Abstract
Background
Digitalis glycosides have been in clinical use for the treatment of heart failure (HF) for longer than 200 years. In recent years, several trials have been conducted to address concerns about their efficacy and toxicity.
Objectives
To examine the effectiveness of digitalis glycosides in treating HF in patients with normal sinus rhythm. To examine the effects of digitalis in patients taking diuretics and angiotensin‐converting enzyme inhibitors; in patients with varying severity and duration of disease; in patients with prior exposure to digitalis versus no prior exposure; and in patients with "HF due to systolic dysfunction" versus "HF with preserved ejection fraction."
Search methods
Searches on the following databases were updated in May 2013: The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and Dissertation Abstracts. Annual meeting abstracts of the American Heart Association, the American College of Cardiology, and the European Society of Cardiology were searched from 1996 to March 2013. In addition, reference lists provided by the pharmaceutical industry (GlaxoSmithKline and Covis Pharma) were searched.
Selection criteria
Included were randomized placebo‐controlled trials of 20 or more adult participants of either sex with symptomatic HF who were studied for seven weeks or longer. Excluded were trials in which the prevalence of atrial fibrillation was 2% or greater, or in which any arrhythmia that might compromise cardiac function or any potentially reversible cause of HF such as acute ischemic heart disease or myocarditis was present.
Data collection and analysis
Articles selected from the searches described above were evaluated in a joint effort of the review authors. The staff of the Cochrane Heart Group ran searches on the Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE.
Main results
No new studies were identified in the updated searches. Thirteen studies (7896 participants) are included, and major endpoints of mortality, hospitalization, and clinical status, based respectively on 8, 4, and 12 of these selected studies, were recorded and analyzed. The data show no evidence of a difference in mortality between treatment and control groups, whereas digitalis therapy is associated with lower rates of both hospitalization and clinical deterioration. The largest study, in which most participants were taking angiotensin‐converting enzyme inhibitors, showed a significant rise in “other cardiac” deaths, possibly due to arrhythmias. However collectively, these findings were based on studies done before beta‐blockers, as well as angiotensin receptor blockers and aldosterone antagonists, became widely used to treat HF.
Authors' conclusions
The literature indicates that digitalis may have a useful role in the treatment of patients with HF who are in normal sinus rhythm. New trials are needed to elucidate the importance of the dosage of digitalis and its usefulness in the era of beta‐blockers and other agents shown to be effective in treating HF.
Keywords: Humans, Heart Rate, Cardiotonic Agents, Cardiotonic Agents/therapeutic use, Cross‐Over Studies, Digitalis Glycosides, Digitalis Glycosides/therapeutic use, Disease Progression, Double‐Blind Method, Heart Failure, Heart Failure/drug therapy, Heart Failure/mortality, Heart Failure/physiopathology, Hospitalization, Hospitalization/statistics & numerical data, Randomized Controlled Trials as Topic
Plain language summary
Digitalis for treatment of heart failure in patients in sinus rhythm
Digitalis is a drug that is extracted from the leaves of the foxglove plant. It contains substances that stimulate heart muscle. The drug has been used for over two centuries to treat heart failure—a condition caused by inability of the injured heart to pump blood adequately. Other drugs that may be useful include diuretics, angiotensin‐converting enzyme inhibitors, and beta‐blockers, but digitalis may also be beneficial. The review of trials found that digitalis reduces hospitalization and can help to relieve symptoms of heart failure. More research is needed to show the full effects of digitalis.
Background
Until the past three decades, no full‐scale randomized trials of digitalis versus placebo were conducted, in part because of concerns about the use of placebo controls to replace the active agent. However, several clinical studies have appeared, showing that digitalis in many instances could be safely withdrawn in participants with HF (Dall 1970; Fonrose 1974; Gheorghiade 1983; Hull 1977; Johnston 1979; McHaffie 1978; Starr 1969). At the same time, studies appeared suggesting that digitalis may have favorable short‐term effects on exercise tolerance, symptoms, and cardiovascular event rates (Arnold 1980; Dobbs 1977; Firth 1980; Fleg 1991; Kirsten 1973; O'Rourke 1976; Vogel 1977). Some concern has focused on possible long‐term toxicity from digitalis, as well as other inotropic agents. The latter was suggested by studies of treatment with the phosphodiesterase inhibitors milrinone (Packer 1991) and enoximone (Cowley 1994), as well as other agents such as the inodilator vesnarinone (Cohn 1998). Digitalis itself was implicated in several nonrandomized trials suggesting that patients with HF treated with digitalis might show excessive mortality rates, particularly when the underlying clinical diagnosis was ischemic heart disease (Bigger 1985; Byington 1985; Moss 1981). Thus conflicting evidence shows that some studies have suggested that digitalis might be discontinued without adverse effects, others have indicated that beneficial effects might indeed be present, and still others have suggested that digitalis might have long‐term toxic effects.
Over the past 31 years, information has become available that helps to settle these points. Since 1982, 12 randomized controlled trials of digitalis in HF participants have been published (Blackwood 1990; Dig captopril 1988; Dig milrinone 1989; Dig xamoterol 1988; DIMT 1993; Fleg 1982; Guyatt 1988; Lee 1982; PROVED 1993; Pugh 1989; RADIANCE 1993; Taggart 1983), indicating that digitalis improves clinical outcomes—a conclusion reached in a meta‐analysis of the first seven of these trials published in 1990 (Jaeschke 1990). In addition, a large randomized controlled trial has been published showing that digitalis, while improving hospitalization rates, has no significant effect on long‐term mortality (DIG study 1997). The current review updates the 1990 meta‐analysis and provides a summary statement about the current status of digitalis in treating HF. Although the utility of digitalis glycosides for controlling heart rate in patients with rapid atrial fibrillation is well recognized, the current review is restricted to the use of digitalis in patients who are in normal sinus rhythm.
Objectives
To examine the effectiveness of digitalis glycosides in treating HF in patients with normal sinus rhythm. To examine the effects of digitalis in patients taking diuretics and angiotensin‐converting enzyme inhibitors; in patients with varying severity and duration of disease; in patients with prior exposure to digitalis versus no prior exposure; and in patients with "HF due to systolic dysfunction" versus "HF with preserved ejection fraction."
Methods
Criteria for considering studies for this review
Types of studies
The central feature of this review is a consideration of the 13 randomized controlled trials selected for inclusion, particularly the data they provide on mortality, hospitalization, and clinical status. Entry criteria were based on the earlier 1990 study (Jaeschke 1990) and included only double‐blind randomized trials with placebo controls, a treatment period of seven weeks or longer, and evaluation of each trial for sensibility of entry criteria, method of randomization, completeness of follow‐up, and method of handling withdrawals.
Types of participants
This review includes adult participants of both sexes with HF, older than 18 years of age, and of any ethnic group. Criteria for diagnosing HF varied among the studies, but all employed clinical criteria such as presence of dyspnea, orthopnea, rales, S3 gallop, neck vein distention, or peripheral edema. In addition, ejection fraction was measured in some studies. For the purposes of this review, the presence of an ejection fraction of 0.45 or less was considered as identifying a subgroup of individuals having "HF due to systolic dysfunction." Patients with an ejection fraction greater than 0.45 were considered to have "HF with preserved ejection fraction." Duration of HF and history of prior treatment with digitalis are noted when stated.
Digitalis is effective in slowing heart rate in patients with atrial fibrillation, although the effect is a modest one (DAAF Trial 1997; Jordaens 1997). This agent was formerly thought to be of value in causing reversion of atrial fibrillation to sinus rhythm, although the latter theory has been disproved (DAAF Trial 1997; Falk 1987; Jordaens 1997). The advent of many newer antiarrhythmic agents, which are also effective in reducing the heart rate in rapid atrial fibrillation, has somewhat restricted the use of acutely administered intravenous or oral digitalis in this condition, although it still is often used as long‐term oral therapy. Because atrial fibrillation is frequently present in patients with HF, exclusion of this group reduces the number of trials available for analysis in the current report. The therapeutic value of digitalis in patients with both HF and atrial fibrillation is, however, an important clinical issue and may be considered in a future review.
Types of interventions
The literature search included participants treated orally with any commonly used digitalis preparation, such as digoxin or digitoxin. For the purposes of this review, the term "digitalis" is used to refer to any of these treatments, although in practice, digoxin was the only agent employed in the 13 included studies. If the information was available, data are included on the incidence of prior treatment with digitalis, dosage levels utilized, and serum or plasma levels achieved. Use of other concurrent cardiac medications, including diuretics, angiotensin‐converting enzyme (ACE) inhibitors, and beta‐blocking agents, is also recorded.
Types of outcome measures
This review focuses on mortality, hospitalization, and clinical status in the 13 trials considered in the analysis. When available, data are also included on New York Heart Association (NYHA) class, quality of life as measured by various instruments, treadmill or bicycle exercise test or walk test performance, and various measures of myocardial size or performance.
The quality of data from trials accepted for the review is assessed by ascertaining the presence of factors such as concealment of randomization, blinding to allocation and outcome assessment, comparability at baseline, and losses to follow‐up. Sources of heterogeneity are identified by analyzing factors such as dose of digoxin, whether titration to blood level was employed, use of concomitant ACE inhibitor therapy, and whether the trial was a withdrawal trial.
Search methods for identification of studies
The searches, originally run in 2008 (Appendix 1) and re‐run for the previous update in April 2011 (Appendix 2), were updated in May 2013 (Appendix 3). The Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 4 of 12), MEDLINE (Ovid) (1946 to May 2013 Week 2), EMBASE (Ovid) (1947 to 2013 Week 20), and Dissertation Abstracts (to February 2013) were searched. For this update, we also searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed) on 21 May 2013 to identify any new records not available through MEDLINE.
Annual meeting abstracts of the American Heart Association, the American College of Cardiology, and the European Society of Cardiology were searched from 1996 to March 2013.
The analysis in MEDLINE was restricted to clinical trials for the period 1966 to 1984. Both clinical trials and reviews from 1985 to 2013 were examined. In addition, reference lists provided by the pharmaceutical industry (GlaxoSmithKline and Covis Pharma) were searched. We also searched the clinical study register of GlaxoSmithKline (http://www.gsk‐clinicalstudyregister.com/). Reference lists of identified studies and reviews were checked.
No language restrictions were applied.
Data collection and analysis
Quantitative information concerning mortality, hospitalization, and clinical deterioration was obtained directly from the articles. Each of these endpoints is presented as a separate meta‐analysis, using the algorithms supplied by Review Manager. The meta‐analyses are based on a fixed‐effect model, and data are presented using the Peto odds ratio.
