Abstract
Background
Gastro‐oesophageal reflux (GOR) is common and usually self‐limiting in infants. Cisapride, a pro‐kinetic agent, was commonly prescribed until reports of possible serious adverse events were associated with its use.
Objectives
To determine the effectiveness of cisapride versus placebo or non‐surgical treatments for symptoms of GOR.
Search methods
We searched the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Specialised Register and Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE, reference lists of relevant review articles and searched in the Science Citation Index for all the trials identified. All searches were updated in February 2009.
Selection criteria
Randomised controlled trials comparing oral cisapride therapy with placebo or other non‐surgical treatments for children diagnosed with GOR were included. We excluded trials with a majority of participants less than 28 days of age.
Data collection and analysis
Primary outcomes were a change in symptoms at the end of treatment, presence of adverse events, occurrence of clinical complications and weight gain. Secondary outcomes included physiological measures of GOR or histological evidence of oesophagitis. We dichotomised symptoms into 'same or worse' versus 'improved' and calculated summary odds ratios (OR). Continuous measures of GOR (for example reflux index) were summarised as a weighted mean difference. All outcomes were analysed using a random‐effects method.
Main results
Ten trials in total met the inclusion criteria. Nine trials compared cisapride with placebo or no treatment, of which eight (262 participants) reported data on symptoms of gastro‐oesophageal reflux. There was no statistically significant difference between the two interventions (OR 0.34; 95% CI 0.10 to 1.19) for 'same or worse' versus 'improved symptoms' at the end of treatment. There was significant heterogeneity between the studies, suggesting publication bias. Four studies reported adverse events (mainly diarrhoea); this difference was not statistically significant (OR 1.80; 95% CI 0.87 to 3.70). Another trial found no difference in the electrocardiographic QTc interval after three to eight weeks of treatment. Cisapride significantly reduced the reflux index (weighted mean difference ‐6.49; 95% CI ‐10.13 to ‐2.85; P = 0.0005). Other measures of oesophageal pH monitoring did not reach significance. One included study compared cisapride with Gaviscon (with no statistically significant difference). One small study found no evidence of benefit on frequency of regurgitation or weight gain after treatment with cisapride versus no treatment, carob bean or corn syrup thickeners.
Authors' conclusions
We found no clear evidence that cisapride reduces symptoms of GOR. Due to reports of fatal cardiac arrhythmias or sudden death, from July 2000 in the USA and Europe cisapride was restricted to a limited access programme supervised by a paediatric gastrologist.
Plain language summary
Cisapride treatment for gastro‐oesophageal reflux in young children
Gastro‐oesophageal reflux is the movement of stomach contents back into the oesophagus. A ring of smooth muscle (sphincter) at the lower end of the oesophagus near the stomach usually prevents this regurgitation. Relaxation of the sphincter, ineffective clearance of food from the oesophagus into the stomach, and delayed emptying of the stomach can all contribute to reflux. The peak incidence of reflux is generally at around four months of age and resolves by one to two years. Parents may seek medical help for the reflux if they are anxious or find the symptoms of regurgitation, crying, irritability, vomiting and, gagging difficult to tolerate. Some young children experience associated respiratory problems of chronic cough, wheezing, hoarseness, recurring bronchitis, pneumonia, apnoea or breath holding; and back‐arching, refusal to feed and sleep disturbance. Inflammation of the oesophagus may be evident with endoscopy or the child may fail to thrive and surgery may be required. Scintigraphy or sonography are used to monitor oesophageal motility.
Attention to the child’s position (by avoiding lying flat or a slumped seated position) and diet (thickened feeds, frequent small meals, non‐prescription stabilisers such as Gaviscon) may be effective in reducing reflux. Medications include prokinetic drugs given before a meal to stimulate gut motility and acid‐secretion inhibitors. Cisapride is a prokinetic drug used to improve symptoms and avoid serious complications of reflux. From this systematic review, we found no clear evidence of reduced symptoms of reflux with cisapride compared to placebo or no treatment. The parent or guardian of the child or the treating physician assessed the symptoms (regurgitation, crying, irritability, vomiting, gagging) at the end of treatment. Nine trials compared cisapride with placebo or no treatment, of which eight (262 participants) reported data on symptoms of gastro‐oesophageal reflux in children aged between five days and five years. They were followed up for two weeks to eight weeks.
Investigations of reflux can include oesophageal pH monitoring for 18 to 24 hours to determine the number of episodes of pH < 4, duration of the longest episode of pH < 4 and the presence of sleep reflux. These pH measurements poorly correlate with symptoms and responses of a child to treatment.
Cisapride significantly reduced the percentage of time the pH < 4 (reflux index) but not other measures of oesophageal pH monitoring
Fatal cardiac arrhythmia or sudden death have been associated with cisapride use in children and it is only used within restricted programmes under specialist supervision. One multicentre study of 134 children found no electrocardiographic QTc interval changes with cisapride.
Background
Description of the condition
Gastro‐oesophageal reflux (GOR), or the passage of gastric contents into the oesophagus, has a multifactorial pathophysiology. Regurgitation of stomach contents into the oesophagus is normally prevented by the action of the lower oesophageal sphincter (LOS). Two different mechanisms contribute to LOS tone. These are the skeletal muscle of the diaphragm which surrounds the oesophagus as it passes through the diaphragm and the smooth muscle at the gastro‐oesophageal junction. Low basal oesophageal sphincter pressure and transient relaxation of the LOS, oesophageal dysmotility resulting in impaired clearance, and delayed emptying of the stomach and duodenum can all contribute to GOR.
In most infants with GOR the outcome is benign. The determining factor in seeking medical assistance may be parental anxiety or intolerance of symptoms rather than the presence of significant complications. In a small minority, GOR is associated with significant problems such as respiratory sequelae (chronic cough, wheezing, apnoea, hoarseness, stridor, recurrent bronchitis, pneumonia), neuro‐behavioural manifestations (back‐arching, feeding refusal, rumination, non‐specific irritability, sleep disturbance), oesophagitis, oesophageal strictures, and failure to thrive. The term gastro‐oesophageal reflux disease (GORD) describes the association of definite pathology, in most cases oesophagitis, with reflux.
Infantile gastro‐oesophageal reflux has a peak incidence around four months and resolves spontaneously by one to two years of age in most patients (Nelson 1997; Rudolph 1996). A much smaller number of children have symptoms of GOR later in childhood; only some of these children will have had GOR in infancy. Less than 50% of children who develop reflux after the age of three years have spontaneous resolution of symptoms (Treem 1991). A substantial proportion of these children will have other problems including neurological or chronic respiratory disease.
A diagnosis of GOR is usually made on clinical grounds. Treatment for GOR, when this is deemed necessary, can be started before performing expensive and often unnecessary investigations (the Working Group of the European Society of Paediatric Gastro‐Enterology and Nutrition (ESPGAN) on Gastro‐Oesophageal Reflux, in Vandenplas 1993). Though not always essential for diagnosis, there are a number of investigations available to assess the cause and quantity of the reflux and to detect the presence of reflux‐related complications. Investigations are extended (18 to 24 hour) oesophageal pH monitoring, upper gastro‐intestinal endoscopy, oesophageal manometry, scintigraphy or sonography. These have low sensitivities and specificities and do not allow correlation of the reflux episodes with the patient's symptoms. Moreover, these investigations are generally poor predictors of how children will respond to treatment (Cucchiara 1996). A few variables, for example sleep reflux, acid clearing time, percentage time pH < 4 and oesophageal motility parameters, may predict which children are likely to have continuing problems despite medical treatment (Colson 1990; Cucchiara 1996; Varty 1993).
Description of the intervention
There are four main types of therapy in infants with GOR: surgery, drugs, dietary measures (thickened feeds, frequent small meals) and positioning (avoidance of slumped seated or supine postures). Surgical treatment is usually reserved for complicated cases, while most cases are treated with some combination of the other options. Pharmacological therapies include acid‐secretion inhibitors (for example cimetidine, ranitidine, omeprazole, lansoprazole) with or without prokinetic agents (for example cisapride, bethanechol, metoclopramide) when oesophagitis is present.
How the intervention might work
Cisapride is administered orally 15 to 30 minutes before a meal to ensure maximum plasma levels of the medication immediately after food intake. It is a gastrointestinal prokinetic agent which stimulates lower oesophageal, gastric, small intestinal and colonic motility, probably acting by enhancing the release of acetylcholine at the level of the myenteric plexus in the gut wall.
