Abstract
Background
A number of tocolytics have been advocated for the treatment of threatened preterm labour in order to delay birth. The rationale is that a delay in birth may be associated with improved neonatal morbidity or mortality. Nitric oxide donors, such as nitroglycerin, have been used to relax the uterus. This review addresses their efficacy, adverse effects and influence on neonatal outcome.
Objectives
To determine whether nitric oxide donors administered in threatened preterm labour are associated with a delay in birth, adverse effects or improved neonatal outcome.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 December 2013).
Selection criteria
Randomised controlled trials of nitric oxide donors administered for tocolysis.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data.
Main results
Twelve trials, including a total of 1227 women at risk of preterm labour, contributed data to this updated review. The methodological quality of trials was mixed; trials comparing nitric oxide donors with other types of tocolytics were not blinded and this may have had an impact on findings.
Three studies compared nitric oxide donors (glyceryl trinitrate (GTN)) with placebo. There was no significant evidence that nitric oxide donors prolonged pregnancy beyond 48 hours (average risk ratio (RR) 1.19, 95% confidence interval (CI) 0.74 to 1.90, two studies, 186 women), and although for most adverse effects there was no significant difference between groups, women in the active treatment group in one study were at higher risk of experiencing a headache. For infant outcomes there was no significant evidence that nitric oxide donors reduced the risk of neonatal death or serious morbidity (stillbirth RR 0.36, 95% CI 0.01 to 8.59, one study, 153 infants; neonatal death RR 0.43, 95% CI 0.06 to 2.89, two studies, 186 infants). One study, using a composite outcome, reported a reduced risk of serious adverse outcomes for infants in the GTN group which approached statistical significance (RR 0.29, 95% CI 0.08 to 1.00, 153 infants). Overall, these studies were underpowered to identify differences between groups for most outcomes.
When nitric oxide donors were compared with other tocolytic drugs there was no significant evidence that nitric oxide donors performed better than other tocolytics (betamimetics, magnesium sulphate, a calcium channel blocker or a combination of tocolytics) in terms of pregnancy prolongation, although nitric oxide donors appeared to be associated with a reduction in most adverse effects, apart from headache. There was no significant difference between groups for infant morbidity or mortality outcomes.
Authors' conclusions
There is currently insufficient evidence to support the routine administration of nitric oxide donors in the treatment of threatened preterm labour.
Keywords: Female; Humans; Pregnancy; Nitric Oxide Donors; Nitric Oxide Donors/therapeutic use; Nitroglycerin; Nitroglycerin/therapeutic use; Obstetric Labor, Premature; Obstetric Labor, Premature/drug therapy; Randomized Controlled Trials as Topic; Tocolysis; Tocolysis/methods; Tocolytic Agents; Tocolytic Agents/therapeutic use
Plain language summary
Nitric oxide donors for the treatment of preterm labour
Preterm birth (birth before 37 weeks) increases the baby's risk of death or disability. Several drugs are available to try and slow down labour so that corticosteroid drugs can be given to help the baby's lungs mature quickly. Nitric oxide donors (glyceryl trinitrate) are drugs that may slow down contractions. They can cause headaches, low blood pressure and increased heart rate for the mother, but they might cause fewer problems than some of the other options. This review gathered the evidence on nitric oxide donors compared with no treatment and compared with other drugs to inhibit preterm labour.
We identified 12 trials involving 1227 women. We found that there is not enough evidence to show whether or not nitric oxide donors can slow down preterm labour.
Background
Preterm birth complicates around 10% of pregnancies and the rate is increasing in almost every country with reliable data. It is the largest cause of death in newborns (Kinney 2012). The consequences of preterm birth for parents and children are enormous. It is a huge financial burden on healthcare resources and, although the costs of immediate neonatal care are high, these are dwarfed by the financial implications of long‐term disability (Stevenson 1996).
Since the perinatal risks are inversely proportional to the gestational age at birth, it has been assumed that delaying preterm birth will reduce perinatal mortality and morbidity. However, preterm labour is multifactorial and it is unlikely that a single treatment will be appropriate for all women. Indeed, the perinatal risks in certain conditions, such as fetal compromise, may even be reduced by expedited birth. The management of preterm labour must therefore be carefully considered on an individual basis.
There are numerous pharmacological treatments available for tocolysis (reducing uterine activity) in uncomplicated preterm labour, which can be used in an attempt to delay birth. The ß‐sympathomimetics (ritodrine, salbutamol, terbutaline) (Anotayanonth 2004) have largely been replaced by other drugs due to potentially serious maternal adverse effects (RCOG 2011). Compared with placebo, other drugs such as calcium channel blockers (King 2002), and prostaglandin synthase inhibitors (non‐selective and selective Cox 2) (King 2005) have been suggested to be effective in terms of pregnancy prolongation, although there is little clear evidence that tocolysis reduces the risk of adverse outcomes for neonates (Haas 2012).
Clinical trials in women with threatened preterm labour are complex. There are no uniform diagnostic criteria. Many women who present with increased uterine activity subsequently deliver at term without treatment and the frequency of threatened preterm labour is higher at later gestational ages when morbidity and mortality are less. Consequently, many studies have reported secondary outcome variables such as delay in birth, mean birthweight or the incidence of preterm birth that may not reflect changes in morbidity or mortality. The situation is further complicated by the relatively short duration of action of a number of treatment options. This, coupled with a failure to demonstrate improved perinatal outcome with long‐term treatment, has led to administration of tocolytics in the short term (up to 48 hours). The rationale is that a short delay in birth may allow interventions to improve perinatal outcome, such as prenatal steroid administration (Roberts 2006) or transfer to a unit with neonatal intensive care facilities.
The basis for the administration of a nitric oxide donor for the treatment of preterm labour is largely derived from observations in animals. L‐arginine (the substrate for nitric oxide synthase) reduces, and inhibition of nitric oxide formation increases, myometrial contractility in the rat (Yallampalli 1993; Yallampalli 1994). In the rabbit and human, nitric oxide synthase activity is increased during pregnancy and reduced at the onset of labour (Arthur 2008; Sladek 1993). Functional experiments in vitro have reported that nitric oxide donors decrease myometrial contractility (Buhimschi 1995; Momohara 2004; Norman 1996) and there may be a reduction in the myometrial response in late pregnancy (Okawa 2005). Thus, there is a scientific rationale for the clinical administration of nitric oxide donors. However, maternal adverse effects due to vasodilatation such as headache, flushing, hypotension and tachycardia may be anticipated. Theoretical fetal adverse effects could result from a direct effect of increased nitric oxide or maternal vasodilatation leading to changes in uterine blood flow. The current review considers the maternal and infant effects of nitric oxide donors for tocolysis in threatened preterm labour. For this review, nitric oxide donors are defined as drugs which increase nitric oxide, either due to an effect on formation or degradation. Nitric oxide donors may be administered intravenously, transdermally or sublingually.
Objectives
To determine the efficacy and safety of nitric oxide donors administered as tocolytics.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised or quasi‐randomised trials were eligible, in which nitric oxide donors were administered for tocolysis in the management of preterm labour.
Types of participants
Pregnant women assessed as being in preterm labour and considered suitable for tocolysis. Preterm labour was defined as uterine contractions (in the absence or presence of ruptured membranes), with or without cervical dilatation.
Types of interventions
Nitric oxide donors, administered by any route, in the management of women in preterm labour, compared with either placebo, no treatment or alternative tocolytics.
Types of outcome measures
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of individual Cochrane reviews.
Consensus was reached on a set of six ‘core’ outcomes, which are highlighted below. These will be included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
Maternal
Interval between randomisation and birth: prolongation of pregnancy greater than 24 hours, 48 hours, 72 hours, seven days, 14 days.
Birth prior to 37, 34, 32 and 28 completed weeks.
Serious maternal outcomes (maternal death, cardiac arrest, respiratory arrest).
Infant
Death unrelated to congenital abnormalities: in utero; in first 28 days of life; after 28 days (perinatal mortality).
Intraventricular haemorrhage (serious infant outcome).
Respiratory distress syndrome.
Use of mechanical ventilation.
Use of mechanical ventilation at 28 days of age.
Length of mechanical ventilation.
Chronic lung disease (defined as the need for supplemental oxygen at 28 days of life).
Long‐term neurological development (general intelligence, hearing, vision, cerebral palsy and disability) (serious infant outcome).
Secondary outcomes
Maternal
Mode of birth.
Adverse drug reactions, e.g. headache, facial flushing, palpitations, hypotension, dizziness, tachycardia.
Women's assessment of the therapy.
Completion of course of maternal steroids (48 hours from first dose).
Infant
Birth for presumed fetal distress: Apgar score of less than seven at five minutes.
Cord pH less than 7.20.
Neonatal intensive care unit (NICU) admission.
Birthweight.
Birthweight under third centile for gestational age.
Use of health services
Length of antenatal hospital stay for mother.
Admission of infant to NICU.
Length of stay of infant in NICU.
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (1 December 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
For details of searching carried out for the previous version of this review (Duckitt 2002), see Appendix 1.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, see Appendix 2.
For this update, we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors (Kirsten Duckitt (KD) and Oliver O'Donovan (OO)) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted Steve Thornton (ST).
Data extraction and management
We designed a form to extract data. For eligible studies, KD and OO extracted data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted ST. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (KD and OO) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor (ST).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We had planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference (MD) as outcomes were measured in the same way between trials. In future updates, if appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials; none were identified for this version of the review. If we identify any in the future, we will adjust their sample sizes using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We have not included cross‐over trials; we did not consider they were a suitable design for this type of intervention.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. If more data become available in the future, we will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used a random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not considered clinically meaningful, we did not combine trials.
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses.
We planned to carry out the following subgroup analyses.
By type and dose of glyceryl trinitrate.
We planned to carry out analyses for primary outcomes only, and to assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We did not carry out this planned analysis as insufficient information was available in the trial reports.
Sensitivity analysis
We had planned sensitivity analysis by trial quality (trials with more or less than 20% loss to follow‐up). In this version of the review none of the trials had a loss to follow‐up of more than 20%.
Results
Description of studies
Results of the search
Our search identified 34 reports corresponding to 20 studies (some of the included studies were described in multiple publications). Twelve studies were assessed as being eligible for inclusion in the review and seven studies were excluded.
One study is awaiting further assessment (Simsek 2000).
Included studies
Twelve trials contributed data to the review. The trials included a total of 1227 women judged to be in preterm labour from clinical centres in countries across the world. Two trials were conducted in Canada (Smith 1999; Smith 2007); one each in Australia (Bisits 1998), Brazil (Amorim 2009), USA (El‐Sayed 1999), Iran (Haghighi 2005), China (He 2002), Germany (Schleussner 2001), Poland (Szulc 2000) and the United Arab Emirates (Wani 2004). Bisits 2004 reported on a multi‐centre trial in Australia, Singapore and HongKong, and Lees 1999 on a trial including centres in the UK, Europe and Indonesia.
Interventions
Three trials compared a nitric oxide donor with placebo (Haghighi 2005; Smith 1999; Smith 2007). The other trials compared a nitric oxide donor with an established tocolytic treatment for preterm labour: sublingual nifedipine (Amorim 2009); intravenous (i.v.) albuterol (obstetric ventolin) (Bisits 1998); i.v. ritodrine or i.v. salbutamol (Bisits 2004); i.v. magnesium sulphate (El‐Sayed 1999); i.v. magnesium sulphate and salbutamol (He 2002); i.v. ritodrine (Lees 1999; Wani 2004); i.v. fenoterol (Schleussner 2001); and i.v. fenoterol with verapamil (Szulc 2000).
All but two of the trials used transdermal administration of nitroglycerin via a patch applied to the skin, while in the trial by El‐Sayed 1999, nitroglycerin was administered intravenously, and in the Haghighi 2005 trial, women received sublingual isosorbide dinitrate.
Participants
All studies included women in preterm labour (less than 37 week's gestation) although the inclusion criteria relating to gestational age at recruitment varied in the individual trials. The lowest gestational age at recruitment was 23 weeks (Szulc 2000; Wani 2004). In most of the remaining trials the lowest gestational age at recruitment was 24 weeks, although Haghighi 2005 only included women between 33 to 36 weeks' gestation, He 2002 between 28 to 37 weeks, and Schleussner 2001 27 to 35 weeks.