Results
Description of studies
Previous searches up to April 2011 identified a total of 1854 articles of possible interest. From these, 19 papers were selected for further examination. Four papers were excluded (see Characteristics of excluded studies). Thirteen studies, reported in 15 papers, were included (see Characteristics of included studies and references for Included studies). Updated searches from May 2013 retrieved 383 new records, for a total of 2237 records examined to date. No new studies were identified.
The 13 studies included a total of 7896 participants. Of these, 7755 contributed to information on mortality, 7262 to information on the incidence of hospitalization due to worsening HF during the course of the study, and 1096 to information on clinical status. The 13 trials varied in size, characteristics of participant populations, duration, drug dosage, ancillary therapy for HF, and experimental design. The largest trial—the one of longest duration—is the study of the Digitalis Investigation Group (DIG study 1997), a multicenter parallel trial in which 6800 participants with "HF due to systolic dysfunction" were randomly assigned to digoxin or placebo and were followed over a period of three to five years. The primary endpoint of this study was mortality, but information was also provided on the incidence of hospitalization. Because of its size, the DIG study provided 87.7% of the participants included in the mortality analysis and 93.6% of those included in the hospitalization analysis. The DIG study also provided 98.0% of the weight to the meta‐analysis for mortality and 97.9% of the weight for hospitalization. However, the DIG study did not provide information on symptoms; these data were derived from the other 12 studies, all of which included this information.
Of the 13 studies, six had a parallel design with drug treatment provided to the active group (Blackwood 1990; Dig captopril 1988; Dig milrinone 1989; DIG study 1997; Dig xamoterol 1988; DIMT 1993), two were drug withdrawal studies (PROVED 1993; RADIANCE 1993), and five had a cross‐over design (Fleg 1982; Guyatt 1988; Lee 1982; Pugh 1989; Taggart 1983). Information derived from withdrawal studies should perhaps be viewed with circumspection (De Bono 1994) because all participants by definition were previously treated with digitalis and were able to tolerate the agent (RADIANCE 1993). Therefore they may represent a group that benefits from the use of digitalis, and this could create some bias in favor of the drug. In fact, this type of bias could also be encountered in parallel trials in which digitalis was initially withdrawn from all participants before randomization (Dig captopril 1988; DIG study 1997). Nonetheless, for the purposes of this review, withdrawal study data are lumped together with data from other groups. Of the 13 studies, eight were multicenter—six with a parallel design and two with a withdrawal design. Each of the other five studies, all with a cross‐over design, was carried out at a single institution. Taken together, data on mortality were derived from eight of the 13 studies, data on hospitalization from four, and data on clinical outcomes from 12. Thus no single study provided information on all three endpoints.
With exclusion of the study of the Digitalis Investigation Group (DIG study 1997), trial duration varied from seven weeks to six months; therefore much of the information reported is relatively short‐term. The size of these smaller trials ranged from 20 to 213 participants, with larger numbers recruited into the multicenter trials. Although in most instances, study duration was clearly specified by the protocol, in one study, it varied somewhat (Lee 1982). Participant characteristics included mean or median age (three studies reported only median age, i.e. Blackwood 1990; Dig xamoterol 1988; Pugh 1989), which ranged from 58 to 69 years, and with three exceptions (Blackwood 1990; Dig xamoterol 1988; Fleg 1982), all showed a predominance of male participants. All studies but two (Lee 1982; Pugh 1989) provided information about NYHA functional class, and most of the participants studied were NYHA Class II or III. Knowledge about severity of illness is of importance in assessing whether the effects of digitalis are dependent on this factor, and comments on this were made in several studies (Guyatt 1988; Lee 1982). On the other hand, inclusion of less severely ill participants, as may have been the case in one of the relevant studies (Dig xamoterol 1988), could limit the value of the study in contributing information on endpoints such as mortality, which clearly is higher in more advanced degrees of HF (CONSENSUS 1987).
All of the 13 studies provided information on the use of diuretics, which were being received by most participants in all but one trial (Dig xamoterol 1988). Five of the thirteen trials provided information on ACE inhibitors, and eight on beta‐blocking agents. Seven of the eight studies published before 1990 did not comment on the use of ACE inhibitors, most likely reflecting the fact that these agents were not yet widely available, and that the benefits of treatment with ACE inhibitors (CONSENSUS 1987; SOLVD 1991) had not yet been recognized. More recently, two trials have provided specific information on the actions of digoxin in the presence or absence of concurrent administration of ACE inhibitors. In the trial reported by Uretsky et al, participants taking ACE inhibitors were deliberately excluded, and the trial was ended in part because of growing evidence that treatment with ACE inhibitors was of value in HF (PROVED 1993). In the trial reported by Packer et al, participants taking ACE inhibitors were intentionally enrolled (RADIANCE 1993). In the large study conducted by the Digitalis Investigation Group, use of ACE inhibitors was encouraged, and 94% of participants were receiving this type of agent (DIG study 1997). However, comparable information is not available concerning the concurrent use of beta‐blocking agents and digoxin. Of the eight trials in which information about use of beta‐blocking agents was given, these agents were not being taken at all in five, and rates of administration in the remaining three trials were low, ranging from 9% to 20% (Fleg 1982; Pugh 1989; Taggart 1983).
The incidence of prior treatment with digitalis, reported in 10 of the 13 studies including by definition the two withdrawal studies, ranged from 46% to 100%. Duration of HF (range 16 months to 3.3 years) was reported in only three of the 13 studies (Dig captopril 1988; DIG study 1997; PROVED 1993). The etiology of HF, which was well characterized in all but one study (Dig xamoterol 1988), was predominantly ischemic. However, the cause was cardiomyopathy in one‐third or more of participants in three series (Lee 1982; PROVED 1993; RADIANCE 1993). The incidence of valvular disease was generally low, and such cases were excluded in three series (DIMT 1993; Guyatt 1988; PROVED 1993), whereas in two studies, the incidence was as high as 34% (Pugh 1989) and 17% (Fleg 1982). Patients with atrial fibrillation were excluded in all but two studies. One study (Dig xamoterol 1988) included a small number of participants with this arrhythmia (four of 213, or 1.9%), and in another, a single participant (one of 108, or 0.9%) had atrial fibrillation (DIMT 1993). It may also be noted that one study, which otherwise might have qualified for inclusion, was excluded because 13 of the 46 participants in the study (28.3%) had atrial fibrillation (Dobbs 1977). The mean or median dose of digoxin employed (one study reported only the median dose, i.e. PROVED 1993) ranged from 0.25 to 0.435 mg/d in the seven studies that provided this information, and mean serum or plasma levels ranged from 0.87 to 1.15 mg/mL in the six studies that provided these data.
Some information is also provided in the study conducted by the Digitalis Investigation Group on the subgroup of participants with "HF with preserved ejection fraction," who have an ejection fraction in the normal or low normal range (DIG study 1997). The cause of HF in this group may relate to restricted diastolic inflow into the left ventricle. The etiology, treatment, and prognosis for this group were less well defined than for participants with low ejection fraction who display "HF due to systolic dysfunction." Although it was thought previously that digitalis might be relatively contraindicated in "HF with preserved ejection fraction" (Gaasch 1994), this hypothesis was put to the test by random assignment of an additional 988 participants with HF who had an ejection fraction greater than 0.45 to treatment with digoxin versus placebo (DIG study 1997).
Risk of bias in included studies
Concealment of randomization was employed in all 13 of the studies included in this review, but blinding of outcome assessment is commented on in only five studies. Baseline comparability between control and treatment groups appeared to be achieved in all eight of the non‒cross‐over studies. Losses to follow‐up were small (less than 2%) in the three studies that provided these data. Titration to the level of the dose of digoxin was carried out in six of the trials. These data, plus information presented earlier, suggest that some heterogeneity in clinical characteristics was present among these trials. However, the Chi2 and I2 values that accompany the meta‐analyses (Analysis 1.1, Analysis 1.2, and Analysis 1.3) do not suggest that the degree of statistical heterogeneity was great.
1.1. Analysis.
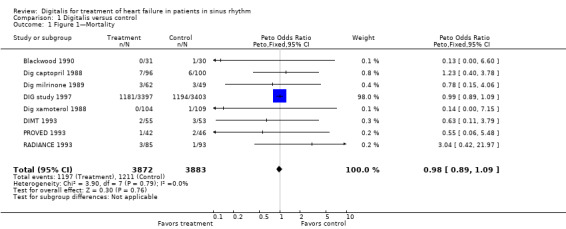
Comparison 1 Digitalis versus control, Outcome 1 Figure 1—Mortality.
1.2. Analysis.
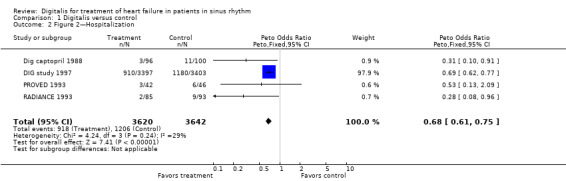
Comparison 1 Digitalis versus control, Outcome 2 Figure 2—Hospitalization.
1.3. Analysis.
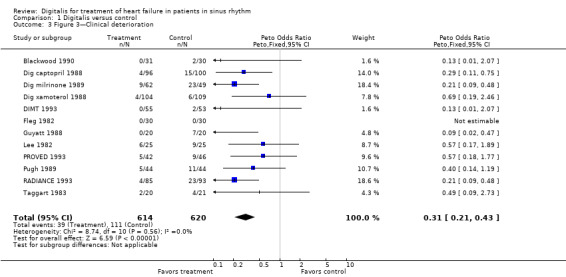
Comparison 1 Digitalis versus control, Outcome 3 Figure 3—Clinical deterioration.
Effects of interventions
Meta‐analyses on mortality, hospitalization, and clinical deterioration
A meta‐analysis of mortality figures for the eight studies that provided these data clearly showed that treatment with digoxin had no effect on death rate (Analysis 1.1). This observation is based almost entirely on findings in the trial conducted by the Digitalis Investigation Group (DIG study 1997). Although the number of deaths in the other seven studies was quite small, the results appear to be in accord with those of the DIG study (Analysis 1.1). Additional information is available from the study conducted by the Digitalis Investigation Group concerning the causes of mortality, which were assigned by investigators to the categories of "worsening heart failure" and "other cardiac" (DIG study 1997), the latter possibly including many deaths attributable to arrhythmia. The group treated with digoxin showed a trend toward a lower death rate due to "worsening heart failure" (P value 0.06) and a significant increase in death due to "other cardiac" causes (P value 0.04), although the latter was not a prespecified endpoint of the study. These two opposite directional changes tended to counterbalance one another, so that the overall effect on survival was neutral. However, the changes are consistent with a small beneficial effect of digoxin in preventing death directly caused by deterioration in cardiac function, which may have been offset by a small increase in mortality resulting from arrhythmias, observations of which are plausible in view of the known pharmacologic and electrophysiologic actions of digitalis. These findings may provide a basis for future investigations of factors that predispose to "other cardiac" death, although none are described in the report (DIG study 1997).
