Abstract
Background
Partner notification (PN) is the process whereby sexual partners of an index patient are informed of their exposure to a sexually transmitted infection (STI) and the need to obtain treatment. For the person (index patient) with a curable STI, PN aims to eradicate infection and prevent re‐infection. For sexual partners, PN aims to identify and treat undiagnosed STIs. At the level of sexual networks and populations, the aim of PN is to interrupt chains of STI transmission. For people with viral STI, PN aims to identify undiagnosed infections, which can facilitate access for their sexual partners to treatment and help prevent transmission.
Objectives
To assess the effects of different PN strategies in people with STI, including human immunodeficiency virus (HIV) infection.
Search methods
We searched electronic databases (the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE) without language restrictions. We scanned reference lists of potential studies and previous reviews and contacted experts in the field. We searched three trial registries. We conducted the most recent search on 31 August 2012.
Selection criteria
Published or unpublished randomised controlled trials (RCTs) or quasi‐RCTs comparing two or more PN strategies. Four main PN strategies were included: patient referral, expedited partner therapy, provider referral and contract referral. Patient referral means that the patient notifies their sexual partners, either with (enhanced patient referral) or without (simple patient referral) additional verbal or written support. In expedited partner therapy, the patient delivers medication or a prescription for medication to their partner(s) without the need for a medical examination of the partner. In provider referral, health service personnel notify the partners. In contract referral, the index patient is encouraged to notify partner, with the understanding that the partners will be contacted if they do not visit the health service by a certain date.
Data collection and analysis
We analysed data according to paired partner referral strategies. We organised the comparisons first according to four main PN strategies (1. enhanced patient referral, 2. expedited partner therapy, 3. contract referral, 4. provider referral). We compared each main strategy with simple patient referral and then with each other, if trials were available. For continuous outcome measures, we calculated the mean difference (MD) with 95% confidence intervals (CI). For dichotomous variables, we calculated the risk ratio (RR) with 95% CI. We performed meta‐analyses where appropriate. We performed a sensitivity analysis for the primary outcome re‐infection rate of the index patient by excluding studies with attrition of greater than 20%. Two review authors independently assessed the risk of bias and extracted data. We contacted study authors for additional information.
Main results
We included 26 trials (17,578 participants, 9015 women and 8563 men). Five trials were conducted in developing countries. Only two trials were conducted among HIV‐positive patients. There was potential for selection bias, owing to the methods of allocation used and of performance bias, owing to the lack of blinding in most included studies. Seven trials had attrition of greater than 20%, increasing the risk of bias.
The review found moderate‐quality evidence that expedited partner therapy is better than simple patient referral for preventing re‐infection of index patients when combining trials of STIs that caused urethritis or cervicitis (6 trials; RR 0.71, 95% CI 0.56 to 0.89, I2 = 39%). When studies with attrition greater than 20% were excluded, the effect of expedited partner therapy was attenuated (2 trials; RR 0.8, 95% CI 0.62 to 1.04, I2 = 0%). In trials restricted to index patients with chlamydia, the effect was attenuated (2 trials; RR 0.90, 95% CI 0.60 to 1.35, I2 = 22%). Expedited partner therapy also increased the number of partners treated per index patient (three trials) when compared with simple patient referral in people with chlamydia or gonorrhoea (MD 0.43, 95% CI 0.28 to 0.58) or trichomonas (MD 0.51, 95% CI 0.35 to 0.67), and people with any STI syndrome (MD 0.5, 95% CI 0.34 to 0.67). Expedited partner therapy was not superior to enhanced patient referral in preventing re‐infection (3 trials; RR 0.96, 95% CI 0.60 to 1.53, I2 = 33%, low‐quality evidence). Home sampling kits for partners (four trials) did not result in lower rates of re‐infection in the index case (measured in one trial), or higher numbers of partners elicited (three trials), notified (two trials) or treated (one trial) when compared with simple patient referral. There was no consistent evidence for the relative effects of provider, contract or other patient referral methods. In one trial among men with non‐gonococcal urethritis, more partners were treated with provider referral than with simple patient referral (MD 0.5, 95% CI 0.37 to 0.63). In one study among people with syphilis, contract referral elicited treatment of more partners than provider referral (MD 2.2, 95% CI 1.95 to 2.45), but the number of partners receiving treatment was the same in both groups. Where measured, there was no statistical evidence of differences in the incidence of adverse effects between PN strategies.
Authors' conclusions
The evidence assessed in this review does not identify a single optimal strategy for PN for any particular STI. When combining trials of STI causing urethritis or cervicitis, expedited partner therapy was more successful than simple patient referral for preventing re‐infection of the index patient but was not superior to enhanced patient referral. Expedited partner therapy interventions should include all components that were part of the trial intervention package. There was insufficient evidence to determine the most effective components of an enhanced patient referral strategy. There are too few trials to allow consistent conclusions about the relative effects of provider, contract or other patient referral methods for different STIs. More high‐quality RCTs of PN strategies for HIV and syphilis, using biological outcomes, are needed.
Keywords: Female, Humans, Male, Chlamydia Infections, Chlamydia Infections/therapy, Chlamydia Infections/transmission, Contact Tracing, Contact Tracing/methods, Gonorrhea, Gonorrhea/therapy, Gonorrhea/transmission, Randomized Controlled Trials as Topic, Sexual Partners, Sexually Transmitted Diseases, Sexually Transmitted Diseases/prevention & control, Sexually Transmitted Diseases/transmission, Urethritis, Urethritis/therapy, Uterine Cervicitis, Uterine Cervicitis/therapy
Plain language summary
Strategies for partner notification for sexually transmitted infections, including HIV.
Sexually transmitted infections (STI) are a major global cause of acute illness, infertility and death. Every year there are an estimated 499 million new cases of the most common curable STIs (trichomoniasis, chlamydia, syphilis and gonorrhoea), and between two and three million new cases of HIV. The presence of several STIs, including syphilis and herpes can increase the risk of acquiring or transmitting HIV.
Partner notification (PN) is a process whereby sexual partners of patients given a diagnosis of STI are informed of their exposure to infection and the need to receive treatment. PN for curable STI may prevent re‐infection of the patient and reduce the risk of complications and further spread.
A review update of the research of the strategies of partner notification in people with STI, including human immunodeficiency virus (HIV) infection was conducted by researchers in the Cochrane Collaboration. After searching for all relevant studies, they found 26 studies. This review covers four main PN strategies: 1) Patient referral means that the patient tells their sexual partners that they need to be treated, either with (enhanced) or without (simple) additional support to enhance outcomes. 2) Expedited partner therapy means that the patient delivers medication or a prescription for medication to their partner(s) without the need for a medical examination of the partner. 3) Provider referral means that health service personnel notify the partners. 4) Contract referral means that the patient is encouraged to notify partners but health service personnel will contact them if they do not visit the health service by a certain date.
The 26 trials in this review included 17,578 participants. Five trials were conducted in developing countries and only two trials were performed among HIV‐positive patients. Expedited partner therapy was more successful than simple patient referral in reducing repeat infection in patients with gonorrhoea, chlamydia or non‐gonococcal urethritis (six trials). Expedited partner therapy and enhanced patient referral resulted in similar levels of repeat infection (three trials). Evidence about the effects of home sampling, where patients with chlamydia received a sample kit for the partner, was inconsistent (three trials). There were too few trials to allow consistent conclusions about the relative effects of provider, contract or other patient referral methods for different STIs. More studies need to be performed on HIV and syphilis and harms need to be measured and reported.
Summary of findings
Summary of findings for the main comparison. Enhanced patient referral compared with simple patient referral for partner notification for STIs, including HIV.
| Enhanced patient referral compared with simple patient referral for partner notification for STIs, including HIV | ||||||
| Health problem: partner notification for STIs, including HIV Settings: people in rural and urban areas, given a diagnosis of STI (clinically or by a laboratory) in health services Intervention: enhanced patient referral Comparison: simple patient referral | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Simple patient referral | Enhanced patient referral | |||||
| Re‐infection in index patient ‐ home sampling vs. simple patient referral Follow‐up: 12 months | Study population | RR 2.14 (0.91 to 5.05) | 220 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 64 per 1000 | 136 per 1000 (58 to 321) | |||||
| Moderate | ||||||
| 64 per 1000 | 137 per 1000 (58 to 323) | |||||
| Re‐infection in index patient ‐ information booklet vs. simple patient referral Follow‐up: 8 weeks | Study population | RR 0.55 (0.22 to 1.33) | 942 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 180 per 1000 | 99 per 1000 (40 to 239) | |||||
| Moderate | ||||||
| 156 per 1000 | 86 per 1000 (34 to 207) | |||||
| Re‐infection in index patient ‐ patient referral (DIS/health advisor) vs. patient referral (nurse) Follow‐up: 6 weeks | Study population | RR 0.35 (0.01 to 8.51) | 140 (1 study) | ⊕⊕⊝⊝ low5 | ||
| 14 per 1000 | 5 per 1000 (0 to 118) | |||||
| Moderate | ||||||
| 14 per 1000 | 5 per 1000 (0 to 119) | |||||
| Re‐infection in index patient ‐ disease‐specific website vs. simple referral Follow‐up: 1 weeks | Study population | RR 3.12 (0.17 to 58.73) | 105 (1 study) | ⊕⊕⊝⊝ low6 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Re‐infection in index patient ‐ additional counselling vs. simple patient referral Follow‐up: 6 months | Study population | RR 0.49 (0.27 to 0.89) | 600 (1 study) | ⊕⊕⊕⊝ moderate7 | ||
| 101 per 1000 | 50 per 1000 (27 to 90) | |||||
| Moderate | ||||||
| 101 per 1000 | 49 per 1000 (27 to 90) | |||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DIS: disease intervention specialist; RR: risk ratio; STI: sexually transmitted infection. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Method of allocation concealment was not reported. 70% completed follow‐up, some were lost to follow‐up and some withdrew from the study, reasons for withdrawal were not reported. Study was not blinded. 2 Assuming alpha of 0.05 and beta of 0.2. For relative risk reduction of 20% with best estimate of control event rate of 0.2 approximately 3000 participants were required. The total sample size was 220 and did not meet the optimal information size. 3 High attrition rate and no information given on method of allocation concealment in one of the studies. Different methods were used for outcome assessment 4 I2 = 76% (P value = 0.06) and minimal overlap of CIs. 5 Sample size less than 400, there were very few events and CIs around both relative and absolute estimates include both appreciable benefit and appreciable harm. 6 Sample size was very small and optimal information size was not met. There were very few events and CIs overlapped, therefore, no effect both for absolute and relative estimates. 7 Risk for selective reporting and unclear method of allocation concealment.
Summary of findings 2. Expedited partner therapy compared with simple patient referral for partner notification for STIs, including HIV.
| Expedited partner therapy compared with simple patient referral for partner notification for STIs, including HIV | ||||||
| Health problem: partner notification for STIs, including HIV Settings: people in rural and urban areas, given a diagnosis of STI (clinically or by a laboratory) in health services Intervention: expedited partner therapy Comparison: simple patient referral | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Simple patient referral | EPT | |||||
| Re‐infection in index patients Follow‐up: 2‐12 months | Study population | RR 0.71 (0.56 to 0.89) | 6018 (6 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 110 per 1000 | 78 per 1000 (62 to 98) | |||||
| Moderate | ||||||
| 84 per 1000 | 60 per 1000 (47 to 75) | |||||
| Re‐infection in index patients ‐ chlamydia Follow‐up: 3‐12 months | Study population | RR 0.9 (0.6 to 1.35) | 2007 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 114 per 1000 | 102 per 1000 (68 to 154) | |||||
| Moderate | ||||||
| 92 per 1000 | 83 per 1000 (55 to 124) | |||||
| Re‐infection in index patients ‐ trichomonas | Study population | RR 0.67 (0.34 to 1.28) | 631 (2 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 67 per 1000 | 45 per 1000 (23 to 85) | |||||
| Moderate | ||||||
| 67 per 1000 | 45 per 1000 (23 to 86) | |||||
| Re‐infection in index patients ‐ chlamydia or gonorrhoea Follow‐up: 4‐18 weeks | Study population | RR 0.61 (0.39 to 0.94) | 3380 (2 studies) | ⊕⊕⊝⊝ low5,6 | ||
| 116 per 1000 | 71 per 1000 (45 to 109) | |||||
| Moderate | ||||||
| 164 per 1000 | 100 per 1000 (64 to 154) | |||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was high attrition rate in three of the studies. Methods of sequence generation and allocation concealment not reported in two of the studies. 2 CI includes possibility of no effect (i.e. RR of 1.0). 3 Method of sequence generation and allocation concealment not reported in one of the studies. There was high attrition rate in one of the studies. 4 Sample size was greater than 400 but CI overlaps, therefore, no effect (i.e. RR of 1.0). 5 There were no details on method of sequence generation and allocation concealment. One of the studies had a high attrition rate. 6 I2 = 74%
Summary of findings 3. Expedited partner therapy compared with enhanced patient referral for partner notification for STIs, including HIV.
| Expedited partner therapy compared with enhanced patient referral for partner notification for STIs, including HIV | ||||||
| Health problem: partner notification for sexually transmitted infections, including HIV Settings: people in rural and urban areas, given a diagnosis of STI (clinically or by a laboratory) in health services Intervention: expedited partner therapy Comparison: enhanced patient referral | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Enhanced patient referral | EPT | |||||
| EPT vs. enhanced patient referral ‐ re‐infection in index patients Follow‐up: 1‐12 months | Study population | RR 0.96 (0.6 to 1.53) | 1220 (3 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 92 per 1000 | 88 per 1000 (55 to 140) | |||||
| Moderate | ||||||
| 86 per 1000 | 83 per 1000 (52 to 132) | |||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EPT: expedited partner therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No details on method of sequence generation in one of the studies. One study had high attrition rate and one study used different methods for outcome assessment. 2 Sample size is high but CI includes appreciable benefit and harms with both relative risk reduction and increase being greater than 25%.
Summary of findings 4. Contract referral compared with expedited partner therapy for partner notification for STIs, including HIV.
| Contract referral compared with expedited partner therapy for partner notification for STIs, including HIV | ||||||
| Health problem: partner notification for sexually transmitted infections, including HIV Settings: people in rural and urban areas, given a diagnosis of STI (clinically or by a laboratory) in health services Intervention: contract referral Comparison: expedited partner therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| EPT | Contract referral | |||||
| Re‐infection in index patient Follow‐up: 3 months | Study population | RR 2 (0.7 to 5.72) | 322 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 99 per 1000 | 198 per 1000 (69 to 565) | |||||
| Moderate | ||||||
| 99 per 1000 | 198 per 1000 (69 to 566) | |||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Method of sequence generation and allocation concealment not reported. The study had high attrition rate. No blinding. 2 Imprecision owing to small sample size.
Background
Description of the condition
Sexually transmitted infections (STI) have a negative impact on the social, health and economic well‐being of a country. Every year an estimated 499 million new cases of the four most common curable STI, trichomoniasis, chlamydia, syphilis and gonorrhoea, are acquired (WHO 2012). Furthermore, two to three million new cases of human immunodeficiency virus (HIV) occur per year (UNAIDS 2010). Up to 4000 infants become blind annually due to eye infections attributable to underlying gonococcal and chlamydial infections in the mother (WHO 2007).
The term STI includes both infections that remain latent or asymptomatic and those that progress to a clinical manifestation (disease). In this update, we used the term STI instead of sexually transmitted diseases (STD), which was used in the original review. STI are more prevalent in countries and communities where socio‐economic conditions are poor (Glasier 2006; Low 2006a). Curable STIs are often overshadowed by the burden of HIV, but are important causes of morbidity in their own right (Table 5).
1. Burden of disease.
| Disease | DALYs |
| HIV | 58.5 million |
| Chlamydia trachomatis | 3.7 million |
| Gonorrhoea | 3.5 million |
| Other | 280,000 |
Source: WHO 2004.
DALY: disability adjusted life years.
Clinical symptoms of STIs can be non‐specific and, where possible, the diagnosis needs to be confirmed by laboratory testing. In lower‐income countries, laboratory testing is not always available and women and men reporting symptoms suggestive of an STI are often treated according to algorithms without confirmatory tests. For male urethritis and genital ulcers, this approach is effective but with vaginal discharge the risk of misdiagnosis is high. Syndromic management of STI can therefore lead to over‐treatment and adverse social consequences such as stigma and intimate partner violence (Trollope‐Kumar 2006). Women are more likely than men to suffer from reproductive tract complications of STIs such as chlamydia and gonorrhoea if the infection ascends to the upper genital tract; pelvic inflammatory disease (PID), ectopic pregnancies and infertility are the most commonly documented complications (Gerbase 1998). STIs are, however, often asymptomatic in both women and men (WHO 2007). As a result, disclosing a diagnosis of an STI to sexual partners and partner treatment play a critical part in the comprehensive management of STI. Willingness to disclose varies according to the STI and gender (Alam 2010). In one study among people with a diagnosis of HIV, 85% of people living with HIV were sexually active, but only 58% revealed their HIV status to recent sexual partners (Simbayi 2007). In a study in Connecticut, US, 25% of females with chlamydia intended not to notify their partners (Niccolai 2007) as most (46%) thought it unimportant and 43% were not willing to discuss the condition. In a study in India, the patient characteristics most likely to increase the odds of referring a partner were having a diagnosis of genital ulcer disease (odds ratio (OR) 2.78, 95% confidence interval (CI) 1.08 to 7.13, P value = 0.033) and having the intention to inform the regular partners (OR 16.9, 95% CI 3.29 to 86.70, P value = 0.001) (Sahasrabuddhe 2002).
Description of the intervention
"Partner notification is a process that includes informing sexual partners of infected people of their exposure, administering presumptive treatment, and providing advice about the prevention of future infection" (UNAIDS 1999). Partner notification (PN) is also known as contact tracing, partner management or partner information. A person with a newly diagnosed STI is often referred to as an 'index case' or 'index patient'. The index patient has one or more sexual partners. The sexual partners of the index patient might have been the source of the infection in the index patient or they might have acquired the infection from the index patient.
A variety of approaches has been used to notify sexual partners and to ensure that they receive treatment. In principle, managing infection in people with more than one current sexual partner should have the greatest impact on the spread of STI (Fenton 1997). The use of different approaches depends partly on the STI for which they were originally intended. There are other influences at the country level, including cultural factors, the structure and financing of health systems, and clinical consensus. At the individual level, factors such as patient choice influence choice of PN strategies. Traditionally, three main approaches have been defined: patient referral, provider referral and contract (or conditional) referral. Definitions and explanations of these PN methods are given below.
Patient referral (patient‐led referral) refers to an approach in which health service personnel encourage index patients to notify their own partners. In this review, we used the term simple patient referral to refer to spoken advice from health service personnel about the need for sexual partners to receive treatment. This can be seen as a minimum standard for a PN intervention. There is, however, no agreement about the content of a consultation for simple patient referral. Patient referral was developed in the 1970s when rates of gonorrhoea in the US were very high and the capacity of specialist PN personnel was exceeded. Patient referral has since become the preferred method of PN for gonorrhoea and subsequently chlamydia in many countries. There has been great interest in developing methods to support index patients so that the outcomes of patient referral can be improved or enhanced (Trelle 2007). Patient referral can, therefore, be split into two categories (simple and enhanced), according to the level of support given to the patient. Expedited partner therapy (EPT) has developed in the US since the late 1990s as a new patient‐led strategy to help index patients to get their partners treated more quickly.
Enhanced patient referral refers to a group of strategies that supplement the spoken advice with the aim of improving patient referral success, including educational material such as videos viewed in waiting rooms, written disease‐specific information for index patients to give to their partners, home sampling kits for partners, disease‐specific websites, theory‐based counselling and reminders by telephone or other means (Trelle 2007).
EPT is a group of strategies to enhance the success of patient referral by increasing the numbers of partners treated and speeding up the time to treatment (CDC 2006). The EPT strategies include: patient‐delivered partner medication (PDPM) or patient‐delivered partner therapy (PDPT), where the index patient receives antibiotics (often in a package with condoms and written information) to give to their partner without the need for a medical examination of the partner (Golden 2005); or additional prescriptions given to index patients for their partner(s). EPT can reduce loss to follow‐up of index cases (Young 2007), and reduce the risk of repeated infection in the index case (Golden 2005). There are, however, disadvantages, including the risk of adverse drug reactions, other underlying disease remaining undetected and a missed opportunity for counselling and testing for other STIs including HIV (Golden 2005). In some countries, such as the UK, EPT is not legal unless the partner is assessed before receiving antibiotic treatment (ECDC 2013).
Provider referral (provider‐led referral) uses third parties (usually specialist health service personnel) to notify partners. The name of these health professionals differs between countries, for example; 'disease intervention specialists' (DIS) in the US; 'health advisers' in the UK and 'Kurators' in Sweden. Provider referral originated in Scandinavia and the UK as a method to trace and refer the sexual partners of people with syphilis when treatment first became available. More recently, it has been used for other clinically severe STIs such as HIV infection and hepatitis B. It can also be used for other STIs such as gonorrhoea and chlamydia when the index patient is unable to notify partners by themselves. Provider referral should only be done with the explicit consent of the index patient. In some countries, for example France, provider referral does not occur because it is seen as an invasion of privacy (ECDC 2013).
Contract referral (conditional referral) refers to an approach in which there is an agreement (contract) between the patient and the health professional. Health service personnel encourage index patients to notify their partners, with the understanding that health service personnel will notify those partners who do not visit the health service by an agreed date. Contract referral is, in practice, difficult to define as a separate PN approach. It can be difficult to distinguish from provider referral if the time window for patient referral is very short (two or three days) (Peterman 1997). In contrast, contract referral is often used as an extension to simple patient referral, rather than a separate strategy, if the index patient has not been able to inform their partner(s) when they are followed up.
How the intervention might work
There are different aims of PN, depending on the level at which it is targeted and the infection (Low 2006a). At the level of the index patient with a curable STI the aim is to provide concurrent antibiotic treatment to the sexual partner(s) so that infection can be eradicated in both people and re‐infection prevented in the index patient, which is a clinical goal. For the sexual partner(s) the aim is to identify and treat infection that might have been the source of infection in the index patient, or might have been acquired from the index patient. At the level of sexual networks and populations, the aim is to interrupt chains of transmission and reduce the spread of STIs, which is a public health goal. For viral STIs, the aim is to identify previously undiagnosed infections, which can provide early access for sexual partners to treatment and prevent onward transmission through behavioural change by the infected person.
To succeed, PN strategies need to first elicit from the index patient details of all sexual partners from whom he/she may have acquired the infection, or whom he/she might have subsequently infected. Identifying partners in the latent period of infection (usually three months for primary syphilis and one month for acute urethritis) (Toomey 1996), should identify those from whom infection was acquired, while identifying partners after the onset of symptoms will identify those who were likely to have been infected by the index case. The time period for identifying partners differs between countries for different STIs.
For most PN strategies, eliciting partner information from infected people is a prerequisite to notifying sexual partners. For example, when health service personnel notify partners, they rely on the index patient to count, name and provide details to enable all his/her partners to be traced. Once partners have been elicited, PN strategies need to provide either the index patient or the health service personnel with the necessary knowledge, skills or resources to enable them to locate, notify, medically evaluate and test or treat these partners.
Communication between partners, during which the index patient encourages them to consider screening or treatment, has been identified as a critical point in effective PN strategies (Young 2007). The communication usually requires the index patient to disclose their STI diagnosis. Disclosure can lead to benefits other than successful partner treatment, such as emotional support and protecting the health of others. Disclosure can also lead to stigma, rejection, physical abuse and discrimination (Arnold 2008).
Why it is important to do this review
PN has been practised as a measure to control STIs since the early 1900s (ECDC 2013), but there is limited evidence of its public health impact. Many evaluations have not been conducted as randomised controlled trials (RCTs) and many were conducted in developed countries before the HIV/acquired immunodeficiency syndrome (AIDS) pandemic. It is not known whether interventions developed for high‐income countries are applicable to resource‐limited settings.
There are several published systematic reviews of PN. The first included only studies conducted in developed countries (Oxman 1994). Another included only published studies conducted in the US after 1980 (Macke 1999). The original Cochrane Review by Mathews et al. was assessed as up to date in July 2001 (Mathews 2001). Trelle et al. systematically reviewed studies of enhanced methods of patient referral, including EPT, to improve the effectiveness of simple patient referral (Trelle 2007). The latest systematic review only studied curable STIs in developing countries (Alam 2010). Considering the ongoing developments in this field, the Cochrane Review was updated in line with recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Objectives
To assess the effects of alternative PN strategies.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that compared at least two PN strategies.
Types of participants
People in rural and urban areas, given a diagnosis of STI (clinically or by a laboratory) in health services with any of the following STI: gonorrhoea (Neisseria gonorrhoeae), chlamydia (Chlamydia trachomatis), trichomoniasis (Trichomonas vaginalis), syphilis (Treponema pallidum), chancroid (Haemophilus ducreyi), genital herpes, hepatitis B and HIV. We also included diagnoses of the following STI syndromes: genital ulcer syndrome ‐ non‐vesicular or vesicular, urethral discharge syndrome, vaginal discharge syndrome and lower abdominal pain in women. Studies conducted in any type of health service were included.
Types of interventions
Strategies directed at patients (patient‐led) or health workers (provider‐led) were included. The following types of strategies were included:
strategies to enhance the effectiveness of patient referral through, for example, health education and counselling, health education materials (such as pamphlets, posters, video and audio productions), patient assistance strategies directed at facilitating patient referral (such as referral cards, incentives, reminders, video and audio productions). EPT was included as a specific type of enhanced patient referral;
contract referral strategies;
provider referral strategies;
combinations of the above.
Types of outcome measures
Primary outcomes
Number of index patients with curable STIs given a clinical or laboratory diagnosis of re‐infection. Re‐infection implies re‐infection of the index patient with the same STI from an untreated sexual partner. In practice, the outcome measured is repeated detection of the STI at some time interval after the index case has been treated. Repeated detection of an STI could also result from a new infection in the index case acquired from a new sexual partner, or treatment failure due to antibiotic resistance or subtherapeutic dosing. These causes cannot be reliably distinguished and the term re‐infection is used to include repeated detection from any cause.
Secondary outcomes
Numbers of partners elicited (sexual partners that the health professional obtains from the index patient for the recall period in question), located (sexual partners that the index patient was able to find; this number is likely to be a subset of partners elicited), notified (sexual partners that the index patient informed of their possible exposure to an STI; this number is likely to be a subset of partners located), presenting for care, testing positive or treated per index case; delay in partners presenting for care; incidence of STIs; changes in the index patient's or partner's behaviour with regard to condom use, abstinence in the presence of symptomatic infections, the number of partners, the number of concurrent partners; emotional impact on the index patient or partner in their relationship; harm to the patient or partners, such as domestic violence, abuse or suicide; ethical outcomes (patient autonomy vs. beneficence).
Search methods for identification of studies
Electronic searches
Search method for original review (Mathews 2001)
The original review authors searched MEDLINE (1966 to 24 July 2001), EMBASE (1974 to 24 July 2001), Psychological Abstracts (1967 to 24 July 2001) and Sociological Abstracts (1963 to 24 July 2001). The Cochrane Controlled Trials register was searched with the text words 'sexual partners', 'partner notification', 'contact‐tracing' and 'contact tracing'. The Effective Practice and Organisation of Care (EPOC) register of studies was searched, as was the register of the HIV and AIDS Cochrane Review Group.
Search method for the review update
We searched three electronic databases, MEDLINE, EMBASE and CENTRAL, from 5 January 2001 to 31 August 2012. Search strategies are shown in Appendix 1, Appendix 2 and Appendix 3.
