Abstract
Background
Few studies examined treatments for amphetamine withdrawal, although it is a common problem among amphetamine users. Its symptoms, in particular intense craving, may be a critical factor leading to relapse to amphetamine use. In clinical practice, medications for cocaine withdrawal are commonly used to manage amphetamine withdrawal although the pharmacodynamic and pharmacokinetic properties of these two illicit substances are different.
Objectives
To assess the effectiveness of pharmacological alone or in combination with psychosocial treatment for amphetamine withdrawals on discontinuation rates, global state, withdrawal symptoms, craving, and other outcomes.
Search methods
MEDLINE (1966 ‐ 2008), CINAHL (1982 ‐ 2008), PsycINFO (1806 ‐ 2008), CENTRAL (Cochrane Library 2008 issue 2), references of obtained articles.
Selection criteria
All randomised controlled and clinical trials evaluating pharmacological and or psychosocial treatments (alone or combined) for people with amphetamine withdrawal symptoms.
Data collection and analysis
Two authors evaluated and extracted data independently. The data were extracted from intention‐to‐treat analyses. The Relative Risk (RR) with the 95% confidence interval (95% CI) was used to assess dichotomous outcomes. The Weighted Mean Difference (WMD) with 95% CI was used to assess continuous outcomes.
Main results
Four randomised controlled trials (involving 125 participants) met the inclusion criteria for the review. Two studies found that amineptine significantly reduced discontinuation rates and improved overall clinical presentation, but did not reduce withdrawal symptoms or craving compared to placebo. The benefits of mirtazapine over placebo for reducing amphetamine withdrawal symptoms were not as clear. One study suggested that mirtazapine may reduce hyperarousal and anxiety symptoms associated with amphetamine withdrawal. A more recent study failed to find any benefit of mirtazapine over placebo on retention or on amphetamine withdrawal symptoms.
Authors' conclusions
No medication is effective for treatment of amphetamine withdrawal. Amineptine showed reduction in discontinuation rates and improvement in clinical presentation compared to placebo, but had no effect on reducing withdrawal symptoms or craving. In spite of these limited benefits, amineptine is not available for use due to concerns over abuse liability when using the drug. The benefits of mirtazapine as a withdrawal agent are less clear based on findings from two randomised controlled trials: one report showed improvements in amphetamine withdrawal symptoms over placebo; a second report showed no differences in withdrawal symptoms compared to placebo. Further potential treatment studies should examine medications that increase central nervous system activity involving dopamine, norepinephrine and/or serotonin neurotransmitters, including mirtazapine.
Plain language summary
Treatment for amphetamine withdrawal
Symptoms of amphetamine withdrawal during the initial days of abstinence from chronic amphetamine use can prompt individuals to return to regular drug use. No medications demonstrate significant effects over placebo in reducing symptoms of acute amphetamine withdrawal. Amphetamines can make people feel more alert, and are prescribed for problems like depression and attention deficit order. Amphetamines can produce euphoria, and so are manufactured for recreational use. Ongoing use can lead to dependence, which can be as hard to recover from as dependence on heroin or cocaine. The only randomized trials of amphetamine withdrawal agents have been of antidepressant drugs (amineptine and mirtazapine). Amineptine was found to have limited benefits, showing improvement only on some subjective effects but is no longer on the market because of concerns over its abuse liability. The benefits of mirtazapine have been less clear based on two randomised controlled trials, with one showing improvements in amphetamine withdrawal symptoms and the other showing no differences in withdrawal outcomes when compared to placebo. More research is needed.
Background
Although there are a variety of amphetamines and amphetamine derivatives, the word "amphetamines" in this review stands for amphetamine, dextroamphetamine, and methamphetamine.
Description of the condition
When chronic heavy users abruptly discontinue amphetamine use, many report a time‐limited withdrawal syndrome that occurs within 24 hours of their last dose. Withdrawal symptoms are sufficiently severe to cause relapse to drug use in the absence of contained environments. The prevalence of this withdrawal syndrome is extremely common (Cantwell 1998; Gossop 1982) with 87.6% of 647 individuals with amphetamine dependence reporting six or more signs of amphetamine withdrawal listed in the DSM when the drug is not available (Schuckit 1999). The DSM‐IV‐TR criteria for diagnosing amphetamine withdrawal include dysphoric mood and two or more symptoms: fatigue, vivid or unpleasant dreams, insomnia or hypersomnia, increased appetite and psychomotor agitation or retardation that occur following discontinuation of the drug (DSM‐IV‐TR 2000). Clinically, amphetamine dependent individuals in acute withdrawal report feeling “severe dysphoria, irritability and melancholia, anxiety, hypersomnia and marked fatigue, intense craving for the drug and paranoia.” Factor analysis of withdrawal symptoms indicate this clinical condition may be comprised of three factors (Srisurapanont 1999b): A hyperarousal factor comprised of drug craving, agitation, and vivid or unpleasant dreams, a reversed vegetative factor comprised of decreased energy, increased appetite, and increased craving for sleep, and an anxiety factor comprised loss of interest or pleasure, anxiety and slowing of movement.
The experience of withdrawal from amphetamines is clinically severe and it is during this period that reports of suicidal ideation and attempts are noted (Meredith 2005; Scott 2007). Amphetamine dependent individuals trying to discontinue or to cut down use of the drug using self‐help or even formal treatment commonly relapse, as a single use of amphetamine immediately removes discomfort and institutes a sense of well‐being or euphoria (Rawson 2002). As the initial phase of treatment requires cessation of use, amphetamine withdrawal compromises long‐term success for some individuals with severe amphetamine dependence to achieve protracted abstinence.
The severity of withdrawal symptoms is greater in amphetamine dependent individuals who are older and who have more extensive amphetamine use disorders (McGregor 2005). Withdrawal symptoms typically present within 24 hours of the last use of amphetamine, with a withdrawal syndrome involving two general phases that can last 3 weeks or more. The first phase of this syndrome is the initial “crash” that resolves within about a week (Gossop 1982; McGregor 2005). The most immediate symptoms occur during this “crash” period and are observed to resolve during the first week of abstinence measured using total scores of the clinician rated Amphetamine Withdrawal Questionnaire, the Amphetamine Selective Severity Assessment and the staff rated Clinical Global Impressions (McGregor 2005). Severe symptoms in the “crash” phase of amphetamine withdrawal include increases in sleep (averaging 2‐3 hours more per night than controls, but with poor sleep quality, light sleep, frequent awakening, and not feeling clearheaded on arising), increases in appetite, and decreases in complaints of depression (McGregor 2005; Newton 2004). A subacute, protracted set of withdrawal symptoms that generally resolve in 3 weeks and that are not as well defined, include continued sleep disturbances (mild hypersomnia or insomnia and continued increased appetite (McGregor 2005;Gossop 1982). Although the most severe symptoms occurring during amphetamine withdrawal resolve in a week or less, some symptoms may continue for weeks or months (Watson 1972; Hofmann 1983).
