Abstract
Background
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the West. Oral steroids are the standard treatment.This is an update of the review published in 2005.
Objectives
To assess treatments for bullous pemphigoid.
Search methods
In August 2010 we updated our searches of the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (Clinical Trials), MEDLINE, EMBASE, and the Ongoing Trials registers.
Selection criteria
Randomised controlled trials of treatments for participants with immunofluorescence‐confirmed bullous pemphigoid.
Data collection and analysis
At least two authors evaluated the studies for the inclusion criteria, and extracted data independently.
Main results
We included 10 randomised controlled trials (with a total of 1049 participants) of moderate to high risk of bias. All studies involved different comparisons, none had a placebo group. In 1 trial plasma exchange plus prednisone gave significantly better disease control at 1 month (0.3 mg/kg: RR 18.78, 95% CI 1.20 to 293.70) than prednisone alone (1.0 mg/kg: RR 1.79, 95% CI 1.11 to 2.90), while another trial showed no difference in disease control at 6 months.
No differences in disease control were seen for different doses or formulations of prednisolone (one trial each), for azathioprine plus prednisone compared with prednisone alone (one trial), for prednisolone plus azathioprine compared with prednisolone plus plasma exchange (one trial), for prednisolone plus mycophenolate mofetil or plus azathioprine (one trial), for tetracycline plus nicotinamide compared with prednisolone (one trial). Chinese traditional medicine plus prednisone was not effective in one trial.
There were no significant differences in healing in a comparison of a standard regimen of topical steroids (clobetasol) with a milder regimen (RR 1.00, 95% 0.97 to 1.03) in one trial. In another trial, clobetasol showed significantly more disease control than oral prednisolone in people with extensive and moderate disease (RR 1.09, 95% CI 1.02 to 1.17), with significantly reduced mortality and adverse events (RR 1.06, 95% CI 1.00 to 1.12).
Authors' conclusions
Very potent topical steroids are effective and safe treatments for BP, but their use in extensive disease may be limited by side‐effects and practical factors. Milder regimens (using lower doses of steroids) are safe and effective in moderate BP. Starting doses of prednisolone greater than 0.75 mg/kg/day do not give additional benefit, lower doses may be adequate to control disease and reduce the incidence and severity of adverse reactions. The effectiveness of adding plasma exchange, azathioprine or mycophenolate mofetil to corticosteroids, and combination treatment with tetracycline and nicotinamide needs further investigation.
Plain language summary
Treatments for bullous pemphigoid
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the West. Incidence figures are not available for most parts of the world but BP appears to be rarer in the Far East. Bullous pemphigoid is usually a disease of the elderly but it can also affect younger people and children. Both sexes are similarly affected. While BP usually resolves within five years, there is a moderate death rate associated with the disease and its treatment. Oral corticosteroid drugs are the most common treatment, but may be associated with serious adverse effects, including some deaths. The most common adverse effects of oral steroids, include weight gain and high blood pressure. Long‐term use is associated with an increased risk of diabetes mellitus and decreased bone density. Topical steroids are also associated with adverse effects, such as thinning of the skin and easy bruising. The risk of experiencing adverse effects of topical steroids depends on the strength of the steroid, how long it is used for, which area of the body it is applied to, and the kind of skin problem; if a high‐strength, potent steroid is used, enough may be absorbed through the skin to cause adverse effects in the rest of the body.
Other treatments include azathioprine, mycophenolate mofetil, dapsone, methotrexate, cyclosporin, cyclophosphamide, plasma exchange, erythromycin, and tetracycline and nicotinamide. Some of these drugs or interventions have the potential for severe adverse effects such as increased susceptibility to serious infections, liver and kidney damage, and bone marrow suppression; and many are very expensive.
Three new studies were included in this update of the review published in 2005 making a total of 10 randomised controlled trials with a total of 1049 participants. All studies involved different comparisons, none had a placebo group. Different doses and formulations of corticosteroids plus azathioprine showed no significant differences in disease control, although azathioprine reduced the amount of prednisone required for disease control. There were no significant differences in healing or disease‐free intervals in participants taking azathioprine compared with mycophenolate mofetil, or in disease response comparing tetracycline plus nicotinamide with prednisolone. One small study using Chinese traditional medicine, 'Jingui Shenqi Pill' (JSP), plus prednisone did not show any benefit in favour of adding this traditional Chinese herbal remedy. Most of the deaths were in participants taking high doses of oral corticosteroids.
The review of trials concluded that lower doses of oral steroids and strong steroid creams seem safe and effective. However, the use of steroid creams in extensive disease may be limited by side‐effects and the practicality of applying creams to large areas of the skin. Milder regimens of topical steroids are safe and effective in moderate BP. More research is needed on treatments for BP, in particular, the effectiveness of adding plasma exchange, azathioprine or mycophenolate mofetil to corticosteroids, and the treatment with tetracyclines and nicotinamide.
Background
Description of the condition
Definition and epidemiology
Bullous pemphigoid (BP) is an acquired autoimmune disorder in which disease‐specific autoantibodies are directed against components of the basement membrane zone of the skin (Morrison 1990; Wojnarowska 1998). It is the most common autoimmune blistering disease in the West with an estimated incidence of six to seven cases per million population per year in France and Germany (Bernard 1995; Zillikens 1995). The figures are probably similar or higher in the United Kingdom. Incidence figures are not available for most parts of the world but bullous pemphigoid appears to be rarer in the Far East (Adam 1992; Jin 1993; Tham 1998). Bullous pemphigoid is usually a disease of the elderly but it can also affect younger people and children (Kirtschig 1994; Orange 1989; Nemeth 1991). Both sexes are similarly affected.
Clinical picture
The characteristic clinical picture is the development of tense blisters, which may arise on inflamed skin or skin of normal appearance. This may be heralded by an urticarial or eczematous rash. The degree of itch varies from none to intense and may precede the appearance of blisters, which contain either clear or bloodstained fluid. The blisters are usually generalised on the body with a tendency to appear on the creases of the limbs. Localised forms also occur. Bullous pemphigoid may affect mucosal surfaces such as the mouth; scarring is usually not observed.
Investigation and diagnosis
The most reliable test to achieve a diagnosis is a skin biopsy for immunopathological investigation. A direct immunofluorescence technique (IF) (on skin) demonstrates deposits of IgG autoantibodies and complement (C3) at the dermo‐epidermal junction. Indirect IF (using serum) demonstrates circulating autoantibodies directed against basement membrane proteins (Morrison 1990; Wojnarowska 1998). When skin tissue is incubated in one molar sodium chloride, separation of the dermis from the epidermis occurs within the lamina lucida level of the basement membrane (visualised on electron microscopic examination). Immunofluorescence techniques performed on such split skin was first shown in the late 1980s to result in a more precise localisation of the antigen‐antibody‐binding site. This helps to separate other autoimmune bullous diseases such as epidermolysis bullosa acquisita and bullous systemic lupus erythematosus (in which fluorescence is at the floor of the blister: dermal binding) from BP (in which fluorescence is usually at the roof: epidermal binding) (Logan 1987). Immunoelectron microscopy and immunoblotting are more specific investigations and in some cases can lead to a change in the diagnosis (Kirtschig 1994). The latter investigations are not yet available for routine clinical use, being largely limited to research centres.
Natural history
The natural history of both treated and untreated BP is of a persistent disease with eventual remission occurring in the majority of cases. Remission is likely to occur within five years, although relapses and exacerbations may occur (Ahmed 1977; Hadi 1988; Nemeth 1991; Person 1977). The mortality rate in the initial 30 cases reported by Lever was 24% at 1 year. This was prior to the use of oral corticosteroids (Lever 1953). The mortality rate in other studies ranges from about 10% to 40% at 1 year (Colbert 2004; Venning 1992; Savin 1979; Savin 1987; Roujeau 1998; Gudi 2005), despite the use of topical and systemic treatments. This might suggest that treatment is at best suppressive (without really altering the prognosis of the disease) or at worst contributes to mortality (e.g. from sepsis secondary to immunosuppression) whilst relieving itch and preventing blisters. Savin suggested that death seemed to be more commonly related to underlying illness in the elderly, debilitation associated with severe illness, or the adverse effects of treatment. The study by Parker et al supports this view, they evaluated 223 participants with pemphigoid and compared mortality data with the general population in the United States (Parker 2008). There was no difference between pemphigoid participants and age‐matched controls in expected mortality. They conclude that mortality of participants with bullous pemphigoid is more likely related to advanced age and associated medical conditions than disease‐specific factors and treatment will not alter the natural disease history but the quality of life.
Description of the intervention
Current treatments include oral steroids (e.g. prednisone or prednisolone), azathioprine, mycophenolate mofetil, dapsone, methotrexate, cyclosporin, cyclophosphamide, plasma exchange, erythromycin, and tetracycline and nicotinamide. Some of these drugs or interventions have the potential for severe adverse effects such as increased susceptibility to serious infections, liver and kidney damage, and bone marrow suppression; and some are very expensive.
High‐potency topical steroids (clobetasol propionate cream) have been demonstrated to improve survival in patients with bullous pemphigoid (Joly 2002). These topical steroids may be safer and more effective than high‐dose oral corticosteroids for controlling BP and therefore may be suitable for treating those patients, often the elderly, who have a poor prognosis because they are at high risk of developing adverse effects with systemic treatments. Topical steroids are not without risk of adverse effects, both locally (increased susceptibility of the skin to damage like skin atrophy and bruising and infections of the skin) and systemically if enough steroid is absorbed through the skin, leading, for example, to Cushing syndrome with fluid retention, increased blood pressure and diabetes mellitus, and adrenal gland suppression.
Prednisone is an inactive drug precursor that is metabolised by the liver and converted to biologically active prednisolone. The two forms are virtually identical therapeutically and can be used interchangeably in many situations. As prednisone is rapidly converted to prednisolone, prednisolone may be preferred in some patients who have liver disease or some other metabolic disorder. There are some differences in the appearance and taste of the two formulations: prednisolone sodium phosphate is very soluble with a not unpleasant taste, whereas prednisone is bitter and poorly soluble. Some reports have suggested that the use of prednisone is preferable to prednisolone in the treatment of BP (Lebrun‐Vignes 1999) and this may account for differences in use of the drug, for example, in France. For the purposes of this review, prednisone and prednisolone are regarded as bio‐equivalent, however, for each of our included studies we have used the drug name which was quoted in the report of the study.
There are emerging reports of some BP cases being treated with biological therapies, in particular, anti‐CD20 monoclonal antibodies (rituximab) (Hertl 2008). CD20 is a molecule which is expressed on the surface of B lymphocytes (the immune cells which produce antibodies) including the autoantibodies which are directed at the skin in BP. Rituximab, a monoclonal antibody, binds specifically to this transmembranous CD20 antigen and the resulting lysis (breaking down) of the B lymphocyte is induced via a number of immune pathways. This limits the immune system's attack by depleting the number of B lymphocytes available to produce antibodies. Rituximab could be used either as an alternative to standard treatments for bullous pemphigoid, in patients that are refractory to standard treatment (Reguiaï 2009) or if the patient is unable to tolerate other treatments.
Why it is important to do this review
Mortality figures, based on uncontrolled studies, have not improved much since the introduction of systemic treatments. This may suggest that BP is a self‐limiting condition (occurring in older people with a higher mortality than the general population) and that the prognosis is not altered by treatment. It is also possible that improved skin care and medical support currently available,compared with the times of Lever (Lever 1953) do significantly lower the mortality rate and that this benefit is masked by the adverse effects of systemic treatments. However, this does not tell us about morbidity and the quality of life of these affected people and whether treatment alters the duration of the lesions. There is also variation in the long‐term toxicity of systemic agents ranging from very little (e.g. antibiotics) to a lot (e.g. prednisolone or cyclophosphamide). Use of very potent topical steroid treatment may be adequate in localised disease and has minimal side‐effects. There is wide variation in practice among clinicians as to which drugs or interventions are used and in what order or combinations.
This review aims to establish:
which are the most effective drugs or interventions, with the least adverse effects;
whether combination therapy (e.g. azathioprine plus steroids) offers any advantages over single drugs (e.g. oral steroids alone);
whether antibiotics such as tetracyclines, erythromycin, dapsone, or sulphonamides are useful; and
whether systemic treatment is better than topical or no treatment.
Objectives
To assess the effects of treatments for bullous pemphigoid.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
People of any age who have received treatment for a diagnosis of bullous pemphigoid, confirmed by immunofluorescence studies.
Types of interventions
Any therapeutic intervention used to treat bullous pemphigoid.
Types of outcome measures
Primary outcomes
(a) Regression or healing of the skin lesions at time periods specified by individual trials.
Secondary outcomes
(a) Effect on the quality of life, e.g. relief of soreness or itching.
(b) Duration of remissions after stopping treatment.
(c) Complications of the primary disease (BP), e.g. localised skin infection.
(d) Systemic infection.
(e) Adverse effects of treatment:
i) organ failure
ii) allergic reactions.
f) Mortality.
Search methods for identification of studies
Electronic searches
We searched the following databases up to 10th August 2010:
The Cochrane Skin Group Specialised Register using the search terms in Appendix 1; and
The Cochrane Central Register of Controlled Trials (Clinical Trials) within The Cochrane Library using the search strategy in Appendix 2; and
MEDLINE and EMBASE using the strategies in Appendix 3 and Appendix 4 respectively.
Ongoing Trials
We searched the following Ongoing Trials registers in August 2010 using the term 'bullous pemphigoid'.
The metaRegister of Controlled Trials www.controlled‐trials.com.
The U.S. National Institutes of Health ongoing trials register www.clinicaltrials.gov.
The Australian and New Zealand Clinical Trials Registry www.anzctr.org.au.
The World Health Organization International Clinical Trials Registry platform www.who.int/trialsearch.
The Ongoing Skin Trials Register on www.nottingham.ac.uk/ongoingskintrials.
Searching other resources
References from published studies
We searched the bibliographies from identified studies.
Data collection and analysis
Selection of studies
We screened the abstracts of potentially relevant studies and obtained full articles if necessary. We assessed articles that were possible RCTs for eligibility using inclusion criteria outlined in the protocol. This was done independently by at least two authors (NP, PM, and GK).
Data extraction and management
We extracted details of eligible studies and summarised them using a data extraction sheet that was based on the outcome measures. Two authors (GK, PM) extracted data independently and subsequently checked for discrepancies. The data of the Chinese article was kindly extracted by Dr Ching‐Chi Chi from Taiwan. We had planned to resolve disagreements by discussion with the other authors (DM and FW), but this was not necessary because there were very few small studies.
Assessment of risk of bias in included studies
Risk of bias for the new studies identified by the updated search was independently assessed by two authors (CB and GK) and we resolved any differences by consensus.
We made an assessment of the risk of bias which includes an evaluation of the following components for each included study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) (details are available in the Characteristics of included studies ('Risk of bias') for each study):
(a) the method of generation of the randomisation sequence; (b) the method of allocation concealment ‐ it was considered 'adequate' if the assignment could not be foreseen; (c) who was blinded or not blinded (participants, clinicians, outcome assessors); and (d) how many participants dropped out of the study overall, and whether participants were analysed in the groups to which they were originally randomised (intention‐to‐treat analysis).
