Abstract:
Since the initial deployment of neonatal extracorporeal membrane oxygenation (ECMO) for respiratory failure, the use of ECMO in this population has diversified. We present a term female infant with carbamoyl phosphate synthetase 1 and partial N-acetylglutamate synthase deficiencies who developed severe hyperammonemia refractory to medical management requiring venoarterial ECMO-driven continuous veno-venous hemodiafiltration for ammonia detoxification. This case report illustrates a subpopulation where neonatal ECMO may improve survival and neurodevelopmental outcomes. To our knowledge, this is the first reported case of a urea cycle defect arising from two proximal enzyme deficiencies. Also, this is one of the few reported patients with UCD associated with peak ammonia levels >2,000 μmol/L who survived to hospital discharge after the successful use of ECMO for ammonia reduction. This case will add to the existing scant literature supporting the use of ECMO as a platform for rapid removal of serum ammonia.
Keywords: hyperammonemia, urea cycle disorder, ECMO (extracorporeal membrane oxygenation), CVVH (continuous veno-venous hemofiltration)
OVERVIEW
Urea cycle disorders (UCDs) are hereditary inborn errors of metabolism that arise from genetic mutations causing variable deficiencies in enzymes and cofactors necessary for the metabolism of nitrogenous waste. These disorders are extremely rare in newborns, with an estimated prevalence of 1:35,000 births in the United States (1). Carbamoyl phosphate synthetase 1 (CPS1) and N-acetylglutamate synthase (NAGS) deficiencies are autosomal recessive forms of UCDs. They represent the rarest and most severe types of this disorder, with an estimated incidence of 1:1,300,000 and 1:2,000,000 births, respectively (1). Both conditions typically present with severe hyperammonemia in the newborn period, which could result in severe neurologic impairment (2).
Although nitrogen-scavenger drugs, hemodialysis (HD), and continuous veno-venous hemofiltration (CVVH) are commonly used in the acute management of UCDs, severe hyperammonemia refractory to these therapies may warrant consideration of extracorporeal membrane oxygenation (ECMO) (3). Currently, limited literature supports the use of ECMO for refractory hyperammonemia (3,4), and despite this technology, survival among neonates with initial or peak ammonia levels >1,860 μmol/L is rare in UCDs or organic acidemias (3,4). We describe a newborn with peak ammonia level >2,000 μmol/L secondary to CPS 1 and partial NAGS deficiencies in whom venoarterial (VA) ECMO was successfully used in conjunction with hemofiltration, resulting in rapid ammonia detoxification.
DESCRIPTION
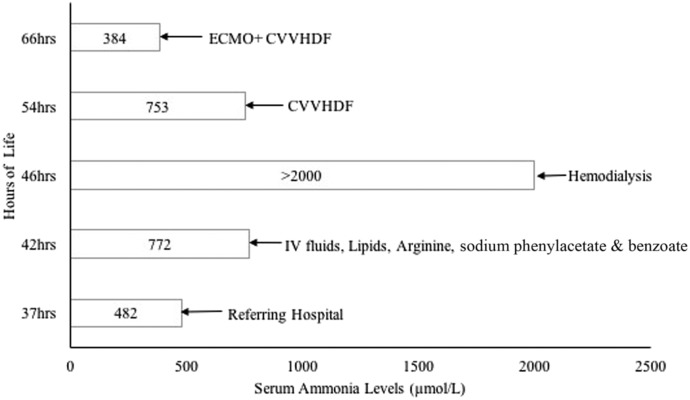
A term 3,508-g female infant was born to a 32-year-old G3P2 female via cesarean section due to failure to progress in labor. Family history was significant for consanguinity and a prior neonatal death of a term female infant with unknown etiology in Bangladesh. Her APGAR scores were 7 and 9 at 1 and 5 minutes of life, respectively. She developed respiratory distress after an initial uneventful 24 hours of life (HOL) in the well-baby nursery. Physical examination revealed an appropriate-for-gestational-age tachypneic infant in mild respiratory distress. She was pale and dusky with global hypotonia and hyporeflexia. A sepsis evaluation was performed, and intravenous (IV) ampicillin and gentamicin were started for suspected sepsis. Her initial workup was remarkable for respiratory alkalosis with an arterial blood gas. She subsequently developed poor feeding and irritability followed by lethargy, with a concurrent ammonia level of 482 μmol/L at 37 HOL. Hiccups and jerking of the limbs ensued with an ammonia level of 772 μmol/L.
