Abstract
Objective:
Mindfulness-based interventions have been found to reduce psychological and physiological stress reactivity. In obesity, however, stress reactivity is complex, with studies showing both exaggerated and blunted physiological responses to stressors. A nuanced view of stress reactivity is the “challenge and threat” framework, which defines adaptive and maladaptive patterns of psychophysiological stress reactivity. We hypothesized that mindfulness training would facilitate increased challenge-related appraisals, emotions, and cardiovascular reactivity, including sympathetic nervous system activation paired with increased cardiac output (CO) and reduced total peripheral resistance (TPR) compared to a control group, which would exhibit an increased threat pattern of psychophysiological reactivity to repeated stressors.
Methods:
Adults (N=194) with obesity were randomized to a 5.5-month mindfulness-based weight loss intervention or an active control condition with identical diet-exercise guidelines. Participants were assessed at baseline and 4.5 months later using the Trier Social Stress Task. Electrocardiogram, impedance cardiography, and blood pressure were acquired at rest and during the speech and verbal arithmetic tasks to assess pre-ejection period (PEP), CO, and TPR reactivity.
Results:
Mindfulness participants showed significantly greater maintenance of challenge-related emotions and cardiovascular reactivity patterns (higher CO and lower TPR) from pre to post-intervention compared to control participants, but groups did not differ in PEP. Findings were independent of changes in body mass index.
Conclusions:
Mindfulness training may increase the ability to maintain a positive outlook and mount adaptive cardiovascular responses to repeated stressors among persons with obesity though findings need to be replicated in other populations and using other forms of mindfulness interventions.
The experience of stress is prevalent in the U.S. In one national survey, 75% of Americans reported at least one symptom of stress in the past month, such as having trouble falling asleep or feeling anxious, irritable, or fatigued (American Psychological Association, 2017). Short-term experiences of stressful events perceived as threatening or beyond one’s ability to cope effectively may increase negative mood and compromise health-related behaviors, such as reducing sleep quality and increasing disordered eating, substance use, and sedentary behavior (Gomez-Bernal et al., 2019; Schneiderman, Ironson, & Siegel, 2005). Physiological stress-related responses, including activation of the hypothalamic-pituitary-adrenal axis and the autonomic nervous system, interact with the cardiovascular, metabolic and immune systems to adapt to current stressors (McEwen et al., 2015). Repetitive exposure to stressors and maladaptive reactions over time, such as hypo or hyper-activation of physiological systems, may lead to bodily “wear and tear” or chronic stress (Juster, McEwen, & Lupien, 2010). Chronic stress may contribute to several health conditions, including obesity, type 2 diabetes, cardiovascular disease, anxiety and depression (Chida & Steptoe, 2010; Chrousos, 2009; Wardle, Chida, Gibson, Whitaker, & Steptoe, 2011).
Mindfulness meditation is increasingly being used to manage stress. It is thought to enhance a form of present-moment, non-evaluative awareness of one’s experience, including thoughts, emotions, and body sensations (Brown, Ryan, & Creswell, 2007). The process of experiencing thoughts and other mental phenomena as passing events in a field of awareness has been referred to as “meta-cognitive awareness” (Teasdale et al., 2002) and is theorized to reduced identification with negative thought patterns and aversive affect and allow for less automatic reactions and more adaptive responses to external stressful events (Hayes-Skelton & Graham, 2013; Kabat-Zinn, 1990).
A growing literature demonstrates promising preliminary outcomes of mindfulness-based interventions for conditions such as chronic pain, anxiety, depression, addiction, and dysregulated eating (Creswell, 2017; Hofmann, Sawyer, Witt, & Oh, 2010; Katterman, Kleinman, Hood, Nackers, & Corsica, 2014; O’Reilly, Cook, Spruijt-Metz, & Black, 2014). A meta-analysis reported small though consistent reductions in psychological stress, including depression, anxiety, and distress (Goyal et al., 2014). Mindfulness meditation has also been shown to reduce psychological stress, anxiety, and rumination in reaction to acute, standardized stress tasks in randomized trials (Britton, Shahar, Szepsenwol, & Jacobs, 2012; Creswell, Pacilio, Lindsay, & Brown, 2014; Hoge et al., 2013; van Vugt, Hitchcock, Shahar, & Britton, 2012). In regards to physiological stress reactivity, mindfulness intervention participants showed greater reductions in blood pressure (Nyklicek, Mommersteeg, Van Beugen, Ramakers, & Van Boxtel, 2013; Steffen & Larson, 2015), adrenocorticotropic hormone (Hoge et al., 2017), and inflammatory markers (Hoge et al., 2017; Rosenkranz et al., 2013) in response to acute standardized stress tasks compared to control participants.
The bulk of studies examining the effects of mindfulness meditation on stress reactivity have, for good reason, adopted a “stress reduction” paradigm in which the aim of mindfulness meditation is to reduce exaggerated responses to stress to improve health. While evidence is accruing that mindfulness training may reduce psychophysiological reactivity to acute stressors, existing randomized trials have not yet addressed whether mindfulness promotes active engagement with acute stressors and adaptive stress responses while simultaneously reducing maladaptive stress reactions. In addition, a “stress reduction” framework becomes problematic for conditions in which blunted reactivity to stress may be associated with disease conditions, such as obesity, depression, and addiction (Brindle, Whittaker, Bibbey, Carroll, & Ginty, 2017; Carroll, Ginty, Whittaker, Lovallo, & de Rooij, 2017). Emerging research suggests that fronto-limbic brain systems that are normally engaged during acute stressors may function less optimally and reduce motivation and successful adaptation in some health conditions (Carroll et al., 2017). In such cases, it may be advantageous to promote healthy responses to stress, as the ability to mount appropriate physiological stress responses to face life’s challenges is essential for health (Chrousos, 2009).
Interestingly, not all stress responses are created equal. Social and health psychology researchers have attempted to differentiate acute stress reactivity that is beneficial and adaptive from that which may be harmful and maladaptive. Several theories have identified psychological antecedents and physiological consequences that differentiate “good” from “bad” stress reactions (e.g., Dienstbier, 1989; Frankenhaeuser, 1986; Henry, 1986). One theory that integrates Dienstbier’s “physiological toughness” theory and Lazarus and Folkman’s stress appraisal theory in the context of acute stressful situations is the biopsychosocial model of challenge and threat (Blascovich & Mendes, 2010). In this model, both challenge and threat states occur during acute “stressful” situations; however, the states differ in their antecedent appraisal process and subsequent downstream cardiovascular reactivity. Event appraisals—how one understands, perceives, and evaluates situations—can influence emotional and physiological reactions to stressful situations, and over time, if such patterns become habitual, may contribute to chronic disease conditions.
More specifically, when appraisals of perceived demands exceed perceived abilities to cope effectively with a stressor, individuals typically feel anxious, defeated, or threatened. When perceived demands are within the perceived ability to cope, individuals feel energized, competent, and challenged. Emotional states of threat and challenge in turn are associated with distinct cardiovascular reactivity profiles. Yet, both states are theorized to activate the sympathetic-adrenal-medullary axis causing the release of catecholamines. Threat states are also predicted to activate the hypothalamic-pituitary-adrenal axis and temper the effects of sympathetic activation. Consequently, challenge-related states are associated with increased cardiac output (CO), the amount of blood ejected from the heart during one minute, and decreased total peripheral resistance (TPR), a measure of overall vascular resistance with increasing levels indicating vasoconstriction (Mendes, 2009). Threat states, in contrast, are characterized by decreased CO and increased TPR (Blascovich & Mendes, 2010). The pre-ejection period (PEP), the time between the left ventricle contracting and the aortic valve opening, is considered a pure measure of sympathetic activation and is expected to be activated during both challenge and threat states (Mendes, 2009).
