Abstract
Background
Smokers have a substantially increased risk of postoperative complications. Preoperative smoking intervention may be effective in decreasing this incidence, and surgery may constitute a unique opportunity for smoking cessation interventions.
Objectives
The objectives of this review are to assess the effect of preoperative smoking intervention on smoking cessation at the time of surgery and 12 months postoperatively, and on the incidence of postoperative complications.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialized Register in January 2014.
Selection criteria
Randomized controlled trials that recruited people who smoked prior to surgery, offered a smoking cessation intervention, and measured preoperative and long‐term abstinence from smoking or the incidence of postoperative complications or both outcomes.
Data collection and analysis
The review authors independently assessed studies to determine eligibility, and discussed the results between them.
Main results
Thirteen trials enrolling 2010 participants met the inclusion criteria. One trial did not report cessation as an outcome. Seven reported some measure of postoperative morbidity. Most studies were judged to be at low risk of bias but the overall quality of evidence was moderate due to the small number of studies contributing to each comparison.
Ten trials evaluated the effect of behavioural support on cessation at the time of surgery; nicotine replacement therapy (NRT) was offered or recommended to some or all participants in eight of these. Two trials initiated multisession face‐to‐face counselling at least four weeks before surgery and were classified as intensive interventions, whilst seven used a brief intervention. One further study provided an intensive intervention to both groups, with the intervention group additionally receiving a computer‐based scheduled reduced smoking intervention. One placebo‐controlled trial examined the effect of varenicline administered one week preoperatively followed by 11 weeks postoperative treatment, and one placebo‐controlled trial examined the effect of nicotine lozenges from the night before surgery as an adjunct to brief counselling at the preoperative evaluation. There was evidence of heterogeneity between the effects of trials using intensive and brief interventions, so we pooled these separately. An effect on cessation at the time of surgery was apparent in both subgroups, but the effect was larger for intensive intervention (pooled risk ratio (RR) 10.76; 95% confidence interval (CI) 4.55 to 25.46, two trials, 210 participants) than for brief interventions (RR 1.30; 95% CI 1.16 to 1.46, 7 trials, 1141 participants). A single trial did not show evidence of benefit of a scheduled reduced smoking intervention. Neither nicotine lozenges nor varenicline were shown to increase cessation at the time of surgery but both had wide confidence intervals (RR 1.34; 95% CI 0.86 to 2.10 (1 trial, 46 participants) and RR 1.49; 95% CI 0.98 to 2.26 (1 trial, 286 participants) respectively). Four of these trials evaluated long‐term smoking cessation and only the intensive intervention retained a significant effect (RR 2.96; 95% CI 1.57 to 5.55, 2 trials, 209 participants), whilst there was no evidence of a long‐term effect following a brief intervention (RR 1.09; 95% CI 0.68 to 1.75, 2 trials, 341 participants). The trial of varenicline did show a significant effect on long‐term smoking cessation (RR 1.45; 95% CI 1.01 to 2.07, 1 trial, 286 participants).
Seven trials examined the effect of smoking intervention on postoperative complications. As with smoking outcomes, there was evidence of heterogeneity between intensive and brief behavioural interventions. In subgroup analyses there was a significant effect of intensive intervention on any complications (RR 0.42; 95% CI 0.27 to 0.65, 2 trials, 210 participants) and on wound complications (RR 0.31; 95% CI 0.16 to 0.62, 2 trials, 210 participants). For brief interventions, where the impact on smoking had been smaller, there was no evidence of a reduction in complications (RR 0.92; 95% CI 0.72 to 1.19, 4 trials, 493 participants) for any complication (RR 0.99; 95% CI 0.70 to 1.40, 3 trials, 325 participants) for wound complications. The trial of varenicline did not detect an effect on postoperative complications (RR 0.94; 95% CI 0.52 to 1.72, 1 trial, 286 participants).
Authors' conclusions
There is evidence that preoperative smoking interventions providing behavioural support and offering NRT increase short‐term smoking cessation and may reduce postoperative morbidity. One trial of varenicline begun shortly before surgery has shown a benefit on long‐term cessation but did not detect an effect on early abstinence or on postoperative complications. The optimal preoperative intervention intensity remains unknown. Based on indirect comparisons and evidence from two small trials, interventions that begin four to eight weeks before surgery, include weekly counselling and use NRT are more likely to have an impact on complications and on long‐term smoking cessation.
Keywords: Humans, Preoperative Care, Smoking Cessation, Benzazepines, Benzazepines/administration & dosage, Nicotine, Nicotine/administration & dosage, Nicotinic Agonists, Nicotinic Agonists/administration & dosage, Postoperative Complications, Postoperative Complications/prevention & control, Quinoxalines, Quinoxalines/administration & dosage, Randomized Controlled Trials as Topic, Smoking, Smoking/adverse effects, Tobacco Use Cessation Devices, Varenicline
Plain language summary
Can people be helped to stop smoking before they have surgery?
Smoking is a well‐known risk factor for complications after surgery. Stopping smoking before surgery is likely to reduce the risk of complications. We reviewed the evidence about the effects of providing smoking cessation interventions to people awaiting surgery on their success in quitting at the time of surgery and longer‐term, and at complications following surgery. The evidence is current to January 2014.
We searched for randomized studies enrolling people who smoked and were awaiting any type of planned surgery. The trials tested interventions to encourage and help them to stop smoking before surgery. Interventions could include any type of support, including written materials, brief advice, counselling, medications such as nicotine replacement therapy (NRT) or varenicline, and combinations of different methods. The control could be usual care or a less intensive intervention.
We found 13 studies which met the inclusion requirements. The overall quality of evidence was moderate, limited by the small number of studies contributing to key analyses. Participants were awaiting a range of different types of surgery. Interventions differed in their intensity, and in how long before surgery they began. Both brief (seven trials, 1141 participants) and intensive (two trials, 210 participants) behavioural interventions were effective in increasing the proportion of smokers who were not smoking at the time they had surgery. The two trials using intensive interventions which started four to eight weeks before surgery had larger effects. Six trials of behavioural interventions assessed postoperative complications. Both trials of intensive interventions (210 participants) detected a reduction in complications in people receiving intervention, but the combined results of the four trials of brief interventions did not show a significant benefit. Only four trials of behavioural interventions followed up participants at twelve months. The two intensive interventions (209 participants) reduced the number of people smoking but the two brief interventions (341 participants) no longer showed a difference in the number of smokers. One trial of varenicline (286 participants), a pharmacotherapy shown to assist quitting in other groups of smokers, showed a benefit on cessation after twelve months, but did not show a benefit at the time of surgery or affect complications. In this trial smokers were only asked to stop the day before surgery.
Summary of findings
Summary of findings for the main comparison. Behavioural intervention versus control for preoperative smoking cessation.
| Behavioural intervention versus control for preoperative smoking cessation | ||||||
| Patient or population: People awaiting surgery Settings: Preoperative assessment clinics and similar settings Intervention: Behavioural intervention (typically including provision or offer of nicotine replacement therapy) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Control | Behavioural intervention versus control | |||||
| Smoking cessation at time of surgery ‐ Intensive behavioural intervention (multiple contacts, initiated at least 4 weeks before surgery) Follow‐up: 0 to 4 weeks | Study population | RR 10.76 (4.55 to 25.46) | 210 (2 studies) | ⊕⊕⊕⊝ moderate2 | Effect on smoking cessation was smaller but still apparent at 12‐month follow‐up (RR 2.96 95% CI 1.57 to 5.55, 209 participants, 2 studies) | |
| 47 per 1000 | 508 per 1000 (215 to 1000) | |||||
| Moderate | ||||||
| 48 per 1000 | 516 per 1000 (218 to 1000) | |||||
|
Smoking cessation at time of surgery ‐ Brief behavioural intervention Follow‐up: 0 to 4 weeks |
Study population | RR 1.3 (1.16 to 1.46) | 1141 (7 studies) | ⊕⊕⊕⊕ high | Effect on smoking cessation was not sustained at 12‐month follow‐up (RR 1.09 95% CI 0.68 to 1.75, 341 participants, 2 studies) | |
| 373 per 1000 | 484 per 1000 (432 to 544) | |||||
| Moderate | ||||||
| 157 per 1000 | 204 per 1000 (182 to 229) | |||||
| Postoperative morbidity: Any complication ‐ Intensive intervention | Study population | RR 0.42 (0.27 to 0.65) | 210 (2 studies) | ⊕⊕⊕⊝ moderate2 | Risk of postoperative complications will depend on type of operation and participants' characteristics | |
| 462 per 1000 | 268 per 1000 (162 to 337) | |||||
| Postoperative morbidity: Any complication ‐ Brief intervention | Study population | RR 0.92 (0.72 to 1.19) | 493 (4 studies) | ⊕⊕⊕⊝ moderate3 | Small effect on smoking cessation was not associated with a reduction in postoperative complications | |
| 308 per 1000 | 283 per 1000 (222 to 367) | |||||
| *The basis for the assumed risk1 (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Assumed control group quit rate based on crude averages. The expected quit rate amongst controls will depend on population characteristics, the definition of cessation, and the level of support for cessation as part of usual care. 2Imprecise estimate based on 2 moderate sized studies 3Imprecise estimate based on 4 moderate sized studies
Background
Complications related to anaesthesia and surgery are important to patients and expensive for the healthcare system. Postoperative complications result in increased morbidity and mortality, and extended hospital stay and convalescence.
Five to ten per cent of a population may annually undergo surgery and anaesthesia. Pulmonary or cardiovascular complications occur in up to 10% of the cases (Pedersen 1994), with people who smoke having a considerably increased risk of intra‐ and postoperative complications (Bluman 1998). In a retrospective study, smokers were found to have a three‐ to six‐fold increased risk of intra‐operative pulmonary complications (Akrawi 1997; Schwilk 1997). Smokers with chronic heart or lung disease have a two‐ to five‐fold increased risk of perioperative complications.
Smoking has many effects on heart function and circulation, both in the short and long term. Short‐term effects may be due to increased amounts of carbon monoxide (CO) and nicotine in the blood. The harmful effects of these substances disappear 24 to 48 hours after smoking cessation (Kambam 1986; Pearce 1984). The long‐term effects include the development of generalized atherosclerotic changes in the vasculature. Short‐term effects are more significant in those who suffer from generalized atherosclerosis (Klein 1984; Nicod 1984; Sheps 1990). The harmful effects of CO are primarily caused by its effect on oxygen metabolism, because CO binds to the haemoglobin molecules instead of oxygen, which reduces the availability of oxygen to the tissues by 3% to 12% (Pearce 1984). Furthermore, CO changes the structure of the haemoglobin molecules, shifting the oxygen‐haemoglobin curve to the left, further reducing oxygen availability, and also increases the risk of cardiac arrhythmias (Sheps 1990). Nicotine stimulates the surgical stress response and increases blood pressure, pulse rate and systemic vascular resistance, increasing the work of the heart. In summary, the effects of nicotine and CO in common tend to create an imbalance between oxygen consumption and oxygen availability in smokers (Kaijser 1985; Roth 1960). The effect of nicotine replacement therapy (NRT) on oxygen consumption is unclear (Benowitz 1997; Keeley 1996). A recent study demonstrated a significant increase in tissue oxygen and a limited vasoactive effect of NRT when administered intravenously (Sørensen 2008). There is no evidence indicating that NRT negatively affects postoperative outcome (Sørensen 2003c; Sørensen 2012). NRT must therefore be considered a better alternative than smoking.
As anaesthesia and surgery cause an increased strain on cardiac and circulatory functions, an existing oxygen imbalance can be worsened in patients who smoke, potentially resulting in hypoxaemia in vital organs.
Smoking also impairs pulmonary function. Smokers have increased mucus production, with damage to the tracheal cilia, which impedes the clearance of mucus. This is the explanation for the accumulation of mucus in the airways, which eventually may lead to pulmonary infections (Lourenco 1971). These effects may be exaggerated by reductions in immune function associated with smoking (Cohen 1993; Pearce 1984; Sørensen 2004). Immobilization during surgery and anaesthesia and in the immediate postoperative period worsens the reduced pulmonary function and the mucus accumulation. Pulmonary function generally improves after approximately eight weeks of smoking cessation (Bode 1975; Buist 1976; Camner 1973; McCarthy 1972, Mitchell 1982). In a retrospective study in people undergoing pulmonary surgery, Nakagawa 2001 found that the risk of postoperative pulmonary complications was significantly higher compared to never‐smokers for both current smokers and for recent smokers who had been smoke‐free for two to four weeks before their operation. Warner 1989 found, in a prospective, descriptive and uncontrolled study, that people who stopped smoking about eight weeks prior to operation reduced their risk of postoperative pulmonary complications. The optimal timing of smoking cessation before surgery to reduce postoperative pulmonary complications remains poorly defined (Mason 2009).
Smoking impairs wound healing after surgery (Haverstock 1998; Jorgensen 1998, Silverstein 1992; Sørensen 2002), and has been shown to increase the risk of anastomotic leakage after colorectal surgery (Sørensen 1999).
