Abstract
Background
Transcutaneous electrical nerve stimulation (TENS) was introduced more than 30 years ago as a therapeutic adjunct to the pharmacological management of pain. However, despite widespread use, its effectiveness in chronic low‐back pain (LBP) is still controversial.
Objectives
To determine whether TENS is more effective than placebo for the management of chronic LBP.
Search methods
The Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, PEDro and CINAHL were searched up to July 19, 2007.
Selection criteria
Only randomized controlled clinical trials (RCTs) comparing TENS to placebo in patients with chronic LBP were included.
Data collection and analysis
Two review authors independently selected the trials, assessed their methodological quality and extracted relevant data. If quantitative meta‐analysis was not possible, a qualitative synthesis was performed, taking into consideration 5 levels of evidence as recommended by the Cochrane Collaboration Back Review Group.
Main results
Four high‐quality RCTs (585 patients) met the selection criteria. Clinical heterogeneity prevented the use of meta‐analysis. Therefore, a qualitative synthesis was completed. There was conflicting evidence about whether TENS was beneficial in reducing back pain intensity and consistent evidence in two trials (410 patients) that it did not improve back‐specific functional status. There was moderate evidence that work status and the use of medical services did not change with treatment. Conflicting results were obtained from two studies regarding generic health status, with one study showing no improvement on the modified Sickness Impact Profile and another study showing significant improvements on several, but not all subsections of the SF‐36 questionnaire. Multiple physical outcome measures lacked statistically significant improvement relative to placebo. In general, patients treated with acupuncture‐like TENS responded similarly to those treated with conventional TENS. However, in two of the trials, an inadequate stimulation intensity was used for acupuncture‐like TENS, given that muscle twitching was not induced. Optimal treatment schedules could not be reliably determined based on the available data. Adverse effects included minor skin irritation at the site of electrode placement.
Authors' conclusions
At this time, the evidence from the small number of placebo‐controlled trials does not support the use of TENS in the routine management of chronic LBP. Further research is encouraged.
Plain language summary
Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low‐back pain
Low‐back pain (LBP) represents a leading cause for work absenteeism and visits to health care professionals. Sixty to 90% of the adult population is at risk of developing LBP. While the majority of episodes appear to resolve within six weeks, recurrences are common. In addition, it is estimated that 10% to 20% of affected adults develop symptoms of chronic LBP (persistent pain lasting longer than three months). Chronic LBP has a significant impact on everyday life.
Transcutaneous electrical nerve stimulation (TENS) is widely used as a supplemental therapy in the management of LBP. It is a relatively safe, non‐invasive and easy to use treatment option. TENS units deliver electrical stimulation to the underlying nerves via electrodes placed over the intact skin surface near the source of maximal pain.
Four high‐quality randomized controlled trials (RCTs; 585 patients) comparing TENS with placebo for chronic low‐back pain were included in this study. Due to conflicting evidence, it is unclear if TENS is beneficial in reducing back pain intensity. However, there was consistent evidence in two trials (410 patients) that TENS did not improve the level of disability due to back pain. There was moderate evidence that use of medical services and work status (e.g. loss of work, sick days) did not change during treatment. Finally, there did not seem to be a difference between conventional and acupuncture‐like TENS.
Some adverse effects were reported, typically minor skin irritations observed equally in the treatment and placebo groups. However, there was one participant who developed a severe rash four days after the start of treatment.
In summary, the review authors found conflicting evidence regarding the benefits of TENS for chronic LBP, which does not support the use of TENS in the routine management of chronic LBP.
Background
Low‐back pain (LBP) represents a leading cause for work absenteeism and visits to healthcare professionals (Andersson 1999; Deveraux 2004). Sixty to 90% of the adult population is at risk of developing LBP at some point in their lifetime (Andersson 1997; Andersson 1999; Coste 1989; Deveraux 2004; Deyo 2006; Deyo 1987a; Sierpina 2002; Skovron 1992; Smeal 2004). While the majority of episodes appear to resolve within six weeks, recurrences are common (Andersson 1999; Pengel 2003; Von Korff 1996). In addition, it is estimated that 10% to 20% of affected adults develop symptoms of chronic LBP, defined as persistent pain occurring on most days and lasting longer than three consecutive months (Hildebrandt 2004; Maher 2004; Von Korff 1996; Waddell 1998). Chronic LBP has a significant impact on functional status, restricting occupational activities with marked socio‐economic repercussions (Deyo 1987b; Van Tulder 1999).
The management of LBP encompasses a diverse range of possible interventions including drug therapy, surgery, exercise, patient education, physiotherapy, cognitive‐behavioural therapy and various other non‐pharmacological therapies. A multidisciplinary approach founded on the biopsychosocial model has been advocated for some patients (Deyo 2001; Hildebrandt 2004; Maher 2004; Sierpina 2002). The goals of treatment are to relieve pain, reduce muscle spasm, increase strength and range of motion, promote an early return to activity and improve overall functional status. The risks and benefits of these treatments vary (Delitto 1993; Ottenbacher 1995; Schlapbach 1991). Acute and chronic LBP warrant separate consideration as they may respond differently to the same interventions (Sierpina 2002; Van Tulder 1999).
Transcutaneous electrical nerve stimulation (TENS) is widely used as a therapeutic adjunct in the management of LBP. It is a relatively safe, non‐invasive and easy to use modality that can be conveniently self‐administered by patients at home, making it an attractive treatment option. TENS units deliver electrical stimulation to the underlying peripheral nerves via electrodes placed over the intact skin surface, near the source of maximal pain (APTA 1993; Barr 1999; Deyo 1990a; Sluka 2003). The development and application of TENS was based on the Gate Control Theory, conceptualized by Melzack and Wall (Melzack 1982). According to this theory, the stimulation of large diameter (A‐beta), primary sensory afferents activates inhibitory interneurons in the substantia gelatinosa of the spinal cord dorsal horn and, thereby, blocks the transmission of nociceptive signals from small diameter A‐delta and C fibres (Melzack 1965; Melzack 1982). Supraspinal mechanisms involving the endogenous opioid system have also been described (Han 1991; Hughes 1984; Kalra 2001; Salar 1981). Overall, TENS is postulated to "close the gate" and dampen the perception of pain (Melzack 1982).
Several types of TENS applications, differing in frequency, amplitude, pulse width and waveform, are used in clinical practice. The two most common application modes include: 1) high frequency or conventional TENS (frequency greater than 80Hz, pulse width less than 150 μsec, low intensity sufficient to produce a comfortable tingling sensation) and 2) low frequency or so called acupuncture‐like TENS (frequency less than 10Hz, pulse width greater than 150 μsec, high intensity sufficient to elicit muscle twitching) (Belanger 2002). Acupuncture‐like TENS is associated with a slower onset and longer duration of analgesia compared to conventional TENS (Belanger 2002). However, whether there is a significant difference in clinical effectiveness between high frequency and low frequency modes is unclear and not well defined (Belanger 2002; Johnson 1991a). Indeed, patient preference for, and response to, different stimulation settings may be highly individualized (Johnson 1991a; Johnson 1991b; Tulgar 1991). Three other standard modes of TENS include: 1) Brief‐Intense TENS (frequency greater than 80Hz, pulse width greater than 150 μsec, brief duration of stimulation, very high intensity sufficient to activate nociceptive fibres in addition to motor fibres and primary sensory afferents), 2) Burst TENS (bursts of high frequency pulses delivered at low frequency (less than 10 Hz) and at a high enough intensity sufficient to activate both motor fibres and primary sensory afferents) and 3) Modulation TENS (one or more parameters are randomly modulated during therapy). Adverse reactions reported with TENS include skin irritation at the site of electrode placement (Deyo 1990a; Rushton 2002). TENS is contraindicated in patients with cardiac pacemakers due to the potential for interfering with pacemaker activity (Belanger 2002; Rushton 2002).
The clinical benefit of TENS for chronic LBP is uncertain. The aim of this update was to re‐evaluate its effectiveness relative to placebo.
Objectives
To determine the effectiveness of TENS versus placebo for the management of chronic LBP.
Methods
Criteria for considering studies for this review
Types of studies
Only RCTs with more than five LBP patients per treatment group were eligible. This sample size limit was applied based on the consensus opinion of the Philadelphia Panel (Philadelphia Panel 2001).
Types of participants
Outpatients, aged 18 years and over with chronic LBP were considered for this review. Chronic was defined as persistent pain (lasting longer than 12 weeks) localized between the inferior gluteal fold and the costal margin in the absence of malignancy, infection, fracture, inflammatory disorder or neurological syndrome. Subjects with symptoms and signs of sciatica or a previous history of back surgery were not specifically excluded from analysis, but had to represent a minority of the study sample to qualify for study selection (the latter criterion was newly defined for the current update in response to reader feedback and to enable generalizability of the results). Trials were excluded if they reported on subjects with a mix of chronic LBP and acute LBP (lasting less than six weeks) or subacute LBP (lasting six to12 weeks), unless the data were presented separately for chronic LBP. Similarly, trials investigating a study population with a mix of LBP and middle or upper back pain were also excluded.
Types of interventions
All standard modes of TENS were considered for this review. Articles were excluded if either the experimental or control groups received electrical stimulation percutaneously using acupuncture needles. We only accepted placebo TENS for the control group, which generally consisted of a TENS device modified so that no electrical current passed to the skin surface electrodes. The use of co‐interventions assigned equally to both the experimental and control groups was permitted. However, head‐to‐head comparisons of TENS with other active treatment modalities were not considered in this review.
Types of outcome measures
The principal outcome measures of interest were taken from a core set of instruments recommended for low‐back pain research and included: 1) Pain (typically measured using a visual analogue scale (VAS)); 2) Back‐specific functional status (e.g. Roland Morris Disability Scale or Oswestry Disability Index); 3) Generic health status (e.g. SF‐36); 4) Work Disability (e.g. loss of work, sick days); and 5) Patient satisfaction (Bombardier 2000; Deyo 1998; Schaufele 2003). Treatment side‐effects also constituted a primary outcome. Physical examination measures such as range of motion, finger‐to‐floor distance, degrees of straight leg raising, and muscle strength were considered secondary outcomes as were medication use and use of medical services.
Search methods for identification of studies
We initially searched the Cochrane Central Register of Controlled Trials (Issue 1, 2005), MEDLINE, EMBASE and the Physiotherapy Evidence Database (PEDro) from their beginning up to April 2005. Conference proceedings and reference lists from guidelines, literature reviews and retrieved articles were screened for further identification of relevant work. Content experts were contacted for additional studies. If sufficient data could not be obtained, abstracts were not used. No language restrictions were applied.
The sensitive search strategy for RCTs described by Haynes 1994 was used and combined with textwords and MeSH terms to identify TENS and low‐back pain. See Appendix 1 for details.
For this update, we consulted with the Trials Search Co‐ordinator from the Cochrane Back Review Group, since guidelines for search strategies have been modified since the original review. Based on the new search strategy, described in Appendix 2, we searched the Cochrane Central Register of Controlled Trials (Issue 3, 2007), MEDLINE, EMBASE and PEDRO from 2004 to July 19, 2007. We also searched CINAHL from its beginning to July 19, 2007 since this database was not used previously. In addition, the International Clinical Trials Registry was searched for ongoing trials.
Data collection and analysis
Two review authors (DO, AK) independently selected the studies to be considered for the review by screening the titles, abstracts and keywords of articles identified in the literature search. The full‐text of all potentially relevant studies was retrieved for closer examination, including studies for which a decision about eligibility could not be reliably made based on the title, abstract and keywords alone. Disagreement about inclusion or exclusion of individual studies was resolved by discussion between the review authors. The review authors were not blinded to the authors, institution, date or journal of publication. There was no selection cut‐off based on methodological quality or source of financial support. From each included trial, we collected information about the study design, study population, treatment characteristics (TENS device, stimulation settings, application method, treatment schedule, concurrent interventions), study outcomes and adverse effects. Differences in data extraction between review authors were resolved by referring back to the original article and establishing consensus. Additional information was sought from the authors of the primary studies when incompletely reported in the publications.
Where appropriate, data on the outcomes from each trial were pooled to arrive at an overall estimate of the effectiveness of TENS. Whenever possible, the analyses were based on intention‐to‐treat data from the individual trials. In cases where trials reported outcomes as graphs, the mean scores and standard deviations were estimated from these graphs.
For continuous data, the results were presented as mean differences (MD). However, when different scales were used to measure the same outcome, standardized mean differences (SMD) were used. For dichotomous data, an odds ratio (OR) was calculated (Petitti 1994). Because the prevalence of the outcome studied is high, the OR cannot be interpreted as being equivalent to the relative risk (Henneken 1987). A test for heterogeneity was calculated using an I2 test. Fixed‐effects models were used throughout, unless statistical heterogeneity was significant, in which case, a random‐effects model was used. Subgroup analysis, sensitivity analysis and tests of publication bias were not performed due to the small number of trials that were included.
Based on a review of the low‐back pain literature on minimal clinically important differences (MCID), we considered a mean difference in VAS scores of between 15 mm and 20 mm on a 0 to 100 mm scale to be clinically important (Hagg 2003; Ostelo 2005; Ostelo 2008). For the Oswestry Disability Index, a mean difference of at least 10 points was considered clinically important (Davidson 2002; Hagg 2003; Ostelo 2005; Ostelo 2008) and for the Roland‐Morris Disability Questionnaire, a mean difference of three points was taken as clinically important (Bombardier 2001; Ostelo 2005). The MCID for the Low Back Pain Outcome Scale has been reported to be 7.5 points (Muller 2006). Pooled effects sizes were considered small for standardized mean differences (SMD) between 0.2 to 0.5, moderate for SMDs between 0.5 to 0.8 and large for SMDs above 0.8 (Cohen 1988). The criteria for clinically relevant outcomes were changed from that of the original protocol, which defined a 15% improvement from baseline relative to placebo as clinically important. The latter criterion was based on the consensus opinion of the Philadelphia Panel and study data regarding multiple rheumatological conditions (Philadelphia Panel 2001). Since then, research on outcome measures for LBP has progressed considerably.
When the statistical pooling of data was not possible, a qualitative synthesis was performed in which five levels of evidence were taken into consideration, as recommended by the Cochrane Back Review Group (Van Tulder 2003).
Strong ‐ consistent findings among multiple high quality RCTs
Moderate ‐ consistent findings among multiple low quality RCTs and/or one high quality RCT
Limited ‐ one low quality RCT
Conflicting ‐ inconsistent findings among multiple RCTs
No evidence from trials ‐ no RCTs.
The criterion for a consistent finding was defined as at least 75% of the studies showing statistically significant and clinically relevant outcomes in the same direction.