Description of the intervention
Symptoms of amphetamine withdrawal are time limited, with most resolving in a week. Thus, a treatment for amphetamine withdrawal needs rapid onset. In clinical practice and in the studies reviewed, treatment is started as soon as possible following the last dose of amphetamine. Medication is continued for up to two weeks to provide symptomatic relief. In addition to medication, it can be helpful to provide psychosocial and/or behavioral treatments for stimulant abuse to assist the patient in amphetamine withdrawal in sustaining abstinence from amphetamine once their treatment is completed (Lee 2008). Following brief exposure to the treatment, the medication is discontinued regardless of response as there is no evidence to suggest a pharmacotherapy for amphetamine withdrawal would have efficacy for amphetamine abuse or dependence.
How the intervention might work
One rationale guiding selection of medications for amphetamine withdrawal involves using a medication to stabilize dopamine, norepinephrine or serotonin neurotransmission to provide relief from withdrawal symptoms. According to this rationale, the neurobiology of the amphetamine withdrawal syndrome and its relief would be related to the cumulative effects of repeated exposure of neurons to high dose amphetamines (Meredith 2005). Initial “highs” mediated by extracellular dopamine and norepinephrine levels in striatum (midbrain) become attenuated. Aspects of the withdrawal syndrome may be mediated by different neurotransmitter systems that include dopamine, norepinephrine and serotonin. Diminished dopamine synaptic transmission in acute withdrawal may be responsible for anhedonia and psychomotor retardation. As well, decreased synaptic serotonin availability may be the substrate for depressed mood, obsessive thoughts about the drug and lack of impulse control (Rothman 2007). Medications that acutely stabilize neurotransmission in these systems may relieve acute withdrawal symptoms and assist the patient in establishing relevant periods of amphetamine abstinence.
Why it is important to do this review
In 2006, 24.7 million individuals aged 15‐64 consumed amphetamine type stimulants (UNODC 2008). Among chronic users of amphetamines, evidence is accruing to describe the range of public health problems attributable to sustained heavy use of the drug. Medical consequences of chronic use of amphetamines include cardiovascular insults, cognitive dysfunction and infectious disease (Meredith 2005; Pasic 2007). Development of one or more medications for amphetamine withdrawal, particularly if implemented with evidence‐based behavioral or counselling interventions, would have great public health significance. Maintaining a review of outcomes from experiences using medications in clinical trials for amphetamine withdrawal is an important method for clinicians to stay current and to seek guidance regarding medication strategies when treating individuals in acute withdrawal from amphetamines.
Objectives
To assess the effectiveness of pharmacological alone or in combination with psychosocial treatment for amphetamine withdrawals on discontinuation rates, global state, withdrawal symptoms, craving, and other outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials (RCTs) and clinical controlled trials (CCTs) were included.
Types of participants
Individuals with amphetamine withdrawal, diagnosed by any set of criteria. Individuals experiencing withdrawal from other substances in addition to amphetamine withdrawal are included only if:
The data for amphetamine withdrawal are reported separately, or
More than half of the participants are amphetamine withdrawal patients.
Types of interventions
Experimental intervention: Any kind of pharmacological treatment, alone or in combination with a psychosocial treatment
Control intervention: Placebo or any kind of psychosocial treatment alone
Types of outcome measures
Primary outcomes
Discontinuation rate measured as number of participants who did not complete the treatment
Average score in global state as measured by global psychiatric rating scales, e.g. Clinical Global Impression
Average score in withdrawal symptoms as measured by withdrawal symptomology assessments, e.g. Amphetamine Withdrawal Questionnaire
Average score in craving as measured by craving rating scales, e.g. Questionnaire for Evaluating Cocaine Craving and Related Responses, Visual Analog Scale, Brief Substance Craving Scale
Patient satisfaction as measured by type and number of adverse events
Secondary outcomes
Duration of adherence to treatment as measured by pill count or self‐report adherence
Death as measured by the number of reported mortality
All outcomes were reported for the short term (4 weeks or 1 month), medium term (more than 4 weeks or 1 month to 12 weeks or 3 months), and long term (more than 3 months). If any outcome was assessed more than once in a particular term, only the results of the longest duration in that term were considered.
Search methods for identification of studies
The search incorporated a number of methods to identify completed and ongoing studies
Electronic searches
We originally searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2000, issue 4, MEDLINE (January 1966 to December 2000) and EMBASE (January 1980 to December 2000).
For this updated version we searched CENTRAL through 2008, Issue 2 of The Cochrane Library, and MEDLINE, PsycINFO and CINAHL through to May 1, 2008. For details seeAppendix 1; Appendix 2; Appendix 3.
Searching other resources
We also searched:
the reference lists of all relevant papers to identify further studies.
some of the main electronic sources of ongoing trials (Current Controlled Trials ‐ http://www.controlled‐trials.com/, Clinical Trials.gov, Trialsjournal.com)
conference proceedings likely to contain trials relevant to the review.
We contracted investigators seeking information about unpublished or incomplete trials.
All searches included non‐English language literature and studies with English abstracts were assessed for inclusion. When considered likely to meet inclusion criteria, studies were translated.
Data collection and analysis
Selection of studies
In the original review, reports identified by the electronic searches were assessed for relevance. Two reviewers (MS & NJ) independently inspected all study citations identified by the electronic searches and full reports of the studies of agreed relevance were obtained. Where disputes arose the full reports were acquired for more detailed scrutiny. The reviewers (MS & NJ) then independently inspected all these full study reports.
For this update of the review, one author (UK) inspected the search hits by reading titles and abstracts. Each potentially relevant study located in the search was obtained in full text and assessed for inclusion independently by two authors (SS & UK). Discrepancies were resolved by discussion between the authors.
The corresponding author was contacted if information necessary for the review was not available in the reports.
Data extraction and management
Data were extracted independently by the authors onto data extraction forms. Again, if disputes arose, these were resolved either by discussion between the two reviewers or the correspondence author of the paper.
Assessment of risk of bias in included studies
We changed the criteria to assess the methodological quality of included studies to conform the review to the recommended methods outlined in the last Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and to the request of RevMan 5. We assessed the new studies included in the updated version and we reassessed the studies already included in the old review using the new criteria. The new criteria were based on the following specific domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues. We evaluated the included studies as follow:
A. Sequence generation was assessed for all outcomes. Studies were considered at low risk of bias if they provided a clear method of generating an allocation sequence to produce comparable groups, i.e. random number table, computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice. Studies were considered at high risk of bias if they used some systematic, non‐random approach, i.e. date of birth, date of admission, clinic record number, by clinician.
B. Allocation concealment was assessed for all outcomes. Studies were considered at low risk of bias if they provided adequate allocation concealment, i.e. central allocation including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. Studies were determined at high risk of bias if they had inadequate allocation concealment, i.e. using an open random allocation schedule such as a list of random numbers; assignment envelopes were used without appropriate safeguards e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered; alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
C. Blinding of participants, personnel, and outcome assessor was considered separately for objective outcomes (discontinuation rates) and subjective outcomes (global state, craving, and withdrawal symptoms). For discontinuation rates, we judged that lack of blinding was unlikely to influence data collection.