Why it is important to do this review
Although cisapride has never been licensed for children under 12 years of age, it has been prescribed to over 36 million children worldwide (Vandenplas 1999) including 19% of preterm newborns in Canadian neonatal units (Ward 1999). In 1999, a consensus statement by the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (Vandenplas 1999) recommended cisapride as the drug of first choice. stating that "the potential benefits far outweigh the potential risks and provide strong justification for its continued use". Since 1993 there have been 175 reports worldwide of fatal cardiac arrhythmia or sudden death associated with cisapride use, including at least two deaths in children, and 261 reports of non‐fatal but serious ventricular arrhythmias (Klausner 1998). These adverse events led to the withdrawal of cisapride from the UK and USA markets in July 2000 (Breckenridge 2000; Henney 2000). To date, the effectiveness of cisapride for the treatment of reflux in children has not been systematically evaluated. As cisapride continues to be used, albeit within restricted programmes in the USA (Henney 2000) and Europe (EMEA 2002), information on its effectiveness is required to enable clinicians and policy makers to decide whether the low risk of serious adverse events is outweighed by the benefits of treatment.
Objectives
The objective of this review was to compare the effectiveness of cisapride in reducing the symptoms of gastro‐oesophageal reflux with: 1. placebo or no treatment; 2. other medical treatments; 3. dietary interventions; 4. positioning; 5. any combination of the other treatments.
Methods
Criteria for considering studies for this review
Types of studies
Searches were carried out for randomised controlled trials comparing oral cisapride therapy with placebo or other non‐surgical treatments (other prokinetic drugs, with or without acid‐secretion inhibitors, dietary measures, positioning) in children with gastro‐oesophageal reflux. Searches for unpublished data are ongoing.
Types of participants
Children (aged less than 18 years) with a diagnosis of gastro‐oesophageal reflux, however defined. We excluded trials in which the majority of participants were aged less than 28 days. This is because cisapride is prescribed to neonates for feed intolerance, which is a different clinical entity from gastro‐oesophageal reflux that may be due to different physiological mechanisms. It may respond differently to cisapride than does GORD in older children (Enriquez 1998).
Types of interventions
Cisapride versus no treatment or placebo
Cisapride versus other medical therapies (bethanechol, metoclopramide, cimetidine, ranitidine, omeprazole, lansoprazole, Gaviscon)
Cisapride versus dietary interventions (small meals, thickened infant feeds)
Cisapride versus positioning (avoidance of slumped seated or supine postures)
Cisapride versus any combination of other non‐surgical therapies
Because of the restriction of surgical treatment to complicated cases of GOR, we did not expect to find randomised controlled trials in this area and they were not sought.
We looked for studies in which cisapride was administered orally for a minimum of one week.
Types of outcome measures
Cisapride treatment usually precedes physiological investigations for GOR and primarily aims to improve symptoms and avoid serious complications of GOR.
Primary outcomes
Symptoms, or changes in symptoms, of gastro‐oesophageal reflux (regurgitation, crying, irritability, vomiting, gagging) assessed subjectively by the parent or guardian of the child or by the treating physician, or both.
Presence of any of the following adverse events: abdominal pain, borborygmi, diarrhoea, headaches, hypersensitivity, convulsions, extrapyramidal effects, increased urinary frequency, liver function abnormalities, increased QTc interval on the electrocardiogram (ECG).
Occurrence of any clinical complications of GOR, e.g. respiratory symptoms.
Weight gain.
Secondary outcomes
Episodes of reflux measured by extended duration oesophageal pH monitoring: percentage of time during which pH < 4 ('reflux index'), number of episodes of pH < 4, number of episodes of pH < 4 lasting > 5 minutes, duration of longest episode of pH < 4.
Lower oesophageal sphincter (LOS) pressure measured by oesophageal manometry.
Histological evidence of oesophagitis on biopsy.
Included studies had to report at least one of the primary outcomes.
Search methods for identification of studies
See: Cochrane Upper Gastrointestinal and Pancreatic Diseases Review Group search strategy
For the first version of this review, searches were conducted of the Cochrane Central Trials Register (CCTR) and the specialised trials register of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group (see Review Group details for more information) using terms related to gastro‐oesophageal reflux and cisapride. In addition, the review authors searched the MEDLINE and EMBASE electronic databases. The search strategy included appropriate MeSH terms and text terms including: cisapride, gastro‐oesophageal reflux, idiopathic gastro‐oesophageal reflux, uncomplicated gastro‐oesophageal reflux, gastro‐oesophageal reflux disease, infantile reflux, regurgitation, excessive regurgitation, and with appropriate truncations and misspellings. These were combined with the use of the most sensitive Cochrane trials filters. Reference lists of relevant review articles and identified trials were scrutinised and forward citation searches were performed in the Science Citation Index on all trials identified. The drug manufacturers were contacted for any unpublished trials.
Searches were updated on The Cochrane Library, pre‐MEDLINE, EMBASE (5 April 2002) using the above strategy. An adapted strategy was used for PubMed. The pharmaceutical company Janssen was contacted for unpublished trials. The searches were re‐run in August 2003, May 2004, June 2005 and June 2006 and no new trials were found.
For the update in 2009, trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, Issue 4), MEDLINE (2005 to February 2009) and EMBASE (2005 to February 2009). We did not confine our search to English language publications. The Cochrane highly sensitive search strategy for identifying randomised trials in MEDLINE, sensitivity maximising version, Ovid format (Higgins 2009) was combined with the search terms in Appendix 1 in order to identify randomised controlled trials in MEDLINE. The MEDLINE search strategy was adapted for use in the other databases that were searched.
Data collection and analysis
Selection of studies
Titles and abstracts of all studies identified by the electronic searches were independently reviewed on screen by two review authors (CA, SM).
Data extraction and management
All potentially eligible studies were retrieved in hard copy and were independently reviewed by two researchers (CA, SM in 2002; SM, RG in 2009) against the inclusion criteria. Disagreements were resolved by consensus with provision for arbitration by a third review author, if required (RG or SL). Additional information was sought for one included trial (Cohen 1999). The reasons for exclusion of trials are given in the table 'Characteristics of excluded studies'.
Assessment of risk of bias in included studies
The methodological quality of the included trials was assessed independently by two review authors (CA, SM in 2002; SM, RG in 2009) using a checklist developed for this purpose. For each included trial, information was collected regarding the method of randomisation, allocation concealment, blinding of outcome assessment, and the relevant interventions and outcomes. Data were extracted independently by two review authors (CA, SM in 2002; RG and LCG in 2009) and any discrepancies were discussed and resolved. Data were then entered into the Review Manager software by one review author (CA or LCG) and accuracy was checked by two other authors (SM, RG).
Measures of treatment effect
For dichotomous data, a random effects model meta‐analysis was performed to determine a summary odds ratio (OR). Where continuous data outcomes were measured in a standard way across studies (for example reflux index), the pooled weighted mean difference (WMD) was calculated again using a random effects model. The denominators for these calculations were all children for whom outcome data had been reported. Analysis was by intention to treat. Sensitivity analyses involved re‐calculation of the summary OR for trials with good allocation concealment. For the primary outcome of 'same or worse' symptoms we analysed funnel plot asymmetry, and hence the likelihood of bias, using the regression method as described by Egger et al (Egger 1997). Evidence of heterogeneity was sought using a standard Chi2 statistic.
Assessment of heterogeneity
To assess heterogeneity, we conducted subgroup analyses according to study quality and produced a funnel plot for the primary outcome 'same or worse symptoms versus improvement' (Figure 1).
1.
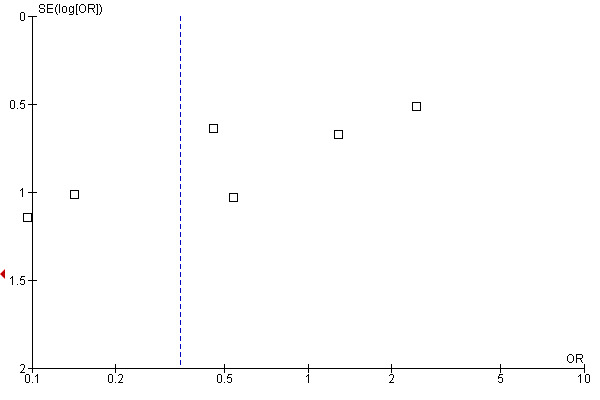
Funnel plot of comparison: 1 Main analysis for cisapride versus no treatment, outcome: 1.1 'Worse, same or slight improvement' versus 'moderate or excellent improvement'.
Data synthesis
We preferentially included symptoms assessed by parents as the parents are likely to have greater contact with their child and therefore have a more accurate view of the change in symptoms. However, we included physician measures of symptoms where parental assessments were not available, as we considered that they were assessing the same entity.
Studies reported symptoms or changes in symptoms at the end of the treatment period in a variety of ways. An a priori decision was made to dichotomise data as 'same or worse' versus 'improvement'. In a sensitivity analysis we examined the effect of redefining the outcomes as 'any symptoms' versus 'no symptoms'. Decisions about how best to dichotomise the data were reached by two review authors (RG, SL) without knowledge of the results. Changes in oesophageal pH measurements at the end of treatment were considered to be a secondary outcome as pH measurements are not reliable (Hampton 1990); they correlate poorly with symptoms and the response to treatment (Cucchiara 1996). We were able to analyse results for the reflux index (percentage of 24 hrs with oesophageal pH < 4) as this was the most commonly reported measure.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses were: in children under and over the age of one year, uncomplicated and complicated gastro‐oesophageal reflux, for those with neurological impairment, and in trials where the assessment of outcomes was blinded versus those in which assessment was non‐blinded. These were not conducted as appropriate trials were not identified.