The infant was transferred to our institution for evaluation and management. She required intubation and mechanical ventilation for apnea and worsening lethargy and was started on IV dextrose-containing fluids, lipids, sodium benzoate, sodium phenylacetate, and arginine for hyperammonemia. Despite medical management, ammonia levels continued to increase rapidly, with peak ammonia level >2,000 μmol/L at 45 HOL (Figure 1). HD with the Prismaflex 2000 system (Baxter International, Deerfield, IL) via the right internal jugular vein with a Medcomp 7-French (Fr) soft line Duo-Flow catheter primed with 5% albumin was carried out for 4 hours. Her clinical course was complicated with worsening hypotension requiring dopamine. She was then transitioned to continuous veno-venous hemodiafiltration (CVVHDF), which ran for 12 hours using Gambro M60 filter (Baxter International, Deerfield, IL) primed with 5% albumin. The CVVHDF blood flow rate was 40–50 mL/min, and PrismaSol BGK 2/3.5 replacement solution (Baxter International) was used. Despite 12 hours of CVVHDF and increasing the blood flow rate to 70 mL/min, the serum ammonia levels remained markedly elevated at 62 HOL with a subsequent increase in circuit hemolysis and worsening hypotension.
Figure 1.
Medical interventions.
Persistently elevated ammonia levels following a combination of maximal medical therapy, HD, and CVVHDF with concurrent hemodynamic instability prompted initiation of ECMO. VA ECMO was used because of pressor needs and anticipated worsening of hypotension from the use of IV nitrogen scavengers and to prevent impending circulatory collapse. The HD cannula in the right internal jugular vein was exchanged for a 12-Fr Medtronic venous cannula, and the right carotid artery was cannulated using a 10-Fr Medtronic arterial cannula. The infant was successfully placed on VA ECMO at 66 HOL after a 100 units/kg heparin bolus using a S5 roller pump (Sorin Group, Deutschland GMBH Munchen, Germany) with a venous compliance reservoir, Quadrox-iD pediatric oxygenator (Maquet Getinge Group, Rastatt, Germany), and a Tygon S-95-E 1⁄4-inch tubing. The circuit was primed with Plasma-Lyte A (pH 7.4), albumin, sodium bicarbonate, calcium gluconate, packed red blood cells, and fresh frozen plasma for a total volume of approximately 400 mL.
