Abstract
Background
High-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT) has been investigated in patients with primary central nervous system lymphoma (PCNSL) and non-Hodgkin lymphoma (NHL) with CNS involvement and has shown promising results.
Patients and Methods
A retrospective analysis was performed of 48 consecutive patients who had undergone HDC/ASCT with TBC (thiotepa, busulfan, cyclophosphamide) conditioning for PCNSL (27 patients), secondary CNS lymphoma (SCNSL) (8 patients), or relapsed disease with CNS involvement (13 patients) from July 2006 to December 2017. Of the 27 patients with PCNSL, 21 had undergone ASCT at first complete remission (CR1).
Results
The 2-year progression-free survival (PFS) rate was 80.5% (95% confidence interval [CI], 69.9-92.9) and the 2-year overall survival (OS) rate was 80.1% (95% CI, 69.2%-92.7%) among all patients. The 2-year PFS and OS rate for patients with PCNSL in CR1 was 95.2% (95% CI, 86.6%-100%) and 95.2% (95% CI, 86.6%-100%), respectively. On univariate analysis of the patients with PCNSL, ASCT in CR1 was the only variable statistically significant for outcome (P = .007 for PFS; P = .008 for OS). Among patients with SCNSL or CNS relapse, the 2-year PFS and OS rate were comparable at 75.9% (95% CI, 59.5%-96.8%) and 75.3% (95% CI, 58.6%-98.6%), respectively. The most common side effects were febrile neutropenia (89.6%; of which 66.7% had an infectious etiology identified), nausea/vomiting (85.4%), diarrhea (93.8%), mucositis (89.6%), and electrolyte abnormalities (89.6%). Four patients (8.3%) died of treatment-related overwhelming infection; of these patients, 3 had SCNSL.
Conclusion
HDC and ASCT using TBC conditioning for both PCNSL and secondary CNS NHL appears to have encouraging long-term efficacy with manageable side effects.
Keywords: ASCT, Non-Hodgkin lymphoma, PCNSL, Relapse, SCNL
Micro-Abstract
We retrospectively analyzed the data from 48 patients with central nervous system lymphoma who had received high-dose chemotherapy and autologous stem cell transplantation using TBC (thiotepa, busulfan, cyclophosphamide) conditioning. The 2-year progression-free and overall survival rate was 80.5% and 80.1%, respectively. Toxicities included nausea/vomiting, diarrhea, mucositis, and febrile neutropenia. Treatment-related mortality was 8.3% in the first 100 days after transplantation. These data support the use of consolidative autologous stem cell transplantation for patients with primary or secondary central nervous system lymphoma.
Introduction
Both primary central nervous system (CNS) lymphoma (PCNSL) and non-Hodgkin lymphoma (NHL) with CNS involvement have historically carried poor prognoses.1 However, the increased efficacy of induction chemotherapy and the addition of consolidation therapy have resulted in improvements in recent years.
Although high-dose methotrexate (HD-MTX), alone or combined with other agents, has become the mainstay of induction therapy,2 the optimal consolidation strategy has not yet been established. Consolidative whole-brain radiation therapy (WBRT) has been used; however, given the low survival benefit and high risk of neurotoxicity with this approach,3 , 4 efforts have ensued to identify alternative options. High-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT) has been investigated for postremission consolidation and for relapsed disease, with promising results.5, 6, 7, 8, 9
HD-MTX–based induction regimens, followed by ASCT, with various conditioning regimens, including BEAM (carmustine, etoposide, cytarabine, melphalan),5 , 6 , 10 thiotepa, busulfan,11 , 12 TBC (thiotepa, busulfan, cyclophosphamide),8 , 9 , 13, 14, 15, 16, 17, 18, 19, 20 and thiotepa, carmustine21, 22, 23, 24, 25, 26 have been evaluated. The data thus far, however, have been limited to single-arm phase II trials, and several important questions remain, including the best candidates for such therapy, timing of its use, possible toxicities, and optimal conditioning regimen. We performed a single institution retrospective analysis of consecutive patients with PCNSL, secondary CNS lymphoma (SCNSL), or relapsed CNS lymphoma who had undergone consolidative ASCT with TBC conditioning based on early experiences using this regimen.19 , 20
Patients and Methods
Patients
From July 2006 to December 2017, the data from all patients with NHL who had undergone ASCT with the TBC conditioning regimen at the University of California, Los Angeles, were retrospectively reviewed. Three groups of patients were included: those with PCNSL, defined as lymphoma confined to the CNS, including intraocular lymphoma; those with SCNSL, defined as lymphoma with systemic disease and CNS involvement; and those with CNS relapse, defined as initial diagnosis of systemic lymphoma, followed by relapse in the CNS, with or without systemic involvement at relapse. The patients with SCNSL and CNS relapse were grouped together as patients with systemic lymphoma with CNS involvement, whether found at the initial diagnosis or on relapse. The primary analysis included PCNSL patients who had undergone ASCT in first complete remission (CR1). Patients with SCNSL or with CNS involvement at relapse were analyzed separately.
Treatment
For patients with PCNSL, a uniform induction therapy was given, consisting of rituximab 375 mg/m2 and HD-MTX 8 g/m2, followed by rituximab 375 mg/m2, cytarabine 2 × 3 g/m2, and thiotepa 40 mg/m2, in accordance with reported data.27 The number of cycles of rituximab/HD-MTX and rituximab/cytarabine/thiotepa was determined by physician preference. The dosages were adjusted at the physicians’ discretion for organ toxicity, tolerance, and/or functional status. For patients with SCNSL or relapsed disease, a variety of chemotherapy regimens were used at the clinician’s discretion with a goal of achieving CR before ASCT (Table 1 ). Remission status was assessed using magnetic resonance imaging and/or positron emission tomography of the brain before transplantation and, ultimately, interpreted by the treating physician.
Table 1.
Pretransplantation Therapy Regimens Stratified by Group
| Regimen | All Patients (n = 48) | PCNSL (n = 27) | SCNSL/CNS Relapse (n = 21) |
|---|---|---|---|
| R-CHOP | 6 (12.5) | 0 (0) | 6 (28.6) |
| R-EPOCH | 9 (18.8) | 0 (0) | 9 (42.9) |
| R-DHAP | 6 (12.5) | 0 (0) | 6 (28.6) |
| R-DHAOx | 2 (4.2) | 0 (0) | 2 (9.5) |
| HyperCVAD | 1 (2.1) | 0 (0) | 1 (4.8) |
| CHOEP | 1 (2.1) | 0 (0) | 1 (4.8) |
| Temozolomide | 4 (8.3) | 3 (11.1) | 1 (4.8) |
| Belinostat | 1 (2.1) | 0 (0) | 1 (4.8) |
| Topotecan | 1 (2.1) | 1 (3.7) | 0 (0) |
| Rituximab | 44 (91.7) | 26 (96.3) | 18 (85.7) |
| HD-MTX | 41 (85.4) | 27 (100) | 14 (66.7) |
| HD-Ara-C | 30 (62.5) | 26 (96.3) | 4 (19.0) |
| Thiotepa | 25 (52.1) | 25 (92.6) | 0 (0) |
| IT-MTX | 8 (16.7) | 0 (0) | 8 (38.1) |
| IT-Ara-C | 7 (14.6) | 0 (0) | 7 (33.3) |
| WBRT before ASCT | 3 (6.3) | 2 (7.4) | 1 (4.8) |
| HD-MTX cycles | |||
| Median | 4 | 4 | 4 |
| Range | 0-14 | 2-14 | 0-10 |
| HD-MTX cycles for PCNSL in CR1 | NA | NA | |
| Median | 4 | ||
| Range | 2-13 | ||
| Ara-C/thiotepa cycles | |||
| Median | 1 | 2 | 0 |
| Range | 0-3 | 0-2 | 0-3 |
| Ara-C/thiotepa cycles for PCNSL in CR1 | NA | NA | |
| Median | 2 | ||
| Range | 1-2 |
Data presented as n (%).