The original protocol of this review stated that the Jadad quality assessment scale would be used, which also similarly assesses randomisation, blinding, withdrawals, and dropouts (Jadad 1996). We assessed all these aspects but reported them individually (see Characteristics of included studies) rather than as a summary score.
Measures of treatment effect
We presented dichotomous measures as risk ratios (RR) with 95% confidence intervals (CI); and continuous measures were presented as mean differences with 95% CI.
Dealing with missing data
We contacted trial investigators to obtain missing data and clarify the specifics of the trial conditions when this was not clear to us from the published report of the trial.
Assessment of heterogeneity
For an assessment of heterogeneity we used the I² statistic. If we found moderate to high levels of heterogeneity (I² > 50%) for the primary outcomes, we explored the possible sources of heterogeneity.
Data synthesis
We had planned to divide data analysis into two groups: (a) trials where the diagnosis of BP was confirmed by immunofluorescence (IF) using intact skin; and (b) trials where split skin was used for IF (this procedures helps, although not completely, to distinguish true BP participants from those with other subepidermal immunobullous diseases), however, this was unnecessary as only one of the studies used IF on split skin (Beissert 2007).
We conducted a narrative synthesis of included trials, and present the characteristics of the trials and results in tables and figures. We were unable to pool data in a meta‐analysis as the studies were heterogenous especially in terms of the treatments used. We did, however, present some of the data in Review Manager 5 (RevMan) in the form of risk ratios and 95% confidence intervals for the results of single trials.
The adverse events are summarised in a separate table "Adverse events in the included studies; Table 11"; some columns are left empty, it would have been misleading to enter "zero" when a paper was silent about a particular adverse event, because we are not sure that all adverse events have been reported.
1. Adverse events in the included studies.
| Study ID | Drug and dose | Infection /Low WCC | Organ impairment | Cardiovascular | Other | Total adverse events | Death |
| Beissert 2007 | Oral methylprednisolone 0.5 mg/kg/day plus azathioprine 2 mg/kg/day (n = 38) | 1 | 7 (1 hyperglycaemia 6 liver) |
0 | 3 | 11 (grade 3/4) |
2 |
| Oral methylprednisolone 0.5 mg/kg/day plus mycophenolate mofetil 2000 mg twice/day (n = 35) | 4 | 6 (5 hyperglycaemia 1 liver) |
0 | 3 | 13 (grade 3/4) |
0 | |
| Burton 1978 | Prednisone 30 to 80 mg/kg/day (n = 13) | 1 | 1 | 3 | 5 | 4 | |
| Azathioprine 2.5 mg/kg + prednisone 30 to 80 mg/kg/day (n = 12) | 2 | 3 | 5 | 3 | |||
| Dreno 1993 | Prednisolone (average) 1.16 mg/kg/day (n = 29) | 1 | 1 | 1 | 3 | 0 | |
| Methylprednisolone (average) 1.17 mg/kg/day (n = 28) | 1 | 1 | 2 | 1 | 5 | 0 | |
| Fivenson 1994 | Prednisone 40 to 80 mg/kg/day (n = 6) | 2 | 3 | 2 | 1 | 8 | 1 |
| Tetracycline 500mg 4x/day + nicotinamide (n = 14) | 1 | 1 | 2 | 4 | 0 | ||
| Guillaume 1993 | Prednisolone 1 mg/kg/day (n = 31) | 10 | 5 | ||||
| Prednisolone 1 mg/kg/day + azathioprine 100 to 150 mg/day (n = 36) | 15 | 6 | |||||
| Prednisolone 1 mg/kg/day + plasma exchange (n = 31) | 6 | 3 | |||||
| Joly 2002 | Moderate disease:
topical steroids (n = 77) Prednisone 0.5 mg/kg/day (n = 76) |
11 16 | 5 14 | 15 16 | 31 46 | 23 23 | |
| Extensive disease: topical steroids (n = 93) Prednisone 1 mg/kg (n=95) | 8 22 | 6 23 | 16 20 | 30 65 | 22 39 P = 0.02 | ||
| Joly 2009 | Mild regimen topical steroids (n = 159) | 27 | 18 (DM) | 21 | 41% (skin) |
194 (grade 3/4) |
60 |
| Standard regimen topical steroids (n = 150) | 32 | 34 (DM) | 35 | 52% (skin) |
227 (grade 3/4) |
58 | |
| Liu 2006 | Jingui Shenqi Pill (JSP) 1# bid plus prednisone 0.5 to 1.0 mg/kg/day (n = 15) | not mentioned | not mentioned | ||||
| Prednisone alone 0.5 to 1.0 mg/kg/day (n = 15) | not mentioned | not mentioned | |||||
| Morel 1984 | Prednisolone 0.75 mg/kg/day (n = 26) | 1 | 2 | 3 | 2 | ||
| Prednisolone 1.25 mg/kg/day (n = 26) | 1 | 1 | 1 | 2 | 5 | 3 | |
| Roujeau 1984 | Prednisolone 0.3 mg/kg/day (n = 17) | 7 | 7 | 0 | |||
| Plasma exchange + prednisolone 0.3 mg/kg/day (n = 24) | 10 | 7 | 7 | 7 | 0 |
Results
Description of studies
Results of the search
We identified 73 abstracts from MEDLINE, 27 from EMBASE, and 7 from the Cochrane Central Register of Controlled Trials (Clinical Trials) at our initial searches. For this update we searched the Cochrane Skin Group Specialised Register, The Cochrane Library (Issue 1, 2009), MEDLINE, and EMBASE on 16th March 2009 and 31 abstracts were identified. Prior to publication we ran another search on 10th August 2010 of the listed databases but found no potentially eligible studies. We assessed 28 of these for eligibility and they were clearly either not RCTs or the participants did not have a diagnosis of bullous pemphigoid. Therefore as none of the studies appeared to meet the eligibility criteria on the surface, no studies appear in the excluded studies table. We included 3 new RCTs in this update (Beissert 2007; Joly 2009; Liu 2006), therefore there are 10 completed RCTs with a total of 1049 participants included in this review; details of all the studies are included in the Characteristics of included studies.
We identified seven ongoing trials from the above mentioned Ongoing Trial registers. We are aware that two of these are ongoing RCTs (ISRCTN13704604; NCT00809822). The remaining five trials will be assessed for eligibility when more details become available.
Included studies
Design
Six of the studies in this review were multicentre French studies (Dreno 1993; Guillaume 1993; Joly 2002; Joly 2009; Morel 1984; Roujeau 1984). Morel was a co‐author in two (Morel 1984; Roujeau 1984), Guillaume in three (Guillaume 1993; Roujeau 1984; Joly 2009), three trial authors (Crickx, Labeille, and Guillot) were in the same two trials (Roujeau 1984; Guillaume 1993), Dreno in three (Dreno 1993; Joly 2002; Joly 2009), and Roujeau in four studies (Guillaume 1993; Joly 2002; Roujeau 1984; Joly 2009). It is not clear if any of the studies included the same groups of participants.
Sample sizes
There were 1049 participants in total. There were 8 small studies (between 20 and 100 participants in each) and 2 larger RCTs including more than half of the participants (653) in this review (Joly 2002; Joly 2009).
Setting
Only four studies in this review were done in centres outside France. Burton 1978 was conducted in the UK, Fivenson 1994 in the USA, Liu 2006 in China, and Beissert 2007 in Germany. The studies were carried out in hospital settings, although it is unclear what the setting was in Liu 2006.
Participants
All participants had confirmed bullous pemphigoid (confirmed by immunofluorescence, except Liu 2006, in which this is unclear). The participants were older men and women (range of mean ages at baseline quoted in the included studies was 65.4 to 84.8 years of age).
Interventions
None of the studies included a placebo group. The interventions tested in the included studies included, oral steroids, with or without other interventions, topical steroids, and tetracycline and nicotinamide (versus prednisolone). All used different interventions with only five studies overlapping; therefore classification by intervention is intended to assist the reader, rather than to attempt to fit different interventions in to broad classification groups. A brief summary of the type of interventions used is presented below. Full details of each trial are given in the Characteristics of included studies.
Oral steroid with or without other interventions, including plasma exchange
Beissert 2007 used oral methylprednisolone plus azathioprine versus (vs) oral methylprednisolone plus mycophenolate mofetil; and Dreno 1993 administered prednisolone versus methylprednisolone. Morel 1984 looked at prednisolone at two doses (0.75 mg/kg versus 1.25 mg/kg). Liu 2006 compared a traditional Chinese medicine, 'Jingui Shenqi Pill' (JSP), plus prednisone (0.5 to 1.0 mg/kg/day) to prednisone alone (0.5 to 1.0 mg/kg/day). In Guillaume 1993 participants received prednisolone versus prednisolone and azathioprine, versus plasma exchange and prednisolone, and Roujeau 1984 also investigated plasma exchange and prednisolone. Burton 1978 compared azathioprine plus prednisone versus prednisone alone. We have used the drug names as reported in the included studies (i.e. prednisone or prednisolone); for the purposes of this review, prednisone and prednisolone are regarded as bio‐equivalent.
Topical steroid treatment
Joly 2002 used topical clobetasol propionate versus oral prednisolone, and in Joly 2009 investigated 2 different regimens of topical clobetasol propionate cream: 40 g clobetasol propionate cream/day versus a mild regimen of 10 to 30 g/day, depending on the body weight, were compared in a large, randomised study. The regimen was chosen according to disease severity.
Tetracycline and nicotinamide
Fivenson 1994 used prednisolone versus nicotinamide and tetracycline.
Outcomes
We specified a number of outcomes of interest for this review in Types of outcome measures. Our primary outcome of regression or healing of skin lesions was reported in all the included studies. Effects of the interventions on quality of life were reported in Dreno 1993, Guillaume 1993, and Roujeau 1984. The duration of remission after stopping treatment was reported in Beissert 2007 and Joly 2009.
Adverse effects were recorded in Beissert 2007, Joly 2002, and Joly 2009, while mortality was reported in Burton 1978, Joly 2002, and Joly 2009. The remaining review outcomes of complications of the primary disease and systemic infection were not reported in any of the included studies.
The reports of the included studies focused on a variety of outcomes, including disease control, survival, and cumulative steroid doses which are summarised as follows:
The outcomes reported in Beissert 2007 were complete healing (complete re‐epithelialisation of all lesions), and cumulative steroid dose. Secondary outcomes were duration of remission (disease‐free interval) and safety.
Dreno 1993 reported the number of blisters, intensity of erythema, and the intensity of pruritus (itch) at days five and ten.
Fivenson 1994 reported the number of bullous, crusted, urticarial lesions as the total highest score possible on each visit per participant.
Only Burton 1978 did not have clearly stated outcome measures. The following outcome measures were obtained from the published report, and include cumulative dose of prednisone in both groups necessary for disease control, mortality, and adverse effects, including whether azathioprine and prednisolone (synergistic immunosuppression) was associated with increased risk of malignancy.
Guillaume 1993 reported disease control in terms of blister formation, resolution of erythema, and no more than minimal pruritus at four weeks and six months after starting treatment.
Joly 2002 and Joly 2009 both reported survival after one year, disease control at three weeks, and occurrence of severe adverse events during the follow‐up year; Joly 2009 also reported occurrence of relapses during follow‐up and cumulative doses of steroid cream.
Liu 2006 reported compete healing at four weeks; Morel 1984 assessed new blister formation at days 21 and 51, and Roujeau 1984 assessed the cumulative and daily corticosteroid dose to achieve disease control in terms of blister formation. Other parameters of disease control were intensity of pruritus and extent of erythema and urticarial lesions.
Excluded studies
A search of the abstracts of potential RCTs for the initial published version of this review and for this update indicated that there were no studies that should be listed in the Characteristics of excluded studies table, e.g. no RCTs that dealt with closely related conditions.
Risk of bias in included studies
Allocation
Some attempt at randomisation was made in all of the studies. Randomisation was not described in detail by Burton 1978, Fivenson 1994, or Liu 2006.
Only Joly 2009 was assessed as having adequate randomisation as both sequence generation and allocation concealment were adequate.
It was not explicitly stated in Burton 1978 that the 25 participants were initially randomised, but this was implied in other sections of the article in that each participant was described as being assigned to treatment by the ward sister who drew a marked paper from an envelope. Since there are no details about how the envelopes were marked, the sequence generation was rated as unclear.
Morel 1984 randomised 50 participants using a table of numbers, but allocation concealment was unclear.
The study reported by Dreno 1993 was randomised, but the method was not described.
Similarly, in Fivenson 1994 randomisation was mentioned but the method used was not explained. A full translation of the study of Liu 2006 provided no details about the randomisation method used.
The prednisolone and plasma exchange versus prednisolone‐only study (Roujeau 1984) had an adequate method of sequence generation (computer‐generated), but allocation was unclear.
The Joly 2002 study on topical versus oral corticosteroids studies (Joly 2002), had an adequate sequence generation method, but seemed marginal for allocation concealment therefore we coded it as unclear.
A three‐arm study (Guillaume 1993) comparing the efficacy of azathioprine or plasma exchange when added to prednisolone used an adequate method of sequence generation (pre‐established lists). But Guillaume 1993 was also marginal regarding allocation concealment as it was not clear if the study co‐ordinator was or was not involved in the allocation process; therefore we have coded it as unclear.
Beissert 2007 had adequate sequence generation by centrally‐generated random numbers to receive oral methylprednisolone plus azathioprine or mycophenolate mofetil.
Blinding
Most of the studies had no blinding of either participants or outcome assessors (see Figure 1). Dreno 1993 employed a double‐blind method although the two products used as interventions were of a different appearance, the supply of the products to the participants was made by a person other than the investigator. Additionally clinical follow‐up after the end of the study was done by a blinded investigator .
1.
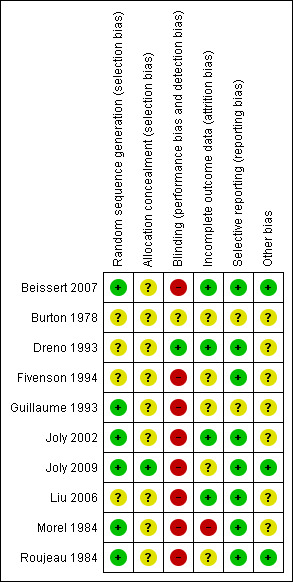
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Two regimens of very potent topical corticosteroids, a standard regimen of 40 g clobetasol propionate cream/day versus a mild regimen of 10 to 30 g/d depending on the body weight, were compared in a large, randomised study (Joly 2009). Blinding was not deemed necessary as the primary outcome was event‐free survival.
The studies by Burton 1978,Guillaume 1993, Fivenson 1994,Liu 2006, and Roujeau 1984 were also not blinded. In Guillaume 1993 blinding could be considered by some as unethical (this was given as a reason for not blinding in one study) because it would mean an invasive procedure (intravenous line) in the control group as well. Joly 2002 was not blinded, however, the primary outcome was survival at one year which was unlikely to be biased by lack of blinding (this was given as the reason for not blinding). However, assessments for disease control and complications were also made which might potentially have been biased by the lack of blinding. In Beissert 2007 complete healing defined as complete re‐epithelialisation of the lesions and cumulative steroid dose at complete healing were primary end points, judgement may have been biased because of the lack of blinding.
Incomplete outcome data
There was one dropout in Dreno 1993: treatment was stopped after eight days of treatment as the participant was in a coma unrelated to treatment.