Over sustained periods of time, higher CO is linked to greater cognitive function and brain volume, as in the Framingham Heart Study (Jefferson et al., 2010), whereas greater TPR is associated with increased blood pressure, a risk factor for coronary heart disease (Mathews, 2005). Threat reactions are considered maladaptive as vascular resistance reduces the delivery of oxygenated blood to the brain and peripheral tissues, which may impair performance in demanding situations and interfere with efficient cardiovascular recovery following acute stressors (Kelsey et al., 1999; McLaughlin, Sheridan, Alves, & Mendes, 2014). Indeed, threat reactivity profiles are related to poorer cognitive and behavioral performance on active tasks (Drach-Zahavy & Erez, 2002; Jamieson, Nock, & Mendes, 2012) and individuals with a history of childhood maltreatment display greater threat-related cardiovascular reactivity which is related to greater externalizing behavioral problems (McLaughlin et al., 2014). In contrast, challenge states are linked to better cognitive performance in domains such as pattern-detection, cooperative games, and decision-making tasks (Blascovich, Mendes, Hunter, & Salomon, 1999; Kassam, Koslov, & Mendes, 2009; Mendes, Major, McCoy, & Blascovich, 2008).
Little is known whether mindfulness training promotes adaptive and reduces maladaptive psychophysiological reactivity to stressors as described by the challenge and threat model, including among persons with obesity. Obesity is often linked to exaggerated stress processes which trigger overeating and contribute to metabolic dysregulation (Rosmond, 2005; Sominsky & Spencer, 2014; Wardle et al., 2011; Yau & Potenza, 2013). However, emerging research suggests that obesity can also be associated with blunted cardiovascular and neuroendocrine reactions to acute stress (Herhaus & Petrowski, 2018; Jones et al., 2012; Tomiyama, Dallman, & Epel, 2011; Torres, Turner, Jayasinghe, Reynolds, & Nowson, 2014; Tryon, DeCant, & Laugero, 2013). These reactions may reflect impairments in fronto-limbic brain networks essential for motivation and behavioral regulation (Carroll et al., 2017). In turn, these impairments may contribute to depression, overeating, and substance use which may increase cardiovascular disease risk over the long term (Bennett, Blissett, Carroll, & Ginty, 2014; Ginty, Kraynak, Fisher, & Gianaros, 2017; Wiggert, Wilhelm, Nakajima, & al’Absi, 2016).
While mindfulness-based interventions have been viewed primarily through the lens of a “stress reduction” model, a more appropriate framework for some health conditions, including obesity, may be one that focuses on increasing “adaptive stress responsiveness.” Given the complexity of stress reactions in obesity, i.e., with studies showing that either exaggerated or blunted reactions to stress may confer disease risk, the challenge and threat framework may be potentially useful for examining effects of mindfulness training on adaptive and maladaptive acute stress processes in obesity. For example, a lack of elevated diastolic or systolic blood pressure during an acute stressor may be characterized as blunted reactivity. However, it is also possible that the cardiovascular system may be shifting from a challenge (myocardial, CO-driven) to a threat (vascular, TPR-driven) response profile due to the dynamic homeostatic relationship between CO and TPR (Palatini & Julius, 2009; Phillips, Ginty, & Hughes, 2013). Within a challenge and threat framework, we can examine nuances in the degree to which cardiovascular reactivity to stress reflect an adaptive challenge profile (increases in CO, reduced TPR) versus a maladaptive threat profile (reduced CO and increased TPR). Little research has examined whether mindfulness also shifts psychological responses from threat to challenge, and whether these shifts are in parallel with shifts in cardiovascular reactivity patterns (Weinstein, BRown, & Ryan, 2009); and, randomized trials of mindfulness-based interventions have yet to use the necessary methodological approaches to address these questions.
We conducted a randomized trial of a mindfulness-based weight loss intervention compared to an active control condition among adults with obesity and reported observed changes in metabolic risk factors elsewhere (Daubenmier et al., 2016). We were interested in the impact of adding mindfulness training to diet and exercise-based weight loss interventions. Therefore, both interventions received identical diet and exercise guidelines and the mindfulness intervention also included mindfulness-based stress management and eating awareness training. To control for attention, social support, expectations of benefit, and a mindfulness approach to stress management, the active control intervention included additional diet-exercise information and limited progressive muscle relaxation and cognitive-behavioral training related to stress eating. We further designed the trial to examine signatures of challenge and threat appraisals, emotions, and cardiovascular reactivity in response to a standardized social-evaluative stress task at baseline and post-intervention. We hypothesized that the mindfulness intervention, compared to the active control condition, would promote increased challenge and decreased threat profiles from pre- to post-intervention.
Methods
Participants
Adults with obesity (>18 years of age; BMI 30-45) and abdominal obesity (waist circumference > 102 cm for men; > 88 cm for women) based on established criteria (National Cholesterol Education Program [NCEP] Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III], 2002) were eligible for the study. Exclusion criteria included prior training in Mindfulness-Based Stress Reduction (MBSR), a current meditation practice, previous mindful eating training, or initiation of a new class of psychiatric medications in the past 2 months. See Daubenmier et al (Daubenmier et al., 2016) for further enrollment and eligibility criteria.
Procedure
Study design.
We randomized adults with obesity in a 1:1 ratio to a two-arm 5.5-month diet-exercise intervention with or without mindfulness training. The University of California, San Francisco (UCSF) Committee on Human Research approved the study procedures and participants provided informed consent. The intervention was provided free of charge at UCSF; participants were recruited from the surrounding area and compensated for assessment visits. Participants completed assessments from July 2009-2012. Planned outcome measures of this trial are listed on Clinicaltrials.gov registration: NCT00960414.
Intervention groups.
Both interventions included 12-weekly group sessions (2-2.5 h), 3-biweekly sessions, 1 follow-up session four weeks later, and an all-day weekend session near the eighth session (5.0 h for the active control, 6.5 h for the mindfulness intervention group) across a 5.5-month period. The mindfulness intervention was co-led by one of three experienced mindfulness instructors and a registered dietitian whereas a single registered dietitian led the control group sessions. Instructors varied across rounds. All mindfulness instructors had a minimum of four years of professional experience teaching mindfulness-based interventions in relation to making health lifestyle changes in group settings. All instructors participated in a 5-day residential training on mindful eating led by a co-investigator (JK) who developed the Mindfulness-Based Eating Awareness Training (MB-EAT) program.
Diet-exercise guidelines were identical across interventions. Participants set goals of reducing daily food intake by 500 calories by focusing on decreasing calorically-dense, nutrient-poor foods such as refined carbohydrates, and increasing fresh fruits and vegetables, healthy oils, and proteins. The exercise component included increasing activity throughout the day and structured aerobic and anaerobic exercise.
Mindfulness intervention.
Meditation practices, modeled on the Mindfulness-based Stress Reduction program, included sitting meditation consisting of mindful awareness of breath, thoughts, feelings, sounds, and body sensations; loving kindness; and yoga postures (Kabat-Zinn, 1990). Mindful eating practices, modeled on the Mindfulness-Based Eating Awareness Training program, were designed to promote awareness and self-regulation of eating-related thoughts and emotions, physical hunger, stomach fullness, taste satisfaction, food cravings, and other eating triggers in the context of reduced caloric intake (Kristeller & Wolever, 2011). Mindful walking included awareness of sensory experience, posture, and alignment (Dreyer & Dreyer, 2006). Home practice guidelines included meditation practice for up to 30 min a day/6 days a week, eating meals mindfully, and use of mini-meditations. Participants kept weekly adherence logs in which they reported the amount of time practicing meditation and mindful eating for each day of the week. As described previously, participants in this arm reported meditating 2.1 (SD=1.2) hours/week (70% of recommendations) and eating 57% of meals mindfully (Daubenmier et al., 2016).
Active control intervention.
