Abstract:
Patients on mechanical circulatory support (MCS) devices are placed on aspirin and may require platelet function testing (PFT) to monitor the adequacy of therapy. Routine laboratory PFT is performed using whole blood aggregation (WBA) which typically has a long turnaround time (4–5 hours) and may not be readily available. By contrast, platelet mapping by thromboelastography (TPM) can provide results within 45 minutes. The objective of this study was to compare the results of TPM with WBA. We compared platelet mapping maximal amplitude (MA) by TPM with that of arachidonic acid (AA) to WBA with AA by impedance. We analyzed paired samples where both TPM and WBA were available. Of 45 paired samples, 34 were from 29 MCS patients and 11 were from non-MCS patients. When applying institutional interpretation guidelines with an MAActivator cutoff of ≤40 mm, WBAAA vs TPM MAAA in non-MCS and MCS patients correlated well with an accuracy of 100 and 94.4%, respectively. MAActivator >40 had poor correlation with an accuracy of 37.5%. Irrespective of MAActivator value, TPM AA inhibition expressed in percent of inhibition had poor accuracy. When used with proper guidelines for interpretation, specifically when MAActivator ≤ 40 mm, TPM is a suitable and reliable test to use for MCS patients on aspirin.
Keywords: thromboelastography platelet mapping (TPM), mechanical circulatory support (MCS) devices, platelet aggregometry by impedance, growing MAActivator
Recent progress in mechanical circulatory support (MCS) technology has extended survival and improved the quality of life for patients with advanced heart failure. MCS devices are used as a bridge to transplant or as destination therapy for those patients ineligible for heart transplantation (1). Device implantation is associated with significant alterations in the hemostatic balance, with both thrombotic and hemorrhagic complications (2). Thrombotic complications are initiated by device-induced systemic inflammatory response and include thrombocytosis with platelet activation, increased rate of thrombin generation, elevated fibrinogen and factor VIII, and hypofibrinolysis (3–6). The imbalance requires individually tailored prophylactic antithrombotic therapy. On the other hand, hemorrhagic complications are due to acquired von Willebrand syndrome, device-associated arteriovenous malformations, and antithrombotic therapy, intended to reduce the risk of stroke and other thrombotic events (6–10). Hemolysis in patients on MCS devices may also contribute to the hemostatic imbalance (11). The need to manage both ends of the hemostatic spectrum varies significantly between different MCS devices and the patient's ongoing hemostatic status, which may undergo daily alterations following implantation. These changes require customization of antithrombotic therapy. Conventional laboratory-based coagulation tests often do not reflect the complexity of hemostatic alterations and do not provide timely information. By contrast, point-of-care viscoelastic hemostasis assays (VHAs) monitor whole blood hemostasis from clot formation to clot lysis, in a timely fashion. Thrombelastography (TEG®) mimics in vivo hemostatic processes described in the cell-based model of hemostasis, which includes interaction of tissue factor expressing cells, platelets, coagulation factors, and inhibitors (12). Thromboelastography platelet mapping® (TPM) is a modification of TEG that measures the effect of specific platelet inhibitors. TPM may be an attractive approach for the evaluation of hemostasis and monitoring of antithrombotic therapy for MCS patients (13–15).
Since 2012, we have been using TPM to monitor and manage anticoagulation (unfractionated heparin and warfarin) and antiplatelet (aspirin and dipyridamole) therapies in patients on MCS devices. In 2012, the coagulation service in conjunction with the transplant team developed a TPM anticoagulation protocol (Table 1). A subsequent pilot study, based on the protocol, demonstrated that TPM was suitable for monitoring antiplatelet therapy in MCS patients when used in accordance with the appropriate guidelines (16). However, a 2014 study using non-MCS patients concluded that TPM was not a suitable tool for monitoring aspirin therapy because of its lack of sensitivity and specificity (17). The aim of this study was to assess the validity of TPM as a monitoring test for aspirin therapy in MCS patients.
Table 1.
Anticoagulation and antiplatelet goals at our institution for patients with MCSD.
| Type of MCSD | Warfarin INR | TEG CI | Aspirin Baseline Dose (mg) | TPM MAAA/ADP (mm) |
|---|---|---|---|---|
| HW | 2.0–3.0 | ≤1.5 | 325 | ≤50 |
| HeartMate II | 2.0–3.0 | ≤1.5 | 81 | ≤50 |
| TAH | 2.0–3.0 | ≤1.2 | 81 | ≤50 |
| BiVAD | 2.5–3.5 | ≤1.5 | 81 | ≤50 |
MCSD, mechanical circulatory support device; MAAA/ADP, maximal amplitude arachidonic acid/adenosine diphosphate; BiVAD, biventricular assisted device.
MATERIALS AND METHODS
Study Population
The study was approved by the Hospital Research Institute Human Subjects Review Committee (Pro 00042830). Between January 2015 and April 2016, 53 MCS patients on aspirin had 538 TPMs. From this group, only 34 TPMs from 29 patients had concurrent whole blood aggregometry (WBA) ordered by physicians. Five of the patients had TPM and WBA assayed twice (29 patients with 34 evaluations). Nineteen evaluations were from patients implanted with continuous flow devices such as HeartWare (HW) and HeartMate II (HM II). Fifteen evaluations were from patients with pulsatile devices such as total artificial heart (TAH).