Results
Description of studies
Overall, the literature search identified 47 potentially relevant studies, four of which were included for this review (N = 585; Cheing 1996; Deyo 1990a; Jarzem 2005a; Topuz 2004). A journal article published by Cheing et al in 1999 and one of their earlier 1996 abstracts, appearing in the conference proceedings of the 8th World Congress of Pain, were based on the same trial, but each reported data at different timepoints (one day versus two weeks) (Cheing 1996). For this study, the outcomes obtained at the end of the treatment phase were considered for analysis. Additional statistical data not reported in the abstract or journal publication were obtained from the primary authors. An ongoing study of 206 subjects entitled, Pain Reducing Effects of Transcutaneous Electrical Nerve Stimulation in Patients with Chronic Low Back Pain or Lumbo‐Radiculalgia, was identified and is expected to be completed by October 2008 (Laurent 2008).
The most common reason for study exclusion was the absence of a placebo‐control group. Several trials were excluded because they assessed a mixed study population with acute, subacute and chronic low‐back pain. Five trials were ineligible because they used needles that were inserted percutaneously. Altogether, six trials were excluded because they were conducted in an inpatient setting, had an inadequate sample size (five subjects per treatment group or less) or recruited subjects with inflammatory conditions such as ankylosing spondylitis. After considerable discussion, a study involving patients with multiple sclerosis (MS) was ultimately excluded because MS is a chronic, inflammatory disorder of the central nervous system, in which non‐mechanical factors, namely demyelinating lesions of the spinal cord, may contribute to back pain. One potentially relevant cross‐over study that did not report the means and standard deviations for its outcomes had to be excluded because requests for additional data were not returned (Jarzem 2005b). A full list of the excluded trials and explanations for their ineligibility are provided in the Characteristics of Excluded Studies Table.
Individually, the four included RCTs (four trials, N = 585) recruited as few as 30 subjects and up to as many as 350 subjects (Cheing 1996; Deyo 1990a; Jarzem 2005a; Topuz 2004). The treatment phase of these trials lasted between two and four weeks, with daily treatment sessions ranging from 20 minutes to three hours per day. Precise stimulation parameters were reported in every trial, except one (Jarzem 2005a). Nu‐wave TENS, which was investigated by Jarzem 2005a, was not considered in this review because it did not constitute a standard form of TENS. Percutaneous neuromodulation therapy, a treatment modality investigated by Topuz 2004, was not considered either because it involved the insertion of acupuncture‐like needles. In two of the studies, subjects were instructed to self‐administer TENS treatments at home (Jarzem 2005a; Deyo 1990a), whereas, in the remaining studies, a therapist was assigned to deliver treatments in the clinic setting (Topuz 2004; Cheing 1996). The stimulating electrodes, ranging between two and four in number, were generally placed over the area of maximal pain or within the same dermatome. However, the positioning of the electrodes was adjusted according to individual preference in one study (Jarzem 2005a) or moved as necessary to maximize pain relief in another study (Deyo 1990a). Since prior exposure to TENS could affect the adequacy of patient blinding, it is notable that Cheing 1996 did not specifically report the exclusion of subjects who had previous exposure to TENS.
Concurrent interventions were assigned in two studies: Jarzem 2005a assigned an exercise program to the experimental and control groups and Deyo 1990a provided local heat and postural advice. Although no restrictions on the use of pain medication were applied in most of the studies, Cheing 1996 demanded that subjects discontinue medication use and physiotherapy two weeks before the start of the trial. Jarzem 2005a excluded subjects receiving either concomitant physiotherapy or chiropractic therapy.
Regarding the study population, a predominantly female sample was recruited by Topuz 2004 and a predominantly male sample was recruited by Cheing 1996. The mean age of subjects ranged from 28 to 51, depending on the particular study and treatment group in question. Two studies included patients with prior back surgery, representing as much as 18% (Jarzem 2005a) and as little as 10% (Deyo 1990a) of the total patient sample. The latter study (Deyo 1990a) also included subjects with sciatica, which, again, constituted a minority of the overall study sample. Outcomes at two‐week and two‐month follow‐up were examined by Deyo 1990a, but the raw data were not presented. No other studies reported long‐term follow‐up outcomes (see Characteristics of Included Studies table).
Risk of bias in included studies
The quality of the studies was assessed independently by two review authors (DO, AK) based on a list of eleven methodological criteria recommended by the Cochrane Back Review Group (Van Tulder 2003) (see Table 7). Differences in scoring were resolved by consensus, which was reached for all trials. A third review author (GW) was consulted for additional guidance.
1. Criteria for Assessment of Methodological Quality.
|
Was the method of randomisation adequate? A random (unpredictable) assignment sequence. Examples of adequate methods are computer‐generated random numbers table and use of sealed opaque envelopes. Methods of allocation using date of birth, date of admission, hospital numbers, or alternation should not be regarded as appropriate. Was the treatment allocation concealed? Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. Was the patient blinded to the intervention? The review author determines if enough information about the blinding is given in order to score a "yes." Was the care provider blinded to the intervention? The review author determines if enough information about the blinding is given in order to score a "yes." Was the outcome assessor blinded to the intervention? The review author determines if enough information about the blinding is given in order to score a "yes." Was the drop‐out rate described and acceptable? The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and drop‐outs does not exceed 20% for immediate and short‐term follow‐ups, 30% for intermediate and long‐term follow‐ups and does not lead to substantial bias a "yes" is scored. Did the analysis include an intention‐to‐treat analysis? All randomized patients are reported/analyzed in the group to which they were allocated by randomization for the most important moments of effect measurement (minus missing values), irrespective of noncompliance and co‐interventions. Were the groups similar at baseline regarding the most important prognostic indicators? In order to receive a "yes," groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of patients with neurological symptoms, and value of main outcome measure(s). Were co‐interventions avoided or similar? Co‐interventions should either be avoided in the trial design or be similar between the index and control groups. Was the compliance acceptable in all groups? The review author determines if the compliance to the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). Was the timing of the outcome assessment in all groups similar? Timing of outcome assessment should be identical for all intervention groups and for all important outcome assessments. |
An arbitrary cut‐off of six out of 11 criteria was used to distinguish studies of higher quality versus lower quality in accordance with the Back Review Group Method Guidelines for Systematic Reviews (Van Tulder 2003). Based on this cut‐off, all of the included studies were considered to be of higher quality (Cheing 1996; Deyo 1990a; Jarzem 2005a; Topuz 2004) with six to eight criteria being met. Although six criteria were initially marked as unclear for the study by Cheing 1996, additional information was sought from and provided by the primary authors and it was determined that many of these criteria were indeed met. See Figure 1.
1.
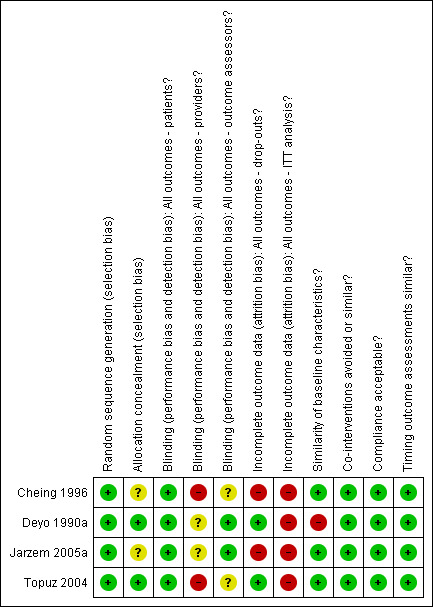
Summary of risks of bias
To summarize the risk of bias assessment, concealment of treatment allocation was unclear in two studies (Cheing 1996; Jarzem 2005a). Subjects were blinded in every trial but, as expected, blinding of the care provider was not clearly achieved in any. The outcome assessor was reported to have been blinded in only two studies (Deyo 1990a; Jarzem 2005a). At the designated three‐month follow‐up, Jarzem 2005a found that only 70% of the subjects returned a diary documenting their visual analogue pain scores and other outcomes; therefore, for this study, the criteria for acceptable drop‐out rate was not met. The drop‐out rate observed for the trial by Cheing 1996 was also large, at just above 26%. Intention‐to‐treat analysis was not clearly performed in any study.
Significant group baseline differences were reported in three studies (Cheing 1996; Deyo 1990a; Jarzem 2005a). Jarzem 2005a found significant differences in marital status between groups. Cheing 1996 found statistically significant differences in age (mean age of 35 years in experimental group versus mean age of 28 years in placebo group). The clinical significance of these differences is likely to be low. A third study by Deyo 1990a found significant differences in mean education level between subjects receiving TENS versus those receiving placebo TENS (13.7 versus 14.9 years). When all four treatment groups assigned in this trial were considered, significant differences were observed for neurologic deficit and previous hospitalization due to back pain. The authors (Deyo 1990a) found no substantial changes in their results when these differences in baseline variables were adjusted for (data not shown).
Effects of interventions
Conventional TENS versus placebo
Pain Intensity
Pain intensity was measured using the visual analogue scale (VAS) in three of the four included studies (N = 235 at randomization) (Deyo 1990a; Cheing 1996Topuz 2004). All three studies were of high methodological quality, meeting at least six out of 11 criteria. Still, they differed in terms of sample size, study population, treatment setting (home versus clinic), treatment schedule and use of concurrent interventions. Because of the clinical heterogeneity among the trials, meta‐analysis was considered inappropriate. Therefore, a qualitative synthesis of the evidence was undertaken.
Individually, the three studies showed inconsistent results regarding the effect of TENS on low‐back pain intensity. Two of the studies showed statistically insignificant and clinically unimportant benefits at the end of two weeks and four weeks of treatment respectively (Cheing 1996; Deyo 1990a). Both Deyo 1990a (N =145 at randomization) and Cheing 1996 (N = 30) assigned subjects to conventional TENS, but offered the choice of switching to acupuncture‐like TENS at the midway‐point of the four‐week trial. Notably, the improvement in VAS scores midway through the four week treatment phase were described as statistically insignificant (data not shown). In contrast to these results, a third study (Topuz 2004) demonstrated both statistically significant and clinically important benefits following two weeks of treatment with conventional TENS (MD ‐21.80; 95% CI ‐33.08 to ‐10.52). What accounts for the discrepancy in results cannot be meaningfully explored due to the small number of trials involved (Higgins 2006).
In summary, there is conflicting evidence about whether TENS improves chronic LBP intensity.
Back‐specific Functional Status
Back‐specific functional status was reported in two of the four studies (N = 410 at randomization; Jarzem 2005a; Topuz 2004), using different, but well‐validated scales. The Oswestry Disability Index and the Low Back Pain Outcome scale were reported in one study (Topuz 2004) and the Roland ‐Morris Disability Questionnaire (Jarzem 2005a) was reported in the other. Again, clinical heterogeneity precluded meta‐analysis and a qualitative analysis was performed. Individually, the smaller study (N = 60 at randomization) by Topuz 2004 showed no statistically significant or clinically important effects of conventional TENS with the Oswestry Disability Index or the Low Back Pain Outcome Scale. Similarly, Jarzem 2005a (N = 350 at randomization) observed no statistically significant or clinically important effects of conventional TENS with the Roland‐Morris Disability Questionnaire.
Regarding acupuncture‐like TENS, Topuz 2004 did not find statistically significant benefits with the Low Back Pain Outcome Scale. At the same time, while statistically significant improvements were found with the Oswestry Disability Index, these were clinically unimportant (MD ‐ 6.07; 95% CI ‐10.52 to ‐1.62). The larger study by Jarzem 2005a found no statistically significant effects of acupuncture‐like TENS with the Roland‐Morris Disability Questionnaire.
There is consistent evidence in individual trials that TENS does not improve back‐specific functional status to a clinically important degree regardless of whether conventional or acupuncture‐like TENS is used.
Generic Health Status
Generic health status was assessed in two studies, using the modified Sickness Impact Profile (Deyo 1990a) and the SF‐36 (Topuz 2004) respectively. Statistical pooling was not possible because of differences in the way these two outcome measures are reported. Whereas, the larger study by Deyo 1990a showed no statistically significant effects with the modified Sickness Impact Profile, Topuz 2004 showed statistically significant benefits for conventional TENS on four out of eight subsections of the SF‐36 (Physical Role Limitations, Emotional Role Limitations, General Mental Health, Vitality). Regarding acupuncture‐like TENS, Topuz 2004 found statistically significant benefits on just two of the eight subsections of the SF‐36 (Emotional Role Limitations, General Mental Health).
Based on the available studies, the effects of TENS on generic health status are conflicting.
Work Status
Work status was assessed in one study (N = 350; Jarzem 2005a) using the McGill Work Scale, which demonstrated no significant differences between TENS and placebo.
Other Outcome Measures
In terms of physical outcome measures, the only two studies that evaluated these outcomes (Deyo 1990a; Jarzem 2005a) found insignificant results, with the exception of the isometric dead‐lift test, which seemed to improve after treatment with acupuncture‐like TENS relative to placebo.
No significant differences between TENS and placebo were identified by Deyo 1990a for the use of medical services or by Jarzem 2005a for the Zung depression scale (data not shown).
With regards to various activity‐related measures, Topuz 2004 demonstrated a statistically significant improvement in activity pain after treatment with either conventional TENS (MD ‐17.20; 95% CI ‐ 27.38 to ‐7.02) or acupuncture‐like TENS (MD ‐12.50; 95% CI ‐24.47 to ‐0.53). However, the latter outcome was not clinically relevant. At the same time, Deyo 1990a found no statistically significant benefits of TENS treatment with respect to self‐rated activity and Jarzem 2005a found no statistically significant benefits from either conventional or acupuncture‐like TENS with the McGill Activity Scale.
Conventional TENS and Acupuncture‐like TENS
What is particularly noteworthy is that the two studies that separately compared conventional TENS and acupuncture‐like TENS to placebo (Jarzem 2005a; Topuz 2004) showed similar results for either TENS mode on most outcomes. The only exceptions included the isometric dead‐lift test, two subsections of the SF‐36 questionnaire (Physical Role Limitations, Vitality) and activity‐pain.
Adverse Effects
In terms of adverse effects, Deyo 1990a found that in a third of the participants, minor skin irritation occurred at the site of electrode placement. These adverse effects were observed equally in the TENS and placebo groups. One participant randomized to the placebo group developed severe dermatitis four days after the start of therapy and was required to withdraw from the trial. The presence or absence of adverse effects was not reported in the other three studies (Cheing 1996; Jarzem 2005a; Topuz 2004). Please note that a short‐term cross‐over trial conducted by Jarzem 2005b could not be included in the analysis because usable data was not reported. The authors of the study described positive results for conventional TENS with regard to pain intensity and various physical outcome measures after a maximum of one or two treatment sessions per subject.