D. Incomplete outcome data were considered for all outcomes except for discontinuation rates. It was assessed for results at the end of the study period. Studies were considered at low risk of bias if they adequately addressed missing data.
E. Selective outcome reporting was considered for all outcomes except for discontinuation rates.
Studies were judged to have unclear risk of bias if there was insufficient information to permit judgment of 'low' or 'high' risk of bias for each of the domains.
Measures of treatment effect
Other than raw data (e.g. death), the outcomes derived from only valid scales were included in the reviews. In this review, a valid scale means a scale that has been published in a scientific journal.
Assessment of heterogeneity
Test of heterogeneity is important to check whether the results of studies are similar within each comparison. The reviewers checked whether differences between the results of trials were greater than could be expected by chance alone. This was done by looking at the graphical display of the results but also by using Chi square tests of heterogeneity. A p‐value being less than 0.05 of a Chi‐square test was indicated the significant heterogeneity of a data set. The statistical methods for dealing with a data set with significant and non significant heterogeneity were described in 'Data synthesis'. In addition, the causes possibly leading to the significant heterogeneity of a data set were discussed.
Data synthesis
Dichotomous data: The Relative Risk (RR) with the 95% confidence interval (95% CI) was used. RR is the ratio of risk in the intervention group to the risk in the control group. The risk (proportion, probability or rate) is the ratio of people with an event in a group to the total in the group. A relative risk of one indicates no difference between comparison groups. For undesirable outcomes a RR that is less than one indicates that the intervention was effective in reducing the risk of that outcome.
In addition, as a measure of efficacy, the number needed to treat (NNT) was also calculated. The reviewers extracted the dichotomous data on an intention‐to‐treat basis by applying the following guidelines to analyse data from included studies: (i) the analysis included all those who entered the trial; and (ii) the analysis maintained the study groups according to the original randomisation procedure. The reviewers assigned people lost to follow‐up to the worst outcome.
Continuous data: The Weighted Mean Difference (WMD) with 95% CI was used. WMD is a method of meta‐analysis used to combine measures on continuous scales (such as weight), where the mean, standard deviation and sample size in each group are known. The weight given to each study (e.g. how much influence each study has on the overall results of the meta‐analysis) is determined by the precision of its estimate of effect and, in the statistical software in RevMan and CDSR, is equal to the inverse of the variance. This method assumes that all of the trials have measured the outcome on the same scale.
For the studies in which the treatment and/or controlled groups were divided into subgroups because of the differences of concurrent treatment, the continuous data of the subgroups receiving more rigorous treatment, e.g., higher doses of drug treatment, more intensive psychotherapy, were extracted.
In conducting a meta‐analysis, a fixed effect model, an analysis that ignores the between‐study variation, can give a narrower confidence interval than a random effect model. It is generally agreed that the fixed effect model is valid as a test of significance of the overall null hypothesis (i.e. 'no effect in all studies'). A statistically significant result obtained by the use of this model indicated that there is an effect in at least one of the studies. Because of these advantages, the fixed effect model was used for the synthesis of a group of data with homogeneity. Although a random effect model can be applied for the synthesis of a group of data with significant heterogeneity, the results obtained by the synthesis of this group of data have to be interpreted with great caution.
As high attrition rate would affect the study results, the studies with the attrition rate of 50% or higher of the total participants were excluded.
Sensitivity analysis
Sensitivity analysis is an analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done. Sensitivity analyses are used to assess how robust the results are to uncertain decisions or assumptions about the data and the methods that were used.
The reviewers examined whether the decision to include the data obtained from studies in which most (50%‐75%) participants were amphetamine dependent or abuse affected the results of review. The sensitivity analyses were done by the inclusion and exclusion of the data obtained from these studies. If both analyses point to the same conclusion in the respect of significant heterogeneity of data, the meta‐analyses including the data obtained from these studies were taken into consideration. Otherwise, the meta‐analyses conducted by the exclusion of the data obtained from these studies were considered.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search strategy resulted in the identification of 1146 studies, 1137 references were excluded on basis of title and abstract and 9 were retrieved for more detailed evaluation, seeFigure 1.
1.
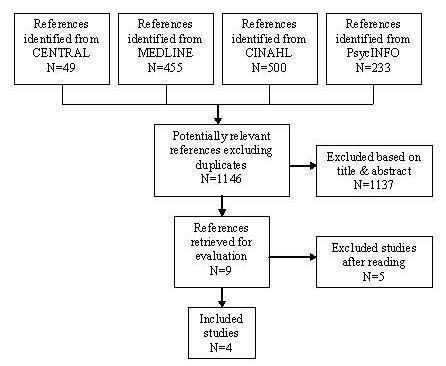
Flow chart showing identification of trials
Included studies
Four studies, involving 125 participants, met the inclusion criteria for this review (seeCharacteristics of included studies). In total, 59 participants received treatment for amphetamine withdrawal (37 amineptine, 22 mirtazapine) compared to 66 participants who received placebo.
Comparisons
Of the four studies that met the inclusion criteria, two studies compared amineptine with placebo (Jittiwutikan 1997; Srisurapanont 1999b) and two studies compared mirtazapine with placebo (Kongsakon 2005; Cruickshank 2008). Amineptine is an atypical tricyclic antidepressant that selectively inhibits the reuptake of dopamine and norepinephrine. Because amineptine has similar mechanism of actions as amphetamines, it was put forth that amineptine could help to relieve amphetamine withdrawal symptoms. Mirtazapine, a noradrenergic and specific serotonergic antidepressant, was also hypothesized to help reduce methamphetamine withdrawal severity by blocking the presynaptic alpha‐2 adrenergic receptors that inhibit the release of norepinephrine and serotonin.
Treatment setting
Of the four studies that met the inclusion criteria, two (Jittiwutikan 1997, Srisurapanont 1999b) were conducted at a drug dependence treatment center, one (Kongsakon 2005) at a probation facility, and one (Cruickshank 2008) in a public drug and alcohol outpatient clinic.
Participant characteristics
The participants of the four included studies were mainly males (110 males, 15 females). In two studies (Jittiwutikan 1997, Srisurapanont 1999b), participants were inpatients at a drug dependence treatment center who met DSM‐IV criteria for amphetamine withdrawal. In the Srisurapanont 1999b study, participants had to meet the additional criteria of having an Amphetamine Withdrawal Questionnaire (AWQ) score of 10 or higher. The combined mean duration of amphetamine use histories and length of time since last use of amphetamine prior to admission for the two studies on amineptine was 23.6 months and 55.2 hours, respectively. The average age of the participants was 19.1 years. Participants in the Kongsakon 2005 study were detainees from a probation facility who were diagnosed with amphetamine dependence by DSM‐IV criteria. All the participants in this study were males and had an average age of 24.3 years. Participants in the Cruickshank 2008 study were those that met DSM‐IV criteria for amphetamine dependence, reported using amphetamine or methamphetamine within the last 72 hours, and were recruited from two drug and alcohol out‐patient clinics.
