Abstract
Background
Bacterial infections are a frequent complication in patients with cirrhosis and upper gastrointestinal bleeding. Antibiotic prophylaxis seems to decrease the incidence of bacterial infections. Oral antibiotics, active against enteric bacteria, have been commonly used as antibiotic prophylaxis in patients with cirrhosis and upper gastrointestinal bleeding. This is an update of a Cochrane review first published in 2002.
Objectives
To assess the benefits and harms of antibiotic prophylaxis in cirrhotic patients with upper gastrointestinal bleeding.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index EXPANDED until June 2010. In addition, we handsearched the references of all identified studies.
Selection criteria
Randomised clinical trials comparing different types of antibiotic prophylaxis with no intervention, placebo, or another antibiotic to prevent bacterial infections in cirrhotic patients with upper gastrointestinal bleeding.
Data collection and analysis
Three authors independently assessed trial quality, risk of bias, and extracted data. We contacted study authors for additional information. Association measures were relative risk (RR) for dichotomous outcomes and mean difference (MD) for continuous outcomes.
Main results
Twelve trials (1241 patients) evaluated antibiotic prophylaxis compared with placebo or no antibiotic prophylaxis. All trials were at risk of bias. Antibiotic prophylaxis compared with no intervention or placebo was associated with beneficial effects on mortality (RR 0.79, 95% CI 0.63 to 0.98), mortality from bacterial infections (RR 0.43, 95% CI 0.19 to 0.97), bacterial infections (RR 0.36, 95% CI 0.27 to 0.49), rebleeding (RR 0.53, 95% CI 0.38 to 0.74), days of hospitalisation (MD ‐1.91, 95% CI ‐3.80 to ‐0.02), bacteraemia (RR 0.25, 95% CI 0.15 to 0.40), pneumonia (RR 0.45, 95% CI 0.27 to 0.75), spontaneous bacterial peritonitis (RR 0.29, 95% CI 0.15 to 0.57), and urinary tract infections (RR 0.23, 95% CI 0.12 to 0.41). No serious adverse events were reported. The trials showed no significant heterogeneity of effects. Another five trials (650 patients) compared different antibiotic regimens. Data could not be combined as each trial used different antibiotic regimen. None of the examined antibiotic regimen was superior to the control regimen regarding mortality or bacterial infections.
Authors' conclusions
Prophylactic antibiotic use in patients with cirrhosis and upper gastrointestinal bleeding significantly reduced bacterial infections, and seems to have reduced all‐cause mortality, bacterial infection mortality, rebleeding events, and hospitalisation length. These benefits were observed independently of the type of antibiotic used; thus, no specific antibiotic can be preferred. Therefore, antibiotic selection should be made considering local conditions such as bacterial resistance profile and treatment cost.
Plain language summary
Antibiotic prophylaxis for prevention of bacterial infections and death in cirrhotic patients with upper gastrointestinal bleeding
Patients with liver cirrhosis have an impaired immune response. Often, liver cirrhosis patients experience complications from portal hypertension, such as gastroesophageal varices. These varices can bleed, increasing the risk of infection and death in a short period of time, despite proper endoscopic management. Patients who develop bacterial infections during hospitalisation for gastroesophageal haemorrhage are at increased risk of dying. Twelve trials (1241 patients) assessing several antibiotic prophylaxis regimens versus no intervention or placebo were analysed, showing that antibiotic prophylaxis successfully reduced the incidence of bacterial infections. Antibiotic prophylaxis was also associated with a reduction in mortality, mortality from bacterial infections, rebleeding rate, and days of hospitalisation. The prophylactic treatment was not associated with important adverse effects. Five trials (650 patients) assessed one antibiotic regimen compared with another. All antibiotic regimens provided similar benefits and none seemed superior. Thus, to this point there is no evidence to recommend one specific antibiotic regimen over the other. All trials analysed were subject to bias; thus, results should be interpreted carefully.
Background
Description of the condition
Chronic liver diseases are characterised by important changes on hepatic physiology with portal hypertension being their haemodynamic expression. The presence of clinically significant portal hypertension (portal pressure gradient ≥10 mmHg) promotes the formation of collateral portosystemic circulation, portal hypertensive gastropathy, gastric varices, and oesophageal varices (Bosch 2009).
Bleeding, secondary to oesophageal or gastro‐oesophageal varices, is observed in up to 30% of the patients with liver cirrhosis during the course of their illness, recurring in 70% of the patients and being fatal in 20% (NIEC 1988). The highest mortality peak is observed during the first six weeks after the bleeding episode (Burroughs 2009). Hepatic functional status (assessed by Child‐Pugh score), renal dysfunction (assessed by creatinine serum levels), and bacterial infections are the most important mortality risk factors (Augustin 2009). Consequently, guidelines for treatment of patients suffering variceal gastrointestinal bleeding include volume expansion, haemorrhage control, use of vasoconstrictors, and short‐term antibiotic prophylaxis (Garcia‐Tsao 2009).
Description of the intervention
The prophylactic use of oral or intravenous antibiotics has been recommended in several consensus guidelines. The recommended drugs are mainly oral quinolones (norfloxacin 400 mg b.i.d for 7 days) or intravenous cephalosporins (ceftriaxone 1 g/day for 7 days) (Garcia‐Tsao 2009). However, other groups of antibiotics have been assessed such as beta‐lactams or aminoglycosides (Bernard 1999).
How the intervention might work
Cirrhosis is characterised by cellular and humoral immune dysfunction as well as increased bacterial translocation from the gut into the bloodstream, facilitating the development of infections (Chavez‐Tapia 2007). The most common bacterial infections are caused by gram‐negative bacteria, producing spontaneous bacterial peritonitis (25%), urinary tract infections (20%), pneumonia (15%), and bacteraemia (12%) (Fernandez 2002). Considering the increased mortality associated with infections, the vulnerability of the immune system and the bacteriological profile, the use of antibiotics is recommended.
Why it is important to do this review
Prophylactic use of antibiotics during an episode of upper gastrointestinal bleeding in cirrhotic patients is considered standard of care. However, differences in the antibiotics used, schedules of administration, duration of therapy, and changes in the bacteriological profile across clinical trials make the evaluation of this intervention difficult. This review systematically assesses these issues and updates the information from a previous published review (Soares‐Weiser 2002).
Objectives
To assess the benefits and harms of antibiotic prophylaxis in cirrhotic patients with upper gastrointestinal bleeding.
Specifically this review was designed to:
Compare the all‐cause mortality and infection mortality between cirrhotic patients with gastrointestinal bleeding receiving antibiotic prophylaxis or no intervention/placebo.
Compare the proportion of bacterial infections in patients with gastrointestinal bleeding receiving antibiotic prophylaxis versus no intervention/placebo.
Determine the most effective antibiotic regimen.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials comparing different types of antibiotic therapy against no intervention, or placebo, or another antibiotic, in the prophylaxis of bacterial infections in cirrhotic patients with upper gastrointestinal bleeding were identified. Trials were included irrespective of publication status, language, or blinding.
Types of participants
Adult patients with cirrhosis and upper gastrointestinal bleeding were included, regardless of the aetiology of cirrhosis or severity of the disease.
Types of interventions
The following interventions, used alone or in combination, and regardless of the mode of administration (intravenous or oral), were considered.
Aminoglycosides (eg, gentamicin, neomycin, tobramycin);
Amoxicillin with or without clavulanic acid;
Cephalosporins (eg, cefotaxime, ceftriaxone, ceftazidime, cefonicid);
Quinolones (eg, ciprofloxacin, ofloxacin, norfloxacin);
Trimethoprim/sulphamethoxazole;
Non‐absorbable antibiotics (eg, colistin, nystatin);
Other antibiotics.
Control groups received no intervention, placebo, or any antibiotic.
Types of outcome measures
Primary outcome measures:
Number of deaths;
Number of patients that developed bacterial infections (bacteraemia, pneumonia, urinary tract infection, spontaneous bacterial peritonitis, and/or other bacterial infections);
Quality of life score (measured by any scale) between groups;
Adverse events (ICH‐GCP 1997):
Any serious adverse events that were fatal, life‐threatening, or requiring inpatient hospitalisation or prolongation of existing hospitalisation;
Any adverse events that resulted in significant disability or incapacity;
Any important medical events that might not be immediately life‐threatening or resulted in death or hospitalisation, but might jeopardise the patient or required intervention to prevent one of the above outcomes;
Any adverse events that required discontinuation of medication.
Secondary outcome measures:
Number of patients who developed bacterial infections after an invasive procedure to stop upper gastrointestinal bleeding;
Number of patients who developed superinfection or antibiotic resistance in at least one of the follow‐up cultures;
Number of patients who developed rebleeding during the follow‐up (overall rebleeding rate and up to seven days rate);
Number of patients who dropped out from the trial after randomisation;
Cost of different types of antibiotics used for prophylaxis;
Number of days of hospitalisation.
Search methods for identification of studies
Electronic searches
Relevant randomised trials were identified by searching The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2010) in The Cochrane Library, MEDLINE (1950 to 21 June 2010), EMBASE (1980 to 21 June 2010), and Science Citation Index EXPANDED (1945 to 21 June 2010) (Royle 2003).
Search strategies and time span of the searches are given in Appendix 1.
Searching other resources
The references of all identified studies were inspected for more trials. Additionally, the first or corresponding author of each included trial, as well as researchers active in the field, were contacted for information regarding unpublished trials and complementary information on their own trial.
References from an existing review on this topic (Bernard 1999) were also checked for any missing trials.
Data collection and analysis
Selection of studies
Three authors (NC, FT, TB) independently inspected each identified reference and applied the inclusion criteria. For potentially relevant articles, or in cases of disagreement between the three reviewers, the full text article was obtained and inspected independently. If resolving disagreement by discussion was not possible, the article was added to those 'awaiting assessment' and the authors of the original study were contacted for clarification. In the event of no reply from the authors within three months, a fourth reviewer (MU or KSW) reviewed the article to solve the disagreement. Justification for study exclusion was documented.
Data extraction and management
Two authors (NC and TB) independently extracted the data from the included trials. In case of disagreement between the two authors, a third author (FT) extracted the data. The data extraction was discussed, decisions documented, and, when necessary, the authors of the original studies were contacted for clarification. Justification for study exclusion was documented. Trials were identified with the last name of the first author and the year in which the trial was first published, and ordered chronologically.
The following data were extracted, verified, and recorded:
Characteristics of trials
Date, location, and setting of trial;
Publication status;
Case definitions used (clinical, serological, bacteriological);
Sponsor of trial (known or unknown; industry or not industry).
Characteristics of participants
Number of participants in each group;
Age, sex, nationality;
Severity of liver disease and cirrhosis according to the aetiology of liver disease, regardless of the criteria used.
Characteristics of interventions
Type of antibiotic, dose, mode of administration, schedule, length of follow‐up (in months).
Number of days that antibiotic prophylaxis was provided.
Characteristics of outcome measures
Whenever possible, the number of events previously listed under Types of outcome measures were recorded in each group of the randomised trials.
Assessment of risk of bias in included studies
Two authors (NC and TB) independently assessed bias risk of the trials, without masking the trial names. For this purpose, instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009) and the Cochrane Hepato‐Biliary Group Module (Gluud 2010) were followed. The risk of overestimation of intervention effects in randomised trials due to inadequate methodological quality (Schulz 1995; Moher 1998; Jüni 2001; Kjaergard 2001; Wood 2008) was assessed using the domains below. Whenever information was not available in the published trial, authors were contacted directly.