Shannon‐Cain 2002 found that surgical patients were not routinely informed of the risk of tobacco use or the potential of benefit of abstinence before their surgery, and concluded that the preoperative period might be a window for smoking intervention. Owen 2007 found that patients were largely not referred preoperatively to smoking cessation services, and that non‐vascular surgeons underestimated the potential benefit of preoperative smoking cessation on postoperative outcome.
The potential reduction of complications would be related to the success rate of a preoperative smoking cessation intervention. Motivation for smoking cessation might be increased, if a potential reduction of complications is possible (Møller 2004; Thomsen 2009). On the other hand, some patients tend to be nervous immediately prior to and after surgery and might feel that they need to smoke during this period, in order to deal with the stress of impending surgery and waiting for the results of the surgery (Møller 2004; Thomsen 2009). Brief smoking interventions delivered within routine daily care may not be powerful enough to influence highly dependent smokers (Hajek 2002). More intensive interventions may be required (Rice 2013; Rigotti 2012).
A successful preoperative smoking intervention could potentially reduce perioperative complications and lead to long‐term health gains if cessation were sustained.
Objectives
The objectives of this review are to assess the evidence for an effect of preoperative smoking intervention on smoking cessation at surgery and 12 months postoperatively, and on the incidence of postoperative complications.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Smokers of any age, who are scheduled for elective surgery.
Types of interventions
Any preoperative intervention to help people awaiting surgery to stop smoking. We considered any intervention, whether brief or more intensive, with or without face‐to‐face contact, using behavioural or pharmacological or combination strategies, initiated at least 48 hours before the operation. We did not consider trials of intra‐operative and postoperative smoking interventions for inclusion.
Types of outcome measures
Smoking cessation:
Prevalence of smoking cessation at the time of surgery, and 12 months postoperatively. We used the most conservative measure of quitting at surgery and at 12 months postoperatively, i.e. we preferred self‐reported continuous abstinence to self‐reported point prevalence abstinence.
Morbidity and mortality:
Wound‐related complications; secondary surgery; cardiopulmonary complications; admission to intensive care; intra‐ and postoperative mortality; length of stay as assessed in included studies.
Search methods for identification of studies
We searched the specialized register of the Cochrane Tobacco Addiction Group, using the topic‐related terms 'pre?operative' or 'post?operative' or 'surgery' or 'operation?' or 'operative' or 'an?esthesia' in text or keyword fields. See Appendix 1 for search strategy. At the time of the most recent search on 14th January 2014 the Register included the results of searches of the Cochrane Central Register of Controlled Trials (CENTRAL), 2013, Issue 12; MEDLINE (via OVID) to update 20140103; EMBASE (via OVID) to week 201352; PsycINFO (via OVID) to update 20131230. See the Specialized Register section of the Tobacco Addiction Group Module in The Cochrane Library for full details of search strategies for this resource. For previous versions of this review there were additional searches of MEDLINE, EMBASE and CINAHL, combining topic‐related and smoking‐related keywords (see Appendix 2). Since these had not retrieved any additional trials in previous updates we did not rerun them for this update.
Data collection and analysis
Three authors (TT, AAM and NV) evaluated all references identified as potentially relevant. Records retrieved by the register search were prescreened by the Tobacco Addiction Group Trials Search Co‐ordinator. All authors retrieved in full and appraised all relevant studies identified from abstracts. We resolved disagreement by consensus.
We report the following information about each study in the table Characteristics of included studies.
Country; site
Type of surgical procedure if reported
Method of randomization and adequacy of concealment
Number and characteristics of study participants
Therapist types
Description of experimental interventions, including timing and duration in relation to operation; description of control interventions
Outcomes: definition of smoking abstinence at each follow‐up point, use of biochemical validation
Data on postoperative complications.
Evaluation of risk of bias
We evaluated studies according to The Cochrane Collaboration's tool for assessing risk of bias. Items in the 'Risk of bias' table were judged to be at 'low risk of bias' , 'unclear risk of bias', or 'high risk of bias' for each study. Blinding of participants in smoking cessation trials involving behavioural interventions alone is not possible, and complete blinding of personnel is considered difficult to uphold. We therefore judged blinding as adequate if outcome assessors were blinded. We considered blinding of participants and personnel prerequisite for a judgement of low risk of bias in studies evaluating pharmacotherapy interventions.
Analysis of data
We have reported relevant outcomes in the text as percentages. For graphical display and pooling we have expressed the outcomes as a risk ratio (RR). For beneficial outcomes a value greater than one indicates that the intervention is better than the control, that is, the rate of quitting is higher in the intervention than in the control group. For unfavourable outcomes such as wound infection, a value less than one indicates that the intervention is better, that is, risk of an unfavourable outcome is lower in the intervention group.
We calculated RRs for smoking cessation and postoperative complications using intention‐to‐treat and available‐case analysis (Higgins 2011). For the smoking cessation outcome, we used as the denominators the number of participants randomized, excluding those whose surgery was cancelled or postponed, those who were erroneously included, those who withdrew from the trial immediately after randomization before receiving any intervention, and finally those who died. We assumed those otherwise lost to follow‐up to be smokers (Higgins 2011). For the complications outcome we used data only on those whose results were known, using as the denominator the total number of people who had data recorded for the outcome in question.
Where it was appropriate to pool studies, we used the Mantel‐Haenszel fixed‐effect method for combining RRs, with 95% confidence intervals (CIs). We used two tests for heterogeneity: the Chi‐squared test for heterogeneity, with P < 0.1 considered significant, and the I² statistic, with values above 75% interpreted as considerable heterogeneity (Higgins 2011). The I² statistic can be interpreted as the proportion of total variation observed between the studies attributable to differences between studies rather than to sampling error (chance) (Higgins 2003).
To assess the impact of missing data, we performed sensitivity analyses excluding trials with more than 20% drop‐out. We also performed sensitivity analyses excluding trials that did not supplement self‐reported smoking cessation with biochemical evaluation in order to explore any potential impact on smoking cessation at the time of surgery and at 12‐month follow‐up.
We pooled trials of behavioural interventions and trials of different pharmacotherapies separately. There is evidence that high‐intensity interventions support successful smoking cessation while briefer interventions have a non‐significant effect on smoking cessation in hospitalised people (Rigotti 2012). We further hypothesized that successful smoking cessation is a prerequisite for reducing complications. In the first version of this review we conducted exploratory subgroup analyses to assess potential differences in smoking cessation and postoperative complications in surgical patients receiving intensive versus brief preoperative interventions. We regarded intensive interventions as consisting of weekly counselling sessions over a period of four to eight weeks. Intensive and brief interventions are now pooled separately for all outcomes, and these subgroups are no longer treated as exploratory.
Earlier versions of this review reported effects as odds ratios. The Tobacco Addiction Group now recommends the use of risk ratios as being easier to interpret.
Results
Description of studies
We retrieved 42 new records by searches of the Tobacco Addiction Group specialized register for this update, of which 10 were potentially relevant reports of trials. The review authors identified one additional study report (Warner 2012). We include five new studies and we add four to the list of excluded studies. (Flow diagram Figure 1). We now include 13 trials conducted in Denmark, Australia, Canada, the USA, the UK and Sweden between 2002 and 2013 in the review. Two studies had different outcomes reported in additional papers. Møller 2002 reported long‐term follow‐up in Villebro 2008, and Lindström 2008 reported postoperative outcomes in Sadr Azodi 2009. We report outcomes using the main study identifiers (Møller 2002; Lindström 2008) with the other papers shown as secondary references.
1.
Flow diagram of searches for 2013 update.
*3 previously excluded reports now listed as secondary references to other studies
Trial participants
The type of surgery and the timing of enrolment in relation to surgery was very varied. Møller 2002 enrolled 120 participants six to eight weeks before scheduled elective hip or knee joint replacement. Sørensen 2003a enrolled 60 participants two to three weeks before colorectal surgery involving an enteric anastomosis. Ratner 2004 enrolled 237 participants attending a presurgical assessment clinic one to three weeks prior to cardiovascular, ophthalmologic, plastic and urologic surgery. Wolfenden 2005 enrolled 210 participants attending a preoperative clinic one to two weeks prior to non‐cardiac surgery (nervous, ear, nose, throat, digestive, hepatobiliary, pancreas, musculoskeletal, connective tissue, skin, subcutaneous tissue, breast, gynaecologic systems). Andrews 2006 enrolled 102 participants four weeks prior to elective surgery (type of surgery not specified). Sørensen 2007 enrolled 180 participants at least four weeks prior to elective open incisional or inguinal day‐case herniotomy. Additionally, they recruited another 64 people who smoked as a control group, some before and some after the trial period. The latter group is not included in the analyses because of the absence of randomization. Lindström 2008 enrolled 117 participants at least four weeks prior to elective inguinal and umbilical hernia repair, laparoscopic cholecystectomy, or a hip or knee prosthesis. Thomsen 2010 enrolled 130 participants at least one week prior to elective breast cancer surgery. Wong 2012 enrolled 286 participants who were scheduled for elective general, orthopedic, urologic, plastic, gynaecologic, ophthalmologic or neurosurgical procedures within eight to 30 days. Warner 2012, a pilot study, enrolled 46 participants scheduled for a wide variety of elective surgical procedures and evaluated in a preoperative evaluation centre; the time from evaluation to surgery was not specified. Lee 2013 enrolled 168 participants at least three weeks prior to elective surgery in connection with their preadmission evaluation. Participants were scheduled for general, gynaecologic, urologic, ophthalmologic, otolaryngologic and orthopaedic surgery. Ostroff 2013 enrolled 185 smokers with newly‐diagnosed cancer and scheduled for surgery no less than seven days from study entry. Participants needed to have sufficient visual acuity and manual dexterity to use a handheld computer. Cancer sites included thoracic, head and neck, breast, gynaecology, urology and other. Shi 2013 enrolled 169 participants attending a preoperative evaluation centre.
Interventions
Eleven trials evaluated behavioural interventions, with pharmacotherapy offered in some. Two trials evaluated pharmacotherapy alone.
Behavioural interventions
Two trials (Møller 2002; Lindström 2008) were classified as tests of intensive interventions because they offered weekly face‐to‐face or telephone counselling over a period of four to eight weeks prior to surgery. Møller 2002 counselled participants face‐to‐face on a weekly basis over a period of six to eight weeks. Lindström 2008 counselled participants either face‐to‐face or by telephone on a weekly basis over a period of four weeks. Additionally, participants were provided with the telephone number to a quitline. Both trials also offered nicotine replacement therapy (NRT) to intervention participants. In both trials the control group participants received standard care with little or no information about smoking cessation or the potential harm of tobacco smoking.
Eight trials provided brief behavioural interventions, of which six also offered NRT to some or all participants (Sørensen 2003a; Sørensen 2007; Ratner 2004; Wolfenden 2005; Thomsen 2010; Lee 2013). Sørensen 2003a called participants the day after expected smoking cessation, provided one counselling session before surgery and informed participants that they were free to call for additional telephone support during normal working hours. Ratner 2004 offered one 15‐minute face‐to‐face counselling session and provided participants with a telephone number to call for further assistance. Wolfenden 2005 offered one interactive counselling session lasting 17 minutes via computer, one telephone counselling call, and nursing and anaesthetic staff were prompted via computer to provide brief advice. Only participants who smoked more than 10 cigarettes per day (CPD) were offered NRT. Andrews 2006 sent a letter stating that stopping smoking before surgery has huge benefits such as less time for recovery, lower chance of wound infection, and containing information on contact details of a smoking cessation service. Sørensen 2007 counselled participants one month before surgery, either face‐to‐face in a 20‐minute meeting, including advice to use NRT, or via a 10‐minute telephone reminder. Thomsen 2010 offered one counselling session lasting between 45 and 90 minutes. Shi 2013 provided all participants with brief advice about smoking cessation from trained counsellors, and informed intervention participants that their exhaled carbon monoxide (CO) level would be checked on the morning of surgery. Lee 2013 provided five minutes of counselling by a trained preadmission nurse and referral to the Canadian Cancer Society's Smokers' Helpline which initiated contact with participants and provided counselling. Various control conditions were offered in these eight trials of brief interventions. Andrews 2006, Sørensen 2007 and Thomsen 2010 gave standard advice about the risks of smoking in relation to surgery. Ratner 2004 and Lee 2013 gave standard care with inconsistent or unco‐ordinated advice. Wolfenden 2005 gave clinical staff the option to provide smoking cessation advice and prescribe NRT to control group participants. Sørensen 2003a asked control group participants to maintain daily smoking habits. Shi 2013 gave the same brief advice to intervention and control groups.
Ostroff 2013 evaluated the additional effect of a scheduled reduced smoking regimen (SRS) as an adjunct to an intensive behavioural intervention The best practice programme consisted of five individual counselling sessions with trained smoking cessation counsellors and NRT at no cost. SInce this intensive intervention was provided to all participants it was not pooled with other behavioural intervention studies.