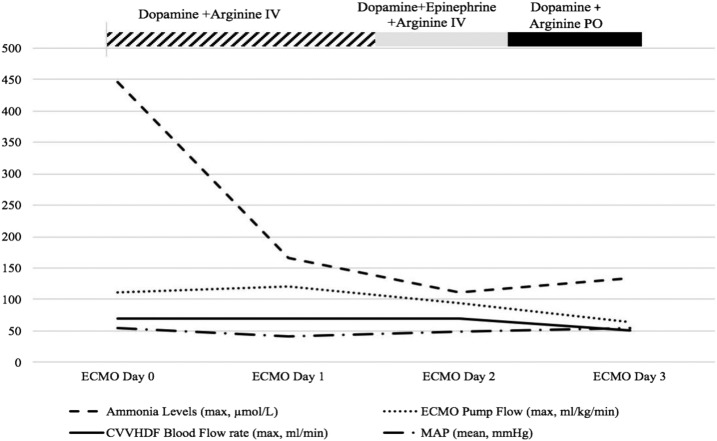
Shortly after going on ECMO, the infant was placed back on CVVHDF via the ECMO circuit. The CVVHDF inlet blood line was connected to the ECMO circuit post-pump, pre-oxygenator, and the CVVHDF blood return line was connected in the same section of the circuit more proximal to the oxygenator. The ECMO pump flow ranged between 77 and 105 mL/kg/min (Figure 2). After 5 hours on ECMO+CVVHDF, the serum ammonia level decreased to 211 μmol/L and subsequently to 115 μmol/L at 10 hours. A transient dilutional effect cannot be underestimated, given the increase in volume of distribution. Over the next 24 hours while on VA-ECMO, hypotension worsened requiring escalation of dopamine therapy and eventual transition to epinephrine. This was carried out to mitigate the vasodilatory effects of IV arginine, which was thought to be partially responsible for the systemic hypotension. Arginine therapy was changed to oral, citrulline was added for suspected proximal UCD by ECMO day 3, and epinephrine was successfully discontinued without adverse effects. Ammonia levels were adequately maintained at <200 μmol/L despite a steadily increasing daily protein challenge. The infant was successfully taken off ECMO after 3.5 days (an 87-hour run).
Figure 2.
Ammonia levels, ECMO/CVVHDF parameters, and medications.
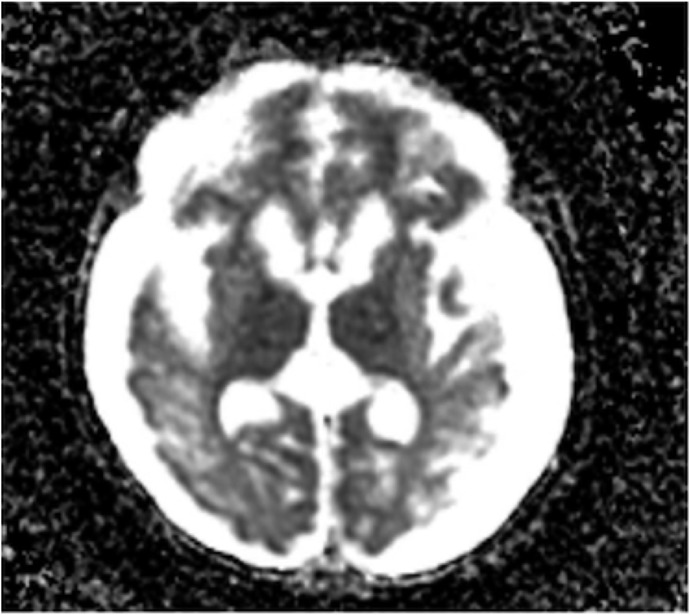
Following ECMO therapy, she was maintained on nitrogen-scavenger drugs with further increase in dietary protein (maximum of 1.2 g/kg/day) using a specialized formula. During her hospitalization, she had recurrent episodes of feeding intolerance associated with severe emesis refractory to antiemetic therapy, and as a result, she had a gastrostomy tube placed for optimization of nutrition and medication delivery. Her hospital course was complicated by rhinovirus infection on day of life (DOL) 60, which required HD followed by CVVHDF because of hyperammonemic crisis (serum ammonia levels of 565 μmol/L). She also had recurrence of seizures, confirmed by video electroencephalogram. Magnetic resonance imaging (MRI) of the brain showed linear T1 and T2 signal abnormality throughout the subcortical white matter (Figure 3). Her serum ammonia levels remained <250 μmol/L post-CVVHDF, and her seizure episodes were controlled with phenobarbital therapy.
Figure 3.
Brain MRI without contrast. Linear T1 and T2 signal abnormality throughout the subcortical white matter; basal ganglia signal abnormality with restriction of diffusion is also seen in the right frontal lobe.