Treatment regimes
Two studies (Srisurapanont 1999b; Jittiwutikan 1997) administered 300 mg of amineptine per day, given orally in two 100 mg capsules after breakfast and one 100 mg capsule after lunch. In these two studies, low dose of lorazepam was administered on occasion to patients with moderate to severe anxiety or insomnia. One study (Kongsakon 2005) administered a dose range of 15‐60 mg per day of mirtazapine with an initial dose of 15 mg. Dose titrations during this study were based on the subjects’ clinical response to the medication. No medication other than mirtazapine was used according to the study report. In another study (Cruickshank 2008), participants were administered 15 mg of mirtazapine on the first two nights and 30 mg mirtazapine every night for the next 12 nights. In all four studies (Srisurapanont 1999b; Jittiwutikan 1997, Kongsakon 2005; Cruickshank 2008), the treatment duration was 14 days.
Excluded studies
Five studies did not meet the criteria for inclusion in this review (Gillin 1994; McGregor 2008a; McGregor 2005; Chan‐Ob 2001; Cox 2004). The reasons for exclusions are described in “Characteristics of excluded studies.”
Risk of bias in included studies
Summary of results across studies for each domain are illustrated in Figure 2 and Figure 3.
2.
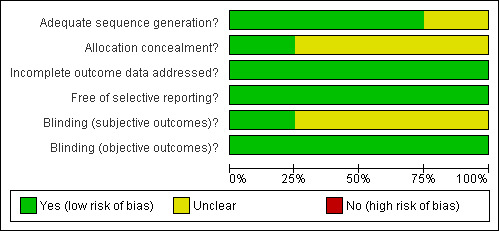
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
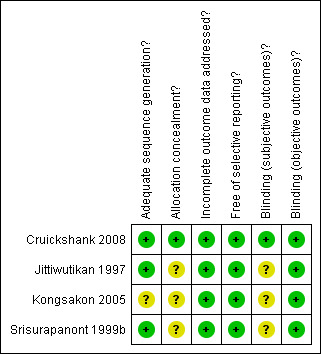
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
One study was judged to be at low risk of bias and three studies were judged to be at unclear risk of bias.
Blinding
For objective outcomes (discontinuation rates), blindness of participants, personnel, and outcome assessors differed across studies. Because we judged that the objective outcome was unlikely to be influenced by the lack of blinding, all studies were determined to be at low risk of bias.
For subjective outcomes (global state, craving, and withdrawal symptoms), blindness of participants, personnel, and outcome assessors were conducted in only one study and was determined to be at low risk of bias. In two studies, it was unclear if the outcome assessors were blinded in addition to the participants and personnel. Hence, we judged them to be at unclear risk of bias. One study did not specify any method of blinding and was determined to be at unclear risk of bias.
Incomplete outcome data
All studies were judged to be at low risk of bias. Three studies performed a last observation carried forward method of intent‐to‐treat analysis and one study had a drop out rate that was balanced across intervention groups.
Selective reporting
All included studies were judged as being low risk of bias. See risk of bias tables in the "Characteristics of included studies" table.
Effects of interventions
Four studies met the criteria to be included in this review (Srisurapanont 1999b; Jittiwutikan 1997; Kongsakon 2005; Cruickshank 2008). The results were summarized, with comparison of quantitative data where possible, between any pharmacological treatments (amineptine, mirtazapine) and placebo at the end of the 14‐day medication period. Outcomes were reported based on available data.
(1) Discontinuation rate
In all four studies involving 125 participants, the discontinuation rate was defined as the number of participants who did not complete the study. Overall, a significant difference in discontinuation rates was observed between treatment and placebo groups (RR 0.52, 95% CI 0.29 to 0.94) (Analysis 1.1). The results indicated that participants receiving any active treatment, but particularly amineptine, were significantly less likely to discontinue the study as compared to those receiving placebo. Hetereogeneity among the four studies was considered moderate but not statistically significant (I2=45%, p=0.14). The moderate level of heterogeneity among the studies was related to the treatment medication used (amineptine versus mirtazapine). See Figure 4
1.1. Analysis.
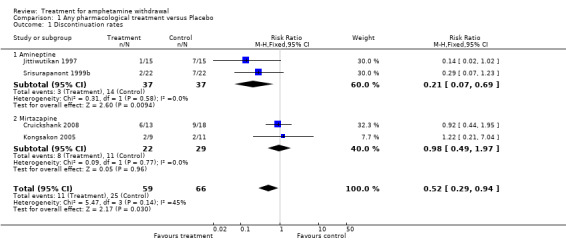
Comparison 1 Any pharmacological treatment versus Placebo, Outcome 1 Discontinuation rates.
4.
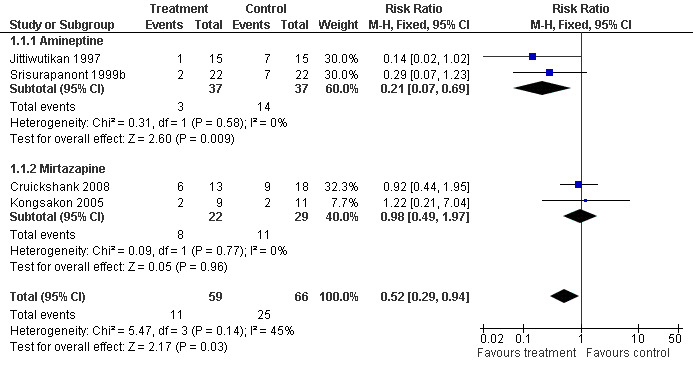
Forest plot of comparison: 1 Any pharmacological treatment versus Placebo, outcome: 1.1 Discontinuation rates.
(2) Average score in global state
The average score in global state was reported in three of the four included studies. Two studies (Srisurapanont 1999b; Jittiwutikan 1997) used the Clinical Global Impression or CGI (Guy 1976) and one (Cruickshank 2008) used the Brief Symptom Inventory Global Severity Index sub scale or BSI‐GSI (Derogatis 1993) to measure global state. For both CGI and BSI‐GSI, a higher score indicates greater severity.
The data from the three studies, involving 103 participants, were significantly heterogenous (I2=69%, p=0.04). The heterogeneity was most likely related to the difference in treatment medication (amineptine versus mirtazapine) and measurements used (CGI versus BSI‐GSI) among the studies, therefore a combined result was not calculated.