Pharmacotherapy interventions
Placebo‐controlled trials evaluated nicotine lozenges (Warner 2012) and varenicline (Wong 2012). Warner 2012 provided brief advice (two minutes) encouraging abstinence from smoking after 7pm the night before surgery and including the potential benefits of abstinence; following this, intervention participants received 16 active nicotine lozenges. Wong 2012 set a target quit date 24 hours before surgery and instructed participants to initiate varenicline exactly one week before the target quit date. Varenicline was provided for 12 weeks. All participants received two 15‐minute standardized counselling sessions, one at the preoperative clinic and one shortly after surgery.
Outcomes
Smoking cessation
Smoking cessation was defined as either self‐reported point prevalence or self‐reported continuous abstinence (see table Characteristics of included studies for further details). Two studies did not biochemically validate self‐reported smoking cessation (Wolfenden 2005; Andrews 2006). All but one study (Sørensen 2003a) assessed smoking status at the time of surgery. Sørensen 2003a did not distinguish between cessation and reduction; we have therefore not included these combined data in the review. Five studies assessed cessation at 12 months (Møller 2002; Ratner 2004; Lindström 2008; Thomsen 2010; Wong 2012).
Postoperative complications
Postoperative complications were defined as a composite outcome, and as wound‐related, cardiopulmonary and other complications requiring treatment. Seven studies assessed complications of surgery (Møller 2002; Sørensen 2003a; Sørensen 2007; Lindström 2008; Thomsen 2010; Wong 2012; Lee 2013).
Excluded studies
Of the possibly eligible studies, six were excluded because they involved preoperative smoking cessation interventions but did not use random allocation to intervention and control groups (Basler 1981; Munday 1993; Haddock 1997); Rissel 2000 used historical controls; Moore 2005 and Sachs 2012 used a prospective cohort design. Four were excluded because the intervention was delivered in the postoperative period (Wewers 1994; Simon 1997; Griebel 1998; Nåsell 2010). One study evaluated a training intervention for surgical residents and did not have patient‐based outcomes (Steinemann 2005). One study evaluated a multicomponent intervention including drinking, obesity and physical activity in addition to smoking, and recruited both smokers and nonsmokers. It did not evaluate perioperative outcomes (McHugh 2001).
We excluded one study (Myles 2004) comparing bupropion to placebo for preoperative cessation because there were high levels of drop‐out in each group, and only a small number of those who remained in the study were admitted for surgery within the six‐month study period. Data on perioperative cessation and complications were available for only 20 of the 47 people originally randomized. Cessation rates and wound infection rates were low and similar in each group.
We excluded one study because the intervention consisted of the application of a nicotine patch immediately before surgery with no additional counselling (Warner 2005).
We excluded Warner 2011 because the primary outcome was the use rate of a quitline service. Abrishami 2010 did not include outcomes relevant for this review.
Risk of bias in included studies
The risk of bias across domains for each study is summarised in Figure 2. All studies reported a method for random sequence generation and allocation concealment that we judged adequate to avoid selection bias. Seven studies explicitly reported blinding of assessors (Møller 2002; Sørensen 2003a; Ratner 2004; Wolfenden 2005; Wong 2012; Warner 2012; Lee 2013). In the remaining six studies, outcome assessment was not regularly blinded; Andrews 2006, Ostroff 2013 and Shi 2013 did not report using blinded outcome assessment; Sørensen 2007 used a study nurse to initially evaluate wound infections; Thomsen 2010 and Lindström 2008 used parallel blinded and unblinded outcome assessment. These studies may therefore be at some risk of detection bias. In all studies, smoking cessation was self‐reported.Nine studies validated self‐reported smoking cessation with measurements of CO in exhaled air and/or cotinine in urine/saliva. Five of nine studies did so at the time of surgery (Møller 2002; Ratner 2004; Sørensen 2007; Lindström 2008; Thomsen 2010; Warner 2012; Lee 2013; Ostroff 2013; Shi 2013); three of five studies at 12‐month follow up (Møller 2002; Ratner 2004; Wong 2012). Møller 2002, however, only did so partly in people participating in focus‐group interviews. Drop‐out rates in the included studies ranged from 1% to 29%. One study (Ratner 2004) had more than 20% drop‐out. All studies recruited participants on the basis of a convenience sample. Participation rates (i.e. the proportion of those eligible and approached who agreed to take part in the trial) were reported in all but one study (Andrews 2006), and ranged from 31% to 96%. Participants were similar across interventions in terms of baseline smoking data and comorbidity. Thomsen 2010 found a longer duration of surgery in intervention participants, which may have introduced a difference between groups in postoperative complications. Lee 2013 likewise found slightly more diabetes, hypertension, and heart disease in the intervention group.
2.
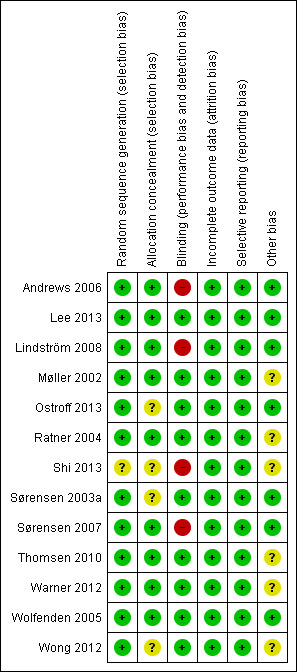
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Effect on smoking behaviours
Cessation at the time of surgery
Of the 10 studies evaluating behavioural interventions versus a control, nine reported cessation outcomes. In six studies (Møller 2002; Ratner 2004; Andrews 2006; Lindström 2008; Thomsen 2010; Lee 2013), the intervention achieved a significant increase in smoking cessation at the time of surgery, and one had a lower confidence interval (CI) of 1 (Wolfenden 2005). We identified substantial heterogeneity (I² = 87%) amongst the nine studies, so we did not consider pooling of all results appropriate. Heterogeneity was lower when we grouped the studies by the intensity of the intervention. We therefore pooled these subgroups (Figure 3; Analysis 1.1). Pooling the two trials (210 participants) using intensive interventions (Møller 2002; Lindström 2008) gave a RR of 10.76; 95% CI 4.55 to 25.46, and no evidence of heterogeneity. The pooled estimate for the six trials of brief interventions was smaller but also excluded no effect (RR 1.30; 95% CI 1.16 to 1.46; 1141 participants). There was still marked heterogeneity (I² = 75%). Exclusion of Shi 2013, in which the only difference between groups was measurement of exhaled CO on the morning of surgery, increased the point estimate to RR 1.47; 95% CI 1.27 to 1.70, and reduced I² to 52%. We suggest that differences in relative effects on smoking cessation at the time of surgery may be in part attributable to the intensity of the support given, and in part to the differing definitions of smoking cessation at the time of surgery. Because the absolute rates for smoking cessation and the definitions used were so varied, we summarise them in Table 2. Excluding two trials (Wolfenden 2005; Andrews 2006) that had no biochemical validation of self‐reported cessation did not substantively change the pooled estimate for brief intervention.
3.
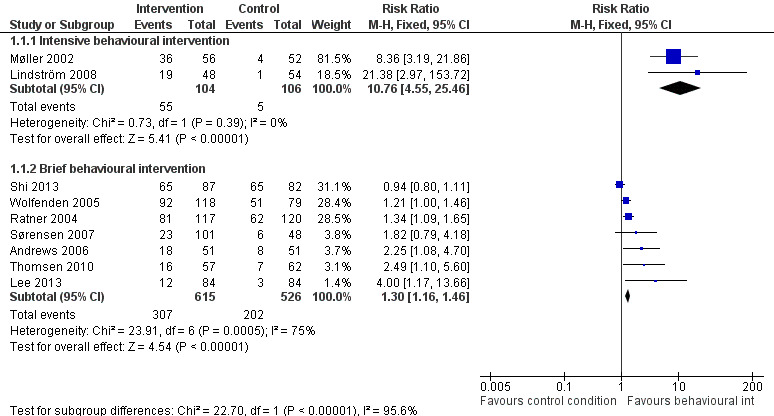
Forest plot of comparison: 1 Behavioural intervention versus control, outcome: 1.1 Smoking cessation at time of surgery.
1.1. Analysis.
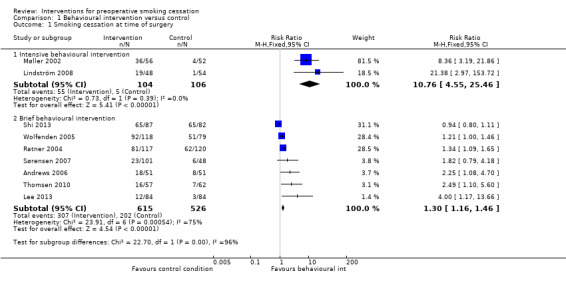
Comparison 1 Behavioural intervention versus control, Outcome 1 Smoking cessation at time of surgery.
1. Short‐term cessation outcomes.
| Study identifier | Intervention % quit | Control % quit | Abstinence Definition | Validation |
| Brief Intervention | ||||
| Ratner 2004 | 69% | 52% | Abstinent at least 24 hours before surgery | CO |
| Wolfenden 2005 | 78% | 64% | Abstinent at least 24 hours before surgery | None |
| Andrews 2006 | 35% | 16% | No puff on day of surgery | None |
| Sørensen 2007 | 23% | 12% | Continuous abstinence at least 1 month before surgery | Saliva cotinine |
| Thomsen 2010 | 28% | 11% | Continuous abstinence from 2 days before to 10 days after surgery | CO |
| Lee 2013 | 14.3% | 3.6% | Continuous abstinence from smoking for at least 7 days before surgery combined with an exhaled CO ≤ 10ppm | CO |
| Shi 2013 | 79% | 75% | Self‐reported abstinence on the day of surgery | CO |
| Intensive intervention | ||||
| Lindström 2008 | 39% | 2% | Continuous abstinence from at least 3 weeks before surgery to 4 weeks after surgery | CO |
| Møller 2002 | 64% | 7.7% | Continuous abstinence for at least 4 weeks prior to surgery | Weekly CO |
| Ostroff 2013 | 45% | 45% | 24‐hour point prevalence abstinence at hospital admission for surgery | CO |
| Pharmacotherapy | ||||
| Wong 2012 | 29.6% | 20% | 7‐day point‐prevalence abstinence rate at admission to hospital | CO & urinary cotinine |
| Warner 2012 | 73% | 54% | Self‐reported abstinence on the morning of surgery | CO |
Ostroff 2013 provided both intervention and control participants with an intensive intervention, and was therefore not pooled with other behavioural interventions. This study found equally high cessation rates at surgery in both intervention and control participants (45% in both groups). There was no evidence of additional benefit from the computer‐based reduced smoking regimen given to intervention participants (RR 1.01; 95% CI 0.73 to 1.39; 184 participants. Analysis 2.1).
2.1. Analysis.
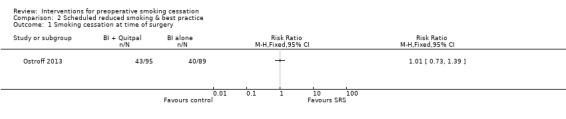
Comparison 2 Scheduled reduced smoking & best practice, Outcome 1 Smoking cessation at time of surgery.
Warner 2012 and Wong 2012 were considered separately as single studies of different pharmacotherapies. The effect of varenicline versus placebo on abstinence on the target quit day, the day before surgery, approached significance; Wong 2012, RR 1.49; 95% CI 0.98 to 2.26; 286 participants, Analysis 3.1), while the effect of nicotine lozenges on abstinence on the morning of surgery was non‐significant; Warner 2012 RR 1.34 (95% CI 0.86 to 2.10; 46 participants. Analysis 4.1)
3.1. Analysis.
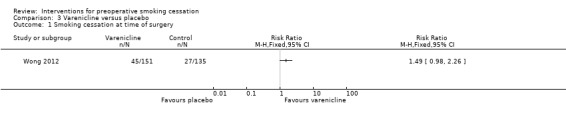
Comparison 3 Varenicline versus placebo, Outcome 1 Smoking cessation at time of surgery.
4.1. Analysis.
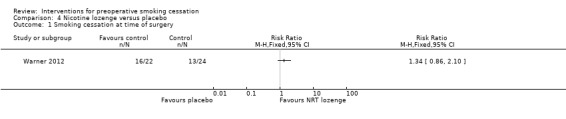
Comparison 4 Nicotine lozenge versus placebo, Outcome 1 Smoking cessation at time of surgery.
Cessation postoperatively at 12‐month follow up
Only five of the 13 studies monitored longer‐term postoperative cessation, i.e. smoking cessation at 12‐month follow‐up. The two trials of intensive interventions retained significantly higher quit rates in intervention versus control group participants; 23% versus 4% (Møller 2002), and 37% versus 17% (Lindström 2008). The pooled RR was 2.96; 95% CI 1.57 to 5.55 (209 participants) for intensive intervention (Analysis 1.2). Quit rates in the two studies using brief interventions decreased over time and significant differences between intervention and control groups were not maintained at 12 months; 20% versus 20% in Ratner 2004; 12% versus 8% in Thomsen 2010, pooled RR 1.09; 95% CI 0.68 to 1.75 (341 participants. Analysis 1.2).