Results
Description of studies
Results of the search
Ten trials met the inclusion criteria (see Characteristics of included studies). Nine of these compared the effects of cisapride with placebo or no treatment (Cohen 1999; Cucchiara 1987; Escobar Castro 1994; Levy 2001; Moya 1999; Scott 1997; Van Eygen 1989(a); Van Eygen 1989(b); Vandenplas 1991). Limited results were reported from an unpublished study of symptomatic children over two months old with biopsy‐proven oesophagitis that compared cisapride with cimetidine and placebo (Orenstein 2000). The results were published only in abstract form but the lead investigator provided a summary in a personal communication. Due to the limited nature of the data provided (see Characteristics of included studies), this study could not be included in our analysis.
Included studies
In two studies (Scott 1997; Vandenplas 1991) positioning or thickened feeds, or both, were given in both the experimental and control arms. One paper (Van Eygen 1989(a),(b)) presented data from three trials. Two of these were on different patient populations and met our inclusion criteria; they were reviewed separately and are identified accordingly. In one trial (Greally 1992) cisapride was compared with Gaviscon with or without Carobel. The most recently identified study was a four‐arm trial with six to eight participants in each arm. This study compared cisapride with no treatment and with thickened feeds using carob bean or corn syrup (Moya 1999). The study reported on two of our primary outcomes: change in the number of regurgitations and weight gain.
Most studies reported outcomes based on a change in symptoms following the intervention. The exceptions were: the Escobar Castro 1994 study which reported symptoms at the end of the treatment period; and the study by Cucchiara 1987 in which improvement in symptoms alone could not be separated from improvement in the pH probe results and histopathological changes. Both these studies were included and their contribution to the overall results was explored in a sensitivity analysis. Moya 1999 did not report overall symptom improvement for each participant but analysed mean number of regurgitations during the treatment period as well as mean daily weight gain.
The study by Levy 2001 only reported data on the QTc interval of the ECG. These data were from a seven‐centre double‐blind, placebo‐controlled study of 134 children on the safety and efficacy of cisapride. It was conducted from 1991 to 1994 with the support of Janssen Pharmaceutical Inc. Levy et al stated that "because efficacy results did not reach statistical significance (possibly because inclusion criteria were too broadly defined), they were not published". The response to a request to Janssen‐Cilag Ltd UK for unpublished data was that they were not aware of any unpublished data. No further information has been provided. The children included in the trials were aged between five days and five years. They had a diagnosis of GOR, defined by clinical symptoms alone or with additional oesophageal pH monitoring. Children were generally excluded from the trials if they: required concomitant therapy with drugs interfering with assessment of the study drug; had reflux caused by known anatomic abnormalities; had underlying disease; had an infection of the gastrointestinal (GI) tract or other organ system; or had neurologic, metabolic, or renal disorders (see table 'Characteristics of included studies').
The dosage of cisapride that was used was 0.8 mg/kg/day, with three exceptions: in one study (Cucchiara 1987) 0.9 mg/kg/day was used, in another (Van Eygen 1989(a)) 0.45 mg/kg/day, and in another study (Levy 2001) 0.6 mg/kg/day. One study (Moya 1999) stated that the usual dosage was given in three divided doses but did not specify the amount. The duration of follow up varied slightly over all studies: four studies followed participants up for two weeks (Cohen 1999; Cucchiara 1987; Moya 1999; Vandenplas 1991); four studies up to four weeks (Escobar Castro 1994; Greally 1992; Van Eygen 1989(a); Van Eygen 1989(b)); one up to six weeks (Scott 1997), and another for eight weeks (Greally 1992). In five of the nine included trials that compared the effects of cisapride with placebo or no treatment cisapride had been supplied by the manufacturing company (Cohen 1999; Greally 1992; Scott 1997; Van Eygen 1989(a); Van Eygen 1989(b)).
Excluded studies
Eight studies identified by the search strategy were excluded: in two (Cucchiara 1990; Saye 1987) cisapride had been given for less than 24 hours; in three (Barnett 2001; Pezzati 2001; McClure 1999) cisapride was administered to preterm infants with feed intolerance; in two studies cisapride was administered prophylactically to preterm infants for feed intolerance (Enriquez 1998; Reddy 2000); and two studies did not report any of the outcomes required for inclusion in the review (Heine 1996; McClure 1999). Please see the 'Characteristics of excluded studies' table for further details.
Risk of bias in included studies
Two trials reported adequate allocation concealment (Cohen 1999; Scott 1997). In the remaining eight trials the method used for allocation concealment was unclear (Figure 2). In one study (Greally 1992) a double‐blind design was not feasible due to the different mode of preparation and time of administration of cisapride and Gaviscon with or without Carobel. Eight trials stated that they were double blind (Cohen 1999; Cucchiara 1987; Escobar Castro 1994; Greally 1992; Levy 2001; Scott 1997; Van Eygen 1989(a); Vandenplas 1991; Van Eygen 1989(b)). Details of blinding of participants were often given but no trial gave explicit information on the blinding of key study personnel for the duration of the study (Figure 2). The most recent study to be included in this review (Moya 1999) was not blinded and provided no information on how randomisation was performed or how allocation was concealed.
2.
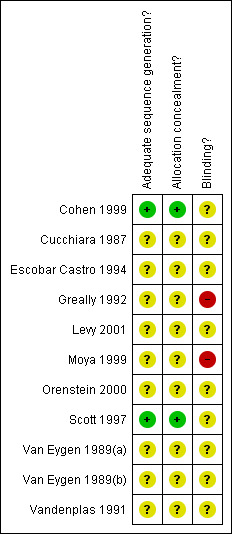
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
In one study all of the randomised infants completed the study (Greally 1992). Losses to follow up in the other studies varied from as little as one and three (Cucchiara 1987; Escobar Castro 1994), respectively, to as much as 30% of the randomised population in three studies (Cohen 1999; Levy 2001; Vandenplas 1991). In the study by Cucchiara 1987 the total number of participants included was reported, as was the number of participants for each group who completed the trial; the number randomised to the treatment and control arms was not. For this study we made the assumption that randomisation had produced equal numbers in each group. Moya 1999 provided no information on losses to follow up but we assumed that all participants completed the study.
Effects of interventions
Cisapride versus placebo or no treatment
Eight trials including 262 participants compared symptoms of GOR after treatment with cisapride or no treatment. Analysis of symptoms was based on parental evaluation in five trials (Cohen 1999; Escobar Castro 1994; Moya 1999; Scott 1997; Vandenplas 1991) and physician assessment in the remaining three trials (Cucchiara 1987; Van Eygen 1989(a); Van Eygen 1989(b)). The pooled OR (random‐effects model) for cisapride versus placebo for 'same or worse' symptoms versus 'improvement' based on seven trials (excluding Moya 1999) was 0.34 (95% CI 0.10 to 1.19) (Analysis 1.1). The trial by Moya 1999 showed no significant difference in the number of regurgitations per day between groups treated with cisapride or no treatment, at 15 days follow up (Analysis 1.2).
1.1. Analysis.
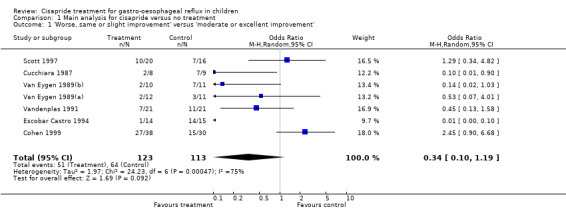
Comparison 1 Main analysis for cisapride versus no treatment, Outcome 1 'Worse, same or slight improvement' versus 'moderate or excellent improvement'.
1.2. Analysis.
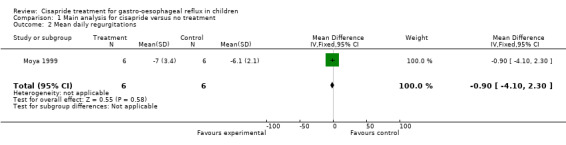
Comparison 1 Main analysis for cisapride versus no treatment, Outcome 2 Mean daily regurgitations.
The OR for cisapride versus placebo for 'same or worse' versus 'improvement' in symptoms after exclusion of the study by Escobar Castro 1994 (OR 0.59; 95% CI 0.22 to 1.59) or Cucchiara 1987 (OR 0.41; 95% CI 0.11 to 1.54) did not change appreciably (data not shown). Analysis of the effects of cisapride after re‐defining outcomes as 'any symptoms' versus 'no symptoms' was restricted to five trials (a total of 156 participants) (Cucchiara 1987; Escobar Castro 1994; Scott 1997; Van Eygen 1989(a); Van Eygen 1989(b); Vandenplas 1991). The pooled OR (random‐effects model) for cisapride versus no treatment was 0.19 (95% CI 0.08 to 0.44) (Analysis 4.3).