We also reviewed 65 TPMs and WBA performed on non-MCS patients between December 2014 and February 2018. Among them, 10 patients were evaluated for aspirin responsiveness by TPM and WBA. One of the patients was assayed twice (10 patients with 11 evaluations).
Laboratory Assays
The TPM analysis was performed using whole blood samples, per the manufacturer’s (TEG 5000, Haemonetics Corporation, Braintree, MA) guidelines. TPM is a modification of the TEG assay that isolates platelet function in the clotting process and allows for the comparison of the maximal amplitude (MA) value, under four different conditions.
Baseline TEG MA (MACK):
Clotting is initiated by the addition of kaolin and calcium generating a strong thrombin response that maximally activates all platelets through the PAR1 receptor, which is unaffected by antiplatelet agents. Thrombin also cleaves all available fibrinogen. MA is a direct measurement of the polymerizing fibrin and activated platelets/fibrinogen interactions via glycoprotein (GP) IIb/IIIa receptors and represents the maximal strength of the clot. The major contributor to clot strength is platelets, contributing 80–90%, and fibrinogen, 10–20%. Therefore, the MACK parameter is a valid proxy for the maximum potential function of platelets present in a whole blood sample. The presence of thrombin overrides inhibition by antiplatelet agents, which is why kaolin-activated TEG samples will not demonstrate the effects of these agents (18,19).
Fibrin TEG MA (MAActivator):
Measurement of fibrin contribution to clot strength requires elimination of platelet activation by thrombin. Thrombin generation is eliminated with the use of heparinized blood. Fibrin formation is enabled with reptilase and Factor XIIIa. Reptilase directly converts fibrinogen to fibrin, and Factor XIIIa cross-links fibrin. The resulting MAActivator represents fibrin contribution to clot strength. In some circumstances, the MAActivator continues to grow wider than expected (>25 mm) (20). This phenomenon is called a “growing” MA. In our experience, the adequacy of the antiplatelet therapy cannot be assessed when MAActivator is >40 mm. The mechanism underlying this phenomenon has not been clarified (20–22).
TEG MAAA:
Maximal platelet activation by thromboxane A2 pathway is measured in a heparinized whole blood sample activated with reptilase, FXIIIa, and arachidonic acid (AA). This test measures residual platelet activity in the presence of aspirin.
TEG MAADP:
Maximal platelet activation by the ADP pathway is measured in a heparinized whole blood sample activated with reptilase, FXIIIa, and ADP. This test measures platelet activity via the ADP/GP IIb/IIIa pathways.
Platelet inhibition in response to the agonist is automatically calculated by the instrument using the following equation:
Although there is no manufacturer recommendation for % AA therapeutic cutoff, TPM % AA inhibition >70% is considered therapeutic by the Berlin Heart EXCOR trial protocol (23).
Determining Adequacy of Aspirin Effect in MCS Patients by TPM
Based on our seven-year experience monitoring platelet inhibition in MCS patients, the adequacy of aspirin-induced platelet inhibition was determined by residual platelet reactivity reflected by the maximum amplitude of AA (MAAA). An MAAA benchmark value of less than 50 mm was chosen to define aspirin efficacy (MAAA <50 mm).
TEG Coagulation Index (CI) is calculated from R, K, angle, and MA and describes the patient’s overall coagulation status. Kaolin CI equation: CI = –.6516Rc – .3772Kc + .1224MAc +.0759αc − 7.7922. In our laboratory, the normal CI range was found to be from −5.3 to 1.5. The target CI values for our anticoagulation protocol (Table 1) was determined from our prior pilot study from MCS patients without thromboembolic events.
Whole blood aggregation (WBA) analysis was performed simultaneous with the TPM assay using Chrono-log instrument. Blood was analyzed according to the manufacturer's instructions using their reagents (Chrono-log Corporation, Havertown, PA).
The platelet count was standardized by diluting blood with saline. Platelet aggregation was measured by submerging an electrode probe into the blood sample with an agonist. Impedance between two wires in the probe changes as platelets aggregate on their surface. This change in electrical impedance was measured in ohms. Agonists used are collagen at 1 µg/mL and 5 µg/mL, AA at .5 mM, and ADP at 10 µM (24,25).
Because less specimen handling is required than in tests using platelet reach plasma, the risk of platelet activation/loss during the specimen processing is minimized. Whole blood aggregation is performed in a more physiologic milieu of red and white blood cells.
The effect of aspirin by WBA (manufacture's guidelines) was determined by the following: reduced aggregation with 1 µg/mL collagen, normal aggregation with 5 µg/mL collagen and ADP, ≥50% difference in aggregation between 1 µg/mL and 5 µg/mL collagen, and absent/greatly reduced (<4 ohms) aggregation with AA.
Statistical Analysis
We used WBA as a gold standard for monitoring aspirin responsiveness and compared it with TPM results.
We divided patients into three different categories:
Non-MCS patients on aspirin: 11 evaluations
MCS patients on aspirin with an MAActivator of ≤40 mm: 18 evaluations
MCS patients on aspirin with an MAActivator of >40 mm: 16 evaluations.
To assess aspirin response, we compared the following result combinations:
WBAAA vs TPM MAAA
WBAAA vs TPM AA % inhibition
For MCS patients, results were correlated with 7 days of clinical outcome for bleeding or thrombotic events.