Abbreviations: ASCT = autologous stem cell transplantation; CHOEP = cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone; CR1 = first complete remission; HD-MTX = high-dose methotrexate; HyperCVAD = cyclophosphamide, vincristine, doxorubicin, dexamethasone; IT-Ara-C = intrathecal cytarabine; IT-MTX = intrathecal methotrexate; NA = not applicable; PCNSL = primary central nervous system lymphoma; PR = partial response; R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-DHAOx = rituximab, dexamethasone, high-dose cytarabine, oxaliplatin; R-DHAP = rituximab, dexamethasone, cytarabine, cisplatin; R-EPOCH = rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; SCNSL = secondary central nervous system lymphoma; WBRT = whole brain radiation therapy.
HDC before ASCT consisted of thiotepa 250 mg/m2 daily for 3 doses on days −9 to −7, busulfan 0.8 mg/kg every 6 hours for 12 doses on days −6 to −4, and cyclophosphamide 60 mg/kg daily for 2 doses on days −3 and −2, with mesna, as previously described by Soussain et al.9 Phenytoin 300 mg daily was given for seizure prophylaxis on days −7 to −2. Peripheral blood stem cells were mobilized and collected per institution protocol.
Toxicity Evaluation
Toxicity was assessed via medical record review. Neutrophil and platelet recovery were assessed according to the Center for International Blood and Marrow Transplant Research (available at: https://www.cibmtr.org/DataManagement/TrainingReference/Manuals/DataManagement/Documents/post-ted-instruction.pdf). Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of ≥ 500 cells/mm2. Platelet recovery was defined as the first of 3 consecutive days with a platelet count of ≥ 20,000/mm2 that was 7 days after the last platelet transfusion. Treatment-related mortality (TRM) was defined as death from any cause other than disease relapse within 100 days after ASCT.
Survival
The Kaplan-Meier method was used to plot the survival curves. Progression-free survival (PFS) was defined from the date of ASCT to the date of relapse, progression, or death from any cause. Overall survival (OS) was calculated from the date of ASCT to death from any cause. The date of disease progression was assigned using the date of the imaging study or biopsy, if available, to confirm disease progression.
Statistical Analysis
Data are summarized as the mean ± standard deviation, median and interquartile range, or number in the group with the percentage of the group, according to the distribution of the data. Univariate comparisons between diagnoses were performed using Fisher’s exact test for discrete data and the Wilcoxon rank sum test for continuous data. OS and PFS were modeled using the Cox proportional hazards model and summarized using hazard ratios (HRs) and 95% confidence intervals (CIs). Time-to-event data were further summarized using Kaplan-Meier curves. All hypotheses were 2-sided, and P < .05 was considered to indicate statistical significance. Analyses were completed using the R Statistical Computing Environment (R Core Team; R Foundation, Vienna, Austria).
Results
Patient Characteristics
From July 2006 to December 2017, 48 consecutive patients with NHL were identified who had undergone ASCT with TBC conditioning. Of these 48 patients, 27 had PCNSL (2 of whom had intraocular lymphoma) and 21 had either relapsed disease with CNS involvement (13 patients) or SCNSL (8 patients). The median follow-up time after ASCT was 23.9 months (interquartile range [IQR], 8.4-59.5 months). Of the 27 patients with PCNSL, 21 had undergone ASCT in CR1. Of the secondary CNSL/CNS relapse group, 17 patients had diffuse large B-cell lymphoma, 1 had anaplastic large cell lymphoma, 1 had primary effusion lymphoma, and 2 had peripheral T-cell lymphoma.
The patient characteristics are listed in Table 2 . The median age of the patients at ASCT was 59.4 years (IQR, 52.1-66.2 years). Of the 48 patients, 23 were women and 25 were men. Two patients had human immunodeficiency virus. Among the total 48 patients, 22 patients had non-germinal center B cell (GCB), 14 patients had GCB, 8 patients had unknown phenotype, and 4 were not applicable. Most patients had a Karnofsky score at ASCT of ≥ 80 (67.0%). Overall, 29 patients (60.0%) had undergone ASCT in CR1 and 19 patients (40.0%) had undergone ASCT not in CR1. Of these 19 patients, 3 had undergone ASCT in partial response (all with PCNSL) and 16 had undergone ASCT in the second or third CR.
Table 2.
Patient Characteristics
| Characteristic | All Patients (n = 48) | PCNSL (n = 27) | SCNSL/CNS Relapse (n = 21) | P Value |
|---|---|---|---|---|
| Age at diagnosis, y | .51 | |||
| Median | 58.0 | 56.7 | 61.4 | |
| IQR | 50.4-64.8 | 49.9-63.9 | 50.8-65.1 | |
| Age group | .56 | |||
| ≤60 y | 26 (54) | 16 (59.3) | 10 (47.6) | |
| >60 y | 22 (46) | 11 (40.7) | 11 (52.4) | |
| Age at ASCT, y | .33 | |||
| Median | 59.4 | 57.2 | 62.0 | |
| IQR | 52.1-66.2 | 50.6-64.8 | 53.5-67.3 | |
| Age group | .77 | |||
| ≤60 y | 25 (52.0) | 15 (55.6) | 10 (47.6) | |
| >60 y | 23 (48.0) | 12 (44.4) | 11 (52.4) | |
| Gender | .77 | |||
| Female | 23 (48.0) | 12 (44.4) | 11 (52.4) | |
| Male | 25 (52.0) | 15 (55.6) | 10 (47.6) | |
| Ethnicity | ||||
| White | 20 (42.0) | 11 (40.7) | 9 (42.9) | .58 |
| Hispanic | 10 (21.0) | 5 (18.5) | 5 (23.8) | |
| Asian | 6 (12.0) | 5 (18.5) | 1 (4.8) | |
| Other | 12 (25.0) | 6 (22.2) | 6 (28.6) | |
| HIV status | ||||
| Negative | 46 (96.0) | 27 (100.0) | 19 (90.5) | .19 |
| Positive | 2 (4.0) | 0 (0) | 2 (9.5) | |
| Cell of origin | ||||
| GCB | 14 (29.0) | 7 (25.9) | 7 (33.3) | .072 |
| Non-GCB | 22 (46.0) | 15 (55.6) | 7 (33.3) | |
| Unknown | 8 (17.0) | 5 (18.5) | 3 (14.3) | |
| NA | 4 (8.0) | 0 (0) | 4 (19.0) | |
| Karnofsky score at ASCT | ||||
| <80 | 16 (33.0) | 10 (37.0) | 6 (28.6) | .76 |
| ≥80 | 32 (67.0) | 17 (63.0) | 15 (71.4) | |
| Disease status at ASCT | .008 | |||
| CR1 | 29 (60) | 21 (77.8) | 8 (38.1) | |
| Beyond CR1 or PR | 19 (40) | 6 (22.2) | 13 (61.9) | |
| Interval from diagnosis to ASCT, mo | .001 | |||
| Median | 7.0 | 6.2 | 16.0 | |
| IQR | 5.2-16.1 | 4.7-9.5 | 7.0-25.3 | |
| Follow-up after ASCT, mo | .33 | |||
| Median | 23.9 | 30.2 | 18.1 | |
| IQR | 8.4-59.5 | 8.9-64.8 | 5.5-50.9 |
Data presented as n (%).