However, in a subgroup analysis between amineptine and placebo, the Weighted Mean Difference (WMD) was ‐0.49 (95% CI ‐0.80 to ‐0.17), showing that participants receiving amineptine was significantly more likely to improve in global state than placebo (Analysis 1.2). No difference was found between mirtazapine and placebo (WMD 0.30, 95% CI ‐0.22 to 0.82) based on the data of one study (Cruickshank 2008). Kongsakon 2005 did not report data on global state, hence it was not included in the analysis. See Figure 5
1.2. Analysis.
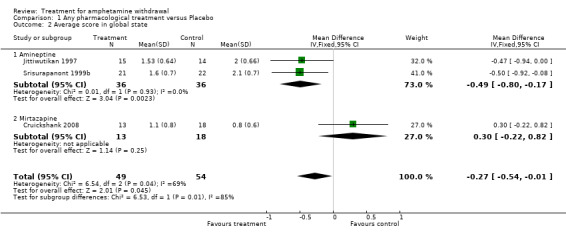
Comparison 1 Any pharmacological treatment versus Placebo, Outcome 2 Average score in global state.
5.
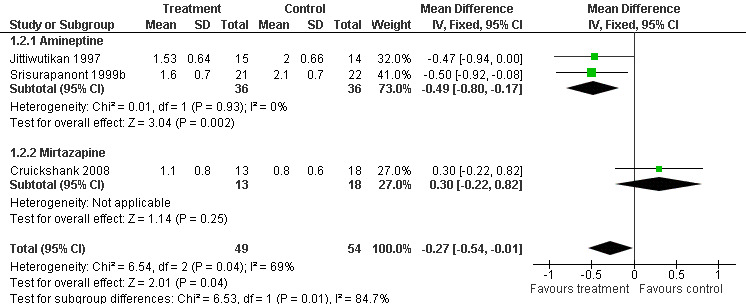
Forest plot of comparison: 1 Any pharmacological treatment versus Placebo, outcome: 1.2 Average score in global state.
(3) Average score in withdrawal symptoms
Although three studies reported data on withdrawal symptoms, only two studies involving 74 participants were included in the analysis (Srisurapanont 1999b; Cruickshank 2008). Data from Kongsakon 2005 for this specific outcome were not used because only median withdrawal scores were reported and means and standard deviations were needed for the comparison. However, the study did report significant improvements in amphetamine withdrawal symptoms as measured by the Amphetamine Withdrawal Questionnaire (AWQ) in the mirtazapine group versus placebo.
Srisurapanont 1999b used the AWQ, which is a 10‐item, self‐administered instrument based on the DSM‐IV withdrawal criteria (Srisurapanont 1999a). Cruickshank 2008, on the other hand, used the Amphetamine Cessation Symptoms Assessment or ACSA (McGregor 2008b) to measure withdrawal symptoms. The ACSA is a 16‐item, self‐administered instrument including 9 of the 10 items from the AWQ and 13 of the 18 items from a modified Cocaine Selective Severity Assessment (the word “cocaine” was replaced with “amphetamines”). For both the AWQ and ACSA, higher numbers indicate greater withdrawal symptom severity.
The Standardised Mean Difference (SMD) for the two studies involving 74 participants was ‐0.08 (95% CI ‐0.54 to 0.38), showing no difference between treatment group and placebo in reducing withdrawal symptoms (Analysis 1.3). SeeFigure 6
1.3. Analysis.
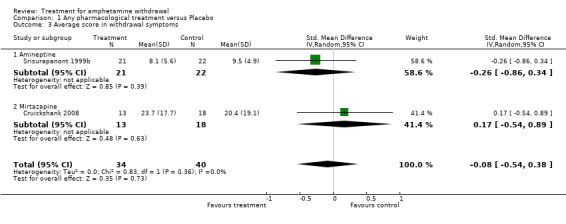
Comparison 1 Any pharmacological treatment versus Placebo, Outcome 3 Average score in withdrawal symptoms.
6.
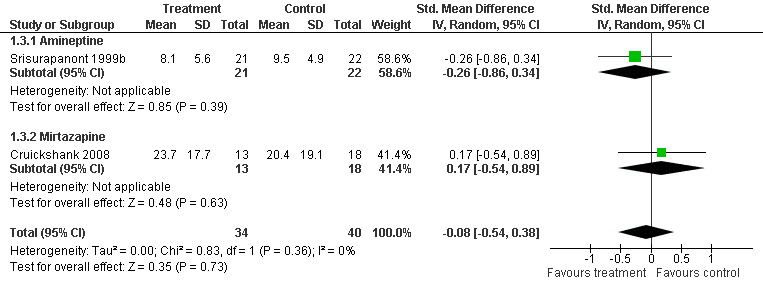
Forest plot of comparison: 1 Any pharmacological treatment versus Placebo, outcome: 1.3 Average score in withdrawal symptoms.
(4) Average score in craving
One study (Jittiwutikan 1997), involving 29 participants, reported data on craving using the Questionnaire for Evaluating Cocaine Craving and Related Responses or QECCRR (Voris 1991). The QECCRR was developed to separately measure four respects of cocaine withdrawal, including craving, depressed mood, no energy and sick feeling. Only the craving score of QECCRR was used in the analysis. For this measure, a high score indicates less severity in craving. The WMD was 0.43 (95% CI ‐1.23 to 2.09) and was not statistically significant (Analysis 1.4). See Figure 7
1.4. Analysis.
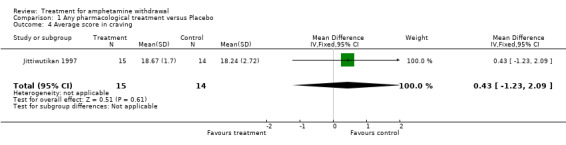
Comparison 1 Any pharmacological treatment versus Placebo, Outcome 4 Average score in craving.
7.
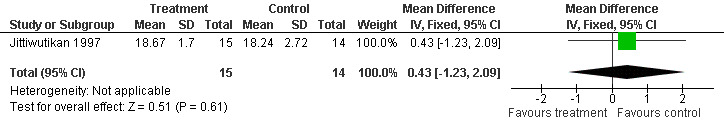
Forest plot of comparison: 1 Any pharmacological treatment versus Placebo, outcome: 1.4 Average score in craving.
In summary, the results showed some benefits of amineptine in the treatment of amphetamine withdrawal, as seen in the discontinuation rate and improvements in the global state as measured by CGI. There were no direct benefits of amineptine on withdrawal symptoms or craving. Mirtazapine was no more effective than placebo in terms of discontinuation rate, improvements on global state, and reduction in withdrawal symptoms based on the results of one study (Cruickshank 2008).