In Morel 1984 four participants were excluded from the analysis, two because they did not fit the inclusion criteria and two due to protocol deviation.
Burton 1978 and Liu 2006 seemed to have no dropouts but the reports were short and no details were given.
In Roujeau 1984 the number and reasons for dropouts, two from each arm of the study, were listed.
In the prednisone (6 participants) versus tetracycline and nicotinamide (14 participants) trial, the report of the trial states that 18 of 20 participants enrolled in the study were treated, the 2 that were unavailable for follow up at 8 weeks were both in the tetracycline/nicotinamide group. The reasons for dropout were not given (Fivenson 1994).
Guillaume 1993 had 3 arms, prednisolone‐only (32 participants with 1 dropout), prednisolone and azathioprine (36 participants, no dropouts) and prednisolone and plasma exchange (32 participants with 1 dropout), the reasons for the 2 dropouts were not given.
Joly 2002 stated reasons for dropouts. This study was the largest study including 341 participants.
In Joly 2009 the intention‐to‐treat analysis was not fulfilled, as only 150 of 153 randomised participants of the standard regimen were analysed, however, this is only a small deviation.
In Beissert 2007 one participant was lost. Two died of causes not related to the treatment, they were included in the intention‐to‐treat analysis.
Selective reporting
All the prospectively stated outcomes were reported in all of the studies except for Burton 1978 and Guillaume 1993. In Burton 1978 the prospectively stated outcome measures were unclear. In Guillaume 1993 outcome measurements of controlled disease were stated to be no more than one new blister occurring four weeks after starting treatment, resolution of erythema, and no more than minimal pruritus. However, only the composite measure of controlled disease was reported.
Other potential sources of bias
Dreno 1993 only had a very short (10 days) follow‐up, which limits the applicability of results to clinical practice, especially in a chronic disease such as BP.
Initial diagnosis confirmed by immunofluorescence was performed in all but one study (a pathological test was mentioned but not described further in Liu 2006).
More participants in the azathioprine group in Beissert 2007 had severe disease; 53% had ≥20% body surface area involvement compared to only 27% in the mycophenolate mofetil (MMF) group, and more participants had raised liver enzyme tests. However, a test to check for thiopurinemethyltransferase activity was not performed. Additionally, only those participants who were likely to attend for follow up were recruited. Eligibility was also determined by the consultant doctor after baseline testing.
In Fivenson 1994 the study was originally designed to randomise 96 participants, but enrolment was terminated when 20 participants were enrolled. No reasons were given for this.
The Guillaume 1993 study was stopped after the interim analysis became available; this showed no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone.
Effects of interventions
The assessment and reporting of disease control, symptoms, and adverse effects of medication were recorded in varying detail in the different trials. Mortality is the only outcome measure documented in all the studies, however, this was not a stated outcome of interest in most studies and it is not always clear whether the deaths were related to treatment.
The following effects of interventions section is organised by comparison, followed by the primary and secondary outcomes stated in the Methods section of this review (Types of outcome measures).
Higher versus lower doses of prednisolone
Primary outcome
Regression or healing of skin lesions
The study Morel 1984 comparing the starting dose of prednisolone 0.75 mg/kg (26 participants) versus prednisolone 1.25 mg/kg (24 participants) reported that 51% versus 64% of participants were clear of skin lesions at day 21 and 33% versus 55% at day 51 (no significant difference in effectiveness, comparing each dose at 21 days and 51 days, data not shown). This was the case for analyses based on completers only as well as on an intention‐to‐treat basis (assuming unknown = not healed). There were no significant differences between doses of prednisolone at 21 days in terms of disease control (healing of skin lesions) (RR 1.20, 95% CI 0.77 to 1.87), or 51 days (RR 1.38, 95% CI 0.69 to 2.79, Analysis 1.1).
1.1. Analysis.
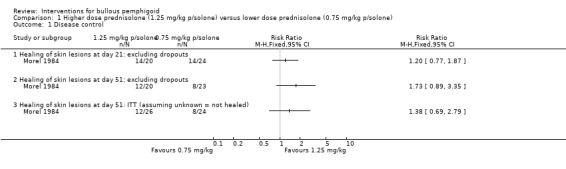
Comparison 1 Higher dose prednisolone (1.25 mg/kg p/solone) versus lower dose prednisolone (0.75 mg/kg p/solone), Outcome 1 Disease control.
Secondary outcomes
Mortality
At day 51, there were 3 deaths out of 22 participants in the higher dose compared to 2 deaths out of 24 participants in the lower dose (RR 1.64, 95% CI 0.30 to 8.90, Analysis 1.2) (Morel 1984).
1.2. Analysis.
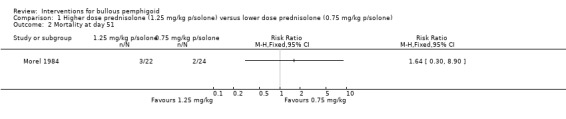
Comparison 1 Higher dose prednisolone (1.25 mg/kg p/solone) versus lower dose prednisolone (0.75 mg/kg p/solone), Outcome 2 Mortality at day 51.
Methylprednisolone versus prednisolone
Primary outcome
Regression or healing of skin lesions
The study comparing different formulations of steroids, methylprednisolone (n = 28) with prednisolone (n = 29) (Dreno 1993), found a large reduction in the number of blisters in both groups. At day 10 the mean number of blisters was 6.0 (SD 19) for methylprednisolone and 13.0 (SD 35) for prednisolone (mean difference ‐7.00) (95% CI ‐21.55 to 7.55, Analysis 2.1).
2.1. Analysis.
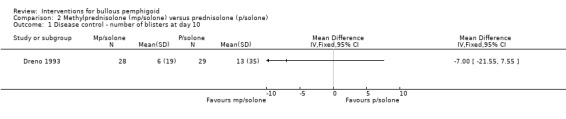
Comparison 2 Methylprednisolone (mp/solone) versus prednisolone (p/solone), Outcome 1 Disease control ‐ number of blisters at day 10.
Collective figures of overall improvement (22 of 28 participants in the methylprednisolone group, 78.6%, versus 18 of 29 participants in the prednisolone group, 62.1%) were reported (RR 1.27, 95% CI 0.90 to 1.79, Analysis 2.2). This was not statistically significant.
2.2. Analysis.
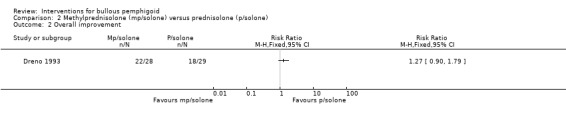
Comparison 2 Methylprednisolone (mp/solone) versus prednisolone (p/solone), Outcome 2 Overall improvement.
Secondary outcomes
Effect on the quality of life
Erythema and pruritus (itch) were each measured by the participant on a scale from zero (absent) to three (severe). No significant difference was seen between the groups for either score: erythema 0.59 (SD 0.69) versus 0.93 (SD 0.72), mean difference ‐0.34 (95% CI ‐0.71 to 0.03, Analysis 2.3), and pruritus 0.59 (SD 0.8) versus 0.86 (SD 0.8), mean difference ‐0.27 (95% CI ‐0.69 to 0.15, Analysis 2.4). The study investigators report that the only statistically significant result was a reduction in pruritis; the statistical significance for this outcome (pruritus) was given as 0.042 by the study investigators, but it was unclear which statistical test they used.
2.3. Analysis.
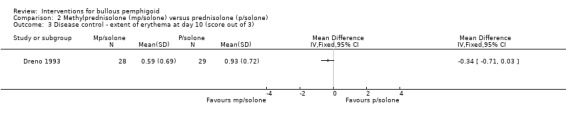
Comparison 2 Methylprednisolone (mp/solone) versus prednisolone (p/solone), Outcome 3 Disease control ‐ extent of erythema at day 10 (score out of 3).
2.4. Analysis.
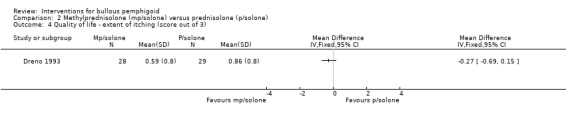
Comparison 2 Methylprednisolone (mp/solone) versus prednisolone (p/solone), Outcome 4 Quality of life ‐ extent of itching (score out of 3).
Mortality
There were no deaths recorded in this study, but the follow‐up period was only 10 days.
Prednisone plus azathioprine versus prednisone
This comparison was evaluated in two small studies, Burton 1978 with a three‐year follow‐up, and Guillaume 1993 with a six‐month follow‐up.
Primary outcome
Regression or healing of skin lesions
The Guillaume 1993 study failed to show improvements in disease control (14/36 vs 13/31; RR 0.93, 95% CI 0.52 to 1.66, Analysis 3.1).
3.1. Analysis.
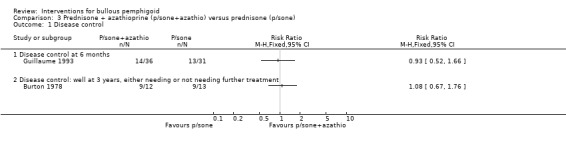
Comparison 3 Prednisone + azathioprine (p/sone+azathio) versus prednisone (p/sone), Outcome 1 Disease control.
In Burton 1978, the prednisone plus azathioprine group, 9/12 participants had their disease controlled at 3 years (7 participants off treatment and 2 still on treatment), and 9/13 participants in the prednisone‐only group had their disease controlled (4 participants off treatment and 5 still on treatment) (RR 1.08, 95% CI 0.67 to 1.76, Analysis 3.1) (P = 0.75: not statistically significant). This study found a 45% reduction in the amount of prednisone required for disease control by the azathioprine group over a 3‐year period (mean total dose 3688 mg in the azathioprine group versus 6732 mg in the prednisone‐only group), which was statistically significant (P < 0.01). The statistical test that was used was not reported.
Secondary outcomes
Adverse effects
In Burton 1978 one of the off‐treatment participants who was originally assigned to the prednisone‐only group withdrew from the prednisone group due to adverse effects and was subsequently successfully treated with azathioprine. In Guillaume 1993 severe complications were more often noted in the azathioprine group (RR 1.29, 95% CI 0.68 to 2.45, Analysis 3.2) (not statistically significant). Unfortunately, the adverse effects were not given in detail for each group (see Adverse Events; Table 11). The study investigators stated that "most of the adverse events could be attributed to corticosteroids". The chief adverse effect associated with azathioprine was a reduction in the white cell count (2 of 12 participants in the Burton 1978 trial and 4 of 36 participants in the Guillaume 1993 trial).
3.2. Analysis.
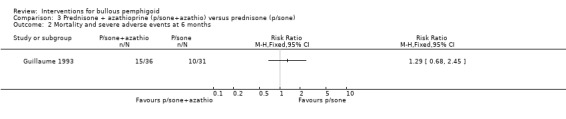
Comparison 3 Prednisone + azathioprine (p/sone+azathio) versus prednisone (p/sone), Outcome 2 Mortality and severe adverse events at 6 months.
Mortality
There was no statistical significance in mortality at 6 months between the prednisone and the prednisone plus azathioprine group in Guillaume 1993 (RR 1.03, 95% CI 0.35 to 3.06, Analysis 3.3). The Burton 1978 study had the longest follow‐up (3 years) and an overall mortality of 7/25 participants (28%). There were 3 deaths in the prednisone plus azathioprine group (n = 12) and 4 in the prednisone‐only group (n = 13) (RR 0.81, 95% CI 0.23 to 2.91, Analysis 3.3) (P = 0.75) after 3 years of treatment.
3.3. Analysis.
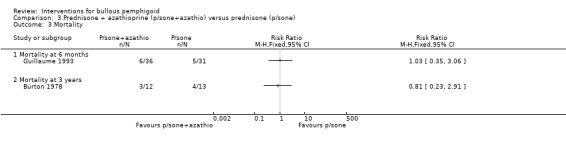
Comparison 3 Prednisone + azathioprine (p/sone+azathio) versus prednisone (p/sone), Outcome 3 Mortality.
Prednisolone plus plasma exchange versus prednisolone
Primary outcome
Regression or healing of skin lesions
This comparison was evaluated in two small studies: Roujeau 1984 with a one‐month follow‐up, and Guillaume 1993 with a six‐month follow‐up.
In the study comparing prednisolone versus prednisolone and plasma exchange (Roujeau 1984), all participants were started on a low dose of prednisolone (0.3 mg/kg/day) which was increased (max 2 mg/kg/day methylprednisolone intramuscular + 2 mg/kg/day oral cyclophosphamide) until disease control was achieved. The addition of plasma exchange appeared to reduce the amount of prednisolone required to achieve disease control. Disease control was achieved with a dose of 0.3 mg/kg/day in 13/22 participants in the prednisolone plus plasma exchange group but in none of the 15 participants in the prednisolone‐only group (risk ratio in favour of prednisolone plus plasma exchange: RR 18.78, 95% CI 1.20 to 293.70, Analysis 4.1) (P = 0.04). This is statistically significant, although the confidence intervals are very wide.
4.1. Analysis.
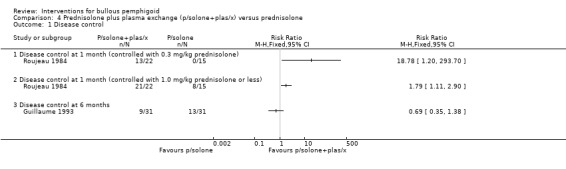
Comparison 4 Prednisolone plus plasma exchange (p/solone+plas/x) versus prednisolone, Outcome 1 Disease control.
Significantly more participants also achieved disease control with prednisolone doses less than or equal to 1 mg/kg: 21/22 for prednisolone plus plasma exchange and 8/15 for prednisolone‐only (RR 1.79, 95% CI 1.11 to 2.90, Analysis 4.1) (P = 0.02). Disease control was achieved with less than half the total prednisolone dose in the plasma exchange group; significantly lower doses of prednisolone were required to achieve disease control, both in terms of the cumulative dose (mean difference ‐1.53 g: 95% CI ‐2.40 to ‐0.66, Analysis 4.2) and the average daily dose: 0.52 (SD 0.28) mg/kg in the plasma exchange group versus 0.97 (SD 0.33) mg/kg in the prednisolone‐only group. They found a similar side‐effect profile in both groups and the disease was controlled within about four weeks in both groups.
4.2. Analysis.
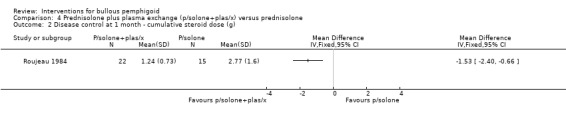
Comparison 4 Prednisolone plus plasma exchange (p/solone+plas/x) versus prednisolone, Outcome 2 Disease control at 1 month ‐ cumulative steroid dose (g).
However, this favourable effect of adding plasma exchange was not seen in the Guillaume 1993 study for disease control at 6 months: 9/31 prednisolone plus plasma exchange vs 13/31 prednisolone (RR 0.69, 95% CI 0.35 to 1.38, Analysis 4.1: see Analysis 4.1.3). The report of the study indicates that the trial was "interrupted after the interim analysis showed no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone" at four weeks or at six weeks follow‐up.