To control for attention, social support, expectation of benefit, food provided during the mindful eating exercises, and home mindfulness practice, the control intervention included additional nutrition and physical activity information, strength training with exercise bands, discussion of societal issues concerning weight loss, snacks, and home activities. To satisfy expectations for stress management training in the control group and to control for a mindfulness approach to stress management, we included progressive muscle relaxation and cognitive-behavioral training in the control intervention, although at a lower dose than in the mindfulness intervention. Progressive muscle relaxation was practiced in four group sessions and participants were provided a CD for optional home practice. Two cognitive behavioral techniques were briefly introduced in two separate sessions. These included replacing distorted thinking patterns related to food (eg, “all or none thinking”) with more realistic thoughts and judgments and identifying alternative behaviors to eating during stress.
Trier Social Stress Test.
The most commonly used laboratory stressor, the Trier Social Stress Task (TSST) (Kirschbaum, Pirke, & Hellhammer, 1993), is designed to induce moderate to high psychological stress in order to examine acute physiological reactivity to a socially evaluative situation. The task requires delivering an impromptu speech and executing a verbal arithmetic task while being socially evaluated by two strangers, which reliably activates the two primary stress systems: hypothalamic-pituitary-adrenocortical and sympathetic adrenal medullary (Dickerson & Kemeny, 2004). The TSST is therefore useful in determining an individual’s typical pattern of arousal under novel, high arousal acute stress conditions.
Participants underwent the TSST at baseline before randomization and approximately 4.5 months after intervention initiation (visits were held during the biweekly sessions and before the last session). Participants were told they would be participating in “thinking and talking tasks” prior to arriving for the first session. The tasks were performed in front of two trained, stoic “evaluators” wearing lab coats and unknown to the participant. The evaluators differed at the pre and post-intervention TSST sessions. The TSST consisted of a 5-minute speech preparation period, a 5-minute public speaking task and a 5-minute serial subtraction task. The speech content differed at the pre and post-intervention assessments to minimize habituation. At the pre-intervention assessment, participants spoke about their “personal strengths and weaknesses.” At the post-intervention assessment, they completed a mock job interview. Additionally, participants were videotaped and told that the tapes would be reviewed by a “panel of experts.” The evaluators interrupted the participants during their speeches, making 2 to 5 “prompts” that were meant to increase the task demands. The serial subtraction task consisted of counting backward from a three-digit number in steps of 13 as quickly and accurately as possible. Alternative sets of numbers with varying difficulty levels were utilized by the evaluators as needed.
Measures
Self-report measures.
Self-report measures were administered at the start of the assessment, after the evaluators were introduced and participants were told that they would be giving a speech and performing a mental arithmetic task. Six items assessed appraisals of the upcoming speech and math tasks in terms of the perceived demands of the task and 6 items assessed resources to cope with the task.(Mendes, Gray, Mendoza-Denton, Major, & Epel, 2007) Items were rated on a 7-point Likert scale from 1 = strongly disagree to 7 = strongly agree. An example of a demand item is: “The upcoming task will take a lot of effort to complete”; an example of a resource item is: “I have the ability to perform the upcoming task successfully.” The internal reliability of the 2 scales using Cronbach’s alpha were .76 and .79, respectively.
The positive challenge emotions were rated on a 5-point scale ranging from 1 = never to 5 = always and included hopeful, eager, excited, and confident. Emotions related to threat included nervous, timid, tense, anxious, and afraid. Cronbach’s alpha for these two emotion scales were .84 and .86, respectively.
Cardiovascular measures.
We used impedance cardiography, electrocardiography, and blood pressure monitors to obtain our primary measures of cardiovascular reactivity. Cardiac impedance was measured using band electrodes that completely encircled the neck and torso (Biopac NICO module) and electrocardiography used a Lead II configuration a (Biopac ECG module). All signals were sampled at 1000Hz and integrated into an MP150 (Biopac). Blood pressure was measured thirteen times during the TSST with the Press-Mate Prodigy® II Non-invasive Blood Pressure Monitor with the cuff placed on the participant’s dominant arm. Post collection, data were visually inspected by trained personnel and Mindware software (IMP 2.6; BP 2.6) was used to edit and ensemble the waveforms (Mendes, 2009). Only scorable data, free of electrical or movement artifacts, with physiologically plausible values were included in analyses. A subset of data (20%) were randomly chosen to be double scored and reliability for all data exceeded r > .90.
We focus on the three cardiovascular variables most commonly used in challenge and threat research: PEP, CO and TPR, with the latter two consistently differentiating challenge from threat profiles. Other cardiovascular measures such as blood pressure and heart rate do not reliably distinguish challenge and threat profiles (McLaughlin et al., 2014). Pre-ejection period is the time between the left ventricle contracting and the aortic valve opening and provides a pure measure of sympathetic activation (Mendes, 2009). CO is the amount of blood ejected from the heart during one minute calculated by estimating stroke volume (the amount of blood ejected per beat) and multiplying by heart rate. TPR is a measure of overall vascular resistance with increasing levels indicating vasoconstriction (Mendes, 2009). TPR was calculated using the standard formula: (mean arterial blood pressure/CO) x 80. All data were scored in one-minute intervals and reactivity values were obtained by subtracting the last minute of the 10-minute resting period in which participants listened to relaxing music (presumably the most de-activated) from the first minute of the speech task and first minute of the math task (presumably the most activated).
At the end of the first TSST, participants were thanked, partially debriefed and questions or concerns were addressed without revealing the purpose of the tasks. At the post-intervention TSST, participants were fully debriefed.
Data Analyses
Independent samples t-tests were used to compare participants with available data for the TSST at baseline and to compare those who completed both TSSTs to those who did not. To assess within and between-group changes over time, mixed linear models were used for repeated measures analyses of self-report emotion and cardiovascular reactivity measures, with a compound symmetry covariance structure, and with factors of group, time (month, with values of 0 and 6) and their interaction. The coefficient for the group x time interaction provides an estimate which can be interpreted as the difference in change scores between groups. Gender, use of beta-blocker medications, centered age, body mass index (BMI), and a month x BMI interaction (to control for changes in BMI over time) were included as covariates in the cardiovascular reactivity models as they directly impact the cardiovascular system. Higher BMI has been related to blunted cardiovascular reactions to stress (Carroll et al., 2017). Within-group change was estimated using least square means. Effect sizes (ES) were also computed using Cohen’s d for within and between-group analyses. We used established guidelines to interpret ES: small = .20; medium = .50; large = .80 (Lakens, 2013). SAS v9.3 was used for analyses.
Results
We randomized 194 participants to the mindfulness or active control intervention (see Figure 1). Groups were similar across baseline demographic, anthropometric, medication use, appraisal, emotion, baseline resting cardiovascular, and cardiovascular reactivity variables (see Tables 1 and 2). The percentage of participants in each group who returned for the post-intervention TSST assessment was similar (72% and 73% in the mindfulness and control conditions, respectively). Among participants who completed both TSSTs, no significant group differences between intervention groups were found in age, gender, baseline BMI, or 3-month weight loss (ps > .21). Participants across both groups who did not complete both TSST assessments were older (48.2 ± 12.6 vs. 43.9 ± 12.6 years, p = .03) and lost less weight at 3 months compared to those who did (−2.2 ± 3.7 vs. −4.0 ± 4.0 kg, p = .02), but had similar baseline BMI and gender (ps > .18). Six and two participants in the mindfulness and control groups, respectively, were taking beta-blocker medications.
Figure 1.