Discussion
Amphetamine withdrawal occurs commonly among amphetamine users and has clinical relevance as the symptoms may prompt relapse to amphetamine use as a means of symptom relief. Yet few well controlled studies have examined pharmacologic treatments for amphetamine withdrawal. To date, only amineptine and mirtazapine have been studied for treating this condition using placebo‐controls, blinding and randomisation. Amineptine is a central stimulant and dopamine reuptake inhibitor with biochemical and pharmacological effects similar to those of amphetamine (Samanin 1977). Amineptine was initially used as an antidepressant in France; availability of amineptine was limited in other countries. Amineptine was voluntarily withdrawn from the market in 1999 due to reports of amineptine abuse. Mirtazapine is an antidepressant with a relatively good tolerance and safety profile. It has been approved by the U.S. Food and Drug Administration and is commonly used to treat moderate to severe depression. Mirtazapine is a tetracyclic piperazinoazepine that enhances central noradrenergic and serotonergic activity by blocking alpha2 receptors and selectively antagonizing 5HT2 and 5HT3 receptors (De Boer 1996). Mirtazapine has also shown to improve suicidal ideation, to show relatively few side effects, and to show little abuse potential. The results of this review suggest that amineptine has some limited benefits in increasing the adherence to treatment and improving general condition but has no direct benefit on specific amphetamine withdrawal symptoms or craving. As amineptine has been withdrawn from the market, additional studies and clinical development of amineptine for amphetamine withdrawal are not warranted. We found no effect for mirtazapine on adherence to treatment, general condition, amphetamine withdrawal symptoms, or cravings. However, this result was based on data of one study (Cruickshank 2008), as the mirtazapine study by Kongsakon 2005 met criteria for inclusion, but their data could not be included due to differences in study methodology. In summary, there are currently no available medications that have been demonstrated to be effective in the treatment of amphetamine withdrawal.
The high prevalence (about 87%) of amphetamine withdrawal in amphetamine users (Cantwell 1998, Schuckit 1999) suggests that clinical trials of potential medications for the treatment of amphetamine withdrawal are needed. Additional clinical studies assessing the natural history of amphetamine withdrawal, the role these symptoms play in relapse to amphetamine use, as well as the validity and reliability of clinical measures to assess amphetamine withdrawal, are also needed. Medications that should be considered for evaluation in future clinical trials include those that increase dopamine, norepinephrine and/or serotonin activities of the brain. Naturalistic studies of amphetamine withdrawal symptoms and course are also crucial for the development of study designs appropriate for further treatment studies of amphetamine withdrawal.
Authors' conclusions
Implications for practice.
The evidence about the treatment for amphetamine withdrawal is very limited. Although amineptine has limited benefits for amphetamine withdrawal, this drug has been withdrawn from the market. Mirtazapine has not been shown to be effective for amphetamine withdrawal, although the number of studies is limited. At present, there is no evidence to guide selection of medications that might relieve symptoms of amphetamine withdrawal for patients in initial abstinence from chronic amphetamine use.
Implications for research.
While there are few medications that have been evaluated, amphetamine withdrawal seems a reasonable target for developing a medication to aid individuals in instilling amphetamine abstinence. Chronic amphetamine abusers seeking treatment must successfully resolve amphetamine withdrawal when establishing sustained abstinence from the drug. It remains unknown whether improved outcomes in successfully resolving amphetamine withdrawal would also correspond with longer term abstinence outcomes.
There is good reason to consider medications for amphetamine withdrawal that demonstrate the propensities to increase central dopamine, norepinephrine and/or serotonin activities. Naturalistic studies of amphetamine withdrawal symptoms and course are also crucial for the development of study designs appropriate for further treatment studies of amphetamine withdrawal.
What's new
| Date | Event | Description |
|---|---|---|
| 16 February 2009 | New search has been performed | updated and conclusions changed |
| 16 February 2009 | New citation required and conclusions have changed | substantially updated |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 25 May 2008 | New citation required and major changes | Substantive amendment |
| 10 May 2008 | Amended | Converted to new review format. Updated and new citation. |
Acknowledgements
Dr. Robert Ali was the contact editor of this update. We wish to thank Dr. Linda Gowing for her guidance and support throughout the review process, Mr. Gregory Victorianne for his assistance in retrieving research articles, and Ms. Suzana Mitrova for her coordination assistance. We also want to thank Dr. Silvia Minozzi and other members of the Cochrane Drug and Alcohol Group for their invaluable comments on the review.
Special thanks to Drs. Manit Srisurapanont, Phunnapa Kittirattanapaiboon, and Ngamwong Jarusuraisin for developing and writing the original protocol and report for the review. We also want to acknowledge Vanna Pistotti, Marica Ferri, and Meredith Cameron for all of their contributions on the earlier reports of this review.
Appendices
Appendix 1. MEDLINE search strategy
randomized controlled trial.pt.
controlled trial.pt.
randomized controlled trials/
controlled clinical trials/
random$.ti,ab.
Double‐blind method/ or Random allocation/
single blind method/
((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$).mp.[mp=title, abstract, registry number word, mesh subject heading]
clinical trial.pt.
clinical trials/
(clinical adj trial$).ti,ab.
placebos/
placebo$,ti,ab.
research design/
exp evaluation studies/
follow‐up studies/
follow up.ti,ab.
prospective studies/
(control$ or prospectiv$ or volunteer$).ti,ab.
or/1‐19
amphetamine/ or dextroamphetamine/ or methamphetamine/
(amphetamine or methamphetamine or dextroamphetamine).ti,ab.
21 or 22
exp substance‐related disorders/dt,px,rh,th [Drug Therapy, Psychology, Rehabilitation, Therapy]
20 and 23 and 24
limit 25 to human
Appendix 2. CINAHL search strategy
amphetamines/
amphetamine/
dextroamphetamine/
methamphetamine/
or/1‐2
dependence/
abuse/
psychosis
withdrawal
or/6‐9
5 and 10
Appendix 3. PsycINFO search strategy
exp Clinical Trials/
exp Drug Therapy/
exp Longitudinal Studies/
prospective studies/
controlled study.mp.
exp Followup Studies/
random$ trial$.mp.
controlled trial$.mp.
randomized controlled trial.mp.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
exp AMPHETAMINE/
exp METHAMPHETAMINE/
exp Dextroamphetamine/
11 or 12 or 13
exp Drug Abuse/
exp Drug Dependency/
15 or 16
exp Drug Withdrawal/
exp PSYCHOSIS/
17 or 18 or 19
14 and 20
10 and 21
limit 22 to human (135)
Data and analyses
Comparison 1. Any pharmacological treatment versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation rates | 4 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.94] |
| 1.1 Amineptine | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.69] |
| 1.2 Mirtazapine | 2 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.97] |
| 2 Average score in global state | 3 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.54, ‐0.01] |
| 2.1 Amineptine | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.80, ‐0.17] |
| 2.2 Mirtazapine | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.22, 0.82] |
| 3 Average score in withdrawal symptoms | 2 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.54, 0.38] |
| 3.1 Amineptine | 1 | 43 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.86, 0.34] |
| 3.2 Mirtazapine | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.54, 0.89] |
| 4 Average score in craving | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐1.23, 2.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cruickshank 2008.