Sequence generation
Low risk of bias, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice were also considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described or insufficient to permit judgement.
High risk of bias, if a system involving dates, names, or admittance numbers were used for the allocation of patients.
Allocation concealment
Low risk of bias, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described or insufficient to permit judgement.
High risk of bias, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding
Low risk of bias, if the trial was described as double blind and the method of blinding involved identical placebo or active drugs.
Unclear, if the trial was described as double blind, but the method of blinding was not described.
High risk of bias, if the trial was not double blind.
Incomplete outcome data
Low risk of bias, if there were no post‐randomisation drop‐outs or withdrawals.
Unclear, if it is not clear whether there are any drop‐outs or withdrawals or if the reasons for these drop‐outs are not clear.
High risk of bias, if the reasons for missing data are likely to be related to true outcomes.
Selective outcome reporting
Low risk of bias, considering that most of the included trials were made before of the obligatory registration on randomised controlled trials databases, and the pre‐specified outcomes are not available. The following outcomes were considered fundamental as outcome to avoid selective reporting; a) mortality, b) response rate, and c) adverse events.
Unclear, there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting.
High risk of bias, not all of the trial's pre‐specified primary outcomes have been reported or similar.
Other sources of bias
Low risk of bias, the trial appears to be free of other sources of bias, considering; a) baseline imbalance, b) source of funding, c) early stopping, and d) interim analysis.
Unclear, there is insufficient information to assess whether other sources of bias are present.
High risk of bias, it is likely that potential sources of bias.
Following the definitions of the above domains, an included trial was judged as a trial with a low risk of bias when the risk of bias was evaluated as 'low' in all domains. If the risk of bias was judged as 'uncertain' or 'high', then the trial was judged as having 'high risk of bias'.
Furthermore, we registered whether or not the randomised clinical trials had used 'intention‐to‐treat' analysis (Gluud 2001), the length of follow‐up, and sample size calculation. Any disagreement was resolved by discussion and settled by a third author (FT). We contacted the trial author for clarification as necessary.
Measures of treatment effect
Dichotomous data were analysed calculating the relative risk (RR) for each trial, expressing the uncertainty with 95% confidence intervals (CI). Continuous data were analysed calculating mean differences between groups of each trial and its 95% CI. Comparisons were made between trials evaluating antibiotic prophylaxis against no intervention or placebo, and trials comparing different antibiotic regimens.
Assessment of heterogeneity
We checked the heterogeneity of effects across trials by visual inspection of the forest plots and Chi2 and I2 tests for heterogeneity (Higgins 2009). Statistical heterogeneity was defined as a P value ≤ 0.10 (Chi2) or I2 > 25%. When heterogeneity existed, subgroup analyses were performed in order to assess the impact of potential sources of heterogeneity over the main results.
Assessment of reporting biases
A funnel plot estimating the precision of trials (plot of logarithm of the RR against the sample size) was examined in order to estimate potential asymmetry. In addition, the standard normal deviate (SND), defined as the RR divided by its standard error, was regressed against the estimate's precision (regression equation: SND = a + b x precision) in order to facilitate the prediction of potential heterogeneity or data irregularities in the meta‐analyses (Egger 1997). In this equation, the SND reflects the degree of funnel plot asymmetry as measured by the intercept from the regression analysis.
Data synthesis
For the statistical analyses, we used RevMan Analyses (RevMan 2010). Dichotomous data were synthesised poling the RR and 95% CI from all trials to estimate the global effect of the intervention. Continous data were synthesised pooling mean differences and 95% CI from each trial to calculate the average mean difference.
In order to compare the RR from different antibiotic groups and detect differences among the antibiotics tested versus no intervention or placebo, a test for interaction was calculated (Altman 2003).
Sensitivity analysis
We analysed data using both fixed‐effect and random‐effects models. When both models produced similar estimates, the fixed‐effect result was reported; otherwise, we reported the results from both analyses. Outcomes were analysed as reported in the trial, that is, either per protocol or as intention‐to‐treat analysis. In order to examine the influence of drop‐outs, we performed both worst‐best‐case (assigning bad outcomes to all of the missing experimental group patients and good outcomes to all of the missing control group patients) and best‐worst‐case (assigning good outcomes to all of the missing experimental group patients and bad outcomes to all of the missing control group patients) analyses.
To assess the reliability of the meta‐analyses on mortality, mortality from bacterial infections, and bacterial infections, the required information size (RIS) was calculated by trial sequential analysis (TSA). We respectively assumed an average event proportion of 22%, 5%, and 36% in the control group of the three meta‐analyses; a 20% relative risk reduction of the experimental intervention, and statistical error levels of 5% alpha and 20% beta (80% power). Whenever the cumulative information size in the meta‐analysis was smaller than the RIS, the threshold to maintain statistical significance was calculated with the O’Brien‐Fleming boundaries (Brok 2008; Wetterslev 2008; Thorlund 2009).
Results
Description of studies
Results of the search
Forty‐one relevant references were initially identified and screened for retrieval. Subsequently, reviews (fourteen references), comments and editorials (four references) were excluded. Twenty‐six studies were considered suitable to be included, but nine studies were excluded because they were not randomised trials (six studies), did not include patients with upper gastrointestinal bleeding (two studies), or did not include any of the outcomes assessed in this review (one study) (See 'Excluded studies' for additional information). Finally, seventeen trials (twenty‐one references) were included for analyses (see 'Included studies' for additional information).
Included studies
Seventeen trials evaluating th effectiveness of antibiotic prophylaxis against bacterial infections in 1891 patients were included in this review (Rimola 1985; Soriano 1992; Rolando 1993; Blaise 1994; Selby 1994; Pauwels 1996; Zacharof 1997; Hsieh 1998; Sabat 1998; Spanish Group 1998; Gulberg 1999; Hong 2002; Lin 2002; Hou 2004; Lata 2005; Fernandez 2006; Jun 2006), in twelve trials (fourteen references) comparison with no intervention or placebo was conducted (Rimola 1985; Soriano 1992; Rolando 1993; Blaise 1994; Selby 1994; Pauwels 1996; Zacharof 1997; Hsieh 1998; Hong 2002; Lin 2002; Hou 2004; Jun 2006).
Five trials (eight references) were head‐to‐head antibiotic comparisons (Sabat 1998; Spanish Group 1998; Gulberg 1999; Lata 2005; Fernandez 2006).
Most trials were retrieved as complete manuscript and two trials as abstracts (Spanish Group 1998; Zacharof 1997). The only non‐English article was written in Korean (Hong 2002).
Trials were conducted in Australia (Selby 1994), Czech Republic (Lata 2005), France (Blaise 1994; Pauwels 1996), Germany (Gulberg 1999), Greece (Zacharof 1997), Korea (Hong 2002; Jun 2006), Spain (Rimola 1985; Soriano 1992; Sabat 1998; Spanish Group 1998; Fernandez 2006), Taiwan (Hsieh 1998; Lin 2002; Hou 2004), and the United Kingdom (Rolando 1993) (see 'Characteristics of included studies' for details).
The source of the haemorrhage was gastroesophageal varices in six trials (Rolando 1993; Blaise 1994; Hong 2002; Hou 2004; Lata 2005; Jun 2006), mixed (variceal and non‐variceal gastrointestinal haemorrhage) in seven trials (Rimola 1985; Soriano 1992; Pauwels 1996; Hsieh 1998; Sabat 1998; Lin 2002; Fernandez 2006), and not specified in four trials (Selby 1994; Zacharof 1997; Spanish Group 1998; Gulberg 1999).
While all trials assessed the use of antibiotic prophylaxis, the primary outcome differed between them. The most common was prevention of bacterial infections in fourteen trials (Rimola 1985; Soriano 1992; Rolando 1993; Blaise 1994; Selby 1994; Pauwels 1996; Zacharof 1997; Hsieh 1998; Sabat 1998; Spanish Group 1998; Gulberg 1999; Hong 2002; Lin 2002; Fernandez 2006), rebleeding rate in two trials (Hou 2004; Jun 2006), and early and late mortality in one trial (Lata 2005).
All trials were performed on hospitalised patients, but four specifically described critical care settings (Rimola 1985; Blaise 1994; Pauwels 1996; Lata 2005). Eleven trials reported the Child‐Pugh score as a categorical variable, from them nine included patients with Child‐Pugh score A/B/C (overall distribution 17/51/32%, respectively), two trials reported only patients with Child Pugh score B/C. Diagnostic and management of the gastrointestinal haemorrhage was done by endoscopy.
The antibiotics compared versus no intervention or placebo were: quinolones (five trials), quinolones plus beta‐lactams (two trials), cephalosporins (three trials), carbapenems (one trial) and non‐absorbable antibiotics (one trial) (Table 2).
1. Group of antibiotics compared versus no intervention or placebo.
| Group of antibiotics | Reference |
| Quinolones | Soriano 1992; Zacharof 1997; Hsieh 1998; Hong 2002; Hou 2004 |
| Quinolones plus beta‐lactams | Blaise 1994; Pauwels 1996 |
| Cephalosporins | Selby 1994; Lin 2002; Jun 2006 |
| Carbapenems | Rolando 1993 |
| Non absorbable | Rimola 1985 |
The head‐to‐head antibiotic comparisons were explored in five trials (Table 3), as follows: combination of antibiotics versus a single antibiotic (Sabat 1998), two antibiotics from the same group (Spanish Group 1998; Gulberg 1999), and different groups of antibiotics in each intervention group (Lata 2005; Fernandez 2006).
2. Trials comparing different antibiotic regimens.
| Reference | Intervention 1 | Intervention 2 |
| Sabat 1998 | Quinolone | Quinolone plus cephalosporin |
| Spanish Group 1998 | Quinolone (Norfloxacin) | Quinolone (Ofloxacin) |
| Gulberg 1999 | Cephalosporin low dose | Cephalosporin high dose |
| Lata 2005 | Quinolone | beta‐lactams |
| Fernandez 2006 | Quinolone | Cephalosporin |
Excluded studies
Nine studies were excluded from this review. The main reason was the retrospective or non‐interventional study design, one study used inadequate allocation concealment (Henrion 1992); another trial did not measure any of the outcomes analysed in this review (Pulanic 1989); two studies were excluded because the patients did not have upper gastrointestinal haemorrhage (Gines 1990; Novella 1997).
Risk of bias in included studies
See Figure 1 and Figure 2 for more information. None of the trials was of low risk of bias.
1.
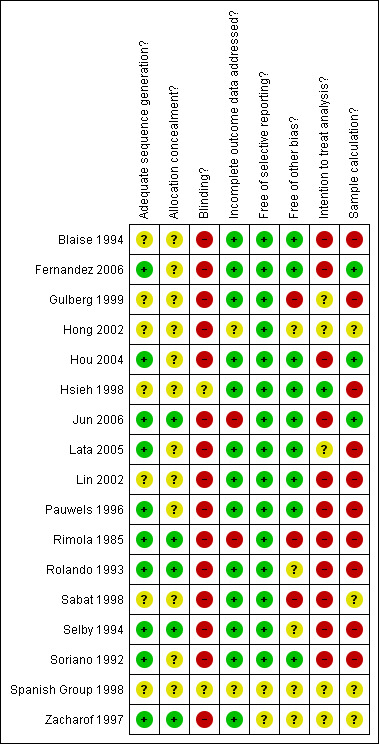
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
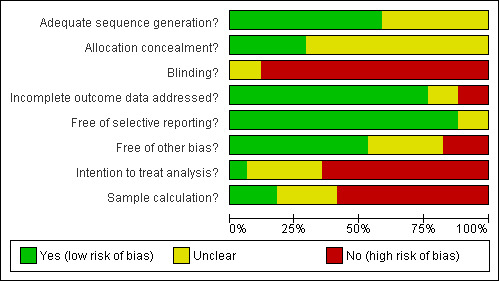
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation sequence was generated by random table number or software in eleven trials (Rimola 1985; Soriano 1992; Rolando 1993; Selby 1994; Pauwels 1996; Zacharof 1997; Sabat 1998; Hou 2004; Lata 2005; Fernandez 2006; Jun 2006). In six trials no clear information about the sequence generation was reported and authors did not provide further information, being classified as unclear. The allocation concealment information was properly indicated in only one trial (Jun 2006), was clarified by direct communication in four trials (Rimola 1985; Rolando 1993; Zacharof 1997; Fernandez 2006), and was unclear in twelve trials. Considering both sequence generation process and allocation concealment, only five trials were classified as having low risk of bias (Rimola 1985; Rolando 1993; Selby 1994; Zacharof 1997; Jun 2006).
