Abstract
Background
Topically applied fluoride gels have been widely used as a caries‐preventive intervention in dental surgeries and school‐based programmes for over three decades. This updates the Cochrane review of fluoride gels for preventing dental caries in children and adolescents that was first published in 2002.
Objectives
The primary objective is to determine the effectiveness and safety of fluoride gels in preventing dental caries in the child and adolescent population.
The secondary objectives are to examine whether the effect of fluoride gels is influenced by the following: initial level of caries severity; background exposure to fluoride in water (or salt), toothpastes, or reported fluoride sources other than the study option(s); mode of use (self applied under supervision or operator‐applied), and whether there is a differential effect between the tray and toothbrush methods of application; frequency of use (times per year) or fluoride concentration (ppm F).
Search methods
We searched the Cochrane Oral Health Group Trials Register (to 5 November 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library 2014, Issue 11), MEDLINE via OVID (1946 to 5 November 2014), EMBASE via OVID (1980 to 5 November 2014), CINAHL via EBSCO (1980 to 5 November 2014), LILACS and BBO via the BIREME Virtual Health Library (1980 to 5 November 2014), ProQuest Dissertations and Theses (1861 to 5 November 2014) and Web of Science Conference Proceedings (1945 to 5 November 2014). We undertook a search for ongoing trials on ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform on 5 November 2014. We placed no restrictions on language or date of publication in the search of the electronic databases. We also searched reference lists of articles and contacted selected authors and manufacturers.
Selection criteria
Randomised or quasi‐randomised controlled trials where blind outcome assessment was stated or indicated, comparing topically applied fluoride gel with placebo or no treatment in children up to 16 years. The frequency of application had to be at least once a year, and study duration at least one year. The main outcome was caries increment measured by the change in decayed, missing and filled tooth surfaces in both permanent and primary teeth (D(M)FS and d(e/m)fs).
Data collection and analysis
At least two review authors independently performed study selection, data extraction and 'Risk of bias' assessment. We contacted study authors for additional information where required. The primary measure of effect was the prevented fraction (PF), that is, the difference in mean caries increments between the treatment and control groups expressed as a percentage of the mean increment in the control group. We performed random‐effects meta‐analyses where we could pool data. We examined potential sources of heterogeneity in random‐effects metaregression analyses. We collected adverse effects information from the included trials.
Main results
We included 28 trials (3 of which are new trials since the original review), involving 9140 children and adolescents. Most of these trials recruited participants from schools. Most of the studies (20) were at high risk of bias, with 8 at unclear risk of bias.
Twenty‐five trials (8479 participants) contributed data for meta‐analysis on permanent tooth surfaces: the D(M)FS pooled prevented fraction (PF) estimate was 28% (95% confidence intervals (CI) 19% to 36%; P < 0.0001; with substantial heterogeneity (P < 0.0001; I2 = 82%); moderate quality evidence). Subgroup and metaregression analyses suggested no significant association between estimates of D(M)FS prevented fractions and the prespecified trial characteristics. However, the effect of fluoride gel varied according to the type of control group used, with D(M)FS PF on average being 17% (95% CI 3% to 31%; P = 0.018) higher in non‐placebo‐controlled trials (the reduction in caries was 38% (95% CI 24% to 52%; P < 0.0001, 2808 participants) for the 10 trials with no treatment as control group, and 21% (95% CI 15% to 28%; P < 0.0001, 5671 participants) for the 15 placebo‐controlled trials. A funnel plot of the 25 trials in the D(M)FS PF meta‐analysis indicated a relationship between prevented fraction and study precision, with an apparent lack of small studies with statistically significant large effects.
The d(e/m)fs pooled prevented fraction estimate for the three trials (1254 participants) that contributed data for the meta‐analysis on primary teeth surfaces was 20% (95% CI 1% to 38%; P = 0.04; with no heterogeneity (P = 0.54; I2 = 0%); low quality evidence).
There was limited reporting of adverse events. Only two trials reported information on acute toxicity signs and symptoms during the application of the gel (risk difference 0.01, 95% CI ‐0.01 to 0.02; P = 0.36; with no heterogeneity (P = 36; I2 = 0%); 490 participants; very low quality evidence). None of the trials reported information on tooth staining, mucosal irritation or allergic reaction.
Authors' conclusions
The conclusions of this updated review remain the same as those when it was first published. There is moderate quality evidence of a large caries‐inhibiting effect of fluoride gel in the permanent dentition. Information concerning the caries‐preventive effect of fluoride gel on the primary dentition, which also shows a large effect, is based on low quality evidence from only three placebo‐controlled trials. There is little information on adverse effects or on acceptability of treatment. Future trials should include assessment of potential adverse effects.
Plain language summary
Fluoride gels for preventing tooth decay in children and adolescents
Review question
The main question was: How effective and safe is the use of fluoride gel for the prevention of tooth decay (dental caries) in children and adolescents compared to placebo (a treatment without the active ingredient fluoride) or no treatment?
Background
Tooth decay is a significant health problem worldwide, affecting not only the vast majority of adults but also 60% to 90% of children. Levels of tooth decay vary between and within countries, but it is generally true that children in lower socioeconomic groups (measured by income, education and employment) have more tooth decay. Over time, untreated tooth decay causes progressive destruction of the tops of teeth (crowns); this is often accompanied by severe pain. Repairing and replacing decayed teeth is extremely costly in terms of time and money and is a major drain on the resources of healthcare systems.
The prevention of tooth decay in children and adolescents is regarded as a priority for dental services and is considered more cost‐effective than its treatment. The use of fluoride, a mineral that prevents tooth decay, is widespread. As well as occurring naturally, fluoride is added to the water supply in some areas, and it is used in most toothpastes and in other products that are available to varying degrees worldwide. As an extra preventive measure there are other ways of applying fluoride directly to teeth, such as mouthrinses, lozenges, varnishes and gels.
Fluoride gel is usually applied by a dental professional, or self applied under supervision (depending on the age of the child), from once a year to several times a year. The gel is usually placed in a tray that the child or young person must keep in their mouth and bite into for about four minutes. It is not uncommon for young people to accidentally swallow some of the gel; feelings of sickness, vomiting, headache and stomach pain have been reported when too much is swallowed. Due to this risk of toxicity, fluoride gel treatment is not generally recommended for children less than six years old.
This review updates the Cochrane review of fluoride gels for preventing tooth decay in children and adolescents that was first published in 2002. We assessed the existing research for the Cochrane Oral Health Group, and the evidence is current up to 5 November 2014.
Study characteristics
We included 28 studies in which over 9000 children (aged 2 to 15 years) were randomised to treatment with fluoride gel or to a control group using placebo gel or receiving no treatment. Study duration ranged from 1 to 4 years (with 13 studies lasting around 2 years). Study reports were published between 1967 and 2005. Thirteen studies took place in the USA, seven in Europe, four in Brazil and one each in Canada, Israel, China and Venezuela.
Key results
This review update confirmed that fluoride gel can reduce tooth decay in children and adolescents. We combined the results of 25 trials and found that on average there is a 28% reduction in decayed, missing and filled tooth surfaces (21% reduction in trials that used a placebo gel in the control group and 38% reduction in trials where the control group received no treatment) in permanent teeth. From the three trials looking at the effect of fluoride gel on first or baby teeth, the evidence suggests that using fluoride gel results in a 20% reduction in decayed, missing and filled tooth surfaces. We found little information about unwanted or harmful effects or how well children and young people were able to cope with the application of the gel.
Conclusion
The application of fluoride gel results in a large reduction in tooth decay in both permanent and baby teeth. We found little information about potential unwanted or harmful effects from accidental swallowing of the gel during treatment. As children often swallow gel during application, more research is needed on these effects.
Quality of the evidence
The evidence available for permanent teeth is of moderate quality. The evidence on baby teeth is low quality because of the small number of studies available. The evidence available for adverse effects is very low quality.
Summary of findings
Summary of findings 1. Summary of findings.
| Fluoride gel compared with placebo or no treatment for caries prevention in children and adolescents | ||||||
|
Patient or population: Children and adolescents Settings: Community (predominantly schools) Intervention: Fluoride gel Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment/placebo | Fluoride gel | |||||
|
Changes in caries on the surfaces of permanent teeth, measured by D(M)FS increment ‐ nearest to 3 years |
The mean increment ranged across control groups from 0.2 to 11.5, median 1.7 | The mean increment in the intervention groups was 0.27 (95% CI 0.18 to 0.37) lower | PF1 28% (95% CI 19% to 36%) | 8479 (25 studies) |
moderate ⊕⊕⊕⊝2 |
|
| Changes in caries on the surfaces of primary teeth, measured by d(m)fs increment ‐ nearest to 3 years | The mean increment ranged across control groups from 1.8 to 5.1, median 1.8 | The mean increment in the intervention groups was 0.52 (95% CI 0.17 to 0.88) lower | PF1 20% (95% CI 1% to 38%) | 1254 (3 studies) |
low ⊕⊕⊝⊝3 |
|
| Signs of acute toxicity ‐ nausea, vomiting | 0 per 1000 | 10 per 1000 (10 fewer to 20 more) | RD | 490 (2 studies) | ⊕⊝⊝⊝ very low4,5, | Risks were calculated from pooled risk differences (RD: 0.01 (95% ‐0.01, 0.02) |
| *The basis for the assumed risk was the range and median in the control groups of the studies included in the review. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; D(M)FS: decayed, (missing) and filled permanent surfaces; PF: prevented fraction; RD: risk difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1PF = 1 ‐ (mean increment in control group/mean increment in treatment group) (expressed as percentages). PF values between 1% to 10% are considered to be a small effect; between 10% to 20%, a moderate effect; values above 20% are considered a large or substantial effect.
2 Quality of evidence was downgraded because of the limitations in study design (at least 85% of information is from studies with unclear sequence generation or allocation concealment or both; about 50% are from studies with no placebo use). Although there was high statistical heterogeneity in this outcome, it was not further downgraded for inconsistency as results consistently showed a large clinical effect in caries reduction.
3 More than 70% of information comes from a study at low risk of bias for key domains of sequence generation, allocation concealment, and blinding. However, evidence was downgraded twice because only 3 out of 28 studies reported this information; there is concern about publication bias, imprecision of results and the relevance of this outcome to clinical practice.
4 Trials either had unclear or high risk of bias in sequence generation and allocation concealment. 5 Information only available from two trials.
Background
Description of the condition
Dental caries is the most prevalent chronic disease, afflicting a significant proportion of the world population, including around 60% to 90% of school‐aged children and the vast majority of adults (Marcenes 2013; Petersen 2004). In general, dental caries levels vary considerably between and within different countries, but children in the lower socioeconomic status groups have higher caries levels than those in the upper socioeconomic status groups, and in high‐income countries the association between socioeconomic position and caries might be stronger (Chen 1995; Reisine 2001; Schwendicke 2015). Untreated caries causes progressive destruction of the crowns of the teeth, often accompanied by severe pain and suffering, especially in children, where it can result in poorer quality of life and general health (Sheiham 2005). Untreated caries in permanent teeth was the most prevalent condition among all evaluated in the Global Burden of Disease 2010 study, affecting 35% of the global population, or 2.4 billion people, and untreated caries in deciduous teeth was the 10th most prevalent condition, affecting 9% of the population, or 621 million children worldwide (Kassebaum 2015). The repair and replacement of carious teeth is excessively time consuming and costly, representing a major drain of resources for healthcare systems. On a population basis, dental caries is the fourth most expensive chronic disease to treat, according to the World Health Organization (Petersen 2008).
Dental caries occurs because of demineralisation of tooth structure by organic acids formed by oral bacteria present in dental plaque through the anaerobic metabolism of dietary sugars. The causal role of sugars in caries is well established (Sheiham 2001). The majority of caries lesions in children’s permanent teeth advance relatively slowly, with an average lesion taking three years to progress through tooth enamel to dentine (Mejare 1998). The dental caries process is influenced by the susceptibility of the tooth surface, the bacterial profile, the quantity and quality of saliva, and the presence of fluoride, which promotes remineralisation and inhibits the demineralisation of the tooth structure.
Description of the intervention
Fluoride gels are widely used in dental surgeries and school‐based caries‐preventive programmes. Although currently recommended only for children with moderate and high caries levels, the cost‐effectiveness of gels has been questioned even for these populations (van Rijkom 1998). Fluoride gels are either administered by a professional or are self applied under supervision. In general, operator‐applied fluoride gels use trays and self applied gels use either a tray or a toothbrush. Fluoride gels must be differentiated from some fluoride toothpastes, which are also available in the form of gels. The 'classical' fluoride gels do not contain abrasives, their fluoride concentration is usually much higher than that of a fluoride toothpaste and they are applied at relatively infrequent intervals. Various methods, concentrations and frequencies of gel applications have been tested, with or without prior dental prophylaxis, and different fluoride compounds have been used. Typically, acidulated phosphate fluoride (APF) gels in the concentration of 12,300 parts per million of fluoride (ppm F) are professionally applied twice a year. The excessive ingestion of fluoride during topical application is not an uncommon occurrence (Whitford 1992); the greatest health hazard is associated with the use of 12,300 ppm F APF gels, where a considerable amount of fluoride may be retained after application. The probable toxic dose of 100 mg of fluoride for a 20 kg (five‐ to six‐year‐old) child is contained in only 8 ml volumes of these gels. Approximately 5 ml is used in a topical application of APF gel in a tray, representing a potential exposure of 61.5 mg of fluoride ion. There is a significant risk of overexposure, which can result in acute toxicity (Ripa 1990). Young people receiving fluoride gel applications have reported nausea, vomiting, headache and abdominal pain. Because of the risk of overingestion, the use of fluoride gels in young children is not generally recommended.
Numerous clinical trials evaluating the caries‐preventive effect of fluoride gels have been reported; these have been the subject of narrative reviews, in Ripa 1989 and Ripa 1991, and of systematic reviews and meta‐analyses (Clark 1985; van Rijkom 1998; Weyant 2013). Although it is evident from these reviews and meta‐analyses that fluoride gels are caries‐inhibitory treatments, they either failed to include a comprehensive and well‐designed search for individual trials or a formal evaluation of the risk of bias in included trials, despite obvious drawbacks in the design and methods in the studies.
How the intervention might work
The most important anticaries effect of fluoride is considered to result from its local action on the tooth/plaque interface, through promoting remineralisation of early caries lesions and by reducing tooth enamel solubility (Featherstone 1988). Enamel demineralisation is markedly inhibited if fluoride is present at the time of the acid challenge because fluoride diffuses with the acid from plaque into the enamel and acts at the crystal surface to reduce mineral loss. When the pH rises following demineralisation, fluoride can combine with dissolved calcium and phosphate ions to precipitate or grow fluorapatite‐like crystalline material within the tooth. Fluoride enhances this mineral gain and provides a material that is more resistant to subsequent acid attack (ten Cate 1999). This occurs with all forms and concentrations of topical fluoride, although to a variable extent. Regular use of fluoride toothpaste or mouthrinse results in sustained elevated fluoride concentrations in the oral fluids during the demineralisation‐remineralisation cycle, but with higher concentration topical fluoride vehicles (such as varnishes and gels), calcium fluoride is precipitated on the enamel surface and in the plaque. This calcium fluoride acts as a fluoride reservoir that is released when the oral pH falls. Thus, gels deliver fluoride to the surface of enamel and to subsurface carious lesions, where it forms deposits of calcium fluoride and provides a reservoir of fluoride ions, and the amount of fluoride deposition in the subsurface lesion is greater after topical applications with such high‐concentration fluoride (Horowitz 1996; Ogaard 1994; Ogaard 2001).
Why it is important to do this review
The Cochrane Oral Health Group undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important ones to maintain on the Cochrane Library (Worthington 2015). Consequently, this review was identified as a priority title by the paediatric expert panel (Cochrane OHG priority review portfolio).
The prevention of dental caries in children and adolescents is generally regarded as a priority for dental services and considered more cost‐effective than its treatment (Burt 1998). Fluoride therapy has been the centrepiece of caries‐preventive strategies since the introduction of water fluoridation schemes over five decades ago (Murray 1991). These were introduced when caries was highly prevalent and severe, and when even modest prevention activities led to considerable reductions in disease levels. In the last 30 years, with the substantial decline in dental caries rates in many Western countries, an increase in dental fluorosis (mottled enamel) levels in some countries, and intensive research on the mechanism of action of fluoride highlighting the primary importance of its topical effect, greater attention has been paid to the appropriate use of other fluoride‐based interventions (Featherstone 1988; Featherstone 1999; Glass 1982; Marthaler 1996; O'Mullane 1994; Ripa 1991).
The use of topically applied fluoride products in particular, which are much more concentrated than the fluoride in drinking water, has increased over recent decades. By definition, the term 'topically applied fluoride' is used to describe those delivery systems that provide fluoride to exposed surfaces of the dentition, at elevated concentrations, for a local protective effect, and are therefore not intended for ingestion. Fluoride‐containing toothpastes (dentifrices), mouthrinses, gels and varnishes are the modalities most commonly used at present, either alone or in combination. Various products are marketed in different countries and a variety of caries‐preventive programmes based on these have been implemented. Toothpastes are by far the most widespread form of fluoride usage (Murray 1991a; Ripa 1991), and although the reasons for the decline in the prevalence of dental caries in children from different countries has been the subject of much debate (de Liefde 1998; Krasse 1996; Marthaler 1996; Marthaler 2004; Nadanovsky 1995), it has been mainly attributed to the use of fluoride in toothpaste and the increase in regular home use of toothpaste (Bratthall 1996; Glass 1982; Marthaler 1994; O'Mullane 1994; Ripa 1991; Rolla 1991).
At the same time, the lower caries prevalence in many countries now and the widespread availability of fluoride from multiple sources have raised the question of whether topically applied fluorides are still effective in reducing caries, and safe, mainly in terms of the potential risk of fluorosis. This is particularly important as nearly all child populations in high‐income countries are exposed to some source of fluoride, notably in toothpaste, and adverse effects may be rare (such as acute fluoride toxicity) or more subtle (such as mild dental fluorosis) (Marthaler 2004; Murray 1991a).
The evidence on the effect of topically applied fluoride products on the prevention of dental caries in children has been extensively reviewed in traditional narrative reviews. A number of systematic reviews focusing on the evaluation of specific fluoride active agents within specific delivery systems have used a quantitative meta‐analytical approach to synthesise trials results (Ammari 2003; Bartizek 2001; Chaves 2002; Clark 1985; Helfenstein 1994; Johnson 1993; Petersson 2004; Stamm 1984; Stamm 1995; Steiner 2004; Strohmenger 2001; Twetman 2004; van Rijkom 1998; Weyant 2013). However, there has been no systematic investigation evaluating and comparing the effects of the main modalities of topically applied fluoride treatments and examining formally the main factors that may influence their effectiveness.
This review is one in a series of systematic reviews of topical‐fluoride interventions and assesses the effectiveness of fluoride gels for the prevention of dental caries in children. It is an update of the review first published in 2002, which showed clear evidence of a caries‐inhibiting effect of fluoride gel in the permanent teeth of children (Marinho 2002). It is generally recognised that blinding is particularly important when outcome measures require specific criteria to improve objectivity in measurement, such as in the assessment of dental caries. Of note in this series of topical‐fluoride reviews is that lack of blinding in main outcome assessment (caries increment), or lack of any indication of blind outcome assessment remains an exclusion criterion – that is, studies are excluded if open outcome assessment is reported or if blind outcome assessment is not reported and is unlikely to have been used.
Objectives
The primary objective is to determine the effectiveness and safety of fluoride gels in preventing dental caries in the child and adolescent population.
The secondary objectives are to examine whether the effect of fluoride gels is influenced by the following:
initial level of caries severity
background exposure to fluoride in water (or salt), toothpastes, or reported fluoride sources other than the study option(s)
mode of use (self applied under supervision or operator‐applied), and whether there is a differential effect between the tray and toothbrush methods of application
frequency of use (times per year) or fluoride concentration (ppm F)
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials where 'blind outcome assessment' was stated or indicated (for example, caries examinations performed independently of previous results, or radiographic examinations registered separately of clinical examinations/added later, or examiners clearly not involved in giving treatment, or use of placebo described), and in which the length of follow‐up was at least one year/school year. We included cluster‐randomised trials, except when only one cluster was assigned to each study group.
We excluded randomised or quasi‐randomised controlled trials with open outcome assessment or no indication of blind assessment of outcome (blind assessment was considered unlikely if there was no description of a caries examination performed independently of previous results, no description of X‐rays registered independently of clinical examination, no description of examiners clearly not involved in giving treatment, and no description of use of a placebo), or lasting less than one year/one school year, or controlled trials where random or quasi‐random allocation was not used or indicated. We also excluded split‐mouth studies as they are unsuitable for fluoride gel due to possible contamination.
Types of participants
Children or adolescents aged 16 or younger at the start of the study, irrespective of initial level of dental caries, background exposure to fluorides, dental treatment level, nationality, setting where intervention is received or time when it started.
We excluded studies where participants were selected on the basis of special (general or oral) health conditions.
Types of interventions
Intervention: Topical fluoride in the form of gels only, operator applied or self applied, using any fluoride agent, at any concentration (ppm F), amount or duration of application, and with any technique of application, prior to or post application. Frequency of application should be at least once a year.
Comparison: The control group is placebo (for any method of gel application) or no treatment (for tray or cotton‐tips methods of gel application, but not for brushing or flossing methods).
The following comparison is therefore of interest: fluoride gel compared with a placebo or no treatment.
We excluded studies where the intervention consisted of any other caries‐preventive agent or procedure (for example, other fluoride‐based measures, chlorhexidine, sealants, oral hygiene interventions, xylitol chewing gums, glass ionomers) used in addition to fluoride gel.
Types of outcome measures
The primary outcome measure in this review is caries increment, as measured by change from baseline in the number of decayed, (missing) and filled permanent tooth surfaces (D(M)FS), or the number of decayed, (extracted/missing) and filled primary tooth surfaces (d(e/m)fs), or both (and in the number of permanent or primary teeth (D(M)FT/d(e/m)ft). We define dental caries here as being clinically and radiographically recorded at the dentin level of diagnosis. If caries data were only reported with both dentine and enamel lesions combined, then we used this in the analysis (see Data collection and analysis for the different ways of recording caries and reporting the D(M)FT/S and d(e/m)ft/s scores in permanent and primary dentitions in clinical trials of caries‐preventive interventions, and for how the data were selected for analysis.)
We excluded studies reporting no dental caries data, reporting only on plaque/gingivitis/gingival bleeding, calculus, dentin hypersensitivity, or on fluoride physiological outcome measures (fluoride uptake by enamel or dentin, salivary secretion levels, etc.).
Primary outcomes
Caries increment in permanent tooth surfaces (D(M)FS), reported as change from baseline (and D(M)FT, whenever reported)
Caries increment in primary tooth surfaces (d(e/m)fs), reported as change from baseline (and d(e/m)ft, whenever reported)
Secondary outcomes
Development of new caries, reported as change in the proportion of children developing new caries
Children not remaining caries‐free, reported as a change in the proportion
Tooth staining, measured as changes in proportion of children
Signs of acute toxicity during application of gel/treatment (such as nausea, gagging, vomiting)
Mucosal irritation/oral soft‐tissue allergic reaction
Overall dropouts or withdrawals during the trial
Search methods for identification of studies
To identify trials for inclusion in this review, we developed detailed search strategies for each database searched. We based these on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free‐text terms and was linked with the Cochrane Highly Sensitive Search Strategy for identifying randomised controlled trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Section 6.4.11.1 and detailed in Box 6.4.c of the Cochrane Handbook forSystematic Reviews of Interventions (Higgins 2011). We have provided details of the MEDLINE search strategy in Appendix 1. The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs.
Electronic searches
We searched the following electronic databases:
The Cochrane Oral Health Group Trials Register (to 5 November 2014) (Appendix 2);
The Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library 2014, Issue 1) (Appendix 3 );
MEDLINE via OVID (1946 to 5 November 2014) (Appendix 1);
EMBASE via OVID (1980 to 5 November 2014) (Appendix 4);
CINAHL via EBSCO (1980 to 5 November 2014) (Appendix 5);
LILACS via BIREME Virtual Health Library (1980 to 5 November 2014) (Appendix 6);
BBO via BIREME Virtual Health Library (1980 to to 5 November 2014) (Appendix 6);
ProQuest Dissertations and Theses (1861 to 5 November 2014) (Appendix 7);
Web of Science Conference Proceedings (1945 to 5 November 2014) (Appendix 8).
We placed no restrictions on language or date of publication in the search of the electronic databases.
Searching other resources
Ongoing trials
We searched the following trial registries for ongoing studies (see Appendix 9 for details of the search strategy):
US National Institutes of Health Trials Register (http://clinicaltrials.gov) (to 5 November 2014);
The World Health Organization Clinical Trials Registry Platform (http://apps.who.int/trialsearch/default.aspx) (to 5 November 2014).
Reference searching
We scanned all eligible trial reports, previous meta‐analyses and review articles for relevant references. For the original version of this review, reference lists of relevant chapters from preventive dentistry textbooks on topically applied fluoride interventions had also been consulted (Ekstrand 1988; Fejerskov 1996; Murray 1991c).
Handsearching
We carried out some handsearching for the original version of this review, in journals identified as having the highest yield of eligible RCTs and controlled clinical trials:
Community Dentistry and Oral Epidemiology (1990 to 1999);
British Dental Journal (1999 to 2000);
Caries Research (1999 to 2000);
Community Dentistry and Oral Epidemiology (1999 to 2000);
Journal of the American Dental Association (1999 to 2000);
Journal of Dental Research (1999 to 2000);
Journal of Public Health Dentistry (1999 to 2000);
European Journal of Oral Sciences (1999 to 2000).
For the update of this review, we did not undertake any handsearching.
Personal contact
For the original review, we contacted experts in the field of preventive dentistry in order to identify any unpublished trials or trial reports that may not have been indexed by the major databases. We sent a letter to the author(s) of each included study published during the 1980s and 1990s in order to obtain information on possible unpublished trials eligible for inclusion. We asked all the authors of trials contacted to clarify reported information to enable assessment of eligibility or to obtain missing data also for unpublished trials. In addition, based on information extracted mainly from included trials, we created a list of manufacturers of fluoride gels for locating unpublished trials and contacted six fluoride gel manufacturers in October 2000. We requested information on any unpublished trials from: GABA AG, Johnson & Johnson, Davies Rose‐Hoyt Pharmaceutical Division, John O. Butler Company, Oral‐B Laboratories, Colgate Oral Pharmaceuticals. GABA provided a list of 409 records from a search performed in GALIDENT (Database of GABA Library in Dentistry) using the keyword 'amine fluoride'. We incorporated the search results from this list of records from GABA in this update.
Data collection and analysis
Selection of studies
At least two review authors did the screening for eligibility in duplicate for all potential reports identified from all searches performed. Trial reports thought to be potentially relevant in languages not known by the review authors were translated and the inclusion criteria form completed by a review author with reference to the translator. We attempted to contact authors of trials that could not be classified to ascertain whether they met the inclusion criteria. We considered it essential to identify all reports related to the same study.