Genetic testing revealed homozygous CPS1 mutation (c.2945G>A, p.G982D) and heterozygous NAGS mutation (c.1024C>T, p.R342C). Following the confirmation of this diagnosis, carglumic acid was added to her medication regimen on DOL 83 after which her ammonia levels were adequately maintained below 100 μmol/L for most of the hospitalization. She was eventually discharged to a chronic care facility at 5.5 months of age, and her ammonia levels remained stable despite an influenza viral infection within 1 week after discharge.
COMMENT
CPS-1 and NAGS deficiencies are rare severe forms of UCDs which present with life-threatening hyperammonemia, and occurrence of these two enzyme mutations in the same individual is uncommon. Both conditions present in the neonatal period with feeding difficulties, vomiting, somnolence, hypotonia, hypothermia, seizures, coma, apnea, and possibly death. Rapid accumulation of ammonia and other precursor metabolites results in acute cerebral edema and neurologic compromise (3). The specific mechanism of ammonia-induced neuropathology remains unclear; however, possible pathogenic mechanisms include glutamine-induced cerebral edema, energy failure, and neurotransmitter alteration (5). Neurologic outcomes depend on the duration of hyperammonemic coma and the extent of ammonia elevation, hence the need for rapid and effective treatment (3,6).
Initial management of mild cases of UCDs includes the use of IV sodium benzoate, sodium phenylacetate, and arginine (6). These medications work primarily to bypass the urea cycle by conjugation of benzoate with glycine to generate hippurate, or phenylacetate with glutamine to generate phenylacetylglutamine (7,8). These conjugates have a higher renal clearance and, therefore, are excreted more rapidly in the urine than ammonia. These medications, while effective, have significant adverse effects that can make long-term tolerability and compliance a challenge (7).
Severe cases of hyperammonemia require dialysis as the primary modality for rapid ammonia clearance (3,5,6). HD has been shown to be effective; however, its prolonged use is limited by the small intravascular volume and the increased risk of hemodynamic instability in newborns (3,6). Peritoneal dialysis (PD) is not effective in these cases because of its slow rate of ammonia clearance of 3–5 mL/min when compared with CVVH, which has a clearance rate of 10–30 mL/min. In addition, PD carries an increased risk of infection. Clearance rates as high as 170–200 mL/min can be achieved when ECMO is used in combination with HD/hemofiltration (6). The combination of both modalities has also been successfully used for ammonia detoxification in some patients with UCDs and supported the decision to use this strategy in this patient (3,6).
This patient suffered from refractory severe hyperammonemia for nearly 24 hours despite aggressive medical management, HD, and CVVHDF. Prolonged duration of plasma ammonia levels above the safe limit, described as >200 μg/dL (>117 μmol/L), is associated with increased mortality and supported the decision to initiate ECMO+CVVHDF while continuing medical management to achieve this goal (9). Measured ammonia levels declined to medically manageable levels within 5 hours and normalized within 10 hours of ECMO+CVVHDF initiation. These findings are consistent with results published by Robinson et al. (3), who demonstrated that the use of ECMO/HD allowed for ammonia reduction to within a medically manageable range with a median of 4.5 hours, median ammonia normalization of 7.3 hours, and median ECMO run time of 50.3 hours. It is important to note that this study only included patients with single enzyme deficiencies. Multienzyme proximal deficiency in this infant may have played a role in prolonging the time required to achieve normalization of ammonia levels after initiation of ECMO (10 hours). In addition, we were faced with the challenge of arginine-associated hypotension, which resulted in the prolonged use of pressors despite VA ECMO support, and both factors could have contributed to the longer than previously reported median ECMO run time. L-Arginine being a precursor of nitric oxide can induce peripheral vasodilation, which can result in hypotension. This systemic effect was mitigated by converting arginine to oral dosing once ammonia levels were normalized.