Four studies provided data on hospitalization for worsening HF (Analysis 1.2). The figure shows that hospitalization for worsening HF was significantly less common among participants taking digoxin than in the control group. Point estimates in terms of odds ratios are shown in the figure, with an overall relative risk reduction (RRR) of 23.4%. The baseline risk of hospitalization in the control groups in these four studies, if constant hazard over the study duration is assumed, ranged from 9.7% to 34.7%, with the larger figure a function of the much longer duration of the DIG study. Values for calculated numbers needed to treat for an additional beneficial outcome (NNTB) ranged from 13 to 17 (Table 1).
1. Hospitalization.
| Study | Baseline risk | Odds ratio | NNTB | Study duration | Comment |
| Dig captopril 1988 | 11.0% | 0.31 | 13 | six months | |
| DIG study 1997 | 34.7% | 0.69 | 13 | mean 37 months | Note NNTB similar despite longer study duration |
| PROVED 1993 | 13.0% | 0.53 | 17 | three months | |
| RADIANCE 1993 | 9.7% | 0.28 | 14 | three months |
Twelve trials presented data on cardiac symptoms (Analysis 1.3). The figure shows that the clinical status of participants taking digoxin was better than that of participants assigned to placebo therapy. Because these 12 smaller trials had many fewer participants than the DIG study, less than 15% of the entire study population was available to contribute to this meta‐analysis. Comparable data were not collected in the DIG study, although the group has published in abstract form a retrospective analysis suggesting that digoxin does ameliorate symptoms in HF (DIG study 1999). Point estimates in terms of odds ratios are shown in the figure, with an overall RRR of 64.5%. The baseline risk of clinical deterioration in the control groups in 11 of these studies in which this calculation could be made, assuming constant hazard over the study duration, ranged from 3.8% to 46.9%, and calculated NNTB from 3 to 61 (Table 2).
2. Clinical deterioration.
| Study | Baseline risk | Odds ratio | NNTB | Study duration |
| Blackwood 1990 | 6.7% | 0.13 | 15 | three months |
| Dig captopril 1988 | 15.0% | 0.29 | 10 | six months |
| Dig milrinone 1989 | 46.9% | 0.21 | 4 | 12 weeks |
| Dig xamoterol 1988 | 5.5% | 0.69 | 61 | three months |
| DIMT 1993 | 3.8% | 0.13 | 27 | six months |
| Guyatt 1988 | 35.0% | 0.09 | 3 | seven weeks |
| Lee 1982 | 36.0% | 0.57 | 9 | mean 53 days |
| PROVED 1993 | 19.6% | 0.57 | 14 | 12 weeks |
| Pugh 1989 | 25.0% | 0.40 | 8 | eight weeks |
| RADIANCE 1993 | 24.7% | 0.21 | 5 | three months |
| Taggart 1983 | 19.0% | 0.49 | 12 | three months |
Other observations
The total series of 13 studies included several other observations (see Characteristics of included studies). One of these is the result of treadmill or bicycle exercise testing and of the six‐minute walk test. Unfortunately, these data were not presented in a consistent manner, and no meta‐analysis could be done. However of the 10 studies using exercise testing (Blackwood 1990; Dig captopril 1988; Dig milrinone 1989; Dig xamoterol 1988; DIMT 1993; Fleg 1982; Guyatt 1988; PROVED 1993; Pugh 1989; RADIANCE 1993), functional capacity was significantly improved by digoxin in four (Dig milrinone 1989; DIMT 1993; PROVED 1993; RADIANCE 1993). Of the three studies in which a six‐minute walk test was carried out (Guyatt 1988; PROVED 1993; RADIANCE 1993), one showed a beneficial effect of digoxin (RADIANCE 1993). Of the eight studies in which assessment of NYHA class or evaluation of a "heart failure score" was performed (Dig captopril 1988; DIMT 1993; Guyatt 1988; Lee 1982; PROVED 1993; Pugh 1989; RADIANCE 1993; Taggart 1983), the results were significantly better with digoxin in four (Guyatt 1988; Lee 1982; Pugh 1989; RADIANCE 1993), whereas no significant change was noted in the other four studies. Quality of life was assessed in four studies (Blackwood 1990; Guyatt 1988; PROVED 1993; RADIANCE 1993) and in a substudy of the trial conducted by the Digitalis Investigation Group (Lader 2003), but a significant result favoring digoxin was noted in only one (RADIANCE 1993). Ejection fraction as a treatment endpoint was measured in five studies (Dig captopril 1988; Dig milrinone 1989; Lee 1982; PROVED 1993; RADIANCE 1993), and the measurement was significantly better with digoxin than placebo therapy in four of them (Dig captopril 1988; Dig milrinone 1989; PROVED 1993; RADIANCE 1993). Cardiothoracic ratio was an endpoint in four studies (Fleg 1982; Guyatt 1988; Lee 1982; Taggart 1983), with a result favoring digoxin noted in two of these studies (Guyatt 1988; Lee 1982). Left ventricular end‐diastolic dimension was measured in six studies (Fleg 1982; Guyatt 1988; Lee 1982; PROVED 1993; Pugh 1989; RADIANCE 1993), with a result favoring digoxin therapy in three of them (Fleg 1982; Lee 1982; RADIANCE 1993). In general, these findings suggest a beneficial effect from digitalis treatment, although the results were by no means uniform or consistent. One may conclude that digitalis does have favorable effects, but that they are not very pronounced and may not be observed in all patients. This point has been made in several of the publications included in this review (Dig xamoterol 1988; Fleg 1982; Guyatt 1988; Taggart 1983).
Additional observations were made in the ancillary study conducted by the Digitalis Investigation Group in 988 participants with "HF with preserved ejection fraction" (DIG study 1997). The pathophysiologic mechanism in this group of participants may relate to restricted diastolic inflow into the left ventricle. Concern has been expressed about whether treatment with digoxin is relatively contraindicated, as the agent could reduce ventricular diastolic compliance (Gaasch 1994). However, findings of the study conducted by the Digitalis Investigation Group do not support this concept (Ahmed 2006; DIG study 1997), as no difference in mortality was seen in participants with "HF with preserved ejection fraction" who received digoxin (N = 492) versus placebo (N = 496). In addition, a trend toward reduction in the combined outcome of death or hospitalization due to worsening heart failure was observed (risk ratio 0.82, 95% confidence interval 0.63 to 1.07).
Discussion
Use of digitalis in the treatment of HF has been considerably attenuated by the widespread acceptance of ACE inhibitors (CONSENSUS 1987; SOLVD 1991) and beta‐blocking agents (Carvedilol 1996; CIBIS II 1999; MERIT‐HF 1999; Packer 2001) for treating this syndrome. Use of ACE inhibitors and beta‐blocking agents is based on randomized clinical trials that conclusively show that both agents decrease mortality. Failure of digoxin to reduce mortality suggests that agents with a mortality benefit—ACE inhibitors and beta‐blocking agents—should be offered to patients in preference to the older drug. However, the present review does show that digoxin therapy can have beneficial effects, even in patients already treated with ACE inhibitors, and most participants in the trials that demonstrated benefits of digoxin for clinical status were also receiving diuretics. Based on these findings, clinicians can offer digoxin to patients who remain symptomatic despite treatment with ACE inhibitors and to those at appreciable risk of hospitalization, with reasonable expectation of benefit. Whether these findings generalize to patients receiving beta‐blockers is unknown at the present time, although a retrospective study showed no benefit of digoxin therapy in a group of predominantly male participants receiving contemporary therapy that included beta‐blockers (Dhaliwal 2008).
Beta‐blocking agents are now, in conjunction with ACE inhibitors, the cornerstone of treatment for HF (CIBIS II 1999; COPERNICUS 2002; MERIT‐HF 1999; SENIORS 2005). Any information yet to be derived in future trials about treatment of HF with digoxin will be obtained in the setting of concurrent treatment with ACE inhibitors plus beta‐blocking agents. Also of note are findings that the aldosterone inhibitors spironolactone (RALES 1999) and eplerenone (Zannad 2011) and the angiotensin receptor blockers valsartan (Cohn 2001) and candesartan (CHARM 2003) have beneficial long‐term effects in HF. All of these agents appear to be effective in groups of participants with substantial levels of digoxin usage: 73% in the spironolactone trial (RALES 1999), 27% in the eplerenone study (Zannad 2011), 67% in the valsartan trial (Cohn 2001), 43% in the candesartan trial (CHARM 2003), and more than 50% in some beta‐blocker trials (CIBIS II 1999; Packer 2001), with demonstrable continuing effectiveness in participants already receiving digitalis.
Although the DIG study showed no reduction in mortality among participants receiving digoxin, the overall effect on reduction of HF hospitalization of 7.9% was substantial (DIG study 1997). This effect occurred within one month of the start of therapy (Bourge 2013) and was sustained (Ahmed 2009). Regarding the use of digitalis in patients with HF of varying severity, individuals with impaired cardiac function who have few or no symptoms (NYHA Class I) and are at low risk for exacerbations or hospitalizations, as occurred in 25% of participants in one of the trials reported here (Dig xamoterol 1988), are very unlikely to receive important benefit from digoxin. The bulk of information available for digoxin therapy pertains to patients whose status is NYHA Class II or III, and this is the group in which digoxin improves symptoms and decreases exacerbations. Some evidence suggests that patients with more advanced symptoms may respond better to digoxin than those with less severe HF (Adams 1998; DIG study 1997; DIMT 1993; Gheorghiade 2013; Guyatt 1988; Lee 1982). In one study it was noted that participants with an S3 gallop and a dilated and poorly functioning ventricle may tend to respond most favorably to digoxin (Lee 1982). Other investigators have sought to confirm this observation but with somewhat less striking results (Guyatt 1988).
