Abstract
Focusing screening and treatment to those most likely to benefit is the promise of precision medicine but inequitable distribution of precision medicine innovations may exacerbate health disparities. We investigated whether complex genomic concepts can be successfully communicated to diverse populations. Incorporating principles of Community-based Participatory Research, we created a precision medicine curriculum tailored to the needs of our predominantly Hispanic community. We administered the curriculum over 26 months, assessed pre- and post-test comprehension of 8 genetics-related terms, and compared comprehension differences based on demography and health literacy. In total, 438 individuals completed pre-/post-test assessments. At pre-test, 45.6% scored ≥75% across 8 major constructs; 66.7% at post-test. Comprehension increased for 7/8 terms with greatest pre/post-test increases for “mutation” (55% to 78%) and “sporadic” (34% to 59%). Mean pre-test comprehension scores (≥75%) were lower for Spanish- vs. English-speakers; mean post-test scores were equivalent. No heterogeneity by demographics or health literacy was observed. We demonstrate that a brief community educational program can improve knowledge of complex genomic concepts. Interventions to increase understanding of genomic concepts underlying precision medicine are key to patients making informed treatment and prevention decisions and may lead to more equitable uptake of precision medicine initiatives.
Keywords: Community, Disparities, Diversity, Education, Underrepresented Populations, Intervention, Public health, Precision medicine, Comprehension, Community-based participatory research, Health education, Hispanic
INTRODUCTION
Precision medicine initiatives focus on targeting treatment, screening and primary prevention to individuals who can most benefit from the intervention. For cancer health outcomes, targeted therapy guided by molecular and histologic features of tumors has proven extremely effective for the treatment of many common cancers including breast (Krop et al., 2017; Nishihara et al., 2013; Rebbeck et al., 2002; Schwaederle et al., 2016). Cancer screening also has a long history of using approaches targeting underlying genetics and family history information in terms of age of screening initiation and frequency of screening and, more recently, targeting other factors, mainly using risk-based methods that are not based on cancer family history (Marcus, Freedman, & Khoury, 2015; Shieh et al., 2017).
A major concern, however, in the use of precision medicine is that there will be a greater emphasis placed on targeted approaches at the expense of population-wide approaches, and as a result health disparities in cancer morbidity and mortality between individuals who have access to the treatment and those that do not will increase. While current research suggests that, in general, the public has some familiarity with genomic terms, understanding of the underlying concepts is lacking (Lea, Kaphingst, Bowen, Lipkus, & Hadley, 2011; Syurina, Brankovic, Probst-Hensch, & Brand, 2011).
How low educational attainment, poor English proficiency, and limited health literacy and numeracy (Giuse et al., 2016) affect the understanding of complex genetic information and, thus, the uptake of these new treatments is of great concern. Accumulating evidence demonstrates that culturally diverse populations have limited understanding of genetics and genetic testing and are concerned about the purpose of genetic testing and the information this testing provides (Canedo, Miller, Myers, & Sanderson, 2019). Minorities fear genetic discrimination and worry how this information may be used/misused (Catz et al., 2005; Hamilton et al., 2016), which impacts genetic testing. Despite concerns, minorities generally have favorable attitudes toward genetic testing and, if provided with actionable health information, many would consider genetic testing (Hamilton et al., 2016; Jagsi et al., 2015; Kinney, Gammon, Coxworth, Simonsen, & Arce-Laretta, 2010; Streicher et al., 2011; Sussner, Jandorf, Thompson, & Valdimarsdottir, 2013).
Materials to assist patients in making decisions about precision medicine and to prepare them for discussions with their physician about genetic testing are few (Collins, Calvo, Greenberg, Forman Neall, & Morrison, 2016). To address this need, the United States National Library of Medicine created the “Genetics Home Reference”, a website to provide consumer-friendly information for the public about precision medicine and diseases that are treated with precision medicine (U. S. National LIbrary of Medicine, 2020). The American Medical Association also developed a comprehensive continuing medical education program to guide physicians in educating patients about genetics in preparation for precision medicine discussions (American Medical Association, 2020; Genetic Alliance, 2008). Both resources provide tools to educate patients about key genetic concepts including the cell, DNA, genes, chromosomes, heredity and laws of inheritance, genetic mutations, abnormalities and variation, and the human genome. Fostering greater communication between providers and patients, and patients and their family members, through a community-based curriculum focused on key genetic concepts could thus provide a key avenue to addressing potential disparities in the use of precision medicine initiatives.
To design a curriculum aimed at building a foundation for the understanding of precision medicine using eight (8) common genetic terms and tailored to the educational needs of minority patients in the community, we used a community-based participatory research methods approach. We tested the effectiveness of this curriculum by assessing comprehension of these eight terms.
METHODS
Preliminary Assessments
We approached the design, implementation, evaluation, and maintenance of our precision medicine curriculum using principles of Community-based Participatory Research (CBPR) (Figure 1) (Israel, Schulz, Parker, Becker, & Community-Campus Partnerships for, 2001; Skinner et al., 2015). Our initial steps included strategic partnering with a local non-profit community-based organization, the Northern Manhattan Improvement Corporation (NMIC) that serves the Washington Heights and Inwood communities of Upper Manhattan to first explore community constituent health concerns overall and specifically related to cancer, a topic previously found to be of concern to the community (Sepulveda-Pacsi & Bakken, 2017). As our second step, we conducted an assessment of educational needs and learning preferences via quantitative surveys and focus groups among 497 adult community members (18+ years) accessing the services of NMIC, our community-based organization partner (Hillyer et al., 2017). Of those who participated, 84.5% were foreign-born, nearly 30% had inadequate health literacy, and 83.5% completed the survey in Spanish. Findings indicated that, in order to effectively communicate key cancer genetics and precision medicine concepts, curriculum development for the adult Hispanic community members of Northern Manhattan would require materials written at a low literacy level in Spanish, tailored to address misperceptions and low knowledge of the basics of genetics in order to provide a foundation to understand precision medicine as treatment for cancer.