Secondary outcomes
Mortality and adverse effects
In Guillaume 1993 mortality at 6 months was assessed as mortality alone (3/31 vs 5/31, RR 0.60, 95% CI 0.16 to 2.30, Analysis 4.4: see analysis 4.4.3), or total adverse events including mortality (10 major adverse events including 5 deaths in the prednisolone, versus 6 major adverse events including 3 deaths in the plasma exchange group) (RR 0.60, 95% CI 0.25 to 1.45, Analysis 4.3) (Guillaume 1993).
4.4. Analysis.
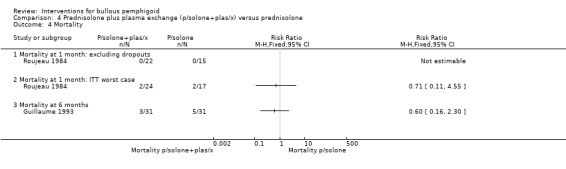
Comparison 4 Prednisolone plus plasma exchange (p/solone+plas/x) versus prednisolone, Outcome 4 Mortality.
4.3. Analysis.
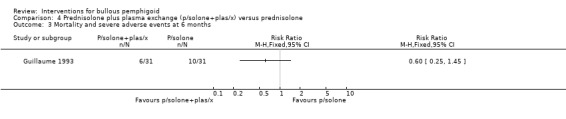
Comparison 4 Prednisolone plus plasma exchange (p/solone+plas/x) versus prednisolone, Outcome 3 Mortality and severe adverse events at 6 months.
In Roujeau 1984 no deaths occurred during the treatment period (Analysis 4.4). There were only 25 of the participants available for follow up, that is after the initial treatment period which included 41 original participants, of which only 37 were analysed by the trial authors because they were lost early in the treatment period. Of those 25 participants available for follow up, 2 participants died in the prednisolone group, and 1 in the prednisolone plus plasma exchange group (the calculation for the worst case scenario includes the 4 lost participants, 2 in each group).
Prednisolone plus azathioprine versus prednisolone plus plasma exchange
Primary outcome
Regression or healing of skin lesions
Comparing the prednisolone plus azathioprine group with the prednisolone plus plasma exchange group (Guillaume 1993), no significant differences were found for disease control at 6 months:14/36 and 9/31 respectively (RR 1.34, 95% CI 0.67 to 2.66, Analysis 5.1).
5.1. Analysis.
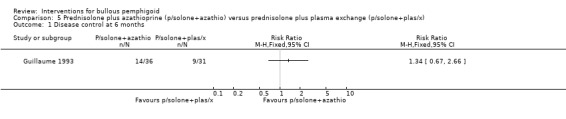
Comparison 5 Prednisolone plus azathioprine (p/solone+azathio) versus prednisolone plus plasma exchange (p/solone+plas/x), Outcome 1 Disease control at 6 months.
Secondary outcomes
Mortality and adverse effects
Mortality at 6 months in Guillaume 1993 was 6/36 (azathioprine) versus 3/31 (plasma exchange) (RR 1.72, 95% CI 0.47 to 6.32, Analysis 5.2). Total adverse events including deaths were more often noted in the azathioprine group (15/36 total adverse effects (including 6 deaths) versus 6/31 (including 3 death) in the plasma exchange group). The results almost reach statistical significance with regard to total adverse events, including mortality at 6 months in favour of the plasma exchange plus prednisolone group (RR 2.15, 95% CI 0.95 to 4.87, Analysis 5.3).
5.2. Analysis.
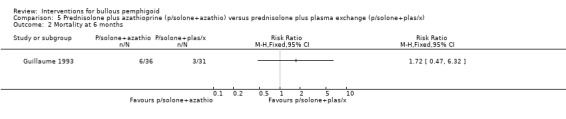
Comparison 5 Prednisolone plus azathioprine (p/solone+azathio) versus prednisolone plus plasma exchange (p/solone+plas/x), Outcome 2 Mortality at 6 months.
5.3. Analysis.
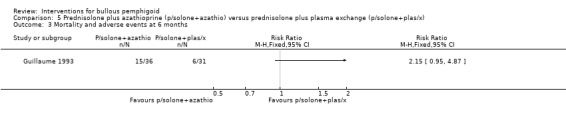
Comparison 5 Prednisolone plus azathioprine (p/solone+azathio) versus prednisolone plus plasma exchange (p/solone+plas/x), Outcome 3 Mortality and adverse events at 6 months.
Prednisone versus tetracycline and nicotinamide
Primary outcome
Regression or healing of skin lesions
Comparing prednisone with tetracycline and nicotinamide (Fivenson 1994), 1 complete and 5 partial responders were reported in the steroid group (n = 6), compared with 5 complete, 5 partial responders, 1 non‐responder, and 1 disease progression in the tetracycline group (n = 12). Two participants in the tetracycline group were unavailable for follow‐up at 8 weeks (together n = 14). The results are not statistically significant for either complete response (RR 2.50, 95% CI 0.37 to 16.89, Analysis 6.1) or complete and/or partial response (RR 0.87, 95% CI 0.62 to 1.22, Analysis 6.1). The data for the non‐responder (n = 1), the participant whose disease progressed (n = 1), and the two participants that were lost to follow up (n = 2) in the tetracycline group are not shown in the table.
6.1. Analysis.
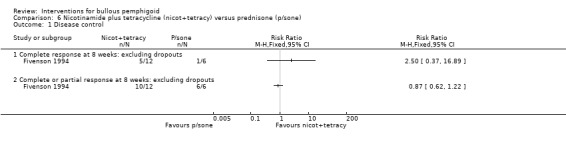
Comparison 6 Nicotinamide plus tetracycline (nicot+tetracy) versus prednisone (p/sone), Outcome 1 Disease control.
Secondary outcomes
Duration of remissions
Of the participants available for long‐term follow‐up in Fivenson 1994, all 5 in the tetracycline group remained disease‐free (mean 17.5 weeks) while 2 of the 3 in the steroid group had repeated flare‐up with tapered off treatment (mean 21.3 weeks). Unfortunately, this trial included very few participants, two thirds of whom were in the tetracycline group (14 of 20 participants). The randomisation in this study was unclear and there was a high dropout rate (2/20 at 8 weeks and a further 10 participants at the end of study). At 10 months there were only 3 participants left in the steroid group (2 of whom had multiple recurrences with tapering of medication), and only 5 participants remained in the nicotinamide plus tetracycline group, all of whom remained disease‐free during medication tapering.
Adverse effects
The report of the study states that "fewer short‐term and long‐term adverse effects occurred in the participants treated with the nicotinamide/tetracycline combination compared with prednisone therapy" (there was also one death due to sepsis in the prednisone group (Analysis 6.2)). Most of the side‐effects in the tetracycline/nicotinamide group in Fivenson 1994 were mild (two participants developed gastrointestinal symptoms which resolved after substitution of tetracycline with minocycline, one of them developed tinnitus on minocycline which resolved despite continuing treatment. One participant in the latter group developed severe tubular necrosis. He had been enrolled in the study with elevated serum creatinine (159 which peaked at 654 micromol/L: normal 60 to 120 micromol/L) and was also taking nonsteroidal anti‐inflammatory drugs (ibuprofen and aspirin). This participant's renal function returned to normal within two weeks of stopping treatment.
6.2. Analysis.
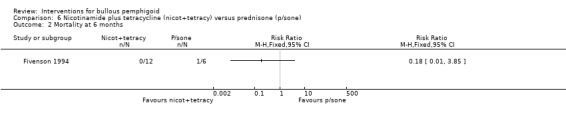
Comparison 6 Nicotinamide plus tetracycline (nicot+tetracy) versus prednisone (p/sone), Outcome 2 Mortality at 6 months.
Very potent topical steroids (clobetasol propionate) versus prednisone
The largest study had two study groups with the study stratified by severity of disease (Joly 2002):
Moderate disease (less than 10 new blisters a day): topical steroids (initial dose of 40 g of 0.05% clobetasol propionate twice daily applied to entire body surface) (77 participants), and oral prednisone 0.5 mg/kg (76 participants).
Extensive disease (more than 10 new blisters a day): topical steroids (93 participants), and oral prednisone 1 mg/kg (95 participants).
Primary outcome
Regression or healing of skin lesions
In the moderate disease group differences were seen between the topical steroid and 0.5 mg/kg oral steroid groups in terms of rate of disease control at 3 weeks: 100% vs 94% (RR 1.06, 95% CI 1.00 to 1.12, Analysis 7.1,: see Analysis 7.1.1) (P = 0.07).
7.1. Analysis.
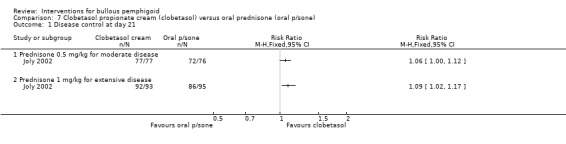
Comparison 7 Clobetasol propionate cream (clobetasol) versus oral prednisone (oral p/sone), Outcome 1 Disease control at day 21.
Both interventions resulted in nearly 100% of participants experiencing disease control. The disease was controlled in 99% of the participants with extensive disease using topical steroids versus 91% of those on oral steroids at 3 weeks. This reached statistical significance (RR 1.09, 95% CI 1.02 to 1.17, Analysis 7.1: see Analysis 7.1.2) (P = 0.01), although this outcome was not assessed blindly, and therefore the possibility of bias exists.
Secondary outcomes
Adverse effects
The incidence of severe complications was reported for people with extensive disease: 29% for topical steroids versus 54% for oral steroids (RR 0.54, 95% CI 0.37 to 0.78, P = 0.001), which is statistically significant, i.e. there were fewer adverse events due to clobetasol (Analysis 7.2: see analysis 7.2.2). But this was not statistically significant in the moderate disease group (32% vs 38%, RR 0.85, 95% CI 0.55 to 1.31, Analysis 7.2) (P = 0.46) (Joly 2002).
7.2. Analysis.
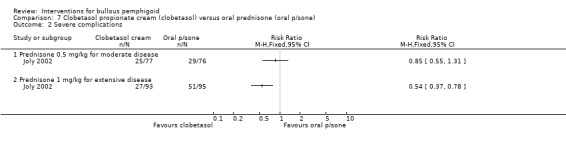
Comparison 7 Clobetasol propionate cream (clobetasol) versus oral prednisone (oral p/sone), Outcome 2 Severe complications.
Mortality (survival)
The major outcome in this study was survival, the study being designed to have 80% power to detect a reduction in the 1‐year mortality rate for both moderate and extensive bullous pemphigoid. To achieve this power, 75 participants were needed in each treatment group, which was accomplished.
In the extensive disease group, those using topical steroids had a better survival rate at 1 year compared to those on oral steroids (76% versus 58%, RR 0.58, 95% CI 0.37 to 0.89, Analysis 7.3: see analysis 7.3.2) (statistically significant: P = 0.01). This was consistent with the incidence of severe complications in the people with extensive disease. In the moderate disease group no significant differences were seen between the topical steroid and 0.5 mg/kg oral steroid groups in terms of overall survival (30% vs 30%, RR 0.99, 95% CI 0.61 to 1.60, Analysis 7.3) (Joly 2002).
7.3. Analysis.
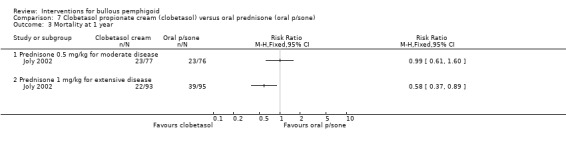
Comparison 7 Clobetasol propionate cream (clobetasol) versus oral prednisone (oral p/sone), Outcome 3 Mortality at 1 year.
Standard dose (40 g/day) of very potent topical steroids versus mild dose (10 to 30 g/day)
A second large study from the same French group was recently published to compare two different regimens of topical steroids (Joly 2009). In the mild regimen of Joly 2009, participants received different amounts of clobetasol propionate cream depending on their body weight and severity of the disease (moderate disease (≤ 10 new blisters/day): 69 participants received 20 g/day if their body weight was > 45 kg and 10 g/day if < 45 kg) (severe disease (> 10 new blisters/day): 90 participants received 30 g/day if their body weight was > 45 kg and 20 g/day if < 45 kg), in the standard regimen all participants received 40 g of the cream/day (moderate disease n = 65, severe disease n = 88).
Primary outcome
Regression or healing of skin lesions
The report of the study has a discrepancy in the number of participants who were evaluated at 21 days and states that 150/153 participants were evaluable, as 3 included participants were lost to follow up early after the initiation of treatment and were not available for evaluation of efficacy at day 21. However, disease control rates at 21 days are given for 153/153 participants. We wrote to the study investigator for clarification (Joly 2010), who confirmed that the published report contains a typographical error. The correct figures are: 153 participants randomised, 150 analysed. We carried out an analysis of the results using the randomised number of participants (n = 153). In the mild regimen 156/159 participants were controlled by day 21 and in the standard regimen 150/153 (RR 1.00, 95% CI 0.97 to 1.03, Analysis 8.1: see Analysis 8.1.1) (not statistically significant P = 0.96).
8.1. Analysis.
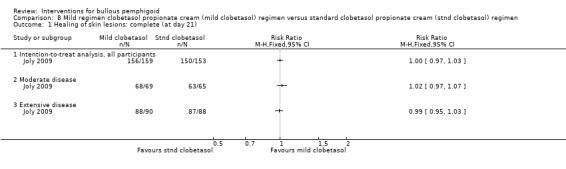
Comparison 8 Mild regimen clobetasol propionate cream (mild clobetasol) regimen versus standard clobetasol propionate cream (stnd clobetasol) regimen, Outcome 1 Healing of skin lesions: complete (at day 21).
In the report of the study, participants and their responses to the treatment regimens were stratified by severity of disease. Using the correct figures supplied by the study investigator (Joly 2010), of those with moderate disease, disease control was achieved in 68/69 using the mild regimen, and with the standard regimen 63/65 were controlled (RR 1.02, 95% CI 0.97 to 1.07, Analysis 8.1: see Analysis 8.1.2). Of those with extensive disease, 88/90 achieved disease control in the mild regimen and 87/88 were controlled with the standard regimen (RR 0.99, 95% CI 0.95 to 1.03, Analysis 8.1: see Analysis 8.1.3).
The median cumulative doses of cream used during the study period were 5760 g in the standard regimen versus 1314 g (mild regimen), which is a 70% reduction in cumulative doses of corticosteroid.
Secondary outcomes
Duration of remissions
There were 67 relapses in 159 participants in the mild regimen and 52 in 153 participants in the standard regimen (RR 1.24, 95% CI 0.93 to 1.65, Analysis 8.2). This was not significantly different between the two groups. There is insufficient evidence to say that one treatment regimen is different from the other in terms of effectiveness.
8.2. Analysis.
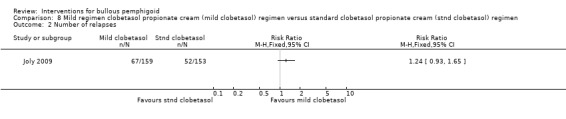
Comparison 8 Mild regimen clobetasol propionate cream (mild clobetasol) regimen versus standard clobetasol propionate cream (stnd clobetasol) regimen, Outcome 2 Number of relapses.