Data extraction and management
At least two review authors extracted data from all included studies in duplicate. We extracted numerical data presented only in graphs and figures whenever possible. We attempted to contact authors through an open‐ended request to obtain missing information or for clarification whenever necessary.
We extracted information related to study methodology including: study design, study duration (overall length of follow‐up in years), objectivity/reliability of primary outcome measurement (diagnostic methods and thresholds/definitions used and included, and monitoring of diagnostic errors). We also recorded information on sponsoring/funding institutions and manufacturers involved.
We extracted characteristics related to participants including: age (mean or range, or both) at start, caries severity at start (average DMFS/dmfs, DFS/dfs, or other caries increment measure, for sample analysed), background exposure to other fluoride sources (toothpaste, water, etc.), year study began, location where study was conducted (country), setting where participants were recruited (and setting of treatment), and total sample randomised (at baseline) and analysed (at relevant final examination).
We extracted characteristics of the intervention including: mode of application (who delivered the intervention), methods (technique/device) of application, information prior‐ and post‐application, fluoride active agents and concentrations used (in ppm F), frequency and duration of application, and amount applied. We also recorded information on what the fluoride gel was compared to (no treatment or placebo), together with numbers in each group. We have described these data in the Characteristics of included studies table.
Different ways of reporting caries increment (change from baseline as measured by the DMF index) were recorded separately and/or combined according to the components of the index chosen and units measured (DMFT/S or DFT/S or DT/S or FT/S), types of tooth/surface considered (primary/permanent teeth/surfaces, first molar teeth approximal surfaces, etc.), state of tooth eruption considered (erupted and/or erupting teeth or surface), diagnostic thresholds used (cavitated/dentin lesions, non‐cavitated incipient lesions, or both), methods of examination adopted (clinical or radiolographical, or both, or other) and approaches to account or not for reversals in caries increment adopted (in a net or observed increment, respectively). In addition, we have recorded caries increment data at all reported time periods (at various follow‐ups).
As we were aware that caries increment would be recorded differently in different trials, we developed a set of a priori rules to choose the main outcome data (D(M)FS) for analysis from each study: DFS data would be chosen over DMFS data and this would be chosen over DS or FS; data for 'all surface types combined' would be chosen over data for 'specific types' only; data for 'all erupted and erupting teeth combined' would be chosen over data for 'erupted' only, and this over data for 'erupting' only; data from 'clinical and radiological examinations combined' would be chosen over data from 'clinical' only, and this over 'radiological' data only; data from 'clinical and FOTI examinations combined' would be chosen over data from 'clinical' examination only; data for dentinal/cavitated caries lesions would be chosen over combined data for dentinal/cavitated and for enamel/non‐cavitated lesions, and these over enamel caries data only; net caries increment data would be chosen over crude (observed) increment data; and follow‐up nearest to three years (often the one at the end of the treatment period) would be chosen over all other lengths of follow‐up, unless otherwise stated. When no specification was provided with regard to the methods of examination adopted, diagnostic thresholds used, groups of teeth and types of tooth eruption recorded, and approaches for reversals adopted, the primary choices described above were assumed.
The Characteristics of included studies table provides a description of all the main outcome data reported from each study, with the chosen primary outcome measure featured at the top. Where assessments of caries increments were made during a postintervention follow‐up period, we noted the length of time over which outcomes were measured after the intervention ended. All other relevant outcomes identified as being assessed in the trials are also listed in this table.
Assessment of risk of bias in included studies
At least two review authors undertook the assessment of the risk of bias in all of the included trials independently. We resolved disagreements by discussion or by the involvement of another review author. We used The Cochrane Collaboration's tool for assessing risk of bias as outlined in the Cochrane Handbook for SystematicReviews of Interventions version 5.1 (Higgins 2011), but according to pre‐defined criteria that were adapted and refined for the Cochrane topical‐fluoride reviews updates. We assessed eight domains, namely sequence generation, allocation concealment, blinding of participants/personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, balance of baseline characteristics, and free from contamination or co‐intervention, according to the tool. Each domain included one or more specific entries in a 'Risk of bias' table. Within each entry, we described information reported in the study and assigned a judgement relating to the risk of bias for that entry. Where the study clearly reported the methodology, we made a judgement of 'low risk of bias' or 'high risk of bias'. Where trial methodology was unclear, we judged a domain as at 'unclear risk of bias' unless and until further information becomes available.
After considering additional information provided by the authors of the trials, we assessed the overall risk of bias in included trials over all eight domains. We assigned studies into the following categories.
Low risk of bias (plausible bias unlikely to seriously alter the results; all eight domains assessed as at low risk of bias).
High risk of bias (plausible bias that seriously weakens confidence in the results; at least one domain assessed as at high risk of bias).
Unclear risk of bias (plausible bias that raises some doubt about the results; at least one domain assessed as at unclear risk of bias, but none at high risk of bias).
Measures of treatment effect
The chosen measure of treatment effect for the primary outcome measure, caries increment, was the prevented fraction (PF), that is (mean increment in the controls minus mean increment in the treated group) divided by mean increment in the controls. For an outcome such as caries increment, where discrete counts are considered to approximate to a continuous scale and are treated as continuous data, we considered this measure more appropriate than the mean difference or standardised mean difference since it allows combination of different ways of measuring caries increment and a meaningful investigation of heterogeneity between trials. It is also simple to interpret.
For outcomes other than caries increment, we summarised continuous data as average mean differences (MD) in treatment effects and their 95% confidence intervals (95% CI), or if different scales had been used to measure the same outcome in different trials, standardised mean differences (SMD) and their 95% CI. We analysed dichotomous outcome data by calculating risk ratios (RR) or, for adverse effects of fluoride treatment, risk differences (RD).
Unit of analysis issues
Trials with multiple treatment arms
In the trials with more than one relevant intervention group and a common control group, such as those comparing different active fluoride agents or concentrations of fluoride ions to a placebo group, we combined summary statistics (the number of children analysed, mean caries increments, and standard deviations) from all relevant experimental groups (and from any relevant control groups, if this was the case) in order to obtain a measure of treatment effect (the PF). This enabled the inclusion of all relevant data in the primary meta‐analysis, although it might have slightly compromised the secondary investigations of dose response.
Cluster‐randomised trials
Where any cluster‐randomised trials did not report results adjusted for the clustering present in the data, we estimated the design effect with the intraclass correlation coefficient (ICC) if reported, or a value of 0.05 (Lawrence 2008; ICC = 0.045). This was then used to modify the numbers in the intervention and control groups by calculating the effective sample size (Higgins 2011).
Dealing with missing data
We decided that missing standard deviations for caries increments that we could not obtain after contacting the original researchers would be imputed through linear regression of log standard deviations on log mean caries increments. This is a suitable approach for caries prevention trials since, as they follow an approximate Poisson distribution, caries increments are closely related (similar) to their standard deviations (van Rijkom 1998).
Assessment of heterogeneity
We assessed heterogeneity by inspection of a graphical display of the estimated treatment effects from the trials along with their 95% CIs and by formal tests of homogeneity undertaken prior to each meta‐analysis (Thompson 1999). This was also quantified by the I2 statistic and classified according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A rough guide to interpretation: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% very substantial ("considerable") heterogeneity.
Assessment of reporting biases
Outcomes reporting bias (within‐study reporting bias)
Within‐study reporting bias (one of the eight 'Risk of bias' domains listed above, as 'selective outcome reporting') would ideally be assessed by comparing the outcomes reported in the published report against the study protocol. As this was not possible, we compared the outcomes listed in the methods section with the results reported. If results were mentioned but not reported adequately in a way that allowed analysis (for example, only mentioned whether the results were statistically significant or not), we sought further information from authors of the study reports. Otherwise, we noted this as 'high risk' of bias. If there was insufficient information to judge the risk of bias, we noted this as unclear (Higgins 2011).
Publication bias (between‐study reporting bias)
Funnel plots (plots of the effect estimates versus the inverse of their standard errors) were drawn where there were sufficient trials (more than 10). Asymmetry of the funnel plot may indicate publication bias and other biases related to sample size, though this may also represent a true relationship between trial size and size of treatment. We performed a formal investigation of the degree of asymmetry using the method proposed by Egger 1997.
Data synthesis
The meta‐analyses for the PFs were conducted as inverse variance weighted averages in Review Manager software (RevMan 2014), where the prevented fraction data PF (SE) were entered using the generic inverse variance option. Variances were estimated using the formula presented in Dubey 1965, which was more suitable for use in a weighted average, and for large sample sizes the approximation should be reasonable. It was noted in a previous review, Marinho 2013) that this formula was inappropriate for studies with small increments, and we excluded the data from such studies from the analysis in this review. We performed random‐effects meta‐analyses. We analysed primary and permanent teeth separately throughout. We also used random‐effects models to calculate a pooled estimate of effect for outcomes other than caries increment data.
Subgroup analysis and investigation of heterogeneity
We specified four potential sources of heterogeneity a priori, as these formed part of the primary objectives of the review. We hypothesised that the effect of fluoride gels differs according to:
the baseline levels of caries severity;
exposure to other fluoride sources (in water, in toothpastes, etc.);
mode (self applied supervised or operator applied) and method (self applied tray or toothbrush) of application; and
frequency of application and fluoride concentration.
We examined the association of these factors with estimated effects (D(M)FS PFs) by performing random‐effects metaregression analyses in Stata version 12.0 (Stata Corporation, USA) using the 'metareg' command (Sharp 1998).
To allow such investigation, we dealt with relevant data as follows: data on 'baseline levels of caries' were calculated from the study sample analysed (final sample) unless otherwise stated, and were averaged among all relevant study groups. Data on 'background exposure to other fluoride sources' combined data on the use of fluoride toothpaste and the consumption of fluoridated water (or salt) and were grouped into two categories: one for studies that were based on samples provided with non‐fluoride toothpaste and that were from non‐fluoridated areas (non‐exposed), and another for studies based on samples using fluoride toothpaste or studies in fluoridated communities, or both. We considered exposure to water fluoridation when fluoride levels in water were stated to be above 0.3 ppm F. Use of fluoride toothpaste reported for 30% or more of the study sample would indicate exposure to fluoridated toothpaste. When use or non‐use of fluoride toothpaste was not clearly indicated in studies carried out in high‐income countries, we assumed that fluoride toothpaste was widely used from the middle of the 1970s (Ripa 1989); we sought this information from authors (or obtained from other sources) when missing from studies carried out in other locations. When data on the year a study had begun was not provided, we calculated this as a 'probable date' by subtracting the duration of the study (in years) plus one extra year, from the publication date of the study. We classified the 'gel application modes/methods' as either operator‐ or self applied under supervision and as self applied supervised application by tray or brush. We have categorised data on 'frequency of application' and 'fluoride concentration applied' (cutoff points used were > twice per year and ≥ 10,000 ppm F, respectively). Since both covariates, fluoride concentration and frequency of application, are unlikely to be linear scales, we chose arbitrary but sensible cut points (unlikely to change results if different cutoffs are used), and felt it was inappropriate to undertake a metaregression analysis multiplying frequency by concentration. We averaged concentrations in multiple‐arm studies over fluoride gel groups.
We investigated further potential sources of heterogeneity by metaregression: for different types of control groups (placebo or no treatment), use or not of prior prophylaxis, length of follow‐up (years) and dropout rate (%), but these 'post hoc' analyses were reported as such and findings should be treated with caution.
Sensitivity analysis
We planned to undertake a sensitivity analysis including the trials with an overall assessment of low risk of bias, but there were no trials satisfying this criterion. We undertook a sensitivity analysis excluding trials where we imputed missing standard deviations. We also undertook a sensitivity analysis excluding trials at high risk of bias for allocation concealment and another excluding trials at high and unclear risk of bias for blinding of outcome assessment. We also performed these meta‐analyses using a random‐effects model.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to rate the overall 'quality of evidence' for each outcome in the studies in the main comparisons; we have presented the primary outcomes in Table 1. This table provides outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes that we rate as important to patient care and decision‐making.
The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct; we apply this in the interpretation of results. The four possible ratings are 'high', 'moderate', 'low', and 'very low'. A rating of 'high' quality of evidence implies that we are confident in our estimate of effect and further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' quality implies that any estimates of effect obtained are very uncertain.
The GRADE approach considers evidence from RCTs that do not have serious limitations as 'high' quality. However, the following factors can decrease the quality of evidence:
study limitations (risk of bias);
inconsistency;
Indirectness of evidence;
imprecision;
publication bias.
Depending on the seriousness, the quality of evidence may be downgraded by one or two levels for each aspect.
Results
Description of studies
Results of the search
We have used the full search conducted as described in Search methods for identification of studies on 5 November 2014 to construct the PRISMA flow chart shown in Figure 1.
1.
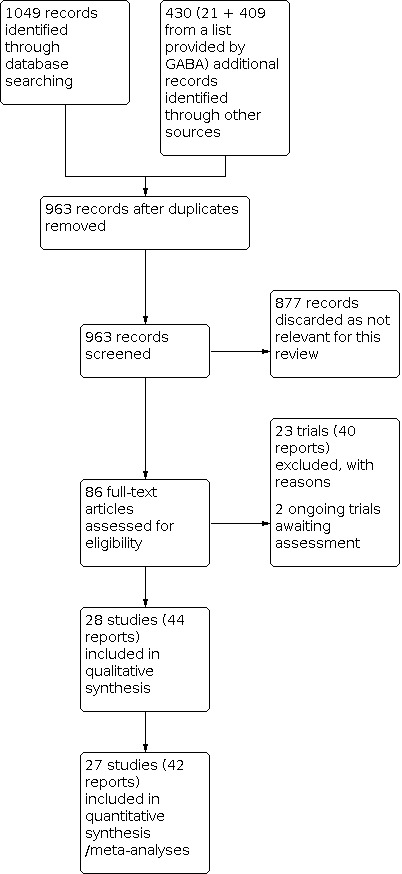
Study flow diagram from 2014 search
For this update, 1479 records were identified by the searches (from databases and other sources), 963 were screened after duplicates were removed and 86 full‐text articles (including some available only as abstracts or summary reports) were assessed as potentially eligible and were considered for this review. Of these 86 reports:
44 reports were related to 28 included trials (including the 25 trials included in the original 2002 review);
40 reports were related to 23 excluded trials (including the 13 trials excluded in the original review);
2 reports were on 2 ongoing studies, which may be eligible in a future update (See Characteristics of ongoing studies).
Included studies
See the Characteristics of included studies tables for details of each study.
We included 28 trials. We treated the study conducted by Marthaler 1970 as two independent trials (Marthaler 1970 and Marthaler 1970a), since the results for the two age groups in the study have been reported separately as distinct studies. All 44 study reports were published between 1967 and 2005. The 25 previously included trials were conducted between 1964 and 1996: 12 during the 1960s, 7 during the 1970s, 5 during the 1980s, and 1 in the 1990s. The 2014 update of this review found another three trials conducted in the late 1990s and early 2000s (Jiang 2005; Truin 2005; Van Rijkom 2004).
Thirteen trials were conducted in the USA (nine of these during the 1960s), seven in Europe, four in Brazil and one in each of the following countries: Canada (Olivier 1992), Israel (Ran 1991), China (Jiang 2005) and Venezuela (Shern 1976). Eleven studies had more than one publication, and one of these studies (USA ‐ Hawaii) had six published reports (Horowitz 1971).
Five trials acknowledged financial support from a fluoride gel manufacturer (Mainwaring 1978; Marthaler 1970; Marthaler 1970a, Ran 1991, Shern 1976); seven trials acknowledged only some assistance or the supply of fluoride gel from manufacturers (Abadia 1978; Cobb 1980; Englander 1967; Englander 1971; Gisselsson 1999; Hagan 1985; Trubman 1973); one trial indicated involvement with a manufacturer by the affiliation of one of the authors (Englander 1978); seven trials acknowledged support from non‐commercial sources (grants) (Cons 1970; DePaola 1980; Jiang 2005; Olivier 1992; Szwejda 1972; Truin 2005; Van Rijkom 2004); and for the remaining eight trials, no information on source of funding or any assistance was available.
Design and methods
All the included studies used parallel‐group designs, one being cluster randomised (Jiang 2005). Eight of these had more than one fluoride gel treatment group compared to a control; among these, one trial had two treatment groups and two placebo control groups (Shern 1976). With regard to type of control group used, 10 trials used a no‐treatment control group, and the remaining 18 used a placebo‐control group, of which 4 used an inactive treatment other than gel ("placebo solution") (Cons 1970; Heifetz 1970; Horowitz 1974; Szwejda 1972). The study duration (indicated by the total length of follow‐up as well as the treatment duration) ranged from 1 to 4 years: 3 trials lasted 4 years (Marthaler 1970a; Truin 2005; Van Rijkom 2004), 9 trials lasted 3 years, 11 trials lasted around 2 years, 2 trials lasted 1.5 years (Bijella 1981; Ran 1991), and 2 trials lasted 1 year (Abadia 1978; Mestrinho 1983).
Participants
All trials reported that participants were aged 15 years or less at the start of the trial. The ages of the children ranged from 2 to 15 years. Fifteen trials included participants who were 12 years old at start, and 3 trials included children younger than 6 years of age (in which deciduous teeth were assessed for caries development) (Englander 1978; Treide 1988; Van Rijkom 2004). There were similar numbers of males and females (where these data were reported), with the exception of Ran 1991, which included male participants only.
Decayed, missing and filled surfaces (D(M)FS) at baseline, reported in all but three of the studies, ranged from 0 to 12.2; in the two studies that reported data for the primary dentition it was 0 dmfs and 3.7 defs (where 'e' is teeth indicated for extraction). With regard to 'background exposure to other fluoride sources', all studies reported whether or not participants had exposure to systemic sources; only five studies were conducted in fluoridated communities: water fluoridation in three studies (Englander 1971, Englander 1978, and Szwejda 1972) and salt fluoridation in two studies (Marthaler 1970; Marthaler 1970a). Of the remaining 23 studies, 4 studies clearly reported no (or very low) exposure to fluoride dentifrices or to other fluoride sources, 1 trial reported some exposure to fluoride toothpaste (43%) (Jiang 2005), and 5 studies reported substantial exposure to fluoride toothpaste (over 95%) (Gisselsson 1999; Hagan 1985; Olivier 1992; Truin 2005; Van Rijkom 2004). In the remaining 13 studies exposure or not to fluoride toothpaste had to be assumed based on study location and year started, as described above.
Studies were large, with only 4 allocating less than 200 children to relevant study groups. The total number of children participating in the 28 included trials (given by the sample analysed at the end of the trial periods) was 9140, ranging from 41 in the smallest trial to 732 in the largest trial (Marthaler 1970a and Van Rijkom 2004, respectively). Participants were recruited from school settings, except in the three trials assessing caries in pre‐school children, where information in one trial, Englander 1978, was unclear, and in the remaining two trials nurseries and paediatric clinics were the settings (Treide 1988 and Van Rijkom 2004, respectively).
Interventions
Seventeen of the included trials reported gel application carried out by professionals (operator applied). In the remaining 11 trials gel was self applied under supervision (by dental personnel in 4 trials, by trained non‐dental personnel in 5 trials, and by mothers and dental personnel in 1 trial; data were not available for 1 of the studies). Gel was usually administered using a tray (18 trials) or a brush (6 trials), but the use of floss was reported in 1 trial (Gisselsson 1999), and cotton‐tip paint application was reported in 2 trials carried out in Brazil and in 1 trial from USA (Abadia 1978; Bijella 1981; Cobb 1980). A variety of fluoride gel types were used, including acidulated phosphate fluoride (APF) (21 trials), sodium fluoride (NaF) (7 trials), amine fluoride (AmF) (5 trials) and stannous fluoride (SnF2) (used in the Gisselsson 1999 study only). The fluoride concentrations ranged from 2425 ppm F (SnF2) to 12,500 ppm F (AmF and NaF). Fourteen trials used the common 12,300 ppm F APF gel concentration. The three studies that did not report the APF gel concentration are likely to have used the standard 12,300 ppm F (Bryan 1970; Ingraham 1970; Szwejda 1972), as they were all carried out in the same country, started in consecutive years, and had APF gel applied by professionals once a year; two studies reported the use of other APF concentrations: 9000 ppm F and 9150 ppm F (Hagan 1985; Mestrinho 1983). The application frequency (times per year) ranged from once a year (reported in 7 studies) to 140 times a year (reported in the study of Englander 1967), but it varied greatly among the studies, with 8 studies reporting the more common twice a year application frequency. With the exception of Shern 1976 (with 5 consecutive once a day or once a week applications in 1 year), all 17 studies where fluoride gel was professionally applied reported a frequency of application of 4 times a year or less. With 1 exception (Trubman 1973), where frequency of application was 4 times a year, the 11 studies of self applied gel reported a frequency of application of 5 times a year or more. Only a few studies reported the amount of gel applied (either in 'ml' or 'gr'), which ranged from 1 ml to 4 ml, and from 1 mg to 3 mg. Reported application times ranged from 2 to 10 minutes, with 16 studies reporting 3 to 5 minutes gel application time. Sixteen trials reported information about the performance of some form of prior (professional or self performed) tooth prophylaxis before administering the gel: 2 trials were performed with no paste (Cobb 1980; Hagan 1985), and 14 trials were performed with a non‐fluoride paste (if with a fluoride paste the trial would have been excluded); we considered the prior tooth cleaning as a possible part of the technique of gel application and not as a separate intervention on its own.
Outcome measures
Caries increment data
All 28 included trials reported caries increment data at the tooth surface level: with D(M)FS reported in 26 trials, de/mfs in 2 trials (Englander 1967; Treide 1988), and both D(M)FS and dmfs in 1 trial (Van Rijkom 2004). Ten of the 26 trials reported caries increment data at the tooth level (D(M)FT), and both trials that reported caries increment data for deciduous teeth only (defs) also reported data at the tooth level (deft) (Englander 1967; Treide 1988). With regard to the components of the DMFS index used (and types of teeth/surface assessed), 21 trials reported DMFS data (2 trials for first molars only, Cons 1970 and Jiang 2005, and 17 trials for all tooth surface types), and 6 trials reported DFS data (1 trial for all approximal surfaces only, Gisselsson 1999, and 5 trials for all tooth surface types); 1 of these trials also reported DS and FS data separately. Three of the 11 trials that reported D(M)FS data on specific teeth or tooth surfaces ‐‐ first molars, occlusal, mesio‐distal (approximal) and/or bucco‐lingual ‐‐ did not report data on all tooth surfaces (Cons 1970; Gisselsson 1999; Jiang 2005). Fourteen trials presented caries increment (D(M)FS) data at a single follow‐up time only, and the remaining 14 trials had data (D(M)FS/de/mfs) reported at more than 1 follow‐up time; overall, 12 trials had caries increment data reported at 1 year follow‐up time, 14 trials at 2 years, 9 trials at 3 years and 2 trials at 4 years (Truin 2005; Van Rijkom 2004). Follow‐up of two years was thus the most common among all trials. In four trials, assessments of D(M)FS increments were also made during a postintervention follow‐up period.
All 28 studies included a visual examination to detect caries; only 5 trials did not report use of a probe including tactile criteria in addition to the visual diagnosis (Gisselsson 1999; Marthaler 1970; Marthaler 1970a; Truin 2005; Van Rijkom 2004). Seven trials used X‐rays in addition to visual examination (DePaola 1980; Gisselsson 1999; Mainwaring 1978; Marthaler 1970; Marthaler 1970a; Truin 2005; Van Rijkom 2004). Clinical (all 28 trials) and radiographic examinations (7 trials) provided the definition of different stages or grades of caries lesions. These have been grouped into two basic grades for each method of examination: NCA = non‐cavitated incipient enamel lesions clinically visible as white spots or discoloured fissures; CA = lesions showing loss of enamel continuity that can be recorded clinically (undermined enamel, softened floor/walls) or showing frank cavitation; ER = any radiolucency in enamel/enamel‐dentin junction; DR = radiolucency into dentin. Fourteen trials presented results using one caries grade only: the dentine cavitation level of diagnosis (CA/DR); the 14 remaining trials either did not report the diagnostic grade/level for caries (8 trials), in which case CA/DR was assumed, or reported both the cavitation (CA) and the non‐cavitation (NCA) grades, in which case CA was chosen. Eleven trials specified data on state of tooth eruption considered: 10 trials reported data for teeth erupted at baseline only and only 1 trial reported combined data for erupting and erupted teeth (Heifetz 1970). Only the two studies of Marthaler 1970 did not use full‐mouth recording.
Other outcomes
One trial reported data on the proportion of children developing new caries (Gisselsson 1999), and two trials reported data on the proportion of children not remaining caries‐free (Englander 1978; Gisselsson 1999). Adverse symptoms (nausea/vomiting) were reported to have been assessed in three trials: two trials had useable data (one reported that there were no events (Mestrinho 1983), and another reported that three participants from the treatment group experienced one event each (Hagan 1985)), but the remaining trial reported no clear data (the event was reported to have occurred 'in many subjects') (Ingraham 1970). Other outcome measures were reported, but without complete or useable data: one trial reported 'no side effects', another trial reported 'no etching of enamel', and two trials reported 'no inadvertent swallowing of fluoride gel'. Data for unacceptability of the treatment regimen and for unacceptability of the treatment effect (as measured by dropouts/exclusions) were fully reported in 8 of the 10 no‐treatment control trials and in 11 of the 18 placebo control trials, respectively.
Excluded studies
See Characteristics of excluded studies for the description of reasons for rejecting each study.
We excluded 23 trials for a variety of reasons. We have categorised these as related to the study design, intervention/comparison, participant, or outcome as given below, based on the main or most obvious reason(s) for exclusion.
Study design
Not RCT or quasi‐RCT or unlikely to be so: 17 studies (Agrawal 2011; Bordoni 1995; Boyd 1985; Cichocka 1981; Ivanova 1990; Kukleva 1983; Kukleva 1998; Kukleva 2001; Loesche 1977; Pinto 1993; Rajic 1977; Ran 1987; Shobha 1987; Spears 1978; Stokes 2011; Szoke 1989; Szwejda 1971).
Open assessment stated or blinded outcome assessment not stated or unlikely: we excluded 2 studies due to the lack of blinding of outcome assessments (Lisiecka 1976; Mellberg 1978); the others (13 studies) also had other features that met the exclusion criteria (Bordoni 1995; Cichocka 1981; Ivanova 1990; Kukleva 1983; Kukleva 1998; Kukleva 2001; Madlena 2002; Pinto 1993; Rajic 1977; Ran 1987; Shobha 1987; Spears 1978; Szoke 1989).
Intervention/comparison
Other intervention with fluoride varnish: two studies (Heifetz 1979; Madlena 2002). Three other studies had other features that met the exclusion criteria (Bellini 1981; Bordoni 1995; Boyd 1985).
Fluoride gel was applied by toothbrushing, and compared to no‐treatment group rather than placebo: one study (Stokes 2011).
Participants
Medically/dentally compromised participants: one study (Stadtler 1982).
Outcome
Followed up for less than one year: we excluded no studies solely on this basis; however, we excluded one study that had a follow‐up of less than one year also because it was clearly not a RCT and had another intervention as well as fluoride gel (Boyd 1985).