Discussion
Despite a strong theoretical framework and widespread use, our synthesis of the currently available evidence (four RCTs, 585 subjects) suggests that TENS is not clearly more effective than placebo for the management of chronic LBP. All four included RCTs were considered to be of reasonably high quality, meeting at least six out of 11 methodological criteria recommended by the Cochrane Back Review Group (Van Tulder 2003). While one smaller study (N = 60; Topuz 2004) described some significant benefits with TENS, the remaining three studies under review did not, including two larger trials with sample sizes of 145 (Deyo 1990a) and 350 subjects (Jarzem 2005a). Larger trials yield more precise estimates of treatment efficacy and are less susceptible to publication bias (Montori 2000; Sterne 2001). Disappointingly, only one of the four trials reported the presence or absence of adverse effects. In this single trial, adverse effects consisted of minor skin irritation at the site of electrode placement that was experienced by approximately a third of the subjects.
The conclusions drawn here are in relative agreement with previous systematic reviews. For example, Van Tulder 1999 and Van Tulder 1997 found contradictory results from three eligible trials and, thereby, concluded that there was no clear evidence to support the use of TENS. Flowerdew 1997 and Gadsby 2000 stated that a definitive study was yet to be conducted after reviewing six eligible trials and finding only limited statistical evidence for a short‐term benefit of TENS treatment. Several clinical guidelines have been produced over the last decade that further reinforce the findings of the current systematic review. The Philadelphia Panel (Philadelphia Panel 2001) found poor evidence to recommend including or excluding TENS in the management of chronic LBP based on an evaluation of five eligible trials. A similar conclusion was drawn by the American Pain Society and the American College of Physicians, which looked at approximately nine studies exploring the benefits of TENS for subacute and chronic LBP (Chou 2007a; Chou 2007b). They considered head‐to‐head studies comparing TENS to other conservative therapies in their review of the evidence. The latest European guidelines on chronic LBP also did not recommend TENS, suggesting that there was strong evidence that TENS was not more effective than placebo and moderate evidence that it was not more effective than acupuncture, electroacupuncture, percutaneous electrical nerve stimulation (PENS) or vertebral axial decompression (Hildebrandt 2004). In contrast to our conclusions, the Quebec Task Force guidelines recommended TENS for chronic LBP (QTF 1987). However, the QTF did not distinguish TENS from other forms of electrotherapy and was convened before any of the currently included studies were published.
It should be emphasized that this review applies only to standard modes of TENS (conventional, acupuncture‐like, brief‐intense, burst, and modulation). No attempt was made to examine the pain‐relieving effects of other forms of electroanalgesia (e.g. PENS, electroacupuncture, neuromuscular electrical stimulation, interferential therapy, electrical spinal cord stimulation or other variant TENS‐like applications). Closer study of these alternative, invasive and non‐invasive forms of electrotherapy is warranted with particular attention given to risk‐benefit ratios.
Optimal stimulation parameters and treatment schedules for TENS in chronic LBP are poorly defined. With few exceptions, the two studies that separately compared conventional TENS and acupuncture‐like TENS to placebo showed similar results for either treatment mode. However, neither study used a sufficient stimulation intensity for the subjects receiving acupuncture‐like TENS, since muscle twitching was not induced (Belanger 2002; Sluka 2003). Because the individual response to various treatment parameters (frequency, pulse width, amplitude) may be quite variable (Johnson 1991a; Johnson 1991b; Tulgar 1991), future RCTs investigating the effects of TENS might consider a trial and error approach using different stimulation modes to determine an individual subject's optimal response before treatment assignment. Of note, Deyo 1990a allowed subjects being treated with conventional TENS to try acupuncture‐like TENS midway through the four week treatment phase and choose which mode they preferred for the remaining half of the study (77% chose acupuncture‐like TENS).
There is little evidence to guide decisions on the optimal treatment duration and the small number of studies reviewed here did not permit meaningful clarification. Since post‐stimulation analgesia following TENS therapy may be limited, especially with conventional TENS (Belanger 2002), there is a rationale for using prolonged application times, divided as multiple sessions throughout the day, to ensure continued and maximal pain relief. It should be noted that the only positive trial in this review assigned just 20 minutes of treatment per day, whereas the three negative trials assigned 60 minutes or more of daily treatment. Tolerance to the analgesic effects of TENS following prolonged stimulation could be argued as a potential contributing factor to the negative outcomes of some of the studies. However, this is, at best, speculative. It may be informative to formally test the effects of different daily treatment durations in a randomized‐controlled trial over a period of four weeks or longer. Modulation TENS, a treatment mode in which the stimulation parameters are randomly altered over the course of a therapy session, has been proposed to reduce the chances of stimulus adaptation (Tulgar 1991) and might be considered if the development of tolerance is an issue. What is significant in this regard is that Deyo 1990a used a modulated pulse rate, where the frequency of stimulation was periodically altered to arrive at a specified average frequency. Still, no therapeutic benefits were observed. Given that cross‐tolerance between the effects of TENS and opioids has been described (Sluka 1999), it would have been interesting to know how many subjects used opioids in the three trials that permitted analgesic medication use.
It is arguable that the use of concurrent interventions in the two larger, negative trials could have masked the effect of TENS relative to placebo. However, determining the additional benefit of TENS in the context of a multi‐modal treatment strategy is much more informative as this better reflects clinical practice. Given that NSAID use for chronic LBP is common and that Topuz 2004 did not specifically restrict the use of analgesic medications, concurrent interventions were not entirely avoided even in this single, positive trial. Additionally, although Cheing 1996 required that subjects terminate the use of pain medications as well as physiotherapy services two weeks prior to the study, the effect of TENS treatment was found to be clinically and statistically insignificant.
Several limitations to this systematic review deserve consideration. First and foremost, there was only a small number of eligible trials from which to draw conclusions. In addition, the same outcome measures were not consistently reported in each of the included trials, making comparisons more difficult. The criteria used in this review to define clinically important differences in outcome between TENS and placebo are still evolving and should be interpreted with caution. Although empirical evidence dealing specifically with LBP exists to support these criteria, the evidence was based partly on changes observed within individual patients and partly on group changes (Bombardier 2001; Davidson 2002; Hagg 2003; Muller 2006; Ostelo 2005, Ostelo 2008). Without ready access to individual patient data, we relied on mean group differences to judge clinically relevant outcomes. Some relevant studies might have been missed in the literature search due to unclear abstracts or the use of different keywords by authors. However, our search strategy was newly revised and an additional electronic database was included so this is unlikely to be a major issue. As previously mentioned at the end of the results section, a short‐term cross‐over trial comparing conventional TENS to placebo in patients with chronic low‐back pain described statistically significant benefits for TENS with respect to pain intensity and various physical outcome measures (Jarzem 2005b). How the inclusion of this trial would have affected the overall conclusions of the review cannot be answered since usable data could not be obtained for analysis. The clinical relevance of this study appears limited given that it was carried out over just a single day with subjects receiving only one or two sessions of active TENS treatment in total.
Given the lack of consistent evidence to support the use of TENS in the more restricted study populations reviewed here, widening the selection criteria to include all causes of chronic LBP might be considered in future updates. Moreover, future updates will look at the effectiveness of TENS relative to other treatment modalities.
In summary, there is inconsistent evidence from a small number of placebo‐controlled trials to support the use of TENS in the routine management of chronic LBP. Further research is encouraged.
Authors' conclusions
Implications for practice.
The evidence from four placebo‐controlled RCTs (585 patients) fails to consistently demonstrate that TENS relieves the symptoms and reduces the disability associated with chronic LBP.
Implications for research.
The possibility that optimal stimulation parameters and treatment schedules exist for TENS in the management of chronic LBP needs to be better defined. The use of standardized outcome measures as recently outlined by Bombardier 2000, Deyo 1998 and Schaufele 2003 greatly facilitates systematic analysis. Due to the natural fluctuations of symptoms in chronic LBP, baseline, end‐of‐treatment and follow‐up outcome measures should ideally be measured over multiple days and at different times of the day (Von Korff 1996). Appropriate reporting of results is encouraged, with means and standard deviations provided for each treatment outcome as well as for relevant baseline characteristics. Monitoring the use of analgesic medications is important since variable and unequal use between groups may represent a confounding factor or, alternatively, a treatment benefit. Reporting the presence or absence of adverse effects is essential. Short‐term treatment trials under two weeks in duration have limited relevance for chronic LBP. Post‐treatment follow‐up assessments should be conducted to determine the durability of treatment effects. Finally, given the increasing recognition of the problem of recurrent low‐back pain as distinct from chronic low‐back pain, investigating the therapeutic benefits of TENS in this population at the time of a recurrence may be useful.
Feedback
February 2005 ‐ refer to Milne 2001 review
Summary
Review conclusions are sensitive to change:
The main problem with this Cochrane review is that conclusions do not adhere to the limited available data. The review authors state that there is no evidence of effect although 2 out of 3 studies found a significant effect. It must also be mentioned that this review replaces a different review by a previous Cochrane Group (Gadsby and Flowerdew) who reached the opposite conclusion on TENS effectiveness.
The first problem with the current review is that the definitions given for TENS are technically specified, but these specifications are unsupported by evidence and different from the Cochrane‐review by Carroll et al. on chronic pain. However, there is evidence that placing electrodes in the same segmental area (dermatome, myotome)and the same side of the body, with frequency range between 1 and 150 Hz and a maximal tolerable stimulation intensity for at least 20 minutes is significantly more effective (32%) than other forms of electrical stimulation (4.2%) for postoperative pain (2003 Eur J Pain, Bjordal JM, Johnson MI, Ljunggren AE). The problem in the review is a that it is very sensitive to changes in interpretation of results the study by Deyo et al. This study is excluded by another Cochrane‐review on TENS for Chronic pain because the results could be confounded by co‐intervention by exercise therapy in the other Cochrane review of TENS for chronic pain. In addition this study is not performed on non‐specific low‐back pain, but also includes patients with radicular pain whom are unevenly distributed in the groups. Thirdly Deyo et al. used a too low fixed setting of stimulation intensity at 15 mA(3) for the high frequency (we have checked this with the specifications of the manufacturer). As long as the study from Deyo et al. contributes with 69.5% in the statistical analysis, this has seriously confounded the review results.
In my opinion, the only possible interpretation of the available data is that the limited material provide weak evidence of some effect from TENS for non‐specific low‐back pain.
1. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta‐analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain 2003;7(2):181‐8.
Reply
I will forward your comments to our lead author and ask that they be addressed. Since this review is due for updating, I'm sure your comments will be taken under advisement. By the way, you are correct that the results are different from the original review by Gatsby and Flowerdew. Data from another trial of 300+ participants were included in this review, which resulted in different conclusions.
Contributors
Jan Magnus Bjordal, Occupational Postdoctoral Research Fellow Victoria Pennick, Back Group Co‐ordinator
August 2005 ‐ refers to Khadilkar 2005 updated review
Summary
I observe that the authors have not taken under advisement my comments about the previous review version. The new review conclusion on 'Implications for practice' is in my opinion misleading: 'The evidence from two RCTs (175 patients) provides inconsistent support for the use of TENS as a single treatment modality in the management of chronic LBP.'
This conclusion refers to the negative trial by Deyo et al. and the positive trial by Cheing et al 1999, of which the latter oddly enough was not included in the previous review version from 2001. Only the trial by Cheing et al. 1999 investigated the effect of TENS as a single treatment while the trial by Deyo et al. used a combination of several common interventions. The Deyo trial was performed with too low stimulation intensity for conventional TENS, according to what is known about optimal stimulation intensity (1). TENS was also administered in combination with exercise therapy, daily hot packs and advice to stay active, which are potent and effective interventions for CLBP. Because of these co‐interventions, the Deyo trial was excluded from another Cochrane review on TENS for chronic pain.
In the new version, the review authors have limited the diagnostic exclusion criteria for chronic LBP from the previous protocol. The new version criteria for chronic LBP and a more specified location of back pain led to exclusion for heterogeneous populations of three positive trials (Gemignani 1991; Marchand 1993a; Moore 1997).
Regarding the modification of diagnostic criteria, it is interesting to observe that the new diagnostic criteria in this version deviate from the criteria governing the new European guidelines for chronic LBP (www.backpaineurope.org). These guidelines differentiate between non‐specific chronic LBP and LBP with nerve root affection, because of differences in their prognosis. From a clinical viewpoint it is also hard to understand why the new review version use an explicit inclusion criteria for trials patients with previous back surgery.
Of the 5 available TENS trials on chronic LBP only the trial by Deyo et al. included patients with a previous history of back pain surgery and nerve root affection (whom were unevenly distributed in the 4 trial groups).
These matters fuel my worries about one vital issue: Was the review protocol truly an a priori protocol, or was it modified later to make the negative trial by Deyo et al. overrule the positive results from the other 4 trials? Although I hope this is not the case, the review authors changed the protocol after they knew the material from the previous version.
In this perspective, where the a priori validity of the review protocol is questioned, what were the clinical considerations behind the new criteria preferences, and why are the new criteria better for answering the most relevant clinical questions about TENS treatment for common CLBP sufferers?
If other related evidence on TENS is considered, the anatomical location should be suitable for TENS treatment as TENS compared to no‐treatment control significantly reduced post‐operative pain after spinal surgery (2) (n=234), while TENS gave considerable pain relief in acute LBP when compared to sham‐TENS (3) (n=72). In other musculoskeletal chronic pain conditions such as knee osteoarthritis, another Cochrane‐review have found significant effect from TENS (4).
I do not disagree that large RCTs are needed to confirm the effect of TENS in CLBP, but in my opinion the available data add weak support to a positive effect from TENS in CLBP.
References:
1.Bjordal, J.M., M.I. Johnson, and A.E. Ljunggreen, Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta‐analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain, 2003. 7(2): p. 181‐8.
2.Rainov, N.G., et al., Transcutaneous electrical nerve stimulation(TENS) for acute postoperative pain after spinal surgery. European Journal of Pain, 1994. 15(2): p. 44‐49.
3.Bertalanffy, A., et al., Transcutaneous electrical nerve stimulation reduces acute low back pain during emergency transport. Acad Emerg Med, 2005. 12(7): p. 607‐11.
4.Osiri, M., et al., Transcutaneous electrical nerve stimulation for knee osteoarthritis. 2001, The Cochrane Library.
Reply
On behalf of the authors, Thank you for your ongoing interest in this review. Their comments are below.