All the studies required sustained, painful, regular contractions as inclusion criteria although studies may also have had inclusion criteria relating to cervical change. Three studies included women with preterm ruptured membranes (Bisits 1998; Bisits 2004; El‐Sayed 1999), while other studies excluded women with ruptured membranes (He 2002; Lees 1999; Smith 1999; Smith 2007; Szulc 2000; Wani 2004), or membrane status was not clear (Amorim 2009; Haghighi 2005; Schleussner 2001).
Seven studies specifically mentioned excluding women with multiple pregnancies (Amorim 2009; Bisits 1998; Bisits 2004; Haghighi 2005; Smith 2007; Szulc 2000; Wani 2004). Only four studies mentioned antenatal administration of corticosteroids. In three studies they were given to all women (Amorim 2009; Smith 2007; Wani 2004) and in the other they were given at the discretion of the attending physician but were given to equal numbers of women in both groups (Smith 1999). In addition, two studies mentioned administration of antibiotics (Smith 1999; Wani 2004).
See tables of Characteristics of included studies for more information on the specific inclusion and exclusion criteria and details of the interventions in the included studies.
Excluded studies
We excluded seven studies. Two studies were excluded because it was not clear that they were randomised trials (Groom 2000; Leszczynska 2001). In two studies, there was insufficient information on methods or results to allow us to assess risk or bias and include the data in our analyses (Bulgay‐Moerschel 2008; Clavin 1996), and three studies did not examine comparisons relevant to the scope of the review (Hogberg 1998; Pasargiklian 1983; Rytlewski 2008).
Risk of bias in included studies
See table of Characteristics of included studies.
Allocation
The method used to generate the randomisation sequence was assessed as being at low risk of bias for all but three of the included studies; computer‐generated or external randomisation services were used by Amorim 2009; He 2002; Smith 1999; and Smith 2007; randomised lists or random number tables were used in the trials by Bisits 2004; Haghighi 2005; Lees 1999; and Szulc 2000; and El‐Sayed 1999 used shuffled cards to determine the sequence. Two trials did not give clear information about the method used (Bisits 1998; Wani 2004) and one trial was assessed as high risk of bias because the order of randomisation was by hospital case‐note number (Schleussner 2001).
Most of the studies used methods to allocate women to treatment groups at the point of randomisation that we judged to be at low risk of bias. Sealed, numbered, opaque envelopes concealing allocations were used by Amorim 2009; Bisits 1998; Bisits 2004; El‐Sayed 1999; Haghighi 2005; Lees 1999; Smith 1999; Smith 2007; and Wani 2004. The methods used by He 2002 and Szulc 2000 were not clear, and Schleussner 2001 used hospital case‐note numbers to allocate women to groups that could be anticipated by staff carrying out randomisation.
Blinding
All but three of these studies compared different treatment regimens and did not attempt to use placebos to mask treatment. In three trials, active treatment was compared with placebo (Haghighi 2005; Smith 1999; Smith 2007) and women would have been aware of treatment allocation. In the Smith 1999 and Smith 2007 trials, outcome assessors were also blind to treatment, although in Haghighi 2005 it was not clear whether or not staff assessing outcomes were aware of the treatment group.
Incomplete outcome data
All but four of the included trials had no, or low levels of sample attrition, and loss to follow‐up and missing data were balanced across groups or an intention‐to‐treat analysis was performed. Loss to follow‐up was unclear in four studies (He 2002; Lees 1999; Schleussner 2001; Szulc 2000) and one trial was assessed as high risk of bias for this domain: in the Haghighi 2005 trial women were excluded from the analyses if they developed hypotension.
Selective reporting
Assessing reporting bias without access to study protocols is not simple, and as most of our 'Risk of bias' assessments were done from published trial reports, studies were predominantly assessed as unclear for this 'Risk of bias' domain.
Other potential sources of bias
Two trials had other possible sources of bias. In the trial by Lees 1999, there were some treatment cross‐overs and some women did not receive treatment as planned, or treatment was discontinued; the impact of these deviations from protocol was not clear. The Smith 2007 trial was stopped early due to recruitment difficulties.
'Risk of bias' assessments for each study and across all studies are set out in Figure 1 and Figure 2.
1.
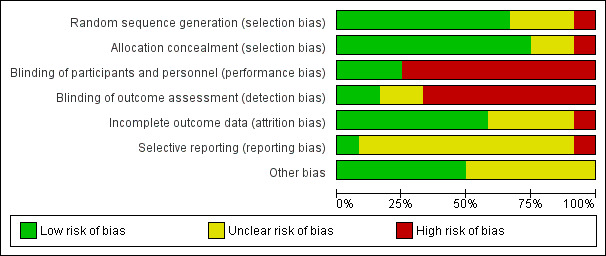
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
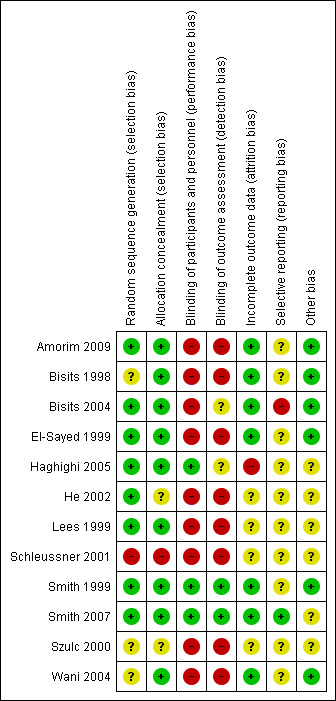
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Comparison 1: Nitric oxide donors versus placebo (three studies with 336 women)
This comparison includes three trials with a total of 336 women randomised (Haghighi 2005; Smith 1999; Smith 2007).
Maternal primary outcomes
Pregnancy prolongation
Two trials examined pregnancy prolongation beyond 48 hours for women randomised to receive glyceryl trinitrate (GTN) patches versus placebo; there were no significant differences between groups (average risk ratio (RR) 1.19, 95% confidence interval (CI) 0.74 to 1.90, two studies, 186 women, I² = 49%, Tau² = 0.07) (Analysis 1.1).
1.1. Analysis.
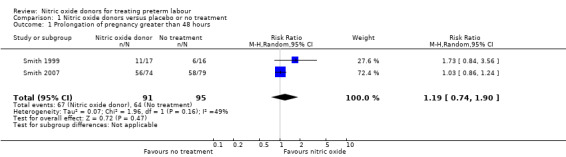
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 1 Prolongation of pregnancy greater than 48 hours.
Birth before 37 weeks completed weeks' gestation was reported for two trials; due to high statistical heterogeneity between trials we did not pool results. In the trial by Smith 2007 the number of women in each group giving birth before 37 weeks' gestation was very similar (RR 1.01, 95% CI 0.73 to 1.40, one study, 153 women), whereas in the Haghighi 2005 trial women receiving a nitric oxide donor were less at risk of birth before 37 weeks (RR 0.30, 95% CI 0.14 to 0.16) (Analysis 1.2).
1.2. Analysis.
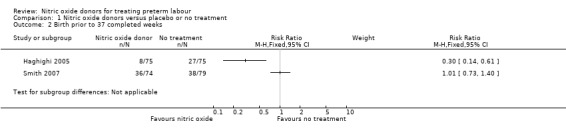
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 2 Birth prior to 37 completed weeks.
Birth before 34 and 28 completed weeks' gestation was reported for one trial; there was no clear difference between groups for either outcome (RR 0.93, 95% CI 0.61 to 1.41, and, RR 0.50, 95% CI 0.23 to 1.09, one trial, 153 women) (Analysis 1.3; Analysis 1.4).
1.3. Analysis.
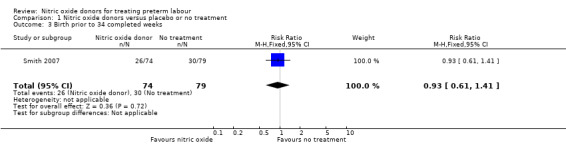
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 3 Birth prior to 34 completed weeks.
1.4. Analysis.
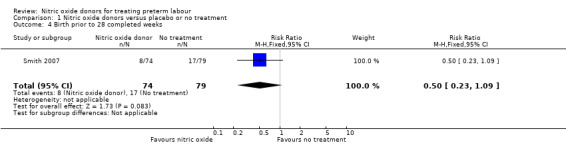
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 4 Birth prior to 28 completed weeks.
Other primary outcomes were not reported.
Maternal secondary outcomes
One study reported mode of birth and showed no difference between GTN and placebo for rates of caesarean birth (RR 0.47, 95% CI 0.14 to 1.57, 33 women) (Analysis 1.5).
1.5. Analysis.
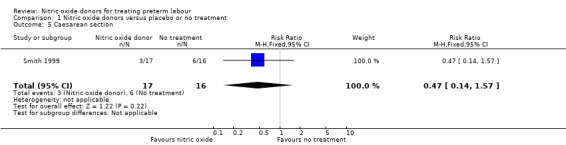
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 5 Caesarean section.
Adverse drug reactions were reported in two studies with data for 186 women; compared with placebo, women receiving GNT were more at risk of adverse effects (RR 1.49, 95% CI 1.14 to 1.94) (Analysis 1.6).
1.6. Analysis.
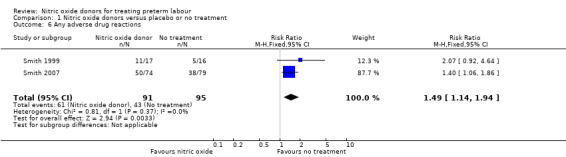
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 6 Any adverse drug reactions.
Smith 2007 reported the number of women in treatment and placebo groups with adverse effects. In this study, with data for 153 women, more women in the GTN group experienced headache (RR 1.95, 95% CI 1.31 to 2.90), but there were no significant differences between groups for other adverse effects (dizziness: RR 1.60, 95% CI 0.60 to 4.28; flushing: RR 0.90, 95% CI 0.43 to 1.89; hypotension: RR 1.20, 95% CI 0.49 to 2.95) (Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10).
1.7. Analysis.
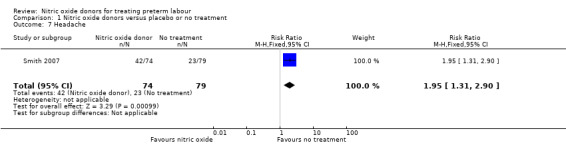
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 7 Headache.
1.8. Analysis.
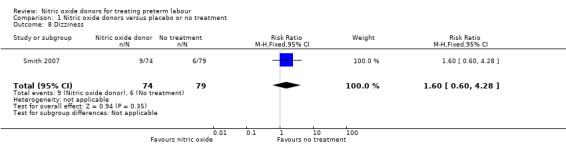
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 8 Dizziness.
1.9. Analysis.
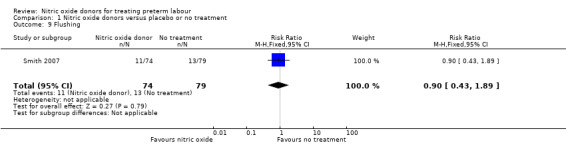
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 9 Flushing.
1.10. Analysis.
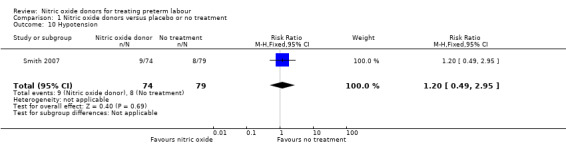
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 10 Hypotension.
Two trials reported whether or not women completed a course of corticosteroids before birth; the number of women receiving a full course of corticosteroids was very similar in the GTN and placebo groups (RR 1.04, 95% CI 0.90 to 1.20, 186 women) (Analysis 1.11).
1.11. Analysis.
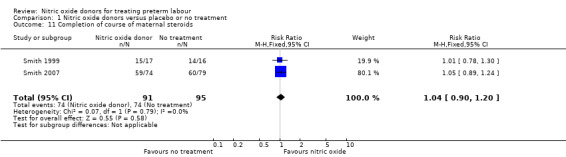
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 11 Completion of course of maternal steroids.