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of TPM were analyzed in comparison to the gold standard WBA, using a conventional two-by-two (2 × 2) table for the diagnostic test. MedCalc Statistical Software version 16.4.3 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org; 2016) was used for calculations. Sensitivity, specificity, PPV, NPV, and accuracy are expressed as percentages. Continuous data are presented as mean ± SD.
RESULTS
The mean age of the cohort was 52.4 ± 14.0 years, and 64% were male. The average time on device was 172.6 ± 291.8 days. For MCS patient's, daily aspirin dose varied: 81 mg (13 evaluations), 162 mg (7 evaluations), 325 mg (9 evaluations), 650 mg (3 evaluations), and 975 mg (2 evaluations). All 10 non-MCS patients were on 81 mg/day of aspirin.
Aspirin Effect
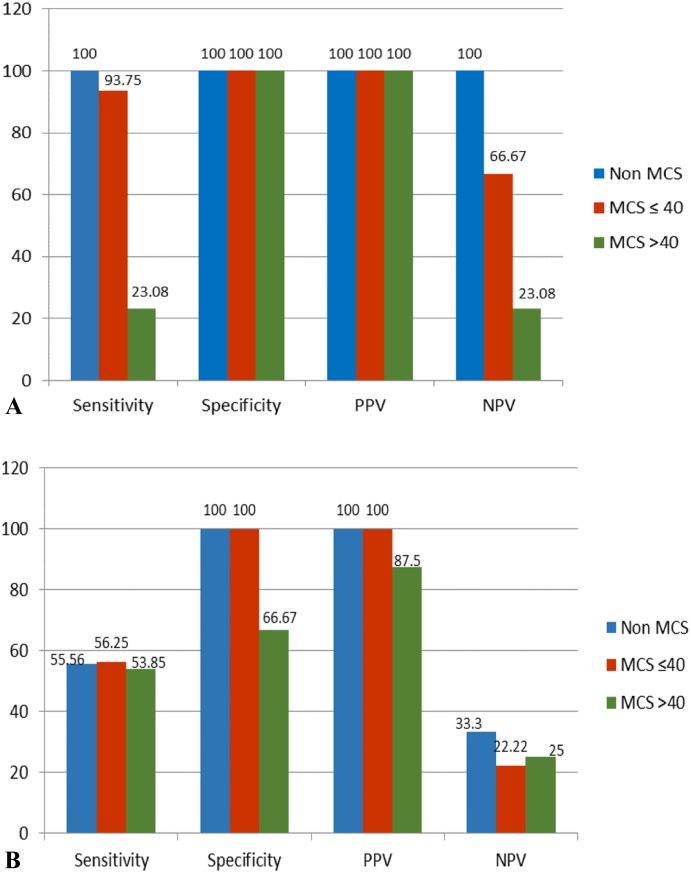
TPM MAAA and WBAAA in non-MCS patients and MCS patients with an MAActivator of ≤40 mm correlated well with an accuracy of 100% and 94.4%, respectively. MCS patients with an MAActivator of >40 mm had poor correlation with WBA with an accuracy of 37.5%. In the MAActivator ≤40 mm group 17/18 evaluations correlated with WBA results (15 true positives and two true negatives (non-responders). Both aspirin non-responders' patients #23 and #24 see Table 2) were on 81 mg of aspirin but showed no AA inhibition by both methods. One patient (1/18) yielded a false-negative result by TPM (NPV 66.67%). In the MAActivator >40 mm group, NPV was 23.08%, and TPM MAAA results showed suboptimal aspirin response in 14/16 evaluations (Table 2 and Figure 1A).
Table 2.
MCS patient’s antithrombotic therapy regiment adjustment based on TPM and or WBA results with one-week outcome.
| Sample # | Device | TEG CI | TEG MAAA (mm) | WBAAA (ohm) | Anticoagulant | INR | Aspirin | Aspirin Dose Change | Anticoagulant Dose Change | 1-Week Event |
|---|---|---|---|---|---|---|---|---|---|---|
| 12 | HW | 1.2 | 34.0 | 2 | W | 1.9 | 81 qd | No | No | No |
| 13 | HW | 1.1 | 41.8 | 0 | W | 2.1 | 325 qd | No | No | No |
| 14 | HW | 0.5 | 13.2 | 2 | W | 2.4 | 162 qd | No | No | No |
| 15 | HMII | 1.7 | 40.2 | 2 | W | 4.1 | 81 qd | No | No | No |
| 16 | HW | −1.1 | 29.7 | 1 | W | 2.8 | 325 qd | No | No | No |
| 17 | HW | 2.0 | 36.3 | 0 | W | 1.9 | 81 qd | No | W dose > W | No |
| 18 | BiVAD | 0.0 | 21.1 | 0 | W | 1.8 | 325 qd | Discontinued | Discontinued | OHT |
| 19 | HMII | 1.9 | 33.6 | 0 | None | 1.4 | 162 qd | No | W added | No |
| 20 | HMII | −0.4 | 35.7 | 0 | W + UFH | 1.3 | 81 qd | No | No | No |
| 21 | HMII | −4.3 | 28.9 | 0 | UFH | 1.2 | 81 qd | No | No | No |
| 22 | LVAD | 1.5 | 49.3 | 0 | W | 2.6 | 325 qd | No | No | No |
| 23 | HMII | 2.0 | 69.6 | 20 | None | 1.9 | 81 qd | 325 qd | UFH added | No |
| 24 | HMII | −0.1 | 68.8 | 15 | W | 2.5 | 81 qd | No | No | No |
| 25 | HMII | 0.8 | 59.4 | 1 | W | 2.7 | 81 qd | No | W dose< | No |
| 26 | TAH | −3.5 | 22.6 | 3 | UFH | 1.8 | 81 qd | No | No | No |
| 27 | TAH | 2.5 | 17.9 | 3 | W | 2.9 | 162 qd | No | W dose> | No |
| 28 | TAH | 1.3 | 27.2 | 0 | W | 2.7 | 162 qd | No | W dose> | No |
| 29 | TAH | 4.2 | 29.0 | 3 | W | 2.3 | 325 qd | No | W dose< | No |
| 30 | HW | 0.5 | 66.5 | 0 | Argatroban | 1.8 | 325 qd | Dipyridamole added | No | No |
| 31 | HW | 2.1 | 55.2 | 1 | Argatroban | 1.5 | 325 qd | No | No | No |
| 32 | HMII | 1.4 | 70.4 | 2 | UFH | 1.2 | 162 qd | 81 qd | No | No |