The inclusion and exclusion criteria used in the current systematic review represent a synthesis of the selection criteria used by the Philadelphia Panel (2001) for its evidence‐based clinical practice guidelines on the management of low back pain and by Milne et al. (2001) for the original version of this Cochrane review [1,2]. Although diagnostic classifications of low‐back pain have not been systematically validated [3,4], nonspecific, mechanical low‐back pain – with or without radiating symptoms – is generally diagnosed by the absence of malignancy, infection, fracture, inflammatory arthritis, cauda equina syndrome and severe or progressive neurological deficit. The methodology of the current review does not specify that patients must have sciatica or a history of previous back surgery to be considered. However, at the same time, these patients were not specifically excluded. According to an international comparison of diagnostic approaches for low‐back pain, "all guidelines propose some form of diagnostic triage in which patients are classified as having (1) nonspecific LBP (low‐back pain), (2) specific LBP ("red flag" conditions such as tumour, infection, or fracture) and (3) sciatica/radicular syndrome. In some guidelines, sciatica is not considered a separate classification but is variously included for management in the category of nonspecific or specific LBP" [5].
For this update, a newly added criterion required that included studies exhibit relative homogeneity with respect to duration and location of pain. Thus, studies that contained a mixed study population with acute and chronic low‐back pain or upper back and lower back pain were excluded. This criterion has been used in previous systematic reviews evaluating the effectiveness of TENS in chronic low‐back pain [6, 7]. In addition, a more explicit definition of mechanical low‐back pain was provided in the current update, which is consistent with several recent clinical review articles [8,9,10].
Three of the five studies included in the original Cochrane review were excluded because of the additional criteria of homogeneity. Gemigniani et al (1991) examined patients with ankylosing spondylitis, a form of inflammatory arthritis [11]. Marchand et al. (1993) included patients with "more specific pathology" such as ankylosing spondylititis and rheumatoid arthritis [12]. Moore et al. (1997) studied seven patients with pain restricted to the upper or mid back out of a total sample size of 24 [13]. Data for these patients were not reported separately. Finally, the abstract by Jarzem et al (1997), which is still unpublished, was felt to have provided insufficient information and statistical data to permit analysis [14].
The fact that the study by Cheing et al (1999) was missed in the literature search of the original Cochrane review (2001) reflects the possibility that even a systematic search strategy can be imperfect [2,15].
Based on the results of a meta‐analysis conducted by Bjordal et al. (2003), the reader states that the stimulation intensities used in the trial by Deyo et al. (1990) were "too low" [6,17]. However, Bjordal et al. (2003) were examining acute, post‐operative pain following spinal surgery, which is very different from chronic low‐back pain [16]. The optimal stimulation parameters for TENS in chronic low‐back pain, including frequency, pulse duration, and intensity, are poorly defined [18,19,20]. A recent RCT comparing the effectiveness of different combinations of stimulation parameters found no significant differences in the reduction of chronic pain [19].
According to the biopsychosocial model, "true multidisciplinary treatment programs have to include medical (pharmacological treatment, education), physical (exercise), vocational and behavioural components and have to be provided by at least by three health care professionals with different clinical backgrounds (physician, physiotherapist, psychologist)" [3]. Based on this definition, the interventions used in the study by Deyo et al (1990) do not qualify as multidisciplinary. In the study, four treatment groups were assigned: (TENS alone), (TENS + exercise), (sham TENS), (exercise + sham TENS) [17]. Since no treatment interaction was found between TENS and exercise, the singular effect of TENS was reported separately, controlling for and independent of any contribution from exercise. It should be noted that heat therapy was provided concurrently to all four treatment groups and, thus, it is unlikely that heat therapy represented a confounding variable [17]. Furthermore, there is as yet no evidence to show that thermotherapy is an effective treatment modality for chronic low‐back pain [1,3].
Finally, the authors wish to acknowledge that, in the Deyo trial, patients with previous back surgery were unevenly distributed among the four treatment groups following randomization [17]. However, the numbers of patients with a history of back surgery were not significantly different in the TENS groups compared to the sham‐TENS groups that formed the primary comparison [17].
1. Philadelphia Panel. Philadelphia Panel evidence‐based clinical practice guidelines on selected rehabilitation interventions for low‐back pain. Phys Ther 2001;81(10):1641‐74.
2. Milne S, Welch V, Brosseau L, Saginur M, Shea B, Tugwell P, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic low‐back pain. In: The Cochrane Database of Systematic Reviews, Issue 2, 2001.
3. COST ACTION B13 Working Group. European Guidelines for the Management of Chronic Non‐specific Low Back Pain. June 14th 2005. Available at www.backpaineurope.org. Accessed September 1, 2005.
4. Van Tulder MW, Waddell G. Evidence‐based medicine for non‐specific low back pain. Best Practice & Research Clinical Rheumatology 2005; 19 (4): vii‐ix.
5. Koes BW, van Tulder MW, Ostelo R, Kim Burton A, Waddell G. Clinical Guidelines for the Management of Low Back Pain in Primary Care: An International Comparison. Spine 2001; 26(22): 2504‐13.
6. Van Tulder MW, Koes BW, Bart W, Bouter LM. Conservative Treatment of Acute and Chronic Nonspecific Low Back Pain: A systematic review of the most common interventions. Spine 1997: 22(18): 2128‐56.
7. Flowerdew MW, Gadsby JG. A review of the treatment of chronic low back pain with acupuncture‐like transcutaneous electrical stimulation and transcutaneous electrical nerve stimulation. Complementary Therapies in Medicine 1997;5:193‐201.
8. Deyo RA, Weinstein JN. Low back pain. New England Journal of Medicine 2001;344(5):363‐70.
9. Carragee EJ, Hannibal M. Diagnostic evaluation of low back pain. Orthopedic Clinics of North America. 2004: 35 (1): 7‐16.
10. Harwood MI, Smith BJ. Low back pain: A primary care approach. Clinics in Family Practice. 2005;l7(2): 279‐303.
11. Gemigniani G. Transcutaneous Electrical Nerve Stimulation in Ankylosing Spondylitis: A Double‐Blind Study. Arth Rheum 1991; 34(6):788‐9.
12. Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, Laurencelle L. Is TENS Purely a Placebo Effect? A Controlled Study on Chronic Low Back Pain. Pain 1993; 54(1):99‐106.
13. Moore SR, Shurman J. Combined Neuromuscular Electrical Stimulation and Transcutaneous Electrical Nerve Stimulation for Treatment of Chronic Back Pain: A Double‐Blind, Repeated Measures Comparison. Arch Phys Med Rehabil 1997; 78:55‐60.
14. Jarzem P, Harvey EJ, Arcaro N, Kazarowski J. Transcutaneous Electrical Nerve Stimulation for Non‐Acute Low Back Pain: A Randomized Double‐Blind Study of Conventional, Nu‐Wavefor, Acupuncture‐Type and Sham Therapies. In: American Academy of Orthopaedic Surgeons Annual Meeting. 1997.
15. Cheing GL. Hui‐Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Archives of Physical Medicine & Rehabilitation 1999;80(3):305‐12.
16. Bjordal, J.M., M.I. Johnson, and A.E. Ljunggreen, Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta‐analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain 2003;7(2):181‐8.
17. Deyo RA, Walsh NE, Martin DC, Schoenfield LS, Ramamurthy S. A Controlled Trial of Transcutaneous Electrical Stimulation (TENS) and Exercise for Chronic Low Back Pain. New England Journal of Medicine 1990;322(23):1627‐34.
18. Belanger AY. Evidence based guide to therapeutic physical agents. Lippincott Williams & Wilkins, 2002.
19. Koke AJA, Schouten JSAG, Lamerichs‐Geelen MJH, Lipsch JSM , Waltje EMH, van Kleef M, Patijn J. Pain reducing effect of three types of transcutaneous electrical nerve stimulation in patients with chronic pain: a randomized crossover trial. Pain 2004; 108: 36–42.
20. Chesterton LS, Barlas P, Foster NE, Lundeberg T, Wright CC, Baxter GD. Sensory stimulation (TENS): effects of parameter manipulation on mechanical pain thresholds in healthy human subjects. Pain 2002;99:253–62.
Contributors
Jan M. Bjordal, Postdoctoral Research Fellow, Institute of Public Health and Primary Health Care, University of Bergen, Norway
Vicki Pennick, Back Review Group Co‐ordinator, in consultation with and on behalf of Amole Khadilkar and the review team
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2013 | Amended | This review is currently being updated by a new review team. This new version of the review will include an expanded set of comparisons, such that TENS will be compared to placebo as well as other active treatments. See Published Notes for additional information. |
| 23 November 2009 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 6 June 2008 | Amended | Converted to new review format. |
| 2 June 2008 | New citation required but conclusions have not changed | Two additional trials were included (Jarzem 2005, Topuz 2004). In addition, an abstract by Cheing et al., 1996 was identified in the conference proceedings of the 8th World Congress of Pain. This abstract was based on the same trial as a previously included journal article (Cheing, 1999), but the outcomes were reported after a longer treatment period. Additional data were obtained from the authors of this study to facilitate analysis. An ongoing trial to be completed by October 2008 awaits review. |
| 19 July 2007 | New search has been performed | For this 2nd update, a revised search strategy was conducted between 2004 and 2007. The CINAHL database was added to the search. |
| 2 August 2005 | Feedback has been incorporated | Feedback on Khadilkar 2005 updated review |
| 30 April 2005 | New search has been performed | The current systematic review represents a substantial update and revision of the original Cochrane Review published in 2001. The search strategy used in original review was re‐executed from 2000 to April 2005. In an effort to retrieve any potentially relevant studies missed in the original review, we also ran a parallel search of MEDLINE, using a modified search strategy from 1966 to April 2005. One article (Cheing 1999) met the eligibility criteria and was included in this update. We modified the inclusion criteria to examine a more homogeneous chronic LBP population. Based on the new criteria, four of the five trials included in the original review were excluded (Gemignani 1991, Jarzem 1997, Marchand 1990, Moore 1997). |
| 1 February 2005 | Feedback has been incorporated | Feedback on Milne 2001 added |
Notes
This review is currently being updated and will be replaced by a review with the following title: Transcutaneous electrical nerve stimulation (TENS) for chronic low‐back pain. The protocol for the new review is now available in The Cochrane Library (Odebiyi 2013).
Acknowledgements
The authors wish to thank Rachel Couban for her assistance with the literature search and Victoria Pennick for important feedback. We would also like to thank Sarah Milne, Vivian (Robinson) Welch, Michael Saginur, Beverley Shea and Peter Tugwell for their contributions to earlier versions of this work.
Appendices
Appendix 1. Keywords and MeSH terms for initial literature search
TENS: exp electric stimulation therapy/ ((electric$ adj nerve) or therapy).tw. electrostimulation.tw. electroanalgesia.tw. (tens or altens).tw. electroacupuncture.tw. (high volt or pulsed or current).tw. (electromagnetic or electrotherap$).tw. Back pain: exp back/ exp back injuries/ exp back pain/ back.hw,tw. (spine or spinal).tw. sacrococcygeal.tw. lumbar.tw. sciatica/ or sciatic$.tw. lumbosacral.tw. cauda equina.hw,tw. backache.tw.
Appendix 2. MEDLINE search strategy (2004‐2007)
1 exp "Clinical Trial [Publication Type]"/ 2 randomized.ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 Animals/ 10 Humans/ 11 9 not (9 and 10) 12 8 not 11 13 dorsalgia.ti,ab. 14 exp Back Pain/ 15backache.ti,ab. 16(lumbar adj pain).ti,ab. 17coccyx.ti,ab. 18 coccydynia.ti,ab. 19 sciatica.ti,ab. 20 sciatica/ 21 spondylosis.ti,ab. 22 lumbago.ti,ab. 23 exp Low Back Pain/ 24 low back pain.mp. 25 or/13‐24 26 Transcutaneous Electric Nerve Stimulation/ 27 TENS.mp. 28 ALTENS.mp. 29 transcutaneous nerve stimulation.mp. 30 TNS.mp. 31 transcutaneous electrical neurostimulation.mp. 32 TENMS.mp. 33 exp Electroacupuncture/ 34 transdermal electrical stimulation.mp. 35 peripheral conditioning stimulation.mp. 36 percutaneous neural stimulation.mp. 37 microamperage electrical stimulation.mp. 38 cranial electrotherapy stimulation.mp. 39 transcutaneous cranial electrical stimulation.mp. 40 transabdominal neurostimulation.mp. 41 exp Electric Stimulation Therapy/ 42 exp Electric Stimulation/ 43 electroanalgesia.mp. 44 electrotherapy.mp. 45 or/26‐44 46 12 and 25 and 45 47 limit 46 to yr="2004 ‐ 2007"
Data and analyses
Comparison 1. Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Intensity , VAS (0‐100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
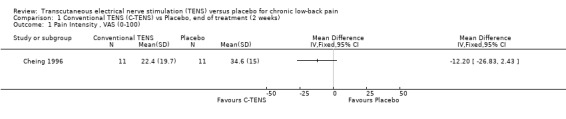
Comparison 1 Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 1 Pain Intensity , VAS (0‐100).
Comparison 2. Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Intensity, VAS (0‐100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain Improvement, VAS (0‐100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pain Improvement, (1‐6, 1=pain entirely gone, 6=much worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Frequency of Pain, (1‐5, 1=never, 5=all the time) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Generic Health Status (Modified Version of Sickness Impact Profile) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Self‐Rated Activity Level (1‐3, 1=more active than baseline, 3=less active) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Flexion ROM (finger‐to‐floor distance (cm)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Flexion ROM (Schober test (cm)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Lasegue's SLR (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Use of Medical Services, (visits to other providers) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
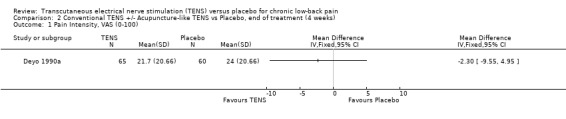
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 1 Pain Intensity, VAS (0‐100).
2.2. Analysis.
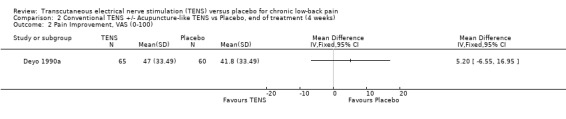
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 2 Pain Improvement, VAS (0‐100).
2.3. Analysis.
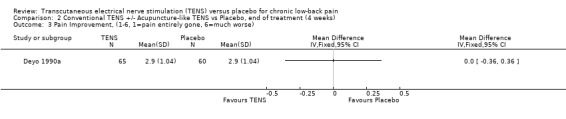
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 3 Pain Improvement, (1‐6, 1=pain entirely gone, 6=much worse).
2.4. Analysis.
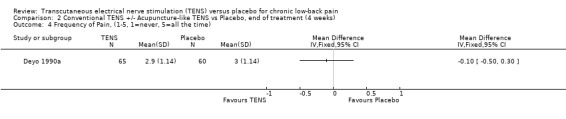
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 4 Frequency of Pain, (1‐5, 1=never, 5=all the time).