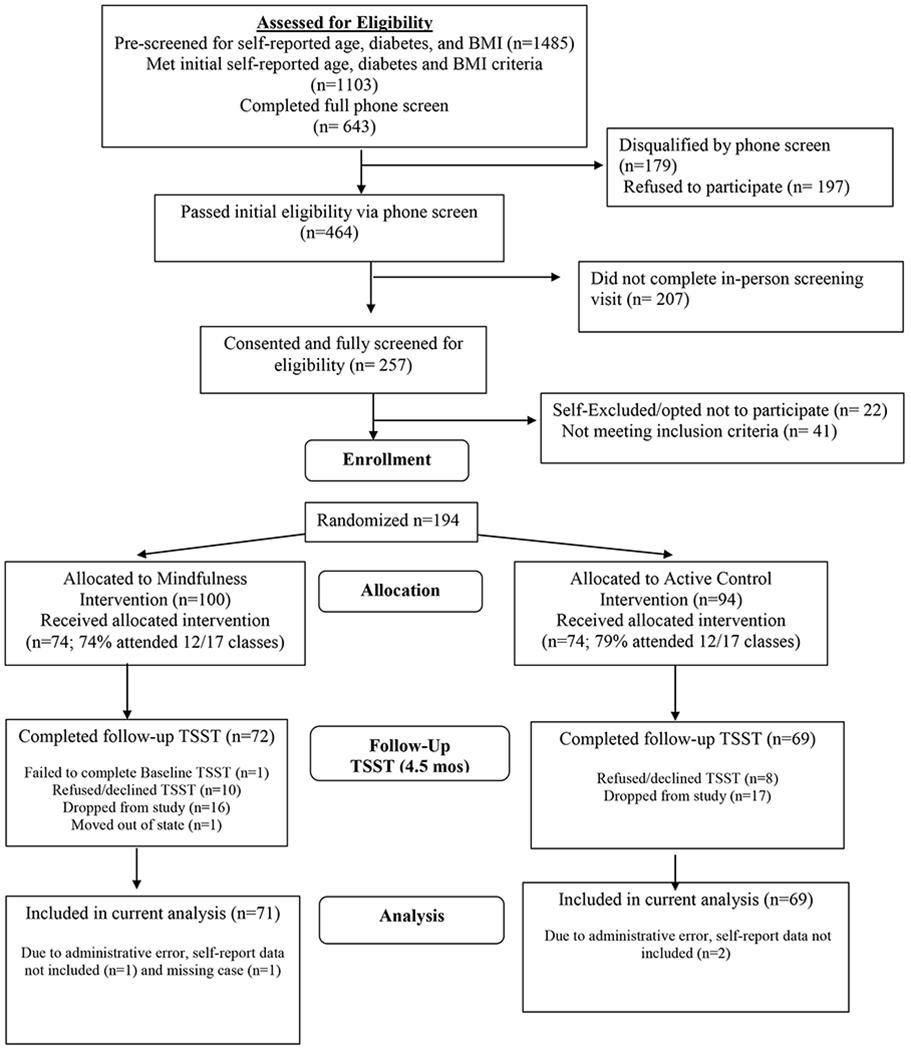
Participant Flow Chart
Table 1.
Baseline Characteristics of Study Participants
| Variable | Mindfulness (n=100) |
Active Control (n=94) |
|---|---|---|
| Age, mean (SD), y | 47.2 (13.0) | 47.8 (12.4) |
| Sex, No. (%), female | 79 (79) | 81 (86) |
| Ethnic Origin, No. (%) | ||
| European | 65 (65.0) | 50 (53.0) |
| African | 13 (13.0) | 12 (12.8) |
| Asian/Pacific Islander | 8 (8.0) | 11 (11.7) |
| Latina/Latino | 7 (7.0) | 16 (17.0) |
| Native American | 0 (0.0) | 2 (2.1) |
| Other | 7 (7.0) | 3 (3.2) |
| Education, No. (%), Bachelor’s degree a | 69 (69.7) | 56 (59.6) |
| Weight, mean (SD), kg | 97.7 (14.1) | 96.7 (14.8) |
| Body mass index, mean (SD)b | 35.4 (3.5) | 35.6 (3.8) |
| Waist circumference, mean (SD), cm | 112.9 (9.7) | 112.7 (10.6) |
| Resting cardiovascular measures, mean (SD)c | ||
| Systolic blood pressure, mmHg | 123.3 (14.8) | 125.5 (12.7) |
| Diastolic blood pressure, mmHg | 72.1 (9.7) | 72.7 (9.3) |
| Mean arterial pressure, mmHg | 88.8 (10.7) | 90.4 (11.1) |
| Heart rate, beats/min | 71.0 (11.5) | 71.1 (9.9) |
| Stroke volume, ml/beat | 113. 1 (36.0) | 105.0 (36.2) |
| Medications, No. (%) | ||
| Beta Blockers | 6 (6.0) | 2 (2.1) |
| Lipid lowering | 11 (11.0) | 9 (9.6) |
| Blood pressure | 16 (16.0) | 21 (22.3) |
| Anti-depressant | 17 (17.0) | 16 (17.0) |
| Metabolic Syndrome, No. (%)d | 28 (28.0) | 27 (28.7) |
One participant in the mindfulness arm did not provide education data.
Calculated as weight in kilograms divided by height in meters squared.
Values reflect the last minute of a 10-minute rest period before the preparation portion of the Trier. Two participants in the mindfulness group have missing data for blood pressure, heart rate, and mean arterial pressure. Eight and twelve participants, in the mindfulness and control groups respectively, have missing data for stroke volume.
The criteria for metabolic syndrome were based on the guidelines developed by the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, 2002). Metabolic syndrome was defined as the presence of three or more risk factors: increased waist circumference (88cm for women; 102 cm for men); elevated triglycerides (≥ 150 mg/dL) or medication use; low HDL cholesterol (<50 mg/dL in women; < 40 mg/dL in men) or medication use; hypertension (≥ 130/≥85 mm Hg) or medication use; and impaired fasting glucose (≥110 mg/dL).
Table 2:
Estimates of Appraisal and Emotion Ratings and Autonomic Reactivity at Baseline and Post-Intervention for Mindfulness and Active Control Participants
| Mindfulness | Active Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||||||
| N | Estimate (SE) | N | Estimate (SE) | p | ES | N | Estimate (SE) | N | Estimate (SE) | p | ES | |
| Appraisals | ||||||||||||
| Demand | 100 | 4.64 (0.10) | 70 | 4.43 (0.12) | .05 | −.23 | 93 | 4.54 (0.11) | 67 | 4.36 (0.12) | .13 | −.19 |
| Resources | 100 | 4.94 (0.09) | 70 | 5.03 (0.11) | .36 | .11 | 93 | 5.07 (0.10) | 67 | 4.91 (0.11) | .12 | −.19 |
| Emotions | ||||||||||||
| Challenge | 99 | 2.82 (0.09) | 70 | 2.72 (0.10) | .30 | −.13 | 94 | 2.89 (0.09) | 66 | 2.42 (0.10) | .0001 | −.58 |
| Threat (anxiety) | 99 | 2.70(0.08) | 70 | 2.38 (0.09) | .0002 | −.46 | 94 | 2.65 (0.08) | 67 | 2.54 (0.09) | .20 | −.16 |
| Pre-ejection period (ms) | ||||||||||||
| Speech | 75 | −16.19 (1.80) | 52 | −15.21 (2.15) | .66 | .06 | 69 | −17.10 (1.86) | 52 | −12.65 (2.12) | .053 | .27 |
| Math | 77 | −16.44 (1.74) | 58 | −13.82 (2.03) | .24 | .15 | 75 | −16.90 (1.76) | 53 | −13.10 (2.06) | .099 | .23 |
| Cardiac Output (L/min) | ||||||||||||
| Speech | 82 | 2.62 (0.34) | 59 | 3.54 (0.40) | .036 | .28 | 73 | 2.57 (0.35) | 58 | 2.30 (0.40) | .53 | −.08 |
| Math | 84 | 2.83 (0.31) | 58 | 3.06 (0.36) | .55 | .08 | 78 | 2.27 (0.32) | 59 | 1.96 (0.37) | .44 | −.10 |
| Total Peripheral Resistance (resistance units) | ||||||||||||
| Speech | 79 | 13.23 (50.80) | 58 | −58.90 (60.21) | .34 | −.13 | 73 | −52.09 (52.41) | 57 | 85.19 (61.57) | .077 | .24 |
| Math | 82 | −33.20 (47.05) | 63 | −86.72 (55.05) | .42 | −.10 | 78 | −60.78 (47.99) | 56 | 99.94 (58.09) | .023 | .31 |
Note: Mixed linear models were used. We adjusted for age, gender, body mass index at baseline, change in body mass index, and betablocker medication use for cardiovascular outcome analyses. No significant differences were observed between groups at baseline using independent samples t-tests (p<0.05). ES = Effect size.