Abbreviations: ASCT = autologous stem cell transplantation; CR1 = first complete remission; GCB = germinal center B cell; HIV = human immunodeficiency virus; IQR = interquartile range; PCNSL = primary central nervous system lymphoma; PR = partial response; SCNSL = secondary central nervous system lymphoma.
The median time from the original diagnosis to ASCT for all patients was 7.0 months (IQR, 5.2-16.1 months). For those patients with PCNSL, the median time was 6.2 months (IQR, 4.7-9.5 months) compared with 16.0 months (IQR, 7.0-25.3) for those with SCNSL or CNS relapse (P = .001).
Pretransplant Therapies
The chemotherapy regimens used for all patients before ASCT are listed in Table 1. Of the 21 patients with PCNSL who had undergone ASCT in CR1, all had received induction therapy with rituximab/HD-MTX for a various number of cycles as determined by the treating physicians (median, 4; range, 2-13), followed by rituximab/cytarabine/thiotepa (median, 2; range, 0-2). One patient with PCNSL did not receive thiotepa with rituximab/cytarabine for unclear reasons. The other 6 patients with relapsed or refractory disease before ASCT had received additional therapies, including temozolomide (3 patients) and topotecan (1 patient), in addition to extra cycles of rituximab/HD-MTX and rituximab/cytarabine/thiotepa.
The patients with SCNSL/CNS relapse had received a variety of therapies before ASCT, including R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; 6 patients; 28.6%), R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; 9 patients; 42.9%), and R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; 6 patients; 28.6%), in addition to other regimens, including R-DHAOx (rituximab, dexamethasone, high-dose cytarabine, oxaliplatin), HyperCVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone), temozolomide, and belinostat. HD-MTX (14 patients; 66.7%) and HD-cytarabine (4 patients; 19.0%) were also given, and intrathecal chemotherapy with IT-MTX (intrathecal methotrexate) and/or intrathecal cytarabine were administered to 7 patients.
The median number of cycles of HD-MTX for all patients was 4 (range, 0-14), and the median number of cycles of cytarabine/thiotepa for all patients was 1 (range, 0-3). Three patients received WBRT before ASCT, including 2 patients with PCNSL without remission after induction chemotherapy and 1 patient with CNS relapse.
Transplant Outcomes
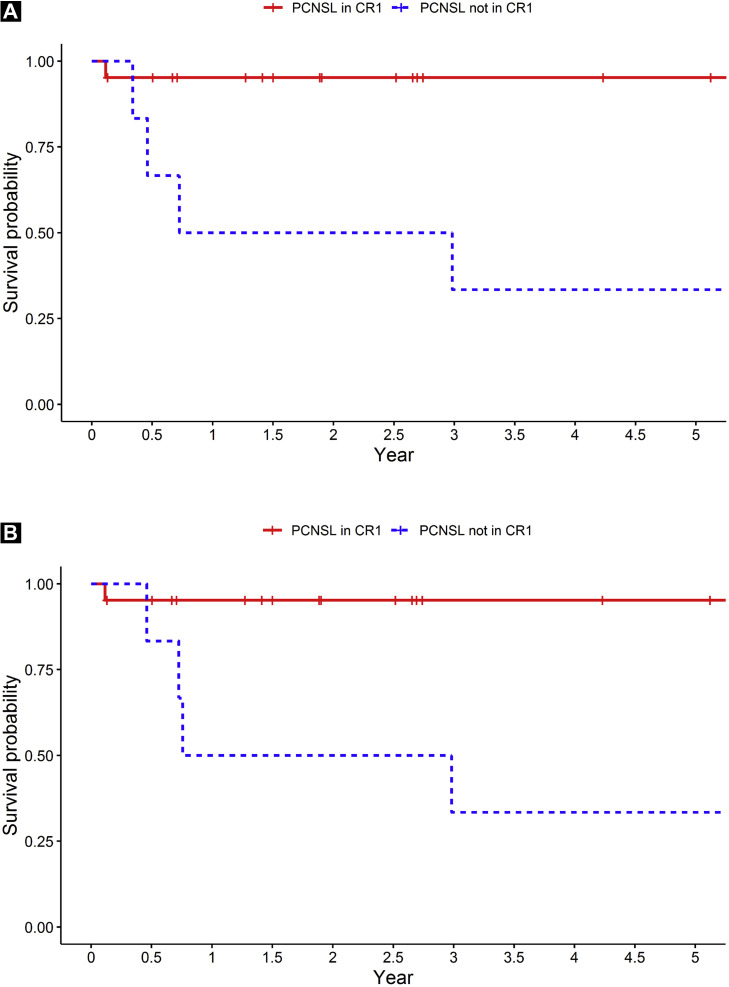
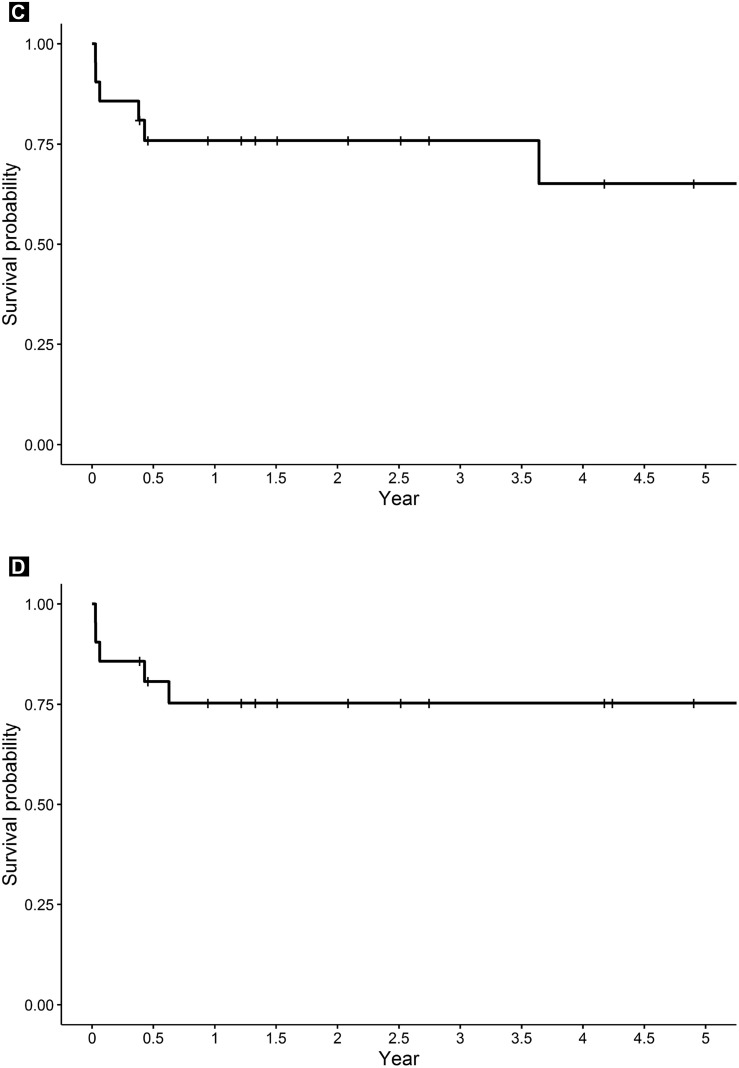
The Kaplan-Meier curves for PFS and OS are displayed in Figure 1 . For all 48 patients, the 2-year PFS rate was 80.5% (95% CI, 69.9%-92.9%) and the 2-year OS rate was 80.1% (95% CI, 69.2%-92.7%). The 2-year PFS rate for the patients with PCNSL and SCNSL/CNS relapse was 84.2% (95% CI, 71.0%-99.8%) and 75.9% (95% CI, 59.5%-96.8%), respectively. The 2-year OS rate for patients with PCNSL and SCNSL/CNS relapse was 83.6% (95% CI, 70.1%-99.8%) and 75.3% (95% CI, 58.6%-96.8%), respectively (Figure 1). The 2-year PFS and OS rate for PCNSL patients in CR1 was 95.2% (95% CI, 86.6%-100%) and 95.2% (95% CI, 86.6%-100%), respectively. For PCNSL patients not in CR1, the median PFS was 1.85 years and the median OS was 1.87 years. The median PFS was not reached for any other patient group (PCNSL patients in CR1 and those with SCNSL/CNS relapse). Only 2 patients had developed a relapse > 1 year after ASCT (1 patient with PCNSL and 1 patient with CNS relapse).