Searching other resources
Original Cochrane review (Mathews 2001)
The original review authors handsearched the Proceedings of the International AIDS Conferences (1996 to 24 July 2001) and the International Society for STD Research meetings (ISSTDR) (1991 to 24 July 2001). Bibliographies of studies and previous reviews were examined for references to other trials. Experts in the field were contacted.
Review update
We searched all reference lists of potential studies and previous reviews for relevant RCTs and contacted experts in the field. We searched the International Clinical Trials Registry Platform (ICTRP) from 18 March 2011 to 31 August 2012 to identify ongoing studies (www.who.int/ictrp/en/). We searched the ICTRP for the protocols of the 16 new studies. Trial registries were not searched for the protocols of the original included studies because these were all published before 1998.
Data collection and analysis
Selection of studies
Two review authors (Cathy Mathews, CM and Riabatu Abdullah, RA (original review); and Adel Ferreira, AF and Taryn Young, TY or CM or Moleen Zunza, MLZ (update)) independently screened titles and abstracts of the electronic search results. We obtained all the eligible abstracts of comparative studies in full‐text format, and two review authors (CM and RA original review and AF and TY or CM update) independently reviewed them for inclusion using prespecified eligibility criteria. We included all studies that reported random allocation. We assessed the risk of bias in the methods of sequence generation and allocation, as described in the section 'Assessment of risk of bias in included studies' and considered risk of bias interpreting the strength of evidence for each intervention.
Data extraction and management
Two review authors (CM and Nicol Coetzee, NC or Merrick Zwarenstein, MZ (original review) and AF and TY or CM or MLZ (update)) independently abstracted study characteristics and outcomes including information on: social context (developing (World Bank classification: countries with low or middle levels of gross national product (GNP) per capita as well as five high‐income developing economies ‐ Hong Kong (China), Israel, Kuwait, Singapore and the United Arab Emirates. These five economies are classified as developing despite their high per‐capita income because of their economic structure or the official opinion of their governments. Several countries with transition economies are sometimes grouped with developing countries based on their low or middle levels of per‐capita income, and sometimes with developed countries based on their high industrialisation (World Bank 2012)) or developed country); access to health services; legislative context (permissive or proscriptive public health legislation); methodological quality of study; type of health facility; type of provider (for example, nurse, physician, DIS); participants; type of interventions; outcome measure; results and correspondence required using a data extraction form.
We resolved disagreements by discussion. We summarised data from included studies in the Characteristics of included studies table and data from excluded studies in the Characteristics of excluded studies table. We summarised studies with insufficient information in the Characteristics of studies awaiting classification table. Where there were missing data, we attempted to contact study authors by email.
Assessment of risk of bias in included studies
Two review authors (AF and CM or MLZ) independently evaluated the risk of bias using The Cochrane Collaboration's tool (Higgins 2011a). We made judgements about the presence of bias by selecting one of three categories of risk of bias: low risk, high risk and unclear risk of bias. We resolved disagreements by discussion. If we could not reach consensus, we involved a third independent review author (TY). We contacted trial authors if there were any unclear issues and, if we received no response, we made a judgement of 'unclear risk of bias'.
We assessed and summarised the following main items in the 'Risk of bias' table: sequence generation, allocation concealment, blinding of participant and personnel, blinding of outcome assessment, whether incomplete outcome data were adequately addressed, selective reporting and any other bias. We searched the ICTRP for protocols of the 16 additional studies to assess selective reporting bias. Figure 1 and Figure 2 show the 'Risk of bias' graphs, which illustrate the proportions of studies with low, high and unclear risk of bias. In the 10 studies of the original review, the ICTRP was not searched; instead, the methods and result sections were compared to evaluate if the same outcomes were reported in these two sections. If the protocol was not available, the methods and results sections were compared to assess selective reporting bias.
1.
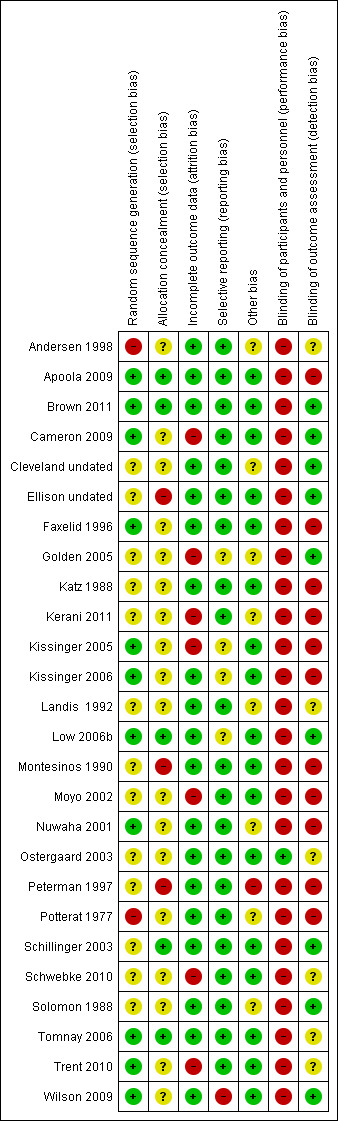
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
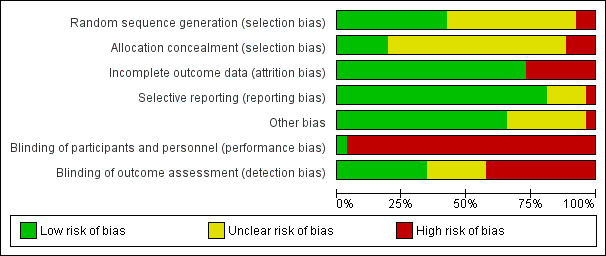
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
The review authors prepared tables summarising the results of each study for each comparison.
We defined re‐infection rate in index patients as the percentage of index patients with a repeated diagnosis of the same STI divided by the number of index patients retested.
Partners elicited, notified, presenting for care, tested, treated or harmed: we assumed that the number of units of each outcome per index patient was a random variable following a Poisson distribution. We assumed that the index patients from the groups within a study had similar distributions for exposure time to partners, for time to notify their partners, and that the same assumption held for partners with respect to the time taken to present to the health service. The value of the mean and the variance of a Poisson distribution are the same.
To calculate a CI for the difference in relevant outcomes, we used the normal approximation to the Poisson distribution since only summarised data from the included RCTs were available.
The approximate 95% CI for the rate difference is given by:
(Lamda1 ‐ Lamda2) ± 1.96√ (lamda1/n1 + lamda2/n2),
where lamda1 and lamda2 are the rates of partners per index patient in two groups, and n1 and n2 the number of index patients.
To calculate the standard error (SE) the formula used was:
(upper limit of 95% CI ‐ lower limit of 95% CI)/3.92.
To calculate the standard deviation (SD) the formula used was:
SE/√ (1/Nexp+ 1/Ncont),
where Nexp is the number of index patients randomised to the experimental group and Ncont is the number of index patients randomised to the control group
For continuous outcomes (number of partners elicited, notified, presenting for care, tested, treated or harmed), we recorded the mean (in number of partners per index patient randomised), SE and sample size. Where the exact numbers of partners were not available, we contacted study authors. If authors did not respond or could not provide the exact numbers, the mean difference (MD) could not be calculated and we reported the study findings descriptively. In studies where the rate of partners elicited per index patient was not reported, we used the number of contact cards given to the index patient as a proxy indicator.
We described the delay in partners presenting for care as the mean or median number of days after index patient enrolment.
Unit of analysis issues
We dealt with studies with multiple intervention groups as recommended in the Cochrane Handbook for Systematic Intervention Reviews (Higgins 2011b). We compared each intervention arm with another.
Where this resulted in shared intervention groups, we did not perform a meta‐analysis to prevent 'double‐counts' of participants. In these studies, we described the results in narrative form (Ellison undated; Montesinos 1990). We did not include any cluster randomised trials and, therefore, no adjustments were necessary.
Dealing with missing data
Where there were missing data, we attempted to obtain the data by contacting study authors by email. We contacted the authors of eight trials and authors provided requested data for five of the eight trials.
Assessment of heterogeneity
We assessed sources of clinical and methodological heterogeneity by looking at characteristics of studies, evaluating similarity between type of participants, intervention used and outcomes. We calculated the Chi2 test for heterogeneity (Deeks 2011), and the I2 statistic to evaluate statistical heterogeneity. Values of the I2 statistic were interpreted as follows (Deeks 2011): 0% to 40%: might not be important; 30% to 60%: might represent moderate heterogeneity; 50% to 90%: might represent substantial heterogeneity; 75% to 100%: might represent considerable heterogeneity.
Assessment of reporting biases
We did not find a sufficient number of studies to produce funnel plots to investigate publication bias for specific comparisons.
Data synthesis
We analysed data according to paired partner referral strategies (Table 6). We organised the comparisons first according to the four main PN strategies (1. enhanced patient referral, 2. EPT, 3. contract referral, 4. provider referral). Each main strategy was compared with simple patient referral and then with each other, if trials were available. We compared each enhanced patient referral with another enhanced patient referral. This resulted in 10 comparisons (Table 6).
2. Summary of comparisons with data available and STI studied.
| Partner notification strategy, intervention | Partner notification comparator, comparison number (number of trials) | STI included in trials | ||||
| Simple patient referral | Enhanced patient referral | Expedited partner therapy | Contract referral | Other enhanced patient referral | ||
| Enhanced patient referral | 1 (16) | ‐ | ‐ | ‐ | 2 (2) | Gonorrhoea, chlamydia, non‐gonococcal urethritis, trichomonas, pelvic inflammatory disease, STI syndromes |
| Expedited partner therapy | 3 (8) | 4 (5)* | ‐ | ‐ | Not applicable | Gonorrhoea, chlamydia, trichomonas, STI syndromes |
| Contract referral | 5 (5) | 6 (1) | 7 (1) | ‐ | Not applicable | Gonorrhoea, trichomonas, HIV |
| Provider referral | 8 (3)† | 9 (1) | No trials | 10 (2) | Not applicable | Non‐gonococcal urethritis, syphilis, HIV |
* Comparison includes one trial comparing combinations of expedited partner therapy and patient referral.
† Comparison Includes one trial comparing a choice between provider or simple patient referral and simple patient referral.
‐ Indicates combinations of an intervention and comparison that are covered elsewhere in the table; HIV: human immunodeficiency virus; STI: sexually transmitted infection.
The largest group of trials (Table 6; comparison 1, enhanced patient referral versus simple patient referral) included several different interventions to enhance the outcomes of patient referral. We grouped these into six categories: (1) patient referral with DIS or health adviser, (2) postal testing kit, (3) information booklet, (4) disease‐specific website, (5) additional counselling or (6) showing a videotape.
We performed meta‐analyses where appropriate using random‐effects models to report the pooled MD (for continuous outcomes) or risk ratio (RR for dichotomous outcomes) with 95% CI. When there was a moderate or low level of heterogeneity (I2 ≤ 50%), we pooled results. If there was more substantial evidence of heterogeneity (I2 > 50%), we pooled the results of individual studies if appropriate or described in the narrative. We reported results of tests for heterogeneity (Tau2, Chi2 test with number of degrees of freedom (df), P value and I2 statistic).
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses to explore possible sources of heterogeneity. These included: age of participant, gender, specific STIs investigated, setting (developed vs. developing country) and category of healthcare worker.
Sensitivity analysis
We performed a sensitivity analysis on the primary outcome, re‐infection rate of index patient with curable STIs. Given the limited numbers of trials and meta‐analyses, the sensitivity analysis examined only the effect of attrition bias. We repeated meta‐analyses excluding trials with more than 20% attrition and compared results with the primary analysis.
'Summary of findings' table
We interpreted results using a 'Summary of findings' table, which provided key information about the quality of evidence for the studies included in a comparison, the magnitude of effect of the interventions examined and the sum of available data on the primary outcome. We imported data from Review Manager 5 (RevMan 2011), using the GRADE profiler (GRADE 2004). We selected the primary outcome of re‐infection in the index case for the 'Summary of findings' table.
Results
Description of studies
Results of the search
The initial search (1966 to 24 July 2001; Mathews 2001) identified 11 RCTs, including 8041 participants. The updated search (5 January 2001 to 31 August 2012) identified an additional 16 RCTs (9597 participants; 6841 women and 2756 men). One study was listed as awaiting classification (Characteristics of studies awaiting classification). In the original review, Levy 1998 (with 60 participants) was listed as under 'Included studies' but, in this update, it was placed under 'Characteristics of studies awaiting classification' because no results were available. We found four ongoing studies in trial registers (Characteristics of ongoing studies).
Included studies
Twenty‐six RCTs (Figure 3) were included in the review including 17,578 participants (Characteristics of included studies). Most of the trials (14) were conducted in the US, four in the UK, two in Denmark, and one each in Australia, Malawi, South Africa, Uganda, Zambia and Zimbabwe. Most trials (21) were based in public health clinics. One was conducted in a large academic medical centre (Trent 2010), three in general practice (Andersen 1998; Low 2006b; Ostergaard 2003), and one on a university campus (Montesinos 1990).
3.
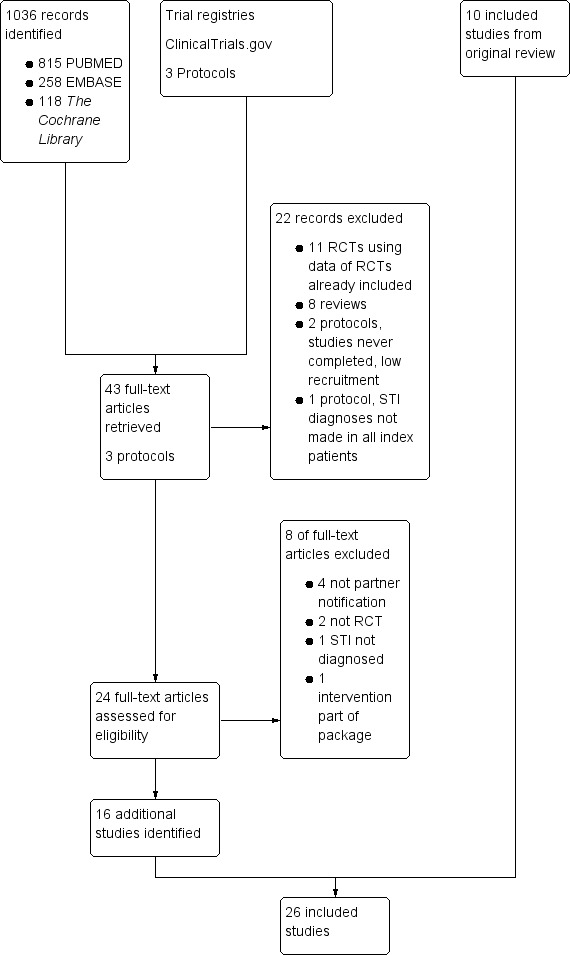
Flow diagram detailing the updated search and selection of studies.
Participants
Trials were conducted among patients with gonorrhoea (three trials, Cleveland undated; Potterat 1977; Solomon 1988); gonorrhoea or non‐gonococcal urethritis (one trial, Montesinos 1990); non‐gonococcal urethritis only (one trial, Katz 1988); chlamydia (six trials, Andersen 1998; Apoola 2009; Cameron 2009; Low 2006b; Ostergaard 2003; Schillinger 2003); syphilis (one trial, Peterman 1997); HIV (two trials, Brown 2011; Landis 1992); chlamydia or gonorrhoea, or both (four trials, Golden 2005; Kerani 2011; Kissinger 2005; Wilson 2009); trichomonas (two trials, Kissinger 2006; Schwebke 2010); PID (one trial, Trent 2010); and chlamydia or non‐gonococcal urethritis (one trial, Tomnay 2006). Four trials in developing countries where syndromic diagnoses are made included patients with any STI syndrome (Ellison undated; Faxelid 1996; Moyo 2002; Nuwaha 2001). In six studies, STI diagnoses were made clinically, based on symptoms or clinic tests (Ellison undated; Faxelid 1996; Katz 1988; Moyo 2002; Nuwaha 2001; Trent 2010). In the other 20 trials, STI diagnoses (other than non‐gonococcal urethritis) were confirmed with laboratory testing. There were no RCTs among patients with laboratory‐diagnosed hepatitis B, genital herpes or chancroid.
Six trials included male patients only, or reported over 90% male index patients (Cleveland undated; Katz 1988; Kerani 2011; Kissinger 2005; Potterat 1977; Solomon 1988). Seven trials included female index patients only (Andersen 1998; Apoola 2009; Cameron 2009; Kissinger 2006; Schillinger 2003; Schwebke 2010; Trent 2010).The remaining trials included male and female index patients. Two trials included men who had sex with men (Kerani 2011; Landis 1992) and one included male and female injecting‐drug users (Landis 1992).
Types of interventions
Included studies investigated the effects of various PN strategies (Table 6; Table 7):
3. Summary of included studies and outcomes reported by authors, according to partner notification strategies and comparisons.
|
Partner notification strategy Comparison number, comparison |
N (studies) |
n (participants) |
Outcomes, as reported in any included RCT | Study ID |
| ENHANCED PATIENT REFERRAL | ||||
| 1. Enhanced patient referral vs. simple patient referral | 16 | 7642 | Index patient returning for a test of cure Knowledge of the index patient Number of partners notified and referral of partners for treatment Proportion of index patients with at least 1 partner tested Proportion of index cases with at least 1 sexual partner treated Proportion of index patients with at least 1 partner positive for C. trachomatis Number of partners treated per index patient 6 weeks after randomisation Number of partners elicited Proportion of index cases with a positive chlamydia test result 6 weeks after randomisation Proportion of index cases with all sexual partners treated Acceptability of Internet for use in standard partner notification Partners located Index re‐infection Harms ‐ adverse effects of medication Index patient 72‐hour follow‐up Medication adherence Temporary abstinence from sexual intercourse as evidence of self care Behavioural change Partners contacted Partners tested Partners testing positive Time until testing of partners Number of partners treated per index case Number of partners identified per index Number of traceable partners Number of partners treated within 28 days Proportion of index patients with at least 1 partner treated within 28 days per index case |
Andersen 1998 Apoola 2009 Cleveland undated Cameron 2009 Ellison undated Kerani 2011 Katz 1988 Kissinger 2005 Kissinger 2006 Low 2005 Moyo 2002 Ostergaard 2003 Solomon 1988 Tomnay 2006 Trent 2010 Wilson 2009 |
| 2. Enhanced patient referral vs. other enhanced patient referral method | 2 | 1336 | Partners presenting for care Partners elicited Partners treated |
Montesinos 1990 Ellison undated |
| EXPEDITED PARTNER THERAPY | ||||
| 3. EPT vs. simple patient referral | 8 | 6537 | Re‐infection rate of index patient Number of partners notified Partner treatment Sexual outcomes such as having unprotected sex before partner took medication, re‐initiated sex with partner, unprotected sex with any partner Partners elicited Index patient 2‐week post‐treatment return Harms ‐ fighting and refusal of intercourse Side effects of drugs Partner testing |
Cameron 2009 Golden 2005 Kerani 2011 Kissinger 2005 Kissinger 2006 Nuwaha 2001 Schillinger 2002 Schwebke 2010 |
| 4.1 EPT vs. enhanced patient referral | 4 | 1253 | Re‐infection rate of index patient Number of partners notified Partner testing Partner treatment Sexual outcome (unprotected sex, re‐initiated sex with untreated partner) |
Cameron 2009 Kerani 2011 Kissinger 2005 Kissinger 2006 |
| 4.2 EPT and enhanced patient referral vs. simple patient referral | 1 | 41 | Number of partners notified Number of partners treated Method (telephone or in person) of partner notification used Partner tested for HIV/syphilis Adverse events |
Kerani 2011 |
| CONTRACT REFERRAL | ||||
| 5 Contract referral vs. simple patient referral | 5 | 2006 | Number of partners notified Partners presenting to health service Partners testing positive |
Brown 2011 Cleveland undated Landis 1992 Potterat 1977 Schwebke 2010 |
| 6. Contract referral vs. enhanced patient referral | 1 | 1266 | Partners presenting for care Partners testing positive |
Cleveland undated |
| 7. Contract referral vs. EPT | 1 | 324 | Re‐infection index patient | Schwebke 2010 |
| 8. PROVIDER REFERRAL | ||||
| 8.1 Provider referral vs. simple patient referral | 2 | 596 | Partners located Partners treated Partner visit to the clinic during the 30 days after index enrolment Harms Partners testing positive |
Brown 2011 Katz 1988 |
| 8.2 Choice between provider or simple patient referral vs. simple patient referral | 1 | 396 | Partners elicited Number of partners notified Partners treated Harms |
Faxelid 1996 |
| 9. Provider referral vs. enhanced patient referral | 1 | 461 | Partners elicited Partners testing positive Partners treated |
Katz 1988 |
| 10. Provider referral vs. contract referral | 2 | 2206 | Partners tested Partners treated Partner presenting for care Harms Partners testing positive |
Brown 2011 Peterman 1997 |
The outcomes listed are those reported by the authors of the RCTs. Not all were named primary or secondary outcomes in the review.
EPT: expedited partner therapy; HIV: human immunodeficiency virus; RCT: randomised controlled trial.
Enhanced patient referral versus simple patient referral;
Enhanced patient referral versus other enhanced patient referral method;
EPT versus simple patient referral;
EPT versus enhanced patient referral;
EPT and enhanced patient referral versus simple patient referral;
contract referral versus simple patient referral;
contract referral versus enhanced patient referral;
contract referral versus EPT;
provider referral versus simple patient referral;
choice between provider or simple patient referral versus simple patient referral;
provider referral versus enhanced patient referral;
provider referral versus contract referral.
Outcomes
Outcomes assessed are reported in Table 7. The comprehensive details of included studies can be seen in the Characteristics of included studies table.
One study from the original review was classified as a study awaiting assessment because there were no results available (Levy 1998) (Characteristics of studies awaiting classification).
Four ongoing studies were identified from the trial register (Characteristics of ongoing studies).
Excluded studies
We excluded 11 studies (see Characteristics of excluded studies for details).
Risk of bias in included studies
The risk of bias for each study is presented in the 'Risk of bias' table in the section Characteristics of included studies. Figure 1 and Figure 2 illustrate the summary of risk of bias in all the studies.
Allocation
Random sequence generation
Eleven trials reported adequate generation of the random allocation sequence (Apoola 2009; Brown 2011; Cameron 2009; Faxelid 1996; Kissinger 2005; Kissinger 2006; Low 2006b; Nuwaha 2001; Tomnay 2006; Trent 2010; Wilson 2009). Of these trials, eight used blocked randomisation (Apoola 2009; Brown 2011; Cameron 2009; Kissinger 2005; Kissinger 2006; Low 2006b; Tomnay 2006; Wilson 2009), two trials used computer‐generated random numbers tables (Nuwaha 2001; Trent 2010), and, in one study, lots were drawn by index patient (Faxelid 1996). Sequence generation was adequate in six of nine trials reporting the primary outcome of re‐infection with a bacterial STI (Cameron 2009; Kissinger 2005; Kissinger 2006; Low 2006b; Tomnay 2006;Wilson 2009).
In 13 trials, random sequence generation was unclear (Cleveland undated; Ellison undated; Golden 2005; Katz 1988; Kerani 2011; Landis 1992; Montesinos 1990; Moyo 2002; Ostergaard 2003; Peterman 1997; Schillinger 2003; Schwebke 2010; Solomon 1988) and two trials reported methods used that can introduce a high risk of bias (Andersen 1998; Potterat 1977). In Andersen 1998, the date of birth of index patient was used and, in Potterat 1977, assignment of index patient was performed alternately to specific intervention arms. Both of these trials reported secondary outcomes only.
Allocation concealment
Five trials reported adequate allocation concealment (Apoola 2009; Brown 2011; Low 2006b; Schillinger 2003; Tomnay 2006). Of these, four trials reported the use of sealed, opaque, sequentially numbered envelopes (Apoola 2009; Brown 2011; Schillinger 2003; Tomnay 2006), and one trial reported the use of a centralised telephone service (Low 2006b). In 18 trials, the methods used for allocation concealment were not adequately described (Andersen 1998; Cameron 2009; Cleveland undated; Faxelid 1996; Golden 2005; Katz 1988; Kerani 2011; Kissinger 2005; Kissinger 2006; Landis 1992; Moyo 2002; Nuwaha 2001; Ostergaard 2003; Potterat 1977; Schwebke 2010; Solomon 1988; Trent 2010; Wilson 2009). Allocation concealment was adequate in three of nine trials reporting the primary outcome of re‐infection with a bacterial STI.
Three studies reported methods that could introduce a high risk of bias (Ellison undated; Montesinos 1990; Peterman 1997). In Ellison et al., the interventions were allocated in turn to each consecutive patient according to a printed schedule, which could have influenced enrolment or exclusion and hence the intervention received by the index patients (Ellison undated). In Montesinos et al., the protocol used in the intervention was colour coded and the counsellor removed the protocol for the next index patient from a randomly ordered set (Montesinos 1990). Peterman et al. reported that the assignment was known to the interviewer before contact with index patients and sequentially adapted (Peterman 1997).
Blinding
Blinding of participants and personnel (performance bias)
Twenty‐five trials did not have blinding of the participants or the personnel (Andersen 1998; Apoola 2009; Brown 2011; Cameron 2009; Cleveland undated; Ellison undated; Faxelid 1996; Golden 2005; Katz 1988; Kerani 2011; Kissinger 2005; Kissinger 2006; Landis 1992; Low 2006b; Montesinos 1990; Moyo 2002; Nuwaha 2001; Peterman 1997; Potterat 1977; Schillinger 2003; Schwebke 2010; Solomon 1988; Tomnay 2006; Trent 2010; Wilson 2009). In one trial, the index patient received identical specimen collection kits to be given to their partners, and was, therefore, blinded to the intervention in which they were taking part (Ostergaard 2003).
Blinding of outcome assessment (detection bias)
Eleven trials did not report blinding of the outcome assessors (Apoola 2009; Faxelid 1996; Katz 1988; Kerani 2011; Kissinger 2005; Kissinger 2006; Montesinos 1990; Moyo 2002; Nuwaha 2001; Peterman 1997; Potterat 1977). In five trials, the outcome assessors were blinded (Cleveland undated; Ellison undated; Low 2006b; Solomon 1988; Wilson 2009). Cameron et al. reported that the laboratory personnel (primary outcome) were blinded but not the interviewers (Cameron 2009). We judged the risk of bias as low. In six studies, the blinding of outcome assessors was unclear (Andersen 1998; Landis 1992; Ostergaard 2003; Schwebke 2010; Tomnay 2006; Trent 2010). In the remaining three studies, the outcome assessor was not blinded but we judged the risk of bias as low because the primary outcome was objectively assessed (Brown 2011; Golden 2005; Schillinger 2003).
Incomplete outcome data
Seven trials had a high (> 20%) attrition rate (Cameron 2009; Golden 2005; Kerani 2011; Kissinger 2005; Moyo 2002; Schwebke 2010; Trent 2010), including four of nine trials reporting re‐infection with a bacterial STI as an outcome. In Cameron 2009, 65% of index patients submitted at least one urine sample in 12 months, while in Golden 2005, 68% of index patients completed the study. In Kerani 2011, 71% of index patients completed baseline and follow‐up interviews. In Kissinger 2005, 79% of index patients had a follow‐up interview but only 37.5% were retested, and in Moyo 2002, only 50% of index patients had a follow‐up interview. In Schwebke 2010, 40% of index patients completed the study. In Trent 2010, 62% of index patients had a follow‐up interview.