The issue of dosage of digitalis and its use in various subgroups has also been explored, with several publications based on retrospective analyses of the large trial carried out by the Digitalis Investigation Group (DIG study 1997). Such findings must be interpreted cautiously and need to be confirmed in prospective studies. Lower doses appear to be associated with beneficial effects (ACC/AHA Guidelines 2005; Adams 2002; ESC Guidelines 2008; HFSA Guideline 2006), as demonstrated in the large DIG study, in which the median baseline dosage of digoxin was 0.25 mg/d (DIG study 1997). A post‐hoc analysis of the DIG study suggested that lower doses and serum digoxin levels were associated with reduced mortality and hospitalization in comparison with the placebo group (Ahmed 2009), and another post‐hoc analysis of the results in the DIG study suggested that higher serum digoxin levels were associated with an increase in mortality (Rathore 2003). On the other hand, evidence shows that larger doses of digoxin are associated with improved efficacy (RADIANCE 1993; Slatton 1997). Furthermore, results from two of the trials (PROVED 1993; RADIANCE 1993) suggest that clinical efficacy was independent of the digoxin dosage (Adams 2002). In summary, results indicate that patients may receive comparable benefits from digoxin dosage regimens that are in current use, although some caution may be required when higher doses are employed.
With regard to differential effects in men and women, a post‐hoc analysis of the DIG study observed that digoxin therapy was associated with an increase in mortality in women, but not in men (Rathore 2002)—an observation supported by a recent cluster analysis of the DIG study data (Ather 2011). The difference was more pronounced in those with higher serum digoxin levels (Adams 2005). This gender differentiation is not, however, consistent (Domanski 2005). No evidence suggests that age (Rich 2001) or duration of HF is important in the decision of who should receive digoxin, and evidence from yet another DIG study post‐hoc analysis suggests that the drug is effective in low doses and with low serum digoxin levels in a geriatric population (Ahmed 2007). No evidence suggests a selective advantage for digoxin therapy in patients whose HF is of ischemic or cardiomyopathic origin.
Based on yet another post‐hoc analysis in a subset of participants in the DIG study (DIG study 1997), evidence that digoxin improves renal function has been observed (Testani 2011). In addition, beneficial effects of digoxin on the combined endpoint of cardiovascular death and hospitalization for worsening HF in the DIG study were comparable to those occurring in HF patients receiving the sinus node If inhibitor ivabradine (Castagno 2012). These findings are perhaps attributable to the bradycardic action of ivabradine comparable to heart rate slowing by digoxin resulting from its known effects on vagal tone. This observation may stimulate further studies to determine the importance of heart rate reduction in treating HF patients.
Although the emphasis in this review is on the beneficial effects of digitalis, it provides more valid data on effectiveness than on harm. However, toxic effects of digitalis therapy are well known and include adverse interactions with other drugs, electrolytes, and various disease states, as well as induction of proarrhythmias. In addressing the issue of postmarketing surveillance, the US Food and Drug Administration (FDA) archives provide an extensive list of these adverse effects but are lacking long‐term follow‐up in large numbers of patients analogous to the RCT approach used to evaluate beneficial effects.
Findings in the ancillary study of the Digitalis Investigation Group in patients having "HF with preserved ejection fraction" raise new questions about this entity (DIG study 1997). Although it is evident that a substantial fraction of patients with HF may share this pathophysiology (Jones 2004; Vasan 1999), the etiology, treatment, and prognosis for this group are less well defined than for patients having "HF due to systolic dysfunction." Some investigators have suggested that the prognosis may be more favorable in patients having "HF with preserved ejection fraction” than in those with "HF due to systolic dysfunction" (Vasan 1999), as was the case in the ancillary study (DIG study 1997). Although the DIG study ancillary trial showed a trend toward better outcomes in the group receiving digoxin for the combined endpoint of death or hospitalization due to worsening heart failure (DIG study 1997), it is still possible that digoxin is not beneficial or may even be harmful in several conditions in which "HF with preserved ejection fraction" is present. These may include such conditions as hypertrophic cardiomyopathy and amyloid infiltration of the myocardium (Chew 1975). Four participants in one of the series included in this review (Lee 1982) had a diagnosis of hypertrophic cardiomyopathy, but none of these participants responded positively to digoxin therapy.
The mechanism of action of digoxin is also of interest. Digitalis has multiple effects on cardiac function at the cellular level (Wasserstrom 2005), but the mechanism by which beneficial effects on the failing heart are produced is not fully understood. The traditional view has been that the drug exerts beneficial inotropic effects on the failing heart, but more recent evidence suggests that reduction of augmented adrenergic tone and renin‐angiotensin system activation in HF may also play a role (Packer 1992; Packer 1999). Evidence indicates that digoxin treatment of patients with HF can reduce sympathetic tone (Ferguson 1989; Gheorghiade 1991), as exemplified in one study among the 13 included in this report that demonstrated a reduction in circulating catecholamine levels in the digoxin‐treated group (DIMT 1993). Digoxin therapy may also inhibit the renin‐angiotensin system (Covit 1983) and augments vagal tone, as evidenced by an increase in heart rate variability (DIMT 1995) and frequent occurrence of sinus node slowing (Castagno 2012). These observations may relate to the question raised earlier regarding the effects of digoxin therapy in patients who are already taking beta‐blocking agents because the latter could abrogate the sympatholytic effects of digoxin (Hauptman 1999). However, ACE inhibitors also reduce sympathetic tone, yet digoxin is known to be effective in this group of patients (RADIANCE 1993).
The key question is whether digoxin added to a treatment regimen that already includes ACE inhibitors and beta‐blocking agents, or some combination of these agents with spironolactone or the angiotensin receptor blockers valsartan or candesartan, produces additional gains. The preferable sequence for adding digoxin, spironolactone, or an angiotensin receptor blocker to established treatment with ACE inhibitors and beta‐blockers remains uncertain at the present time. However currently available data indicate that spironolactone (RALES 1999), candesartan (CHARM 2003), and eplerenone (Zannad 2011) may reduce mortality and morbidity in patients with HF, and both digoxin (DIG study 1997) and valsartan (Cohn 2001), as well as candesartan (CHARM 2003), reduce hospitalization. Digoxin is as least as efficacious as valsartan and candesartan in this respect. Another issue is whether there are different indications for these agents in NYHA Class IV HF. Finally, there is the question of whether digoxin truly has a different mechanism of action in patients having "HF due to systolic dysfunction" versus "HF with preserved ejection fraction," and whether this is dependent on the underlying cause of the two syndromes. In patients having "HF with preserved ejection fraction," does digoxin actually have any therapeutic potential?
Authors' conclusions
Implications for practice.
The results of the present meta‐analyses further strengthen the concept that digoxin may have beneficial effects in treating patients with HF who remain symptomatic despite therapy with ACE inhibitors, diuretics, and possibly beta‐blockers. The agent has been shown to improve clinical status and to reduce hospitalizations. Although some authors have stated that the agent is of limited value, and its ultimate role in the current era of improved pharmacotherapy for HF remains untested, the agent may still have value as adjunctive therapy. The significant rise in ”other cardiac” deaths possibly due to arrhythmias warrants cautious use in patients at risk for such events.
Implications for research.
Several unanswered questions may ultimately deserve further investigation. Concerns about dosage and specific indications for digoxin therapy in different subgroups have led to the suggestion that additional clinical trials in the future may be warranted (Ahmed 2009; van Veldhuisen 2002). It has also been recommended that studies of the drug be carried out in acute heart failure syndromes (Gheorghiade 2009). Of considerable interest is the question whether there is truly a slight decrease in mortality due to "worsening heart failure" and an increase in mortality due to "other cardiac" causes in patients taking digoxin (DIG study 1997), but further studies on the point are lacking.
Despite more than two centuries of use, up‐to‐date information about digitalis is still not available in the current era, when several newer first‐line agents have been identified for the treatment of HF. The DIG study is now 16 years old, and although most of the study participants were taking ACE inhibitors, definitive information about interactions with beta‐blockers and with angiotensin receptor blockers and aldosterone inhibitors is lacking. Perhaps in the future, studies will be carried out to address these unknowns.
What's new
| Date | Event | Description |
|---|---|---|
| 12 April 2016 | Review declared as stable | No new studies since 2006 |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 30 July 2013 | New citation required but conclusions have not changed | No new studies were identified by the updated search. Minor changes were made to the Discussion. Conclusions have not changed. |
| 30 July 2013 | New search has been performed | Searches were re‐run in May 2013. |
| 13 April 2011 | New search has been performed | The search was re‐run in April 2011. No new studies were identified. Minor changes were made to the Discussion. Conclusions not changed. |
| 17 December 2008 | New search has been performed | The search was updated to November 2008. No new studies were identified. Minor changes were made to discussion. Conclusions not changed. |
| 7 September 2008 | Amended | Converted to new review format. |
| 3 October 2006 | Amended | The search was updated to August 2006. No new studies were found. Minor changes have been made to the discussion to take account of newly identified references. |
| 1 February 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors gratefully acknowledge the input of Professor Shah Ebrahim of the London School of Hygiene and Tropical Medicine, London, UK.
We also thank Dr. Fiona Taylor, Dr. Juan‐Pablo Casas, and Nicole Martin of the Cochrane Heart Group Office for their contributions to this update in 2013. We thank Dr. Jonathan Sterne, Theresa Moore, and Margaret Burke of the University of Bristol, UK, and Dr. Joey Kwong and Claire Williams of the Cochrane Heart Group Office for their support with previous versions of this review.