Figure 1.
Kaplan-Meier Curves of Progression-free Survival and Overall Survival According to Subgroup. (A) Progression-free Survival of Patients With Primary Central Nervous System Lymphoma (PCNSL) According to Transplantation in First Complete Response (CR1). (B) Overall Survival in Patients With PCNSL According to Transplantation in CR1. (C) Progression-free Survival of Patients With Secondary Central Nervous System (CNS) Lymphoma (SCNSL)/CNS Relapse. (D) Overall Survival for Patients With SCNSL/CNS Relapse
On univariate analysis of the patients with PCNSL (Table 3 ), ASCT in CR1 versus not in CR1 was the only statistically significant variable (PFS: HR, 13.36; 95% CI, 1.48-120.18, P = .007; OS: HR, 12.89; 95% CI, 1.43-115.88; P = .008). On univariate analysis of SCNSL/CNS relapse (Table 4 ), patient age ≤ 60 years and Karnofsky score < 80 were statistically significant factors for PFS (patient age: HR, 6.55; 95% CI, 0.75-57.22; P = .046; Karnofsky score: HR, 0.17; 95% CI, 0.03-0.94; P = .036) but not for OS (age: HR, 4.22; 95% CI, 0.47-37.84; P = .148; Karnofsky score: HR, 0.22; 95% CI, 0.04-1.32; P = .097).
Table 3.
Univariable Cox Proportional Hazard Regression Analysis for PFS and OS in PCNSL
| Parameter | PFS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Gender | .185 | .192 | ||
| Female | Reference | Reference | ||
| Male | 3.77 (0.42-33.8) | 3.70 (0.41-33.15) | ||
| Ethnicity | .092 | .102 | ||
| Other | Reference | Reference | ||
| White | 5.35 (0.59-48.55) | 5.10 (0.56-46.17) | ||
| Age, y | .061 | .051 | ||
| ≤60 | Reference | Reference | ||
| >60 | 6.32 (0.70-57.04) | 6.79 (0.75-61.23) | ||
| Karnofsky score | .060 | .067 | ||
| <80 | Reference | Reference | ||
| ≥80 | 0.16 (0.02-1.42) | 0.17 (0.02-1.49) | ||
| Transplant in CR1 | .007 | .008 | ||
| No | Reference | Reference | ||
| Yes | 13.36 (1.48-120.18) | 12.89 (1.43-115.88) | ||
Abbreviations: CI = confidence interval; CR1 = first complete remission; HR = hazard ratio; OS = overall survival; PCNSL = primary central nervous system lymphoma; PFS = progression-free survival.
Table 4.
Univariable Cox Proportional Hazard Regression Analysis for PFS and OS in SCNSL/CNS Relapse
| Parameter | PFS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Gender | .997 | .592 | ||
| Female | Reference | Reference | ||
| Male | 1.0 (0.20-5.12) | 1.62 (0.27-9.74) | ||
| Ethnicity | .523 | .263 | ||
| Other | Reference | Reference | ||
| White | 0.58 (0.11-3.20) | 0.32 (0.04-2.88) | ||
| Age, y | .046 | .148 | ||
| ≤60 | Reference | Reference | ||
| >60 | 6.55 (0.75-57.22) | 4.22 (0.47-37.84) | ||
| Karnofsky score | .036 | .097 | ||
| <80 | Reference | Reference | ||
| ≥80 | 0.17 (0.03-0.94) | 0.22 (0.04-1.32) | ||
| Transplantation in CR1 | .263 | .178 | ||
| No | Reference | Reference | ||
| Yes | 2.58 (0.50-13.23) | 3.41 (0.56-20.66) | ||
Abbreviations: CI = confidence interval; CNS = central nervous system; CR1 = first complete remission; HR = hazard ratio; OS = overall survival; PFS = progression-free survival; SCNSL = secondary central nervous system lymphoma.
ASCT-related Toxicities
The ASCT-related toxicities that occurred with the TBC conditioning regimen are listed in Table 5 . The median interval to neutrophil recovery and platelet recovery was 9.5 days (IQR, 9.0-10.0 days) and 15.0 days (IQR, 12.5-19.5 days), respectively. Most patients (89.6%) experienced febrile neutropenia, and 66.7% were found to have infection. Bacterial infection was present in 58.3% and included pneumonia (18.8%), colitis (16.7%; 10.4% with Clostridium difficile), bacteremia (10.4%; with organisms that included Escherichia coli, Staphylococcus epidermidis, and coagulase-negative staphylococcus), urinary tract infection (6.3%; with organisms that included coagulase-negative staphylococcus and E. coli), and sinusitis (2.1%). Viral infection was present in 45.8% of patients, including 33.3% with herpes simplex virus mucositis, 2.1% with herpes simplex virus vaginitis, 2.1% with coronavirus, 2.1% with rhinovirus, 2.1% with parainfluenza, 2.1% with human herpesvirus 6 bacteremia, and 2.1% with viral pneumonia. Fungal infection was present in 8.3% of patients, including 6.3% with Candida glabrata urinary tract infection and 2.1% with Candida dubliniensis bacteremia. Almost all patients developed nausea and vomiting (85.4%) and diarrhea (93.8%). Mucositis was present in 89.6% of patients; 35.4% with grade 3 or higher. Dermatologic sequelae, including rashes, bullae, desquamation, discoloration, and pruritus, were found in 31.3% of the patients. Acute neurotoxicity occurred in 27.1% of patients, with 4.2% developing seizure. Four patients (8.3%) developed hemorrhagic cystitis as a result of cyclophosphamide. Renal injury occurred in 16.7% of patients, and electrolyte abnormalities occurred in 89.6% of patients. Cardiac toxicity developed in 16.7% of patients, including 2.1% with cardiomyopathy presumed secondary to cyclophosphamide and 14.6% with arrhythmia that included supraventricular tachycardia and atrial fibrillation with a rapid ventricular response. Liver function abnormalities occurred in 18.8% of patients. Four patients (8.3%) died of treatment-related causes resulting from overwhelming infection, 3 of whom had SCNSL. All 4 patients had died within 1.5 months after ASCT. No evidence of pulmonary toxicity or veno-occlusive disease was noted.