Adverse effects
Eighty‐nine participants in each group had severe adverse events. This consisted of 194 events in 89 participants in the mild regimen group and 227 in the standard regimen group. There were 42 life‐threatening adverse effects in 33 participants. The main severe side‐effects in both groups were diabetes mellitus (n = 34 standard, n = 18 mild), cardiovascular and neurovascular disorders in n = 35 (standard) and n = 21 participants (mild), and severe infections in 32 and 27 participants, in the standard and mild regimen groups respectively. There were also cutaneous side‐effects which included purpura, severe skin atrophy, and striae.
Mortality
In the mild regimen 60/159 participants had died by year 1 (moderate disease 19/69, severe disease 41/90) and in the standard regimen 58/153 (moderate disease 21/65, severe disease 37/88). This was not statistically significant between the 2 groups for those participants with moderate (RR 0.85, 95% CI 0.51 to 1.43) or extensive (RR 1.08, 95% CI 0.78 to 1.51) disease (Analysis 8.3).
8.3. Analysis.
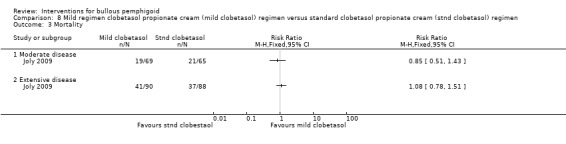
Comparison 8 Mild regimen clobetasol propionate cream (mild clobetasol) regimen versus standard clobetasol propionate cream (stnd clobetasol) regimen, Outcome 3 Mortality.
The report of the study gives an adjusted analysis (Cox model adjusted for age and Karnofsky score), after which a beneficial effect of the mild regimen was observed in participants with moderate BP, with an almost twofold decrease in the risk of death or life‐threatening adverse events relative to the standard regimen (hazard ratio = 0.54, 95% confidence interval, 0.30 to 0.97; P = 0.039).
Jingui Shenqi Pill (JSP) 1# bid plus prednisone versus prednisone
The Jingui Shenqi Pill (JSP) 1# bid plus prednisone 0.5 to 1.0 mg/kg/day was compared to prednisone alone 0.5 to 1.0 mg/kg/day in a small trial (Liu 2006). Thirty participants with bullous pemphigoid were included, the primary clinical outcome was healing of the skin lesions after 4 weeks treatment.
A cure was defined as > 90% of the total number of lesions being healed, moderate healing if only 60% to 89% of the affected area had healed, improved if 30% to 59% of the lesions had healed, and not effective if less than 30% of the lesions had healed.
Primary outcome
Regression or healing of skin lesions
Complete healing of the lesions at 4 weeks was achieved in 1 participant receiving the Jingui Shenqi Pill (1/15) and none in the prednisone group (0/15) (RR 3.00, 95% CI 0.13 to 68.26, Analysis 9.1). Partial healing was achieved in 13 of 15 with JSP treatment compared with 11 of 15 participants with prednisone‐only treatment (RR 1.18, 95% CI 0.82 to 1.70, Analysis 9.1) .
9.1. Analysis.
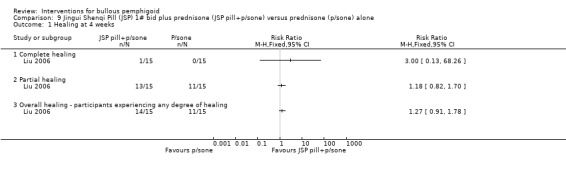
Comparison 9 Jingui Shenqi Pill (JSP) 1# bid plus prednisone (JSP pill+p/sone) versus prednisone (p/sone) alone, Outcome 1 Healing at 4 weeks.
Overall, the treatment was effective (some degree of healing) in 14/15 participants (93.33%) of the treatment group compared to 11/15 (73.33%) of the prednisone group (RR 1.27, 95% CI 0.91 to 1.78, Analysis 9.1). None of the results were statistically significant.
Secondary outcomes
Mortality
No deaths were reported during the four‐week follow‐up (Liu 2006).
Azathiopine plus corticosteroid versus mycophenolate mofetil plus corticosteroid
Primary outcome
Regression or healing of skin lesions
Comparing azathioprine (2 mg/kg/day) and mycophenolate mofetil (MMF) (2000 mg twice/day) both in addition to oral methylprednisolone (0.5 mg/kg/day), all participants achieved some degree of healing (either partial or complete).
In this trial complete healing and disease remission was defined as complete re‐epithelialisation of all lesions. 35/38 of the azathioprine group and 35/35 of the MMF group showed complete healing (92% vs 100%) (RR 0.92, 95% CI 0.83 to 1.03, Analysis 10.1) (Beissert 2007). Participants showed complete healing after 23.8 ± 18.9 days and 42.0 ± 55.3 days for azathioprine and mycophenolate mofetil respectively, but this difference was not statistically significant (unpaired t‐test).
10.1. Analysis.
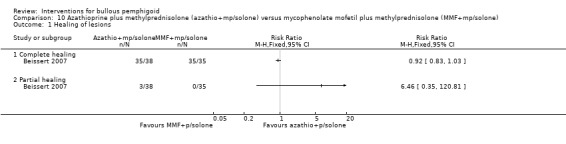
Comparison 10 Azathioprine plus methylprednisolone (azathio+mp/solone) versus mycophenolate mofetil plus methylprednisolone (MMF+mp/solone), Outcome 1 Healing of lesions.
Three participants in the azathioprine group showed partial healing (RR 6.46, 95% CI 0.35 to 120.81, Analysis 10.1) (not statistically significant), 2 of whom died of unrelated cause, leading to premature discontinuation of the treatment, and 1 was lost to follow up before treatment was completed.
Secondary outcomes
Duration of remissions (weeks)
The disease‐free interval between complete remission and recurrence of lesions (new blister formation) was 23.5 weeks ± 19.4 weeks for azathioprine and 18 weeks ± 12.8 weeks for MMF, that is 5.50 more weeks of remission for those participants treated with azathioprine, but this difference was not statistically significant (unpaired t‐test).
Adverse effects
Nine (24%) grade 3/4 adverse effects are described in the azathioprine group, and 6 (17%) in the MMF group. There were more elevated liver function tests in the azathioprine group (6/37 vs 1/35), however, participants were not checked for thiopurinemethyltransferase activity prior to treatment. There was no statistical difference between treatments for any of the outcomes.
Mortality
There were two deaths in the azathioprine group, described as not treatment‐related.
Discussion
Summary of main results
Ten studies were included in this review (7 studies were previously included; in this updated review 3 new studies were found and included: Beissert 2007,Joly 2009, and Liu 2006). The studies in this review used oral prednisolone or prednisone in the control group (there were no comparisons with placebo) and were all small trials apart from two studies comparing different amounts of topical steroids and oral steroids (Joly 2002; Joly 2009). For the purposes of this review prednisone and prednisolone are regarded as bio‐equivalent.
No meta‐analysis was possible because of the clinical heterogeneity of the studies in terms of interventions, measures of disease control, and follow‐up. The three studies that had overlapping treatments compared prednisone versus prednisone plus azathioprine (Burton 1978), prednisolone versus prednisolone plus plasma exchange (Roujeau 1984), and prednisolone alone, with azathioprine and with plasma exchange (Guillaume 1993), but they were heterogenous especially in terms of the doses of treatment used.
Overall completeness and applicability of evidence
The outcome measures in these studies are very varied, as can be seen when looking at the definition of disease control and the interventions used (see Characteristics of included studies). Our primary outcome was regression or healing of skin lesions; we did not pre‐specify the follow‐up times in our protocol as there is no established optimum treatment for BP (Wojnarowska 2002) and we did not want to exclude potentially effective therapies from our analyses because they did not meet strict inclusion criteria in relation to follow up times. We found that there were relatively few reports of trials for this rare disease and that there is variation in the time points reported.
Some of the included studies reported short follow‐up periods (only 10 days in Dreno 1993), which makes judgment of the practical significance of the results difficult, especially in view of the chronic nature of this disease. One study (Morel 1984) compared the starting dose of prednisolone, and reported results at 21 and 51 days, so perhaps the follow‐up of 51 days may be more reasonable. We reported the results of that study at both time points in this review, although there were no significant differences in healing when the length of time or the dose given were compared. The Burton 1978 trial had the longest follow‐up period but unfortunately details on how disease control was evaluated are not given and few clinical data were available. Participants with contraindications to oral steroids or azathioprine and those "unlikely to attend follow‐up" were excluded from the trial.
The Fivenson 1994 study had an unclear method of randomisation, a high dropout rate, and small numbers, but may suggest some merit in the use of tetracycline and nicotinamide. However, further study is needed to confirm these findings.
Probably the most interesting feature of the Roujeau 1984 study was the lower doses of prednisolone used in both treatment groups. Strict measures of disease control were used (complete disappearance of blisters, pruritus, and erythema) and in both groups the disease was controlled within about 4 weeks in all participants; however, higher doses than the initial low dose of 0.3 mg/kg prednisone were needed in all participants of the prednisolone‐only group and in two thirds of the participants in the plasma exchange group to achieve disease control. There were no deaths during the study but this may be partly because of the exclusion of participants older than 80 years of age. This study found that the plasma exchange group required much less prednisolone than the prednisolone‐only group. This benefit was, however, not confirmed by Guillaume 1993. This latter study also failed to confirm the benefit of the addition of azathioprine to prednisolone. Beissert 2007 added either azathioprine or mycophenolate mofetil to an initial dose of 0.5 mg methylprednisolone/kg/day; there was no difference in effectiveness. The cumulative steroid dose until the end of the documentation (> 720 days) was 4967 ± 12191mg for the azathioprine group and 5754 ± 9693mg for the MMF group. The similarity between the two groups possibly reflects a comparable immunosuppressive effect of the two drugs. Interestingly, there were more participants with severe disease in the azathioprine group: 53% had ≥ 20% body surface area involvement compared to only 27% in the MMF group.
In a small, methodologically unclear trial Liu 2006 added the Jingui Shenqi Pill to oral steroids and described a beneficial effect after four weeks treatment compared to the control group. They found an increased expression of glucocorticosteroid receptor (GCr) α and a decreased expression of GCr β in skin lesions of the treatment group which may improve the sensitivity of the skin to glucocorticosteroids. However, the effectiveness of this intervention was not proven in our analyses.
As it is unlikely that future studies on interventions for bullous pemphigoid including a placebo group would be ethically justifiable, a comparison of low‐dose prednisolone with tetracyclines and nicotinamide (or potent topical corticosteroids, for mild and/or localised disease) may prove a worthy alternative. Uncontrolled studies have suggested the successful use of topical steroids as first‐line for the treatment of both localised and mild disease (Garg 1994; Rollin 1993; Zimmermann 1999), and two recent randomised controlled trials (Joly 2002; Joly 2009) confirm this view. The use of potent topical steroids is favoured because they have minimal side‐effects and a limited number of contraindications. Joly 2002 showed a significant benefit of 40 g 0.05% clobetasol propionate cream/day over 1 mg/kg of prednisone in extensive disease for disease control, adverse events, and mortality. No statistically significant differences between clobetasol propionate cream and 0.5 mg/kg prednisone were found in the moderate disease group for disease control, adverse events, and mortality.
However, even though there were less severe adverse effects (pneumonia, diabetes requiring insulin, myocardial infarction, psychiatric symptoms, stroke, thrombosis, bone fracture) noted in the group of participants treated with prednisone 1 mg/kg, it is not mentioned if participants in the different groups had similar adverse effects regarding, for example, blood pressure and bone mineral density. Also, the effort needed in applying the creams twice daily to the whole body is a major limitation in people with BP, who are mostly elderly and may have other co‐existing disease. Another aspect is that topical preparations may be more costly than oral cortisone preparations and nursing care may be costly too.
It is likely that very potent topical corticosteroids applied in such large quantities may have systemic effects (perhaps comparable to 0.5 mg/kg/day prednisone). However, we do expect that there is also a local immunosuppressive and anti‐inflammatory effect of the topically applied corticosteroids, because there are reports of participants with localised BP effectively treated with potent topical steroids only. In fact, a later trial by the same group shows that less topical steroids (≤ 30 g 0.05% clobetasol propionate cream/day) are as effective in disease control after 21 days, and that the mean cumulative dose was 71% lower in a mild treatment regimen than in a standard treatment regimen. The mild regimen was associated with less severe adverse effects (Joly 2009).
An important research question for the future is to evaluate whether a lower dose of steroid (0.5 mg/kg/day) would be adequate for disease control in extensive disease when compared with standard higher doses.
Quality of the evidence
Most of the studies were very small and of poor methodological quality because of:
an unclear method of randomisation;
lack of blinding in the majority of studies; and
exclusion of dropouts from the analysis in most studies.
This is summarised in Figure 1.
Some studies which did not describe the method of randomisation were published some time ago (e.g. Burton 1978;Dreno 1993; Morel 1984); we did not attempt to gain further information as it was unlikely that further details of the studies would be available.
The main concern with Liu 2006 is that in the published report of this study it was not absolutely clear that the diagnosis of BP was confirmed by immunofluorescence. The report of the trial was translated from Chinese and refers to the confirmation of the diagnosis listing the usual clinical features, histology, direct immunofluorescence, indirect IF on salt split skin and immuno EM, using a method given in a reference. The source of the methods in the reference was translated but it was unclear which of the methods listed were used. We contacted the trial investigators on two occasions, regarding the method used to confirm diagnosis, but did not receive a reply. Given that the description of the diagnosis of BP in the published report is not precise, it is possible that inclusion of the trial into a meta‐analysis could introduce bias. However, Chinese traditional medicine plus prednisone was not shown to be effective in this single trial.
In Burton 1978 there may have been selection bias, as participants were "started on oral prednisone 30 to 80 mg/day, to suppress new blisters" and only then "did the consultant decide whether to include the participant in the trial". Also Joly 2002 switched three participants from one intervention group to another because of side‐effects of treatment, although this was done in accordance with the study protocol.
The Guillaume 1993 study was stopped after the interim analysis became available which showed no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone. The trial investigators calculated that "the inclusion of 120 participants as initially scheduled, could not change these negative results". The Fivenson 1994 study was terminated after 20 participants were enrolled (the study was originally designed to randomise 96 participants in 4 centres). No reasons were given for this early ending but a further randomised, double‐blind, multicentre trial was mentioned as being 'underway' in the published report of the trial. We have attempted to contact the trial investigators but have been unsuccessful in obtaining any further details or data from the later study.
Overall, there were relatively few included studies of and these were of variable methodological quality, therefore some caution should be exercised in interpreting the results. Additionally no statistical pooling of the data was possible because of the clinical heterogeneity of the studies in terms of interventions, measures of disease control, and follow‐up.
Agreements and disagreements with other studies or reviews
The last published version(Khumalo 2005) of this review had seven included randomised controlled trials. The authors concluded at that time that oral corticosteroid drugs were the most common treatment, but may have been associated with serious adverse effects, including some deaths. The review concluded that more research was needed on treatments for BP; however, strong corticosteroid creams appeared safe and effective and lower doses of oral corticosteroids could be effective also in severe disease with fewer adverse effects than usual doses.
