Abstract
Liver pericytes, commonly named hepatic stellate cells (HSCs), reside in the space between sinusoidal endothelial cells (LSECs) and hepatocytes. They display important roles in health and disease. HSCs ensure the storage of the majority of vitamin A in a healthy body, and they represent the major source of fibrotic tissue in liver disease. Surrounding cells, such as LSECs, hepatocytes and Kupffer cells, present a significant role in modulating HSC behavior. Therapeutic strategies against liver disease are being currently developed, where HSCs represent an ideal target. In this chapter, we will discuss HSC quiescence and activation in the context of healthy liver and diseases, such as fibrosis, steatohepatitis and hepatocellular carcinoma.
Keywords: liver, hepatic stellate cells, pericytes, healthy liver, regeneration, fibrosis, NASH, NAFLD, hepatocellular carcinoma, hepatocytes, sinusoidal endothelial cells, Kupffer cells
Introduction
The liver specific pericytes are called hepatic stellate cells (HSCs). They reside in the subendothelial space of Disse, defined as the space between the liver sinusoidal endothelial cells and the parenchymal cells. HSCs were first described in 1876 by the German anatomist Carl von Kupffer who visualized them using gold chloride preparations of human liver. He called these cells « sternzellen » meaning stellate cells due to their shape (Kupffer 1876). In 1952, Toshito Ito described perisinusoidal cells as fat-storing cells based on their capacity to accumulate lipid droplets (Ito and Nemoto 1952), also called later lipocytes or Ito cells. The term HCS was reused in 1971 by the Japanese Kenjiro Wake (Wake 1971), who also described the storage of vitamin A in rat quiescent HSCs (Wake 1974) and who opened the era of their characterization. The existence of many names describing the same cell, such as Ito cell, fat-storing cell, lipocyte, perisinusoidal or parasinusoidal cell, prompted the investigators of the field in 1996 to standardize the term HSC when referring to these cells (Hepatology 1996;23:193). In this chapter, we will discuss the origin of HSCs, their role in the adult healthy liver and finally their implication in liver diseases where HSCs are designated as the main mediators of fibrosis.
The origin of HSCs during development
The developmental origin of HSCs has been debated since they express both neuronal and mesenchymal cell markers, such as nestin, glial fibrillary acidic protein (GFAP), p75 neutrophin receptor (p75NTR), desmin, type 1 collagen and vimentin. For this reason, it has been thought that HSCs derive from neural crest and from the mesenchyme (Asahina 2012). To investigate the neural crest origin of HSCs, yellow fluorescent protein (YFP)+ neural crest descendants were obtained by crossing wingless-type MMTV integration site family member 1 (Wnt1)Cre mice with Rosa26YFP reporter mice (Cassiman et al. 2006). In this model, HSCs failed to express the YFP leading to the conclusion that they do not derive from the neural crest (Cassiman et al. 2006). Nevertheless, a more recent study shows that in zebrafish, HSCs express heart and neural crest derivatives expressed 2 (hand2), a mesoderm and neural crest marker suggesting a link between HSCs and neural crest (Yin et al. 2012). This controversy may be explained by inter-species differences and needs further investigation.
The most accepted developmental origin for HSCs is a sheet of mesoderm that develops along the foregut endoderm near the cardiac mesoderm, called the septum transversum (Asahina et al. 2009, 2011, Loo and Wu 2008, Pérez-Pomares et al. 2004, Toi et al. 2018). The septum transversum is also important for the pre-natal development of the liver by secreting bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs) (Zaret 2002). To test whether septum transversum contributes to HSC population in mice, Wilm’s tumor 1 (WT1) was used as a marker. Before liver development, WT1 is only expressed in the septum transversum, while in later stages of liver morphogenesis WT1 is expressed in mesothelial and submesothelial cells. These cells give rise to WT1−/β-galactosidase+ HSCs (Asahina et al. 2011). The septum transversum origin of HSCs is also demonstrated in rats and in developing human liver (Toi et al. 2018, Loo and Wu 2008). Moreover, in human fetuses a cell population expressing hematopoietic stem cell markers (CD34 and cytokeratin 7/8) and HSC markers (desmin and α-smooth muscle actin (αSMA)) was identified suggesting a link between hematopoietic and hepatic systems (Suskind and Muench 2004). In addition to these studies, further investigation is necessary to understand the developmental origin of HSCs in different species and whether there exist several sub-populations of HSCs deriving from different precursors.
