distinct parts of the manuscript and critically read, reviewed, and approved the final version of the manuscript.
Conflict of Interest
For CS: Consultancy and research funding, Hycor Biomedical and Thermo Fisher Scientific; Consultancy, Bencard Allergie; Research Funding, Mead Johnson Nutrition (MJN). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group on Respiratory Viruses (ESGREV) for providing a platform for scientific discussion on the topic and Debbie Jordan for proofreading the manuscript.
Footnotes
Funding. CS is supported by Universities Giessen and Marburg Lung Center (UGMLC), the German Center for Lung Research (DZL), the Rhön-Klinikum (UKGM), and the Deutsche Forschungsgemeinschaft (DFG)-funded-SFB 1021 (C04), -KFO 309 (P10), and SK 317/1-1 (Project number 428518790).
References
- 1.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. (2002) 2:417–26. 10.1038/nri820 [DOI] [PubMed] [Google Scholar]
- 2.Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. (1998) 188:1705–15. 10.1084/jem.188.9.1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. (2010) 235:244–66. 10.1111/j.0105-2896.2010.00897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Regenmortel MH. Specificity, polyspecificity, and heterospecificity of antibody-antigen recognition. J Mol Recognit. (2014) 27:627–39. 10.1002/jmr.2394 [DOI] [PubMed] [Google Scholar]
- 5.Barnett LA, Fujinami RS. Molecular mimicry: a mechanism for autoimmune injury. FASEB J. (1992) 6:840–4. 10.1096/fasebj.6.3.1740233 [DOI] [PubMed] [Google Scholar]
- 6.Selin LK, Cornberg M, Brehm MA, Kim S-K, Calcagno C, Ghersi D, et al. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin Immunol. (2004) 16:335–47. 10.1016/j.smim.2004.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie S, Lin S-J, Kim S-K, Welsh RM, Selin LK. Pathological features of heterologous immunity are regulated by the private specificities of the immune repertoire. Am J Pathol. (2010) 176:2107–12. 10.2353/ajpath.2010.090656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netea MG, van der Meer JW. Trained immunity: an ancient way of remembering. Cell Host Microbe. (2017) 21:297–300. 10.1016/j.chom.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. (2016) 352:aaf1098. 10.1126/science.aaf1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, James E, Gates TJ, DeLong JH, LaFond RE, Malhotra U, et al. CD4+ T cells recognize unique and conserved 2009. H1N1 influenza hemagglutinin epitopes after natural infection and vaccination. Int Immunol. (2013) 25:447–57. 10.1093/intimm/dxt005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder T, Jørgensen LG, Knudsen L, Perko M. Haemodynamisk vurdering af det cerebrale kredsløb med transkranial doppler-ultralyd hos patienter med carotisstenose. Ugeskr Laeg. (1990) 152:2110–3. [PubMed] [Google Scholar]
- 12.Paquette SG, Huang SS, Banner D, Xu L, Leȯn A, Kelvin AA, et al. Impaired heterologous immunity in aged ferrets during sequential influenza a H1N1 infection. Virology. (2014) 464–5:177–83. 10.1016/j.virol.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J, Feng Y, Barnes P, Huang F-F, Idell S, Su D-M, et al. Deletion of FoxN1 in the thymic medullary epithelium reduces peripheral T cell responses to infection and mimics changes of aging. PLoS ONE. (2012) 7:e34681. 10.1371/journal.pone.0034681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josset L, Engelmann F, Haberthur K, Kelly S, Park B, Kawoaka Y, et al. Increased viral loads and exacerbated innate host responses in aged macaques infected with the 2009 pandemic H1N1 influenza A virus. J Virol. (2012) 86:11115–27. 10.1128/JVI.01571-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muto NA, Sunden Y, Hattori T, Fujikura D, Nakayama Y, Miyazaki T, et al. Pathological examination of lung tissues in influenza A virus-infected mice. Jpn J Infect Dis. (2012) 65:383–91. 10.7883/yoken.65.383 [DOI] [PubMed] [Google Scholar]
- 16.Pica N, Langlois RA, Krammer F, Margine I, Palese P. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge. J Virol. (2012) 86:10293–301. 10.1128/JVI.01131-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bender BS, Small PA. Heterotypic immune mice lose protection against influenza virus infection with senescence. J Infect Dis. (1993) 168:873–80. 10.1093/infdis/168.4.873 [DOI] [PubMed] [Google Scholar]