Appendices
Appendix 1. Search strategies 2008
CENTRAL
#1 DIGITALIS‐GLYCOSIDES*:ME #2 DIGITALIS #3 DIGOXIN #4 DIGITOXIN #5 (#1 or #2 or #3 or #4) #6 HEART‐FAILURE‐CONGESTIVE*:ME #7 (HEART near FAILURE) #8 (CARDIAC near FAILURE) #9 (#6 or #7 or #8) #10 (#5 and #9)
MEDLINE on Ovid (2008 November 13)
1 Digitalis/ 2 exp Digitalis Glycosides/ 3 digitalis.tw. 4 digoxin.tw. 5 digitoxin.tw. 6 or/1‐5 7 exp Heart Failure, Congestive/ 8 heart failure.tw. 9 cardiac failure.tw. 10 or/7‐9 11 6 and 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 Randomized controlled trials/ 15 random allocation/ 16 double blind method/ 17 single‐blind method/ 18 or/12‐17 19 exp animal/ not humans/ 20 18 not 19 21 clinical trial.pt. 22 exp Clinical Trials as Topic/ 23 (clin$ adj25 trial$).ti,ab. 24 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 25 placebos/ 26 placebo$.ti,ab. 27 random$.ti,ab. 28 research design/ 29 or/21‐28 30 29 not 19 31 30 or 20 32 11 and 31
EMBASE on Ovid (2008 week 44)
1 Digitalis Glycoside/ 2 Digoxin/ 3 Digitalis/ 4 Digitoxin/ 5 digitalis.tw. 6 digoxin.tw. 7 digitoxin.tw. 8 or/1‐7 9 exp Heart Failure/ 10 heart failure.tw. 11 cardiac failure.tw. 12 or/9‐11 13 8 and 12 14 controlled clinical trial/ 15 random$.tw. 16 randomized controlled trial/ 17 follow‐up.tw. 18 double blind procedure/ 19 placebo$.tw. 20 placebo/ 21 factorial$.ti,ab. 22 (crossover$ or cross‐over$).ti,ab. 23 (double$ adj blind$).ti,ab. 24 (singl$ adj blind$).ti,ab. 25 assign$.ti,ab. 26 allocat$.ti,ab. 27 volunteer$.ti,ab. 28 Crossover Procedure/ 29 Single Blind Procedure/ 30 or/14‐29 31 (exp animals/ or nonhuman/) not human/ 32 30 and 13
Appendix 2. Search strategies 2011
CENTRAL
#1 MeSH descriptor Digitalis Glycosides explode all trees #2 digitalis #3 digoxin #4 digitoxin #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Heart Failure explode all trees #7 heart near failure #8 cardiac near failure #9 (#6 OR #7 OR #8) #10 (#5 AND #9)
MEDLINE on Ovid (2011 March week 5)
1. Digitalis/ 2. exp Digitalis Glycosides/ 3. digitalis.tw. 4. digoxin.tw. 5. digitoxin.tw. 6. or/1‐5 7. exp Heart Failure/ 8. heart failure.tw. 9. cardiac failure.tw. 10. or/7‐9 11. 6 and 10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. Randomized controlled trials/ 15. random allocation/ 16. double blind method/ 17. single‐blind method/ 18. or/12‐17 19. exp animal/ not humans/ 20. 18 not 19 21. clinical trial.pt. 22. exp Clinical Trials as Topic/ 23. (clin$ adj25 trial$).ti,ab. 24. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 25. placebos/ 26. placebo$.ti,ab. 27. random$.ti,ab. 28. research design/ 29. or/21‐28 30. 29 not 19 31. 30 or 20 32. 11 and 31 33. ("20081114" or "20081115" or "20081116" or "20081117" or "20081118" or "20081119" or 2008112* or 2008113* or 2009* or 2010* or 2011*).ed. 34. 32 and 33
EMBASE on Ovid (2011 Week 14)
1. digitalis glycoside/ 2. digoxin/ 3. digitalis/ 4. digitalis/ 5. digitalis.tw. 6. digoxin.tw. 7. digitoxin.tw. 8. or/1‐7 9. exp heart failure/ 10. heart failure.tw. 11. cardiac failure.tw. 12. or/9‐11 13. 8 and 12 14. random$.tw. 15. factorial$.tw. 16. crossover$.tw. 17. cross over$.tw. 18. cross‐over$.tw. 19. placebo$.tw. 20. (doubl$ adj blind$).tw. 21. (singl$ adj blind$).tw. 22. assign$.tw. 23. allocat$.tw. 24. volunteer$.tw. 25. crossover procedure/ 26. double blind procedure/ 27. randomized controlled trial/ 28. single blind procedure/ 29. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. (animal/ or nonhuman/) not human/ 31. 29 not 30 32. 13 and 31 33. ("200845" or "200846" or "200847" or "200848" or "200849" or "200850" or "200851" or "200852" or 2009* or 2010* or 2011*).em. 34. 32 and 33 35. limit 34 to embase
Appendix 3. Search strategies 2013
CENTRAL
#1MeSH descriptor: [Digitalis Glycosides] explode all trees #2MeSH descriptor: [Digitalis] this term only #3digitalis #4digoxin #5digitoxin #6#1 or #2 or #3 or #4 or #5 #7MeSH descriptor: [Heart Failure] explode all trees #8heart near/6 failure #9cardiac near/6 failure #10#7 or #8 or #9 #11#6 and #10 #12((cardi* or heart* or myocard*) near/2 (failure* or incompet* or insufficien* or decompensat*)) #13#7 or #12 #14#13 and #6 #15#11 or #14
MEDLINE
1. Digitalis/ 2. exp Digitalis Glycosides/ 3. digitalis.tw. 4. digoxin.tw. 5. digitoxin.tw. 6. or/1‐5 7. exp Heart Failure/ 8. heart failure.tw. 9. cardiac failure.tw. 10. or/7‐9 11. 6 and 10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomized.ab. 15. placebo.ab. 16. drug therapy.fs. 17. randomly.ab. 18. trial.ab. 19. groups.ab. 20. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. exp animals/ not humans.sh. 22. 20 not 21 23. 11 and 22 24. (201104* or 201105* or 201106* or 201107* or 201108* or 201109* or 201110* or 201111* or 201112* or 2012* or 2013*).ed. 25. 23 and 24 26. ((heart or cardia* or myocard*) adj2 (fail* or insufficienc*)).tw. 27. 7 or 26 28. 6 and 22 and 27 29. 28 not 23 30. 25 or 29
EMBASE
1. digitalis glycoside/ 2. digoxin/ 3. digitalis/ 4. digitalis.tw. 5. digoxin.tw. 6. digitoxin.tw. 7. or/1‐6 8. exp heart failure/ 9. heart failure.tw. 10. cardiac failure.tw. 11. or/8‐10 12. 7 and 11 13. random$.tw. 14. factorial$.tw. 15. crossover$.tw. 16. cross over$.tw. 17. cross‐over$.tw. 18. placebo$.tw. 19. (doubl$ adj blind$).tw. 20. (singl$ adj blind$).tw. 21. assign$.tw. 22. allocat$.tw. 23. volunteer$.tw. 24. crossover procedure/ 25. double blind procedure/ 26. randomized controlled trial/ 27. single blind procedure/ 28. 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29. (animal/ or nonhuman/) not human/ 30. 28 not 29 31. 12 and 30 32. ("201115" or "201116" or "201117" or "201118" or "201119" or 20112* or 20113* or 20114* or 20115* or 2012* or 2013*).em. 33. 31 and 32 34. ((heart or cardia* or myocard*) adj2 (fail* or insufficienc*)).tw. 35. 8 or 34 36. 7 and 30 and 35 37. 36 not 31 38. 33 or 37 39. limit 38 to embase
PubMed
#4 #3 Filters: Publication date from 2011/01/01 to 2013/05/21 #3 #1 not #2 #2 (digitalis or digoxin or digitoxin) and (heart failure) Filters: MEDLINE #1 (digitalis or digoxin or digitoxin) and (heart failure)
Data and analyses
Comparison 1. Digitalis versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Figure 1—Mortality | 8 | 7755 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.89, 1.09] |
| 2 Figure 2—Hospitalization | 4 | 7262 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.61, 0.75] |
| 3 Figure 3—Clinical deterioration | 12 | 1234 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.21, 0.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blackwood 1990.
| Methods | three‐mo multicenter parallel trial with third xamoterol arm; endpoints—ET, QOL | |
| Participants | 61 pts; *median age 60; 50% male; 94% Class II, 6% Class III; 56% on diuretics; dx—˜67% ischemic *Note: Figures shown in "Participants" section show composite data from all three study arms, except for number of pts | |
| Interventions | dig dosage 0.25 mg/d | |
| Outcomes | for dig vs pla, no significant difference in ET, QOL | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—yes loss to follow‐up—? dig titrated to level—no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Dig captopril 1988.
| Methods | six‐mo multicenter parallel trial with third captopril arm; primary endpoint—ET; secondary endpoints—NYHA class, hospitalization, EF | |
| Participants | 196 pts; mean age 58; 83% male; 80% Class II; 87% on diuretics, none on beta‐blockers; mean EF 0.25; mean duration of HF 3.0 yrs; prior dig Rx in 68%; dx—61% ischemic, 31% cardiomyopathic | |
| Interventions | dig dosage of 0.125 to 0.375 mg/d titrated to serum levels of 0.7 to 2.5 ng/mL | |
| Outcomes | for dig versus pla, ET +10% & +6% (P value NS), EF +17% & +3% (P < 0.01); NYHA class improved in 31% of dig versus 22% of pla (P value NS) | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—yes loss to follow‐up—? dig titrated to level—yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Dig milrinone 1989.
| Methods | 12‐wk multicenter parallel trial with third milrinone arm & fourth dig + milrinone arm; endpoints—ET, EF | |
| Participants | 111 pts; mean age 60; 76% male; 32% Class II, 66% Class III; 100% on diuretics, none on beta‐blockers; mean EF 0.25; dx—53% ischemic, 32% idiopathic | |
| Interventions | dig dosage 0.125 to 0.5 mg/d | |
| Outcomes | ET +14% for dig (P < 0.03 compared with pla); EF +6.7% for dig, ‐8.2% for pla (P < 0.01) | |
| Notes | blind allocation—yes blinded to outcomes—yes baselines comparable—yes loss to follow‐up—? dig titrated to level—no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
DIG study 1997.
| Methods | Three‐ to five‐yr (mean follow‐up 37 mo) multicenter parallel trial; primary endpoint—mortality; secondary endpoint—hospitalization for HF | |
| Participants | 6800 pts with EF ≤ 0.45 in main trial; mean age 63; 88% male; 54% Class II, 31% Class III; 82% on diuretics, 94% on ACEI; EF 0.29 & 0.28 (dig & pla); median duration of HF 17 & 16 mo (dig & pla); prior dig Rx in 44%; dx—71% ischemic, 15% idiopathic. Note: ancillary trial carried out in 988 additional pts with EF > 0.45 | |
| Interventions | dig dosage at investigators' discretion; median baseline dosage in main trial of 0.25 mg/d | |
| Outcomes | main trial mortality, dig versus pla: overall 34.8% & 35.1% (P value 0.80), from "worsening heart failure" 11.6% & 13.2% (P value 0.06), from "other cardiac" 15.0% & 13.0% (P value 0.04); main trial hospitalization, dig versus pla: 26.8% & 34.7% (P < 0.001); ancillary trial mortality 23.4% in both groups | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—yes main trial loss to follow‐up—1.4% dig titrated to level—no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Dig xamoterol 1988.