| 33 | HW | 1.9 | 64.2 | 0 | W | 2.4 | 162 qd | No | No | No |
| 34 | HMII | −5.6 | 50.0 | 0 | W + UFH | 1.1 | 325 qd | No | W dose>, UFH discontinued | No |
| 35 | TAH | 0.0 | 53.0 | 0 | W | 2.0 | 81 qd | No | W dose> | No |
| 36 | TAH | 1.0 | 47.0 | 1 | W | 2.9 | 162 qd | No | W dose<, UFH added | OHT |
| 37 | TAH | −8.9 | 79.1 | 5 | UFH | 1.1 | 325 tid | No | No | No |
| 38 | TAH | 0.2 | 51.5 | 0 | W | 3.1 | 325 qd | No | W dose> | No |
| 39 | TAH | −0.6 | 73.3 | 1 | W | 2.9 | 325 bid | No | W dose< | No |
| 40 | TAH | −6.2 | 72.1 | 0 | W | 3.0 | 325 bid | No | W dose< | No |
| 41 | TAH | 2.8 | 48.5 | 1 | W | 3.2 | 325 tid | No | No | Expired |
| 42 | TAH | −0.2 | 61.4 | 2 | W | 3.1 | 325 bid | Dipyridamole added | No | GI bleed, rectal ulcers |
| 43 | TAH | 1.8 | 71.0 | 19 | UFH | 2.0 | 81 qd | 162 qd | W added | Venous thrombosis |
| 44 | TAH | −2.9 | 76.9 | 24 | W | 4.1 | 81 qd | Aspirin held | W held | No |
| 45 | TAH | 3.7 | 67.1 | 0 | W | 2.7 | 81 qd | No | No | No |
BiVAD, biventricular assisted device; W, warfarin; UFH, unfractionated heparin; +, additional anticoagulant added; >, dose increase; <, dose decrease; OHT, orthotopic heart transplant; GI, gastrointestinal. Interpretation guidelines for aspirin responsiveness: WBAAA cutoff <4 ohms; TPM cutoff for MAAA <50 mm. The range for a normocoagulable state (free from thrombotic events) for patients with HM II and HW devices should be maintained at a CI value ≤1.5, whereas patients on TAH devices should be maintained at a value ≤1.2.
Figure 1.
Statistical evaluation for TPM when compared with WBA, in all three patient categories. (A) compares TPM MAAA with WBAAA aggregation. (B) compares TPM % AA inhibition with WBAAA aggregation.
From these groups, by WBA, two patients (#43 and #44) showed no aspirin response and one patient (#37) showed suboptimal response, despite 975 mg of aspirin (Table 2). Most of these patients were on high aspirin doses, 162 mg daily (3 evaluations), 325 mg daily (4 evaluations), 325 mg twice daily (6 evaluations), and 325 mg three times daily (2 evaluations). When comparing WBA results with TPM % AA inhibition, the accuracy was significantly decreased across all three patient categories: 63.6%, 61.1%, and 56.3%, respectively, mainly affecting NPV (Figure 1B).
Correlation of Results with Clinical Outcome
In our institution's protocol in assessing antithrombotic therapy with TPM, we also take into consideration MAADP and TEG CI in combination with international normalized ratio (INR) targets (Table 1). TPM results, corresponding INR, anticoagulant/antiplatelet dose adjustment, and seven-day clinical outcome data are presented in Table 2.
TPM Evaluations with MAActivator ≤ 40 mm (#12–#29)
Among the 15 patients with good aspirin response by TPM and WBA, antithrombotic therapy was discontinued in one patient (#18) in anticipation of heart transplant, and for the remaining 14 evaluations, aspirin dose was not adjusted. For seven evaluations, warfarin doses were changed. There were no bleeding or thrombotic events in any patients within 7 days. Three evaluations (#23–#25) showed suboptimal aspirin response with TPM. Patients # 23 and #24 had also adequate aspirin response by WBA. Aspirin dose for patient #23 was increased, and heparin was added. There were no thrombotic or bleeding events for patients #23–#25.