Appraisal and Emotion Variables
Mindfulness compared to active control participants reported similar reductions in demand appraisals of the social stress test from pre to post-intervention (p = .80; ES = −.02). However, mindfulness participants tended to report having greater resources to cope with the stressor from pre to post-intervention compared to active control participants, although the effect did not reach statistical significance (p = .08; ES = .15; see Tables 2 and 3 and Figure 2). Mindfulness participants reported significantly greater increases in challenge emotions compared to control participants from pre to post-intervention (p = .008; ES = .23) and tended to report greater decreases in threat-related emotions of anxiety compared to control participants, although this effect did not reach statistical significance (p = .08; ES = −.15; see Tables 2 and 3 and Figure 2).
Table 3:
Mean Differences in Pre to Post-intervention Changes Between Intervention Groups on Appraisals, Emotions, and Cardiovascular Reactions During the Trier Social Stress Test
| Mean difference estimate (Mindfulness – Control) (SE) | df | t | p | 95% CI (lower, upper) | Effect Size | |
|---|---|---|---|---|---|---|
| Appraisals | ||||||
| Demand | −0.04 (0.16) | 134 | −0.25 | .80 | (−0.36, 0.27) | −.02 |
| Resources | 0.25 (0.14) | 134 | 1.76 | .08 | (−0.03, 0.52) | .15 |
| Emotions | ||||||
| Challenge | 0.37 (0.14) | 134 | 2.67 | .008 | (0.09, 0.64) | .23 |
| Threat-Anxiety | −0.21 (0.12) | 135 | −1.76 | .081 | (−0.44, 0.03) | −.15 |
| Cardiovascular Reactions | ||||||
| Pre-ejection period (ms) | ||||||
| Speech | −3.47 (3.08) | 77 | −1.13 | .26 | (−9.6, 2.67) | −.11 |
| Math | −1.17 (3.08) | 85 | −0.38 | .70 | (−7.30, 4.95) | −.04 |
| Cardiac Output (L/min) | ||||||
| Speech | 1.20 (0.60) | 88 | 2.00 | .049 | (0.005,2.40) | .19 |
| Math | 0.54 (0.53) | 97 | 1.00 | .32 | (−0.52, 1.60) | .09 |
| Total Peripheral Resistance (resistance units) | ||||||
| Speech | −209.42 (105.29) | 85 | −1.99 | .0499 | (−418.76, −0.08) | −.19 |
| Math | −214.25 (93.96) | 93 | −2.28 | .02 | (−400.83, −27.68) | −.21 |
Note: Mixed linear models were used to estimate mean differences in pre to post-intervention changes between mindfulness and control group participants. Models testing cardiovascular outcomes adjusted for age, gender, beta-blocker medication use, body mass index at baseline, and body mass index x month interaction. A positive value, for example on challenge-related emotions, means that mindfulness participants, on average, reported greater increases in challenge-related emotions related to the Trier Social Stress Test compared to the control group, or that declines in challenge-related emotions were lower among mindfulness compared to control participants.
Figure 2.
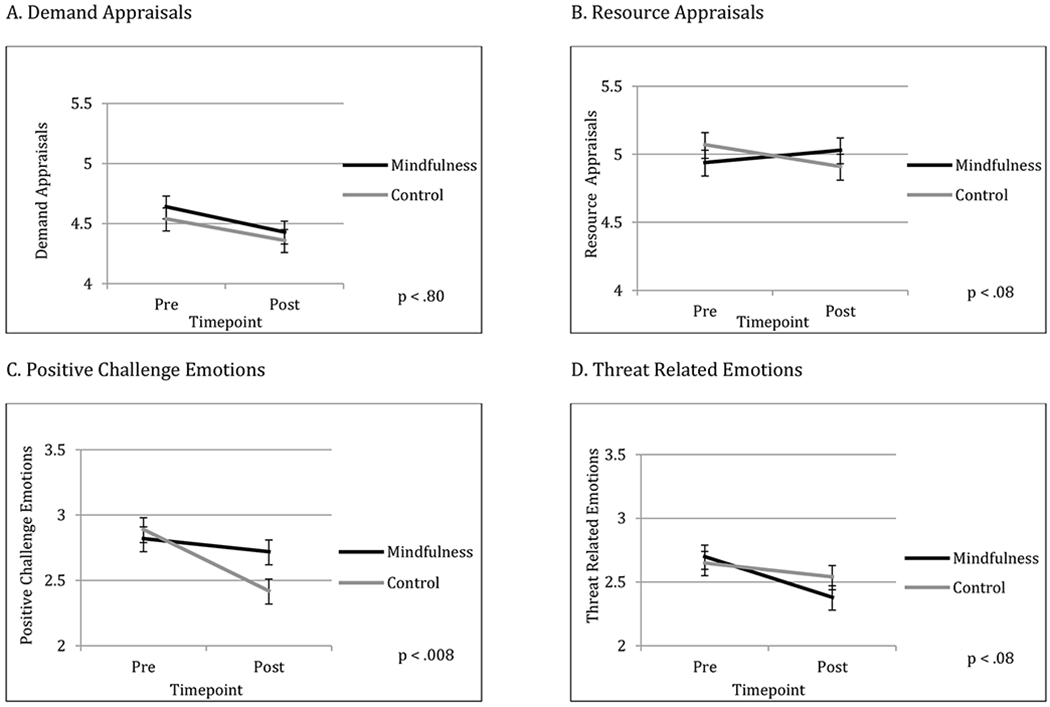
Changes in appraisals and emotions over time. Each panel shows changes from pre to post-intervention, with the mindfulness participants represented by black lines and the active control group represented by grey lines. Standard error bars are shown. Panel A shows changes in demand appraisals, panel B shows changes in resource appraisals, panel C shows changes in positive challenge emotions, and panel D shows changes in threat-related emotions. See Table 2 for means and standard errors and Table 3 for statistical tests between timepoints.
Cardiovascular Reactivity
We first examined changes in pre-ejection period to determine if both groups experienced sympathetic nervous system activation, a requirement for examining the challenge-threat distinction. Given the intense nature of the TSST, we observed significant average increases in sympathetic activation, indicated by shortened PEP, for TSSTs (pre and post) across both groups (all ps < .001). Mindfulness and control groups did not significantly differ in PEP changes from pre to post-intervention during the speech (p = .26) and math tasks (p = .70; see Tables 2 and 3).
We next examined group differences in changes in CO and TPR. Mindfulness participants exhibited significantly greater increases in CO during the speech task from pre to post-intervention compared to active control participants (p = .049; ES = .19; see Tables 2 and 3 and Figure 2), though no statistically significant differences between groups were found during the math task (p = .32; ES = .09). Participants in the mindfulness group also showed significantly lower TPR during the speech and math tasks from pre to post-intervention compared to the active control participants (p = .0499, ES = −.19 and p = .02, ES = −.21, respectively; see Tables 2 and 3 and Figure 2).
Discussion
In this study, we examined appraisals, emotions, and cardiovascular reactivity in response to repeated acute social stressors among participants with obesity randomly assigned to a mindfulness-based weight loss intervention or active control condition. Specifically, we hypothesized that mindfulness participants would respond to a socially-evaluative stressor with greater challenge-oriented appraisals, emotions and cardiovascular reactions compared to active control participants from pre to post-intervention. Overall, we found a pattern of evidence consistent with this hypothesis for cardiovascular responses and more limited support for self-reports of appraisals and emotions during the repeated social stress tasks.