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of risk of bias of the 28 studies included in the review.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
We considered none of the included studies to be at low risk of bias overall. We considered eight studies to be at unclear risk of bias (Hagan 1985; Mainwaring 1978; Marthaler 1970; Olivier 1992; Ran 1991; Shern 1976; Truin 2005; Van Rijkom 2004). We considered the remaining 20 studies to be at high risk of bias.
Allocation
Participants were the unit of randomisation in 27 studies; only 1 study randomised based on school classes (Jiang 2005).
We considered only two studies to have a low risk of selection bias overall, that is low risk of bias for both sequence generation and allocation concealment (Truin 2005; Van Rijkom 2004). Most had an unclear risk of bias for sequence generation and allocation concealment.
We considered another four studies to be at low risk of bias relating to random sequence generation (Bryan 1970; Marthaler 1970; Marthaler 1970a; Szwejda 1971), but the adequacy of allocation concealment was unclear. Marthaler 1970 and Marthaler 1970a had paired students according to their sequence in class lists, and used a table of random digits to randomise participants. For an odd digit, the first child got the fluoride gel, the second child got the placebo. However, these studies did not state whether they had concealed the random tables or the code to the allocation. Bryan 1970 and Szwejda 1972 also did not specify whether they had taken any measures to conceal allocation.
We assessed 17 trials as being at unclear risk of selection bias; there was either inadequate or no information provided on how sequence generation or allocation concealment was done, or it was difficult to judge if the method used (particularly for allocation concealment) was effective (Cobb 1980; Cons 1970; DePaola 1980; Englander 1967; Englander 1971; Englander 1978; Hagan 1985; Ingraham 1970; Jiang 2005; Mainwaring 1978; Olivier 1992; Ran 1991; Shern 1976; Treide 1988; Trubman 1973).
We considered seven trials to be quasi‐randomised and assigned them a high risk of bias for both sequence generation and allocation concealment (Abadia 1978; Bijella 1981; Gisselsson 1999; Heifetz 1970; Horowitz 1971; Horowitz 1974; Mestrinho 1983).
There were attempts to stratify by baseline characteristics in 10 studies: Abadia 1978, Bijella 1981, and Mestrinho 1983 ordered participants by number of permanent teeth present and DMF levels; Englander 1967, Englander 1978, and Mainwaring 1978 stratified by age and sex; Gisselsson 1999 by numeric value of caries experience; Heifetz 1970, Horowitz 1971, and Horowitz 1974 by sex, age and caries experience.
Blinding
Ten studies used either a no‐treatment arm as control or a placebo that was inadequate and easily distinguishable from the active treatment (Abadia 1978; Bijella 1981; Bryan 1970; Cobb 1980; Englander 1967; Englander 1971; Horowitz 1971; Ingraham 1970; Jiang 2005; Mestrinho 1983). Five others suggested that some blinding or placebo was used but either provided insufficient information of its description, or the effectiveness of these 'placebos' was unclear Cons 1970; Heifetz 1970; Horowitz 1974; Olivier 1992; Szwejda 1972).
We excluded studies that clearly did not have any blinding for assessment and were therefore at high risk of detection bias.
Sixteen of the included studies had descriptions that suggested adequate blinding of personnel involved in assessment and were rated as at low risk of bias for outcome assessment (Cons 1970; DePaola 1980; Englander 1978; Gisselsson 1999; Hagan 1985; Heifetz 1970; Horowitz 1974; Mainwaring 1978; Olivier 1992; Ran 1991; Shern 1976; Szwejda 1972; Treide 1988; Trubman 1973; Truin 2005; Van Rijkom 2004). Of these, 11 also had adequate blinding of personnel and participants, while 5 studies were at unclear risk of bias (Cons 1970; Heifetz 1970; Horowitz 1974; Olivier 1992; Szwejda 1972).
The remaining 12 studies had unclear risk of blinding for outcome assessment (Abadia 1978; Bijella 1981; Bryan 1970; Cobb 1980; Englander 1967; Englander 1971; Horowitz 1971; Ingraham 1970; Jiang 2005; Marthaler 1970; Marthaler 1970a; Mestrinho 1983). All but two of these studies, Marthaler 1970 and Marthaler 1970a, were also at high risk of bias for participant and personnel blinding; most of these either used no treatment as the control arm or a placebo that could easily be distinguished from the active intervention (for example, distilled water, Cons 1970)
Incomplete outcome data
We focused our assessment of attrition bias on the data points used for the analyses in the review, that is how much was missing compared to what would be available if all participants were included in the analysis. There was considerable variation in dropout rates, ranging from 8% at one year to 55% at three years.
Three trials were at low risk of bias (Olivier 1992; Truin 2005; Van Rijkom 2004), 9 were at unclear risk (Abadia 1978; Bryan 1970; Gisselsson 1999; Hagan 1985; Ingraham 1970; Mainwaring 1978; Marthaler 1970; Ran 1991; Shern 1976), and the remaining 16 trials were at high risk of bias (Bijella 1981; Cobb 1980; Cons 1970; DePaola 1980; Englander 1967; Englander 1971; Englander 1978; Heifetz 1970; Horowitz 1971; Horowitz 1974; Jiang 2005; Marthaler 1970; Marthaler 1970a; Szwejda 1972; Treide 1988; Trubman 1973). Most studies rated as at high risk of bias either had large or differential dropout percentages, or both.
Where this information was supplied, the most common reason for attrition was that participants were not available for follow‐up examination at the end of the study; nine trials reported exclusions based on presence in all follow‐up examinations, and three trials reported exclusions based on compliance. Other reasons for exclusions (when given) included characteristics of participants that should have been used as eligibility criteria before randomisation (use of orthodontic bands, lifetime exposure to fluoridated water). Only one trial reported the numbers excluded according to reason for attrition.
Selective reporting
Ideally we should have compared the outcomes listed in each study protocol with the outcomes reported in the papers, but this was seldom possible. Most of the studies in this review were published before the year 2000 and provided very little information. We compared the results reported in the studies against what was stated in the methods section and used clinical judgement to consider whether studies had reported data as expected, and considered the majority of studies (21 trials, 75%) as at low risk of bias. We considered the other seven studies, which included all three studies published after 2000, to be at unclear risk of bias (Heifetz 1970; Horowitz 1971; Jiang 2005; Mestrinho 1983; Treide 1988; Truin 2005; Van Rijkom 2004).
Other potential sources of bias
Baseline imbalance
We also assessed whether there was a balance of important prognostic factors (baseline caries level) between the arms of the included trials. We assessed 25 trials (89%) as at low risk of bias for this domain. In three trials we were unclear whether the baseline differences for caries posed an important clinical difference and classified these trials as at unclear risk of bias (DePaola 1980; Mestrinho 1983; Treide 1988).
Contamination/co‐intervention
We assessed only seven trials as at low risk of bias due to co‐intervention. These trials provided information to suggest that there was no difference between groups in co‐interventions that could have affected the outcomes observed, such as supervised brushing, oral hygiene instructions, or gel application procedures (DePaola 1980; Englander 1967; Englander 1978; Heifetz 1970; Ran 1991; Truin 2005; Van Rijkom 2004). In the other studies the risk of bias was unclear as no or not enough information was provided.
Effects of interventions
See: Table 1
Effect on dental caries increment
The included trials reported the effects of fluoride gels on dental caries increment in a variety of different ways. We did not include one study, Ran 1991 in the meta‐analysis, as the formula for estimating the standard error of the prevented fraction (PF) was inappropriate, but we retained it in the review as part of the qualitative data synthesis only (we have described its characteristics in the Characteristics of included studies table). We have extracted data from the other trials as appropriate to produce pooled estimates, as described in the Methods section. We have reported the PF results separately for:
Decayed, Missing and Filled Surface Prevented Fraction (D(M)FS PF); (Analysis 1.1; 25 trials)
Decayed, Missing and Filled Teeth Prevented Fraction (D(M)FT PF); (Analysis 1.2; 10 trials)
Decayed, (extraction indicated/missing), and filled surfaces prevented fraction (d(e/m)fs PF); (Analysis 1.3; 3 trials)
1.1. Analysis.

Comparison 1: Fluoride gel versus placebo or no treatment, Outcome 1: D(M)FS increment ‐ nearest to 3 years (25 trials)
1.2. Analysis.

Comparison 1: Fluoride gel versus placebo or no treatment, Outcome 2: D(M)FT increment ‐ nearest to 3 years (10 trials)
1.3. Analysis.

Comparison 1: Fluoride gel versus placebo or no treatment, Outcome 3: d(e/m)fs increment ‐ nearest to 3 years (3 trials)
Imputation of missing standard deviations
In the original version of this review, we estimated unreported standard deviations (SD) from an analysis of the 179 available treatment arms for the series of topical‐fluoride reviews with complete information (as of October 1999). This resulted in a regression equation of: log (SD caries increment) = 0.64 + 0.55*log (mean caries increment), (R2 = 77%). We applied this equation to results of the trials where SDs of mean caries increment data were missing to estimate them in 3 of the 25 trials reporting D(M)FS data (Abadia 1978; Bijella 1981; Mestrinho 1983), in 2 of the 10 trials reporting D(M)FT data (Bijella 1981; Mestrinho 1983) and in 2 of the 3 trials reporting d(e/m)fs data (Englander 1978).
Effective sample size for cluster‐randomised trials
One cluster‐randomised trial reported the results not accounting for clustering of the data (Jiang 2005). We used an intraclass correlation coefficient of 0.05 (using the value reported in a similar trial, Lawrence 2008, included in the series of topical‐fluoride reviews) to estimate the design effect. This was then used to adjust the sample size of the control and intervention groups (to calculate the effective sample size) in order to estimate the PF for the cluster‐randomised trial.
Effect on tooth surfaces ‐ permanent dentition: D(M)FS PF
For all 25 trials combined (8479 participants), the D(M)FS PF pooled estimate was 0.28 (95% confidence interval (CI) 0.19 to 0.36; P < 0.0001), suggesting a large caries‐preventive benefit from the use of fluoride gel. The CIs were not wide, but we could observe substantial heterogeneity in results graphically (Chi2 = 136 on 24 degrees of freedom, P < 0.0001, I2 = 82%; Analysis 1.1).
Metaregression, subgroup and sensitivity analyses: D(M)FS PF
Univariate metaregression suggested no significant association between estimates of D(M)FS PFs and the prespecified trial characteristics: baseline levels of caries, background exposure to other fluoride sources, background exposure to fluoridated water, background exposure to fluoride toothpaste, gel application mode (operator/self applied), gel application self applied method (tray or paint/brush or floss), frequency of gel application and fluoride concentration. Further univariate metaregression analyses on other characteristics not specified a priori showed no significant association between estimates of D(M)FS PFs and length of follow‐up (duration of study in years), prior prophylaxis, or dropout rate; however, the pooled estimated treatment effect was 17% greater (95% CI 3% to 31%; P = 0.018) in trials with no treatment rather than placebo control groups.
The pooled estimate of treatment effect on D(M)FS PF from the 10 trials (2808 participants) with a no‐treatment control group was 0.38 (95% CI 0.24 to 0.52; P < 0.0001), while that from the 15 placebo‐controlled trials (5671 participants) was 0.21 (95% CI 0.15 to 0.28; P < 0.0001). There was no statistically significant heterogeneity in the analysis of the 15 trials with placebo control groups (Chi2 = 23 on 14 degrees of freedom, P = 0.07, I² = 38%); heterogeneity was substantially less than that observed when all trials were included in the meta‐analysis. Although this was a post hoc analysis and thus should be viewed with caution, we have decided to present the results of the D(M)FS PF meta‐analyses subgrouped by type of control group, due to the clear influence of this covariate (Analysis 1.1.1 and Analysis 1.1.2) .
We have also presented the results of the random‐effects meta‐analyses of D(M)FS PFs (all trials and trials subgrouped by type of control group) in Table 2: Meta‐analyses of prevented fractions. We have provided metaregression results for all potential effect modifiers investigated in Table 3: Random‐effects metaregression analyses of prevented fractions: D(M)FS (results not adjusted for type of control group). These metaregression results must be interpreted with caution given the observational nature of the comparisons and the large number of comparisons made. Note that differences between subgroups from metaregression may differ from differences between separate meta‐analyses in separate subgroups (Table 2) due to an assumption of similar residual heterogeneity in the metaregression.
1. Meta‐analyses of prevented fractions: D(M)FS and D(M)FT.
| Analysis | Number of studies | RE estimate | 95% CI | Meta‐analysis P‐values | Heterogeneity test |
| D(M)FS ‐ all studies | 25 | 28% | (19% to 36%) | P < 0.0001 | Chi2 = 136 (24 df); P < 0.0001; I² = 82% |
| D(M)FS ‐ placebo control | 15 | 21% | (15% to 28%) | P < 0.0001 | Chi2 = 23 (14 df); P = 0.07; I² = 38% |
| D(M)FS ‐ no‐treatment control | 10 | 38% | (24% to 52%) | P < 0.0001 | Chi2 = 63 (9 df); P < 0.0001; I² = 86% |
| D(M)FT ‐ all studies | 10 | 32% | (19% to 46%) | P < 0.0001 | Chi2 = 103 (9 df); P < 0.0001; I² = 91% |
| D(M)FT ‐ placebo control | 4 | 18% | (9% to 27%) | P < 0.0001 | Chi2 = 3.2 (3 df); P = 0.37; I² = 6% |
| D(M)FT ‐ no‐treatment control | 6 | 43% | (29% to 57%) | P < 0.0001 | Chi2 = 48 (5 df); P < 0.0001; I² = 90% |
CI = confidence interval D(M)FS/T = decayed, (missing) and filled permanent surfaces or teeth df = degrees of freedom RE = random effects
2. Random‐effects metaregression analyses of prevented fractions: D(M)FS.
| Objective | Characteristic | Number of trials | Slope estimate | 95% CI | Slope interpretation | P value | I2 residual variation for heterogeneity |
| (2) | Mean baseline caries | 23 | 1.15% | (‐0.97% to 3.26%) | Increase per unit increase in mean baseline caries DM(F)S | 0.27 | 82% |
| (3) | Fluoridated water | 25 | ‐4.63% | (‐27.13% to 17.88%) | Lower PF in presence of background water fluoridation | 0.68 | 83% |
| (3) | Dentifrice use | 25 | ‐10.01% | (‐26.74% to 6.71%) | Lower PF in presence of dentifrice use | 0.23 | 81% |
| (3) | Any background fluorides | 25 | ‐10.65% | (‐25.86% to 4.56%) | Lower PF in presence of other fluorides | 0.16 | 81% |
| (4) | Self versus operator application | 25 | 8.17% | (‐7.99% to 24.33%) | Higher PF if self applied | 0.31 | 81% |
| (4) | Paint or tray versus toothbrush or floss application | 25 | 3.79% | (‐16.99% to 24.56%) | Higher PF with tray | 0.71 | 83% |
| (5) | Frequency of application > twice per year | 25 | 6.33% | (‐9.48% to 22.14%) | Higher PF if application > twice per year |
0.42 | 81% |
| (5) |
Concentration of fluoride ≥ 10,000 ppm F | 22 | 12.90% | (‐31.48% to 5.67%) | Higher PF if fluoride concentration is ≥ 10,000 ppm F |
0.16 | 80% |
| Placebo versus control | 25 | 17.26% | (3.24% to 31.29%) | Lower PF in presence of placebo group | 0.018 | 73% |
|
| Years of follow‐up | 25 | ‐2.11% | (‐11.50% to 7.27%) | Decrease per unit increase in years follow‐up | 0.65 | 81% | |
| Prior prophylaxis vs no prophylaxis | 25 | ‐2.48% | (‐18.71% to 13.75%) | Lower PF in presence of no prophylaxis | 0.75 | 82% | |
| Dropouts (%) | 23 | ‐0.06% | (‐0.67% to 0.54%) | _ | 0.82 | 82% |
CI = confidence interval D(M)FS = decayed, (missing) and filled permanent surfaces PF = prevented fraction ppm F = parts per million of fluoride
In order to determine the potential influence of data imputation and approximation, we undertook a sensitivity analysis, restricting the pooling of trials to those that were fully reported and suitable for analysis (21 trials). The results of this gave rise to a very similar D(M)FS PF value as the results of the full meta‐analysis (PF = 0.27, 95% CI 0.18 to 0.37), and there was no change in the indicator of heterogeneity (83%). We also performed sensitivity analyses excluding the 7 trials at high risk of bias for allocation concealment (Abadia 1978; Bijella 1981; Gisselsson 1999; Heifetz 1970; Horowitz 1974; Horowitz 1971; Mestrinho 1983) and excluding the 12 trials at high and unclear risk of bias for blinding of outcome assessment (Abadia 1978; Bijella 1981; Bryan 1970; Cobb 1980; Englander 1967; Englander 1971; Horowitz 1971; Ingraham 1970; Jiang 2005; Marthaler 1970; Marthaler 1970a; Mestrinho 1983). For allocation concealment, results were quite similar to those of the full meta‐analysis (PF = 0.28, 95% CI 0.17 to 0.39) with very little change in the indicator of heterogeneity (from 82% to 84%); for blind outcome assessment, results showed smaller PF values than the results of the full meta‐analysis (PF = 0.22, 95% CI 0.16 to 0.29) and a somewhat reduced indicator of heterogeneity (from 82% to 75%).
Funnel plot and test for funnel plot asymmetry: D(M)FS PF
A funnel plot of the 25 included studies reporting D(M)FS PFs indicated a possible relationship between PF and precision (related to sample size) (Figure 4).The funnel plot was asymmetrical and the Egger formal test for asymmetry suggested statistically significant asymmetry intercept: 0.56 (95% CI 0.36 to 0.75; P < 0.0001) (Egger 1997). If this was a reflection of publication bias, it would imply that small studies with especially large beneficial effects of fluoride gel were missing. The clinical significance of this result is unclear, and it appears to be related to the effects of an outlier, a large study suggesting the largest positive effect of fluoride gel.
4.

Funnel plot of comparison: 1 Fluoride gel versus placebo or no treatment, outcome: 1.1 D(M)FS increment ‐ nearest to 3 years (25 trials)
Effect on whole teeth ‐ permanent dentition: D(M)FT PF
Ten trials reported data that allowed the calculation of the D(M)FT PF. All of these trials are also included in the analysis of D(M)FS PF. The pooled estimate of D(M)FT PF was 0.32 (95% CI 0.19 to 0.46; P < 0.0001). The CIs were relatively wide, and there was considerable heterogeneity between trials (Chi2 = 102.50 on 9 degrees of freedom, P < 0.0001, I2 = 91%; Analysis 1.2).
As with the estimates of the effects of gels on D(M)FS PF, a metaregression suggested that the estimates from trials using no treatment control as opposed to placebo control were substantially higher, in this case by 26% (95% CI 4% to 48%; P = 0.02). The pooled estimate of the D(M)FT PF from the six trials with a no‐treatment control group was 0.43 (95% CI 0.29 to 0.57; P < 0.0001), while that from the four placebo‐controlled trials was 0.18 (95% CI 0.09 to 0.27; P < 0.0001). When the meta‐analysis was confined to those trials with placebo controls, there was no statistically significant heterogeneity (Chi2 = 3.18 on 3 degrees of freedom, P = 0.36, I2 = 6%).
We have also presented the results of the random‐effects meta‐analyses of D(M)FT PFs (all trials and trials subgrouped by type of control group) in Table 2: Meta‐analyses of prevented fractions.
Effect on tooth surfaces ‐ primary dentition: d(e/m)fs PF
Three trials reported data that allowed the calculation of the d(e/m)fs PF. Only one of these, Van Rijkom 2004, was also included in the analysis of D(M)FS PF. The pooled estimate of d(e/m)fs PF was 0.20 (95% CI 0.01 to 0.38; P = 0.04), suggesting a benefit of fluoride gel in the primary dentition, albeit with large CIs, but there was no statistically significant heterogeneity between trials (Chi2 = 124 on 2 degrees of freedom, P = 0.54, I2 = 0; Analysis 1.3). These results should be viewed with a degree of caution given that SDs were imputed in two of the three trials. In addition, the test for heterogeneity has low power to detect excess variability between the results of the trials when only a few trials are included.
In order to determine the potential influence of data imputation for SD, we undertook a sensitivity analysis, restricting the result to the single trial where SDs were fully reported (Van Rijkom 2004). This trial indicated a very similar d(e/m)fs PF value to the result of the meta‐analysis (d(e/m)fs PF = 0.20, 95% CI ‐0.01 to 0.42; P = 0.07), although this was a non‐significant result with a larger CI.
Effect on whole teeth ‐ primary dentition: d(m)ft PF
There were no data on d(m)ft available.
Effect of fluoride gel on other outcomes
Few trials reported data for other relevant outcomes.
Development of new caries: risk ratio
The only trial reporting on the proportion of children developing one or more new caries (tooth surface in the permanent dentition ‐ new DFS) reported a risk ratio (RR) of 0.82 (95% CI 0.68 to 0.99; 280 participants) (Gisselsson 1999).
Not remaining caries‐free: risk ratio
Two trials reported the proportion of children not remaining caries‐free, one for tooth surfaces in the permanent dentition (RR 0.72, 95% CI 0.46 to 1.14; 280 participants; Gisselsson 1999) and another in the primary dentition (RR 0.53, 95% CI 0.26 to 1.07; 145 participants; Englander 1978).
Tooth staining
None of the studies reported on staining of tooth surfaces (not even in Gisselsson 1999, the only trial where stannous fluoride was one of the fluoride gels being tested).
Signs of acute toxicity during application of of gel/treatment (nausea, gagging, vomiting): risk difference
Only 2 trials (490 participants) reported useable data on adverse events (Mestrinho 1983; Hagan 1985), but one of these had no events in either arm (Analysis 1.4). The pooled estimate of the risk difference between the gel and placebo arms was 0.01 (95% CI ‐0.01 to 0.02; Chi2 for heterogeneity 0.8 on 1 degree of freedom, P = 0.36, I2 = 0), that is marginally favouring the placebo/no‐treatment (PL/NT) arms, although the results were consistent with no difference.
1.4. Analysis.

Comparison 1: Fluoride gel versus placebo or no treatment, Outcome 4: Signs of acute toxicity ‐ nausea, vomiting (2 trials)
Mucosal irritation/oral soft‐tissue allergic reaction
None of the studies reported this outcome.
Dropouts/exclusions during the trial period: risk ratio
The pooled estimate of the RR of dropping out from the fluoride gel arm as opposed to the control‐group arm in the 19 trials that reported dropouts was 1.03 (95% CI 0.89 to 1.19), that is there was no difference in the risk of dropping out. There was substantial heterogeneity in these results (Chi2 = 56.04 on 18 degrees of freedom, P < 0.00001, I2 = 68%). (Analysis 1.5)
1.5. Analysis.

Comparison 1: Fluoride gel versus placebo or no treatment, Outcome 5: Dropouts or withdrawals
We explored and presented the results for this outcome by type of control group (placebo or no treatment) in order to investigate the hypothesis that while the no‐treatment subset results may indirectly reflect unacceptability of the treatment regimen, the placebo subset results are likely to reflect unacceptability of the fluoride treatment itself. We found no evidence of a difference between these subgroups:
When a placebo was used, the pooled RR was 1.05 (95% CI 0.91 to 1.22; participants = 6067, studies = 11, I2 = 61%).
When no‐treatment was the control group, the pooled RR was 1.03 (95% CI 0.73 to 1.47; participants = 2628, studies = 8, I2 = 76%).
Discussion
Summary of main results
We have presented the key findings for the primary outcomes in Table 1.
The main aim of this review was to estimate the effects on caries of using fluoride gel in children compared to placebo or no treatment. In this updated review, we have included 3 new trials, giving us a total of 28 included trials published between 1967 and 2005. A total of 9140 children were randomised in these trials to fluoride gel or placebo/no treatment. Twenty‐five trials contributed data for the permanent tooth surfaces meta‐analysis: over 5670 children were included in the 15 trials comparing a fluoride gel with a placebo and over 2800 in the 10 trials comparing fluoride gel with no treatment. For the great majority of children, the fluoride gel received was acidulated phosphate fluoride. There was moderate quality evidence that fluoride gel has a caries‐inhibiting effect in the permanent dentition of about 28% (95% CI 19% to 36%). We performed a meta‐analysis of the three trials assessing the effect of fluoride gel on the primary dentition. This low quality evidence suggested that fluoride gel leads to a 20% (95% CI 1% to 38%) reduction in decayed, missing and filled tooth surfaces; there was no heterogeneity in this estimate.
A secondary aim of this review was to examine whether there was any relationship between the caries‐preventive effectiveness of fluoride gel and a number of factors including the initial level of caries severity, background exposure to fluoride, mode of use (operator versus self applied under supervision, and tray/paint versus brush/floss), frequency of use or fluoride concentration. We were unable to detect a clear relationship between any of these factors and the magnitude of the treatment effect despite substantial variation in these factors between trials. However, this result should be interpreted with caution. Even a meta‐analysis including 25 trials has very limited power to detect such relationships and, like all analyses of observational data, is subject to the problem of potential confounding. For some factors, such as 'background exposure to fluoride', there is, in addition, the problem of potential misclassification due to the poor quality of the reported data. We were forced to make a number of assumptions, for instance classifying 'use of fluoride toothpaste' for 13 of the studies on the basis of the year when the study was conducted. We also treated this as a dichotomous variable (before/after mid‐1970s), although it is likely that the use of fluoridated toothpaste gradually increased during the 1960s, 1970s and 1980s. Similarly, we grouped exposure to fluoride in toothpaste and fluoride in water into a single dichotomous variable, which was likely to group studies whose participants had quite different levels of baseline exposure. These problems will bias any estimates of effect towards the null hypothesis.
Although we did not observe significantly higher treatment effect with self application of fluoride gels, nor with frequency of applications of fluoride gel greater than twice per year, it should be noted that studies assessing the self application of fluoride gels tended to employ higher frequencies of application. With the exception of one study where frequency of application was four times a year (Trubman 1973), the nine studies of self applied fluoride gel reported a frequency of application of five times a year or more. By contrast, the 17 studies where fluoride gel was professionally applied, with the exception of 1 study in which 5 consecutive daily or weekly applications in 1 year were performed (Shern 1976), reported a frequency of application of 4 times a year or less. Nevertheless, little/no difference of treatment effects should be expected between the once‐ and the twice‐a‐year frequency of application range with the traditional professionally applied mode of acidulated phosphate fluoride gel treatment, since such lower frequencies of application are considered. More robust investigations of these aspects of the intervention require direct, head‐to‐head comparisons of different frequencies of application, which were not within the scope of this review.
Subgroup and metaregression analyses showed that the effect of fluoride gel varied according to type of control group used, with D(M)FS PF on average being 17% (95% CI 3% to 31%; P = 0.018) higher in non‐placebo‐controlled trials; whether the study employed a placebo or a no‐treatment control group was the only factor that was significantly related to heterogeneity of effect. Nevertheless, this was a post hoc analysis, and must be interpreted with caution given its observational nature, and the large number of other factors examined.