- 18.Decman V, Laidlaw BJ, Dimenna LJ, Abdulla S, Mozdzanowska K, Erikson J, et al. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol. (2010) 184:5151–9. 10.4049/jimmunol.0902063 [DOI] [PubMed] [Google Scholar]
- 19.Pizzolla A, Wakim LM. Memory T cell dynamics in the lung during influenza virus infection. J Immunol. (2019) 202:374–81. 10.4049/jimmunol.1800979 [DOI] [PubMed] [Google Scholar]
- 20.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. (2016) 1:e85832. 10.1172/jci.insight.85832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant E, Wu C, Chan K-F, Eckle S, Bharadwaj M, Zou QM, et al. Nucleoprotein of influenza A virus is a major target of immunodominant CD8+ T-cell responses. Immunol Cell Biol. (2013) 91:184–94. 10.1038/icb.2012.78 [DOI] [PubMed] [Google Scholar]
- 22.Gutiérrez AH, Rapp-Gabrielson VJ, Terry FE, Loving CL, Moise L, Martin WD, et al. T-cell epitope content comparison (EpiCC) of swine H1 influenza A virus hemagglutinin. Influenza Other Respir Viruses. (2017) 11:531–42. 10.1111/irv.12513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant EJ, Josephs TM, Loh L, Clemens EB, Sant S, Bharadwaj M, et al. Broad CD8+ T cell cross-recognition of distinct influenza A strains in humans. Nat Commun. (2018) 9:5427. 10.1038/s41467-018-07815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J Virol. (2001) 75:11392–400. 10.1128/JVI.75.23.11392-11400.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbani S, Amadei B, Fisicaro P, Pilli M, Missale G, Bertoletti A, et al. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med. (2005) 201:675–80. 10.1084/jem.20041058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clute SC, Naumov YN, Watkin LB, Aslan N, Sullivan JL, Thorley-Lawson DA, et al. Broad cross-reactive TCR repertoires recognizing dissimilar epstein-barr and influenza A virus epitopes. J Immunol. (2010) 185:6753–64. 10.4049/jimmunol.1000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skevaki C, Hudemann C, Matrosovich M, Möbs C, Paul S, Wachtendorf A, et al. Influenza-derived peptides cross-react with allergens and provide asthma protection. J Allergy Clin Immunol. (2018) 142:804–14. 10.1016/j.jaci.2017.07.056 [DOI] [PubMed] [Google Scholar]
- 28.Slon Campos JL, Mongkolsapaya J, Screaton GR. The immune response against flaviviruses. Nat Immunol. (2018) 19:1189–98. 10.1038/s41590-018-0210-3 [DOI] [PubMed] [Google Scholar]
- 29.Elong Ngono A, Shresta S. Cross-reactive T cell immunity to dengue and zika viruses: new insights into vaccine development. Front Immunol. (2019) 10:1316. 10.3389/fimmu.2019.01316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grifoni A, Pham J, Sidney J, O'Rourke PH, Paul S, Peters B, et al. Prior dengue virus exposure shapes T cell immunity to zika virus in humans. J Virol. (2017) 91:e01469-17. 10.1128/JVI.01469-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim MQ, Kumaran EA, Tan HC, Lye DC, Leo YS, Ooi EE, et al. Cross-reactivity and anti-viral function of dengue capsid and NS3-specific memory T cells toward zika virus. Front Immunol. (2018) 9:2225. 10.3389/fimmu.2018.02225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regla-Nava JA, Elong Ngono A, Viramontes KM, Huynh A-T, Wang Y-T, Nguyen A-VT, et al. Cross-reactive dengue virus-specific CD8+ T cells protect against zika virus during pregnancy. Nat Commun. (2018) 9:3042. 10.1038/s41467-018-05458-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saron WA, Rathore AP, Ting L, Ooi EE, Low J, Abraham SN, et al. Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci Adv. (2018) 4:eaar4297. 10.1126/sciadv.aar4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balamurugan A, Ng HL, Yang OO. Cross-reactivity against multiple HIV-1 epitopes is characteristic of HIV-1-specific cytotoxic T lymphocyte clones. J Virol. (2018) 92:JVI.00617–18. 