Role of HSCs in healthy liver
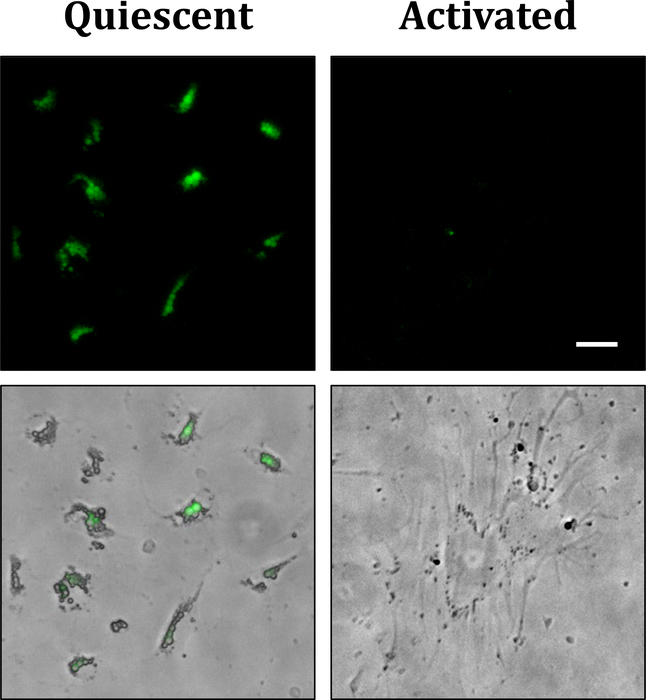
In a healthy adult liver, quiescent HSCs represent ~10% of total resident cells (Tsuchida and Friedman 2017). They are mainly recognized for their role in storage and controlled release of vitamin A. Storage of retinols or vitamin A in cytoplasmic lipid droplets is the most distinctive feature of quiescent HSCs. These lipid droplets are routinely used for HSC isolation and are autofluorescent when excited with the light of ~328 nm (Popper 1944) (Figure 1). In a healthy liver, 50–80% of total body retinol is stocked in the liver (Blomhoff et al. 1990), of which 80–90% is stored in HSCs (Hendriks et al. 1985). While it remains unknown how retinol is transferred to HSCs, more studies exist regarding retinol metabolism in HSCs. Upon entering in HSCs, retinol is esterified and mostly stored as retinyl esters by lecithin retinol acyltransferase (LRAT) (Friedman 2008). Retinyl esters constitute 30–50% of the lipids of HSC lipid droplets (Senoo 2004). Vitamin A is important for maintaining HSCs in quiescence since treatment of cultured HSCs with vitamin A induced its accumulation in cytoplasmic lipid droplets and decreased the expression of HSC activation markers (Yoneda et al. 2016). Moreover, all-trans retinoic acid, a derivate of vitamin A, was demonstrated to reduce HSC activation (Shimizu et al. 2017). However, the absence of lipid droplets in LRAT-deficient mice was not associated with liver fibrosis (Kluwe et al. 2011). This controversy raises the question whether vitamin A loss is the cause or the consequence of HSC activation, leading to the need of further studies to elucidate this question.
Figure 1:
Left: primary quiescent HSCs isolated from mouse liver presenting autofluorescent lipid droplets (day 1 after isolation). Right: primary activated HSC isolated from mouse liver (day 10 after isolation). It presents several processes, and lipid droplets are absent. Upper panels: autofluorescent lipid droplets. Lower panels: brightfield capture of the cells merged with the autofluorescent lipid droplets. Scale bar: 10 μm.
HSCs also secrete molecules that act in an autocrine or paracrine manner, part of which are lipoproteins, growth factors and cytokines (Friedman 2008). As other quiescent perivascular cells, HSCs secrete apolipoprotein E (ApoE) (Ramadori et al. 1989). At basal level, ApoE-deficient mice showed increased transforming growth factor beta (TGFβ), monocyte chemoattractant protein (MCP-1) and tissue inhibitor of metalloproteinase 1 (TIMP-1) expression in the liver when compared to wild-type (WT) mice (Ferré et al. 2009), suggesting a role for ApoE in maintaining liver homeostasis. It would have been interesting to investigate whether HSC-specific ApoE deletion would have an incidence on liver injury. Follistatin and interleukin 10 (IL-10) are other molecules secreted by HSCs that have shown anti-fibrotic effects (Friedman 2008). Indeed, treatment of carbon tetrachloride (CCl4)-exposed rats with follistatin reduced liver fibrosis and constrained HSC proliferation (Patella et al. 2006). Nevertheless, another study showed that follistatin is expressed only by activated HSCs and could be a marker of liver fibrosis (Boers et al. 2006). HSCs can also secrete IL-10, a hepatoprotective cytokine recognized for its role in reducing liver injury (Thompson KC et al. 1998, Byun et al. 2013). Indeed, IL-10-deficient mice develop more severe liver fibrosis following CCl4 administration (Thompson K et al. 1998). The anti-fibrotic molecules produced by HSCs deserve more elucidation as they have a therapeutic potential.
HSCs have also been shown to have an important role in liver regeneration following a partial hepatectomy (Preziosi and Monga 2017). In the earlier phase of liver regeneration, they regulate matrix degradation by secreting matrix metalloproteinase and several proteoglycans and induce hepatocyte proliferation by secreting pleiotrophin and the potent mitogen hepatocyte growth factor (HGF) (Asahina et al. 2002, Taub 2004, Preziosi and Monga 2017). When HSC death was caused by gliotoxin 24 hours before partial hepatectomy, hepatocyte proliferation was significantly impaired due to a lack of HGF (Nejak-Bowen et al. 2013). At later phases of liver regeneration, HSCs secrete the mitoinhibitory TGFβ to inhibit hepatocyte proliferation (Preziosi and Monga 2017). When gliotoxin was administered 5 days after partial hepatectomy, hepatocyte proliferation was prolonged due to decreased HSC-derived TGFβ and collagen deposition (Nejak-Bowen et al. 2013). An increasing interest has been shown on the aspect of HSCs as progenitor cells. Several groups have demonstrated that HSCs can differentiate in hepatocytes during liver regeneration (Yang et al. 2008b, Kordes et al. 2014), suggesting a mesenchymal stem cell role. All these studies show that HSC presence is essential for normal liver function and regeneration. Nevertheless, further investigation is needed to elucidate the existing controversy on the role of HSC-derived signaling in healthy liver.
Role of HSCs in liver disease
HSCs have mainly been studied in liver diseases, due to their capacity to transdifferentiate in myofibroblasts, the major source of collagen deposition during fibrogenesis. In this sub-chapter, the HSC behavior will be discussed in the context of liver fibrosis, steatohepatitis and hepatocellular carcinoma.