TPM Evaluations with MAActivator >40 mm (#30–#45)
Most evaluations were from patients with TAH devices (11/16). Despite significantly higher aspirin dose, 14 showed suboptimal aspirin response by TPM. When WBAAA was considered, only three of 16 evaluations showed suboptimal aspirin response (#37, #43, and #44). Patient #37 was on 325 three times a day dose, had borderline WBAAA response and was overall hypocoagulable per TEG (low CI of −8.9). No adjustments to antithrombotic therapy were made, and no bleeding events were observed in this group of patients. Despite the increase in aspirin dose to 162 mg and warfarin therapy initiation, patient #43 developed deep venous thrombosis (the response to dose adjustment was not reassessed). Patient #44 was readmitted with sternal incision infection and was placed on multiple antibiotics. Because of supratherapeutic INR 4.1 and overall hypocoagulable CI of −2.9, his antithrombotic therapy was placed on hold with no complications. In the group of patients with MAActivator >40 mm, 13 had no complications, one had deep venous thrombosis, one had gastrointestinal bleeding, and one expired.
We also investigated the MAActivator variability in our cohort of non-MCS and MCS patients.
MAActivator Variability in Non-MCS Patients
We had 65 TPMs performed on non-MCS patients between December 2014 and February 2018. Average MAActivator was 17.9 mm ± 16.6 mm. Eight evaluations (12%) had MAActivator >40 mm. Most of the patients with MAActivator >40 mm had preexisting conditions that potentially could explain the high MAActivator (e.g. s/p recent surgery, recent DVT, metastatic cancer, and myeloproliferative neoplasm). One evaluation was from a patient with thrombocytosis (MAActivator 70.3 mm and platelet count 932K) and one with thrombocytopenia (MAActivator 46.3 mm and platelet count 146K). One of the patients in this group had an MAActivator of 53.6 mm during active thrombosis and treatment with thrombolytic agents, which normalized to 6.6 mm when tested one week later.
MAActivator Variability in MCS Patients
Fifty-three MCS patients had 538 TPMs. From this patient group, we reviewed 182 tracings: baseline (44), within 1 week of post-MCS device implantation (51), midpoint of MCS implant (37), and the last available TEG tracings (50). Average MAActivator for MCS patients was 21.1 mm ± 17.0 mm at baseline, 31.8 mm ± 15.8 mm 1 week after device implantation, 28.5 mm ± 17.5 mm at the midpoint, and 27.0 mm ± 15.6 mm during last available TPM evaluation.
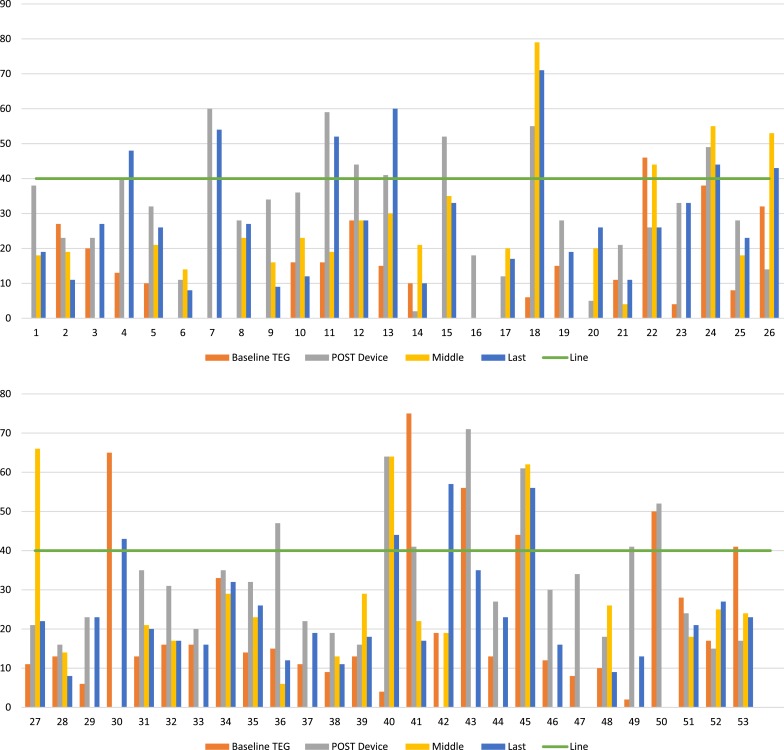
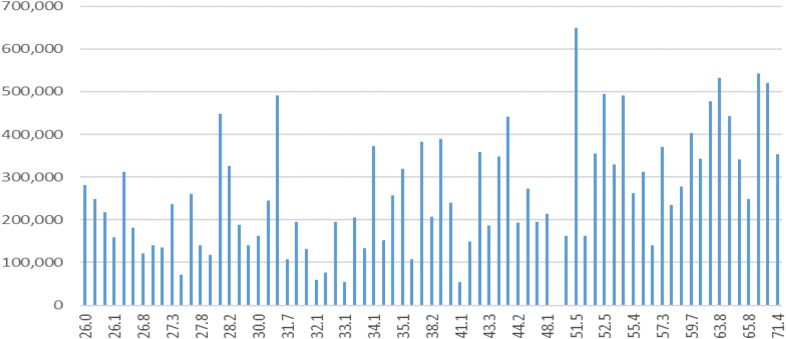
From 182 tracings, 29 (21%) had MAActivator >40 mm. Similar to non-MCS patients, MAActivator of patients on devices was affected by preexisting conditions and fluctuated during the implant (Figure 2). Patients with baseline elevated MAActivator were either on short-term MCS devices [ECMO (#34, #41, and #53), Impella (#30)] or had a condition that also activated the coagulation cascade: recent surgery (#2, #12, and #24), infection (#26 and #50), cardiogenic shock (#43), and amyloidosis (#45). Immediate postimplantation period was another reason for elevated MAActivator. When available, the platelet count did not seem to correlate with elevated MAActivator (Figure 3). Fibrinogen was not routinely measured.