2.5. Analysis.
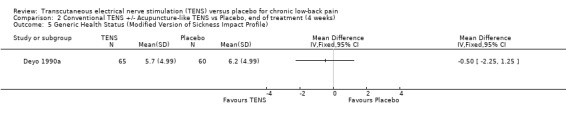
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 5 Generic Health Status (Modified Version of Sickness Impact Profile).
2.6. Analysis.
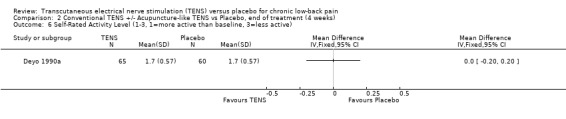
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 6 Self‐Rated Activity Level (1‐3, 1=more active than baseline, 3=less active).
2.7. Analysis.
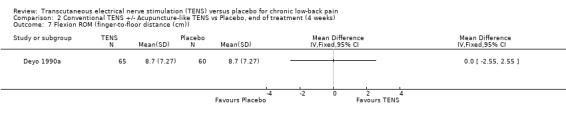
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 7 Flexion ROM (finger‐to‐floor distance (cm)).
2.8. Analysis.
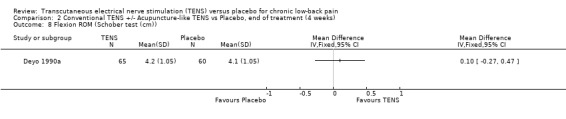
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 8 Flexion ROM (Schober test (cm)).
2.9. Analysis.
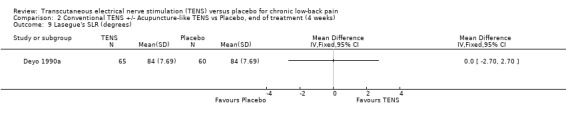
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 9 Lasegue's SLR (degrees).
2.10. Analysis.
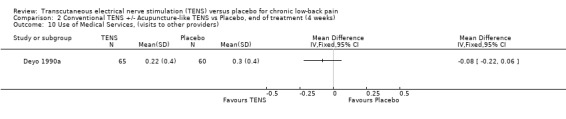
Comparison 2 Conventional TENS +/‐ Acupuncture‐like TENS vs Placebo, end of treatment (4 weeks), Outcome 10 Use of Medical Services, (visits to other providers).
Comparison 3. Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Intensity, VAS (0‐100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Activity Pain, VAS (0‐100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Oswestry Disability Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Low Back Pain Outcome Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of Life (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Physical Function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Social Functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Physical Role Limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Emotional Role Limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 General Mental Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Bodily Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 General Health Perception | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
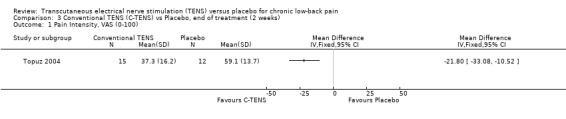
Comparison 3 Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 1 Pain Intensity, VAS (0‐100).
3.2. Analysis.
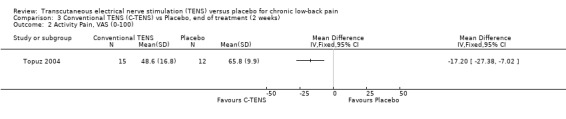
Comparison 3 Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 2 Activity Pain, VAS (0‐100).
3.3. Analysis.
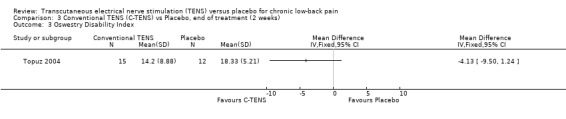
Comparison 3 Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 3 Oswestry Disability Index.
3.4. Analysis.
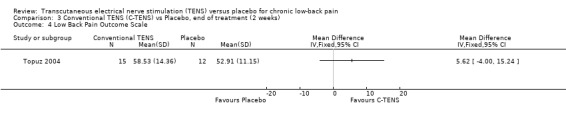
Comparison 3 Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 4 Low Back Pain Outcome Scale.
3.5. Analysis.
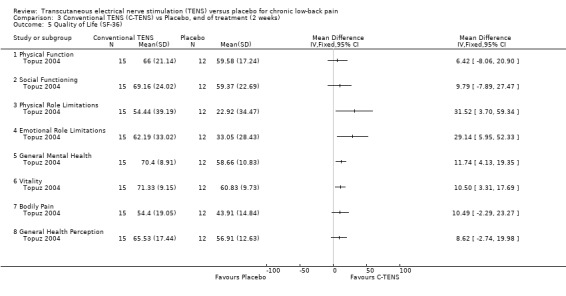
Comparison 3 Conventional TENS (C‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 5 Quality of Life (SF‐36).
Comparison 4. Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (2 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain Intensity, VAS (0‐100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Activity Pain, VAS (0‐100) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Oswestry Disability Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Low Back Pain Outcome Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of Life (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Physical Function | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Social Functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Physical Role Limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Emotional Role Limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 General Mental Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Bodily Pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 General Health Perception | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
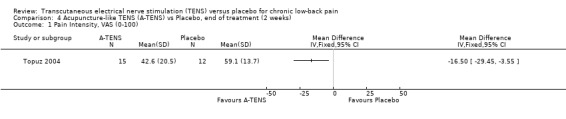
Comparison 4 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 1 Pain Intensity, VAS (0‐100).
4.2. Analysis.
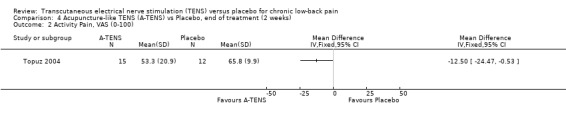
Comparison 4 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 2 Activity Pain, VAS (0‐100).
4.3. Analysis.
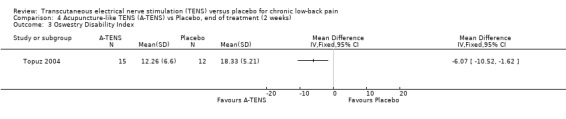
Comparison 4 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 3 Oswestry Disability Index.
4.4. Analysis.
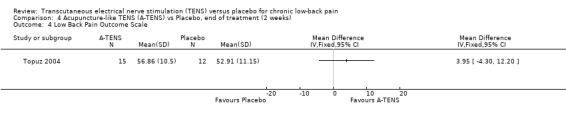
Comparison 4 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 4 Low Back Pain Outcome Scale.
4.5. Analysis.
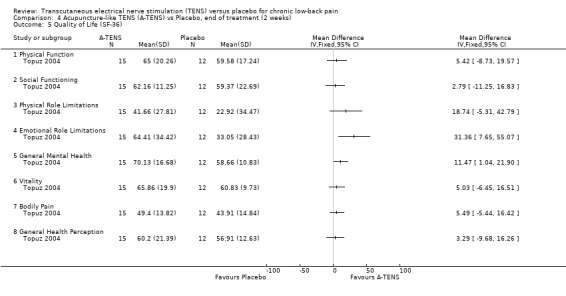
Comparison 4 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (2 weeks), Outcome 5 Quality of Life (SF‐36).
Comparison 5. Conventional TENS (C‐TENS) vs Placebo, end of treatment (4 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Roland Disability Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 McGill Work Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Physical Measures | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Extension | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Straight Leg Raise (Right) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Straight Leg Raise (Left) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Isolift | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 McGill Activity Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.
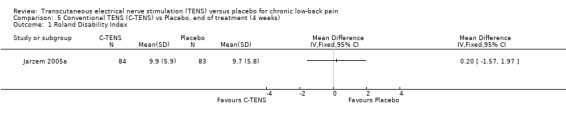
Comparison 5 Conventional TENS (C‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 1 Roland Disability Index.
5.2. Analysis.
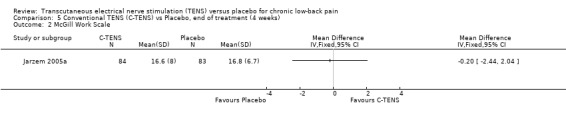
Comparison 5 Conventional TENS (C‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 2 McGill Work Scale.
5.3. Analysis.
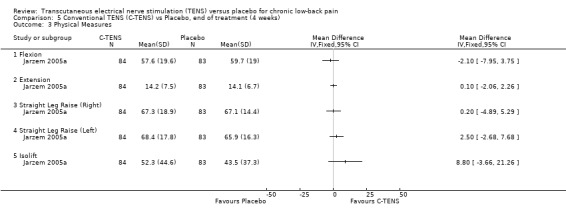
Comparison 5 Conventional TENS (C‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 3 Physical Measures.
5.4. Analysis.
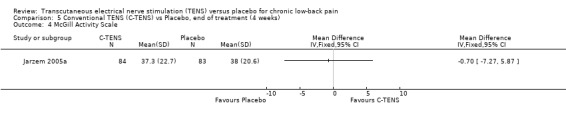
Comparison 5 Conventional TENS (C‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 4 McGill Activity Scale.
Comparison 6. Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (4 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Roland Disability Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 McGill Work Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Physical Measures | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Extension | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Straight Leg Raise (Right) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Straight Leg Raise (Left) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Isolift | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 McGill Activity Scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.
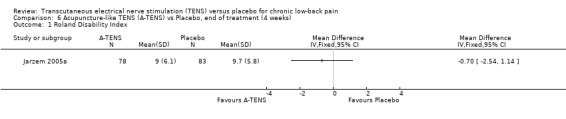
Comparison 6 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 1 Roland Disability Index.
6.2. Analysis.
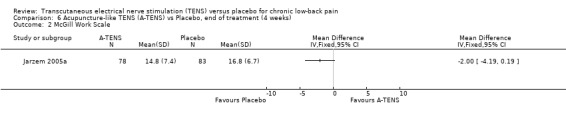
Comparison 6 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 2 McGill Work Scale.
6.3. Analysis.
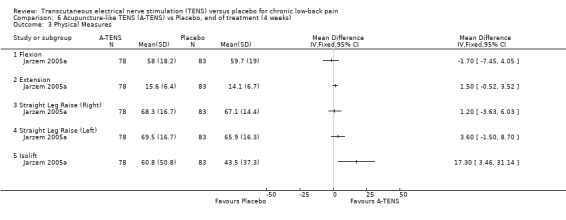
Comparison 6 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 3 Physical Measures.
6.4. Analysis.
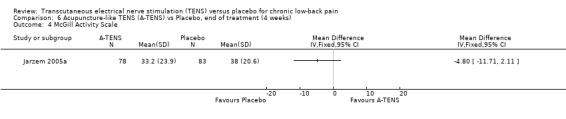
Comparison 6 Acupuncture‐like TENS (A‐TENS) vs Placebo, end of treatment (4 weeks), Outcome 4 McGill Activity Scale.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cheing 1996.
| Methods | Randomized, placebo‐controlled trial, parallel design
Participants stratified by gender, duration of pain and severity of pain prior to randomization
Sample size: N = 30 (group 1: N = 15, group 2: N = 15); after 8 dropouts and withdrawals, 22 subjects remained (group 1: N = 11; group 2: N = 11)
Treatment duration: two weeks (data for 1st treatment session reported in 1999 journal article, data at the end of two weeks reported in 1996 abstract)
No follow‐up reported after the end of the two‐week treatment period
TENS administered by researcher in clinic
Subjective outcomes measured at home over three days prior to study and, then, in the clinic setting Note: Exclusion of subjects with prior TENS exposure not specifically reported, prior exposure may affect adequacy of blinding |
|
| Participants | Inclusion: age 18 to 50 years, low‐back pain for at least six months, moderate to severe pain (greater than or equal to 30% on Visual Analogue Scale), daily pain, stable flexion reflex capable of being induced by painful electrical stimulus to plantar surface of foot Exclusion: pregnancy, neuromuscular or neurological disorders, muscle atrophy in the lower extremities, a history of back surgery, a consistent sciatica symptoms, cardiac pacemaker, spondylolisthesis > 1 cm Mean age: group 1: 34.7±9.1; group 2 28.2 ±7.2 Pain duration: group 1: 6.3±5.7 yrs; group 2: 5.7±4.3 yrs Pain severity (SD): group 1: 40.1 (18.7); group 2: 42.7 (12.2) Gender: group 1: four women, eleven men; group 2: five women, ten men No withdrawals or dropouts up to first treatment session; however, there were a total of eight dropouts/withdrawals (four from each of the two groups) by the end of the two‐week treatment period, representing 26.6% of the original sample ‐ two dropouts due to time conflicts, two dropouts due to dislike of experimental pain induction, four dropouts due increase in pain or lack of pain improvement during study period |
|
| Interventions | Group 1: TENS
Group 2: Placebo TENS TENS device: Staodyn MAXIMA III generating continuous trains of biphasic square pulses Stimulation mode: Conventional TENS Frequency: 80 Hz Pulse Width: 140 μsec Amplitude: adjusted to produce tingling sensation at two to three times above sensory threshold Electrode Placement: two surface electrodes (16.5 cm X 3.2 cm each) were placed over the lumbosacral area (L4‐S2) paraspinally Treatment schedule: 60 minutes/day for five days/week over two weeks (only data for first treatment session reported in 1999 journal article) Total treatment time: 600 minutes (10 hours) Concurrent treatment: participants were required to discontinue physiotherapy or pain medication two weeks before day of treatment Subjects in both experimental and control groups were told that they might or might not perceive the electrical stimulation |
|
| Outcomes | Pain Intensity (20‐cm visual analogue scale) ‐ measured three times a day, over three consecutive days prior to starting treatment to determine baseline Additional statistical data not reported in the abstract was obtained from the primary authors with VAS scores converted to a 100mm scale. No report of presence or absence of adverse effects |
|
| Notes | Quality 6/11 see Table 1 for questions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | No withdrawals or dropouts up to first treatment session; however, there were a total of eight dropouts/withdrawals (four from each of the two groups) by the end of the two‐week treatment period, representing 26.6% of the original sample ‐ two dropouts due to time conflicts, two dropouts due to dislike of experimental pain induction, four dropouts due increase in pain or lack of pain improvement during study period |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Low risk | |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
Deyo 1990a.