Table 5.
ASCT Toxicity With TBC Conditioning Regimen
| Variable | All Patients (n = 48) | PCNSL (n = 27) | SCNSL/CNS Relapse (n = 21) |
|---|---|---|---|
| Interval to neutrophil recovery, d | |||
| Median | 9.5 | 9.0 | 10.0 |
| IQR | 9.0-10.0 | 8.0-10.0 | 9.0-10.0 |
| Interval to platelet recovery, d | |||
| Median | 15.0 | 15.0 | 14.0 |
| IQR | 12.5-19.5 | 14.0-21.0 | 11.0-16.0 |
| Toxicity | |||
| Febrile neutropenia | 43 (89.6) | 25 (52.1) | 18 (37.5) |
| Infection | 32 (66.7) | 18 (37.5) | 14 (29.2) |
| Bacterial | 28 (58.3) | 20 (41.7) | 8 (16.7) |
| Viral | 22 (45.8) | 10 (20.8) | 12 (25.0) |
| Fungal | 4 (8.3) | 3 (6.3) | 1 (2.1) |
| Nausea/vomiting | 41 (85.4) | 22 (45.8) | 19 (39.6) |
| Diarrhea | 45 (93.8) | 26 (54.2) | 19 (39.6) |
| Mucositis | 43 (89.6) | 22 (45.8) | 21 (43.8) |
| Grade 1 or 2 | 26 (54.2) | 12 (25.0) | 14 (29.2) |
| Grade 3 or 4 | 17 (35.4) | 10 (20.8) | 7 (14.6) |
| Dermatologic | 15 (31.3) | 15 (31.3) | 0 (0) |
| Acute neurotoxicity | 13 (27.1) | 10 (20.8) | 3 (6.3) |
| AMS/delirium | 13 (27.1) | 10 (20.8) | 3 (6.3) |
| Seizure | 2 (4.2) | 1 (2.1) | 1 (2.1) |
| Hemorrhagic cystitis | 4 (8.3) | 2 (4.2) | 2 (4.2) |
| Renal injury | 8 (16.7) | 3 (6.3) | 5 (10.4) |
| Electrolyte abnormalities | 43 (89.6) | 24 (50.0) | 19 (39.6) |
| Hypokalemia | 38 (79.2) | 22 (45.8) | 16 (33.3) |
| Hypomagnesemia | 7 (14.6) | 1 (2.1) | 6 (12.5) |
| Hypophosphatemia | 18 (37.5) | 9 (18.8) | 9 (18.8) |
| Hyponatremia | 8 (16.7) | 3 (6.3) | 5 (10.4) |
| Hypernatremia | 6 (12.5) | 4 (8.3) | 2 (4.2) |
| Metabolic alkalosis | 4 (8.3) | 1 (2.1) | 3 (6.3) |
| Metabolic acidosis | 6 (12.5) | 4 (8.3) | 2 (4.2) |
| Cardiac | 8 (16.7) | 5 (10.4) | 3 (6.3) |
| Cardiomyopathy | 1 (2.1) | 0 (0) | 1 (2.1) |
| Arrhythmia | 7 (14.6) | 5 (10.4) | 2 (4.2) |
| Liver function abnormalities | 9 (18.8) | 8 (16.7) | 1 (2.1) |
| Respiratory compromise | 4 (8.3) | 1 (2.1) | 3 (6.3) |
| Transplant-related mortality | 4 (8.3) | 1 (2.1) | 3 (6.3) |
Data presented as n (%).
Abbreviations: AMS = altered mental status; ASCT = autologous stem cell transplantation; CNS = central nervous system; IQR = interquartile range; PCNSL = primary central nervous system lymphoma; SCNSL = secondary central nervous system lymphoma; TBC = thiotepa, busulfan, cyclophosphamide.
Discussion
Despite data that support the use of HDC/ASCT for patients with NHL involving the CNS as both postremission consolidation5 , 6 , 8 , 10, 11, 12, 13, 14, 15, 16, 17, 18 , 21, 22, 23, 24, 25, 26 and in the relapsed/refractory setting,8 , 9 , 19 , 20 , 28, 29, 30 several unanswered questions remain regarding the ideal patient candidates, optimal conditioning, and timing of transplantation. Furthermore, the efficacy and toxicity compared with those of WBRT or nonmyeloablative chemotherapy are incompletely characterized.31 Our study, which evaluated HDC/ASCT in the postremission and relapsed/refractory settings using a uniform TBC preparative regimen, in accordance with previously reported data by Soussain et al,9 , 19 , 20 aimed to provide insight for identifying patients who might benefit most from this therapy.
The first trials examining HDC/ASCT for PCNSL used the BEAM (carmustine, etoposide, cytarabine, melphalan) conditioning regimen with disappointing results.5 , 6 , 10 Abrey et al5 reported a relapse rate of 57.1% at a median of 2.3 months after transplant using BEAM conditioning. Colombat et al6 reported a projected event-free survival (EFS) rate of 66% at 3 years and a projected OS rate of 64% at 4 years. These results, in part, were attributed to the limited penetration of the blood–brain barrier by BEAM chemotherapy and led to trials investigating busulfan and thiotepa, both reported to have CNS penetration of > 80%, as alternative myeloablative agents.32 , 33 Studies of TBC have shown higher efficacy than that with BEAM conditioning. Omuro et al14 reported a PFS and an OS rate of 85% (95% CI, 64%-94%) and 88% (95% CI, 68%-96%) at 1 year after ASCT for patients with PCNSL, respectively. Chen et al15 reported a PFS and an OS rate of 81% (95% CI, 59%-92%) and 93% (95% CI, 76%-98%) at 2 years after ASCT in a cohort of patients with PCNSL and SCNSL, respectively. Our data add to these findings, with disease control and survival rates comparable to those reported in these studies, with a 2-year PFS rate of 80.5% (95% CI, 69.9%-92.9%) and 2-year OS rate of 80.1% (95% CI, 69.2%-92.7%).
In the subset of patients with PCNSL who had undergone ASCT in CR1, the data might be even more favorable, with our institution reporting a 2-year PFS and OS rate of 95.2% (95% CI, 86.6%-100%). These results mimic the data reported by DeFilipp et al,17 which noted PFS and OS of 92% and 95% at 2 years for patients with PCNSL who had undergone transplantation in CR1. Furthermore, on univariate analysis of the data from patients with PCNSL, ASCT in CR1 was the only variable that was statistically significant for PFS (HR, 13.36; 95% CI, 1.48-120.18; P = .007) or OS (HR, 12.89; 95% CI, 1.43-115.88; P = .008). These data are not surprising, given the clear survival benefit and lower relapse rates for patients with NHL in CR before ASCT.34 , 35
Of the patients with SCNSL and CNS-relapsed NHL who had undergone ASCT with TBC conditioning, the 2-year PFS and OS rates in our study were 75.9% (95% CI, 59.5%-96.8%) and 75.3% (95% CI, 58.6%-96.8%), respectively. These findings are equivalent or superior to the results reported by other studies and compare favorably with the results for patients with relapsed/refractory PCNSL and intraocular lymphoma.20 In a subset analysis of 12 patients with SCNSL who had undergone HDC/ASCT, also with TBC conditioning, Chen et al15 reported 2-year PFS and OS of 51% (95% CI, 18%-77%) and 83% (95% CI, 48%-96%), respectively. Another study by Qualls et al36 of 20 patients with SCNSL who had also undergone HDC/ASCT with TBC conditioning reported a 4-year PFS and OS rate of 77% (95% CI, 48%-91%) and 82% (95% CI, 54%-94%), respectively. Given that CNS involvement by NHL has historically been associated with poor outcomes,1 , 37 our data are encouraging and demonstrate that HDC/ASCT can be safe and efficacious for these patients. Maziarz et al38 conducted a large analysis of 151 patients and noted comparable long-term outcomes for patients with NHL with and without CNS involvement who had undergone ASCT. Other studies have reported less impressive results, with a noted EFS rate of 46% ± 26% and OS rate of 41% ± 28% at 5 years for 13 patients with SCNSL who had undergone HDC/ASCT with carmustine, etoposide, and cyclophosphamide conditioning39 and a disease-free survival rate of 23% ± 19% at 2 years for 24 patients with SCNSL who had undergone HDC/ASCT with TBC conditioning.40 However, both of these studies had included patients with active CNS disease, which is known to result in poorer outcomes than those for patients with CNS lymphoma in remission.38 , 41 All the patients with SCNSL/CNS relapse in our study and in the study by Chen et al15 and Qualls et al36 had undergone transplantation while in CR, which likely contributed to the favorable survival outcomes.