Figure 1:
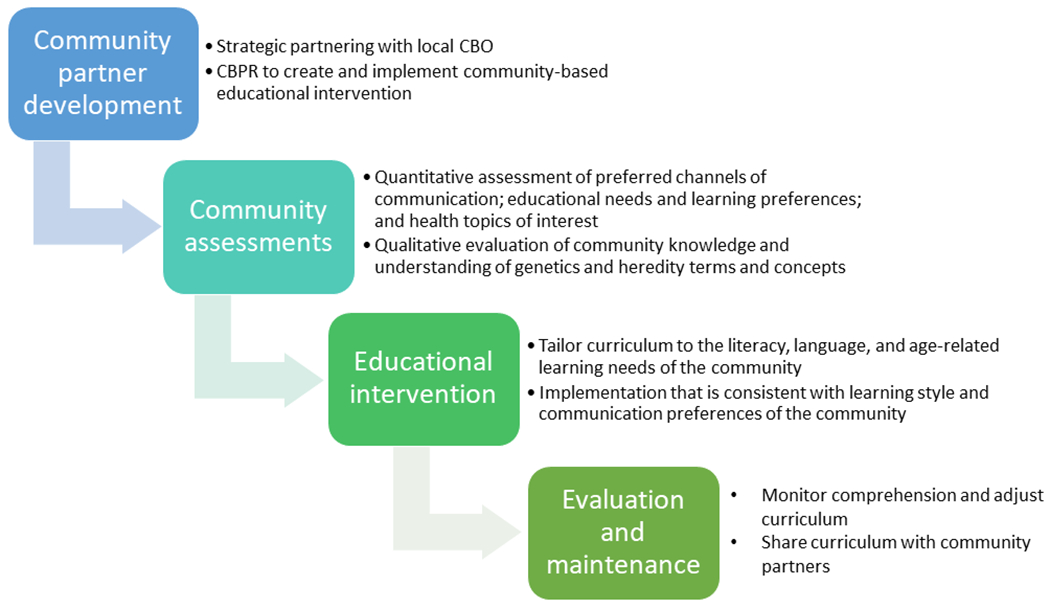
Theoretical framework and study design based on concepts of Community Based Participatory Research (CBPR)
Through a combination of individual recruiting and hosting small group events, we supplemented information learned through our surveys by gathering qualitative data to support our curriculum development using a focus group-based approach. We employed a purposive recruitment sampling scheme to select focus group participants that was representative of the population surveyed with regard to language (84% Spanish-speaking vs. 16% English), age (40% <45 years vs. 60% ≥45 years), and gender (32% male vs. 68% female) (Hillyer G et al., 2016). In total, 26 NMIC community clients recruited from NMIC waiting rooms and adult classes participated in 8 focus groups led by an experienced, bilingual group moderator. During the sessions, we presented multiple images showing genetic variability across humans, plants, and animals and models representing the scientific genetic concepts (e.g., DNA model, mitosis board educational prop illustrating the process of mitosis) from copyright-free sources, to assess understanding of basic and key biological concepts underlying genetics upon which to build a precision medicine curriculum, and recorded reactions to these images and models. Images that resonated with the audience were retained for use in the curriculum. The DNA model and mitosis board were thought by the participants to be confusing and were not used in the final curriculum.
Using an inductive approach and a thematic analysis process to assess the dominant and recurring themes, and overarching and underlying patterns in our data, we evaluated discussion content and identified salient themes for use in the development of the curriculum. Our analysis revealed a lack of understanding of basic biology and mechanisms of heredity and a lack of comprehension of the connection between genes and inheritance, as well as misperceptions related to genetics and risk of disease.
Educational Intervention Curriculum Development and Delivery
Based on the findings of our quantitative and qualitative assessments, we incorporated into our curriculum concepts related to personalized medicine, cell structure, chromosomes and DNA, genetics, genetic variation, acquired, hereditary, and sporadic DNA mutations, the Human Genome Project, biospecimens, and genetic testing. Material used in the curriculum was derived from expert sources including the National Cancer Institute (National Cancer Institute, 2013), the American Cancer Society (American Cancer Society, 2014), the National Human Genome Research Institute (National Human Genome Research Institute, 2015), and ASCO Cancer.Net (American Society for Clinical Oncology (ASCO), 2017, 2018). Learning objectives for the curriculum included being able to: 1) recognize the parts of the cell including chromosomes, genes, and DNA; 2) understand how human characteristics vary because of genes including response to medication; 3) remember means by which genes can become abnormal or mutated (e.g., acquired, hereditary, and sporadic mutation) and cause disease; 4) recall lifestyle and environmental exposures that increase susceptibility to the development of a genetic mutation; and 5) become familiar with concepts of biospecimens, genetic testing, and precision medicine.
Text was written at the 4th grade reading level (ReadabilityFormulas.com, 2018) to accommodate our low literacy community population (29.6% with inadequate health literacy). All study documents (e.g., consent, curriculum, pre- and post-test assessments, and the fact sheet) were translated into Spanish by the Herbert Irving Comprehensive Cancer Center Community Outreach and Engagement (COE) core Translation Team. This team employs bilingual native Spanish-speakers who are certified as “qualified” translators by the Columbia University Medical Center IRB and are proficient in providing guidance to non-Spanish-speaking research team members with regard to contextualizing Hispanic cultural references and phrases. Although the Spanish translation of study material was consistent in content, style, and level of readability of the English text, phrases were translated in a manner relevant to the culture and customs of the countries of origin of the majority of the target population (predominantly from the Dominican Republic). Back translation to English was conducted to ensure content equivalency.
Prior to implementation, we pilot tested English and Spanish versions of the curriculum and the pre/post-test assessments among community members, health professionals, health administrators, study recruiters, community leaders, genetic counselors, and academics (n = 80). With feedback from these groups received via email correspondence and telephone discussion, we made minor adjustments to further refine the basic cellular and genetics concepts addressed in the curriculum.
Educational workshops to deliver the curriculum in English or Spanish were scheduled during day, evening, and weekend hours and accommodated up to 25 participants at our community partner site. Scheduling of the workshops was facilitated by NMIC staff and educators in collaboration with our health educator. Workshops were approximately 1 hour in duration, and presented in the language preferred by the participants (English or Spanish), determined prior to the start of the workshop, by the study team community health educator (CHE) and community health worker (CHW) (who were both native Spanish-speakers). All materials including pre- and post-test surveys were provided in the language of the workshop. The majority of workshops held in Spanish were attended by adult students in the English as a Second Language (ESL) classes. A bilingual research assistant was also available to assist participants who had vision issues or preferred the questions and response choices be read to them. Answers were then recorded on the test sheet for the participant by the research assistant.
Using a PowerPoint presentation in classrooms equipped with smart screens at our community-based organization partner site, NMIC, concepts related to eight key genetic terms (genetics, chromosomes, susceptibility, mutation, variation, abnormality, heredity, and sporadic mutation) were introduced in individual subsections. The “Inside our cells” section described different cell types (e.g., muscle, nerve, skin), the structures inside the nucleus of a cell including genes and explained how genes determine human characteristics and impact our body’s reaction to our environment. The term “biospecimen” was also introduced in this section and we discussed how genetic information contained in our blood, urine, saliva and tissue cells can be collected for genetic testing. In the section called “Genetics,” we defined genetics as the study of our genes and presented multiple examples of genetic differences in humans that can be observed (e.g., eye color, hair color, allergic reactions to food and medications like penicillin). How genes become altered or mutated was covered in the section “Genetic Mutation”. Visual examples of inherited (e.g., famous actress with inherited BRCA breast cancer mutation), acquired (e.g., exposure to ultraviolet radiation from the sun or cigarette smoking), and sporadic mutations (e.g., images of families with children of both average size and those with dwarfism) were presented to demonstrate each concept. In the last section, precision medicine was discussed as it relates to cancer treatment based on our individual genetic differences.
At the end of each section, a review slide was presented, and participants were given the opportunity to ask questions before moving on to the next section of curriculum. Similarly, at the conclusion of the presentation, a question and answer session was held and conversations were encouraged to allow the health educator to conduct “myth-busting”. To each participant we provided a fact sheet that covered all the key concepts with corresponding images and graphics in English or Spanish as preferred by the participant and website links for further exploration at home (See Supplementary Material).