Selective reporting
We compared the trial protocols with published trial results sections to assess reporting bias. If the trial protocol was not available, we compared the methods and results sections of the trial. We searched three trial registries for the protocols of the 16 additional studies included in this update. Protocols were available for five of these studies (Apoola 2009; Kissinger 2006; Low 2006b; Schwebke 2010; Wilson 2009).
We judged 21 trials to have a low risk of reporting bias either because the primary outcome stated in the protocol was reported in the trial result sections (Apoola 2009; Schwebke 2010), or the outcomes stated in the method sections were reported in the result sections (Andersen 1998; Brown 2011; Cameron 2009; Cleveland undated; Ellison undated; Faxelid 1996; Katz 1988; Kerani 2011; Landis 1992; Montesinos 1990; Moyo 2002; Nuwaha 2001; Ostergaard 2003; Peterman 1997; Potterat 1977; Schillinger 2003; Schwebke 2010; Solomon 1988; Tomnay 2006; Trent 2010).
We considered four trials to have an unclear risk of reporting bias because the outcomes reported in the results sections differed from those stated in the method sections (Golden 2005; Kissinger 2005), or protocols (Kissinger 2006; Low 2006b). In Kissinger 2006, the protocol had primary and secondary outcomes whereas in the trial report outcomes were not divided into primary and secondary. Furthermore, additional sexual and behavioural outcomes were reported. Low et al. reported some outcomes in the published paper that differed from the protocol (Low 2006b).
We assessed one trial as being at high risk of reporting bias. In Wilson 2009, the primary outcomes stated in the protocol differed from those stated in trial report; in the protocol there were also three intervention arms described but only two were reported in the trial publication.
Other potential sources of bias
One study had a high potential for other bias (Peterman 1997). The authors of the study reported contamination between the three groups caused by overlap of partners common to index patients. In eight studies, it was unclear if there was any other potential source of bias (Andersen 1998; Cleveland undated; Golden 2005; Kerani 2011; Landis 1992; Nuwaha 2001; Potterat 1977; Solomon 1988). Of these seven studies, in five no comparisons of baseline characteristics between study arms were given (Andersen 1998; Cleveland undated; Landis 1992; Potterat 1977; Solomon 1988). In Golden 2005, selective reporting of subgroups might have introduced bias and in Nuwaha 2001, partners of the patient referral group could have been treated elsewhere leading to misclassification bias. In the remainder of the studies, the risk for potential sources of bias was low.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Enhanced patient referral
1. Enhanced patient referral versus simple patient referral
Sixteen studies looked at different types of enhanced patient referral compared with simple patient referral among patients with gonorrhoea (Cleveland undated; Solomon 1988), chlamydia (Andersen 1998; Apoola 2009; Cameron 2009; Low 2006b; Ostergaard 2003), non‐gonococcal urethritis (Katz 1988), gonorrhoea or chlamydia (Kerani 2011; Kissinger 2005; Wilson 2009), trichomoniasis (Kissinger 2006), chlamydia or non‐gonococcal urethritis (Tomnay 2006), PID (Trent 2010), or any STI syndrome (Ellison undated; Moyo 2002).
There were seven different types of enhanced patient referral interventions for patients or partners: 1) an additional counselling session (Cleveland undated; Ellison undated; Moyo 2002; Wilson 2009); 2) a home testing kit for the partners to use and send back to a laboratory (Andersen 1998; Cameron 2009; Ostergaard 2003), or for the partners to bring back to the clinic (Apoola 2009); 3) an additional information booklet to be given to the partner (Kissinger 2005; Kissinger 2006); 4) a videotape shown to the index patient (Solomon 1988; Trent 2010); 5) a disease‐specific website was available to the partner (Kerani 2011; Tomnay 2006); 6) health education messages for the index case (Ellison undated); and 7) health education plus counselling for the index patient (Ellison undated). In addition, two studies compared patient referral performed by a contact tracer (DIS or health adviser) with patient referral performed by a nurse (Katz 1988; Low 2006b).
Primary outcome
Six studies (2007 participants) assessed the index patient re‐infection rate (Cameron 2009; Kissinger 2005; Kissinger 2006; Low 2006b; Tomnay 2006; Wilson 2009) (Figure 4). Owing to substantial heterogeneity (Tau2 = 0.38; Chi2 = 16.86, df = 5 (P value = 0.005); I2 = 70%), the results of individual studies were not pooled. In one comparison, the risk of re‐infection in the index patients was 51% lower in the enhanced patient referral (additional counselling) compared with the simple patient referral group (RR 0.49, 95% CI 0.27 to 0.89) (Wilson 2009). In two smaller studies, the risk of re‐infection was higher in index patients receiving the enhanced patient referral strategy but CIs included the possibility of no difference (Cameron 2009; Tomnay 2006). In the other three studies, there was no statistical evidence of a difference between enhanced and simple patient referral (Table 8).
4.
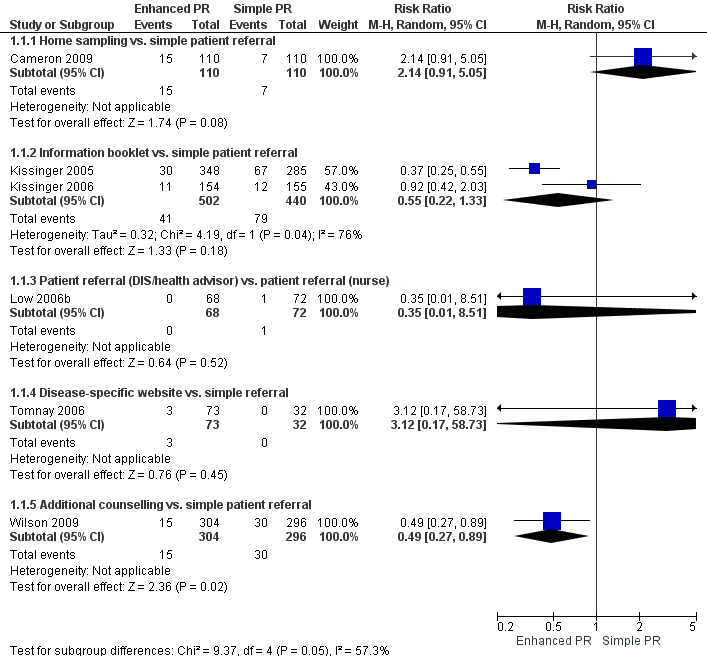
Forest plot: 1 Enhanced patient referral versus simple patient referral, outcome: 1.1 Re‐infection in index patient, by STI.
4. Enhanced patient referral versus simple patient referral, re‐infection in the index patient, effect size.
| Comparison |
N (studies) |
n (participants) |
Study ID |
RR (95% CI) |
Test for heterogeneity I2; Chi2, P value |
| Home sampling kit vs. simple patient referral | 1 | 220 | Cameron 2009 | 2.14 (0.91 to 5.05) | n/a |
| Information booklet vs. simple patient referral | 2 | 942 | Kissinger 2005; Kissinger 2006 | 0.55 (0.22 to 1.33) | 76%; 4.19, P value = 0.04 |
| Patient referral (DIS/health adviser) vs. patient referral (nurse) | 1 | 140 | Low 2005 | 0.35 (0.01 to 8.51) | n/a |
| Disease‐specific website vs. simple patient referral | 1 | 105 | Tomnay 2006 | 3.12 (0.17 to 58.73) | n/a |
| Additional counselling vs. simple patient referral | 1 | 600 | Wilson 2009 | 0.49 (0.27 to 0.89) | n/a |
Enhanced patient referral is taken as the experimental group. Risk ratio (RR) < 1 indicates a lower re‐infection risk after enhanced patient referral than simple patient referral. If RR = 1, the risk of re‐infection is the same in both groups. If RR > 1, there is a higher risk of re‐infection in the enhanced patient referral group. In the trial by Low et al., the outcome was assessed in a minority of index patients.
CI: confidence interval; DIS: disease intervention specialist; n/a: not applicable; RR: risk ratio.
We judged the quality of evidence for the primary outcome, using the GRADE approach, as low for four of the five enhanced patient referral interventions. We judged additional counselling to provide moderate evidence of a beneficial effect when compared with simple patient referral but there was only one trial in this group (Wilson 2009) (Table 1).
Secondary outcomes
Twelve studies (6045 participants) used five different comparisons and assessed the number of partners elicited (Andersen 1998; Apoola 2009; Cameron 2009; Cleveland undated; Ellison undated; Katz 1988; Kerani 2011; Kissinger 2005; Low 2006b; Moyo 2002; Solomon 1988; Tomnay 2006). There was no evidence of clinically relevant differences between enhanced and simple patient referral strategies (Table 9). When simple patient referral delivered by a nurse was compared with specialist contact tracer (DIS or health adviser) (Katz 1988; Low 2006b), the number of partners elicited was slightly higher in the simple patient referral (nurse) group. We conducted a sensitivity analysis, removing the trial by Andersen 1998 (high risk of bias in random sequence generation), but there was no appreciable difference in the results.
5. Enhanced patient referral versus simple patient referral, number of partners elicited per index patient randomised, effect size.
| Comparison |
N (studies) |
n (participants) |
Study ID |
MD (95% CI) |
Test for heterogeneity I2; Chi2, P value |
| Home sampling kit vs. simple patient referral | 3 | 516 | Cameron 2009; Andersen 1998; Apoola 2009 | 0.00 (‐0.19 to 0.19) | 0%; 0.32, P value = 0.85 |
| Additional counselling vs. simple patient referral | 3 | 2401 | Cleveland undated; Ellison undated; Moyo 2002 | 0.1 (0.00 to 0.19) | 0%; 1.17, P value = 0.56 |
| Patient referral (DIS) vs. patient referral (nurse) | 2 | 597 | Katz 1988; Low 2005 | ‐0.40 (‐0.57 to ‐0.24) | 0%; 0.03, P value = 0.87 |
| Information booklet vs. simple patient referral | 1 | 633 | Kissinger 2005 | 0.0 (‐0.22 to 0.22) | n/a |
| Disease‐specific website vs. simple patient referral | 2 | 140 | Kerani 2011; Tomnay 2006 | ‐0.15 (‐0.72 to 0.42) | 13%; 1.15, P value = 0.28 |
Enhanced patient referral is taken as the experimental group. Mean difference (MD) < 0 indicates that simple patient referral resulted in more partners elicited; MD = 0 indicates no difference between groups; MD > 0 indicates more partners elicited in the enhanced patient referral group.
CI: confidence interval; MD: mean difference; n/a indicates not applicable.
In Ellison et al. there were four intervention arms comparing three different enhanced patient referral methods with simple patient referral: (1) patient referral with a health education message, (2) patient referral with counselling and (3) patient referral with health education message and counselling (Ellison undated). Small increases in the number or partners elicited per index patient were observed in the enhanced patient referral strategy with a health education message (MD 0.25, 95% CI 0.10 to 0.39) and health education message plus counselling (MD 0.6, 95% CI 0.45 to 0.76). In Solomon et al. the authors reported that there was no evidence of differences between enhanced patient referral (videotape) and simple patient referral group for number of partners elicited (Solomon 1988).
Six studies (1885 participants) assessed number of partners notified (Cameron 2009; Moyo 2002; Ostergaard 2003; Tomnay 2006; Trent 2010; Wilson 2009). In Trent 2010 and Wilson 2009, the exact number of partners notified was not reported so we could not calculate the MD. In three studies (Table 10), there was no evidence of a difference in the number of partners notified per index patient between the groups (Cameron 2009; Ostergaard 2003; Tomnay 2006). In Moyo et al. additional counselling resulted in slightly more partners being notified (Moyo 2002).
6. Enhanced patient referral versus simple patient referral, number of partners notified per index patient randomised, effect size.
| Comparison |
N (studies) |
n (participants) |
Study ID |
MD (95% CI) |
Test for heterogeneity I2; Chi2, P value |
| Home sampling kit vs. simple patient referral | 2 | 782 | Cameron 2009; Ostergaard 2003 | 0.01 (‐0.12 to 0.14) | 0%; 0.01, P value = 0.93 |
| Additional counselling vs. simple patient referral | 2 | 272 | Moyo 2002; Wilson 2009 |
0.21 (0.05 to 0.36) data not available |
n/a |
| Disease‐specific website vs. simple patient referral | 1 | 105 | Tomnay 2006 | ‐0.17 (‐0.68 to 0.35) | n/a |
| Videotape vs. simple patient referral | 1 | 77 | Trent 2010 | data not available | n/a |
Enhanced patient referral group is taken as the experimental group. Mean difference (MD) < 0 indicates that simple patient referral resulted in more partners notified; MD = 0 indicates no difference between groups; MD > 0 indicates more partners notified in the enhanced patient referral group.
CI: confidence interval; MD: mean difference; n/a indicates not applicable.
Five studies (2684 participants) assessed the number of partners who presented for care (Andersen 1998; Apoola 2009; Cameron 2009; Cleveland undated; Solomon 1988). Data were only available for four studies (Andersen 1998; Apoola 2009; Cameron 2009; Cleveland undated). There was no evidence that one group resulted in more partners who presented for care compared with another (MD 0.1, 95% CI ‐0.08 to 0.28; heterogeneity: Tau2 = 0.02; Chi2 = 12.59, df = 3 (P value = 0.006); I2 = 76%). In Solomon 1988), the authors reported no difference in number of partners presenting for care when a videotape was used.
Five studies (2601 participant) assessed the number of partners who tested positive (Andersen 1998; Cameron 2009; Cleveland undated; Katz 1988; Ostergaard 2003). There was no evidence that there were more partners testing positive in one group than the other (MD 0.04, 95% CI ‐0.01 to 0.09; heterogeneity: Tau2 = 0.00; Chi2 = 8.49, df = 4 (P value = 0.08); I2 = 53%).
Six studies (3275 participants) assessed the number of partners treated (Table 11) (Apoola 2009; Ellison undated; Katz 1988; Kissinger 2005; Low 2006b; Trent 2010). In Trent 2010, the exact number of partners treated was not reported so we could not calculate the MD. The enhanced group receiving the information booklet had slightly more partners treated compared with simple patient referral (Kissinger 2005). The combination of a health education message and counselling also resulted in slightly more partners treated (Ellison undated) (MD 0.08, 95% CI 0.01 to 0.14). There was no evidence of a difference in partners treated with the other enhanced patient referral strategies.
7. Enhanced patient referral versus simple patient referral, number of partners treated per index patient randomised, effect size.
| Comparison |
N (studies) |
n (participants) |
Study ID |
MD (95% CI) |
Test for heterogeneity I2; Chi2, P value |
| Home sampling kit vs. simple patient referral | 1 | 200 | Apoola 2009 | ‐0.03 (‐0.25 to 0.19) | n/a |
| Additional counselling vs. simple patient referral | 1 | 863 | Ellison undated | 0.04 (‐0.02 to 0.1) | n/a |
| Patient referral (DIS) vs. patient referral (nurse) | 2 | 597 | Katz 1988; Low 2005 | ‐0.05 (‐0.13 to 0.03) | 0%; 0.71, P value = 0.40 |
| Information booklet vs. simple patient referral | 1 | 633 | Kissinger 2005 | 0.22 (0.08 to 0.36) | n/a |
| Videotape vs. simple patient referral | 1 | 12,677 | Trent 2010 | not reported | n/a |
Enhanced patient referral group is taken as the experimental group. Mean difference (MD) < 0 indicates that simple patient referral resulted in more partners treated; MD = 0 indicates no difference between groups; MD > 0 indicates more partners treated in the enhanced patient referral group.
CI: confidence interval; MD: mean difference; n/a indicates not applicable.
In one study (902 participants), 14.5% of partners in the simple patient referral group and 3.3% in the enhanced group (videotape) attended the clinic eight or more days after the index patient (Solomon 1988).
Five studies (1138 participants) assessed the number of harmful events reported (Kerani 2011; Moyo 2002; Tomnay 2006; Trent 2010; Wilson 2009). In two of these, no harms were reported (Kerani 2011; Tomnay 2006). In Wilson et al., no evidence of a difference of the amount of harm (argument, fight or physical violence) was found in the group receiving the enhancement (additional counselling) compared with simple patient referral group (Wilson 2009). In the fourth trial, complications due to medicine or symptoms worsening were equally distributed between two groups (Trent 2010). The fifth study did not specify the number of harms (physical and verbal abuse) reported but stated that it was not associated with the study arm assignment (Moyo 2002).
No information was available for incidence of STI, changes in behaviour emotional impact and ethical outcomes.
2. Enhanced patient referral versus other enhanced patient referral method
Two studies (1351 participants) compared one enhanced patient referral method with another enhanced patient referral method among patients with any STI syndrome (Ellison undated) and gonorrhoea or non‐gonococcal urethritis (Montesinos 1990).
Secondary outcomes
Both studies assessed the number of partners elicited. In Ellison et al., a health education message plus counselling elicited a slightly higher number of partners compared with counselling alone (MD 0.48, 95% CI 0.32 to 0.64) or to a health education message alone (MD 0.35, 95% CI 0.19 to 0.52) (Ellison undated). There was no difference between the groups receiving health education messages alone compared with the group receiving counselling alone (MD ‐0.12, 95% CI ‐0.27 to 0.03). In Montesinos 1990, there was no evidence of differences in the number of partners elicited when counselling was compared with a combination of counselling plus incentive plus contact cards, and with counselling plus no incentive plus follow‐up call.
One study (65 participants) assessed the number of partners who presented for care (Montesinos 1990), and found no difference between groups when index patients received counselling plus follow‐up call plus no incentive plus contact cards compared with counselling alone or counselling plus incentive plus contact cards.
One study (1286 participants) assessed number of partners treated (Ellison undated). There was no difference between the groups receiving counselling plus health education message compared with health message alone (MD 0.05, 95% CI ‐0.01 to 0.12) or with counselling alone (MD 0.03, 95% CI ‐0.03 to 0.1). No evidence of a difference between groups receiving health message alone compared with counselling alone was found (MD 0.02, 95% CI ‐0.04 to 0.08).
No information was available for index patient re‐infection rate, partners notified, delay in partners presented for care, partners testing positive, incidence of STI, changes in behaviour, emotional impact, harms or ethical outcomes.
Expedited partner therapy
3. Expedited partner therapy versus simple patient referral
Eight studies compared EPT versus simple patient referral among patients with chlamydia (Cameron 2009; Schillinger 2003), trichomoniasis (Kissinger 2006; Schwebke 2010), gonorrhoea or chlamydia (Golden 2005; Kerani 2011; Kissinger 2005) and any STI syndrome (Nuwaha 2001).
Primary outcome
Six studies (6018 participants) assessed the index patient re‐infection rate (Cameron 2009; Golden 2005; Kissinger 2005; Kissinger 2006; Schillinger 2003; Schwebke 2010). Index patients in the EPT group had a 29% lower risk of being re‐infected compared with index patients in simple patient referral group (RR 0.71, 95% CI 0.56 to 0.89; heterogeneity: Tau2 = 0.03; Chi2 = 8.15, df = 5 (P value = 0.15), I2 = 39%) (Figure 5). When a sensitivity analysis was performed and only studies with attrition less than 20% were included (Kissinger 2006; Schillinger 2003), the effect of EPT was attenuated and CIs were wider (RR 0.8, 95% CI 0.62 to 1.04; heterogeneity: Tau2 = 0.00; Chi2 = 18, df = 1 (P value = 0.67), I2 = 0%).
5.
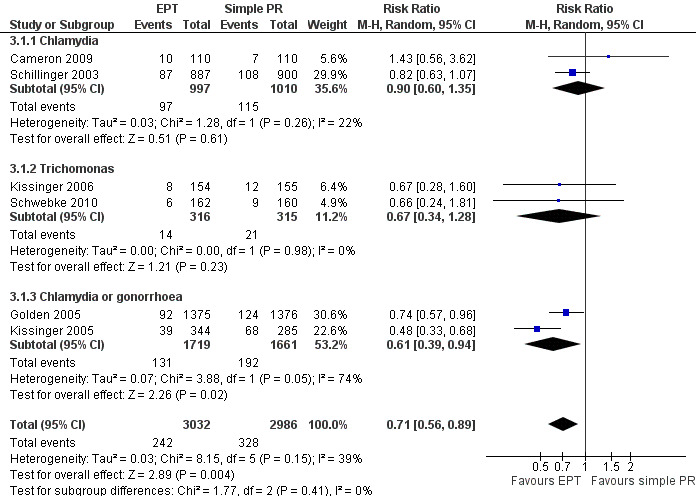
Forest plot: 3 Expedited partner therapy versus simple patient referral, outcome 3.1 Re‐infection in index patients, by STI.
The GRADE quality of the overall evidence for six studies reporting the primary outcome of re‐infection was moderate. We downgraded the quality of the evidence because of the serious risk of bias resulting from attrition and from inadequately described methods in several of the studies. When stratified according to type of STI (two studies each), there was low‐quality evidence suggesting no difference between EPT and simple patient referral for chlamydia, and low‐quality evidence favouring EPT for trichomonas and a combined outcome of either chlamydia or gonorrhoea (Table 2).
Secondary outcomes
Six studies (4339 participants) assessed the number of partners elicited (Cameron 2009; Golden 2005; Kerani 2011; Kissinger 2005; Nuwaha 2001; Schwebke 2010). There was no evidence of a difference between the two groups (MD ‐0.02, 95% ‐0.09 to 0.04; heterogeneity: Tau2 = 0.00; Chi2 = 1.05, df = 5 (P value = 0.96); I2 = 0% (Figure 6).
6.
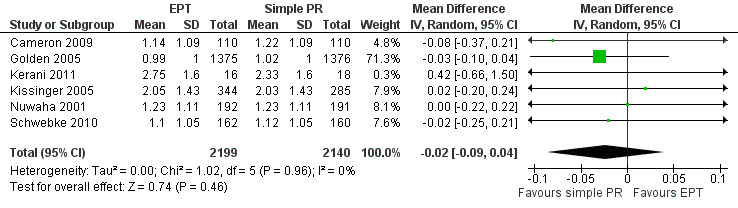
Forest plot: 3 Expedited partner therapy versus simple patient referral, outcome 3.2 Number of partners elicited.
In one small study of men who have sex with men (75 men, Kerani 2011), a slightly higher number of partners was elicited when the index patient received EPT compared with simple patient referral (MD 0.42, 95% CI 0.05 to 0.79).
Three studies (3600 participants) assessed number of partners notified (Cameron 2009; Golden 2005; Kissinger 2005). These three studies showed inconsistent results (heterogeneity: Tau2 = 0.07; Chi2 = 29.71, df = 2 (P value < 0.001); I2 = 93%) (Figure 7). Heterogeneity was explored by setting, STI and gender, and it could not be explained by subgroup analysis. In one study, slightly more partners of index patients in the EPT group were notified (MD 0.45, 95% CI 0.28 to 0.62) (Kissinger 2005). In two studies, there was no significant difference (Cameron 2009: MD 0.13, 95% CI ‐0.06 to 0.32; Golden 2005: MD ‐0.05, 95% CI ‐0.12 to 0.01).
7.
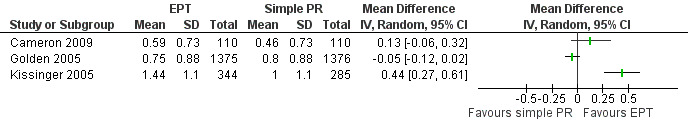
Forest plot: 3 Expedited partner therapy versus simple patient referral, outcome 3.3 Number of partners notified.
One study (220 participants) found no evidence of a difference in the number of partners who presented for care between the groups (MD 0.05, 95% CI ‐0.12 to 0.23) (Cameron 2009).
Four studies (4085 participants) assessed the number of partners treated (Golden 2005; Kissinger 2005; Nuwaha 2001; Schwebke 2010). The studies showed results in the same direction but were very heterogeneous (heterogeneity: Tau2 = 0.07; Chi2 = 59.57, df = 3 (P value < 0.001); I2 = 95%) (Figure 8). Subgroup analysis (setting, STI, gender) did not explain the heterogeneity. In three of the four trials, there was a moderate difference favouring EPT (Kissinger 2005: MD 0.43, 95% CI 0.28 to 0.58; Nuwaha 2001: MD 0.50, 95% CI 0.34 to 0.67; Schwebke 2010: MD 0.51, 95% CI 0.35 to 0.67). The difference between groups was very small in the fourth trial (Golden 2005: MD 0.06, 95% CI 0.01 to 0.12).
8.
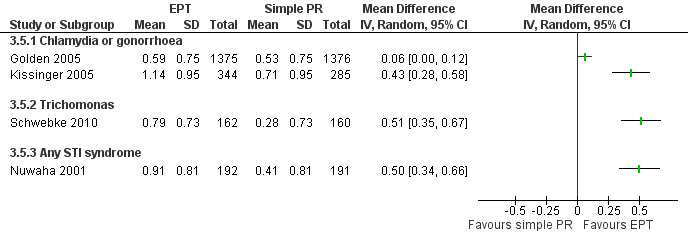
Forest plot: 3. Expedited partner therapy versus simple patient referral, outcome 3.5 Number of partners treated.
One of the studies included a measure of harm (Nuwaha 2001). This study (383 participants) found no statistical evidence of a difference in harm between simple patient referral and EPT (MD 0.06, 95% CI 0.0 to 0.12). The index patients in the EPT group reported 23 incidents of quarrelling compared with 11 incidents of quarrelling reported in simple patient referral group. Side effects were reported by index patients in 20 partners in the EPT group and in 10 partners in the simple patient referral group.
No information was available for: partners testing positive, changes in behaviour, emotional impact, ethical outcomes, delay in partners presenting for care or incidence of STI.
4. Expedited partner therapy versus enhanced patient referral
Four studies compared EPT versus enhanced patient referral among patients with gonorrhoea or chlamydia (Kerani 2011; Kissinger 2005), trichomoniasis (Kissinger 2006) or chlamydia (Cameron 2009).
Primary outcome
Three studies (1220) assessed the index patient re‐infection rate (Cameron 2009; Kissinger 2005; Kissinger 2006). There was no evidence of a difference between the two groups (RR 0.96, 95% CI 0.6 to 1.53; heterogeneity: Tau2 = 0.06; Chi2 = 2.99, df = 2 (P value = 0.22); I2 = 33%) (Figure 9). Sensitivity analysis including only studies with attrition less than 20% (Kissinger 2006) also found no evidence of a difference between the two groups (RR 0.73, 95% CI 0.30 to 1.76).
9.
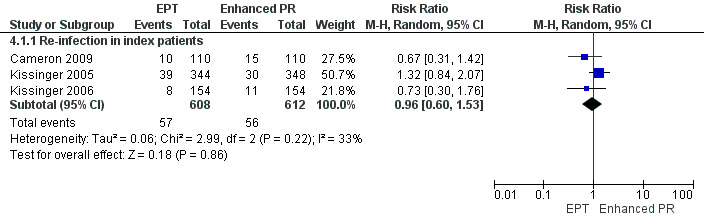
Forest plot: 4 Expedited partner therapy versus enhanced patient referral, outcome: 4.1 Re‐infection in index patients.
The GRADE assessment suggests low‐quality evidence that there was no difference between EPT and enhanced patient referral for preventing re‐infection in patients with curable STI (three studies). The evidence was downgraded because of the risk of bias in the methods and imprecision in the effect estimate (Table 3).
Secondary outcomes
Three studies (945 participants) assessed the number of partners elicited (Cameron 2009; Kerani 2011; Kissinger 2005). There was no evidence of a difference between the two groups (MD 0.07, 95% CI ‐0.180 to 0.32; heterogeneity: Tau² = 0.02; Chi² = 3.33, df = 2 (P = 0.19); I² = 40%) (Figure 10).
10.