Overall completeness and applicability of evidence
Although there is evidence that fluoride gel has a caries‐inhibiting effect, we found little useful information about the effects of fluoride gels on a number of other clinically important outcomes such as acute side effects (nausea/vomiting) and on epidemiologically important outcomes such as the proportion of children developing caries or remaining caries‐free. We found no information on other adverse effects such as tooth staining or oral allergic reactions. Only three studies measured adverse effects and reported that there were none. This scarcity of evidence about adverse effects makes it more difficult for clinicians and policymakers to weigh the benefits of fluoride gels in preventing caries against the possible shortcomings of the procedure. Data for unacceptability of the treatment regimen and the treatment effect (as measured indirectly by dropouts in no‐treatment trials and exclusions in placebo‐controlled trials) were more fully reported.
The trials included in this review used a variety of fluoride gel frequencies, methods, and techniques of application, and various fluoride agents and concentrations. In the studies with more than one relevant intervention group and a common control group, such as those comparing different active fluoride agents or concentrations of fluoride ions to a placebo group, we combined summary statistics from the studies (number of children analysed, mean caries increments, and standard deviations) from all relevant intervention groups in order to obtain a measure of treatment effect. This enabled the inclusion of all relevant data in the primary meta‐analyses assessing the caries‐inhibiting effect of fluoride gel on children’s permanent tooth surfaces, but has limited a secondary investigation of dose‐response.
The trials included in this review were conducted with participants who were at differing levels of caries risk, as evidenced by the variability of the caries increments in the control groups, and who were based in different locations with variability in exposure to other sources of fluoride.
The caries increment prevented fraction appeared to be consistent across different populations, levels of caries risk and exposure to other fluoride sources. The absolute benefit from fluoride gel will, of course, depend on the expected caries increment in the target population. Where the expected caries increment is small, the absolute benefit of fluoride gel will be very small.
An important issue in this review is whether the body of evidence, which mainly consists of older studies with participants who were probably not exposed to fluoride toothpaste, is applicable today, when fluoridated toothpastes are widely available and use is generally high. Regarding this, we included three new studies in this update, and in two of them, which were well conducted and carried out in the Netherlands in the the early 2000s, the treatment effects observed where participants would have had lifetime use of fluoride toothpaste were very similar to the overall pooled results.
We have found little information about the adverse effects of topical fluoride gel; only two RCTs reported on this. Substantial information on a particular type of adverse effect (fluorosis) of topically applied fluoride treatments (especially toothpaste) can be found in a Cochrane review on topical fluoride and the risk of fluorosis (Wong 2010).
Quality of the evidence
We assessed none of the trials included in this review as being at low risk of bias; most (20) were at high risk of bias. The domains most commonly found to be at high risk of bias were random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias) and incomplete outcome data (attrition bias).
For the primary outcome, DMFS increment, we downgraded the quality of the evidence because of these limitations in study design (in particular, at least 85% of information was from studies with unclear sequence generation or allocation concealment, or both, and about 50% was from studies with no placebo use). We also observed substantial heterogeneity in the main outcome (DMFS increment) in this review, but the results consistently pointed to the caries‐inhibiting effectiveness of fluoride gel in the permanent dentition, so we did not downgrade the quality of the evidence for inconsistency. In addition, a substantial amount of evidence could be included in the DMFS PF meta‐analysis, and the size of treatment effect for the effectiveness outcomes (caries increment) was clinically important. We therefore consider the quality of evidence for the caries increment outcome for the permanent dentition to be moderate; we are moderately confident in the effect estimate. The true effect is likely to be close to the effect estimate, but there is a possibility that it could be substantially different. The evidence quality for the caries‐preventive effect on the primary dentition, however, is low because only three trials reported the results of the effect on the primary dentition, and the applicability of these results was less certain.
Only 2 out of 28 studies reported on acute toxicity (490 participants). Both of these studies have serious limitations in their methodology, with either unclear or high risk of bias for sequence generation and allocation concealment. It is unclear whether the other studies measured this outcome at all, and so we cannot rule out a possibility of reporting bias. We downgraded further for imprecision because of the small number of events and participants. The quality of evidence for this outcome is therefore very low. Our confidence in the effect estimate is very limited, and further research is very likely to have an important impact and likely to change this estimate.
The quality of the evidence for the number of dropouts in the studies, used as an outcome in this review, was also low. Apart from the limitations of study design, we also had concerns about selective reporting. Of the nine studies that did not report the dropout by study arm, six provided the total number of dropouts, and this ranged from 18% to 38%; we could not rule out differential dropout in these studies. Nevertheless, it is unclear how dropout as an outcome is linked to participant's acceptability of the treatment regimen, or adverse events experienced.
Potential biases in the review process
We used a sensitive search strategy to identify trials for inclusion in this review and placed no restrictions on publication status or language. Many references were translated in order to determine whether or not they reported trials eligible for inclusion in this review.
We observed a degree of asymmetry in the funnel plot, which suggests an association between smaller studies and a lower estimate of treatment effect. Publication bias is usually reported to result in a lower probability of publication of small studies with negative effects, the reverse of what is observed here. This asymmetry was strongly influenced by a large study, which reported the largest positive effect. There may be other reasons for differences between the average effects in small and large studies, and this result may well represent the effects of confounding by other study characteristics.
As most of the included studies (25 out of 28) were published before the year 2000, the majority in the 1970s, most papers provided very little information in areas that are considered important for assessment of bias. This meant that we rated many studies as 'unclear'. In the assessment of selective reporting bias, we rated most studies as 'low', simply because there was no protocol and little information to suggest that there was selective reporting, and clinically it seems expected outcomes were reported. However, should more information be made available, this may not be the case. We rated all three newer studies (published after the year 2000) as unclear for selective reporting bias because information was provided to suggest that results were not reported exactly in the way planned.
We made a thorough attempt to investigate sources of heterogeneity in this review, examining factors related to participants, interventions, and study design quality. None of the a priori specified factors above were clearly related to heterogeneity. When we examined whether there was any relationship between the caries‐preventive effectiveness of fluoride gel and a few other factors posed post hoc, we also found no significant associations for length of follow‐up (duration of study in years), prior prophylaxis and dropout rate. The only factor that was significantly related to heterogeneity of effect was whether the study employed a placebo or a no‐treatment control group. The latter group of studies was associated with a significant 17% greater estimate of treatment effect on the main outcome than those with a placebo control group (a finding that informed the way results were presented in both the original and this updated review). Blind assessment of outcome was an inclusion criterion for this review, but clearly participants could not have been blinded in trials with no‐treatment controls. Although it is unclear why this should have been associated with differences in outcome in these particular circumstances, type of control group can be considered a useful 'proxy' for the use or not of double‐blinding in included studies, a key methodological feature that probably represents the best indicator of study quality in this review. However, lack of blinding in outcome assessment was not a major criterion for exclusion of studies; we excluded only two studies for this reason alone ‐ most studies had multiple features that met our exclusion criteria, such as lack of randomisation.
We also performed sensitivity analyses for the main meta‐analysis to take into account the uncertainty we had about the imputations for the missing standard deviations; the clustering where this had not been done (in one cluster‐randomised trial); and the uncertainty we had about the inclusion of trials at high risk of bias for allocation concealment and for blinding of outcome assessment. The sensitivity analyses showed similar results and levels of heterogeneity to those of the full meta‐analysis, except for blind outcome assessment, where the effect estimate was smaller than the full meta‐analysis and with a lower level of heterogeneity. The unchanged sensitivity analysis result obtained for the key domain of allocation concealment was possibly due to the fact that this process was poorly described in the studies included in general.
Agreements and disagreements with other studies or reviews
The findings of this updated Cochrane review did not differ from those of the initial review, published first in 2002. The general direction of findings presented is in keeping with those of other reviews (for example van Rijkom 1998 and Weyant 2013), which also found evidence for the effectiveness of fluoride gel.
The estimate of caries reduction in this review remains very similar to that reported in the meta‐analysis on the caries‐preventive effect of fluoride gels from the late 1990s (van Rijkom 1998), which found a pooled D(M)FS PF estimate of a 22% (95% CI 18% to 25%) reduction in caries increment. It is also similar to that reported in the most recent meta‐analysis (Weyant 2013), where treatment effects were presented as pooled D(M)FS standardised mean differences (SMD), and a pooled estimate of ‐0.25 (95% CI ‐0.33 to ‐0.16) was obtained (due to the character of D(M)FS data, mean caries increments are closely related to their standard deviations).
Nevertheless, there were substantial differences in selection criteria and methods between the reviews, and consequently the number and types of studies included. Of the 19 studies included in the review by van Rijkom 1998, we did not include 10 in this review: in 4 of the these trials fluoride toothpaste in gel form was applied daily by toothbrush in standard concentrations (found in toothpastes) of less than 1500 ppm F, and the other 6 studies were excluded for a variety of reasons. We identified a further 13 studies for this review, including 4 published after the van Rijkom 1998 review (Gisselsson 1999; Jiang 2005; Truin 2005; Van Rijkom 2004).
As for the other review (Weyant 2013), of the 12 studies included in its D(M)FS SMD meta‐analysis, 11 were also included in this review; in the trial that did not meet the inclusion criteria for our review (Agrawal 2011), only 2 schools were assigned by coin tossing to 1 of the 2 groups compared. We identified a further 13 studies for inclusion in this review, all published before the Weyant 2013 review.
This updated Cochrane review includes an additional three RCTs compared to the previous version (Marinho 2002). None of these three included trials are thus included in the van Rijkom 1998 review, and one trial is included in the Weyant 2013 review (Jiang 2005).
The large body of evidence contained in this updated Cochrane review provides the best available evidence of the effectiveness of fluoride gel compared to either placebo or no treatment (the comparative effectiveness of topical‐fluoride interventions is addressed in another review in this series (Marinho 2004)).
Authors' conclusions
Implications for practice.
This review suggests that the application of fluoride gels, either by professionals or self applied, is associated with a large reduction in caries increment in permanent teeth in children (the quality of evidence is moderate). We are less certain of the large reduction observed in the primary dentition (low quality evidence). Unfortunately, there was little information on the risk of adverse effects with this treatment.
We found no evidence that this relative effect was dependent on the baseline caries level, or exposure to other fluoride sources in the population, or to application features such as mode/method or frequency of gel application, or fluoride concentration. We also found that this relative effect was not dependent on length of follow‐up, whether a prophylaxis was undertaken prior to application of the gel, or according to dropout rate, although these results should be interpreted with caution (even a meta‐analysis including 25 trials has very limited power to detect such relationships and, like all analyses of observational data, is subject to the problem of potential confounding). The effect of fluoride gel varied according to type of control group used, with larger caries reductions in non‐placebo‐controlled trials.
The evidence seems applicable to current clinical practice. For example, with regards to exposure to other fluoride sources in the population, although the evidence base for fluoride gel is mainly from older studies conducted when fluoridated toothpaste was not widely available, we have found no evidence of smaller treatment effects in the trials conducted more recently.
Implications for research.
We have identified a large number of trials, but the quality of the trials included in this review is relatively poor, with many reports lacking important methodological details. This is likely to be due in part to the fact that most of the trials are relatively old. Many characteristics considered crucial for excluding bias, such as clearly stated randomisation and allocation concealment, have been emphasised more in recent years, after most of the gel trials were reported. Researchers should pay particular attention to reporting the methods of randomisation and the history and reasons for dropouts and exclusions throughout the course of the study. Nevertheless, given the clarity of the results for the permanent dentition (and general lack of recommendation for fluoride gel use in young children), further randomised comparisons of fluoride gel and placebo/no treatment alone would be hard to justify.
If further trials are considered, head‐to‐head comparisons of fluoride gels and other caries‐preventive strategies may provide more useful information. It is important that future trials should include the assessment of potential adverse effects and acceptability. Planning and conducting an economic analysis alongside the clinical trial may also be considered. The evaluation of possible differences in effect associated to fluoride gel application features, such as frequency of application, should be based on available/future trials that directly address the comparison of such features. Further trials should be well‐designed RCTs (adequate sequence generation and allocation concealment methods, blinding of participants and outcome assessors) and reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement (www.consort-statement.org). Core outcomes on the assessment of caries and impact of caries, which may be available through the Core Outcome Measures in Effectiveness Trials (COMET) initiative (www.comet-initiative.org) should be used.
What's new
| Date | Event | Description |
|---|---|---|
| 8 February 2021 | Review declared as stable | This review has stable conclusions and will not be further updated. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 3 June 2015 | New citation required but conclusions have not changed | Three new studies added. Methods updated. Fuller 'Risk of bias' assessment completed. 'Summary of findings' table added. |
| 5 November 2014 | New search has been performed | New search carried out. Three of the original authors not involved in the update. |
| 27 August 2008 | Amended | Converted to new review format. |
Notes
This review has stable conclusions and will not be further updated.
Acknowledgements
We would like to acknowledge the considerable amount of work undertaken by Julian Higgins, Stuart Logan, and Aubrey Sheiham, who were authors on the previous version of this review published in 2002. We gratefully acknowledge the help of the following investigators who provided additional information about their trials: H Gisselsson (Eslöv Public Dental Service), M Olivier (University of Montreal), H D Mestrinho (University of Brasilia), H Horowitz (NIDR, Bethesda), A Treide (University of Leipzig), M Kukleva (Plovdiv Higher Medical Institute); thanks to B Kiene and B Egger‐Heigold (GABA, Switzerland) who supplied reports of trials. The help and expertise of the following is also gratefully acknowledged: R Wents, A Schreiber, H Pikhart, K Turai, T Janicki (German, Russian, Bulgarian, Polish and Hungarian translations); B Anagnostelys, L Jones (Systematic Reviews Training Unit, London), E Tavender and S Bickley, A Littlewood, L Fernandez ‐Mauleffinch and L MacDonald (Cochrane Oral Health Group), O Onwood (QMUL, London). We would also like to thank those who have provided comments and editorial input into this review: D Richards (University of Dundee), C Deery (University of Sheffield), R Weyant (University of Pittsburgh), R Holt (UCL), M Lennon (University of Liverpool), S Poulsen (University of Aarhus), A Rugg‐Gunn (Newcastle University Dental School), L Hooper (Cochrane Oral Health Group), J Clarkson, A‐M Glenny, A Littlewood and P Riley (Cochrane Oral Health Group).
Appendices
Appendix 1. MEDLINE via OVID search strategy
1. exp Tooth demineralization/ 2. (carie$ or carious or DMF).ti,ab. 3. ((dental or tooth or teeth or enamel or dentin$) and (decay$ or cavit$ or deminerali$ or reminerali$ or "white spot$")).ti,ab. 4. or/1‐3 5. exp Fluorides/ 6. (fluorid$ or fluor or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat$ F" or "acidulat$ F" or "phosphat$ fluor$" or fluorphosphat$ or "amin$ fluor$" or "sodium fluor$" or "stannous fluor$" or SMFP or MFP or monofluor$).ti,ab. 7. 5 or 6 8. exp gels/ 9. (gel$ or gelee$ or tray$ or foam$).ti,ab. 10. (Malvatricin or Elmex or Topol or Fluormex or Oralgene or Dentagel or Fluoridex or Phos‐Flur or Prevident or Fluorigard or Gel‐Kam or Flo‐Gel).ti,ab. 11. 8 or 9 12. 7 or 10 13. 4 and 11 and 12
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Section 6.4.11.1 and detailed in Box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health Group’s Trials Register search strategy
1 (deminerali* or caries or carious or DMF or fissure* or decay* or cavit* or "white spot*"):ti,ab 2 (fluorid* or fluor* or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat* F" or "acidulat* F" or "phosphat* fluor*" or fluorphosphat* or "amin* fluor*" or "sodium fluor*" or "stannous fluor*" or SMFP or MFP or monofluor*):ti,ab 3 (gel* or gelee* or tray* or foam*):ti,ab 4 (Malvatricin or Elmex or Topol or Fluormex or Oralgene or Dentagel or Fluoridex or Phos‐Flur or Prevident or Fluorigard or Gel‐Kam or Flo‐Gel):ti,ab 5 #2 or #4 6 #1 and #3 and #5
Appendix 3. CENTRAL search strategy
#1 [mh "Tooth demineralization"] #2 (carie* or carious or DMF) #3 ((dental or tooth or teeth or enamel or dentin*) and (decay* or cavit* or deminerali* or reminerali* or "white spot*")) #4 {or #1‐#3} #5 [mh Fluorides] #6 (fluorid* or fluor or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat* F" or "acidulat* F" or "phosphat* fluor*" or fluorphosphat* or "amin* fluor*" or "sodium fluor*" or "stannous fluor*" or SMFP or MFP or monofluor*) #7 #5 or #6 #8 (gel* or gelee* or tray* or foam*) #9 (Malvatricin or Elmex or Topol or Fluormex or Oralgene or Dentagel or Fluoridex or Phos‐Flur or Prevident or Fluorigard or Gel‐Kam or Flo‐Gel) #10 #7 or #9 #11 #4 and #8 and #10
Appendix 4. EMBASE via OVID search strategy
1. exp Dental caries/ 2. (carie$ or carious or DMF).ti,ab. 3. ((dental or tooth or teeth or enamel or dentin$) and (decay$ or cavit$ or deminerali$ or reminerali$ or "white spot$")).ti,ab. 4. or/1‐3 5. exp Fluoride/ 6. (fluorid$ or fluor or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat$ F" or "acidulat$ F" or "phosphat$ fluor$" or fluorphosphat$ or "amin$ fluor$" or "sodium fluor$" or "stannous fluor$" or SMFP or MFP or monofluor$).ti,ab. 7. 5 or 6 8. exp gel/ 9. (gel$ or gelee$ or tray$ or foam$).ti,ab. 10. (Malvatricin or Elmex or Topol or Fluormex or Oralgene or Dentagel or Fluoridex or Phos‐Flur or Prevident or Fluorigard or Gel‐Kam or Flo‐Gel).ti,ab. 11. 8 or 9 12. 7 or 10 13. 4 and 11 and 12
The above subject search was linked to the Cochrane Oral Health Group filter for identifying randomised controlled trials in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. CINAHL via EBSCO search strategy
S12 S3 and S10 and S11 S11 S7 or S8 S10 S6 or S9 S9 (Malvatricin or Elmex or Topol or Fluormex or Oralgene or Dentagel or Fluoridex or Phos‐Flur or Prevident or Fluorigard or Gel‐Kam or Flo‐Gel) S8 (gel* or gelee* or tray* or foam*) S7 (MH "Gels+") S6 S4 or S5 S5 (fluoride* or fluor or "PPM F" or PPMF or APF or NAF or "Sodium F" or "Amine F" or SNF2 or "Stannous F" or "phosphat* F" or "acidulat* F" or "acidulat* fluor*" or "phosphat* fluor*" or fluorphosphat* or "amin* fluor*" or "sodium* fluor*" or "stannous* fluor*" or SMFP or MFP or monofluor*) S4 (MH "Fluorides+") S3 S1 or S2 S2 (carie or caries or carious or DMF* or cavit* or deminerali* or reminerali* or "white spot"*) S1 (MH "Tooth demineralization+")
Appendix 6. LILACS/BBO via BIREME Virtual Health Library search strategy
((Mh Fluorides or fluoride$ or fluoruro$ or fluoreto$) AND (gel$ or foam$ or espuma$)) [Words] and (Mh Dental caries or carie$ or carious)
Appendix 7. ProQuest Dissertation and Theses search strategy
all(fluoride) AND all(gel) AND all(caries or carious or decay)
Appendix 8. Web of Science Conference Proceedings search strategy
#4 #1 and #2 and #3 #3 TS=(fluoride* or "PPM F" or "PPMF" or "APF" or "NAF" or "sodium F" or "amine F" or "SNF2" or "stannous F" or acidulat* or "phosphat* fluorid*" or "fluorophosphat* sodium fluorid*" or "amine* fluorid*" or"stannous* fluorid*" or SMFP or "MFP" or monofluor*) # 2 TS=(gel* or foam*) # 1 TS=(deminerali* or caries or carious or DMF* or fissure* or decay* or cavit* or "white spot*")
Appendix 9. US National Institutes of Health Trials Registry and WHO Clinical Trials Registry Platform search strategy
fluoride gel
Data and analyses
Comparison 1. Fluoride gel versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 D(M)FS increment ‐ nearest to 3 years (25 trials) | 25 | 8479 | Prevented Fraction (IV, Random, 95% CI) | 0.28 [0.19, 0.36] |
| 1.1.1 Placebo control | 15 | 5671 | Prevented Fraction (IV, Random, 95% CI) | 0.21 [0.15, 0.28] |
| 1.1.2 No‐treatment control | 10 | 2808 | Prevented Fraction (IV, Random, 95% CI) | 0.38 [0.24, 0.52] |
| 1.2 D(M)FT increment ‐ nearest to 3 years (10 trials) | 10 | 3198 | Prevented Fraction (IV, Random, 95% CI) | 0.32 [0.19, 0.46] |
| 1.2.1 Placebo control | 4 | 1525 | Prevented Fraction (IV, Random, 95% CI) | 0.18 [0.09, 0.27] |
| 1.2.2 No‐treatment control | 6 | 1673 | Prevented Fraction (IV, Random, 95% CI) | 0.43 [0.29, 0.57] |
| 1.3 d(e/m)fs increment ‐ nearest to 3 years (3 trials) | 3 | 1254 | Prevented fraction (IV, Random, 95% CI) | 0.20 [0.01, 0.38] |
| 1.3.1 Placebo control | 3 | 1254 | Prevented fraction (IV, Random, 95% CI) | 0.20 [0.01, 0.38] |
| 1.3.2 No‐treatment control | 0 | 0 | Prevented fraction (IV, Random, 95% CI) | Not estimable |
| 1.4 Signs of acute toxicity ‐ nausea, vomiting (2 trials) | 2 | 490 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 1.5 Dropouts or withdrawals | 19 | 8695 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.89, 1.19] |
| 1.5.1 Placebo control | 11 | 6067 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.91, 1.22] |
| 1.5.2 No‐treatment control | 8 | 2628 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.73, 1.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abadia 1978.
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT (quasi), non‐placebo controlled Study duration: 1 year |
|
| Participants | 254 children analysed at 1 year (available at final examination) Participants randomised (N = 291) Age range 11‐12 years Surfaces affected: 12.2 DMFS Exposure to other fluoride: none assumed Year study began: 1977 Location: Brazil Setting of recruitment and treatment: school and school clinic, respectively |
|
| Interventions | FG + ptc (2 groups)* versus NT
(APF group 1 = 12,300 ppm F
APF group 2 = 12,300 ppm F) Operator applied, with cotton‐paint tip, for 4 min, once a year (one FG group had rubber dam isolation, the other had no rubber dam, only cotton rolls isolation) Prior to application = tooth cleaning performed with rotating rubber cup with a non‐F prophy paste Postop instruction = refrain from rinsing, eating and drinking for 30 min |
|
| Outcomes | 1‐year net DMFS increment ‐ (CA)(E)
Reported at 1 year follow‐up O‐DMFS MD‐DMFS BL‐DMFS Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | [MSc Dissertation] Gel manufacturer mentioned ‐ Rescue Squad, United Corp, USA, not as sponsors though. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included = E; diagnostic errors NR *Prior prophylaxis with non‐F paste carried out in FG groups only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Children's records were ordered by the number of permanent teeth present, and then condensed in groups of 3, and allocated 'at random' to 3 groups designated I, II, and III. Then lists were prepared with the names of the children with an indication of the groups to which they belonged” Comment: Method unclear, quasi method likely. |
| Allocation concealment (selection bias) | High risk | Quote: “Children's records ... then condensed in groups of 3, and allocated 'at random' to 3 groups designated I, II, and III. Then, lists were prepared with the names of the children with an indication of the groups to which they belonged” Comment: no concealment of allocation indicated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Group III received no treatment and served as control" Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The examiner had no knowledge of the children's group assignment, neither was this information available in their records, which were brought for examination at random, without consideration of group assignment ....Topical applications were supervised by 3 dentists, one of them the author" “Group III received no treatment and served as control" Comment: Blind caries assessment described, but indication that examiner may have been involved in supervising treatment AND no placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 13% in 1 year. Dropout by group: 14/97 FG1 (14.4%), 15/96 FG2 (15.6%), 8/98 NT (8.2%). Reasons for losses: Not reported. Comment: Numbers lost not unduly high for length of follow‐up, almost no differential losses (treatment groups vs NT). It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA)(E)CL, reported at 1‐year follow‐up. ODMFS, MDDMFS, BLDMFS, incisorsDMFS, pre‐mDMFS, mDMFS Comment: Trial protocol available (thesis). All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported (for sample randomised and final/analysed at 1 year): DMFS: 12.7 FG1, 11.6 FG2, 12.3 NT Comment: Initial caries appears balanced between groups. Dental age and gender are other characteristics reported and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided on inadvertent application of the intervention to people in the control group (contamination), or on possibility of additional treatment given to one of the groups differentially (co‐intervention). |
Bijella 1981.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group quasi RCT (only 2 relevant arms used); non‐placebo controlled Study duration: 1.5 years |
|
| Participants | 320 children analysed at 1.5 years (after exclusions, available at final examination)
Participants randomised (N = 401) Age range 7‐10 years Surfaces affected: 6.6 DMFS Exposure to other fluoride: none assumed Year study began: 1979 Location: Brazil Setting of recruitment and treatment: school and school clinic, respectively |
|
| Interventions | FG + ptc* versus NT
(APF group = 12,300 ppm F) Operator applied, with cotton‐paint tip, 2 ml applied for 4 min, once a year Prior to application = tooth cleaning performed with rotating rubber cup + floss with a non‐F prophy paste Postop instruction = refrain from rinsing, eating, and drinking for 30 min |
|
| Outcomes | 1.5‐year DMFS increment ‐ (CA)(E)
Reported at 1.5 years follow‐up O‐DMFS BL‐DMFS MD‐DMFS DMFT(CA)(E) |
|
| Declaration of interest | No information provided. | |
| Source of funding | [PhD Dissertation]. No information provided. | |
| Notes | Clinical (VT) caries assessment by 4 examiners; diagnostic threshold = CA; state of tooth eruption included = E; reversal rate = 2.2% and 0.7% of observed DMFS increment in FG and control groups, respectively. *Prior prophylaxis with non‐F paste carried out in FG group only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Children were initially ordered by the number of permanent teeth present, then by level of DMF, and then, they were, each 4, distributed 'at random', to form each one of the 4 groups designated I, II, III and IV. Each group formed in this way were assigned 'at random' to the treatments (3 groups) and control (1 NT group).” Comment: Method unclear, quasi method likely. |
| Allocation concealment (selection bias) | High risk | Quote: “Children were initially ordered by the number of permanent teeth present, then by level of DMF, and then, they were grouped, each 4, distributed 'at random', to form each one of the 4 groups designated I, II, III and IV. Each group formed in this way were assigned 'at random' to the treatments (3 groups) and control (1 NT group).” Comment: No concealment of allocation indicated/likely. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Group I received no treatment and served as the control group" Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The examiners had no knowledge of the children's group assignment, neither was this information available in their records, which were brought for examination at random, without consideration of group assignment .... Each child was re‐examined by the same dentist of previous examination. Author was involved in providing topical applications" “Group I received no treatment and served as the control group" Comment: Blind caries assessment described, but indication that examiner may have been involved in providing treatment AND no placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 81/401 (20.2%) in 1.5 years. Dropout by group: 41/201 (20.4%) FG, 40/200 (20%) NT. Reasons for losses: Exclusions based on 'statistical reasons' (made at random to keep groups of equal size, after 11% 'natural loss'). Comment: Numbers lost were not high for the length of follow‐up, but it is unclear if there were differential losses between groups (since the numbers above were produced after 'statistical' exclusions). It is unclear if all reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations, after exclusions were made at random to keep groups balanced in size (not all participants available at follow‐up were actually analysed). |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS and DMFT increment ‐ (CA)CL, reported at 1.5 years follow up. ODMFS, MDDMFS, BLDMFS Comment: Trial protocol (thesis) available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported (for sample randomised and final/analysed at 1.5 years): DMFS: 6.7 FG, 6.6 NT DMFT: 4.1 FG, 4 NT Comment: Initial caries appears balanced between groups. Dental age and gender are other characteristics reported and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided on inadvertent application of the intervention to people in the control group (contamination), or on possibility of additional treatment given to one of the groups differentially (co‐intervention). |
Bryan 1970.