One concern with the TBC regimen is nonhematologic toxicity. The most common therapy-related toxicities noted in most studies using this regimen have been febrile neutropenia, mucositis, and diarrhea.14 , 15 , 18 Our study also found a high rate of nausea/vomiting and electrolyte abnormalities. The incidence of septic complications with TBC conditioning appears to be greater than that with other conditioning regimens and has also led to higher rates of treatment-related mortality. In our study, the TRM rate was 8.3% (all from septic complications), and other institutions have reported a range of 0% to 14.3% with TBC conditioning in patients with PCNSL.8 , 15 , 18 , 42 The reason for this increased toxicity is not clear. Although some studies have found age > 60 years to be a factor,8 others have found no such association.18 Scordo et al18 hypothesized that the high TRM rate might be secondary to busulfan if the levels are greater than the therapeutic range. However, they found that patients with elevated first-dose busulfan area under the curve values did not experience more toxicity.18
Given that CNS penetration of cyclophosphamide is much lower (< 30%) than what can be achieved with thiotepa and busulfan,32 the additional benefit is unclear and might instead contribute to increased toxicity. Four patients (8.3%) developed hemorrhagic cystitis in our study. One study by Montemurro et al,11 in 2006, examined thiotepa and busulfan without cyclophosphamide for conditioning as postremission consolidation for 16 patients. The estimated 2-year EFS was 45%,11 and a long-term follow-up study showed an OS rate of 35%.12 They reported a greater relapse rate (4 patients; 25%) than that reported by other studies with TBC conditioning14 , 15 and also a high TRM (2 patients; 12.5%).11 Neurotoxicity occurred in 88.9% of the patients who had undergone WBRT, although no such cases were reported for patients who had undergone ASCT without previous WBRT. Therefore, the data thus far show no superior outcomes with the removal of cyclophosphamide from the TBC conditioning regimen. However, given that only 1 study has examined thiotepa and busulfan alone and it had a small sample size of only 16 patients, the true benefit of cyclophosphamide remains undetermined.
An alternative option for a CNS-directed conditioning regimen is the combination of carmustine and thiotepa. Carmustine is another agent with substantial CNS penetration (15%-70%), although not as high as busulfan.32 Two studies by Illerhaus et al7 , 21 evaluated this conditioning regimen with favorable results. One study reported a PFS and an OS rate of 83% at 5 years,21 , 22 and the second study reported a projected PFS rate of 73% and OS rate of 82% at 5 years.7 , 24 Both studies reported no TRM, making this regimen another promising treatment option. As discussed in further detail in subsequent paragraphs, the conditioning regimen of carmustine and thiotepa was also evaluated in the randomized study, IELSG32 (International Extranodal Lymphoma Study Group-32), which reported a 2-year OS of 77% and a TRM of 3%.43 A meta-analysis of HDC/ASCT for PCNSL found carmustine/thiotepa to be the conditioning regimen with the lowest risk of TRM.44 However, the TBC regimen had numerically superior PFS and OS rates.44
Neurotoxicity appears to be less common with HDC/ASCT than with WBRT consolidation. We found a rate of 27.1% for reversible neurotoxicity, which most commonly consisted of delirium and, rarely, seizure (4.2%). Scordo et al18 reported a similar finding of neurotoxicity in 10% of patients (including delirium, seizure, anxiety/depression, neuropathy, syncope, headache, and mania) who had undergone HDC/ASCT. Long-term neurotoxic effects were not evaluated in our study, although other reports have documented some delayed effects.15 , 17 However, the rates of long-term neurotoxic effects appear to be lower than those with WBRT, which has been reported as a 5-year cumulative incidence of neurotoxicity of 24%.3 , 4 , 45 , 46
Our study had several limitations, including the limited sample size and its retrospective nature. Although we did not have precise data on the number of patients who had not received HDC/ASCT, the vast majority of patients with these diagnoses in whom we initiated therapy did ultimately undergo ASCT. In our experience, it was rare for patients with these diagnoses to not be candidates for ASCT after receiving induction or salvage therapy once they had achieved a best response. Additionally, although patients with PCNSL who underwent ASCT in CR1 received a uniform induction regimen, the patients with refractory or relapsed PCNSL and SCNSL/CNS received heterogeneous induction and salvage therapies (Table 1), making definitive conclusions on the efficacy of HDC/ASCT in this cohort of patients difficult. Finally, toxicity evaluation was not performed using the Common Terminology for Adverse Events or objective scoring systems, and toxicity was arbitrarily defined using medical record review. Also, the long-term neurotoxic effects and minimal residual disease status were not evaluated.
Several randomized clinical trials have been investigating the role of HDC/ASCT consolidation therapy compared with WBRT47 , 48 and nonmyeloablative chemotherapy.31 , 49 In the PRECIS trial,16 which included only patients aged ≤ 60 years, those patients randomized to HDC/ASCT with TBC conditioning after induction chemotherapy (n = 70) had a 2-year PFS of 87% (95% CI, 77%-98%) compared with 63% (95% CI, 49%-81%) for those receiving WBRT (n = 70). However, the OS after 4 years was similar in both arms (66% vs. 64%). The TRM rate was greater in the HDC/ASCT group (5 deaths vs. 1 death in the WBRT group). Cognitive impairment was documented after WBRT; however, the cognitive function improved or remained stable after HDC/ASCT.48 The IELSG32 trial compared WBRT (n = 55 patients) versus ASCT (n = 58 patients) with carmustine/thiotepa conditioning for consolidation and showed similar 2-year PFS (76% after WBRT vs. 75% after ASCT; P = .62) and 2-year OS (82% after WBRT vs. 77% after ASCT; P = .91).43 The TRM rate was 3% in the ASCT group, with a nonsignificant trend toward impaired attention and executive function 2 years after therapy for those given WBRT.43 For nonmyeloablative chemotherapy, 2 trials are in process. The CALGB (Cancer and Leukemia Group B) 51101 trial will compare dose-intensive etoposide and cytarabine chemotherapy with HDC/ASCT using carmustine and thiotepa conditioning,31 , 49 and the European MATRix/IELSG43 will compare consolidation chemotherapy, consisting of R-DeVIC (rituximab, dexamethasone, etoposide, ifosfamide, carboplatin) with HDC/ASCT using thiotepa and carmustine conditioning.31 Data are also available on the use of novel agents targeting components of the B-cell receptor pathway (ie, the Bruton tyrosine kinase inhibitor ibrutinib) and immunomodulatory drugs (eg, lenalidomide and pomalidomide).50 These agents have shown promising high response rates but have currently only been tested in the salvage setting.51
Conclusion
Overall, our results support the use of HDC/ASCT with CNS-directed conditioning as consolidative therapy for PCNSL with long-term efficacy and manageable side effects. Patients with PCNSL who had undergone ASCT in CR1 appeared to have a survival benefit compared with those patients who had undergone ASCT after CR1. Data for SCNSL and in the relapse setting also appear encouraging. Longer follow-up and comparison with other consolidative therapies will provide further insight and should be reported in the near future.