10.1128/JVI.00617-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vali B, Tohn R, Cohen MJ, Sakhdari A, Sheth PM, Yue FY, et al. Characterization of cross-reactive CD8+ T-cell recognition of HLA-A2-restricted HIV-Gag (SLYNTVATL) and HCV-NS5b (ALYDVVSKL) epitopes in individuals infected with human immunodeficiency and hepatitis C viruses. J Virol. (2011) 85:254–63. 10.1128/JVI.01743-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang X-H, et al. Cross-genotype immunity to hepatitis C virus. J Virol. (2004) 78:1575–81. 10.1128/JVI.78.3.1575-1581.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince AM, Brotman B, Lee D-H, Pfahler W, Tricoche N, Andrus L, et al. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis. (2005) 192:1701–9. 10.1086/496889 [DOI] [PubMed] [Google Scholar]
- 38.Giugliano S, Oezkan F, Bedrejowski M, Kudla M, Reiser M, Viazov S, et al. Degree of cross-genotype reactivity of hepatitis C virus-specific CD8+ T cells directed against NS3. Hepatology. (2009) 50:707–16. 10.1002/hep.23096 [DOI] [PubMed] [Google Scholar]
- 39.Ziegler S, Skibbe K, Walker A, Ke X, Heinemann FM, Heinold A, et al. Impact of sequence variation in a dominant HLA-A*02-restricted epitope in hepatitis C virus on priming and cross-reactivity of CD8+ T cells. J Virol. (2014) 88:11080–90. 10.1128/JVI.01590-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fytili P, Dalekos GN, Schlaphoff V, Suneetha PV, Sarrazin C, Zauner W, et al. Cross-genotype-reactivity of the immunodominant HCV CD8 T-cell epitope NS3-1073. Vaccine. (2008) 26:3818–26. 10.1016/j.vaccine.2008.05.045 [DOI] [PubMed] [Google Scholar]
- 41.Antunes DA, Rigo MM, Silva JP, Cibulski SP, Sinigaglia M, Chies JA, et al. Structural in silico analysis of cross-genotype-reactivity among naturally occurring HCV NS3-1073-variants in the context of HLA-A*02:01 allele. Mol Immunol. (2011) 48:1461–7. 10.1016/j.molimm.2011.03.019 [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Bakshi RK, Suneetha PV, Fytili P, Antunes DA, Vieira GF, et al. Frequency, private specificity, and cross-reactivity of preexisting hepatitis C virus (HCV)-specific CD8+ T cells in HCV-seronegative individuals: implications for vaccine responses. J Virol. (2015) 89:8304–17. 10.1128/JVI.00539-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agrawal B, Singh S, Gupta N, Li W, Vedi S, Kumar R. Unsolved puzzles surrounding HCV immunity: heterologous immunity adds another dimension. Int J Mol Sci. (2017) 18:1626. 10.3390/ijms18081626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy PT, Urbani S, Moses RA, Amadei B, Fisicaro P, Lloyd J, et al. The influence of T cell cross-reactivity on HCV-peptide specific human T cell response. Hepatology. (2006) 43:602–11. 10.1002/hep.21081 [DOI] [PubMed] [Google Scholar]
- 45.Wei R, Yang C, Zeng M, Terry F, Zhu K, Yang C, et al. A dominant EV71-specific CD4+ T cell epitope is highly conserved among human enteroviruses. PLoS ONE. (2012) 7:e51957. 10.1371/journal.pone.0051957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gern JE, Dick EC, Kelly EA, Vrtis R, Klein B. Rhinovirus-specific T cells recognize both shared and serotype-restricted viral epitopes. J Infect Dis. (1997) 175:1108–14. 10.1086/516449 [DOI] [PubMed] [Google Scholar]
- 47.Muehling LM, Mai DT, Kwok WW, Heymann PW, Pomés A, Woodfolk JA. Circulating memory CD4+ T cells target conserved epitopes of rhinovirus capsid proteins and respond rapidly to experimental infection in humans. J Immunol. (2016) 197:3214–24. 10.4049/jimmunol.1600663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao J, Zhao J, Mangalam AK, Channappanavar R, Fett C, Meyerholz DK, et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. (2016) 44:1379–91. 10.1016/j.immuni.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gisa A, Suneetha PV, Behrendt P, Pischke S, Bremer B, Falk CS, et al. Cross-genotype-specific T-cell responses in acute Hepatitis E Virus (HEV) infection. J Viral Hepat. (2016) 23:305–15. 10.1111/jvh.12495 [DOI] [PubMed] [Google Scholar]