4.3. Analysis.
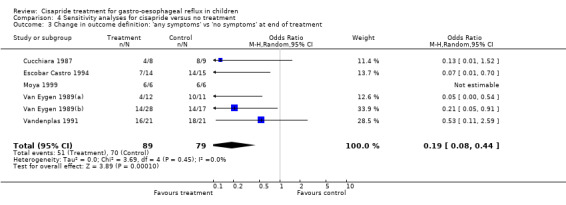
Comparison 4 Sensitivity analyses for cisapride versus no treatment, Outcome 3 Change in outcome definition: 'any symptoms' vs 'no symptoms' at end of treatment.
The analysis of 'same or worse' versus 'improvement' in symptoms showed significant heterogeneity (Chi2 = 24.23; df = 6; P = 0.0005; I2 = 75%) (Analysis 1.1). The reasons for the heterogeneity were not obvious from examination of the included trials, which were conducted in clinically similar populations and reported similar baseline event rates. The funnel plot was asymmetrical, suggesting an absence of small studies showing small or no benefit of cisapride. The intercept was ‐5.07 (95% CI ‐7.18 to ‐2.95). This result is consistent with publication bias favouring studies that showed a positive effect of cisapride. The results regarding the benefits of cisapride should therefore be interpreted with caution.
Adverse events
Adverse events (principally diarrhoea) were reported in four trials (a total of 190 participants). There were fewer adverse events in the non‐treatment group than in the cisapride group but the difference was not statistically significant (OR for cisapride versus placebo 1.86; 95% CI 0.88 to 3.93) (Analysis 1.5). One trial (Levy 2001) reported data on the QTc interval following three to eight weeks of either cisapride or placebo. No statistically significant differences were found between the two groups in either total QTc interval duration or change in QTc interval compared to baseline. Mean QTc was 408 ± 21 ms in the cisapride group and 399 ± 21 ms in the placebo group; the change in QTc was 1.7 ± 18 ms and 2.4 ± 20 ms, respectively.
1.5. Analysis.
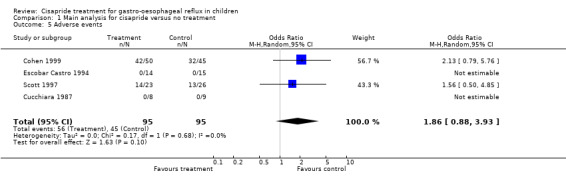
Comparison 1 Main analysis for cisapride versus no treatment, Outcome 5 Adverse events.
A primary outcome for our study was weight gain following the intervention but only two studies reported this outcome (Cohen 1999; Moya 1999). For the study by Cohen 1999 the mean difference in weight gain at the end of the two week trial was based on data provided by the authors. In the study by Cohen 1999 the difference between the two groups was not statistically significant (0.9 kg; 95% CI ‐0.38 to 2.18) (data not shown). Moya 1999 reported no difference in mean daily weight gain between infants given cisapride and those with no treatment (Analysis 1.3).
1.3. Analysis.
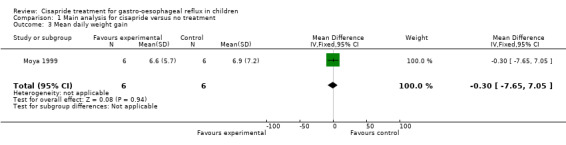
Comparison 1 Main analysis for cisapride versus no treatment, Outcome 3 Mean daily weight gain.
Use of cisapride was associated with a reduction in the reflux index, as measured by oesophageal pH monitoring, in five studies. The weighted mean difference for cisapride versus no treatment was ‐6.49 (95% CI ‐10.13 to ‐2.85) (Analysis 1.4). Several other physiological measured were reported. The differences between the cisapride and the placebo groups were not statistically significant for: number of episodes of pH < 4 in 24 hours (data available from two studies), number of reflux episodes lasting more than 5 min (data from three studies), or number over the course of a day (one study).
1.4. Analysis.
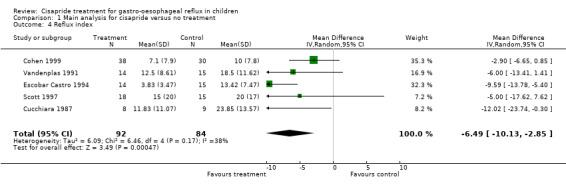
Comparison 1 Main analysis for cisapride versus no treatment, Outcome 4 Reflux index.
For this review we also looked for histological evidence of oesophagitis at biopsy. Three trials reported data. In Cohen 1999 only six of 68 participants underwent endoscopy and there was no difference in the presence of 'mild histologic oesophagitis' between the cisapride and the placebo group (2/4 versus 1/2). In Cucchiara 1987 the degree of oesophagitis was histologically defined as 'mild', 'moderate' or 'severe' and transformed into a score. Cisapride was more effective than placebo but only three of the 17 participants included in the study had severe oesophagitis at the beginning. In Scott 1997 the biopsy was repeated at the end of the trial only if oesophagitis had been present at baseline. The difference in abnormal findings between the cisapride and the placebo groups (7/11 versus 5/9) was not statistically significant.
Cisapride versus other medical interventions (Gaviscon)
One study comparing cisapride with Gaviscon (or Gaviscon and carob bean thickener in 21 of the 24 cases) was identified (Greally 1992). The 'same or worse symptoms' were slightly more common in the cisapride group than in the Gaviscon group but this difference was not significant (OR 3.26; 95% CI 0.93 to 11.38) (Analysis 2.1). The outcomes in this study were based on evaluations by parents who were not blind to the intervention.
2.1. Analysis.
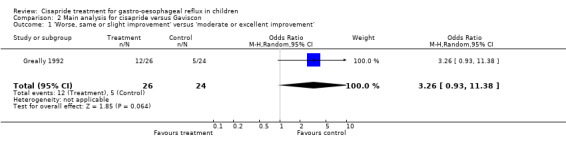
Comparison 2 Main analysis for cisapride versus Gaviscon, Outcome 1 'Worse, same or slight improvement' versus 'moderate or excellent improvement'.
Cisapride versus dietary interventions
One study compared cisapride with two dietary interventions, carob bean thickener and corn syrup (Moya 1999). As the authors did not provide details of overall symptom improvement for each patient, we were not able to calculate an OR for 'same or worse' versus 'improvement' in symptoms (Analysis 3.1). There was no significant difference in the number of regurgitations per day at the end of 15 days follow up between infants treated with cisapride, carob bean or corn syrup (Analysis 3.2; Analysis 3.4). There was a greater mean daily weight gain in infants given corn syrup compared with cisapride (MD ‐3.70; 95% CI ‐10.46 to 3.06) (Analysis 3.5) but no significant difference between carob bean and cisapride (Analysis 3.3). No adverse events were reported when comparing cisapride against the other interventions.
3.2. Analysis.
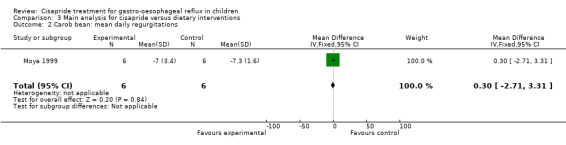
Comparison 3 Main analysis for cisapride versus dietary interventions, Outcome 2 Carob bean: mean daily regurgitations.
3.4. Analysis.
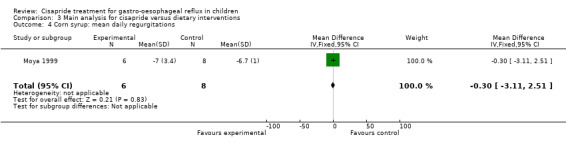
Comparison 3 Main analysis for cisapride versus dietary interventions, Outcome 4 Corn syrup: mean daily regurgitations.
3.5. Analysis.
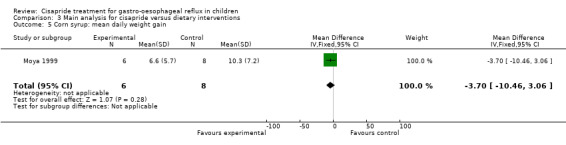
Comparison 3 Main analysis for cisapride versus dietary interventions, Outcome 5 Corn syrup: mean daily weight gain.
3.3. Analysis.
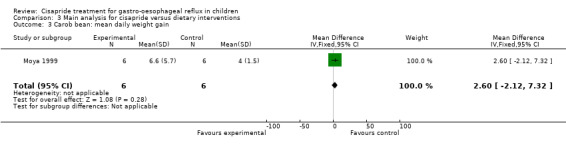
Comparison 3 Main analysis for cisapride versus dietary interventions, Outcome 3 Carob bean: mean daily weight gain.