HSCs and liver fibrosis
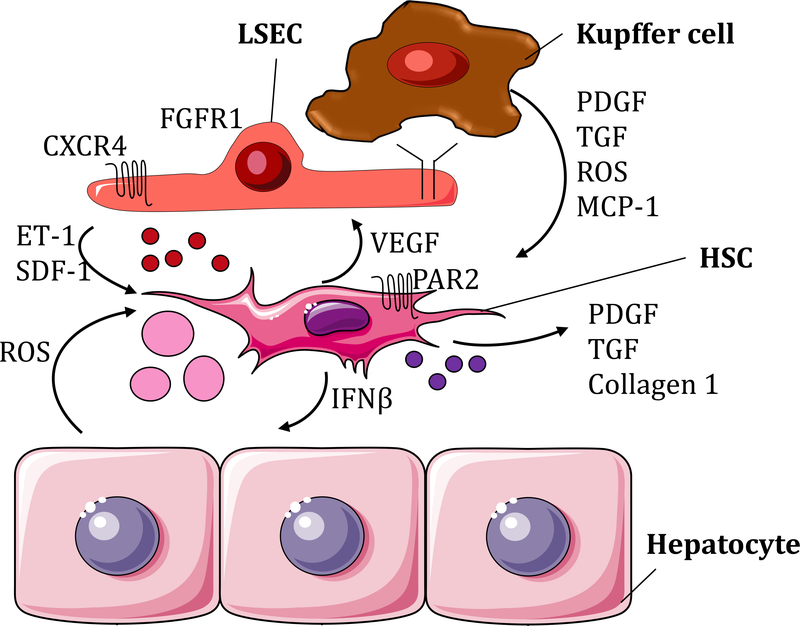
Liver fibrosis is the deposition of excessive scar tissue in response to a chronic injury. The identification of HSC activation in hepatic fibrosis has been a significant discovery in the understanding of liver’s response to injury. HSC activation refers to the passage from a quiescent vitamin-rich cell to a proliferative, contractile, migratory and fibrogenic cell (Friedman 2008) (Figure 1). Some of the most studied signals that activate HSCs are platelet growth factor (PDGF), TGFβ, Fas cell surface death receptor (Fas), apoptotic bodies, extracellular vesicles and stiffness (Friedman 2008, Kostallari and Shah 2016, Dou et al. 2018). These signals derive from several cell types, such as sinusoidal endothelial cells (SECs) (Kostallari and Shah 2016), Kupffer cells (Bilzer et al. 2006), injured hepatocytes (Canbay et al. 2002, 2003), and HSCs (Kostallari et al. 2018) (Figure 2).
Figure 2:
Cross-talking between HSCs and the surrounding cells in the context of liver fibrosis. Defenestrated LSEC upregulate the expression of CXCR4 and FGFR1 and secretion of ET-1, SDF-1 and SK-1-containing EVs (small red circles), which induce HSC activation. Kupffer cells participate in this process by secreting PDGF, TGF, ROS and MCP-1. ROS are also released by injured hepatocytes. HSCs secrete PDGF, TGF collagen 1 and PDGFRα-enriched EVs (small purple circles) which act in an autocrine manner. In addition, activated HSCs release IFNβ that induces hepatocyte apoptosis and hepatocyte-derived apoptotic bodies (pink circles) implicated in HSC further activation.
In liver injury, SECs become activated or capillarized by losing their fenestrae (Brunt et al. 2014). Defenestrated SECs increase endothelin 1 (ET-1) secretion and decrease nitric oxide (NO) release, which contribute to HSC contraction (Kostallari and Shah 2016, Tsuchida and Friedman 2017). ET-1 is found to also be secreted by HSCs and to act in an autocrine manner (Pinzani et al. 1996). SECs stimulate HSC migration by producing stromal derived factor 1 (SDF-1)/C-X-C motif chemokine ligand 12 (CXCL12) (Kordes and Häussinger 2013) and releasing sphingosine kinase (SK)-1-containing EVs (Wang et al. 2015). Moreover, in liver fibrogenesis, SECs upregulate fibroblast growth factor receptor 1 (FGFR1) and C-X-C motif chemokine receptor 4 (CXCR4) expressions, enforcing the fibrogenic phenotype of HSCs (Ding et al. 2014). Another cell type influencing HSC behavior is the Kupffer cell. Kupffer cells are the liver resident macrophages lying in the sinusoids and penetrating between the hepatocytes during liver injury. They have an important role in HSC migration through PDGF secretion and HSC differentiation into myofibroblasts through TGFβ and reactive oxygen species (ROS) release (Tsuchida and Friedman 2017, Kiagiadaki et al. 2018). Moreover, Kupffer cells secrete MCP-1 in a SK-1-dependent manner (Lan et al. 2018). MCP-1 binds to C-C motif chemokine receptor 2 (CCR2), which is expressed by both HSCs and Kupffer cells, leading to their respective migration (Marra et al. 1999, Lan et al. 2018) and ultimately to liver fibrosis (Seki et al. 2009). Treatment of mice with a specific inhibitor of SK-1 prevented liver fibrosis in CCl4− and bile duct ligation (BDL)-induced liver injuries (Lan et al. 2018). Hepatocytes, which constitute 70–85% of the liver mass, also have a role in HSC activation. In a healthy liver, hepatocytes are polarized cells presenting microvilli, which contribute to the absorption of proteins and other molecules coming from the blood. Injured hepatocytes lose their microvilli and can participate to HSC activation (Canbay et al. 2003, Tsuchida and Friedman 2017). Indeed, injured hepatocytes underwent apoptosis through a Fas-dependent mechanism and released apoptotic bodies, which were engulfed by HSCs (Canbay et al. 2002, 2003). This induced HSC fibrogenic activity by increasing collagen α1, TGFβ1 and αSMA expression (Canbay et al. 2003). Moreover, damaged hepatocytes release ROS, which activate HSCs and lead to liver fibrogenesis (Jiang et al. 2010, Tsuchida and Friedman 2017).