1.2. Analysis.
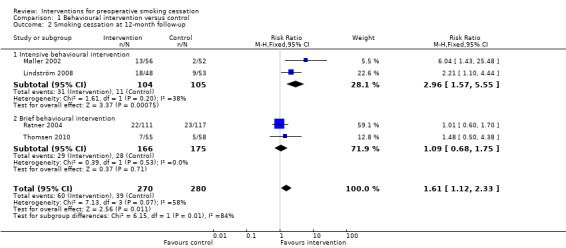
Comparison 1 Behavioural intervention versus control, Outcome 2 Smoking cessation at 12‐month follow‐up.
Sensitivity analyses excluding Ratner 2004 which had over 20% loss to follow‐up, or excluding two studies that did not biochemically evaluate smoking cessation at 12‐month follow‐up (Lindström 2008; Thomsen 2010), did not substantially affect the subgroup estimates, but left only a small amount of data.
Contrary to the non‐significant effect of varenicline at the time of surgery, Wong 2012 showed a significant increase in smoking cessation at 12 months; RR 1.45; 95% CI 1.01 to 2.07 (286 participants. Analysis 3.2).
3.2. Analysis.
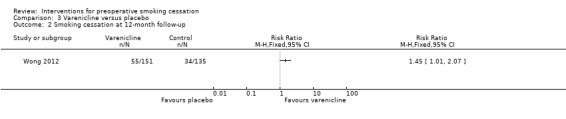
Comparison 3 Varenicline versus placebo, Outcome 2 Smoking cessation at 12‐month follow‐up.
Effect on postoperative morbidity and mortality
Any complications
Seven studies reported these outcomes. Two studies, both offering intensive preoperative smoking cessation interventions, found a reduced incidence of postoperative complications. In Møller 2002, 18% intervention versus 52% control participants developed any complication, (P = 0.0003). In Lindström 2008, the corresponding figures were 21% versus 41%, (P = 0.03). None of the four studies offering brief interventions (Sørensen 2003a; Sørensen 2007; Thomsen 2010; Lee 2013) detected significant differences between intervention and control participants in the incidence of postoperative complications. In Sørensen 2003a, 41% intervention versus 43% control participants developed any type of complication, in Thomsen 2010 61% intervention and 61% control participants, and in Lee 2013 13.1% intervention and 16.7% control participants. Lee 2013 monitored intra‐ and postoperative complications. Sørensen 2007 specifically monitored wound infections and did not detect any difference between the intervention and control groups. Pooling intensive and brief interventions separately, the RR for developing any complication was 0.42; 95% CI 0.27 to 0.65 (210 participants) using intensive interventions and 0.92; 95% CI 0.72 to 1.19 (493 participants) for brief interventions (Figure 4; Analysis 1.3). There was no evidence of statistical heterogeneity in either subgroup.
4.
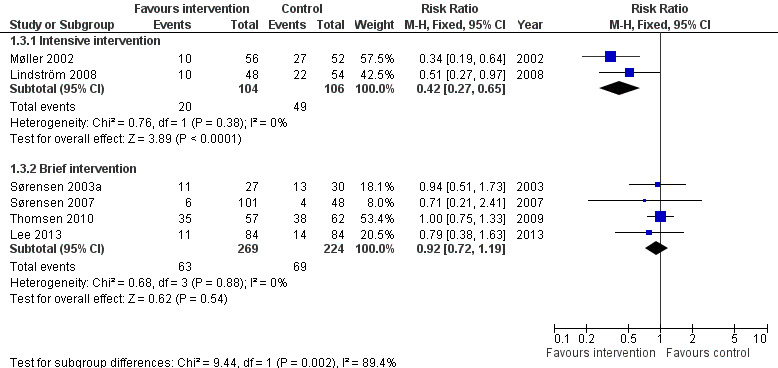
Behavioural intervention versus control: Postoperative morbidity: Any complication.
1.3. Analysis.
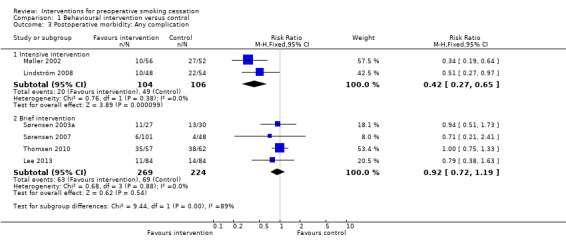
Comparison 1 Behavioural intervention versus control, Outcome 3 Postoperative morbidity: Any complication.
Wong 2012 found no effect of varenicline on postoperative complications; RR 0.94; 95% CI 0.52 to 1.72 (286 participants. Analysis 3.2).
Wound complications
Møller 2002 found a significantly reduced incidence of wound‐related complications in the intervention group (5% versus 31%, P = 0.001). Wound complications were divided into infections (positive culture and antibiotics prescribed), wound haematoma, and wound complication with subfascial involvement. Sørensen 2003a found non‐significant differences in wound‐related complications in 33% of the intervention group and 27% of the control group. In this study wound‐related complications were divided into the following subgroups: anastomotic leakage, fascial dehiscence, wound infection, necrotic stoma, haematoma. Sørensen 2007 monitored wound infections as a secondary outcome and found no significant difference between intervention and control groups in the incidence of these (6% versus 8%). Lindström 2008 likewise found no significant difference between intervention and control groups in the incidence of wound‐related complications (13% versus 26%, P = 0.13). In this study, wound‐related complications were divided into the following sub‐groups: haematoma, wound infection, seroma, other wound complication requiring intervention. Thomsen 2010 found identical incidences of wound‐related complications in intervention and control participants (44% versus 45%). Wound‐related complications were divided into the following subgroups: wound infection, haematoma, seroma, epidermolysis/necrosis requiring intervention.
Pooling studies according to intervention intensity, there was an effect of intensive interventions on wound complications: RR 0.31; 95% CI 0.16 to 0.62 (210 participants) but not for brief interventions: RR 0.99; 95% CI 0.70 to 1.40 (325 participants. Analysis 1.4).
1.4. Analysis.
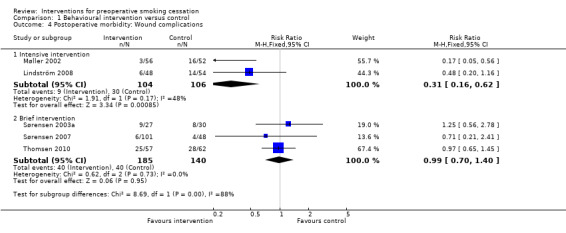
Comparison 1 Behavioural intervention versus control, Outcome 4 Postoperative morbidity: Wound complications.
Again, Wong 2012 did not detect any effect of varenicline on wound complications; RR 0.89; 95% CI 0.32 to 2.48 (286 participants. Analysis 3.4).
3.4. Analysis.
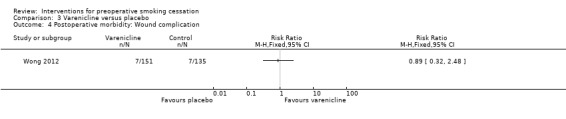
Comparison 3 Varenicline versus placebo, Outcome 4 Postoperative morbidity: Wound complication.
Other surgical outcomes
Secondary surgery was performed in 4% of the intervention group and in 15% of the control group participants in Møller 2002. In the intervention group, one participant had reposition of the prosthesis, and one had wound‐related secondary surgery. In the control group seven participants (13%) had wound‐related secondary surgery, and one had vascular‐related secondary surgery. Although it is evident that some participants in Sørensen 2003a had secondary surgery, no data on this are given in the paper. Sørensen 2007 and Lindström 2008 did not report data on secondary surgery. Thomsen 2010 found no difference between groups in the need for secondary surgery due to complication; one participant in the intervention group due to haematoma versus no participants in the control group.
Cardiopulmonary complications
No studies detected significant differences between groups in regard to postoperative pulmonary or cardiovascular complications. Møller 2002 found 2% intervention versus 2% control group participants suffering from respiratory insufficiency, and 0% versus 10% suffering from cardiovascular insufficiency, needing either ventilatory support or cardiological treatment. Sørensen 2003a found 11% with pulmonary complications in the intervention group versus 16% in the control group. No cardiac complications were recorded in this study. Lindström 2008 found 0% with pulmonary complications in the intervention group versus 2% in the control group, and 2% with cardiovascular complications in both the intervention and control groups. Thomsen 2010 found 30% with pulmonary complications in the intervention group versus 34% in the control group. Pulmonary complications were all minor, primarily desaturation requiring supplemental oxygen after transfer from the postoperative recovery room. Furthermore, Thomsen 2010 found 3% intervention participants with cardiovascular complications versus 2% control participants. Wong 2012 found no differences between groups in pulmonary complications (0% intervention versus 0.7% control) or in cardiovascular complications (3% intervention versus 1.3% control).
Intensive care admissions
Møller 2002 states the number of days spent in intensive care in the two groups as two days in the intervention group versus 32 days in the control group. The number of participants was not stated.
Length of stay
No studies detected significant differences in duration of hospital admission. Duration of hospital admission was 11 days (range 7 to 55) in the intervention group and 13 (range 8 to 65) in the control group in Møller 2002. Sørensen 2003a found that the median duration of hospital admission was 11 days in both groups (range 8 to 14). Lindström 2008 reported a median duration of hospital admission of one day (range 0 to 10) for the intervention group versus one day for the control group (range 0 to 11). The corresponding numbers in Thomsen 2010 were two days (range one to seven) in the intervention group versus three days (range one to eight) in the control group. Lee 2013 reported 1.75 days (Interquartile Range (IQR) 1.1 to 3.1) in intervention participants versus 2.1 (IQR 1.4 to 3.2) in control participants.
Mortality
There were two deaths in the control group during the perioperative period in Sørensen 2003a. In Ostroff 2013, one intervention participant and two control participants died postoperatively.
Adverse events
No studies reported serious adverse events. Wong 2012 found nausea to be the most common adverse event reported by participants; 13.3% among those receiving varenicline versus 3.7% among those receiving placebo (P = 0.004).
Discussion
Two questions need to be answered in order to investigate the possible prevention of smoking‐related postoperative complications.The first is whether preoperative smoking intervention, behavioural or pharmacological or a combination, reduces smoking by people before surgery. The second is whether successful preoperative smoking cessation reduces the incidence of postoperative complications. This review includes 12 studies addressing the first question, but only seven of them address the second. Of these, two trials offering intensive smoking cessation interventions (Møller 2002; Lindström 2008), one of which (Møller 2002) was conducted by two of the authors of this review, achieved a large change in smoking behaviour in the intervention group, and a lower incidence of complications. Among the remaining eight trials offering brief interventions, four had a modestly significant effect on smoking cessation at the time of surgery (Ratner 2004; Andrews 2006; Thomsen 2010; Lee 2013), and the pooled effect of brief interventions supported an effect on abstinence, but not on postoperative complications. Thomsen 2010 was also conducted by two of the authors of this review. Wong 2012 identified no effect of varenicline initiated one week prior to surgery on smoking cessation at surgery or on postoperative complications. In this study, participants were not asked to quit until the day before surgery. The effect on long‐term cessation was consistent with the results of trials in other populations (Cahill 2012). The difference in effects on complications between intensive and brief interventions may be due to both the intensity and the timing of the intervention. The smoking cessation intervention began six to eight weeks before scheduled surgery in Møller 2002, and in Lindström 2008 it began four weeks before surgery and continued for four weeks postoperatively. In Sørensen 2003a and Lee 2013, participants had access to counselling two to three weeks before surgery; in Sørensen 2007, a brief intervention was provided one month before surgery; and in Thomsen 2010 participants received a brief intervention shortly before surgery. This suggests that intensive counselling, and in parallel with this a longer period of preoperative varenicline are needed to support and sustain smoking cessation; furthermore, a longer period of abstinence may be required to achieve a reduction in some or all types of complication. Based on indirect comparisons, the effects of brief interventions are likely to be smaller than those of more intensive ones. Although we detected no significant effects on postoperative complications or long‐term cessation, the confidence intervals do not exclude small effects of brief interventions on these outcomes. The comparisons between intensity subgroups were initially exploratory, but the increased number of studies strengthens our confidence in a difference in effect attributable to timing and duration of support..
Pathophysiologically, smoking‐induced reduction in lung function may be significantly improved by six to eight weeks of smoking abstinence (Buist 1976). The smoking‐related impairment of immune function may likewise be reversed by six to eight weeks of abstinence (Beckers 1991; Akrawi 1997). These studies suggest that smoking cessation interventions are likely to be more beneficial when offered at least six weeks before surgery than in the immediate preoperative period, if possible. This complements the results of this review. Intensive intervention for four to eight weeks preoperatively, including provision of NRT, supported smoking cessation and reduced postoperative complications. Rigotti 2012 concluded similarly in a review of the effect of interventions on smoking cessation in hospitalized patients. Such interventions may, however, be difficult to achieve unless there is a partnership between surgical services and other branches of the health service, particularly primary care.