| Methods | Randomised, double‐blind, 14‐day study. Recruited from two public inner‐city drug and alcohol outpatient clinics. | |
| Participants | 31 (20 males, 11 females) who met DSM‐IV criteria for amphetamine dependence; mean age = 31 years | |
| Interventions | (1) mirtazapine 15 mg nocte for 2 days followed by 30 mg nocte for 12 days (n = 13) (2) placebo (n = 18). Narrative therapy counseling was offered to both groups. | |
| Outcomes | Discontinuation rate, ACSA score, BSI‐GSI score, Athens Insomnia Scale, DASS, Severity of Dependence scale, Opiate Treatment Index Drug Use subscale | |
| Notes | Intent‐to‐treat analysis. Country of origin: Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomisation was conducted independently by pharmacists at each site using the service of Randomization.com" |
| Allocation concealment? | Low risk | "Randomisation was conducted independently by pharmacists at each site using the service of Randomization.com" |
| Incomplete outcome data addressed? All outcomes | Low risk | Missing outcome data were similar across groups. |
| Free of selective reporting? | Low risk | |
| Blinding (subjective outcomes)? All outcomes | Low risk | "Clinical staff, research staff and participants were blinded to the outcome of the randomisation." |
| Blinding (objective outcomes)? All outcomes | Low risk | "Clinical staff, research staff and participants were blinded to the outcome of the randomisation." |
Jittiwutikan 1997.
| Methods | Randomised, double‐blind, 14‐day study. Recruited from the inpatients of a drug dependence treatment center. | |
| Participants | 30 (29 males, 1 females) who met DSM‐IV criteria for amphetamine withdrawal; mean age = 18.5 years; mean duration of amphetamine use = 23.6 months; mean duration of amphetamine abstinence = 57.7 hours | |
| Interventions | (1) amineptine 300 mg/day (n = 15) (2) placebo (n = 15); trial medication was given in 1‐3 capsules/day. Dose titration done during first 5 days. Occasional use of lorazepam in patients with anxiety or insomnia | |
| Outcomes | Discontinuation rate, QECCRR score, CGI score | |
| Notes | Used a last observation carried forward method for intent‐to‐treat analysis. Country of origin: Thailand |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "the investigators . . .randomly assigned either amineptine or placebo to the subjects." "Block randomization was applied by the use of tossing‐a‐coin technique" |
| Allocation concealment? | Unclear risk | "the investigators . . .randomly assigned either amineptine or placebo to the subjects." Method of allocation concealment not specified. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome analysis was conducted using a last observation carried forward method of intent‐to‐treat analysis. |
| Free of selective reporting? | Low risk | |
| Blinding (subjective outcomes)? All outcomes | Unclear risk | "To blind the subjects and raters, the investigators filled either 100 mg of amineptine or placebo into an unmarked, identical capsule." Unclear of investigators' role with study subjects or raters. Insufficient information to determined risk of bias. |
| Blinding (objective outcomes)? All outcomes | Low risk | "To blind the subjects and raters, the investigators filled either 100 mg of amineptine or placebo into an unmarked, identical capsule." Unclear of investigators' role with study subjects or raters. Insufficient information to determined risk of bias. |
Kongsakon 2005.
| Methods | Randomised, double‐blind, 14‐day study. Recruited from a probation center. | |
| Participants | 20 males who met DSM‐IV criteria for amphetamine dependence; mean age = 24.3 years | |
| Interventions | (1) mirtazapine 15‐60 mg/day with an initial dose of 15 mg (n = 9) (2) placebo (n = 11); dose titrations during the study was based on subjects clinical response to medication | |
| Outcomes | Discontinuation rate, AWQ score, MADRS score | |
| Notes | Used a last observation carried forward method for intent‐to‐treat analysis. Country of origin: Thailand |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Twenty cases . . .were enrolled and randomized." |
| Allocation concealment? | Unclear risk | "Twenty cases . . .were enrolled and randomized." Method of allocation concealment not specified. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome analysis was conducted using a last observation carried forward method of intent‐to‐treat analysis. |
| Free of selective reporting? | Low risk | |
| Blinding (subjective outcomes)? All outcomes | Unclear risk | Blindness of participants, personnel, and assessors were not specified. |
| Blinding (objective outcomes)? All outcomes | Low risk | Blindness of participants, personnel, and assessors were not specified. |
Srisurapanont 1999b.
| Methods | Randomised, double‐blind, 14‐day study. Recruited from the inpatients of a drug dependence treatment center. | |
| Participants | 44 (41 males, 3 females) who met DSM‐IV criteria for amphetamine withdrawal and had AWQ score = 10 or more; mean age = 19.6 years; mean duration of amphetamine use = 23.5 months; mean duration of amphetamine abstinence = 52.7 hours | |
| Interventions | (1) amineptine 300 mg/day (n = 22) (2) placebo (n = 22); occasional use of lorazepam in patients with anxiety or insomnia | |
| Outcomes | Discontinuation rate, AWQ score, CGI score | |
| Notes | Used a last observation carried forward method for intent‐to‐treat analysis. Country of origin: Thailand |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Blocked randomisation by using tossing‐a‐coin technique" |
| Allocation concealment? | Unclear risk | "Blocked randomisation by using tossing‐a‐coin technique" Method of allocation concealment not specified. |
| Incomplete outcome data addressed? All outcomes | Low risk | Outcome analysis was conducted using a last observation carried forward method of intent‐to‐treat analysis. |
| Free of selective reporting? | Low risk | |
| Blinding (subjective outcomes)? All outcomes | Unclear risk | "To ensure blinding of the patients and raters, either 100 mg amineptine or placebo was enclosed within an unmarked, identical capsule." Blindness of outcome assessor not specified. |
| Blinding (objective outcomes)? All outcomes | Low risk | "To ensure blinding of the patients and raters, either 100 mg amineptine or placebo was enclosed within an unmarked, identical capsule." Blindness of outcome assessor not specified. |
Abbreviation: AWQ = Amphetamine Withdrawal Questionnaire, CGI = Clinical Global Impression, MADRS = Montgomery‐Asberg Depression Rating Scale, ACSA=Amphetamine Cessation Symptoms Assessment, BSI‐GSI = Brief Symptom Inventory Global Severity Index, DASS = Depression‐Anxiety‐Stress Scale, and QECCRR = Questionnaire for Evaluating Cocaine Craving and Related Responses
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chan‐Ob 2001 | Excluded as study design were not in the inclusion criteria: not RCT, but a case report. |
| Cox 2004 | Excluded as study design were not in the inclusion criteria: not RCT, but an open label case series report |
| Gillin 1994 | Excluded for the type of participants not in the inclusion criteria: majority of the participants were cocaine dependent. |
| McGregor 2005 | Excluded as the objective and study design were not in the inclusion criteria: not RCT, but an observational study characterizing the natural history of methamphetamine withdrawal. |
| McGregor 2008a | Excluded as the objective and study design were not in the inclusion criteria: not RCT, used historical comparison group and enrolled subjects sequentially rather than randomly allocated. |
Contributions of authors
Steven Shoptaw and Uyen Kao conducted the article searches, study selection, data extraction, data analysis, and write up of updated review. Keith Heinzerling provided feedback on the data analysis and assisted in writing the discussion section. Walter Ling provided overall guidance and assisted in writing the report.
*Manit Srisurapanont, Phunnapa Kittirattanapaiboon, and Ngamwong Jarusuraisin were involved in the protocol development and writing of the original review.