Blinding
The risk of bias was high or unclear in all trials included in this review. Only one trial used placebo (Hsieh 1998), but blinding procedures were not reported. Most trials were designed to compare antibiotic prophylaxis versus no intervention, complicating the blinding process. The Cochrane Handbook for Systematic Reviews or Interventions (Higgins 2009) allows considering a trial with a low risk of bias if the outcome is not directly affected by the lack of blinding. Thus, when mortality was the outcome, the trials were considered to be at low risk of bias. Mortality secondary to infections and overall infections as the outcomes could have been affected by the lack of blinding, therefore, considering these outcomes, all trials were considered at high risk of bias.
Incomplete outcome data
Common exclusion criteria from the trials included bacterial infections at admission, positivity of biological cultures during follow‐up and death, or surgery during the first twelve to twenty‐four hours. The trial by Rimola 1985 was considered at high risk of bias because patients excluded were not balanced across groups; similarly, Jun 2006 reported a higher proportion of missing outcomes in the non‐prophylaxis group.
Selective reporting
Except for the trials reported only in abstract form (Zacharof 1997; Spanish Group 1998), all the trials reported at least the primary outcome in their methods.
Other potential sources of bias
Lack of sample size calculation was the most frequently observed source of other bias. Sample size calculation was described in four trials (Sabat 1998; Hou 2004; Fernandez 2006; Jun 2006).
Intention‐to‐treat analysis was performed in one trial (Hsieh 1998). There was a clear rule to exclude some patients from the analysis in certain trials (Blaise 1994; Lin 2002; Fernandez 2006), without considering the intention‐to‐treat analysis (Higgins 2009).
Three other trials could have been subjected to other forms of bias. In Rimola 1985, the antibiotic used was changed over the course of the trial; Gulberg 1999 used surrogates for infection in several analysis; and Sabat 1998 stopped the trial prematurely due to differences in economical outcomes.
Effects of interventions
Mortality
Twelve trials reported overall mortality in 1241 patients. No significant heterogeneity of effects was observed and a slight asymmetry towards the left side of the funnel plot suggested a potential overestimation of effects from small sized trials. The effect of antibiotic prophylaxis on overall mortality was significant (RR 0.79, 95% CI 0.79 to 0.98).
To explore the impact of dropouts on mortality, best‐worst‐case and worst‐best‐case analyses were performed (worst‐best‐case analysis RR 1.45, 95% CI 1.04 to 2.02; best‐worst‐case analysis RR 0.48, 95% CI 0.38 to 0.60).
The TSA showed a trend towards beneficial effects of the intervention to reduce mortality, but the cumulative Z‐score did not cross the O'Brien‐Fleming boundaries (Figure 3); therefore, no clear conclusion can be drawn and more trials are needed to confirm the result of this outcome.
3.
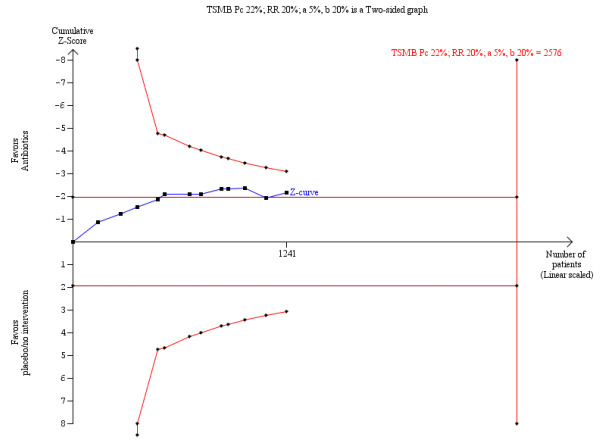
O’Brien‐Fleming monitoring boundaries for assessing statistical significance for overall mortality. The solid blue curve presents the cumulative meta‐analysis test‐score and the inward sloping red curves present the adjusted threshold for statistical significance ‐ the two‐sided O'Brien‐Fleming boundaries.
Mortality from bacterial infections
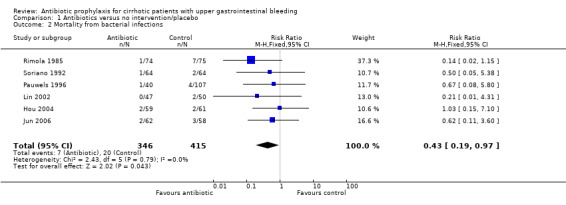
Six trials reported mortality from bacterial infections, comparing prophylaxis with no intervention or placebo (Rimola 1985; Soriano 1992; Pauwels 1996; Lin 2002; Hou 2004; Jun 2006). Antibiotic prophylaxis was associated with a significant decrease in mortality from bacterial infections (RR 0.43, 95% CI 0.19 to 0.97).
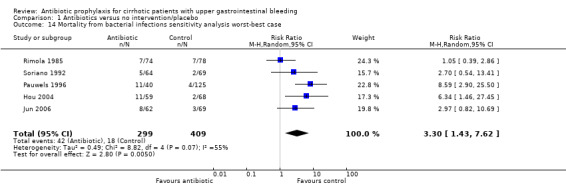
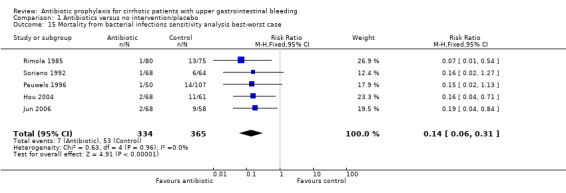
The sensitivity analysis showed this estimation could have been biased by differential drop‐out rates (worst‐best‐case analysis RR 3.30, 95% CI 1.43 to 7.62; best‐worst‐case analysis RR 0.14, 95% CI 0.06 to 0.31).
The TSA demonstrated that the few number of trials included in the analysis were not enough to conclude a beneficial effect of prophylaxis over mortality from bacterial infections (Figure 4).
4.
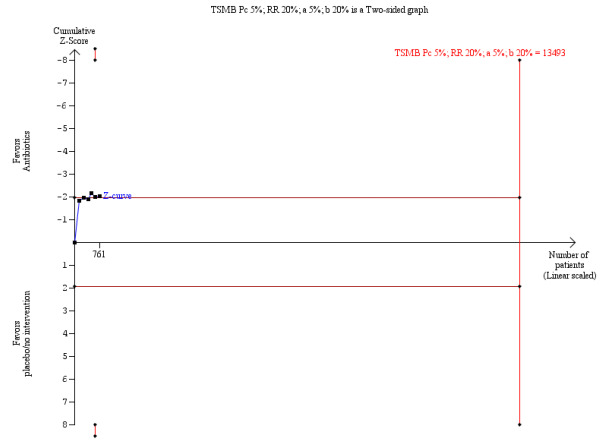
O’Brien‐Fleming monitoring boundaries for assessing statistical significance for mortality from bacterial infections. The solid blue curve presents the cumulative meta‐analysis test‐score and the inward sloping red curves present the adjusted threshold for statistical significance ‐ the two‐sided O'Brien‐Fleming boundaries.
Bacterial infections
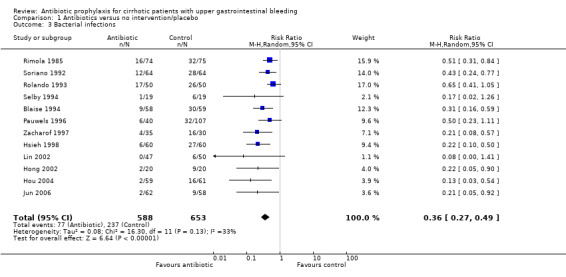
All trials comparing antibiotic prophylaxis versus no intervention or placebo reported incidence of bacterial infections. For this review, only confirmed bacterial infections were considered. A total of 1241 patients were included in the trials, showing antibiotic prophylaxis to be significantly beneficial to reduce bacterial infections (RR 0.36, 95% CI 0.27 to 0.49). Heterogeneity of effects across trials was significant. However, all estimators remained statistically significant independently of the type of analyses used (random or fixed‐effects), strengthening the evidence for the proposed effect.
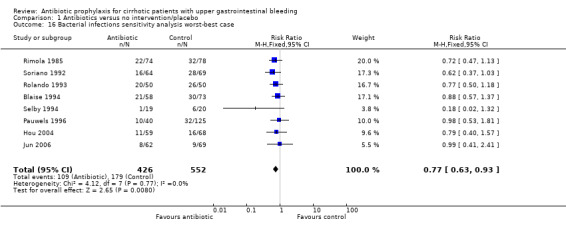
Bacterial infections were not affected by the sensitivity analysis (worst‐best‐case analysis RR 0.77, 95% CI 0.63 to 0.93; best‐worst‐case analysis RR 0.26, 95% CI 0.26 to 0.43), and also the TSA showed significant benefit of antibiotic prophylaxis over no intervention or placebo (Figure 5).
5.

O’Brien‐Fleming monitoring boundaries for assessing statistical significance for bacterial infections. The solid blue curve presents the cumulative meta‐analysis test‐score and the inward sloping red curves present the adjusted threshold for statistical significance ‐ the two‐sided O'Brien‐Fleming boundaries.
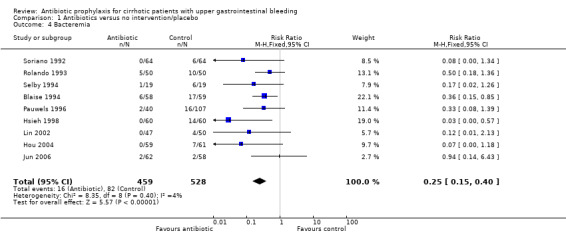
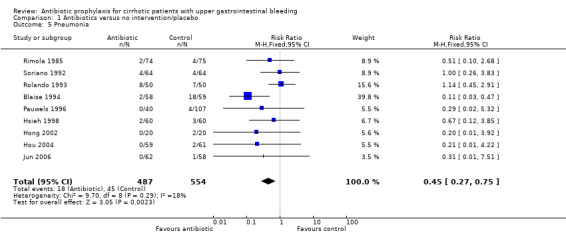
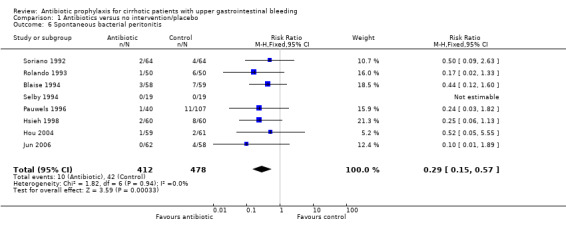
Bacteremia was reported in nine trials with a significant risk reduction in patients under antibiotic prophylaxis (RR 0.25, 95% CI 0.15 to 0.40). Similarly, other infectious outcomes were significantly reduced with the use of antibiotic prophylaxis: pneumonia in nine trials (RR 0.45, 95% CI 0.27 to 0.75), spontaneous bacterial peritonitis in eight trials (RR 0.29, 95% CI 0.15 to 0.57), and urinary tract infections in nine trials (RR 0.23, 95% CI 0.12 to 0.41).