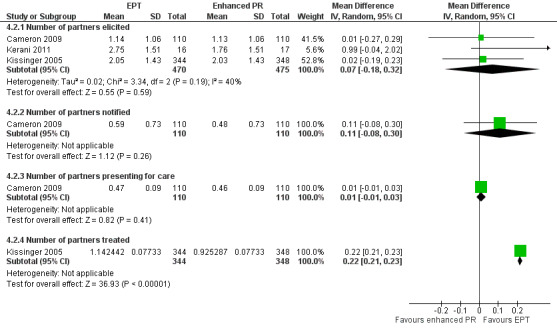
Forest plot: 4 Expedited partner therapy versus enhanced patient referral: 4.2 Secondary outcomes.
One study (220 participants) measured the number of partners notified and found no evidence of a difference between the groups (MD 0.11, 95% CI ‐0.08 to 0.3) (Cameron 2009).
One study (220 participants) measured the effect on number of partners presenting for care. There was no evidence of a difference between groups (MD 0.01, 95% CI ‐0.02 to 0.03) (Cameron 2009).
One study (692 participants) found a small increase in the number of partners treated per index patient randomised to the EPT group compared with the enhanced patient referral group (MD 0.22, 95% CI 0.21 to 0.23) (Kissinger 2005).
No information was available for delay in partners presenting for care, partners testing positive, changes in behaviour, emotional impact, harms, ethical outcomes and incidence of STI.
One study compared EPT plus enhanced patient referral or simple patient referral among men who have sex with men with chlamydia or gonorrhoea (Kerani 2011). A website, 'inSPOT' was used to enhance the patient referral intervention. The primary outcome assessed was the number of partners treated or notified. In the comparison of EPT and inSPOT (41 participants), a moderately higher number of partners was elicited in the combination group compared with inSPOT alone (MD 1.15, 95% CI 0.22 to 2.08). There was no evidence of differences in the number of partners treated or notified for the comparisons of EPT and inSPOT versus EPT alone (40 participants; MD 0.17, 95% CI ‐0.89 to 1.23); or EPT and inSPOT versus simple patient referral (42 participants, MD 0.58, 95% CI ‐0.4 to 1.57).
No information was available for index patient re‐infection rate, incidence of STI, partners notified, partners presenting for care, number of partners tested, number of partners testing positive, partners treated, delay in partners presented for care, changes in behaviour, emotional impact, harms or ethical outcomes.
Contract referral
5. Contract referral versus simple patient referral
Five trials compared contract referral versus simple patient referral among patients with HIV (Brown 2011; Landis 1992), gonorrhoea (Cleveland undated; Potterat 1977) or trichomoniasis (Schwebke 2010).
Primary outcome
The index patient re‐infection rate was assessed in one trial (322 participants) among women with trichomoniasis (Schwebke 2010). There was no statistical evidence of a difference in the risk of re‐infection in the women receiving contract referral or simple patient referral at either one month (RR 1.65, 95% CI 0.74 to 3.65) or three months (RR 1.65, 95% CI 0.4 to 6.77).
The GRADE level of evidence was very low because the findings were from one small trial with a serious risk of bias in the methods (Table 4).
Secondary outcomes
All five studies (2006 participants) assessed the number of partners elicited per index patient. Slightly fewer partners were elicited in the contract referral than the simple patient referral group (MD ‐0.22, 95% CI ‐0.37 to ‐0.06; heterogeneity: Tau2 = 0.01; Chi2 = 5.27, df = 4 (P value = 0.26); I2 = 24%) (Figure 11). We conducted a sensitivity analysis, removing the trial by Potterat et al. (high risk of bias in random sequence generation), but there was no appreciable difference in the results.
11.
Forest plot: 5 Contract referral versus simple patient referral, outcome: 5.1 Number of partners elicited.
One study (74 participants) assessed the number of partners notified per index patient among patients with HIV (Landis 1992). There were more partners notified per index patient in the contract referral group than those that were asked to refer partners themselves (MD 1.71, 95% CI 1.24 to 2.19).
Three studies (1610 participants) assessed the number of partners who presented for care (Brown 2011; Cleveland undated; Potterat 1977). Contract referral resulted in slightly more partners presenting for care (MD 0.25, 95% CI 0.18 to 0.32; heterogeneity: Tau2 = 0.00; Chi2 = 0.85, df = 2 (P value = 0.65); I2 = 0%). We conducted a sensitivity analysis, removing the trial by Potterat 1977 (high risk of bias in random sequence generation), but there was no appreciable difference in the results.
Two studies (481 participants) assessed the time delay between enrolment of the index patient and presentation of the partner for care (Brown 2011; Schwebke 2010). In both studies, authors reported that the partner presented sooner in the simple patient referral than the contract referral group. In one study, the median time between enrolment of the index patient and partner presentation was 3 days (interquartile range (IQR) 2 to 7 days) in the simple patient referral group compared with 7 days (IQR 3 to 11 days) in the contract referral group (Brown 2011), and, in the other trial, the mean time was 5 days in the simple patient referral group and 7.25 days in the contract referral group (P value = 0.19) (Schwebke 2010).
Four studies (1684 participants) assessed the number of partners who tested positive (Brown 2011; Cleveland undated; Landis 1992; Potterat 1977). Contract referral resulted in slightly more partners who tested positive (MD 0.12, 95% CI 0.07 to 0.17; Heterogeneity: Tau2 = 0.00; Chi2 = 2.73, df = 3 (P value = 0.44); I2 = 0%). We conducted a sensitivity analysis, removing the trial by Potterat 1977 (high risk of bias in random sequence generation), but there was no appreciable difference in the results.
Two studies (509 participants) assessed the number of partners treated (Potterat 1977; Schwebke 2010). In one study, slightly more partners of women with trichomoniasis were treated in contract referral than in the simple patient referral group (MD 0.28, 95% CI 0.14 to 0.42) (Schwebke 2010). In the trial of men with gonorrhoea (Potterat 1977), there was no evidence of a difference between groups (MD 0, 95% CI ‐0.04 to 0.03). These two studies were not summarised in a meta‐analysis (heterogeneity: Tau2 = 0.04; Chi2 = 14.61, df = 1 (P value = 0.0001); I2 = 93%). These two studies were both performed in the US but in different gender groups reporting different STI.
One study (159 participants) reported on harms with one event in each group (Brown 2011). One episode of abandonment was reported in the simple patient referral group and the police were contacted to placate the partner of one index patient in the contract referral group.
No information was available for incidence of STI, changes in behaviour and emotional impact.
6. Contract referral versus enhanced patient referral
One study (1266 participants) compared contract referral versus enhanced patient referral (counselling) among patients with gonorrhoea (Cleveland undated).
Secondary outcomes
The number of partners elicited per index patient randomised was moderately lower in contract referral group than the enhanced patient referral (counselling) group (MD ‐0.40, 95% CI ‐0.59 to ‐0.21).
The number of partners who presented for care per index patient (MD 0.25, 95% CI 0.17 to 0.33) and the number of partners who tested positive per index patient (MD 0.11, 95% CI 0.05 to 0.18) were slightly higher in the contract referral group than the enhanced patient referral (counselling) group.
7. Contract referral versus expedited partner therapy
One study (324 participants) compared contract referral with EPT among patients with trichomoniasis (Schwebke 2010).
Primary outcome
There was no statistical evidence of a difference in index patient re‐infection rate at one or three months after treatment comparing EPT with contract referral (one month: RR 0.40, 95% CI 0.16 to 1.01 and three months: RR 2.0, 95% CI 0.7 to 5.72).
Secondary outcomes
There was no statistical evidence of a difference between the two groups in the number of partners elicited per index patient (MD 0.11, 95% CI ‐0.11 to 0.33). The number of partners treated per index patient was slightly higher in the EPT group compared with the contract referral group (MD 0.23, 95% CI 0.05 to 0.41).
No information was available for incidence of STI, changes in behaviour, emotional impact, harms, ethical outcomes, partners notified, partners presented for care, delay in partners presented for care or partners testing positive.
Provider referral
8. Provider referral versus simple patient referral
Two studies compared provider referral versus simple patient referral among patients with HIV (Brown 2011), and non‐gonococcal urethritis (Katz 1988). One study compared a choice between simple patient or provider referral with counselling versus simple patient referral among patients with STI syndromes (Faxelid 1996). None of these studies rinvestigated the primary outcome.
Secondary outcomes
Both studies comparing provider referral with simple patient referral (Brown 2011; Katz 1988) (596 participants) assessed the number of partners elicited per index patient. The results of these two studies showed effects in the opposite direction (heterogeneity: Tau2 = 0.14; Chi2 = 7.76, df = 1 (P value = 0.005); I2 = 87%). Subgroup analysis showed that these two studies included index patients with different STI, different settings and participants, and these studies were reported individually. Among women and men with HIV infection (Brown 2011), there was no evidence of a difference in the number of partners elicited per index patient (MD 0.21, 95% CI ‐0.15 to 0.57). Among men with non‐gonococcal urethritis (Katz 1988), those receiving provider referral reported fewer partners (MD ‐0.36, 95% CI ‐0.55 to ‐0.17).
In one study (158 participants), the time delay in partners presenting for care was measured (Brown 2011). Partners presented sooner for care in the simple patient referral group (median time from index patient enrolment to partners presenting for care 3 days (IQR 2 to 7 days)) compared with the provider referral group (median time 4 days (IQR 2 to 8 days)).
In both trials, there was a small increase in the number of partners testing positive per index patient in the provider group compared with the simple patient referral group (MD 0.06, 95% CI 0.02 to 0.11; heterogeneity: Tau2 = 0.00; Chi2 = 0.35, df = 1 (P value = 0.55); I2 = 0%).
Among men with non‐gonococcal urethritis (438 participants) there was a moderate increase in the number of partners treated per index patient in the provider referral group compared with the simple patient referral group (MD 0.5, 95% CI 0.37 to 0.63) (Katz 1988).
One trial (158 participants) reported harms among patients with HIV infection (Brown 2011). In the provider referral group, no harms were reported and in the simple patient referral group one episode of abandonment was reported.
No information was available for index patient re‐infection rate, partners notified, partners presenting for care, incidence of STI, changes in behaviour, emotional impact or ethical outcomes.
In the study that compareda choice between simple patient or provider referral with counselling versus simple patient referral (Faxelid 1996) (396 participants), there was evidence of a difference between the two groups in the number of partners elicited (MD ‐0.03, 95% CI ‐0.3 to 0.23).
The number of partners notified per index patient (MD 0.41, 95% CI 0.18 to 0.64) and the number of partners who presented for care per index patient (MD 0.46, 95% CI 0.24 to 0.69) were moderately higher in those given a choice than the simple patient referral group. The number of harms reported per male index patient randomised was slightly higher in choice option compared with patient referral option (MD 0.15, 95% CI 0.06 to 0.25). The trial authors did not report individual data but stated that there was no difference between the two groups in the number of harms reported.
No information was available for delay in partners presenting for care, partners testing positive, partners treated, changes in behaviour, emotional impact or ethical outcomes.
9. Provider referral versus enhanced patient referral
One study (461 participants) compared provider referral versus enhanced patient referral (contact tracer (DIS)) among men with non‐gonococcal urethritis (Katz 1988). This study did not investigate the primary outcome.
Secondary outcomes
No evidence of a difference was found in the two groups when comparing the number of partners elicited per index patient (MD ‐0.05, 95% CI ‐0.21 to 0.11). The number of partners who tested positive per index patient (MD ‐0.06, 95% CI ‐0.11 to ‐0.02) and number of partners treated per index patient (MD ‐0.54, 95% CI ‐0.66 to ‐0.42) were slightly lower in the provider referral group than the enhanced patient referral (contact tracer) group.
No information was available for index patient re‐infection rate, partners notified, partners presented for care, delay in partners presented for care, incidence of STI, changes in behaviour, emotional impact, harms or ethical outcomes.
10. Provider referral versus contract referral
Two studies (1491 participants) compared provider referral versus contract referral among patients with HIV (Brown 2011), and syphilis (Peterman 1997). Peterman et al. also compared a strategy of enhanced provider referral (field testing) with contract referral (1224 participants) and with provider referral alone (1380 participants). Neither of these studies investigated the primary outcome.
Secondary outcomes
Both studies that compared provider referral with contract referral (Brown 2011; Peterman 1997) assessed the number of partners elicited. The results were inconsistent (heterogeneity: Tau2 = 3.03; Chi2 = 127.21, df = 1 (P value < 0.001); I2 = 100%). Subgroup analysis (setting, STI, gender) could not explain heterogeneity. Brown et al. (163 participants) found no evidence of a difference between the two groups in patients with HIV infection (MD ‐0.27, 95% CI ‐0.62 to 0.07). The other study (1328 participants) found that the number of partners elicited per index patient with syphilis was higher in the contract referral group than the provider referral group (MD 2.2 95% CI 1.95 to 2.45) (Peterman 1997).
Both studies (1491 participants) assessed the number of partners located. No evidence of a difference between the two groups was found (MD 0.10, 95% CI ‐0.01 to 0.2; heterogeneity: Tau2 = 0.00; Chi2 = 0.05, df = 1 (P value = 0.82); I2 = 0%).
One of these studies (163 participants) compared the number of partners who presented for care and found no evidence of a difference between the two groups (MD 0.03, 95% CI ‐0.19 to 0.25) (Brown 2011).
Peterman et al. (1328 participants) assessed the number of partners tested per index patient and found no evidence of a difference between two groups (MD 0.05, 95% CI ‐0.05 to 0.15) (Peterman 1997).
Both studies (1491 participants) compared the number of partners testing positive (Brown 2011; Peterman 1997). No evidence was found of a difference between two groups (MD 0.02, 95% CI ‐0.03 to 0.06; heterogeneity: Tau2 = 0.00; Chi2 = 0.07, df = 1 (P value = 0.79); I2 = 0%).
Peterman et al. (1328 participants) compared the number of partners treated per index patient and found no evidence of a difference between the two groups (MD 0.06, 95% CI ‐0.03 to 0.15) (Peterman 1997).
One of these studies (163 participants) reported on harms (Brown 2011). The study reported no harms reported in the provider referral arm and one episode of abandonment reported in the contract referral arm (MD 0.01, 95% CI ‐0.01 to 0.04).
One study (163 participants) assessed the time delay in partners presented for care after enrolment of index patient (Brown 2011). The study found that the partners of the index patient in the provider referral arm presented sooner for care (median time between enrolment of index patient and partner presenting 4 days (IQR 2 to 8 days) compared with the contract referral arm (median time between enrolment of index patient and partner presenting for care 7 days (IQR 3 to 11 days).
No information was available for ethical outcomes, index patient re‐infection rate, partners presenting for care, incidence of STI, changes in behaviour or emotional impact.
In Peterman et al., the number of partners elicited per index patient was moderately higher in the enhanced provider (field testing) referral group compared with the contract referral group (MD 0.5, 95% CI 0.21 to 0.79) and much higher than in the group receiving provider referral alone (MD 2.7, 95% CI 2.45 to 2.95) (Peterman 1997).
There was no evidence of a difference between enhanced provider (field testing) referral group compared with contract referral or provider referral alone in the number of partners located per index patient (contract referral: MD ‐0.10, 95% CI ‐0.22 to 0.02; provider referral: MD 0.0, 95% CI ‐0.11 to 0.11), in the number of partners who were tested (contract referral: MD ‐0.06, 95% CI ‐0.17 to 0.05; provider referral: MD ‐0.01, 95% CI ‐0.11 to 0.09), in the number of partners testing positive (contract referral: MD ‐0.02 95% CI ‐0.07 to 0.03; provider referral: MD 0.0; 95% ‐0.05 to 0.04) or in the number of partners receiving treatment (contract referral: MD ‐0.05, 95% CI ‐0.14 to 0.04; provider referral: MD 0.01, 95% CI ‐0.07 to 0.09).
No information was available for incidence of STI, partners notified, index patient re‐infection rate, partners presenting for care, delay in partners presenting for care, changes in behaviour, emotional impact, harms, and ethical outcomes.
Discussion
Summary of main results
Twenty‐six RCTs including 17,578 participants (9015 women and 8563 men) conducted in 10 countries were included in this systematic review.
Summary of evidence according to type of partner notification strategy
EPT for index patients, in trials including those with gonorrhoea, chlamydia, gonorrhoea or chlamydia, or trichomonas (six trials) was better than simple patient referral for the prevention of re‐infection of the index patient. EPT also increased the number of partners treated per index patient (four trials). The re‐infection rate after EPT was similar to that with enhanced patient referral (three trials) but EPT resulted in more partners treated (one trial). When contract referral was compared with EPT (one trial), there was no difference in re‐infection rates among index patients but EPT resulted in more partners being treated. There was insufficient evidence to determine the most effective components of an enhanced patient referral intervention.
We found some evidence that more partners were treated with provider referral (one trial of non‐gonococcal urethritis) compared with simple patient referral. In patients with syphilis (one trial), contract referral elicited more partners than provider referral but the number of partners presenting for care and receiving treatment was the same in the two groups. There was no consistent evidence for the relative effects of provider, contract or patient referral for other STI.
The results of four trials comparing home sampling kits for partners with simple patient referral found no evidence of a reduction in re‐infection rates in index cases or higher numbers of partners elicited, notified or treated. We found no studies evaluating provider training. Only seven trials assessed potential harms; we could not combine the results but there was no evidence of differences in the incidence of adverse effects in any of the individual trials.
Summary of evidence, by infection
There were 11 different categories of STI included in the review. Fifteen studies assessed strategies for PN in individual STI and 11 studies assessed combinations of STI or syndromic diagnoses. There were no RCTs among patients with laboratory‐diagnosed hepatitis B, genital herpes or chancroid.
HIV
Only two studies evaluated PN strategies among patients with HIV (314 participants) (Brown 2011; Landis 1992). Both contract referral and provider referral resulted in more partners presenting for care and testing positive than simple patient referral.
Chlamydia
There was no evidence of a difference in index patient re‐infection rates in two trials that compared EPT with simple patient referral (2007 participants) (Cameron 2009; Schillinger 2003). Four studies compared home sampling kits for partners with simple patient referral (1058 participants) (Andersen 1998; Apoola 2009; Cameron 2009; Ostergaard 2003). One study found no reduction in re‐infection in index patients (Cameron 2009). There was no difference between groups in numbers of partners elicited, notified or treated.
Gonorrhoea
There was no evidence about index patient re‐infection rates. Three studies were performed among patients with gonorrhoea (Cleveland undated; Potterat 1977; Solomon 1988). One study compared simple patient referral versus contract referral (Potterat 1977), another study compared simple patient referral versus enhanced patient referral (videotape) (Solomon 1988), and the third study compared simple patient referral versus contract referral versus enhanced patient referral (additional counselling) (Cleveland undated). Simple patient referral elicited a slightly higher number of partners if compared with contract referral (Cleveland undated; Potterat 1977). The authors of one study using the enhanced patient referral (videotape) did not report results (Solomon 1988).
Chlamydia or gonorrhoea
In trials that included index patients with either chlamydia or gonorrhoea, there was evidence that EPT reduced the index patient re‐infection rate compared with simple patient referral (3380 participants) (Golden 2005; Kissinger 2005). There was also evidence from one trial (600 participants) that index patient re‐infection rates were reduced by patient referral enhanced by additional counselling compared with simple patient referral (Wilson 2009). In one trial among men who had sex with men, more partners were elicited when a combination of EPT and enhanced patient referral (inSPOT website) was used compared with enhanced patient referral (inSPOT website) alone (Kerani 2011).
Trichomonas
There was no statistical evidence that EPT resulted in a lower re‐infection rate in female index patients in comparisons of: EPT versus patient‐booklet enhanced patient referral versus simple patient referral (463 participants) (Kissinger 2006); or EPT versus contract referral versus simple patient referral (484 participants) (Schwebke 2010). Slightly more partners were treated when EPT was used compared with contract referral (Schwebke 2010).
Non‐gonococcal urethritis
One study (678 participants) compared simple patient referral delivered by a nurse versus enhanced patient referral delivered by a DIS versus provider referral (Katz 1988). Provider referral resulted in slightly more partners who tested positive and who received treatment when compared with simple patient referral delivered by a nurse. Simple patient referral by a nurse was superior to enhanced patient referral in the number of partners elicited, but there was no evidence of a difference between groups in number of partners who tested positive.
Non‐gonococcal urethritis or gonorrhoea
There was no evidence about index patient re‐infection rates. One study (65 participants) compared patient referral enhanced by additional counselling alone, counselling with incentives and counselling with a follow‐up telephone call (Montesinos 1990). There was no evidence of superiority of any of the different method assessed in eliciting partners or increasing the number of partners who presented for care.
Non‐gonococcal urethritis or chlamydia
In one study (105 participants), there was no evidence that the use of a website reduced index patient re‐infection rates, or increased the number of partners elicited or notified (Tomnay 2006).
Syphilis
One study (1966 participants) was performed among patients with syphilis (Peterman 1997). This study compared contract referral versus provider referral versus enhanced provider referral (with field testing). Contract referral elicited more partners than provider referral. There was no difference between the numbers of partners who were tested, who tested positive or who received treatment between the contract and provider referral group.
Pelvic inflammatory disease
There was no evidence about index patient re‐infection rates. One study (126 participants) was included on PID, but exact numbers of partners notified were not available from trial authors (Trent 2010).
Any sexually transmitted infections syndrome
Four studies (2770 participants) in developing countries in Africa were performed among patients with a syndromic diagnosis of a STI (Ellison undated; Faxelid 1996; Moyo 2002; Nuwaha 2001). One study (396 participants) found that index patients given a choice between patient and provider referral, compared with simple patient referral resulted in slightly more partners notified and presenting for treatment (Faxelid 1996). One study (383 participants) found that EPT resulted in slightly more partners treated (Nuwaha 2001). In two studies (1991 participants), simple patient referral was compared with enhanced patient referral with additional counselling (Ellison undated; Moyo 2002). In one study (858 participants), a combination of giving additional counselling and health education messages compared with simple patient referral resulted in slightly more partners elicited and treated (Ellison undated).
Overall completeness and applicability of evidence
We identified 16 new trials in the update in addition to the 11 in the first Cochrane review (Mathews 2001). We found studies on the four most common curable STIs: chlamydia (six trials), gonorrhoea (three trials), chlamydia or gonorrhoea (four trials), trichomoniasis (two trials) and syphilis (one trial). We included only two trials among people with HIV and we identified no studies on chancroid, genital herpes or hepatitis B. Only five of the 26 trials were conducted in developing countries. Only one trial included in this review enrolled men who had sex with men who were infected with chlamydia or gonorrhoea (Kerani 2011). One of the trials among people with HIV infection included men who had sex with men (Landis 1992). We added EPT as a new strategy to enhance the effectiveness of patient referral in this update. In addition, we separated patient referral interventions into those that added components such as counselling, written information, websites and specimen testing kits (enhanced patient referral), and those restricted to spoken advice about the need for partners to receive treatment (simple patient referral). We found no studies on provider training. Nine studies reported index patient re‐infection rate, the primary outcome for curable STIs. Few of the studies assessed the proportion of partners who were infected, but both studies of patients with HIV infection reported this outcome. Instead, most studies relied on surrogate outcomes such as partners presenting for medical evaluation, or reports by index patients of partners presenting. Secondary outcomes reported on infrequently or not at all included delays in partners presenting for care, incidence of STIs, changes in behaviour, emotional impact and ethical outcomes.
Quality of the evidence
In every study, there were risks to the validity of the findings and assessment of risk of bias was hampered by incomplete reporting in more than half of the included studies. Sequence generation was adequate in 11 studies while allocation concealment was only adequate in five studies. Inadequate methods of allocation concealment are an important source of potential bias for RCTs of PN interventions, where those enrolling participants might preferentially allocate selected patients to one particular intervention. Blinding of investigators and patients was not feasible for the types of interventions studied and only six studies reported blinding of outcome assessors. Where outcomes can be subjective, for example judging patient‐reported outcomes, unblinded outcome assessment could introduce bias. Re‐infection is an objective biological outcome, so lack of blinding of outcome assessors would be less important. Seven studies reported loss to follow‐up of more than 20%. Most studies had a low risk of selective outcome reporting. In addition, methods and sensitivity of tests used to diagnose STIs varied across studies.
When the body of evidence about PN strategies was considered, there were only four comparisons reporting the primary outcome of re‐infection of index patients with curable STI. EPT compared with simple patient referral was the comparison with the largest number of trials, showing moderate‐quality evidence that EPT reduces re‐infection more than simple patient referral when we pooled results from trials of all curable STIs. We downgraded the quality of evidence because of the risk of bias resulting from attrition and inadequately described methods. There was also low‐quality evidence (limited by the small number of studies and attrition bias), that effect size might differ for different STI. There was also low‐quality evidence from three trials that the effect of EPT was similar to that of enhanced patient referral strategies. Comparisons of enhanced versus simple patient referral were limited to one or two trials for each strategy. There was moderate‐quality evidence that additional counselling reduced re‐infection more than simple patient referral. There was low‐quality evidence from one trial that the effect of contract referral was similar to patient referral.
Potential biases in the review process
We conducted an extensive and comprehensive search strategy with no language restrictions of electronic databases to identify all published and unpublished trials. We contacted experts in the field and searched trial registries to identify ongoing studies. We contacted trial authors, where necessary, to obtain missing data. To minimise bias in the review process, two review authors independently performed all study selection, eligibility assessment, data extraction and assessment of risk of bias. If consensus could not be reached, we consulted a third review author. We used standardised eligibility and data extraction forms.
Agreements and disagreements with other studies or reviews
Our findings were consistent with the findings of the two most recently published systematic reviews (Alam 2010; Trelle 2007). The first found that counselling increased partner referral and was reasonable for developing countries where it was well received by index patient, easily integrated and cost effective (Alam 2010). It also found that EPT resulted in more partners treated compared with simple patient referral alone. Barriers to partner referral were mainly cultural and psychosocial (fear of rejection and abuse). The second review also found that EPT resulted in fewer re‐infections of the index patient and more partners treated than simple patient referral and that the outcomes of EPT were similar to those with enhanced patient referral (Trelle 2007). Consistent with this update, both Trelle 2007 and Alam 2010 reported the inappropriateness of summarising the evidence in a meta‐analysis due to the differences in PN methods used and the way outcomes were reported. Two observational studies reported on adverse effects, 9% of index patients reported physical violence (Kissinger 2003), and 44% reported negative emotional reactions by partners (Rosenthal 1995). In Trelle et al., the authors suggested that labour‐intensive methods, such as provider and contract referral, could be considered for more serious conditions, such as HIV and syphilis, even though evidence for their superiority was inconsistent (Trelle 2007).
Furthermore, Trelle et al. argued for more studies on the use of EPT in chlamydia and gonorrhoea, as well as large RCTs on PN and HIV and syphilis, and that adverse effects need to be reported specifically (Trelle 2007).
Authors' conclusions
Implications for practice.
The evidence assessed in this systematic review does not identify a single optimal strategy for partner notification (PN) for any particular sexually transmitted infection (STI). Few studies evaluated syphilis and human immunodeficiency virus (HIV), most were conducted in developed countries for STIs acquired heterosexually and few studies assessed adverse events.
It is important that expedited partner therapy (EPT) interventions include all the components that were part of the EPT package in trials to achieve the outcomes expected. The EPT interventions in the trials in this review included condoms, details of STI clinics, and written information for patients and partners in addition to treatment with antibiotics. In practice, many physicians report giving additional courses of antibiotics or prescriptions to index patients, but it is not clear whether they also give additional support (CDC 2006). EPT is more successful than simple patient referral in preventing re‐infection of the index patient and resulted in more partners treated when compared with simple patient referral and contract referral. The effect of EPT was attenuated when we excluded studies with high attrition (> 20%) from the analysis. In addition, in many countries, EPT is not legal and, therefore, not an available option at present. Provider referral and contract referral identified slightly more new infections in partners of patients with HIV compared with simple patient referral. These strategies are more labour and cost intensive than simple patient referral but are considered worthwhile for serious conditions such as HIV and syphilis (Trelle 2007).