Since the review was last updated we have found and included three new RCTs but our conclusions have not changed greatly: very potent topical steroids are effective and safe treatments for bullous pemphigoid. Their use in extensive disease may be limited by side‐effects and practical factors; new evidence suggests that milder regimens are safe and effective in moderate BP (Joly 2009). Starting doses of prednisolone greater than 0.75 mg/kg/day do not give additional benefit; lower doses may be adequate to control disease and reduce the incidence and severity of adverse reactions. One new included study investigated the addition of a Chinese herbal medicine (Jinqui Shui pill) to prednisone, but this was not more effective (Liu 2006). The third newly included study, Beissert 2007, compared oral methylprednisolone plus azathioprine versus oral methylprednisolone plus mycophenolate mofetil, but no significant differences between the two therapies were observed. The effectiveness of adding plasma exchange, or azathioprine to corticosteroids has therefore not been established, a conclusion which is unchanged from the previous published version of this review. There was no new information about combination treatment with tetracycline and nicotinamide to add to this review, therefore further research is needed to determine if this therapy might be helpful.
We identified seven ongoing trials from the updated searches: ACTRN12607000104459 (rituximab), NCT00286325 (rituximab), NCT00472030 (omalizumab), NCT00525616 (rituximab (Mabthéra)), NCT00802243 (leflunomide), NCT00809822 (NPB‐01(intravenous immunoglobulin)), and ISRCTN13704604 (doxycycline versus prednisolone).
The latter study (ISRCTN13704604) investigates the effectiveness of doxycycline versus 0.5 mg/day prednisolone in the treatment of bullous pemphigoid. The primary outcomes are effectiveness measured by a blinded blister count at week six and safety, measured by the number of adverse events related to the trial medication which occur during the year of follow‐up; results will be available by October 2013.
Monoclonal antibody therapies could offer alternatives to long‐term steroid use, or may permit the dose of steroids or immune suppressive drugs to be reduced. Four of these ongoing studies are investigating the use of such biological agents. The use of rituximab as an adjuvant treatment in BP is being studied in ACTRN12607000104459, and the efficacy and tolerance of a single cycle of rituximab in the control of bullous pemphigoid is being examined in NCT00525616. The safety and efficacy of omalizumab is being tested in NCT00472030, and the safety of treatment of bullous pemphigoid in participants resistant to therapy with systemic corticosteroids, with rituximab plus systemic corticosteroids in NCT00286325. However, results from randomised controlled trials were not available at the time of this update, and while monoclonal antibody therapy may have a role to play in treatment of BP, it is not without adverse reactions including infusion reactions, fever, neutropenia, chills, increased risk of infection, weakness, and fatigue. Participants would potentially also require re‐treatment with monoclonal antibodies, and there is a risk of neutralising antibodies that would interfere with therapeutic efficacy. Furthermore, it is a very costly treatment and will probably be reserved for treatment‐resistant cases. The efficacy and safety of newer biology therapies (monoclonal antibodies) such as rituximab should be investigated in the context of randomised controlled trials.
Authors' conclusions
Implications for practice.
Starting doses of prednisolone greater than 0.75 mg/kg/day do not seem to give additional benefit, and it would appear that lower doses (0.5 mg/kg/day) may be adequate for disease control in more participants than was previously believed. This would be expected to reduce the incidence and severity of adverse reactions (especially death) associated with treatment.
Very potent topical steroids (clobetasol propionate) are an effective treatment for bullous pemphigoid. They seem to have less serious adverse effects compared to high‐dose systemic steroids, however, their use in extensive disease may be limited by practical factors (ability of participant or availability of carer to apply the treatment). When feasible they should be considered for first‐line treatment, especially in localised disease. However, if large quantities are needed to be applied topically, they may be associated with systemic absorption and adverse events. It would be helpful to determine serum cortisol levels with the use of topical steroid therapies.
The effectiveness of the addition of plasma exchange, azathioprine, or mycophenolate mofetil to prednisolone or prednisone, has not been established. The addition of a Chinese traditional herbal medicine to prednisone was not beneficial.
Combination treatment with tetracycline and nicotinamide may be useful, although this needs further validation.
Implications for research.
Double‐blind studies are needed to confirm the appropriate oral steroid doses for the treatment of bullous pemphigoid.
Blinded RCTs comparing topical steroids with low doses of prednisolone/prednisone and with (less toxic) systemic treatments such as tetracycline and nicotinamide are needed.
The efficacy and safety of newer biologic therapies (monoclonal antibodies) such as rituximab, should be investigated in the context of randomised controlled trials.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2015 | Amended | Author information (affiliation) updated. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 11 September 2013 | Amended | Contact author's out‐of‐date email address removed and current email address and second affiliation added. Another author's affiliation also updated |
| 7 November 2011 | Amended | Correction made to the data relating to the Beissert 2007 study ('1000' mg MMF amended to '2000' mg MMF) |
| 6 September 2010 | New citation required but conclusions have not changed | Change in authorship |
| 6 September 2010 | New search has been performed | Review updated with 3 new studies |
| 5 February 2010 | New citation required but conclusions have not changed | New studies found and included or excluded, authors changed |
| 5 February 2010 | New search has been performed | Updated |
| 8 August 2008 | Amended | Converted to new review format |
| 5 June 2008 | New citation required but conclusions have not changed | New studies found and included or excluded |
| 16 May 2005 | New citation required and conclusions have changed | Substantive amendment |
| 5 June 2003 | New search has been performed | Minor update |
Acknowledgements
The authors thank Tina Leonard and Hywel Williams for their patience, without whose help the original review may never have been completed. Nonhlanhla P Khumalo thanks the Nuffield Trust Fellowship Scheme for the financial support in the preparation of this review. Sally Hollis contributed to the first version of the review as follows: extraction of the data, analysis of the results (statistics), and writing of the systematic review. The editorial base would like to thank the following people who were the external referees for this review: Grant Anhalt, Vicky Werth (content experts), and Jean Sangster (consumer).
GK thanks the "Deutsche Forschungsgemeinschaft" (DFG) for their financial support in the past, which enabled her to build up her knowledge of autoimmune blistering diseases. This knowledge was of great value when conducting this review.
For this update of the review the authors would like to thank Dr Ching‐Chi Chi from Taiwan who translated the Chinese article by Liu 2009 and extracted the data of this article; Dr Ningning Dang, Consultant Dermatologist, Jinan Hospital, Department of Dermatology, China who translated the Chinese textbook section on the diagnosis of bullous pemphigoid, and Ingrid I. Riphagen, Medisch Informatiespecialist, VUmc Amsterdam, Netherlands who assisted in updating the review.
The Cochrane Skin Group editorial base would also like to thank Sue Jessop and Jo Leonardi‐Bee who acted as the Key and Statistical Editors respectively, Vicky Werth who was the clinical referee and Alison Devine who was the consumer referee.
Appendices
Appendix 1. CSG Specialised Register search strategy
((bullous and pemphigoid) OR (pemphigoid AND NOT gestationis)) AND ((drug AND therapy) OR treatment* OR medication* OR predniso* OR corticosteroid* OR steroid* OR azathioprine OR immunosuppres* OR dapsone OR erythromycin* OR tetracyclin* OR nicotinamide OR cyclophosphamide OR cyclosporin* OR sulph* OR methotrexate OR plasmaph* OR (mycophenolate and mofetil))
Appendix 2. Cochrane Library search strategy
#1(bullous pemphigoid) OR (pemphigoid NOT gestationis) #2MeSH descriptor Pemphigoid, Bullous explode all trees in MeSH products #3(#1 OR #2) #4SR‐SKIN in All Fields in all products #5(#3 AND NOT #4)
Appendix 3. MEDLINE search strategy
1. randomised controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. (animals not (human and animals)).sh. 10. 8 not 9 11. bullous pemphigoid.mp. or exp Pemphigoid, Bullous/ 12. pemphigoid gestationis.mp. or exp Pemphigoid Gestationis/ 13. 11 not 12 14. 13 and 10
Appendix 4. EMBASE search strategy
1. random$.mp. 2. factorial$.mp. 3. (crossover$ or cross‐over$).mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. (assign$ or allocat$).mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. bullous pemphigoid.mp. or exp Bullous Pemphigoid/ 15. pemphigoid gestationis.mp. or exp Pemphigoid Gestationis/ 16. 14 not 15 17. 16 and 13
Data and analyses
Comparison 1. Higher dose prednisolone (1.25 mg/kg p/solone) versus lower dose prednisolone (0.75 mg/kg p/solone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Healing of skin lesions at day 21: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Healing of skin lesions at day 51: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Healing of skin lesions at day 51: ITT (assuming unknown = not healed) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mortality at day 51 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Methylprednisolone (mp/solone) versus prednisolone (p/solone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control ‐ number of blisters at day 10 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Overall improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Disease control ‐ extent of erythema at day 10 (score out of 3) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Quality of life ‐ extent of itching (score out of 3) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Prednisone + azathioprine (p/sone+azathio) versus prednisone (p/sone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Disease control at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Disease control: well at 3 years, either needing or not needing further treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mortality and severe adverse events at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Mortality at 3 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Prednisolone plus plasma exchange (p/solone+plas/x) versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Disease control at 1 month (controlled with 0.3 mg/kg prednisolone) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Disease control at 1 month (controlled with 1.0 mg/kg prednisolone or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Disease control at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Disease control at 1 month ‐ cumulative steroid dose (g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality and severe adverse events at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mortality at 1 month: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Mortality at 1 month: ITT worst case | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Prednisolone plus azathioprine (p/solone+azathio) versus prednisolone plus plasma exchange (p/solone+plas/x).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality and adverse events at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Nicotinamide plus tetracycline (nicot+tetracy) versus prednisone (p/sone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Complete response at 8 weeks: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Complete or partial response at 8 weeks: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Clobetasol propionate cream (clobetasol) versus oral prednisone (oral p/sone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease control at day 21 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Prednisone 0.5 mg/kg for moderate disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Prednisone 1 mg/kg for extensive disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Severe complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Prednisone 0.5 mg/kg for moderate disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Prednisone 1 mg/kg for extensive disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mortality at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Prednisone 0.5 mg/kg for moderate disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Prednisone 1 mg/kg for extensive disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Mild regimen clobetasol propionate cream (mild clobetasol) regimen versus standard clobetasol propionate cream (stnd clobetasol) regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Healing of skin lesions: complete (at day 21) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Intention‐to‐treat analysis, all participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Moderate disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Extensive disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of relapses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Moderate disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Extensive disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Jingui Shenqi Pill (JSP) 1# bid plus prednisone (JSP pill+p/sone) versus prednisone (p/sone) alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Healing at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Complete healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Overall healing ‐ participants experiencing any degree of healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Azathioprine plus methylprednisolone (azathio+mp/solone) versus mycophenolate mofetil plus methylprednisolone (MMF+mp/solone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Healing of lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Complete healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beissert 2007.
| Methods | Multicentre in Germany, central randomisation, not blinded 2 parallel groups; initial dose was maintained until blister formation ceased and re‐epithelialisation started. Corticosteroid dose was then reduced every 2 weeks; after discontinuation of corticosteroid, azathioprine, or mycophenolate mofetil (MMF) dose was maintained for 4 more weeks, then reduced (see taper regimen page 1537) Follow‐up of 720 days |
|
| Participants | 73 participants with bullous pemphigoid, confirmed by direct and indirect immunofluorescence on salt split skin | |
| Interventions | A: 38/38 oral methylprednisolone 0.5 mg/kg/day plus azathioprine sodium 2 mg/kg/day. B: 35/35 oral methylprednisolone 0.5 mg/kg/day plus mycophenolate mofetil 2000 mg/day (tapering described on page 1537). |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Cumulative corticosteroid dose: described as primary outcome until complete healing was achieved (page 1537); on page1539 the cumulative corticosteroid dose was defined as corticosteroid dose until the end of the documentation period (> 720 days) (Table 2, Table 3). We presumed that calculated cumulative corticosteroid dose is calculated until the end of the documentation period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was stratified according to the clinical centre and performed centrally with the use of random number of three for each stratum." (Page 1537) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not well described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Intentionally not blinded by trial investigators. Quote: "Since complete healing was a primary outcome measure, blinding was not considered necessary." (Page 1537) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes. In Beissert 2007 1 participant was lost, 2 died of treatment unrelated causes ‐ they were included in the intention‐to‐treat analysis. The same number of participants who started the trial were analysed at the end of the trial. (Figure 1, page 1538) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported for both outcome measures. Primary:
Secondary:
|
| Other bias | Low risk | No other bias. |
Burton 1978.
| Methods | Randomised but not blind Intervention took place over 3 years, treatment maintained at starting doses until 'rash suppressed' at which point prednisone doses were gradually reduced in both groups. If rash did not recur then also azathioprine was gradually withdrawn in the azathioprine group Follow‐up: at the end of the 3‐year treatment period |
|
| Participants | 25 participants with BP confirmed by IF studies | |
| Interventions | A: 12/12 participants prednisone (30 to 80 mg/day) + azathioprine (2.5 mg/kg/day). B: 13/13 participants prednisone (30 to 80 mg/kg/day). |
|
| Outcomes | Unclear outcome measures:
|
|
| Notes | After 1 week of prednisolone to suppress lesions, consultant decided whether to include in trial. Not clear how prednisolone dose decided or numbers of participants on lower or higher doses in each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Once included, each participant was randomly assigned ......by the ward sister who drew a marked paper from an envelope." (Page 1190) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear. Unlikely as the intervention group (azathioprine) were monitored by frequent blood testing. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. The results section states that 25 participants completed a 3‐year follow‐up, but it is unclear how many were randomised to each group at the start. Table 1 page 1190 reports outcomes over 3 years for all 25 participants. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures were not clearly stated. |
| Other bias | Unclear risk | Only those participants who were likely to attend for follow up were recruited. Elibility was also determined by the consultant doctor after baseline testing. Baseline imbalance: There were more participants with severe disease in the azathioprine group, 53% had ≥ 20% body surface area involvement compared to only 27% in the MMF group. |
Dreno 1993.
| Methods | Randomised, double‐blind Disease control = reduction of blisters, redness & itch > 50% Follow‐up: 10 days (= treatment period) |
|
| Participants | 57 participants with BP confirmed by IF studies | |
| Interventions | A: 29/29 participants prednisolone (1.16 mg/kg/day). B: 28/28 participants methylprednisolone (1.17 mg/kg/day). |
|
| Outcomes |
|
|
| Notes | Problem: 'scale' for measuring symptoms & signs. Very short study duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Yes: double‐blind. Quote: "Taking into account the difference in presentation between the 2 products, the supply of the products to the participants was made by a person other than the investigator; additionally clinical follow‐up after the end of the study was done by a masked (blinded) investigator." (Translation, page 518) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29/29 evaluated at both time points for prednisolone group. 27/28 evaluated at both times points for the methylprednisolone group. 1 participant dropped out (ceased treatment) after 8 days of treatment, due to a coma unrelated to the treatment (page 519). |
| Selective reporting (reporting bias) | Low risk | All outcomes (number of blisters, itching, and redness) reported on at 5 and 10 days of treatment. |
| Other bias | Unclear risk | Short study duration. Non‐validated assessment scales. |
Fivenson 1994.