Drop‐outs and sensitivity analysis
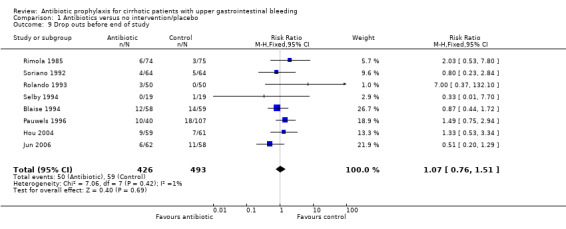
There was no significant difference in drop‐out rates across eight trials reporting this outcome (RR 1.07, 95% CI 0.76 to 1.51).
We analysed overall mortality and bacterial infections stratifying by sample size (larger than 100 versus smaller than 101 patients), RR were homogeneous across strata.
Adverse events
No significant adverse events were reported.
Quality of life
No information regarding this outcome was reported in the trials.
Antibiotic regimens
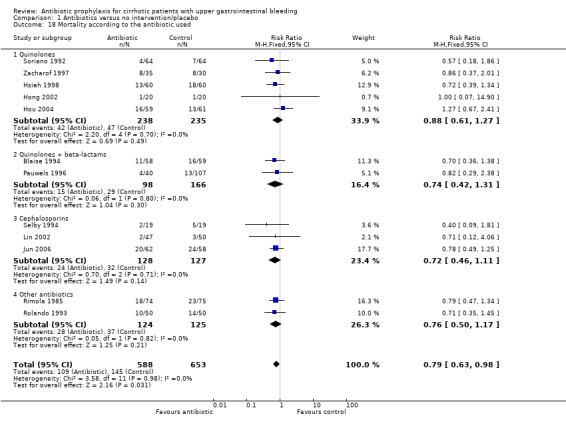
Although a beneficial effect of antibiotic prophylaxis was observed when pooling all trials, stratifying by group of antibiotic diluted the effect and no beneficial effects on mortality or mortality from bacterial infections was observed.
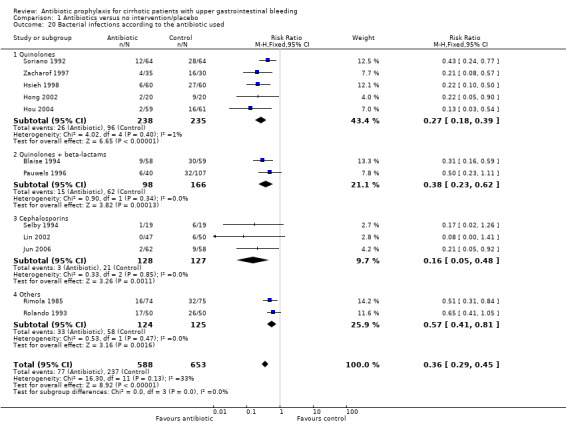
Regarding bacterial infections, all the antibiotics showed a risk reduction, the beneficial effect seemed to be higher in trials using cephalosporins (RR 0.16, 95% CI 0.05 to 0.48), followed by quinolones (RR 0.27, 95% CI 0.18 to 0.39), quinolones plus beta‐lactams (RR 0.38, 95% CI 0.23 to 0.62), and other antibiotics (RR 0.57, 95%CI 0.41 to 0.81). However, the test for interaction demonstrated that only the group of 'other antibiotics' significantly differed from all other drugs (quinolones versus other antibiotics P value = 0.004, and cephalosporins versus other antibiotics P value 0.03). No significant difference between quinolones and cephalosporins was observed (Table 4).
3. Test of interaction for antibiotic regimens (assessing bacterial infections).
| Antibiotic regimen 1 | Antibiotic regimen 1 | P‐value |
| Cephalosporins | Quinolones | 0.39 |
| Cephalosporins | Quinolones + beta‐lactams | 0.17 |
| Cephalosporins | Other antibiotics | 0.03 |
| Quinolones | Quinolones + beta‐lactams | 0.29 |
| Quinolones | Other antibiotics | 0.004 |
| Quinolones + beta‐lactams | Other antibiotics | 0.19 |
Rebleeding
In three trials (Hong 2002; Hou 2004; Jun 2006) rebleeding was reported. A significant reduction was observed among patients under antibiotic prophylaxis in overall rebleeding (RR 0.53, 95% CI 0.38 to 0.74), and rebleeding after up to seven days of follow‐up (RR 0.24, 95% CI 0.12 to 0.50).
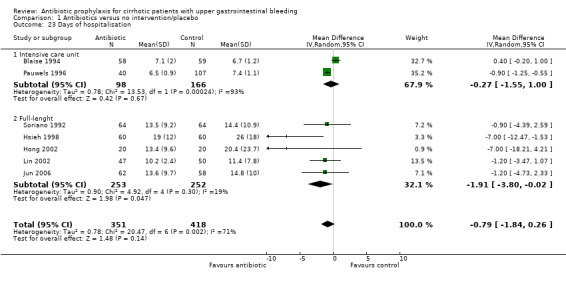
Days of hospitalisation
Seven trials reported hospitalisation length of stay, two trials during intensive care hospitalisation only (Blaise 1994; Pauwels 1996), and five trials during full‐length of hospitalisation (Soriano 1992; Hsieh 1998; Hong 2002; Lin 2002; Jun 2006). The overall effect of the intervention was not significant (MD ‐0.79 days, 95% CI ‐1.84 to 0.26) ‐ however, this estimate is highly influenced by the intensive care hospitalisation trials. When full‐length hospitalisation trials were considered alone, a beneficial effect from the intervention was observed (MD ‐1.91 days, 95% CI ‐3.80 to ‐0.02).
Antibiotic regimens versus other antibiotic regimens
Five trials compared different antibiotic regimens. No significant differences between regimens were observed for the outcomes under study. See Table 5 for more information.
4. Data on trials comparing two different regimens of antibiotic prophylaxis.
| Trial ID | Outcome | Experimental (n/N) | Control (n/N) | Relative Risk | 95% CI |
| Sabat 1998 | Mortality | Norfloxacin + ceftriaxone (1/24) | Norfloxacin (2/22) | 0.46 | 0.04 to 4.71 |
| Sabat 1998 | Bacterial infections | Norfloxacin + ceftriaxone (3/24) | Norfloxacin (4/22) | 0.69 | 0.17 to 2.73 |
| Sabat 1998 | Cost | Norfloxacin + ceftriaxone ‐ US $99.3 to 220.1 | Norfloxacin ‐ US $1.9 to 745.5 | ‐ | ‐ |
| Sabat 1998 | Drop‐outs | Norfloxacin + ceftriaxone (4/28) | Norfloxacin (6/28) | 0.67 | 0.21 to 2.11 |
| Gulberg 1999 | Mortality | Ceftriaxone 1g (0/40) | Ceftriaxone 2g (0/42) | Risk difference: 0.00 | ‐0.05 to 0.05 |
| Gulberg 1999 | Bacterial infections | Ceftriaxone 1g (1/40) | Ceftriaxone 2g (1/42) | 1.05 | 0.11 to 9.80 |
| Spanish Group 1998 | Bacterial infections (proven) | Norfloxacin 800mg (26/183) | Ofloxacin 400mg (27/182) | 0.96 | 0.58 to 1.58 |
| Spanish Group 1998 | Bacterial infections (suspected) | Norfloxacin 800mg (51/183) | Ofloxacin 400mg (53/182) | 0.96 | 0.69 to 1.32 |
| Lata 2005 | Mortality | Ampicillin and sulbactam 3g (12/21) | Norfloxacin 800 mg (7/25) | 2.04 | 0.98 to 4.23 |
| Fernandez 2006 | Mortality | Ceftriaxone 1g (8/54) | Norfloxacin 800 mg (6/57) | 1.41 | 0.52 to 3.79 |
| Fernandez 2006 | Mortality from bacterial infections | Ceftriaxone 1g (1/54) | Norfloxacin 800 mg (1/57) | 1.06 | 0.07 to 16.46 |
| Fernandez 2006 | Bacterial infections | Ceftriaxone 1g (6/54) | Norfloxacin 800 mg (5/57) | 1.27 | 0.41 to 3.94 |
Discussion
Summary of main results
This systematic review is an update of a previous systematic review and meta‐analysis published in 2002 (Soares‐Weiser 2002). From that date, new trials supporting the use of antibiotic prophylaxis on cirrhotic patients with upper gastrointestinal haemorrhage became available. Six new trials ‐ four comparing antibiotics against no‐intervention, and two comparing different antibiotic regimens ‐ were included in this update.
The most clinically relevant outcomes assessed in this review were mortality, mortality from bacterial infections, bacterial infections, and rebleeding. The inclusion of new trials did not modify a previously observed beneficial effect of antibiotic prophylaxis on mortality and bacterial infections. Rebleedingwas included as a new secondary outcome as it is an important outcome in clinical practice. We observed a statistically significant beneficial effect of the intervention on rebleeding. However, studies assessing rebleeding are still scarce and our presented results are based on only three trials. To confirm this observation, more data are required. In this updated review, antibiotic prophylaxis reduced the hospitalisation length of stay, but not the time in critical care.
The effects observed were more robust for prevention of bacterial infections, which remained significant after sensitivity analysis and TSA. This could be explained by the fact that all the trials included were designed and powered to evaluate this outcome.
The evolution of the intervention goes from non‐absorbable antibiotics (one trial), to quinolones (five trials), and to more recent cephalosporins (three trials), but there is no solid evidence to prefer one antibiotic regimen over the other. This was also observed in trials exploring several antibiotic regimens simultaneously. Use of quinolones was first explored by Soriano 1992 and quinolones have been broadly used since then, despite rising concerns of a potential reduction of their effects due to bacterial resistance. However, considering that bacterial resistance pattern vary by location, use of quinolones for antibiotic prophylaxis will have to be assessed in specific local settings.
Overall completeness and applicability of evidence
The current evidence to support antibiotic prophylaxis is based on twelve randomised trials, and, except for America and Africa, the intervention has been assessed in heterogeneous populations, providing external validity to this review. This review aimed to evaluate the effects of antibiotic prophylaxis on bacterial infections and mortality, and although a beneficial effect was observed, caution should be exerted when interpreting mortality results, since all included trials were not specifically designed to evaluate this outcome. Moreover, the significance of the observation could not be confirmed in trial sequential analysis (Wetterslev 2008; Brok 2008; Thorlund 2009).
Several issues were not answered in this review. Adverse events, quality of life, and the economic impact of the intervention were not explored in the trials included, remaining important areas of uncertainty and requiring further data to establish an evidence‐based conclusion.
Quality of the evidence
This review included 1891 cirrhotic patients with upper gastrointestinal bleeding; 1241 of them participated in randomised trials comparing antibiotic prophylaxis versus no intervention or placebo, and the remaining 650 participants in trials comparing different antibiotic prophylactic regimens. All trials presented methodological weaknesses and should be considered at risk of bias. Lack of blinding and lack of proper sample size calculations were the most common sources of bias. Although less subjective outcomes were considered in this review (mortality, mortality from bacterial infections, confirmed bacterial infections, and rebleeding) the influence of these sources of bias cannot be determined precisely.