Figure 2.
MAActivator variability in 53 MCS patients at various stages of pre- and post-device implantation. Each set of columns on X axes represent results of MAActivator for one patient. Baseline TEG, preoperative MAActivator result; post-device, MAActivator result within seven days of implantation; middle, MAActivator result at the midpoint of implantation; last, last available MAActivator result; line, MAActivator value of 40 mm.
Figure 3.
MAActivator value (X axes) showing no correlation with the platelet count (Y axes) in patients on MCS devices.
Reliability of TPM Results for Aspirin Effect
In our cohort of 29 MCS patients, we had four patients who were tested by TPM on two different dates, as shown in Table 3. The MAAA parameter on two different dates for each of the four patients was similar as was the diagnostic interpretation. By contrast, % AA inhibition results significantly varied between the two dates in two of the four patients.
Table 3.
Intraindividual variation of TPM assay parameters.
| Patient | Date | Device | Aspirin (mg) | % Inhibition by AA | MAAA |
|---|---|---|---|---|---|
| #1 | April 8, 2015 | HW | 325 | 72 | 34 |
| #1 | April 21, 2015 | HW | 325 | 48 | 41.8 |
| #2 | March 11, 2015 | TAH | 325 | 100 | 73.3 |
| #2 | April 27, 2015 | TAH | 325 | 20 | 72.1 |
| #3 | April 21, 2015 | TAH | 81 | 100 | 17.8 |
| #3 | May 4, 2015 | TAH | 81 | 99 | 27.2 |
| #4 | January 6, 2016 | HW | 325 | 93 | 66.5 |
| #4 | January 11, 2016 | HW | 325 | 100 | 55.2 |
DISCUSSION
Since 2012, we have been using a TPM-based protocol to monitor and manage anticoagulation (unfractionated heparin and warfarin) and antiplatelet (aspirin and dipyridamole) therapies in patients supported on MCS devices. One study has concluded that TPM was the least suitable test to monitor aspirin therapy in MCS patients. These study results were based on normal donor population on aspirin (17).
Five platelet function assays were compared in the study: platelet function assay (PFA-100), light transmittance aggregometry (LTA), VerifyNow, vasodilator-stimulated phosphoprotein (VASP) flow cytometry, Multiplate aggregometry, and TPM.
The PFA-100 is designed to measure platelet adhesion and aggregation under high shear conditions. The PFA test result is dependent on the platelet number and function, fibrinogen levels, von Willebrand factor (vWF) level, and hematocrit. Anemia and vWF loss will affect the accuracy of results. The test is designed to detect the presence of aspirin but gives no information about its efficacy. The sensitivity of PFA-100 for detecting the aspirin presence is shown to be only 60–70% (26,27). Traditionally, LTA has been the gold standard for the evaluation of platelet function. The assay is based on the optical measurement of platelet aggregation in platelet-rich plasma. Hemolysis is present in all MCS patients, therefore making LTA an unsuitable method for monitoring aspirin therapy. Similarly, VerifyNow is based on the turbidimetric optical detection method, and results may be affected by hemolysis. VASP flow cytometry provides information only on ADP-induced platelet function, which is irrelevant for patients on aspirin. Multiplate impedance aggregometry was found to demonstrate an acceptable reliability when monitoring aspirin therapy. Multiplate is no longer available in the United States.
Because of the complexity of hemostatic challenges in patients implanted with MCS devices, the comparison of aspirin testing in MCS patients with normal patients may not be appropriate. The hemostatic conditions such as hemolysis, anemia, and acquired von Willebrand multimer loss possibly present in MCS patients are likely to influence platelet function tests.
Whole blood aggregometry, used at our medical center, is based on impedance and evaluates platelet function in its natural milieu. Using TPM and, if necessary, WBA, we have been able to monitor MCS patients on aspirin therapy.
Unlike the normal population, which may not require continuous aspirin monitoring, patients implanted with MCS devices are subject to conditions that may cause daily fluctuations in platelet function; therefore, they require frequent assessment of their hemostatic status. This is especially important during the first two weeks of the immediate postoperative and recovery period, when the risk of potentially avoidable adverse thromboembolic cerebral and hemorrhagic events is highest (28). Recently published long-term MCS expert consensus advocates for patient-tailored antithrombotic therapy. It also recognizes the current lack of assays necessary for in-depth patient hemostatic profile evaluation (29). Laboratory tests that assess the adequacy of antithrombotic therapy for those patients must be simple to interpret, cost-effective, and have defined target parameters. Our TPM-based antithrombotic protocol has four defined target parameters (CI, INR, MAAA, and MAADP) performed with two assays (TPM and INR, Table 1).