| Methods | Three‐mo multicenter parallel trial with third xamoterol arm; endpoints—EX, symptoms, signs | |
| Participants | 213 pts; median age 62; 38% male; 25% Class I, 62% Class II, 13% Class III; 22% on diuretics, none on ACEI or beta‐blockers; prior dig Rx in 46%; dx—unspecified in 76% of pts | |
| Interventions | dig dosage 0.25 mg/d, mean plasma dig level 0.87 ng/mL | |
| Outcomes | for dig versus pla, no significant difference in EX; symptoms: Likert but not VAS improved by dig; signs: edema & rales improved by dig, but not JVP or hepatomegaly | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—yes loss to follow‐up—? dig titrated to level—no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
DIMT 1993.
| Methods | Six‐mo multicenter parallel trial with third ibopamine arm; primary endpoint—EX; secondary endpoints—"heart failure score," change in plasma norepinephrine | |
| Participants | 108 pts; mean age 61; 86% male; 80% Class II, 20% Class III; 100% on diuretics, none on ACEI or beta‐blockers; dx—69% ischemic, 31% idiopathic; valvular disease excluded | |
| Interventions | dig dosage 0.25 mg/d, mean plasma level 0.94 ng/mL | |
| Outcomes | for dig versus pla, EX +1.6% & ‐5.8% (P value 0.008); no significant difference in "heart failure score;" plasma norepinephrine values changed by ‐106 pg/mL for dig & +62 pg/mL for pla (P < 0.001) | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—yes loss to follow‐up—? dig titrated to level—no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Fleg 1982.
| Methods | Three‐mo cross‐over trial; endpoints—ET, CT ratio, LVED, FS, Vcf | |
| Participants | 30 pts; mean age 69; 47% male; 53% Class II, 43% Class III; 77% on diuretics, 13% on beta‐blockers; prior dig Rx in 87%; dx—63% ischemic, 17% valvular | |
| Interventions | dig dosage titrated to serum dig level of 1.0 to 2.0 ng/mL | |
| Outcomes | for dig versus pla, no difference in ET, CT ratio, FS; LVED 55.8 & 57.6 mm (P < 0.001), Vcf 0.90 & 0.82 circ/s (P < 0.05) | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—NA loss to follow‐up—? dig titrated to level—yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Guyatt 1988.
| Methods | Seven‐wk cross‐over trial; endpoints—"heart failure score," six‐minute walk test, CT ratio, QOL, LVED, FS, EX | |
| Participants | 20 pts; mean age 63; 90% male; 50% Class II, 40% Class III; 90% on diuretics; prior dig Rx in 85%; dx—85% ischemic; valvular disease excluded | |
| Interventions | dig dosage titrated to serum level of 1.54 to 2.56 nmol/L (1.2 to 2.0 ng/mL), mean dig dosage 0.391 mg/d | |
| Outcomes | for dig versus pla, "heart failure score" 2.3 & 4.4 (P value 0.001), six‐minute walk test 411 & 392 m (P value 0.055), CT ratio 0.53 & 0.58 (P value 0.04), FS 21% & 17% (P value 0.04); no significant difference in QOL profile, LVED, EX | |
| Notes | blind allocation—yes blinded to outcomes—yes baselines comparable—NA loss to follow‐up—? dig titrated to level—yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Lee 1982.
| Methods | cross‐over trial, mean treatment duration 53 days for both dig & pla; endpoints—"heart failure score," CT ratio, LVED, EF | |
| Participants | 25 pts; mean age 61; 72% male; 88% on diuretics; mean EF 0.29; prior dig Rx in 96%; dx—60% ischemic, 24% cardiomyopathic, 16% hypertrophic cardiomyopathy | |
| Interventions | dig dosage titrated to mean serum level of 1.15 ng/mL, mean dig dosage 0.435 mg/d | |
| Outcomes | for dig versus pla, "heart failure score" 2.0 & 3.6 (P < 0.05), CT ratio 0.51 & 0.53 (P value 0.00027), LVED 31 & 33 mm/sq m (P value 0.0026), EF 0.30 & 0.29 (P value NS) | |
| Notes | blind allocation—yes blinded to outcomes—yes baselines comparable—NA loss to follow‐up—? dig titrated to level—yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
PROVED 1993.
| Methods | 12‐wk multicenter withdrawal trial; primary endpoint—ET, six‐minute walk test; secondary endpoints—QOL, "heart failure score," global evaluation of progress, LVED, EF | |
| Participants | 88 pts; mean age 64; 85% male; 83% Class II or III; 100% on diuretics, none on ACEI or beta‐blockers; mean EF 0.28; mean duration of HF 3.3 yrs; prior dig Rx in 100%; dx—64% ischemic, 36% cardiomyopathic; valvular disease excluded | |
| Interventions | dig dosage titrated to mean serum level of 1.2 ng/mL, median digoxin dosage 0.375 mg/d | |
| Outcomes | for dig versus pla, median ET change +1% & ‐18% (P value 0.003); no significant difference in six‐minute walk test, QOL profile, "heart failure score," global evaluation of progress, LVED; EF +7% for dig & ‐10% for pla (P value 0.016) | |
| Notes | blind allocation—yes blinded to outcomes—yes baselines comparable—yes loss to follow‐up—1.1% dig titrated to level—yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Pugh 1989.
| Methods | Eight‐wk cross‐over trial; endpoints—clinical score, systolic time intervals (LVET, PEP, PEP/LVET, LVED, FS, Vcf, ET) | |
| Participants | 44 pts; median age 62; 73% male; 75% on diuretics, 20% on beta‐blockers; prior dig Rx in 100%; dx—61% ischemic, 34% valvular | |
| Interventions | dig dosage was "the patient's usual dose" | |
| Outcomes | for dig versus pla, worsened clinical score in 11% versus 25% of pts (P < 0.04); borderline decrease in PEP (P ≤ 0.08), no significant differences in LVED, PEP/LVET, FS, Vcf, ET | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—NA loss to follow‐up—? dig titrated to level—no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
RADIANCE 1993.
| Methods | Three‐mo multicenter withdrawal trial; primary endpoints—clinical deterioration, ET, six‐minute walk test; secondary endpoints—symptoms, QOL, functional class, LVED, EF | |
| Participants | 178 pts; mean age 60; 76% male; 73% Class II, 27% Class III; 100% on diuretics & ACEI; mean EF 0.27; prior dig Rx in 100%; dx—60% ischemic, 38% cardiomyopathic | |
| Interventions | dig dosage titrated to mean serum level of 1.2 ng/mL, mean dig dosage 0.38 mg/d | |
| Outcomes | for dig versus pla, ET 43 seconds greater (P value 0.033), six‐minute walk test 41 m further (P value 0.01), better self‐assessed symptoms (P value 0.007) & QOL profile (P value 0.04), less deterioration in class (10% vs 27%, P value 0.019); LVED ‐1.4% versus +3.0% (P value 0.04), EF ‐3.7% versus ‐13.3% (P value 0.001) | |
| Notes | blind allocation—yes blinded to outcomes—yes baselines comparable—yes loss to follow‐up—1.7% dig titrated to level—yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Taggart 1983.
| Methods | Three‐mo cross‐over trial; endpoints—"heart failure score," CT ratio, systolic time intervals (LVET, PEP, PEP/LVET) | |
| Participants | 22 participants; mean age 65; 64% male; 82% Class II; 95% on diuretics, 9% on beta‐blockers; prior dig Rx in 100%; dx—77% ischemic, 9% valvular | |
| Interventions | mean plasma dig level 1.2 ng/mL | |
| Outcomes | for dig versus pla, no significant difference in "heart failure score," CT ratio; LVET 388 & 403 msec (P < 0.001), PEP 128 & 138 msec (P < 0.001), PEP/LVET 0.39 & 0.41 (P < 0.02) | |
| Notes | blind allocation—yes blinded to outcomes—? baselines comparable—NA loss to follow‐up—? dig titrated to level—no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A—Adequate |
Abbreviations: ET = treadmill exercise time. class = NYHA functional class. EF = ejection fraction. pts = participants. HF = heart failure. dig = digoxin. Rx = treatment. dx = diagnosis. pla = placebo. ACEI = angiotensin‐converting enzyme inhibitors. EX = bicycle exercise. VAS = visual analogue scale. JVP = jugular venous pulse. CT ratio = cardiothoracic ratio. QOL = quality of life. LVED = left ventricular end‐diastolic dimension. FS = fiber‐shortening fraction. Vcf = circumferential fiber shortening velocity. LVET = left ventricular ejection time. PEP = pre‐ejection period.
mo = month.
wk = week.
yr = year. Note: For studies of dig versus pla with additional arms, all data shown are for the dig and pla groups only.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dobbs 1977 | Atrial fibrillation in 28.3% of participants |
| Fleg 1991 | Ten participants, studied using cross‐over design over two four‐week periods |
| Just 1993 | Study in 133 participants directed at individuals "without significantly reduced ejection fraction at rest" |
| Kostis 1994 | Seven participants randomly assigned to digitalis and six to placebo |
Contributions of authors
All review authors participated in preparation of the final review by preparing critiques of the protocol and of various drafts of the review. Statistical input was also provided (GHG and RJ).
Sources of support
Internal sources
University of Washington, USA.
College of Medicine, University of the Philippines, Philippines.
McMaster University, Canada.
University of Glasgow, Western Infirmary Glasgow, UK.
External sources
University of Rochester, USA.