The interventions were tested in heterogeneous surgical populations which increases the external validity of the review. However, different surgical procedures and underlying pathologies may have diverse impacts on the incidence of postoperative complications and on motivation for and ability to stop smoking. This might have influenced the type of complications likely to occur as well as smoking cessation rates. Ostroff 2013, for example, found that participants with thoracic cancers were more likely to quit smoking than those with other cancer sites.
Overall, the studies were assessed to be at low risk of bias. However, assessment of postoperative complications may have been subject to intra‐ and interobserver variation (Bruce 2001). Self report of smoking cessation by participants without biochemical validation may similarly introduce a risk of performance and detection bias, given the lack of blinding (Higgins 2011). Differences between studies in definitions of postoperative complications and smoking cessation, specifically smoking cessation at the time of surgery, may be a potential source of heterogeneity affecting the strength of the conclusions that can be drawn. Interventions were furthermore primarily provided by research nurses or assistants specifically allocated to this task. This raises the question of whether intervention effects will persist when administered by staff within routine clinical settings.
The studies were conducted between 2002 and 2013. Within this time frame, attitudes to smoking have changed and many countries, and hence hospitals, have implemented restrictive smoking policies, including those from which the studies originate. This may have influenced control interventions in the more recent studies, with control participants receiving brief cessation advice and nicotine replacement therapy (NRT) ( Wolfenden 2005; Sørensen 2007; Thomsen 2010; Wong 2012; Warner 2012). This could potentially have rendered the relative additional effect of brief interventions smaller, thus making detection of any incremental benefit more difficult. In Ostroff 2013, quit rates were high in both groups, probably because both groups received intensive intervention, the only difference being the scheduled reduced smoking (SRS) in the intervention group. Detection of any incremental effect of the SRS was therefore likely to be difficult. Small sample sizes may further aggravate the detection of smaller but potentially clinically important, intervention effects. The small sample sizes and the relatively small number of studies contributing data to the meta‐analyses contribute to a judgement that the overall quality of evidence is moderate rather than high.
The validity of using composite outcomes to assess the effect of smoking intervention on postoperative morbidity is also debatable (Montori 2005). Two studies (Møller 2002; Lindström 2008) identified a significant effect on composite outcomes for postoperative complications and wound complications. When assessing intervention effects, careful consideration should be given to those complications comprising the composite outcomes that are of clinical significance and greatest importance to patients (Montori 2005).
The results support the view that interventions that help people to stop smoking in other settings also work for perioperative patients. These include measures to increase motivation and treat nicotine dependence, and intensive behavioural support (Rigotti 2012), NRT (Stead 2012) and varenicline (Cahill 2012).
Whether the perioperative period is a particularly suitable time for smoking interventions, however, warrants further investigation. Schwartz 1987 demonstrated that people may be more likely to comply with smoking cessation advice during the time of an acute illness. Ostroff 2013 found that participants with thoracic cancers were more likely to quit smoking than those with other cancer sites. Recently Shi 2010 reported an association between undergoing surgery and an increased likelihood for smoking cessation in older US citizens, with a particularly marked association in those undergoing major surgery. Participants reported that the possibility of reducing perceived vulnerability to postoperative complications promoted motivation to quit or reduce smoking prior to operation (Møller 2004; Thomsen 2009). Lindström 2010 found that participants randomized to the control intervention were disappointed with this allocation. On the other hand, some smokers found it more difficult to quit when facing the stress of an operation (Møller 2004; Thomsen 2009). Two studies recruited substantially fewer participants than planned (Sørensen 2003a; Lindström 2008), and Thomsen 2010 recruited only 51% of eligible patients. This may reflect a lack of motivation among some smokers to stop smoking in relation to surgery.
None of the studies included in this review reported serious adverse effects of preoperative smoking intervention, supporting the safety of short‐term preoperative smoking cessation. This is consistent with a recent meta‐analysis that found no adverse effects on surgical outcomes of stopping smoking shortly before surgery (Myers 2011). It has previously been claimed that recent quitters may suffer from pulmonary symptoms such as cough and sputum production.
Authors' conclusions
Implications for practice.
Intensive interventions initiated at least four weeks before surgery and including multiple contacts for behavioural support and the offer of pharmacotherapy are beneficial for changing smoking behaviour perioperatively and in the long term, and for reducing the incidence of complications. Brief interventions offered closer to the time of surgery are likely to have a small benefit on smoking behaviour, but have not been demonstrated to reduce complications. The current evidence supports giving smokers scheduled for surgery advice to quit and offering them effective interventions, including behavioural support and pharmacotherapy, at least four weeks ahead of surgery if possible.
Implications for research.
We need to establish the effect on postoperative morbidity and smoking cessation of interventions that are initiated immediately before or after surgery, for example subacute and acute surgery, and continued for at least eight weeks. The effect on postoperative complications of intensive interventions before higher morbidity surgical procedures, for example upper abdominal and thoracic surgery, also needs to be established. We also need to know how preoperative smoking intervention affects long‐term smoking abstinence rates, so future studies should include at least 12‐month follow‐up. In addition we need to evaluate the effect of different methods of smoking intervention, including other pharmacotherapies than NRT (Hughes 2014; Cahill 2012) in order to find the most effective way of supporting smoking cessation in people undergoing surgery. Varenicline may have potential as an intervention for smoking cessation in relation to surgery but more trials are needed in this population. Finally, the perspectives of smokers who decline to participate in perioperative smoking cessation trials warrant research.
What's new
| Date | Event | Description |
|---|---|---|
| 30 January 2014 | New citation required but conclusions have not changed | No major change to conclusions, stronger evidence about brief behavioural interventions, two new trials evaluating pharmacotherapy. |
| 30 January 2014 | New search has been performed | Searches updated; 5 new included studies. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 15 July 2010 | Amended | Minor edits including change to abstract; 5 trials (rather than 6) detected significantly increased smoking cessation at the time of surgery. |
| 18 May 2010 | New citation required and conclusions have changed | Updated for Issue 7, 2010 with 4 new trials and clearer evidence on short‐term outcomes. Change to authorship. |
| 3 September 2008 | Amended | Converted to new review format. |
| 17 May 2005 | New citation required and minor changes | Four new trials included. |
Acknowledgements
Thanks to Tom Pedersen who was an author on the first version of this review, and to the Managing Editor/Trial Search Co‐ordinator Lindsay Stead, Tobacco Addiction Group, for invaluable help with literature search.
Appendices
Appendix 1. Specialized Register search strategy
Register search strategy using Cochrane Register of Studies (CRS)
#1 (pre?operative or post?operative):TI,AB,MH,EMT,XKY,KY,KW #2 (surgery or operation? or operative or an?esthesia):TI,MH,EMT,KY,KW #3 (surgery or operation? or operative or an?esthesia):XKY #4 #1 or #2 OR #3
MH, EMT, KY & KW are keyword fields from electronic database records. XKY includes keywords assigned as part of internal indexing
Appendix 2. MEDLINE, EMBASE & CINAHL search strategies
These strategies were last run in April 2010
MEDLINE STRATEGY (via OVIDSP)
1. RANDOMIZED‐CONTROLLED‐TRIAL.pt. 2. CONTROLLED‐CLINICAL‐TRIAL.pt. 3. CLINICAL‐TRIAL.pt. 4. exp Clinical Trial/ 5. Random‐Allocation/ 6. randomized‐controlled trials/ 7. smoking cessation.mp. or exp Smoking Cessation/ 8. "Tobacco‐Use‐Cessation"/ 9. "Tobacco‐Use‐Disorder"/ 10. exp Smoking/pc, th [Prevention & Control, Therapy] 11. (surgery or operation or operativ: or an?esthesia).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 12. exp Postoperative complication/ 13. exp Preoperative care/ 14. exp Patient education/ 15. 12 and (13 or 14) 16. 11 or 15 [topic related terms] 17. 1 or 2 or 3 or 4 or 5 or 6 [design terms] 18. 8 or 7 or 9 or 10 [smoking terms] 19. 16 and 17 and 18
EMBASE STRATEGY (via OVIDSP)
1. smoking cessation.mp. or Smoking Cessation/ 2. smoking/ 3. ((smok* or tobacco or cigar*) adj3 (stop* or quit* or giv* or refrain* or reduc*)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 4. 1 or (2 and 3) 5. (surgery or surgical or operation or operativ* or preoperativ* or an?esthesia).ti,an,de. 6. 4 and 5
CINAHL STRATEGY
1. "Smoking‐Cessation" OR "Smoking‐Cessation‐Programs" OR "Smoking"/ prevention‐and‐control OR (smoking cessation) OR ((smok* or tobacco or cigar*) near (stop* or quit*)) 2. surgery or operation or operativ* or an?esthesia 3. #1 AND #2
Data and analyses
Comparison 1. Behavioural intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at time of surgery | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intensive behavioural intervention | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.76 [4.55, 25.46] |
| 1.2 Brief behavioural intervention | 7 | 1141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.16, 1.46] |
| 2 Smoking cessation at 12‐month follow‐up | 4 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.12, 2.33] |
| 2.1 Intensive behavioural intervention | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [1.57, 5.55] |
| 2.2 Brief behavioural intervention | 2 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.68, 1.75] |
| 3 Postoperative morbidity: Any complication | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intensive intervention | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.27, 0.65] |
| 3.2 Brief intervention | 4 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.19] |
| 4 Postoperative morbidity: Wound complications | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Intensive intervention | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.16, 0.62] |
| 4.2 Brief intervention | 3 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.70, 1.40] |
Comparison 2. Scheduled reduced smoking & best practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at time of surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Varenicline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at time of surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Smoking cessation at 12‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Postoperative morbidity: Any complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Postoperative morbidity: Wound complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.3. Analysis.
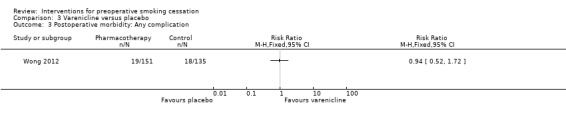
Comparison 3 Varenicline versus placebo, Outcome 3 Postoperative morbidity: Any complication.
Comparison 4. Nicotine lozenge versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at time of surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrews 2006.
| Methods | Country: United Kingdom Randomized controlled trial | |
| Participants | 102 smoking participants (51 intervention, 51 control) routinely attending the preoperative ward 4 weeks before surgery. The types of surgery were not specified. | |
| Interventions | Intervention: In addition to booklet and nurse advice given to all participants when they are routinely seen 4 weeks prior to surgery, intervention group participants received a letter from the participant's consultant stating that stopping smoking 1 ‐ 2 weeks before surgery has huge benefits. Participants were furthermore provided with contact details for Stop Smoking Service. Control: Booklet and nurse advice. | |
| Outcomes | Self‐reported abstinence from smoking, defined as not smoking a single puff on the day of surgery. No biochemical conformation of smoking status. | |
| Notes | Smoking cessation defined as point prevalence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Numbers drawn from opaque bag |
| Allocation concealment (selection bias) | Low risk | "Each patient agreeing to participate on the pre‐operative ward that day was numbered sequentially. The corresponding numbers were put in an opaque bag and the first number drawn out was assigned to intervention status." Although this system is open to manipulation we did not judge the risk of bias to be high in this study. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of participants or personnel, assessors of smoking status not stated as blinded, |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All intervention group participants completed, 1/51 missing from control group |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Low risk | |
Lee 2013.
| Methods | Country: Canada Randomized controlled trial |
|
| Participants | 168 daily smokers (84 intervention/84 control) scheduled for elective surgery and having a preadmission clinic appointment at least 3 weeks before their surgical date. The primary types of surgery were general surgery, gynaecologic, urologic, ophthalmologic, otolaryngologic and orthopaedic. | |
| Interventions | Intervention: 5‐minute counselling by a trained preadmission nurse, brochures on smoking cessation, referral to the Canadian Cancer Society's Smokers' Helpline. The Smoker's Helpline initiated contact with participants (up to 4 attempts) and subsequent counselling was agreed on with the participant, generally aiming at having at least 4 contacts with each person. 6‐week supply of free transdermal nicotine replacement. Control: Standard care implying inconsistent smoking cessation advice from nurses, surgeons or anaesthesiologists. |
|
| Outcomes | Self‐reported abstinence from smoking for at least 7 days before surgery combined with an exhaled CO ≤ 10 ppm. Inaccurately reported preoperative smoking cessation (self‐reported 7‐day abstinence with exhaled CO > 10 ppm). A composite of all intraoperative and immediate postoperative complications (those occurring in PACU) Total duration of care in PACU and time to PACU discharge readiness. Unanticipated hospital admission (participants scheduled for day surgery and subsequently admitted to hospital). Hospital length of stay for inpatients. Self‐reported smoking cessation for 7 days before the 30‐day postoperative phone call. Self‐reported smoking reduction (by ≥ 50% of baseline) at the 30‐day postoperative phone call. |
|
| Notes | Smoking cessation only as 7‐day point prevalence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded outcome assessment. Participants not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 intervention and 6 control participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | |
Lindström 2008.