Besides SECs, Kupffer cells and hepatocytes, activated HSCs can also release signals in a paracrine manner, which contribute to fibrosis. Activated HSCs are well recognized to secrete the canonical pro-fibrogenic molecules such as collagen 1α1, TGFβ and PDGF-BB (Drinane et al. 2017, Friedman 2008). Moreover, a decrease of retinoic acid signaling in HSCs is associated with induction of TGFβ1 expression and activation of HSCs, suggesting a role for retinoic acid in HSCs quiescence (Ohata et al. 1997). The presence of bacterial lipopolysacharide (LPS), due to increased gut permeability in liver disease, has been shown to have a significant role in retinoic acid signaling decrease by inducing the autophagy of HSC lipid droplets and leading to HSC activation (Chen et al. 2017). LPS-dependent activation of HSCs induces interferon beta (IFNβ) release, which promotes hepatocyte apoptosis (Dangi et al. 2016). In turn, apoptotic hepatocytes participate to further activation of HSCs (Canbay et al. 2002, 2003). Moreover, activated HSCs and myofibroblasts secrete vascular endothelial growth factor (VEGF), a potent angiogenic molecule that binds to VEGF receptor (VEGFR) expressed by SECs, and induce fibrosis-associated angiogenesis (Das et al. 2010). In a recent study, activated HSCs also secrete PDGFRα-enriched EVs, which have a paracrine effect in promoting in vitro cell migration and in vivo fibrogenesis (Kostallari et al. 2018). The PDGFRα enrichment in HSC-derived EVs is SHP2 dependent and treatment of mice with SHP2 inhibitor, SHP099, reduced significantly liver fibrosis (Kostallari et al. 2018). Interestingly, G protein-coupled receptors (GPCRs) are emerging as new targets in liver fibrosis. Indeed, protease-activated receptor-2 (PAR2) is expressed in activated HSCs. Inhibiting PAR2 by PZ-235 decreased HSC proliferation and collagen deposition (Shearer et al. 2016).
Another interesting stimulator of HSC activation is liver stiffness. Indeed, when plated on a soft substrate freshly isolated HSCs maintain their lipid droplets and high levels of PPARγ. However, when plated on a stiff substrate HSCs lose their lipid droplets and upregulate αSMA and collagen1 expression (et al. 2014). Moreover, in a recent study, it has been demonstrated that substrate stiffness induces HSC activation through translocation into the nucleus of histone acetyltransferase p300, which upregulated transcription of HSC activation genes (Dou et al. 2018). In line with these studies and in a therapeutic perspective, it could be of interest to further investigate the cause of the stiffness in order to modulate it in vivo and to explore the role of other GPCRs in liver fibrosis.
HSCs and steatohepatitis
Steatohepatitis is one of the leading causes of liver diseases in the United States. It can occur due to a high consumption of alcohol leading to alcoholic steatohepatitis (ASH), or a high fat diet leading to non-alcoholic steatohepatitis (NASH). Despite the different etiologies, these two entities have similar pathogenic mechanisms. They usually start with steatosis, which is the fat accumulation within the hepatocytes (ballooned hepatocytes), followed by hepatocyte cell death response, inflammation and ultimately fibrogenesis (Greuter et al. 2017, Ibrahim et al. 2018). HSCs have a significant role in driving inflammation and fibrogenesis in ASH and NASH by receiving and emitting signals. Lipotoxicity-induced hepatocyte-derived extracellular vesicles promote HSC activation, proliferation and migration by inhibiting the quiescence marker peroxisome proliferator-activated receptor gamma (PPARγ) through miR-128–3p (Povero et al. 2015). Lipotoxicity-induced hepatocyte can also secrete sonic hedgehog (Shh), which is an activator of HSCs (Yang et al. 2008a, Rangwala et al. 2011). In turn, activated HSCs can increase the inflammatory reaction by secreting pro-inflammatory cytokines such as MCP1, IL6, TGFβ and neural cell adhesion molecules (Friedman 2008, Lee and Jeong 2012).
In alcoholic and non-alcoholic liver disease, intestinal epithelial permeability is increased, which can be detected by a high level of bacterial LPS in the liver. LPS can stimulate HSCs through Toll-like receptors (TLRs) (Tsuchida and Friedman 2017). HSCs express several TLRs, such as TLR2, TLR3, TLR4, TLR7 and TLR9 (Seki et al. 2007, Gäbele et al. 2008, Chou et al. 2012, Miura et al. 2013, Byun et al. 2013). In some mouse models of steatohepatitis, activation of TLR4 induces Kupffer cell chemotaxis and inflammation (Seki et al. 2007, Guo et al. 2009). Moreover TLRs activation in HSCs induces liver fibrosis (Huang et al. 2007, Seki et al. 2007). HSCs nuclear receptors, such as farnesoid X receptor (FXR), peroxisome proliferator-activated receptor (PPAR) or vitamin D3 receptor (VDR), are other factors involved in liver disease. Indeed, deficiency of FXR in HSCs promotes hepatic inflammation and fibrosis (Kong et al. 2009). Furthermore, a dual PPARα-PPARδ agonist, GFT505 that may target both hepatocytes and HSCs, protects liver from steatosis, inflammation and fibrosis in several mouse models (Staels et al. 2013). In line with this, inhibition of VDR signaling by sequestosome 1 (SQSTM1/p62) knock out attenuates liver inflammation and fibrosis in experimental steatohepatitis (Beilfuss et al. 2015, Duran et al. 2016).
