Blood transfusions happen in more than 10% of all hospital stays that include a procedure. The American Medical Association1 identified overuse of five medical treatments—blood transfusions, along with cardiac stents, ear tubes, antibiotics, and the induction of birth in pregnant women—and highlighted the danger of unnecessary transfusions. Although blood transfusions are believed to be lifesaving, this assumption has never been shown in a controlled clinical trial; blood transfusions have been described as “unavoidably, unsafe, and inherently dangerous” in the US Blood Shield laws.2 Thus, perceived benefits relative to the known risks of a blood transfusion are important elements in discussions with patients about informed consent, especially in management of anaemia with alternatives to blood transfusion.3
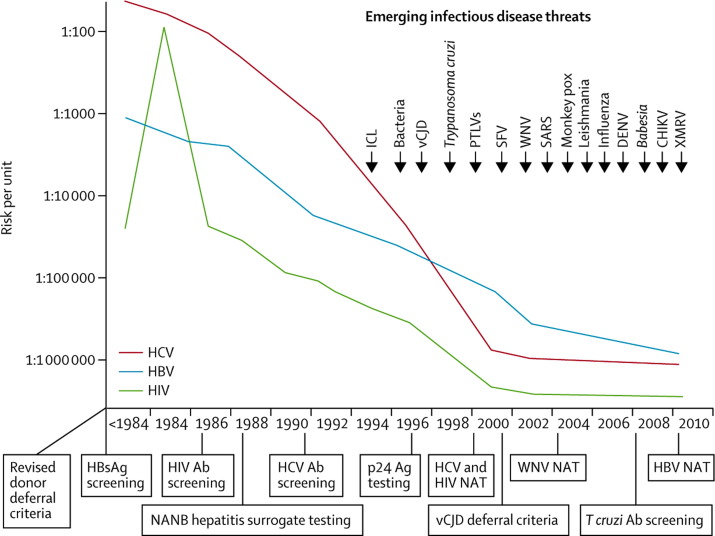
Transfusion-transmitted infections have been a worry, especially in the 1980s with the recognition of transfusion-associated HIV and the risk of transmission of hepatitis C virus.4 Transmission of known viral agents has decreased and responses to emerging infectious diseases transmitted by blood transfusion have been rapid (figure ). Concomitantly, the 2011–12 annual report of transfusion fatalities by the US Food and Drug Administration Center for Biologics Evaluation and Research showed a decrease in all-cause deaths related to transfusion, with only 30 deaths attributable to transfusion reported in the USA in 2011.5 Between 20×07 and 2011, transfusion-related acute lung injury caused the highest percentage (43%) of reported fatalities, followed by haemolytic transfusion reactions (23%) caused by non-ABO (13%) or ABO (10%) incompatibilities.
Figure.
Risks of major transfusion-transmitted viruses related to interventions, and accelerating rate of emerging infectious diseases of concern to blood safety
Evolution of the risks of transmission by blood transfusion for HIV, hepatitis B virus, and hepatitis C virus. Major interventions to reduce risks are shown below the time line on the X axis. Emerging infectious disease threats in the past 20 years are shown above in the top right quadrant of the figure. HBsAg=hepatitis B surface antigen. Ab=antibody. NANB=non-A, non-B hepatitis. Ag=antigen. HCV=hepatitis C virus. NAT=nucleic acid testing. HBV=hepatitis B virus. ICL=idiopathic CD4 T-lymphocytopenia. vCJD=variant Creutzfeldt-Jakob disease. PTLVs=primate T lymphotropic viruses. SFV=simian foamy virus. WNV=West Nile virus. SARS=severe acute respiratory syndrome. DENV=dengue virus. CHIKV=chikungunya virus. XMRV=xenotropic murine leukaemia virus-related virus. Reproduced with permission from reference 4.
Increasing evidence suggests that patients have additional adverse clinical outcomes (ie, increased morbidity and mortality) associated with blood transfusions.6 The panel lists risks that include not only known transmissible pathogens for infectious disease, transfusion reactions, transfusion-related acute lung injury, errors in blood administration, and circulatory overload; but also potential, as yet undefined, risks such as immunomodulation (eg, perioperative infection or tumour progression), unknown risks (emerging pathogens such as new variant Creutzfeldt-Jakob disease and West Nile virus),4 and risks associated with duration of blood storage lesions in patients undergoing cardiac surgery.7 The potential threat of xenotropic murine leukemia virus-related virus (XMRV), for example, required extensive research to determine that its presence in the blood of patients with chronic fatigue syndrome could not be reproducibly confirmed, and that blood donor screening is not warranted.8
Panel. Potential risks of blood transfusion.