| Methods | Country: Sweden Randomized controlled trial | |
| Participants | 117 daily smokers undergoing elective surgery for primary hernia repair, laparoscopic cholecystectomy and hip or knee prosthesis. | |
| Interventions | Intervention: weekly sessions, face‐to‐face or by telephone, with a trained smoking cessation counsellor and NRT 4 weeks pre‐ and 4 weeks postoperatively. Control: Standard care. | |
| Outcomes | Smoking cessation from 3 weeks before to 4 weeks after surgery, and at 1 year (not validated). Postoperative complications requiring intervention within 30 days postoperatively. Wound complications requiring intervention within 30 days postoperatively. |
|
| Notes | Smoking cessation was validated by CO in exhaled air. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomization. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcomes assessed by study nurses who were not blinded and by the study physicians who were unaware of group allocation. No blinding of participants or personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to 30‐day follow‐up. 7/48 intervention and 3/54 controls lost to 12‐month follow‐up. |
| Selective reporting (reporting bias) | Low risk | Reports all prespecified outcomes. |
| Other bias | Low risk | |
Møller 2002.
| Methods | Country: Denmark Randomized controlled trial | |
| Participants | 120 daily smokers (60 intervention, 60 control) who underwent elective hip or knee replacement surgery. | |
| Interventions | Intervention: Weekly meetings initiated 6 ‐ 8 weeks prior to surgery. Personalized nicotine substitution schedule. Participants were strongly encouraged to stop smoking but also had the option to reduce tobacco consumption by at least 50%. Advice about smoking cessation/reduction, benefits, side effects, how to manage withdrawal symptoms, and how to keep weight gain to a minimum. Participants could also discuss other issues related to smoking intervention or hospitalization. The intervention was provided by a research nurse trained as a smoking cessation counsellor. Control: Standard care, which was little or no information about the risks of tobacco smoking or smoking cessation counselling. | |
| Outcomes | Smoking cessation before surgery, 4 weeks after surgery and 1 year after surgery. Outcome assessor blinded. Long‐term smoking cessation was validated in those who participated in focus group interviews by measurements of CO in exhaled air. | |
| Notes | Randomized participants who did not have surgery are not included in denominators; Long‐term cessation is reported in Villebro 2008 and included in the text and analyses under this study identifier. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomization. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded outcome assessment. No blinding of participants or personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/60 intervention participants and 8/60 control participants missing due to cancellation of surgery; 0/56 intervention and 11/52 controls were missing at 1‐year follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Unclear risk | Reports all prespecified outcomes. |
Ostroff 2013.
| Methods | Country: USA Randomized controlled trial |
|
| Participants | 185 smokers (minimum 8 CPD within the past week) with newly diagnosed cancer who were scheduled for hospitalization and surgical resection no less than 7 days from study entry, with sufficient visual acuity and manual dexterity to use a handheld computer. Cancer sites included thoracic, head and neck, breast, gynaecology, urology, other. | |
| Interventions | Intervention: Best Practice (BP) and Scheduled Reduced Smoking (SRS).
BP = 5 individual counselling sessions with trained smoking cessation counsellors and nicotine replacement therapy at no cost. 2 sessions prior to surgery, 1 during hospitalization, 2 sessions during the month after hospital discharge. Apart from the 3rd session during hospitalization, counselling was provided by telephone.
SRS = individually tailored presurgical gradual tapering regimen prompted by QuitPal. A quit date was planned at least 24 hours prior to hospital admission. Control: BP as described above |
|
| Outcomes | Abstinence at hospital admission verified by exhaled CO. Also reported; Primary: 7‐day point prevalence abstinence at 6 months post‐hospitalization verified by saliva cotinine. Secondary: abstinence at 3 months post‐hospitalization verified by saliva cotinine, cigarettes smoked per day at the same follow‐up times. |
|
| Notes | All participants received intensive intervention so not pooled with other trials. Test of tailored tapering. Requirement for sufficient visual acuity and manual dexterity to use a handheld computer may reduce generalisability of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized permuted‐block randomization stratified by daily cigarette consumption (≥ 20 CPD vs < 20 CPD). |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and researchers not blinded but self‐reported abstinence biochemically validated at all follow‐ups. Missing biochemical validation resulted in categorization as not abstinent. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis, Equal attrition in groups (15% intervention/16% control). |
| Selective reporting (reporting bias) | Low risk | Reports all prespecified outcomes. |
| Other bias | Low risk | |
Ratner 2004.
| Methods | Country: Canada Randomized controlled trial | |
| Participants | 237 participants attending a presurgical assessment clinic 1 ‐ 3 weeks prior to cardiovascular, ophthalmologic, plastic and urologic surgery. | |
| Interventions | Intervention: One 15‐minute face‐to‐face counselling from a trained study nurse 1 ‐ 3 weeks before surgery and written materials, nicotine gum, quit kit, hotline number. Postoperative counselling in hospital and via telephone. Control: usual care. | |
| Outcomes | Smoking cessation (abstinence for at least 24 hours before surgery, 6 months, 12 months). Validated by CO (face‐to‐face) or urine cotinine. Postoperative complications not assessed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with computer‐generated random allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded assessment of smoking status. No blinding of participants, not stated whether personnel were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24/117 intervention participants and 11/120 control participants missing at 6‐month follow‐up. 36/117 and 32/120 control participants missing at 12‐month follow‐up. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes as prespecified in the article are reported. |
| Other bias | Unclear risk | 50% of participants returned nicometer strips at 6‐month follow‐up, 45% returned nicometer strips at 12‐month follow‐up. |
Shi 2013.
| Methods | Country: USA Randomized controlled trial |
|
| Participants | 169 participants (82 intervention/87 control) attending a preoperative evaluation centre who were current smokers with > 100 cigarettes lifetime consumption and self report of smoking daily or sometimes. | |
| Interventions | Intervention: Brief advice and monitoring of exhaled CO on the morning of surgery. Control: Brief advice. |
|
| Outcomes | CO levels, intent to quit, time from last cigarette, smoking on the morning of surgery. | |
| Notes | Sample size determined on the basis of CO level, no flow chart. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomization, not stated who generated the randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants not blinded, clinical personnel not blinded (clinical personnel measured CO). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes monitored for 80 intervention/84 control participants. |
| Selective reporting (reporting bias) | Low risk | Reports all prespecified outcomes. |
| Other bias | Unclear risk | Not stated whether there were baseline differences between intervention and control group participants. |
Sørensen 2003a.
| Methods | Country: Denmark Randomized controlled trial | |
| Participants | 60 participants awaiting colorectal surgery. 57 completed, 3 withdrew from intervention group. | |
| Interventions | Intervention: Initiated 15 days (inter quartile range 8 ‐ 24) before surgery from a research nurse. 1 telephone support call + 1 additional support session + telephone number to research nurses during normal working hours. NRT available up to 24 hours before surgery. Control: told to continue smoking. | |
| Outcomes | Smoking cessation defined as abstaining or reduction by more than half of daily tobacco smoking on day before surgery, at suture removal, validated by CO and cotinine. Postoperative complications up to 30 days requiring medical or surgical intervention. | |
| Notes | The authors did not distinguish between smoking cessation and reduction, so smoking cessation outcomes are not included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized, envelopes in blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | Sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Primary outcome assessed by blinded assessor. No blinding of participants or personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/30 intervention participants and 0/30 control participants missing at 30 days follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Low risk | |
Sørensen 2007.
| Methods | Country: Denmark Randomized controlled trial with a supplemental non‐randomized consecutive group of smokers | |
| Participants | 244 participants: 180 who were daily smokers and scheduled for elective open incisional or inguinal day‐case herniotomy randomized to one of 2 interventions or control; 64 consecutive, non‐randomized control group participants who were daily smokers and who underwent inguinal or incisional herniotomy without advice to quit smoking. 46 of these participants were recruited prior to the trial period, and 18 were recruited 3 months after the trial period. | |
| Interventions | Interventions: 1) Standard advice + 1 telephone reminder to stop smoking (10‐minute conversation with a study nurse) 1 month before surgery. 2) Standard advice + 1 reminder to stop smoking in the outpatient clinic 1 month before surgery (face‐to‐face counselling for 20 minutes with a study nurse) including NRT until 24 hours before surgery. Control: Standard advice to stop smoking at least 1 month before surgery and until removal of skin sutures 10 days after surgery. Non‐randomized, consecutive historical control received no advice to stop smoking (data from this group are not included in the review). | |
| Outcomes | Self‐reported smoking on day of surgery, day of skin suture removal and at 3‐month follow‐up validated by CO in expired air at all contacts with the study nurse, sputum cotinine on the day of surgery. Postoperative complications defined as swollen, red, hot, painful wound with or without pus discharge and postoperative clinical intervention including antibiotics, extensive wound care or re‐operation. Linear analogue self‐assesment scale (LASA‐scale) to assess participants' motivation for smoking cessation. | |
| Notes | The 64 consecutive, non‐randomized control group participants are not included in the meta‐analyses. Participants receiving standard advice to stop smoking are included as a control group. Participants receiving interventions 1 and 2 are pooled and included as an intervention group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, consecutively arranged envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Self‐reported smoking status validated by CO/cotinine measurements administered by the study nurse. Postoperative wound infection initially assessed by the study nurse and in the event of clinical signs of infection referred for further blinded assessment; no blinding of participants or personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/120 intervention participants and 12/60 intervention participants missing at 3‐month follow‐up. Reasons for withdrawal reported but not related to the specific interventions. Drop‐out analysis showed no significant difference between those who dropped out or had their surgery cancelled and the included participants ‐ these data are not shown. |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Low risk | |
Thomsen 2010.
| Methods | Country: Denmark Randomized controlled trial |
|
| Participants | 130 women who were smokers and scheduled for elective breast cancer surgery, (65 randomized to intervention and 65 to control). | |
| Interventions | Intervention: Brief one‐time preoperative smoking cessation counselling 3 ‐ 7 days preoperatively with a trained smoking cessation counsellor supplemented by free NRT. Control: Standard preoperative information and care entailing no or inconsistent advice about the risks of smoking in relation to surgery. |
|
| Outcomes | Primary outcome: death or postoperative morbidity requiring treatment within 30 days after surgery (including seroma requiring aspiration). Secondary outcomes: self‐reported smoking cessation from 2 days before to 10 days after surgery, long‐term smoking cessation defined as smoking cessation from 2 days before to 12 months after surgery, length of hospital stay (LOS), need for secondary surgery, readmission to hospital within 30 days postoperatively due to complication of primary surgery. |
|
| Notes | Smoking cessation from 2 days before to 10 days after surgery was biochemically validated using exhaled CO; long‐term smoking cessation was not biochemically validated. First included in 2012 update as Thomsen 2009, based on PhD thesis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence generated by a research secretary with no other involvement in the study. |
| Allocation concealment (selection bias) | Low risk | Stratified block randomization using sealed, opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. 58/65 control participants and 55/65 intervention participants completed the trial. Numbers and reasons for drop‐out did not differ across interventions. |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Unclear risk | 129/310 eligible participants declined to participate in the study. Long‐term smoking cessation was not biochemically validated. |
Warner 2012.
| Methods | Country: USA Double‐blind randomized controlled trial |
|
| Participants | 46 participants (22 intervention/24 control) who were daily smokers evaluated at the Mayo Clinic Rochester PreOperative Evaluation Center in preparation for elective surgery. | |
| Interventions | Intervention: 2‐minute intervention advising smoking cessation from 7 pm the night before surgery, benefits of smoking cessation and encouragement to use lozenges when they usually smoked, including the morning before surgery. Active nicotine lozenges according to nicotine dependence (4 mg or 2 mg lozenges) ‐ all intervention participants received 16 lozenges to cover at least the time from 7pm the night before surgery to the time of admission to the surgical facility. Control: 2‐minute intervention advising smoking cessation from 7 pm the night before surgery, benefits of smoking cessation and encouragement to use lozenges when they usually smoked, including the morning before surgery. Placebo lozenges. |
|
| Outcomes | Self‐reported abstinence the morning of surgery (outcome used in review) and postoperative day 8. Also reported: expired CO in the preoperative holding area. Time to last cigarette. |
|
| Notes | No sample size calculation. No flow chart ‐ therefore no information on eligible versus included participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated stratified block randomization. |
| Allocation concealment (selection bias) | Low risk | Packets according to strata specific subject ID numbers. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs. |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Unclear risk | No sample size calculation. No flow chart ‐ therefore no information on eligible versus included participants. |
Wolfenden 2005.
| Methods | Country: Australia Randomized controlled trial | |
| Participants | 210 participants awaiting surgery, 197 included in preoperative assessment. | |
| Interventions | Intervention: 1 ‐ 2 weeks before surgery 1 interactive counselling session lasting 17 minutes via computer, one telephone counselling, and nursing and anaesthetic staff were prompted via computer to provide intervention participants with brief advice. NRT in the dependent group (> 10 CPD). Control: staff could provide advice and NRT at their discretion. |
|
| Outcomes | Smoking cessation, for > 24 hours before admission, at 3‐month follow‐up. No validation. Outcomes reported to a blinded assessor at 3 months. Postoperative complications not assessed. | |
| Notes | 13 randomized participants who did not have surgery are not included in denominators for perioperative abstinence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Web‐based randomization. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Primary and secondary outcomes assessed by blinded assessor. Personnel and participants not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16/124 intervention and 10/86 control group participants missing at 3‐month follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Low risk | |
Wong 2012.