Discussion
Summary of main results
We found no clear evidence for a significant effect of cisapride compared with placebo on symptoms of GOR in children. However, the midpoint estimate of the summary OR was 0.34. This result is likely to be an overestimate of the benefits of cisapride.
Overall completeness and applicability of evidence
The analysis of funnel plot asymmetry showed that the intercept from the regression analysis was ‐5.07 (Figure 1). In an analysis of 75 meta‐analyses published in The Cochrane Library and in four leading journals, Egger 1997 reported intercepts varying from ‐3.5 to +3.5. In comparison, our result shows extreme asymmetry reflecting an inverse association between study precision and an apparent beneficial effect of cisapride. While this relationship may reflect real differences between large and smaller trials, it may be explained by publication bias favouring submission or publication of small positive studies rather than small negative studies. This possibility is supported by the report of a multicentre trial of 96 children randomised to either cisapride or placebo (with or without cimetidine) which was not published "because efficacy results did not reach statistical significance (possibly because inclusion criteria were too broadly defined)" (Levy 2001). The six smallest studies in this review had unclear allocation concealment, which has been reported to be associated with an overestimate of treatment effect (Schulz 1995). If the analysis was restricted to the two trials with good allocation concealment the summary OR was 1.94 (95%CI 0.87 to 4.31) (data not shown).
Quality of the evidence
There was substantial heterogeneity of results between trials. The source of this heterogeneity is unclear as there was no obvious clinical heterogeneity (age, method of GOR diagnosis, dose and duration of interventions were broadly similar).
We found a statistically significant reduction in the reflux index with cisapride compared to no treatment. The reflux index is generally taken to be the percentage of time with pH < 4 over 24 hours of pH monitoring; the reduction suggested by this analysis was equivalent to 1.5 hours (with 95% CI of 40 minutes to almost 2.5 hours). This finding however has to be seen in the context of a lack of correlation between the reflux index and clinical symptoms and the evidence of publication bias.
A small study was found which compared the use of cisapride with Gaviscon or a combination of Gaviscon and carob bean thickener (Greally 1992), the results did not reach conventional levels of statistical significance. A further study showed no difference between cisapride and carob bean thickener or corn syrup for mean daily regurgitation frequency or daily weight gain (Moya 1999).
This review did not find a statistically significant difference in adverse events between cisapride and no treatment. However, serious adverse events are rare and are unlikely to be detected by small trials. Recent reports from surveillance studies of death and life threatening events potentially related to cisapride, together with the uncertain benefits of cisapride, have led to the decision to stop marketing cisapride in the United States (Henney 2000).
Authors' conclusions
Implications for practice.
Our findings of a lack of evidence for a beneficial effect of cisapride contradict previous widely held opinions to the contrary (Shulman 2000; Vandenplas 1999). This review found no statistically significant effect of cisapride on symptoms of GOR, although the results were consistent with a substantial reduction, no effect, or even an increase in symptoms associated with cisapride treatment compared with placebo. In this review there was evidence of substantial funnel plot asymmetry which may be explained by publication bias. There was also statistical heterogeneity between the trials that may be related to variation in study quality. For these reasons, the results are uncertain and should be interpreted with caution. Finally, this review has highlighted the paucity of randomised controlled trial information for such a widely prescribed drug. However, due to the potential for serious adverse events, large randomised trials of cisapride that have long‐term follow up are unlikely to be conducted.
Implications for research.
The literature search did not find sufficient trials to fulfil all the objectives of this review. Due to the restricted use of cisapride since July 2000, a larger study to determine the effectiveness of cisapride for children with GOR is no longer possible.
What's new
| Date | Event | Description |
|---|---|---|
| 12 October 2010 | Review declared as stable | Review no longer being updated due to stability of evidence. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 26 May 2010 | Amended | Plain language summary revised |
| 2 March 2010 | New search has been performed | Updated, one new study included, conclusions not changed. |
| 5 February 2010 | New citation required but conclusions have not changed | Review declared stable. |
| 5 November 2009 | Amended | Converted to new review format. |
| 13 June 2006 | New search has been performed | Minor update new studies sought but none found |
| 5 April 2002 | New search has been performed | New studies found and included or excluded |
Notes
All authors contributed to the overall design of the review and the development of the protocol for study inclusion and data extraction.
Acknowledgements
We thank Professor Geoffrey P Davidson for providing additional information for one study trial; Dr Billy Bourke, Professor B Drumm and Dr Jaqui Dalby‐Payne for their comments on the first draft of the original review; Susan Orenstein for her comments on a subsequent version and for a personal communication about the results of her unpublished study; and Melissa Harden for assisting in the search for unpublished trials from the pharmaceutical company Janssen.
Appendices
Appendix 1. MEDLINE search strategy
1. randomised controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. humans.sh. 11. 9 and 10 12. exp esophageal motility disorders/ 13. exp esophagitis/ 14. esophagitis.tw. 15. oesophagitis.tw. 16. exp gastroesophageal reflux/ 17. (gastro?esophageal adj5 reflux).tw. 18. (gastro esophageal adj5 reflux).tw. 19. (gastro oesophageal adj5 reflux).tw. 20. infantile reflux.tw. 21. regurgitat$.tw. 22. GORD.tw. 23. GERD.tw. 24. GER.tw. 25. exp proton pump inhibitors/ 26. (proton adj3 pump adj3 inhibitor$).tw. 27. PPI$.tw. 28. exp omeprazole/ 29. omeprazole.tw. 30. (lansoprazole or lanzoprazole).tw. 31. pantoprazole.tw. 32. rabeprazole.tw. 33. exp Histamine h2 antagonists/ 34. h2 receptor antagonist$.tw. 35. exp cimetidine/ 36. exp ranitidine/ 37. exp famotidine/ 38. exp nizatidine/ 39. cimetidine.tw. 40. ranitidine.tw. 41. famotidine.tw. 42. nizatidine.tw. 43. (prokinetic or prokinetics).tw. 44. metoclopramide.tw. 45. exp metoclopramide/ 46. domperidone.tw. 47. exp domperidone/ 48. bethanechol.tw. 49. exp bethanechol/ 50. exp cisapride/ 51. cisapride.tw. 52. (Acenalin or Alimix or Arcasin or Cisaprid or Prepulsid or Propulsid or Propulsin or Propulsit or R 51 619 or R 51,619 or R 51619 or R51619 or Risamol).tw. 53. or/12‐24 54. or/25‐49 55. 53 or 54 56. or/50‐52 57. 55 and 56 58. exp infant, newborn/ 59. exp infant/ 60. exp child/ 61. exp adolescent/ 62. infan$.tw. 63. child$.tw. 64. neonat$.tw. 65. newborn$.tw. 66. pediatric$.tw. 67. paediatric$.tw. 68. juvenile$.tw. 69. (young adj3 people).tw. 70. youth$.tw. 71. adolescen$.tw. 72. or/58‐71 73. 57 and 72 74. 11 and 73 75. limit 74 to yr="2005‐2009"
Data and analyses
Comparison 1. Main analysis for cisapride versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 'Worse, same or slight improvement' versus 'moderate or excellent improvement' | 7 | 236 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.10, 1.19] |
| 2 Mean daily regurgitations | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐4.10, 2.30] |
| 3 Mean daily weight gain | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐7.65, 7.05] |
| 4 Reflux index | 5 | 176 | Mean Difference (IV, Random, 95% CI) | ‐6.49 [‐10.13, ‐2.85] |
| 5 Adverse events | 4 | 190 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.88, 3.93] |
Comparison 2. Main analysis for cisapride versus Gaviscon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 'Worse, same or slight improvement' versus 'moderate or excellent improvement' | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 3.26 [0.93, 11.38] |
Comparison 3. Main analysis for cisapride versus dietary interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 'Worse, same or slight improvement' versus 'moderate or excellent improvement' | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Carob bean: mean daily regurgitations | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.71, 3.31] |
| 3 Carob bean: mean daily weight gain | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐2.12, 7.32] |
| 4 Corn syrup: mean daily regurgitations | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐3.11, 2.51] |
| 5 Corn syrup: mean daily weight gain | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐10.46, 3.06] |
Comparison 4. Sensitivity analyses for cisapride versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Best case scenario | 8 | 302 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.54] |
| 2 Worst case scenario | 7 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.18, 2.65] |
| 3 Change in outcome definition: 'any symptoms' vs 'no symptoms' at end of treatment | 6 | 168 | Odds Ratio (M‐H, Random, 95% CI) | 0.19 [0.08, 0.44] |
4.1. Analysis.
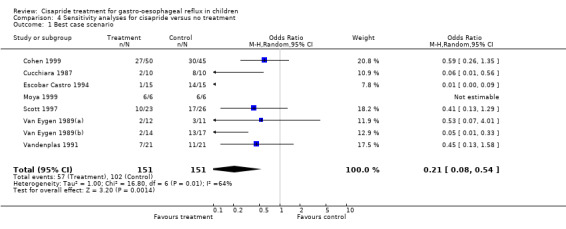
Comparison 4 Sensitivity analyses for cisapride versus no treatment, Outcome 1 Best case scenario.