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, non‐placebo controlled Study duration: 2 years |
|
| Participants | 208 children analysed at 2 years (available at final examination)
Participants randomised (N = 287) Age range 8‐12 years (average = 9.5) Surfaces affected: 8.3 DMFS Exposure to other fluoride: none assumed Year study began: assumed in/before 1966 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc versus NT + ptc
(APF, concentration NR) Operator applied, with tray, once a year, applied for 4 min Prior to application = tooth cleaning performed Postop instruction = refrain from eating and drinking for 30 min |
|
| Outcomes | 2‐year net DMFS increment ‐ (CA)
Reported at 1 and 2 year follow‐ups DMFT(CA) Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included NR; diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Students were assigned to the control and treatment groups by using classroom rosters and a table of random number.” |
| Allocation concealment (selection bias) | Unclear risk | Unclear if an open list was used that would not allow for allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: “The control group ... also received an examination and prophylaxis, but had no fluoride application.” Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: “... the examiner was unaware to which group the children were assigned.” “The control group ... received an examination and prophylaxis.” Comment: Blind outcome assessment, but no placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 27.5% in 2 years. Dropout by group: 36/139 (25.9%) FG, 43/148 (29.05%) PL. Reasons for losses: Not reported. Comment: Numbers lost not unduly high for length of follow‐up; no differential losses. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA), reported at 1 and 2 year follow‐ups. DMFT(CA) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 8.37(5.99) FG, 8.14(5.98) NT DMFT: 4.27(2.67) FG, 4.37(2.28) NT Comment: Initial caries appears balanced between groups. Gender is the other characteristic reported and is balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information. |
Cobb 1980.
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT (only 2 relevant arms used); non‐placebo controlled Study duration: 2 years |
|
| Participants | 193 children analysed at 2 years (available at final examination) Participants randomised (N = 237) Age range 11‐14 years Surfaces affected: 5.7 DMFS (data from original sample only) Exposure to other fluoride: toothpaste assumed Year study began: in/before 1977 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc versus NT + ptc
(APF group = 12,300 ppm F) Operator applied, with cotton‐paint tip, twice a year, applied for 4 min Prior to application = tooth cleaning of stained plaque (with disclosing solution) performed with brush and floss (no paste/toothpaste used) Postop instruction = refrain from eating and drinking for 30 min |
|
| Outcomes | 2‐year DMFS increment ‐ (CA)
Reported at 0.5, 1, 1.5, and 2 year follow‐ups Dropout |
|
| Declaration of interest | No information provided | |
| Source of funding | The trial authors thank Pacemaker Corp for supplying the gel. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included NR; diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “... students were randomly assigned to one of three groups.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Subjects in group C also had plaque removed from their teeth but had no fluoride application.” Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “The protocol was designed so that the examiner did not know which group the subjects were assigned to until data collection was complete.” “Subjects in group C had plaque removed from their teeth but had no fluoride application.” Comment: Blind outcome assessment, but no placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 18.56% in 2 years. Dropout by group: 15/130 (11.54%) FR, 29/107 (27.10%) NT. Reasons for losses: Not reported. Comment: Numbers lost not unduly high for length of follow‐up, with differential losses between groups (even though reported as NS difference). It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA), reported at 0.5, 1, 1.5, and 2 year follow‐ups. Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 6.01(5.24) FG, 5.38(4.76) NT Age, gender, ethnicity, regularity of dental care described as ’balanced’ (values not reported). Comment: Initial caries appears balanced between groups. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Cons 1970.
| Study characteristics | ||
| Methods | Study design: 5‐arm parallel‐group RCT (only 2 relevant arms used), placebo controlled Study duration: 3 years |
|
| Participants | 589 children analysed at 3 years (present for all examinations)
Participants randomised (N = 795) Age range 6‐11 years (average = 8) Surfaces affected: 3 DMFS (first molar) Exposure to other fluoride: none assumed Year study began: 1964 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc versus 'PL' + ptc
(APF group = 12,300 ppm F) Operator applied, with tray, once a year, applied for 4 min Prior to application = tooth cleaning performed with pumice Postop instruction = refrain from eating and drinking for 30 min |
|
| Outcomes | 3‐year net 1stm DMFS increment ‐ (E)
Reported at 3 years follow‐up NetDMFT(E) Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by a Public Health Service Grant DH00065. | |
| Notes | Clinical (VT) caries assessment by 4 examiners; diagnostic threshold NR; state of tooth eruption included = E; diagnostic errors NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children were randomly assigned within school ...” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: “Control ... topical application of distilled water ...” Comment: Use of a control as 'placebo' was described, but it is likely personnel (and perhaps participants) were able to distinguish between gel and water. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “Examiners did not know to which groups the patients belonged.” “Control ... topical application of distilled water ...” Comment: Blind outcome assessment and use of 'placebo' described. It was unclear if patients could tell a difference. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 25.91% in 3 years. Dropout by group: 134/412 FG, 72/383 ‘PL’. Reasons for losses: Exclusions based on presence at all follow‐up examinations. Comment: Numbers lost not unduly high for length of follow‐up, with differential losses between groups (32.52% FG, 18.80% ‘PL’). It is unclear if reasons for dropout are acceptable. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (E), reported at 3 years follow‐up DMFT(E) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 3.16 FG, 2.86 ‘PL’ DMFT: 1.99 FG, 1.75 ‘PL’ Comment: Initial caries appears balanced between groups: "adjustment made little difference in the magnitude of caries increment" |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
DePaola 1980.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo controlled Study duration: 2 years, + 1‐year postintervention period (but only 1‐year results used) |
|
| Participants | 270 children analysed at 1* year (after exclusions, present for both examinations)
Participants randomised (numbers NR) Age range 12‐14 years (average = 13) Surfaces affected: NR Exposure to other fluoride: toothpaste assumed Year study began: in/before 1977 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG versus PL
(APF group = 12,300 ppm F) Self applied under supervision, with tray, 10 consecutive applications (days) in 1st year, applied for 5 min Prior to application = no tooth cleaning performed Postop instruction = no information provided |
|
| Outcomes | 1‐year* net DFS increment ‐ (CA) CL + XR Reported at 1 and 2 year follow‐ups (and 1 year post‐treatment) | |
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by National Institute of Dental Research contract No NOI‐DE42445. | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by 2 examiners (diagnostic threshold NR); diagnostic errors NR. *Intervention applied during 1st year of study only (final 2 years results not considered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomly assigned to 1 examiner and 1 of 4 treatment groups at the time of the clinical examination” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: “A strict double‐blind routine was maintained throughout the course of the investigation.” “The same procedure as group 1 except that placebo gel was used ...” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “A strict double‐blind routine was maintained throughout the course of the investigation.” “The same procedure as group 1 except that placebo gel was used ...” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: Not reported. Dropout by group: Not reported. Reasons for losses: Exclusions based on compliance and presence at all exams. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DFS increment ‐ (CA) CL + XR, reported at 1 and 2 year follow‐ups (and 1 year post‐treatment). Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Unclear risk | Prognostic factors reported: DFS, dental age and age reported as balanced (values not reported). |
| Free of contamination/co‐intervention? | Low risk | Quote: “Special care was taken to be sure that each subject received the proper agent ...” Comment: There is sufficient indication overall of prevention of contamination/co‐intervention. |
Englander 1967.
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, non‐placebo controlled Study duration: 1.8 years, + 1.9 years postintervention period |
|
| Participants | 500 children analysed at 1.8 years (present for all examinations)
Participants randomised (N = 574) Age range 11‐14 years (average = 12) Surfaces affected: 10.1 DMFS Exposure to other fluoride: no Year study began: 1964 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG (2 groups) versus NT
(APF group = 5000 ppm F, NaF group = 5000 ppm F) Self applied under supervision, with tray, 140 times a year (average), 1 mg ‐ 2 mg F (5‐10 drops) applied for 6 min* Prior to application = no tooth cleaning performed Postop instruction = no brushing after application |
|
| Outcomes | 1.8‐year DMFS increment ‐ (CA)
Reported at 1.8 years follow‐up (and 1.9 years post‐treatment) DMFT(CA) Dropout Etching of enamel; inadvertent swallowing, by total increase in fluoride urinary excretion |
|
| Declaration of interest | No information provided. | |
| Source of funding | The trial authors thank Davies, Rose Hoyt Pharmaceutical Div, the Kendall Co, Needham, Mass, 02194 for supplying the gels. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included NR; diagnostic errors NR *Gel application started 7 weeks after baseline examination. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children ... were stratified by age and sex and assigned at random ...” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: “The third group was the control group ... no NaF gels were applied.” Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: “The third group was the control group ... no NaF gels were applied.” “All clinical examinations were conducted by one of the authors .... He did not know to which group a child belonged at the time of the examination.” Comment: Blind outcome assessment, but no placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 12.9% in 1.8 years. Dropout by group: 64/369 (17.3%) for both FG groups, 10/205 (4.9%) NT. Reasons for losses: Moved away or could not participate, exclusions based on presence in all follow‐up examinations. Comment: Numbers lost not unduly high for length of follow‐up, with differential losses between groups (17.34% FG (both groups), 4.89% NT). It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA), reported at 1.8 years follow‐up (and 1.9 years post‐treatment) DMFT(CA) Etching of enamel; inadvertent swallowing, by total increase in fluoride urinary excretion Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 10.23 FG1, 10.19 FG2, 10.00 NT DMFT: 6.05 FG1, 5.99 FG2, 5.95 NT Comment: Initial caries appears balanced between groups. Age and gender reported as balanced (values for age not reported). |
| Free of contamination/co‐intervention? | Low risk | Quote: “All children in the three groups were provided with a generous supply of fluoride‐free dentifrice for home use throughout the study.” Comment: There is sufficient indication overall of prevention of contamination/co‐intervention. |
Englander 1971.
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, non‐placebo controlled Study duration: 2.5 years |
|
| Participants | 557 children analysed at 2.5 years (available at final examination)
Participants randomised (N = 896) Age range 11‐15 years (average = 12.2) Surfaces affected: 3.7 DMFS Exposure to other fluoride: water Year study began: 1967 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG vs NT
(APF group = 5000 ppm F) Self applied under supervision, with tray, 85 times a year (average), 1 mg ‐ 2 mg applied for 3 min Prior to application = no tooth cleaning performed Postop instruction = no information provided |
|
| Outcomes | 2.5‐year net DMFS increment ‐ (CA)(E) Reported at 2.5 years follow‐up | |
| Declaration of interest | No information provided. | |
| Source of funding | Davies, Rose Hoyt Pharmaceutical Div, the Kendall Co, Needham, Mass, 02194 supplied the gels. | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA; state of tooth eruption included = E; diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “They were assigned at random into two study groups.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: “The second group acted as controls. Applicators were not constructed for these children, and no NaF gel‐drops were applied.” Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: “Neither examiner knew to which group a child belonged at any time of any examination.” “The second group acted as controls. Applicators were not constructed for these children, and no NaF gel‐drops were applied.” Comment: Blind outcome assessment, but no placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 37.83% in 2.5 years. Dropout by group: Not reported. Reasons for losses: Not reported. Comment: Numbers lost high for length of follow‐up; any differential losses not assessable. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA)(E) reported at 2.5 years follow‐up. Inadvertent swallowing, by total increase in fluoride urinary excretion (no data reported). Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 3.98 FG, 3.35 NT Comment: Initial caries appears balanced between groups. Age, gender, ethnicity, SAR also reported, and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Englander 1978.
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT; placebo controlled Study duration: 2.3 years (but only 1.5 years results used) |
|
| Participants | 145 children analysed at 1.5* years (available at 2nd examination)
Participants randomised (N = 231) Age range 2‐6 years (average = 4.8) Surfaces affected: 3.7 defs (data from original sample only) ‐ 43% caries‐free Exposure to other fluoride: water Year study began: in/before 1974 Location: USA Setting of recruitment: no clear information provided. Setting of treatment: clinic and grammar school |
|
| Interventions | FG versus PL
(APF group = 5000 ppm F) Self applied under supervision, with tray, 76 times a year (average), 1 mg (5 drops) applied for 3 min Prior to application = no tooth cleaning performed Postop instruction = no information provided |
|
| Outcomes | 1.5‐year *defs increment ‐ (CA)(E)
Reported at 0.5, 1.5, and 2.3 year follow‐ups deft (CA)(E) Proportion of children remaining caries‐free Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. One of the authors is affiliated with Colgate Research Centre. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included = E; diagnostic errors NR *Dramatic dropout rate after 1.5 years of treatment (final 2.3 years results not considered) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “These children were then stratified by age and sex and randomly assigned to two groups.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: “... the children in group 2 were placebo treated and similarly applied neutral fluoride free and phosphate free gel drops ...” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “... the children in group 2 were placebo treated and similarly applied neutral fluoride free and phosphate free gel drops ...” “The examiner did not know to which group a child belonged at any time of any examination.” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 37.23% in 1.5 years. Dropout by group: 45/119 (37.8%) FG, 41/112 PL (36.7%). Reasons for losses: Moved away. Comment: Numbers lost unduly high for length of follow‐up, although with no differential losses between groups. Reason for dropout is acceptable and balanced. Caries data used in analysis pertain to participants present at 2nd follow‐up examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: defs increment ‐ (CA)(E), reported at 0.5, 1.5, and 2.3 year follow‐ups deft (CA)(E) Proportion of children remaining caries‐free Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: defs: 3.65 FG, 3.65 PL deft: 2.06 FG, 2.40 PL Comment: Initial caries appears balanced between groups. Age, TAR, SAR, % caries‐free also reported, and balanced. |
| Free of contamination/co‐intervention? | Low risk | Quote: “All families were provided with a continuous supply of toothbrushes and fluoride free dentifrices.” Comment: There is sufficient indication overall of prevention of contamination/co‐intervention. |
Gisselsson 1999.
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group quasi RCT, placebo controlled Study duration: 3 years, + 1.9 years postintervention period |
|
| Participants | 280 children analysed at 3 years (available at final examination)
Participants randomised (N = 317) Average age 13 years Surfaces affected: 0.24 DFS* ‐ 39% caries‐free Exposure to other fluoride: toothpaste Year study began: 1993 Location: Sweden Setting of recruitment and treatment: school and public dental clinic, respectively |
|
| Interventions | FG (2 groups) vs PL
(NaF group = 4500 ppm F, SnF2 group = 2425 ppm F) Operator applied, with syringe + floss, 1 ml applied to approximal surfaces of all teeth, in 10 min, 4 times a year Prior to application = no tooth cleaning performed Postop instruction = no information provided |
|
| Outcomes | 3‐year MD‐DFS increment ‐ (CA/NCA)CL+ (DR/ER)XR
Reported at 3 years follow‐up DS FS Proportion of children remaining caries‐free, proportion with 1 or more new DFS (at NCA/ER level) Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by Patentmedelsfonden for odontologisk prophylaxforskning, Malmohus County Council, and the Faculty of Odontology, Goteborg University. Apoteksbolaget for manufacturing the gels used. |
|
| Notes | Clinical caries assessment by 11 examiners; diagnostic threshold = CA and NCA; state of tooth eruption included NR. Radiographic assessment (postBW) by 1 examiner; diagnostic threshold = DR and ER. Diagnostic errors NR. *Gel application started 12 weeks before (2nd) baseline examination. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from correspondence: “The children were ranked at 12 years of age to the numeric values of caries prevalence ... from this list, from top to bottom, the children were distributed in respective group ... first name distributed to a group, second to B, third C, fourth A and so on ...” Comment: Non‐random method used. |
| Allocation concealment (selection bias) | High risk | The non‐random method used for sequence generation would not allow for allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The gels were marked 1, 2, 3, and the study was carried out double‐blind. All gels were manufactured and packed in identical bottles ... The code was not broken until all caries data had been analysed.” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The gels were marked 1, 2, 3, and the study was carried out double‐blind. All gels were manufactured and packed in identical bottles ... The code was not broken until all caries data had been analysed.” Comment: Use of placebo and blind outcome assessment described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 11.67% in 3 years. Dropout by group: 10/107 FG1, 16/101 FG2, 11/109 PL. Reasons for losses: Refused to participate (8), moved away or preferred to visit a private dentist (29). Comment: Numbers lost were not unduly high given length of follow‐up, almost no differential losses between groups 9.35% FG1, 15.84% FG2, 10.09% PL. It is unclear if reasons for the dropout are acceptable and balanced. Caries data used in the analysis pertain to participants present at final examination. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: MD‐DFS increment ‐ (CA/NCA)CL + (DR/ER)XR, reported at 3 years follow‐up DS FS Proportion of children remaining caries‐free, proportion with 1 or more new DFS (at NCA/ER level) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DFS (CA)(from correspondence): 0.25(0.91) FR1, 0.18(0.56) FR2, 0.28(1.00) PL DS (CA)(from correspondence): 0.11(0.50) FR1, 0.09(0.33) FR2, 0.16(0.68) PL Comment: Initial caries (FS and % caries‐free also reported) with some imbalance (reported as NS difference). |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Hagan 1985.
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, placebo controlled Study duration: 2 years |
|
| Participants | 316 children analysed at 2 years (available at final examination)
Participants randomised (N = 428) Age range 11‐15 years (average = 12.5) Surfaces affected: 4.6 DMFS Exposure to other fluoride: toothpaste Year study began: 1981 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG (2 groups) + ptc vs PL + ptc
(APF group 1 = 12,300 ppm F, APF group 2 = 6000 ppm F) Operator applied, with tray, twice a year, 2.5 ml applied Prior to application = tooth cleaning of stained plaque (with disclosing solution) performed with brush and floss (no paste/toothpaste used) Postop instruction = excess saliva spitted, refrain from eating and drinking for 30 min |
|
| Outcomes | 2‐year DMFS increment ‐ (E)
Reported at 2 years follow‐up PF‐DMFS MD‐BL‐DMFS Nausea/vomiting within 15 min of gel application |
|
| Declaration of interest | No information provided. | |
| Source of funding | Coopercare manufacturer supplied gel. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold NR; state of tooth eruption included = E; reversal rate less than 0.002% of observed caries increment in all groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Students ... were assigned randomly to 1 of 3 groups ...” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: “... and those to receive a placebo gel containing no fluoride.” “The study followed the classic double‐blind protocol in that examiner, subjects and dental auxiliaries were unaware of which agent was applied in each subject.” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “... and those to receive a placebo gel containing no fluoride.” “The study followed the classic double‐blind protocol in that examiner, subjects and dental auxiliaries were unaware of which agent was applied in each subject.” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 26.17% in 2 years. Dropout by group: Not reported, said to range from 24% to 26%. Reasons for losses: Moved to other locations Comment: Numbers lost not unduly high for length of follow‐up with an indication of no differential losses between groups. Reason for dropout is acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (E), reported at 2 years follow‐up. PF‐DMFS MD‐BL‐DMFS Nausea/vomiting within 15 min of gel application Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 5.05(4.88) FG1, 4.41(4.41) FG2, 4.41(4.87) PL Age, sex, regularity of dental care, exposure to other F sources also reported as 'balanced'. Comment: Initial caries appears balanced between groups. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Heifetz 1970.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group quasi RCT (only 2 relevant arms used); placebo controlled Study duration: 2 years |
|
| Participants | 309 children analysed at 2 years (after exclusions, present for all examinations)
Participants randomised (N = 525) Age range 12‐13 years Surfaces affected: 8.2 DMFS Exposure to other fluoride: none assumed Year study began: 1966 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc vs 'PL' + ptc
(APF group = 12,300 ppm F) Self applied under supervision, with toothbrush, 5 times a year, 4 ml applied for 5 min Prior to application = tooth cleaning (supervised toothbrushing) performed with non‐F prophy paste Postop instruction = No information provided. |
|
| Outcomes | 2‐year net DMFS increment ‐ (E + U)
Reported at 1 and 2 year follow‐ups NetDMFT(E + U) Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold NR; state of tooth eruption included = E + U; reversal rate approximately 4% of observed DMFS increment for all groups combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “The baseline records were stratified according to the sex, age and previous caries experience ... within each stratum, the children were separated into four study groups ...” Quote from correspondence: “... after stratification, the children were systematically divided into four groups ...” Comment: Non‐random method used. |
| Allocation concealment (selection bias) | High risk | The non‐random method used for sequence generation would not allow for adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: “The controls ... then brushed with a flavoured solution as a placebo.” Comment: Use of 'placebo' described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “The examiners did not know the groups to which any child was assigned.” “The controls ... then brushed with a flavoured solution as a placebo.” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 41.14% in 2 years. Dropout by group: 102/263 (38.8%) FG, 114/262 (43.5%) ‘PL’. Reasons for losses: Exclusions based on compliance and presence at all follow‐up examinations. Comment: Numbers lost unduly high for length of follow‐up, though with no differential losses between groups. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: DMFS increment ‐ (E + U), reported at 1 and 2 year follow‐ups DMFT(E + U) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 8.26 FG, 8.08 ‘PL’ DMFT: 4.94 FG, 4.80 ‘PL’ Comment: Initial caries appears balanced between groups. |
| Free of contamination/co‐intervention? | Low risk | Quote: “To prevent children from carrying out the wrong procedure or from exchanging the material of treatment, only children in the same study group assembled and brushed at any one time.” Comment: There is sufficient indication overall of prevention of contamination/co‐intervention. |
Horowitz 1971.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group quasi RCT (only 2 relevant arms used); non‐placebo controlled Study duration: 3 years, + 2 years postintervention period |
|
| Participants | 352 children analysed at 3 years (available at final examination)
Participants randomised (N = 552) Age range 10‐12 years Surfaces affected: 8.9 DMFS Exposure to other fluoride: none assumed Year study began: 1965 Location: Hawaii Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc vs NT + ptc
(APF group = 12,300 ppm F) Operator applied, with tray, once a year, applied for 4 min Prior to application = tooth cleaning performed with rotating rubber cup + bristle brush + floss with a pumice paste Postop instruction = refrain from eating and drinking for 30 min |
|
| Outcomes | 3‐year net DMFS increment ‐ (E)
Reported at 1, 2, and 3 year follow‐ups (and 2 years post‐treatment) O‐DMFS BL‐DMFS MD‐DMFS NetDMFT(E) Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold NR; state of tooth eruption included = E; diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “The participants were then assigned randomly into four groups.” “The baseline record cards were separated according to sex and dental age, and then arranged in ascending order of previous caries experience .... the cards were then placed in sequence in one of the four following groups ...” Comment: Non‐random method used. |
| Allocation concealment (selection bias) | High risk | The non‐random method used for sequence generation would not allow for adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Control group also had annual prophylaxis with use of standard abrasive (nonfluoride) paste but no fluoride application ...” Comment: Blind outcome assessment, but no placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Control, also had annual prophylaxis with use of standard abrasive (non fluoride) paste but no fluoride application.” “The examiner did not know the group assignment of any child on any examination.” Comment: Blind outcome assessment, but no placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 36.23% in 3 years; Dropout by group: 106/276 (38.41%) NT, 94/276 (34.06%) FG; Reasons for losses: Moved away, absent at examination, had teeth bonded for orthodontic treatment Comment: Numbers lost unduly high for length of follow‐up with no differential losses between groups. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: DMFS increment ‐ (E), reported at 1, 2 and 3 year follow‐ups (and 2 years post‐treatment) O‐DMFS BL‐DMFS MD‐DMFS DMFT(E) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 9.03 FG, 8.81 NT DMFT: 5.15 FG, 4.96 NT Comment: Initial caries appears balanced between groups. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Horowitz 1974.
| Study characteristics | ||
| Methods | Study design: 5‐arm parallel‐group quasi RCT (only 2 relevant arms used), placebo controlled Study duration: 3 years |
|
| Participants | 233 children analysed at 3 years (present for all examinations)
Participants randomised (N = 512) Age range 11‐14 years (average = 11.5) Surfaces affected: 11.4 DMFS Exposure to other fluoride: no Year study began: 1967 Location: Brazil Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc vs 'PL' + ptc
(APF group = 12,300 ppm F) Self applied under supervision, with toothbrush, 5 times a year, 4 ml applied for 2 min Prior to application = tooth cleaning (supervised toothbrushing) performed with non‐F prophy paste Postop instruction = No information provided. |
|
| Outcomes | 3‐year net DMFS increment ‐ (CA)
Reported at 1, 2, and 3 year follow‐ups Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA; state of tooth eruption included NR; diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “... within each stratum, the subjects were allocated systematically to one of five treatment groups.” Quote from correspondence: “To use your term, we used a ‘quasi‐random' method of alternate allocation.” Comment: Non‐random method used. |
| Allocation concealment (selection bias) | High risk | The non‐random method used for sequence generation would not allow for adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “A double blind technique was followed.” “Controls brushed their teeth with a commercial non fluoride dental prophylaxis paste and then brushed with a flavoured placebo solution.” Comment: Use of 'placebo' described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “A double blind technique was followed.” “Controls brushed their teeth with a commercial non fluoride dental prophylaxis paste and then brushed with a flavoured placebo solution.” Comment: Blind outcome assessment, and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 54.49% in 3 years. Dropout by group: 139/256 (54.3%) 'PL', 140/256 (54.7%) FG. Reasons for losses: Left school Comment: Numbers lost unduly high for length of follow‐up with no differential losses between groups. Reason for dropout is acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA), reported at 1, 2 and 3 year follow‐ups Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 11.44(6.93) FG, 11.37(7.86) ‘PL’ Comment: Initial caries appears balanced between groups. Age and dental age also reported to be balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Ingraham 1970.