| Methods | Country: Canada Double‐blind, randomized, placebo‐controlled trial |
|
| Participants | 286 participants who were smokers and scheduled for elective ambulatory or inpatient general surgical, orthopaedic, urologic, plastic, gynaecologic, ophthalmologic or neurosurgical procedures (151 varenicline /135 placebo) | |
| Interventions | Intervention: varenicline initiated 1 week before the target quit date (24 hours before surgery) and continued for a total of 12 weeks, including a 1‐week titration as follows: days 1 ‐ 3: 0.5 mg once daily; days 4 ‐ 7: 0.5 mg twice daily; and days 8 ‐ 12 weeks: 1 mg twice daily. Control: Placebo following the schedule described for varenicline above. Both intervention and control participants received 2 15‐minute standardized counselling sessions by trained research co‐ordinators. The first counselling session occurred in the preoperative clinic, the second before discharge for ambulatory participants or 24 hours after surgery for inpatients. |
|
| Outcomes | Abstinence on target quit day/at admission to hospital. 7‐day point‐prevalence abstinence rate at 12 months after start of treatment (Primary outcome for study). Also reported: 7‐day point‐prevalence abstinence rate at 3 and 6 months after the target quit date, self‐reported changes in the number of CPD, stage of change at 3, 6 and 12 months. |
|
| Notes | Perioperative complications and adverse events were recorded, as documented in hospital charts. Smoking abstinence was biochemically validated using exhaled CO and urinary cotinine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list. |
| Allocation concealment (selection bias) | Unclear risk | Stratified block randomization in blocks of 40. Participant assignments were placed in sequentially‐numbered opaque, sealed envelopes and kept at each centre by an independent research pharmacist who was not involved in participant care or outcome assessments. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, healthcare personnel and research staff were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Numbers and reasons for drop‐out similar across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes as prespecified in the article are reported. |
| Other bias | Unclear risk | 666/965 eligible participants declined to participate in the study. Perioperative complications were recorded; however the authors do not present a definition of perioperative complications. |
CO: carbon monoxide CPD: cigarettes per day NRT: nicotine replacement therapy PACU: perioperative anaesthetic care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrishami 2010 | Outcomes not relevant (prevalence of smoking and readiness for smoking cessation). |
| Basler 1981 | Participant allocation not randomized. |
| Griebel 1998 | Intervention takes place during the course of postoperative recovery. |
| Haddock 1997 | Quasi‐experimental design. |
| McHugh 2001 | Not all participants allocated to treatment and control group were smokers. The intervention was directed not only at smoking habits, but also drinking habits, obesity, physical activity etc. |
| Moore 2005 | Not a randomized trial. Compared perioperative complication rates between nonsmokers and smokers who received a perisurgical cessation programme using a prospective cohort design. |
| Munday 1993 | Participant allocation not randomized. |
| Myles 2004 | Outcome assessment not immediately prior to surgery. Change of protocol within the study. |
| Nåsell 2010 | The intervention was not preoperative but initiated after surgery. |
| Rissel 2000 | Historical controls. |
| Sachs 2012 | Not a randomized controlled trial. |
| Simon 1997 | Intervention postoperative, not preoperative. |
| Steinemann 2005 | Intervention was training of surgical residents. No participant‐related outcomes assessed. |
| Sørensen 2003b | Not a clinical trial ‐ experimental test of surgical procedures on volunteers. A secondary reference, Yang 2003, is a commentary on this trial |
| Warner 2005 | Intervention consisted of a nicotine patch applied immediately prior to surgery with no additional counselling or support. |
| Warner 2011 | Primary outcome quitline use. |
| Wewers 1994 | Intervention postoperative, not preoperative. |
Differences between protocol and review
In updating the review in 2010, we introduced subgroup analyses of intervention effects on smoking cessation and postoperative complications according to the intensity of interventions. In the 2014 update we introduced a subgroup for varenicline, nicotine lozenges and intensive intervention + scheduled reduced smoking (SRS). Additionally, we have used sensitivity analyses to assess the impact of studies with high drop‐out rates and lacking biochemical evaluation of self‐reported smoking cessation. Earlier versions of this review reported effects as odds ratios. The Tobacco Addiction Group now recommends the use of risk ratios as being easier to interpret.
Contributions of authors
In the updated version Lindsay Stead did the searches and prescreened the results and AMM, NV and TT evaluated the identified studies. AMM, NV and TT extracted data and wrote the review.
In the previous version NV and AMM did searches, scanned the results for relevant studies and evaluated the studies found. AMM and NV did data extraction and wrote the review. Tom Pedersen was an author of the first version.
Declarations of interest
The authors of the review are also authors of two of the included trials (Møller 2002; Thomsen 2010).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andrews 2006 {published data only}
- Andrews K, Bale P, Chu J, Cramer A, Aveyard P. A randomized controlled trial to assess the effectiveness of a letter from a consultant surgeon in causing patients to stop smoking pre‐operatively. Public Health 2006;120(4):356‐8. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee SM, Landry J, Jones PM, Buhrmann O, Morley‐Forster P. The effectiveness of a periooperative smoking cessation program: A randomized clinical trial. Anesthesia & Analgesia 2013;117(3):605‐13. [DOI: 10.1213/ANE.0b013e318298a6b0] [DOI] [PubMed] [Google Scholar]
Lindström 2008 {published data only}
- Lindström D, Azodi OS, Wladis A, Tønnesen H, Linder S, Nåsell H, et al. Effects of a perioperative smoking cessation intervention on postoperative complications. Annals of Surgery 2008;248(5):739‐45. [DOI] [PubMed] [Google Scholar]
- Sadr Azodi O, Lindström D, Adami J, Tønnesen H, Nåsell H, Gilljam H, et al. The efficacy of a smoking cessation programme in patients undergoing elective surgery: a randomised clinical trial. Anaesthesia 2009;64(3):259‐65. [DOI] [PubMed] [Google Scholar]
Møller 2002 {published data only}
- Møller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: A randomised clinical trial. Lancet 2002;359(9301):114‐7. [DOI] [PubMed] [Google Scholar]
- Villebro NM, Pedersen T, Møller AM, Tonnesen H. Long‐term effects of a preoperative smoking cessation programme. Clinical Respiratory Journal 2008;2(3):175‐82. [DOI] [PubMed] [Google Scholar]
Ostroff 2013 {published data only}
- Ostroff J, Burkhalter J, Cinciripini P, Li Y, Shiyko M, Lam C, et al. Randomized trial of a pre‐surgical, scheduled reduced smoking intervention for patients newly diagnosed with cancer. Psycho‐oncology 2013;22(Suppl 2):74‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff JS, Burkhalter JE, Cinciripini PM, Li Y, Shiyko PM, Lam CY, et al. Randomized trial of a presurgical scheduled reduced smoking intervention for patients newly diagnosed with cancer. Health Psychology 2013; Vol. Jul 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Ratner 2004 {published data only}
- Bottorff JL, Johnson JL, Moffat B, Fofonoff D, Budz B, Groening M. Synchronizing clinician engagement and client motivation in telephone counseling. Qualitative Health Research 2004;14(4):462‐77. [DOI] [PubMed] [Google Scholar]
- Ratner PA, Johnson JL, Richardson CG, Bottorff JL, Moffat B, Mackay M, et al. Efficacy of a smoking‐cessation intervention for elective‐surgical patients. Research in Nursing and Health 2004;27(3):148‐61. [DOI] [PubMed] [Google Scholar]
Shi 2013 {published data only}
- Shi Y, Ehlers S, Hinds R, Baumgartner A, Warner DO. Monitoring of exhaled carbon monoxide to promote preoperative smoking abstinence. Health Psychology 2013;32(6):714‐7. [DOI: 10.1037/a0029504] [DOI] [PubMed] [Google Scholar]
- Warner DO, Shi Y, Ehlers SL, Baumgartner A, Hinds RF. Monitoring of exhaled carbon monoxide to promote pre‐operative smoking abstinence (POS3‐111). Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13 ‐ 16 Houston, Texas. 2012:122.
Sørensen 2003a {published data only}
- Sørensen LT, Jørgensen T. Short‐term preoperative smoking cessation intervention does not affect postoperative complications in colorectal surgery: A randomised clinical trial. Colorectal Disease 2003;5(4):347‐52. [DOI] [PubMed] [Google Scholar]
Sørensen 2007 {published data only}
- Sørensen LT, Hemmingesen U, Jørgensen T. Strategies of smoking cessation intervention before hernia surgery ‐ effect on perioperative smoking behaviour. Hernia 2007;11(4):327‐33. [DOI] [PubMed] [Google Scholar]
Thomsen 2010 {published data only}
- Thomsen T. Effect of a brief preoperative smoking cessation intervention shortly before breast cancer surgery on postoperative complications and smoking cessation: a randomized controlled trial.. PhD Thesis 2009.
- Thomsen T, Tønnesen H, Okholm M, Kroman N, Maibom A, Sauerberg ML, et al. Brief smoking cessation intervention in relation to breast cancer surgery: a randomized controlled trial. Nicotine & Tobacco Research 2010;12(11):1118‐24. [DOI] [PubMed] [Google Scholar]
Warner 2012 {published data only}
- Warner DO, Kadimpati S. Nicotine lozenges to promote brief preoperative abstinence from smoking: pilot study. Clinical Health Promotion 2012;2(3):85‐8. [Google Scholar]
Wolfenden 2005 {published data only}
- Wolfenden L, Wiggers J, Knight J, Campbell E, Rissel C, Kerridge R, et al. A programme for reducing smoking in pre‐operative surgical patients: randomised controlled trial. Anaesthesia 2005;60(2):172‐9. [DOI] [PubMed] [Google Scholar]
Wong 2012 {published data only}
- Wong J, Abrishami A, Yang Y, Zaki A, Friedman Z, Selby P, et al. A perioperative smoking cessation intervention with varenicline. A double‐blind, randomized placebo‐controlled trial. Anesthesiology 2012;117(4):755‐64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrishami 2010 {published data only}
- Abrishami A. Prevalence of smoking and stage of readiness for smoking cessation in surgical patients. Canadian Journal of Anesthesia 2010;57(S1):S44‐S45. [Google Scholar]
Basler 1981 {published data only}
- Basler HD, Wilcke I. The change of smoking habits of vaso operated patients [Die Veränderung der Rauchgewohnheiten gefassoperierter Raucher]. Medizinische Psychologie 1981;7:27‐43. [Google Scholar]
Griebel 1998 {published data only}
- Griebel B, Wewers ME, Baker CA. The effectiveness of a nurse‐managed minimal smoking cessation intervention among hospitalized patients with cancer. Oncology Nursing Forum 1998;25(5):897‐902. [PubMed] [Google Scholar]
Haddock 1997 {published data only}
- Haddock J, Burrows C. The role of the nurse in health promotion: an evaluation of a smoking cessation programme in surgical pre‐admission clinics. Journal of Advanced Nursing 1997;26(6):1098‐110. [PubMed] [Google Scholar]
McHugh 2001 {published data only}
- McHugh F, Lindsay GM, Hanlon P, Hutton I, Brown MR, Morrison C, et al. Nurse led shared care for patients on the waiting list for coronary artery bypass surgery: a randomised controlled trial. Heart 2001;86(3):317‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2005 {published data only}
- Moore SM, Mills BB, Moore RD, Miklos JR, Mattox TF. Perisurgical smoking cessation and reduction of postoperative complications. American Journal of Obstetrics and Gynecology 2005;192(5):1718‐21. [DOI] [PubMed] [Google Scholar]
Munday 1993 {published data only}
- Munday IT, Desai PM, Marshall CA, Jones RM, Phillips ML, Rosen M. The effectiveness of pre‐operative advice to stop smoking: a prospective controlled trial. Anaesthesia 1993;48(9):816‐8. [DOI] [PubMed] [Google Scholar]
Myles 2004 {published data only}
- Myles PS, Leslie K, Angliss M, Mezzavia P, Lee L. Effectiveness of bupropion as an aid to stopping smoking before elective surgery: A randomised controlled trial. Anaesthesia 2004;59(11):1053‐8. [DOI] [PubMed] [Google Scholar]
Nåsell 2010 {published data only}
- Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial.. Journal of Bone and Joint Surgery. American volume 2010;92(6):1335‐42. [DOI: 10.2106/JBJS.I.00627] [DOI] [PubMed] [Google Scholar]
Rissel 2000 {published data only}