Other prespecified maternal secondary outcomes were not reported.
Infant primary outcomes
Perinatal death not related to congenital abnormalities was reported in two trials with 186 infants; overall there were no significant differences in mortality between groups; there was one neonatal death in the treatment group and three neonatal deaths and one stillbirth in the control group (Analysis 1.12).
1.12. Analysis.
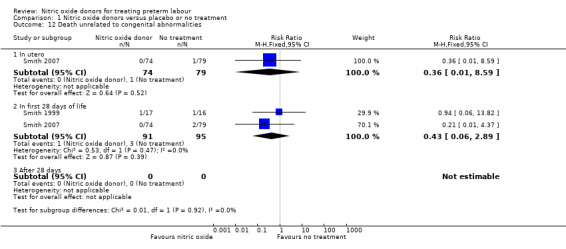
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 12 Death unrelated to congenital abnormalities.
Serious neonatal morbidity was reported by Smith 2007 with data for 153 infants and by Smith 1999 with data for 33 infants; there were no significant differences between groups for intraventricular haemorrhage (RR 2.14, 95% CI 0.20 to 23.06; one study; 153 infants); respiratory distress syndrome (RR 0.47, 95% CI 0.14 to 1.57; one study; 33 infants) or chronic lung disease (RR 0.15, 95% CI 0.02 to 1.21; ) (Analysis 1.13; Analysis 1.14; Analysis 1.15).
1.13. Analysis.
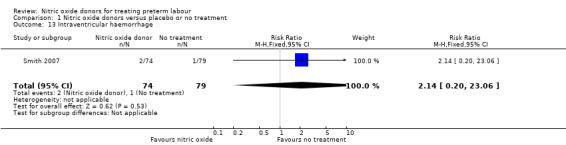
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 13 Intraventricular haemorrhage.
1.14. Analysis.
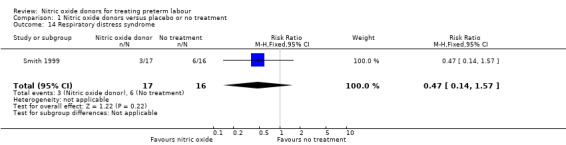
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 14 Respiratory distress syndrome.
1.15. Analysis.
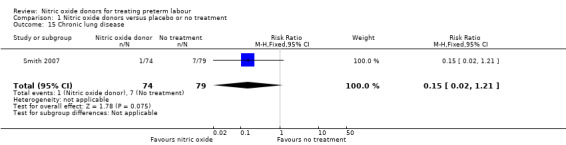
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 15 Chronic lung disease.
One trial (Smith 2007) reported on an outcome that we had not prespecified in our review protocol: a composite outcome incorporating serious neonatal morbidity, long‐term morbidity or perinatal mortality. Despite the relatively small sample size, there was a trend towards a reduced risk of serious infant outcome in the GTN group (RR 0.29, 95% CI 0.08 to 1.00, data for 153 infants; P = 0.05) (Analysis 1.17).
1.17. Analysis.
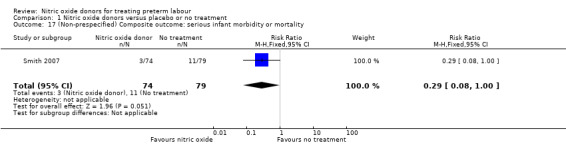
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 17 (Non‐prespecified) Composite outcome: serious infant morbidity or mortality.
Infant secondary outcomes
Most infant secondary outcomes were not reported. Mean birthweight was reported for one trial with data for 33 infants; there was no significant difference between groups (mean difference (MD) 327.00 g, 95% CI ‐272.13 to 926.13).
Comparison 2: Nitric oxide donors versus any betamimetic (five studies with 691 women)
Five trials contributed data to this comparison (Bisits 1998; Bisits 2004; Lees 1999; Schleussner 2001; Wani 2004). Trials compared nitric oxide donors with a range of betamimetic drugs including salbutamol/albuterol, fenoterol and ritodrine.
Maternal primary outcomes
Pregnancy prolongation
Two trials examined pregnancy prolongation beyond 24 hours for women randomised to receive GTN patches versus betamimetic tocolytic treatment; there were no significant differences between groups (average RR 0.94, 95% CI 0.82 to 1.08, 264 women) (Analysis 2.1). Three trials reported pregnancy prolongation beyond 48 hours, again, there was no clear difference between the nitric oxide donors and betamimetics (RR 0.96, 95% CI 0.87 to 1.05, 420 women) (Analysis 2.2).
2.1. Analysis.
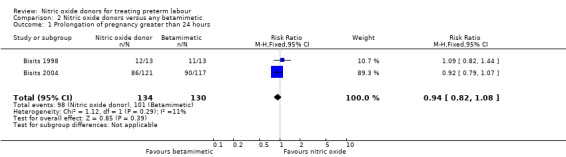
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 1 Prolongation of pregnancy greater than 24 hours.
2.2. Analysis.
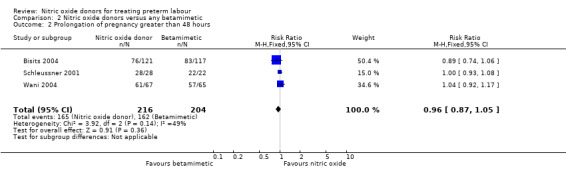
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 2 Prolongation of pregnancy greater than 48 hours.
Prolongation of pregnancy beyond seven and 14 days was similar for GTN and betamimetic therapies (average RR 1.03, 95% CI 0.92 to 1.15, five studies, 679 women, Tau² = 0.01, I² = 41%; average RR 1.07, 95% CI 0.82 to 1.39, two studies, 365 women, Tau² = 0.03, I² = 80%, respectively) (Analysis 2.3; Analysis 2.4).
2.3. Analysis.
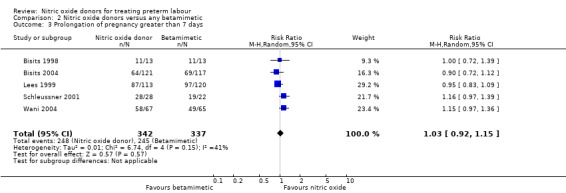
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 3 Prolongation of pregnancy greater than 7 days.
2.4. Analysis.
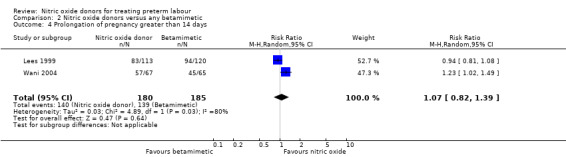
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 4 Prolongation of pregnancy greater than 14 days.
We did not identify significant differences between groups for preterm or very preterm birth. For birth before 37 completed weeks' gestation, the difference between groups receiving GTN versus betamimetics did not reach statistical significance, although there was considerable variation in the findings of the studies contributing data, and we used a random‐effects analysis for this outcome (average RR 0.73, 95% CI 0.50 to 1.05, five studies, 679 women, I² = 70%, Tau² = 0.10) (Analysis 2.5). There were also no clear differences between groups for birth before 34 or 32 weeks (average RR 0.71, 95% CI 0.36 to 1.42, two studies, 365 women, Tau² = 0.17, I² = 67%; RR 1.00, 95% CI 0.54 to 1.85, one study, 233 women, respectively) (Analysis 2.6; Analysis 2.7).
2.5. Analysis.
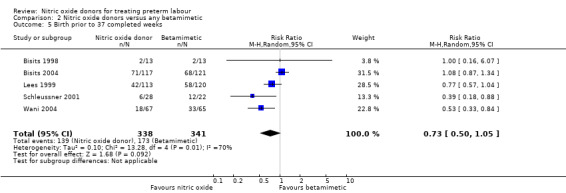
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 5 Birth prior to 37 completed weeks.
2.6. Analysis.
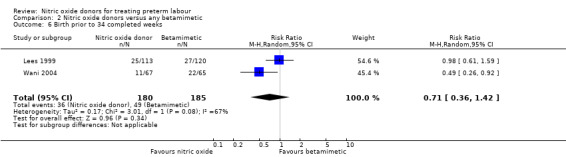
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 6 Birth prior to 34 completed weeks.
2.7. Analysis.
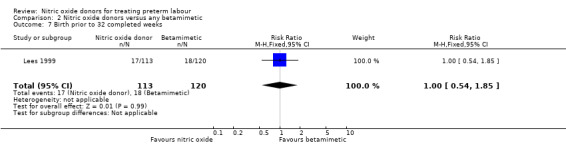
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 7 Birth prior to 32 completed weeks.
No trials reported on birth prior to 28 completed weeks.
Maternal secondary outcomes
Mode of birth
Rates of caesarean section were reported in two trials; there was no strong evidence to suggest that rates of caesarean section were different for women receiving GTN versus treatment with betamimetics (RR 0.87, 95% CI 0.53 to 1.43, 283 women) (Analysis 2.8).
2.8. Analysis.
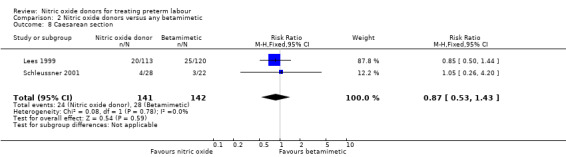
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 8 Caesarean section.
Adverse effects
Only one trial reported the overall number of women experiencing any adverse effects; in this study with data for 132 women, rates of adverse effects were reduced in the GTN group compared with the betamimetic group (RR 0.45, 95% CI 0.30 to 0.68) (Analysis 2.9).
2.9. Analysis.
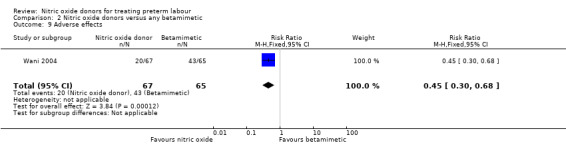
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 9 Adverse effects.
For individual adverse effects, compared with betamimetics GTN appeared to be associated with a reduction in most adverse effects apart from headache. For headaches there was no clear difference between groups (average RR 4.35, 95% CI 0.91 to 20.71, three studies, 349 women, Tau² = 1.37, I² = 73%) there was considerable variation in effect size in different studies (Analysis 2.10).
2.10. Analysis.
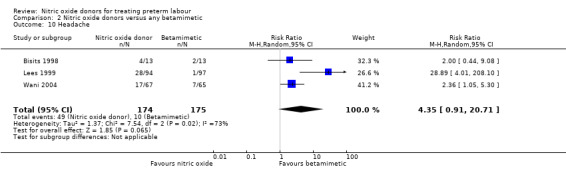
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 10 Headache.
For other adverse effects women were at reduced risk in the GTN group compared with betamimetics; they were less likely to experience palpitations (RR 0.06, 95% CI 0.01 to 0.30, two studies, 323 women) (Analysis 2.12), shortness of breath (RR 0.09, 95% CI 0.02 to 0.46, two studies, 217 women) (Analysis 2.13), nausea (RR 0.47, 95% CI 0.22 to 0.98, three studies, 349 women) (Analysis 2.14), tachycardia (RR 0.03, 95% CI 0.01 to 0.10, two studies, 323 women) (Analysis 2.15) or chest pain/tightness (RR 0.12, 95% CI 0.02 to 0.64, two studies, 323 women) (Analysis 2.16). For dizziness, there were no clear differences between groups (RR 1.55, 95% CI 0.26 to 9.06, one study, 191 women) (Analysis 2.11).
2.12. Analysis.
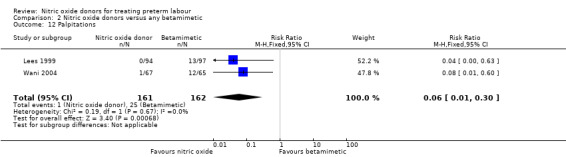
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 12 Palpitations.
2.13. Analysis.
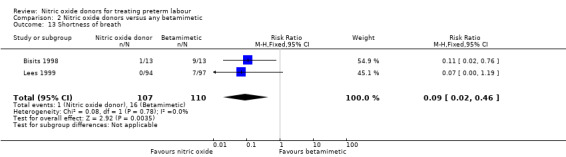
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 13 Shortness of breath.