Curriculum Assessment
We conducted the study at NMIC, our community-based organization partner that provides a wide range of services to the predominantly underprivileged Hispanic, Spanish-speaking population of the Washington Heights/Inwood neighborhoods in Northern Manhattan. Participants were identified among clients utilizing the services of NMIC, as previously described for our preliminary evaluations. The research team CHWs embedded in NMIC approached participants directly in several NMIC waiting rooms and through contact with NMIC instructors of programs and courses including General Education Development (GED) and ESL. We invited anyone who was aged 18 years and older, who were either Spanish- or English-speaking and obtained informed consent prior to the start of each workshop. Participants were allowed to attend more than one educational session, but to complete only one pre/post-test assessment. Individuals who participated in any of the preliminary assessments were excluded from the curriculum assessment. A total of 513 adults participated in this curriculum, and of these, 438 (85.4%) completed pre- and post-comprehension testing. The institutional review board at Columbia University Irving Medical Center approved all study procedures.
We collected information related to sociodemographic characteristics including gender, age, race/ethnicity, nativity, education, health insurance status, and whether the participant had ever consulted with a geneticist in the past. Additionally, participants were asked about what sources they used for health information including print (e.g., books, pamphlets, magazine/newspaper), broadcast (e.g., TV/radio), friends and family, doctor/healthcare professional, community center, and the internet. Health literacy (Chew, Bradley, & Boyko, 2004) was assessed using two questions “How often do you understand all of the information you receive from your doctor?” and “How often do you need to have someone help you to read instructions and information from your doctor or pharmacy?” (Chew et al., 2004). Reponses were “all the time,” “sometimes,” “almost never,” and “never”.
Comprehension of genetic terms communicated in the curriculum was assessed prior to and immediately following the intervention using the Genetics Short REAL-G (Rapid Estimate of Adult Literacy in Genetics) (Hooker et al., 2014; Rodriguez, Roter, Castillo-Salgado, Hooker, & Erby, 2015). For these assessments, we asked participants to complete eight fill-in-the-blank sentences, one for each of the following terms: genetics, chromosome, susceptibility, mutation, variation/variant, abnormality, heredity, and sporadic mutation. Four response choices were provided for each sentence from which the participant could choose. Only one response was correct and was coded as “1” with incorrect responses coded as “0.”
Statistical Analysis
We examined descriptive statistics stratified first by language of class (Spanish, English) and compared the post-test to pre-test scores using paired t tests. We summed the correct responses for each of the 8 items for a total score ranging from 0 correct answers to 8 correct answers. We examined the overall distribution as well as compared post- to pre-test scores based on a “passing” score of 75% or greater (6 correct responses of 8). We calculated the aggregate score for the entire cohort (n = 438) and for Spanish- (n = 289) and English-speakers (n = 149) separately. We further examined whether these pre/post differences differed based on participant characteristics (education, ethnicity, age, gender, language of survey, years in the US if foreign-born, and health literacy) using stratified analyses and relative risk regressions. We also conducted multivariable analyses to examine relationships between each genetic term tested, gender, and level of health literacy, controlling for significant covariates. P values <0.05 were considered statistically significant. All analyses were performed using IBM SPSS, version 25 (IBM Corp., 2017).
RESULTS:
In total, 438 community adults completed the education and pre-/post-testing for comprehension (Table 1). Of these, 352 (80.4%) surveys were completed in Spanish. The majority of participants were female (61.2%), between the ages of 18 and 59 years (84.4%), Hispanic (86.1%), and foreign-born (77.4%). Of the foreign-born, 56.4% were born in the Dominican Republic (data not shown) and 41.9% had resided in the United States (U.S.) for 0-3 years. Just over 76% of participants scored <75% on the comprehension pre-test, compared to 60.3% at post-test.
Table 1:
Characteristics of Participants Attending our Community Cancer Genomics and Precision Medicine Educational Workshops.
| Total n = 438 |
Spanish n = 352 |
English n = 176 |
P value | |
|---|---|---|---|---|
| N (%) | ||||
| Survey Language | -- | |||
| Spanish | 289 (66.0) | -- | -- | |
| English | 149 (34.0) | -- | -- | |
| Sex | <0.001 | |||
| Male | 170 (38.8) | 72 (24.9) | 98 (65.8) | |
| Female | 268 (61.2) | 217 (75.1) | 51 (34.2) | |
| Age | ||||
| Mean [SD] (years) | 42.7 [13.9] | 43.5 [13.5] | 41.1 [14.7] | 0.09 |
| Range (years) | 18-82 | 18-82 | 18-77 | |
| 18-39 | 185 (42.2) | 113 (39.8) | 72 (49.7) | 0.15 |
| 40-59 | 185 (42.2) | 128 (45.1) | 57 (39.3) | |
| 60-79 | 57 (13.0) | 41 (14.4) | 146 (11.0) | |
| 80+ | 2 (0.5) | 2 (0.7) | 0 (0.0) | |
| Missing | 9 (2.1) | -- | --- | |
| Ethnicity | <0.001 | |||
| Non-Hispanic | 58 (13.2) | 2 (0.7) | 56 (38.4) | |
| Hispanic | 377 (86.1) | 287 (99.3) | 90 (61.6) | |
| Missing | 3 (0.7) | -- | -- | |
| Race | <0.001 | |||
| African American/Afro Caribbean, Black | 83 (18.9) | 39 (14.9) | 44 (34.4) | |
| Alaskan Native/American Indian | 13 (3.0) | 3 (1.1) | 10 (7.8) | |
| Asian/Pacific Islander | 5 (1.1) | 1 (0.4) | 4 (3.1) | |
| White | 38 (8.7) | 21 (8.0) | 17 (13.3) | |
| Mixed race | 171 (39.0) | 145 (54.9) | 26 (20.3) | |
| Don’t know | 32 (7.3) | 23 (8.7) | 9 (7.0) | |
| Other | 50 (11.4) | 32 (12.1) | 18 (14.1) | |
| Missing | 46 (10.5) | -- | -- | |
| Nativity | ||||
| U.S. born | 99 (22.6) | 3 (1.0) | 96 (64.4) | <0.001 |
| Foreign born | 339 (77.4) | 286 (99.0) | 53 (35.6) | |
| Missing | 14 (3.2) | -- | -- | |
| Number of years in the U.S. | ||||
| Mean | 10.1 [12.5] | 8.3 [10.8] | 19.4 [16.1] | <0.001 |
| Range | 0-67 | 0-54 | 0-67 | |
| 0-3 | 129 (41.9) | 121 (47.1) | 8 (15.7) | |