| Methods | Randomized, double‐blind, placebo‐controlled, parallel design
Sample size: N = 145; after 20 withdrawals and dropouts, 125 subjects remained (group 1: N = 31; group 2: N = 34; group 3: N = 31; group 4: N = 29)
Treatment duration: four weeks
Follow‐up at two weeks and two months post‐treatment (raw data not reported)
TENS was self‐administered at home
Subjective outcomes measured in the clinic setting Note: Subjects with prior exposure to TENS were excluded |
|
| Participants | Inclusion: low‐back pain longer than three months Exclusion: history of cancer, use of corticosteroids/anticoagulant, maximal pain above T12, age over 70 years or under 18 years, cardiac pacemaker, known heart disease, severe coexisting disease, previous unevaluated neurologic deficit, previous use of TENS, seeking or receiving disability compensation, factors that would impair follow‐up (plan to move within three months, inability to speak English, inaccessibility by telephone, inability to keep twice‐weekly appointments) Mean age : total sample = 51.4 (group 1 = 53.7, group 2 = 53, group 3 = 48.1, group 4 = 50.6) Mean VAS score: total sample = 41.3; group 1 = 39.9; group 2 = 43.1; group 3 = 37.9; group 4 = 44.2 Median pain duration (months): total sample = 60; group 1 = 84; group 2 = 66; group 3 = 60; group 4 = 36 % females: total = 58, group 1 = 58, group 2 = 59, group 3 = 58, group 4 = 59 Withdrawals and dropouts: 20 dropped out representing 14% of the sample (five from group 1; three from group 2; five from group 3; seven from group 4) ‐ eleven dropouts were due to inconvenience and difficulties with transportation, one dropped out because of impression that treatments were of no help, one subject randomized to sham TENS developed severe dermatitis requiring discontinuation of treatment after four days; reasons for other dropouts not specified) By the two‐month follow‐up, there were three further dropouts, representing an additional 2% of the sample. |
|
| Interventions | Group 1: TENS
Group 2: TENS + exercise (12 sequential exercises: three relaxation exercises followed by nine stretching exercises for flexibility of spine, hip, lower extremities)
Group 3: no exercise + sham TENS
Group 4: sham TENS + exercise TENS device: Epix 982 units Stimulation modes: Conventional TENS for first two weeks, then Conventional TENS or Acupuncture‐like TENS for next two weeks depending on patient preference (23% chose to continue using Conventional TENS till the end of the study) Average Pulse Frequency: Conventional TENS: 80 to 100 Hz, Acupuncture‐like TENS: 2 to 4 Hz; note that a modulated‐pulse‐rate mode was used in which the rate of stimulation was periodically altered to reach the specified average frequency. Pulse Width: not available Amplitude: Conventional TENS: 30 (units not reported); Acupuncture‐like TENS: 100 (units not reported) Electrode placement: four electrodes (5.5 cm in diameter) were initially placed over the area of most severe pain, but were then moved as necessary to optimize pain relief; in sciatica, electrodes were placed on leg and back Duration of each treatment session: 45 minutes Schedule of treatment sessions: three sessions/day for four weeks Cumulative application time: 3780 min (63 hours) Concurrent treatments: hot packs and electric heating pads; written and oral advice for lifting, standing, resting positions; usual pain medications continued; no restriction on use of new medications or physical therapy during study Subjects were told that the electrical stimulation was sometimes below the threshold of perception and that they might or might not perceive it; placebo units had "on" lights that flashed at the selected frequency. |
|
| Outcomes | Pain (10 cm visual analogue scale used to measure pain intensity and improvement, six‐point scale was also used to measure self‐rated improvement)
Functional status (modified Sickness Impact Profile, Self‐Rated activity)
Physical measures (finger‐to‐floor distance, Schober test, Straight Leg Raise)
Use of medical services (days in hospital, visits to other providers)
Data for TENS and placebo TENS reported as adjusted means after controlling for baseline values and the effect of exercise Data at two‐month follow‐up not provided in sufficient detail to permit analysis for that time period Adverse effects: skin irritation at the site of electrode placement was reported in a third of subjects (equal proportions affected in the TENS and sham TENS groups); one subject receiving sham TENS was required to withdraw due to the development of severe dermatitis four days after therapy |
|
| Notes | Quality: 8/11 see Table 1 for questions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | 20 dropped out representing 14% of the sample (five from group 1; three from group 2; five from group 3; seven from group 4) ‐ eleven dropouts were due to inconvenience and difficulties with transportation, one dropped out because of impression that treatments were of no help, one subject randomized to sham TENS developed severe dermatitis requiring discontinuation of treatment after four days; reasons for other dropouts not specified) By the two‐month follow‐up, there were three further dropouts, representing an additional 2% of the sample. |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | |
| Similarity of baseline characteristics? | High risk | |
| Co‐interventions avoided or similar? | Low risk | |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
Jarzem 2005a.
| Methods | Randomized , placebo‐controlled, parallel design
Sample size : N = 350; after 26 withdrawals, 324 subjects remained (group 1: N = 84; group 2: N = 84; group 3: N = 78; group 4: N = 79)
Treatment duration: four weeks
Follow‐up: three months (outcomes not reported)
TENS administered by subjects at home; outcomes measured at home and in the clinic setting Subjects with prior TENS exposure were excluded |
|
| Participants | Inclusion: Continuous low‐back pain without leg symptoms for at least three months; age between 18 and 70; able to make all required visits Exclusion: Maximal pain above T12; previous use of TENS; patient currently seeking to obtain disability compensation; history of cancer; corticosteroids or anticoagulant use; implanted pacemaker; sciatica; concomitant physiotherapy or chiropractic therapy; recent surgery in the previous three months; onset of major illness; pregnancy Age: total sample = 45.1 Pain duration in years: total sample = 10.1 (Group 1 = 10.1; group 2: 9.0; group 3 = 9.4; group 4 = 12.2) Baseline pain intensity not reported Gender breakdown: 50% female, 50% male Withdrawals and dropouts: 26 withdrawals (7.4% of sample) due to inability to return to clinic for all evaluation sessions; distribution of dropouts according to treatment group was not reported; note that only 70% returned questionnaires and diaries at the three‐months follow‐up (data contained in the questionnaires and diaries were not reported) |
|
| Interventions | Group 1: Placebo TENS
Group 2: Conventional TENS
Group 3: Acupuncture‐like TENS
Group 4: NuWave TENS (not considered in this review) TENS device not reported Stimulation parameters not reported Electrode number and size not reported Electrode placement: adjusted to the patient's preference Treatment schedule: daily treatment for four weeks, average of 188 minutes of use per day Total treatment time: 5264 minutes or about 88 hours Concurrent treatment: exercise programs were assigned by physiotherapists to all subjects; medication use was monitored; subjects undergoing other physiotherapy or chiropractic therapy were excluded The placebo TENS devices had indicator lights to mimic operation; all subjects were told that some might or might not feel the stimulation |
|
| Outcomes | Roland‐Morris Disability Questionnaire
McGill work scale
McGill activity scale
Zung depression scale
Physical measures (flexion, extension, straight leg raise, isometric dead‐lift score) Patient Diaries tracking pain intensity (VAS), frequency of pain medication usage, ancillary care, general medical concerns and usage of the TENS unit were not returned in 30% of cases and the data was not reported Outcomes not reported at three months follow‐up (30% loss to follow‐up) No reporting of adverse effects |
|
| Notes | Quality 8/11 See Table 1 for questions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | 26 withdrawals (7.4% of sample) due to inability to return to clinic for all evaluation sessions; distribution of dropouts according to treatment group was not reported; note that only 70% returned questionnaires and diaries at the three‐months follow‐up (data contained in the questionnaires and diaries were not reported) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Low risk | |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
Topuz 2004.
| Methods | Randomized, placebo‐controlled trial, parallel design
Sample size: N = 60; after five dropouts, 55 subjects remained (group 1: N = 12; group 2: N = 15; group 3: N = 15; group 4: N = 13)
Treatment duration: two weeks
No follow‐up
TENS administered by researcher in clinic Subjects with prior TENS exposure were excluded |
|
| Participants | Inclusion: low‐back pain for at least three months and ambulatory Exclusion: history of cancer; use of corticosteroids or anticoagulants; use of cardiac pacemaker; prior lumbar spine surgery; known heart disease; severe co‐existing disease; vertebral fracture; spinal infection; spinal tumour; severe orthopedic abnormalities; nerve root findings; previous use of a therapeutic electrical stimulation modality Mean age (SD): group 1 = 41.92 (7.70); group 2 = 45.20 (11.19); group 3 = 50.13 (11.97) Pain severity (SD): group 1 = 5.75 (1.35); group 2 = 6.53 (1.18); group 3 = 6.86 (1.24) Pain duration in months (SD): group 1 = 16.81 (8.75); group 2 = 16.46 (9.78); group 3 = 20.53 (14.42) Gender (% female): group 1 = 91.7%; group 2 = 60%; group 3 = 73.3% No significant differences in the Beck Depression Inventory between treatment groups Withdrawals and Dropouts ‐ five dropouts (8.3% of sample) for personal reasons; three of the dropouts were in the Placebo TENS group and two were in the percutaneous neuromodulation therapy group |
|
| Interventions | Group 1: Placebo TENS
Group 2: Conventional TENS
Group 3: Low‐frequency TENS
Group 4: Percutaneous neuromodulation therapy (not considered) TENS device: Trio 300 units generating symmetric, biphasic, rectangular pulses Stimulation modes: Conventional TENS and Low Frequency TENS Pulse Frequency: 80Hz for Conventional TENS group; 4Hz for Low‐frequency TENS group Pulse Width: 100 μsec Amplitude: For the conventional TENS group, the amplitude was increased up to the subjects' perception of paresthesia; for the low frequency TENS group, amplitude was increased to a maximum tolerated level without inducing muscle contraction Electrode Placement: four electrodes (2x2 cm) were placed in a standard dermatomal pattern over the most painful lumbar region Treatment duration: 20 minutes per session Treatment schedule: five sessions per week for two weeks Cumulative stimulation time: 200 minutes or just over three hours Concurrent treatment: No specific restrictions on the use of analgesic medications, except corticosteroids Subjects were told that they might or might not perceive the electrical stimulation and that it was sometimes below a patient's threshold of perception |
|
| Outcomes | Pain intensity (10 cm visual analogue scale)
Activity Pain (10 cm visual analogue scale)
Oswestry Disability Index
Low Back Pain Outcome Scale
Health Status Survey Short Form (SF‐36) No reporting of adverse effects |
|
| Notes | Quality: 8/11 see Table 1 for questions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | |
| Blinding (performance bias and detection bias) All outcomes ‐ providers? | High risk | |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | five dropouts (8.3% of sample) for personal reasons; three of the dropouts were in the Placebo TENS group and two were in the percutaneous neuromodulation therapy group |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | |
| Similarity of baseline characteristics? | Low risk | |
| Co‐interventions avoided or similar? | Low risk | |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Smadi 2003 | Pilot study involving only five subjects per treatment group; Study sample confined to patients with multiple sclerosis, a chronic inflammatory disorder of the central nervous system. Potential contribution of neuropathic pain (e.g. demyelinating lesions involving the spinal cord) to etiology of low‐back symptoms cannot be excluded |
| Biedermann 1987 | Trial investigated EMG biofeedback, not TENS |
| Bloodworth 2004 | Sample composed exclusively of patients with chronic, electromyographically‐documented lumbosacral radiculopathy |
| Cheng 1987 | No appropriate control (TENS versus electroacupuncture) |
| Cubucku 2004 | Most subjects had meralgia paresthetica, a painful neuropathy of the lateral femoral cutaneous nerve |
| Fox 1976 | No appropriate control (TENS versus acupuncture) |
| Gemignani 1991 | Mixed sample of acute, subacute, and chronic low‐back pain; Study confined to subjects with ankylosing spondylitis (inflammatory arthritis) |
| Ghoname 1999a | No appropriate control (four modalities were compared: TENS, percutaneous electrical nerve stimulation (PENS), sham PENS, exercise) |
| Ghoname 1999b | No appropriate control (three modalities were compared: TENS, PENS, sham PENS); study confined to subjects with sciatica |
| Glaser 2001 | Mixed sample of subacute and chronic low‐back pain; investigation of electrical muscle stimulation, not TENS (subthreshold TENS served as a placebo control) |
| Grant 1999 | No appropriate control (TENS versus acupuncture) |
| Hackett 1988 | Intervention involved electroacupuncture, not TENS |
| Hamza 1999 | Investigation of percutaneous electrical nerve stimulation |
| Herman 1994 | Study included subjects with acute and subacute low‐back pain Mixed sample of subjects with acute, subacute, and chronic low‐back pain |
| Hsieh 2002 | Mixed sample of acute, subacute, and chronic low‐back pain |
| Hurley 2001 | Subjects had subacute low‐back pain (one to three months); Intervention involved Interferential therapy, not TENS |
| Jarzem 2005b | Inadequate statistical data |
| Jeans 1979 | Fewer than five patients with chronic low‐back pain per study group |
| Laitinen 1976 | No appropriate control (TENS versus acupuncture) |
| Lampe 1987 | Duration and location of back pain unclear; no appropriate control (Conventional TENS versus nonstandard, experimental waveform) |
| Lehmann 1983 | Inpatient program |
| Lehmann 1986 | Inpatient program; based on the same trial as Lehmann 1983 |
| Lundeberg 1984 | Study involved patients with chronic myalgia for which etiology was not clearly defined; study sample not specifically limited to subjects with chronic low‐back pain |
| Macdonald 1995 | Intervention involved superficial acupuncture, not TENS |
| Marchand 1993 | Study included subjects with inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis) and other specific diagnoses, for which exact numbers were not provided |
| Melzack 1980 | No appropriate control (TENS versus ice massage) |
| Melzack 1983 | No appropriate control (TENS versus massage) |
| Moore 1997 | Mixed sample of upper, middle and low‐back pain |
| Pressor 2000 | Trial examined the analgesic effect of TENS on the pain of epidural steroid injection, not chronic back pain itself |
| Puranik 2002 | Biophysical parameters used for stimulation not comparable to standard forms of TENS (device known as the Action Potential Stimulator) |
| Rutkowski 1977 | Intervention involved electroacupuncture, not TENS |
| Schuster 1980 | Inpatients; investigation of the relief of postoperative pain following back surgery |
| Sherry 2001 | No appropriate control (TENS versus vertebral axial decompression) |
| Shimoji 2007 | Included patients with pain above L1, at the middle and/or upper back |
| Sternbach 1976 | Not randomized; subjects had chronic pain of multiple etiologies and locations |
| Stonnington 1976 | No appropriate control; pilot study on chronic pain, not specific for chronic low‐back pain |
| Thorsteinsson 1978 | Could not separate data for chronic low‐back pain from data for other causes of chronic pain. |
| Tsukayama 2002 | No appropriate control (TENS vs electroacupuncture) Study sample included subjects with acute low‐back pain |
| Warke 2004 | Duplicate report. Based on the same trial as Al‐Smadi 2003, except with longer follow‐up. Only five subjects per treatment group. |
| Warke 2006 | Study sample confined to patients with multiple sclerosis, a chronic inflammatory disorder of the central nervous system. Potential contribution of neuropathic pain (e.g. demyelinating lesions involving the spinal cord) to etiology of low‐back symptoms cannot be excluded. |
| Weiner 2003 | Study evaluated percutaneous electrical stimulation not TENS |
| Werners 1999 | Mixed sample of acute, subacute and chronic low‐back pain; Intervention involved Interferential therapy, not TENS; no appropriate control |
| Yokuyama 2004 | Head‐to‐head study comparing percutaneous electrical nerve stimulation to TENS |
Characteristics of ongoing studies [ordered by study ID]
Laurent 2008.
| Trial name or title | Pain reducing effect of transcutaneous electrical nerve stimulation in patients with chronic low‐back pain or lumbo‐radiculalgia |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
Amole Khadilkar participated in the selection of trials, assessment of methodological quality, data extraction, statistical analysis and conclusions for the current update.