2.14. Analysis.
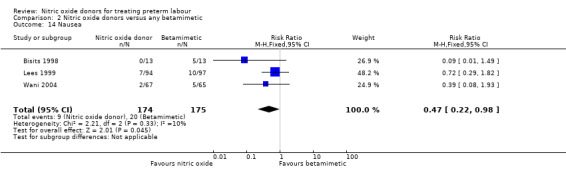
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 14 Nausea.
2.15. Analysis.
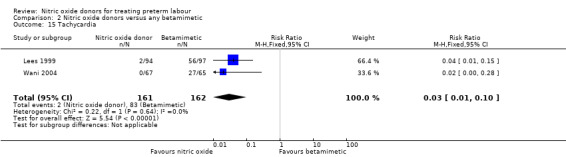
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 15 Tachycardia.
2.16. Analysis.
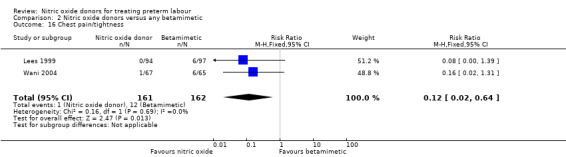
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 16 Chest pain/tightness.
2.11. Analysis.
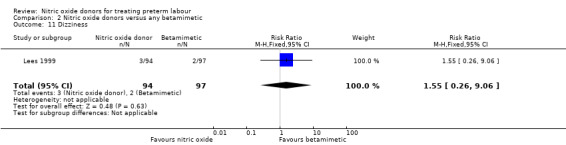
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 11 Dizziness.
One study reported on treatment cessation due to adverse effects; betamimetics were associated with an increased risk of treatment cessation compared with GTN (RR 0.05, 95% CI 0.00 to 0.86, 132 women) (Analysis 2.17). One study reported pulmonary oedema; there were no events in the GTN group and a single case in the group receiving the betamimetic ritodrine (RR 0.34, 95% CI 0.01 to 8.34, 191 women) (Analysis 2.18).
2.17. Analysis.
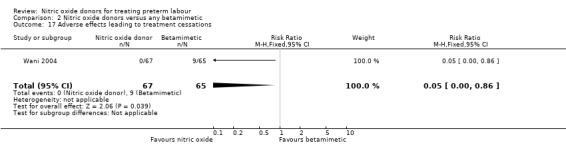
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 17 Adverse effects leading to treatment cessations.
2.18. Analysis.
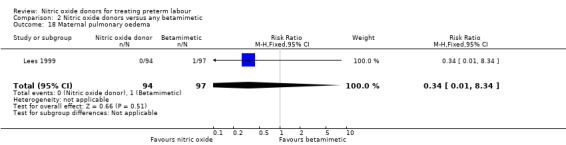
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 18 Maternal pulmonary oedema.
None of our other secondary maternal outcomes were reported.
Infant primary outcomes
Perinatal death and serious infant morbidity
Only one of the trials with data for 191 infants (Lees 1999) reported infant mortality. There were no significant differences between groups; there were two neonatal deaths in the GTN group and one stillbirth and one neonatal death in the control group (Analysis 2.19).
2.19. Analysis.
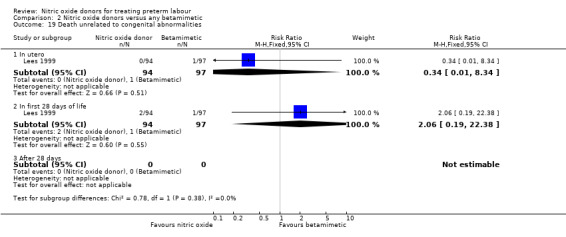
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 19 Death unrelated to congenital abnormalities.
Few outcomes relating to serious infant morbidity were reported. In one study (132 infants), there were no differences between groups for use of mechanical ventilation (RR 0.42, 95% CI 0.11 to 1.54) (Analysis 2.20). In one study rates of chronic lung disease were very similar in the two groups (RR 1.03, 95% CI 0.43 to 2.51) (Analysis 2.21). Admission to NICU was reported in two studies for a total of 181 infants; admission was reduced in the GTN group (RR 0.50, 95% CI 0.31 to 0.80) (Analysis 2.22).
2.20. Analysis.
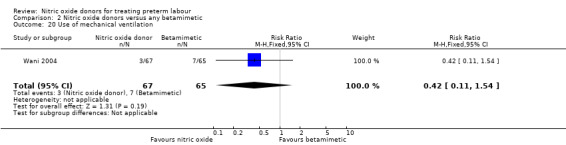
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 20 Use of mechanical ventilation.
2.21. Analysis.
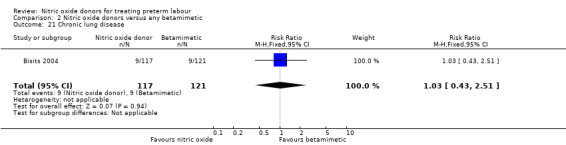
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 21 Chronic lung disease.
2.22. Analysis.
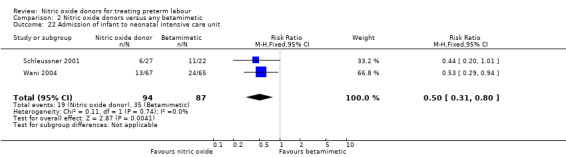
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 22 Admission of infant to neonatal intensive care unit.
Secondary outcomes
Very few of our secondary outcomes were reported including serious infant morbidity such as intraventricular haemorrhage or respiratory distress syndrome. Infants in the GTN group had, on average, higher birthweights (MD 440.39 g, 95% CI 237.35 to 643.44, two studies with 182 infants) (Analysis 2.23).
2.23. Analysis.
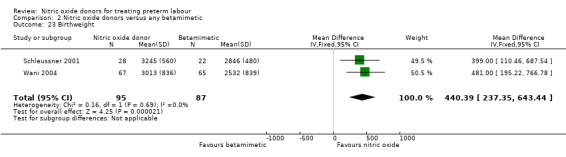
Comparison 2 Nitric oxide donors versus any betamimetic, Outcome 23 Birthweight.
There were no data on use of health services.
Comparison 3: Nitric oxide donors versus magnesium sulphate (one study with 30 women)
A single trial with a small sample size (30 women) contributed data to this comparison (El‐Sayed 1999). This trial reported on only five of our prespecified outcomes and there were no data on infant and maternal mortality or serious morbidity outcomes.
Primary outcomes
Primary maternal and neonatal outcomes were not reported.
Secondary outcomes
Adverse effects
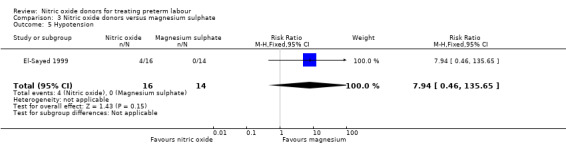
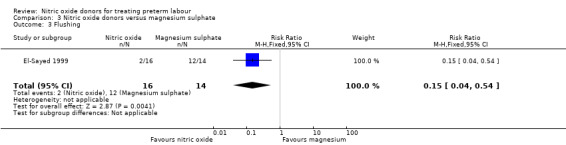
There was insufficient evidence to demonstrate any significant differences between women receiving nitric oxide versus magnesium sulphate for most the adverse effects reported (headache (RR 2.41, 95% CI 0.99 to 5.87); dizziness (RR 3.06, 95% CI 0.76 to 12.40); palpitations (RR 0.44, 95% CI 0.04 to 4.32); and hypotension (RR 7.94, 95% CI 0.46 to 135.65) (Analysis 3.1; Analysis 3.2; Analysis 3.4; Analysis 3.5). Women receiving magnesium sulphate appeared more likely to experience flushing (RR 0.15, 95% CI 0.04 to 0.54) (Analysis 3.3). Other prespecified secondary outcomes were not reported.
3.1. Analysis.
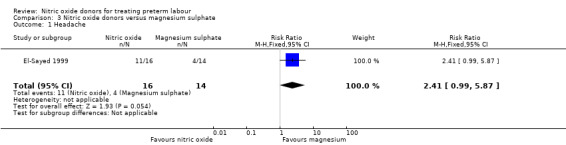
Comparison 3 Nitric oxide donors versus magnesium sulphate, Outcome 1 Headache.
3.2. Analysis.
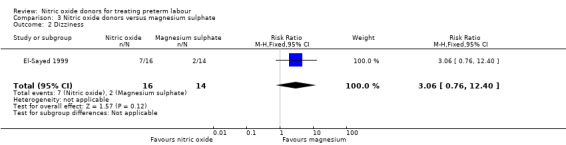
Comparison 3 Nitric oxide donors versus magnesium sulphate, Outcome 2 Dizziness.
3.4. Analysis.
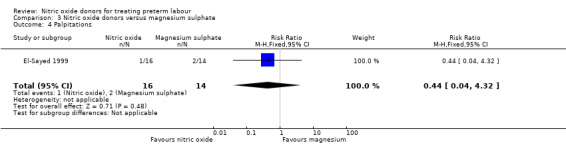
Comparison 3 Nitric oxide donors versus magnesium sulphate, Outcome 4 Palpitations.
3.5. Analysis.
Comparison 3 Nitric oxide donors versus magnesium sulphate, Outcome 5 Hypotension.
3.3. Analysis.
Comparison 3 Nitric oxide donors versus magnesium sulphate, Outcome 3 Flushing.
Comparison 4: Nitric oxide donor versus calcium channel blocker (one study with 50 women)
A single trial with 50 women contributed data to this comparison (Amorim 2009). The trial reported very few of our prespecified outcomes and there were no data on infant and maternal mortality or serious morbidity outcomes.
Primary outcomes
Pregnancy prolongation
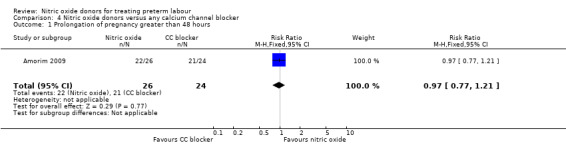
There was insufficient evidence to demonstrate any difference between groups receiving a nitric oxide donor versus magnesium sulphate for pregnancy prolongation beyond 48 hours (RR 0.97, 95% CI 0.77 to 1.21) (Analysis 4.1). Other primary maternal and neonatal outcomes were not reported.
4.1. Analysis.
Comparison 4 Nitric oxide donors versus any calcium channel blocker, Outcome 1 Prolongation of pregnancy greater than 48 hours.
Secondary outcomes
Adverse effects
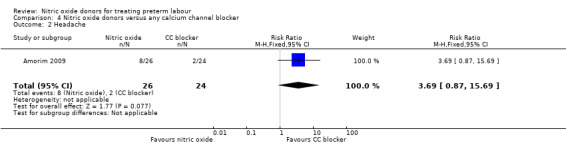
There was insufficient evidence to demonstrate any significant differences between women receiving nitric oxide versus a calcium channel blocker for any of the adverse effects reported (headache (RR 3.69, 95% CI 0.87 to 15.69); flushing (RR 1.85, 95% CI 0.18 to 19.08); hypotension (RR 0.31, 95% CI 0.03 to 2.76); nausea (RR 0.62, 95% CI 0.11 to 3.37), and tachycardia (RR 0.31, 95% CI 0.01 to 7.23) (Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6). Other prespecified secondary outcomes were not reported.
4.2. Analysis.
Comparison 4 Nitric oxide donors versus any calcium channel blocker, Outcome 2 Headache.
4.3. Analysis.
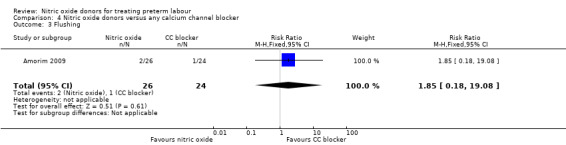
Comparison 4 Nitric oxide donors versus any calcium channel blocker, Outcome 3 Flushing.
4.4. Analysis.
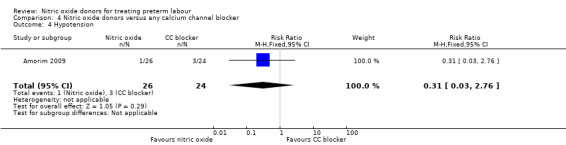
Comparison 4 Nitric oxide donors versus any calcium channel blocker, Outcome 4 Hypotension.