| 4-10 | 78 (25.3) | 69 (26.8) | 9 (17.6) | |
| 11+ | 101 (32.8) | 67 (26.1) | 34 (66.7) | |
| Missing | -- | -- | ||
| Education | 0.014 | |||
| 0-8 | 53 (12.1) | 42 (14.6) | 11 (7.4) | |
| 9-12 | 178 (40.6) | 105 (36.6) | 73 (49.0) | |
| >12 | 205 (46.8) | 140 (48.8) | 65 (43.6) | |
| Missing | 2 (0.5) | -- | -- | |
| Health insurance status | 0.04 | |||
| Uninsured | 77 (17.6) | 58 (20.7) | 19 (12.8) | |
| Insured | 351 (80.1) | 222 (79.3) | 129 (87.2) | |
| Missing | 10 (2.3) | -- | -- | |
| Geneticist | 0.02 | |||
| Yes | 44 (10.0) | 22 (7.7) | 22 (14.8) | |
| No | 334 (76.3) | 230 (81.0) | 104 (69.8) | |
| Don’t know | 55 (12.6) | 32 (11.3) | 23 (15.4) | |
| Missing | 5 (1.1) | -- | -- | |
| Source of health information | ||||
| Books | 140 (32.0) | 100 (34.6) | 40 (26.8) | 0.10 |
| Pamphlets | 104 (23.7) | 71 (24.6) | 33 (22.1) | 0.57 |
| Friends and family | 115 (26.3) | 82 (28.4) | 33 (22.1) | 0.16 |
| Doctor/healthcare professional | 244 (55.7) | 140 (48.4) | 104 (69.8) | <0.001 |
| Magazine/newspaper | 85 (19.4) | 54 (18.7) | 31 (20.8) | 0.60 |
| TV/radio | 137 (31.3) | 107 (37.0) | 30 (20.1) | <0.001 |
| Community center | 72 (16.4) | 54 (18.7) | 18 (12.1) | 0.08 |
| Internet | 231 (52.7) | 162 (56.1) | 69 (46.3) | 0.053 |
| Health literacy | ||||
| Understand verbal health information | 0.07 | |||
| All the time | 260 (59.4) | 169 (59.3) | 91 (62.3) | |
| Sometimes | 146 (33.3) | 94 (33.0) | 52 (35.6) | |
| Almost never | 12 (2.7) | 11 (3.9) | 1 (0.7) | |
| Never | 13 (3.0) | 11 (3.9) | 2 (1.4) | |
| Missing | 7 (1.6) | -- | -- | |
| Need help with written health instructions | <0.001 | |||
| Never | 122 (27.9) | 45 (15.7) | 77 (52.0) | |
| Almost never | 87 (19.9) | 58 (20.2) | 29 (19.6) | |
| Sometimes | 177 (40.4) | 142 (49.5) | 35 (23.6) | |
| Always | 49 (11.2) | 42 (14.6) | 7 (4.7) | |
| Missing | 3 (0.7) | -- | ||
| Comprehension Test Score | ||||
| Pre-test | <0.001 | |||
| <75% correct | 334 (76.4) | 238 (82.6) | 96 (64.4) | |
| ≥75% correct | 103 (23.6) | 50 (17.4) | 53 (35.6) | |
| Post-test | ||||
| <75% correct | 263 (60.3) | 181 (63.1) | 82 (55.0) | 0.10 |
| ≥75% correct | 173 (39.7) | 106 (36.9) | 67 (45.0) |
By preferred language of the survey, Spanish-speakers were more often female (75.1% vs. 34.2%, p <0.001), Hispanic (99.3% vs. 61.6%, p <0.001), foreign-born (99.0% vs. 35.6%, p <0.001), and had 0-8 years of education (14.6% vs. 7.4%, p = 0.014) compared to English-speakers (Table 1). Sources of health information varied by language as well with English-speakers more often reporting their doctor as the source of health information (69.8% vs. 48.4%, p <0.001) and Spanish-speakers preferring TV/radio (37.0% vs. 20.1%, p <0.001); there was no difference in the use of the internet for health-related information (p = 0.053) between English- and Spanish-speakers. We found no difference between English- and Spanish-speakers’ reported understanding of verbal health information “all the time” (59.3% vs. 62.3%, p = 0.07) but the need for help with written materials was much greater for Spanish-speakers. Compared to 52.0% of English-speakers, only 15.7% of Spanish-speakers stated they “never” needed help with written instructions (p <0.001). A pre-test comprehension score of <75% was recorded for 82.6% of Spanish-speaking participants compared to 64.4% of English-speakers (p <0.001); post-test scores were comparable.
Table 2a displays change in comprehension (mean difference in difference) of the 8-item panel and each of the 8 genetics-related concepts individually prior to and following the educational intervention. At pre-test, 45.6% of participants scored ≥75% and 66.7% at post-test; mean number of correct responses increased by approximately one question (post-pre aggregate score = 0.99, standard deviation [SD] 1.70, p <0.001). A statistically significant increase in comprehension was observed for 7 of the 8 measures, with greatest increases in comprehension for “mutation” with a 23% increase in the number of participants correctly using this term post-test vs pre-test (p <0.001) and for “sporadic” where we observed a 24% increase in comprehension (p <0.001). No change in comprehension of “chromosome” was found (p = 0.65). Similar increases in comprehension among Spanish-speakers were observed with greatest increases with the terms “mutation” (29% increase, p <0.001) and “sporadic” (24% increase, p <0.001) (Table 2b). Pre-test comprehension for English-speakers was generally higher (e.g., “mutation” 73.2% vs. 46.4% for Spanish-speakers) with gains in comprehension for 4 of the 8 terms. An increase of 17% in comprehension was noted for “abnormality” (p <0.001) and 26% increase for “sporadic” (p <0.001) (Table 2c). Independent sample t test showed a significant difference in the pre-test comprehension score (≥75%) between Spanish- and English-speakers (4.76 vs. 5.32, p = 0.003) but post-test score was equivalent (5.92 vs. 5.99, p = 0.66) (data not shown).
Table 2a:
Change in comprehension pre- to post-test for eight genetics-related concepts (both English- and Spanish-speakers).
| Total (n = 438) | |||||||
|---|---|---|---|---|---|---|---|
| Pre-test N (%) correct |
Post-test N (%) correct |
Mean correct [SD] | Mean difference pre- to post-test | 95% CI | P value | ||
| Pre-test | Post-test | ||||||
| 8-item panel | 195 (45.6)* | 292 (66.7)* | 4.94 [1.92] | 5.94 [1.56] | 0.99 [1.70] | 0.83, 1.15 | <0.001 |
| Genetics** | 366 (83.6) | 402 (91.8) | 0.84 [0.37] | 0.92 [0.44] | 0.08 [0.41] | 0.04, 0.12 | <0.001 |
| Chromosome** | 307 (70.1) | 302 (68.9) | 0.70 [0.46] | 0.69 [0.46] | −0.01 [0.52] | −0.06, 0.04 | 0.65 |
| Susceptibility** | 228 (52.1) | 265 (60.5) | 0.52 [0.50] | 0.61 [0.49] | 0.08 [0.57] | 0.03, 0.14 | 0.002 |
| Mutation** | 243 (55.5) | 343 (78.3) | 0.55 [0.50] | 0.78 [0.41] | 0.23 [0.49] | 0.18, 0.27 | <0.001 |
| Variation** | 255 (58.2) | 290 (66.2) | 0.58 [0.49] | 0.66 [0.47] | 0.08 [0.59] | 0.02, 0.13 | 0.005 |
| Abnormality** | 285 (65.1) | 362 (82.6) | 0.65 [0.48] | 0.83 [0.38] | 0.18 [0.47] | 0.13, 0.22 | <0.001 |
| Heredity ** | 334 (76.3) | 381 (87.0) | 0.76 [0.43] | 0.87 [0.34] | 0.11 [0.42] | 0.07, 0.15 | <0.001 |
| Sporadic** | 150 (34.2) | 257 (58.7) | 0.34 [0.48] | 0.59 [0.49] | 0.24 [0.58] | 0.19, 0.30 | <0.001 |
Overall score = 6/8 correct responses = 75%, range = 0-8;
Individual score, range = 0-1.