4.2. Analysis.
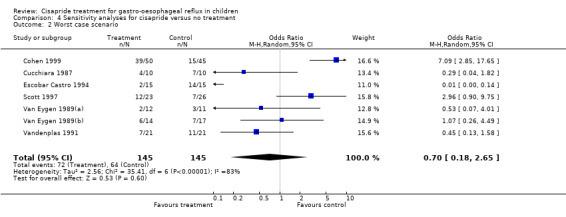
Comparison 4 Sensitivity analyses for cisapride versus no treatment, Outcome 2 Worst case scenario.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cohen 1999.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Age < 36 months Clinical diagnosis of GOR: frequent V or R often associated with feeding difficulties and/or excessive crying. Baseline 24‐h oesophageal pH monitoring: RI ≥5% OR GOR score (Euler and Byrne) ≥50. Exclusion criteria: anatomic abnormality of the GI tract, previous GI surgery, treatment with anticholinergics, theophylline, other diagnosis which could explain vomiting. | |
| Interventions | 2 weeks of either: cisapride suspension (1mg/ml) 0.2 mg/kg qid (n=50*) placebo (n=45*) | |
| Outcomes | Parental evaluation at 2 weeks:
overall symptom intensity on VAS 0‐10 cm (0=absence of symptoms, 10= could not be worse)
improvement (marked=complete or near complete resolution of symptoms, moderate=partial resolution, minimal=slight improvement, unchanged, deterioration).
Evaluation during the 2 weeks of treatment:
presence of vomiting, gagging, crying (score 0‐3).
AE: any, withdrawals due to AE.
Investigator assessment at 2 weeks:
24‐h oesophageal pH
oesophagitis at biopsy. Data (means, SD) also obtained for complications of GOR (apnoea, wheezing, nocturnal cough, haematemesis) and weight gain (Davidson 2009). |
|
| Notes | 68 participants (38, 30) completed the trial. Withdrawn if: consent was withdrawn, serious AE, further investigations necessitated a change in treatment. A high proportion of participants had received prior treatment with: thickened feeds, positional therapy, cisapride, H2 antagonists, antacids, metoclopramide, other. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Further details obtained from one of the trial investigators (GD) by one of the review authors (SM). Randomisation was centrally controlled (outside of the three participating centres), by computer generated code (Davidson 2009). |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Unclear risk | "matched placebo (both provided by Janssen Research Foundation, Belgium)" p288. Further details not given. |
Cucchiara 1987.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Age 75 days ‐ 47 months Reflux oesophagitis in all (endoscopy and biopsy). Diagnosis of GOR made by oesophageal pH (pH<4 for ≥20min) and manometry. Exclusion criteria: infections, neurologic, metabolic, renal disorders, abnormalities of the GI tract. | |
| Interventions | 8 weeks of either: cisapride syrup (1mg/ml) 0.3 mg/kg tid or placebo syrup. | |
| Outcomes | Assessed at 8 weeks by investigator: 24‐h pH LOS pressure oesophagitis at biopsy. Improvement at end of treatment: cured (clinical, pH‐metric and histological variables normalised), improved (at least one of the three variables had improved), unchanged, worsened. | |
| Notes | 3 participants were withdrawn: 2 febrile URTI, 1 failed to take drug continuously. Other outcomes measured: peristalsis amplitude, clinical score. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | "placebo syrup, which was identical to cisapride in taste and appearance" p454. Further details not given. |
Escobar Castro 1994.
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | Age 3 months ‐ 5 yrs V and R present GOR at oesophageal pH monitoring (RI <3.5% considered normal). No organic pathology to justify the reflux. | |
| Interventions | 4 weeks of either: cisapride 0.2 mg/kg (n=15) placebo (n=15). | |
| Outcomes | Assessment at 2 and 4 weeks (probably by parents) of digestive symptoms: severe (R and/or V after each meal of an important part of the meal), moderate (R of a small quantity more than once a day), mild (R of a very small quantity once a day or sometime during the week), absent. Investigator assessment at 4 weeks: 24‐h oesophageal pH, AE, complications. | |
| Notes | 1 drop‐out in the cisapride group ('lack of motivation'). Other outcomes measured: radiological image, endoscopy, respiratory symptoms improvement (nil, slight, good, excellent). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | Details not given. |
Greally 1992.
| Methods | Randomised study Double‐blind design was not feasible (see text for details). | |
| Participants | Age 2‐18 months Chronic vomiting and GOR confirmed by 24h pH oesophageal monitoring (pH<4 for ≥5% of the recording period). No neurological, respiratory, metabolic, GI disease, treatment with H2 antagonists, theophylline, anticholinergic drugs. | |
| Interventions | 4 weeks of either: cisapride p.o. 0.2 mg/kg qid (n=26) Gaviscon 1/2 sachet to each 90 ml feed qid (n=24, 21 also had Carobel). | |
| Outcomes | Parental evaluation at 4 weeks of improvement (improved, not improved). Investigator evaluation at 4 weeks of 24‐h pH (RE was defined as pH<4 for ≥15min). | |
| Notes | All 50 infants completed the study. Other outcomes measured at 4 weeks: daily parental evaluation of severity of V: 0 (absent), 1(1‐4 episodes/day), 2 (>4 episodes/day), leading to a final symptoms score (range 0‐1); improvement in diary scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Double‐blind design was not feasible (see text of review for details). |
Levy 2001.
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | Age 6 months ‐ 4 years (mean age 14.4 months) Minimum 3 months of symptomatic GOR with failure to respond to at least 6 weeks of non‐surgical treatment other than cisapride. | |
| Interventions | 3‐8 weeks of either cisapride 0.6 mg/kg/day or placebo. | |
| Outcomes | Data on QTc retained in 4 (68 participants) of 7 study centres (134 participants in total) in the trial. 19/68 excluded as ECGs recorded after 8 weeks of treatment. Mean QTc reported at 3‐8 weeks of treatment and mean difference in QTc from baseline. | |
| Notes | Data on symptoms of GOR not published "because efficacy results did not reach statistical significance". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | "blindly assigned to receive either placebo or cisapride" p459. Further details not given. |
Moya 1999.
| Methods | Randomised controlled trial, unblinded | |
| Participants | Age 1‐4 months, total 26 infants No previous illness, with frequent regurgitation (>5 regurgitations per day). Exclusion criteria: receiving antireflux medication, breast fed. |
|
| Interventions | Two weeks of either: no treatment˜original formula (n= 6) carob bean gum with formula (n=8) corn syrup with formula (n=6) cisapride with formula (n=6). |
|
| Outcomes | Outcomes recorded by a daily diary by parents up to day 15 Mean daily number of regurgitations Mean daily weight gain. No information on how daily means were calculated. |
|
| Notes | Cisapride dosage unclear ‐ only noted as “usual dosage in 3 divided doses". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | High risk | Blinding not feasible, due to nature of different interventions (see above). |
Orenstein 2000.
| Methods | Multicentre, randomised controlled trial | |
| Participants | 96 children > 2 months old with biopsy‐proven oesophagitis. | |
| Interventions | 1. cisapride 0.2 mg/kg qid and placebo 2. cimetidine 10 mg/kg qid and placebo 3. cisapride + cimetidine 4. placebo + placebo. | |
| Outcomes | Measured at 2, 6 and 12 months. Initial findings reported for 2 months. 1.Symptoms measured by parents using the Infant Gastroesophageal Reflux Questionnaire 2. Vomiting 3. Crying 4. Biopsy measuring histological parameters of papillary height and basal layer thickness | |
| Notes | Trial finished but only published as an abstract. Personal communication provided by Dr SR Orenstein, Academic Hospital of Pittsburgh, USA (Orenstein 2009). Cisapride versus no cisapride: symptom questionnaire showed 86% with cisapride were well or better at 2 months compared with 70% for no cisapride groups (if 3 lost to follow up in cisapride group and 8 given no cisapride were assumed to have worse symptoms). No significant improvement in vomiting (frequency or volume). This paper was never published in article form as cisapride was taken off the market in the US amid concerns about its toxicity. 100 infants were enrolled, and nearly all of them completed the 12 months of the protocol, although follow up was discontinued for the last few infants in concordance with the wishes of the FDA and the company. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "randomised, double‐blind in blocks of 8" pA20. Further details not given. |
| Allocation concealment? | Unclear risk | Unclear ‐ details not given |
| Blinding? All outcomes | Unclear risk | "blindly assigned to 1 of 4 arms", "At any visit with both Sx & Bx unimproved, infants were rescued to un‐masked Cm‐Cs, without un‐masking prior Rx." P0791. Further details not given. |
Scott 1997.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Age 6 weeks ‐ 2 years Daily R or V during a 1 week baseline period AND ≥1 episode of GOR (pH<4 for >20min) at 18‐h pH monitoring. Exclusion criteria: not meeting the inclusion criteria, premature, previous GI surgery (excluding for appendicitis), illnesses and drugs that could interfere with cisapride, reflux due to known anatomic abnormalities, underlying disease, infection of the GI tract; parents who couldn't express concern, comply with study, complete diaries. | |
| Interventions | 6 weeks of positioning and thickened feeds (where appropriate) and either: cisapride suspension (1mg/ml) 0.2 mg/kg qid (n=23) or placebo suspension (n=26). | |
| Outcomes | Assessed at 2, 4, 6 weeks by parent and investigator: global evaluation of condition on VAS 0‐100 mm (0=the worst it's ever been, 100=completely recovered); any AE, specific AE. Assessed at 6 weeks by parent and investigator: global evaluation of overall treatment (deterioration=symptoms worse, poor=no improvement, fair=slight improvement, persistence of some symptoms, good=improvement, occasional symptoms, excellent=complete relief of symptoms). Assessed at 6 weeks by investigator: 24‐h pH, LOS pressure, oesophagitis at biopsy. | |
| Notes | 45 participants (21 cisapride, 24 placebo) were evaluated (4 were non‐compliant or had violated protocol). Other outcomes: various at 24‐h pH, swallow pressure, daily diary recording of each episode (none, mild, moderate, severe), score for R and V. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "randomisation code for the allocation of patients to cisapride or placebo suspension was generated by computer" (p501). |
| Allocation concealment? | Low risk | Adequate |
| Blinding? All outcomes | Unclear risk | "matching suspension of placebo" p500. "The double‐blind code was to be broken only in the event of an emergency." p501. Further details not given. |
Van Eygen 1989(a).