Interestingly, we found that both groups reported similar reductions in the perceived demands of the task from pre to post-intervention as may be expected based on prior research on habituation to the TSST (Jonsson et al., 2010; Kelsey et al., 1999). However, mindfulness compared to control participants tended to report greater resources to meet those demands from pre-to post intervention. This pattern of changes in resource appraisals is consistent with changes in emotional responses between groups, such that the mindfulness participants reported significantly greater maintenance of positive, challenge-related emotions and tended to report greater reductions in threat-related emotions compared to control participants from pre to post-intervention. Only one of the appraisals and emotions measures was statistically significant, though the pattern was consistent with hypotheses. Effect sizes were small though meaningful, in line with a review of effect sizes of mindfulness interventions for trait levels of psychological distress in randomized controlled trials (Goyal et al, 2014).
These patterns of more adaptive appraisal and emotional responses to the social-evaluative stress task over time are also consistent with the patterns of cardiovascular reactivity showing that mindfulness participants exhibited greater increases in CO during the speech task compared to control participants from pre to post-intervention. Mindfulness participants also showed greater decreases in TPR during the speech and math tasks over time compared to control participants who showed increased TPR. Other research has found that vascular resistance actually increases in response to repeated stress contrary to the notion of habituation (Kelsey et al., 1999); thus, mindfulness training may have prevented such increases from occurring. These results held after controlling for demographic variables, baseline BMI, and changes in BMI. Higher BMI has been related to blunted cardiovascular reactions to stress (Carroll et al., 2017); thus weight loss is an important covariate to ensure effects are not due to differential weight loss between groups.
Overall, the pattern of findings supports the notion that the mindfulness intervention increased ‘adaptive stress responsiveness’ rather than reduced stress reactivity across repeated, high-arousal social stress tasks, as groups did not differ in changes in PEP, a measure of sympathetic nervous system arousal. These results suggest that mindfulness training may not necessarily result in less sympathetic activation but may facilitate an adaptive physiological responsivity to repeated stressful events. For example, challenge profiles have been found to result in increased brain and periphery blood-oxygenation and are often associated with improved cognitive and cooperative performance (Buhle et al., 2014; Creswell, 2017; Hofmann et al., 2010; Katterman et al., 2014).
From this study, we did not assess how mindfulness training may have shifted psychological and cardiovascular reactivity to repeated stressors. A recent “mindfulness-to-meaning” theory posits that mindfulness facilitates positive reappraisals through reduced identification with and disengagement from negative appraisals and attentional biases which free up cognitive resources to broaden the scope of attention and develop meaningful interpretations of stressful events that enhance purposeful engagement (Garland, Farb, Goldin, & Fredrickson, 2015). Stressful events initially perceived as threatening may be re-construed as benign, meaningful, or growth promoting (Lazarus & Folkman, 1984). As the mindfulness participants reported stable levels of challenge-related emotions and tended to report greater decreases in threat-related emotions during the second social-evaluative stress test relative to control participants, our data support this theory.
The current challenge and threat paradigm indicates that shifts in appraisals can, at least to some extent, lead to shifts in cardiovascular profiles. It is important to note that mindfulness meditation may shift cardiovascular responses through pathways other than direct reappraisal. Our study design did not allow us to directly assess these pathways, but several plausible pathways could be examined in future research. As one possible pathway, the development of meta-cognitive awareness of threat-related appraisals and emotions itself may reduce the impact of these appraisals or emotions on cardiovascular reactivity. This idea is consistent with an earlier study in which we found a significant association between anxiety and the stress-reactive measure of the cortisol awakening response among individuals low in the acceptance dimension of dispositional mindfulness but not among individuals who reported higher levels of acceptance (Daubenmier, Hayden, Chang, & Epel, 2014). Other research reports a similar decoupling of stress-related psychological and physiological responses as a function of mindfulness (Creswell et al., 2014; Feldman, Lavalle, Gildawie, & Greeson, 2016).
A second possible mechanism by which mindfulness meditation may alter physiological responses is through increased positive affect, which broadens attentional resources to notice and appreciate positive aspects of one’s experience (Garland et al., 2015). Given that participants had obesity, a third possibility is that mindfulness specifically reduced internalized weight stigma or perceptions of weight bias through processes of meta-cognitive awareness as they were evaluated during the social stress task. Research from our group suggests that weight stigma independent of weight is associated with physiological stress markers (Tomiyama et al., 2014). The degree to which mindfulness may modulate the impact of threat-related appraisals on cardiovascular reactivity to social stress and how these processes may occur are areas in need of further inquiry.
The results of the current study suggest mindfulness training may be especially beneficial for persons with obesity in promoting more adaptive psychological and cardiovascular responses to repeated socially-evaluative stressors. More adaptive stress responses may have positive effects on health behaviors as some research indicates that blunted cardiovascular reactivity to stress may reflect impairments in brain systems essential for motivation and behavioral regulation (Carroll et al., 2017). The potential impact of adaptive stress responses on health behaviors and longer-term health outcomes is an area worthy of further research.
Limitations and Future Directions
The majority of our sample was women, thus future research may wish to examine these processes in men. Secondly, our study sample was predominately White and college educated, and future research may specifically examine how racial/ethnic minority-related stress and economic background may impact the effects of mindfulness training on exposure to repeated stress in the context of the challenge and threat framework. Thirdly, it is possible our results do not fully apply to persons of normal weight. As individuals who are above normal weight tend to have blunted autonomic reactions to acute stress, it is possible that normal weight individuals might show greater improvements in cardiovascular reactivity after mindfulness training. Fourth, although participants were masked to the design of the trial involving mindfulness meditation before randomization and even though we took great care to control for type of stress management training, attention, social support, and expectations of benefit, our findings nevertheless may have resulted from differing expectations across the two groups as the study progressed. Fifth, we found that older participants and those who lost less weight during the intervention were less likely to complete the post-intervention TSST; thus, results may generalize less to these subgroups. Future studies may need to address potential concerns that older adults and those who show less improvement on primary outcomes may have with completing post-intervention stress reactivity assessments. Sixth, although our training included meditations and components drawing on Mindfulness-based Stress Reduction, it also included components of Mindfulness-based Eating-Awareness Training (Kristeller & Wolever, 2011), entailing more sessions than either program. Thus, our results may differ from either of these interventions alone. Finally, we note that results should be interpreted in the context of multiple hypothesis testing and future studies should aim to replicate findings.
In conclusion, mindfulness training may promote adaptive psychological and cardiovascular responses and reduce the development of maladaptive stress reactions in the face of acute, repeated stressors in obesity. Here, we show mindfulness intervention effects on challenge and threat psychophysiological states under standardized repeated stress conditions. Our results suggest that mindfulness training may lead to subtle changes in stress reactivity over time which could have salutary benefits to health, and this possibility deserves direct examination in future studies.
Figure 3.