The interaction between HSCs and neutrophils also seems to play an important role in the establishment of steatohepatitis through HSCs. Indeed, neutrophils stimulate the secretion of HSC-derived GM-CSF and IL-15, which in turn prolong neutrophil survival and may serve as a positive forward loop to promote liver damage and fibrosis (Zhou et al. 2018). Cholesterol, a component of high-fat diet, is another factor that can accumulate in HSCs and induce their activation (Tomita et al. 2014). Cholesterol-lowering drugs, such as ezetimibe or statins, attenuate steatohepatitis and fibrosis in a mouse model of NASH (Van Rooyen et al. 2013). Nevertheless, the effect of activated HSCs on recruiting pro-inflammatory cells is not fully understood and deserves further investigation.
HSCs and hepatic metastases
The combination of the unique microenvironment and its hemodynamics characteristics makes the liver one of the most targeted organs of cancer metastasis. Furthermore, liver metastases are dependent on the interactions between the tumor cells and the hepatic stromal cells, such as HSCs. HSCs have several important roles in liver tumors, such as promoting tumor growth, releasing growth factors and cytokines, regulating extracellular matrix (ECM) turn-over, tumor-associated angiogenesis and suppressing anti-tumor immune response (Kang et al. 2011). In 130 cases of hepatocellular carcinoma, peritumoral activated HSCs independently contributed to high recurrence or death rates (Ju et al. 2009). Similarly to liver fibrosis, tumor-stimulated HSCs differentiate into myofibroblasts, upregulate αSMA expression, proliferate, migrate and contribute significantly to collagen deposition (Vidal-Vanaclocha 2008). This behavior is stimulated by tumor cells through paracrine signaling, including secretion of TGFβ (Kang et al. 2011). In turn, activated HSCs induce tumor cell proliferation, migration and invasion through secretion of several secreted growth factors and cytokines, such as TGFβ, HGF, PDGF, SDF-1, VEGF and CXCL12 (Okabe et al. 2009, Amann et al. 2009, Zhao et al. 2011, Kang et al. 2011, Yaqoob et al. 2012, Dou et al. 2018) and by downregulating other molecules such as endosialin (Mogler et al. 2017). Indeed, conditioned media of activated HSCs promoted tumor cell invasion and proliferation in vitro (Okabe et al. 2009, Amann et al. 2009) and in vivo (Dou et al. 2018). Furthermore, HSCs cultured on a stiff environment induced a higher tumor growth than HSCs cultured on soft matrix (Dou et al. 2018), suggesting a role for stiffness in tumor progression.
Activated HSCs at the invasive front of liver metastasis can regulate the ECM by producing matrix metalloproteinase 2 (MMP2), TIMP2 and a disintegrin and metalloproteinase 9 (ADAM9) allowing cancer cells to increase their invasive behavior (Musso et al. 1997, Mazzocca et al. 2005). Furthermore, HSC-derived ECM components, such as collagen, fibronectin, laminin and CCN family member 1 (CCN1), regulate adhesion, migration and survival of tumor cells by activating the integrins on tumor cell surface (Kang et al. 2011, Azzariti et al. 2016, Li et al. 2018). For example, inhibiting α3 integrin on HCC cells abrogated the anti-apoptotic effect of laminin-332 (Azzariti et al. 2016). Moreover, the expression of neuropilin-1 by HSC-derived myofibroblasts promoted the secretion of fibronectin and therefore the increase of tumor microenvironment stiffness leading to a greater tumor growth (Yaqoob et al. 2012).
Activated HSCs can also promote tumor-associated angiogenesis since they release VEGF, angiopoietins and IL-8 (Semela et al. 2008, Taura et al. 2008, Das et al. 2010, Zhu et al. 2015). Indeed, IL-8 derives mainly from activated HSCs and IL-8 inhibition with a blocking antibody significantly reduced angiogenesis (Zhu et al. 2015). Moreover, activated HSCs protect the hepatic tumor by inhibiting anti-tumor cytotoxic T cells (Xia et al. 2017). Indeed, tumor-associated HSCs induce dendritic cell-derived immunoglobulin receptor 2 (DIgR2) in dendritic cells, which in turn inhibit the inflammatory response of T cells (Xia et al. 2017). It could be of interest to better understand the role of activated HSCs on the inflammatory cell behavior in the context of HCC.
Conclusion
In this chapter, we have identified HSCs as a crucial cell for liver development, homeostasis and disease through paracrine and autocrine signaling, which includes growth factors, cytokines and extracellular vesicles. While most of the studies describe HSC-dependent ECM regulation in response to microenvironment stimuli, less attention is brought to the understanding of other HSC-derived signals and their effect on the surrounding cell types. The HSC-derived signaling study remains incomplete, especially in the context of HSC heterogeneity. The isolation of different HSC sub-populations and specifically targeting the most fibrogenic ones are essential for developing novel therapies. Several encouraging antifibrotic strategies aiming the prevention or reversal of HSC-induced liver fibrosis have been or are being developed (Schuppan et al. 2018). Recently, a dual C-C chemokine receptors type 2 and 5 (CCR2/CCR5) antagonist, cenicriviroc, is in phase 2 clinical trial and is being evaluated in liver inflammation and fibrosis in the context of NASH (Friedmann et al. 2016). This is the first prospective study of an oral agent in patients with exclusively NASH and liver fibrosis, which aims to assess correlations between inflammation and fibrosis (Friedmann et al. 2016). In parallel, other clinical trials are ongoing (clinicaltrials.gov) and throw a glimmer of hope in finding treatments for liver diseases.