Table 2b:
Change in comprehension pre- to post-test for eight genetics-related concepts.
| Spanish-speakers (n = 289) | |||||||
|---|---|---|---|---|---|---|---|
| Pre-test N (%) correct |
Post-test N (%) correct |
Mean correct [SD] | Mean difference pre- to post-test | 95% CI | P value | ||
| Pre-test | Post-test | ||||||
| 8-item panel | 116 (40.1)* | 194 (67.1)* | 4.76 [1.83] | 5.92 [1.48] | 1.16 [1.71] | 0.96, 1.36 | <0.001 |
| Genetics** | 237 (82.0) | 268 (92.7) | 0.82 [0.38] | 0.93 [0.26] | 0.11 [0.42] | 0.06, 0.16 | <0.001 |
| Chromosome** | 195 (67.5) | 198 (68.5) | 0.67 [0.47] | 0.69 [0.47] | 0.01 [0.56] | −0.05, 0.07 | 0.75 |
| Susceptibility** | 133 (46.0) | 166 (57.4) | 0.46 [0.50] | 0.57 [0.50] | 0.11 [0.59] | 0.5, 0.18 | 0.001 |
| Mutation** | 134 (46.4) | 219 (75.8) | 0.46 [0.50] | 0.76 [0.43] | 0.29 [0.51] | 0.23, 0.35 | <0.001 |
| Variation** | 157 (54.3) | 183 (63.3) | 0.54 [0.50] | 0.63 [0.48] | 0.09 [0.61] | 0.02, 0.16 | 0.013 |
| Abnormality** | 193 (66.8) | 244 (84.4) | 0.67 [0.47] | 0.84 [0.36] | 0.18 [0.49] | 0.12, 0.23 | <0.001 |
| Heredity ** | 219 (75.8) | 256 (88.6) | 0.76 [0.43] | 0.89 [0.32] | 0.13 [0.43] | 0.08, 0.18 | <0.001 |
| Sporadic** | 107 (37.0) | 176 (60.9) | 0.37 [0.48] | 0.61 [0.49] | 0.24 [0.59] | 0.17, 0.31 | <0.001 |
Overall score = 6/8 correct responses = 75%, range = 0-8;
Individual score, range = 0-1.
Table 2c:
Change in comprehension pre- to post-test for eight genetics-related concepts.
| English-speakers (n = 149) | |||||||
|---|---|---|---|---|---|---|---|
| Pre-test N (%) correct |
Post-test N (%) correct |
Mean correct [SD] | Mean difference pre- to post-test | 95% CI | P value | ||
| Pre-test | Post-test | ||||||
| 8-item panel | 79 (53.0)* | 98 (65.8)* | 5.32 [2.04] | 5.99 [1.17] | 0.66 [1.64] | 0.40, 0.93 | <0.001 |
| Genetics** | 129 (86.6) | 134 (89.9) | 0.87 [0.34] | 0.90 [0.30] | 0.03 [0.38] | −0.02, 0.09 | 0.28 |
| Chromosome** | 112 (75.2) | 104 (69.8) | 0.75 [0.43] | 0.70 [0.46] | −0.05 [0.43] | −0.12, 0.02 | 0.13 |
| Susceptibility** | 95 (63.8) | 99 (66.4) | 0.64 [0.48] | 0.66 [0.47] | 0.03 [0.52] | −0.06, 0.11 | 0.53 |
| Mutation** | 109 (73.2) | 124 (83.2) | 0.73 [0.44] | 0.83 [0.37] | 0.10 [0.42] | 0.03, 0.17 | 0.004 |
| Variation** | 98 (65.8) | 107 (71.8) | 0.66 [0.48] | 0.72 [0.45] | 0.06 [0.54] | −0.02, 0.15 | 0.17 |
| Abnormality** | 92 (61.7) | 118 (79.2) | 0.62 [0.49] | 0.79 [0.41] | 0.17 [0.45] | 0.10, 0.25 | <0.001 |
| Heredity ** | 115 (77.2) | 125 (83.9) | 0.77 [0.42] | 0.84 [0.37] | 0.07 [0.40] | 0.003, 0.13 | 0.04 |
| Sporadic** | 43 (28.9) | 81 (54.4) | 0.29 [0.45] | 0.54 [0.50] | 0.26 [0.56] | 0.16, 0.34 | <0.001 |
Overall score = 6/8 correct responses = 75%, range = 0-8;
Individual score, range = 0-1.
We further examined whether the results differed by participant characteristics (age, gender, and language of survey) and as well as other factors that could be potentially modifiable through additional educational offerings (e.g., written health literacy). As Table 3 shows, we did find some differences by participant characteristics such as education, age and sex when the pre-test score was incorrect followed by a correct post-test score. Hispanics were more likely to correctly use “heredity” at post-test after using the term incorrectly at pre-test (OR 4.24, 95% CI 1.03-17.51). For the term “mutation,” we observed a positive linear association between education and the correct use of this term; those with 9th-12th grade education were 2.4 times more likely and those with greater than 12 years of education were 3.5 times more likely to correctly use this term after education (OR 2.40, 95% CI 1.09-5.26 and OR 3.55, 95% CI 1.58-7.99, respectively) than those with less than an 9th grade education. However, participants over the age of 50 years were much less likely to correctly use the term “mutation” (OR 0.47, 95% CI 0.26-0.86) compared to younger participants (<50 years of age). Spanish-speakers were less likely to correctly use the term “chromosome” (OR 0.37, 95% CI 0.16-0.85) at post-test after an incorrect response in pre-test compared to English-speakers. Females were also less likely to correctly use the term “abnormality” compared to males (OR 0.60, 95% CI 0.37-0.96). In multivariable analysis (data not shown), controlling for education and age, female sex was significantly associated with increased comprehension of “mutation” (OR 1.79, 95% CI 1.12-2.89) and “sporadic” (OR 2.31, 95% CI 1.49-3.58). There were no differences by written health literacy.