| Study characteristics | ||
| Methods | Study design: 5‐arm parallel‐group RCT (3 relevant arms used), non‐placebo controlled Study duration: 2 years |
|
| Participants | 119 children analysed at 2 years (available at final examination)
Participants randomised (N = 155) Age range 6‐11 years (average = 9) Surfaces affected: 2.4 DMFS Exposure to other fluoride: none assumed Year study began: 1965 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG (2 groups) + ptc vs NT + ptc
(APF groups 1 and 2, concentration(s) NR) Operator applied, with tray (beeswax vs foam rubber), once a year (data extracted from Bryan 1968 (in Bryan 1970), applied for 4 min Prior to application = tooth cleaning performed Postop instruction = refrain from eating and drinking for 30 min |
|
| Outcomes | 2‐year net DMFS increment ‐ (CA)
Reported at 1 and 2 year follow‐ups NetDMFT(CA) Nausea/vomiting on application seen as a reaction according to type of tray used (no data). Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included NR; diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: “Children ... were randomly assigned to control and treatment groups ...” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: “Controls also received a prophylaxis, but no fluoride applications.” Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: “Controls received a prophylaxis.” “... the examiner did not know the group assignment of the children at the time of the examination.” Comment: Blind outcome assessment, but no placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 23.23% in 2 years. Dropout by group: 17/73 (23.3%) FG, 19/82 (23.2%) NT. Reasons for losses: Not reported Comment: Numbers lost not unduly high for length of follow‐up with no differential losses between groups. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA), reported at 1 and 2 year follow‐ups DMFT(CA) Nausea/vomiting on application seen as a reaction according to type of tray used (no data). Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 2.32(3.57) FG, 2.55(3.66) NT DMFT: 1.29(1.68) FG, 1.49(1.76) NT Comment: Initial caries appears balanced between groups. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Jiang 2005.
| Study characteristics | ||
| Methods | Study design: 3‐arm cluster RCT (only 2 relevant arms used); non‐placebo controlled Study duration: 2 years |
|
| Participants | Year study began: 2000 Location: China (Wuhan City, Hubei Province) Setting of recruitment and treatment: 3 primary schools Numbers randomised: 456 Numbers analysed: 421 children at 2 years (available at final examination) Age: mean 6.5 SD 0.5 years (range: 6 to 7 years) Mean surfaces affected: 1stm DMFS = 0.11 (SD 0.41) Background exposure to other fluoride: assumed yes (drinking‐water fluoride in water level 0.1 ppm F to 0.3 ppm F, about 43% of participants self reported use of fluoridated toothpaste at baseline) |
|
| Interventions | Comparison: FG versus NT Group 1 (n = 200): APF gel (Xiao Tianshi), pH 3.5, 1.23% APF, 12,300 ppm F Group 2 (n = 221): No treatment Professionally applied, with sponge‐lined tray, for 4 min, 2 times a year. The amount of APF foam or APF gel placed in the tray was no more than 40% of the tray’s volume. Child seated in an upright position with the head inclined forward and downward to reduce swallowing and told not to swallow. Prior to application: No professional prophylaxis, dentition not dried by compressed air. Postop instruction: Expectorate the mixture of saliva or foam/gel for 1 min after tray was removed. Refrain from rinsing, eating, and drinking for 30 min. |
|
| Outcomes | At 2 years: 1stm DMFS increment ‐ (CA)CL Smooth 1stm DMFS and pit and fissure 1stm DMFS Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | Supported by the Hubei Committee for Oral Health, People’s Republic of China | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA. Intra‐examiner K statistics/Kappa values of the duplicate examination was over 0.90 at both baseline and follow‐up examinations. State of tooth eruption included NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children were randomly allocated to the three groups based on school class. A total 13 classes from three primary schools were numbered from 1 to 13. Then, all classes were randomly assigned to APF foam group (n = 4 classes), APF gel group (n = 4 classes) and control group (n = 5 classes) by using blocked randomisation." Comment: Not enough information (no description of sequence generation). |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not enough information is provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "The subjects were blinded to the assignment of group and two dental examiners were blind to all group allocations". Comment: Blinding of treatment intervention could not have been possible for the no‐treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: “The subjects were blinded to the assignment of group and two dental examiners were blind to all group allocations". Comment: It would not have been possible to blind the participants. Unclear how blinding of examiners could be effectively achieved. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "... total of 421 children completed the study (relevant groups data only); the drop‐out rate was 7.7% in 2 years. Most children who were lost from the study were caused by transfer to other schools. ... Only those children who were present at both baseline and follow‐up examinations, and all four treatments (the maximum number of applications a child could have during the trial) were included in the analysis" Comment: 200/210 (95.2%) in FG and 221/246 (89.8%) in NT groups were available for examination at 2 years (reported for individuals within clusters only). Quite low dropout rates, but proportion seems to be higher in the control group. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations, and all 4 treatment times (exclusions made based on a threshold for treatment uptake). |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: 1stm DMFS increments ‐ CL, at 2 years follow‐up (and these according to specific tooth sites ‐ pits and smooth) Comment: Trial protocol not available. Not clear if all pre‐specified outcomes (in Methods) were reported in the pre‐specified way (dmfs and dmft data only reported at baseline). |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported:
age, gender, toothbrushing frequency, use of F toothpaste, family socioeconomic status, dental visits Comment: Initial caries appears balanced between groups (for individuals within clusters). |
| Free of contamination/co‐intervention? | Unclear risk | No information on inadvertent application of the intervention to people in the no‐treatment control group (contamination), or on possibility of additional treatment given to 1 of the groups differentially (co‐intervention). |
Mainwaring 1978.
| Study characteristics | ||
| Methods | Study design: 5‐arm parallel‐group RCT (only 2 relevant arms used), placebo controlled Study duration: 3 years |
|
| Participants | 631 children analysed at 3 years (available at final examination)
Participants randomised (numbers, for 2 relevant groups, NR) Age range 11‐12 years Surfaces affected: 7.9 DFS Exposure to other fluoride: no Year study began: in/before 1974 Location: UK Setting of recruitment and treatment: school and clinic |
|
| Interventions | FG + ptc versus PL + ptc
(APF group = 12,300 ppm F) Operator applied, with tray, twice a year, applied for 4 min Prior to application = tooth cleaning of stained plaque (with disclosing tablets) performed with toothbrush with a non‐F paste Postop instruction = no information provided |
|
| Outcomes | 3‐year Net/Crude DFS increment ‐ (CA)(E)CL + (ER)XR
Reported at 3 years follow‐up PF‐DFS CL postMD‐DFS XR DFS (U) CL + XR CIR |
|
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by a grant from Beecham Group Ltd (SmithKline Beecham merged with Glaxo Wellcome to become GlaxoSmithKline). | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included = E. Radiographic assessment (2 postBW) by 1 examiner; diagnostic threshold = ER. Intra‐examiner reproducibility checks for DFS in 10% sample (ICC for VT/XR over 0.95); error variance less than 5% of total variance; reversal rate less than 4% of observed DFS increment in all groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were stratified according to age, sex and then randomly assigned to one of the treatment groups; children from the same family were assigned to the same group.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The study was of double‐blind design, neither examiner nor participants knowing the identity of the treatment group to which the subjects had been allocated” “... control group had applications of fluoride free gel.” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The study was of double‐blind design, neither examiner nor participants knowing the identity of the treatment group to which the subjects had been allocated” “... control group had applications of fluoride free gel.” Comment: Blind outcome assessment, and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 18% in 3 years (for all 5 groups combined). Dropout by group: NR. Reasons for losses: NR Comment: Numbers lost were not unduly high given length of follow‐up, but any differential loss between groups was not assessable. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in the analysis pertain to participants who completed the study. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DFS increment ‐ (E)(CA)CL + (ER)XR, reported at 3 years follow‐up PF‐DFS postMD‐DFS Caries incidence rate Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DFS: 8.19(6.01) FG, 7.59(5.56) PL Comment: Initial caries appears balanced between groups. Age and SAR also reported, and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Marthaler 1970.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo controlled Study duration: 3 years |
|
| Participants | 120 children analysed at 3 years (present for all examinations)
Participants randomised (numbers for relevant groups NR) Age range 6‐7 years Surfaces affected: 0.81 DFS Exposure to other fluoride: salt Year study began: 1966 Location: Switzerland Setting of recruitment and treatment: school |
|
| Interventions | FG vs PL
(AmF/NaF group = 12,500 ppm F) Self applied under supervision, with toothbrush, 20 times a year, 1 g applied for 6 min Prior to application = no tooth cleaning performed Postop instruction = children not allowed to rinse, simply emptied the mouth after brushing |
|
| Outcomes | 3‐year net DFS increment ‐ (NCA/CA)CL + (DR/ER)XR
Reported at 1 and 3 year follow‐ups 1stmPF‐DFS (CA)CL 1stmMD‐DFS (CA)XR |
|
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by GABA AG, Basel. | |
| Notes | Clinical (V) caries assessment by 2 examiners; diagnostic threshold = CA and NCA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by 2 examiners; diagnostic threshold = DR and ER; partial recording. 'Sufficient agreement of the two examiners known from earlier work'. (quote from the report) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Children were paired according to their sequence in the class lists. The first and second child of each pair was allocated control and fluoride respectively when, in a table of random digits, an even digit was present. In the case of an odd random digit, the first child was allocated fluoride, and the second one control. A few siblings were found ... were taken into the same pair to guarantee that they did not receive the same treatment at school.” |
| Allocation concealment (selection bias) | Unclear risk | Not enough information provided. Unclear if there was concealment of the allocation code. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Control group received exactly the same, just without fluoride.” "the type of ingredient was unknown to the supervisors" Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Use of placebo described. It is unclear if the examiners were blind to treatment allocations, although it is probable that clinical and radiographic exams were done independently. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 18% in 3 years. Dropout by group: NR. Reasons for losses: Exclusions based on use of orthodontic bands and presence in all follow‐up examinations. Comment: Numbers lost not unduly high for length of follow‐up; any differential losses not assessable. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DFS increment (CA)CL + (DR)XR, reported at 1 and 3 year follow‐ups 1stmPF‐DFS 1stmMD‐DFS Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 0.78 (FD), 0.84 (PL) 1stmDMFS: 0.03 FD, 0.04 PL Comment: Initial caries appears balanced between groups. Age also reported, and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Marthaler 1970a.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo controlled Study duration: 4 years (but only 2 years results used) |
|
| Participants | 41 children analysed at 2* years (present for all examinations)
Participants randomised (numbers for relevant groups NR) Age range 7‐9 years Surfaces affected: 2.5 DFS Exposure to other fluoride: salt Year study began: 1966 Location: Switzerland Setting of recruitment and treatment: school |
|
| Interventions | FG vs PL
(AmF/NaF group = 12,500 ppm F) Self applied under supervision, with toothbrush, 22 times a year, 1 g applied for 6 min Prior to application = no tooth cleaning performed Postop instruction = no information provided |
|
| Outcomes | 2‐year* net DFS increment ‐ (NCA/CA)CL + (DR/ER)XR
Reported at 2 and 4 year follow‐ups 1stmPF‐DFS (CA) CL 1stmMD‐DFS (DR) XR |
|
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by GABA AG, Basel | |
| Notes | Clinical (V) caries assessment by 2 examiners; diagnostic threshold = CA and NCA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by 2 examiners; diagnostic threshold = DR and ER; partial recording. 'Sufficient agreement of examiners known from earlier work' (quote from the report) *FG replaced by F solution after 2 years (final 4 years results not considered) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Children were paired according to their sequence in the class lists. The first and second child of each pair was allocated control and fluoride respectively when, in a table of random digits, an even digit was present. In the case of an odd random digit, the first child was allocated fluoride, and the second one control. A few siblings were found ... were taken into the same pair to guarantee that they did not receive the same treatment at school.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The first and second child of each pair was allocated control and fluoride respectively.” “Control group received exactly the same, just without fluoride.” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “The first and second child of each pair was allocated control and fluoride respectively.” Comment: Use of placebo described. It is unclear if the examiners were blind to treatment allocations, although it is probable that clinical and radiographic exams were done independently. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 30% in 2 years. Dropout by group: NR. Reasons for losses: Exclusions based on use of orthodontic bands and presence in all follow‐up examinations. Comment: Numbers lost unduly high for length of follow‐up; any differential losses not assessable. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DFS increment (CA)CL + (DR)XR, reported at 1 and 3 year follow‐ups. 1stmPF‐DFS 1stmMD‐DFS Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 2.24 (FD), 2.75 (PL) 1stmDMFS: 0.1 FD, 0.1 PL Comment: Initial caries appears balanced between groups. Age also reported, and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Mestrinho 1983.
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group quasi RCT; non‐placebo controlled Study duration: 1 year |
|
| Participants | 174 children analysed at 1 year (after exclusions, available at final examination)
Participants randomised (N = 218) Age range 7‐10 years Surfaces affected: NR Exposure to other fluoride: none assumed Year study began: 1981 Location: Brazil Setting of recruitment and treatment: school and school clinic, respectively |
|
| Interventions | FG + ptc* vs NT
(APF group = 9150 ppm F) Operator applied, with tray, 2.5 ml applied, twice a year Prior to application = tooth cleaning (supervised toothbrushing) performed with a non‐F toothpaste + abrasive paste Postop instruction = spit excess saliva, refrain from rinsing, eating, and drinking for 30 min |
|
| Outcomes | 1‐year DMFS increment
Reported at 1 year follow‐up O‐DMFS BL‐DMFS MD‐DMFS DMFT Nausea on application, discomfort in using trays Dropout (no data by group) |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. | |
| Notes | Clinical (VT) caries assessment by 3 examiners; diagnostic threshold NR; state of tooth eruption included NR; diagnostic errors NR *Prior toothbrushing with non‐F toothpaste and abrasive paste performed in FG group only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Children were initially ordered by the number of permanent teeth present, then by level of DMF, and then, they were distributed 'at random', into 2 groups designated I and II” Comment: Method unclear, quasi method likely |
| Allocation concealment (selection bias) | High risk | “Children were initially ordered by the number of permanent teeth present, then by level of DMF, and then, they were distributed 'at random', into 2 groups designated I and II” Comment: No concealment of allocation indicated/likely. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Group I received no treatment and served as the control group" Comment: No placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote from correspondence: “The study was carried out blind, the examiners had no knowledge of the group a child belonged to" “Group I received no treatment and served as the control group" Comment: Blind caries assessment described, but no placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 44/218 (20.2%) in 1 year. Dropout by group: NR. Reasons for losses: Exclusions based on "statistical reasons" (made at random to keep groups of equal sizes, after 8% "natural loss"). Comment: Numbers lost were high for the length of follow‐up, unclear if differential losses were shown between groups, and it is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations, after exclusions were made at random to keep groups balanced in size. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: DMFS increment ‐ (CA)CL, reported at 1 year follow‐up ODMFS, MDDMFS, BLDMFS Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Unclear risk | Prognostic factors reported: dental age, and DMFS described as "balanced" (but data NR) |
| Free of contamination/co‐intervention? | Unclear risk | No information on inadvertent application of the intervention to people in the control group (contamination), or on possibility of additional treatment given to 1 of the groups differentially (co‐intervention). |
Olivier 1992.
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT; placebo controlled Study duration: 2 years |
|
| Participants | 431 children analysed at 2 years (available at final examination)
Participants randomised (N = 488) Age range 6‐7 years Surfaces affected: 0.68 DMFS Exposure to other fluoride: toothpaste Year study began: 1985 Location: Canada Setting of recruitment and treatment: school |
|
| Interventions | FG vs PL
(APF group = 12,300 ppm F) Operator applied, with a foam tray, twice a year, applied for 4 min Prior to application = no tooth cleaning performed Postop instruction = excess saliva removed by saliva ejector, refrain from eating and drinking for 30 min |
|
| Outcomes | 2‐year DMFS increment ‐ (CA) (by 2 levels of initial defs)
Reported at 2 years follow‐up Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | Supported by a grant (66052247‐43) from the National Health Research and Development Program, Health and Welfare, Canada | |
| Notes | Clinical (VT) caries assessment by 5 examiners; diagnostic threshold = CA; state of tooth eruption included NR; inter‐ and intra‐examiner reproducibility checks for DMFS in 10% sample (ICC over 0.96) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...and were randomly assigned to experimental and control groups.” Quote from correspondence: “A research assistant who never met the subjects proceeded to their random assignation into one of two groups.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote from correspondence: “A research assistant who never met the subjects proceeded to their random assignation into one of two groups.” Comment: Not enough information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes: “A double blind clinical field trial...” “...the control group received a placebo” “...each dentist, blinded to the exposure, examined the same number of subjects.” Comment: Blind outcome assessment and use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “A double blind clinical field trial ...” “... the control group received a placebo” “... each dentist, blinded to the exposure, examined the same number of subjects.” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall dropout for length of follow‐up: 11.68% in 2 years. Dropout by group: 24/248 (9.7%) FG, 33/240 (13.8%) PL. Reasons for losses: Moved away, absent from school on day of final examination. Comment: Numbers lost not unduly high for length of follow‐up with no differential losses. Reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA) (by 2 levels of initial defs), reported at 2 years follow‐up. Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factor reported: DMFS: 0.59(1.44) FG, 0.74(1.68) PL defs: 21.75(12.43) FG, 22.31(13.30) PL Comment: Initial caries appears balanced between groups. Age, gender, daily sugar consumption, daily toothbrushing, exposure to other fluoride, etc. reported and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Ran 1991.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (3 relevant arms used); placebo controlled Study duration: 1.5 years |
|
| Participants | Numbers analysed (at 1.5 years); all male: 83
Numbers randomised: 140 for all 4 groups combined (numbers for each relevant group NR) Average age 13 years Surfaces affected: 6.5 DMFS Exposure to other fluoride: data not obtained for dentifrice Year study began: in/before 1989 Location: Israel Setting of recruitment and treatment: school (educational institution) |
|
| Interventions | FG (2 groups) vs PL
(AmF group 1 = 4000 ppm F
AmF group 2 = 12,500 ppm F) Self applied under supervision, with toothbrush, 25 times a year, 1 g applied for 4 min Prior to application = no tooth cleaning performed Postop instruction = spit after brushing, refrain from eating and drinking for 30 min |
|
| Outcomes | 1.5‐year net DMFS increment ‐ (CA) Reported at 0.5 and 1.5 year follow‐ups (and 0.5 year post‐treatment) | |
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by GABA AG, Basel. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included NR; intra‐examiner reproducibility checks for DMFS (ICC reaching 0.97) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children were randomly assigned to four class groups ...” "Each boy was assigned to one of four groups" (from additional abstract) Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: “The study was conducted in a double blind manner.” “... brushed their teeth with gel containing 0% fluoride (placebo) ...” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “The study was conducted in a double blind manner.” “... brushed their teeth with gel containing 0% F (placebo) ...” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 20% in 1.5 years (all groups). Dropout by group: NR. Reasons for losses: NR Comment: Numbers lost not unduly high for length of follow‐up; any differential losses not assessable. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA) Reported at 0.5 and 1.5 year follow‐ups (and 0.5 year post‐treatment) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 6.0(6.5) FG1, 6.1(6.3) FG2, 7.1(6.1) PL Comment: Initial caries appears balanced between groups (NS differences). |
| Free of contamination/co‐intervention? | Low risk | Quote: "Each class group ... brushed their teeth under the supervision of an instructor." Comment: There is sufficient indication overall of prevention of contamination/co‐intervention. |
Shern 1976.
| Study characteristics | ||
| Methods | Study design: 5‐arm RCT (all arms are relevant); placebo controlled Study duration: 2 years (but only 1 year results used) |
|
| Participants | 562 children analysed at 1* year (available at 1st examination)
Participants randomised (N = 614) Age range 6‐13 years Surfaces affected: 2.7 DMFS (data from original sample only) Exposure to other fluoride: none assumed Year study began: in/before 1973 Location: Venezuela Setting of recruitment and treatment: school |
|
| Interventions | FG (3 groups) + ptc vs PL (2 groups) + ptc
(APF group 1 = 12,300 ppm F, AmF group 2 = 12,500 ppm F, AmF group 3 = 12,500 ppm F) Operator applied, with tray, 5 consecutive applications (every day/week) in 1st year, 3 mg (about 14 drops) applied for 5 min Prior to application = tooth cleaning performed with rotating rubber‐cup with non‐F abrasive paste Postop instruction = refrain from rinsing and eating for 30 min |
|
| Outcomes | 1‐year* net DMFS increment
Reported at 1 and 2 year follow‐ups O‐DMFS MD‐BL‐DMFS Side effects Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by GABA AG, Basel. | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold NR; state of tooth eruption included NR; diagnostic errors NR *Intervention applied during 1st year of study only (final 2 years results not considered). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...were assigned from classroom rosters at random to one of five groups.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “To assure a double‐blind study, the examiner was not informed about the group assignment of any child or the results of the child’s previous examinations, and the gels were similar in physical characteristics.” Comment: Blind outcome assessment and use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “To assure a double‐blind study, the examiner was not informed about the group assignment of any child or the results of the child’s previous examinations, and the gels were similar in physical characteristics.” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall dropout for length of follow‐up: 8.47% in 1 year. Dropout by group: 14/144 FG1, 12/143 FG2, 10/138 FG3, 4/90 PL1, 12/99 PL2. "losses distributed evenly among groups". Reasons for losses: NR Comment: Numbers lost not unduly high for length of follow‐up with almost no differential losses between groups (9.72% FG1, 8.39% FG2, 7.25% FG3, 4.44% PL1, 12.12% PL2). It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment, reported at 1 and 2 year follow‐ups O‐DMFS, MD‐BL‐DMFS, Side effects. Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported (sample at baseline only): DMFS: 2.80(6.63) FG1, 2.85(4.54) FG2, 2.51(3.41) FG3, 2.46(3.23) PL1, 2.99(4.68) PL2 Comment: Initial caries appears balanced between groups. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Szwejda 1972.
| Study characteristics | ||
| Methods | Study design: 8‐arm parallel‐group RCT (only 2 relevant arms used), placebo controlled Study duration: 3 years |
|
| Participants | 316 children analysed at 3 years (after exclusions, present for all examinations)
Participants randomised (numbers NR) Age range 7‐9 years Surfaces affected: 0.86 DMFS Exposure to other fluoride: water Year study began: in/before 1968 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc vs 'PL' + ptc
(APF, concentration NR) Operator applied, with tray, once a year Prior to application = tooth cleaning performed with pumice paste in the FG group, and with a bland prophy paste in the 'PL' group Postop instruction = no information provided |
|
| Outcomes | 3‐year net DMFS increment ‐ (E/U)
Reported at 3 years follow‐up O‐DMFS MD‐DMFS BL‐DMFS NetDMFT(E/U) |
|
| Declaration of interest | No information provided. | |
| Source of funding | The study was supported by US Public Health Service Grant DH00018 from the Division of Dental Health. | |
| Notes | Clinical (VT) caries assessment by more than 1 examiner; diagnostic threshold NR; state of tooth eruption included = E and U; reversal rate 3.9% and 2.2% of observed DMFT increment, and 3.2% and 1.5% of observed DMFS increment in FG and 'PL' groups, respectively (3rd‐year results only). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...children were allocated randomly...” “The randomized allocation was based on the instructions of the statistical consultant.” Comment: Most likely a random method used. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “The examiner was unaware of the status of the child or the results of previous examinations.” “Group H received a prophylaxis with a bland prophylactic paste and an application of a solution as a placebo.” Comment: Blind outcome assessment and use of 'placebo' described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The examiner was unaware of the status of the child or the results of previous examinations.” “Group H received a prophylaxis with a bland prophylactic paste and an application of a solution as a placebo.” Comment: Blind outcome assessment and use of 'placebo' described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: NR. Dropout by group: NR. Reasons for losses: Exclusions based on lifetime exposure to fluoridated water, compliance to treatment, and presence in all follow‐up examinations. Comment: Numbers lost and any differential losses not assessable. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (E/U), reported at 3 years follow‐up O‐DMFS MD‐DMFS BL‐DMFS DMFT(E/U) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 0.86 FG, 0.85 ‘PL’ DMFT: 0.61 FG, 0.67 ‘PL’ Comment: Initial caries appears balanced between groups. TAR, SAR, age also reported, and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Treide 1988.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (all 4 used); placebo controlled Study duration: 3 years |
|
| Participants | 433 children analysed at 3 years
Participants randomised (N = 643) Average age 3.5 years Surfaces affected: NR (but dmft data reported from original sample only = 0.8) Exposure to other fluoride: no Year study began: 1983 Location: GDR Setting of recruitment and treatment: nurseries |
|
| Interventions | FG (3 groups) + ptc vs PL + ptc
(NaF + hexaf group = 12,500 ppm F, NaF group = 12,500 ppm F, AmF group = NR) Self applied under supervision, with toothbrush, approximately 130 times a year Prior to application = tooth cleaning performed Postop instruction = no information |
|
| Outcomes | 3‐year dmfs increment ‐ (E)
Reported at 1, 2, and 3 year follow‐ups dmft (E) Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | No information provided. | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold NR; state of tooth eruption included = E | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from translation of correspondence: “Distribution of participants was random, not using any mathematical model.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The study was of double‐blind design," "Gel B: placebo" Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The study was of double‐blind design," "Gel B: placebo" Comment: Blind assessment reported, and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 33% in 3 years. Dropout by group: 59/162 (36.4%) FG1, 49/160 (30.6%) FG2, 49/157 (31.2%) FG3, 53/164 (32.3%) PL. Reasons for losses: NR. Comment: Numbers lost not unduly high for length of follow‐up; no differential losses between groups. It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations. However, an overall loss of > 30% of original participants is still considered high risk. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: dmft and dmfs increments ‐ CL, at 3 years follow‐up Comment: Trial protocol not available. Not clear if all pre‐specified outcomes (in Methods) were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Unclear risk | Prognostic factors reported (sample at baseline only): dmft was 0.81, 0.87, 0.68 in the 3 fluoride groups, and 0.73 in the placebo group Initial caries (dmft) appears balanced between groups. |
| Free of contamination/co‐intervention? | Unclear risk | Translation of report not detailed enough to make a categorical decision regarding risk of contamination/co‐intervention. |
Trubman 1973.
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo controlled Study duration: 3 years |
|
| Participants | 311 children analysed at 3 years (present for all examinations)
Participants randomised (N = 575) Average age 8.1 years Surfaces affected: 2.1 DMFS Exposure to other fluoride: water Year study began: in/before 1969 Location: USA Setting of recruitment and treatment: school |
|
| Interventions | FG + ptc vs PL + ptc
(APF group = 12,300 ppm F) Self applied under supervision, with tray, 4 times a year, applied for 4 min (children could expectorate during application if needed) Prior to application = tooth cleaning (supervised toothbrushing) performed with with non‐F prophy paste Postop instruction = no information provided |
|
| Outcomes | 3‐year net DMFS increment ‐ (CA)
Reported at 2 and 3 year follow‐ups NetDMFT(CA) Dropout |
|
| Declaration of interest | No information provided. | |
| Source of funding | US Public Health Service Grants DH00122‐01 through ‐04 [The authors thank Davies, Rose Hoyt Pharmaceutical Div, the Kendall Co, Needham, Mass, 02194 for supplying the gels] |
|
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA; state of tooth eruption included NR; reversal rate 18.4% and 9.2% of observed DMFT increment in FG and PL groups, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After baseline examinations, children were randomly assigned to one of four groups ...” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: “The examiners had no knowledge of group assignments of children or of results of previous examinations.” “Children in group 1 applied a non fluoride gel.” Comment: Blind outcome assessment and use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “The examiners had no knowledge of group assignments of children or of results of previous examinations.” “Children in group 1 brushed with non fluoride prophylaxis paste and applied a non fluoride gel.” Comment: Blind outcome assessment and use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall dropout for length of follow‐up: 45.9% in 3 years. Dropout by group: 141/286 FG, 123/289 PL. Reasons for losses: Exclusions based on presence at all follow‐up examinations. Comment: Numbers lost unduly high for length of follow‐up with no differential group losses (49.30% FG, 42.56% PL). It is unclear if reasons for dropout are acceptable and balanced. Caries data used in analysis pertain to participants present at all examinations. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: DMFS increment ‐ (CA), reported at 2 and 3 year follow‐ups. DMFT(CA) Comment: Trial protocol not available. All pre‐specified outcomes (in Methods) were reported and were reported in the pre‐specified way. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported: DMFS: 2.36 FG, 1.92 PL DMFT: 1.51 FG, 1.17 PL Comment: Initial caries with some imbalance between groups (stats adjustment had trivial effect). Age also reported, and balanced. |
| Free of contamination/co‐intervention? | Unclear risk | No information provided. |
Truin 2005.