| Methods | Randomised open‐label, but randomisation method not stated, not blind Disease control after 8 weeks Rx: complete response = 100% clearing of all lesions, partial response ≥ 50%, no response < 25%. Pruritus and physician's global assessment were also recorded. Disease recurrence in the follow up period was recorded if: new blisters, urticarial lesions and/or crusts were noted. Follow‐up: 10 months (= treatment period) |
|
| Participants | 20 participants with BP confirmed by IF studies | |
| Interventions | A: 6/6 participants prednisone 40 to 80 mg/day. B: 14/14 participants nicotinamide 1500 mg/day in 3 divided doses + tetracycline 2 g/day 4 divided doses. |
|
| Outcomes |
|
|
| Notes | Not clear how the prednisone dose was decided or number of participants on higher or lower dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given. |
| Allocation concealment (selection bias) | Unclear risk | Unclear; no details given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blind, described as "open‐label" (page 753). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 were randomised, 18 were treated. 2 were unavailable for follow up within the initial 8 weeks, both from the tetracycline/nicotinamide group. There was 1 death in the prednisone group due to sepsis complicated by aspiration pneumonia, the time point was not given but the participant was available for follow up at week 8 as the results for all 8 participants who received prednisolone is given in Table 1 (page 755). At longer term follow‐up, only n = 3 in the prednisolone group and n = 5 in the tetracycline/nicotinamide group are reported, no detail of the reasons for loss to follow up at this later follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported at each time point. |
| Other bias | Unclear risk | Unclear if the participant groups were equivalent with respect to disease severity or demographics at the start of the therapy. |
Guillaume 1993.
| Methods | Randomised, not blind Disease control = no new blisters for 4 weeks, prednisolone dose decreased gradually to 0.5 mg/kg at 3 months and 0.2 mg/kg at 6 months Follow‐up: 6 months ( = treatment period) |
|
| Participants | 100 participants with BP confirmed by IF studies | |
| Interventions | 31/32 participants prednisolone 1 mg/kg/day versus 36/36 participants azathioprine + prednisolone 1 mg/kg/day versus 31/32 participants plasma exchange + prednisolone 1 mg/kg/day. | |
| Outcomes |
|
|
| Notes | Trial stopped at interim period due to no appreciable benefit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. Quote: "According to pre‐established randomisation lists equilibrated in blocks of 3 for each center." (Page 50) |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No details given about how the allocation was concealed from the study investigator. Quote: "After determining a patient's eligibility, the attending physician telephoned the study co‐ordinator who assigned the patient." (Page 50) |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was not blind. See "Treatment Groups", page 50. "Techinically difficult and theoretically debatable to perform 'sham' plasma exchanges". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for 2 dropouts not clear. Quote: "unavailable for follow‐up after having withdrawn their consent" (page 51) (not clear if this was before or after starting treatment). Dropouts not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Controlled disease was stated to be no more than 1 new blister occurring 4 weeks after starting treatment, resolution of erythema, and no more than minimal pruritis. Only the composite measure of controlled disease was reported (Table 2, page 52). |
| Other bias | Unclear risk | Trial stopped early. Quote: "Our trial was interrupted after the interim analysis showed no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone in the initial (at 4 weeks) and maintenance (at 6 months) treatments of BP" (page 52). |
Joly 2002.
| Methods | Randomised, not blinded Disease control = number of new blisters after 3 weeks (21 days) of treatment. (Not clear if participant kept record of new blisters daily or if all new blisters since previous visit were averaged out to get a daily rate) |
|
| Participants | 341 participants with BP confirmed by IF studies | |
| Interventions | Moderate disease: A: 40 g topical clobetasol propionate cream twice daily to entire body 77/77 versus 0.5 mg/kg/day oral prednisone 76/76. Extensive disease: A: topical clobetasol 93/93 versus prednisone 1 mg/kg/day 95/95. |
|
| Outcomes |
|
|
| Notes | Originally there were 364 participants recruited: 14 did not meet the inclusion criteria, 8 did not give consent, 1 withdrew his consent in the beginning of the study: left with 341 study participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally with the use of random numbers in permuted blocks of four within each stratum." (Page 322) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not well described. Probably adequate as it was done centrally. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was not blinded. A nurse not otherwise associated with the study assessed the number of new bullae, daily. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants and outcomes adequately reported. |
| Selective reporting (reporting bias) | Low risk | Adequately reported. |
| Other bias | Unclear risk | Quote: "In accordance with the study protocol, the investigators switched three patients with moderate bullous pemphigoid and four with extensive bullous pemphigoid from the oral‐prednisone group to the topical‐corticosteroid group because of side‐effects of treatment." (Page 324) 'Compliance with treatment and adverse effects'. |
Joly 2009.
| Methods | Multicentre in France, centrally randomised; not blinded 2 different regimens were applied. In the mild regimen participants received doses depending on their body weight; follow‐up of 360 days |
|
| Participants | 312 participants with bullous pemphigoid; confirmed by direct immunofluorescence test | |
| Interventions | Mild regimen: clobetasol propionate cream 10 to 30 g/day, until 15 days after disease control, thereafter corticosteroid tapering over 4 months (159) moderate disease (≤10 new blisters/day): 20 g/day if body weight > 45 kg, 10 g/day if < 45 kg severe disease (>10 new blisters/day): 30 g/day if body weight > 45 kg, 20 g/day if < 45 kg. Standard regimen: clobetasol propionate cream 40 g/day initially, until 15 days after disease control, corticosteroid tapering over 12 months (page 1686) (153 participants). |
|
| Outcomes | Primary:
Secondary end points:
|
|
| Notes | Page 1686 typing error: should be 0.05% clobetasol propionate cream, not 0.005% Discrepancy between the numbers of participants as follows: the numbers in figure 1 (153 participants randomised, 150 participants analysed), text (153 participants randomised, 150 participants analysed), and table 2 (153 randomised and 153 analysed) for the standard regimen do not match Clarification from the study investigator (Joly 2010): numbers in table 2 on page 1684, are wrong, should be 150 in the standard regimen Only 150 were analysed, therefore intention‐to‐treat (ITT) analysis is not fulfilled Worst case scenario calculation (none of the 3 missing cases in the standard group had complete healing): ‐ 156/159 v 150/153 (ITT analysis), Analysis 8.1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally with the use of random numbers in permuted blocks of four within each stratum." (Page 1686) |
| Allocation concealment (selection bias) | Low risk | Allocation concealment is not well described. Probably adequate as it was done centrally. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Intentionally not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not all participants were accounted for at each stage of the trial. See Figure 1, page 1683, and Table 2, page 1686: typing error in table 2, only 150 participants were analysed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported adequately. |
| Other bias | Low risk | No other bias. |
Liu 2006.
| Methods | Healing of skin lesions (complete, partial, or no healing depending on the area affected) after 4 weeks treatment | |
| Participants | 30 participants with bullous pemphigoid; diagnosis confirmed by pathological tests | |
| Interventions | A: Jingui Shenqi Pill (JSP) 1# bid plus prednisone 0.5 to 1.0 mg/kg/day (15 participants). B: prednisone alone 0.5 to 1.0 mg/kg/day (15 participants). |
|
| Outcomes |
|
|
| Notes | Article in Chinese. Translation obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomisation details in English abstract; nor in paper. |
| Allocation concealment (selection bias) | Unclear risk | No details in English abstract; nor in paper. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded, no details in English abstract; nor in paper. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequately reported. |
| Selective reporting (reporting bias) | Low risk | Outcome is adequately reported. |
| Other bias | Unclear risk | Few details about any methods in translated paper. Precise method of diagnosis not specified (referred to as 'clinical' and 'pathological' tests). No further details available from the trial investigator. |
Morel 1984.
| Methods | Randomised but not blind Disease control = number new blisters between days 21 to 51 Follow‐up: 51 days ( = treatment period) |
|
| Participants | 50 participants with BP confirmed by IF studies. | |
| Interventions | A: 24/26 prednisolone 0.75 mg/kg/day. B: 22/24 prednisolone 1.25 mg/kg/day. |
|
| Outcomes |
|
|
| Notes | 2 dropouts in each group, no reasons given, and not included in analysis Erythromycin used for infection but its anti‐inflammatory effect not evaluated or commented upon |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a single table of pre‐established and balanced randomisation for all 8 patients." (Translation, page 926). |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No. No details given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 dropouts in each group, no reasons given, and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | New blister formation at days 21 and 51 reported. |
| Other bias | Unclear risk | Some participants that could have been recruited were excluded on the grounds that they were able to take part in a parallel study involving plasma exchanges. |
Roujeau 1984.
| Methods | Randomised but not blinded Disease control = complete disappearance of blisters, itch, and erythema Follow‐up: 6 months ( = treatment period) |
|
| Participants | 41 participants with BP confirmed by IF studies, age > 80 years excluded | |
| Interventions | A: 15/17 prednisolone 0.3 mg/kg/day. B: 22/24 plasma exchange + prednisolone 0.3 mg/kg/day. Both groups were treated with an identical protocol based on weekly dose adjustments according to the therapeutic results observed. Therapy was started at a dose of 0.3 mg/kg oral prednisolone daily for 1 week. If the treatment was ineffective, the dose was increased weekly to 1.5 mg/kg/day oral prednisolone, 2 mg/kg/day intramuscular methylprednisolone, and 2 mg/kg/day intramuscular methylprednisolone with (maximum permissible) 3 mg/kg/day oral cyclophosphamide. |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. Quote: "Assigned to groups according to lists drawn by microcomputer for each participating centre and equilibrated after every 4 patients." (Page 487) |
| Allocation concealment (selection bias) | Unclear risk | Probably done as randomisation was computerised. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 41 randomised, 2 from each group were excluded from analysis, reasons accounted for in Results (page 487). 2 participants from each group withdrawn from study, before treatment was initiated (1 had no BP, 2 accidentally received higher initial doses of prednisolone, 1 no plasma exchange). Dropouts not included in the analysis (they were randomised, but did not really start proper trial treatment and were excluded). Outcomes (disease control) are reported for the remaining 37 participants the groups they were randomised to. (Table 2, page 488). Side‐effects and mortality are reported in the text. (Table II and III). |
| Selective reporting (reporting bias) | Low risk | Yes. Control of blister formation, erythema, and pruritus, although this is only reported as a composite when bullae, pruritis, and erythema has resolved; this was reported as disease control. |
| Other bias | Low risk | No other bias. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12607000104459.
| Trial name or title | Rituximab in the adjuvant treatment of bullous pemphigoid: A prospective open‐label pilot study in three patients to study remission of disease with rituximab |
| Methods | A prospective open‐label pilot study in three participants to study remission of disease with rituximab |
| Participants | Bullous pemphigoid |
| Interventions | Rituximab 375 mg m‐2 weekly for 4 weeks |
| Outcomes | Primary:
Secondary:
These secondary outcomes were measured at 1 and 3 months post‐rituximab and 3‐monthly thereafter until study completion. Completion of study was at 17 months post‐rituximab but in 1 participant, follow‐up was ceased at 13 months post‐rituximab when they demonstrated clinical remission |
| Starting date | February 2007 |
| Contact information | Professor H. Miles Prince (miles.prince@petermac.org) Chair, Haematology Services Peter MacCallum Cancer Centre Locked Bag 1, A'Beckett St, East Melbourne Victoria 8006 Australia |
| Notes | ‐ |
ISRCTN13704604.
| Trial name or title | A randomised controlled trial to compare the safety and effectiveness of doxycycline (200 mg/day) with prednisolone (0.5 mg/kg/day) for initial treatment of bullous pemphigoid |
| Methods | Prospective, 2‐arm, single‐blind, parallel group, multicentre randomised controlled trial |
| Participants | 256 participants with bullous pemphigoid, confirmed by immunofluorescence tests |
| Interventions | Doxycycline (200 mg/day) versus prednisolone (0.5 mg/kg/day) |
| Outcomes | Primary:
Secondary: Differences in the 2 treatment arms in the:
Tertiary: Differences in the 2 treatment arms in the:
|
| Starting date | March 2009 |
| Contact information | Mrs Caroline Onions (blister@nottingham.ac.uk) Clinical Trials Unit Office, B39 Medical School Queens Medical Centre Nottingham NG7 2UH England |
| Notes | ‐ |
NCT00286325.
| Trial name or title | Rituximab in the treatment of participants with bullous pemphigoid |
| Methods | Non‐randomised, open label, active control, single group assignment, safety/efficacy study |
| Participants | Bullous pemphigoid |
| Interventions | Infusion of 1000 mg of rituximab on day 0 and day 14 |
| Outcomes |
|
| Starting date | March 2005 |
| Contact information | Russell Hall, III, MD Duke University Medical Center Durham, North Carolina 27710 United States |
| Notes | ‐ |
NCT00472030.
| Trial name or title | An open label case series on the effects of Xolair (Omalizumab) in bullous pemphigoid |
| Methods | An open label case series on the effects of Xolair (Omalizumab) in bullous pemphigoid |
| Participants | Bullous pemphigoid |
| Interventions | Subcutaneous administration of omalizumab, dose dependent on weight and circulating levels of IgE. Omalizumab will be administered every 4 weeks for 12 weeks. Additional injections will be given on weeks 2, 6,10, and 14 if required based on weight and IgE levels Versus prednisone |
| Outcomes |
|
| Starting date | August 2007 |
| Contact information | Janet A Fairley (janet‐fairley@uiowa.edu) & Debra S Brandt (debra‐brandt@uiowa.edu) Department of Dermatology University of Iowa USA |
| Notes | janet‐fairley@uiowa.edu |
NCT00525616.
| Trial name or title | Assessment of rituximab efficiency and tolerance in treatment of bullous pemphigoid |
| Methods | Non‐randomised, open label, uncontrolled, single group assignment, safety/efficacy study |
| Participants | 20 participants with bullous pemphigoid |
| Interventions | 2 IV perfusions of 1000 mg at 15 day intervals |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | December 2008 |
| Contact information | Pascal Joly, MD, PhD (pascal.joly@chu‐rouen.fr) Rouen University Hospital Direction de la Recherche et de l'Innovation Rouen 76031 France |
| Notes | pascal.joly@chu‐rouen.fr |
NCT00802243.
| Trial name or title | Leflunomide associated with topical corticosteroids for bullous pemphigoid. An open prospective study |
| Methods | Open label, uncontrolled, single group assignment, efficacy study |
| Participants | 54 participants with bullous pemphigoid |
| Interventions | 40 g topical clobetasol propionate cream per day plus 20 mg leflunomide per day |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | September 2007 |
| Contact information | Christophe Bedane (christophe.bedane@chu‐limoges.fr) Limoges University Hospital Limoges 87042 France |
| Notes | Christophe |
NCT00809822.
| Trial name or title | NPB‐01 (intravenous immunoglobulin) therapy for patients with bullous pemphigoid unresponsive to corticosteroids: Randomized, double‐blind, placebo control, parallel assignment study (phase II) |
| Methods | Randomised, double‐blind (subject, investigator), placebo‐control, parallel assignment, safety/efficacy study |
| Participants | 20 participants with bullous pemphigoid |
| Interventions | NPB‐01 Intravenous immunoglobulin versus placebo physiological saline |
| Outcomes |
|
| Starting date | December 2008 |
| Contact information | Yasumasa Ogawa (kaihatsu@nihon‐pharm.co.jp) Nihon Pharmaceutical Co. Ltd Japan |
| Notes | ‐ |
Differences between protocol and review
We changed the wording of the primary outcome "regression or healing of skin lesions" which referred to the "rate of" and "when/how soon?" as these are time‐to‐event measures which are complicated to measure and analyse (and not often reported in trials). We added "at time periods specified by individual trials".