- 50.Daniels KA, Hatfield SD, Welsh RM, Brehm MA. MHC basis of T cell-dependent heterologous immunity to arenaviruses. Virology. (2014) 464–5:213–7. 10.1016/j.virol.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci USA. (2010) 107:16922–7. 10.1073/pnas.1010867107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mangada MM, Endy TP, Nisalak A, Chunsuttiwat S, Vaughn DW, Libraty DH, et al. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J Infect Dis. (2002) 185:1697–703. 10.1086/340822 [DOI] [PubMed] [Google Scholar]
- 53.Wijeratne DT, Fernando S, Gomes L, Jeewandara C, Ginneliya A, Samarasekara S, et al. Quantification of dengue virus specific T cell responses and correlation with viral load and clinical disease severity in acute dengue infection. PLoS Negl Trop Dis. (2018) 12:e0006540. 10.1371/journal.pntd.0006540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon-Lorière E, Duong V, Tawfik A, Ung S, Ly S, Casadémont I, et al. Increased adaptive immune responses and proper feedback regulation protect against clinical dengue. Sci Transl Med. (2017) 9:eaal5088. 10.1126/scitranslmed.aal5088 [DOI] [PubMed] [Google Scholar]
- 55.Aaby P, Martins CL, Garly M-L, Rodrigues A, Benn CS, Whittle H. The optimal age of measles immunisation in low-income countries: a secondary analysis of the assumptions underlying the current policy. BMJ Open. (2012) 2:e000761. 10.1136/bmjopen-2011-000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aaby P, Samb B, Simondon F, Seck AM, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ. (1995) 311:481–5. 10.1136/bmj.311.7003.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aaby P, Bhuiya A, Nahar L, Knudsen K, Francisco A de, Strong M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol. (2003) 32:106–16. 10.1093/ije/dyg005 [DOI] [PubMed] [Google Scholar]
- 58.Aaby P, Biai S, Veirum JE, Sodemann M, Lisse I, Garly M-L, et al. DTP with or after measles vaccination is associated with increased in-hospital mortality in Guinea-Bissau. Vaccine. (2007) 25:1265–9. 10.1016/j.vaccine.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 59.Blok BA, Arts RJ, van Crevel R, Benn CS, Netea MG. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. (2015) 98:347–56. 10.1189/jlb.5RI0315-096R [DOI] [PubMed] [Google Scholar]
- 60.Jensen KJ, Benn CS, van Crevel R. Unravelling the nature of non-specific effects of vaccines-A challenge for innate immunologists. Semin Immunol. (2016) 28:377–83. 10.1016/j.smim.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 61.Higgins JP, Soares-Weiser K, Reingold A. Systematic Review of the Non-Specific Effects of BCG, DTP and Measles Containing Vaccines. (2014). Available online at: https://www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf
- 62.Higgins JP, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. (2016) 355:i5170. 10.1136/bmj.i5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. (2011) 204:245–52. 10.1093/infdis/jir240 [DOI] [PubMed] [Google Scholar]
- 64.Aaby P, Gustafson P, Roth A, Rodrigues A, Fernandes M, Sodemann M, et al. Vaccinia scars associated with better survival for adults. An observational study from Guinea-Bissau. Vaccine. (2006) 24:5718–25. 10.1016/j.vaccine.2006.04.045 [DOI] [PubMed] [Google Scholar]
- 65.Villumsen M, Sørup S, Jess T, Ravn H, Relander T, Baker JL, et al. Risk of lymphoma and leukaemia after bacille calmette-guérin and smallpox vaccination: a Danish case-cohort study. Vaccine. (2009) 27:6950–8. 10.1016/j.vaccine.2009.08.103 [DOI] [PubMed] [Google Scholar]
- 66.Sørup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA. (2014) 311:826–35. 10.1001/jama.2014.470 [DOI] [PubMed] [Google Scholar]
- 67.Fish EN, Flanagan KL, Furman D, Klein SL, Kollmann TR, Jeppesen DL, et al. Changing oral vaccine to inactivated polio vaccine might increase mortality. Lancet. (2016) 387:1054–5. 10.1016/S0140-6736(16)00661-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aaby P, Rodrigues A, Biai S, Martins C, Veirum JE, Benn CS, et al. Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine. (2004) 22:3014–7. 10.1016/j.vaccine.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 69.Andersen A, Fisker AB, Rodrigues A, Martins C, Ravn H, Lund N, et al. National immunization campaigns with oral polio vaccine reduce all-cause mortality: a natural experiment within seven randomized trials. Front Public Health. (2018) 6:13. 