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo controlled Study duration: 4 years |
|
| Participants | Year study began: 1995 Location: The Netherlands Setting of recruitment and treatment: dental clinics Numbers randomised: 594 Numbers analysed: 530 children at 4 years (available at final examination ‐ 'ITT' sample) Age: mean 10.4 (SD = 0.6) years (range 9.5 to 11.5 years) Mean surfaces affected: D2S = 3.9 (SD = 3.0) (not clear if data are from final sample analysed as 'ITT') Background exposure to other fluoride: yes (fluoride in water level < 0.3 ppm F, but exposure to fluoridated toothpaste ‐ “Both groups received oral hygiene instruction, followed by supervised brushing with fluoride toothpaste at semi‐annual check‐ups“) |
|
| Interventions | Comparison: FG vs PL Group 1 (n = 305): Neutral 1% NaF gel, 4500 ppm F Group 2 (n = 289): Placebo gel Identical application method in both groups: professionally applied, with flexible tray, for 4 min, 2 times a year Prior to application = no professional prophylaxis, dentition not dried by compressed air Postop instruction = refrain from rinsing, eating and drinking for 30 min |
|
| Outcomes | At 4 years:
|
|
| Declaration of interest | No information provided. | |
| Source of funding | Supported by a grant (SGZ/16524/95) from the College van Zorgverzekeringen, Amstelveen, the Netherlands. | |
| Notes | Clinical (V) caries assessment by 11 examiners; diagnostic threshold = CA and NCA. Radiographic assessment (postBW) by 1 examiner; diagnostic threshold = DR and ER; intra‐examiner K statistics/Kappa values was 0.96 for permanent dentition (clin + radiog combined for principal examiner), and inter‐examiner values varied from 0.90 to 0.98. State of tooth eruption included NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The participants were randomly assigned to either the placebo or the fluoride treatment group by drawing a random unmarked envelope containing the allocation to one of both treatments.” Comment: Not enough information provided. |
| Allocation concealment (selection bias) | Low risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The gels were identical regarding packing, taste, colour, and consistency.” Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The design of the study was a double blind randomised controlled trial.” Comment: Blind assessment reported, though unclear what procedures were used, but use of placebo described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Non‐adherence" (defined as participants refusing further treatment, treatment not performable, and non‐regular attenders) participants were excluded in the per protocol analysis (77/594, 13%) from the 4‐year examination, but a small portion of these participants ("non adhering patients who were prepared to participate at 4 year evaluation") were recalled, and they formed the ITT analysis with a dropout of 11% (64/594, 10.8%). Comment: Numbers lost relatively low for the length of follow‐up, with similar losses between groups (36/305, 11.8% FG; 28/289, 9.7% PL). Caries data used in the analysis pertain to participants present at final examination (ITT dataset), those participating according to the protocol for the entire study duration (the per protocol dataset), and those with no caries and no sealants at baseline (caries‐free/sealant‐free subgroup). |
| Selective reporting (reporting bias) | Unclear risk | Comment: Trial protocol not available. Only D3MFS for primary dentition and second molars and the associated prevented fraction, incident cases and attributable risk reported for the ITT population. Mean number of sealants, in the permanent and primary dentition, and mean percentages of enamel lesions (permanent dentition, occlusal surfaces excluded) were mentioned as outcomes analysed in the Methods section, but not reported for the ITT population. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported:
Comment: Initial caries appears to have some small imbalance between groups. The only other baseline characteristic reported is age. |
| Free of contamination/co‐intervention? | Low risk | Comment: Both groups had identical supervised brushing, oral hygiene instruction and gel application procedures. |
Van Rijkom 2004.
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo controlled Study duration: 4 years |
|
| Participants | Year study began: 1996 Location: the Netherlands, 3 cities Setting of recruitment and treatment: paediatric clinics Numbers randomised: 773 children Numbers analysed: 732 children at 4 years (available at final examination‐ 'ITT' sample) Age: mean 5.5 (SD = 0.6) years (range 4.5 to 6.5 years) Mean surfaces affected: D3MFS = 0, d3mfs = 0 Background exposure to other fluoride: yes (99% reported F toothpaste use, 70% some F tablets use, and F toothpaste applied semi‐annually during STB at check‐ups, but no F in water – “< 0.3 ppm F”) |
|
| Interventions | Comparison: FG vs PL Group 1 (n = 372): Neutral 1% NaF gel, 4500 ppm F Group 2 (n = 360): Placebo gel Identical application method in both groups; professionally applied, with flexible tray, for 4 min, 2 times a year Prior to application = no professional prophylaxis, dentition not dried by compressed air Postop instruction = refrain from rinsing, eating, and drinking for 30 min |
|
| Outcomes | At 4 years:
|
|
| Declaration of interest | No information provided. | |
| Source of funding | Supported by a grant (SGZ/16524/95) from the College van Zorgverzekeringen, Amstelveen, the Netherlands. | |
| Notes | Clinical (V) caries assessment by 10 examiners; diagnostic threshold = CA and NCA. Radiographic assessment (postBW) by 10 examiners; diagnostic threshold = DR and ER; intra‐examiner K statistics/Kappa values were 0.96 and 0.94 for permanent and primary dentitions, respectively, and inter‐examiner values were 0.95 and 0.90, respectively. State of tooth eruption included NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... participants were randomly assigned to either the placebo or fluoride treatment group by drawing a random unmarked envelope containing the allocation to one of the treatment groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "... unmarked envelope containing the allocation ..." Comment: No mention of sealed and opaque envelope, but probably still at low risk of bias considering the overall precautions taken in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes: "The placebo as well as the fluoride treatment were represented by 2 colour codes. All gels were identical regarding taste, colour and consistency. Only the pharmacist and the chief analyst of the laboratory were acquainted with the content of each gel." Comment: Use of placebo described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was conducted double‐blind. After all analyses were carried out, the data set was frozen and and the code referring to the placebo and fluoride groups subsequently broken." Comment: Blind outcome assessment reported (though not how it was achieved, but placebo used). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall dropout for length of follow‐up: 'ITT' = 41/773 (5.3%) in 4 years; per protocol = (97/773, 12.6%) in 4 years*. Dropout by group ('ITT'): 15/387 (3.9%) FG, 26/386 (6.7%) PL. Reasons for losses: refusing further treatment, treatment not performable, and non‐regular attenders (e.g. moving). *"Non‐adherence" (defined as participants refusing further treatment, treatment not performable, and non‐regular attenders) participants were excluded in the 4‐year examination per protocol analysis (97/773, 12.6%), but a small portion of these participants ("non adhering patients who were prepared to participate at final examination") had a 4‐year follow‐up examination (56/773, 7.2%), forming the ITT analysis, with a dropout of 5.3% (41/773). Comment: Numbers lost were low for the length of follow‐up, no differential losses shown between groups, and reasons for the dropout appear to be acceptable and balanced between groups. Caries data used in the analysis pertain to 3 sets of participants: those present at final examination (ITT), those participating according to the protocol for the entire study duration (the per protocol dataset), and those with no caries and no sealants at baseline (caries‐free/sealant‐free subgroup). |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported: DMFS and dmfs increments ‐ (CA)CL + (DR)XR, at 4 years follow‐up (and these according to specific sites ‐ pits, occlusal, approximal, and smooth). D12MFS (incl sealants)/d12mfs reported at baseline only. Comment: Trial protocol not available. Not all pre‐specified outcomes (in Methods) were reported in the pre‐specified way; although results for the permanent dentition (D3MFS) are reported for all datasets analysed (the ITT, the per protocol, and the caries‐free/sealant‐free subgroups), results for the primary dentition (d3mfs) are reported only for the per protocol and caries‐free subgroups. |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported (for per protocol population only, at 4 years exam): Mean age: 5.5 (0.6) FG, 5.5 (0.6) PL D3MFS and d3mfs = 0 (both FG and PL groups) Comment: Initial caries balanced between groups. D12MFS/d12mfs (incl sealants), dental plaque, toothbrushing frequency, use of F tablets, % X‐ray, socioeconomic status, gender also reported. Quote: "no relevant differences found between the treatment groups for these variables/factors". |
| Free of contamination/co‐intervention? | Low risk | Quote: "Preventive treatment at the semi‐annual check‐ups included oral hygiene instruction, followed by supervised toothbrushing with fluoride toothpaste." Comment: No indication of inadvertent application of the intervention to people in the control group (no apparent contamination) or of any additional treatment given to 1 of the groups differentially (no risk of co‐intervention). |
Dropout rate data based only on groups (arms) relevant to the review, on relevant follow‐ups, unless otherwise stated. Baseline caries experience averaged among relevant study arms, and based on the study sample analysed at the end of treatment period (final sample), unless otherwise stated. Age range (+ average age when reported) at the time the study was begun based on all study participants or only on relevant groups when data were available.
1stm = first permanent molar 'A' = classified as double blind, but participants may not be blind ('PL' used) AmF = amine fluoride APF = acidulated phosphate fluoride BL = bucco and lingual surfaces CA = lesions showing loss of enamel continuity that can be recorded clinically (undermined enamel, softened floor/walls) or showing frank cavitation CIR = caries incidence rate CL = clinical examination deft/s = decayed, needing extraction, and filled deciduous teeth or surface DMFS/T = decayed, missing, and filled permanent surfaces or teeth dmft/s = decayed, missing, and filled deciduous teeth or surface DR = radiolucency into dentin E = teeth erupted at baseline ER = any radiolucency in enamel/enamel‐dentin junction F = fluoride FG = fluoride gel treatment ICC = intraclass correlation coefficient ITT = intention‐to‐treat MD = mesio and distal surfaces mDMFS = permanent molar DMFS N = numbers NaF = sodium fluoride NCA = non‐cavitated enamel lesions visible as white spots or discolored fissures NR = not reported NS = not significant NT = no treatment O = occlusal surfaces PF = pit and fissure surfaces PL = placebo gel 'PL' = not a true placebo (inactive treatment other than gel used) postBW = posterior bite‐wing X‐ray assessment postMD = posterior mesio‐distal ppm F = parts per million of fluoride pre‐mDMFS = permanent pre‐molar DMFS ptc = prior tooth cleaning performed with or without a non‐fluoride paste RCT = randomised controlled trial SAR = surfaces at risk SD = standard deviation SnF2 = stannous fluoride STB = supervised toothbrushing TAR = teeth at risk U = teeth unerupted at baseline VT = visual‐tactile assessment XR = radiographic examination
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agrawal 2011 | Not a randomised or quasi‐randomised trial (selection of 2 clusters (schools) only, each assigned (by coin tossing) to 1 of the 2 groups). |
| Bellini 1981 | Additional fluoride‐based intervention associated to fluoride gel. Note ‐ no relevant outcome reported. |
| Bordoni 1995 | Additional fluoride‐ or non‐fluoride‐based interventions associated to fluoride gel. No random or quasi‐random allocation used. Blind outcome assessment not stated and unlikely. |
| Boyd 1985 | Additional fluoride‐based intervention associated to fluoride gel. Clearly not a randomised or quasi‐randomised trial. Length of follow‐up of less than 1 year/school year. |
| Cichocka 1981 | No random or quasi‐random allocation used (selected group comparisons). Blind outcome assessment not stated and unlikely. |
| Heifetz 1979 | Additional fluoride‐based intervention associated to fluoride gel. Note ‐ inappropriate 'placebo' used. |
| Ivanova 1990 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely. |
| Kukleva 1983 | Random or quasi‐random allocation not stated or indicated. Open outcome assessment reported after contacting author. |
| Kukleva 1998 | Random or quasi‐random allocation not stated or indicated. Open outcome assessment reported after contacting author. |
| Kukleva 2001 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely. |
| Lisiecka 1976 | Blind outcome assessment not stated and unlikely in any element/phase of assessment. |
| Loesche 1977 | Random or quasi‐random allocation not stated or indicated. Note ‐ abstracts only; full text not obtainable; insufficient information available to include in review. |
| Madlena 2002 | Additional fluoride‐based interventions associated to fluoride gel. Blind outcome assessment not stated and unlikely. |
| Mellberg 1978 | Blind outcome assessment not stated and unlikely in any element/phase of assessment. |
| Pinto 1993 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely. |
| Rajic 1977 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely. |
| Ran 1987 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely. Unclear comparisons with fluoride gel. Note ‐ abstract only; full text not obtainable; insufficient information available to include in review. |
| Shobha 1987 | Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely. Note ‐ main outcome data not reported in control group (and not obtainable). |
| Spears 1978 | No random or quasi‐random allocation used (non‐random concurrent control). Blind outcome assessment not stated and unlikely. Note ‐ dramatic dropout rate during the study period. |
| Stadtler 1982 | Medically compromised group of institutionalised children selected. |
| Stokes 2011 | Fluoride gel applied by toothbrushing is compared with a no‐treatment control group rather than placebo (no‐treatment rather than placebo group when fluoride gel is applied through brushing or flossing). |
| Szoke 1989 | Additional fluoride‐ or non‐fluoride‐based intervention associated to fluoride gel. Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely. |
| Szwejda 1971 | No random or quasi‐random allocation used (concurrent control taken from another study). |
Characteristics of ongoing studies [ordered by study ID]
NCT00670618.
| Study name | A Prospective, Randomized Clinical Study on the Effects of CPP‐ACP Paste on Plaque, Gingivitis and White Spot Lesions in Orthodontic Patients ‐ Part 2 |
| Methods | Single‐blinded (participant) parallel randomised controlled trial |
| Participants | 10 to 60 years old |
| Interventions |
|
| Outcomes | Prevention of the opacity of white spots during orthodontic treatment with fixed appliances [time frame: after 2 years] |
| Starting date | June 2008 |
| Contact information | Silvia Dauwe, email: silvia.dauwe@ugent.be |
| Notes | Expected completion May 2015 |
NCT01329731.
| Study name | Remineralisation of White Spot Lesions by Elmex® gelée in Post‐orthodontic Patients |
| Methods | Double‐blinded randomised controlled trials |
| Participants | Orthodotic patients, healthy volunteers (≥ 11 years) scheduled for bracket removal |
| Interventions |
|
| Outcomes | White spot lesions, caries, up to 24 weeks |
| Starting date | March 2011 |
| Contact information | Dr. Christian Heumann, Gaba International PG |
| Notes | Study completed |
Differences between protocol and review
In the 2015 update, we further defined the outcomes for clarity. We also trimmed the list of outcomes to those that are more relevant to patients. Use of health service resources (such as visits to dental‐care units, length of dental treatment time) were not available from the studies and will no longer be collected. These data have limited applicability across settings.
Other changes implemented in this update are the addition of a full ‘Risk of bias’ assessment, and the development of a ‘Summary of findings’ table for the primary outcomes in the review.
Finally, there were changes in the investigations of heterogeneity performed through metaregression and subgroup analyses, and in the investigations of sensitivity analyses, including changes to the way a few covariates were analysed in each. We have reported these changes and the rationale for them in the relevant sections of the review.
Contributions of authors
For the 2015 update, all members of the new review team decided on the updated methods to be used for this review. Valeria Marinho (VM) and Lee Yee Chong (LYC) undertook the study selection, data extraction, 'Risk of bias' assessments and analyses. Tanya Walsh (TW) and Helen Worthington (HW) provided advice when consulted throughout the update and undertook some of the extra analyses. VM and LYC prepared the full review, and all review authors were active in its revision and approval.
For the original review, all four authors contributed to the development of the protocol. VM wrote the protocol, conducted searches, selected studies and extracted data. Julian Higgins duplicated study selection and data extraction in a sample of studies, and Stuart Logan or Aubrey Sheiham were consulted when necessary. VM entered and analysed the data in consultation with Julian Higgins. VM prepared the full review, and all review authors were active in its revision and approval.
Sources of support
Internal sources
Queen Mary University of London, UK
Department of Epidemiology and Public Health (UCL), UK
Systematic Reviews Training Unit, Institute of Child Health (UCL), UK
Medical Research Council, UK
School of Dentistry, The University of Manchester, UK
External sources
-
National Institute for Health Research (NIHR), UK
The NIHR is the largest single funder of the Cochrane Oral Health Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Group Global Alliance, Other
The production of all our reviews is assisted by funding from our Global Alliance partners (http://ohg.cochrane.org/): British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK
CAPES ‐ Ministry of Education, Brazil
Declarations of interest
Valeria CC Marinho: none known Helen Worthington: none known Tanya Walsh: none known Lee Yee Chong: none known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Abadia 1978 {published data only}
- Abadia SMS. Prevenção da cárie dentária através da aplicação tópica de gel de flúor fosfato ácido, utilizando-se isolamento relativo e absoluto [dissertation]. Baurú (SP): Universidade de São Paulo, 1978. [Google Scholar]
Bijella 1981 {published data only}
- Bijella MF, Bijella VT, Lopes ES, Bastos JR. Comparison of dental prophylaxis and toothbrushing prior to topical fluoride applications. Community Dentistry and Oral Epidemiology 1985;13(4):208-11. [DOI] [PubMed] [Google Scholar]
- Bijella MFTB. Prevenção da cárie dentária através da aplicação tópica de gel e solução de fuor fosfato acidulado com e sem profilaxia prévia [dissertation]. Bauru (SP): Universidade de São Paulo, 1981. [Google Scholar]
Bryan 1970 {published data only}
- Bryan ET, Williams JE. The cariostatic effectiveness of a phosphate-fluoride gel administered annually to school children; final results. Journal of Public Health Dentistry 1970;30(1):13-6. [DOI] [PubMed] [Google Scholar]
- Bryan ET, Williams JE. The cariostatic effectiveness of a phosphate-fluoride gel administered annually to school children. I. The results of the first year. Journal of Public Health Dentistry 1968;28(3):182-5. [DOI] [PubMed] [Google Scholar]
Cobb 1980 {published data only (unpublished sought but not used)}
- Cobb BH, Rozier GR, Bawden JW. A clinical study of the caries preventive effects of an APF solution and APF thixotropic gel. Pediatric Dentistry 1980;2(4):263-6. [PubMed] [Google Scholar]
Cons 1970 {published data only}
- Cons NC, Janerich DT, Senning RS. Albany topical fluoride study. Journal of the American Dental Association 1970;80(4):777-81. [DOI] [PubMed] [Google Scholar]
DePaola 1980 {published data only (unpublished sought but not used)}
- DePaola PF, Soparkar M, Van Leeuwen M, DeVelis R. The anticaries effect of single and combined topical fluoride systems in school children. Archives of Oral Biology 1980;25(10):649-53. [DOI] [PubMed] [Google Scholar]
- Lu KH, Porter DR, Pickles TH. Separate and combined cariostatic effects of fluoride gel and rinse [abstract No 239]. Journal of Dental Research 1980;59:947. [Google Scholar]
Englander 1967 {published data only}
- Englander HR, Carlos JP, Senning RS, Mellberg JR. Residual anticaries effect of repeated topical sodium fluoride applications by mouthpieces. Journal of the American Dental Association 1969;78(4):783-7. [DOI] [PubMed] [Google Scholar]
- Englander HR, Keyes PH, Gestwicki M, Sultz HA. Clinical anticaries effect of repeated topical sodium fluoride applications by mouthpieces. Journal of the American Dental Association 1967;75(3):638-44. [DOI] [PubMed] [Google Scholar]
Englander 1971 {published data only}
- Englander HR, Sherrill LT, Miller BG, Carlos JP, Mellberg JR, Senning RS. Incremental rates of dental caries after repeated topical sodium fluoride applications in children with lifelong consumption of fluoridated water. Journal of the American Dental Association 1971;82(2):354-8. [DOI] [PubMed] [Google Scholar]
Englander 1978 {published data only}
- Englander HR, Mellberg JR, Engler WO. Observations on dental caries in primary teeth after frequent fluoride toplications in a program involving other preventives. Journal of Dental Research 1978;57(9-10):855-60. [DOI] [PubMed] [Google Scholar]
Gisselsson 1999 {published and unpublished data}
- Gisselsson H, Birkhed D, Emilson CG. Effect of professional flossing with NaF or SnF2 gel on approximal caries in 13-16-year-old schoolchildren. Acta Odontologica Scandinavica 1999;57(2):121-5. [DOI] [PubMed] [Google Scholar]
Hagan 1985 {published data only (unpublished sought but not used)}
- Hagan PP, Rozier RG, Bawden JW. The caries-preventive effects of full-strength and half-strength topical acidulated phosphate fluoride. Pediatric Dentistry 1985;7(3):185-91. [PubMed] [Google Scholar]
Heifetz 1970 {published and unpublished data}
- Heifetz SB, Horowitz HS, Driscoll WS. Evaluation of a self-administered procedure for the topical application of acidulated phosphate-fluoride. Journal of Dental Research 1968;102:Abstr No 257 (Abstract). [DOI] [PubMed] [Google Scholar]
- Heifetz SB, Horowitz HS, Driscoll WS. Two-year evaluation of a self-administered procedure for the topical application of acidulated phosphate-fluoride; final report. Journal of Public Health Dentistry 1970;30(1):7-12. [DOI] [PubMed] [Google Scholar]
Horowitz 1971 {published and unpublished data}
- Horowitz HS, Doyle J. The effect on dental caries of topically applied acidulated phosphate-fluoride: results after three years. Journal of the American Dental Association 1971;82(2):359-65. [DOI] [PubMed] [Google Scholar]
- Horowitz HS, Kau MC. Retained anticaries protection from topically applied acidulated phosphate-fluoride: 30- and 36-month post-treatment effects. Journal of Preventative Dentistry 1974;1(1):22-7. [PubMed] [Google Scholar]
- Horowitz HS. Effect of topically applied acidulated phosphate-fluoride on dental caries in Hawaiian school children [abstract No 256]. Journal of Dental Research 1968;101:Abstr No 256.(Abstract). [Google Scholar]
- Horowitz HS. Effect of topically applied acidulated phosphate-fluoride on dental caries in Hawaiian school children [abstract No 275]. Journal of Dental Research 1967;105:Abstr No 275.(Abstract). [Google Scholar]
- Horowitz HS. Effect on dental caries of topically applied acidulated phosphate- fluoride: results after two years. Journal of the American Dental Association 1969;78(3):568-72. [DOI] [PubMed] [Google Scholar]
- Horowitz HS. The effect on dental caries of topically applied acidulated phosphate-fluoride: results after one year. Journal of Oral Therapeutics and Pharmacology 1968;4:286-91. [PubMed] [Google Scholar]
Horowitz 1974 {published and unpublished data}
- Horowitz HS, Heifetz SB, McClendon BJ, Viegas AR, Guimaraes LO, Lopes ES. Evaluation of self-administered prophylaxis and supervised toothbrushing with acidulated phosphate fluoride. Caries Research 1974;8(1):39-51. [DOI] [PubMed] [Google Scholar]
Ingraham 1970 {published data only}
- Ingraham RQ, Williams JE. An evaluation of the utility of application and cariostatic effectiveness of phosphate-fluorides in solution and gel states. Journal of Tennessee State Dental Association 1970;50(1):5-12. [PubMed] [Google Scholar]
Jiang 2005 {published data only}
- Jiang H, Tai B, Du M, Peng B. Effect of professional application of APF foam on caries reduction in permanent first molars in 6-7-year-old children: 24-month clinical trial. Journal of Dentistry 2005;33(6):469-73. [DOI] [PubMed] [Google Scholar]
Mainwaring 1978 {published data only}
- Mainwaring PJ, Naylor MN. A three-year clinical study to determine the separate and combined caries-inhibiting effects of sodium monofluorophosphate toothpaste and an acidulated phosphate-fluoride gel. Caries Research 1978;12(4):202-12. [DOI] [PubMed] [Google Scholar]
Marthaler 1970 {published data only}
- Marthaler TM, Konig KG, Muhlemann HR. The effect of a fluoride gel used for supervised toothbrushing 15 or 30 times per year. Helvetica Odontologica Acta 1970;14(2):67-77. [PubMed] [Google Scholar]
- Marthaler TM. Fluoride gel applied in 60 toothbrushings, caries after seven years. Journal of Dental Research 1995;74(SI):411 (Abstr 83). [Google Scholar]
Marthaler 1970a {published data only}
- Marthaler TM, Konig KG, Muhlemann HR. The effect of a fluoride gel used for supervised toothbrushing 15 or 30 times per year. Helvetica Odontologica Acta 1970;14(2):67-77. [PubMed] [Google Scholar]
- Marthaler TM. Fluoride gel applied in 60 toothbrushings, caries after seven years. Journal of Dental Research 1995;74(SI):411 (Abstr 83). [Google Scholar]
Mestrinho 1983 {published and unpublished data}
- Mestrinho HD, Bijella MFTB, Bijella VT, Lopes ES. Prevention of dental caries through topical application of APF gel with plastic trays [Prevenção da cárie dental pela aplicação tópica de gel de flúor fosfato acidulado, através de moldeiras plásticas]. Odontologo Moderno 1983;10(1-2):29-32. [Google Scholar]
Olivier 1992 {published and unpublished data}
- Olivier M, Brodeur JM, Simard PL. Efficacy of APF treatments without prior toothcleaning targeted to high-risk children. Community Dentistry and Oral Epidemiology 1992;20(1):38-42. [DOI] [PubMed] [Google Scholar]
Ran 1991 {published data only (unpublished sought but not used)}
- Ran F, Fried M, Hadani P, Gedalia I. Caries rate after fortnightly toothbrushing with gel containing aminofluoride. Journal of Dental Research 1990;69(SI):829 (Abstr 12). [Google Scholar]
- Ran F, Gedalia I, Fried M, Hadani P, Tved A. Effectiveness of fortnightly tooth brushing with amine fluorides in caries-prone subjects. Journal of Oral Rehabilitation 1991;18(4):311-6. [DOI] [PubMed] [Google Scholar]
Shern 1976 {published data only}
- Shern RJ, Duany LF, Senning RS, Zinner DD. Clinical study of an amine fluoride gel and acidulated phosphate fluoride gel. Community Dentistry and Oral Epidemiology 1976;4(4):133-6. [DOI] [PubMed] [Google Scholar]
Szwejda 1972 {published data only}
- Szwejda LF. Fluorides in community programs; a study of four years of various fluorides applied topically to the teeth of children in fluoridated communities. Journal of Public Health Dentistry 1972;32(1):25-33. [DOI] [PubMed] [Google Scholar]
Treide 1988 {published and unpublished data}
- Treide A, Treide B. The anticaries effectiveness of newly developed fluoride-containing gels following 3 years of clinical use in preschool children. Stomatologie der DDR 1988;38(10):708-12. [PubMed] [Google Scholar]
Trubman 1973 {published data only}
- Trubman A, Crellin JA. Effect on dental caries of self-application of acidulated phosphate fluoride paste and gel. Journal of the American Dental Association 1973;86(1):153-7. [DOI] [PubMed] [Google Scholar]
Truin 2005 {published data only}
- Truin GJ, van't Hof M. The effect of fluoride gel on incipient carious lesions in a low caries child population. Community Dentistry and Oral Epidemiology 2007;35(4):250-4. [DOI] [PubMed] [Google Scholar]
- Truin GJ, van't Hof MA. Professionally applied fluoride gel in low-caries 10.5-year olds. Journal of Dental Research 2005;84(5):418-21. [DOI] [PubMed] [Google Scholar]
Van Rijkom 2004 {published data only}
- Truin GJ, van't Hof MA. Caries prevention by professional fluoride gel application on enamel and dentinal lesions in low-caries children. Caries Research 2005;39(3):236-40. [DOI] [PubMed] [Google Scholar]
- Rijkom HM, Truin GJ, van't Hof MA. Caries inhibiting effect of professional fluoride application in children with a low caries activity. Caries Research (ORCA Abstract) 2002;36(3):185.