Daniel Oluwafemi Odebiyi assisted with the study selection, methodological quality assessment and data extraction for the current update.
George Wells provided statistical consultation and overall guidance.
Lucie Brosseau developed the original protocol.
Sarah Milne, Vivian (Robinson) Welch, Lucie Brosseau, Michael Saginur, Beverley Shea, Peter Tugwell and George Wells were involved in the original review
Sources of support
Internal sources
No sources of support supplied
External sources
CIGNA Foundation provided an educational grant, USA.
Lucie Brosseau is an Ontario Ministry of Health Career Scientist, Canada.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Cheing 1996 {published and unpublished data}
- Cheing GL, Hui‐Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Archives of Physical Medicine & Rehabilitation 1999;80(3):305‐12. [DOI] [PubMed] [Google Scholar]
- Cheing GLY, Hui‐Chan CWY. Repeated application of TENS produce cumulative effects on chronic clinical pain but not acute experimental pain in chronic low back patients. The 8th World Congress on Pain. 1996; Vol. 8:85.
Deyo 1990a {published data only}
- Deyo RA, Walsh NE, Martin DC, Schoenfield LS, Ramamurthy S. A controlled trial of transcutaneous electrical stimulation (TENS) and exercise for chronic low back pain. New England Journal of Medicine 1990;322(23):1627‐34. [DOI] [PubMed] [Google Scholar]
Jarzem 2005a {published data only}
- Jarzem P, Harvey EJ, Arcaro N, Kazarowski J. Transcutaneous electrical nerve stimulation for non‐acute low back pain: A randomized double‐blind study of conventional, Nu‐Wavefor, acupuncture‐type and sham therapies. American Academy of Orthopaedic Surgeons Annual Meetiing. 1997.
- Jarzem PF, Harvey EJ, Arcaro N, Kaczorowski J. Transcutaneous electrical nerve stimulation (TENS) for chronic low back pain. Journal of Musculoskeletal Pain 2005;13(2):3‐8. [Google Scholar]
Topuz 2004 {published data only}
- Topuz O, Ozfidan E, Ozgen M, Ardic F. Efficacy of transcutaneous electrical nerve stimulation and percutaneous electrical neuromodulation therapy in chronic low back pain. Journal of Back and Musculoskeletal Rehabilitation 2004;17:127‐33. [Google Scholar]
References to studies excluded from this review
Al‐Smadi 2003 {published data only}
- Al‐Smadi J, Warke K, Wilson I, Cramp AF, Noble G, Walsh DM, Lowe‐Strong AS. A pilot investigation of the hypoalgesic effects of transcutaneous electrical nerve stimulation upon low back pain in people with multiple sclerosis. Clinical Rehabilitation 2003;17(7):742‐9. [DOI] [PubMed] [Google Scholar]
Biedermann 1987 {published data only}
- Biedermann HJ, McGhie A, Monga TN, Shanks GL. Perceived and actual control in EMG treatment of back pain. Behaviour Research & Therapy 1987;25(2):137‐47. [DOI] [PubMed] [Google Scholar]
Bloodworth 2004 {published data only}
- Bloodworth DM, Nguyen BN, Garver W, Moss F, Pedroza C, Tran T, Chiou‐Tan FY. Comparison of stochastic vs. conventional transcutaneous electrical stimulation for pain modulation in patients with electromyographically documented radiculopathy. American Journal of Physical Medicine & Rehabilitation 2004;83(8):584‐91. [DOI] [PubMed] [Google Scholar]
Cheng 1987 {published data only}
- Cheng RSS, Pomeranz B. Electrotherapy of chronic musculoskeletal pain: comparison of electroacupuncture and acupuncture‐like transcutaneous electrical stimulation. Clinical Journal of Pain 1987;2:143‐9. [Google Scholar]
Cubucku 2004 {published data only}
- Cubucku S, Karsli B, Alimoglu MK. Meralgia Paresthetica and low back pain. Journal of Back and Musculoskeletal Rehabilitation 2004;17(3‐4):135‐9. [Google Scholar]
Fox 1976 {published data only}
- Fox EJ, Melzac R. Transcutaneous electrical stimulation and acupuncture: comparison treatment for low back pain. Pain 1976;2:141‐8. [PubMed] [Google Scholar]
Gemignani 1991 {published data only}
- Gemigniani G. Transcutaneous Electrical Nerve Stimulation in Ankylosing Spondylitis: a Double‐Blind Study. Arth Rheum 1991;34(6):788‐9. [DOI] [PubMed] [Google Scholar]
Ghoname 1999a {published data only}
- Ghoname EA, Craig WF, White PF, Ahmed HE, Hamza MA, Henderson BN, Gajraj NM, Huber PJ, Gatchel RJ. Percutaneous Electrical Nerve Stimulation for Low Back Pain. JAMA 1999;281(9):818‐23. [DOI] [PubMed] [Google Scholar]
Ghoname 1999b {published data only}
- Ghoname EA, White PF, Hamza MA, Craig WF, Noe CE. Percutaneous electrical nerve stimulation: an alternative to TENS in the management of sciatica. Pain 1999;83(2):193‐9. [DOI] [PubMed] [Google Scholar]
Glaser 2001 {published data only}
- Glaser JA, Baltz MA, Nietart PJ, Bensen CV. Electrical muscle stimulation as an adjunct to exercise therapy in the treatment of nonacute low back pain: a randomized trial. The Journal of Pain 2001;2(5):295‐300. [DOI] [PubMed] [Google Scholar]
Grant 1999 {published data only}
- Grant D, Bishop‐Miller J, Winchester D. A randomized comparative trial of acupuncture versus transcutaneous electrical nerve stimulation for chronic back pain in the elderly. Pain 1999;82:9‐13. [DOI] [PubMed] [Google Scholar]
Hackett 1988 {published data only}
- Hackett GI, Seddon D, Kaminski D. Electroacupuncture compared with paracetamol for acute low back pain. Practitioner 1988;232(1443):163‐4. [PubMed] [Google Scholar]
Hamza 1999 {published data only}
- Hamza M, Ghoname E, White P, Craig W, Ahmed H, Gajraj N, Vakharia AS, Noe CE. Effect of the duration of electrical stimulation on the analgesic response in patients with low back pain. Anesthesiology 1999;91(6):1622‐7. [DOI] [PubMed] [Google Scholar]
Herman 1994 {published data only}
- Herman E, Williams R, Stratford P, Fargas‐Babjak A, Trott M. A randomized controlled trial of transcutaneous electrical nerve stimulation (CODETRON) to determine its benefits in a rehabilitation program for acute occupational low back pain. Spine 1994;19(5):561‐8. [DOI] [PubMed] [Google Scholar]
Hsieh 2002 {published data only}
- Hsieh RL, Lee WC. One‐shot percutaneous electrical nerve stimulation vs. transcutaneous electrical nerve stimulation for low back pain: comparison of therapeutic effects. American Journal of Physical Medicine & Rehabilitation 2002;81(11):838‐43. [DOI] [PubMed] [Google Scholar]
Hurley 2001 {published data only}
- Hurley DA, Minder PM, McDonough SM, Walsh, DM, Moore AP, Baxter DG. Interferential therapy electrode placement technique in acute low back pain: a preliminary investigation. Archives of Physical Medicine and Rehabilitation 2001;82:485‐93. [DOI] [PubMed] [Google Scholar]
Jarzem 2005b {published data only}
- Jarzem PF, Harvey EJ, Arcaro N, Kaczorowski J. transcutaneous electrical nerve stimulation (TENS) for short‐term treatment of low back pain ‐ randomized double blind crossover study of sham versus conventional TENS. Journal of Musculoskeletal Pain 2005;13(2):11‐7. [Google Scholar]
Jeans 1979 {published data only}
- Jeans ME. Relief of chronic pain by brief, intense transcutaneous electrical stimulation ‐ A double‐blind study. Advances in Pain Research and Therapy 1979;3:601‐6. [Google Scholar]
Laitinen 1976 {published data only}
- Laitinen J. Acupuncture and transcutaneous electric stimulation in the treatment of chronic sacrolumbalgia and ischialgia. American Journal of Chinese Medicine 1976;4(2):169‐75. [DOI] [PubMed] [Google Scholar]
Lampe 1987 {published data only}
- Lampe J, Dunn B. Symmetrical biphasic TENS waveform for the treatment of back pain. The Clinical Journal of Pain 1987;3:145‐51. [Google Scholar]
Lehmann 1983 {published data only}
- Lehmann TR, Russell DW, Spratt KF. The impact of patients with nonorganic physical findings on a controlled trial of transcutaneous electrical nerve stimulation and electroacupuncture. Spine 1983;8(6):625‐34. [DOI] [PubMed] [Google Scholar]
Lehmann 1986 {published data only}
- Lehmann TR, Russel DW, Spratt KF, Colby H, Liu YK, Fairchild ML, Christensen S. Efficacy of electroacupuncture and TENS in the rehabilitation of chronic low back patients. Pain 1986;26:277‐90. [DOI] [PubMed] [Google Scholar]
Lundeberg 1984 {published data only}
- Lundeberg T. A comparative study of the pain alleviating effect of vibratory stimulation, transcutaneous electrical nerve stimulation, electroacupuncture and placebo. American Journal of Chinese Medicine 1984;12:72‐9. [DOI] [PubMed] [Google Scholar]
Macdonald 1995 {published data only}
- Macdonald AJR, Coates TW. The discovery of transcutaneous spinal electroanalgesia and its relief of chronic pain. Physiotherapy 1995;81(11):653‐61. [Google Scholar]
Marchand 1993 {published data only}
- Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain 1993;54(1):99‐106. [DOI] [PubMed] [Google Scholar]
Melzack 1980 {published data only}
- Melzack R, Jeans ME, Stratford JG, Monks RC. Ice massage and transcutaneous electrical stimulation: comparison of treatment for low‐back pain. Pain 1980;9:209‐17. [DOI] [PubMed] [Google Scholar]
Melzack 1983 {published data only}
- Melzack R, Vetere P, Finch L. Transcutaneous electrical nerve stimulation for low back pain ‐ A comparison of TENS for pain and range of motion. Physical Therapy 1983;63(4):489‐93. [DOI] [PubMed] [Google Scholar]
Moore 1997 {published data only}
- Moore SR, Shurman J. Combined neuromuscular electrical stimulation and transcutaneous electrical nerve stimulation for treatment of chronic back pain: A double‐blind, repeated measures comparison. Archives of Physical Medicine and Rehabilitation 1997;78(1):55‐60. [DOI] [PubMed] [Google Scholar]
Pressor 2000 {published data only}
- Presser M, Birkhan J, Adler R, Hanani A, Eisenberg E. Transcutaneous electrical nerve stimulation (TENS) during epidural steroids injection: A randomized controlled trial. The Pain Clinic 2000;12(2):77‐80. [Google Scholar]
Puranik 2002 {published data only}
- Puranik S, Fozard J, Paremain G, Kilminster S, Hughes D, Williams E. A randomised, single blind study to evaluate the effects of action potential stimulator therapy compared with placebo in patients with chronic back pain. The Pain Clinic 2002;14(1):69‐73. [Google Scholar]
Rutkowski 1977 {published data only}
- Rutkowski B, Niedzialkowska T, Otto J. Electrical stimulation in chronic low‐back pain. British Journal of Anaesthesia 1977;49(6):629‐32. [DOI] [PubMed] [Google Scholar]
Schuster 1980 {published data only}
- Schuster GD, Infante MC. Pain relief after low back surgery: the efficacy of transcutaneous electrical nerve stimulation. Pain 1980;8(3):299‐302. [DOI] [PubMed] [Google Scholar]
Sherry 2001 {published data only}
- Sherry E, Kitchener P, Smart R. A prospective randomised controlled study of VAX‐D and TENS for the treatment of chronic low back pain. Neurological Research 2001;23:780‐4. [DOI] [PubMed] [Google Scholar]
Shimoji 2007 {published data only}
- Shimoji K, Takahashi N, Nishio Y, Koyanagi M, Aida S. Pain relief by transcutaneous electric nerve stimulation with bidirectional modulated sine waves in patients with chronic back pain: A randomized, double‐blind, sham‐controlled study. Neuromodulation 2007;10(1):45‐51. [DOI] [PubMed] [Google Scholar]
Sternbach 1976 {published data only}
- Sternbach RA, Ignelzi RJ, Deems LM, Timmermans G. Transcutaneous electrical analgesia: A follow‐up analysis. Pain 1976;2:35‐41. [DOI] [PubMed] [Google Scholar]
Stonnington 1976 {published data only}
- Stonnington HH, Stillwell GK, Ebersold MJ, Thorsteinsson G, Laws ER. Transcutaneous electrical stimulation for chronic pain relief: A pilot study. Minnesota Medicine 1976;59(10):681‐3. [PubMed] [Google Scholar]
Thorsteinsson 1978 {published data only}
- Thorsteinsson G, Stonnington HH, Stillwell GK, Elveback LR. The placebo effect of transcutaneous electrical stimulation. Pain 1978;5:31‐41. [DOI] [PubMed] [Google Scholar]
Tsukayama 2002 {published data only}
- Tsukayama H, Yamashita H, Amagai H, Tanno Y. Randomised controlled trial comparing the effectiveness of electroacupuncture and TENS for low back pain: A preliminary study for a pragmatic trial. Acupuncture in Medicine 2002;20(4):175‐80. [DOI] [PubMed] [Google Scholar]
Warke 2004 {published data only}
- Warke K, Al‐Smadhi J, Walsh DM, Lowe‐Strong AS. Use of self‐applied TENS for low back pain in people with multiple sclerosis. International Journal of Therapy and Rehabilitation 2004;11(6):275‐80. [Google Scholar]
Warke 2006 {published data only}
- Warke K, Al‐Smadi J, Baxter D, Walsh DM, Lowe‐Strong AS. Efficacy of TENS for chronic low back pain in a multiple sclerosis population: a randomized, placebo‐controlled clinical trial. The Clinical Journal of Pain 2006;22(9):812‐9. [DOI] [PubMed] [Google Scholar]
Weiner 2003 {published data only}
- Weiner DK, Rudy TE, Glick RM, Boston JR, Lieber SJ, Morrow LA, Taylor S. Efficacy of percutaneous electrical nerve stimulation for the treatment of chronic low back pain in older adults. Journal of the American Geriatrics Society 2003;51(5):599‐608. [DOI] [PubMed] [Google Scholar]
Werners 1999 {published data only}
- Werners R, Pynsent PB, Bulstrode CJK. Randomized trial comparing interferential therapy with motorized lumbar traction and massage in the management of low back pain in a primary care setting. Spine 1999;24(15):1579‐84. [DOI] [PubMed] [Google Scholar]
Yokuyama 2004 {published data only}
- Yokoyama M, Sun X, Oku S, Taga N, Sato K, Mizobuchi S, Takahashi T, Morita Kiyoshi. Comparison of percutaneous electrical nerve stimulation with transcutaneous electrical nerve stimulation for long‐term relief in patients with chronic low back pain. Analgesia and Anesthesia 2004;98(6):1552‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Laurent 2008 {unpublished data only}
- Laurent B, Navez ML, Nayme P, Joux‐Ruesch, Buchmuller A. Pain reducing effect of transcutaneous electrical nerve stimulation in patients with chronic low back pain or lumbo‐radiculalgia. NCT00452010.