When considering the use of enhanced patient referral in chlamydia, gonorrhoea or trichomonas infections or non‐gonococcal urethritis, most methods were only investigated in one trial and there was no strong evidence of differences in specific outcomes when compared with simple patient referral. The most effective components in the enhanced patient referral strategy could not be identified.
Implications for research.
There is a need for more evaluations of interventions combining provider training and patient education, and for evaluations conducted in developing countries. The use of syndromic diagnosis in trials needs to be discouraged especially where vaginal discharge is the concern. Self sampling and self testing need to be evaluated in low‐income communities relying heavily on syndromic management. Evaluations of interventions to improve the training in delivering PN for healthcare providers and interventions combining both training and patient education would be valuable.
Large randomised controlled trials (RCTs) for PN in syphilis and HIV are needed and could compare the outcomes of provider referral with methods of enhanced patient referral. Trials conducted in the future should strongly consider using biological outcomes, such as re‐infection of the index patient for curable STI and numbers of infected partners identified for HIV. The effect of PN strategies on changes in the behaviour of index patients or partners should also be assessed, particularly for HIV patients. Furthermore, they need to consider measuring to what extent strategies are successful at reaching partners who have a high potential for onward transmission of STI as opposed to monogamous partners. The acceptability of various PN strategies to index patients and partners needs to be assessed, and the costs and potential harms of PN need to be measured and compared. A proposed question for primary research is: "In patients given a diagnosis of HIV in developing countries, will provider referral when compared with enhanced patient referral increase the number of infected partners identified?"
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2013 | New citation required but conclusions have not changed | The conclusions of the review are essentially unchanged from the previously published version of the review. |
| 11 September 2012 | New search has been performed | Major update completed which include a new search, 16 new studies, new review format and methodology. |
Acknowledgements
We would like to thank Rabiatu Abdullah for her general assistance and for reviewing abstracts and developing the data extraction form for the original review. In addition, we are grateful to Elizabeth Pienaar, Joy Olivier, Susan Hansen, Miguel Diaz Ortega and Gail Kennedy for their assistance with the search for trials. Authors of the original review: Catherine Mathews, Nicol Coetzee, Merrick Zwarenstein, Carl Lombard, Sally Guttmacher, Andrew D Oxman, George Schmid. For statistical support Carl Lombard and Tonya Esterhuizen.
The Medical Research Council and the Public Health Department of the University of Cape Town supported and funded the original review. The update was supported by funding from the Effective Health Care Research Consortium, which is funded by UK Aid from the Department for International Development.
Part of the text of this review contributed to a project supported by the National Institute for Health Research (NIHR) Health Technology Assessment programme (project number 07/42/02). The project will be published in full in the Health Technology Assessment journal series. Visit the HTA programme website for more details (www.hta.ac.uk/1722). The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Database: PubMed (2001‐2012)
Date: 18 March 2011, 29 January 2012, and 31 August 2012
| Search | Most Recent Queries |
| #7 | Search #3 AND #4 AND #5 Limits: Publication Date from 10 May 2001 to 18 March 2011 |
| #6 | Search #3 AND #4 AND #5 |
| #5 | Search partner notification[tiab] OR partner notifications[tiab] OR contact tracing[mh] OR contact tracing[tiab] OR (expedited[tiab] AND partner[tiab]) OR patient delivered[tiab] OR referral[tiab] OR referrals[tiab] OR partner tracing[tiab] |
| #4 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #3 | Search #1 OR #2 |
| #2 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) |
| #1 | Search sexually transmitted infections[mh] OR sexually transmitted disease*[tiab] OR sexually transmissible disease*[tiab] OR sexually transmitted infection*[tiab] OR sexually transmissible infection*[tiab] OR sexually transmitted infectious disease*[tiab] OR sexually transmissible infectious disease*[tiab] OR sexually transmitted disorder*[tiab] OR sexually transmissible disorder*[tiab] OR STI[tiab] OR STIs[tiab] OR STD[tiab] OR STIs[tiab] OR venereal disease*[tiab] OR venereal infection*[tiab] OR venereal disorder*[tiab] OR genital herpes[tiab] OR herpes genitalis[mh] OR herpes genitalis[tiab] OR genital infection*[tiab] OR genital disorder*[tiab] OR herpes simplex[tiab] OR herpes virus[tiab] OR HSV‐1[tiab] OR HSV‐2[tiab] OR chancroid[mh] OR chancroid* [tiab] OR haemophilus ducreyi[tiab] OR chlamydia infection*[tiab] OR chlamydia trachomatis[mh] OR chlamydia trachomatis[tiab] OR gonorrhea[mh] OR gonorrhoea*[tiab] OR gonorrhea*[tiab] OR syphilis[mh] OR syphilis[tiab] OR syphillis[tiab] OR condylomata lata[tiab] OR chancre*[tiab] OR lymphogranuloma venereum[mh] OR lymphogranuloma venereum[tiab] OR granuloma Inguinale[mh] OR granuloma inguinale[tiab] OR donovania[tiab] OR donovanosis[tiab] OR calymmatobacterium[mh] OR calymmatobacterium granulomatis[tiab] OR klebsiella granulomatis[tiab] OR klebsiella granulomatis[tiab] OR treponema pallidum[mh] OR treponema pallidum[tiab] OR genital wart*[tiab] OR venereal wart*[tiab] OR condylomata acuminata[mh] OR human papillomavirus 6[mh] OR hpv‐6[tiab] OR hpv‐11[tiab] OR hpv6[tiab] OR human papillomavirus[tiab] OR hepatitis b[mh] OR hepatitis b[tiab] OR trichomonas vaginitis[mh] OR trichomonas vaginitis[tiab] OR genital ulcer*[tiab] OR anogenital ulcer*[tiab] OR anorectal ulcer*[tiab] OR anorectal ulcer*[tiab] OR penile ulcer*[tiab] |
SEXUALLY TRANSMITTED DISEASES[MH] OR HERPES GENITALIS[MH] OR GONORRHEA[MH] OR SYPHILIS[MH] OR GRANULOMA INGUINALE[MH] OR CONDYLOMATA ACUMINATA[MH] OR LYMPHOGRANULOMA VENEREUM[MH]
Appendix 2. EMBASE search strategy
Database: EMBASE (2001‐2012)
Date: 18 March 2011, 29 January 2012 and 31 August 2012
| No. | Query |
| #7 | #3 AND #4 AND #5 AND [humans]/lim AND [EMBASE]/lim AND [1‐5‐2001]/sd NOT [18‐3‐2011]/sd |
| #6 | #3 AND #4 AND #5 |
| #5 | 'contact examination'/syn OR 'contact detection':ab,ti OR 'contact tracing':ab,ti OR 'partner notification':ab,ti OR 'partner notifications':ab,ti OR 'expedited partner':ab,ti OR 'patient delivered':ab,ti OR referral*:ab,ti OR 'partner tracing':ab,ti |
| #4 | #1 OR #2 |
| #3 | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial'/de OR 'randomized controlled trial' |
| #2 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
| #1 | 'sexually transmitted infections'/exp OR 'sexually transmitted infections, bacterial'/exp OR 'sexually transmitted infections, viral'/exp OR (sexually AND transmitted AND disease*:ti OR sexually AND transmitted AND disease*:ab) OR (sexually AND transmissible AND disease*:ti OR sexually AND transmissible AND disease*:ab) OR (sexually AND transmitted AND infection*:ti OR sexually AND transmitted AND infection*:ab) OR (sexually AND transmissible AND infection*:ti OR sexually AND transmissible AND infection*:ab) OR (sexually AND transmitted AND infectious AND disease*:ti OR sexually AND transmitted AND infectious AND disease*:ab) OR (sexually AND transmissible AND infectious AND disease*:ti OR sexually AND transmissible AND infectious AND disease*:ab) OR (sexually AND transmitted AND disorder*:ti OR sexually AND transmitted AND disorder*:ab) OR (sexually AND transmissible AND disorder*:ti OR sexually AND transmissible AND disorder*:ab) OR sti:ti OR sti:ab OR std:ti OR std:ab OR (genital AND ulcer*:ti OR genital AND ulcer*:ab) OR (genital AND 'ulcer'/exp AND disease*:ti OR genital AND 'ulcer'/exp AND disease*:ab) OR (ulcerative AND sexually AND transmitted*:ti OR ulcerative AND sexually AND transmitted*:ab) OR (genital AND infection*:ti OR genital AND infection*:ab) OR (genital AND disorder*:ti OR genital AND disorder*:ab) OR (venereal AND disease*:ti OR venereal AND disease*:ab) OR (venereal AND infection*:ti OR venereal AND infection*:ab) OR (venereal AND disorder*:ti OR venereal AND disorder*:ab) OR 'herpes simplex'/exp OR 'herpes genitalis'/exp OR ('herpes'/exp AND simplex:ti OR 'herpes'/exp AND simplex:ab) OR ('herpes'/exp AND genitalis:ti OR 'herpes'/exp AND genitalis:ab) OR (genital AND herpes:ti OR genital AND herpes:ab) OR ('herpes'/exp AND virus:ti OR 'herpes'/exp AND virus:ab) OR 'hsv 1':ti OR 'hsv 1':ab OR 'hsv 2':ti OR 'hsv 2':ab OR donovanosis:ti OR donovanosis:ab OR ('granuloma'/exp AND inguinale:ti OR 'granuloma'/exp AND inguinale:ab) OR ('calymmatobacterium'/exp AND granulomatis:ti OR 'calymmatobacterium'/exp AND granulomatis:ab) OR donovania:ti OR donovania:ab OR ('klebsiella'/exp AND granulomatis:ti OR 'klebsiella'/exp AND granulomatis:ab) OR 'syphilis'/exp OR syphilis:ti OR syphilis:ab OR syphillis:ab OR syphillis:ti OR ('treponema'/exp AND pallidum:ti OR 'treponema'/exp AND pallidum:ab) OR chancre:ti OR chancre:ab OR ('condylomata'/exp AND lata:ti OR 'condylomata'/exp AND lata:ab) OR chancroid:ti OR chancroid:ab OR ('haemophilus'/exp AND ducreyi:ti) OR (soft AND chancre:ti OR soft AND chancre:ab) OR 'chlamydia trachomatis'/exp OR 'lymphogranuloma venereum'/exp OR (lymphogranuloma AND venereum:ti OR lymphogranuloma AND venereum:ab) OR ('chlamydia'/exp AND trachomatis:ti OR 'chlamydia'/exp AND trachomatis:ab) OR ('chlamydia'/exp AND infections:ti OR 'chlamydia'/exp AND infections:ab) OR lgv:ti OR lgv:ab OR (vaginal AND ulcer*:ti OR vaginal AND ulcer*:ab) OR (anogenital AND ulcer*:ti OR anogenital AND ulcer*:ab) OR (anorectal AND ulcer*:ti OR anorectal AND ulcer*:ab) OR (penile AND ulcer*:ti OR penile AND ulcer*:ab) OR (genital AND wart*:ti OR genital AND wart*:ab) OR (venereal AND wart*:ti OR venereal AND wart*:ab) OR 'condyloma acuminatum'/exp OR 'human papillomavirus 6'/exp OR ('hpv 6':ti OR 'hpv 6':ab OR hpv6:ti OR hpv6:ab OR human AND papillomavirus:ti OR human AND papillomavirus:ab) OR 'hepatitis b'/exp OR 'hepatitis b':ti OR 'hepatitis b':ab OR 'gonorrhea'/exp OR gonorrhea*:ti OR gonorrhea*:ab OR gonorrhoea*:ti OR gonorrhoea*:ab |
Appendix 3. CENTRAL search strategy
Database: The Cochrane Library 2011, Issue 1 (2001‐2012)
Date: 22 March 2011, 29 January 2012 and 31 August 2012
Number of clinical trials retrieved: 191 records
| ID | Search |
| #1 | MeSH descriptor Sexually Transmitted Diseases explode all trees |
| #2 | MeSH descriptor Herpes Genitalis, this term only |
| #3 | MeSH descriptor Chancroid, this term only |
| #4 | MeSH descriptor Chlamydia trachomatis, this term only |
| #5 | MeSH descriptor Gonorrhea, this term only |
| #6 | MeSH descriptor Syphilis, this term only |
| #7 | MeSH descriptor Lymphogranuloma Venereum, this term only |
| #8 | MeSH descriptor Granuloma Inguinale, this term only |
| #9 | MeSH descriptor Calymmatobacterium, this term only |
| #10 | MeSH descriptor Treponema pallidum, this term only |
| #11 | MeSH descriptor Condylomata Acuminata, this term only |
| #12 | MeSH descriptor Human papillomavirus 6 explode all trees |
| #13 | MeSH descriptor Hepatitis B explode all trees |
| #14 | MeSH descriptor Trichomonas Vaginitis, this term only |
| #15 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) |
| #16 | sexually transmitted disease*:ti,ab,kw OR sexually transmissible disease*:ti,ab,kw OR sexually transmitted infection*:ti,ab,kw OR sexually transmissible infection*:ti,ab,kw OR sexually transmitted infectious disease*:ti,ab,kw OR sexually transmissible infectious disease*:ti,ab,kw OR sexually transmitted disorder*:ti,ab,kw OR sexually transmissible disorder*:ti,ab,kw OR STI:ti,ab,kw OR STIs:ti,ab,kw OR STD:ti,ab,kw OR STIs:ti,ab,kw OR venereal disease*:ti,ab,kw OR venereal infection*:ti,ab,kw OR venereal disorder*:ti,ab,kw OR genital herpes:ti,ab,kw OR herpes genitalis:ti,ab,kw OR genital infection*:ti,ab,kw OR genital disorder*:ti,ab,kw |
| #17 | herpes simplex:ti,ab,kw OR herpes virus:ti,ab,kw OR HSV‐1:ti,ab,kw OR HSV‐2:ti,ab,kw OR chancroid*:ti,ab,kw OR haemophilus ducreyi:ti,ab,kw OR chlamydia infection*:ti,ab,kw OR chlamydia trachomatis:ti,ab,kw OR gonorrhoea*:ti,ab,kw OR gonorrhea*:ti,ab,kw OR syphilis:ti,ab,kw OR syphillis:ti,ab,kw OR condylomata lata:ti,ab,kw OR chancre*:ti,ab,kw OR lymphogranuloma venereum:ti,ab,kw OR granuloma inguinale:ti,ab,kw OR donovania:ti,ab,kw OR donovanosis:ti,ab,kw OR calymmatobacterium granulomatis:ti,ab,kw OR klebsiella granulomatis:ti,ab,kw OR klebsiella granulomatis:ti,ab,kw OR treponema pallidum:ti,ab,kw OR genital wart*:ti,ab,kw OR venereal wart*:ti,ab,kw OR hpv‐6:ti,ab,kw OR hpv‐11:ti,ab,kw OR hpv6:ti,ab,kw OR human papillomavirus:ti,ab,kw OR hepatitis b:ti,ab,kw OR trichomonas vaginitis:ti,ab,kw OR genital ulcer*:ti,ab,kw OR anogenital ulcer*:ti,ab,kw OR anorectal ulcer*:ti,ab,kw OR anorectal ulcer*:ti,ab,kw OR penile ulcer*:ti,ab,kw |
| #18 | (#15 OR #16 OR #17) |
| #19 | MeSH descriptor HIV Infections explode all trees |
| #20 | MeSH descriptor HIV explode all trees |
| #21 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME |
| #22 | MeSH descriptor Lymphoma, AIDS‐Related, this term only |
| #23 | (#19 OR #20 OR #21 OR #22) |
| #24 | (#18 OR #23) |
| #25 | MeSH descriptor Contact Tracing, this term only |
| #26 | partner notification:ti,ab,kw OR partner notifications:ti,ab,kw OR contact tracing:ti,ab,kw OR expedited partner:ti,ab,kw OR patient delivered:ti,ab,kw OR referral:ti,ab,kw OR referrals:ti,ab,kw OR partner tracing:ti,ab,kw |
| #27 | (#25 OR #26) |
| #28 | (#24 AND #27) |
| #29 | (#24 AND #27), from 2001 to 2011 |
Data and analyses
Comparison 1. Enhanced patient referral versus simple patient referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐infection in index patient | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Home sampling vs. simple patient referral | 1 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.91, 5.05] |
| 1.2 Information booklet vs. simple patient referral | 2 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.22, 1.33] |
| 1.3 Patient referral (DIS/health advisor) vs. patient referral (nurse) | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.51] |
| 1.4 Disease‐specific website vs. simple referral | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [0.17, 58.73] |
| 1.5 Additional counselling vs. simple patient referral | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.89] |
| 2 Number of partners elicited | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Home sampling vs. patient referral | 3 | 516 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.19, 0.18] |
| 2.2 Additional counselling vs. patient referral | 3 | 4108 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.03, 0.43] |
| 2.3 Patient referral (DIS) vs. patient referral (nurse) | 2 | 597 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.57, ‐0.24] |
| 2.4 Information booklet vs. patient referral | 1 | 633 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.22, 0.22] |
| 2.5 Disease‐specific website vs. patient referral | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.72, 0.42] |
| 3 Number of partners notified | 5 | 1236 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.06, 0.20] |
| 3.1 Home sampling vs. patient referral | 2 | 782 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 3.2 Additional counselling vs. patient referral | 1 | 272 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.06, 0.36] |
| 3.3 Disease‐specific website vs. patient referral | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.68, 0.34] |
| 3.4 Videotape vs. patient referral | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of partners presenting for care | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Home sampling vs. patient referral | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Additional counselling vs. patient referral | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of partners testing positive | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Home sampling vs. patient referral | 3 | 878 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.05, 0.17] |
| 5.2 Additional counselling vs. patient referral | 1 | 1266 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
| 5.3 Patient referral (DIS) vs. patient referral (nurse) | 1 | 457 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6 Number of partners treated | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1 Enhanced patient referral versus simple patient referral, Outcome 1 Re‐infection in index patient.
1.2. Analysis.
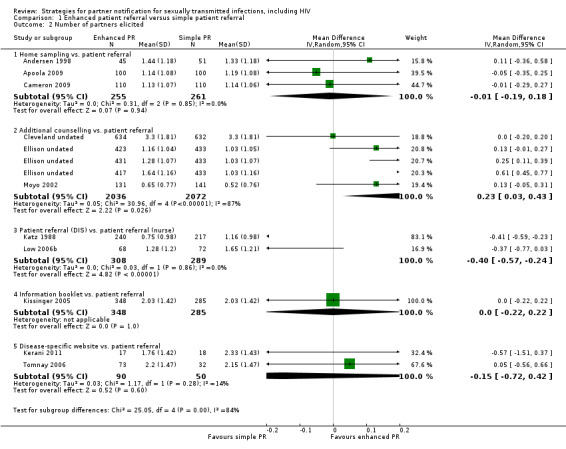
Comparison 1 Enhanced patient referral versus simple patient referral, Outcome 2 Number of partners elicited.
1.3. Analysis.

Comparison 1 Enhanced patient referral versus simple patient referral, Outcome 3 Number of partners notified.
1.4. Analysis.
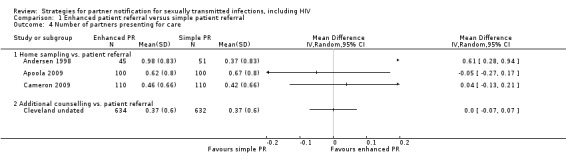
Comparison 1 Enhanced patient referral versus simple patient referral, Outcome 4 Number of partners presenting for care.
1.5. Analysis.

Comparison 1 Enhanced patient referral versus simple patient referral, Outcome 5 Number of partners testing positive.
1.6. Analysis.
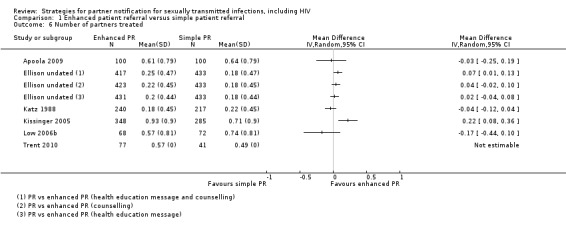
Comparison 1 Enhanced patient referral versus simple patient referral, Outcome 6 Number of partners treated.
Comparison 2. Enhanced patient referral versus other enhanced patient referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Partners elicited | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Number of partners presenting for care | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Number of partners treated | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2 Enhanced patient referral versus other enhanced patient referral, Outcome 1 Partners elicited.
2.2. Analysis.
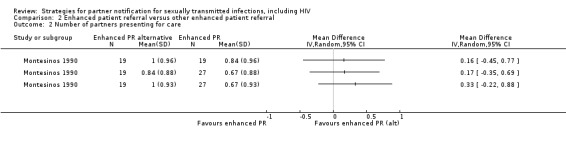
Comparison 2 Enhanced patient referral versus other enhanced patient referral, Outcome 2 Number of partners presenting for care.
2.3. Analysis.

Comparison 2 Enhanced patient referral versus other enhanced patient referral, Outcome 3 Number of partners treated.
Comparison 3. Expedited partner therapy (EPT) versus simple patient referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐infection in index patients | 6 | 6018 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.89] |
| 1.1 Chlamydia | 2 | 2007 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.60, 1.35] |
| 1.2 Trichomonas | 2 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.28] |
| 1.3 Chlamydia or gonorrhoea | 2 | 3380 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.39, 0.94] |
| 2 Number of partners elicited | 6 | 4339 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.09, 0.04] |
| 3 Number of partners notified | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Number of partners presenting for care | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Number of partners treated | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Chlamydia or gonorrhoea | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Trichomonas | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Any STI syndrome | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of harmful events reported | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
3.1. Analysis.
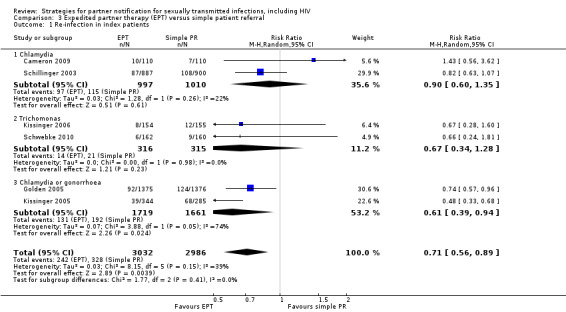
Comparison 3 Expedited partner therapy (EPT) versus simple patient referral, Outcome 1 Re‐infection in index patients.
3.2. Analysis.
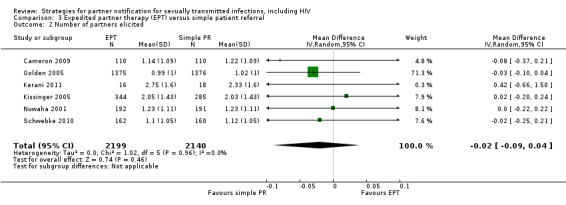
Comparison 3 Expedited partner therapy (EPT) versus simple patient referral, Outcome 2 Number of partners elicited.
3.3. Analysis.

Comparison 3 Expedited partner therapy (EPT) versus simple patient referral, Outcome 3 Number of partners notified.
3.4. Analysis.

Comparison 3 Expedited partner therapy (EPT) versus simple patient referral, Outcome 4 Number of partners presenting for care.
3.5. Analysis.
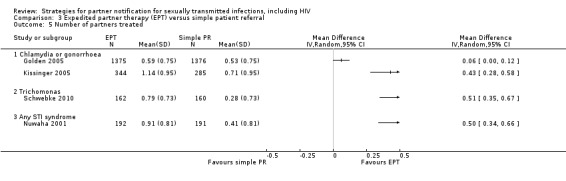
Comparison 3 Expedited partner therapy (EPT) versus simple patient referral, Outcome 5 Number of partners treated.
3.6. Analysis.
Comparison 3 Expedited partner therapy (EPT) versus simple patient referral, Outcome 6 Number of harmful events reported.
Comparison 4. Expedited partner therapy (EPT) versus enhanced patient referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 EPT vs. enhanced patient referral | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Re‐infection in index patients | 3 | 1220 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.60, 1.53] |
| 2 EPT vs. enhanced patient referral | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Number of partners elicited | 3 | 945 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.32] |
| 2.2 Number of partners notified | 1 | 220 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.08, 0.30] |
| 2.3 Number of partners presenting for care | 1 | 220 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 2.4 Number of partners treated | 1 | 692 | Mean Difference (IV, Random, 95% CI) | 0.22 [0.21, 0.23] |
| 3 Enhanced patient referral plus EPT vs. simple patient referral | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Number of partners elicited | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 Expedited partner therapy (EPT) versus enhanced patient referral, Outcome 1 EPT vs. enhanced patient referral.
4.2. Analysis.

Comparison 4 Expedited partner therapy (EPT) versus enhanced patient referral, Outcome 2 EPT vs. enhanced patient referral.
4.3. Analysis.
Comparison 4 Expedited partner therapy (EPT) versus enhanced patient referral, Outcome 3 Enhanced patient referral plus EPT vs. simple patient referral.
Comparison 5. Contract referral versus simple patient referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of partners elicited | 5 | 2006 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.37, ‐0.06] |
| 2 Number of partners notified | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Number of partners presenting for care | 3 | 1610 | Mean Difference (IV, Random, 95% CI) | 0.25 [0.18, 0.32] |
| 4 Number of partners testing positive | 4 | 1684 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.07, 0.18] |
| 5 Number of partners treated | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Number of harmful events reported | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
5.1. Analysis.
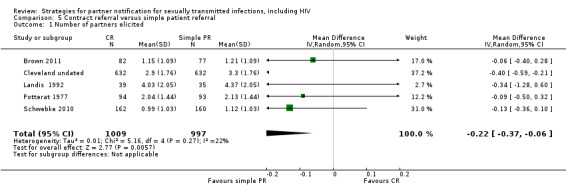
Comparison 5 Contract referral versus simple patient referral, Outcome 1 Number of partners elicited.
5.2. Analysis.

Comparison 5 Contract referral versus simple patient referral, Outcome 2 Number of partners notified.
5.3. Analysis.

Comparison 5 Contract referral versus simple patient referral, Outcome 3 Number of partners presenting for care.
5.4. Analysis.
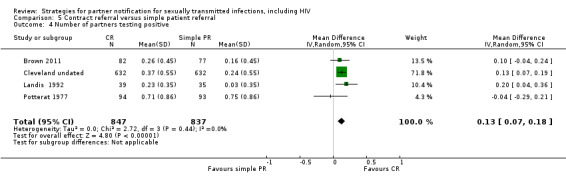
Comparison 5 Contract referral versus simple patient referral, Outcome 4 Number of partners testing positive.
5.5. Analysis.

Comparison 5 Contract referral versus simple patient referral, Outcome 5 Number of partners treated.
5.6. Analysis.
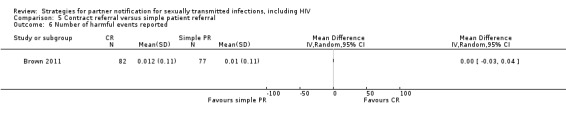
Comparison 5 Contract referral versus simple patient referral, Outcome 6 Number of harmful events reported.
Comparison 6. Contract referral versus enhanced patient referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of partners elicited | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Partners presenting for care | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Partners testing positive | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
6.1. Analysis.

Comparison 6 Contract referral versus enhanced patient referral, Outcome 1 Number of partners elicited.