- Rijkom HM, Truin GJ, van't Hof MA. Caries-inhibiting effect of professional fluoride gel application in low-caries children initially aged 4.5-6.5 years. Caries Research 2004;38(2):115-23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agrawal 2011 {published data only}
- Agrawal N, Pushpanjali K. Feasibility of including APF gel application in a school oral health promotion program as a caries-preventive agent: a community intervention trial. Journal of Oral Sciences 2011;53(2):185-91. [DOI] [PubMed] [Google Scholar]
Bellini 1981 {published data only}
- Bellini HT, Campi R, Denardi JL. Four years of monthly professional toothcleaning and topical fluoride application in Brazilian schoolchildren. I. Effect on gingivitis. Journal of Clinical Periodontology 1981;8(3):231-8. [DOI] [PubMed] [Google Scholar]
Bordoni 1995 {published data only}
- Bordoni N, Bellagamba H, Dono R, Piovano S, Marcantoni M, Squassi A. Effect of self-brushing with acidulated phosphate fluoride (pH 5.6) on dental caries in children. Acta Odontologica Latinoamericana 1994-5;8(2):217-25. [PubMed] [Google Scholar]
- Bordoni N, Paternosto de Bellagamba H, Doño R, Piovano S, Marcantoni M, Squassi A. Efecto del autocepillado con fosfato flúor acidulado pH 5.6 sobre la caries dental en niños. Boletin de la Asociacion Argentina de Odontologia para Ninos 1999;28(1):14-8. [Google Scholar]
Boyd 1985 {published data only}
- Boyd CH, Boyd CM, Gallien GS Jr. A preliminary report: the effectiveness of 0.4% stannous fluoride on controlling dental caries. Arkhiv Dental Journal 1985;56(4):14-5. [PubMed] [Google Scholar]
Cichocka 1981 {published data only}
- Cichocka E. Economic evaluation of selected methods of contact fluoridation of the teeth [Ocena ekonomiczna wybranych metod kontaktowego fluorkowania zebow]. Czasopismo Stomatologiczne 1981;34:245-50. [PubMed] [Google Scholar]
- Cichocka E. Oral hygiene and the efficacy of selected methods of contact tooth fluoridation [Higiena jamy ustnej a skutecznosc wybranych metod kontaktowego fluorkowania zebow]. Czasopismo Stomatologiczne 1981;34(2):145-53. [PubMed] [Google Scholar]
Heifetz 1979 {published data only}
- Heifetz SB, Franchi GJ, Mosley GW, MacDougall O, Brunelle J. Combined anti-cariogenic effect of fluoride gel-trays and fluoride mouthrinsing in a fluoridated community [abstract No 811]. Journal of Dental Research 1978;57:277. [Google Scholar]
- Heifetz SB, Franchi GJ, Mosley GW, MacDougall O, Brunelle J. Combined anticariogenic effect of fluoride gel-trays and fluoride mouthrinsing in an optimally fluoridated community. Clinical Preventative Dentistry 1979;1(1):21-23, 28. [PubMed] [Google Scholar]
Ivanova 1990 {published data only (unpublished sought but not used)}
- Ivanova EN. The comparative efficacy of local anticaries agents [Sravnitel'naia effektivnost' mestnykh protivokarioznykh sredtsv]. Stomatologiia Moskva 1990;69(2):60-1. [PubMed] [Google Scholar]
Kukleva 1983 {published and unpublished data}
- Kukleva M, Palabikjan V, Stoilova R, Skuleva V, Nenkovsca J. Prevention of dental caries using Kerr fluoride gel (preliminary communication) [Profilaktika na zubniia karies s floren gel "Kerr" (predvaritelno sobshchenie)]. Stomatologiia Sofiia 1983;65(6):5-7. [PubMed] [Google Scholar]
Kukleva 1998 {published and unpublished data}
- Kukleva M, Kondeva V, Kotsikas M. Group caries prophylactic with fluoride gel. 3rd Balkan Congress of Medicine and Dentistry for Students and Young Doctors 1999 Nov:5-7.
- Kukleva M. Prevention of dental caries on the first permanent molars with fluoride gel in the first year after eruption. Folia Medica Plovdiv 1998;40(4):60-4. [PubMed] [Google Scholar]
- Kukleva MP, Kondeva VK. Dynamics of caries acivity and caries reduction in a group prophylaxis with fluoride gel. Folia Medica 2001;43(1-2):12-5. [PubMed] [Google Scholar]
Kukleva 2001 {published data only}
- Kukleva MP. Changes in the appearance and form of the spots of macula cariosa alba in treatment with fluoride gel. Folia Medica 2002;44(1-2):64-9. [PubMed] [Google Scholar]
- Kukleva MP. Dynamics of some parameters of macula cariosa alba treated with fluoride gel. Folia Medica 2001;XLIII(1-2):5-8. [PubMed] [Google Scholar]
- Kukleva MP. Treatment of incipient caries in children with fluoride gel. Folia Medica 2002;44(1-2):50-5. [PubMed] [Google Scholar]
Lisiecka 1976 {published data only (unpublished sought but not used)}
- Lisiecka K. 2-year follow-up studies of the efficiency of various fluorine preparations in the prevention of dental caries in school children. Czasopismo stomatologiczne 1977;30(4):298. [PubMed] [Google Scholar]
- Lisiecka K. 2-year observation of the efficacy of various fluoride preparations used in toothbrushing in the prevention of dental caries in children. Czasopismo stomatologiczne 1978;31(11):1009-12. [PubMed] [Google Scholar]
- Lisiecka K. Evaluation of the effectiveness of certain fluorine compounds with the use of brushing in school-children caries prophylaxis. Annales Academiae Medicae Stetinensis 1976;22:231-52. [Google Scholar]
Loesche 1977 {published data only (unpublished sought but not used)}
- Loesche J, Pink T. Reduction of decay following 1 week application of a 1.2% neutral NaF gel in rampant caries in children. Journal of Dental Research 1977;56(Special Issue B):B209 (Abs 631).
- Loesche J, Pink T. Reduction of decay following 1 week unsupervised application of 1.2% neutral NaF gel in rampant caries in children. Journal of Dental Research 1979;58(Special issue A):296 (Abs 815).
- Loesche J, Pink T. Reduction of decay following 1 week unsupervised application of neutral fluoride gels (1.2%) in rampant caries in children. Interim Report. Journal of Dental Research 1980;59(Special issue A):408 (Abs 564).
Madlena 2002 {published data only}
- Madléna M, Nagy G, Gábris K, Márton S, Keszthelyi G, Bánóczy J. Effect of aminefluoride toothpaste and gel in high risk groups of Hungarian adolescents: results of a longitudinal study. Caries Research 2002;36(2):142-6. [DOI] [PubMed] [Google Scholar]
- Madléna M, Nagy G, Gábris K, Márton S, Keszthelyi G, Bánóczy J. Effect of amine fluoride toothpaste and gel in high-risk groups of Hungarian adolescents [ORCA Abstract]. Caries Research 2001;35(4):307. [DOI] [PubMed] [Google Scholar]
Mellberg 1978 {published data only (unpublished sought but not used)}
- Mellberg JR, Franchi GJ, Englander HR, Mosley GW, Nicholson CR. Short intensive topical APF applications and dental caries in a fluoridated area. Community Dentistry and Oral Epidemiology 1978;6(3):117-20. [DOI] [PubMed] [Google Scholar]
Pinto 1993 {published data only (unpublished sought but not used)}
- Pinto IL. Dental caries prevention through APF Gel-Tray applications each six months [Prevenção da cárie dental com aplicaçõe tópicas semestrais de fluor fosfato]. Revista de Saude Publica 1993;27(4):277-90. [DOI] [PubMed] [Google Scholar]
Rajic 1977 {published data only (unpublished sought but not used)}
- Rajic Z, Rajic A. The effect of amine fluoride - Elmex gel on the inhibition of dental caries. Stomatol-Kongress Belgrad, Serbien 1977:18-20.
Ran 1987 {published data only (unpublished sought but not used)}
- Ran F, Toeg A, Hadani P, Gedalia I. Caries rate, enamel fluoride concentration after fortnightly toothbrushing with aminofluoride gel. IADR-CED 24th Meeting, Regensburg, Deutschland 1987:Abstr 167. [Google Scholar]
Shobha 1987 {published data only (unpublished sought but not used)}
- Shobha T, Nandlal B, Prabhakar AR, Sudha P. Fluoride varnish versus acidulated phosphate fluoride for schoolchildren in Manipal. Journal of the Indian Dental Association 1987;59(6-9):157-60. [PubMed] [Google Scholar]
Spears 1978 {published data only}
- Spears ND, Goldstein C, Gordinier N, Crysler C. Effects of a thrice yearly application of fluoride gel. Dental Hygiene (Chicago) 1978;52(12):569-72. [PubMed] [Google Scholar]
Stadtler 1982 {published data only}
- Busse H, Stadtler P. A statistical model for caries incidence from a double-blind NaF-gel study. Caries Research 1990;24:427(Abstract). [Google Scholar]
- Stadtler P. Results of a 3-year clinical, experimental double-blind study of weekly supervised brushing with a sodium fluoride gel [Ergebnisse einer dreijahrigen klinisch experimentellen Doppelblindstudie mit wochentlichem uberwachtem Einbursten eines natriumfluoridhaltigen Gels]. Osterreichische Zeitschrift fur Stomatologie 1982;79(3):83-99. [PubMed] [Google Scholar]
- Stadtler P. Results of a 3-year clinical, experimental double-blind study of weekly supervised brushing with a sodium fluoride gel [Ergebnisse einer dreijahrigen klinisch-experimentellen Doppelblindstudie mit wochentlichem uberwachtem Einbursten eines natriumfluoridhaltigen Gels]. Osterreichische Zeitschrift fur Stomatologie 1982;79(4):132-54. [PubMed] [Google Scholar]
Stokes 2011 {published data only}
- Stokes E, Ashcroft A, Burnside G, Mohindra T, Pine CM. Randomised controlled trial of the efficacy of a high-fluoride gel self-applied by toothbrushing in children at high caries risk. Caries Research 2011;45(5):475-85. [DOI] [PubMed] [Google Scholar]
Szoke 1989 {published data only}
- Szoke J, Kozma M. Results of 3-year study of toothbrushing with a fluoride amine gel [Ergebnisse einer dreijahrigen Untersuchung uber Zahneputzen mit einem Aminfluorid-Gelee]. Oral-prophylaxe 1989;11(4):137-43. [PubMed] [Google Scholar]
- Szoke J, Kozma M. Results of a three-year group test of the use of Elmex gel [Csoportosan vegzett Elmex zsele bedorzsoles harom eves eredmenyei]. Fogorvosi Szenile 1988;81(6):161-7. [PubMed] [Google Scholar]
Szwejda 1971 {published data only}
- Szwejda LF, Tossy CV, Below DM. Fluorides in community programs; results from a fluoride gel applied topically. Bulletin of Michigan Dental Hygienists Association 1968;14(1):13-5. [PubMed] [Google Scholar]
- Szwejda LF, Tossy CV, Below DM. Fluorides in community programs; results from a fluoride gel applied topically. Journal of Public Health Dentistry 1967;27:192-4. [DOI] [PubMed] [Google Scholar]
- Szwejda LF. Fluorides in community programs: results after two years from a fluoride gel applied topically. Journal of Public Health Dentistry 1971;31(4):241-2. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00670618 {published data only}
- De Pauw G. A prospective, randomized clinical study on the effects of CPP-ACP Paste on plaque, gingivitis and white spot lesions in orthodontic patients - Part 2 [A prospective, randomized clinical study on the effects of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Paste on plaque, gingivitis and white spot lesions in orthodontic patients - Part 2]. http://clinicaltrials.gov/ct2/show/NCT00670618 2008 (accessed on 29 December 2014).
NCT01329731 {published data only}
- Heumann C. Remineralisation of white spot lesions by Elmex® gelée in post-orthodontic patients [White spot lesion development in post-orthodontic patients following weekly application of a 1.25% fluoride gel compared to placebo over 6 months]. http://clinicaltrials.gov/ct2/show/study/NCT01329731 2011 (accessed on 29 December 2014).
Additional references
Ammari 2003
- Ammari AB, Bloch-Zupan A, Ashley PF. Systematic review of studies comparing the anti-caries efficacy of children's toothpaste containing 600 ppm of fluoride or less with high fluoride toothpastes of 1,000 ppm or above. Caries Research 2003;37(2):85-92. [DOI] [PubMed] [Google Scholar]
Bartizek 2001
- Bartizek RD, Gerlach RW, Faller RV, Jacobs SA, Bollmer BW, Biesbrock AR. Reduction in dental caries with four concentrations of sodium fluoride in a dentifrice: a meta-analysis evaluation. Journal of Clinical Dentisty 2001;12(3):57-62. [PubMed] [Google Scholar]
Bratthall 1996
- Bratthall D, Hansel Petersson G, Sundberg H. Reasons for the caries decline: what do the experts believe? European Journal of Oral Sciences 1996;104(4):416-22. [DOI] [PubMed] [Google Scholar]
Burt 1998
- Burt BA. Prevention policies in the light of the changed distribution of dental caries. Acta Odontologica Scandinavica 1998;56(3):179-86. [DOI] [PubMed] [Google Scholar]
Chaves 2002
- Chaves SC, Vieira-da-Silva LM. Anticaries effectiveness of fluoride toothpaste: a meta-analysis. Revista de Saúde Pública 2002;36(5):598-606. [DOI] [PubMed] [Google Scholar]
Chen 1995
- Chen MS. Oral health of disadvantaged populations. In: Disease Prevention and Oral Health Promotion. 152-212 edition. Munksgaard: Cohen LK, Gift CH, 1995. [Google Scholar]
Clark 1985
- Clark DC, Hanley JA, Stamm JW, Weinstein PL. An empirically based system to estimate the effectiveness of caries-preventive agents. A comparison of the effectiveness estimates of APF gels and solutions, and fluoride varnishes. Caries Research 1985;19(1):83-95. [DOI] [PubMed] [Google Scholar]
de Liefde 1998
- Liefde B. The decline of caries in New Zealand over the past 40 years [see comments]. New Zealand Dental Journal 1998;94(417):109-13. [PubMed] [Google Scholar]
Dubey 1965
- Dubey SD, Lehnhoff RW, Radike AW. A statistical confidence interval for true per cent reduction in caries-incidence studies. Journal of Dental Research 1965;44(5):921-23. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekstrand 1988
- Ekstrand J, Fejerskov O, Silverstone LM. Fluoride in Dentistry. Copenhagen: Munksgaard, 1988. [Google Scholar]
Featherstone 1988
- Featherstone JDB, Ten Cate JM. Physicochemical aspects of fluoride-enamel interactions. In: Ekstrand J, Fejerskov O, Silverstone LM, editors(s). Fluoride in Dentistry. Copenhagen: Munksgaard, 1988:125-49. [Google Scholar]
Featherstone 1999
- Featherstone JD. Prevention and reversal of dental caries: role of low level fluoride. Community Dentistry and Oral Epidemiology 1999;27:31-40. [DOI] [PubMed] [Google Scholar]
Fejerskov 1996
- Fejerskov O, Ekstrand J, Burt BA. Fluoride in Dentistry. 2nd edition. Copenhagen: Munksgaard, 1996. [Google Scholar]
Glass 1982
- Glass RL. The first international conference on the declining prevalence of dental caries. Journal of Dental Research 1982;61(Special Issue):1301-83. [Google Scholar]
Helfenstein 1994
- Helfenstein U, Steiner M. Fluoride varnishes (Duraphat): a meta-analysis. Community Dentistry and Oral Epidemiology 1994;22(1):1-5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Horowitz 1996
- Horowitz HS, Ismail AI. Topical fluorides in caries prevention. In: Fejerskov O, Ekstrand J, Burt BA, editors(s). Fluoride in Dentistry. Copenhagen: Munksgaard, 1996:311-27. [Google Scholar]
Johnson 1993
- Johnson MF. Comparative efficacy of NaF and SMFP dentifrices in caries prevention: a meta-analytic overview. Caries Research 1993;27(4):328-36. [DOI] [PubMed] [Google Scholar]
Kassebaum 2015
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: A systematic review and metaregression. Journal of Dental Research 2015;94(5):650-658. [DOI] [PubMed] [Google Scholar]
Krasse 1996
- Krasse B. The caries decline: is the effect of fluoride toothpaste overrated? European Journal of Oral Sciences 1996;104(4):426-9. [DOI] [PubMed] [Google Scholar]
Lawrence 2008
- Lawrence HP, Binguis D, Douglas J, McKeown L, SwitzerB, Figueiredo R, et al. A 2-year community-randomized controlled trial of fluoride varnish to prevent early childhood caries in Aboriginal children. Community Dentistry and Oral Epidemiology 2008;36(6):503–16. [DOI] [PubMed] [Google Scholar]
Marcenes 2013
- Marcenes W, Kassebaum NJ, Bernabé E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990-2010: a systematic analysis. Journal of Dental Research 2013;92(7):592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2004
- Marinho VCC, Higgins JPT, Sheiham A, Logan S. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No: CD002780. [DOI: 10.1002/14651858.CD002780.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marinho 2013
- Marinho VCC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD002279. [DOI: 10.1002/14651858.CD002279.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marthaler 1994
- Marthaler TM, Steiner M, Menghini G, Bandi A. Caries prevalence in Switzerland. International Dental Journal 1994;44(4 Suppl 1):393-401. [PubMed] [Google Scholar]
Marthaler 1996
- Marthaler TM, O'Mullane DM, Vrbic V. The prevalence of dental caries in Europe 1990-1995. ORCA Saturday afternoon symposium 1995. Caries Research 1996;30(4):237-55. [DOI] [PubMed] [Google Scholar]
Marthaler 2004
- Marthaler TM. Changes in dental caries 1953-2003. Caries Research 2004;38(3):173-81. [DOI] [PubMed] [Google Scholar]
Mejare 1998
- Mejàre I, Källestål C, Stenlund H, Johansson H. Caries development from 11 to 22 years of age: a prospective radiographic study. Prevalence and distribution. Caries Research 1998;32(1):10-6. [DOI] [PubMed] [Google Scholar]
Murray 1991
- Murray JJ, Rugg-Gunn AJ, Jenkins GN, editors. A history of water fluoridation. In: Fluorides in Caries Prevention. Oxford: Wright, 1991:7-37. [Google Scholar]
Murray 1991a
- Murray JJ, Rugg-Gunn AJ, Jenkins GN, editors. Fluoride toothpastes and dental caries. In: Fluorides in Caries Prevention. Oxford: Wright, 1991:127-60. [Google Scholar]
Murray 1991c
- Murray JJ, Rugg-Gunn AJ, Jenkins GN. Fluorides in Caries Prevention. 3rd edition. Oxford: Butterworth-Heinemann, 1991. [Google Scholar]
Nadanovsky 1995
- Nadanovsky P, Sheiham A. Relative contribution of dental services to the changes in caries levels of 12-year-old children in 18 industrialized countries in the 1970s and early 1980s. Community Dentistry and Oral Epidemiology 1995;23(6):331-9. [DOI] [PubMed] [Google Scholar]
O'Mullane 1994
- O'Mullane DM. Introduction and rationale for the use of fluoride for caries prevention. International Dental Journal 1994;44(3 Suppl 1):257-61. [PubMed] [Google Scholar]
Ogaard 1994
- Ogaard B, Seppa L, Rolla G. Professional topical fluoride applications-clinical efficacy and mechanism of action. Advances in Dental Research 1994;8(2):190-201. [DOI] [PubMed] [Google Scholar]
Ogaard 2001
- Ogaard B. CaF2 formation: cariostatic properties and factors of enhancing the effect. Caries Research 2001;35(Suppl 1):40-4. [DOI] [PubMed] [Google Scholar]
Petersen 2004
- Petersen PE, Lennon MA. Effective use of fluorides for the prevention of dental caries in the 21st century: the WHO approach. Community Dentistry and Oral Epidemiology 2004;32(5):319-21. [DOI] [PubMed] [Google Scholar]
Petersen 2008
- Petersen PE. World Health Organization global policy for improvement of oral health: World Health Assembly 2007. International Dental Journal 2008;58:115–21. [DOI] [PubMed] [Google Scholar]
Petersson 2004
- Petersson LG, Twetman S, Dahlgren H, Norlund A, Holm AK, Nordenram G, et al. Professional fluoride varnish treatment for caries control: a systematic review of clinical trials. Acta Odontologica Scandinavica 2004;62(3):170-6. [DOI] [PubMed] [Google Scholar]
Reisine 2001
- Reisine ST, Psoter W. Socioeconomic status and selected behavioral determinants as risk factors for dental caries. Journal of Dental Education 2001;65(10):1009-16. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripa 1989
- Ripa LW. Review of the anticaries effectiveness of professionally applied and self-applied topical fluoride gels. Journal of Public Health Dentistry 1989;49(5):297-309. [DOI] [PubMed] [Google Scholar]
Ripa 1990
- Ripa LW. An evaluation of the use of professional (operator-applied) topical fluorides. Journal of Dental Research 1990;69(Spec Issue):786-96. [DOI] [PubMed] [Google Scholar]
Ripa 1991
- Ripa LW. A critique of topical fluoride methods (dentifrices, mouthrinses, operator-, and self-applied gels) in an era of decreased caries and increased fluorosis prevalence. Journal of Public Health Dentistry 1991;51(1):23-41. [DOI] [PubMed] [Google Scholar]
Rolla 1991
- Rolla G, Ogaard B, Cruz R-d-A. Clinical effect and mechanism of cariostatic action of fluoride-containing toothpastes: a review. International Dental Journal 1991;41(3):171-4. [PubMed] [Google Scholar]
Schwendicke 2015
- Schwendicke F, Dörfer CE, Schlattmann P, Page LF, Thomson WM, Paris S. Socioeconomic inequality and caries: a systematic review and meta-analysis. Journal of Dental Research 2015;94(1):10-8. [DOI] [PubMed] [Google Scholar]
Sharp 1998
- Sharp S. Meta-analysis regression. Stata Technical Bulletin 1998;7(42):16-22.
Sheiham 2001
- Sheiham A. Dietary effects on dental diseases. Public Health Nutrition 2001;4(2B):569–91. [DOI] [PubMed] [Google Scholar]
Sheiham 2005
- Sheiham A. Oral health, general health and quality of life. Bulletin of the World Health Organization 2005;83(9):644. [PMC free article] [PubMed] [Google Scholar]
Stamm 1984
- Stamm JW, Bohannan HM, Graves RC, Disney JA. The efficiency of caries prevention with weekly fluoride mouthrinses. Journal of Dental Education 1984;48(11):617-26. [PubMed] [Google Scholar]
Stamm 1995
- Stamm JW. Clinical studies of neutral sodium fluoride and sodium monofluorophosphate dentifrices. In: Bowen WH, editors(s). Relative Efficacy of Sodium Fluoride and Sodium Monofluorophosphate as Anti-Caries Agents in Dentifrices. London: The Royal Society of Medicine Press Limited, 1995:43-58. [Google Scholar]
Steiner 2004
- Steiner M, Helfenstein U, Menghini G. Effect of 1000 ppm relative to 250 ppm fluoride toothpaste. American Journal of Dentistry 2004;17(2):85-8. [PubMed] [Google Scholar]
Strohmenger 2001
- Strohmenger L, Brambilla E. The use of fluoride varnishes in the prevention of dental caries: a short review. Oral Diseases 2001;7(2):71-80. [PubMed] [Google Scholar]
ten Cate 1999
- ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontologica Scandinavica 1999;57(6):325-9. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693-708. [DOI] [PubMed] [Google Scholar]
Twetman 2004
- Twetman S, Petersson L, Axelsson S, Dahlgren H, Holm AK, Källestål C, et al. Caries-preventive effect of sodium fluoride mouthrinses: a systematic review of controlled clinical trials. Acta Odontologica Scandinavica 2004;62(4):223-30. [DOI] [PubMed] [Google Scholar]
van Rijkom 1998
- Rijkom HM, Truin GJ, 't Hof MA. A meta-analysis of clinical studies on the caries-inhibiting effect of fluoride gel treatment. Caries Research 1998;32(2):83-92. [DOI] [PubMed] [Google Scholar]
Weyant 2013
- Weyant RJ, Tracy SL, Anselmo TT, Beltrán-Aguilar ED, Donly KJ, Frese WA, et al for the American Dental Association (ADA) Council on Scientific Affairs Expert Panel on topical fluoride caries preventive agents. Topical fluoride for caries prevention: Full report of the updated clinical recommendations and supporting systematic review. A report of the Council on Scientific Affairs. November 2013. http://ebd.ada.org/~/media/EBD/Files/Topical_fluoride_for_caries_prevention_2013_update.ashx (accessed on 29 December 2014).
Whitford 1992
- Whitford GM. Acute and chronic fluoride toxicity. Journal of Dental Research 1992;71(5):1249-54. [DOI] [PubMed] [Google Scholar]
Wong 2010
- Wong MCM, Glenny AM, Tsang BWK, Lo ECM, Worthington HV, Marinho VCC. Topical fluoride as a cause of dental fluorosis in children. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD007693. [DOI: 10.1002/14651858.CD007693.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision-making. Evidence-based Dentistry 2015;16(3):69-71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Marinho 2002
- Marinho VCC, Higgins J, Logan S, Sheiham A. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD002280. [DOI: 10.1002/14651858.CD002280] [DOI] [PubMed] [Google Scholar]