Additional references
Andersson 1997
- Andersson GBJ. The epidemiology of spinal disorders. In: J.W. Frymoyer editor(s). The adult spine: principles and practice. 2nd Edition. New York: Raven Press, Ltd, 1997:93‐141. [Google Scholar]
Andersson 1999
- Andersson GBJ. Epidemiologic features of chronic low‐back pain. Lancet 1999;354:581‐5. [DOI] [PubMed] [Google Scholar]
APTA 1993
- American Physical Therapy Association. American Physical Therapy Association Anthology. 1993; Vol. Vol 2.
Barr 1999
- Barr JO. Transcutaneous Electrical Nerve Stimulation for pain management. In: Nelson RM, Hayes KW, Currier DP editor(s). Clinical electrotherapy. 3rd Edition. Appleton & Lange, 1999:291‐354. [Google Scholar]
Belanger 2002
- Belanger AY. Evidence based guide to therapeutic physical agents. Lippincott Williams & Wilkins, 2002. [Google Scholar]
Bombardier 2000
- Bombardier C. Outcome Assessments in the Evaluation of Treatment of Spinal Disorders: Summary and General Recommendations. Spine 2000;25(24):3100‐3. [DOI] [PubMed] [Google Scholar]
Bombardier 2001
- Bombardier C, Hayden J, Beaton DE. Minimally Clinically Important Difference: low back pain: outcome measures. Journal of Rheumatology 2001;28:431‐8. [PubMed] [Google Scholar]
Chou 2007a
- Chou R, Qaseem R, Snow V, Casey D, Cross JT, Shekelle P, Owens DK. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annals of Internal Medicine 2007;147:478‐91. [DOI] [PubMed] [Google Scholar]
Chou 2007b
- Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society ‐ American College of Physicians clinical practice guideline. Annals of Internal Medicine 2007;147:492‐504. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioural sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Coste 1989
- Coste J, Paolaggi JB. Critical review of the epidemiology of backache [Revue critique de l'épidémiologie des lombalgies]. Revue D'épidémiologie et de Santé Publique 1989;37:371‐83. [PubMed] [Google Scholar]
Davidson 2002
- Davidson M, Keating JL. A comparison of five low back pain disability questionnaires: reliability and responsiveness. Physical therapy 2002;82(1):8‐24. [DOI] [PubMed] [Google Scholar]
Delitto 1993
- Delitto A, Cibulka MT, Erhard RE, Bowling RW, Tenhula JA. Evidence for use of an extension‐mobilization category in acute low back syndrome: a prescriptive validation pilot study. Physical Therapy 1993;73:216‐28. [DOI] [PubMed] [Google Scholar]
Deveraux 2004
- Deveraux MW. Low Back Pain. Primary Care: Clinics in Office Practice 2004;31:33‐51. [Google Scholar]
Deyo 1987a
- Deyo RA, Tsui‐Wu YJ. Descriptive epidemiology of low‐back pain and its related medical care in the United States. Spine 1987;12:264‐8. [DOI] [PubMed] [Google Scholar]
Deyo 1987b
- Deyo RA, Tsui‐Wu YJ. Functional disability due to low‐back pain. Arthritis Rheumatism 1987;30:1247‐53. [DOI] [PubMed] [Google Scholar]
Deyo 1998
- Deyo R, Battie M, Beurskens A, Bombardier C, Croft P, Koes B, Malmivaara A, Roland M, Korff M, Waddell G. Outcome Measures for Low Back Pain Research: a proposal for standardized use. Spine 1998;23(18):2003‐13. [DOI] [PubMed] [Google Scholar]
Deyo 2001
- Deyo RA, Weinstein JN. Low back pain. New England Journal of Medicine 2001;344(5):363‐70. [DOI] [PubMed] [Google Scholar]
Deyo 2006
- Deyo R, Mirza S, Martin BI. Back pain prevalence and visit rates: estimates from US national surveys, 2002. Spine 2006;31(23):2724‐7. [DOI] [PubMed] [Google Scholar]
Flowerdew 1997
- Flowerdew MW, Gadsby JG. A review of the treatment of chronic low back pain with acupuncture‐like transcutaneous electrical stimulation and transcutaneous electrical nerve stimulation. Complementary Therapies in Medicine 1997;5:193‐201. [Google Scholar]
Gadsby 2000
- Gadsby JG, Flowerdew MW. Transcutaneous electrical nerve stimulation and acupuncture‐like transcutaneous like nerve stimulation for chronic low back pain. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000210.pub2] [DOI] [PubMed] [Google Scholar]
Hagg 2003
- Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. European Spine Journal 2003;12:12‐20. [DOI] [PubMed] [Google Scholar]
Han 1991
- Han JS, Chen XH, Sun SL. Effect of low‐ and high frequency TENS on metenkephalin‐Arg‐Phe and dynorphin: an immunoreactivity in human lumbar CSF. Pain 1991;47:295‐8. [DOI] [PubMed] [Google Scholar]
Haynes 1994
- Haynes RB, Wilczynski N, McKibbon KA, Walker CJ, Sinclair JC. Developing optimal search strategies for detecting clinically sound studies in MEDLINE. Journal of the American Medical Informatics Association 1994;1(6):447‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henneken 1987
- Henneken C, Buring J. Epidemiology in Medicine. Boston: Little Brown & Company, 1987. [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook of Systematic Reviews for Interventions, Version 4.2.6 [updated September 2006]. The Cochrane Library. Chichester, UK: John Wiley & Sons, Ltd, 2006, issue 4.
Hildebrandt 2004
- Hidebrandt J, Ursin H, Mannion AF, Aireksinin O, Brox JI, Cedraschi C et al. (COST B13 working group on guidelines for chronic low back pain). European guidelines for the management of chronic non‐specific low back pain. www. backpaineurope.org/web/files/WG2_Guidelines.pdf November 2004 (accessed Jan 2008).
Hughes 1984
- Hughes GS, Lichstein PR, Whitlock D, Harker C. Response of plasma beta‐endorphins to transcutaneous electrical stimulation in healthy subjects. Physical Therapy 1984;64:1062‐6. [DOI] [PubMed] [Google Scholar]
Johnson 1991a
- Johnson MI, Ashton CH, Thompson JW. The consistency of pulse frequencies and pulse patterns of transcutaneous electrical nerve stimulation (TENS) used by chronic pain patients. Pain 1991;44(3):231‐4. [DOI] [PubMed] [Google Scholar]
Johnson 1991b
- Johnson MI, Ashton CH, Thompson JW. An in‐depth study of long‐term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain 1991;44(3):221‐9. [DOI] [PubMed] [Google Scholar]
Kalra 2001
- Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical stimulation (TENS). JPET 2001;298:257‐63. [PubMed] [Google Scholar]
Maher 2004
- Maher CG. Effective physical treatment for chronic low back pain. Orthopedic Clinics of North America 2004;35(1):57‐64. [DOI] [PubMed] [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain Mechanisms: a new theory. Science 1965;150:971‐9. [DOI] [PubMed] [Google Scholar]
Melzack 1982
- Melzack R, Wall PD. The challenge of pain. New York: Penguin Books Ltd, 1982. [Google Scholar]
Montori 2000
- Montori VM, Smiega M, Guyatt GH. Publication bias: a brief review for clinicians. Mayo Clinic Proceedings 2000;75:1284‐8. [DOI] [PubMed] [Google Scholar]
Muller 2006
- Muller U, Roder C, Greenough CG. Back related outcome instruments. European Spine Journal 2006;15:s25‐s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostelo 2005
- Ostelo RWJ, Vet HCW. Clinically important outcomes in low back pain. Best practice & research in clinical rheumatology 2005;19 (4):693‐707. [DOI] [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RWJG, Deyo RA, Stratford P, Waddell G, Croft P, Korff M, Bouter LM, Vet HC. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimally important change. Spine 2008;33(1):90‐4. [DOI] [PubMed] [Google Scholar]
Ottenbacher 1995
- Ottenbacher KJ. Why rehabilitation research does not work (As well as we think it should). Arch Phys Med Rehabil 1995;76:123‐9. [DOI] [PubMed] [Google Scholar]
PEDro
- Australian Physiotherapy Association, Cochrane Rehabilitation, Related Therapies Field. School of Physiotherapy of the University of Sydney. PEDro: Physiotherapy Evidence Database. http://ptwww.cchs.usyd.edu (accessed July 2007).
Pengel 2003
- Pengel LHM, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. British Medical Journal 2003;327:323‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petitti 1994
- Petitti D. Meta‐analysis, decision analysis, and cost‐effectiveness analysis: methods for quantitative synthesis in medicine. New York: Oxford University Press, 1994. [Google Scholar]
Philadelphia Panel 2001
- Philadelphia Panel. Philadelphia Panel evidence‐based clinical practice guidelines on selected rehabilitation interventions for low back pain. Phys Ther 2001;81(10):1641‐74. [PubMed] [Google Scholar]
QTF 1987
- Québec Task Force on Spinal Disorders. Scientific approach to the assessment and management of activity‐related spinal disorder: a monograph for clinicians. Spine 1987;12:51‐9. [PubMed] [Google Scholar]
Rushton 2002
- Rushton DN. Electrical stimulation in the treatment of pain. Disability and Rehabilitation 2002;24(8):407‐15. [DOI] [PubMed] [Google Scholar]
Salar 1981
- Salar G, Job I, Mingrino S, Bosio A, Trabucchi M. Effect of transcutaneous electrotherapy on CSF B‐endorphin content in patients without pain problems. Pain 1981;10:169‐72. [DOI] [PubMed] [Google Scholar]
Schaufele 2003
- Schaufele MK, Boden SB. Outcome research in patients with low back pain. Orthopedic Clinics of North America 2003;34(2):231‐7. [DOI] [PubMed] [Google Scholar]
Schlapbach 1991
- Schlapbach P, Gerber NJ. Rheumatology. Physiotherapy: Controlled trials and facts. Vol. 14, Basel: S Karger AG, 1991. [Google Scholar]
Sierpina 2002
- Sierpina VS, Curtis P, Doering J. An integrative approach to low back pain. Clinics in Family Practice 2002;4(4):817‐31. [Google Scholar]
Skovron 1992
- Skovron ML. Epidemiology of low back pain. Baillieres Clinical Rheumatologia 1992;6:559‐73. [DOI] [PubMed] [Google Scholar]
Sluka 1999
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. JPET 1999;289:840‐6. [PubMed] [Google Scholar]
Sluka 2003
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation basic science mechanisms and clinical effectiveness. Journal of Pain 2003;4(3):109‐21. [DOI] [PubMed] [Google Scholar]
Smeal 2004
- Smeal WL, Tyburski M, Alleva J, Prather H, Hunt D. Conservative management of low back pain. Part I. Discogenic/radicular pain. Dis Mon 2004;50(12):636‐69. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Mathias E, Smith GD. Investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001;323:101‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tulgar 1991
- Tulgar M, McGlone F, Bowsher D, Miles JB. Comparative effectiveness of different stimulation modes in relieving pain. Part II: a double randomized controlled long term clinical trial. Pain 1991;47:157‐62. [DOI] [PubMed] [Google Scholar]
Van Tulder 1997
- Tulder MW, Koes BW, Bouter LM. Conservative treatment of acute and chronic nonspecific low back pain: a systematic review of randomized controlled trials of the most common conservative interventions. Spine 1997;22(18):2126‐58. [DOI] [PubMed] [Google Scholar]
Van Tulder 1999
- Tulder MW, Koes BW, Assendelft WJJ, Bouter LM. The effectiveness of conservative treatment of acute and chronic low back pain. Amsterdam: EMGO Institute, 1999. [Google Scholar]
Van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L, Editorial Board of of the Cochrane Collaboration Back Review Group. Updated Method Guidelines for Systematic Reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Von Korff 1996
- Korff M, Saunders K. The course of back pain in primary care. Spine 1996;21(24):2833‐7. [DOI] [PubMed] [Google Scholar]
Waddell 1998
- Waddell G. The clinical course of low back pain. In: Waddell G editor(s). The back pain revolution. Edinburgh: Churchill Livingstone, 1998:103‐17. [Google Scholar]
References to other published versions of this review
Brosseau 2002
- Brosseau L, Milne S, Robinson V, Marchand S, Shea B, Wells G, et al. Efficacy of the Transcutaneous Electrical Nerve Stimulation for the Treatment of Chronic Low Back Pain: A Meta‐Analysis. Spine 2002;27(6):596‐03. [DOI] [PubMed] [Google Scholar]
Khadilkar 2005
- Khadilkar A, Milne S, Brosseau L, Robinson V, Saginur M, Shea B, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation (TENS) for chronic low back pain. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003008.pub2] [DOI] [PubMed] [Google Scholar]
Milne 2001
- Milne S, Welch V, Brosseau L, Saginur M, Shea B, Tugwell P, et al. Transcutaneous electrical nerve stimulation (TENS) for chronic low‐back pain. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003008.] [DOI] [PubMed] [Google Scholar]
Odebiyi 2013
- Odebiyi DO, Henschke N, Chesterton L, Ferreira ML, Tella A. Transcutaneous electrical nerve stimulation (TENS) for chronic low‐back pain. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010500] [DOI] [Google Scholar]