10.3389/fpubh.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frankild S, Boer RJ de, Lund O, Nielsen M, Kesmir C. Amino acid similarity accounts for T cell cross-reactivity and for “holes” in the T cell repertoire. PLoS ONE. (2008) 3:e1831. 10.1371/journal.pone.0001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. (2013) 34:431–9. 10.1016/j.it.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 72.Aaby P, Benn C, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open. (2012) 2:e000707. 10.1136/bmjopen-2011-000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freyne B, Donath S, Germano S, Gardiner K, Casalaz D, Robins-Browne RM, et al. Neonatal BCG vaccination influences cytokine responses to toll-like receptor ligands and heterologous antigens. J Infect Dis. (2018) 217:1798–808. 10.1093/infdis/jiy069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nissen TN, Birk NM, Blok BA, Arts RJ, Andersen A, Kjærgaard J, et al. Bacillus calmette-guérin vaccination at birth and in vitro cytokine responses to non-specific stimulation. A randomized clinical trial. Eur J Clin Microbiol Infect Dis. (2018) 37:29–41. 10.1007/s10096-017-3097-2 [DOI] [PubMed] [Google Scholar]
- 75.Aaby P, Jensen H, Walraven G. Age-specific changes in the female-male mortality ratio related to the pattern of vaccinations: an observational study from rural Gambia. Vaccine. (2006) 24:4701–8. 10.1016/j.vaccine.2006.03.038 [DOI] [PubMed] [Google Scholar]
- 76.Noho-Konteh F, Adetifa JU, Cox M, Hossin S, Reynolds J, Le MT, et al. Sex-differential non-vaccine-specific immunological effects of diphtheria-tetanus-pertussis and measles vaccination. Clin Infect Dis. (2016) 63:1213–26. 10.1093/cid/ciw492 [DOI] [PubMed] [Google Scholar]
- 77.Ndure J, Noho-Konteh F, Adetifa JU, Cox M, Barker F, Le MT, et al. Negative correlation between circulating CD4+FOXP3+CD127− regulatory T cells and subsequent antibody responses to infant measles vaccine but not diphtheria-tetanus-pertussis vaccine implies a regulatory role. Front Immunol. (2017) 8:921. 10.3389/fimmu.2017.00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samb B, Whittle H, Aaby P, Seck AM, Bennett J, Markowitz L, et al. No evidence of long-term immunosuppression after high-titer edmonstron-zagreb measles vaccination in Senegal. J Infect Dis. (1995) 171:506–8. 10.1093/infdis/171.2.506 [DOI] [PubMed] [Google Scholar]
- 79.Lisse IM, Aaby P, Knudsen K, Whittle H, Andersen H. Long term impact of high titer edmonston-zagreb measles vaccine on T lymphocyte subsets. Pediatr Infect Dis J. (1994) 13:109–12. 10.1097/00006454-199402000-00006 [DOI] [PubMed] [Google Scholar]
- 80.León ME, Ward B, Kanashiro R, Hernández H, Berry S, Vaisberg A, et al. Immunologic parameters 2 years after high-titer measles immunization in Peruvian children. J Infect Dis. (1993) 168:1097–104. 10.1093/infdis/168.5.1097 [DOI] [PubMed] [Google Scholar]
- 81.Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. (2016) 254:228–36. 10.1016/j.atherosclerosis.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 82.Novakovic B, Messina NL, Curtis N. The heterologous effects of bacillus calmette-guérin (BCG) vaccine and trained innate immunity. In: Faustman D. editor. The Value of BCG and TNF in Autoimmunity. London, UK; San Diego, CA: Academic Press; (2018). p. 71–90. [Google Scholar]
- 83.Krone B, Kölmel KF, Grange JM, Mastrangelo G, Henz BM, Botev IN, et al. Impact of vaccinations and infectious diseases on the risk of melanoma—evaluation of an EORTC case–control study. Euro J Cancer. (2003) 39:2372–8. 10.1016/S0959-8049(03)00625-7 [DOI] [PubMed] [Google Scholar]
- 84.Boisgerault N, Guillerme J-B, Pouliquen D, Mesel-Lemoine M, Achard C, Combredet C, et al. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed Res Int. (2013) 2013:387362. 10.1155/2013/387362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bree LC de, Koeken VA, Joosten LA, Aaby P, Benn CS, van Crevel R, et al. Non-specific effects of vaccines: current evidence and potential implications. Semin Immunol. (2018) 39:35–43. 10.1016/j.smim.2018.06.002 [DOI] [PubMed] [Google Scholar]