Potential biases in the review process
All trials included in this review presented incomplete data to adequately assess their methodological strength and usefulness. Several attempts were made to contact original authors, but only in few cases an answer was obtained.
Agreements and disagreements with other studies or reviews
The current treatment guidelines consider antibiotic prophylaxis as standard of care (Garcia‐Tsao 2007; Bosch 2008; Garcia‐Tsao 2009) based on the beneficial effects reported on mortality. However, this effect is less well‐supported compared to the effect of prophylaxis to prevent bacterial infections. The information provided in this review will help the practitioner to weight the most important effects from antibiotic prophylaxis.
Authors' conclusions
Implications for practice.
The use of prophylactic antibiotics in patients with cirrhosis and upper gastrointestinal bleeding significantly reduce bacterial infections and seems to reduce all‐cause mortality, bacterial infection mortality, incidence of rebleeding events, and length of hospitalisation. These benefits were observed indistinctly of the antibiotic assessed. No specific antibiotic regimen can be recommended over another.Thus, antibiotic selection should be made considering local conditions such as bacterial resistance profile and treatment cost.
Implications for research.
The sensitivity analysis and the TSA, in addition to the meta‐analysis, demonstrate the robustness of data regarding antibiotic prophylaxis to prevent bacterial infections. However, data are not as conclusive concerning the other outcomes. Considering the benefits of antibiotic prophylaxis for bacterial infection prevention it will seem unwise to further conduct trials with placebo or no intervention as comparators, although specific conditions in clinical settings could justify their conduct.
The information regarding the benefits and harms from different antibiotic regimens is still scarce. Critical information such as the differential impact of each regimen over quality of life or their pharmaco‐economical advantages are still unclear and require further investigation.
Future research must include adequate sample size calculations and proper blinding processes. Additionaly, information about other significant outcomes, particularly adverse events, should also be considered in the future. Finally, trials should follow the recommended guidelines for the reporting of clinical trials (CONSORT ‐ Consolidated Standards of Reporting Trials: www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2010 | New citation required but conclusions have not changed | Six new trials were added to the review. |
| 29 June 2010 | New search has been performed | This review was updated: the list of authors was changed, six new trials were included, the Background section was changed, the Methods section was modified according the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009), the discussion was modified. The primary outcomes were arranged to include quality of life and adverse events as primary outcomes in concordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). |
Acknowledgements
We thank Dimitrinka Nikolova and Christian Gluud of The Cochrane Hepato‐Biliary Group for ongoing support for this review. Thanks to J. Fernandez, P. Gines, A. Rimola, N. Rolando, and A. Zacharof, who kindly supplied additional information on their own trials.
We would like thank M Brezis, R Tur‐Kaspa, and L Leibovici who participated in the previous version of this review (Soares‐Weiser 2002). Their work was supported by the Tel Aviv University, Hadassah University Hospital ‐ Mount Scopus, and Rabin Medical Center ‐ Beilinson Campus, (Israel). The Danish Medical Research Council's Grant on Getting Research into Practice (GRIP), Copenhagen Hospital Corporation's Research Grant on Getting Research into Practice (GRIP), and The 1991 Pharmacy Foundation (Denmark
Peer Reviewers: Wolfgang Fleig, Germany; Gennaro D'Amico, Italy. Contact Editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search Strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | June 2010 | (antibiotic* OR antibacteri*) AND (bleed* OR hemorr* or haemorr*) AND cirrho* |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 2, 2010 | #1 MeSH Descriptor antibiotic‐prophylaxis explode all trees in MeSH products #2 antibiotic* in All Fields in all products #3 antibacteri* NEAR prophyl* in All Fields in all products #4 (#1 OR #2 OR #3) #5 MeSH descriptor Gastrointestinal Hemorrhage explode all trees in MeSH products #6 bleed* in All Fields in all products #7 haemorr* in All Fields in all products #8 hemorr* in All Fields in all products #9 (#5 OR #6 OR #7 OR #8) #10 MeSH Descriptor Liver Cirrhosis explode all tress in MeSH products #11 cirrho* in All Fields in all products #12 (#10 OR #11) #13 (#4 AND #9 AND #12) |
| MEDLINE (Ovid SP) | 1950 to June 2010 | #1 explode antibiotic‐prophylaxis /All subheadings #2 antibiotic* prophyl* #3 antibiotic* pre *#4 #1 or #2 or #3 #5 explode liver‐cirrhosis /All subheadings #6 liver cirrho* #7 hepatic cirrho* #8 liver fibro* #9 #5 or #6 or #7 or #8 #10 explode gastrointestinal‐hemorrhage /All subheadings #11 gastr* hemorrhage #12 gastr* haemorrhage #13 gastr* bleeding #14 #10 or #11 or #12 or #13 #15 random* or blind* or placebo* or meta‐analysis #16 #4 and #9 and #14 and #15 |
| EMBASE (Ovid SP) | 1980 to June 2010 | #1 explode “antibiotic‐prophylaxis”/all subheadings #2 antibiotic* #3 antibacteri* prophy* #4 #1 or #2 or #3 #5 explode “gastrointestinal‐hemorrhage”/all subheadings #6 hemorr* #7 haemorr* #8 bleed* #9 #5 or #6 or #7 or #8 #10 explode “liver‐cirrhosis”/all subheadings #11 cirrho* #12 #10 or #11 #13 #4 and #9 and #12 #14 random* or blind* or placebo* or meta‐analysis #13 #13 and #14 |
| Science Citation Index EXPANDED (http://apps.isiknowledge.com) | 1945 to June 2010 | #1 TS=(antibiotic* OR antibacteri* prophyl*) #2 TS=(bleed* or hemorr* or haemorr*) #3 TS=(cirrho*) #4 #3 AND #2 AND #1 #5 TS=(random* or blind* or placebo* or meta‐analysis) #6 #5 AND #4 |
Data and analyses
Comparison 1. Antibiotics versus no intervention/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 12 | 1241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.63, 0.98] |
| 2 Mortality from bacterial infections | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.97] |
| 3 Bacterial infections | 12 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.27, 0.49] |
| 4 Bacteremia | 9 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.15, 0.40] |
| 5 Pneumonia | 9 | 1041 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.75] |
| 6 Spontaneous bacterial peritonitis | 8 | 890 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.15, 0.57] |
| 7 Urinary tract infections | 9 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.13, 0.41] |
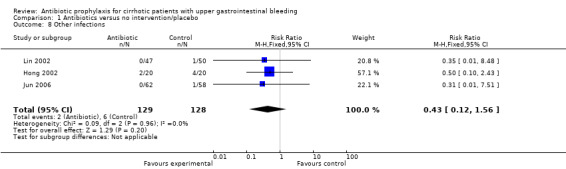
| 8 Other infections | 3 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.56] |
| 9 Drop outs before end of study | 8 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.76, 1.51] |
| 10 Mortality according to trial sample size | 12 | 1241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.63, 0.98] |
| 10.1 Sample size smaller than 101 patients | 5 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.44, 1.15] |
| 10.2 Sample size larger than 100 patients | 7 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.03] |
| 11 Bacterial infections according to trial sample size | 12 | 1241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.30, 0.46] |
| 11.1 Sample size smaller than 101 patients | 5 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.29, 0.62] |
| 11.2 Sample size larger than 100 patients | 7 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.27, 0.46] |
| 12 Mortality sensitivity analysis worst‐best case | 8 | 978 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.04, 2.02] |
| 13 Mortality sensitivity analysis best‐worst case | 8 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.38, 0.60] |
| 14 Mortality from bacterial infections sensitivity analysis worst‐best case | 5 | 708 | Risk Ratio (M‐H, Random, 95% CI) | 3.30 [1.43, 7.62] |
| 15 Mortality from bacterial infections sensitivity analysis best‐worst case | 5 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.06, 0.31] |
| 16 Bacterial infections sensitivity analysis worst‐best case | 8 | 978 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.63, 0.93] |
| 17 Bacterial infections sensitivity analysis best‐worst case | 8 | 969 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.16, 0.43] |
| 18 Mortality according to the antibiotic used | 12 | 1241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.63, 0.98] |
| 18.1 Quinolones | 5 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.27] |
| 18.2 Quinolones + beta‐lactams | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.31] |
| 18.3 Cephalosporins | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.46, 1.11] |
| 18.4 Other antibiotics | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.50, 1.17] |
| 19 Mortality from bacterial infections according to the antibiotic used | 6 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.97] |
| 19.1 Quinolones | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.18, 3.34] |
| 19.2 Quinolones + beta lactam | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.08, 5.80] |
| 19.3 Cephalosporins | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.10, 1.95] |
| 19.4 Others | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.15] |
| 20 Bacterial infections according to the antibiotic used | 12 | 1241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.29, 0.45] |
| 20.1 Quinolones | 5 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.18, 0.39] |
| 20.2 Quinolones + beta‐lactams | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.23, 0.62] |
| 20.3 Cephalosporins | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.48] |
| 20.4 Others | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.41, 0.81] |
| 21 Rebleeding | 3 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.38, 0.74] |
| 22 Early rebleeding (up to 7 days) | 3 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.12, 0.50] |
| 23 Days of hospitalisation | 7 | 769 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.84, 0.26] |
| 23.1 Intensive care unit | 2 | 264 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.55, 1.00] |
| 23.2 Full‐lenght | 5 | 505 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐3.80, ‐0.02] |
1.1. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 2 Mortality from bacterial infections.
1.3. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 3 Bacterial infections.
1.4. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 4 Bacteremia.
1.5. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 5 Pneumonia.
1.6. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 6 Spontaneous bacterial peritonitis.
1.7. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 7 Urinary tract infections.
1.8. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 8 Other infections.
1.9. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 9 Drop outs before end of study.
1.10. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 10 Mortality according to trial sample size.
1.11. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 11 Bacterial infections according to trial sample size.
1.12. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 12 Mortality sensitivity analysis worst‐best case.
1.13. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 13 Mortality sensitivity analysis best‐worst case.
1.14. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 14 Mortality from bacterial infections sensitivity analysis worst‐best case.
1.15. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 15 Mortality from bacterial infections sensitivity analysis best‐worst case.
1.16. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 16 Bacterial infections sensitivity analysis worst‐best case.
1.17. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 17 Bacterial infections sensitivity analysis best‐worst case.
1.18. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 18 Mortality according to the antibiotic used.
1.19. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 19 Mortality from bacterial infections according to the antibiotic used.
1.20. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 20 Bacterial infections according to the antibiotic used.
1.21. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 21 Rebleeding.
1.22. Analysis.

Comparison 1 Antibiotics versus no intervention/placebo, Outcome 22 Early rebleeding (up to 7 days).