References
- Hepatic stellate cell nomenclature. Hepatology 1996;23:193 No authors listed. [PubMed] [Google Scholar]
- Amann T, Bataille F, Spruss T, Mühlbauer M, Gäbele E, Schölmerich J, Kiefer P, Bosserhoff AK, Hellerbrand C. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009. April;100(4):646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K Hepatic stellate cell progenitor cells. J Gastroenterol Hepatol. 2012. March;27 Suppl 2:80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Tsai SY, Li P, Ishii M, Maxson RE Jr, Sucov HM, Tsukamoto H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology. 2009. March;49(3):998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Zhou B, Pu WT, Tsukamoto H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011. March;53(3):983–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Sato H, Yamasaki C, Kataoka M, Shiokawa M, Katayama S, Tateno C, Yoshizato K. Pleiotrophin/heparin-binding growth-associated molecule as a mitogen of rat hepatocytes and its role in regeneration and development of liver. Am J Pathol. 2002. June;160(6):2191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzariti A, Mancarella S, Porcelli L, Quatrale AE, Caligiuri A, Lupo L, Dituri F, Giannelli G. Hepatic stellate cells induce hepatocellular carcinoma cell resistance to sorafenib through the laminin-332/α3 integrin axis recovery of focal adhesion kinase ubiquitination. Hepatology. 2016. December;64(6):2103–2117. [DOI] [PubMed] [Google Scholar]
- Beilfuss A, Sowa JP, Sydor S, Beste M, Bechmann LP, Schlattjan M, Syn WK, Wedemeyer I, Mathé Z, Jochum C, Gerken G, Gieseler RK, Canbay A. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut. 2015. May;64(5):791–9. [DOI] [PubMed] [Google Scholar]
- Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006. December;26(10):1175–86. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990. October 19;250(4979):399–404. [DOI] [PubMed] [Google Scholar]
- Boers W, Aarrass S, Linthorst C, Pinzani M, Elferink RO, Bosma P. Transcriptional profiling reveals novel markers of liver fibrogenesis: gremlin and insulin-like growth factor-binding proteins. J Biol Chem. 2006. June 16;281(24):16289–95. [DOI] [PubMed] [Google Scholar]
- Brunt EM, Gouw AS, Hubscher SG, Tiniakos DG, Bedossa P, Burt AD, Callea F, Clouston AD, Dienes HP, Goodman ZD, Roberts EA, Roskams T, Terracciano L, Torbenson MS, Wanless IR. Pathology of the liver sinusoids. Histopathology. 2014. June;64(7):907–20. [DOI] [PubMed] [Google Scholar]
- Byun JS, Suh YG, Yi HS, Lee YS, Jeong WI. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J Hepatol. 2013. February;58(2):342–9. [DOI] [PubMed] [Google Scholar]
- Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002. October;123(4):1323–30. [DOI] [PubMed] [Google Scholar]
- Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003. May;83(5):655–63. [DOI] [PubMed] [Google Scholar]
- Cassiman D, Barlow A, Vander Borght S, Libbrecht L, Pachnis V. Hepatic stellate cells do not derive from the neural crest. J Hepatol. 2006. June;44(6):1098–104. [DOI] [PubMed] [Google Scholar]
- Chen M, Liu J, Yang W, Ling W. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy. 2017;13(11):1813–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MH, Huang YH, Lin TM, Du YY, Tsai PC, Hsieh CS, Chuang JH. Selective activation of Toll-like receptor 7 in activated hepatic stellate cells may modulate their profibrogenic phenotype. Biochem J. 2012. October 1;447(1):25–34. [DOI] [PubMed] [Google Scholar]
- Dangi A, Huang C, Tandon A, Stolz D, Wu T, Gandhi CR. Endotoxin-stimulated Rat Hepatic Stellate Cells Induce Autophagy in Hepatocytes as a Survival Mechanism. J Cell Physiol. 2016. January;231(1):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Shergill U, Thakur L, Sinha S, Urrutia R, Mukhopadhyay D, Shah VH. Ephrin B2/EphB4 pathway in hepatic stellate cells stimulates Erk-dependent VEGF production and sinusoidal endothelial cell recruitment. Am J Physiol Gastrointest Liver Physiol. 2010. June;298(6):G908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014. January 2;505(7481):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou C, Liu Z, Tu K, Zhang H, Chen C, Yaqoob U, Wang Y, Wen J, van Deursen J, Sicard D, Tschumperlin D, Zou H, Huang WC, Urrutia R, Shah VH, Kang N. P300 Acetyltransferase Mediates Stiffness-Induced Activation of Hepatic Stellate Cells Into Tumor-Promoting Myofibroblasts. Gastroenterology. 2018. June;154(8):2209–2221.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinane MC, Yaqoob U, Yu H, Luo F, Greuter T, Arab JP, Kostallari E, Verma VK, Maiers J, De Assuncao TM, Simons M, Mukhopadhyay D, Kisseleva T, Brenner DA, Urrutia R Lomberk G, Gao Y, Ligresti G, Tschumperlin DJ, Revzin A, Cao S, Shah VH. Synectin promotes fibrogenesis by regulating PDGFR isoforms through distinct mechanisms. JCI Insight. 2017. December 21;2(24). pii: 92821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S, Roberts LR, Kisseleva T, Karin M, Diaz-Meco MT, Moscat J. p62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer. Cancer Cell. 2016. October 10;30(4):595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré N, Martínez-Clemente M, López-Parra M, González-Périz A, Horrillo R, Planagumà A, Camps J, Joven J, Tres A, Guardiola F, Bataller R, Arroyo V, Clària J. Increased susceptibility to exacerbated liver injury in hypercholesterolemic ApoE-deficient mice: potential involvement of oxysterols. Am J Physiol Gastrointest Liver Physiol. 2009. March;296(3):G553–62. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008. January;88(1):125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016. March;47:356–65. [DOI] [PubMed] [Google Scholar]
- Gäbele E, Mühlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, Wiest R, Schölmerich J, Obermeier F, Hellerbrand C. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008. November 14;376(2):271–6. [DOI] [PubMed] [Google Scholar]