6.2. Analysis.
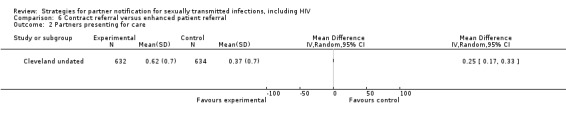
Comparison 6 Contract referral versus enhanced patient referral, Outcome 2 Partners presenting for care.
6.3. Analysis.
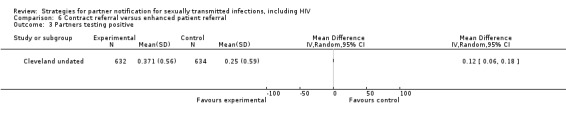
Comparison 6 Contract referral versus enhanced patient referral, Outcome 3 Partners testing positive.
Comparison 7. Contract referral versus expedited partner therapy (EPT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐infection in index patient | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
7.1. Analysis.
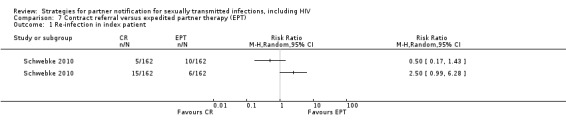
Comparison 7 Contract referral versus expedited partner therapy (EPT), Outcome 1 Re‐infection in index patient.
Comparison 8. Provider referral versus simple patient referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Provider referral vs. simple patient referral | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Number of partners elicited | 2 | 596 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.65, 0.46] |
| 1.2 Number of partners testing positive | 2 | 596 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 1.3 Number of partners treated | 1 | 438 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.37, 0.63] |
| 1.4 Number of harmful events reported | 1 | 158 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 2 Choice between provider or simple patient referral vs. simple patient referral | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Number of partners elicited | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Number of partners notified | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Number of partners presenting for care | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
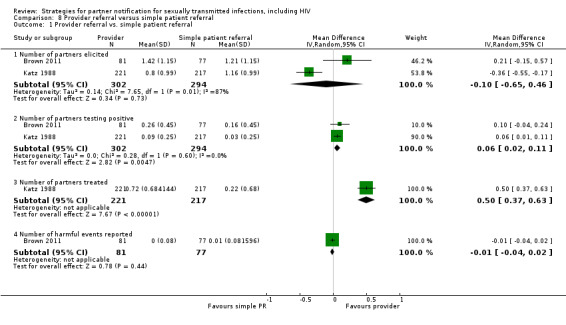
Comparison 8 Provider referral versus simple patient referral, Outcome 1 Provider referral vs. simple patient referral.
8.2. Analysis.
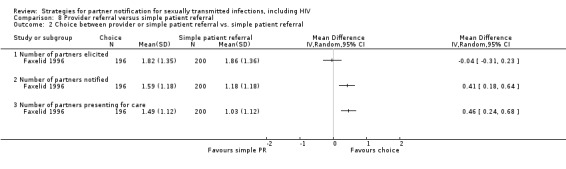
Comparison 8 Provider referral versus simple patient referral, Outcome 2 Choice between provider or simple patient referral vs. simple patient referral.
Comparison 9. Provider referral versus enhanced patient referral (disease intervention specialist).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of partners elicited | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Number of partners testing positive | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Number of partners treated | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
9.1. Analysis.

Comparison 9 Provider referral versus enhanced patient referral (disease intervention specialist), Outcome 1 Number of partners elicited.
9.2. Analysis.
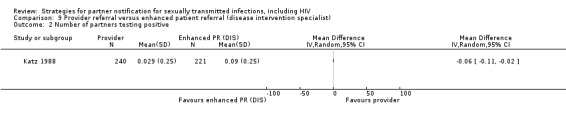
Comparison 9 Provider referral versus enhanced patient referral (disease intervention specialist), Outcome 2 Number of partners testing positive.
9.3. Analysis.
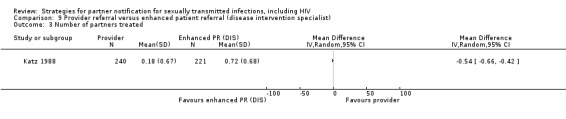
Comparison 9 Provider referral versus enhanced patient referral (disease intervention specialist), Outcome 3 Number of partners treated.
Comparison 10. Provider referral versus contract referral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of partners elicited | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Number of partners presenting for care | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.19, 0.25] |
| 3 Number of partners located | 2 | 2129 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.00, 0.20] |
| 4 Number of partners tested | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Partners testing positive | 2 | 2129 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 6 Number of partners treated | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Number of harmful events reported | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
10.1. Analysis.
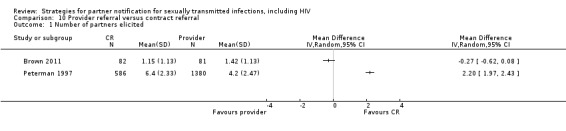
Comparison 10 Provider referral versus contract referral, Outcome 1 Number of partners elicited.
10.2. Analysis.

Comparison 10 Provider referral versus contract referral, Outcome 2 Number of partners presenting for care.
10.3. Analysis.
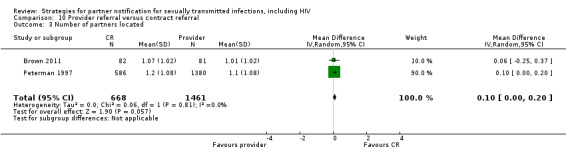
Comparison 10 Provider referral versus contract referral, Outcome 3 Number of partners located.
10.4. Analysis.

Comparison 10 Provider referral versus contract referral, Outcome 4 Number of partners tested.
10.5. Analysis.

Comparison 10 Provider referral versus contract referral, Outcome 5 Partners testing positive.
10.6. Analysis.
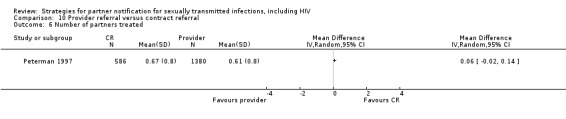
Comparison 10 Provider referral versus contract referral, Outcome 6 Number of partners treated.
10.7. Analysis.
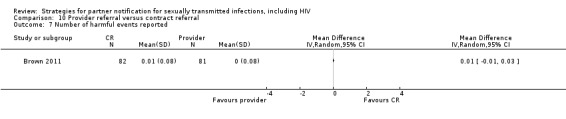
Comparison 10 Provider referral versus contract referral, Outcome 7 Number of harmful events reported.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersen 1998.
| Methods |
Setting: general practices in Aarhus, Denmark Enrolment: women who tested positive for Chlamydia trachomatis were randomised ‐ no specific date given Follow‐up: no follow‐up was recorded |
|
| Participants | 96 women with C. trachomatis were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Patient referral with home sampling (n = 45) Index patients were given a questionnaire about numbers of sexual partners. Index patients were given an envelope with a urine sample home test kit for each partner. The sample was to be sent by the partner to the study laboratory in the provided prepaid envelope Patient referral with office sampling (n = 51) Not stated if index patients completed questionnaire. Index patients were given an envelope containing a contact slip and a request to partner to visit his doctor to request sampling by urethral swab. The doctor was to send a sample in a prepaid envelope to the study laboratory |
|
| Outcomes |
|
|
| Notes | It is not known how many of the partners who tested positive were treated Ethical approval was obtained but no details given Unclear whether consent was obtained |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Used date of birth "Ninety six women with C trachomatis infection seen in general practices in Aarhus County, Denmark, were randomly divided according to their date of birth into an intervention group (45 patients) and a control group (51 patients)" |
| Allocation concealment (selection bias) | Unclear risk | Envelopes used for both groups but not stated if they appeared identical. Envelopes for the intervention group contained a 10 mL container that may be palpable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Test outcome for each partner of every index patient who was randomised was available |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Trial registries were not searched |
| Other bias | Unclear risk | No comparison of baseline characteristics between study arms |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Samples were sent to the laboratory in provided envelopes. It is not stated whether the laboratory personnel knew which procedure was allocated to which group. For urine sample a PCR was performed, for urethral swab enzyme immune assay and if inconclusive a PCR to confirm |
Apoola 2009.
| Methods |
Setting: STI clinic at a single study site in Derbyshire, UK Enrolment: participants recruited by health adviser ‐ recruitment period not given Follow‐up: no follow‐up of index patient |
|
| Participants | 200 index patients with a diagnosis of genital chlamydia were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Patient referral with swab testing (clinic) (n = 100) Index patients were seen by a health adviser and details of contacts recorded. Contact slips coded with the diagnosis were given to the index patient to give to the male partners, who were to bring this to the clinic for testing by urethral swab and treatment Patient referral with home sampling urine kit (n = 100) Index patients were seen by health advisers and details of contacts recorded. Contact slips coded with the diagnosis and a urine sampling kit, for the partner, with instructions, on collecting a first pass urine sample at home, were given to the index patient. Sampling kits included directions to clinic where the samples would be tested and partners would be treated if they tested positive |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Ethical approval was obtained from the Derbyshire Research Ethics Committee When the study was originally designed, the PN rate at the study site was 0.3 contacts per index case of chlamydia and the study was powered to detect a difference of 0.2 contacts per index case. However, during the study period, the PN rates improved significantly making it more difficult to detect 0.2 contacts per case difference Authors were contacted regarding blinding, consent and exact numbers reported. Authors reported that investigators were not blinded, oral consent was obtained and they gave the number of partners elicited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation based on random numbers |
| Allocation concealment (selection bias) | Low risk | Allocated group concealed in sealed opaque numbered envelopes opened sequentially |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome for each partner of every index patient who was randomised was available |
| Selective reporting (reporting bias) | Low risk | Protocol was available from trial registry. Only primary outcome was stated in protocol, no secondary outcomes were stated. Primary outcome in protocol same as in trial. Outcomes in method section of trial are the same outcomes reported |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel (health adviser) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators were not blinded. Nucleic acid amplification tests with high specificity and sensitivity were used on urine specimens. Test used for urethral swab not specified. Details on blinding not given but obtained from authors directly who advised that investigators were not blinded |
Brown 2011.
| Methods |
Setting: 2 hospitals in Malawi, outpatient STI clinics Enrolment: participants enrolled from 2 October 2008 to 2 September 2009 Follow up: 2 weeks after initial diagnosis follow‐up was scheduled but authors did not report number of index patients returning for follow‐up |
|
| Participants | 240 newly diagnosed HIV‐positive men (n = 100) and women (n = 140) from 2 Malawian hospitals were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | All index patients were provided with referral cards, all counselled on importance of safe sex behaviour, staged according to WHO, blood drawn for CD4 count Simple patient referral (n = 77) Index patients notify partners themselves Contract referral (n = 82) Index patients were given 7 days to notify their partners after which a healthcare provider contacted partners, who had not reported to the clinic, for counselling and testing Provider referral (n = 81) Notification of partners within 48 hours by community outreach workers who were trained HIV testing counsellors or nurses |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Authors did not report the number of index patients who came for 2‐week follow‐up. Authors were contacted but data from Malawi on 2‐week follow‐up were not available Ethics approval from Institutional Review Board at the University of North Carolina, Chapel Hill and the National Health Sciences Research Committee in Malawi Power was set at 85% to detect an absolute difference of 25% between passive referral and the 2 active referral study arms ‐ therefore need 80 index patients in each arm ‐ respective arms had 77, 82, 81 therefore sufficient |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a permuted block design with randomly allocated block sizes of 6, 9 and 12 stratified by sex and study site |
| Allocation concealment (selection bias) | Low risk | Was concealed in a sealed envelope until the end of the enrolment visit (after all partner data and locator information had been collected) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clinic visit and test outcome data were available for each partner of every index patient who was randomised Harms ‐ number of index patients returning for 2‐week follow‐up was not given, therefore, loss to follow‐up cannot be calculated |
| Selective reporting (reporting bias) | Low risk | Outcome for each partner of every index patient who was randomised was available. Protocol not available in 3 trial registries |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel not blinded to which group index patient or partner belonged ‐ partner identified if presented with a patient referral card or if their name was found on the log. Index patient returned 2 weeks after enrolment and were asked if partners were notified, how they were notified and what their behaviour was like (harms). HIV antibody‐negative or antibody‐indeterminate specimens were tested for the presence of HIV RNA using the ultrasensitive Roche Amplicor Monitor HIV RNA assay. Primary outcome low risk |
Cameron 2009.
| Methods |
Setting: city centre FPC, GUM or a hospital termination of pregnancy in Edinburgh, UK Enrolment: participants enrolled from May 2004 to December 2006 Follow‐up: index patients agreed to submit a urine sample at 3‐monthly intervals over 12 months |
|
| Participants | 330 index patients who tested positive for Chlamydia trachomatis were randomised in Edinburgh Inclusion criteria
Exclusion criteria
|
|
| Interventions | All index patients received written and verbal information about chlamydia and the importance of partner treatment Simple patient referral (n = 110) Index patients provided details of partners of past 6 months. Index patients contacted partners themselves and were given standard contact slips to be given to partners. Index patients also received information leaflet about chlamydia with details of GUMs. After 4 weeks, index patient was contacted by study personnel to check if partners were successfully contacted Patient referral with postal testing urine kit (n = 110) Index patient provided details of partners of past 6 months. Index patient received 1 postal testing kit to deliver to each partner to collect a urine sample in. Postal testing kit consisted of a universal container for the urine sample, laboratory form with preferred contact method, an instruction leaflet and a postage paid pre‐addressed envelope to send sample to laboratory. The kit also included a leaflet about chlamydia, information about the study and contact details of study nurse if further information required. EPT (n = 110) Index patient provided details of partners of past 6 months. Index patient was given 1 treatment pack to give to each partner. The treatment pack contained azithromycin 1 g, an information leaflet about the study with contact details for study nurse, information about chlamydia, drug safety leaflet and details of GUMs they could attend for testing/treatment if they preferred. The study information leaflet contained a 'tear‐off' slip that the partner was asked to complete and return (in a pre‐addressed postage paid envelope) to confirm that they had taken the medication. There was also an 'objection' slip that could be completed and returned, if the partner objected to treatment in this way |
|
| Outcomes |
Primary outcome Re‐infection in index patient (all index patients received a postal testing kit for themselves, and were asked to post a urine sample to laboratory for re‐testing at 3 months' post‐treatment, further postal testing kits were sent to index patient at 6, 9 and 12 months for repeat testing) Secondary outcomes Partner testing/treatment rates (laboratory and clinic databases were checked) |
|
| Notes | Ethical approval obtained from the Lothian Research Ethics Committee. Approval was also obtained from both the Research and Developmental Department and the Chief Pharmacist of the Responsible Health Care Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation numbers in blocks, stratified for each recruitment site |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes, not clear if sequentially numbered |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Primary outcome: 215/303 participants submitted at least 1 urine sample in the 12‐month follow‐up (70%) period ‐ 13 woman informed the study personnel that they did not want to take part anymore (reasons not given), other 75 loss to follow‐up no details given. No details given on ITT For secondary outcomes the partners of every index patient who was randomised had an outcome |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Protocol not available in 3 trial registries |
| Other bias | Low risk | No other bias detected |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research nurse and doctor were not blinded. They were involved in the baseline interview and did the 6‐month follow‐up call to record whether partners were notified ‐ to validate treatment and testing rates the laboratory, FPC and GUM clinic databases were checked Primary outcome assessment was blinded. The postal testing kit samples of the participating woman were labelled with non‐identifying subject study codes so the laboratory staff who reported the results did not know to which intervention the woman belonged The COBAS Amplicor CT test was used on urine samples Partner treatment/test rates: outcome assessment not blinded but validated by records |
Cleveland undated.
| Methods |
Setting: Dade County Department of Public Health, Georgia, US Enrolment: once the study criteria were met, participants were enrolled ‐ details not given Follow‐up: a test of cure was performed 3‐5 days after treatment. A re‐screening interview was performed 28 days after treatment |
|
| Participants | 1898 index patients with gonorrhoea were randomised, 1786 men and 112 women Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Patient referral with pamphlet and health worker interview (n = 634) Index patient received an informational pamphlet. A health worker used the pamphlet to explain asymptomatic partners, re‐infection and complications. The patient was also encouraged to ask questions. Index patient was advised to refer his partners of the previous 30 days to the clinic. Index patient was offered 4 referral cards to be given to partners and where asked if he/she needed more or less. The number of cards taken was recorded Contract referral with interview from health worker (n = 632) Index patient received a standard interview to offer medical information, allow rapport building and to elicit contact details of partners. Index patient was advised to refer his partners of the previous 30 days to the clinic and was told that if partners did not present at the health service after 3 days, then the health worker would contact them Simple patient referral standard message (n = 632) Index patient only received a message to say that he/she had been diagnosed with gonorrhoea, that it was contracted sexually and that sexual partners of the previous 3‐4 weeks needed examination and treatment. Index patient was offered 4 referral cards to be given to partners and where asked if he/she needed more. No contact details of partners recorded |
|
| Outcomes |
|
|
| Notes | No details on ethics approval or consent from participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned through random selection to an intervention ‐ no specific details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Test outcome available for all partners of every index patient who was randomised |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Trial registries not searched |
| Other bias | Unclear risk | Baseline comparability not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinic worker showed partner a referral card and asked them whether they have seen one of these, it was coded to what mode of interview was used originally |
Ellison undated.
| Methods |
Setting: Alexandra Health Centre and University Clinic, a community health clinic and principle provider of health care to the township of Alexandra, South Africa Enrolment: participants enrolled from 23 June to 12 September 1997 Follow‐up: no follow‐up of index patient scheduled |
|
| Participants | 1719 index patients, 811 men and 908 females, with any STI syndromically diagnosed were enrolled Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Simple patient referral (n = 433) Index patient received a standard clinical consultation given by a nurse and received a contact card to be given to partner Patient referral and health education message (n = 431) Index patient received a standard clinical consultation, contact card and standardised verbal health education message given by nurse Patient referral and counselling (n = 430) Index patient received a standard clinical consultation, contact card and patient‐centred counselling in a private room, conducted by trained lay‐counsellors of same gender Patient referral with health education message and counselling (n = 425) Index patient received a standard clinical consultation, contact card and both interventions (health education by the nurse and counselling by lay‐counsellors) |
|
| Outcomes |
|
|
| Notes | Ethical approval from Committee for Research on Human Subjects of the University of the Witwatersrand in Johannesburg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each consecutive patient received an anonymous consecutive number ‐ no specific details on how these numbers were delivered |
| Allocation concealment (selection bias) | High risk | Research nurse allocated alternate patients to 1 of 4 groups. Research nurse allocated alternate interventions to each consecutive patient according to a printed schedule (drawn up by project co‐ordinator). Authors acknowledge that research nurse could unwittingly or deliberately influence which patient received each intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk for main outcome, partner treated |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section |
| Other bias | Low risk | No other risk of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel and participant not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research personnel at the pharmacy and casualty unit who collected the PN slips were masked to which intervention the participant received Masked bivariate analysis, unmasked multivariate statistical analysis took place |
Faxelid 1996.
| Methods |
Setting: urban health centre, Lusaka, Zambia Enrolment: participants were enrolled from October 1992 to March 1993 Follow up: interview and follow‐up 2 weeks after enrolment of index patient |
|
| Participants | 396 index patients (94 women, 302 men) with clinically or laboratory diagnosed STI were randomised Inclusion criteria
Exclusion
|
|
| Interventions |
Simple patient referral (n = 200) Index patient received standard care, no contact cards were given Choice between patient and provider referral with counselling (n = 196) Index patient received individual counselling (10‐20 minutes) from same‐gender nurse (female) or clinical officer (male). Index patient was given health education, information on importance of completing treatment, advise on abstinence and how to inform partners of previous 3 months of their exposure. Index patients received contact cards with the index patient's file number on to be given to partners. Names and address of partners taken. Provider referral offered if patient did not want to talk to partner |
|
| Outcomes |
|
|
| Notes | The policy at this health service was not to treat an index patient unless they bring a partner. This may affect the generalisability of the study to other settings No details on ethical approval given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors state that patients were randomised. Patients drew lots ‐ in each box 4 cards with "intervention" and 4 cards with "non‐intervention" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 188/196 (96%) index patients in intervention group and 189/200 (94.5%) index patients in control group returned for follow‐up |
| Selective reporting (reporting bias) | Low risk | Same outcomes in methods as in results section. Trial registries were not searched |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Outcomes are subjective and therefore risk of detection bias |
Golden 2005.
| Methods |
Setting: participants were interviewed at the Public Health‐Seattle and King County (PHSKC) STI clinic and one other PHSKC clinic in King County, Washington, US Enrolment: patients who received a diagnosis of gonorrhoea or genital chlamydial infection between 29 September 1998 and 7 March 2003 were identified through laboratory reporting, case reports from healthcare providers and onsite case ascertainment were identified. Clinicians who made diagnosis were contacted to seek permission and potential participants were contacted for an interview Follow‐up: interview of index patient 10‐18 weeks after treatment |
|
| Participants | 2751 index patients, 646 men and 2105 women, with either gonorrhoea or chlamydia or both infections were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | Before randomisation, study personnel offered to contact partners who index patients were unable or unwilling to contact themselves Simple patient referral (n = 1376) Index patients were advised to tell their partners to seek care and that care was available at no cost at the STI clinic EPT (n = 1375) Index patients were offered medication to give to up to 3 partners, study staff members offered medication to partners they contacted themselves. Partner packages were distributed to patients or their partners through commercial pharmacies, the PHSKC STI Clinic or direct mailing. Packets also contained condoms, information on medication, warning for adverse effects, telephone contact for study staff and brochure. Pharmacies were contacted 1 week after medication prescribed to determine whether it was picked up ‐ if not picked up within 1 week patient received a telephone call reminder |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Ethical approval obtained from the institutional review board of the University of Washington and Group Health Cooperative | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | At enrolment, 2751 patients reported having untreated partners they could contact and underwent randomisation. No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 1375 assigned to the expedited treatment arm, 929 (68%) completed study. Of 1376 assigned to partner referral arm, 931 (68%) completed study. Only participants completing the study were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Persistent or recurrent gonorrhoea or chlamydia were the primary outcome stated in the methods section. Behavioural outcomes were reported in the outcome section. Adverse events were not reported and unclear whether no adverse events were reported or whether authors failed to record them. Protocol was not available from 3 trial registries |
| Other bias | Unclear risk | Selective reporting of subgroups, this might have been a potential bias but there is insufficient information to assess whether an important risk of bias exist |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. Urine test use LCx (Abbott) ligase chain reaction used for primary outcome ‐ objective. Self report on behavioural outcome ‐ subjective |
Katz 1988.
| Methods |
Setting: public STI clinic, Indianapolis, Indiana, US Enrolment: male participants with NGU enrolled between July and December 1985 Follow‐up: no follow‐up stated for index patients |
|
| Participants | 678 index patients with NGU were randomised to 1 of 3 interventions Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Simple patient referral with nurse (n = 217) Nurse providing health education and referral letters. No contact details of partners were requested Patient referral with contact tracer (DIS) (n = 240) Counselling with contact tracer, partners names recorded but no referral letters given and no partner contact details elicited Provider referral by contact tracer (n = 221) Interview with contact tracer, contact details of partners taken, attempt to contact by phone calls, letters or visits |
|
| Outcomes |
|
|
| Notes | Ethical approval details not mentioned The effectiveness of interventions 1 and 2 underestimated due to bias in outcome assessment: partners choosing to be treated at other health services were not counted for these groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised ‐ no details given |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for all partners of index patients randomised available |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Trial registries not searched |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Partners were matched to the index patient by the referral letter or the clinic's computerised database in group 1 and 2. In group 3, the contact details were taken and partners were contacted by the provider |
Kerani 2011.
| Methods |
Setting: Public Health‐Seattle & King County (PHSKC), Washington State, US Enrolment: men who had sex with men who were given a diagnosis of gonorrhoea or chlamydia or both were enrolled at the time they were contacted to provide them with partner services between 1 July 2007 and 31 March 2009 Follow‐up: index patients completed a follow‐up interview approximately 2 weeks after enrolment |
|
| Participants | 75 men with gonorrhoea or chlamydia or both were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Simple patient referral (n = 18) Index patients notify partners themselves Enhanced patient referral (n = 17) Index patient used inSPOT (inspot.org), an Internet‐based PN service. Index patients received a printed card with the site's Internet address or telephonic instructions if not present in STI clinic EPT (n = 16) Index patient received prepackaged medicine to give to 3 different partners. The package also included information on STI, importance of HIV testing, allergy warning to medication, condoms and a free visit to STI clinic. If not present in STI clinic, index patient was telephoned and informed to pick up similar packages at several local pharmacies Combination of EPT and enhanced patient referral (n = 24) Index patient received EPT and inSPOT |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Ethical approval was received from University of Washington Institutional Review Board. Authors were contacted to clarify type of allocation concealment and whether protocol was available. Exact numbers of partners treated and notified per intervention arm were also requested and the type of adverse events. Authors failed to provide any of the above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A computer was used to randomly assign participants ‐ no details given how this was performed |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 53/75 (70.6%) participants completed the study. Only participants completing the study were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported in methods section were reported in results section. The protocol was not available from 3 trial registries |
| Other bias | Unclear risk | Baseline imbalances (race, type of STI) evident but insufficient to assess whether an important risk of bias existed. Early stopping due to low recruitment rate are not more likely to show extreme results and not considered to be prone to bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants and personnel not blinded, outcomes subjective |
Kissinger 2005.
| Methods |
Setting: public STI clinic in New Orleans, US Enrolment: participants enrolled from December 2001 to March 2004 Follow‐up: index patients were asked to return 4 weeks after the initial clinic visit (with a window of 2‐8 weeks) for a follow‐up interview and a urine specimen |
|
| Participants | 977 index patients with diagnosis of urethritis were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Simple patient referral (n = 285) Index patients were instructed to tell their partners that they needed to go to either the public STI clinic or the clinic of their choice for STI evaluation and treatment Patient referral booklet enhanced (n = 348) Index patients were given a wallet‐sized booklet that contained 4 tear‐out cards with information for the partner and treatment guidelines for professionals. If they had more than 4 partners they were given additional booklets EPT (n = 344) Index patients were given packages containing medication, written instructions about how to take medication, warning about adverse effects, 24‐h nurse's pager number to call if any enquiries and asked to give package to each of their partners |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Institutional review board approval was obtained from all participating institutions Authors were contacted for statistical analysis (sample size calculations, power) details and exact numbers, authors replied that sample size calculations were performed, but could not provide exact details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by month in which they attended the clinic to 1 of 3 study arms. Randomisation of months was conducted using a blocked scheme of 3 to 6 units using Microsoft Excel software |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 770/977 (79%) participants returned for follow‐up interview but only 37.5% were retested. At follow‐up interview, index patients were asked outcome questions for each partner. Outcome of interest was the response to the question: "Did baseline partner tell you that he or she took the medicine?" |
| Selective reporting (reporting bias) | Unclear risk | Same outcomes in the methods section (re‐infection index patient and partners treated) were reported in the results section. With additional sexual outcomes (unprotected sex before partner treatment, re‐initiated sex with baseline partner, unprotected sex with any partner) not stated in the methods section. Protocol not available from 3 trial registries |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The 1‐month interview was performed either by computer‐assisted self interview or study staff The outcomes were assessed by an interview either computer‐assisted self interview (24.3%), telephonic (35.4%) or face‐to‐face (40.2%). The interviewer was not blinded. An in‐person interview has the potential for information bias. No details given whether laboratory personnel were blinded but outcome measure was objective |
Kissinger 2006.