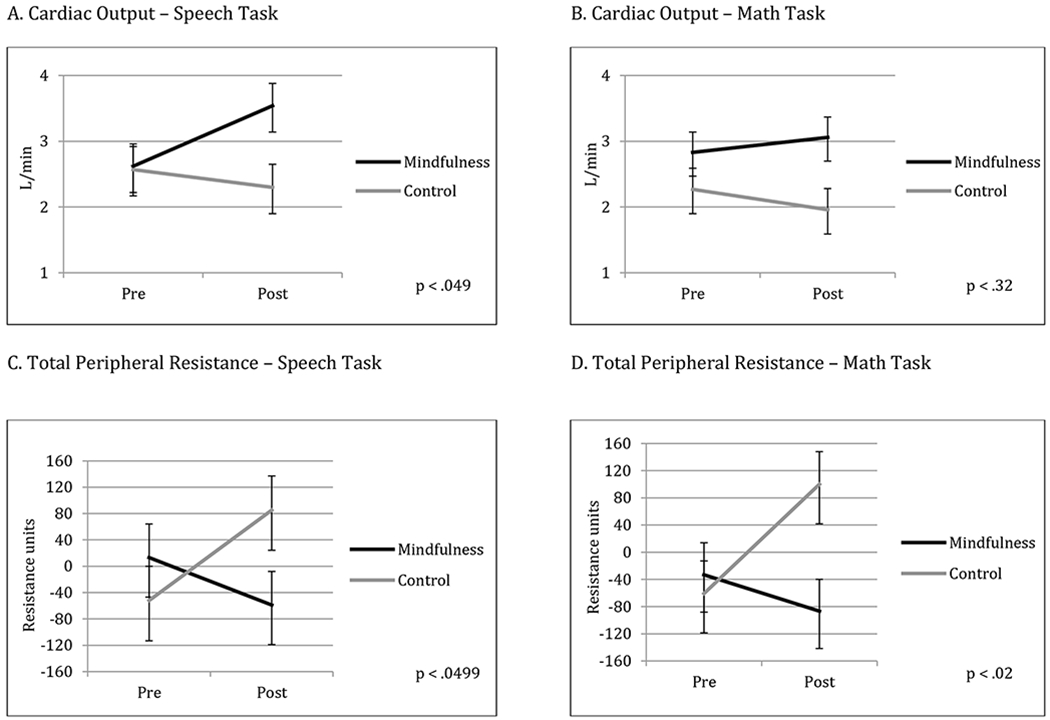
Changes in cardiovascular reactivity over time. Each panel shows changes from pre to post-intervention, with the mindfulness participants represented by black lines and the active control group represented by grey lines. Standard error bars are shown. Panel A shows changes in cardiac output during the speech task, panel B shows changes in cardiac output during the math task, panel C shows changes in total peripheral resistance during the speech task, and panel D shows changes in total peripheral resistance during the math task. See Table 2 for means and standard errors and Table 3 for statistical tests between timepoints.
Acknowledgments
Funding: This study was supported by NIH grants from the National Center for Complementary and Integrative Health (NCCIH) P01AT005013, K24AT007827, and K01AT004199, as well as the National Center for Advancing Translational Sciences, UCSF-CTSI Grant Number UL1 TR000004.
Conflicts of Interest and Sources of Funding: No conflicts of interest reported. This study was supported by NIH grants from the National Center for Complementary and Integrative Health (NCCIH) P01AT005013 (Hecht),K24AT007827 (Hecht), and K01AT004199 (Daubenmier), as well as the National Center for Advancing Translational Sciences, UCSF-CTSI Grant Number UL1 TR000004.
Footnotes
Conflict of Interest: No authors report any conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were approved by University of California, San Francisco in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- American Psychological Association (2017). Stress in America: The State of Our Nation. Stress in America Survey; Retrieved from https://www.apa.org/images/state-nation_tcm7-225609.pdf. [Google Scholar]
- Bennett C, Blissett J, Carroll D, & Ginty AT (2014). Rated and measured impulsivity in children is associated with diminished cardiac reactions to acute psychological stress. Biological Psychology, 102, 68–72. doi: 10.1016/j.biopsycho.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Blascovich J , & Mendes WB (2010). Social psychophysiology and embodiment . In Fiske ST (Ed.), The Handbook of Social Psychology (5th ed., pp. 194–227). New York, NY: Wiley. [Google Scholar]
- Blascovich J, Mendes WB, Hunter SB, & Salomon K (1999). Social “facilitation” as challenge and threat. Journal of Personality and Social Psychology, 77(1), 68–77. [DOI] [PubMed] [Google Scholar]
- Brindle RC, Whittaker AC, Bibbey A, Carroll D, & Ginty AT (2017). Exploring the possible mechanisms of blunted cardiac reactivity to acute psychological stress. International Journal of Psychophysiology, 113, 1–7. doi: 10.1016/j.ijpsycho.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Britton WB, Shahar B, Szepsenwol O, & Jacobs WJ (2012). Mindfulness-based cognitive therapy improves emotional reactivity to social stress: results from a randomized controlled trial. Behavior Therapy, 43(2), 365–380. doi: 10.1016/j.beth.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, & Creswell JD (2007). Mindfulness: Theoretical foundations and evidence for its salutary effects. Psychological Inquiry, 18(4), 211–237. [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. doi: 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Whittaker AC, Lovallo WR, & de Rooij SR (2017). The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neuroscience and Biobehavioral Reviews, 77, 74–86. doi: 10.1016/j.neubiorev.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, & Steptoe A (2010). Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension, 55(4), 1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621 [DOI] [PubMed] [Google Scholar]
- Chrousos GP (2009). Stress and disorders of the stress system. Nature Reviews Endocrinology, 5(7), 374–381. doi: 10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- Creswell JD (2017). Mindfulness Interventions. Annual Review of Psychology, 68, 491–516. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Pacilio LE, Lindsay EK, & Brown KW (2014). Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology, 44, 1–12. doi: 10.1016/j.psyneuen.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Daubenmier J, Hayden D, Chang V, & Epel E (2014). It’s not what you think, it’s how you relate to it: dispositional mindfulness moderates the relationship between psychological distress and the cortisol awakening response. Psychoneuroendocrinology, 48, 11–18. doi: 10.1016/j.psyneuen.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Moran PJ, Kristeller J, Acree M, Bacchetti P, Kemeny ME, … Hecht FM (2016). Effects of a mindfulness-based weight loss intervention in adults with obesity: A randomized clinical trial. Obesity, 24(4), 794–804. doi: 10.1002/oby.21396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. doi: 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dienstbier RA (1989). Arousal and physiological toughness: Implications for mental and physical health. Psychological Review, 96(1), 84–100. 10.1037/0033-295X.96.1.84 [DOI] [PubMed] [Google Scholar]
- Drach-Zahavy A, & Erez M (2002). Challenge versus threat effects on the goalperformance relationship. Organizational Behavior and Human Decision Processes, 88, 667–682. [Google Scholar]
- Dreyer D , & Dreyer K (2006). Chi Walking: The Five Mindful Steps for Lifelong Health and Energy. New York, NY: Simon and Schuster. [Google Scholar]
- Feldman G, Lavalle J, Gildawie K, & Greeson JM (2016). Dispositional Mindfulness Uncouples Physiological and Emotional Reactivity to a Laboratory Stressor and Emotional Reactivity to Executive Functioning Lapses in Daily Life. Mindfulness, 7(2), 527–541. doi: 10.1007/s12671-015-0487-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeuser M (1986). A psychobiological framework for research on human stress and coping In Appley M & Trumbull R (Eds.), Dynamics of stress: Physiological, psychological, and social perspectives (pp. 101–116). New York, NY: Plenum Press. [Google Scholar]
- Garland EL, Farb NA, Goldin P, & Fredrickson BL (2015). Mindfulness Broadens Awareness and Builds Eudaimonic Meaning: A Process Model of Mindful Positive Emotion Regulation. Psychological Inquiry, 26(4), 293–314. doi: 10.1080/1047840X.2015.1064294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Kraynak TE, Fisher JP, & Gianaros PJ (2017). Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Autonomic Neuroscience. doi: 10.1016/j.autneu.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Bernal F, Madva EN, Puckett J, Amonoo HL, Millstein RA, & Huffman JC (2019). Relationships Between Life Stressors, Health Behaviors, and Chronic Medical Conditions in Mid-Life Adults: A Narrative Review. Psychosomatics, 60(2), 153–163. doi: 10.1016/j.psym.2018.12.007 [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA (2014). Meditation Programs for Psychological Stress and Well-being: A Systematic Review and Meta-analysis. JAMA Internal Medicine. doi: 10.1001/jamainternmed.2013.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes-Skelton S, & Graham J (2013). Decentering as a common link among mindfulness, cognitive reappraisal, and social anxiety. Behavioral and Cognitive Psychotherapy, 41, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP (1986). Neuroendocrine patterns of emotional response In Plutchik R & Kellerman H (Eds.), Emotion, theory, research, and experience (Vol. 3, pp. 37–60). Orlando, FL: Academic Press. [Google Scholar]