- Greuter T, Malhi H, Gores GJ, Shah VH. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: exploiting similarities and differences in pathogenesis. JCI Insight. 2017. September 7;2(17). pii: 95354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, Tarocchi M, Abar OT, Huang H, Sninsky JJ, Friedman SL. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009. March;49(3):960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvendiren M, Perepelyuk M, Wells RG, Burdick JA. Hydrogels with differential and patterned mechanics to study stiffness-mediated myofibroblastic differentiation of hepatic stellate cells. J Mech Behav Biomed Mater. 2014. October;38:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985. September;160(1):138–49. [DOI] [PubMed] [Google Scholar]
- Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ, Layden TJ, Wright TL, White T, Cheung RC. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007. August;46(2):297–306. [DOI] [PubMed] [Google Scholar]
- Ibrahim SH, Hirsova P, Gores GJ. Non-alcoholic steatohepatitis pathogenesis: sublethal hepatocyte injury as a driver of liver inflammation. Gut. 2018. May;67(5):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nemoto M. Kupfer’s cells and fat storing cells in the capillary wall of human liver. Okajimas Folia Anat Jpn. 1952. October;24(4):243–58. [DOI] [PubMed] [Google Scholar]
- Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, Adamson R, Devaraj S, Shah V, Gershwin ME, Friedman SL, Török NJ. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010. October;139(4):1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, Zhou J, Li YW, Tang ZY. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009. April;131(4):498–510. [DOI] [PubMed] [Google Scholar]
- Kang N, Gores GJ, Shah VH. Hepatic stellate cells: partners in crime for liver metastases Hepatology. 2011. August;54(2):707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiagiadaki F, Kampa M, Voumvouraki A, Castanas E, Kouroumalis E, Notas G. Activin-A causes Hepatic stellate cell activation via the induction of TNFα and TGFβ in Kupffer cells. Biochim Biophys Acta. 2018. March;1864(3):891–899. [DOI] [PubMed] [Google Scholar]
- Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, Jiang H, Siddiqi M, Piantedosi R, O’Byrne SM, Blaner WS, Schwabe RF. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011. September;60(9):1260–8. [DOI] [PubMed] [Google Scholar]
- Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009. January;328(1):116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordes C, Häussinger D. Hepatic stem cell niches. J Clin Invest. 2013. May;123(5):1874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordes C, Sawitza I, Götze S, Herebian D, Häussinger D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J Clin Invest. 2014. December;124(12):5503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostallari E, Shah VH. Angiocrine signaling in the hepatic sinusoids in health and disease. Am J Physiol Gastrointest Liver Physiol. 2016. August 1;311(2):G246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, Roberts LR, Shah VH. Hepatic stellate cell-derived PDGFRα-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology. 2018. January 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupffer CV (1876) Ueber Sternzellen der Leber. Briefliche Mitteilung an Prof. Waldeyer. Arch Mikr Anat 12:353–358. [Google Scholar]
- Lan T, Li C, Yang G, Sun Y, Zhuang L, Ou Y, Li H, Wang G, Kisseleva T, Brenner D, Guo J. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology. 2018. March 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Jeong WI. Retinoic acids and hepatic stellate cells in liver disease. J Gastroenterol Hepatol. 2012. March;27 Suppl 2:75–9. [DOI] [PubMed] [Google Scholar]
- Li ZQ, Wu WR, Zhao C, Zhao C, Zhang XL, Yang Z, Pan J, Si WK. CCN1/Cyr61 enhances the function of hepatic stellate cells in promoting the progression of hepatocellular carcinoma. Int J Mol Med. 2018. March;41(3):1518–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CK, Wu XJ. Origin of stellate cells from submesothelial cells in a developing human liver. Liver Int. 2008. December;28(10):1437–45. [DOI] [PubMed] [Google Scholar]
- Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999. January;29(1):140–8. [DOI] [PubMed] [Google Scholar]
- Mazzocca A, Coppari R, De Franco R, Cho JY, Libermann TA, Pinzani M, Toker A. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 2005. June 1;65(11):4728–38. [DOI] [PubMed] [Google Scholar]
- Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013. February;57(2):577–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogler C, König C, Wieland M, Runge A, Besemfelder E, Komljenovic D, Longerich T, Schirmacher P, Augustin HG. Hepatic stellate cells limit hepatocellular carcinoma progression through the orphan receptor endosialin. EMBO Mol Med. 2017. June;9(6):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso O, Théret N, Campion JP, Turlin B, Milani S, Grappone C, Clément B. In situ detection of matrix metalloproteinase-2 (MMP2) and the metalloproteinase inhibitor TIMP2 transcripts in human primary hepatocellular carcinoma and in liver metastasis. J Hepatol. 1997. March;26(3):593–605. [DOI] [PubMed] [Google Scholar]
- Nejak-Bowen KN, Orr AV, Bowen WC Jr, Michalopoulos GK. Gliotoxin-induced changes in rat liver regeneration after partial hepatectomy. Liver Int. 2013. August;33(7):1044–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata M, Lin M, Satre M, Tsukamoto H. Diminished retinoic acid signaling in hepatic stellate cells in cholestatic liver fibrosis. Am J Physiol. 1997. March;272(3 Pt 1):G589–96. [DOI] [PubMed] [Google Scholar]
- Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, Komori H, Ishikawa S, Watanabe M, Takamori H, Iyama K, Baba H. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009. September;16(9):2555–64. [DOI] [PubMed] [Google Scholar]
- Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006. January;290(1):G137–44. [DOI] [PubMed] [Google Scholar]
- Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, Gentilini P. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996. February;110(2):534–48. [DOI] [PubMed] [Google Scholar]
- Popper H Distribution of vitamin A in tissue as visualized by flourescence microscopy. Physiol Rev 1944;24:205–224. [Google Scholar]
- Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, Pinatel EM, Alisi A, Nobili V, Feldstein AE. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell Mol Gastroenterol Hepatol. 2015. November 1;1(6):646–663.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosi ME, Monga SP. Update on the Mechanisms of Liver Regeneration. Semin Liver Dis. 2017. May;37(2):141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Rieder H, Theiss F, Meyer zum Büschenfelde KH. Fat-storing (Ito) cells of rat liver synthesize and secrete apolipoproteins: comparison with hepatocytes. Gastroenterology. 1989. July;97(1):163–72. [DOI] [PubMed] [Google Scholar]
- Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, Chen W, Diehl AM. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011. July;224(3):401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018. April 12 pii: S0945–053X(18)30160–4. [DOI] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007. November;13(11):1324–32. [DOI] [PubMed] [Google Scholar]
- Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009. July;50(1):185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo H Structure and function of hepatic stellate cells. Med Electron Microsc. 2004. March;37(1):3–15. [DOI] [PubMed] [Google Scholar]
- Shearer AM, Rana R, Austin K, Baleja JD, Nguyen N, Bohm A, Covic L, Kuliopulos A. Targeting Liver Fibrosis with a Cell-penetrating Protease-activated Receptor-2 (PAR2) Pepducin. J Biol Chem. 2016. October 28;291(44):23188–23198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Tsubota T, Kanki K, Shiota G. All-trans retinoic acid ameliorates hepatic stellate cell activation via suppression of thioredoxin interacting protein expression. J Cell Physiol. 2018. January;233(1):607–616. [DOI] [PubMed] [Google Scholar]
- Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013. December;58(6):1941–52. [DOI] [PubMed] [Google Scholar]
- Suskind DL, Muench MO. Searching for common stem cells of the hepatic and hematopoietic systems in the human fetal liver: CD34+ cytokeratin 7/8+ cells express markers for stellate cells. J Hepatol. 2004. February;40(2):261–8. [DOI] [PubMed] [Google Scholar]
- Taub R Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004. October;5(10):836–47. Review. [DOI] [PubMed] [Google Scholar]
- Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, Brenner DA. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008. November;135(5):1729–38. [DOI] [PubMed] [Google Scholar]
- Thompson KC, Trowern A, Fowell A, Marathe M, Haycock C, Arthur MJ, Sheron N. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation in vitro. Hepatology. 1998. December;28(6):1518–24. [DOI] [PubMed] [Google Scholar]
- Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998. December;28(6):1597–606. [DOI] [PubMed] [Google Scholar]
- Toi M, Hayashi Y, Murakami I. Hepatic stellate cells derived from the nestin-positive cells in septum transversum during rat liver development. Med Mol Morphol. 2018. January 29. [DOI] [PubMed] [Google Scholar]
- Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, Usui S, Furuhashi H, Kimura A, Nishiyama K, Maejima T, Okada Y, Kurihara C, Shimamura K, Ebinuma H, Saito H, Yokoyama H, Watanabe C, Komoto S, Nagao S, Sugiyama K, Aosasa S, Hatsuse K, Yamamoto J, Hibi T, Miura S, Hokari R, Kanai T. Acyl-CoA:cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. J Hepatol. 2014. July;61(1):98–106. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017. July;14(7):397–411. [DOI] [PubMed] [Google Scholar]
- Van Rooyen DM, Gan LT, Yeh MM, Haigh WG, Larter CZ, Ioannou G, Teoh NC, Farrell GC. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J Hepatol. 2013. July;59(1):144–52. [DOI] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F The prometastatic microenvironment of the liver. Cancer Microenviron. 2008. December;1(1):113–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake K “Sternzellen” in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat. 1971. December;132(4):429–62. [DOI] [PubMed] [Google Scholar]
- Wake K Development of vitamin A-rich lipid droplets in multivesicular bodies of rat liver stellate cells. J Cell Biol. 1974. November;63(2 Pt 1):683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, Hirsova P, Cao S, Mukhopadhyay D, Huebert RC, Shah VH. Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration. J Biol Chem. 2015. December 25;290(52):30684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia YH, Lu Z, Zhao M, Dai WT, Ding L, Hu LX, Jiang GL. Tumor-specific hepatic stellate cells (tHSCs) induces DIgR2 expression in dendritic cells to inhibit T cells. Oncotarget. 2017. July 5;8(33):55084–55093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008a. January;48(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Jung Y, Omenetti A, Witek RP, Choi S, Vandongen HM, Huang J, Alpini GD, Diehl AM. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cells. 2008b. August;26(8):2104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob U, Cao S, Shergill U, Jagavelu K, Geng Z, Yin M, de Assuncao TM, Cao Y, Szabolcs A, Thorgeirsson S, Schwartz M, Yang JD, Ehman R, Roberts L, Mukhopadhyay D, Shah VH. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 2012. August 15;72(16):4047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Evason KJ, Maher JJ, Stainier DY. The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver. Hepatology. 2012. November;56(5):1958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Sakai-Sawada K, Niitsu Y, Tamura Y. Vitamin A and insulin are required for the maintenance of hepatic stellate cell quiescence. Exp Cell Res. 2016. February 1;341(1):8–17. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002. July;3(7):499–512. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang L, Yin Z, Su W, Ren G, Zhou C, You J, Fan J, Wang X. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer. 2011. December 1;129(11):2651–61. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Xu MJ, Cai Y, Wang W, Jiang JX, Varga ZV, Feng D, Pacher P, Kunos G, Torok NJ, Gao B. Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018. January 8;5(3):399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Lin N, Zhang M, Zhu Y, Cheng H, Chen S, Ling Y, Pan W, Xu R. Activated hepatic stellate cells promote angiogenesis via interleukin-8 in hepatocellular carcinoma. J Transl Med. 2015. November 22;13:365. [DOI] [PMC free article] [PubMed] [Google Scholar]