| Methods |
Setting: the Orleans Women's Health Clinic in New Orleans, US Enrolment: participants enrolled from December 2001 to August 2004 Follow‐up: participants were asked to return 4 weeks after the initial visit (with a window of 2‐8 weeks) |
|
| Participants | 463 index patients with a culture‐confirmed diagnosis of Trichomonas vaginalis were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | Study staff counselled women in all study arms about T. vaginalis and the importance of partner treatment before randomisation Simple patient referral (n = 155) Index patients were instructed to tell their partners that they need to go to a clinic for STI evaluation and treatment Booklet enhanced partner referral (n = 154) Index patients were given a wallet‐sized booklet containing tear‐out cards with information for the partner and treatment guidelines for providers EPT (n = 154) Index patients were given packages for their partners, containing medicine, written instructions on how to take medicine, warnings about side effects and nurse's pager number for enquiries |
|
| Outcomes |
|
|
| Notes | Ethical approval from Institutional review board from Tulane University Health Sciences Center, CDC and the Louisiana Office of Public Health Author was contacted and provided details on consent (oral) and exact numbers of how many women returned for follow‐up and testing. Details to what intervention arm the woman with re‐infection belonged to was also provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked scheme of 3 or 6 units using Microsoft Excel |
| Allocation concealment (selection bias) | Unclear risk | Previously prepared envelopes. Not specified if these were sealed or identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 412/463 (89%) index patients were re‐interviewed (some interviews done by telephone, and, therefore, no sample submitted) but only 376/463 (81%) index patients were retested and re‐interviewed (data from author directly) |
| Selective reporting (reporting bias) | Unclear risk | Protocol available from trial registries Outcomes stated in the protocol: Primary ‐ Index patient report of partner taking medicine at 6‐8 weeks Secondary ‐ Index patient re‐infection at 6‐8 weeks, cost‐effectiveness outcomes Outcome reported in actual study: Outcomes were not reported as primary and secondary. Additional sexual and behavioural outcomes reported Re‐interview scheduled for 4 weeks after treatment (window of 2‐8 weeks) Outcome in method section same as results section but differs from protocol |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding Although some outcomes were subjective, the outcome of interest in the telephone interview was the response to this question: "Did partner tell you that he took the medicine?" Different methods were used for outcome assessment (i.e. telephone or computer‐assisted self interview) that may have introduced detection bias, outcomes assessors were unlikely blinded Assessment of T. vaginalis culture result was not blinded but is an objective outcome |
Landis 1992.
| Methods |
Setting: 3 large county health departments in North Carolina, US Enrolment: participants enrolled from 16 November 1988 to 30 June 1990 Follow up: no follow‐up of index patient reported |
|
| Participants | 74 HIV‐infected men (51) and women (23) were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | Public health counsellor revealed diagnosis, provided standard counselling and explained study before randomisation. After consent partner information was obtained Simple patient referral (n = 35) Index patient had interview with counsellor, discussing the process of notification. Index patient received coloured cards with identification codes to be given to their partner. After 1 month, the counsellor attempt to contact any partner not yet contacted Contract referral (n = 39) Index patient could choose to notify some or all of their partners themselves. Index patient received coloured cards with identification codes to be given to their partner. The remaining partners, as well as those not presenting at the health service after 2 weeks were contacted by the counsellors |
|
| Outcomes |
|
|
| Notes | Ethical approval from the Ethics Committee on the protection of the rights of human subjects of the University of North Carolina School of Medicine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned no specifications |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome for each partner of every index patient who was randomised was available |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Trial registries were not searched |
| Other bias | Unclear risk | Baseline comparability unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Coded cards were used but it is unclear if it was obvious to personnel whether they belonged to intervention or control group. Unclear who collected the cards and whether person had involvement in study findings |
Low 2006b.
| Methods |
Setting: 27 general practices in Bristol and Birmingham, UK Enrolment: participants enrolled from March 2001 to October 2002 Follow‐up: 6 weeks after randomisation there was telephone follow‐up of index patient |
|
| Participants | 140 index patients (92 woman and 48 men) with Chlamydia trachomatis were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | All participants received antibiotic treatment before randomisation Simple patient referral with counselling from practice nurse (n = 72) Nurses received 1 day of training about sexual history taking, management of chlamydia and PN. The index patient had a PN interview with the trained nurse. This interview involved taking of sexual history of the previous 6 months, patient referral using contact slips, abstinence and information about being screened for other STIs. Contact slips included details of the study GUM clinics and requested the treatment centre to return the slip to the study centre. Practise nurses did not follow‐up the index patient Referral to GUM clinic for partner referral from specialist health advisor (n = 68) At randomisation, index patients were referred to GUM clinic. If clinic had not been contacted by telephone within 1 week by index patient, the health adviser made 2 attempts to contact them. PN was performed according to standardised protocols and contact slips were issued. The index patient was also offered a consultation for screening for other STIs. Follow‐up was by telephone |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Ethical approval South West multicentre research ethics committee Only 72 in nurse arm and 47 in clinic arm Study author was contacted to clarify clustering and replied. The author replied that the trial was individually randomised. However, there was often more than 1 participant from a single general practice (i.e. clustering), and it means that there are likely to be similarities between patients within the same practice |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, permuted blocks, stratified by practice |
| Allocation concealment (selection bias) | Low risk | Central computerised telephone system |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The PR (nurse) group had PN interview on same day. In PR (GUM) 21/68 (31%) did not attend PN interview. Authors used ITT analysis and assumed those lost to follow‐up were not treated |
| Selective reporting (reporting bias) | Unclear risk | Protocol available from trial registries. In protocol, adherence to advice to abstain from sexual intercourse until both partners completed treatment was stated as a secondary outcome but not reported in trial. Outcome "Cases with all partners treated" was not prespecified in study protocol but reported |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A researcher not involved in the participant's PN did the follow‐up |
Montesinos 1990.
| Methods |
Setting: the health service of the Southern Illinois University, a large mid‐western university, Illinois, US Enrolment: participants were enrolled from July 1984 to June 1985 Follow‐up: no follow‐up recorded of index patients |
|
| Participants | 65 index patients (48 men and 17 females) with gonorrhoea or NGU were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Patient referral with counselling (nurse of physician) (n = 27) Index patient received counselling from a physician (in his office) or a nurse (designated private area) following a written protocol. Counsellor ascertained the reason for seeking treatment, gave information on STI, obtained names of sexual partners in previous 6 weeks, advise index patient to notify partner and assured index patient of confidentiality Patient referral with counselling, incentive and cards (n = 19) Index patient received counselling from a physician (in his office) or a nurse (designated private area) following a written protocol. Counsellor ascertained the reason for seeking treatment, gave information on STI, obtained names of sexual partners in previous 6 weeks, advised index patient to notify partner and assured index patient of confidentiality. In addition, counsellor advised index patient that USD 3 charge, for index patient and partner, will be waived if partner successfully referred. A card with naming specific STI and advise to seek treatment given to index patient to give to partner Patient referral with counselling, cards, follow‐up call after 5 days, no incentive (n = 19) Index patient received counselling from a physician (in his office) or a nurse (designated private area) following a written protocol. Counsellor ascertained the reason for seeking treatment, gave information on STI, obtained names of sexual partners in previous 6 weeks, advised index patient to notify partner and assured index patient of confidentiality. A card naming specific STI and advise to seek treatment given to index patient to give to partner. Index patient did not receive any financial incentive. Counsellor told index patient that if partner failed to arrive at health service within 5 working days the index patient would be contacted by telephone |
|
| Outcomes |
|
|
| Notes | Ethical approval from Southern Illinois University ‐ Committee for Research involving Human Subjects 17 females vs. 48 males. 2 different time periods. Group 1 was interviewed from July to December 1984 and groups 2 and 3 received intervention in January to June 1985 ‐ possibility that holidays can play a role on who is available during that time |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | High risk | The protocol was colour coded. The counsellor removed the next protocol for the next patient from a randomly ordered set |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for all partners of index patients available |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Trial registries not searched |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Names of partners were recorded on the counselling protocols. A list for these identified partners were maintained for up to 1 month after index patient was seen to see if partners returned |
Moyo 2002.
| Methods |
Setting: 2 large public STI clinics in Harare, Zimbabwe Enrolment: index patients were consecutively recruited from July to September 2000. Follow‐up: index patient was interviewed for 15 minutes at the routine 1‐week clinic follow‐up visit |
|
| Participants | 272 index patients (135 men and 137 women) with a syndromically diagnosed bacterial STI were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | All index patients completed a standard STI treatment and counselling consultation, a clinic nurse or doctor explained the objectives and procedure. The same gender counsellor explained the basic procedure to all, then conducted the 30‐minute baseline interview with each patient. All participants were given reminder cards to visit the study counsellor for a 15‐minute follow‐up interview when returning for routine 1‐week clinic follow‐up visit Patient referral with additional counselling session (n = 131) Counsellor conducted an additional individualised session with the index patient lasting approximately 30 minutes. Session included identification of likely sources and spread of STI, approaches to notification, role playing, motivating factors, barriers and domestic violence. Session also include health education. Index patients were also allocated coupons to give to partners for free treatment at the study clinic Simple patient referral (n = 141) Counsellor did a 30‐minute baseline interview with index patient. No coupons were given for partners free treatment |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Ethics approved by the Committee on Human Research at the University of California, San Francisco, and by the Medical Research Council of Zimbabwe Authors were contacted without success regarding discrepancies in numbers reported and distribution of harms in intervention arms |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | The nurse or doctor selected a sealed, opaque envelope from a box that randomly assigned the patient to intervention or control. Envelopes were constructed prior to any recruitment. An equal number of the allocation slips with the words 'intervention' or 'control' were placed in the box and manually mixed. All participants brought the envelope to the study counsellor, whereupon it was opened in the presence of both study counsellor and patient. Unclear whether these envelopes were sequentially numbered |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Self reported notification and referral of partners to treatment were assessed at follow‐up interview. 137/272 (50%) participants completed the follow‐up interview. ITT analyses were performed. However, the high loss to follow‐up is potentially a source of bias |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. No protocol available from trial registries |
| Other bias | Low risk | No other bias identified The randomisation scheme produced approximately equivalent numbers in the intervention and control groups for men and women. Of note, people randomly allocated to the intervention arm were slightly older, more likely to be working in the formal economy, and more likely to be currently married or co‐habiting. These findings may indicate a problem with randomisation It may also be due to the small sample size that baseline differences occurred by chance |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Index patient reports about PN can introduce bias The same study counsellor who did the counselling session did the 1‐week follow‐up interview ‐ this might have introduced detection bias |
Nuwaha 2001.
| Methods |
Setting: Mulago Hospital STI clinic in Kampala, Uganda treats patients free of charge. Clinic is the main STI reference centre, mainly serves as a walk‐in primary care STI treatment centre Enrolment: consecutive patients with STI symptoms enrolled between November 1999 and January 2000 Follow‐up: index patients were asked to return to the clinic within 2 weeks |
|
| Participants | 383 index patients (196 men, 187 women) with STI symptoms were randomised Inclusion criteria:
Exclusion criteria
|
|
| Interventions | All index patients were given information, education and communication for 5‐10 minutes. Trained research assistants performed interviews using a pre‐tested questionnaire Simple patient referral (n = 191) Index patients were given contact slips to take to sexual partners. Index patient asked to return 2 weeks later EPT (n = 192) Index patients were given medications to take to sexual partners. Index patient were asked to return after 2 weeks. Index patients were request to return medication if their partners refused them or if they could not trace the partner |
|
| Outcomes |
|
|
| Notes | Ethics approval by Mbarara University, the Faculty of Medicine Research Committee, the Uganda AIDS Committee, the Uganda National Council for Science and Technology, and the Ethics Research Committee at Karolinska Institute (Stockholm, Sweden). Permission to conduct the study was obtained from the Mulago Hospital administration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number between 0 and 999; even numbers to EPT group, and odd numbers were assigned to the patient‐based partner referral group. Stratified randomisation according to the sex of the index patient was used |
| Allocation concealment (selection bias) | Unclear risk | No detail of allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was used. In EPT, 187/192 (97%) index patients returned after 2 weeks and in simple patient referral 117/191 (61%) returned. On return, participants reported on partner treatment and partner reaction. Attrition bias in the simple patient referral arm |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Protocol not available from 3 trial registries |
| Other bias | Unclear risk | Partners of participants in simple patient referral group could have been treated elsewhere leading to misclassification bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Partners in simple patient referral group returned coded slips. Clinic workers checked clinic records for all patients who said they had been referred by a partner to attempt to link them to an index patient. In addition, they collected reports from index patients on partner referral (not analysed in this review) In the EPT participants, the outcome was index patient reports whether partner took medication. This can introduce detection bias |
Ostergaard 2003.
| Methods |
Setting: 4 counties in Denmark Enrolment: participants enrolled between February 1999 to March 2000 Follow up: no follow‐up of index patient reported |
|
| Participants | 562 index patients ( 414 women and 148 men) with a positive chlamydia swab were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | Specimen collection package was posted to the index patient's home address. There were 5 specimen collection kits in this package. The index patient was instructed to give collection kits to his/her sexual partners of the previous 12 months. The collection kits were identical. For male partners the kit contained 10 mL tube to collect first void urine sample. The female partners received a vaginal pipette containing 5 mL sterile normal saline to be inserted into the vagina, flushed and aspirated Patient referral with home sampling (n = 304) Samples collected by the partners at home had to be posted directly to the diagnostic laboratory in postage paid and pre‐addressed envelopes Patient referral with office sampling (n = 258) Partners had to bring specimen collection kit into the office of a healthcare provider to obtain sample. Partners also brought a letter with them, explaining the study and the importance that the healthcare provider used the provided specimen collection kit to collect sample. The healthcare provider posted the sample to the laboratory |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Ethical approval by Danish ethics committee system Implied consent |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The index patient was randomised based on a positive swab sample ‐ no details given |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for all partners of index patients available |
| Selective reporting (reporting bias) | Low risk | Outcomes in method same as in results. Protocol not available from 3 trial registries |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Specimen collection kits for the 2 study groups were identical and the index patient was blinded to content of the specimen collection kit. However there is no guarantee that the index patient did not open the package before forwarding to partner The healthcare provider, who did the office sampling, was not part of the study. They only collected the samples and posted it to the study centre |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not mentioned whether laboratory personnel were blinded. However, the chlamydia test is an objective outcome measure |
Peterman 1997.
| Methods |
Setting: public health services in Broward County, Florida; Tampa, Florida; Patterson, New Jersey, US Enrolment: participants were enrolled from December 1990 to March 1993 Follow up: no follow‐up recorded of index patients |
|
| Participants | 1966 index patients with syphilis were randomised, 1042 male and 924 female Inclusion criteria
Exclusion criteria
|
|
| Interventions | After syphilis diagnosis all index patients were interviewed by DIS to identify sexual partners Contract referral (n = 586) Index patient to notify partners within 2 days, or a DIS would notify them on the third day Provider referral (n = 742) Partner notified immediately by DIS and referral of partner for testing Provider referral and field test (n = 638) Partner notified immediately by DIS who could draw blood for testing in the field, if it seemed unlikely for partner to come in for testing |
|
| Outcomes |
|
|
| Notes | Details on ethical approval not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Individual index patients were randomly assigned. Every day the study co‐ordinator at each site generated a list of assignments by using a random number table. The total number of patients in each arm differed significantly from 742, 638 and 586, this raises suspicion about whether randomisation was performed appropriately |
| Allocation concealment (selection bias) | High risk | The assignment was known to the interviewer before contact with the patient and the method was sequentially adapted by the interviewer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for all partners of index patients |
| Selective reporting (reporting bias) | Low risk | Same outcomes in methods section compared to results section. Trial registries not searched |
| Other bias | High risk | Deviation from protocol was reported by authors Some contamination was reported by the authors and this would have reduced the difference between the 3 groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The DIS was not blinded, DIS did the interview before randomisation and also the intervention. No blinding of data entry personnel or data analyst No control in place to ensure 2‐day waiting period |
Potterat 1977.
| Methods |
Setting: El Paso, City‐county health department, Colorado, US Enrolment: participants were enrolled from February to September 1975 Follow up: index patient in patient referral group was re‐interviewed 7‐10 days after enrolment |
|
| Participants | 187 index patients with gonorrhoea were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Simple patient referral (n = 93) Study personnel had a short interview (3‐5 minutes) with index patient where the disease and importance of PN were discussed. Index patient received contact cards to be given to partners. Study personnel did not elicit any partner details Contract referral (n = 94) Study personnel had a longer interview (15‐20 minutes) with index patient and partner contact details were elicited. Index patient was informed that health services personnel would contact partners if they did not present at the health service within 7‐10 days |
|
| Outcomes |
|
|
| Notes | Ethical approval details not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternately assigned "During the period February‐September 1975, we assigned all heterosexual male patients with gonorrhea diagnosed at the El Paso City‐County Health Department (Colorado) alternately to a Study or Control group" |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for partners of all index patients available. In simple patient referral group, a second interview was performed to record contact details (91/93 index patients re‐interviewed). These details were used to contact partners to find out their subsequent clinical course and fate of contact slips |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Trial registries were not searched |
| Other bias | Unclear risk | Baseline comparability unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The personnel knew to what group the participant belonged and, due to longer time spent with control group, this could have introduced detection bias. No specifics on test used. 9 contacts in the study group were also identified through field effort although field effort was not part of the original intervention in the study group ‐ detection bias |
Schillinger 2003.
| Methods |
Setting: FPCs (Southern California (SC), Seattle (S) and New Orleans (NO)), adolescent clinics (Birmingham (B), Indianapolis (I), Northern California (NC) and S), primary care clinics (I) and STD clinics (B, I, NO, SC, NC, S) or emergency and other hospital departments (B), US Enrolment: participants enrolled between September 1996 and June 2000 Follow‐up: index patients returned for a follow‐up at 1 and 3 months after enrolment for an interview and urine test |
|
| Participants | 1889 index patients with laboratory confirmed Chlamydia trachomatis were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | At enrolment all women were treated for chlamydia infection and were advised to abstain from intercourse until 7 days after partner's treatment Simple patient referral (n = 943) Index patients were instructed to tell their partners that they had been exposed to chlamydial infection and to recommend that they seek treatment. They were given an information sheet for each partner and list of clinics where the partner could obtain free care EPT (n = 946) Index patients were provided with up to 4 doses of medication for their partners, instructed to tell their partners of their exposure, and to give a package with the medication, instructions, warnings, fact sheet on chlamydia and telephone number to contact if partners had any questions. Index patients were advised to abstain from intercourse until 7 days after each partner's treatment |
|
| Outcomes |
|
|
| Notes | Ethical approval by investigational review boards at each of participating institutions and the CDC Limited power as only 1454 participants completed study to 1 follow‐up. With 0.05 significance, this study only had 62% power to detect a 30% reduction in infection. For a 20% difference in infection rate (as was observed in this study), there was only 37% power to detect a significant difference between 2 interventions. In order to have 80% power, need 2035 women in each arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study allocations were made with use of "randomly sized blocks" |
| Allocation concealment (selection bias) | Low risk | Study arm assignments were printed on cards and placed in sequentially numbered, opaque envelopes and sealed at the study co‐ordination centre |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1454/1787 (81%) participants came for at least 1 follow‐up visit and gave a urine sample for the outcome measure. There was a similar proportion in each study arm. ITT was not followed because index patients who did not return for follow‐up or for whom no urine test result existed were excluded |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Protocol not available from 3 trial registries |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 1 month after treatment, women were interviewed again and urine tested with LCx/PCR. Assessor knew assignment but outcome measure was objective |
Schwebke 2010.
| Methods |
Setting: Jefferson County Department of Health in Birmingham, AL, US Enrolment: participants were enrolled between February 2003 and June 2008 Follow‐up: index patients were asked to return to clinic 5‐9 days after enrolment for a "test of cure". Follow‐up visits to detect repeat infections were performed at the clinic, 1 and 3 months after "test‐of‐cure". At these visits an examination was performed, including culture for Trichomonas vaginalis and a follow‐up questionnaire completed |
|
| Participants | 484 index patients with Trichomonas vaginalis were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Simple patient referral (n = 160) Simple patient referral: usual care ‐ index patient were given a standard message on the importance of PN and asked to tell partners to come for treatment. If the partner did present to the clinic they were offered participation in the male substudy Contract referral (n = 162) Index patients were interviewed by a DIS who took the details of partners of previous 60 days, then entered in to a verbal contract with DIS to refer their partners to the clinic for treatment, partners were telephoned within 1‐2 days of index patient's enrolment. The partners were informed that they will be eligible for remuneration if participate in male study. If treatment of partner could not be verified within 2 working days the DIS attempted to notify partner by telephone or field visits EPT (n = 162) Index patients were given medication for up to 4 partners. The index patients were also given a list of contraindications of the medication and a 24‐hour phone number for partners if they had any questions regarding medication, indications for therapy and further evaluation of symptoms |
|
| Outcomes |
|
|
| Notes | Ethical approval by the Institutional Review Boards of the University of Alabama and the Jefferson County Department of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were data available on 296/484 (61%) index patients at 1‐month follow‐up and 194/484 (40%) participants completed the study |
| Selective reporting (reporting bias) | Low risk | Protocol available from trial registries. In the protocol, the only outcome was the recurrence of trichomonas in index patient at 6 weeks. In the trial, the authors reported re‐infection in index patient at 1 and 3 months post treatment |
| Other bias | Low risk | Study authors planned to recruit 330 participants in each arm but after 4 years were only able to recruit about 50%. Early stopping due to lower than expected recruitment rate are not considered to be prone to bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. The primary outcome was repeat infection in index patient ‐ an objective outcome measure (positive culture or presence of motile trichomonads microscopically) |
Solomon 1988.
| Methods |
Setting: Eastern Clinic of the Baltimore City Health Department, MD, US Enrolment: index patients were enrolled between May 1984 and January 1985 Follow up: index patients returned to clinic 14 days after treatment |
|
| Participants | 902 index patients, with a positive Gram stain for gonorrhoea, were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | All index patients received a DIS contact tracing interview and treatment from a nurse. At the time of test of cure examination an 18‐item, oral test to assess the videotape's impact on knowledge and beliefs of the index patient was performed Patient referral and videotape (n = 456) Index patient was interviewed by DIS to get the contact details of their partners, and was given contact cards and was invited to view a video‐tape promoting PN Simple patient referral (n = 446) Index patient was interviewed by DIS to get the contact details of their partners, and was given contact cards |
|
| Outcomes |
|
|
| Notes | Ethical approval details not given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given except that the research assistant assigned patients at random to group 1 (watching the videotape) and group 2 (not watching the videotape) |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data available for all partners of index patients |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in methods section were reported in results section. Trial registries not searched |
| Other bias | Unclear risk | Baseline comparability unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | If a partner came to the clinic with a referral card, a clerk noted the participant number on registration. The clerk was blinded to what experimental study the colour coding belonged to. The research assistant, who performed the oral test at the test of cure evaluation, was blinded to whether participant saw the video tape or not |
Tomnay 2006.
| Methods |
Setting: a publicly funded sexual health clinic in Melbourne, Vic, Australia Enrolment: participants were enrolled between July 2003 and July 2004 Follow‐up: 1 week after attending the clinic all index patients were contacted via telephone and interviewed by an experienced "contact tracer" |
|
| Participants | 105 index patients with chlamydia or NGU (76 men and 29 women) were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Simple patient referral (n = 32) Each index patient received a sealed envelope. In each envelope there were 5 standard partner letters used for contact tracing. Each index patient was asked to pass a letter to each partner Patient referral with website (n = 73) Each index patient received a sealed envelope. In each envelope there were 5 standard letters used for contact tracing with addition of a uniform resource locator address to a disease‐specific website. Each index patient was asked to pass a letter to each partner. The sites provided information for the partners about the infection to which they had been exposed. A printable letter for the partner to take to their own doctor and an anonymous questionnaire were available on the website. Contact details of the researchers and ethics committee were available to report any complaints |
|
| Outcomes |
Primary outcome
Secondary outcome
|
|
| Notes | Ethical approval by the Department of Human Services, Victoria and the University of Melbourne | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistical package for Social Sciences (SPSS Inc., Chicago, USA) to generate random numbers between 1 and 27. Block randomisation was used (blocks of 27), with 18 randomised to the website and 9 to the standard letter. This was performed so that each clinic room had 1 randomised block |
| Allocation concealment (selection bias) | Low risk | The envelopes with the website or standard pack were identical. Thickened opaque paper and were thoroughly sealed. No opened or missing envelopes were identified during the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 97/105 (92%) index patients completed study up to telephone interview. Only 48/105 (46%) index patients returned to the clinic to evaluate re‐infection |
| Selective reporting (reporting bias) | Low risk | Same outcomes reported that is stated in methods. No protocol available from 3 trial registries |
| Other bias | Low risk | No other risk of bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding Contact tracer: not clear if contact tracer was blinded. Participants were contacted via telephone 1 week after attending the clinic and were interviewed by an experienced contact tracer regarding the number of partners contacted, the method used whether the letter had been passed on and the reaction of the partner(s) to the method used. A questionnaire was used but no details given on whether this was a structured questionnaire Study personnel: to assess re‐infection of index patient, the study personnel looked at medical files in the 2‐12 week period post‐treatment |
Trent 2010.
| Methods |
Setting: 5 clinical sites in 2 institutions ‐ a large academic medical centre (John Hopkins School of Medicine) and a community hospital (Saint Agnes Hospital), Baltimore, MD, US The 5 sites of recruitment included the paediatrics and adult emergency department at both centres and the combined general paediatrics and adolescent medicine clinic in the large academic centre Enrolment: trained research assistants screened patients with mild‐to‐moderate PID regarding inclusion and exclusion criteria to determine eligibility ‐ from 14 February 2006 to 25 July 2008 Follow‐up: index patients returned for a 72‐hour follow‐up after treatment, and a 2‐week post‐treatment, face‐to‐face interview with DIS |
|
| Participants | 162 index patients with mild‐to‐moderate PID, were approached about recruitment, 131 were enrolled, data gathered from 126 participants were successfully transferred at enrolment and could be randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions | Care of patients in both arms included detailed discharge instructions, a full 14‐day course of medication and a written hand‐out to facilitate self care Patient referral with video (n = 61) Index patient watched a 6‐minute video that tells the story of PID as related by a universal patient created by the voices and images of 7 different female adolescents. The video portrays the patient's interface with health provider and the male partner's interface and allows the universal girl to acknowledge the barriers and benefits of PID self care while providing cues for action Simple patient referral (n = 65) Index patient received standardised discharge instructions based on the 2006 CDC STI treatment guidelines |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | The study was approved by the John Hopkins School of Medicine Institutional Review Board and the Saint Agnes Hospital Institutional Review Board. Additional approval was obtained from the Maryland State Attorney General for recruitment of children who were wards of the state at the time of diagnosis To reach 80% power to detect a statistically significant difference for the 72‐hour follow‐up visit at the P value = 0.05 level an additional 240 study subjects would have been needed. The authors were contacted for exact numbers of partners notified and treated but these numbers were not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Envelopes containing the group assignment and pertinent information materials were opened by participants after informed consent to participate had been obtained from each of them |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 81/126 (62%) index patients had a 2‐week follow‐up interview where information on PN were collected |
| Selective reporting (reporting bias) | Low risk | Outcomes in method section same as in results. No protocol available from 3 trial registries |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of DIS unclear. The DIS performed the follow‐up standardised interview and completed a form. The DIS was not involved with randomisation or initial interaction with participant. Face‐to‐face interview can introduce bias |
Wilson 2009.
| Methods |
Setting: 2 STI clinics in Brooklyn, NY, US. One was a non‐Department of Health clinic for STI and the other a Department of Health STI clinic Enrolment: index patients enrolled between January 2002 and December 2004 Follow‐up: index patient was interviewed at 1 and 6 months after baseline. Testing of index patient for re‐infection with Neisseria gonorrhoeae and Chlamydia trachomatis at 6 months after baseline |
|
| Participants | 600 index patients (245 women and 355 men), with chlamydia or gonorrhoea, were randomised Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Patient referral with 2 counselling sessions (4 weeks apart) (n = 304) The first session was designed to occur in the clinic at the time of STI diagnosis. This was a one‐on‐one counselling session with health educator discussing risk behaviour, identification of eligible sexual partners, development of a notification plan, role‐play exercises and completion of a signed behavioural contract to notify partners. Index patients received support material including written pamphlet on PN and referral slips to give to partner with information on where to access free confidential STI testing and treatment. The second session was designed to take place by telephone or in person, 4 weeks after initial session. Review of progress and any remaining barriers to notification process were discussed Simple patient referral (n = 296) Index patient met with health educator at the time of STI diagnosis. The health educator asked the index patient if there were any questions related to the clinic visit, diagnosis, treatment or prevention. A brief discussion period followed. Index patient was given referral slips to give to partner with information on where to access free confidential STI testing and treatment |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Ethical approval by institutional review board at participating sites and at the CDC Author was contacted and they were unable to account for reasons for unequal distribution of STIs at baseline Authors could not provide exact numbers of partners for outcomes. Distribution of harms between 2 groups and detail on protocol obtained from authors. The randomisation process was implemented throughout recruitment as described in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation algorithm, with stratifications by site of recruitment and gender within site. Computerised random number generator |
| Allocation concealment (selection bias) | Unclear risk | The principal investigator pre‐assigned sequential study identification numbers according to the random number generated sequence. Participants were assigned study identification numbers sequentially as they enrolled in the study. There was no explicit mention of safeguards to concealment such as opaque sealed envelopes, or signing consent before randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 263/296 (88%) in simple patient referral group completed 1 and 6 month after baseline interview and had a valid urine test result. In the patient referral group with 2 counselling sessions, 253/304(83%) completed 1 and 6 month after baseline and had a valid urine test result |
| Selective reporting (reporting bias) | High risk | Protocol obtained from trial registries Outcome in protocol: Primary outcomes in protocol was PN and re‐infection of index patient at 6 months Outcome in actual study: Primary outcomes in actual study are PN and harms In the protocol, 3 intervention arms were described, in the actual study only 2 arms were reported |
| Other bias | Low risk | No other bias identified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participant or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of study interviewers was performed. The study interviewers were not employees of the study clinics neither did they engage in any health education activities. Study interviewers were not informed of participant group assignment. Laboratory personnel were blinded |
CDC: Centers for Disease Control and Prevention; DIS: disease intervention specialist; DNA: deoxyribonucleic acid; EPT: expedited partner therapy; FPC: family planning clinic; GUM: genitourinary medicine; HIV: human immunodeficiency virus; ITT: intention to treat; NGU: non‐gonococcal urethritis; PCR: polymerase chain reaction; PID: pelvic inflammatory disease; PN: partner notification; RNA: ribonucleic acid; STI: sexually transmitted infection; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Colvin 2006 | PN was part of a package given to the index patient and the effect of PN alone cannot be evaluated |
| Garcia 2003 | Study not on PN |
| Hogben 2005 | Study was discontinued due to low recruitment |
| Marion 2009 | Study not on PN |
| Okonofua 2003 | No STI diagnosis was made |
| Richens 2010 | Study not on PN |
| Shain 2004 | Study not on PN |
| Sherman 2005 | Study was discontinued due to low recruitment |
| Thurman 2008 | Not an RCT |
| Wu 2009 | STI diagnosis not made in all index patients |
| Young 2007 | Not an RCT |
PN: partner notification; RCT: randomised controlled trial; STI: sexually transmitted infections.