TPM AA Percent Inhibition versus TPM MAAA
Platelet inhibition in response to the agonist is reported by TEG software as TPM AA percent inhibition, but there is no manufacturer recommendation for % AA therapeutic cutoff. Many publications have suggested that TPM AA percent inhibition to monitor aspirin therapy is unreliable. Our experience agrees with the results of these studies (17,22,30,31). Platelet inhibition in response to the agonist is automatically calculated by the TEG instrument using complex calculations from results of two samples collected in different anticoagulants (citrate and heparin). Using two different types of specimens increases the inaccuracy of calculations. Another important issue is the variability of MAActivator, the “growing” MAActivator. This phenomenon has been observed by other investigators and is frequently present in MCS patients. The mechanism for this response has not been determined (20–22). “Growing” MAActivator, in our experience, was not dependent on platelet count or heparin. However, we observed that MAActivator in MCS patients was affected by preexisting conditions and fluctuated during the implant (Figure 2). Patients with baseline preimplantation elevated MAActivator were either on short-term MCS devices (ECMO, Impella) or had a condition that also activated the coagulation cascade: recent surgery, infection, cardiogenic shock, and amyloidosis. Immediate postimplantation period was another reason for elevated MAActivator. There is a growing amount of clinical experience and scientific literature supporting the successful use of TPMAA/ADP in monitoring the effect of platelet inhibitors (13,14,32).
Assessment of Antiplatelet Therapy Adequacy
Per our guideline, we used TPM results only when MAActivator was ≤40 mm. Accuracy, when compared with WBA, was >90%, and patients had no adverse events during the week of follow-up. In the MAActivator >40 mm group, accuracy dropped to <40%, with a very low NPV of 23%. This cohort of patients were implanted mostly with TAH and were on high aspirin doses (>81 mg daily). TPM results were unreliable and showed suboptimal aspirin response. Dose adjustments were made based on WBA results. In this cohort of patients, there were three adverse events (see Results section). These events were not related to our reported interpretation of results.
Our experience has found that evaluating the efficacy of antiplatelet therapy in MCS patients requires examination of multiple TPM parameters (CI, TPMAA, and TPMADP) at least twice a week within first two weeks. Even if there is an adequate aspirin response, but MAADP is high, another antiplatelet agent (dipyridamole) is advocated. When patients are stable on antithrombotic therapy and MAActivator is ≤40 mm, precision and reliability of results are within acceptable limits as demonstrated by a patient in Table 4. When patient's postimplantation condition was stabilized, MAAA results varied minimally with the coefficient of variation (CV) 4% when results were expressed as TPMAA. Once the patient is stabilized, TPM can be performed only if necessary (in case of infection, bleeding or thrombotic event).
Table 4.
Patient’s TPM MAAA results over multiple days.
| Patient | Date of TPM | MAAA (mm) |
|---|---|---|
| Baseline | March 30, 2015 | 24 |
| Immediate postimplantation | April 9, 2015 | 45.4 |
| Postimplantation | April 21, 2015 | 17.9 |
| Postimplantation | April 22, 2015 | 19.9 |
| Postimplantation | May 4, 2015 | 27.2 |
| Postimplantation | May 15, 2015 | 22.3 |
| Postimplantation | May 18, 2015 | 25.8 |
| Postimplantation | May 20, 2015 | 25.3 |
| Postimplantation | May 22, 2015 | 25.9 |
STUDY LIMITATIONS
This is a retrospective analysis of data of TPM and WBA on a small group of patients supported by various types of MCS devices. This was intended as an exploratory analysis; therefore, clinical outcome was included.
CONCLUSION
We have found TPM to be a useful tool to monitor aspirin therapy in patients implanted with an MCS. TPM provides a complete assessment of a patient's hemostatic status, including the rate of thrombin generation, platelet function potential, degree of platelet inhibition, and fibrinolysis. TPM provides reliable and reproducible results in patients with an MAActivator ≤40 mm, which accounts for 79% of patients on MCS in our cohort. The successful use of TPM requires proper training and expertise in operating the TEG instruments and performing the assays in the laboratory. When used with proper device-specific guidelines for interpretation, TPM allows the MCS team to individualize patient's antithrombotic therapy. For an MAActivator > 40 mm, other approaches, such as WBA, should be considered to monitor platelet activity.
Footnotes
Presented in part as poster presentation at the Eurothrombosis Summit, Como, Italy, October 2015.