4.5. Analysis.
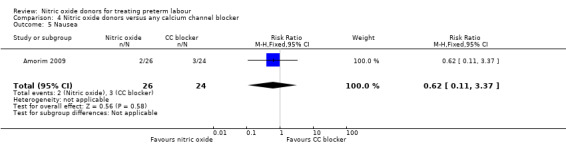
Comparison 4 Nitric oxide donors versus any calcium channel blocker, Outcome 5 Nausea.
4.6. Analysis.
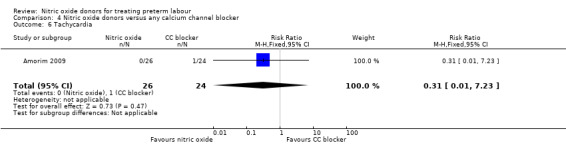
Comparison 4 Nitric oxide donors versus any calcium channel blocker, Outcome 6 Tachycardia.
Comparison 5: Nitric oxide donors versus a combination of tocolytics (two studies with 120 women)
Two trials compared nitric oxide donors with combinations of tocolytic drugs.
He 2002, in a study with 60 women compared a nitric oxide donor with magnesium sulphate plus salbutamol. Only one of our prespecified outcomes was reported; there was no clear difference between groups for prolongation of pregnancy beyond 48 hours (RR 1.13, 95% CI 0.91 to 1.39) (Analysis 5.1).
5.1. Analysis.
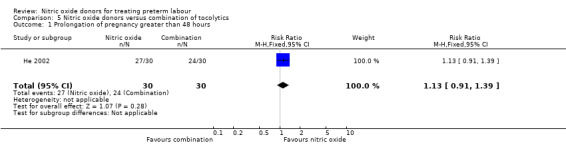
Comparison 5 Nitric oxide donors versus combination of tocolytics, Outcome 1 Prolongation of pregnancy greater than 48 hours.
In a study with 60 women, Szulc 2000 reported on mode of birth and the frequency of side effects in women receiving nitric oxide donors versus fenoterol with verapamil. There was no clear evidence that the type of tocolytic affected risk of caesarean section (RR 1.50, 95% CI 0.47 to 4.78) (Analysis 5.2). While women receiving nitric oxide donors were at higher risk of headache (RR 13.00, 95% CI 3.38 to 49.96), they appeared at less risk of tachycardia (RR 0.05, 95% CI 0.01 to 0.32) compared with women receiving a combination of tocolytics (Analysis 5.3; Analysis 5.6). There was no clear difference between groups for nausea, flushing or chest pain (Analysis 5.4; Analysis 5.5; Analysis 5.7).
5.2. Analysis.
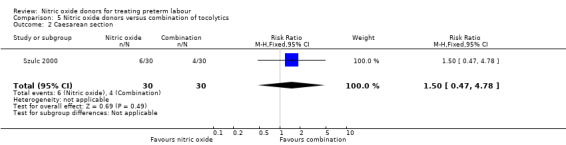
Comparison 5 Nitric oxide donors versus combination of tocolytics, Outcome 2 Caesarean section.
5.3. Analysis.
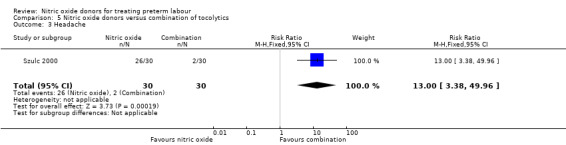
Comparison 5 Nitric oxide donors versus combination of tocolytics, Outcome 3 Headache.
5.6. Analysis.
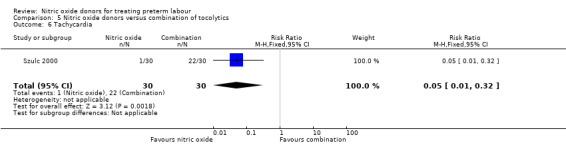
Comparison 5 Nitric oxide donors versus combination of tocolytics, Outcome 6 Tachycardia.
5.4. Analysis.
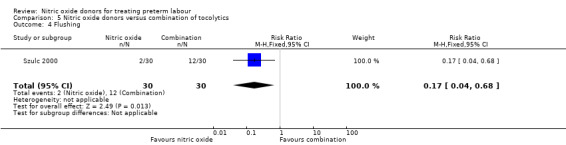
Comparison 5 Nitric oxide donors versus combination of tocolytics, Outcome 4 Flushing.
5.5. Analysis.
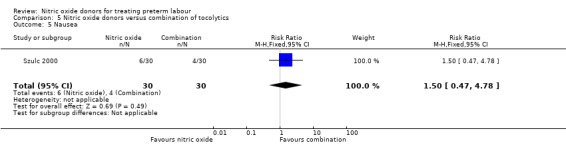
Comparison 5 Nitric oxide donors versus combination of tocolytics, Outcome 5 Nausea.
5.7. Analysis.
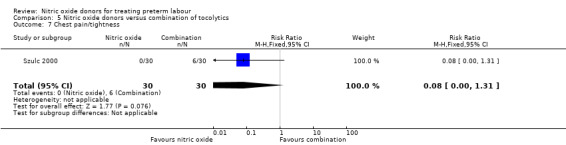
Comparison 5 Nitric oxide donors versus combination of tocolytics, Outcome 7 Chest pain/tightness.
Subgroup analysis
It was not possible to perform any of the planned subgroup analyses as the data required could not be extracted from the published information.
Discussion
Summary of main results
Compared with placebo, there was no significant evidence that nitric oxide donors prolonged pregnancy, and although for most adverse effects there was no significant difference between groups, women in the active treatment group were at higher risk of experiencing a headache. For infant outcomes there was no significant evidence that nitric oxide donors reduced the risk of neonatal death or serious morbidity, although one study, using a composite outcome, reported a reduced risk of serious adverse outcomes for infants in the glyceryl trinitrate group which approached statistical significance. Overall, these studies were underpowered to identify differences between groups for most outcomes.
When nitric oxide donors were compared with other tocolytic drugs there was no clear evidence that nitric oxide donors performed better than other tocolytics (betamimetics, magnesium sulphate, a calcium channel blocker or a combination of tocolytics) in terms of pregnancy prolongation although nitric oxide donors appeared to be associated with a reduction in most adverse effects apart from headache. There was no significant difference between groups for infant morbidity or mortality outcomes.
Overall completeness and applicability of evidence
This review update demonstrates that, despite several new studies, there is insufficient evidence to support the use of nitric oxide donors for the treatment of uncomplicated threatened preterm labour. The purpose of maternal administration of a tocolytic is to improve neonatal outcome. This has not been conclusively demonstrated for any tocolytic and most studies have used surrogate outcomes such as the rate of preterm birth or an increase in gestational age. Future demonstration of benefit will require high quality placebo or comparator trials. Nitric oxide donors (compared with placebo and other tocolytics) are associated with an increased incidence of headache but may be associated with fewer other adverse effects compared with other tocolytics. The only difference in perinatal morbidity or mortality is a decrease in admission to NICU with nitric oxide donors (compared with betamimetics) although the numbers are small. In the absence of clear beneficial effects on perinatal morbidity or mortality, routine administration of nitric oxide donors cannot be advocated.
Quality of the evidence
The studies included in the review were of mixed methodological quality; studies comparing different treatment regimens were not blinded and this may have had an impact on outcomes, lack of blinding may have affected other aspects of care, clinical decision‐making and may possibly have affected responses for more subjective outcomes (some adverse effects).
Potential biases in the review process
We are aware that the review process may be subject to bias and we took steps to minimise this. At least two of the review authors independently assessed risk of bias in the included trials and carried out data extraction.
Authors' conclusions
Implications for practice.
There are insufficient data to determine whether nitric oxide donors are effective in the treatment of preterm labour. Therefore, their use should be limited to randomised, controlled trials.
Implications for research.
There is currently insufficient evidence to support the routine administration of nitric oxide donors in the treatment of threatened preterm labour. The most recent trial included in this version of the review was published in 2009, and up to that time nitric oxide donors had been compared with only a limited number of other types of tocolytics. Future trials should investigate whether compared with other tocolytics, nitric oxide donors improve perinatal morbidity and mortality as well as surrogate outcomes such as the effect on preterm birth. Such trials should be adequately powered and of high quality. They should also record whether other therapy (such as steroids and antibiotics) have been given and to how many women since such therapy may influence outcome. Placebo‐controlled trials are the gold standard in this context , but other trials comparing nitric oxide donors with other commonly used tocolytics such as calcium channel blockers and atosiban would be helpful.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2013 | New citation required but conclusions have not changed | Search updated on 1 December 2013. Seven new trials added (Amorim 2009; Bisits 2004; Haghighi 2005; He 2002; Schleussner 2001; Smith 2007; Szulc 2000). |
| 16 December 2013 | New search has been performed | The updated version of the review now includes data from 12 randomised controlled trials. Core outcomes for tocolysis reviews added. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 5 July 2011 | Amended | Search updated. Twenty‐four reports added to Studies awaiting classification. |
| 4 November 2008 | Amended | Converted to new review format. |
Acknowledgements
Thank you to Elizabeth Whiteley, Derek Scoins and Karin Gerlach for their help with translations.
Therese Dowswell's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search methods used in the previous version of the review
For the Duckitt 2002 review, we used the search strategy developed for the Cochrane Pregnancy and Childbirth Group as a whole. The full list of journals and conference proceedings as well as the search strategies for the electronic databases, which are searched by the Group on behalf of its reviewers, are described in detail in the 'Search strategies for the identification of studies section' within the editorial information about the Cochrane Pregnancy and Childbirth Group. Briefly, the Group searches on a regular basis MEDLINE, the Cochrane Controlled Trials Register and reviews the Contents tables of a further 38 relevant journals received via ZETOC, an electronic current awareness service.
Relevant trials, which are identified through the Group's search strategy, are entered into the Group's Specialised Register of Controlled Trials. Please see Review Group's details for more detailed information. Date of last search: March 2002.
In addition, the Cochrane Controlled Trials Register (CCTR) (The Cochrane Library, Issue 1, 2002) was searched using the following terms:
#1 NITROGLYCERIN*:ME #2 NITROGLYCERIN #3 (GLYCERYL next TRINITRATE*) #4 GTN #5 (NITRIC next (OXIDE next DONOR*)) #6 (NITRIC next OXIDE) #7 (SODIUM next NITROPRUSSIDE) #8 ISOSORBIDE‐DINITRATE*:ME #9 (ISOSORBIDE next DINITRATE) #10 NONOATES* #11 NITROSOGLUTATHIONE #12 FK409 #13 LABOR‐PREMATURE*:ME #14 (PRETERM near LABOR) #15 (PRETERM near LABOUR) #16 (PREMATURE near LABOR) #17 (PREMATURE near LABOUR) #18 TOCOLY* #19 (((((((((((#1 or #2) or #3) or #4) or #5) or #6) or #7) or #8) or #9) or #10) or #11) or #12) #20 (((((#13 or #14) or #15) or #16) or #17) or #18) #21 (#19 and #20)
#1 NITROGLYCERIN*:ME #2 NITROGLYCERIN #3 (GLYCERYL next TRINITRATE*) #4 GTN #5 (NITRIC next (OXIDE next DONOR*)) #6 (NITRIC next OXIDE) #7 (SODIUM next NITROPRUSSIDE) #8 ISOSORBIDE‐DINITRATE*:ME #9 (ISOSORBIDE next DINITRATE) #10 NONOATES* #11 NITROSOGLUTATHIONE #12 FK409 #13 LABOR‐PREMATURE*:ME #14 (PRETERM near LABOR) #15 (PRETERM near LABOUR) #16 (PREMATURE near LABOR) #17 (PREMATURE near LABOUR) #18 TOCOLY* #19 (((((((((((#1 or #2) or #3) or #4) or #5) or #6) or #7) or #8) or #9) or #10) or #11) or #12) #20 (((((#13 or #14) or #15) or #16) or #17) or #18) #21 (#19 and #20)
Appendix 2. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Bisits 1998; El‐Sayed 1999; Lees 1999; Smith 1999; Wani 2004 (1999 paper); Lees 1999 (1999 paper by Black et al); Hogberg 1998.