Table 3:
Relative risk assessment of the association of participant characteristics and a correct post-test response following an incorrect pre-test response compared to participants with incorrect responses at both pre- and post-test.
| Comprehension terms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Participant characteristics | Genetics | Chromosome | Susceptibility | Mutation | Variation | Abnormality | Heredity | Sporadic |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Education | ||||||||
| 0-8 years | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 9-12 years | 1.21 (0.34-4.24) | 0.73 (0.29-1.88) | 1.94 (0.81-4.66) | 2.40 (1.09-5.26)* | 0.96 (0.42-2.15) | 1.67 (0.72-3.87) | 1.23 (0.39-3.90) | 0.87 (0.42-1.78) |
| >12 years | 1.76 (0.37-8.32) | 1.79 (0.67-4.78) | 1.17 (0.49-2.80) | 3.55 (1.58-7.99)* | 0.50 (0.22-1.18) | 2.20 (0.87-5.54) | 1.91 (0.55-6.60) | 1.12 (0.55-2.31) |
| Ethnicity | ||||||||
| Non-Hispanic | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hispanic | 2.35 (0.50-11.15) | 1.38 (0.38-4.96) | 0.62 (0.20-1.92) | 2.23 (0.61-8.17) | 0.65 (0.24-1.77) | 2.12 (0.85-5.28) | 4.24 (1.03-17.51)* | 1.20 (0.59-2.42) |
| Age | ||||||||
| <50 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥50 | 0.57 (0.18-1.77) | 0.91 (0.43-1.91) | 1.00 (0.57-1.76) | 0.47 (0.26-0.86)* | 1.34 (0.74-2.44) | 1.76 (1.08-2.87) | 0.53 (0.23-1.19) | 0.70 (0.43-1.15) |
| Gender | ||||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 1.52 (0.49-4.70) | 1.09 (0.54-2.18) | 0.96 (0.53-1.75) | 1.44 (0.79-2.65) | 0.78 (0.43-1.43) | 0.60 (0.37-0.96)* | 0.88 (0.39-1.97) | 1.07 (0.67-1.70) |
| Language | ||||||||
| English | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Spanish | 0.39 (0.12-1.25) | 0.37 (0.16-0.85)* | 0.89 (0.48-1.67) | 0.74 (0.37-1.48) | 0.98 (0.51-1.87) | 0.96 (0.58-1.59) | 0.49 (0.21-1.13) | 0.76 (0.47-1.23) |
| Years in country | ||||||||
| <10 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥10 | 1.47 (0.29-7.45) | 0.87 (0.37-2.06) | 0.95 (0.48-1.86) | 0.81 (0.42-1.57) | 1.47 (0.73-2.93) | 1.74 (0.98-3.08) | 1.03 (0.39-2.76) | 0.96 (0.54-1.71) |
| Health Literacy | ||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| High | 0.98 (0.30-3.27) | 0.86 (0.43-1.72) | 0.80 (0.46-1.38) | 1.39 (0.77-2.53) | 1.39 (0.77-2.52) | 0.69 (0.43-1.11) | 1.66 (0.73-3.80) | 0.90 (0.57-1.44) |
P-value <0.05
DISCUSSION
Using a curriculum informed by CBPR, we found improvement in comprehension of 8 genetics-related terms. The two terms for which we observed the greatest increase in comprehension pre- to post-test for all participants were “mutation” and “sporadic” (23% and 24% increase in post-test compared to pre-test, respectively). By language, comprehension of “mutation” increased 29% for Spanish-speakers vs. 10% for English-speakers and, for “sporadic” we observed a 24% increase in comprehension among Spanish-speakers and 26% among English-speakers. Differences in comprehension for a few terms varied by age and sex. Reasons for this are not clear but are possibly related to educational attainment. Surprisingly, comprehension of the term “chromosome” that was understood by approximately 70% of participants at pre-test, did not improve statistically. This may be related to poor foundational understanding of biological science.
Overall, comprehension test scores at pre-test were low with only 23.6% of participants scoring 75% or greater (correctly answering 6 of the 8 test questions). When evaluating comprehension by language in which the education was conducted, Spanish-speaking participants scored significantly lower at pre-test than did English-speakers. Recent large national studies assessing knowledge surrounding genetic testing found that compared to Whites, minorities are less knowledgeable of genetic testing (Canedo et al., 2019; Haga, O’Daniel, Tindall, Lipkus, & Agans, 2012; Singer, Couper, Raghunathan, Van Hoewyk, & Antonucci, 2008). A retrospective evaluation of the General Social Survey at three time points (1990, 1996, and 2004) showed that while the proportion of respondents claiming to know “a great deal” about genetic testing has increased (from 12.8% in 1990 to 18.1% in 2004), 67.4% of respondents know “not very much” and 14.1% know “nothing at all”. Non-Whites were much less likely to know “a great deal” (β −0.632, SE 0.138) (Singer et al., 2008). In another study of the attitudes toward pharmacogenetic testing among a sample of 1139 U.S. adults, Whites were 74% more likely to be aware of genetic testing vs. non-Whites (OR 1.74, 95% CI 1.22-2.49) (Haga et al., 2012). Two additional studies comparing differences in genetic knowledge using scored tests similarly reported lower average knowledge for Blacks and Hispanic/Latinos compared to Whites (Singer, Antonucci, & Van Hoewyk, 2004; Suther & Kiros, 2009). Using a series of seven factual questions related to genetic testing (e.g., “Genetic testing can be used in adults to find out if they have a greater than average chance of developing certain kinds of cancer (True/false question; correct response = True)”), Singer et al.(2004) found that mean accurate responses varied significantly by race/ethnicity, with Hispanics scoring lower than Whites, (p <0.01) and concluded that lower levels of genetic awareness and knowledge could lead to greater health disparities in the uptake of precision medicine initiatives.
Among cancer patients, knowledge of cancer genetics is also low. One study evaluating knowledge, attitudes, and expectations toward genomic testing in cancer (GTC) among 98 patients with advanced cancer found the median knowledge of GTC was 67% (8 of 12 items correct). Less than half (48%) of the patients believed they had enough knowledge to make an informed decision to pursue GTC (Blanchette et al., 2014). In another study, patients enrolled in Lung-MAP, a precision medicine randomized controlled trial examining somatic mutations associated with lung cancer, were asked about somatic and germline mutations (Kosko, 2019; Yasinski, 2020). Of the 123 patients with advanced non-small cell lung cancer enrolled onto the trial who completed this survey, only 8% knew that the tests done would not reveal any information about cancer risk for their family members and 12% knew their test results could not predict their own increased risk of other diseases. These studies demonstrate that patients with cancer who have access to information about genetics from their healthcare providers lack understanding of key genetic concepts. Supporting these findings, a recent scoping review of 29 papers from 25 studies synthesized what was know about healthcare professionals’ communication about genetic testing for hereditary breast and ovarian cancer (Jacobs, Patch, & Michie, 2019). The authors concluded that genetic counseling tended to focus on biomedical information and that patients’ need for cancer-focused, personalized information was not being met, further emphasizing the importance of basic genetic educational curricula such as ours.