Declarations of interest
None.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Blackwood 1990 {published data only}
- Blackwood R, Mayou RA, Garnham JC, Armstrong C, Bryant B. Exercise capacity and quality of life in the treatment of heart failure. Clinical Pharmacology & Therapeutics 1990;48:325‐32. [DOI] [PubMed] [Google Scholar]
Dig captopril 1988 {published data only}
- The Captopril‐Digoxin Multicenter Research Group. Comparative effects of therapy with captopril and digoxin in patients with mild to moderate heart failure. JAMA 1988;259:539‐44. [PubMed] [Google Scholar]
Dig milrinone 1989 {published data only}
- DiBianco R, Shabetai R, Kostuk W, Moran J, Schlant RC, Wright R. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. New England Journal of Medicine 1989;320:677‐83. [DOI] [PubMed] [Google Scholar]
DIG study 1997 {published data only}
- The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long‐term trial to evaluate the effect of digitalis on mortality in heart failure. Controlled Clinical Trials 1996;17:77‐97. [DOI] [PubMed] [Google Scholar]
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. New England Journal of Medicine 1997;336:525‐33. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Garg R, Held P, Gorlin R. Need for a large randomized trial to evaluate the effects of digitalis on morbidity and mortality in congestive heart failure. American Journal of Cardiology 1992;69:64G‐70G. [DOI] [PubMed] [Google Scholar]
Dig xamoterol 1988 {published data only}
- The German and Austrian Xamoterol Study Group. Double‐blind placebo‐controlled comparison of digoxin and xamoterol in chronic heart failure. Lancet 1988;1:489‐93. [PubMed] [Google Scholar]
DIMT 1993 {published data only}
- Veldhuisen DJ, Man in 't Veld AJ, Dunselman PHJM, Lok DJA, Dohmen HJM, Poortermans JC, et al. Double‐blind placebo‐controlled study of ibopamine and digoxin in patients with mild to moderate heart failure: results of the Dutch Ibopamine Multicenter Trial (DIMT). Journal of the American College of Cardiology 1993;22:1564‐73. [DOI] [PubMed] [Google Scholar]
Fleg 1982 {published data only}
- Fleg JL, Gottlieb SH, Lakatta EG. Is digoxin really important in treatment of compensated heart failure? A placebo‐controlled crossover study in patients with sinus rhythm. American Journal of Medicine 1982;73:244‐50. [DOI] [PubMed] [Google Scholar]
Guyatt 1988 {published data only}
- Guyatt GH, Sullivan MJJ, Fallen EL, Tihal H, Rideout E, Halcrow S, et al. A controlled trial of digoxin in congestive heart failure. American Journal of Cardiology 1988;61:371‐5. [DOI] [PubMed] [Google Scholar]
Lee 1982 {published data only}
- Lee DC, Johnson RA, Bingham JB, Leahy M, Dinsmore RE, Goroll AH, et al. Heart failure in outpatients. A randomized trial of digoxin versus placebo. New England Journal of Medicine 1982;306:699‐705. [DOI] [PubMed] [Google Scholar]
PROVED 1993 {published data only}
- Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. Journal of the American College of Cardiology 1993;22:955‐62. [DOI] [PubMed] [Google Scholar]
Pugh 1989 {published data only}
- Pugh SE, White NJ, Aronson JK, Grahame‐Smith DG, Bloomfield JG. Clinical, hemodynamic, and pharmacological effects of withdrawal and reintroduction of digoxin in patients with heart failure in sinus rhythm after long term treatment. British Heart Journal 1989;61:529‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
RADIANCE 1993 {published data only}
- Packer M, Gheorghiade M, Young JB, Costantini PJ, Adams KF, Cody RJ, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin‐converting‐enzyme inhibitors. RADIANCE study. New England Journal of Medicine 1993;329:1‐7. [DOI] [PubMed] [Google Scholar]
Taggart 1983 {published data only}
- Taggart AJ, Johnston GD, McDevitt DG. Digoxin withdrawal after cardiac failure in patients with sinus rhythm. Journal of Cardiovascular Pharmacology 1983;5:229‐34. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dobbs 1977 {published data only}
- Dobbs SM, Kenyon WI, Dobbs RJ. Maintenance digoxin after an episode of heart failure: placebo‐controlled trial in outpatients. British Medical Journal 1977;1:749‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleg 1991 {published data only}
- Fleg JL, Rothfeld B, Gottlieb SH. Effect of maintenance digoxin therapy on aerobic performance and exercise left ventricular function in mild to moderate heart failure due to coronary artery disease; a randomized, placebo‐controlled, crossover trial. Journal of the American College of Cardiology 1991;17:743‐51. [DOI] [PubMed] [Google Scholar]
Just 1993 {published data only}
- Just H, Drexler H, Taylor SH, Siegrist J, Schulgen G, Schumacher M, for the CADS Study Group. Captopril versus digoxin in patients with coronary artery disease and mild heart failure. Herz 1993;18 Suppl 1:436‐43. [PubMed] [Google Scholar]
Kostis 1994 {published data only}
- Kostis JB, Rosen RC, Cosgrove NM, Shindler DM, Wilson AC. Nonpharmacologic therapy improves functional and emotional status in congestive heart failure. Chest 1994;106:996‐1001. [DOI] [PubMed] [Google Scholar]
Additional references
ACC/AHA Guidelines 2005
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005;112:e154‐235. [DOI] [PubMed] [Google Scholar]
Adams 1998
- Adams KF, Gheorghiade M, Uretsky BF, Young JB, Patterson JH, Tomasko L, et al. Clinical predictors of worsening heart failure during withdrawal from digoxin therapy. American Heart Journal 1998;135:389‐97. [DOI] [PubMed] [Google Scholar]
Adams 2002
- Adams KF Jr, Gheorghiade M, Uretsky BF, Patterson JH, Schwartz TA, Young JB. Clinical benefits of low serum digoxin concentrations in heart failure. Journal of the American College of Cardiology 2002;39:946‐53. [DOI] [PubMed] [Google Scholar]
Adams 2005
- Adams KF Jr, Patterson JH, Gattis WA, O'Connor CM, Lee CR, Schwartz TA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the Digitalis Investigation Group Trial. Journal of the American College of Cardiology 2005;46:497‐504. [DOI] [PubMed] [Google Scholar]
Ahmed 2006
- Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure. The Ancillary Digitalis Investigation Group Trial. Circulation 2006;114:397‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2007
- Ahmed A. Digoxin and reduction in mortality and hospitalization in geriatric heart failure: importance of low doses and low serum concentrations. Journal of Gerontology A Biological Sciences and Medical Sciences 2007;62:323‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2009
- Ahmed A, Waagstein F, Pitt B, White M, Zannad F, Young JB, et al. Effectiveness of digoxin in reducing one‐year mortality in chronic heart failure in the Digitalis Investigation Group trial. American Journal of Cardiology 2009;103:82‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnold 1980
- Arnold SB, Byrd RC, Meister W, Melmon K, Cheitlin MD, Bristow JD, et al. Long‐term digitalis therapy improves left ventricular function in heart failure. New England Journal of Medicine 1980;303:1443‐8. [DOI] [PubMed] [Google Scholar]
Ather 2011
- Ather S, Peterson LF, Divakaran VG. Deswal A, Ramasubbu K, Giorgberidze I, et al. Digoxin treatment in heart failure—Unveiling risk by cluster analysis of DIG data. International Journal of Cardiology 2011;150:264‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bigger 1985
- Bigger JT Jr, Fleiss JL, Rolnitzky LM, Merab JP, Ferrick KJ. Effect of digitalis treatment on survival after acute myocardial infarction. American Journal of Cardiology 1985;55:623‐30. [DOI] [PubMed] [Google Scholar]
Bourge 2013
- Bourge RC, Fleg JL, Fonarow GC, Cleland JGF, McMurray JJV, Veldhuisen DJ, et al. Digoxin reduces 30‐day all‐cause hospital admission in older patients with chronic systolic heart failure. American Journal of Medicine 2013;126:701‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byington 1985
- Byington R, Goldstein S. Association of digitalis therapy with mortality in survivors of acute myocardial infarction: observations in the Beta‐Blocker Heart Attack Trial. Journal of the American College of Cardiology 1985;6:976‐82. [DOI] [PubMed] [Google Scholar]
Carvedilol 1996
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. New England Journal of Medicine 1996;334:1349‐55. [DOI] [PubMed] [Google Scholar]
Castagno 2012
- Castagno D, Petrie MC, Claggert B, McMurray J. Should we SHIFT our thinking about digoxin? Observations on ivabradine and heart rate reduction in heart failure. European Heart Journal 2012;33:1137‐41. [DOI] [PubMed] [Google Scholar]
CHARM 2003
- Pfeffer MA. Swedberg K. Granger CB, Held P, McMurray JJV, Michelson EL, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM‐Overall programme. Lancet 2003;362:759‐66. [DOI] [PubMed] [Google Scholar]
Chew 1975
- Chew C, Ziady GM, Raphael MJ, Oakley CM. The functional defect in amyloid heart disease: the "stiff heart" syndrome. American Journal of Cardiology 1975;36:438‐44. [DOI] [PubMed] [Google Scholar]
CIBIS II 1999
- CIBIS II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999;353:9‐13. [PubMed] [Google Scholar]
Cohn 1998
- Cohn JN, Goldstein SO, Greenberg BH, Lorell BH, Bourge RC, Jaski BE, et al. Vesnarinone Trial Investigators. A dose‐dependent increase in mortality with vesnarinone among patients with severe heart failure. New England Journal of Medicine 1998;339:1810‐6. [DOI] [PubMed] [Google Scholar]
Cohn 2001
- Cohn JN, Tognoni G, for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. New England Journal of Medicine 2001;345:1667‐75. [DOI] [PubMed] [Google Scholar]
CONSENSUS 1987
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). New England Journal of Medicine 1987;316:1429‐35. [DOI] [PubMed] [Google Scholar]
COPERNICUS 2002
- Packer M, Fowler MB, Roecker EB, Coats AJS, Katus HA, Krum H, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106:2194‐9. [DOI] [PubMed] [Google Scholar]
Covit 1983
- Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin‐angiotensin system by intravenous digoxin in chronic congestive heart failure. American Journal of Medicine 1983;75:445‐7. [DOI] [PubMed] [Google Scholar]
Cowley 1994
- Cowley AJ, Skene AM, Enoximone Investigators. Treatment of severe heart failure: quantity or quality of life? A trial of enoximone. British Heart Journal 1994;72:226‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
DAAF Trial 1997