Studies were reviewed by two independent reviewers (S Thornton, K Duckitt) to determine if they met the inclusion criteria and to grade the methodological quality. Any disagreement was resolved by discussion until a consensus was reached. Methods for the consideration of trials for inclusion, evaluation of methodological quality, trial data extraction and processing was undertaken as described in the Cochrane Handbook (Clarke 2000). Methodological quality was categorised as low, moderate or high risk of bias depending on randomisation methods, allocation concealment, blinding and use of placebo. All eligible trials were included in the initial analysis. It was planned to assess the influence of trial quality on the findings of the review by conducting a sensitivity analysis of high versus low quality. High quality trials were those classified as having a low risk of bias (Clarke 2000). An a priori decision was made to exclude trials when outcome data were unavailable for more than 20% of participants, in order to minimise attrition bias. Data were abstracted independently by the two reviewers and checked and entered by one (K Duckitt). Results are presented using risks (RR) for categorical data and weighted mean difference (WMD) for variables measured on a continuous scale and will include 95% confidence intervals.
The review will assess the effects of nitric oxide donors when compared with:
‐ placebo or no treatment; ‐ any other tocolytic agent.
It was planned to carry out subgroup analyses as determined below to try and clarify in which particular circumstances surrounding preterm labour, if any, nitric oxide donors may exert an effect.
A priori sub‐group analyses:
treatment commenced prior to 24 weeks' gestation;
treatment commenced between 24 and 34 weeks' gestation;
treatment commenced at or greater than 34 weeks' gestation;
treatment commenced with cervical dilatation < 3 cm;
treatment commenced with cervical dilatation equal to or > 3 cm;
treatment commenced prior to membrane rupture;
treatment commenced after membrane rupture;
single gestation;
multiple gestation;
non transdermal administration;
transdermal administration.
Data and analyses
Comparison 1. Nitric oxide donors versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolongation of pregnancy greater than 48 hours | 2 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.74, 1.90] |
| 2 Birth prior to 37 completed weeks | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Birth prior to 34 completed weeks | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.61, 1.41] |
| 4 Birth prior to 28 completed weeks | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.23, 1.09] |
| 5 Caesarean section | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.14, 1.57] |
| 6 Any adverse drug reactions | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.14, 1.94] |
| 7 Headache | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.31, 2.90] |
| 8 Dizziness | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.60, 4.28] |
| 9 Flushing | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.43, 1.89] |
| 10 Hypotension | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.49, 2.95] |
| 11 Completion of course of maternal steroids | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.20] |
| 12 Death unrelated to congenital abnormalities | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 In utero | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.59] |
| 12.2 In first 28 days of life | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.06, 2.89] |
| 12.3 After 28 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Intraventricular haemorrhage | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.20, 23.06] |
| 14 Respiratory distress syndrome | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.14, 1.57] |
| 15 Chronic lung disease | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.21] |
| 16 Birthweight (grams) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 327.0 [‐272.13, 926.13] |
| 17 (Non‐prespecified) Composite outcome: serious infant morbidity or mortality | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.00] |
1.16. Analysis.
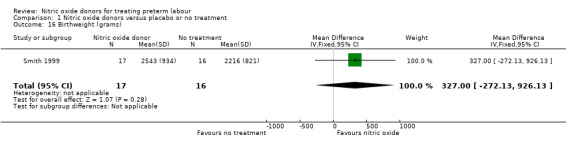
Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 16 Birthweight (grams).
Comparison 2. Nitric oxide donors versus any betamimetic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolongation of pregnancy greater than 24 hours | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 2 Prolongation of pregnancy greater than 48 hours | 3 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.87, 1.05] |
| 3 Prolongation of pregnancy greater than 7 days | 5 | 679 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.92, 1.15] |
| 4 Prolongation of pregnancy greater than 14 days | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.82, 1.39] |
| 5 Birth prior to 37 completed weeks | 5 | 679 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.05] |
| 6 Birth prior to 34 completed weeks | 2 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.42] |
| 7 Birth prior to 32 completed weeks | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.85] |
| 8 Caesarean section | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.43] |
| 9 Adverse effects | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.30, 0.68] |
| 10 Headache | 3 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [0.91, 20.71] |
| 11 Dizziness | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.26, 9.06] |
| 12 Palpitations | 2 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.30] |
| 13 Shortness of breath | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.46] |
| 14 Nausea | 3 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 0.98] |
| 15 Tachycardia | 2 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.10] |
| 16 Chest pain/tightness | 2 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.64] |
| 17 Adverse effects leading to treatment cessations | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.86] |
| 18 Maternal pulmonary oedema | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.34] |
| 19 Death unrelated to congenital abnormalities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 In utero | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.34] |
| 19.2 In first 28 days of life | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.19, 22.38] |
| 19.3 After 28 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Use of mechanical ventilation | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.54] |
| 21 Chronic lung disease | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.43, 2.51] |
| 22 Admission of infant to neonatal intensive care unit | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.80] |
| 23 Birthweight | 2 | 182 | Mean Difference (IV, Fixed, 95% CI) | 440.39 [237.35, 643.44] |
Comparison 3. Nitric oxide donors versus magnesium sulphate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.99, 5.87] |
| 2 Dizziness | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.76, 12.40] |
| 3 Flushing | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.54] |
| 4 Palpitations | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.04, 4.32] |
| 5 Hypotension | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.94 [0.46, 135.65] |
Comparison 4. Nitric oxide donors versus any calcium channel blocker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolongation of pregnancy greater than 48 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.21] |
| 2 Headache | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.69 [0.87, 15.69] |
| 3 Flushing | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.18, 19.08] |
| 4 Hypotension | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.76] |
| 5 Nausea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.11, 3.37] |
| 6 Tachycardia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.23] |
Comparison 5. Nitric oxide donors versus combination of tocolytics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolongation of pregnancy greater than 48 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.91, 1.39] |
| 2 Caesarean section | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.47, 4.78] |
| 3 Headache | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [3.38, 49.96] |
| 4 Flushing | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.68] |
| 5 Nausea | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.47, 4.78] |
| 6 Tachycardia | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.32] |
| 7 Chest pain/tightness | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amorim 2009.
| Methods | Randomisation performed by opaque, sealed envelopes. Computer‐generated, random‐number sequence. Not blinded. Prospective power calculation. Intention‐to‐treat analysis. | |
| Participants | Brazil. 50 pregnant women in preterm labour (defined as at least 4 contractions in 30 minutes, cervical dilatation at 4 cm and associated cervical changes of position, length, consistency or dilatation) between 24 and 34 weeks with a singleton pregnancy. | |
| Interventions | 1. GTN patch 10 mg/24 hrs, 2nd patch added after 6 hrs if contractions had not reduced. If contractions settled, removed at 12 hrs; if not settled by 12 hrs, 250 mg subcutaneous terbutaline was given (n = 26). 2. Nifedipine (Adalat) 10 mg capsule sublingual, repeated after 30 minutes; and if contractions continued, 20 mg every 6 hrs (n = 24). | |
| Outcomes | Primary outcome was time required to obtain effective tocolysis. Secondary outcomes were inhibition of labour within 12 hrs, time taken for tocolysis (up to 6 hrs), recurrence of labour within 24 hrs and premature birth within 48 hrs. Maternal adverse effects (headache; flushing; hypotension; nausea; tachycardia). | |
| Notes | Comment that terbutaline was given to nitroglycerin group at 12 hrs if contractions had not reduced, but no comment on how often this was done. All women got steroids but there is no mention of antibiotics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Envelopes sequentially numbered, sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants, doctors or assessors. Feasible if used placebo patch with active tablet and vice versa. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | As above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Bisits 1998.
| Methods | Unclear how allocation sequence generated, but allocation sequence concealed using sealed, opaque envelopes. No blinding or power calculation. | |
| Participants | Australia. 26 pregnant women in preterm labour (defined as painful, regular contractions at < 5‐minute intervals) with singleton pregnancies between 24‐34 weeks' gestation with cervical dilation < 5 cm with intact or ruptured membranes. | |
| Interventions | (1) GTN patch 10 mg/24 hrs (n = 13). (2) Intravenous albuterol at an initial rate of 25 micrograms/minute (n = 13). | |
| Outcomes | Birth within 24 hrs, birth within 7 days, birth at term, adverse drug reactions, plasma corticotrophin releasing hormone levels. | |
| Notes | Two participants changed from GTN group to albuterol because of persistent contractions. No information on whether women got steroids and/or antibiotic therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how allocation sequence generated. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence concealed using sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Baseline characteristics were described as similar. |
Bisits 2004.
| Methods | Randomisation performed by opaque, sealed envelopes. Each centre had own local random‐number sequence. Not blinded. Prospective power calculation. Intention‐to‐treat analysis. | |
| Participants | Australia, Singapore and Hong Kong. 238 pregnant women in preterm labour (defined as at least 2 contractions in 10 minutes and either positive fibronectin or ruptured membranes but cervical dilatation less than 5 cm) between 24 and 35 weeks with a singleton pregnancy. | |
| Interventions | GTN patch 10 mg/24 hrs , 2nd patch added after one hr if contractions had not stopped. If contractions settled removed at 12 hours, if not settled by 2 hrs rescue treatment with β2 sympathomimetic treatment. (n = 121). The control group received β2 sympathomimetic treatment with either i.v. ritodrine or i.v. salbutamol depending on local practice. | |
| Outcomes | Primary outcome was latency period (number of days from randomisation to birth). Secondary outcomes were maternal strength of contraction, adverse effects and neonatal outcome (short and long term). | |
| Notes | 1/3 GTN rescued with β2 sympathomimetic. No information on whether women got steroids and/or antibiotic therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number tables. |
| Allocation concealment (selection bias) | Low risk | Envelopes sequentially numbered, sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and doctors not blinded. Feasible if placebo patch with active i.v. therapy and vice versa. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups and intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | Not all of the studies pre‐specified outcomes were reported. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
El‐Sayed 1999.
| Methods | Randomisation by independent third party using envelope shuffling to generate allocation sequence. Concealment maintained by opaque, sealed envelopes. No blinding. No power calculation. | |
| Participants | USA. 30 pregnant women in preterm labour (defined as the occurrence of at least 2 contractions in 10 minutes, with cervical change or ruptured membranes) under 35 weeks' gestation with singleton or multiple pregnancies with cervical dilation less than 4 cm with intact or ruptured membranes. | |
| Interventions | (1) Nitroglycerin i.v. 100 microgram bolus then 1 microgram/kg/minute up to maximum of 10 micrograms/kg/minute (n = 16). (2) Intravenous magnesium sulphate 4 g bolus, then 2 g/hr up to maximum of 4 g/hr (n = 14). | |
| Outcomes | 12 hrs of successful tocolysis, maternal adverse effects (headache; dizziness; flushing; palpitations; hypotension), serial maternal BP and heart rate changes and fetal heart rate changes. | |
| Notes | 1 woman in each group discontinued treatment because of adverse effects. All participants received hydration with Ringers lactate solution as well as randomised therapy. No information on whether women got steroids and/or antibiotic therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelope shuffling. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups appeared similar at baseline. |
Haghighi 2005.
| Methods | Randomised by sequential use of opaque, sealed envelopes numbered using random‐number tables. Double blinding implied. No power calculation. No intention‐to‐treat analysis. | |
| Participants | Iran. 150 pregnant women in preterm labour (defined as more than 8 uterine contractions per hr that lasted longer than 30 seconds and progressive cervical dilatation z1 cm during a 3.5‐h observation) between 33 and 36 weeks with a singleton pregnancy. | |
| Interventions | 1. Isosorbide dinitrate sublingual tablet 5 mg, repeated every 30 minutes up to 40 mg or stop of contractions. 10 mg 1 hour after halt of contractions and every 6 hrs for 48 hrs (n = 75). 2. Placebo (n = 75). |
|
| Outcomes | Primary outcome was preterm birth. Secondary outcomes were adverse effects and Apgar scores. | |
| Notes | Both groups first administered 50 mg i.v. of meperidine in 500 mL of Ringer solution over 30 minutes, followed by 100 mL per hr of the same for 3 hrs. No information on whether women got steroids and/or antibiotic therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number tables. |
| Allocation concealment (selection bias) | Low risk | Envelopes sequentially numbered, sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded, unclear re personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 81 women randomised to isosorbide dinitrate group, 6 were excluded for reasons that may have related to treatment allocation (hypotension). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | Unclear risk | Little information on participant characteristics. |
He 2002.