Sources of support
Internal sources
UCLA Integrated Substance Abuse Programs, USA.
External sources
No sources of support supplied
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Cruickshank 2008 {published and unpublished data}
- Cruickshank CC, Montebello ME, Dyer KR, Quigley A, Blaszczyk J, Tomkins S, Shand D. A placebo‐controlled trial of mirtazapine for the management of methamphetamine withdrawal. Drug Alcohol Review 2008;27(3):326‐33. [DOI] [PubMed] [Google Scholar]
Jittiwutikan 1997 {published data only}
- Jittiwutikan J, Srisurapanont M, Jarusuraisin N. Amineptine in the treatment of amphetamine withdrawal: a placebo‐controlled, randomised, double‐blind study. Journal of the Medical Association of Thailand 1997;80(9):587‐91. [PubMed] [Google Scholar]
Kongsakon 2005 {published data only}
- Kongsakon R, Papadopoulos K, Saguansiritham R. Mirtazapine in amphetamine detoxification: a placebo‐controlled pilot study. International Clinical Psychopharmacology 2005;20(5):253‐256. [DOI] [PubMed] [Google Scholar]
Srisurapanont 1999b {published and unpublished data}
- Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: II. A placebo‐controlled, randomised, double‐blind study of amineptine treatment. Australian and New Zealand Journal of Psychiatry 1999b;33:94‐98. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chan‐Ob 2001 {published data only}
- Chan‐Ob T, Kuntawongse N, Boonyanaruthee V. Bupropion for amphetamine withdrawal syndrome. Journal of the Medical Association of Thailand 2001;84(12):1763‐5. [PubMed] [Google Scholar]
Cox 2004 {published data only}
- Cox D, Bowers R, McBride A. Reboxetine may be helpful in the treatment of amphetamine withdrawal. British Journal of Clinical Pharmacology 2004;58(1):100‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillin 1994 {published data only}
- Gillin JC, Pulvirenti L, Withers N, Golshan S, Koob G. The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. Biological Psychiatry 1994;35(11):843‐9. [DOI] [PubMed] [Google Scholar]
McGregor 2005 {published data only}
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005;100(9):1320‐9. [DOI] [PubMed] [Google Scholar]
McGregor 2008a {published data only}
- McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: A comparison of mirtazapine and modafinil with treatment as usual. Journal of Substance Abuse Treatment 2008a;35:334‐342. [PUBMED: 18329221 Epub March 6 2008] [DOI] [PubMed] [Google Scholar]
Additional references
Cantwell 1998
- Cantwell B, McBride AJ. Self detoxification by amphetamine dependent patients: a pilot study. Drug and Alcohol Dependence 1998;49:157‐63. [DOI] [PubMed] [Google Scholar]
De Boer 1996
- Boer T. The pharmacologic profile of mirtazapine. Journal of Clinical Psychiatry 1996;57((supple 4)):19‐25. [PubMed] [Google Scholar]
Derogatis 1993
- Derogatis LR. Brief symptom inventory administration, scoring, and proedures manual. Brief symptom inventory administration, scoring, and procedures manual. Minneapolis: The Psychological Corporation, National Computer Systems, Inc., 1993. [Google Scholar]
DSM‐IV‐TR 2000
- DSM‐IV‐TR. Diagnositic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Gossop 1982
- Gossop MR, Bradley BP, Brewis RK. Amphetamine withdrawal and sleep disturbance. Drug and Alcohol Dependence 1982;10(2‐3):177‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU assessment manual for psychopharmacology, revised. Rockville, Maryland: National Institute of Mental Health, 1976. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0. The Cochrane Collaboration 2008:Available from www.cochrane‐handbook.org.
Hofmann 1983
- Hofmann FG. A handbook on drug and alcohol abuse: the biomedical aspects. 2nd Edition. New York: Oxford University Press, 1983. [Google Scholar]
Lee 2008
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev 2008;27(3):309‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGregor 2008b
- McGregor C, Srisurapanont M, Mitchell A, Longo MC, Cahill S, White JM. Psychometric evaluation of the Amphetamine Cessation Symptom Assessment. Journal of Substance Abuse Treatment 2008b;34(4):443‐9. [PUBMED: 17629443 Epub 13 July 2007] [DOI] [PubMed] [Google Scholar]
Meredith 2005
- Meredith CW, Jaffe C, Ang‐Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harvard Review of Psychiatry 2005;13(3):141‐54. [DOI] [PubMed] [Google Scholar]
Newton 2004
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. American Journal on Addictions 2004;13(3):248‐55. [DOI] [PubMed] [Google Scholar]
Pasic 2007
- Pasic J, Russo JE, Ries RK, Roy‐Byrne PP. Methamphetamine users in the psychiatric emergency services: A case‐control study. Am J Drug Alcohol Abuse 2007;33(5):675‐86. [DOI] [PubMed] [Google Scholar]
Rawson 2002
- Rawson RA, Gonzales R, Brethen P. Treatment of methamphetamine use disorders: an update. Journal of Substance Abuse Treatment 2002;23(2):145‐50. [DOI] [PubMed] [Google Scholar]
Rothman 2007
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. American Association of Pharmaceutical Scientists Journal 2007;9(1):E1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samanin 1977
- Samanin R, Jori A, Bernasconi S, Morpugo E, Garattini S. Biochemical and pharmacological studies on amineptine (S 1694) and (+)‐amphetamine in the rat. Journal of Pharmacy and Pharmacology 1977;29:555‐558. [DOI] [PubMed] [Google Scholar]
Schuckit 1999
- Schuckit MA, Daeppen J‐B, Danko GP, et al. Clinical implications for four drugs of the DSM‐IV distinction between substance dependence with and without a physiological component. American Journal of Psychiatry 1999;156:41‐9. [DOI] [PubMed] [Google Scholar]
Scott 2007
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta‐analysis. Neuropsychology Review 2007;17(3):275‐97. [DOI] [PubMed] [Google Scholar]
Srisurapanont 1999a
- Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: I. reliability, validity and factor structure of a measure. Australian and New Zealand Journal of Psychaitry 1999a;33:89‐93. [DOI] [PubMed] [Google Scholar]
UNODC 2008
- United Nations Office on Drugs and Crime. World Drug Report 2008. www.unodc.org/documents/wdr/WDR_2008/WDR_2008_eng_web.pdf. Accessed on 9‐07‐08.
Voris 1991
- Voris J, Elder I, Sebastian P. A simple test of cocaine craving and related responses. Journal of Clinical Psychology 1991;47:320‐3. [DOI] [PubMed] [Google Scholar]
Watson 1972
- Watson R, Hartmann E, Schildkraut JJ. Amphetamine withdrawal: affective state, sleep patterns, and MHPG excretion. American Journal of Psychiatry 1972;129(3):263‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Srisurapanont 2001
- Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon P. Treatment for amphetamine withdrawal. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI] [PubMed] [Google Scholar]


