ABSTRACT
Targeted degradation is a promising new modality in drug discovery that makes it possible to reduce intracellular protein levels with small molecules. It is a complementary approach to the conventional protein knockdown typically used in laboratories and may offer a way to approach the currently undruggable human proteome. Recently, the first autophagy-mediated degraders, called AUTACs, were developed based on observations in a xenophagy study.
KEYWORDS: AUTAC, drug discovery, guanylation, mitophagy, PROTAC, targeted degradation
The ubiquitin-proteasome system (UPS) and macroautophagy/autophagy are the primary intracellular degradation mechanisms. Traditionally, inhibitors of these mechanisms have been investigated for the treatment of cancer but the promoters specifically degrading a protein of interest are just recently attracting attentions.
PROTACs and SNIPER are small-molecule degraders that rely on the UPS. These small molecules connect a ubiquitin ligase with the substrate of interest and thereby promote ubiquitination. Arvinas Inc. is currently investigating an orally-available PROTAC in a phase 1 clinical study. Degraders using autophagy may offer unique advantages over the UPS-based degraders because autophagy degrades a wider range of substrates than the UPS. However, our understanding about which ubiquitin ligases accelerate clearance of biomolecules via selective autophagy remains insufficient.
Autophagy-targeting chimera (AUTAC) is the first degrader using the autophagy mechanism (Figure 1) [1]. AUTAC originated from our previous observations in xenophagy. Autophagic clearance of group A Streptococcus (GAS) is a ubiquitin-mediated selective autophagy, although the ubiquitin ligase involved has not been identified. Guanylation of the bacterial surface by an endogenous nucleotide (8-nitro-cGMP) was found to promote ubiquitination of GAS. This led us to investigate in this study the possibility of recruiting the autophagy machinery using guanine derivatives as a tag. A typical AUTAC is made of a small unit that binds to a substrate and a guanine derivative (degradation tag) connected by a flexible linker. AUTAC can penetrate cell membranes.
Figure 1.
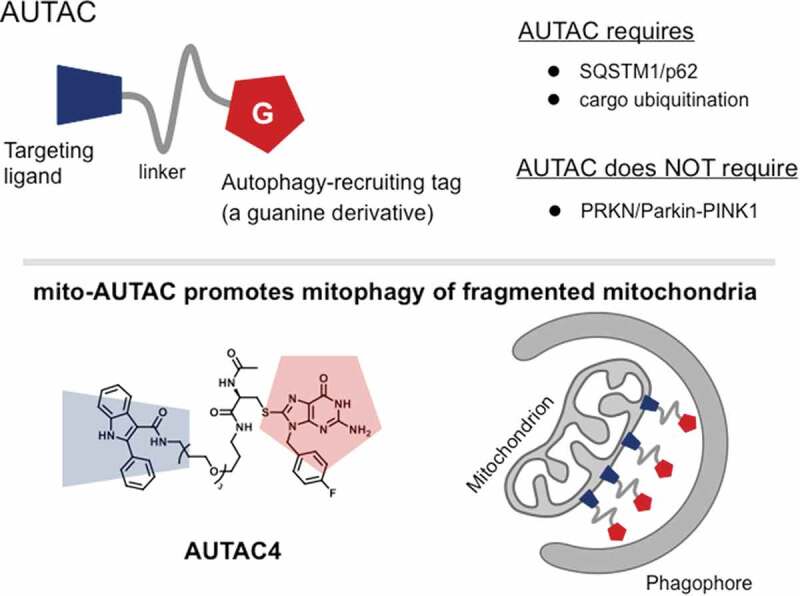
AUTAC is a heterobifunctional compound with a targeting warhead and a tag that recruits the autophagy system. AUTACs are demonstrated to promote autophagic clearance of cargos including damaged mitochondria.
AUTACs were successfully employed to degrade several cytosolic proteins. Furthermore, mitochondria-targeted AUTAC (AUTAC4) promotes mitophagy of small, fragmented mitochondria. Diseases and aging are demonstrated to trend toward mitochondrial fragmentation. Thus, removal of fragmented mitochondria may provide beneficial effects. In accordance with this idea, cell functions such as mitochondrial membrane potential and ATP production are restored, when human fibroblasts from a Down syndrome patient with mitochondrial fragmentation are treated with AUTAC4 for 3 days. Notably, the mitophagy induction by an AUTAC4 treatment also increases the level of PPARGC1A/PGC-1α a master regulator of mitochondrial biogenesis. The mitophagy induction by AUTAC is ubiquitin-mediated but does not require either PRKN/Parkin or PINK1.
These results constituted the first proof of concept of AUTAC that may have broad application in autophagy-based drug discovery. However, a number of important questions remain to be addressed. Along these lines, the potential of the AUTAC approach have not fully investigated. For instance, the application of the AUTAC methodology for removal of protein aggregates has not yet been examined. Obviously, insight into the mechanism by which AUTAC works is needed. In selective autophagy, cargo selectivity is usually achieved by a substrate’s ubiquitination or a direct recognition by autophagy receptors. The authors currently think that the mode of action of AUTAC is distinct from that of PROTACs that induce proximity between a ubiquitin ligase and the substrate. The mechanism of how the AUTAC’s degradation tag is recognized will be reported in due course.
Funding Statement
This work is supported by supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan Society for the Promotion of Science (MEXT, JSPS), KAKENHI (grant numbers JP17K19190, JP18H04604, JP18H04377, JP19H02844).
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Takahashi D, Moriyama J, Nakamura T, et al. AUTACs: cargo-specific degraders using selective autophagy. Mol Cell. 2019;76(5):797–810.e10. [DOI] [PubMed] [Google Scholar]