| Methods | Randomisation by computer. No blinding. No mention of power calculation or intention‐to‐treat analysis. | |
| Participants | China. 60 pregnant women in preterm labour (defined as contracting every 5‐10 minutes lasting 30 seconds with cervix more than 2 cm dilated) between 28 and 37 weeks with intact membranes. No comment on multiple pregnancies. Not blinded. No power calculation mentioned and no intention‐to‐treat analysis mentioned. | |
| Interventions | 1. GTN patch 5 mg/24 hrs with another patch added every hr until contractions stopped or 25 mg maximum dose reached. Patches changed every 24 hrs (n = 30). 2. Magnesium sulphate and salbutamol, no doses stated (n = 30). |
|
| Outcomes | Percentage of women in whom contractions stopped, days delay of labour, pre and post‐treatment corticotrophin releasing hormone levels. | |
| Notes | No information on whether women got steroids and/or antibiotic therapy. Translated by Cochrane. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition and exclusions to permit judgement, e.g. number randomised not stated or no reasons for missing data provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | Unclear risk | Not addressed in translation. |
Lees 1999.
| Methods | Random lists prepared centrally used to generate allocation sequence. Randomisation was by random‐permuted blocks, stratified by centre. Central randomisation and sealed envelopes then used to conceal allocation sequence. No blinding. Power calculation performed prospectively. Intention‐to‐treat analysis. | |
| Participants | UK, Europe and Indonesia. 245 pregnant women in preterm labour (defined as painful, regular uterine contractions > 2 in 10 for 1 hr or more) between 24 and 36 weeks' gestation with singleton or multiple pregnancies with intact membranes. | |
| Interventions | (1) GTN patch 10 mg/24 hrs (46%) or 20 mg/24 hrs (54%) n = 120. (2) i.v. ritodrine starting at 50 microgram/minute (n = 125). | |
| Outcomes | Prolongation of pregnancy expressed as a percentage of the time from entry to 37 weeks, proportion of women who delivered the same day, next day, within 7 or 14 days and by 32, 34 and 37 weeks. Maternal adverse effects (headache; dizziness; palpitations; shortness of breath; nausea; tachycardia; chest pain/tightness; cessation of drug). | |
| Notes | Outcome data missing for 4.8% women. No information on whether women got steroids and/or antibiotic therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random lists prepared centrally used to generate allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Central randomisation and sealed envelopes then used to conceal allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 245 randomised, some loss to follow‐up, 233/245 followed up to birth. There were missing data for adverse effects. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Baseline characteristics were comparable. There some treatment cross‐overs and some women did not receive treatment as planned or treatment was discontinued. |
Schleussner 2001.
| Methods | Randomised. No blinding. No mention of power calculation or intention‐to‐treat analysis. | |
| Participants | 50 pregnant women between 27 and 35 weeks. Germany. | |
| Interventions | 1. GTN patches 0.4‐0.8 mg/hr (n = 28). 2. Fenoterol 60‐120 micrograms/hr (n = 22). |
|
| Outcomes | Primary outcomes were prolongation of pregnancy and neonatal outcome. Secondary outcomes were cervical ripening and maternal adverse effects. | |
| Notes | Only abstract available in English. No information on whether women got steroids and/or antibiotic therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by hospital number. |
| Allocation concealment (selection bias) | High risk | Allocation on basis of hospital number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Different treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Different treatment regimens. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clear. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from translated notes. |
| Other bias | Unclear risk | Assessment from translated notes. |
Smith 1999.
| Methods | Randomisation with stratification by gestational age (< and > 30 weeks) and blocking in groups of 2 were performed before the study by the clinical investigations unit of the hospital. Sealed study envelopes coded with the randomisation number and used in order of randomisation number, contained the study patches, instructions for the nurses and bags to collect all used and unused patches. Therapy blinded to participant, doctor and outcome assessor although not to nurses who either peeled off the impermeable protective backing to use the active patch or kept the backing on if placebo was required. Both patches were covered with non‐transparent tape so would appear similar to all participants. Intention‐to‐treat analysis. Designed as pilot study. Post‐trial power calculation performed to determine numbers required for larger multi‐centre trial. | |
| Participants | Canada. 33 pregnant women in preterm labour (defined as evidence of cervical change over 1‐2 hrs) between 24 and 34 weeks' gestation with singleton or twin pregnancies with intact membranes and cervical dilatation < 4 cm. | |
| Interventions | (1) GTN transdermal patch 9.6 mg/24 hrs for 48 hrs (n = 17). (2) Placebo (n = 16). | |
| Outcomes | Prolongation of pregnancy > 48 hrs, completed course of maternal steroids, adverse drug reactions. | |
| Notes | Both groups also received a 1000 mL i.v. infusion of 0.9% saline over 1‐2 hrs before randomisation applying the patch. Women received betamethasone and antibiotics at discretion of attending physician but numbers same in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by pharmacy using randomisation number. |
| Allocation concealment (selection bias) | Low risk | Envelopes sequentially numbered, sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and doctors blinded, not nurses who applied the patch. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33 women randomised and all accounted for in the analysis. Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Baseline characteristics of groups appeared similar. |
Smith 2007.
| Methods | Web‐based randomisation by independent company, stratified by centre and gestation with random‐block sizes of 2, 4 or 6. Opaque envelopes. Double blinded. Prospective power calculation performed. Intention‐to‐treat analysis. | |
| Participants | Canada.153 pregnant women in preterm labour (defined as 4 painful uterine contractions per 20 minutes and evidence of cervical change, i.e. change in Bishop score or Bishop score 6) between 24 and 32 weeks with a singleton pregnancy and intact membranes. | |
| Interventions | 1. GTN patch 0.4 mg/hr, with another added 1 hr later if still labouring, patches replaced after 24 hrs (n = 74). 2. Placebo patch (n = 79). |
|
| Outcomes | Composite outcome of serious neonatal morbidity and perinatal mortality, chronic lung disease, necrotising enterocolitis, grade III or i.v. intraventricular haemorrhage, periventricular leukomalacia, birth at less than 48 hrs, and before 28, 34, and 37 weeks' gestation, completion of course of corticosteroids. | |
| Notes | Recruitment stopped early due to unavailability of ritodrine. Many units switched to GTN patch as standard practice. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Envelopes sequentially numbered, sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and doctors blinded, not nurses who applied the patch. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced across intervention groups |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and all prespecified outcomes that are of interest have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Study stopped early due to recruitment difficulties. |
Szulc 2000.
| Methods | Randomised. Not blinded. No power calculation. Intention‐to‐treat analysis. | |
| Participants | Poland. 60 pregnant women in preterm labour (defined as contracting 4/20, cervical shortening 60%) between 23 and 34 weeks with a singleton pregnancy and intact membranes. | |
| Interventions | 1. GTN patch 10 mg/24 hrs , 2nd 5 mg patch added after 1 hr if contractions had not stopped. Replaced after 24 hrs (n = 30). 2. Fenoterol 1 mg and Isoptin 10 mg i.v., then 5 mg fenoterol and 40 mg Isoptin PO repeated 4‐6 times per day (n = 30). |
|
| Outcomes | Mean prolongation of pregnancy, maternal adverse effects (headache; flushing; nausea; tachycardia; chest pain/tightness) and neonatal outcome. | |
| Notes | Translated from Polish. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised coding. |
| Allocation concealment (selection bias) | Unclear risk | Unclear what method used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition or exclusions to permit judgement, e.g. number randomised not stated or no reasons for missing data provided. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (assessment from translated notes). |
| Other bias | Unclear risk | Original article in Polish, assessment from translated notes. |
Wani 2004.
| Methods | Randomisation by sequentially numbered, opaque envelopes. Not blinded. No power calculation. Intention‐to‐treat analysis. | |
| Participants | United Arab Emirates. 132 pregnant women in preterm labour (defined as painful, regular contractions (> 20/hrs) and/or cervical dilatation of 2 cm or more) between 23 and 34 weeks with a singleton pregnancy and intact membranes. | |
| Interventions | GTN patch 10mg/24 hrs, 2nd patch added after 1 hr if contractions had not stopped, replaced after 24 hrs (maximum 5 days) (n = 67). i.v. ritodrine150 mg/minute with 50 mg increment until contractions ceased, a maximum dose of 350 mg/minute was reached or the occurrence of adverse effects (maximum 3 days) (n = 65). |
|
| Outcomes | Prolongation of pregnancy, neonatal outcome and maternal adverse effects (headache; palpitations; nausea; tachycardia; chest pain/tightness; cessation of drug). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no mention of how sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Envelopes sequentially numbered, sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dropouts accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
BP: blood pressure GTN: glyceryl trinitrate hr(s): hour(s) i.v.: intravenous PO: oral
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bulgay‐Moerschel 2008 | Not clear how many women assigned to each intervention group. |
| Clavin 1996 | Preliminary data. Denominator of total women randomised unclear. Author contacted. Data lost in Hurricane Katrina. |
| Groom 2000 | Letter, not RCT. |
| Hogberg 1998 | Does not include relevant comparisons as both groups received terbutaline as a tocolytic with 1 group having additional nitroglycerin and the other having terbutaline alone. |
| Leszczynska 2001 | Not RCT. |
| Pasargiklian 1983 | NO donor not used. |
| Rytlewski 2008 | Both intervention groups received standard tocolysis with MgSo4 and a B2 agonist. Later, 1 group received L‐arginine, a NO precursor, or placebo. |
NO: nitric oxide RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Simsek 2000.
| Methods | Little information on study methods. Stated that women were "randomly selected" to receive 1 of the 2 treatments. |
| Participants | 24 women admitted to hospital with preterm labour with gestational age of 20‐34 weeks. |
| Interventions | Glyceryl trinitrate (patches) or ritodrine HCL infusion. |
| Outcomes | "The interval of therapy to birth was found to be 29.2+/‐20.8 (mean+/‐SD) in GTN and 18.0+/‐16.2 (mean+/‐SD) for the ritodrine HCL group." |
| Notes | Brief abstract identified by search. We are seeking the full text article. |
GTN: glyceryl trinitrate HCL: hydrochloride
Differences between protocol and review
Analysis 1.17 assessed a non‐prespecified composite outcome: serious infant morbidity or mortality. This is one of the core outcomes, which has been added to this review for the 2014 update.
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of individual Cochrane reviews. Consensus was reached on a number of ‘core’ outcomes, as outlined in the methods section. These will be included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Contributions of authors
ST wrote the background. ST and KD wrote the original protocol and first edition of the review. KD and OO assessed the trials for inclusion and exclusion, performed data extraction and assessed risk of bias for this review update. KD was responsible for data input. TD checked data input, updated the methods section and wrote the results section. All authors contributed to the final editing and take responsibility for the overall discussion and conclusions of the review.
Sources of support
Internal sources
-
Nuffield Department of Obstetrics and Gynaecology, Oxford University, UK.
For the first edition of this review
-
University of Warwick, UK.