1.23. Analysis.
Comparison 1 Antibiotics versus no intervention/placebo, Outcome 23 Days of hospitalisation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blaise 1994.
| Methods | Data collection: 09/1990 to 01/1992. Exclusion from analysis: patients with no oesophageal varices or infected. Follow‐up period: 14 days. | |
| Participants | France Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage, without infection, or treatment with antibiotics in the previous two weeks. Etiology of bleeding: oesophageal varices. Initial infection assessment: chest X‐ray, blood count, urine/sputum/blood/ascites culture. Endoscopy scheduled within 12 h after enrolment. Sclerotherapy performed together with endoscopy to stop bleeding. Child‐Pugh class A/B/C: 0/20/7. | |
| Interventions | Experimental: intravenous + oral ofloxacin, 400 mg/day, 10 days; amoxicillin + clavulanic acid (bolus, 1g) before each endoscopy procedure. Control: no antibiotic prophylaxis. |
|
| Outcomes | Prevent bacterial infections. | |
| Notes | Authors were contacted for the first version of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Missing data are balanced in number across the intervention groups. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The excluded participants are balanced and with a justifiable reason. |
| Sample calculation? | High risk | No sample calculation was done. |
Fernandez 2006.
| Methods | Data collection 02/2000 to 04/2004. Exclusion from analysis: occult infection (positive blood cultures obtained prior to randomisation) and less than two signs of liver failure (Norfloxacin group 6/63; Ceftriaxone group 7/61). Follow‐up period: 10 days. | |
| Participants | Spain Multicenter trial on hospitalised patients in patients with cirrhosis and upper gastrointestinal haemorrhage. Etiology of bleeding: portal hypertension related in 77% of participants. Initial infection assessment: blood cultures, ascitic fluid polymorphonuclear count, urine sediment and culture, chest X‐ray. Emergency endoscopy within 24 h after onset of the haemorrhage, plus somatostatin or terlipressin on portal hypertension related haemorrhage. Sclerotherapy or banding was performed to stop bleeding. Chil‐Pugh class A/B/C: 0/52/59. |
|
| Interventions | Experimental: intravenous ceftriaxone 1g per day for 7 days. Control: oral norfloxacin 400 mg b.i.d. for 7 days. |
|
| Outcomes | Prevention of bacterial infections. | |
| Notes | The authors were contacted via e‐mail and response was received (28‐June‐2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a random number software. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Missing data are balanced in number across the intervention groups. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The excluded participants are balanced and with a justifiable reason. |
| Sample calculation? | Low risk | The sample was calculated considering the expected incidence of proved and possible infections. |
Gulberg 1999.
| Methods | Date of collection: no information provided. Exclusion from analysis: no information provided. Follow‐up period: 7 days. | |
| Participants | Germany
Cirrhotic patients with upper gastrointestinal haemorrhage, without infection, or treatment with antibiotics in the previous week, who underwent TIPS.
Etiology of bleeding: no information provided.
Initial infection assessment: fever, white blood cells, C‐reactive protein. Child‐Pugh class A/B/C: 29/39/14. |
|
| Interventions | Experimental: intravenous ceftriaxone, 1g, single dose before TIPS. Control: intravenous ceftriaxone, 2g, single dose before TIPS. |
|
| Outcomes | Bacterial infection after TIPS procedure. | |
| Notes | Authors were contacted for the first version of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | High risk | The report support the intervention effects on surrogates of infection. |
| Intention to treat analysis? | Unclear risk | No information provided. |
| Sample calculation? | High risk | No sample calculation procedure. |
Hong 2002.
| Methods | Data collection: 12/1998 to 9/2001. Exclusion from analysis: death or surgery within 24 h. Follow‐up period: 30 days. | |
| Participants | Korea Hospitalised cirrhotic patients with oesophageal variceal bleeding, without infection or previous use of antibiotics. Initial infection assessment: physical examination, white blood cells count, liver function test, ascitic fluid analysis and culture. All patients were enrolled after emergency endoscopic oesophageal variceal ligation. Child‐Pugh class: 8.8 ± 1.6 vs 9.1 ± 1.4. |
|
| Interventions | Experimental: intravenous ciprofloxacin 200 mg b.i.d. for 3 days. Control: no antibiotic prophylaxis. |
|
| Outcomes | Prevention of bacterial infections. | |
| Notes | The authors were contacted via e‐mail and no response was received (23‐June‐2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No information provided. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Unclear risk | No information provided. |
| Intention to treat analysis? | Unclear risk | No information provided. |
| Sample calculation? | Unclear risk | No information provided. |
Hou 2004.
| Methods | Data collection: 01/2001 to 02/2003. Exclusion from analysis: positive initial bacteriological sample. Follow‐up period: until death or 3 months. | |
| Participants | Taiwan Hospitalised patients with endoscopy‐proven gastroesophageal variceal bleeding without signs of infection. Etiology of bleeding: endoscopy‐proven gastroesophageal variceal bleeding. Endoscopic procedure was completed within 24 h of admission or bleeding onset. Endoscopic variceal ligation or sclerotherapy was preceded by vasoactive agent or balloon tamponade. Child‐Pugh class (A/B/C): 20/64/27. |
|
| Interventions | Experimental: intravenous ofloxacin 200 mg b.i.d. for 2 days followed by oral ofloxacin 200 mg b.i.d. for 5 days. Control: no antibiotic prophylaxis. |
|
| Outcomes | Rebleeding rate. | |
| Notes | The authors were contacted via e‐mail and no response was received (23‐June‐2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a random number software. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Missing data are balanced in number across the intervention groups. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The exclusion of the patients was not based on an exclusion rule. |
| Sample calculation? | Low risk | The sample was calculated considering the expected rebleeding rate. |
Hsieh 1998.
| Methods | Data collection: 07/95 to 07/96. Exclusion from analysis: no information. Follow‐up period: 30 days. | |
| Participants | Taiwan Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage, without infection, or treatment with antibiotics in the previous two weeks. Etiology of bleeding: oesophageal or gastric varices, peptic ulcers, others. Initial infection assessment: chest X‐ray, blood count, urine/blood/ascites culture. Source of bleeding determined by endoscopy scheduled within 24 h after enrolment. Sclerotherapy, or band ligation performed within 48 h after enrolment. Child‐Pugh class A/B/C: 11/64/45. | |
| Interventions | Experimental: oral ciprofloxacin, 1 g/day, 7 days. Control: placebo. |
|
| Outcomes | Prevention of bacterial infections. | |
| Notes | Authors were contacted for the first version of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | Low risk | All randomised participants were included in the analysis. |
| Sample calculation? | High risk | No sample calculation procedure. |
Jun 2006.
| Methods | Data collection: 01/2010 to 12/2004. Exclusion from the analysis: past history of previous gastroesophageal variceal bleeding, or surgical or endoscopic treatment of gastroesophageal varices; or antibiotics use in the previous two weeks; or terminal illness (or non hepatic malignancy); or other causes of gastrointestinal bleeding. Follow‐up period: 22 ± 14 months. | |
| Participants | Korea Hospitalised cirrhotic patients with endoscopy‐proven bleeding from oesophageal or gastric varices, with no signs of infection at admission. Etiology of the bleeding: oesophageal or gastric varices. Initial infection assessment: complete blood cell count, chest X‐ray, urine analysis and culture, blood culture, and ascitic fluid neutrophil count with culture. Endoscopy procedure was performed within 12h of admission to emergency room. Before endoscopy octreotide was used, followed by endoscopic treatment (variceal ligation or sclerotherapy). Child‐Pugh class A/B/C: 8.7 ± 1.9 and 8.3 ± 2.1. |
|
| Interventions | Experimental: intravenous cefotaxime 2 g t.i.d for 7 days. Control: no antibiotic prophylaxis. |
|
| Outcomes | Rebleeding rate. | |
| Notes | The authors were contacted via e‐mail and no response was received (23‐June‐2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a table of random numbers. |
| Allocation concealment? | Low risk | The concealment was done using numbered envelopes. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | High risk | The proportion of missing outcomes is higher in the no prophylaxis group. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The exclusion of the patients was not based on an exclusion rule. |
| Sample calculation? | Low risk | The sample was calculated considering the expected rebleeding rate. |
Lata 2005.
| Methods | Data collection: 05/2001 to 11/2003. Exclusion from analysis: Past history of previous variceal bleeding within 3 months. Follow‐up period: 42 days. |
|
| Participants | Czech Republic. Hospitalised (intensive care unit) patients with endoscopy proven oesophageal varices. Etiology of bleeding: oesophageal varices. Initial infection assessment: aerobic blood culture, anaerobic blood culture, perianal smear, urine examination, throat smear, ascites examination (>250 neutrophils/mL). The endoscopic treatment was performed within 3 h after admission to intensive care unit and was preceded by the administration of terlipressin. Schlerotherapy performed together with endoscopy to stop bleeding. Child‐Pugh class A/B/C: 4/19/23. |
|
| Interventions | Experimental:intravenous ampicillin/sulbactam 1.5 g b.i.d. for 7 days. Control: oral or through nasogastric tube norfloxacin 400 mg b.i.d. for 7 days. |
|
| Outcomes | Early and late mortality. | |
| Notes | The authors were contacted via e‐mail and no response was received (23‐June‐2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a system of accidental numbers. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | Unclear risk | No information provided. |
| Sample calculation? | High risk | No sample calculation procedure. |
Lin 2002.
| Methods | Data collection: 7/1999 to 8/2000. Exclusion from analysis: life expectancy less than 7 days, fever or infection on entry, positive bacterial culture on entry, and previous use of antibiotics within 2 weeks prior to admission. Initial infection assessment: blood culture, urine culture cell, chest X‐ray, sputum culture, and ascitic fluid culture. Follow‐up period: 7 days. | |
| Participants | Taiwan Hospitalised cirrhotic patients with endoscopy proven upper gastrointestinal bleeding, without infection or previous use of antibiotics. Endoscopy was performed 12 h within of hospitalisation. The haemostatic procedure include band ligation plus somastotatin, sandostatin or terlipressin (variceal bleeding) or endoscopic injection of water or diluted epinephrine (peptic ulcer bleeding). Child‐Pugh class A/B/C: 27/50/20. |
|
| Interventions | Experimental: intravenous cefazolin 1 g tid during 3 days and then shift to oral cephalexin 500 mg qid for 4 days. Control: no antibiotic prophylaxis. |
|
| Outcomes | Prevention of bacterial infections. | |
| Notes | The authors were contacted via e‐mail and no response was received (23‐June‐2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Missing data are balanced in number across the intervention groups. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The exclusion of the patients was based an exclusion rule. |
| Sample calculation? | High risk | No sample calculation procedure. |
Pauwels 1996.
| Methods | Data collection: 12/89 to 03/92. Exclusion from analysis: infection on admission, undergoing surgery within 24 hs after admission, or death in the first 12 h. Follow‐up period: up to 10 days after stopping bleeding (four weeks). | |
| Participants | France
Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage, without infection, or treatment with antibiotics in the previous week.
Etiology of bleeding: oesophageal or gastric varices, gastric or duodenal ulceration, and others. Initial infection assessment: chest X‐ray, white blood cell count, urine/blood/ascites culture. Source of bleeding determined by endoscopy scheduled within 12 hs after enrolment. Emergency sclerotherapy performed for patients bleeding from varices. Child‐Pugh class A/B/C: 16/54/49. |
|
| Interventions | Experimental (Group III): intravenous + oral ciprofloxacin 400mg per day, amoxicillin‐clavulanic acid 3g per day, until three days after cessation of haemorrhage. Control (Group I and II): no antibiotic prophylaxis. |
|
| Outcomes | Prevention of bacterial infections. | |
| Notes | Two group of patients received no intervention and were combined for the purpose of this review. Authors were contacted for the first version of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a table of random numbers. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Missing data are balanced in number across the intervention groups. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The exclusion of the patients was based on an exclusion rule. |
| Sample calculation? | High risk | No sample calculation procedure. |
Rimola 1985.
| Methods | Data collection: no information provided. Exclusion from analysis: underwent surgery, or died within 24 hs after admission. Follow‐up period: up to 10 days after stopping bleeding (four weeks). | |
| Participants | Spain Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage, without infection, or treatment with antibiotics in the previous two weeks. Etiology of bleeding: oesophageal varices (128 patients), gastric haemorrhage ( 10 patients), peptic ulcers (8 patients). Initial infection assessment: chest X‐ray, white blood cell count, urine/blood/ascites culture. Source of bleeding determined by endoscopy scheduled within 24 hs after enrolment. Emergency sclerotherapy performed for patients bleeding from varices. Child‐Pugh class A/B/C: not reported. | |
| Interventions | Experimental (non‐absorbable antibiotics):
Group Ia ‐ oral gentamicin (200mg) + vancomycin (500 mg) + nystatin (10^6 UI) every six hs, until two days after cessation of haemorrhage.