| Methods | Three trials: I open trial (n=69) II (Van Eygen 1989a) randomised, double‐blind, placebo‐controlled trial (n=23) III (Van Eygen 1989b) dose‐response trial (n=50). | |
| Participants | Age 5 days ‐ 12 months Excessive R or V at least twice a day in all children. In trial II: GOR at radiology or pH monitoring in all children. In trial III: GOR at radiology, endoscopy or pH monitoring in 16 children. Non‐pharmacologic measures (e.g. positioning, food thickening) had failed to improve the reflux. | |
| Interventions | 4 weeks of either Trial II: cisapride oral suspension 0.15 mg/kg tid (n=12) placebo oral suspension (n=11). Trial III: cisapride 0.1 mg/kg tid (n=14) (not used in the analysis) cisapride 0.2 mg/kg tid (n=14) placebo tid (n=17). | |
| Outcomes | Assessed at 2 and 4 weeks by the investigator: AE, global therapeutic result (poor=no change, fair=distinct but slight improvement, good=marked reduction in R, excellent=virtually complete symptomatic cure). | |
| Notes | In trial III: analysis based on 45 of 50 participants. There were 4 early drop‐outs and 1 protocol violation and a further 10 drop‐outs (4 in the cisapride 0.2 mg/kg group and 6 in the placebo group. Other outcomes assessed at 2 and 4 weeks by investigator: severity of R (severe=the major part of the meal is R, moderate=effortless R of a mouthful of feeding, slight=R of rather excessive saliva only, no R); frequency of R (after each meal, at least twice a day, once a day or several times a week, never). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | "under double‐blind conditions, the medications being identical in appearance and taste" p670. Further details not given. |
Van Eygen 1989(b).
| Methods | Trial III referred to above. Please see above for details. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | "under double‐blind conditions, the medications being identical in appearance and taste" p670. Further details not given. |
Vandenplas 1991.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Age 2 ‐ 4 months Pathological GOR of V and R for > 2 weeks, >6 times/day AND abnormal oesophageal pH monitoring. Exclusion criteria: reflux secondary to diseases (e.g. infections, allergy, pyloric stenosis). | |
| Interventions | 13‐16 days of positional therapy and either: cisapride (1 mg/ml) 0.2 mg/kg qid (n=21) or placebo (n=21). | |
| Outcomes | Parental evaluation of GOR severity at 2 weeks: 0 (no V at all), 1 (1‐3 episodes of V or R/day), 2 (4‐6 episodes of V or R/day), 3 (>6 episodes of V or R/day). NB: all had grade 3 at the beginning. Investigator evaluation at 2 weeks: 24‐h pH. | |
| Notes | None of the infants received milk‐thickening products. 29 completed the study, 13 exclusions post‐randomisation: unexpected weaning (3,3), withdrawal of permission for second pH monitoring because symptoms had improved (4,1), lack of improvement and parents refused to continue (0,2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Details not given. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? All outcomes | Unclear risk | "Both products (cisapride, 1mg/ml, and placebo) were prepared in the same way and could not be recognised by taste or aspect." p45. "The randomisation code was broken after the second pH monitoring" p45. Further details not given. |
* Number of participants in brackets represents number randomised to respective treatment group. AE = adverse events, GI = gastrointestinal, GOR = gastro‐oesophageal reflux, LOS = lower oesophageal sphincter, R = regurgitation, RE = reflux episode, RI = reflux index (percentage of time in 24 h during which pH < 4), URTI = upper respiratory tract infection, V = vomiting, VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnett 2001 | Study participants were preterm neonates. |
| Cucchiara 1990 | Cisapride given intravenously in a single dose over 5 minutes. No mention of study being randomised. |
| Enriquez 1998 | Cisapride was administered prophylactically during the introduction of enteral feeding by nasogastric tube to preterm infants of less than 33 weeks gestation at birth. |
| Heine 1996 | The outcomes reported by this study (i.e. drooling) are not relevant to the review. |
| McClure 1999 | The outcomes reported in this study (i.e. half gastric emptying time, whole gastrointestinal transit time) are not relevant to the review. Cisapride was prescribed for clinically diagnosed gastro‐oesophageal reflux or poor feed tolerance in very preterm infants (less than 32 weeks of gestation). |
| Pezzati 2001 | Study participants were preterm neonates less than 34 weeks gestation. Cisapride was administered for feed intolerance by naso‐gastric tube. |
| Reddy 2000 | Preterm neonates less than 34 weeks gestation. Cisapride administered prophylactically during the introduction of enteral feeding. |
| Saye 1987 | All infants and children were suspected of having GOR (only 5 of them had digestive symptoms) and cisapride treatment was given for only 16 hours. |
Contributions of authors
All review authors contributed to the design of the study, the development of the protocol, the writing of the original version of this review. All authors, apart from CA, contributed to the 2009 update.
Sources of support
Internal sources
Systematic Reviews Training Unit, UK.
Professor Stuart Logan was supported in this work by funding through PenCLAHRC, the NIHR Collaboration for Leadership in Applied Health Research and Care of the South West Peninsula, UK.
External sources
No sources of support supplied
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Cohen 1999 {published and unpublished data}
- Cohen RC, O'Loughlin EV, Davidson GP, Moore DJ, Lawrence DM. Cisapride in the control of symptoms in infants with gastroesophageal reflux. A randomized, double‐blind, placebo‐controlled trial. Journal of Pediatrics 1999;134:287‐92. [DOI] [PubMed] [Google Scholar]
- Davidson GP. Personal communication [Clarification of trial characteristics and additional data]. Meeting 14.09.99.
Cucchiara 1987 {published data only}
- Cucchiara S, Staiano A, Capozzi C, Lorenzo C, Boccieri A, Auricchio S. Cisapride for gastro‐oesophageal reflux and peptic oesophagitis. Archives of Disease in Childhood 1987;62:454‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Escobar Castro 1994 {published data only}
- Escobar Castro H, Bettas Ferrero G, Suarez Cortina L, Camarero Salces C, Lima M. Efficacy of cisapride in the treatment of gastroesophageal reflux (GER) in children. Evaluation of a double blind study [Efectividad del Cisapride en el tratamiento del reflujo gastroesofagico (R.G.E.) en ninos. Valoracion de un estudio a doble ciego]. Anales Espanoles de Pediatria 1994;40(1):5‐8. [Google Scholar]
Greally 1992 {published data only}
- Greally P, Hampton FJ, MacFadyen UM, Simpson H. Gaviscon and Carobel compared with cisapride in gastro‐oesophageal reflux. Archives of Disease in Childhood 1992;67:618‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2001 {published data only}
- Levy J, Hayes C, Kern J, Harris J, Flores A, Hyams J, et al. Does cisapride influence cardiac rhythm? Results of a United States multicenter, double‐blind, placebo‐controlled pediatric study. Journal of Pediatric Gastroenterology and Nutrition 2001;32:458‐63. [DOI] [PubMed] [Google Scholar]
Moya 1999 {published data only}
- Moya M, Juste M, Cortes E, Auxina A, Ortiz L. Clinical evaluation of the different therapeutic possibilities in the treatment of infant regurgitation. [Valoracion clinica de las distintas posibilidades terapeuticas en el manejo de las regurgitaciones del lactante]. Revista Espanola de Pediatria 1999;55(3):219‐23. [Google Scholar]
Orenstein 2000 {published and unpublished data}
- Orenstein SR. Personal communication [Additional data and details regarding completion of the study]. Email 11.06.09.