For the first edition of this review
University of Liverpool, UK.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
ST provides commercial consultancy advice. None of these companies make any of the drugs included in the review. KD has received honoraria from Merck, Bayer and Actavis. None of these companies produce tocolytics. TD has been paid from a grant from WHO for work on this review; WHO has had no influence over the findings or conclusions of the review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Amorim 2009 {published data only}
- Amorim MM, Lippo LA, Costa AA, Coutinho IC, Souza AS. Transdermal nitroglycerin versus oral nifedipine administration for tocolysis: a randomized clinical trial [Nitroglicerina transdermica versus nifedipina oral para inibicao do trabalho de parto prematuro: ensaio clinico randomizado]. Revista Brasileira de Ginecologia e Obstetricia 2009;31(11):552‐8. [PubMed] [Google Scholar]
Bisits 1998 {published data only}
- Bisits A, Madsen G, McLean M, O'Callaghan S, Smith R, Giles W. Corticotrophin‐releasing hormone: a biochemical predictor of preterm delivery in a pilot randomized trial of the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1998;178:862‐6. [DOI] [PubMed] [Google Scholar]
Bisits 2004 {published data only}
- Bisits A, Madsen G, Knox M, Gill A, Smith R, Yeo G, et al. The Randomized Nitric Oxide Tocolysis Trial (RNOTT) for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 2004;191(3):683‐90. [DOI] [PubMed] [Google Scholar]
- Giles W, Knox M, Madsden G, Bisits A, Gill A, Smith R, et al. The randomised nitric oxide tocolysis trial. American Journal of Obstetrics and Gynecology 2001;184(1):S6. [DOI] [PubMed] [Google Scholar]
- Gill A, Giles W, Bisits A, Madsen G, Knox M, Tudehope D, et al. Neonatal neurodevelopmental outcomes following tocolysis with glycerol trinitrate patches and iv beta 2 agonist therapy [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S117. [DOI] [PubMed] [Google Scholar]
- Gill A, Madsen G, Knox M, Bisits A, Giles W, Tudehope D, et al. Neonatal neurodevelopmental outcomes following tocolysis with glycerol trinitrate patches. American Journal of Obstetrics and Gynecology 2006;195(2):484‐7. [DOI] [PubMed] [Google Scholar]
El‐Sayed 1999 {published data only}
- El‐Sayed YY, Riley ET, Holbrook RH, Cohen SE, Chitkara U, Druzin ML. Randomized comparison of intravenous nitroglycerin and magnesium sulfate for treatment of preterm labour. Obstetrics and Gynecology 1999;93:79‐83. [DOI] [PubMed] [Google Scholar]
Haghighi 2005 {published data only}
- Haghighi L, Akbarian A. Isosorbide dinitrate for treatment of preterm labor. International Journal of Gynecology & Obstetrics 2005;89:274‐5. [DOI] [PubMed] [Google Scholar]
He 2002 {published data only}
- He Q, Sha J, Gu Q, Gu H, Chen X, Yang Z, et al. Clinical effect and mechanism of nitroglycerin patch on arresting preterm labor. [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2002;37(3):134‐5. [PubMed] [Google Scholar]
Lees 1999 {published data only}
- Black RS, Lees C, Thompson C, Pickles A, Campbell S. Maternal and fetal cardiovascular effects of transdermal glyceryl trinitrate and intravenous ritodrine. Obstetrics and Gynecology 1999;94(4):572‐6. [DOI] [PubMed] [Google Scholar]
- Lees CC, Lojacono A, Thompson C, Danti L, Black RS, Tanzi P, et al for the GTN preterm labour investigation group. Glyceryl trinitrate and ritodrine in tocolysis: an international multicenter randomized study. Obstetrics and Gynecology 1999;94(3):403‐8. [DOI] [PubMed] [Google Scholar]
Schleussner 2001 {published data only}
- Schleussner E, Moller A, Gross W, Kahler C, Moller U, Richter S, et al. Maternal and fetal side effects of tocolysis using transdermal nitroglycerin or intravenous fenoterol combined with magnesium sulfate. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:14‐9. [DOI] [PubMed] [Google Scholar]
- Schleussner E, Richter S, Gross W, Kahler C, Moller A, Moller U, et al. Glyceryl trinitrate patches versus fenoterol intravenous in tocolysis ‐ a prospective randomized trial [Nitroglyzerinpflaster zur Tocolyse ‐ ein prospectiv randomisierter Vergleich mit fenoterol per infusionem]. Zeitschrift fur Geburtshilfe und Neonatologie 2001;205:189‐94. [DOI] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith GN, Walker MC, McGrath MJ. Randomized double‐blind, placebo controlled trial assessing nitroglycerin as a tocolytic. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S181. [Google Scholar]
- Smith GN, Walker MC, McGrath MJ. Randomized double‐blind, placebo controlled trial assessing nitroglycerin as a tocolytic. British Journal of Obstetrics and Gynaecology 1999;106(7):736‐9. [DOI] [PubMed] [Google Scholar]
Smith 2007 {published data only}
- Guo Y, Longo CJ, Xie R, Wen SW, Walker MC, Smith GN. Cost‐effectiveness of transdermal nitroglycerin use for preterm labor. Value in Health 2011;14(2):240‐6. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xie R, Wen SW, Walker MC, Smith GN. Maternal transdermal nitroglycerin use and early childhood development. Journal of Obstetrics and Gynaecology Canada 2010;32(12):1147‐52. [DOI] [PubMed] [Google Scholar]
- Smith G. Canadian preterm labour nitroglycerin trial. Personal communication 2002.
- Smith G. The Canadian preterm labour nitroglycerin trial. Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 2005).
- Smith G, Walker M, Ohlsson A, O'Brien K, Rory W. Transdermal nitroglycerin for preterm labor [abstract]. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco, CA, USA. San Francisco, 2006.
- Smith GN, Guo Y, Wen SW, Walker MC. Secondary analysis of the use of transdermal nitroglycerin for preterm labor. American Journal of Obstetrics and Gynecology 2010;203(6):565.e1‐565.e6. [DOI] [PubMed] [Google Scholar]
- Smith GN, Walker MC, Ohlsson A, O'Brien K, Windrim R, for the Canadian Preterm Labour Nitroglycerin Trial Group. Randomized double‐blind placebo‐controlled trial of transdermal nitroglycerin for preterm labor. American Journal of Obstetrics and Gynecology 2007;196(1):37.e1‐37.e8. [DOI] [PubMed] [Google Scholar]
Szulc 2000 {published data only}
- Szulc E, Leibschang J. Comparative evaluation of efficiency and tolerance of two alternative methods for premature uterine contractions suppression using fenoterol and nitroglycerin [Polish] [Ocena porownawcza skutecznoSci i tolerancji dwoch alternatywnych metod hamowania przedwczesnej czynnoSci skurczowej macicy za pomocy fenoterolu i nitrogliceryny.]. Medycyna Wieku Rozwojowego 2000;4(3):307‐16. [PubMed] [Google Scholar]
Wani 2004 {published data only}
- Rath S. Use of glyceryl trinitrate for preterm labour in a maternity hospital in United Arab Emirates [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:49.
- Wani M, John S, Graham I. A comparison of glyceryl trinitrate and ritodrine for the treatment of threatened preterm labour. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. London, UK: RCOG Press, 1999:62.
- Wani MP, Barakzai N, Graham I. Glyceryl trinitrate vs ritodrine for the treatment of preterm labor. International Journal of Gynecology & Obstetrics 2004;85:165‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bulgay‐Moerschel 2008 {published data only}
- Bulgay‐Moerschel M, Schneider U, Schleussner E. Tocolysis with nitroglycerin patches vs. fenoterol i.v. ‐ results of a randomized multicenter study. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):115. [Google Scholar]
Clavin 1996 {published data only}
- Clavin DK, Bayhi DA, Nolan TE, Rigby FB, Cork RC, Miller JM. Comparison of intravenous magnesium sulfate and nitroglycerin for preterm labor: preliminary data [abstract]. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):307. [Google Scholar]
Groom 2000 {published data only}
- Groom KM, Bennett PR, Shennan AH. Randomised, double‐blind, placebo controlled pilot study assessing nitroglycerin as a tocolytic [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1182‐3. [DOI] [PubMed] [Google Scholar]
Hogberg 1998 {published data only}
- Hogberg U. Nitroglycerin and terbutalin versus placebo and terbutalin ‐ a randomized controlled study for preterm labour. Personal communication January 21 1998.
Leszczynska 2001 {published data only}
- Leszczynska‐Gorzelak B, Laskowska M, Marciniak B, Oleszczuk J. Nitric oxide for treatment of threatened preterm labour. International Journal of Gynecology and Obstetrics 2001;73(3):201‐6. [DOI] [PubMed] [Google Scholar]
Pasargiklian 1983 {published data only}
- Pasargiklian R, Monti G, Bertulessi C. Clenbuterol in the treatment of premature labor [Italian] [Il clenbuterolo nel trattamento del parto pretermine]. Minerva Ginecologica 1983;35:423‐9. [PubMed] [Google Scholar]
Rytlewski 2008 {published data only}
- Rytlewski K, Olszanecki R, Lauterbach R, Grzyb A, Kiec‐Wilk B, Dembinska‐Kiec A, et al. Effects of oral L‐arginine on the pulsatility indices of umbilical artery and middle cerebral artery in preterm labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;138(1):23‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Simsek 2000 {published data only}
- Simsek T, Ozgur K, Sarlak O, Trak B, Uner M. Gliseryl trinitrate patches are as effective as ritodrin HCL in the treatment of preterm labor. Jinekoloji Ve Obstetri Bulteni 2000;9:63‐5. [Google Scholar]
Additional references
Anotayanonth 2004
- Anotayanonth S, Subhedar NV, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004352.pub2] [DOI] [PubMed] [Google Scholar]
Arthur 2008
- Arthur P, Taggart MJ, Zielnik B, Wong S, Mitchell BF. Relationship between gene expression and function of uterotonic systems in the rat during gestation, uterine activation and both term and preterm labour. Journal of Physiology 2008;586:6063‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buhimschi 1995
- Buhimschi I, Yallampalli C, Dong YL, Garfield RE. Involvement of a nitric oxide‐cyclic guanosine‐monophosphate pathway in control of human uterine contractility in pregnancy. American Journal of Obstetrics and Gynecology 1995;172:1577‐84. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1. Oxford, England: The Cochrane Collaboration, 2000.
Haas 2012
- Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta‐analysis. BMJ 2012;345:e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
King 2002
- King JF, Flenady V, Papatsonis D, Dekker G, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002255] [DOI] [PubMed] [Google Scholar]
King 2005
- King J, Flenady V, Cole S, Thornton S. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001992.pub2] [DOI] [PubMed] [Google Scholar]
Kinney 2012
- Kinney MV, Howson CP, McDougall L, Lawn JE (editors). March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
Momohara 2004
- Momohara Y, Sakamoto S, Obayashi S, Aso T, Goto M, Azuma H. Roles of endogenous nitric oxide synthase inhibitors and endothelin‐1 for regulating myometrial contractions during gestation in the rat. Molecular Human Reproduction 2004;7:505‐12. [DOI] [PubMed] [Google Scholar]
Norman 1996
- Norman J. Nitric oxide and the myometrium. Pharmacology and Therapeutics 1996;70:91‐100. [DOI] [PubMed] [Google Scholar]
Okawa 2005
- Okawa T, Asano K, Takahashi H, Sato A, Vedernikov YP, Saade GR, et al. Nitric oxide donor‐induced inhibition of pregnant rat uterine spontaneous contractile activity and release of nitric oxide from uterus measured by microdialysis. Journal of Endocrinological Investigation 2005;11:998‐1002. [DOI] [PubMed] [Google Scholar]
RCOG 2011
- Guidelines Committee of the Royal College of Obstetricians and Gynaecologists. Tocolysis for Women in Preterm Labour. Green‐top Guidelines 1B. London: RCOG, 2011. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Sladek 1993
- Sladek SM, Regenstein AC, Lykins D, Roberts JM. Nitric oxide synthase activity in pregnant rabbit uterus decreases on the last day of pregnancy. American Journal of Obstetrics and Gynecology 1993;169:1285‐91. [DOI] [PubMed] [Google Scholar]
Stevenson 1996
- Stevenson RC, Pharoah PO, Stevenson CJ, McCabe CJ, Cooke RW. Cost of care for a geographically determined population of low birthweight infants to age 8‐9 years. II. Children with disability. Archives of Disease in Childhood 1996;74:F118‐F121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yallampalli 1993
- Yallampalli C, Garfield RE, Byam‐Smith M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology 1993;133:1899‐902. [DOI] [PubMed] [Google Scholar]
Yallampalli 1994
- Yallampalli C, Izumi H, Byam‐Smith M, Garfield RE. An l‐arginine‐nitric oxide‐cyclic guanosine monophosphate system exists in the uterus and inhibits contractility during pregnancy. American Journal of Obstetrics and Gynecology 1994;170:175‐85. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Duckitt 2002
- Duckitt K, Thornton S. Nitric oxide donors for the treatment of preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002860] [DOI] [PubMed] [Google Scholar]