REFERENCES
- 1.Stewart GC, Givertz MM. Mechanical circulatory support for advanced heart failure: Patients and technology in evolution. Circulation. 2012;125:1304–15. [DOI] [PubMed] [Google Scholar]
- 2.Susen S, Rauch A, Van Belle E, et al. Circulatory support devices: Fundamental aspects and clinical management of bleeding and thrombosis. J Thromb Haemostasis. 2015;10:1757–67. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen VG, Kirklin JK, Holman WL, et al. Mechanical circulatory device thrombosis: A new paradigm linking hypercoagulation and hypofibrinolysis. Am Soc Artif Intern Organs J. 2008;54:351–8. [DOI] [PubMed] [Google Scholar]
- 4.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–720. [DOI] [PubMed] [Google Scholar]
- 5.Thompson LO, Loebe M, Noon GP. What price support? Ventricular assist device induced systemic response. Am Soc Artif Intern Organs J. 2003;49:518–26. [DOI] [PubMed] [Google Scholar]
- 6.John R, Lee S. The biological basis of thrombosis and bleeding in patients with ventricular assist devices. J Cardiovasc Transl Res. 2009;2:63–70. [DOI] [PubMed] [Google Scholar]
- 7.Suarez J, Patel CB, Felker GM, et al. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail. 2011;4:779–84. [DOI] [PubMed] [Google Scholar]
- 8.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–9. [DOI] [PubMed] [Google Scholar]
- 9.Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137:208–15. [DOI] [PubMed] [Google Scholar]
- 10.Reich HJ, Morgan J, Arabia F, et al. Comparative analysis of von Willebrand factor profiles after implantation of left ventricular assist device and total artificial heart. J Thromb Haemostasis. 2017;15:1620–4. [DOI] [PubMed] [Google Scholar]
- 11.Heilmann C, Geisen U, Benk C, et al. Haemolysis in patients with ventricular assist devices: Major differences between systems. Eur J Cardiothorac Surg. 2009;36:580–4. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman M, Monroe DM 3rd. A cell-based model of hemostasis. Thromb Haemostasis. 2001;85:958–65. [PubMed] [Google Scholar]
- 13.Yang Z, Xie Z, Pei X, et al. Effect of thrombelastography on timing of coronary artery bypass grafting. Exp Ther Med. 2018;16:579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahla E, Suarez TA, Bliden KP, et al. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: The timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG(TARGET-CABG) study. Circ Cardiovasc Interv. 2012;5:261–9. [DOI] [PubMed] [Google Scholar]
- 15.Bochsen L, Wiinberg B, Kjelgaard-Hansen M, et al. Evaluation of the TEG® platelet mapping TM assay in blood donors. Thromb J. 2007;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volod O, Lam LD, Lin G, et al. Role of thromboelastography platelet mapping and international normalized ratio in defining “normocoagulability” during anticoagulation for mechanical circulatory support devices: A pilot retrospective study. Am Soc Artif Intern Organs J. 2017;63:24–31. [DOI] [PubMed] [Google Scholar]
- 17.Karon BS, Tolan NV, Koch CD, et al. Precision and reliability of 5 platelet function tests in healthy volunteers and donors on daily antiplatelet agent therapy. Clin Chem. 2014;60:1524–31. [DOI] [PubMed] [Google Scholar]
- 18.Craft RM, Chavez JJ, Bresee SJ, et al. A novel modification of the thrombelastograph assay, isolating platelet function, correlates with optical platelet aggregation. J Lab Clin Med. 2004;143:301–9. [DOI] [PubMed] [Google Scholar]
- 19.Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26:1–13. [DOI] [PubMed] [Google Scholar]
- 20.Haemonetics Corporation. Customer letter CL101323, 2015.
- 21.Nelles NJ, Chandler WL. Platelet mapping assay interference due to platelet activation in heparinized samples. Am J Clin Pathol. 2014;142:331–8. [DOI] [PubMed] [Google Scholar]
- 22.Giorni C, Costopoulos M, Bachelot-Loza C, et al. Platelet-mapping assay for monitoring antiplatelet therapy during mechanical circulatory support in children: A retrospective observational study. Res Pract Thromb Haemost. 2017;1:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almond CS, Buchholz H, Massicotte P, et al. Berlin heart EXCOR pediatric ventricular assist device investigational device exemption study: study design and rationale. Am Heart J. 2011;162:425–35. [DOI] [PubMed] [Google Scholar]
- 24.Manoharan A, Gemmell R, Hartwell T. Use of whole blood platelet lumi-aggregometry to optimize anti-platelet therapy in patients with chronic myeloproliferative disorders. Am J Hematol. 2006;81:676–83. [DOI] [PubMed] [Google Scholar]
- 25.Israels SJ, Robertson C, McNicol A. Identification of patients with storage pool deficiency using ATP release and dense granule counts. Hematology. 1997;2:161–7. [DOI] [PubMed] [Google Scholar]
- 26.Kweon OJ, Lim YK, Kim B, et al. Effectiveness of platelet function analyzer-100 for laboratory detection of anti-platelet drug-induced platelet dysfunction. Ann Lab Med. 2018;39:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels A, Sarpong Y, Coberly J, et al. Failure of the platelet function assay (PFA)-100 to detect antiplatelet agents. Surgery. 2015;158:1012–8. [DOI] [PubMed] [Google Scholar]
- 28.Volod O, Lam LD, Mirocha J, et al. , TEG platelet mapping (TEG PM) guided management of patients with total artificial heart (TAH): A single center 5-year experience. Artif Organs. 2018;42:A26. [Google Scholar]
- 29.Potapov EV, Antonides C, Crespo-Leiro MG, et al. 2019 EACTS expert consensus on long-term mechanical circulatory support. Eur J Cardio Thorac Surg. 2019;56:230–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandler WL, Brown AF, Chen D, et al. External quality assurance of platelet function assays: Results of the college of American pathologists proficiency testing program. Arch Pathol Lab Med. 2019;143:472–82. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson LP, Duong P, Pearce KF, et al. Monitoring of antiplatelet therapy in children on ventricular assist device support: Comparison of multiplate and thromboelastography platelet mapping. Am Soc Artif Intern Organs J. 2019;65:84–93. [DOI] [PubMed] [Google Scholar]
- 32.Sivapalan P, Bäck AC, Ostrowski SR, et al. Transfusion requirements in elective cardiopulmonary bypass surgery patients: Predictive value of multiplate and thromboelastography (TEG) platelet mapping assay. Scand J Clin Lab Invest. 2017;77:345–51. [DOI] [PubMed] [Google Scholar]