Group Ib ‐ neomycin (1 gm) + colistin (1.5 x 10^6 UI) + nystatin (10^6 UI) every six hs, until two days after cessation of haemorrhage. Control (Group II): no antibiotic prophylaxis. |
|
| Outcomes | Prevent bacterial infections. | |
| Notes | The antibiotic therapy regimen was modified after inclusion of the first 40 patients in the experimental group because of budget constraints.
The two group of patients treated with antibiotics were combined for the purpose of this review. Authors were contacted for the first version of this review, and additional information was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using random number table. |
| Allocation concealment? | Low risk | Adequate, this item keeps as the first review. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | High risk | The proportion of missing outcomes is not balanced among the groups. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | High risk | The antibiotic therapy regimen was modified after inclusion of the first 40 patients in the experimental group because of budget constraints. |
| Intention to treat analysis? | High risk | The exclusion of the patients was based on an exclusion rule. |
| Sample calculation? | High risk | No sample calculation procedure. |
Rolando 1993.
| Methods | Data collection: no information provided. Exclusion from analysis: bleeding not related to variceal bleeding. Follow‐up period: 7 days. | |
| Participants | UK Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage and high risk of infection, without infection, or treatment with antibiotics in the previous two weeks. Etiology of bleeding: all patients had oesophageal varices. Endoscopic sclerotherapy was performed after randomisation. Initial infection assessment: chest X‐ray, white blood cell count, urine/blood/ascites culture. Child‐Pugh class A/B/C: not reported. | |
| Interventions | Experimental: intravenous imipenem + cilastin, 500 mg before and after the sclerotherapy. Control: intravenous dextrose‐saline solution. |
|
| Outcomes | Prevent bacterial infections. | |
| Notes | Authors were contacted for the first version of this review, and additional information was received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a computer‐based table of random numbers. |
| Allocation concealment? | Low risk | Patients assigned sequentially, sealed opaque envelopes used. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Reassons for missing data are based on the violation of the protocol. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Unclear risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The exclusion of the patients was based on an exclusion rule. |
| Sample calculation? | High risk | No sample calculation procedure. |
Sabat 1998.
| Methods | Data collection: 06/93 to 06/95. Exclusion from analysis: occult infection, or underwent surgery, or died within 24 hs after admission. Follow‐up period: up to three weeks. | |
| Participants | Spain Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage and high risk of infection, without infection, or treatment with antibiotics in the previous two weeks. Etiology of bleeding: no information. Initial infection assessment: chest X‐ray, white blood cell count, urine/blood/ascites culture. Source of bleeding determined by endoscopy scheduled within four hs after enrolment. Emergency sclerotherapy performed for patients bleeding from varices. Child‐Pugh class A/B/C: 4/31/11. | |
| Interventions | Experimental: oral norfloxacin 800 mg/day, during seven days plus intravenous ceftriaxone 2g/day the first three days. Control: oral norfloxacin 800 mg/day, seven days. |
|
| Outcomes | Prevent bacterial infections. | |
| Notes | The initial sample size was calculated to be 152 patients. An interim analysis was done after 1/3 of patients were included, a statistical significant result was found and the trial was stopped. Authors were contacted for the first version of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Reassons for missing data are based on the violation of the protocol. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | High risk | The trials was stopped due to economical differences and not due to primary outcome differences. |
| Intention to treat analysis? | High risk | The exclusion of the patients was based on an exclusion rule. |
| Sample calculation? | Unclear risk | The was calculated considering the primary outcome, but the trial was stopped considering differences in secondary outcomes. |
Selby 1994.
| Methods | Data collection: 08/89 to 12/91. Exclusion from analysis: no information provided. Follow‐up period: up to 24 hs. | |
| Participants | Australia Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage and high risk of infection, without infection, or treatment with antibiotics in the previous two weeks. Etiology of bleeding: no information provided. All patients underwent emergency section of sclerotherapy, followed by further sections in weekly intervals. Initial infection assessment: chest X‐ray, white blood cell count, urine/blood/ascites culture. Child‐Pugh class A/B/C: 8/18/13. | |
| Interventions | Experimental: intravenous cefotaxime, 1 g immediately before sclerotherapy. Control: no antibiotic prophylaxis. |
|
| Outcomes | Prevent bacterial infections. | |
| Notes | Authors were contacted for the first version of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a table of random numbers. |
| Allocation concealment? | Low risk | The treatment was contained in sealed envelopes. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Reassons for missing data are based on the violation of the protocol. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Unclear risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The exclusion of the patients was based on an exclusion rule. |
| Sample calculation? | High risk | No sample calculation procedure. |
Soriano 1992.
| Methods | Data collection: 08/89 to 06/91. Exclusion from analysis: underwent surgery, or died within 24 hs after admission. Follow‐up period: up to four weeks. | |
| Participants | Spain Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage, without infection or treatment with antibiotics in the previous two weeks. Etiology of bleeding: oesophageal varices, gastric haemorrhage, peptic ulcers, and others. Initial infection assessment: chest X‐ray, white blood cell count, urine/blood/ascites culture. Source of bleeding determined by endoscopy scheduled within four hs after enrolment. Emergency sclerotherapy performed for patients bleeding from varices. Child‐Pugh class A/B/C: 40/55/24. | |
| Interventions | Experimental: oral norfloxacin 800 mg/day during seven days. Control: no antibiotic prophylaxis. |
|
| Outcomes | Prevent bacterial infection. | |
| Notes | Authors were contacted for the first version of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a table of random numbers. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | Reassons for missing data are based on the violation of the protocol and are balanced across the groups. |
| Free of selective reporting? | Low risk | The report include all the expected outcomes. |
| Free of other bias? | Low risk | The trial appears to be free of other sources of bias. |
| Intention to treat analysis? | High risk | The exclusion of the patients was based on an exclusion rule. |
| Sample calculation? | High risk | No sample calculation procedure. |
Spanish Group 1998.
| Methods | Data collection: no information provided. Exclusion from analysis: no information provided. Follow‐up period: first 10 days of the bleeding episode. | |
| Participants | Spain Cirrhotic patients with upper gastrointestinal haemorrhage. Etiology of bleeding: no information provided. Initial infection assessment: no information provided. Source of bleeding determined by: no information. Child‐Pugh class A/B/C: no information provided. | |
| Interventions | Experimental: oral norfloxacin, 800 mg/day, five days. Control: oral ofloxacin, 400 mg/day, five days. |
|
| Outcomes | Prevent bacterial infections. | |
| Notes | Data extracted from an abstract, no publication available. Authors were contacted for the first version of this review, and additional information was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information provided. |
| Allocation concealment? | Unclear risk | No information provided. |
| Blinding? All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Insufficient information to permit judgement. |
| Free of selective reporting? | Unclear risk | Insufficient information to permit judgement. |
| Free of other bias? | Unclear risk | Insufficient information to permit judgement. |
| Intention to treat analysis? | Unclear risk | No information provided. |
| Sample calculation? | Unclear risk | No information provided. |
Zacharof 1997.
| Methods | Data collection: no information provided. Exclusion from analysis: no information provided. Follow‐up period: no information provided. | |
| Participants | Greece Hospitalised cirrhotic patients with upper gastrointestinal haemorrhage. Etiology of bleeding: no information provided. Source of bleeding determined by emergency endoscopy. Child‐Pugh class A/B/C: not information provided. | |
| Interventions | Experimental: oral ciprofloxacin 500 mg/day during seven days. Control: no antibiotic prophylaxis. |
|
| Outcomes | Prevent bacterial infections. | |
| Notes | Data extracted from an abstract, and confirmed by the authors. Authors were contacted for the first version of this review, and additional information was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The sequence was generated using a table of random numbers. |
| Allocation concealment? | Low risk | Sequentially administered by a pharmacist not involved in the trial. |
| Blinding? All outcomes | High risk | Not a blinded trial. |
| Incomplete outcome data addressed? All outcomes | Low risk | The report include all the expected outcomes. |
| Free of selective reporting? | Unclear risk | Insufficient information to permit judgement. |
| Free of other bias? | Unclear risk | Insufficient information to permit judgement. |
| Intention to treat analysis? | Unclear risk | No information provided. |
| Sample calculation? | Unclear risk | No information provided. |
b.i.d. = twice (two times) a day. hs = hours.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gines 1990 | Spain Randomised clinical trial. Through communication with the first author it was clarified that the patients did not have upper gastrointestinal bleeding. |
| Goulis 1998 | UK Observational study. Not a randomised trial. |
| Henrion 1992 | Belgium Controlled clinical study. Inadequate allocation concealment, alternate method. |
| Husova 2005 | Czech Republic Observational study. Not a randomised trial. |
| Novella 1997 | Spain Randomised clinical trial. Through communication with the first author it was clarified that the patients did not have upper gastrointestinal bleeding. |
| Pohl 2004 | Germany Retrospective cohort study. |
| Pulanic 1989 | Yugoslavia Randomised clinical trial. No clinical outcomes, only laboratory variables after antibiotic prophylaxis. |
| Wilbur 2005 | Canada Retrospective cohort study. |
| Zhao 2002 | China Not a randomised trial. |
RCT ‐ randomised clinical trial. CCT ‐ controlled clinical trial.
Differences between protocol and review
During the update process, the condition assessed changed from gastrointestinal bleeding to upper gastrointestinal bleeding. Rebleeding was included as a secondary outcome, and quality of life and adverse events were included as primary outcomes.
Test of interaction and TSA were included.
Contributions of authors
Norberto C. Chavez‐Tapia: update co‐ordination, data collection, data management, data interpretation, and review writing. Tonatiuh Barrientos‐Gutierrez: data collection, data management, data analysis, and review writing. Felix I Tellez‐Avila: data analysis and interpretation. Karla Soares‐Weiser: conception and co‐ordination of the previous version of this review, provided general advice on this updated review. Misael Uribe: data interpretation, provided general advice on the review, and secured funding for the review.