- Orenstein SR, Shalaby TM, Frankel EA, Kelsey SF. Cisapride, cimetidine, both or neither for infantile esophagitis: symptomatic and histologic results of 2‐months randomized, double‐blind, placebo‐controlled therapy in 100 babies. Gastroenterology 2000;118(4):A20. [Google Scholar]
- Orenstein SR, Shalaby TM, Kelsey SF, Frankel E. Natural history of infant reflux esophagitis: symptoms and morphometric histology during one year without pharmacotherapy. American Journal of Gastroenterology 2006;101(3):628. [DOI] [PubMed] [Google Scholar]
- Orenstein SR, Shalaby TM, Kelsey SF, Frankel EA. Cimetidine, cisapride, both or neither for infant esophagitis: symptomatic response to 6m randomized, double blind, placebo‐controlled therapy in 100 babies. Gastroenterology 2004;126 Suppl 2(4):A508. [Google Scholar]
- Orenstein SR, Shalaby TM, Kelsey SF, Frankel EA. Cimetidine, cisapride, both or neither for infantile esophagitis: symptomatic response to 4m randomized, double‐blind, placebo‐controlled therapy in 100 babies [Abstracts: Poster session abstracts]. Journal of Pediatric Gastroenterology and Nutrition 2004;39 Suppl 1:P0791. [Google Scholar]
Scott 1997 {published data only}
- Scott RB, Ferreira C, Smith L, Jones AB, Machida H, Lohoues MJ, et al. Cisapride in pediatric gastroesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition 1997;25:499‐506. [DOI] [PubMed] [Google Scholar]
Van Eygen 1989(a) {published data only}
- Eygen M, Ravensteyn H. Effect of cisapride on excessive regurgitation in infants. Clinical Therapeutics 1989;11(5):669‐77. [PubMed] [Google Scholar]
Van Eygen 1989(b) {published data only}
- Eygen M, Ravensteyn H. Effect of cisapride on excessive regurgitation in infants. Clinical Therapeutics 1989;11(5):669‐77. [PubMed] [Google Scholar]
Vandenplas 1991 {published data only}
- Vandenplas Y, Roy C, Sacre L. Cisapride decreases prolonged episodes of reflux in infants. Journal of Pediatric Gastroenterology and Nutrition 1991;12:44‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barnett 2001 {published data only}
- Barnett CP, Omari T, Davidson GP, Goodchild L, Lontis R, Dent J, et al. Effect of cisapride on gastric emptying in premature infants with feed intolerance. Journal of Paediatrics and Child Health 2001;37:559‐63. [DOI] [PubMed] [Google Scholar]
Cucchiara 1990 {published data only}
- Cucchiara S, Staiano A, Boccieri A, Stefano M, Capozzi C, Manzi G, et al. Effects of cisapride on parameters of oesophageal motility and on the prolonged intraoesophageal pH test in infants with gastro‐oesophageal reflux disease. Gut 1990;31:21‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enriquez 1998 {published data only}
- Enriquez A, Bolisetty S, Patole S, Garvey PA, Campbell PJ. Randomised controlled trial of cisapride in feed intolerance in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79:F110‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heine 1996 {published data only}
- Heine RG, Catto‐Smith AG, Reddihough DS. Effect of antireflux medication on salivary drooling in children with cerebral palsy. Developmental Medicine and Child Neurology 1996;38:1030‐6. [DOI] [PubMed] [Google Scholar]
McClure 1999 {published data only}
- McClure RJ, Kristensen JH, Grauaug A. Randomised controlled trial of cisapride in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1999;80:F174‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pezzati 2001 {published data only}
- Pezzati M, Dani C, Biadaioli R, Gambi B, Lachina L, Rubaltelli FF. Randomised controlled trial of the effect of cisapride on the pyloric muscle in preterm infants. European Journal of Pediatrics 2001;160:572‐5. [DOI] [PubMed] [Google Scholar]
Reddy 2000 {published data only}
- Reddy PS, Deorari AK, Bal CS, Paul VK, Singh M. A double‐blind placebo‐controlled study on prophylactic use of cisapride on feed intolerance and gastric emptying in preterm neonates. Indian Pediatrics 2000;37:837‐44. [PubMed] [Google Scholar]
Saye 1987 {published data only}
- Saye ZN, Forget P, Geubelle F. Effect of Cisapride on gastroesophageal reflux in children with chronic bronchopulmonary disease: A double‐blind cross‐over pH‐monitoring study. Pediatric Pulmonology 1987;3:8‐12. [DOI] [PubMed] [Google Scholar]
Additional references
Breckenridge 2000
- Breckenridge A. Suspension of cisapride (Prepulsid) licences: Product withdrawal. Committee on Safety of Medicines, London 2000.
Colson 1990
- Colson DJ, Campbell CA, Wright VA, Watson BW. Predictive value of oesophageal pH variables in children with gastro‐oesophageal reflux. Gut 1990;31:370‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucchiara 1996
- Cucchiara S, Campanozzi A, Greco L, Franco MT, Emiliano M, Alfieri E, et al. Predictive value of esophageal manometry and gastroesophageal pH monitoring for responsiveness of reflux disease to medical therapy in children. American Journal of Gastroeneterology 1996;91(4):680‐5. [PubMed] [Google Scholar]
Davidson 2009
- Davidson GP. Personal communication [Clarification of trial characteristics and additional data]. Meeting 14.9.99.
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMEA 2002
- European Medicines Agency. Committee for proprietary medicinal products (CPMP) opinion following and article 31 referral. Cisapride. EMEA/CPMP/24844/02. http://www.emea.europa.eu/pdfs/human/referral/cisapride/2484402en.pdf London 7 October 2002. Accessed 26/01/10.
Hampton 1990
- Hampton FJ, MacFayden UM, Simpson H. Reproducibility of 24 hour oesophageal pH studies in infants. Archives of Diseases in Childhood 1990;65:1249‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henney 2000
- Henney JE. Withdrawal of troglitazone and cisapride. JAMA 2000;283:2228. [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Klausner 1998
- Klausner MA. Jansen Pharmaceutical Research Foundation. Important safety and efficacy information. http://pharminfo.com/medwatch/mwrpt50.html (accessed June 2000) 1998.
Nelson 1997
- Nelson SP, Chen EH, Syniar GM, Kaufer Christoffel K. Prevalence of symptoms of gastroesophageal reflux during infancy. Archives of Pediatrics and Adolescent Medicine 1997;151:569‐72. [DOI] [PubMed] [Google Scholar]
Orenstein 2009
- Orenstein SR. Personal communication [Additional data and details regarding completion of the study]. Email 11.06.09.
Rudolph 1996
- Rudolph CD. Gastroesophageal Reflux. In: Rudolph AM, Hoffman JIE, Rudolph CD editor(s). Rudolph's Pediatrics. Stamford, Connecticut: Appleton and Lange, 1996:1058‐60. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Shulman 2000
- Shulman RJ, Boyle JT, Colletti RB, Friedman R, Heyman MB, Kearns G, et al. The North American Society for Pediatrics Gastroenterology and Nutrition. An updated medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2000;31(2):232‐3. [DOI] [PubMed] [Google Scholar]
Treem 1991
- Treem WR, Davis PM, Hyams JS. Gastroesophageal reflux in the older child: presentation, response to treatment and long‐term follow‐up. Clinical Pediatrics 1991;30(7):435‐40. [DOI] [PubMed] [Google Scholar]
Vandenplas 1993
- Vandenplas Y, Ashkenazi A, Belli D, Boige N, Bouquet J, Cadranel S, et al. A proposition for the diagnosis and treatment of gastro‐oesophageal reflux disease in children: a report from a working group on gastro‐oesophageal reflux disease. European Journal of Pediatrics 1993;152:704‐11. [DOI] [PubMed] [Google Scholar]
Vandenplas 1999
- Vandenplas Y, Belli DC, Benatar A. The role of cisapride in the treatment of pediatric gastroesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition 1999;28:518‐28. [DOI] [PubMed] [Google Scholar]
Varty 1993
- Varty K, Evans D, Kapila L. Paediatric gastro‐oesophageal reflux: prognostic indicators from pH monitoring. Gut 1993;34:1478‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 1999
- Ward RM, Lemons JA, Molteni RA. Cisapride: a survey of the frequency of use and adverse events in premature newborns. Pediatrics 1999;103:469‐72. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gilbert RE et al
- Gilbert RE, Augood C, MacLennan S, Logan S. Cisapride treatment for gastro‐oesophageal reflux: a systematic review of randomized controlled trials. Journal of Paediatrics and Child Health 2000;36:525‐9. [DOI] [PubMed] [Google Scholar]