Clinical Practice Points
-
•
HDC with ASCT has been investigated in patients with PCNSL or NHL with CNS involvement as postremission consolidation or in the relapse setting, with promising results.
-
•
Our study included 48 patients with PCNSL (27 patients) or SCNSL/CNS relapse (21 patients) who had undergone HDC/ASCT with TBC conditioning; of the 27 patients with PCNSL, 21 had undergone ASCT in CR1.
-
•
Overall, our data have shown encouraging long-term efficacy with a 2-year PFS rate of 80.5% (95% CI, 69.9%-92.9%) and a 2-year OS rate of 80.1% (95% CI, 69.2%-92.7%) for all patients.
-
•
The 2-year PFS and OS rate for patients with PCNSL was 84.2% (95% CI, 71.0%-99.8%) and 83.6% (95% CI, 70.1%-99.8%), respectively.
-
•
There appears to be a PFS and OS benefit for patients with PCNSL who undergo transplantation in CR1 compared with those who undergo transplantation beyond CR1 (PFS: HR, 13.36; 95% CI, 1.48-120.18; P = .007; OS: HR, 12.89; 95% CI, 1.43-115.88; P = .008).
-
•
The most common side effects were febrile neutropenia and infection, nausea/vomiting, diarrhea, mucositis, and electrolyte abnormalities, with 4 (8.3%) dying of treatment-related overwhelming infection.
-
•
Reversible neurotoxicity occurred in 27.1% of our patients.
-
•
Our study adds to the increasing data suggesting encouraging disease control and survival for HDC/ASCT with CNS-directed conditioning in PCNSL and secondary CNS NHL.
-
•
Randomized trials are ongoing to compare HDC/ASCT with other consolidative therapies, including WBRT and nonmyeloablative chemotherapy.
Disclosure
The authors have stated that they have no conflicts of interest.
Footnotes
H.A.E. and J.T. share senior authorship.
References
- 1.Rubenstein J.L., Gupta N.K., Mannis G.N., Lamarre A.K., Treseler P. How I treat CNS lymphomas. Blood. 2013;122:2318–2330. doi: 10.1182/blood-2013-06-453084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Citterio G., Reni M., Gatta G., Ferreri A.J.M. Primary central nervous system lymphoma. Crit Rev Oncol Hematol. 2017;113:97–110. doi: 10.1016/j.critrevonc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Thiel E., Korfel A., Martus P. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 4.Korfel A., Thiel E., Martus P. Randomized phase III study of whole-brain radiotherapy for primary CNS lymphoma. Neurology. 2015;84:1242–1248. doi: 10.1212/WNL.0000000000001395. [DOI] [PubMed] [Google Scholar]
- 5.Abrey L.E., Moskowitz C.H., Mason W.P. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151–4156. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Colombat P., Lemevel A., Bertrand P. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38:417–420. doi: 10.1038/sj.bmt.1705452. [DOI] [PubMed] [Google Scholar]
- 7.Illerhaus G., Muller F., Feuerhake F., Schafer A.-O., Ostertag C., Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93:147–148. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
- 8.Alimohamed N., Daly A., Owen C., Duggan P., Stewart D.A. Upfront thiotepa, busulfan, cyclophosphamide, and autologous stem cell transplantation for primary CNS lymphoma: a single centre experience. Leuk Lymphoma. 2012;53:862–867. doi: 10.3109/10428194.2011.633250. [DOI] [PubMed] [Google Scholar]
- 9.Soussain C., Suzan F., Hoang-Xuan K. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742–749. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]
- 10.Brevet M., Garidi R., Gruson B., Royer B., Vaida I., Damaj G. First-line autologous stem cell transplantation in primary CNS lymphoma. Eur J Haematol. 2005;75:288–292. doi: 10.1111/j.1600-0609.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 11.Montemurro M., Kiefer T., Schuler F. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2006;18:665–671. doi: 10.1093/annonc/mdl458. [DOI] [PubMed] [Google Scholar]
- 12.Kiefer T., Hirt C., Spath C. Long-term follow-up of high-dose chemotherapy with autologous stem-cell transplantation and response-adapted whole-brain radiotherapy for newly diagnosed primary CNS lymphoma: results of the multicenter Ostdeutsche Studiengruppe Hamatologie und Onkologie OSHO-53 phase II study. Ann Oncol. 2012;23:1809–1812. doi: 10.1093/annonc/mdr553. [DOI] [PubMed] [Google Scholar]
- 13.Cheng T., Forsyth P., Chaudhry A. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant. 2003;31:679–685. doi: 10.1038/sj.bmt.1703917. [DOI] [PubMed] [Google Scholar]
- 14.Omuro A., Correa D.D., DeAngelis L.M. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125:1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y.-B., Batchelor T., Li S. Phase 2 trial of high-dose rituximab with high-dose cytarabine mobilization therapy and high-dose thiotepa, busulfan, and cyclophosphamide autologous stem cell transplantation in patients with central nervous system involvement by non-Hodgkin lymphoma. Cancer. 2015;121:226–233. doi: 10.1002/cncr.29023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houillier C., Taillandier L., Lamy T. Whole brain radiotherapy (WBRT) versus intensive chemotherapy with haematopoietic stem cell rescue (IC + HCR) for primary central nervous system lymphoma (PCNSL) in young patients: an Intergroup Anocef-Goelams randomized phase II trial (PRECIS) Blood. 2016;128:782. [Google Scholar]
- 17.DeFilipp Z., Li S., El-Jawahri A. High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission. Cancer. 2017;123:3073. doi: 10.1002/cncr.30695. [DOI] [PubMed] [Google Scholar]
- 18.Scordo M., Bhatt V., Hsu M. A comprehensive assessment of toxicities in patients with central nervous system lymphoma undergoing autologous stem cell transplantation using thiotepa, busulfan, and cyclophosphamide conditioning. Biol Blood Marrow Transplant. 2017;23:38–43. doi: 10.1016/j.bbmt.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soussain C., Hoang-Xuan K., Taillandier L. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 20.Soussain C., Choquet S., Fourme E. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97:1751–1756. doi: 10.3324/haematol.2011.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Illerhaus G., Marks R., Ihorst G. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–3870. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 22.Kasenda B., Schorb E., Fritsch K., Finke J., Illerhaus G. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma—a long-term follow-up study. Ann Oncol. 2012;23:2670–2675. doi: 10.1093/annonc/mds059. [DOI] [PubMed] [Google Scholar]
- 23.Bojic M., Berghoff A.S., Troch M. Haematopoietic stem cell transplantation for treatment of primary CNS lymphoma: single-centre experience and literature review. Eur J Haematol. 2015;95:75–82. doi: 10.1111/ejh.12482. [DOI] [PubMed] [Google Scholar]