-
1
Infectious agents
-
•Transfusion-transmitted disease for which donors are tested*
-
•Hepatitis B virus (1970 [surface antigen]; 1986–87 [core antibody]; 2009 [nucleic acid])
-
•HIV (1985 [antibody]; 1999 [nucleic acid])
-
•Hepatitis C virus (1986–87 [alanine aminotransferase]; 1990 [antibody]; 1999 [nucleic acid])
-
•Human T-cell lymphotropic virus (1988 [antibody])
-
•West Nile virus (2003 [nucleic acid])
-
•Bacteria (in platelets only; 2004)
-
•Trypanosoma cruzi (2007 [antibody])
-
•Cytomegalovirus
-
•Syphilis
-
•
-
•Transfusion-transmitted disease for which donors are not routinely tested
-
•Hepatitis A virus
-
•Parvovirus B19
-
•Dengue fever virus
-
•Malaria
-
•Babesia spp
-
•Plasmodium spp
-
•Leishmania spp
-
•Brucella spp
-
•New variant Creutzfeldt-Jakob disease prions
-
•Unknown pathogens
-
•
-
•
-
2
Transfusion reactions
-
3
Alloimmunisation
-
4
Medical errors (wrong blood to patient because of mislabelled specimen or patient misidentification)
-
5
Transfusion-associated acute lung injury
-
6
Transfusion-associated circulatory overload
-
7
Iron overload
-
8
Immunomodulation
-
9
Storage lesions (age of blood)
These considerations have given rise to the specialty of blood management, supported by corresponding initiatives to “promote the appropriate use of blood and blood components, with a goal of minimizing their use”. This movement has been motivated by the need to improve blood safety and patient outcomes, preserve the blood inventory, and constrain escalating costs.9 Patient blood management has been recognised by WHO as a means to “promote the availability of transfusion alternatives”.10 In 2010, blood management was cited as one of the ten key advances in transfusion medicine in the past 50 years.11
Awareness of risks, costs, and the effect on blood inventory has led providers to look at institution-based initiatives in patient blood management, such as the use of guidelines restricting use of transfusion to improve blood use.12 Patient blood management encompasses an evidence-based medical and surgical approach that is multidisciplinary (ie, including transfusion medicine specialists, surgeons, anaesthesiologists, and critical care specialists) and multiprofessional (ie, including physicians, nurses, pump technologists, and pharmacists). In this approach, preventive strategies are emphasised to identify, assess, and manage anaemia in medical13 and surgical14 patients, including use of pharmacological interventions15 and the avoidance of unnecessary diagnostic teting to minimise iatrogenic blood loss;16 to optimise homoeostasis17 and use of point-of-care testing;18 and to establish clinical practice guidelines for blood transfusions. With recent development of quality–performance indicators for patient blood management by health-care institutions and accreditation organisations,3 the accompanying Clinical Series in The Lancet is appropriate and timely, and looks at the effect of patient blood management on three areas of transfusion medicine: blood utilisation, alternatives to blood, and inventory management of the blood supply.
Acknowledgments
I declare that I have no conflicts of interest.
Footnotes
The target of the screening assay (antibody, microbial antigen, or microbial nucleic acid) and the year of assay implementation are shown in parentheses. Updated from reference 3.
References
- 1.The Joplin Globe Decline in need for blood leads to staff cuts at center. http://www.joplinglobe.com/local/x2015922821/Decline-in-need-for-blood-leads-to-staff-cuts-at-center (accessed April 10, 2013).
- 2.Zuck TF. Legal liability for transfusion injury in the acquired immunodeficiency syndrome era. Arch Pathol Lab Med. 1990;114:309–315. [PubMed] [Google Scholar]
- 3.Goodnough LT, Shander A. Patient blood management. Anesthesiology. 2012;116:1367–1376. doi: 10.1097/ALN.0b013e318254d1a3. [DOI] [PubMed] [Google Scholar]
- 4.Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080–2099. doi: 10.1111/j.1537-2995.2010.02851.x. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Center for Biologics Evaluation and Research Fatalities reported to FDA following blood collection and transfusion. Annual summary for fiscal year 2011. http://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/UCM300764.pdf (accessed Jan 8, 2013).
- 6.Pattakos G, Koch CG, Brizzio ME. Outcome of patients who refuse transfusion after cardiac surgery: a natural experiment with severe blood conservation. Arch Intern Med. 2012;172:1154–1160. doi: 10.1001/archinternmed.2012.2449. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons G, Glynn SA, Komaroff AL, for the Blood XMRV Scientific Research Working Group (SRWG) Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Science. 2011;334:814–817. doi: 10.1126/science.1213841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy WG. Of mad cows and bolted horses: the economics of blood safety. Transfusion. 2012;52:2278–2281. doi: 10.1111/j.1537-2995.2012.03931.x. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization 63rd World Health Assembly. Availability, safety and quality of blood products. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R12-en.pdf (accessed April 10, 2013).
- 11.McCullough J. Innovation in transfusion medicine and blood banking: documenting the record in 50 years of TRANSFUSION. Transfusion. 2010;50:2542–2546. doi: 10.1111/j.1537-2995.2010.02787.x. [DOI] [PubMed] [Google Scholar]
- 12.The New York Times Approaching Illness as a Team. Dec 24, 2012. http://www.nytimes.com/2012/12/25/opinion/approaching-illness-as-a-team-at-the-cleveland-clinic.html (accessed April 10, 2013).
- 13.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 14.Goodnough LT, Maniatis A, Earnshaw P. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodnough LT, Shander A. Special article: current status of pharmacologic therapies in patient blood management. Anesth Analg. 2013;116:15–34. doi: 10.1213/ANE.0b013e318273f4ae. [DOI] [PubMed] [Google Scholar]
- 16.Salisbury AC, Reid KJ, Alexander KP. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653. doi: 10.1001/archinternmed.2011.361. [DOI] [PubMed] [Google Scholar]
- 17.Goodnough LT, Shander A. How I treat warfarin-associated coagulopathy in patients with intracerebral hemorrhage. Blood. 2011;117:6091–6099. doi: 10.1182/blood-2010-11-316075. [DOI] [PubMed] [Google Scholar]
- 18.Despotis GJ, Joist JH, Goodnough LT. Monitoring of hemostasis in cardiac surgical patients: impact of point-of-care testing on blood loss and transfusion outcomes. Clin Chem. 1997;43:1684–1696. [PubMed] [Google Scholar]