Study Limitations:
While our study demonstrated significant increases in knowledge of key terms associated with genetics, it is limited in that it was an observational study conducted at a single site in a largely Hispanic neighborhood of New York City and may not be generalizable to other geographic locations and Hispanic subpopulations. The validated measure of comprehension tested a limited number of items. Knowledge or lack of knowledge of terms outside those tested may have influenced our measure of comprehension positively or negatively, although this instrument has been used successfully by others as part of a cancer decision support tool (Giuse et al., 2016). Furthermore, pre- and post-test assessments utilized a “fill in the blank” format with which some participants educated in countries other than the U.S. may not have been familiar. This may have resulted in incomplete or missing responses thus potentially lowering test scores despite the presence of a trained bilingual research assistant to assist with test-taking.
Practice Implications:
Our results, however, demonstrate the effectiveness of our community-based curriculum to educate a predominantly Hispanic population in Northern Manhattan about key concepts related to precision medicine and emphasize the importance of tailoring materials to the literacy level of Hispanic and minority, disadvantaged populations and conducting education directly in the community. Our findings are especially poignant in light of substantial evidence in the literature demonstrating lower comprehension of genetics among minorities compared to non-Hispanic Whites. Most importantly, our curriculum increased comprehension of the key concepts of genetic mutations in general and the difference between sporadic and germline mutations – both of which are critically important concepts when communicating genetic risk to patients.
Opportunities to deploy a genetic curriculum such as ours in settings other than the community are as varied as precision medicine applications in oncology. For example, in the primary care setting where family history may reveal a potential risk for cancers with a known genetic determinant (e.g., Lynch syndrome) or when cancer surgery and/or treatment decisions are made and clinical trial options are presented may be an opportune time to introduce genetic education. Although designed for a Hispanic community audience, our curriculum not only lends itself to settings outside of the community but also to adaptation to the educational needs of other racial and ethnic groups and varied geographic locations.
Research Recommendations:
Further research to expand and optimize educational offerings to diverse communities will be essential to the application of precision medicine and assurance that these initiatives are applied equitably across all patient groups. Building an understanding of basic key genomic concepts lays the foundation for future investigations related to developing best practices to educate diverse cultural groups about the genetic referral and counseling process, interpretation of testing results, and health implications for themselves and family members. Additional research is also needed to examine barriers to minority enrollment to genetic research and clinical trials in order to tailor and target recruitment strategies to specific needs of diverse populations.
CONCLUSIONS
As genomic research transforms our understanding of health and disease, our approaches to disease prevention and treatment are rapidly changing. Unequal health care access and social determinants of health experienced by Hispanics and racial minority groups may result in the unequal dissemination of precision medicine, thus exacerbating the health disparity gap (Canedo et al., 2019). There now exists an increasing need to effectively translate this complex information to patients in a meaningful and culturally sensitive manner that accounts for existing knowledge and English proficiency. Our curriculum was tailored to the educational and linguistic needs of a predominantly Hispanic population based on preliminary work that revealed a lack of understanding of basic genetic concepts and the connection between genes, inheritance, and risk of disease. That comprehension was increased for 7 of 8 genetics-related terms demonstrates the utility of this education to prepare this population for future conversations with healthcare providers about disease prevention and treatment using precision medicine.
Supplementary Material
ACKNOWLEDGEMENTS:
This work was supported by a grant from the National Cancer Institute (5P30CA013686-44S3).
Footnotes
CONFLICT OF INTEREST: Grace Clarke Hillyer, Karen M. Schmitt, Andria Reyes, Alejandro Cruz, Maria Lizardo, Gary K. Schwartz, and Mary Beth Terry have no conflicts of interest to declare.
HUMAN STUDIES AND INFORMED CONSENT: All procedures followed were approved by the Columbia University Irving Medical Center Institutional Review Board and conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all participants for being included in the study.
ANIMAL STUDIES: No non-human animal studies were carried out by the authors for this article.
REFERENCES
- American Cancer Society. (2014). Oncogenes and tumor suppressor genes. Retrieved from https://www.cancer.org/cancer/cancer-causes/genetics/genes-and-cancer/oncogenes-tumor-suppressor-genes.html
- American Medical Association. (2020). Precision medicine: Education & resources in genetics & personalized medicine. Retrieved from https://www.ama-assn.org/delivering-care/precision-medicine/education-resources-genetics-personalized-medicine
- American Society for Clinical Oncology (ASCO). (2017). What is personalized cancer medicine? Retrieved from https://www.cancer.net/navigating-cancer-care/how-cancer-treated/personalized-and-targeted-therapies/what-personalized-cancer-medicine
- American Society for Clinical Oncology (ASCO). (2018). The genetics of cancer. Retrieved from https://www.cancer.net/navigating-cancer-care/cancer-basics/genetics/genetics-cancer
- Blanchette PS, Spreafico A, Miller FA, Chan K, Bytautas J, Kang S, … Siu LL (2014). Genomic testing in cancer: patient knowledge, attitudes, and expectations. Cancer, 120(19), 3066–3073. doi: 10.1002/cncr.28807 [DOI] [PubMed] [Google Scholar]
- Canedo JR, Miller ST, Myers HF, & Sanderson M (2019). Racial and ethnic differences in knowledge and attitudes about genetic testing in the US: Systematic review. Journal of Genetic Counseling, Epub 2019/01/22. doi: 10.1002/jgc4.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz DS, Green NS, Tobin JN, Lloyd-Puryear MA, Kyler P, Umemoto A, … Wolman F (2005). Attitudes about Genetics in Underserved, Culturally Diverse Populations. Public Health Genomics, 8(3), 161–172. doi: 10.1159/000086759 [DOI] [PubMed] [Google Scholar]
- Chew LD, Bradley KA, & Boyko EJ (2004). Brief questions to identify patients with inadequate health literacy. Family Medicine, 36(8), 588–594. [PubMed] [Google Scholar]
- Collins H, Calvo S, Greenberg K, Forman Neall L, & Morrison S (2016). Information Needs in the Precision Medicine Era: How Genetics Home Reference Can Help. Interactive Journal of Medical Research, 5(2), e13–e13. doi: 10.2196/ijmr.5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetic Alliance. (2008). Chapter 1: Genetics 101. In Understanding Genetics: A New York - Mid-Atlantic guide for patients and health professionals. Washington, D.C. [PubMed] [Google Scholar]