- The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group. Intravenous digoxin in acute atrial fibrillation. Results of a randomized placebo‐controlled multicentre trial in 239 patients. European Heart Journal 1997;18:649‐54. [DOI] [PubMed] [Google Scholar]
Dall 1970
- Dall JLC. Maintenance digoxin in elderly patients. British Medical Journal 1970;2:705‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Bono 1994
- Bono D. Digoxin in eurhythmic heart failure: PROVED or "not proven?". Lancet 1994;343:128‐9. [DOI] [PubMed] [Google Scholar]
Dhaliwal 2008
- Dhaliwal AS, Bredikis A, Habib G, Carabello BA, Ramasubbu K, Bozkurt B. Digoxin and clinical outcomes in systolic heart failure patients. American Journal of Cardiology 2008;102:1256‐60. [DOI] [PubMed] [Google Scholar]
DIG study 1999
- Tsuyuki RT, Yusuf S, Montague TJ, Teo KK, Arnold JMO, Davies RF, et al. Effects of digoxin on worsening of symptoms of congestive heart failure in the Digitalis Investigation Group (DIG) Trial. Circulation 1999;100:I‐537. [Google Scholar]
DIMT 1995
- Brouwer J, Veldhuisen DJ, Man in 't Veld AJ, Dunselman PH, Boomsma F, Haaksma J, et al. Heart rate variability in patients with mild to moderate heart failure: effects of neurohormonal modulation by digoxin and ibopamine. The Dutch Ibopamine Multicenter Trial (DIMT) Study Group. Journal of the American College of Cardiology 1995;26:983‐90. [DOI] [PubMed] [Google Scholar]
Domanski 2005
- Domanski M, Fleg J, Bristow M, Knox S. The effect of gender on outcome in digitalis‐treated heart failure patients. Journal of Cardiac Failure 2005;11:83‐6. [DOI] [PubMed] [Google Scholar]
ESC Guidelines 2008
- Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). European Journal of Heart Failure 2008;10:933‐99. [DOI] [PubMed] [Google Scholar]
Falk 1987
- Falk RH, Knowlton AA, Bernard SA, Gottlieb NE, Battinelli NJ. Digoxin for converting recent‐onset atrial fibrillation to sinus rhythm. A randomized double‐blinded trial. Annals of Internal Medicine 1987;106:503‐6. [DOI] [PubMed] [Google Scholar]
Ferguson 1989
- Ferguson DW, Berg WJ, Sanders JS, Roach PJ, Kempf JS, Kienzle MG. Sympathoinhibitory responses to digitalis glycosides in heart failure patients: direct evidence from sympathetic neural recordings. Circulation 1989;80:65‐77. [DOI] [PubMed] [Google Scholar]
Firth 1980
- Firth BG, Dehmer GJ, Corbet JR, Lewis SE, Parkey RW, Willerson JT. Effect of chronic oral digoxin therapy on ventricular function at rest and peak exercise in patients with ischemic heart disease. American Journal of Cardiology 1980;46:481‐90. [DOI] [PubMed] [Google Scholar]
Fonrose 1974
- Fonrose HA, Ahlbaurn N, Bugatch E, Cohen M, Genovese C, Kelly J. The efficacy of digitalis withdrawal in an institutional aged population. Journal of the American Geriatric Society 1974;22:208‐11. [DOI] [PubMed] [Google Scholar]
Gaasch 1994
- Gaasch WH. Diagnosis and treatment of heart failure based on left ventricular systolic or diastolic dysfunction. JAMA 1994;271:1276‐80. [PubMed] [Google Scholar]
Gheorghiade 1983
- Gheorghiade M, Beller GA. Effects of discontinuing maintenance digoxin therapy in patients with ischemic heart disease and congestive heart failure in sinus rhythm. American Journal of Cardiology 1983;51:1243‐50. [DOI] [PubMed] [Google Scholar]
Gheorghiade 1991
- Gheorghiade M, Ferguson D. Digoxin. A neurohumoral modulator in heart failure?. Circulation 1991;84:2181‐6. [DOI] [PubMed] [Google Scholar]
Gheorghiade 2009
- Gheorghiade M, Braunwald E. Reconsidering the role for digoxin in the management of acute heart failure syndromes. JAMA 2009;302:2146‐7. [DOI] [PubMed] [Google Scholar]
Gheorghiade 2013
- Gheorghiade M, Patel K, Fillippatos G, Anker SD, Veldhuisen DJ, Cleland JGF, et al. Effect of oral digoxin in high‐risk heart failure patients: a pre‐specified subgroup analysis of the DIG trial. European Journal of Heart Failure 2013;15:551‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauptman 1999
- Hauptman PJ, Garg R, Kelly RA. Cardiac glycosides in the next millennium. Progress in Cardiovascular Diseases 1999;41:247‐54. [DOI] [PubMed] [Google Scholar]
HFSA Guideline 2006
- Heart Failure Society of America. HFSA 2006 comprehensive heart failure practice guideline. Section 7: Heart failure in patients with left ventricular systolic dysfunction. Journal of Cardiac Failure 2006;12:38‐57. [Google Scholar]
Hull 1977
- Hull SM, Mackintosh A. Discontinuation of maintenance digoxin therapy in general practice. Lancet 1977;2:1054‐55. [DOI] [PubMed] [Google Scholar]
Jaeschke 1990
- Jaeschke R, Oxman AD, Guyatt GH. To what extent do congestive heart failure patients in sinus rhythm benefit from digoxin therapy? A systematic overview and meta‐analysis. American Journal of Medicine 1990;88:279‐86. [DOI] [PubMed] [Google Scholar]
Johnston 1979
- Johnston GD, McDevitt DG. Is maintenance digoxin necessary in patients with sinus rhythm?. Lancet 1979;1:567‐70. [DOI] [PubMed] [Google Scholar]
Jones 2004
- Jones RC, Francis GS, Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis Investigation Group Trial. Journal of the American College of Cardiology 2004;44:1025‐9. [DOI] [PubMed] [Google Scholar]
Jordaens 1997
- Jordaens L, Trouerbach J, Calle P, Tavernier R, Derycke E, Vertongen P, et al. Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. European Heart Journal 1997;18:643‐8. [DOI] [PubMed] [Google Scholar]
Kirsten 1973
- Kirsten E, Rodstein M, Luster Z. Digoxin in the aged. Geriatrics 1973;28:95‐101. [PubMed] [Google Scholar]
Lader 2003
- Lader E, Egan D, Hunsberger S, Garg R, Czajkowski S, McSherry F. The effect of digoxin on the quality of life in patients with heart failure. Journal of Cardiac Failure 2003;9:4‐12. [DOI] [PubMed] [Google Scholar]
McHaffie 1978
- McHaffie D, Purcell H, Mitchell‐Heggs P, Guz A. The clinical value of digoxin in patients with heart failure and sinus rhythm. Quarterly Journal of Medicine 1978;47:401‐19. [PubMed] [Google Scholar]
MERIT‐HF 1999
- MERIT‐HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999;353:2001‐7. [PubMed] [Google Scholar]
Moss 1981
- Moss AJ, Davis HT, Conard DL, DeCamilla JJ, Odoroff CL. Digitalis‐associated cardiac mortality after myocardial infarction. Circulation 1981;64:1150‐6. [DOI] [PubMed] [Google Scholar]
O'Rourke 1976
- O'Rourke RA, Henning H, Theroux P, Crawford MH, Ross J Jr. Favorable effects of orally administered digoxin on left heart size and ventricular wall motion in patients with previous myocardial infarction. American Journal of Cardiology 1976;37:708‐15. [DOI] [PubMed] [Google Scholar]
Packer 1991
- Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Research Group. New England Journal of Medicine 1991;325:1468‐75. [DOI] [PubMed] [Google Scholar]
Packer 1992
- Packer M. The neurohumoral hypothesis: a theory to explain the mechanism of disease progression in heart failure. Journal of the American College of Cardiology 1992;20:248‐54. [DOI] [PubMed] [Google Scholar]
Packer 1999
- Packer M, Cohn JN, Abraham WT, Colucci WS, Fowler MB, Greenberg BH, et al. Consensus recommendations for the management of chronic heart failure. On behalf of the membership of the advisory council to improve outcomes nationwide in heart failure. American Journal of Cardiology 1999;83:1A‐38A. [PubMed] [Google Scholar]
Packer 2001
- Packer M, Coats AJS, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. New England Journal of Medicine 2001;344:1651‐8. [DOI] [PubMed] [Google Scholar]
RALES 1999
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. New England Journal of Medicine 1999;341:709‐17. [DOI] [PubMed] [Google Scholar]
Rathore 2002
- Rathore SS, Wang Y, Krumholz HM. Sex‐based differences in the effect of digoxin for the treatment of heart failure. New England Journal of Medicine 2002;347:1403‐11. [DOI] [PubMed] [Google Scholar]
Rathore 2003
- Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 2003;289:871‐8. [DOI] [PubMed] [Google Scholar]
Review Manager [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rich 2001
- Rich MW, McSherry F, Williford WO, Yusuf S. Effect of age on mortality, hospitalizations, and response to digoxin in patients with heart failure: the DIG study. Journal of the American College of Cardiology 2001;38:806‐13. [DOI] [PubMed] [Google Scholar]
SENIORS 2005
- Flather MD, Shibata MC, Coats AJS, Veldhuisen DJ, Parkhomenko A, Borbola J, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). European Heart Journal 2005;26:215‐25. [DOI] [PubMed] [Google Scholar]
Slatton 1997
- Slatton ML, Irani WN, Hall SA, Marcoux LG, Pae RL, Grayburn PA, et al. Does digoxin provide additional hemodynamic and autonomic benefit at higher doses in patients with mild to moderate heart failure and normal sinus rhythm?. Journal of the American College of Cardiology 1997;29:1206‐13. [DOI] [PubMed] [Google Scholar]
SOLVD 1991
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New England Journal of Medicine 1991;325:293‐302. [DOI] [PubMed] [Google Scholar]
Starr 1969
- Starr I, Luchi RJ. Blind study on the action of digitoxin on elderly women. American Heart Journal 1969;78:740‐51. [DOI] [PubMed] [Google Scholar]
Testani 2011
- Testani JM, Brisco MA, Wilson Tang WH, Kimmel SE, Tiku‐Owens A, Forfia PR, et. al. Potential effects of digoxin on long‐term renal and clinical outcomes in chronic heart failure. Journal of Cardiac Failure 2011;191:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Veldhuisen 2002
- Veldhuisen DJ. Low‐dose digoxin in patients with heart failure. Less toxic and at least as effective?. Journal of the American College of Cardiology 2002;39:954‐6. [DOI] [PubMed] [Google Scholar]
Vasan 1999
- Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population‐based cohort. Journal of the American College of Cardiology 1999;33:1948‐55. [DOI] [PubMed] [Google Scholar]
Vogel 1977
- Vogel R, Frischknecht J, Steele P. Short‐ and long‐term effects of digitalis on resting and posthandgrip hemodynamics in patients with coronary artery disease. American Journal of Cardiology 1977;40:171‐6. [DOI] [PubMed] [Google Scholar]
Wasserstrom 2005
- Wasserstrom JA, Aistrup GL. Digitalis: new actions for an old drug. American Journal of Physiology, Heart and Circulatory Physiology 2005;289:H1781‐93. [DOI] [PubMed] [Google Scholar]
Zannad 2011
- Zannad F, McMurray JJV, Krum H, Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. New England Journal of Medicine 2011;364:12‐21. [Google Scholar]