The threshold for initiating ECMO in patients with hyperammonemia is not clearly defined in the literature, and it is postulated that early utilization of this modality may reduce the duration of coma because of its efficacy in rapid ammonia clearance. This may potentially improve neurodevelopmental outcomes; however, ECMO-related risks must also be taken into consideration.
The outcome of patients with peak ammonia levels >2,000 μmol/L has been reported to be invariably poor, with no infant surviving to discharge in a cohort of 25 infants despite ECMO/HD (3). Contrary to this finding, this infant survived with ECMO despite having peak ammonia levels >2,000 μmol/L during the initial metabolic crisis. In addition to ECMO, another pivotal point in the management of this infant was commencement of carglumic acid despite uncertainty about the phenotypic significance of heterozygosity for NAGS mutation (representing carrier status versus partial deficiency) after diagnostic confirmation. Carglumic acid was approved in 2010 for use in NAGS deficiency. UCD guidelines by Häberle et al. proposed the use of carglumic acid plus ammonia scavengers as initial therapy for undiagnosed patients who present with acute hyperammonenemia, given its relatively safe drug profile (7). It has also been proposed as a therapeutic option for several other urea cycle disorders as it is a structural analog of human NAG, directly replacing NAG and acting as a urea cycle activator. However, it is important to note that its routine use is not indicated in CPS-1 deficiency. Furthermore, it may not be readily available for use in all facilities; hence, emergent initiation of extracorporeal detoxification with CVVHDF and/or ECMO should not be delayed in severe hyperammonemic episodes (10).
UCD is a neonatal emergency when complicated by moderate to severe hyperammonemia. The goal of initial therapy is to normalize ammonia levels rapidly to mitigate neurologic sequela. Medical therapy and HD remain the mainstay of therapy; however, ECMO can serve as a platform for rapid ammonia clearance to reduce neurologic compromise. Early treatment with carglumic acid is beneficial in patients with NAGS deficiency and should be strongly considered.
REFERENCES
- 1.Summar ML, Koelker S, Freedenberg D, et al. The incidence of urea cycle disorders. Mol Genet Metab. 2013;110:179–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessler P, Buchal P, Schwenk HU, et al. Favorable long-term outcome after immediate treatment of neonatal hyperammonemia due to N-acetylglutamate synthase deficiency. Eur J Pediatr. 2010;169:197–9. [DOI] [PubMed] [Google Scholar]
- 3.Robinson JR, Conroy PC, Hardison D, et al. Rapid resolution of hyperammonemia in neonates using extracorporeal membrane oxygenation as a platform to drive hemodialysis. J Perinatol. 2018;38:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gander JW, Rhone ET, Wilson WG, et al. Veno-venous extracorporeal membrane oxygenation for continuous renal replacement in a neonate with propionic acidemia. J Extra Corpor Technol. 2017;49:64–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Batshaw ML. Inborn errors of urea synthesis. Ann Neurol. 1994;35:133–41. [DOI] [PubMed] [Google Scholar]
- 6.Summar M, Pietsch J, Deshpande J, et al. Effective hemodialysis and hemofiltration driven by extracorporeal membrane oxygenation pump in infants with hyperammonemia. J Pediatr. 1996;128:379–82. [DOI] [PubMed] [Google Scholar]
- 7.Haberle J, Boddaert N, Burlina A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrapani A, Valayannopoulos V, Segarra NG, et al. Effect of carglumic acid with or without ammonia scavengers on hyperammonaemia in acute decompensation episodes of organic acidurias. Orphanet J Rare Dis. 2018;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ring E, Zobel G, Stockler S. Clearance of toxic metabolites during therapy for inborn error of metabolism. J Pediatr. 1990;117:349–50. [DOI] [PubMed] [Google Scholar]
- 10.Blair H. Carglumic acid in hyperammonaemia due to organic acidurias: A profile of its use in the EU. Drugs Ther Perspect. 2019;35:101–8. [Google Scholar]