We made minor changes to the secondary outcomes of systemic infection and mortality. We had originally intended to look at systemic infection and mortality as a result of the primary disease and as a result of treatment. At the time of the first published version of the review, we decided that these data were unlikely to be available and we no longer include them.
The original protocol of this review stated that the Jadad quality assessment scale would be used, which also similarly assesses randomisation, blinding, withdrawals, and dropouts (Jadad 1996). We assessed all these aspects but reported them individually (see 'Risk of bias' tables in the Characteristics of included studies) rather than as a summary score, as the use of scales for assessing quality or risk of bias is explicitly discouraged in Cochrane reviews (Higgins 2008; section 8.3.3) .
We have reclassified the outcomes as primary and secondary outcomes.
We have changed the measures of treatment effect to risk ratio (RR) from odds ratio (OR) in accordance with Cochrane Skin Group policy.
Contributions of authors
GK contributed to the writing of the protocol, extraction of data, analysis of the results, the writing of the systematic review, and updating the review.
PM contributed to the extraction of the data, analysis of the results (statistics), writing of the systematic review, and provided comments on the updated review.
CB contributed to the 'Risk of bias' tables for the updated review, corresponded with trial investigators, contributed to writing and editing the text, revised some of the analyses, and (with GK) responded to the peer referees' comments.
DFM contributed to the writing of the protocol, extraction of data, and analysis of the results of the first version of the systematic review and editing the text of the current version.
FW contributed to the writing of protocol and analysis of the results of the first version of the systematic review.
NPK was the first author of the first version of the systematic review and was the main contributor to that version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Nuffield Trust Fellowship scheme, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Beissert 2007 {published data only}
- Beissert S, Werfel T, Frieling U, Böhm M, Sticherling M, Stadler R, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Archives of Dermatology 2007;143(12):1536‐42. [DOI] [PubMed] [Google Scholar]
Burton 1978 {published data only}
- Burton JL, Harman RR, Peachy RD, Warin RP. Azathioprine plus prednisone in treatment of pemphigoid. British Medical Journal 1978;2:1190‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreno 1993 {published data only}
- Dreno B, Sassolas B, Lacour P, Montpoint S, Lota I, Giordano F, et al. Methylprednisolone versus prednisolone methylsulfobenzoate in pemphigoid: A comparative multicenter study. Annales de Dermatologie et de Vénéréologie 1993;120:518‐21. [PubMed] [Google Scholar]
Fivenson 1994 {published data only}
- Fivenson DP, Breneman DL, Rosen GB, Hersh CS, Cardone S, Mutasim D. Nicotinamide and tetracycline therapy of bullous pemphigoid. Archives of Dermatology 1994;130(6):753‐8. [PubMed] [Google Scholar]
Guillaume 1993 {published data only}
- Guillaume JC, Vaillant L, Bernard P, Picard C, Prost C, Labeille B, et al. Controlled trial of azathioprine and plasma exchange in addition to prednisolone in the treatment of bullous pemphigoid. Archives of Dermatology 1993;129(1):49‐53. [PubMed] [Google Scholar]
Joly 2002 {published data only}
- Joly P, Benichou J, Lok C, Hellot MF, Saiag P, Trancrede‐Bohin E, et al. Prediction of survival for patients with bullous pemphigoid. A prospective study. Archives of Dermatology 2005;141:691‐98. [DOI] [PubMed] [Google Scholar]
- Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. New England Journal of Medicine 2002;346(5):321‐7. [DOI] [PubMed] [Google Scholar]
Joly 2009 {published data only}
- Joly P. Personal communication. Email communication clarifying the published report. 30 January 2010.
- Joly P, Roujeau JC, Benichou J, Delaporte E, D'Incan M, Dreno B, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: A multicenter randomized study. Journal of Investigative Dermatology 2009;129(7):1681‐7. [DOI] [PubMed] [Google Scholar]
Liu 2006 {published data only}
- Liu BG, Li ZY, Du M. Effects of Jingui Shenqi Pill combined prednisone on expression of glucocorticoid receptor and its clinical effect in treating bullous pemphigoid patients. Chinese Journal of Integrated Traditional and Western Medicine 2006;26(10):881‐4. [PubMed] [Google Scholar]
Morel 1984 {published data only}
- Morel P, Guillaume JC. Treatment of bullous pemphigoid with prednisolone only: 0.75 mg/kg/day versus 1.25 mg/kg/day. A multicenter randomized study. Annales de Dermatologie et de Vénéréologie 1984;111(10):925‐8. [PubMed] [Google Scholar]
Roujeau 1984 {published data only}
- Roujeau JC, Guillaume JC, Morel P, Crickx B, Dalle E, Doutre MS, et al. Plasma exchange in bullous pemphigoid. Lancet 1984;2(8401):486‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12607000104459 {published data only}
- ACTRN12607000104459. Rituximab in the adjuvant treatment of bullous pemphigoid: A prospective open‐label pilot study in three patients to study remission of disease with rituximab. http://www.anzctr.org.au/ACTRN12607000104459.aspx (accessed 27 July 2010).
ISRCTN13704604 {published data only}
- ISRCTN13704604. A randomised controlled trial to compare the safety and effectiveness of doxycycline (200 mg/day) with prednisolone (0.5 mg/kg/day) for initial treatment of bullous pemphigoid. http://www.controlled‐trials.com/ISRCTN13704604/Bullous+Pemphigoid (accessed 20 April 2009).
NCT00286325 {published data only}
- NCT00286325. Rituximab in the treatment of patients with bullous pemphigoid. http://www.clinicaltrials.gov/ct2/show/NCT00286325 (accessed 20 April 2009).
NCT00472030 {published data only}
- NCT00472030. An open label case series on the effects of Xolair (Omalizumab) in bullous pemphigoid. http://clinicaltrials.gov/show/NCT00472030 (accessed 20 April 2009).
NCT00525616 {published data only}
- NCT00525616. Assessment of rituximab efficiency and tolerance in treatment of bullous pemphigoid. http://www.clinicaltrials.gov/show/NCT00525616 (accessed 20 April 2009).
NCT00802243 {published data only}
- NCT00802243. Leflunomide associated with topical corticosteroids for bullous pemphigoid. An open prospective study. http://clinicaltrials.gov/show/NCT00802243 (accessed 20 April 2009).
NCT00809822 {published data only}
- NCT00809822. NPB‐01 (intravenous immunoglobulin) therapy for patients with bullous pemphigoid unresponsive to corticosteroids: Randomized, double‐blind, placebo control, parallel assignment study (phase II). http://clinicaltrials.gov/show/NCT00809822 (accessed 20 April 2009).
Additional references
Adam 1992
- Adam BA. Bullous diseases in Malaysia: Epidemiological and natural history. International Journal of Dermatology 1992;31(1):42‐5. [DOI] [PubMed] [Google Scholar]
Ahmed 1977
- Ahmed AR, Maize JC, Provost TT. Bullous pemphigoid: Clinical and immunologic follow‐up after successful treatment. Archives of Dermatology 1977;113(8):1043‐6. [DOI] [PubMed] [Google Scholar]
Bernard 1995
- Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Archives of Dermatology 1995;131(1):48‐52. [PubMed] [Google Scholar]
Colbert 2004
- Colbert RL, Allen DM, Eastwood D, Fairley JA. Mortality rate of bullous pemphigoid in a US medical center. Journal of Investigative Dermatology 2004;122(5):1091‐5. [DOI] [PubMed] [Google Scholar]
Garg 1994
- Garg V, Jusko WJ. Bioavailability and reversible metabolism of prednisone and prednisolone in man. Biopharmaceutics and Drug Disposition 1994;15(2):163‐72. [DOI] [PubMed] [Google Scholar]
Gudi 2005
- Gudi VS, White MI, Cruickshank N, Herriot R, Edwards SL, Nimmo F, et al. Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North‐east Scotland. British Journal of Dermatology 2005;153(2):424‐7. [DOI] [PubMed] [Google Scholar]
Hadi 1988
- Hadi SM, Barnetson RS, Gawkrodger DJ, Saxena U, Bird P, Merrett TG. Clinical, histological and immunological studies in 50 patients with bullous pemphigoid. Dermatologica 1988;176(1):6‐17. [DOI] [PubMed] [Google Scholar]
Hertl 2008
- Hertl M, Zillikens D, Borradori L, Bruckner‐Tuderman L, Burckhard H, Eming R, et al. Recommendations for the use of rituximab (anti‐CD20 antibody) in the treatment of autoimmune bullous skin diseases. Journal der Deutschen Dermatologischen Gesellschaft 2008;6(5):366‐373. [DOI: 10.1111/j.1610‐0387.2007.06602.x] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. Available from www.cochrane‐handbook.org.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jin 1993
- Jin P, Shao C, Ye G. Chronic bullous dermatoses in China. International Journal of Dermatology 1993;32(2):89‐92. [DOI] [PubMed] [Google Scholar]
Joly 2010
- Joly P, et al. Personal communication. Email communication clarifying the published report. 30 January 2010.
Kirtschig 1994
- Kirtschig G, Wojnarowska F, Marsden RA, Edwards S, Bhogal B, Black MM. Acquired bullous disease of childhood: Re‐evaluation of diagnosis by indirect immunofluorescence examination on 1 M NaCl split skin and immunoblotting. British Journal of Dermatology 1994;130(5):610‐6. [DOI] [PubMed] [Google Scholar]
Lebrun‐Vignes 1999
- Lebrun‐Vignes B, Roujeau JC, Bernard P, Delaporte E, Joly P, Prost C, et al. Prednisone is more effective than prednisolone metasulfobenzoate in the treatment of bullous pemphigoid. Archives of Dermatology 1999;135(1):89‐90. [DOI] [PubMed] [Google Scholar]
Lever 1953
- Lever WF. Pemphigus. Medicine 1953;32(1):1‐123. [DOI] [PubMed] [Google Scholar]
Logan 1987
- Logan RA, Bhogal B, Das AK, McKee PM, Black MM. Localization of bullous pemphigoid antibody ‐ An indirect immunofluorescence study of 228 cases using a split‐skin technique. British Journal of Dermatology 1987;117(4):471‐8. [DOI] [PubMed] [Google Scholar]
Morrison 1990
- Morrison LH, Diaz L, Anhalt GJ. Bullous pemphigoid. In: Wojnarowska F, Briggaman RN (Editors) editor(s). Management of blistering diseases. London: Chapman and Hall Medical, 1990:63‐82. [Google Scholar]
Nemeth 1991
- Nemeth AJ, Klein AO, Gould EW, Schachner LA. Childhood bullous pemphigoid. Clinical and immunologic features, treatment, and prognosis. Archives of Dermatology 1991;127(3):378‐86. [DOI] [PubMed] [Google Scholar]
Orange 1989
- Orange AP, Joost T. Pemphigoid in children. Pediatric Dermatology 1989;6(4):267‐74. [DOI] [PubMed] [Google Scholar]
Parker 2008
- Parker SR, Dyson S, Brisman S, Pennie M, Swerlick RA, Khan R, et al. Mortality of bullous pemphigoid: An evaluation of 223 patients and comparison with the mortality in the general population in the United States. Journal of the American Academy of Dermatology 2008;59(4):582‐8. [DOI] [PubMed] [Google Scholar]
Person 1977
- Person JR, Rogers RS. Bullous and cicatricial pemphigoid: Clinical, pathological, and immunologic collerations. Mayo Clinic Proceedings 1977;52:54‐66. [PubMed] [Google Scholar]
Reguiaï 2009
- Reguiaï Z, Tchen T, Perceau G, Bernard P. Efficacy of rituximab in a case of refractory bullous pemphigoid [Efficacite du rituximab dans une pemphigoide bulleuse en echec therapeutique.]. Annales de dermatologie et de Vénéréologie 2009;136(5):431‐4. [PUBMED: 19442800] [DOI] [PubMed] [Google Scholar]
Rollin 1993
- Rollin C, Chosidow O, Diquet B, Dutreuil C, Herson S, Revuz J, et al. Comparative study of availability of prednisolone after intestinal infusion of prednisolone metasulfobenzoate and prednisone. European Journal of Clinical Pharmacology 1993;44(4):395‐9. [DOI] [PubMed] [Google Scholar]
Roujeau 1998
- Roujeau JC, Lok C, Bastuji‐Garin S, Mhalla S, Enginger V, Bernard P. High risk of death in elderly patients with extensive bullous pemphigoid. Archives of Dermatology 1998;134(4):465‐9. [DOI] [PubMed] [Google Scholar]
Savin 1979
- Savin JA. The events leading to the death of patients with pemphigus and pemphigoid. British Journal of Dermatology 1979;101(5):521‐34. [DOI] [PubMed] [Google Scholar]
Savin 1987
- Savin JA. Death in bullous pemphigoid. Clinics in Dermatology 1987;5(1):52‐9. [DOI] [PubMed] [Google Scholar]
Tham 1998
- Tham SN, Thirumoorthy T, Rajan VS. Bullous pemphigoid: Clinico‐epidemiological study of patients seen at a Singapore skin hospital. Australasian Journal of Dermatology 1998;25(2):68‐72. [DOI] [PubMed] [Google Scholar]
Venning 1992
- Venning VA, Wojnarowska F. Lack of predictive factors for the clinical course of bullous pemphigoid. Journal of the American Academy of Dermatology 1992;26(4):585‐9. [DOI] [PubMed] [Google Scholar]
Wojnarowska 1998
- Wojnarowska F, Eady RAJ, Burge SM. Bullous pemphigoid. In: Champion RH, Burton JL, Burns DA, Breathnach SM editor(s). Textbook of Dermatology. 6. Vol. 3, Oxford: Blackwell Science Ltd, 1998:1866‐73. [Google Scholar]
Wojnarowska 2002
- Wojnarowska F, Kirtschig G, Highet AS, Venning VA, Khumalo NP. Guidelines for the management of bullous pemphigoid. British Journal of Dermatology 2002;147(2):214‐221. [DOI] [PubMed] [Google Scholar]
Zillikens 1995
- Zillikens D, Wever S, Roth A, Weidenthaler‐Barth B, Hashimoto T, Bröcker EB. Incidence of autoimmune subepidermal blistering dermatoses in a region of central Germany (Comment). Archives of Dermatology 1995;131(1):48‐52. [DOI] [PubMed] [Google Scholar]
Zimmermann 1999
- Zimmermann R, Faure M, Claudy A. Prospective study of treatment of bullous pemphigoid by a class I topical corticosteroid. Annales de Dermatologie et de Vénéréologie 1999;126(1):13‐6. [PubMed] [Google Scholar]
References to other published versions of this review
Khumalo 2002
- Khumalo NP, Murrell DF, Wojnarowska F, Kirtschig G. A systematic review of treatments for bullous pemphigoid. Archives of Dermatology 2002;138(3):385‐9. [DOI] [PubMed] [Google Scholar]
Khumalo 2005
- Khumalo N, Kirtschig G, Middleton P, Hollis S, Wojnarowska F, Murrell DF. Interventions for bullous pemphigoid. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002292.pub3] [DOI] [PubMed] [Google Scholar]