Sources of support
Internal sources
Medica Sur Clinic & Foundation, Mexico.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Blaise 1994 {published data only}
- Blaise M, Pateron D, Trinchet JC, Levacher S, Beaugrand M, Pourriat JL. Systemic antibiotic therapy prevents bacterial infection in cirrhotic patients with gastrointestinal hemorrhage. Hepatology 1994;20(1 Pt 1):34‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fernandez 2006 {published data only}
- Fernandez J, Ruiz del Arbol L, Gomez C, Durandez R, Serradilla R, Guarner C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006;131(4):1049‐56; quiz 1285. [DOI] [PubMed] [Google Scholar]
Gulberg 1999 {published data only}
- Gulberg V, Deibert P, Ochs A, Rossle M, Gerbes AL. Prevention of infectious complications after transjugular intrahepatic portosystemic shunt (TIPS) in patients with cirrhosis of the liver with a single dose of ceftriaxone (AASLD Abstract). Hepatology 1997;26(4):518A. [PubMed] [Google Scholar]
- Gulberg V, Deibert P, Ochs A, Rossle M, Gerbes AL. Prevention of infectious complications after transjugular intrahepatic portosystemic shunt in cirrhotic patients with a single dose of ceftriaxone. Hepato‐Gastroenterology 1999;46(26):1126‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Hong 2002 {published data only}
- Hong SN, Kim BJ, Lee SY, Lee CY, Ryu MK, Choi MS, et al. [Prospective randomized trial of intravenous ciprofloxacin for prevention of bacterial infection in cirrhotic patients with esophageal variceal bleeding]. Taehan Kan Hakhoe Chi 2002;8(3):288‐96. [PubMed] [Google Scholar]
Hou 2004 {published data only}
- Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology 2004;39(3):746‐53. [DOI] [PubMed] [Google Scholar]
Hsieh 1998 {published data only}
- Hsieh W, Lin H, Hwang S, Hou M, Lee F, Chang F, et al. The effect of ciprofloxacin in the prevention of bacterial infection in patients with cirrhosis after upper gastrointestinal bleeding. American Journal of Gastroenterology 1998;93(6):962‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jun 2006 {published data only}
- Jun CH, Park CH, Lee WS, Joo YE, Kim HS, Choi SK, et al. Antibiotic prophylaxis using third generation cephalosporins can reduce the risk of early rebleeding in the first acute gastroesophageal variceal hemorrhage: a prospective randomized study. Journal of Korean Medical Science 2006;21(5):883‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lata 2005 {published data only}
- Lata J, Jurankova J, Husova L, Senkyrik M, Dite P, Dastych M, et al. Variceal bleeding in portal hypertension: bacterial infection and comparison of efficacy of intravenous and per‐oral application of antibiotics ‐ a randomized trial. European Journal of Gastroenterology & Hepatology 2005;17(10):1105‐10. [DOI] [PubMed] [Google Scholar]
Lin 2002 {published data only}
- Lin YT, Lo GH, Lai KH, Chen TA, Lin WJ. Prophylactic antibiotics in cirrhotics with upper gastrointestinal hemorrhage: a prospective, controlled trial. Zhonghua Yi Xue Za Zhi (Taipei) 2002;65(8):365‐71. [PubMed] [Google Scholar]
Pauwels 1996 {published data only}
- Pauwels A, Mostefa Kara N, Debenes B, Degoutte E, Levy VG. Systemic antibiotic prophylaxis after gastrointestinal hemorrhage in cirrhotic patients with a high risk of infection. Hepatology 1996;24(4):802‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rimola 1985 {published and unpublished data}
- Rimola A, Bory F, Teres J, Perez‐Ayuso R, Arroyo V, Rodes J. Oral, nonabsorbable antibiotics prevent infection in cirrhotics with gastrointestinal hemorrhage. Hepatology 1985;5(3):463‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rolando 1993 {published and unpublished data}
- Rolando N, Gimson A, Philpott‐Howard J, Sahathevan M, Casewell M, Fagan E, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: role of antibiotic prophylaxis. Journal of Hepatology 1993;18(3):290‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sabat 1998 {published data only}
- Sabat M, Kolle L, Ortiz J, Pamplona J, Novella MT, Villanueva C, et al. Parental antibiotic prophylaxis in cirrhotic patients with gastrointestinal bleeding (AASLD abstract). Hepatology 1996;24(4 Pt 2):448A. [DOI] [PubMed] [Google Scholar]
- Sabat M, Kolle L, Soriano G, Ortiz J, Pamplona J, Novella MT, et al. Parenteral antibiotic prophylaxis of bacterial infections does not improve cost‐efficacy of oral norfloxacin in cirrhotic patients with gastrointestinal bleeding. American Journal of Gastroenterology 1998;93(12):2457‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Selby 1994 {published data only}
- Selby WS, Norton ID, Pokorny CS, Benn RA. Bacteremia and bacterascites after endoscopic sclerotherapy for bleeding esophageal varices and prevention by intravenous cefotaxime: a randomized trial. Gastrointestinal Endoscopy 1994;40(6):680‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Soriano 1992 {published data only}
- Soriano G, Guarner C, Tomas A, Villanueva C, Torras X, Gonzalez D, et al. Norfloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage (see comments). Gastroenterology 1992;103(4):1267‐72. [DOI] [PubMed] [Google Scholar]
- Soriano G, Guarner G, Tena F, Villanueva C, Fabrega E, Gonzalez D, et al. Prophylaxis of infections with norfloxacin in cirrhotic patients with gastrointestinal bleeding (EASL abstract). Journal of Hepatology 1990;11(Suppl 2):S56. [MEDLINE: ] [Google Scholar]
Spanish Group 1998 {published data only}
- Spanish Group for the Study of Bacterial Infections in Cirrhosis. Norfloxacin versus ofloxacin in the prophylaxis of infection in cirrhotic patients with gastrointestinal hemorrhage. Journal of Hepatology 1998;28(Suppl 1):80. [Google Scholar]
Zacharof 1997 {published and unpublished data}
- Zacharof A, Petrogiannopoulos C, Flevaris C, Deliousis A, Poulikakos J. Ciprofloxacin prevents bacterial infection in cirrhotics with gastrointestinal hemorrhage (EASL abstract). Journal of Hepatology 1997;26(Suppl 1):12. [Google Scholar]
- Zacharof A, Petrogiannopoulos C, Soutos D, Katsaros D, Zacharof H. Bacterial infection is prevented by ciprofloxacin in cirrhotics with gastrointestinal hemorrhage (Abstract). Gut 1997;41(Suppl 3):A189. [Google Scholar]
References to studies excluded from this review
Gines 1990 {published and unpublished data}
- Gines P, Rimola A, Planas R, Vargas V, Forne M, Miranda ML, et al. Norfloxacin prevents spontaneous bacterial peritonitis (SBP) in cirrhosis. Final results of a multicenter double‐blind placebo controlled study (EASL abstract). Journal of Hepatology 1990;11(Suppl2):S26. [DOI] [PubMed] [Google Scholar]
- Gines P, Rimola A, Planas R, Vargas V, Llach J, Salmeron JM, et al. Norfloxacin for prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhosis: results of a double‐blind, placebo‐controlled trial (AASLD Annual Meeting). Hepatology 1989;10(4):587. [DOI] [PubMed] [Google Scholar]
- Gines P, Rimola A, Planas R, Vargas V, Marco F, Almela M, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double‐blind, placebo‐controlled trial. Hepatology 1990;12:716‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goulis 1998 {published data only}
- Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology 1998;27(5):1207‐12. [DOI] [PubMed] [Google Scholar]
Henrion 1992 {published data only}
- Henrion J, Schapira M, Derue G, Heller FR. Prevention of bacterial infection using selective intestinal decontamination in patients with cirrhosis admitted to intensive care. Controlled study in 120 patients [Prevention de l'infection bacterienne par decontamination intestinale selective chez des patients cirrhotiques admis en soins intensifs Etude controlee chez 120 malades]. Acta Gastroenterologica Belgica 1992;55(4):333‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Husova 2005 {published data only}
- Husova L, Lata J, Husa P, Senkyrik M, Jurankova J, Dite P. Bacterial infection and acute bleeding from upper gastrointestinal tract in patients with liver cirrhosis. Hepato‐Gastroenterology 2005;52(65):1488‐90. [PubMed] [Google Scholar]
Novella 1997 {published data only}
- Novella M, Sola R, Soriano G, Andreu M, Gana J, Ortiz J, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology 1997;25(3):532‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ortiz J, Vila M, Soriano G, Minana J, Gana J, Mirelis B, et al. Infections caused by Escherichia coli resistant to norfloxacin in hospitalized cirrhotic patients. Hepatology 1999;29(4):1064‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pohl 2004 {published data only}
- Pohl J, Pollmann K, Sauer P, Ring A, Stremmel W, Schlenker T. Antibiotic prophylaxis after variceal hemorrhage reduces incidence of early rebleeding. Hepato‐Gastroenterology 2004;51(56):541‐6. [PubMed] [Google Scholar]
Pulanic 1989 {published data only}
- Pulanic R, Vrhovac B, Jereb B, Jokic N. Controlled trial of the prophylactic administration of antibiotics in sclerotherapy of esophageal varices. Journal of Chemotherapy 1989;1(4):261‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilbur 2005 {published data only}
- Wilbur K, Sidhu K. Antimicrobial therapy in patients with acute variceal hemorrhage. Canadian Journal of Gastroenterology 2005;19(10):607‐11. [DOI] [PubMed] [Google Scholar]
Zhao 2002 {published data only}
- Zhao C, Chen SB, Zhou JP, Xiao W, Fan HG, Wu XW, et al. Prognosis of hepatic cirrhosis patients with esophageal or gastric variceal hemorrhage: multivariate analysis. Hepatobiliary & Pancreatic Diseases International 2002;1(3):416‐9. [PubMed] [Google Scholar]
Additional references
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ (Clinical Research Ed.) 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustin 2009
- Augustin S, Muntaner L, Altamirano JT, Gonzalez A, Saperas E, Dot J, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clinical Gastroenterology and Hepatology 2009;7(12):1347‐54. [DOI] [PubMed] [Google Scholar]
Bernard 1999
- Bernard B, Grange JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta‐analysis. Hepatology 1999;29(6):1655‐61. [DOI] [PubMed] [Google Scholar]
Bosch 2008
- Bosch J, Berzigotti A, Garcia‐Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. Journal of Hepatology 2008;48(Suppl 1):S68‐S92. [DOI] [PubMed] [Google Scholar]
Bosch 2009
- Bosch J, Abraldes JG, Berzigotti A, Garcia‐Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nature Reviews Gastroenterology & Hepatology 2009;6(10):573‐82. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Burroughs 2009
- Burroughs AK, Triantos CK, O'Beirne J, Patch D. Predictors of early rebleeding and mortality after acute variceal hemorrhage in patients with cirrhosis. Nature Clinical Practice Gastroenterology & Hepatology 2009;6(2):72‐3. [DOI] [PubMed] [Google Scholar]
Chavez‐Tapia 2007
- Chavez‐Tapia NC, Torre‐Delgadillo A, Tellez‐Avila FI, Uribe M. The molecular basis of susceptibility to infection in liver cirrhosis. Current Medicinal Chemistry 2007;14(28):2954‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez 2002
- Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002;35(1):140‐8. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2007
- Garcia‐Tsao G, Sanyal A J, Grace N D, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46(3):922‐38. [DOI] [PubMed] [Google Scholar]
Garcia‐Tsao 2009
- Garcia‐Tsao G, Lim J K. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. American Journal of Gastroenterology 2009;104(7):1802‐29. [DOI] [PubMed] [Google Scholar]
Gluud 2001
- Gluud C. Alcoholic hepatitis: no glucocorticosteroids?. FALK Symposium 121. Steatohepatitis (NASH and ASH), 2001:322‐42. [Google Scholar]
Gluud 2010
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2010, Issue 7. Art. No.: LIVER.
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Colloboration, 2009. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ (Clinical Research Ed.) 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
NIEC 1988
- North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. The New England Journal of Medicine 1988;319(15):983‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2010 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2010.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux P J, Wetterslev J, Guyatt G, Ioannidis J P, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Soares‐Weiser 2002
- Soares‐Weiser K, Brezis M, Tur‐Kaspa R, Leibovici L. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002907] [DOI] [PubMed] [Google Scholar]