- Giuse NB, Kusnoor SV, Koonce TY, Naylor HM, Chen SC, Blasingame MN, … Lovly CM (2016). Guiding oncology patients through the maze of Precision Medicine. Journal of Health Communication, 21 Suppl, 5–17. doi: 10.1080/10810730.2015.1131772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB, O’Daniel JM, Tindall GM, Lipkus IR, & Agans R (2012). Survey of US public attitudes toward pharmacogenetic testing. The Pharmacogenomics Journal, 12(3), 197–204. doi: 10.1038/tpj.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JG, Shuk E, Arniella G, Gonzalez CJ, Gold GS, Gany F, … Hay JL. (2016). Genetic Testing Awareness and Attitudes among Latinos: Exploring Shared Perceptions and Gender-Based Differences. Public Health Genomics, 19(1), 34–46. doi: 10.1159/000441552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer G, Schmitt KM, Lizardo M, Reyes A., Bazan M, Sandoval R, … MA O. (2016). Examination of e-communication and health information preferences among Hispanics in Northern Manhattan: Prelude to delivering a public-centered precision medicine curriculum. [Google Scholar]
- Hillyer GC, Schmitt KM, Lizardo M, Reyes A, Bazan M, Alvarez MA, … Orjuela MA (2017). Electronic communication channel use and health information source preferences among Latinos in Northern Manhattan. Journal of Community Health, 42(2), 349–357. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27655586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker GW, Peay H, Erby L, Bayless T, Biesecker BB, & Roter DL (2014). Genetic literacy and patient perceptions of IBD testing utility and disease control: a randomized vignette study of genetic testing. Inflammatory Bowel Diseases, 20(5), 901–908. doi: 10.1097/MIB.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, Becker AB, & Community-Campus Partnerships for, H. (2001). Community-based participatory research: policy recommendations for promoting a partnership approach in health research. Education for Health (Abingdon), 14(2), 182–197. doi: 10.1080/13576280110051055 [DOI] [PubMed] [Google Scholar]
- Jacobs C, Patch C, & Michie S (2019). Communication about genetic testing with breast and ovarian cancer patients: a scoping review. European Journal of Human Genetics, 27(4), 511–524. doi: 10.1038/s41431-018-0310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagsi R, Griffith KA, Kurian AW, Morrow M, Hamilton AS, Graff JJ, … Hawley ST (2015). Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. Journal of Clinical Oncology, 33(14), 1584–1591. doi: 10.1200/jco.2014.58.5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney AY, Gammon A, Coxworth J, Simonsen SE, & Arce-Laretta M (2010). Exploring attitudes, beliefs, and communication preferences of Latino community members regarding BRCA1/2 mutation testing and preventive strategies. Genetics in Medicine, 12(2), 105–115. doi: 10.1097/GIM.0b013e3181c9af2d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosko K (2019). Lung-MAP trial reveals genetic testing knowledge gaps among patients. Retrieved from https://www.curetoday.com/publications/cure/2019/lung-2-2019/lungmap-trial-reveals-genetic-testing-knowledge-gaps-among-patients
- Krop I, Ismaila N, Andre F, Bast RC, Barlow W, Collyar DE, … Stearns V (2017). Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. Journal of Clinical Oncology, 35(24), 2838–2847. doi: 10.1200/jco.2017.74.0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea DH, Kaphingst KA, Bowen D, Lipkus I, & Hadley DW (2011). Communicating genetic and genomic information: health literacy and numeracy considerations. Public Health Genomics, 14(4–5), 279–289. doi: 10.1159/000294191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus PM, Freedman AN, & Khoury MJ (2015). Targeted cancer screening in average-risk individuals. American Journal of Preventive Medicine, 49(5), 765–771. doi: 10.1016/j.amepre.2015.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. (2013). Genetic testing for hereditary cancer syndromes. Retrieved from http://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet
- National Human Genome Research Institute. (2015). Chromosomes. Retrieved from https://www.genome.gov/26524120/chromosomes-fact-sheet/ [Google Scholar]
- Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, … Ogino S (2013). Aspirin use and risk of colorectal cancer according to BRAF mutation status. Journal of the American Medical Association, 309(24), 2563–2571. doi: 10.1001/jama.2013.6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReadabilityFormulas.com (2018). The Flesch Reading Ease Readability Formula. Retrieved from http://www.readabilityformulas.com/flesch-reading-ease-readability-formula.php
- Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, … Weber BL (2002). Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. New England Journal of Medicine, 346(21), 1616–1622. doi: 10.1056/NEJMoa012158 [DOI] [PubMed] [Google Scholar]
- Rodriguez SA, Roter DL, Castillo-Salgado C, Hooker GW, & Erby LH (2015). Translation and validation of a Spanish-language genetic health literacy screening tool. Health Psychology, 34(2), 120–129. doi: 10.1037/hea0000162 [DOI] [PubMed] [Google Scholar]
- Schwaederle M, Zhao M, Lee JJ, Lazar V, Leyland-Jones B, Schilsky RL, … Kurzrock R (2016). Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: A meta-analysis. Journal of the American Medical Association Oncology, 2(11), 1452–1459. doi: 10.1001/jamaoncol.2016.2129 [DOI] [PubMed] [Google Scholar]
- Sepulveda-Pacsi AL, & Bakken S (2017). Correlates of Dominicans’ Identification of Cancer as a Worrisome Health Problem. Journal of Immigrant and Minority Health, 19(5), 1227–1234. doi: 10.1007/s10903-016-0509-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y, Eklund M, Madlensky L, Sawyer SD, Thompson CK, Stover Fiscalini A, … Athena Breast Health Network, I. (2017). Breast cancer screening in the Precision Medicine era: Risk-based screening in a population-based trial. Journal of the National Cancer Institute, 109(5). doi: 10.1093/jnci/djw290 [DOI] [PubMed] [Google Scholar]
- Singer E, Antonucci T, & Van Hoewyk J (2004). Racial and ethnic variations in knowledge and attitudes about genetic testing. Genetic Testing, 8(1), 31–43. doi: 10.1089/109065704323016012 [DOI] [PubMed] [Google Scholar]
- Singer E, Couper MP, Raghunathan TE, Van Hoewyk J, & Antonucci TC (2008). Trends in U.S. attitudes toward genetic testing, 1990-2004. Public Opinion Quarterly, 72(3), 446–458. doi: 10.1093/poq/nfn033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HG, Calancie L, Vu MB, Garcia B, DeMarco M, Patterson C, … Schisler JC (2015). Using community-based participatory research principles to develop more understandable recruitment and informed consent documents in genomic research. PLoS One, 10(5), e0125466. doi: 10.1371/journal.pone.0125466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher SA, Sanderson SC, Jabs EW, Diefenbach M, Smirnoff M, Peter I, … Richardson LD (2011). Reasons for participating and genetic information needs among racially and ethnically diverse biobank participants: a focus group study. Journal of Community Genetics, 2(3), 153–163. doi: 10.1007/s12687-011-0052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussner KM, Jandorf L, Thompson HS, & Valdimarsdottir HB (2013). Barriers and facilitators to BRCA genetic counseling among at-risk Latinas in New York City. Psychooncology, 22(7), 1594–1604. doi: 10.1002/pon.3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suther S, & Kiros GE (2009). Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genetics in Medicine, 11(9), 655–662. doi: 10.1097/GIM.0b013e3181ab22aa [DOI] [PubMed] [Google Scholar]
- Syurina EV, Brankovic I, Probst-Hensch N, & Brand A (2011). Genome-based health literacy: a new challenge for public health genomics. Public Health Genomics, 14(4–5), 201–210. doi: 10.1159/000324238 [DOI] [PubMed] [Google Scholar]
- U. S. National LIbrary of Medicine. (2020). Genetics Home Reference: Your guide to understanding genetic conditions. Retrieved from https://ghr.nlm.nih.gov/
- Yasinski E (2020). Misunderstandings about cancer DNA tests. Retrieved from https://www.cancertodaymag.org/Pages/cancer-talk/Misunderstandings-About-Cancer-DNA-Tests.aspx [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