- 24.Schorb E., Kasenda B., Atta J. Prognosis of patients with primary central nervous system lymphoma after high-dose chemotherapy followed by autologous stem cell transplantation. Haematologica. 2013;98:765–770. doi: 10.3324/haematol.2012.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreri A.J.M., Illerhaus G. The role of autologous stem cell transplantation in primary central nervous system lymphoma. Blood. 2016;127:1642–1649. doi: 10.1182/blood-2015-10-636340. [DOI] [PubMed] [Google Scholar]
- 26.Illerhaus G., Kasenda B., Ihorst G. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol. 2016;3:e388–e397. doi: 10.1016/S2352-3026(16)30050-3. [DOI] [PubMed] [Google Scholar]
- 27.Illerhaus G., Fritsch K., Egerer G. Sequential high dose immuno-chemotherapy followed by autologous peripheral blood stem cell transplantation for patients with untreated primary central nervous system lymphoma—a multicentre study by the collaborative PCNSL Study Group Freiburg. Blood. 2012;120:302. [Google Scholar]
- 28.Cote G.M., Hochberg E.P., Muzikansky A. Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2012;18:76–83. doi: 10.1016/j.bbmt.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Welch M.R., Sauter C.S., Matasar M.J. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma. 2015;56:361–367. doi: 10.3109/10428194.2014.916800. [DOI] [PubMed] [Google Scholar]
- 30.Choi M.K., Kang E.S., Kim D.W. Treatment outcome of relapsed/refractory primary central nervous system diffuse large B-cell lymphoma: a single-center experience of autologous stem cell transplantation. Int J Hematol. 2013;98:346–354. doi: 10.1007/s12185-013-1403-z. [DOI] [PubMed] [Google Scholar]
- 31.Fraser E., Gruenberg K., Rubenstein J.L. New approaches in primary central nervous system lymphoma. Chin Clin Oncol. 2015;4:11. doi: 10.3978/j.issn.2304-3865.2015.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiebe V.J., Smith B.R., DeGregorio M.W., Rappeport J.M. Pharmacology of agents used in bone marrow transplant conditioning regimens. Crit Rev Oncol Hematol. 1992;13:241–270. doi: 10.1016/1040-8428(92)90092-5. [DOI] [PubMed] [Google Scholar]
- 33.Muldoon L.L., Soussain C., Jahnke K. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25:2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 34.Gulati S., Yahalom J., Acaba L. Treatment of patients with relapsed and resistant non-Hodgkin’s lymphoma using total body irradiation, etoposide, and cyclophosphamide and autologous bone marrow transplantation. J Clin Oncol. 1992;10:936–941. doi: 10.1200/JCO.1992.10.6.936. [DOI] [PubMed] [Google Scholar]
- 35.Rapoport A.P., Rowe J.M., Kouides P.A. One hundred autotransplants for relapsed or refractory Hodgkin’s disease and lymphoma: value of pretransplant disease status for predicting outcome. J Clin Oncol. 1993;11:2351–2361. doi: 10.1200/JCO.1993.11.12.2351. [DOI] [PubMed] [Google Scholar]
- 36.Qualls D., Sullivan A., Li S. High-dose thiotepa, busulfan, cyclophosphamide, and autologous stem cell transplantation as upfront consolidation for systemic non-Hodgkin lymphoma with synchronous central nervous system involvement. Clin Lymph Myeloma Leuk. 2017;17:884–888. doi: 10.1016/j.clml.2017.08.100. [DOI] [PubMed] [Google Scholar]
- 37.Herr M.M., Barr P.M., Rich D.Q., Mohile N. Clinical features, treatment, and survival of secondary central nervous system lymphoma. Blood. 2014;124:5389. doi: 10.1016/j.clml.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Maziarz R.T., Wang Z., Zhang M.-J. Autologous haematopoietic cell transplantation for non-Hodgkin lymphoma with secondary CNS involvement. Br J Haematol. 2013;162:648–656. doi: 10.1111/bjh.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarnas J.C., Negrin R.S., Horning S.J. High-dose therapy with hematopoietic cell transplantation for patients with central nervous system involvement by non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2000;6:352–358. doi: 10.1016/s1083-8791(00)70060-7. [DOI] [PubMed] [Google Scholar]
- 40.van Besien K., Przepiorka D., Mehra R. Impact of preexisting CNS involvement on the outcome of bone marrow transplantation in adult hematologic malignancies. J Clin Oncol. 1996;14:3036–3042. doi: 10.1200/JCO.1996.14.11.3036. [DOI] [PubMed] [Google Scholar]
- 41.Williams C.D., Pearce R., Taghipour G., Green E.S., Philip T., Goldstone A.H. Autologous bone marrow transplantation for patients with non-Hodgkin’s lymphoma and CNS involvement: those transplanted with active CNS disease have a poor outcome—a report by the European Bone Marrow Transplant Lymphoma Registry. J Clin Oncol. 1994;12:2415–2422. doi: 10.1200/JCO.1994.12.11.2415. [DOI] [PubMed] [Google Scholar]
- 42.Kasenda B., Ferreri A.J.M., Marturano E. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)—a systematic review and individual patient data meta-analysis. Ann Oncol. 2015;26:1305–1313. doi: 10.1093/annonc/mdv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreri A.J.M., Cwynarski K., Pulczynski E. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4:e510–e523. doi: 10.1016/S2352-3026(17)30174-6. [DOI] [PubMed] [Google Scholar]
- 44.Alnahhas I., Jawish M., Alsawas M. Autologous stem-cell transplantation for primary central nervous system lymphoma: systematic review and meta-analysis. Clin Lymph Myeloma Leuk. 2019;19:e129–e141. doi: 10.1016/j.clml.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 45.DeAngelis L.M., Seiferheld W., Schold S.C., Fisher B., Schultz C.J., Radiation Therapy Oncology Group Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group study 93-10. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Omuro A.M.P., Ben-Porat L.S., Panageas K.S. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62:1595–1600. doi: 10.1001/archneur.62.10.1595. [DOI] [PubMed] [Google Scholar]
- 47.Ferreri A.J.M., Cwynarski K., Pulczynski E.J. Effects on survival and neurocognitive functions of whole-brain radiotherapy (WBRT) and autologous stem cell transplantation (ASCT) as consolidation options after high-dose methotrexate-based chemoimmunotherapy in patients with newly diagnosed primary CNS lymphoma (PCNSL): results of the second randomization of the IELSG32 trial. Blood. 2016;128:511. [Google Scholar]
- 48.Houillier C., Taillandier L., Dureau S. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the Intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol. 2019;37:823–833. doi: 10.1200/JCO.18.00306. [DOI] [PubMed] [Google Scholar]
- 49.Rubenstein J.L., Hsi E.D., Johnson J.L. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202) J Clin Oncol. 2013;31:3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grommes C., Nayak L., Tun H.W., Batchelor T.T. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro Oncol. 2019;21:306–313. doi: 10.1093/neuonc/noy193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendez J.S., Grommes C. Treatment of primary central nervous system lymphoma: from chemotherapy to small molecules. Am Soc Clin Oncol Educ Book. 2018;38:604–615. doi: 10.1200/EDBK_200829. [DOI] [PubMed] [Google Scholar]