- Rissel C, Salmon A, Hughes AM. Evaluation of a (pilot) stage tailored brief smoking cessation intervention among hospital patients presenting to a hospital preadmission clinic. Australian Health Review 2000;23(3):83‐93. [DOI] [PubMed] [Google Scholar]
Sachs 2012 {published data only}
- Sachs R, Wild C, Thomas L, Hammal F, Finegan BA. Smoking cessation interventions in the pre‐admission clinic: assessing two approaches. Canadian Journal of Anesthesia 2012;59(7):662‐9. [DOI: 10.1007/s12630-012-9716-6] [DOI] [PubMed] [Google Scholar]
Simon 1997 {published data only}
- Simon JA, Solkowitz SN, Carmody TP, Browner WS. Smoking cessation after surgery ‐ A randomized trial. Archives of Internal Medicine 1997;157:1371‐6. [PubMed] [Google Scholar]
Steinemann 2005 {published data only}
- Steinemann S, Roytman T, Chang J, Holzman J, Hishinuma E, Nagoshi M, et al. Impact of education on smoking cessation counseling by surgical residents. American Journal of Surgery 2005;189:44‐6. [DOI] [PubMed] [Google Scholar]
Sørensen 2003b {published data only}
- Sørensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Annals of Surgery 2003;238(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GP, Longaker MT. Abstinence from smoking reduces incisional wound infection: a randomised controlled trial. Annals of Surgery 2003;238(1):6‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warner 2005 {published data only}
- Warner DO, Patten CA, Ames SC, Offord KP, Schroeder DR. Effect of nicotine replacement therapy on stress and smoking behaviour in surgical patients. Anesthesiology 2005;102(6):1138‐46. [DOI] [PubMed] [Google Scholar]
Warner 2011 {published data only}
- Warner DO, Klesges RC, Dale LC, Offord KP, Schroeder DR, Shi Y, Vickers KS, Danielson DR. Clinician‐delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology 2011;114(4):847‐855. [DOI] [PubMed] [Google Scholar]
Wewers 1994 {published data only}
- Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse‐delivered smoking cessation intervention among hospitalized patients ‐ influence of a smoking‐related diagnosis: A pilot study. Heart & Lung 1994;23(2):151‐6. [PubMed] [Google Scholar]
Additional references
Akrawi 1997
- Akrawi W, Benumof JL. A pathophysiological basis for informed preoperative smoking cessation counseling. Journal of Cardiothoracic and Vascular Anesthesia 1997;11(5):629‐40. [DOI] [PubMed] [Google Scholar]
Beckers 1991
- Beckers S, Camu F. The anesthetic risk of tobacco smoking. Acta Anaesthesiologica Belgica 1991;42(1):45‐56. [PubMed] [Google Scholar]
Benowitz 1997
- Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. Journal of the American College of Cardiology 1997;29(7):1422‐31. [DOI] [PubMed] [Google Scholar]
Bluman 1998
- Bluman LG, Mosca L, Newman N, Simon DG. Preoperative smoking habits and portoperative pulmonary complications. Chest 1998;113(4):883‐9. [DOI] [PubMed] [Google Scholar]
Bode 1975
- Bode FR, Dosman J, Martin RR, Macklem PT. Reversability of pulmonary function abnormalities in smokers ‐ a prospective study of early diagnostic tests of small airway disease. American Journal of Medicine 1975;59(1):43‐52. [DOI] [PubMed] [Google Scholar]
Bruce 2001
- Bruce J, Russell EM, Mollison J, Krukowski ZH. The measurement and monitoring of surgical adverse events. Health Technology Assessment 2001;5(22):1‐194. [DOI] [PubMed] [Google Scholar]
Buist 1976
- Buist AS, Sexton GJ, Nagy JM, Ross BB. The effect of smoking cessation and modification on lung function. American Review of Respiratory Disease 1976;114(1):115‐22. [DOI] [PubMed] [Google Scholar]
Cahill 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
Camner 1973
- Camner P, Philipson K. Some studies of tracheobronchial clearance in man. Chest 1973;63:23. [DOI] [PubMed] [Google Scholar]
Cohen 1993
- Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to common cold. American Journal of Public Health 1993;83(9):1277‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2002
- Hajek P, Taylor TZ, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: randomised controlled trial. BMJ 2002;324(7329):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haverstock 1998
- Haverstock BD, Mandracchia VJ. Cigarette smoking and bone healing: implications in foot and ankle surgery. Journal of Foot and Ankle Surgery 1998;37(1):69‐74. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorgensen 1998
- Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery 1998;123(4):450‐5. [PubMed] [Google Scholar]
Kaijser 1985
- Kaijser L, Berglund B. Effect of nicotine on coronary blood flow in man. Clinical Physiology 1985;5(6):541‐52. [DOI] [PubMed] [Google Scholar]
Kambam 1986
- Kambam JR, Chen LH, Hyman SA. Effects of short‐term smoking halt on carboxyhemoglobin levels and p50 values. Anesthesia and Analgesia 1986;65(11):1186‐8. [PubMed] [Google Scholar]
Keeley 1996
- Keeley EC, Pirwitz MJ, Landau C, Lange RA, Hillis LD, Foerster EH, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. American Journal of Medicine 1996;101(4):357‐63. [DOI] [PubMed] [Google Scholar]
Klein 1984
- Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Tiechholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary heart disease. Journal of the American College of Cardiology 1984;3(4):879‐86. [DOI] [PubMed] [Google Scholar]
Lindström 2010
- Lindström D, Sundberg‐Petersson I, Adami J, Tønnesen H. Disappointment and drop‐out rate after being allocated to control group in a smoking cessation trial. Contemporary Clinical Trials 2010;31(1):22‐6. [DOI] [PubMed] [Google Scholar]
Lourenco 1971
- Lourenco RV, Klimek MF, Borowski CJ. Deposition and clearance of two micron particles in the trachiobronchial tree of normal subjects ‐ smokers and non‐smokers. Journal of Clinical Investigation 1971;50(7):1411‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2009
- Mason DP, Subramanian S, Nowicki ER, Grab JD, Murthy SC, Rice TW, et al. Impact of smoking cessation before resection of lung cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Study. Annals of Thoracic Surgery 2009;88(2):362‐70. [DOI] [PubMed] [Google Scholar]
McCarthy 1972
- McCarthy DS, Spencer R, Greene R, Milic‐Emili J. Measurement of "closing volume" as a simple and sensitive test for early detection of small airway disease. American Journal of Medicine 1972;52(6):747‐53. [DOI] [PubMed] [Google Scholar]
Mitchell 1982
- Mitchell C, Garrahy P, Peake P. Postoperative respiratory morbidity: identification of risk factors. Australian and New Zealand Journal of Surgery 1982;52(2):203‐9. [DOI] [PubMed] [Google Scholar]
Montori 2005
- Montori VM, Permanyer‐Miralda G, Ferreira‐Gonzalez I, Busse JV, Pachego‐Huergo V, Bryant D, et al. Validity of composite endpoints in clinical trials. BMJ 2005;330(7491):594‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2011
- Myers K, Hajek P, Hinds C, McRobbie H. Stopping smoking shortly before surgery and postoperative complications: A systematic review and meta‐analysis. Archives of Internal Medicine 2011;171(11):983‐9. [DOI] [PubMed] [Google Scholar]
Møller 2004
- Møller AM, Villebro NM. Preoperative smoking intervention: What do patients think? [Præoperativ rygeintervention: Hvad mener patienterne?]. Ugeskrift for Læger 2004;166(42):3714‐8. [PubMed] [Google Scholar]
Nakagawa 2001
- Nakagawa M, Tanaka H, Tsukuma H, Kishi Y. Relationship between the duration of the preoperative smoke‐free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest 2001;120(3):705‐10. [DOI] [PubMed] [Google Scholar]
Nicod 1984
- Nicod P, Rehr R, Winniford MD, Campbell WP, Firth BG, Hillis LD. Acute systemic and coronary hemodynamic and serologic response to cigarette smoking in long term smokers with arteriosclerotic coronary artery disease. Journal of the American College of Cardiology 1984;4(5):964‐71. [DOI] [PubMed] [Google Scholar]
Owen 2007
- Owen D, Bicknell C, Hilton C, Lind J, Jalloh I, Owen M, et al. Preoperative smoking cessation: a questionnaire study. International Journal of Clinical Practice 2007;61(12):2002‐4. [DOI] [PubMed] [Google Scholar]
Pearce 1984
- Pearce AC, Jones RM. Smoking and anaesthesia: preoperative abstinence and perioperative morbidity. Anesthesiology 1984;61(5):576‐84. [DOI] [PubMed] [Google Scholar]
Pedersen 1994
- Pedersen T. Complications and death following anaesthesia; A prospective study with special reference to the influence of patient‐, anaesthesia‐, and surgery‐related risk factors. Danish Medical Bulletin 1994;41(3):319‐31. [PubMed] [Google Scholar]
Rice 2013
- Rice VH, Hartmann‐Boyce J, Stead LF. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001188.pub4] [DOI] [PubMed] [Google Scholar]
Rigotti 2012
- Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roth 1960
- Roth GM, Shick RM. The cardiovascular effects of smoking with special reference to hypertension. Annals of the New York Academy of Science 1960 Sep 27;90:308‐16. [DOI] [PubMed] [Google Scholar]
Sadr Azodi 2009
- Sadr Azodi O, Lindström D, Adami J, Tønnesen H, Nåsell H, Gilljam H, et al. The efficacy of a smoking cessation programme in patients undergoing elective surgery: a randomised clinical trial. Anaesthesia 2009;64(3):259‐65. [DOI] [PubMed] [Google Scholar]
Schwartz 1987
- Schwartz JL. Review and evaluation of smoking cessation methods; The United States and Canada 1975‐1987. Publication no. 79‐8369. Washington: Government Printing Office, 1987. [Google Scholar]
Schwilk 1997
- Schwilk B, Bothner U, Schraag S, Georgieff M. Perioperative respiratory events in smokers and non‐smokers undergoing general anaesthesia. Acta Anaesthesiologica Scandinavica 1997;41(3):348‐55. [DOI] [PubMed] [Google Scholar]
Shannon‐Cain 2002
- Shannon‐Cain J, Webster SF, Cain BS. Prevalence of and reasons for preoperative tobacco use. American Association of Nurse Anesthetists Journal 2002;70(1):33‐40. [PubMed] [Google Scholar]
Sheps 1990
- Sheps DS, Herbst MC, Hinderliter AL, Adams KF, Ekelund LG, O'Neill JJ. Production of arrhythmias by elevated carboxyhemoglobin in patients with coronary artery disease. Annals of Internal Medicine 1990;113(5):343‐51. [DOI] [PubMed] [Google Scholar]
Shi 2010
- Shi Y, Warner D. Surgery as a teachable moment for smoking cessation. Anesthesiology 2010;112(1):102‐7. [DOI] [PubMed] [Google Scholar]
Silverstein 1992
- Silverstein P. Smoking and wound healing. American Journal of Medicine 1992;93(1A):22S‐24S. [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Sørensen 1999
- Sørensen LT, Jørgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille‐Jørgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. British Journal of Surgery 1999;86(7):927‐31. [DOI] [PubMed] [Google Scholar]
Sørensen 2002
- Sørensen LT, Hørby J, Friis E, Pilsgaard B, Jørgensen T. Smoking as a risk factor for wound healing and infection in breast cancer surgery. European Journal of Surgical Oncology 2002;28(8):815‐20. [DOI] [PubMed] [Google Scholar]
Sørensen 2003c
- Sørensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Annals of Surgery 2003;238(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sørensen 2004
- Sørensen LT, Nielsen HB, Kharazmi A, Gottrup F. Effect of smoking and abstention on oxidative burst and reactivity of neutrophils and monocytes. Surgery 2004;136(5):1047‐53. [DOI] [PubMed] [Google Scholar]
Sørensen 2008
- Sørensen LT, Jørgensen S, Petersen LJ, Hemmingsen U, Bülow J, Loft S, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. Journal of Surgical Research 2008;152(2):224‐30. [DOI] [PubMed] [Google Scholar]
Sørensen 2012
- Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Annals of Surgery 2012;255(6):1069‐79. [DOI] [PubMed] [Google Scholar]
Thomsen 2009
- Thomsen T, Esbensen BA, Samuelsen S, Tønnesen H, Møller AM. Brief preoperative smoking cessation counselling in relation to breast cancer surgery: A qualitative study. European Journal of Oncology Nursing 2009;13(5):344‐9. [DOI] [PubMed] [Google Scholar]
Villebro 2008
- Villebro NM, Pedersen T, Møller AM, Tønnesen H. Long‐term effects of a preoperative smoking cessation programme. The Clinical Respiratory Journal 2008;2(3):175‐82. [DOI] [PubMed] [Google Scholar]
Warner 1989
- Warner MA, Offord KP, Warner ME, Lennon RL, Conover A, Jansson‐Schumacher U. Role of preoperative smoking cessation and other factors in postoperative pulmonary complications: a blinded prospective study of coronary artery bypass patients. Mayo Clinic Proceedings 1989;64(6):609‐16. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Møller 2001
- Møller AM, Villebro N, Pederson T. Interventions for preoperative smoking cessation. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD002294] [DOI] [PubMed] [Google Scholar]
Møller 2005
- Møller AM, Villebro N. Interventions for preoperative smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002294.pub2] [DOI] [PubMed] [Google Scholar]
Thomsen 2010
- Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD002294.pub3] [DOI] [PubMed] [Google Scholar]