Characteristics of studies awaiting assessment [ordered by study ID]
Levy 1998.
| Methods |
Setting: US, poor, high‐crime urban area, neighbourhood‐based service in converted store front Enrolment: over the first 12 months of the study ‐ 386 intravenous drug users were recruited by outreach team from the streets Follow‐up: re‐interview 3 months later |
| Participants | 60 HIV‐positive participants were randomised Inclusion criteria
|
| Interventions | All index patients receive referral to case management services, help in identifying and naming at‐risk partners, reasons to inform their partners and counselling in how to do so Simple patient referral Index patients receive help in identifying and naming partners and are counselled about notification Choice patient referral or provider referral Index patients receive help in identifying and naming partners and are counselled about notification. Outreach team notify those partners the patient does not want to notify themselves, without revealing the identity of index patient |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | This study is still ongoing, and apart from limited data on patient preferences, there are no data on other outcomes The only study conducted outside of the formal health services Harms are being compared |
HIV: human immunodeficiency virus.
Characteristics of ongoing studies [ordered by study ID]
Cassell 2010.
| Trial name or title | Different Approaches to Partner Notification in Primary Care |
| Methods | Cluster randomised trial |
| Participants | Practices from the MRC General Practice Research Framework, South East Care Research Network or the Primary Care Research Network Greater London, UK Patients with curable STIs |
| Interventions | Patient referral, contract referral and provider referral |
| Outcomes | Number of partners treated. Proportion of index patients testing negative for the relevant STI at 3 months |
| Starting date | 1 May 2010 |
| Contact information | j.cassell@bsms.ac.uk +044 (0) 1273 641924 |
| Notes | Trial registration number: ISRCTN24160819 |
Falk 2012.
| Trial name or title | Home‐Sampling in Partner Notification of Chlamydia |
| Methods | Multicentre cluster‐randomised controlled trial |
| Participants | Sexual partners to chlamydia‐infected index patients |
| Interventions | Home sampling |
| Outcomes | Difference in time, measured as days from the meeting between the index patient and the counsellor until the date of testing of partners |
| Starting date | November 2006 |
| Contact information | Not reported |
| Notes | Trial registration number: NCT01596946 |
Farquhar 2012.
| Trial name or title | Assisted‐Partner Notification Services |
| Methods | Randomised controlled trial |
| Participants | Newly diagnosed HIV‐infected patients |
| Interventions | Assisted partner notification |
| Outcomes | Rate of HIV testing of partners, newly identified HIV‐infected partners, rate of linkage to HIV care, cost‐effectiveness. |
| Starting date | June 2012 |
| Contact information | cfarq@u.washington.edu |
| Notes | Trial registration number: NCT01616420 |
Golden 2012.
| Trial name or title | Washington State Community Expedited Partner Treatment (EPT) Trial |
| Methods | Cluster randomised trial |
| Participants | Male or females given a diagnosis of chlamydia or gonorrhoea. Inclusion criteria Aged over 14 years, not men who have sex with men Setting: 23 Washington state local health jurisdictions Enrolment: medical providers will refer selected persons for partner services Follow‐up: no follow‐up scheduled but report through public health surveillance |
| Interventions | Patient‐delivered partner therapy packages including antibiotics, condom, written information |
| Outcomes | Primary outcomes: test positivity for chlamydia in women at family planning clinics, incidence of gonorrhoea among women Secondary outcomes: re‐infection of index patient, adverse drug reactions; use of patient‐delivered partner therapy by medical providers |
| Starting date | July 2007 |
| Contact information | Matthew Golden, MD, University of Washington |
| Notes | Trial registration number: NCT01665690 |
HIV: human immunodeficiency virus; MRC: Medical Research Council; STI: sexually transmitted infection.
Differences between protocol and review
This was an update of an existing Cochrane Review.
Contributions of authors
Adel Ferreira co‐ordinated the update of the review. She was involved in screening the results and eligibility assessment of the studies. She was involved in data extraction, data management, risk of bias assessment and data interpretation. She performed the data analysis and was responsible for writing the first draft of the update.
Taryn Young provided methodological support for the review update. She was involved in eligibility assessment of studies, conducted data extraction and risk of bias assessment, assisted in resolving disagreements, contributed to data analysis and interpretation of results, and commented on and revised the manuscript.
Catherine Mathews was involved in the conception and design of the original review. In the update, she was involved in the screening of search results, analysis and interpretation of the data, and commented on and revised the manuscript.
Moleen Zunza was involved in screening of search results, assessing eligibility of studies, data extraction and risk of bias assessment. She commented on and revised the manuscript.
Nicola Low contributed to the analysis and interpretation of the results. She commented on and revised the manuscript.
All authors approved the final version of the updated review.
Sources of support
Internal sources
Centre for Evidence‐based Health Care, Stellenbosch University, South Africa.
External sources
Effective Health Care Research Consortium, UK.
Declarations of interest
Taryn Young and Catherine Mathews are co‐authors of studies cited in the Background section.
Nicola Low is a co‐author on one of the included RCTs and a systematic review that is cited in the Background and Discussion section.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andersen 1998 {published data only}
- Andersen B, Ostergaard L, Moller JK, Olesen F. Home sampling versus conventional contact tracing for detecting Chlamydia trachomatis infection in male partners of infected women: randomised study. BMJ 1998;316:350‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Apoola 2009 {published data only}
- Apoola A, Beardsley J. Does the addition of a urine testing kit to use of contact slips increase the partner notification rates for genital chlamydial infection. International Journal of STD & AIDS 2009;20:775‐7. [DOI] [PubMed] [Google Scholar]
Brown 2011 {published data only}
- Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub‐Saharan Africa: opportunities for HIV treatment and prevention. Journal of Acquired Immune Deficiency Syndromes 2011;56(5):437‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 2009 {published data only}
- Cameron ST, Glasier A, Scott G, Young, Melvin L, Johnstone A, et al. Novel interventions to reduce re‐infection in women with chlamydia: a randomized controlled trial. Human Reproduction 2009;24(4):888‐95. [DOI] [PubMed] [Google Scholar]
- Melvin L, Cameron ST, Glasier A, Scott G, Johnstone A, Elton R. Preferred strategies of men and women for managing chlamydial infection. BJOG 2009;116:357‐65. [DOI] [PubMed] [Google Scholar]
Cleveland undated {unpublished data only}
- Cleveland JQ. A cost‐effective study of alternate methods for gonorrhea contact referral and rescreening. Data on file.
Ellison undated {unpublished data only}
- Ellison GTH, Moniez V, Stein J. Improving partner notification for sexually transmitted disease using a standardised health message and patient‐centred counselling. Data on file.
Faxelid 1996 {published data only}
- Faxelid E, Tembo G, Ndulo J, Krantz I. Individual counselling of patients with sexually transmitted diseases: a way to improve partner notification in a Zambian setting?. Sexually Transmitted Diseases 1996;23:289‐92. [PubMed] [Google Scholar]
Golden 2005 {published data only}
- Golden MR, Whittington WLH, Handsfield HH, Clark A, Malinski C, Helmers JR, et al. Failure of family‐planning referral and high interest in advanced provision emergency contraception among women contacted for STD partner notification. Contraception 2004;69:241‐6. [DOI] [PubMed] [Google Scholar]
- Golden MR, Whittington WLH, Handsfield HH, Hughes JP, Stamm WE, Hogben M, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. New England Journal of Medicine 2005;352:676‐85. [DOI] [PubMed] [Google Scholar]
- Golden MR, Whittington WLH, Handsfield HH, Malinski C, Clark A, Hughes JP, et al. Partner management for gonococcal and chlamydial infection expansion of public health services to the private sector and expedited sex partner treatment through a partnership with commercial pharmacies. Sexually Transmitted Disease 2001;28(11):658‐65. [DOI] [PubMed] [Google Scholar]
- Shiely F, Hayes K, Thomas KK, Kerani RP, Hughes JP, Whittington WLH, et al. Expedited partner therapy: a robust intervention. Sexually Transmitted Diseases 2010;37(10):602‐7. [DOI] [PubMed] [Google Scholar]
Katz 1988 {published data only}
- Katz BP, Danos CS. Efficiency and cost‐effectiveness of field follow‐up for patients with chlamydia trachomatis infection in a sexually transmitted disease clinic. Sexually Transmitted Diseases 1988;15(1):11‐6. [DOI] [PubMed] [Google Scholar]
Kerani 2011 {published data only}
- Kerani RP, Fleming M, DeYoung B, Golden MR. A randomized, controlled trial of inSPOT and patient‐delivered partner therapy for gonorrhea and chlamydial infection among men who have sex with men. Sexually Transmitted Diseases 2011;38(10):941‐6. [DOI] [PubMed] [Google Scholar]
Kissinger 2005 {published data only}
- Kissinger P, Mohammed H, Richardson‐Alston G, Leichliter JS, Taylor SN, Martin DH, et al. Patient‐delivered partner treatment for male urethritis: a randomized, controlled trial. Clinical Infectious Diseases 2005;41:623‐9. [DOI] [PubMed] [Google Scholar]
- Mohammed H, Leichliter JS, Schmidt N, Kissinger P. Does patient‐delivered partner treatment improve disclosure for treatable sexually transmitted diseases?. AIDS Patient Care and STDs 2010;24(3):183‐8. [DOI] [PubMed] [Google Scholar]
Kissinger 2006 {published data only}
- Kissinger P, Schmidt N, Mohammed H, Leichliter JS, Gift TL, Meadors B, et al. Patient‐delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sexually Transmitted Diseases 2006;33(7):445‐50. [DOI] [PubMed] [Google Scholar]
Landis 1992 {published data only}
- Landis SE, Schoenbach VJ. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. New England Journal of Medicine 1992;326(2):101‐6. [DOI] [PubMed] [Google Scholar]
Low 2006b {published data only}
- Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection (executive summary). Health Technology Assessment 2007;11(8):1‐165. [DOI] [PubMed] [Google Scholar]
- Low N, McCarthy A, Macleod J, Salisbury C, Horner PJ, Roberts TE, et al. The chlamydia screening studies: rationale and design. Sexually Transmitted Infections 2004;80:342‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low N, McCarthy A, Roberts TE, Huengsberg M, Sanford E, Sterne JAC, et al. Partner notification of chlamydia infection in primary care: randomised controlled trial and analysis of resource use. BMJ 2006;332:14‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montesinos 1990 {published data only}
- Montesinos L, Frisch LE, Greene BF, Hamilton M. An analysis of and intervention in the sexual transmission of disease. Journal of Applied Behavior Analysis 1990;23(3):275‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyo 2002 {published data only}
- Moyo W, Chirenje ZM, Mandel J, Schwarcz SK, Klausner J, Rutherford G, et al. Impact of a single session of counseling on partner referral for sexually transmitted disease treatment, Harare, Zimbabwe. AIDS and Behavior 2002;6(3):237‐43. [Google Scholar]
Nuwaha 2001 {published data only}
- Nuwaha F, Kambugu F, Nsubuga PSJ, Hojer B, Faxelid E. Efficacy of patient‐delivered partner medication in the treatment of sexual partners in Uganda. Sexually Transmitted Diseases 2001;28:105‐10. [DOI] [PubMed] [Google Scholar]
Ostergaard 2003 {published data only}
- Ostergaard L, Andersen B, Moller JK, Olesen F, Worm AM. Managing partners of people diagnosed with Chlamydia trachomatis: a comparison of two partner testing methods. Sexually Transmitted Infections 2003;79:358‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterman 1997 {published data only}
- Peterman TA, Toomey KE, Dicker LW, Zaidi AA, Wroten JE, Carolina J. Partner notification for syphilis: a randomised controlled trial of three approaches. Sexually Transmitted Diseases 1997;24(9):511‐18. [DOI] [PubMed] [Google Scholar]
Potterat 1977 {published data only}
- Potterat JJ, Rothenberg RR. The case‐finding effectiveness of a self‐referral system for gonorrhea: a preliminary report. American Journal of Public Health 1977;67(2):174‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schillinger 2003 {published data only}
- Magnus M, Schillinger JA, Fortenberry JD, Berman SM, Kissinger P. Partner age not associated with recurrent Chlamydia trachomatis infection, condom use, or partner treatment and referral among adolescent women. Journal of Adolescent Health 2006;39:396‐403. [DOI] [PubMed] [Google Scholar]
- Schillinger JA, Kissinger P, Calvet H, Whittington WLH, Ransom RL, Sternberg MR, et al. Patient‐delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women. Sexually Transmitted Diseases 2003;30:49‐56. [DOI] [PubMed] [Google Scholar]
Schwebke 2010 {published data only}
- Schwebke JR, Desmond RA. A randomized controlled trial of partner notification methods for prevention of trichomoniasis in women. Sexually Transmitted Diseases 2010;37(6):392‐6. [DOI: 10.1097/OLQ.0b013e3181dd1691] [DOI] [PubMed] [Google Scholar]
Solomon 1988 {published data only}
- Solomon MZ, DeJong W. The impact of a clinic‐based educational videotape on knowledge and treatment behavior of men with gonorrhea. Sexually Transmitted Diseases 1988;15:127‐32. [DOI] [PubMed] [Google Scholar]
Tomnay 2006 {published data only}
- Tomnay JE, Pitts MK, Kuo TC, Fairley CK. Does the Internet assist clients to carry out contact tracing? A randomized controlled trial using web‐based information. International Journal of STD & AIDS 2006;17(6):391. [DOI] [PubMed] [Google Scholar]
Trent 2010 {published data only}
- Trent M, Chung S, Burke M, Walker A, Ellen J. Results of a randomized controlled trial of a brief behavioral intervention for pelvic inflammatory disease in adolescents. Journal of Pediatric and Adolescent Gynecology 2010;23:96‐101. [DOI] [PubMed] [Google Scholar]
Wilson 2009 {published data only}
- Hoffman S, Beckford Jarrett ST, Keivin EA, Wailace SA, Augenbraun M, Hogben M, et al. HIV and sexually transmitted infection risk behaviors and beliefs among black West Indian immigrants and US‐born blacks. American Journal of Public Health 2008;98:2042‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Hogben M, Malka ES, Liddon N, McCormack WM, Rubin SR, et al. A randomized controlled trial for reducing risks for sexually transmitted infections through enhanced patient‐based partner notification. American Journal of Public Health 2009;99(S1):S104‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Colvin 2006 {published data only}
- Colvin M, Bachmann MO, Homan RK, Nsibande D, Nkwanyana NM, Connolly C, et al. Effectiveness and cost effectiveness of syndromic sexually transmitted infection packages in South African primary care: cluster randomised trial. Sexually Transmitted Infections 2006;82:290‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2003 {published data only}
- Garcia P, Hughes J, Carcamo C, Holmes KK. Training pharmacy workers in recognition, management, and prevention of STDs: district randomised control trial. Bulletin of the World Health Organization 2003;81(11):806‐14. [PMC free article] [PubMed] [Google Scholar]
Hogben 2005 {published data only}
- Hogben M, Madico G. The Participant Agreement for Contact Tracing (PACT) study: enhancing partner notification services. clinicaltrials.gov/show/NCT00207493 (accessed 17 June 2013).
Marion 2009 {published data only}
- Marion LN, Finnegan L, Campbell RT, Szalacha LA. The Well Woman Program: a community‐based randomized trial to prevent sexually transmitted infections in low‐income African American women. Research in Nursing & Health 2009;32:274‐85. [DOI] [PubMed] [Google Scholar]
Okonofua 2003 {published data only}
- Okonofua FE, Coplan P, Collins S, Oronsaye F, Ogunsaki D, Ogonor JT, et al. Impact of an intervention to improve treatment‐seeking behavior and prevent sexually transmitted diseases among Nigerian youths. International Journal of Infectious Diseases 2003;7:61‐73. [DOI] [PubMed] [Google Scholar]
Richens 2010 {published data only}
- Richens J, Copas A, Sadiq ST, Kingori P, McCarthy O, Jones V, et al. A randomised controlled trial of computer‐assisted interviewing in sexual health clinics. Sexually Transmitted Infections 2010;86(4):310‐37. [DOI] [PubMed] [Google Scholar]
Shain 2004 {published data only}
- Shain RN, Piper JM, Holden AEC, Champion JD, Perdue ST, Korte JE, et al. Prevention of gonorrhea and chlamydia through behavioral intervention results of a two‐year controlled randomized trial in minority women. Sexually Transmitted Diseases 2004;31(7):401‐8. [DOI] [PubMed] [Google Scholar]
Sherman 2005 {published data only}
- Sherman C, Hogben M. Computer‐assisted STD partner notification. clinicaltrials.gov/ct2/show/NCT00207571 (accessed 17 June 2013).
Thurman 2008 {published data only}
- Thurman AS, Holden AEC, Shain R, Perdue S, Piper J. Partner notification of sexually transmitted infections among pregnant women. International Journal of STD & AIDS 2008;19:309‐15. [DOI] [PubMed] [Google Scholar]
Wu 2009 {published data only}
- Wu Z, Yen W. A randomized community intervention trial on reducing HIV infection among drug users attending methadone maintenance treatment (MMT) and preventing secondary transmission from HIV positive clients to their sexual partners in China. http://clinicaltrials.gov/show/NCT01108614 (Accessed 14 September 2013).
Young 2007 {published data only}
- Young IT, Kock A, Jones, Altini L, Ferguson T, Wijgert J. A comparison of two methods of partner notification for sexually transmitted infections in South Africa: patient‐delivered partner medication and patient‐based partner referral. International Journal of STD & AIDS 2007;18:338‐40. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Levy 1998 {published data only}
- Levy JA, Fox SE. The Outreach‐Assisted Model of partner notification with IDUs. Public Health Reports 1998;113 Suppl 1:160‐9. [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Cassell 2010 {published data only}
- Different Approaches to Partner Notification in Primary Care. Ongoing study 1 May 2010.
Falk 2012 {published data only}
- Home‐Sampling in Partner Notification of Chlamydia. Ongoing study November 2006.
Farquhar 2012 {published data only}
- Assisted‐Partner Notification Services. Ongoing study June 2012.
Golden 2012 {published data only}
- Washington State Community Expedited Partner Treatment (EPT) Trial. Ongoing study July 2007.
Additional references
Alam 2010
- Alam N, Chamot E, Vermund SH, Streatfield K, Kristensen S. Partner notification for sexually transmitted infections in developing countries: a systematic review. BioMed Central Public Health 2010;10(19):1‐11. [DOI: 10.1186/1471-2458-10-19] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnold 2008
- Arnold EM, Rice E, Flannery D, Rotherham‐Borus MJ. HIV disclosure among adults living with HIV. Aids Care 2008;20(1):80‐92. [DOI] [PubMed] [Google Scholar]
CDC 2006
- Centers for Disease Control and Prevention. Expedited partner therapy in the management of sexually transmitted diseases. US Department of Health and Human Services 2006.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ECDC 2013
- European Centre for Disease Prevention and Control. Public health benefits of partnernotification for sexually transmitted infections and HIV, 2013. http://optimisation.ecdc.europa.eu/en/publications/publications/partner‐notification‐for‐hiv‐sti‐june‐2013.pdf.. Stockholm: ECDC, (accessed 16 September 2013).
Fenton 1997
- Fenton KA, Peterman TA. HIV partner notification: taking a new look. AIDS 1997;11:1535‐46. [DOI] [PubMed] [Google Scholar]
Gerbase 1998
- Gerbase AC, Rowley JT, Heymann DHL, Berkley SFB, Piot P. Global prevalence and incidence estimate of selected curable STDs. Sexually Transmitted Infections 1998;74 Suppl 1:S12‐6. [PubMed] [Google Scholar]
Glasier 2006
- Glasier A, Gűlmezoglu AM, Schmid GP, Moreno CG, Look PFA. Sexual and reproductive health: a matter of life and death. Lancet 2006;368:1595. [DOI: 10.1016/S0140-6736(06)69478-6)] [DOI] [PubMed] [Google Scholar]
GRADE 2004 [Computer program]
- Brozek J, Oxman A, Schunemann H. GRADEpro. Version 3.2 for Windows. 2008.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kissinger 2003
- Kissinger PJ, Niccolai LM, Magnus M. Partner notification for HIV and syphilis: effects on sexual behaviors and relationship stability. Sexually Transmitted Diseases 2003;30:75‐82. [DOI] [PubMed] [Google Scholar]
Low 2006a
- Low N, Broutet N, Adu‐Sarkodie Y, Barton P, Hassain M, Hawkes S. Global control of sexually transmitted infections. Lancet 2006;368(9551):2001‐16. [DOI] [PubMed] [Google Scholar]
Macke 1999
- Macke BA, Maher JE. Partner notification in the United States: an evidence‐based review. American Journal of Preventive Medicine 1999;17(3):230‐42. [DOI] [PubMed] [Google Scholar]
Mathews 2001
- Mathews C, Coetzee N, Zwarenstein M, Lombard C, Guttmacher S, Oxman AD, et al. Strategies for partner notification for sexually transmitted diseases. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD002843] [DOI] [PubMed] [Google Scholar]
Niccolai 2007
- Niccolai L, Livingston K, Teng F, Pettigrew M. Behavioral intentions in sexual partnerships following a diagnosis of Chlamydia trachomatis. Preventive Medicine 2007;46(2008):170‐6. [DOI] [PubMed] [Google Scholar]
Oxman 1994
- Oxman AD, Scott EAF, Sellors JW, Clarke JH, Millson ME, Rasooly I, et al. Partner notification for sexually transmitted diseases: an overview of the evidence. Canadian Journal of Public Health 1994;85:127‐32. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenthal 1995
- Rosenthal SL, Baker JG, Biro FM. Secondary prevention of STD transmission during adolescence: partner notification. Adolescent & Pediatric Gynecology 1995;8:183‐7. [Google Scholar]
Sahasrabuddhe 2002
- Sahasrabuddhe VV, Gholap TA, Jethava YS, Joglekar NS, Brahme RG, Gaikwad BA, et al. Patient‐led partner referral in a district hospital based STD clinic. Journal of Postgraduate Medicine 2002;48(2):105‐8. [PubMed] [Google Scholar]
Simbayi 2007
- Simbayi L, Strebel A, Cloete A, Henda N, Mqeketo A, Kalichman SC. HIV status disclosure to sex partners and sexual risk behaviours among HIV‐positive men and women in Cape Town, South Africa. Sexually Transmitted Infections 2007;83:29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toomey 1996
- Toomey KE, Latif AS, Steen RC. Partner management. In: Dallabetta GA, Laga M, Lamptey PR editor(s). Control of Sexually Transmitted Diseases. A Handbook for the Design and Management of Programs. Arlington, VA: Family Health International, AIDS Control and Prevention Project, 1996:211‐224. [Google Scholar]
Trelle 2007
- Trelle S, Shang A, Nartey L, Cassell JA, Low N. Improved effectiveness of partner notification for patients with sexually transmitted infections: systematic review. BMJ 2007;334(7589):354‐60. [10.1136/bmj.39079.460741.7C] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trollope‐Kumar 2006
- Trollope‐Kumar K, Guyatt G. Syndromic approach for treatment of STIs: time for a change. Lancet 2006;367:1380‐1. [DOI] [PubMed] [Google Scholar]
UNAIDS 1999
- UNAIDS/World Health Organization. Sexually transmitted diseases: policies and principles for prevention and care, 1999. www.who.int/hiv/pub/sti/pubstiprevcare/en/index.html (accessed 17 June 2013).
UNAIDS 2010
- UNAIDS. UNAIDS report on the global AIDS epidemic, 2010. www.unaids.org/globalreport/global_report.htm (accessed 17 June 2013).
WHO 2004
- World Health Organization. Global burden of disease, 2004. www.who.org (accessed 17 June 2013).
WHO 2007
- World Health Organization. Global strategy for the prevention and control of sexually transmitted infections: 2006‐2015: breaking the chain of transmission. www.who.int/reproductivehealth/publications/rtis/9789241563475/en/ (accessed 17 June 2013).
WHO 2012
- World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infections ‐ 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/ (accessed 17 June 2013).
World Bank 2012
- World Bank. World Bank Data, 2012. http://data.worldbank.org/income‐level/MIC (accessed 14 September 2013).