- Herhaus B, & Petrowski K (2018). Cortisol Stress Reactivity to the Trier Social Stress Test in Obese Adults. Obesity Facts, 11(6), 491–500. doi: 10.1159/000493533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, & Oh D (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology, 78(2), 169–183. doi: 10.1037/a0018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Bui E, Marques L, Metcalf CA, Morris LK, Robinaugh DJ, … Simon NM (2013). Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. Journal of Clinical Psychiatry, 74(8), 786–792. doi: 10.4088/JCP.12m08083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Bui E, Palitz SA, Schwarz NR, Owens ME, Johnston JM, … Simon NM (2017). The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Research. doi: 10.1016/j.psychres.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JP, Nock MK, & Mendes WB (2012). Mind over matter: reappraising arousal improves cardiovascular and cognitive responses to stress. Journal of Experimental Psychology: General, 141(3), 417–422. doi: 10.1037/a0025719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, … Manning WJ (2010). Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation, 122(7), 690–697. doi: 10.1161/CIRCULATIONAHA.109.905091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, McMillan MR, Jones RW, Kowalik GT, Steeden JA, Deanfield JE, … Muthurangu V (2012). Adiposity is associated with blunted cardiovascular, neuroendocrine and cognitive responses to acute mental stress. PLoS One, 7(6), e39143. doi: 10.1371/journal.pone.0039143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson P, Wallergard M, Osterberg K, Hansen AM, Johansson G, & Karlson B (2010). Cardiovascular and cortisol reactivity and habituation to a virtual reality version of the Trier Social Stress Test: a pilot study. Psychoneuroendocrinology, 35(9), 1397–1403. doi: 10.1016/j.psyneuen.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. doi: 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full Catastrophe Living. New York, NY: Dell Publishing. [Google Scholar]
- Kassam KS, Koslov K, & Mendes WB (2009). Decisions under distress: stress profiles influence anchoring and adjustment. Psychological Science, 20(11), 1394–1399. doi: 10.1111/j.1467-9280.2009.02455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katterman SN, Kleinman BM, Hood MM, Nackers LM, & Corsica JA (2014). Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: a systematic review. Eating Behavior, 15(2), 197–204. doi: 10.1016/j.eatbeh.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Blascovich J, Tomaka J, Leitten CL, Schneider TR, & Wiens S (1999). Cardiovascular reactivity and adaptation to recurrent psychological stress: effects of prior task exposure. Psychophysiology, 36(6), 818–831. [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1-2), 76–81. doi: 119004 [DOI] [PubMed] [Google Scholar]
- Kristeller JL, & Wolever RQ (2011). Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eating Disorders, 19(1), 49–61. [DOI] [PubMed] [Google Scholar]
- Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psycholology, 4, 863. doi: 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R, & Folkman S (1984). Stress, Appraisal, and Coping. New York, NY: Springer. [Google Scholar]
- Mathews KA (2005). Psychological perspectives on the development of coronary heart disease American Psychologist, 60, 783–796. 10.1037/0003-066X.60.8.783 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, & Nasca C (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18(10), 1353–1363. doi: 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Alves S, & Mendes WB (2014). Child maltreatment and autonomic nervous system reactivity: identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosomatic Medicine, 76(7), 538–546. doi: 10.1097/PSY.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB (2009). Assessing the autonomic nervous system In Harmon-Jones E & Beer JS (Eds.), Methods in Social Neuroscience. (pp. 119–147). New York: NY: Guildford Press. [Google Scholar]
- Mendes WB, Gray H, Mendoza-Denton R, Major B, & Epel E (2007). Why egalitarianism might be good for your health: Physiological thriving during stressful intergroup encounters. Psychological Science, 18, 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Major B, McCoy S , & Blascovich J (2008). How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of Personality and Social Psychology 94, 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). (2002). Third report of the National Cholesterol Education PRogram (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation, 106, 3142–3421. [PubMed] [Google Scholar]
- Nyklicek I, Mommersteeg PM, Van Beugen S, Ramakers C, & Van Boxtel GJ (2013). Mindfulness-based stress reduction and physiological activity during acute stress: a randomized controlled trial. Health Psychology, 32(10), 1110–1113. doi: 10.1037/a0032200 [DOI] [PubMed] [Google Scholar]
- O’Reilly GA, Cook L, Spruijt-Metz D, & Black DS (2014). Mindfulness-based interventions for obesity-related eating behaviours: a literature review. Obes Rev, 15(6), 453–461. doi: 10.1111/obr.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P, & Julius S (2009). The role of cardiac autonomic function in hypertension and cardiovascular disease. Currrent Hypertension Reports, 11(3), 199–205. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, & Hughes BM (2013). The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International Journal of Psychophysiology, 90(1), 1–7. doi: 10.1016/j.ijpsycho.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, & Lutz A (2013). A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behavior and Immunity, 27(1), 174–184. doi: 10.1016/j.bbi.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R (2005). Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology, 30(1), 1–10. doi: 10.1016/j.psyneuen.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, & Siegel SD (2005). Stress and health: psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology, 1, 607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sominsky L, & Spencer SJ (2014). Eating behavior and stress: a pathway to obesity. Frontiers in Psychology, 5, 434. doi: 10.3389/fpsyg.2014.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen PR, & Larson MJ . (2015). A brief mindfulness exercise reduces cardiovascular reactivity during a laboratory stressor paradigm. Mindfulness, 6, 803–811. 10.1007/s12671-014-0320-4 [DOI] [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, & Segal ZV (2002). Metacognitive awareness and prevention of relapse in depression: empirical evidence. Journal of Consulting and Clinical Psychology, 70(2), 275–287. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, & Epel ES (2011). Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology, 36(10), 1513–1519. doi: 10.1016/j.psyneuen.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama AJ, Epel ES, McClatchey TM, Poelke G, Kemeny ME, McCoy SK, & Daubenmier J (2014). Associations of weight stigma with cortisol and oxidative stress independent of adiposity. Health Psychology, 33(8), 862–867. doi: 10.1037/hea0000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres SJ, Turner AI, Jayasinghe SU, Reynolds J, & Nowson CA (2014). The effect of overweight/obesity on cardiovascular responses to acute psychological stress in men aged 50-70 years. Obesity Facts, 7(6), 339–350. doi: 10.1159/000369854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon MS, DeCant R, & Laugero KD (2013). Having your cake and eating it too: a habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiology and Behavior, 114-115, 32–37. doi: 10.1016/j.physbeh.2013.02.018 [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Hitchcock P, Shahar B, & Britton W (2012). The effects of mindfulness-based cognitive therapy on affective memory recall dynamics in depression: a mechanistic model of rumination. Frontiers in Human Neuroscience, 6, 257. doi: 10.3389/fnhum.2012.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Chida Y, Gibson EL, Whitaker KL, & Steptoe A (2011). Stress and adiposity: a meta-analysis of longitudinal studies. Obesity,, 19(4), 771–778. doi: 10.1038/oby.2010.241 [DOI] [PubMed] [Google Scholar]
- Weinstein N, BRown KW , & Ryan RM (2009). A multi-method examination of the effects of mindfulness on stress attribution, coping, and emotinal well-being. Journal on Research and Personality 43, 374–385. 10.1016/j.jrp.2008.12.008 [DOI] [Google Scholar]
- Wiggert N, Wilhelm FH, Nakajima M, & al’Absi M (2016). Chronic Smoking, Trait Anxiety, and the Physiological Response to Stress. Substance Use and Misuse, 51(12), 1619–1628. doi: 10.1080/10826084.2016.1191511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau YH, & Potenza MN (2013). Stress and eating behaviors. Minerva Endocrinologica, 38(3), 255–267. [PMC free article] [PubMed] [Google Scholar]


